
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Neurosci. , 17 August 2022
Sec. Auditory Cognitive Neuroscience
Volume 16 - 2022 | https://doi.org/10.3389/fnins.2022.858576
Auditory stimuli, encompassing a continually expanding collection of musical genres and sonic hues, present a safe and easily administrable therapeutic option for alleviating cognitive deficits associated with neuropsychological disorders, but their effects on executive control are yet to be completely understood. To better understand how the processing of certain acoustic properties can influence conflict processing, we had a large of cohort of undergraduate students complete the Stroop colour and word test in three different background conditions: classical music, white noise, and silence. Because of pandemic guidelines and the necessity to run the experiment remotely, participants also completed the Wisconsin card sorting test (WCST), so that the reliability and consistency of acquired data could be assessed. We found that white noise, but not classical music increased the response time difference between congruent (low conflict) and incongruent (high conflict) trials (conflict cost), hence impairing performance. Results from the WCST indicated that home-based data collection was reliable, replicating a performance bias reported in our previous laboratory-based experiments. Both the auditory stimuli were played at a similar intensity, thus their dissociable effects may have resulted from differing emotional responses within participants, where white noise, but not music elicited a negative response. Integrated with previous literature, our findings indicate that outside of changes in tempo and valence, classical music does not affect cognitive functions associated with conflict processing, whilst white noise impairs these functions in a manner similar to other stressors, and hence requires further research before its implementation into neuropsychiatric care.
The ability to efficiently process conflicting information is a core feature of executive control (Miller and Cohen, 2001; Mansouri et al., 2020b), and is believed to recruit a wide network of prefrontal regions (Botvinick et al., 2001; Kerns et al., 2004; Mansouri et al., 2017b,2022; Mansouri and Buckley, 2018). The colour-word matching variant of the Stroop test offers an easily reproducible method of assessing an individual’s ability to separate information pertaining to colour and word meaning (Stroop, 1935; MacLeod and MacDonald, 2000; Zysset et al., 2001). Deficits in Stroop performance have been observed in several psychiatric disorders (Bannon et al., 2002; Lansbergen et al., 2007; Kravariti et al., 2009; Joyal et al., 2019), which suggests impaired conflict processing may underlie some of their symptoms. Current treatment options for these conditions are limited, consisting primarily of psychotherapy or pharmacological interventions, which generally have poor efficacy rates (Thase, 2007; Kirsch et al., 2008; Fournier et al., 2010; Stafford et al., 2015; Cuijpers et al., 2018), waning compliance (Velligan et al., 2009; Semahegn et al., 2020), and/or a myriad of potential debilitating side effects (Henderson, 2008; Serretti et al., 2013; Hilt et al., 2014; Stafford et al., 2015). Therefore, it is evident there is a need for additional treatment options that are both safe, and easily administrable to alleviate the cognitive deficits associated with these conditions. Changing the context in which information processing takes place provides a relatively simple avenue for potentially modulating performance in various cognitive domains. Background auditory stimuli, ranging from various genres of music to differing power spectrums of noise signals (sonic hues), are some of the most commonly explored contextual factors, yet their effects on cognitive processes remain inconclusive, with all of positive (Miller and Schyb, 1989; Day et al., 2009; Manan et al., 2012; Masataka and Perlovsky, 2013; Rausch et al., 2014; Proverbio et al., 2015; Angwin et al., 2017; Feizpour et al., 2018; Othman et al., 2019), null (Kämpfe et al., 2010; Wais and Gazzaley, 2011; Jing et al., 2012; Bottiroli et al., 2014; Jäncke et al., 2014; Herweg and Bunzeck, 2015; Kou et al., 2017; Lehmann and Seufert, 2017; Burkhard et al., 2018; Fehring et al., 2019b), and negative (Brodsky, 2001; Furnham and Strbac, 2002; Cassidy and MacDonald, 2007; Dobbs et al., 2011; Jing et al., 2012; Brodsky and Slor, 2013; Masataka and Perlovsky, 2013; Proverbio et al., 2015; Musliu et al., 2017; Feizpour et al., 2018; Cloutier et al., 2020) outcomes having been reported in cognitive task performance for healthy populations. Underlying these differences may be significant variation in study designs (between-subject or within-subject), presented stimuli, and cognitive tasks. Significant research is necessary to understand these distinctions, and thus determine their suitability for use within neuropsychiatric care.
One of the foremost difficulties in studying music is its conceptual broadness, which has particularly expanded in the past century with the rapid development of new musical styles and genres. From a quantifiable perspective, music can be differentiated from other sounds by the pitch, loudness, and timbre of its constituent tones, and the ordered timing (rhythms) in which these tones are arranged (Roederer, 2008). In a more psychological context, music has been considered to relate to appetitive urges, consummatory expression, drive, and satisfaction; allowing the communication of these bodily states and information among members of the same species through the modulation of each other’s emotional states (Dewey, 1958). Research into music’s effects on physiology has since identified that the valence (e.g., joyous/smooth, sad/harsh) and tempo (slow, fast) of songs can induce dissociable effects in measures of emotional state, and related cortical activation (Blood et al., 1999; Schmidt and Trainor, 2001; Carpentier and Potter, 2007; Arjmand et al., 2017; Mansouri et al., 2017a). Accordingly, behavioural studies have centred on describing how these emotional alterations can affect cognitive task performance, generally making comparisons between different music conditions (e.g., positive vs. negative valence, slow vs. fast tempo), and to silence. Although such research has proven insightful, fewer behavioural studies have included other, task-irrelevant sounds in their designs, where those having done so have often reported contrasting outcomes depending on the relevant task (Furnham and Strbac, 2002; Bottiroli et al., 2014), or musical genre/type (Cassidy and MacDonald, 2007; Bottiroli et al., 2014; Proverbio et al., 2015). Thus, whilst it is well established that music can alter an individual’s emotional state (and subsequently their higher cognitive function, albeit with various uncertainties/task-dependencies) through variation in valence and tempo, it remains unclear if there exists dissociable effects on executive control, when compared to silence, between music and other non-musical sounds. Considering results from previous studies have differed between genres, classical music could be considered suitable for continuing such investigations, as tempo and valence can vary considerably between songs, it is often non-lyrical, and contains a wide array of rhythmic patterns and melodies. Whilst classical genres have been extensively used in behavioural research (particularly in investigating the “Mozart effect”), most studies use only select songs with an identifiable valence or tempo, rather than a varied/expansive playlist. Therefore, we postulated that by playing a wide and varied collection of classical songs, any emotional alterations produced from the valence and/or tempo of songs would fluctuate, and hence not significantly affect overall performance. Inclusion of another task-irrelevant, non-musical sound in our design would then allow further understanding of the interaction between executive control and the perception of music.
Outside of music, one of the most commonly used auditory stimuli in neuroscience research is white noise, which consists of sound at every frequency of the human hearing rage (20 Hz–20 kHz) played at equal intensities. Its use within the field primarily stems from the phenomenon of stochastic resonance, where moderate levels of random noise can improve information transfer and processing in man-made and naturally occurring non-linear systems (Moss et al., 2004; McDonnell and Abbott, 2009). In the context of neural processing, it was originally found the addition of white noise at moderate levels could improve the detection of proprioceptive, tactile, and visual stimuli (Manjarrez et al., 2007; Lugo et al., 2008). Electroencephalography (EEG) examination subsequently revealed that white noise can improve neural synchronisation within and between brain regions, expanding to regions typically distinguished from sensory processing such as the superior frontal gyrus and posterior cingulate cortex (Ward et al., 2010). Behavioural research has since shown that low (50–60 dB) and moderate (70–80 dB) levels of white noise can improve performance in auditory working memory and semantic memory tasks (Manan et al., 2012; Rausch et al., 2014; Herweg and Bunzeck, 2015; Angwin et al., 2017; Othman et al., 2019), with functional magnetic resonance imaging (fMRI) taken during performance of these tasks finding increased blood oxygen level dependent (BOLD) signal in various cortical, midbrain, and brainstem regions (Manan et al., 2012; Rausch et al., 2014; Othman et al., 2019). However, performance in other cognitive domains has failed to replicate these benefits, with outcomes in visual working memory, set-shifting, and phonemic fluency tasks unchanged (Bottiroli et al., 2014; Herweg and Bunzeck, 2015), or even impaired (Herweg and Bunzeck, 2015). Moreover, investigations in school children have found improvements only in those diagnosed with attention-deficit/hyperactivity disorder (ADHD) and not healthy comparisons (Söderlund et al., 2007, 2010, 2016). These contrasting findings suggest any cognitive facilitation provided by white noise may be sensitive to both differences between tasks, and study cohorts.
Considering white noise is beginning to be implemented into healthcare and research environments for a variety of purposes (Patterson-Kane and Farnworth, 2006; Farokhnezhad Afshar et al., 2016), including in patients suffering from neuropsychological disorders (Kaneko et al., 2013; Lin et al., 2018), the factors underlying these distinctions must be better understood. One outstanding question is whether processes underlying executive control can benefit from white noise, where there exist contrasting findings between working memory tasks (Manan et al., 2012; Bottiroli et al., 2014; Herweg and Bunzeck, 2015; Othman et al., 2019), hence highlighting a need for a greater variety of executive functions to be tested in its presence. Because improvements from white noise have indicated relatively small effect sizes (Manan et al., 2012; Rausch et al., 2014; Herweg and Bunzeck, 2015; Angwin et al., 2017; Othman et al., 2019), it is also necessary to consider the sensitivity of related outcome measures in conducting such testing. The Stroop test, which under the umbrella of conflict processing assesses cognitive flexibility, response inhibition, and selective attention (Grandjean et al., 2012; Scarpina and Tagini, 2017), as well as containing sensitive outcome measures such as conflict cost and adaptation (Gratton et al., 1992; Botvinick et al., 1999, 2004; Mayr et al., 2003; Carter and van Veen, 2007; Mansouri et al., 2017b,2022), satisfies these criteria, and as such may be appropriate for better understanding the parameters of white noise’s influence on executive control.
Due to pandemic-related social distancing orders, we had to reformat our planned experiment to a home-based study, which has remained a relatively unexplored area in psychophysics research (Semmelmann and Weigelt, 2017). For accessibility to participants, we decided to use a well-established and reliable platform for online behavioural and cognitive testing paradigms, PsyToolkit, which has been developed by a researcher within the field (Stoet, 2010, 2016). Whilst our change to the platform was forced due to the COVID pandemic, the use and advancement of remote testing within psychophysics may prove beneficial for mobility-limited or geographically isolated individuals, such as the elderly or those living outside major metropolitan areas. The primary concern surrounding these external platforms is the reliability of sensitive measures such as response time, and adherence to experimental protocols and procedures. Accounting for these factors, we included an analogue of the Wisconsin card sorting test (WCST) into our design, where a response time bias to colour-matching over shape-matching has been consistently reported in previous studies (Mansouri et al., 2020a,b). Because both version of the WCST (laboratory-based and home-based) included both colour and shape matching, and the two cohorts were similar in background (age, education), we could compare these biases between studies to assess the sensitivity and reliability of remote data acquisition.
Therefore, to further explore the effects exerted by background acoustic stimuli within the context of conflict processing, we designed a three-session, two-staged, repeated-measures experiment using both the Stroop test and WCST (Figure 1). In each session, participants were to perform the tests in the presence of a varied and expansive classical playlist, white noise, or silence. Both tests were readily available for use and modification through the PsyToolkit experiment library. The inclusion of a pre-post design allowed for the consideration of within-session learning effects, which have been reported in previous psychophysical studies (Fehring et al., 2019a,2022), and may even be modulated by contextual factors during conflict processing (Fehring et al., 2019b). The order in which background acoustic stimuli were presented was counterbalanced to offset any influence of across-session learning, and the order of task performance was also counterbalanced to negate any potential advantage or disadvantage of performing one test before the other.
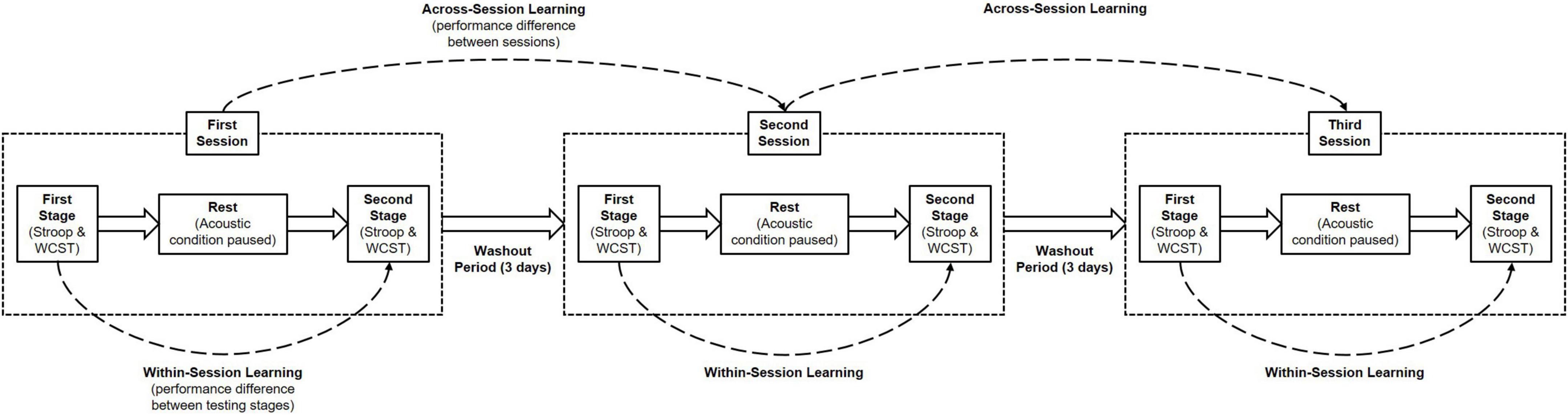
Figure 1. Experimental protocol for assessing the effects of background acoustic conditions on cognitive functions. Participants performed both the Stroop test and Wisconsin card sorting test in three separate daily sessions, each separated by a 3-day washout period. Within a daily session there were two testing stages, where each test was completed once over, separated by a ten-minute rest period. A different background acoustic condition was played in each daily session. This protocol allows for the assessment of within-session learning (the behavioural changes occurring between the first and second testing stages in the same daily session). The order of both background acoustic conditions and task performance was counterbalanced across the three daily sessions, accounting for the influence of across-session learning.
In using classical music and white noise, our study design encompassed a great breadth of acoustic properties; from complex rhythmic combinations and intricate melodies, to a random distribution of sound within each frequency of the Human hearing range (20Hz-20kHz), and hence their investigation may serve as a foundation for enquiry into the higher-order cognitive effects of more nuanced acoustic stimuli. This design compliments current literature regarding background auditory stimuli and the Stroop test, which have centred around certain musical types (high/low tempo, positive/negative valence) and more naturalistic noises (a range of traffic, office, and voice-related sounds) (Cassidy and MacDonald, 2007; Masataka and Perlovsky, 2013). Due to such significant differences between the stimuli, we hypothesised that they may differentially affect participant’s conflict processing capabilities. In particular, we expected white noise may impair Stroop performance, given the sensitivity of the tests outcome measures, combined with the neutral (Bottiroli et al., 2014; Herweg and Bunzeck, 2015) and negative (Herweg and Bunzeck, 2015) results reported in other visual-based tasks assessing aspects of executive control. Overall, we aimed to further elucidate the interaction between auditory processing and executive control processes, alongside helping to explore new effective, safe, and well-tolerated treatments for cognitive deficits associated with neuropsychological disorders.
Sixty-seven Monash University undergraduate students (45 females, 22 males) chose to participate in the project as part of their coursework. A priori power analysis was performed based on the effect size observed in our previous studies in which we examined the effects of background acoustic conditions (music) in the context of cognitive tasks (Feizpour et al., 2018). With the significance level of 0.05 and the power at 0.80, the estimated sample size for this study was 61 participants (using G*Power 3.1; Faul et al., 2007). However, 67 participants allowed for full counterbalancing and maintenance of an adequate sample in the circumstance some participants could not complete their data collection. Participants were instructed to complete the Stroop test and WCST at home (during the COVID-related lockdown period in 2020) in three different background acoustic conditions, each separated by at least three days. The order of acoustic conditions and cognitive tests (Stroop/WCST) was counterbalanced. We also aimed to counterbalance the number of males and females in each condition; however, due to the uneven sex ratio we could not achieve a perfect equilibrium. All participants were between the ages of 18 and 29 (21.06 ± 1.82; mean ± standard error), with similar educational level (third-year University science students), and had no history of any neurological disorders, nor any medical conditions that may interfere with performing the tests or listening to the acoustic stimuli (checked by a screening questionnaire). Approval was obtained from the Monash University Human Research Ethics Committee. Informed consent was obtained from all participants.
To facilitate remote testing, we used PsyToolkit, a free web-based service that can be edited to run various cognitive tests and accessed from participants’ personal computers (Stoet, 2016). The programs within PsyToolkit use an efficient Linux-based scripting language which allows for millisecond timing precision, as is required when recording response times in cognitive tests (Stoet, 2010). This online platform for behavioural testing has been developed and validated by neuroscientists (Stoet, 2010, 2016).
Participants were provided with clear instructions (explanatory written documents for setting the hardware, software, and two online tutorial sessions) which detailed how they should prepare for and conduct the two tasks. This included keeping time of day (which was the quietest possible time), distance from their monitor, and mouse position constant, the intensity level they should use among acoustic conditions, turning off mobile phones, closing other programs, and alerting any household members they are not to be disturbed during the session. After completing each task, participants were instructed to copy the resulting data output into a provided spreadsheet, and at the end of their three testing sessions, they sent this spreadsheet via email to investigators. A detailed explanation of how the experiment and related classes were run can be found in Jaberzadeh and Mansouri (2021).
Each of the three daily sessions required participants to complete both tests twice, with a 10-min break between their repetitions (Figure 1). Participants were told they could pause the current acoustic condition and have a drink of water or juice (no caffeinated or alcoholic beverages) during this break period. As a whole, sessions should have lasted no longer than one hour, with 70 Stroop trials and 60 WCST trials required for completion each time. Information regarding the PsyToolkit WCST can be found in the Supplementary material (Appendix 1) and Supplementary Figure 1.
The PsyToolkit variant of the Stroop test (Figure 2) is mostly analogous to the standard colour-word matching Stroop test. Trials begin with the presentation of a white cross against a black background, which after 300 ms is replaced by the name of a colour printed in coloured ink. Participants are instructed to respond to the print colour, which can be the same (congruent) or different (incongruent) to the colour name, by pressing the corresponding key on their keyboard. These keys are “R” for red, “Y” for yellow, “G” for green, and “B” for blue. Feedback is delivered to participants by the appearance of a grey box, with text consisting of “CORRECT” or “WRONG.” This textbox lasts for 500 ms, before the commencement of the following trial. If participants fail to respond within 2,000 ms following word presentation, their response is recorded as a “timeout.” Participants performed 70 trials each testing stage; 15–30% of these trials were congruent (low congruent), and 70–85% were incongruent (high conflict).
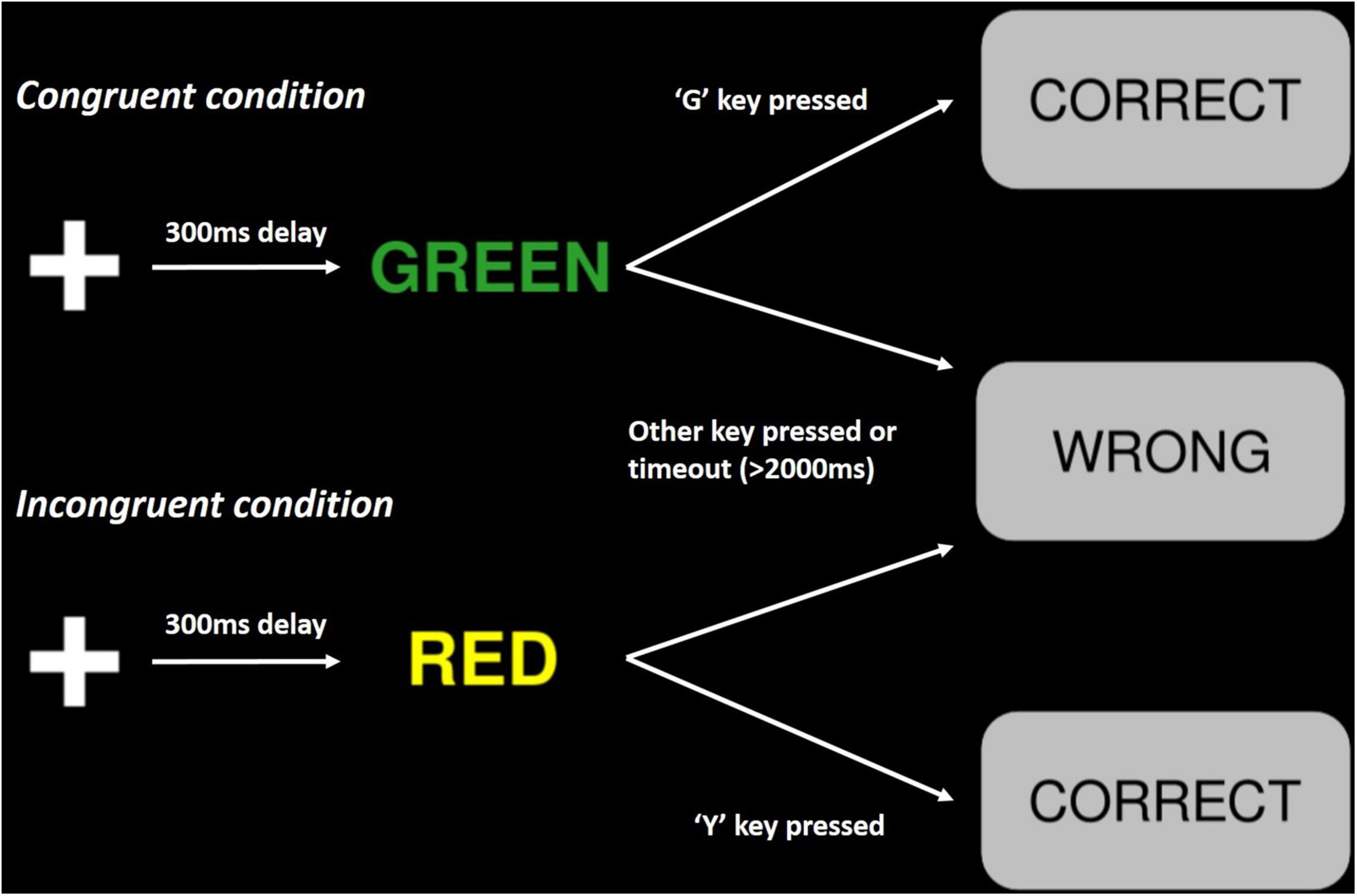
Figure 2. Schematic representation of the experimental paradigm in the PsyToolkit Stroop test. Trials begin with on the onset of a white cross, followed by the presentation of a colour name printed in coloured ink. A grey text box displaying either “CORRECT” or “WRONG” indicates a correct or incorrect response, respectively (Stoet, 2010, 2016).
Participants were provided with links to download the required acoustic conditions and therefore all participants listened to the same set of songs and white noise. They were instructed to play these songs or files by their preferred medium, whether earphones, headphones, or speakers, at a moderate intensity where they could hear the stimuli clearly, but not have it bother them; similar to how they would normally listen to music. It was also emphasised that this intensity level should remain constant between the two stimuli (music and white noise), and that their attention remain solely on the cognitive task. There was no particular sound level set across participants, but instead participants were instructed (both in the preceding classes and written instruction) to listen to both acoustic stimuli at a level similar to that they would listen to music; a moderate intensity where they can hear the stimuli clearly without being annoyed by loud sound. It is important to consider some individuals may be accustomed to listening to music and other auditory stimuli at higher intensities than others, and therefore it may be beneficial to have participants choose their own definition of moderate. In our previous laboratory-based experiments, we have also allowed participants to adjust volume levels to their preference (Mansouri et al., 2017a; Feizpour et al., 2018; Fehring et al., 2019) because some participants found pre-set intensities to be too loud or inaudible. Setting a particular sound intensity for all participants might lead to non-specific effects, such as being annoyed by loud sound, and become a confounding factor.
For the music condition, a free online playlist, via archive.org (a non-profit digital library) was provided; “100 Classical Music Masterpieces.” This playlist was chosen as it includes predominantly instrumental pieces dating from 1685 to 1928, with no distinct preference to a given valence or tempo between songs. A full list of the included songs can be found in the Supplementary material (Appendix 3). For the white noise condition, a MP3 audio file, which encoded 2 h of continuous white noise (20–20 kHz, same intensity throughout), was provided to participants. This file was generated using Audacity, a free open-source audio editor and recorder.
Repeated-measures ANOVAs were used to assess the effects of practice, trial type (conflict level or rule), background acoustic condition, and sex on various behavioural measures. Within-session practice-related learning could be assessed due to the two-stage design, where each test was completed twice per session. Including Practice and Sex factors into the repeated-measures ANOVAs was necessary considering they have both been found to influence performance in several cognitive tasks (Mansouri et al., 2016b; Feizpour et al., 2018; Fehring et al., 2019a,2021, 2022), and may also interact differentially with background acoustic conditions (Feizpour et al., 2018). For the Stroop test, response time was measured as the time from presentation of the colour name to the registration of keyboard input. For the WCST, response time was measured as the time from trial onset to registration of a mouse click on one of four target items. The timeout thresholds for Stroop and WCST trials were 2,000 and 10,000 ms, respectively, therefore all response time data were limited to these ranges. Implementing arbitrary procedures for removing outlying data points may bias results and outcome of statistical analyses, thus we included all data points in the statistical analyses. Considering the variance in mean response time between participants, and in order to ease comparison of testing stages and acoustic conditions, response time data was normalised by dividing each value by the grand average for all conditions in each individual. This normalisation procedure has been implemented in previous behavioural studies (Mansouri et al., 2016a,2017a,2020a; Fehring et al., 2019b,a, 2022). Response accuracy for both tests was calculated as the percentage of correct trials (correct responses/total responses), and was analysed without any normalisation. Normalisation was not required as there was a high level of accuracy (majority > 80%) and consistency across all stages and conditions in both tests.
Three participants, however, were excluded from analyses. One was taking prescribed psychoactive medication and reported feeling ill during two sessions, whilst for the other two the results were incomplete. Therefore, in total results from 64 participants, 43 females and 21 males, were included in the analyses. Using repeated-measures ANOVAs, including predominantly within-subject factors, reduces the effects of non-specific factors such as sleep level, food-drink, and emotional or motivational state. The order of cognitive tasks and acoustic conditions were counterbalanced to control for any confounding effect of across-session learning or its interaction with other factors.
Sphericity was examined (Mauchly’s test) for all ANOVA measures and if violated, Greenhouse-Geisser correction was implemented. Significance level was set at 0.05 for all statistical tests. For significant effects, partial eta squared (ηp2) is reported, which indicates the proportion of variance explained by the effect in the ANOVA analysis. Where significant interactions were detected, pairwise comparisons were conducted. Pairwise comparisons consisted of a two-tailed t-test with Bonferroni adjustment for multiple comparisons.
Within-session practice-related learning, differing levels of conflict, and acoustic environment may interactively affect cognitive control, and consequently performance in cognitive tasks. To examine the interaction between these factors and conflict processing, a multifactorial repeated-measures ANOVA was applied to the mean normalised response time and mean percentage of correct trials. The ANOVA included Practice (first/second stage), Conflict (congruent/incongruent), and Sound (music/noise/silence) as within-subject factors, and Sex (female/male) as a between-subject factor.
A multifactorial repeated-measures ANOVA [Practice (first/second stage of testing, within-subject factor) × Conflict (congruent/incongruent trials, within-subject factor) × Sound (music/noise/silence, within-subject factor) × Sex (female/male, between-subject factor)] applied to mean normalised response time revealed a significant main effect of Practice [F(1,64) = 78.86; p < 0.001, ηp2 = 0.56]: response time decreased from the first to second stage of testing within the same session (within-session learning). The main effect of Conflict was highly significant [F(1,64) = 74.10; p < 0.001, ηp2 = 0.54]: response time was longer in incongruent trials (Figure 3A). Importantly, the main effect of Sound was not significant [F(2,128) = 1.10; p = 0.34]: background acoustic condition did not influence overall response time. There was, however, a significant interaction between Conflict and Sound factors, [F(2,128) = 3.23; p = 0.043, ηp2 = 0.050], indicating that the background acoustic condition differentially affected response time depending on the level of conflict encountered (Figure 4A). We also performed the same multi-factorial repeated-measures ANOVA on the raw (non-normalised) response time values. Similar to the results from the normalised data, there were significant main effects for Practice [F(1,64) = 61.89; p < 0.001, ηp2 = 0.50] and Conflict [F(1,64) = 57.81; p < 0.001, ηp2 = 0.48], and a significant interaction between Conflict and Sound factors [F(2,128) = 3.15; p = 0.046, ηp2 = 0.048], whilst the main effect of Sound was not significant [F(2,128) = 1.01; p = 0.37].
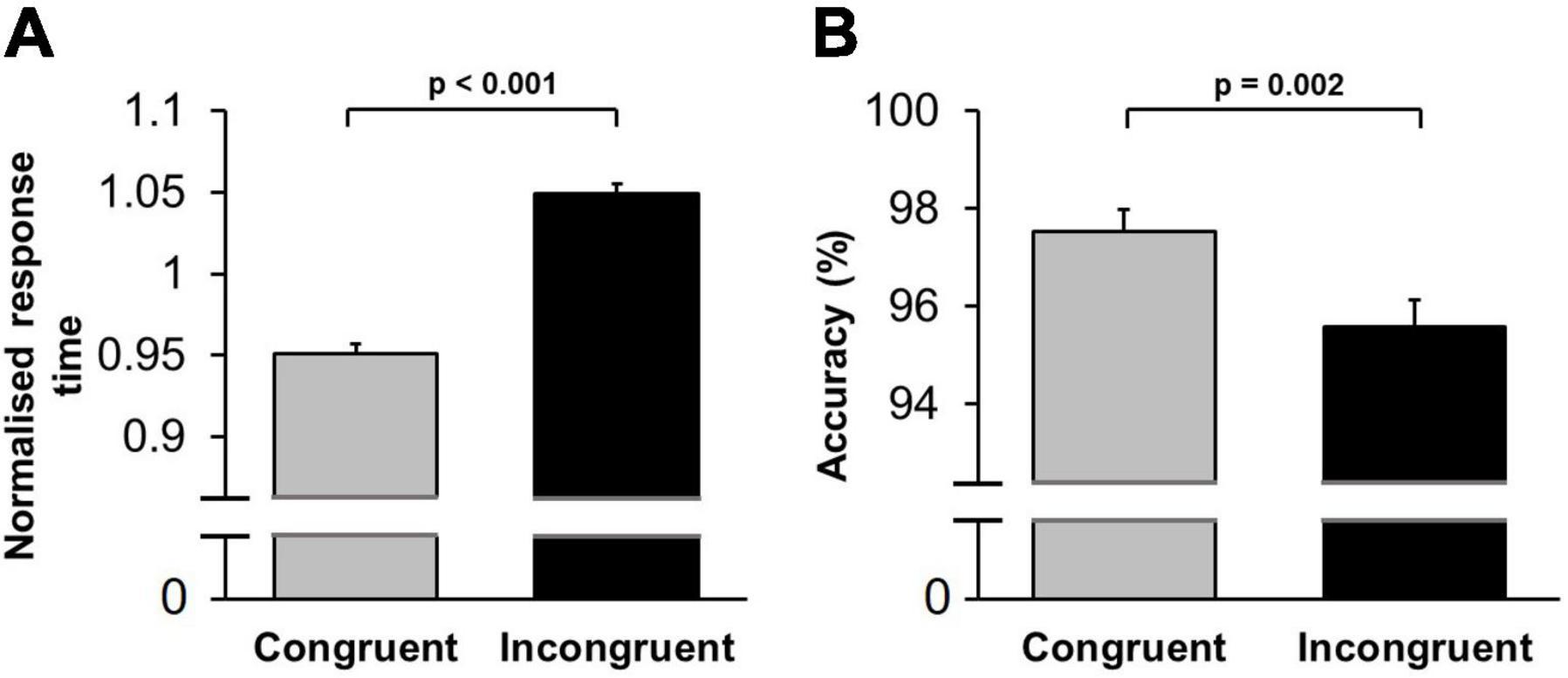
Figure 3. Conflict-induced behavioural adjustment in the Stroop test. (A) Mean normalised response time is shown for congruent and incongruent trials. Response time was significantly shorter in low conflict (congruent trials), compared to high conflict (incongruent trials). (B) The mean percentage of correct responses (accuracy) is shown for congruent and incongruent trials. Accuracy was significantly higher in congruent trials compared to incongruent trials. Error bars represent standard error of the mean (SEM).
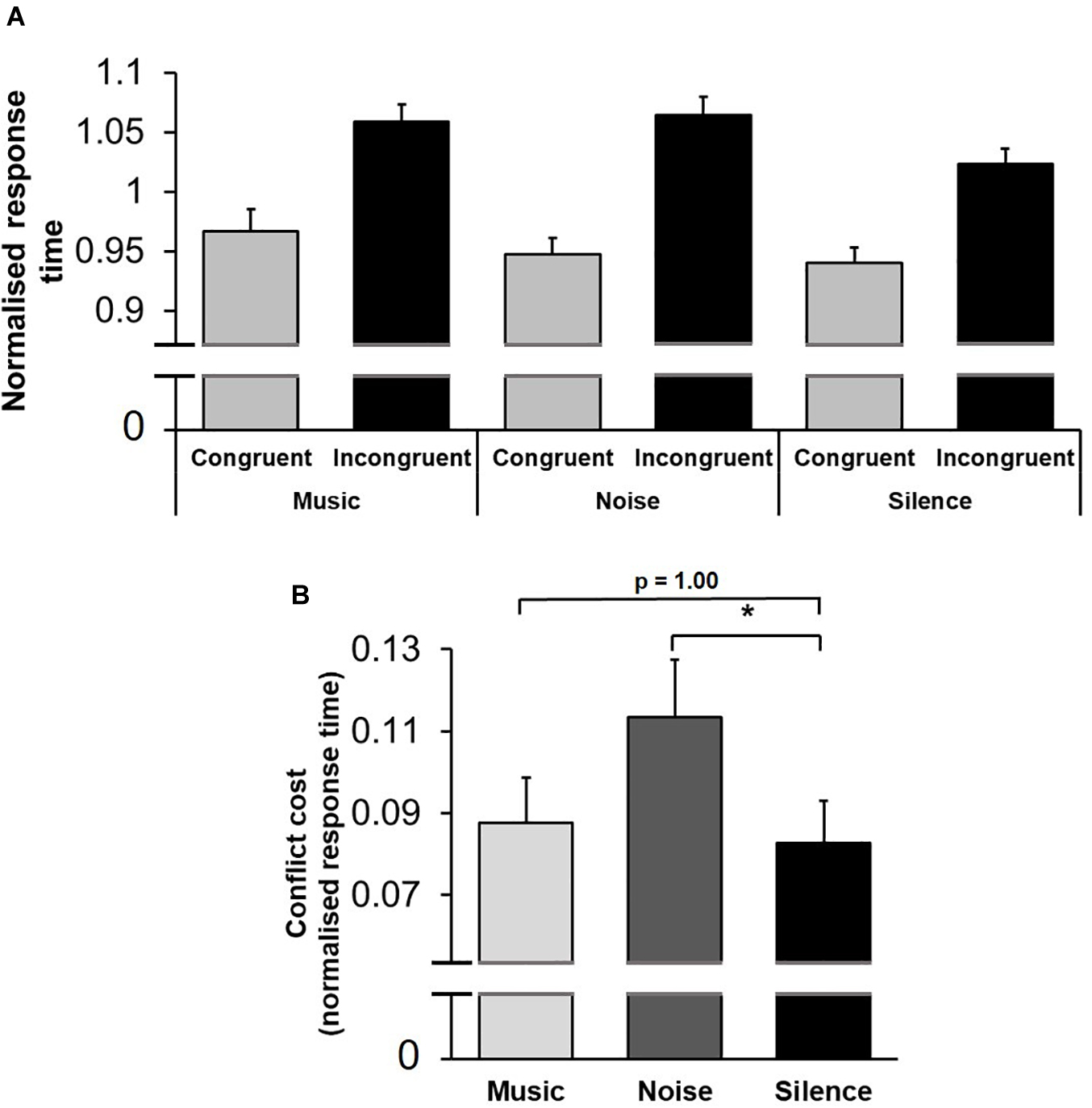
Figure 4. Conflict cost was modulated by the background acoustic condition. (A) Mean normalised response time is shown for congruent and incongruent trials in each background acoustic condition. The acoustic environment modulated the conflict-induced alterations in response time. (B) The difference in mean normalised response time between congruent and incongruent trials (conflict cost) is shown for each background acoustic condition. Compared to silence, white noise increased the conflict cost. *represents p < 0.05. Error bars represent standard error of the mean (SEM).
Conflict cost refers to the difference in performance between congruent and incongruent trials, reflecting the higher processing demands for resolving conflict between competing options (Botvinick et al., 2004; Carter and van Veen, 2007; Mansouri et al., 2017b). To further investigate the interaction of Conflict and Sound factors, conflict cost was calculated by subtracting mean response time in congruent trials from those in incongruent trials for each sound condition. In a planned comparison, the conflict cost for music and noise conditions were contrasted with that of silence in separate pairwise comparisons (paired two-tailed t-test with Bonferroni adjustment for multiple comparisons). The conflict cost was not significantly different between the music and silence conditions [t(63) = 0.38; p = 1.00]; however, conflict cost was significantly different between noise and silence conditions (t(63) = 2.52; p = 0.029]. This indicates that the conflict cost in the presence of white noise was increased (Figure 4B). The same paired two-tailed t-tests performed using the conflict cost calculated from non-normalised response time values returned similar results, where the difference between music and silence was not significant [t(63) = 0.0052; p = 1.00], whilst the difference between noise and silence was significant [t(63) = 2.67; p = 0.019].
The same multifactorial repeated-measures ANOVA [Practice (first/second stage of testing, within-subject factor) × Conflict (congruent/incongruent trials, within-subject factor) × Sound (music/noise/silence, within-subject factor) × Sex (female/male, between-subject factor)] applied to mean percentage of correct trials showed an insignificant main effect of Practice [F(1,64) = 0.41, p = 0.52, ηp2 = 0.007]: accuracy did not change between the first and second testing stages of each session. Meanwhile, the main effect of Conflict was significant [F(1,64) = 11.00, p = 0.002, ηp2 = 0.15]: accuracy was lower in incongruent trials (Figure 3B). The main effect of Sound was not significant [F(2,128) = 0.54; p = 0.58]: background acoustic condition did not change accuracy. The interaction between Sound and Conflict factors was also not significant [F(2,128) = 13.68; p = 0.56], indicating that accuracy in both conflict conditions was equally unaffected by the background acoustic condition.
The effects of conflict on performance are not limited to the current trial in which the conflict is experienced, but can also be observed in the subsequent trial, where a behavioural improvement can occur if the subject is presented with a high level of conflict again. This conflict-induced behavioural change is known as conflict adaptation (Gratton et al., 1992; Botvinick et al., 1999; Mayr et al., 2003; Mansouri and Buckley, 2018; Mansouri et al., 2022). In the context of the Stroop test, it can be examined through contrasting incongruent trials that were immediately preceded by another incongruent trial (ii sequence) to those preceded by a congruent trial (ci sequence). Therefore, to detect if conflict adaptation was evident in this study, and if acoustic environment modulated its effect, we applied a repeated-measures ANOVA to mean normalised response time in the second trial of each sequence (ci/ii). The multifactorial repeated-measures ANOVA [Practice (first/second stage of testing, within-subject factor) × Trial Sequence (ci/ii, within-subject factor) × Sound (music/noise/silence, within-subject factor) × Sex (female/male, between-subject factor)] applied to mean normalised response time in the second trial of each sequence revealed a significant main effect of Trial Sequence [F(1,64) = 6.33; p = 0.014, ηp2 = 0.093]: response time was shorter in ii sequences. Notably, the interaction between Sound and Trial Sequence factors was not significant [F(2,128) = 0.76; p = 0.47, ηp2 = 0.012], indicating that acoustic environment did not influence conflict adaptation. Performing an identical multifactorial repeated-measure ANOVA using non-normalised response time values returned alike results, where the main effect of Trial Sequence was significant [F(1,64) = 6.017, p = 0.017, ηp2 = 0.088], and the interaction between Sound and Trial factors was insignificant [F(2,128) = 0.71; p = 0.49, ηp2 = 0.011].
In this study, participants performed both Stroop test and the WCST. Inclusion of the WCST allowed for the assessment of reliability and sensitivity of the measurements in remotely collected data by comparison of results with those obtained in our laboratory-based studies (Mansouri et al., 2020a). One of the consistent findings in our laboratory-based testing with a computerised WCST is that young adults show a significant behavioural bias to colour matching over shape matching, which appears as a shorter response time and higher accuracy in the colour-matching blocks (Mansouri et al., 2020a,b). Therefore, to examine whether this bias could also be detected in home-based studies, we applied a multifactorial repeated-measures ANOVA to mean normalised response time in correct trials and mean percentage of correct trials. The ANOVA included Practice (first/second stage), Rule (colour/shape/number), and Sound (music/noise/silence) as within-subject factors, and Sex (female/male) as a between-subject factor. We found that the previously reported dimensional bias in laboratory settings was replicated in this cohort tested using an online platform (Supplementary Figures 2A,B). For further information about these results, please see the Supplementary material (Appendix 1).
The aim of this study was to investigate how potential cognitive effects produced by background music and white noise may influence conflict processing. Our findings indicate that in the context of the Stroop test, white noise, but not classical music significantly affected the conflict cost (the difference in response time between congruent and incongruent trials), whereby participant’s ability to resolve conflict was impaired (Figure 4B). These results provide important reference for previous behavioural studies assessing both background music and noise (Furnham and Strbac, 2002; Cassidy and MacDonald, 2007; Bottiroli et al., 2014; Proverbio et al., 2015), where even when a large, varied playlist of complex music is employed, thus reducing the influence of emotionally salient properties within songs such as valence and tempo, and noise is made to be stochastic (white noise), there still exists a dissociable influence between the stimuli on a core component of executive control (conflict processing) when compared to silence. Remarkably, such findings are also homologous with a previous study in a non-human primate species (macaque monkeys) where similar, dissociable task-dependent effects between classical music and white noise were reported (Zarei et al., 2019; Supplementary material Appendix 2). Additionally, because of the necessity to run the experiment remotely, we assessed the reliability of remote data collection by comparing rule-based performance differences in the WCST between the present (home-based), and one of our previous laboratory-based studies. In the home-based study, we found a significant bias toward the colour dimension over shape, which replicates consistent observations made in our laboratory-based experiments with a comparable version of the WCST (Supplementary Figures 2A,B). Below, we discuss the implications of these findings, and how they can help to clarify outstanding questions in the current literature.
By using a varied playlist of classical music and white noise, we sought to investigate how music and white noise may differentially affect executive control when compared to silence. In the Stroop test, background music did not significantly alter participant’s response time or accuracy, nor did it modulate the conflict cost or conflict adaptation effects. This absence of effect contrasts white noise, which increased the conflict cost, indicating that the processing of each stimuli lead to dissociable effects that became apparent when participants had to resolve conflict (incongruent trials). Considering individuals have been shown to consistently rank various noises lower in scales of emotional preference compared to musical excerpts (Gomez and Danuser, 2004), it is possible that a negative emotional state was induced by the white noise, but not the classical music. This suggestion is supported by previous findings in the Stroop test where a dissonant rendition of a Mozart piece, but not the original version, caused a similar increase in conflict cost (Masataka and Perlovsky, 2013). In the case of music, its unique or pleasurable acoustic properties, where an ordered arrangement of different harmonic tones exist (Roederer, 2008), may have prevented such an impairment, by which individuals failed to be concerned or distressed by its presence. Alternatively, the range of valences and tempos present throughout the classical playlist may have resulted in continuing, contrasting alterations to their emotional state (Schmidt and Trainor, 2001; Carpentier and Potter, 2007; Arjmand et al., 2017; Mansouri et al., 2017a), which subsequently summed to no overall effect. In either circumstance, our findings do not support the idea that the perception and processing of classical music can modulate conflict processing.
Despite failing to provide clear recommendations for therapeutic use, our results may help to reconcile earlier comparisons made between music and other non-musical sounds in the context of cognitive task performance. The dichotomy in the effects of listening to the classical playlist and white noise suggests music, in its most general form, can be considered less detrimental to cognitive performance compared to other non-musical sounds. Previous reports, where music has been claimed to equally impair performance compared to noise (Furnham and Strbac, 2002), may instead be explained by emotional modulation related to the tempo and valence of chosen songs, which in the aforementioned study was ‘garage’ music of high intensity/tempo, and the subsequent influence of these modulations on processes involved with performing the cognitive task. This is supported by findings where high tempo music and noise produce near parallel (negative or neutral) effects in task performance, but low tempo music conversely facilitates performance (Cassidy and MacDonald, 2007; Proverbio et al., 2015). However, the directionality of effects induced by these aspects of music on cognitive performance has yet to be consistently established, with studies reporting opposing effects in response to high tempo (Brodsky, 2001; Day et al., 2009; Brodsky and Slor, 2013) and low tempo (Cassidy and MacDonald, 2007; Proverbio et al., 2015; Cloutier et al., 2020) conditions, whilst definitions of valence have varied considerably between studies (e.g., consonant vs. dissonant intervals, major vs. minor chords/scales, positive vs. negative lyrical content).
Moreover, it remains unclear if inter-individual differences may modulate these effects, where sex (Jing et al., 2012; Feizpour et al., 2018) and personality type (Furnham and Strbac, 2002; Dobbs et al., 2011) have been found to differentiate music’s influence in several cognitive tasks. Thus, there is still significant effort to be expended in determining how music can be applied to improve executive control. Despite our results showing classical music has no effect on conflict processing, future studies may consider extending our findings to other, more nuanced musical genres, particularly modern forms of music which can depart significantly from classical composition. It is also crucial to better understand the task-dependent and inter-individual differences between studies assessing changes in tempo and valence, particularly by further uncovering their neural substrate and parameters surrounding their relevant emotional changes.
In using the Stroop test, we hoped to gain insight into whether white noise can affect performance in a visual-based task assessing a core component of executive control (conflict processing). We found that white noise increased the conflict cost, therefore indicating its continuing presence diminished the ability of participants to perform the relevant functions involved in resolving conflict, which involves a network of prefrontal regions (Botvinick et al., 2001; Kerns et al., 2004; Mansouri et al., 2017b,2022; Mansouri and Buckley, 2018). At first, it may be considered that any task-irrelevant information, such as environmental sound, might act as an extra-task distracting feature, engaging parts of the limited cognitive resources that could otherwise be directed toward the current task (Beaman and Jones, 1998; Jones et al., 1999; Elliott and Cowan, 2005; Kerzel and Schönhammer, 2013). However, the failure of music, which was also task-irrelevant and played at the same intensity, to cause similar impairments instead suggests other mechanisms may be responsible.
The prefrontal cortex is highly sensitive to stress (Arnsten, 2009), where despite short-lived levels potentially ameliorating its functioning (Hartley and Adams, 1974; Fehring et al., 2019b), prolonged exposure to negative emotional stimuli, alongside public speaking tasks, has been found to significantly decrease prefrontal activation and impair performance in several of its functional domains (Dolcos and McCarthy, 2006; Alexander et al., 2007; Luethi et al., 2009; Qin et al., 2009). Continuous exposure to loud levels of white noise (≥ 85 dB) has also been used as an acute stressor for behavioural studies in both humans and monkeys, where it has been found to impair higher cognitive function in a manner consistent with observations from other stress-inducing stimuli (Arnsten and Goldman-Rakic, 1998; Hillier et al., 2006; Banis and Lorist, 2012). Whilst such findings may primarily stem from the intensity of the stimulus, it has also been reported that individuals consistently rank various noises (played at a moderate level) lower in terms of emotional preference compared to musical excerpts (Gomez and Danuser, 2004), hence it is likely that continued exposure to moderate levels of white noise might also lead to higher levels of stress and a negative emotional state, albeit to a lesser degree than that observed at higher intensity levels. Subsequently, when considering cognitive task performance and moderate levels of white noise, it is possible there may exist a ‘trade off’ between facilitation caused by stochastic resonance, and impairment arising from a negative response to its presence, where the balance depends not only on the involved brain regions, but also on different factors such as the mode of stimulus presentation and task difficulty.
In this context, distinctions between working memory tasks, where performance involving auditory, but not visual stimuli is improved by white noise (Manan et al., 2012; Bottiroli et al., 2014; Herweg and Bunzeck, 2015; Othman et al., 2019), may arise from the crossmodal nature of stochastic resonance, through which it is posited white noise’s ability to improve tactile, visual, and proprioceptive processing originates from facilitation in multisensory regions such as superior colliculus and posterior parietal cortex (Manjarrez et al., 2007; Lugo et al., 2008). Whereas the linear facilitation in auditory processing regions and upstream activity may be sufficient to overcome, or be greater in magnitude than any impairment resulting from a negative response to the white noise, its more indirect influence on visual processing and subsequent functions may not be of the same degree, and hence fail to significantly improve performance. Findings in healthy children, where performance remained unchanged in an auditory working memory task during white noise exposure (Söderlund et al., 2016), may initially appear to contradict this reasoning, however, such failure may instead likely result from their continuing auditory development, where the ability to accurately separate acoustic stimuli, alongside narrowing of frequency sensitivities, do not reach completion until teenage years (Maxon and Hochberg, 1982; Trehub et al., 1989; Werner, 2007).
Regarding task difficulty, animal and human studies have consistently found that during periods of stress, behavioural responses switch from involving adaptive, albeit slow processes originating from higher order regions such as the prefrontal cortex, to reflexive and rapid processing utilising primarily subcortical regions (Murphy et al., 1996; Lupien et al., 1997; Elliott and Packard, 2008; Luethi et al., 2009). Therefore, the degree by which a given cognitive task requires flexible behaviour and novel decision making, as opposed to repetitive, previously learnt, or solely reflexive responding may also contribute to whether performance is inhibited, or facilitated by exposure to white noise. Previous findings, where performance in tasks that assess an individual’s ability to form stimulus associations or recall previously displayed stimuli is improved by the presence of white noise (Rausch et al., 2014; Herweg and Bunzeck, 2015; Angwin et al., 2017), but not in tasks that require more transient goal-related memory (except for auditory information, as explained above), directed shifting of attention, or complex phonemic construction (Bottiroli et al., 2014; Herweg and Bunzeck, 2015) supports this notion. Our present results, where white noise increased response time in incongruent (high conflict/task load), but not congruent (low conflict/task load) trials may also be interpreted in this context. It is important to note that such distinctions may not apply to ADHD-diagnosed individuals, where improvements have been found in prefrontal domains such as working memory and response inhibition (Helps et al., 2014; Söderlund et al., 2016), likely due to physiological/neurochemical differences as outlined in the authors’ moderate brain arousal hypothesis (Söderlund et al., 2007, 2010, 2016).
Altogether, it is evident that the cognitive influence of white noise is dependent on the abilities/tasks, individuals, and stimulus intensities involved. From our present findings and previous literature, however, only a rudimentary understanding of the parameters by which these factors alter its influence can be proposed. Accordingly, its use in the context of psychiatric care should remain narrowly targetted to particular patient groups where benefit has been clearly described, such as children diagnosed with ADHD (Söderlund et al., 2007, 2010, 2016). Crucial areas for future research include examining how changes in stimulus intensities (outside of signal-to-noise ratios in auditory-based tasks) and cognitive tasks can modulate its influence on behavioural measures, alongside identifying the relevant alterations in underlying physiological processes through functional imaging methods (EEG, fMRI).
By comparing rule-based biases in the WCST, we found that the results obtained by this home-based study were closely compatible with those obtained within previous laboratory-based studies (Supplementary Figures 2A,B). Recently, the COVID-19 pandemic and related social-distancing measures have forced many researchers to rethink how they conduct research, particularly for behavioural studies which require considerable face-to-face interactions. In view of our results, and complementing previous studies (Semmelmann and Weigelt, 2017), remote testing may provide a viable alternative for researchers faced with these challenges. This can include online testing platforms, as well as the development of offline software that can easily be used by individuals on non-specialised computers. The benefits of remote testing, however, can extend beyond pandemic-related situations. For individuals where laboratory access may be unfeasible, such as those residing in aged care facilities and schools, are mobility-limited, or are geographically isolated, advances in remote testing may foster their inclusion in cognitive and behavioural studies. In the context of Australian healthcare, Aboriginal and Torres Strait Islander individuals are 25% more likely to suffer from psychiatric illness (Australian Bureau of Statistics, 2016a,b), and experience burden from mental and substance use issues at a rate of 2.4 times the overall population (measured through disability-adjusted life years) (Australian Institute of Health and Welfare, 2016). Concurrently, the majority (65%) of aboriginal and Torres Strait islander individuals live away from capital city metropolitans (compared to 25% of Australia’s overall population) (Australian Bureau of Statistics, 2016c), and thus there may exist a significant disparity in their research participation. Similar health inequalities may also be present in other isolated (geographically, health-related, or politically) populations globally, therefore alternative reliable and validated approaches to laboratory-based testing should be considered a viable option for addressing such inequalities, alongside preparing for any potential future disruptions in travel and social contact.
Within each daily session, participants’ performance markedly improved from the first to the second stage in both tests. These practice-related, within-session learning effects have been previously reported in the context of other cognitive tasks (Mansouri et al., 2017a; Fehring et al., 2019a,2022), and the WCST (Fehring et al., 2019b). We did not find any interactions with task conditions, or acoustic environment in either test, suggesting that task-related learning remained unaffected by classical music and white noise in their respective sessions. Investigations into whether listening to music during study can ameliorate learning processes have generally indicated that when individuals feel positive toward its presence, benefit in a variety of tasks can be derived (Miskovic et al., 2008; Dosseville et al., 2012; Kang and Williamson, 2013). Our findings, where classical music of mixed valence (and therefore unlikely to consistently appease listeners) did not affect within-session learning, further supports the idea that these reported improvements arose from participants’ subjective enjoyment of the music, and not from other aspects of its perception and processing. Furthermore, the similar failure of white noise suggests that the apparent task-dependencies of its cognitive effects extend to related learning processes, where our results contrast findings that white noise augments performance improvements over consecutive testing stages (within the same day) in a semantic memory task (Angwin et al., 2017).
In this study, all participants were young undergraduate students within a limited age range (18–29 years old), and hence were a reasonably homogenous cohort. Accordingly, our findings cannot be directly applied to individuals outside this age range, where further research may be required. Since participants completed the tests from their homes, there may have been significant differences in the background noise levels (e.g., traffic levels). Although they were instructed to perform the tests at the quietest possible time of day, and our design was repeated-measures, variations in environmental noise may have impacted participants’ test performance and perception of acoustic stimuli. Further studies may serve to validate our results in more controlled laboratory environments. Participants also used different mediums for playing the acoustic stimuli (e.g., headphones, earphones, speakers), and hence the quality of acoustic stimuli may have fluctuated between individuals. Whilst such alterations to quality would have been constant for each participant because of the repeated-measures design, it is possible they may have led to different effects between individuals. Future home-based studies may endeavour to provide participants the same acoustic medium to account for such possibilities. Lastly, there was no direct measure of biological activity to accompany the behavioural data in this study. Therefore, we could not provide direct evidence for the potential physiological underpinnings of our observed results, particularly for whether the dissociable effects of music and white noise observed in this study did in fact arise from differing emotional reactions, or stress responses. Future studies should consider attempting to validate (or disprove) our hypothesis with the inclusion of such measures.
Our findings indicate that compared to silence, background classical music and white noise differentially affect performance in the Stroop test, where classical music has no influence, and white noise increases the response time difference between congruent and incongruent trials (conflict cost). Such effects cannot be explained by white noise acting as task-irrelevant information and thus occupying limited cognitive resources (Beaman and Jones, 1998; Jones et al., 1999; Elliott and Cowan, 2005; Kerzel and Schönhammer, 2013), as music was played at a similar intensity, yet did not produce a similar impairment. Therefore, it is possible participant’s may have experienced a negative emotional response to the presence of the white noise, hence impairing their ability to resolve conflict, whilst it is unclear whether the presence of certain pleasurable acoustic qualities, or fluctuating alterations to their emotional state may have prevented a similar response to the music.
The absence of effect in the music condition suggests varied (no specific tempo, valence) classical music may not be an appropriate acoustic medium for improving conflict processing, but it is important to note these findings cannot be extended to other, more nuanced or modern genres of music, which requires further investigation. Meanwhile, the impairment caused by white noise indicates its cognitive effects may be nuanced and particularly sensitive to differences between tasks and individuals. In particular, these adverse effects highlight the necessity for further research on white noise’s interactions with cognitive functions before it can be recommended for addressing other symptoms of neuropsychological disorders (Kaneko et al., 2013; Lin et al., 2018). Non-human primate models may be suitable for continuing such investigations, given the similarity of our present results and those reported in one of our previous studies (Zarei et al., 2019). Lastly, the correspondence of rule biases within the WCST between home-based, and laboratory-based experiments (Mansouri et al., 2020a) indicates remote testing may be a reliable avenue for psychophysical testing when in-person contact cannot be achieved.
The original contributions presented in the study are included in the Supplementary material, further inquiries can be directed to the corresponding author/s.
The studies involving human participants were reviewed and approved by Monash Human Ethics Committee. The patients/participants provided their written informed consent to participate in this study.
AP conducted the study, analysed the data, and wrote the manuscript. ZH and RS participated in data collection, analysis, and writing the manuscript. DF and FM participated in analysis and writing the manuscript. All authors contributed to the article and approved the submitted version.
This work was supported by ARC Centre of Excellence for Integrative Brain Function.
We thank all the student participants within this study for their flexibility during a challenging period.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnins.2022.858576/full#supplementary-material
Alexander, J., Hillier, A., Smith, R., Tivarus, M., and Beversdorf, D. (2007). Beta-adrenergic modulation of cognitive flexibility during stress. J. Cogn. Neurosci. 19, 468–478. doi: 10.1162/jocn.2007.19.3.468
Angwin, A. J., Wilson, W. J., Arnott, W. L., Signorini, A., Barry, R. J., and Copland, D. A. (2017). White noise enhances new-word learning in healthy adults. Sci. Rep. 7:13045. doi: 10.1038/s41598-017-13383-3
Arjmand, H.-A., Hohagen, J., Paton, B., and Rickard, N. S. (2017). Emotional responses to music: Shifts in frontal brain asymmetry mark periods of musical change. Front. Psychol. 8:2044. doi: 10.3389/fpsyg.2017.02044
Arnsten, A. F. T. (2009). Stress signalling pathways that impair prefrontal cortex structure and function. Nat. Rev. Neurosci. 10, 410–422. doi: 10.1038/nrn2648
Arnsten, A. F. T., and Goldman-Rakic, P. S. (1998). Noise stress impairs prefrontal cortical cognitive function in monkeys: Evidence for a hyperdopaminergic mechanism. Arch. Gen. Psychiatry 55, 362–368. doi: 10.1001/archpsyc.55.4.362
Australian Bureau of Statistics (2016b). 2018-19 National aboriginal and torres strait islander health survey. Canberra: ABS.
Australian Bureau of Statistics (2016c). Census of population and housing: Reflecting australia - stories from the census, 2016. Canberra: ABS.
Australian Institute of Health and Welfare (2016). Australian burden of disease study: Impact and causes of illness and death in aboriginal and torres strait islander people 2011. Canberra: AIHW.
Banis, S., and Lorist, M. M. (2012). Acute noise stress impairs feedback processing. Biol. Psychol. 91, 163–171. doi: 10.1016/j.biopsycho.2012.06.009
Bannon, S., Gonsalvez, C. J., Croft, R. J., and Boyce, P. M. (2002). Response inhibition deficits in obsessive–compulsive disorder. Psychiatry Res. 110, 165–174. doi: 10.1016/S0165-1781(02)00104-X
Beaman, C. P., and Jones, D. M. (1998). Irrelevant sound disrupts order information in free recall as in serial recall. Q. J. Exp. Psychol. A 51, 615–636. doi: 10.1080/713755774
Blood, A. J., Zatorre, R. J., Bermudez, P., and Evans, A. C. (1999). Emotional responses to pleasant and unpleasant music correlate with activity in paralimbic brain regions. Nat. Neurosci. 2, 382–387. doi: 10.1038/7299
Bottiroli, S., Rosi, A., Russo, R., Vecchi, T., and Cavallini, E. (2014). The cognitive effects of listening to background music on older adults: Processing speed improves with upbeat music, while memory seems to benefit from both upbeat and downbeat music. Front. Aging Neurosci. 6:284. doi: 10.3389/fnagi.2014.00284
Botvinick, M., Nystrom, L. E., Fissell, K., Carter, C. S., and Cohen, J. D. (1999). Conflict monitoring versus selection-for-action in anterior cingulate cortex. Nature 402, 179–181. doi: 10.1038/46035
Botvinick, M. M., Cohen, J. D., and Carter, C. S. (2004). Conflict monitoring and anterior cingulate cortex: An update. Trends Cogn. Sci. 8, 539–546. doi: 10.1016/j.tics.2004.10.003
Botvinick, M. M., Braver, T. S., Barch, D. M., Carter, C. S., and Cohen, J. D. (2001). “Conflict monitoring and cognitive control,” in Psychological review, ed. E. L. Grigorenko (Washington, D.C: American Psychological Association).
Brodsky, W. (2001). The effects of music tempo on simulated driving performance and vehicular control. Transp. Res. Part F Traffic Psychol. Behav. 4, 219–241. doi: 10.1016/S1369-8478(01)00025-0
Brodsky, W., and Slor, Z. (2013). Background music as a risk factor for distraction among young-novice drivers. Accid. Anal. Prev. 59, 382–393. doi: 10.1016/j.aap.2013.06.022
Burkhard, A., Elmer, S., Kara, D., Brauchli, C., and Jäncke, L. (2018). The effect of background music on inhibitory functions: An ERP study. Front. Hum. Neurosci. 12:293. doi: 10.3389/fnhum.2018.00293
Carlson, S., Rämä, P., Artchakov, D., and Linnankoski, I. (1997). Effects of music and white noise on working memory performance in monkeys. Neuroreport 8, 2853–2856.
Carpentier, F., and Potter, R. (2007). Effects of music on physiological arousal: Explorations into tempo and genre. Media Psychol. 10, 339–363. doi: 10.1080/15213260701533045
Carter, C. S., and van Veen, V. (2007). Anterior cingulate cortex and conflict detection: An update of theory and data. Cogn. Affect. Behav. Neurosci. 7, 367–379. doi: 10.3758/CABN.7.4.367
Cassidy, G., and MacDonald, R. A. R. (2007). The effect of background music and background noise on the task performance of introverts and extraverts. Psychol. Music 35, 517–537. doi: 10.1177/0305735607076444
Cloutier, A., Fernandez, N. B., Houde-Archambault, C., and Gosselin, N. (2020). Effect of background music on attentional control in older and young adults. Front. Psychol. 11:557225. doi: 10.3389/fpsyg.2020.557225
Cuijpers, P., Karyotaki, E., Reijnders, M., and Ebert, D. (2018). Was Eysenck right after all? A reassessment of the effects of psychotherapy for adult depression. Epidemiol. Psychiatr. Sci. 28, 21–30. doi: 10.1017/S2045796018000057
Day, R.-F., Lin, C.-H., Huang, W.-H., and Chuang, S.-H. (2009). Effects of music tempo and task difficulty on multi-attribute decision-making: An eye-tracking approach. Comput. Hum. Behav. 25, 130–143. doi: 10.1016/j.chb.2008.08.001
Dobbs, S., Furnham, A., and McClelland, A. (2011). The effect of background music and noise on the cognitive test performance of introverts and extraverts. Appl. Cogn. Psychol. 25, 307–313. doi: 10.1002/acp.1692
Dolcos, F., and McCarthy, G. (2006). Brain systems mediating cognitive interference by emotional distraction. J. Neurosci. 26, 2072–2079. doi: 10.1523/JNEUROSCI.5042-05.2006
Dosseville, F., Laborde, S., and Scelles, N. (2012). Music during lectures: Will students learn better? Learn. Individ. Differ. 22, 258–262. doi: 10.1016/j.lindif.2011.10.004
Elliott, A. E., and Packard, M. G. (2008). Intra-amygdala anxiogenic drug infusion prior to retrieval biases rats towards the use of habit memory. Neurobiol. Learn. Mem. 90, 616–623. doi: 10.1016/j.nlm.2008.06.012
Elliott, E. M., and Cowan, N. (2005). Coherence of the irrelevant-sound effect: Individual profiles of short-term memory and susceptibility to task-irrelevant materials. Mem. Cogn. 33, 664–675. doi: 10.3758/BF03195333
Farokhnezhad Afshar, P., Bahramnezhad, F., Asgari, P., and Shiri, M. (2016). Effect of white noise on sleep in patients admitted to a coronary care. J. Caring Sci. 5, 103–109. doi: 10.15171/jcs.2016.011
Faul, F., Erdfelder, E., Lang, A.-G., and Buchner, A. (2007). G*Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav. Res. Methods 39, 175–191. doi: 10.3758/BF03193146
Fehring, D. J., Pascoe, A. J., Haque, Z. Z., Samandra, R., Yokoo, S., Abe, H., et al. (2022). Dimension of visual information interacts with working memory in monkeys and humans. Sci. Rep. 12:5335. doi: 10.1038/s41598-022-09367-7
Fehring, D. J., Samandra, R., Haque, Z. Z., Jaberzadeh, S., Rosa, M., and Mansouri, F. A. (2021). Investigating the sex-dependent effects of prefrontal cortex stimulation on response execution and inhibition. Biol. Sex Differ. 12:47. doi: 10.1186/s13293-021-00390-3
Fehring, D. J., Illipparampil, R., Acevedo, N., Jaberzadeh, S., Fitzgerald, P. B., and Mansouri, F. A. (2019a). Interaction of task-related learning and transcranial direct current stimulation of the prefrontal cortex in modulating executive functions. Neuropsychologia 131, 148–159. doi: 10.1016/j.neuropsychologia.2019.05.011
Fehring, D. J., Samandra, R., Rosa, M. G., and Mansouri, F. A. (2019b). Negative emotional stimuli enhance conflict resolution without altering arousal. Front. Hum. Neurosci. 13:282. doi: 10.3389/fnhum.2019.00282
Feizpour, A., Parkington, A. H. C., and Mansouri, F. A. (2018). Cognitive sex differences in effects of music in Wisconsin Card Sorting Test. Psychol. Music 48, 252–265. doi: 10.1177/0305735618795030
Fournier, J. C., DeRubeis, R. J., Hollon, S. D., Dimidjian, S., Amsterdam, J. D., Shelton, R. C., et al. (2010). Antidepressant drug effects and depression severity: A patient-level meta-analysis. JAMA 303, 47–53. doi: 10.1001/jama.2009.1943
Furnham, A., and Strbac, L. (2002). Music is as distracting as noise: The differential distraction of background music and noise on the cognitive test performance of introverts and extraverts. Ergonomics 45, 203–217. doi: 10.1080/00140130210121932
Gomez, P., and Danuser, B. (2004). Affective and physiological responses to environmental noises and music. Int. J. Psychophysiol. 53, 91–103. doi: 10.1016/j.ijpsycho.2004.02.002
Grandjean, J., D’Ostilio, K., Phillips, C., Balteau, E., Degueldre, C., Luxen, A., et al. (2012). Modulation of brain activity during a Stroop inhibitory task by the kind of cognitive control required. PLoS One 7:e41513. doi: 10.1371/journal.pone.0041513
Gratton, G., Coles, M. G. H., and Donchin, E. (1992). Optimizing the use of information: Strategic control of activation of responses. J. Exp. Psychol. Gen. 121, 480–506.
Hartley, L. R., and Adams, R. G. (1974). Effect of noise on the Stroop test. J. Exp. Psychol. 102, 62–66.
Helps, S. K., Bamford, S., Sonuga-Barke, E. J. S., and Söderlund, G. B. W. (2014). Different effects of adding white noise on cognitive performance of sub-, normal and super-attentive school children. PLoS One 9:e112768. doi: 10.1371/journal.pone.0112768
Henderson, D. (2008). Managing weight gain and metabolic issues in patients treated with atypical antipsychotics. J. Clin. Psychiatry 69:e04. doi: 10.4088/JCP.0208e04
Herweg, N. A., and Bunzeck, N. (2015). Differential effects of white noise in cognitive and perceptual tasks. Front. Psychol. 6:1639. doi: 10.3389/fpsyg.2015.01639
Hillier, A., Alexander, J. K., and Beversdorf, D. Q. (2006). The effect of auditory stressors on cognitive flexibility. Neurocase 12, 228–231. doi: 10.1080/13554790600878887
Hilt, R. J., Chaudhari, M., Bell, J. F., Wolf, C., Koprowicz, K., and King, B. H. (2014). Side effects from use of one or more psychiatric medications in a population-based sample of children and adolescents. J. Child Adolesc. Psychopharmacol. 24, 83–89. doi: 10.1089/cap.2013.0036
Jaberzadeh, S., and Mansouri, F. A. (2021). Short-term research projects in cognitive neuroscience for undergraduate students: A contingency plan to maintain quality teaching during COVID-19 pandemic. Adv. Physiol. Educ. 45, 376–383. doi: 10.1152/advan.00012.2021
Jäncke, L., Brügger, E., Brummer, M., Scherrer, S., and Alahmadi, N. (2014). Verbal learning in the context of background music: No influence of vocals and instrumentals on verbal learning. Behav. Brain Funct. 10:10. doi: 10.1186/1744-9081-10-10
Jing, Y., Jing, S., Huajian, C., Chuangang, S., and Yan, L. (2012). “The gender difference in distraction of background music and noise on the cognitive task performance,” in Proceedings of the 2012 8th international conference on natural computation. Chongqing: IEEE.
Jones, D. M., Banbury, S. P., Tremblay, S., and Macken, W. J. (1999). The effect of task-irrelevant sounds on cognitive performance. Proc. Hum. Fact. Ergon. Soc. Annu. Meet. 43, 261–265. doi: 10.1177/154193129904300328
Joyal, M., Wensing, T., Levasseur-Moreau, J., Leblond, J., Sack, A., and Fecteau, S. (2019). Characterizing emotional Stroop interference in posttraumatic stress disorder, major depression and anxiety disorders: A systematic review and meta-analysis. PLoS One 14:e0214998. doi: 10.1371/journal.pone.0214998
Kämpfe, J., Sedlmeier, P., and Renkewitz, F. (2010). The impact of background music on adult listeners: A meta-analysis. Psychol. Music 39, 424–448. doi: 10.1177/0305735610376261
Kaneko, Y., Butler, J. P., Saitoh, E., Horie, T., Fujii, M., and Sasaki, H. (2013). Efficacy of white noise therapy for dementia patients with schizophrenia. Geriatr. Gerontol. Int. 13, 808–810. doi: 10.1111/ggi.12028
Kang, H. J., and Williamson, V. J. (2013). Background music can aid second language learning. Psychol. Music 42, 728–747. doi: 10.1177/0305735613485152
Kerns, J. G., Cohen, J. D., MacDonald, A. W., Cho, R. Y., Stenger, V. A., and Carter, C. S. (2004). Anterior cingulate conflict monitoring and adjustments in control. Science 303, 1023–1026. doi: 10.1126/science.1089910
Kerzel, D., and Schönhammer, J. (2013). Salient stimuli capture attention and action. Attent. Percept. Psychophys. 75, 1633–1643. doi: 10.3758/s13414-013-0512-3
Kirsch, I., Deacon, B. J., Huedo-Medina, T. B., Scoboria, A., Moore, T. J., and Johnson, B. T. (2008). Initial severity and antidepressant benefits: A meta-analysis of data submitted to the Food and Drug Administration. PLoS Med. 5:e45. doi: 10.1371/journal.pmed.0050045
Kou, S., McClelland, A., and Furnham, A. (2017). The effect of background music and noise on the cognitive test performance of Chinese introverts and extraverts. Psychol. Music 46, 125–135. doi: 10.1177/0305735617704300
Kravariti, E., Schulze, K., Kane, F., Kalidindi, S., Bramon, E., Walshe, M., et al. (2009). Stroop-test interference in bipolar disorder. Br. J. Psychiatry 194, 285–286. doi: 10.1192/bjp.bp.108.052639
Lansbergen, M. M., Kenemans, J. L., and van Engeland, H. (2007). Stroop interference and attention-deficit/hyperactivity disorder: A review and meta-analysis. Neuropsychology 21, 251–262. doi: 10.1037/0894-4105.21.2.251
Lehmann, J. A. M., and Seufert, T. (2017). The influence of background music on learning in the light of different theoretical perspectives and the role of working memory capacity. Front. Psychol. 8:1902. doi: 10.3389/fpsyg.2017.01902
Lin, L.-W., Weng, S.-C., Wu, H.-S., Tsai, L.-J., Lin, Y.-L., and Yeh, S.-H. (2018). The effects of white noise on agitated behaviors, mental status, and activities of daily living in older adults with dementia. J. Nurs. Res. 26, 2–9. doi: 10.1097/JNR.0000000000000211
Luethi, M., Meier, B., and Sandi, C. (2009). Stress effects on working memory, explicit memory, and implicit memory for neutral and emotional stimuli in healthy men. Front. Behav. Neurosci. 2:5. doi: 10.3389/neuro.08.005.2008
Lugo, E., Doti, R., and Faubert, J. (2008). Ubiquitous crossmodal stochastic resonance in humans: Auditory noise facilitates tactile, visual and proprioceptive sensations. PLoS One 3:e2860. doi: 10.1371/journal.pone.0002860
Lupien, S. J., Gaudreau, S., Tchiteya, B. M., Maheu, F., Sharma, S., Nair, N. P. V., et al. (1997). Stress-induced declarative memory impairment in healthy elderly subjects: Relationship to cortisol reactivity1. J. Clin. Endocrinol. Metab. 82, 2070–2075. doi: 10.1210/jcem.82.7.4075
MacLeod, C. M., and MacDonald, P. A. (2000). Interdimensional interference in the Stroop effect: Uncovering the cognitive and neural anatomy of attention. Trends Cogn. Sci. 4, 383–391. doi: 10.1016/S1364-6613(00)01530-8
Manan, H. A., Franz, E. A., Yusoff, A. N., and Mukari, S. Z.-M. S. (2012). Hippocampal-cerebellar involvement in enhancement of performance in word-based BRT with the presence of background noise: An initial fMRI study. Psychol. Neurosci. 5:247.
Manjarrez, E., Mendez, I., Martinez, L., Flores, A., and Mirasso, C. R. (2007). Effects of auditory noise on the psychophysical detection of visual signals: Cross-modal stochastic resonance. Neurosci. Lett. 415, 231–236. doi: 10.1016/j.neulet.2007.01.030
Mansouri, F. A., and Buckley, M. J. (2018). “Context-dependent adjustments in executive control of goal-directed behaviour: Contribution of frontal brain areas to conflict-induced behavioural adjustments in primates,” in Systems Neuroscience, eds A. Y. Cheung-Hoi and L. Li (Cham: Springer International Publishing), 71–83. doi: 10.1007/978-3-319-94593-4_4
Mansouri, F. A., Buckley, M. J., and Tanaka, K. (2022). The neural substrate and underlying mechanisms of executive control fluctuations in primates. Prog. Neurobiol. 209:102216. doi: 10.1016/j.pneurobio.2022.102216
Mansouri, F. A., Acevedo, N., Illipparampil, R., Fehring, D. J., Fitzgerald, P. B., and Jaberzadeh, S. (2017a). Interactive effects of music and prefrontal cortex stimulation in modulating response inhibition. Sci. Rep. 7:18096. doi: 10.1038/s41598-017-18119-x
Mansouri, F. A., Egner, T., and Buckley, M. J. (2017b). Monitoring demands for executive control: Shared functions between human and non-human primates. Trends Neurosci. 40, 15–27. doi: 10.1016/j.tins.2016.11.001
Mansouri, F. A., Fehring, D. J., Feizpour, A., Gaillard, A., Rosa, M., Rajan, R., et al. (2016a). Direct current stimulation of prefrontal cortex modulates error-induced behavioral adjustments. Eur. J. Neurosci. 44, 1856–869. doi: 10.1111/ejn.13281
Mansouri, F. A., Fehring, D. J., Gaillard, A., Jaberzadeh, S., and Parkington, H. (2016b). Sex dependency of inhibitory control functions. Biol. Sex Differ. 7:11. doi: 10.1186/s13293-016-0065-y
Mansouri, F. A., Freedman, D. J., and Buckley, M. J. (2020b). Emergence of abstract rules in the primate brain. Nat. Rev. Neurosci. 21, 595–610. doi: 10.1038/s41583-020-0364-5
Mansouri, F. A., Buckley, M. J., Fehring, D. J., and Tanaka, K. (2020a). The role of primate prefrontal cortex in bias and shift between visual dimensions. Cereb. Cortex 30, 85–99. doi: 10.1093/cercor/bhz072
Masataka, N., and Perlovsky, L. (2013). Cognitive interference can be mitigated by consonant music and facilitated by dissonant music. Sci. Rep. 3:2028. doi: 10.1038/srep02028
Maxon, A. B., and Hochberg, I. (1982). Development of psychoacoustic behavior: Sensitivity and discrimination. Ear Hear. 3, 301–308.
Mayr, U., Awh, E., and Laurey, P. (2003). Conflict adaptation effects in the absence of executive control. Nat. Neurosci. 6, 450–452. doi: 10.1038/nn1051
McDonnell, M. D., and Abbott, D. (2009). What is stochastic resonance? Definitions, misconceptions, debates, and its relevance to biology. PLoS Comput. Biol. 5:e1000348. doi: 10.1371/journal.pcbi.1000348
Miller, E. K., and Cohen, J. D. (2001). An integrative theory of prefrontal cortex function. Annu. Rev. Neurosci. 24, 167–202. doi: 10.1146/annurev.neuro.24.1.167
Miller, L. K., and Schyb, M. (1989). Facilitation and interference by background music. J. Music Ther. 26, 42–54. doi: 10.1093/jmt/26.1.42
Mingle, M., Eppley, T., Campbell, M., Hall, K., Horner, V., and W-aal, F. (2014). Chimpanzees prefer African and Indian music over silence. J. Exp. Psychol. Anim. Learn. Cogn. 40, 502–505. doi: 10.1037/xan0000032
Miskovic, D., Rosenthal, R., Zingg, U., Oertli, D., Metzger, U., and Jancke, L. (2008). Randomized controlled trial investigating the effect of music on the virtual reality laparoscopic learning performance of novice surgeons. Surg. Endosc. 22, 2416–2420. doi: 10.1007/s00464-008-0040-8
Moss, F., Ward, L. M., and Sannita, W. G. (2004). Stochastic resonance and sensory information processing: A tutorial and review of application. Clin. Neurophysiol. 115, 267–281. doi: 10.1016/j.clinph.2003.09.014
Murphy, B. L., Arnsten, A. F., Goldman-Rakic, P. S., and Roth, R. H. (1996). Increased dopamine turnover in the prefrontal cortex impairs spatial working memory performance in rats and monkeys. Proc. Natl. Acad. Sci. U.S.A. 93, 1325–1329. doi: 10.1073/pnas.93.3.1325
Musliu, A., Berisha, B., and Latifi, D. (2017). The impact of music in memory. Eur. J. Soc. Sci. Educ. Res. 4:2017.
Othman, E., Yusoff, A. N., Mohamad, M., Abdul Manan, H., Giampietro, V., Abd Hamid, A. I., et al. (2019). Low intensity white noise improves performance in auditory working memory task: An fMRI study. Heliyon 5:e02444. doi: 10.1016/j.heliyon.2019.e02444
Patterson-Kane, E., and Farnworth, M. (2006). Noise exposure, music, and animals in the laboratory: A commentary based on laboratory animal refinement and enrichment forum (LAREF) discussions. J. Appl. Anim. Welf. Sci. 9, 327–332. doi: 10.1207/s15327604jaws0904_7
Proverbio, A. M., Lozano Nasi, V., Alessandra Arcari, L., De Benedetto, F., Guardamagna, M., Gazzola, M., et al. (2015). The effect of background music on episodic memory and autonomic responses: Listening to emotionally touching music enhances facial memory capacity. Sci. Rep. 5:15219. doi: 10.1038/srep15219
Qin, S., Hermans, E., Marle, H., Luo, J., and Fernández, G. (2009). Acute psychological stress reduces working memory-related activity in the dorsolateral prefrontal cortex. Biol. Psychiatry 66, 25–32. doi: 10.1016/j.biopsych.2009.03.006
Rausch, V. H., Bauch, E. M., and Bunzeck, N. (2014). White noise improves learning by modulating activity in dopaminergic midbrain regions and right superior temporal sulcus. J. Cogn. Neurosci. 26, 1469–1480. doi: 10.1162/jocn_a_00537
Roederer, J. G. (2008). The physics and psychophysics of music: An introduction. Berlin: Springer Science & Business Media.
Scarpina, F., and Tagini, S. (2017). The stroop color and word test. Front. Psychol. 8:557. doi: 10.3389/fpsyg.2017.00557
Schmidt, L. A., and Trainor, L. J. (2001). Frontal brain electrical activity (EEG) distinguishes valence and intensity of musical emotions. Cogn. Emot. 15, 487–500. doi: 10.1080/02699930126048
Semahegn, A., Torpey, K., Manu, A., Assefa, N., Tesfaye, G., and Ankomah, A. (2020). Psychotropic medication non-adherence and its associated factors among patients with major psychiatric disorders: A systematic review and meta-analysis. Syst. Rev. 9:17. doi: 10.1186/s13643-020-1274-3
Semmelmann, K., and Weigelt, S. (2017). Online psychophysics: Reaction time effects in cognitive experiments. Behav. Res. Methods 49, 1241–1260. doi: 10.3758/s13428-016-0783-4
Serretti, A., Chiesa, A., Calati, R., Fabbri, C., Sentissi, O., De Ronchi, D., et al. (2013). Side effects associated with psychotropic medications in patients with bipolar disorder: Evidence from two independent samples. J. Psychopharmacol. 27, 616–628. doi: 10.1177/0269881113485143
Söderlund, G., Sikström, S., and Smart, A. (2007). Listen to the noise: Noise is beneficial for cognitive performance in ADHD. J. Child Psychol. Psychiatry 48, 840–847. doi: 10.1111/j.1469-7610.2007.01749.x
Söderlund, G. B. W., Björk, C., and Gustafsson, P. (2016). Comparing auditory noise treatment with stimulant medication on cognitive task performance in children with attention deficit hyperactivity disorder: Results from a pilot study. Front. Psychol. 7:1331. doi: 10.3389/fpsyg.2016.01331
Söderlund, G. B. W., Sikström, S., Loftesnes, J. M., and Sonuga-Barke, E. J. (2010). The effects of background white noise on memory performance in inattentive school children. Behav. Brain Funct. 6:55. doi: 10.1186/1744-9081-6-55
Stafford, M. R., Mayo-Wilson, E., Loucas, C. E., James, A., Hollis, C., Birchwood, M., et al. (2015). Efficacy and safety of pharmacological and psychological interventions for the treatment of psychosis and schizophrenia in children, adolescents and young adults: A systematic review and meta-analysis. PLoS One 10:e0117166. doi: 10.1371/journal.pone.0117166
Stoet, G. (2010). PsyToolkit: A software package for programming psychological experiments using Linux. Behav. Res. Methods 42, 1096–1104. doi: 10.3758/BRM.42.4.1096
Stoet, G. (2016). PsyToolkit: A novel web-based method for running online questionnaires and reaction-time experiments. Teach. Psychol. 44, 24–31. doi: 10.1177/0098628316677643
Stroop, J. R. (1935). Studies of interference in serial verbal reactions. J. Exp. Psychol. 18, 643–662.
Sugimoto, T., Kobayashi, H., Nobuyoshi, N., Kiriyama, Y., Takeshita, H., Nakamura, T., et al. (2009). Preference for consonant music over dissonant music by an infant chimpanzee. Primates 51, 7–12. doi: 10.1007/s10329-009-0160-3
Thase, M. E. (2007). STEP-BD and bipolar depression: What have we learned? Curr. Psychiatry Rep. 9, 497–503. doi: 10.1007/s11920-007-0068-9
Trehub, S. E., Schneider, B. A., Morrongiello, B. A., and Thorpe, L. A. (1989). Developmental changes in high-frequency sensitivity. Audiology 28, 241–249. doi: 10.3109/00206098909081629
Velligan, D., Weiden, P., Sajatovic, M., Scott, J., Carpenter, D., Ross, R., et al. (2009). The expert consensus guideline series: Adherence problems in patients with serious and persistent mental illness introduction. J. Clin. Psychiatry 70, 6–46. doi: 10.4088/JCP.7090su1cj
Wais, P. E., and Gazzaley, A. (2011). The impact of auditory distraction on retrieval of visual memories. Psychon. Bull. Rev. 18, 1090–1097. doi: 10.3758/s13423-011-0169-7
Ward, L. M., MacLean, S. E., and Kirschner, A. (2010). Stochastic resonance modulates neural synchronization within and between cortical sources. PLoS One 5:e14371. doi: 10.1371/journal.pone.0014371
Werner, L. A. (2007). Issues in human auditory development. J. Commun. Disord. 40, 275–283. doi: 10.1016/j.jcomdis.2007.03.004
Zarei, S. A., Sheibani, V., and Mansouri, F. A. (2019). Interaction of music and emotional stimuli in modulating working memory in macaque monkeys. Am. J. Primatol. 81:e22999. doi: 10.1002/ajp.22999
Keywords: executive control, conflict processing, music, white noise, Stroop test, online testing
Citation: Pascoe AJ, Haque ZZ, Samandra R, Fehring DJ and Mansouri FA (2022) Dissociable effects of music and white noise on conflict-induced behavioral adjustments. Front. Neurosci. 16:858576. doi: 10.3389/fnins.2022.858576
Received: 20 January 2022; Accepted: 25 July 2022;
Published: 17 August 2022.
Edited by:
Isabelle Peretz, Université de Montréal, CanadaReviewed by:
Natalia B. Fernandez, Geneva University Hospitals (HUG), SwitzerlandCopyright © 2022 Pascoe, Haque, Samandra, Fehring and Mansouri. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Farshad A. Mansouri, RmFyc2hhZC5NYW5zb3VyaUBtb25hc2guZWR1
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.