
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Neurosci., 23 March 2022
Sec. Auditory Cognitive Neuroscience
Volume 16 - 2022 | https://doi.org/10.3389/fnins.2022.857855
This article is part of the Research TopicNew Discoveries in the Benefits and Outcomes of Cochlear ImplantationView all 18 articles
 Lifang Zhang1,2†
Lifang Zhang1,2† Biao Chen1,2†
Biao Chen1,2† Ying Kong2,3
Ying Kong2,3 Natalia Liau1,2
Natalia Liau1,2 Xingmei Wei1,2
Xingmei Wei1,2 Ying Shi1,2
Ying Shi1,2 Jingyuan Chen1,2
Jingyuan Chen1,2 Mengge Yang1,2
Mengge Yang1,2 Anandhan Dhanasingh4
Anandhan Dhanasingh4 Yongxin Li1,2*
Yongxin Li1,2*
Object: To investigate the long-term development of auditory and speech in patients with common cavity deformity (CCD) after cochlear implantation (CI) and its relationship to imaging characteristics.
Methods: Twenty-three CCD patients and 59 age- and sex-matched CI children with normal inner ear structure were recruited. The auditory and speech development of these two groups were evaluated at 0, 1, 3, 6, 12, and 18 months after CI activation using four parent reports questionnaires [Categories of Auditory Performance (CAP), Speech Intelligibility Rating (SIR), Meaningful Auditory Integration Scale/Infant-Toddler Meaningful Auditory Integration Scale (MAIS/ITMAIS), and Meaningful Use of Speech Scale (MUSS)]. Computed tomography-based 3-dimensional reconstruction of the surgical side of 18 CCD children was performed, the volume and surface area were calculated. Correlation analysis was performed on the imaging performance and post-operative outcomes.
Results: The percentages of MAIS/IT-MAIS scores and CAP scores at different evaluation time points are significantly different (p < 0.05). When comparing SIR results across time points, significant growth was observed in most of the comparisons. In addition, significant differences (p < 0.05) are observed among the percentages of MUSS scores at different time points except the comparison between 0 and 1 month after CI activation. Patients in the CCD group had poorer auditory and speech performances at different stages after CI compared with those in the control group. According to the reconstruction of CCD patients, the volume ranged from 12.21 to 291.96 mm3; the surface area ranged from 27.81 to 284.7 mm2. When the lumen surface area was <190.45 mm2 or the volume was <157.91 mm3, the survival time for CCD children to achieve a CAP score of 4 after CI was significantly shorter.
Conclusion: Cochlear implantation are less effective in CCD patients than in patients with normal inner ear structures, but they can still achieve significant improvement post-operatively. The morphology and size of the inner ear vary in CCD patients, which reflects the degree of inner ear development influences the outcome after CI surgery.
Common cavity deformity (CCD) is characterized by the presence of an abnormally ovoid or round chamber formed by the cochlea and vestibule and is generally associated with profound sensorineural hearing loss (SNHL) (Sennaroğlu and Bajin, 2017). This condition occurs due to the arrest of otocyst development during the fourth week of embryonic development (Jackler et al., 1987). CCD is diagnosed primarily on the basis of computed tomography (CT) or magnetic resonance imaging (MRI), showing a single fluid-filled cavity of the cochlea and vestibule (Casselman et al., 2001). This malformation varies in size and shape as well as in the location of the internal auditory canal and its size is often assumed to be related to the arrest time of the cochlear vestibule development; that is, the larger the cystic cavity, the later the arrest (Brotto et al., 2019).
For CCD patients, we often choose a cochlear implant (CI) or an auditory brainstem implant (ABI) to help them restore their hearing. Since the ABI was first used in a patient with neurofibromatosis type 2 in 1979, its indications have been constantly updated. In recent years, ABI has been applied to patients with profound SNHL who suffer from conditions such as cochlear sclerosis and severe cochlear malformations (Colletti et al., 2005; Bozorg Grayeli et al., 2007; Sennaroğlu et al., 2016). Sennaroğlu et al. (2016) performed ABI surgeries on seven patients with CCD and found that these patients achieved better hearing threshold and language outcome scores compared with patients with other types of severe cochlear malformations. Nevertheless, given the complications following ABI, such as cerebrospinal fluid leakage, electrode displacement, and limited post-operative benefit (Toh and Luxford, 2008), cochlear implantation (CI) is currently the primary intervention for CCD.
In 1986, McElveen et al. (1997) reported the first CI in a patient with CCD using the transmastoid facial recess approach. Since then, an increasing number of CCD patients have received CI surgery, and the surgical approach and electrodes have continually improved (Molter et al., 1993; Tucci et al., 1995; McElveen et al., 1997; Beltrame et al., 2000, 2005, 2013; Sennaroglu et al., 2014). In 2017, our team proposed that custom-made electrodes could be implanted via the transmastoid slotted labyrinthotomy approach (TSLA) for CCD patients (Wei et al., 2018). Instead of conventional electrodes, we used custom electrodes made by MED-EL, with 12 electrodes in the middle of the electrode array and extension wires made of inert silicone carriers containing platinum wires. This strategy allows the electrodes to remain as attached to the lumen as possible, allowing them to stimulate a larger area, which may mean that more spiral ganglion cells are stimulated, resulting in better post-operative outcomes.
Common cavity deformity patients could have long-term benefits with CI (Al-mahboob et al., 2022). Although most studies have concluded that post-operative outcomes in CCD patients are worse than in children with normal inner ear structures and mild malformations, specific auditory speech rehabilitation outcomes are inconsistent (Ahmad et al., 2005; Papsin, 2005; Ahn et al., 2011). In addition, a previous study showed a correlation between the effect of CI implantation and imaging performance (Wei et al., 2017). However, to date, no study has investigated the relationship between post-operative CI outcomes and imaging performance in CCD patients. The present study aims to understand the post-operative auditory-speech performance and developmental patterns of the CCD children implanted with custom electrodes via the TSLA approach by analyzing the long-term post-operative outcomes of these children and their relationship with imaging characteristics.
A total of 23 pediatric patients (12 female and 11 male) with CCD were recruited from April 2016 to January 2020 at the Department of Otolaryngology Head and Neck Surgery of our hospital. High-resolution computed tomography (HRCT) and inner ear MRI were performed before surgery in all cases, and the diagnosis of CCD was confirmed by two or more physicians from the Radiology and Otorhinolaryngology departments.
Inclusion criteria were as follows.
(1) Bilateral severe or profound SNHL patients diagnosed with CCD.
(2) Available for post-operative follow-up.
Exclusion criteria were as follows.
(1) Patients with contraindications to cochlear implantation.
(2) Patients with history of serious systemic diseases or intellectual disorders.
The age at implantation ranged from 0 to 7 years (mean: 27.65 months, standard deviation: 13.79 months).
A total of 59 congenitally severe or profound SNHL children who met the inclusion criteria and who had normal inner ear structure, matched for age and sex, were recruited as a control group. The age range was 0–8 years (mean, 29.00 months; standard deviation, 20.14 months).
In compliance with ethical standards for human subjects, written informed consent was obtained from the guardians of all participants before proceeding with the study procedures. This study was approved by the Institutional Review Board of our hospital.
All participants underwent routine otorhinolaryngological examination, followed by audiological tests, CT, and MRI scans before the CI surgery. To investigate the audiological status in terms of hearing level, function of the central auditory system, and the development of the auditory system, the following audiological tests were performed: (1) Behavioral hearing assessment, (2) Auditory Steady State Response, (3) Auditory Brainstem Response, (4) Distorted Product Otoacoustic Emissions, (5) conventional low 226 Hz tympanometry, and (6) 40-Hz auditory-evoked related potential.
In the CCD group, all children were implanted with customized electrodes using a transmastoid slotted labyrinthotomy approach (TSLA) (Wei et al., 2018). In the TSLA, the bony wall of the cavity was exposed after mastoidectomy, and a slot was made in the area where the lateral semicircular canal is commonly situated away from the facial nerve. A customized electrode was placed in the cavity toward the cochlear side after the perilymph flow abated (Shi et al., 2019). The electrodes were fully implanted in all children except for one child who had two extra cochlear electrodes because of the small size of the common cavity. None of the children had post-operative complications, such as facial paralysis or cerebrospinal fluid leakage.
In the control group, electrodes were implanted using the conventional transmastoid facial recess approach. All electrodes were successfully implanted.
The assessment of the child’s auditory and speech development was performed using the categories of auditory performance (CAP), Speech Intelligibility Rating (SIR), Meaningful Auditory Integration Scale/Infant-Toddler Meaningful Auditory Integration Scale (MAIS/ITMAIS), and Meaningful Use of Speech Scale (MUSS). The parents or guardians of the infants were asked to complete the questionnaires. The questionnaires were evaluated at the activation of CI and at 1, 3, 6, 9, 12, 18, 24, and 36 months after activation. For the MAIS/IT-MAIS and MUSS, we converted the actual scores into percentages as final statistics, and the result was expressed as a percentage using the following equation: total score/40 × 100%.
All patients underwent a temporal bone CT scan (GE 64-row helical CT, Boston, MA, United States) and inner ear MRI (Philips Ingenia 3.0 T MRI scan, Amsterdam, Netherlands) at our hospital before surgery. The E-3D digital medical design system (Liao, 2018) was applied to reconstruct the lumen on the CI side in the CCD group to obtain the volume and surface area of the cavity.
Statistical analyses were performed using SPSS 22.0. The results of the MAIS/IT-MAIS and MUSS for both groups did not comply with the normal distribution by the Shapiro-Wilk test. The results of the non-normal distribution were described using the median (25th percentile, 75th percentile) and a non-parametric test was used to compare whether their differences were significant between the two groups and between the various assessment stages. Since the results of the cap questionnaire as well as the SIR questionnaire were rank data, the Wilcoxon test was used to compare whether there was a significant difference between the results of the two groups and between the various assessment stages. For lumen volume and surface area, Kaplan-Meier survival analysis was used to analyze their correlation with post-operative outcomes. Considering that patients with a CAP score of 4 could discriminate speech sounds without the aid of lip-reading, we defined a CAP score of 4 as the endpoint event. The post-operative time for a child to reach a CAP score of 4 was defined as the survival time. The median volume and median surface area were used as criteria for grouping. At the same time, p < 0.05 was considered a statistically significant difference.
• Demographic information of the CCD group
Table 1 summarizes the subject information, including gender, age at implantation, and length of follow-up. In the CCD group, there were 11 males and 12 females, with an implantation age of 0–7 years and a mean implantation age of 28 months, while the control group consisted of 31 males and 28 females, with an implantation age of 0–8 years and a mean implantation age of 29 months. The groups were not statistically different in terms of age and gender after non-parametric testing (p < 0.05).
• Development of auditory ability in the CCD Group
As shown in Tables 2, 3, both the percentage of MAIS/IT-MAIS scores and the CAP scores were significantly different between each time point in the CCD group (p < 0.05).
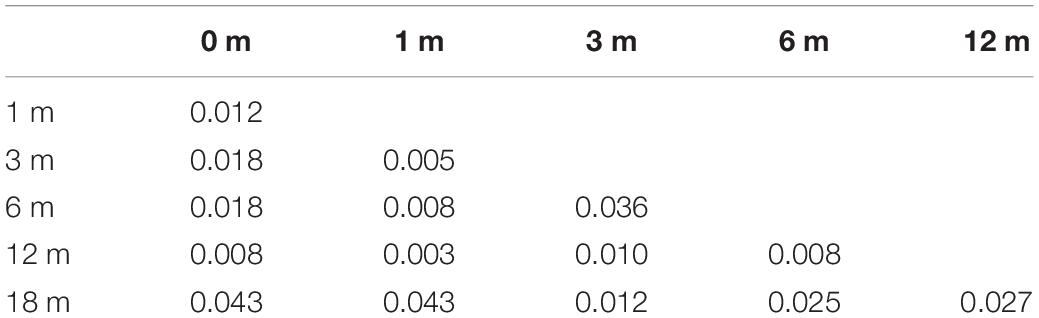
Table 2. Comparison of the percentage of Meaningful Auditory Integration Scale/Infant-Toddler Meaningful Auditory Integration Scale (MAIS/IT-MAIS) scores for each time point in the common cavity deformity (CCD) group.
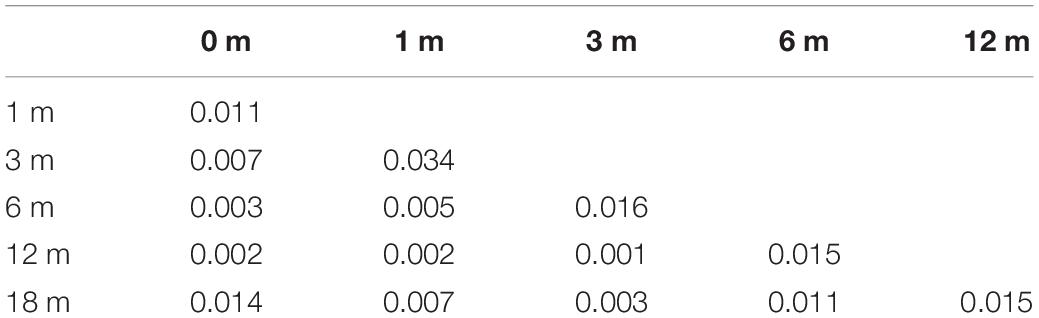
Table 3. Comparison of the categories of auditory performance (CAP) scores for each time point in the common cavity deformity (CCD) group.
• Development of speech in the CCD Group
For the percentage of MUSS scores (Table 4), there was no significant difference between the results of each time point except between 0 and 1 month after cochlear activation in the CCD group (p < 0.05). The SIR scores of the CCD group (Table 5) were significantly different between 0 and 6 months, 1 and 6 months, 0 and 12 months, 1 and 12 months, 3 and 12 months, 6 and 12 months, 0 and 18 months, 1 and 18 months, 3 and 18 months, and 6 and 18 months after cochlear activation (p < 0.05).
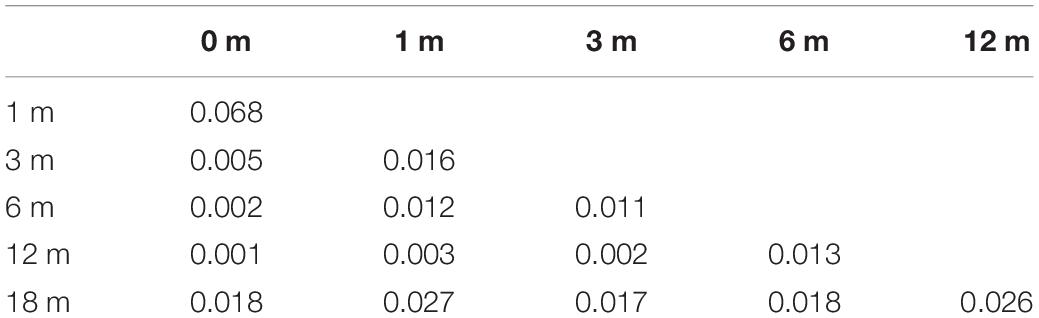
Table 4. Comparison of the percentage of Meaningful Use of Speech Scale (MUSS) scores for each time point in the common cavity deformity (CCD) group.

Table 5. Comparison of the Speech Intelligibility Rating (SIR) scores for each time point in the common cavity deformity (CCD) group.
• Characteristics of IT-MAIS/MAIS in the CCD and control groups
Figure 1A shows the mean, median, and 25th and 75th percentiles of the MAIS/ITMAIS score percentages for the CCD and control groups. The median scores were significantly different between the two groups at 1, 6, 12, and 18 months after cochlear activation (p < 0.05). Furthermore, trend of the average MAIS/ITMAIS score percentages of the two groups as the follow-up time increased.
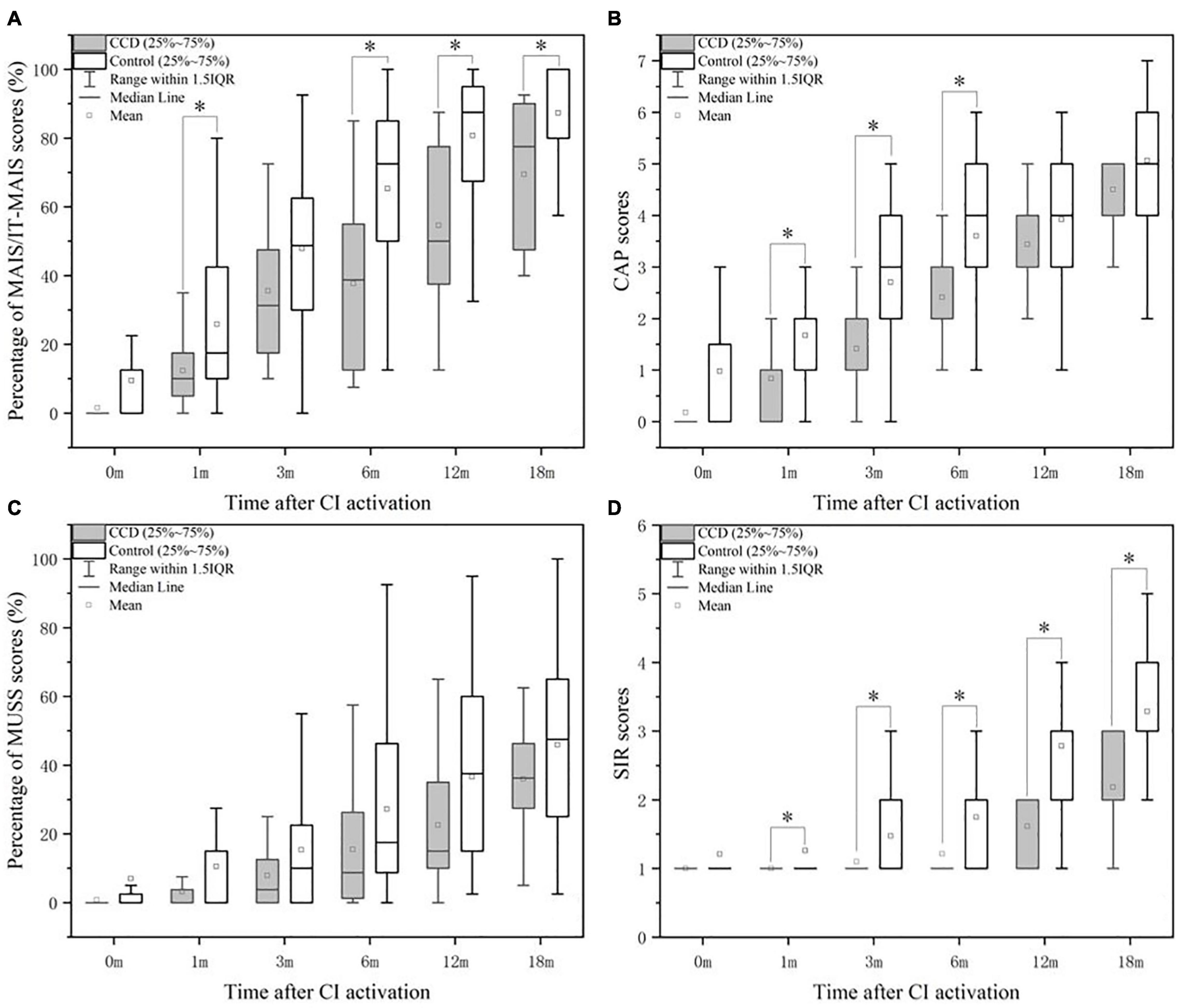
Figure 1. Comparison of the performance in MAIS/IT-MAIS (A), CAP (B), MUSS (C), and SIR (D) between the CCD and control groups. CAP, Categories of auditory performance; CCD, common cavity deformity; MAIS/IT-MAIS, Meaningful Auditory Integration Scale/Infant-Toddler Meaningful Auditory Integration Scale; MUSS, Meaningful Use of Speech Scale; SIR, Speech Intelligibility Rating. *indicates significant (p < 0.05).
• Characteristics of CAP in the CCD and control groups
Similar results were found in the comparison of CAP scores. Significant differences between the two groups were observed at 1, 3, and 6 months after CI activation (Figure 1B). The average CAP scores of the two groups increased over the follow-up period, but the average scores of the control group were higher than those of the CCD group.
• Characteristics of MUSS in the CCD and Control Groups
As for the median percentage of MUSS scores, there was no significant difference between the two groups at each time point after CI activation (Figure 1C). Nevertheless, the average percentage of MUSS scores increased slowly over time in both groups, and the CCD group obtained lower scores than the normal group (Figure 1C).
• Characteristics of SIR in the CCD and control groups
After statistically analyzing the differences in median SIR scores between the CCD and control groups, we observed that the differences were significant at 1, 3, 6, 12, and 18 months after CI activation (Figure 1D). Similar to the other questionnaires, the mean SIR score in the CCD group increased gradually over time and was worse than that in the control group.
• Correlations between Imaging Characteristics and CAP results
As shown in Figure 2, we reconstructed CT images of the surgical side of the cavity in 18 CCD patients, calculated their volume and surface area (Table 6), and performed a Kaplan-Meier survival analysis with the CAP results (Figure 3). Considering that patients with a CAP score of 4 could discriminate speech sounds without the aid of lip-reading, we defined a CAP score of 4 as the endpoint event. The post-operative time for a child to reach a CAP score of 4 was defined as the survival time. When the lumen surface area was ≥190.45 mm2, the mean survival time for CCD children to achieve a CAP score of 4 after surgery was 20.57 months, and the median survival time was 18.00 months; when the lumen surface area was <190.45 mm2, the mean survival time for CCD children to reach a CAP score of 4 after surgery was 12 months, and the median survival time was 12.00 months, with a statistically significant difference between the two groups (p = 0.02) (Figure 3A). When the lumen volume was ≥157.91 mm3, the mean survival time was 20.571 months, and the median time was 18.00 months; when it was <157.91 mm3, the mean and median survival times were 13.142 and 12.00 months, respectively. There was a significant difference between the two groups (p = 0.022) (Figure 3B).
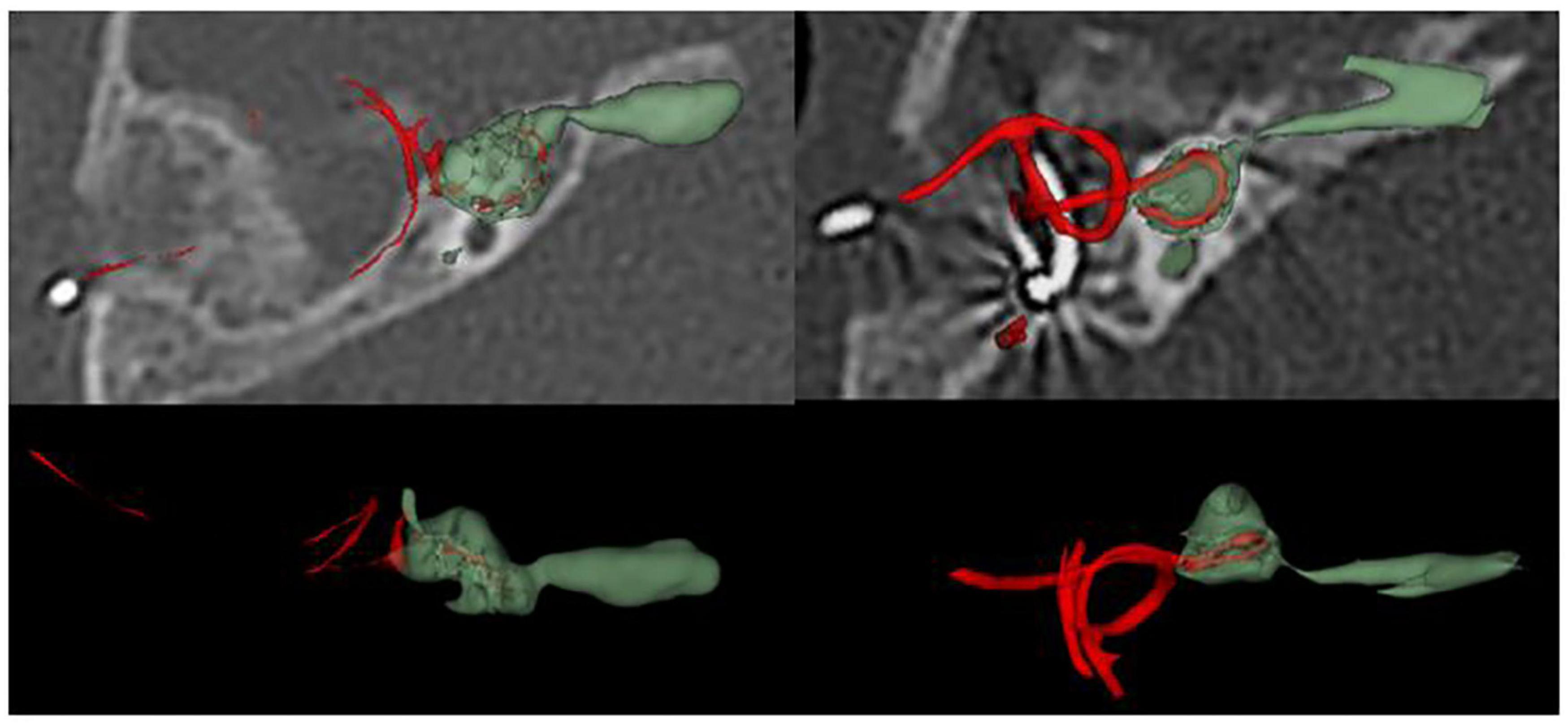
Figure 2. Computed tomography (CT) reconstruction of surgical side in patients with common cavity deformity (CCD).
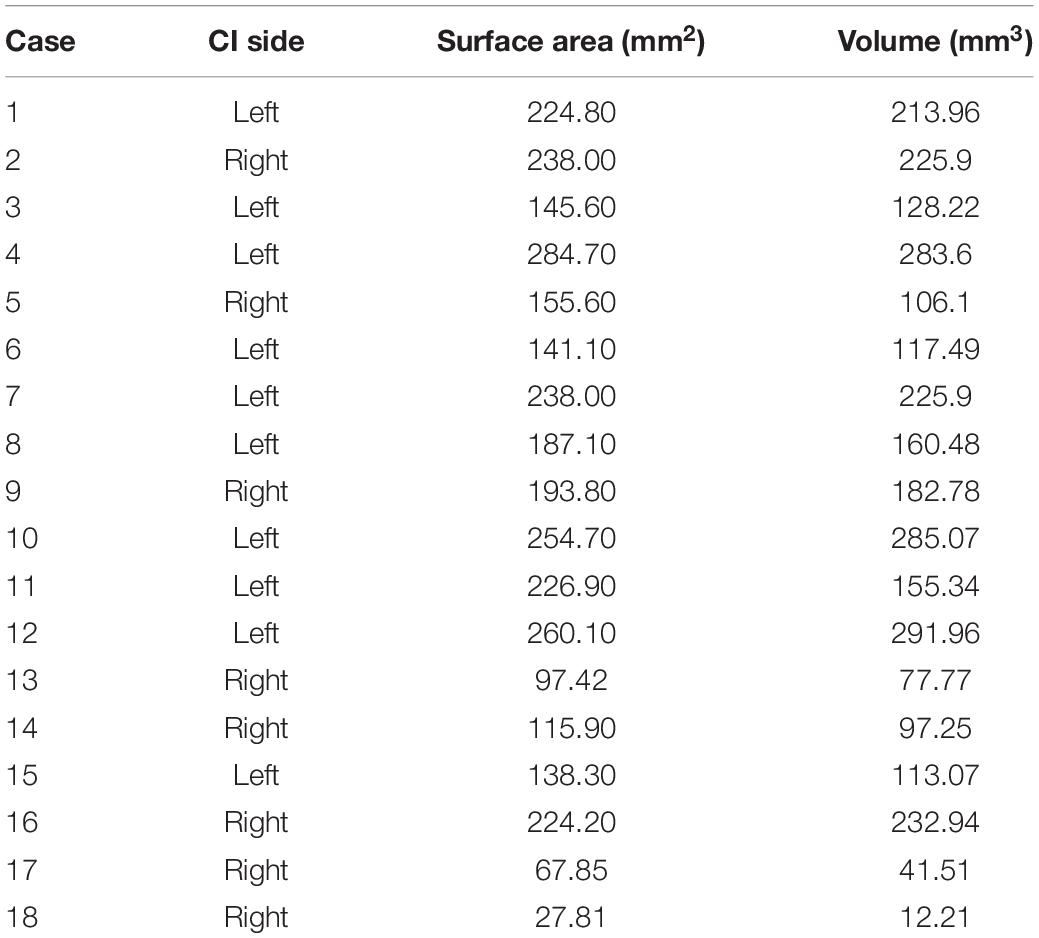
Table 6. Surface area and volume after three-dimensional reconstruction of the lumen in common cavity deformity (CCD) patients.
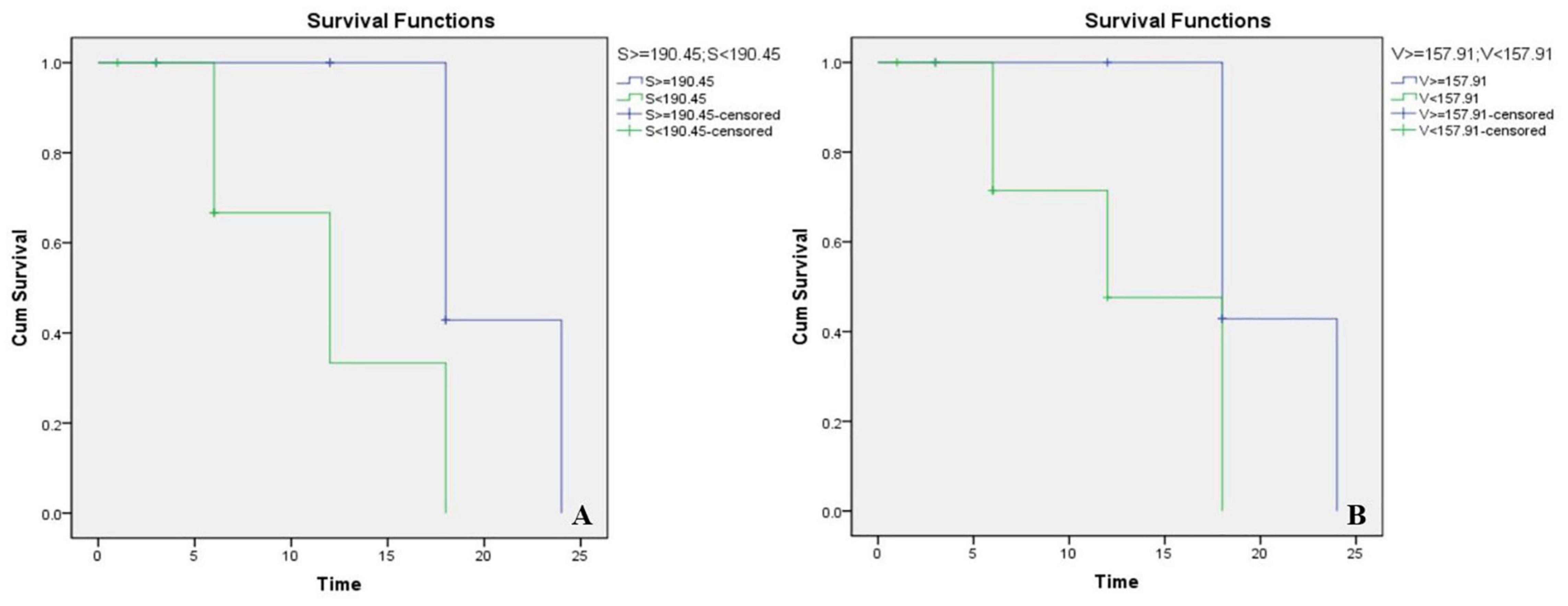
Figure 3. Kaplan-Meier survival chart of different lumen surface areas (A) and volumes (B). S, surface areas; V, volumes.
Common cavity deformity is regarded as a severe inner ear malformation, and the post-operative outcome of CI is generally considered to be worse in patients with CCD than in children with normal cochlear structures. In this study, 23 children with CCD were followed up using IT-MAIS/MAIS, MUSS, CAP, and SIR to assess their post-operative outcomes and correlations with imaging performance.
The MAIS is a common tool used to assess functional hearing in hearing-impaired children (Robbins et al., 1991). Each child was assessed by the answers provided by a parent or guardian familiar with the child’s condition. It consists of 10 questions that assess the use of hearing aids and the ability to perceive and understand sounds. The CAP is a scale used to assess the auditory ability of pediatric cochlear implant users in their daily lives (Archbold et al., 1998). The above two questionnaires are effective for evaluating the development of auditory stimuli in children who received cochlear implants or hearing aids. In relation to this, stimulation of the remaining spiral ganglion cells in the cochlea has been found to activate hearing (Fayad and Linthicum, 2006) and it has been suggested that spiral ganglion cells are distributed in the cavity walls of patients with CCD. This is the foundation for hearing acquisition after CI in CCD patients (Brotto et al., 2019). ITMAIS/MAIS and CAP scores were lower in the CCD group than in the normal group after activation. Similar results were reported by Xia et al. (2015). According to Tables 2, 3, the auditory development of CCD patients continuously improved up to 18 months after activation; that is, in the CCD group, there was no significant platform phase of auditory development in the 18 months after the activation of CI. Ahn et al. (2011) also observed an increase in the auditory performance of CCD patients with prolonged follow-up after 48 months of post-cochlear surgery evaluation, with a mean percentage MAIS score of 90.3 ± 18.1% and a mean CAP score of 4.9 ± 1.6. Although auditory development after CI is slower in CCD patients than in CI patients with normal cochlear structures, progress has been consistently made, suggesting the need for long-term post-operative rehabilitation in these patients.
For the assessment of speech development, MUSS and SIR are commonly used instruments. As shown in Tables 4, 5, there were significant differences in both the percentage of MUSS score and the SIR score when comparing the assessment results at different time points. However, the SIR score did not show a significant difference compared to the previous assessment results until 1 year after activation, whereas the percentage of MUSS score showed a significant difference at 3 months after activation, indicating that CCD patients showed a faster increase in MUSS performance compared to SIR. This may be related to the various aspects of speech development assessed by the two questionnaires. Like the MAIS, the MUSS is a parental report scale. It assesses the use of speech and consists of 10 questions designed to evaluate three aspects of speech development: vocalizing behavior, oral communication skills, and oral clarification skills (Archbold et al., 1998; Fayad and Linthicum, 2006). The SIR has been regularly used to evaluate the intelligibility of spontaneous speech in patients with cochlear implants (Xia et al., 2015). That is, the MUSS questionnaire evaluates speech skills, while SIR rates the intelligibility of pronunciation. However, the young age of the children in our study, with a follow-up period of only 1.5 years, made it difficult to demonstrate significant improvements in speech intelligibility. Nevertheless, since the MUSS includes an evaluation of vocalizing behavior, the children were given the opportunity to improve their scores. Additionally, this study observed that the percentage of MUSS scores in children with CCD was higher at 18 months than at 12 months after the activation, and the difference was significant, indicating that speech ability was still improving at 1.5 year after surgery. Xia et al. (2015) also observed a sustained increase in SIR scores for 4 years after CI. Therefore, post-operative speech development in patients with CCD is slow and requires long-term rehabilitation training.
Based on the comparison between the CCD group and the control group, it is obvious that auditory and speech development after CI was poorer in the CCD group than in the CI children with normal cochlear structures. This may be related to the structure malformation, lack of sufficient spiral ganglion cells (Brotto et al., 2019), or developmental delays (Buchman et al., 2004).
However, we observed good outcomes in some children. By 18 months after the activation, 66.67% of children with CCD had a CAP score of 5, but 16.67% achieved a score of only 3. Among the four children with CCD who had been followed for 3 years, only one reached a score of 40 on the IT-MAIS at 3 years after CI activation. We suspect that this may be related to the distribution and number of spiral ganglion cells in the cavity and the location of the electrode. The higher the number of spiral ganglion cells the electrode can stimulate, the greater the benefit to the child.
Furthermore, the post-operative outcome of CCD patients is also related to cerebrospinal fluid gusher, partial electrode insertion, and fewer active electrodes and the contact of the electrode with the inner wall (Dettman et al., 2011; Bae et al., 2022). The present study explored the relationship of post-operative outcomes with the degree of inner ear development in these patients.
With the development of imaging technology, techniques to assess the development of inner ear structures have become more sophisticated (Skinner et al., 2002; Verbist et al., 2010). After evaluating 36 cochleae with inner ear malformations using volume-rendering technique reconstruction and MPR, Ma et al. (2008) concluded that cochlear development could be more clearly assessed using volume-rendering technique reconstruction and MPR. We calculated the lumen volume and the surface area of 18 CCD patients using three-dimensional reconstruction techniques. The volume ranged from 12.21 to 291.96 mm3, with a mean volume of 163.98 ± 84.02 mm3; the surface area ranged from 27.81 to 284.7 mm2, with a mean value of 178.99 ± 71.85 mm2. This result implies that patients with CCD have variable cochlear morphology and size differences, which is consistent with the findings of Dhanasingh et al. (2019). This difference may require serious consideration of the surgical approach and the choice of electrodes.
Furthermore, after analyzing the correlations between volume, surface area, and post-operative outcomes of CCD patients, we found that the smaller the lumen, the shorter the time to reach a 4-point post-operative CAP. We speculate that this may be due to the smaller lumen, whose spiral ganglion cells may be more densely distributed, thus providing a larger effective area of electrode stimulation, which results in a greater likelihood of stimulation to ganglion cells. Earlier achievement of 4 points in CAP indicates a faster speed of rehabilitation within a year and a half after surgery but is not indicative of higher scores in the distant future.
Therefore, further studies using larger sample sizes and longer follow-up periods are needed to explore the distribution of intracochlear spiral ganglion cells in conjunction with the post-operative electrode location. These studies will provide guidance in the selection of treatment strategies for CCD patients.
The present study investigated the imaging performance and long-term auditory speech outcomes of 23 children with CCD, who were found to have poorer auditory and speech development and slower progress after CI than the control group. However, CCD patients still showed improvement in auditory and speech abilities at 1.5 year after CI; hence, they required long-term rehabilitation. The reconstruction of the temporal bone CT showed that the size, volume, and morphology of the cavity in CCD patients varied widely, and a small lumen size is associated with shorter time needed to reach a 4-point post-operative CAP. Further studies should be conducted to verify these results and clarify the relationship and mechanism using a larger sample of patients with CCD.
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
The studies involving human participants were reviewed and approved by the Ethics Committee of Beijing Tongren Hospital, Capital Medical University. Written informed consent to participate in this study was provided by the participants’ legal guardian/next of kin.
LZ and BC performed experiments, acquired and analyzed the data, drafted and revised the manuscript. YK programmed the CI and critically revised the manuscript. NL performed experiments and revised the manuscript. XW and YS conceived and designed the study, revised the manuscript. JC and MY acquired data and revised the manuscript. AD reconstructed the CT image of CCD patients. YL supervised the study, interpreted the result and critically revised the manuscript. All authors contributed to the article and approved the final and submitted version.
This work was supported by the National Natural Science Foundation of China (Grant No. 81870716).
AD was employed by MED-EL Medical Electronics GmbH.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
We thank all the subjects for their participation in this study.
Ahmad, R. L., Lokman, S., and Raja, A. A. K. (2005). Cochlear implantation in congenital cochlear abnormalities. Med. J. Malaysia 60, 379–382.
Ahn, J. H., Lim, H. W., and Lee, K. S. (2011). Hearing improvement after cochlear implantation in common cavity malformed cochleae: long-term follow-up results. Acta Otolaryngol. 131, 908–913. doi: 10.3109/00016489.2011.570786
Al-mahboob, A., Alhabib, S. F., Abdelsamad, Y., and Alzhrani, F. (2022). Cochlear implantation in common cavity deformity: a systematic review. Eur. Arch. Oto-Rhino-Laryngol. 279, 37–48. doi: 10.1007/s00405-021-06884-6885
Archbold, S., Lutman, M. E., and Nikolopoulos, T. (1998). Categories of auditory performance: inter-user reliability. Br. J. Audiol. 32, 7–12. doi: 10.3109/03005364000000045
Bae, S. H., Choi, J., and Choi, J. Y. (2022). Cochlear implants for patients with common cavity deformities and the impact of electrode positioning. Clin. Exp. Otorhinolaryngol. Online ahead of print. doi: 10.21053/ceo.2021.00745
Beltrame, M. A., Birman, C. S., Cervera Escario, J., Kassouma, J., Manolidis, S., Pringle, M. B., et al. (2013). Common cavity and custom-made electrodes: speech perception and audiological performance of children with common cavity implanted with a custom-made MED-EL electrode. Int. J. Pediatr. Otorhinolaryngol. 77, 1237–1243. doi: 10.1016/j.ijporl.2013.04.008
Beltrame, M. A., Bonfioli, F., and Frau, G. N. (2000). Cochlear implant in inner ear malformation: double posterior labyrinthotomy approach to common cavity. Adv. Otorhinolaryngol. 57, 113–119. doi: 10.1159/000059162
Beltrame, M. A., Frau, G. N., Shanks, M., Robinson, P., and Anderson, I. (2005). Double posterior labyrinthotomy technique: results in three Med-El patients with common cavity. Otol. Neurotol. 26, 177–182. doi: 10.1097/00129492-200503000-200503008
Bozorg Grayeli, A., Kalamarides, M., Bouccara, D., Ben Gamra, L., Ambert-Dahan, E., and Sterkers, O. (2007). Auditory brainstem implantation to rehabilitate profound hearing loss with totally ossified cochleae induced by pneumococcal meningitis. Audiol. Neurotol. 12, 27–30. doi: 10.1159/000096155
Brotto, D., Avato, I., Lovo, E., Muraro, E., Bovo, R., Trevisi, P., et al. (2019). Epidemiologic, imaging, audiologic, clinical, surgical, and prognostic issues in common cavity deformity: a narrative review. JAMA Otolaryngol. Head Neck Surg. 145, 72–78. doi: 10.1001/jamaoto.2018.2839
Buchman, C. A., Copeland, B. J., Yu, K. K., Brown, C. J., Carrasco, V. N., and Pillsbury, H. C. (2004). Cochlear implantation in children with congenital inner ear malformations. Laryngoscope 114, 309–316. doi: 10.1097/00005537-200402000-200402025
Casselman, J. W., Offeciers, E. F., De Foer, B., Govaerts, P., Kuhweide, R., and Somers, T. (2001). CT and MR imaging of congential abnormalities of the inner ear and internal auditory canal. Eur. J. Radiol. 40, 94–104. doi: 10.1016/S0720-048X(01)00377-371
Colletti, V., Carner, M., Miorelli, V., Guida, M., Colletti, L., and Fiorino, F. (2005). Auditory brainstem implant (ABI): new frontiers in adults and children. Otolaryngol. Head Neck Surg. 133, 126–138. doi: 10.1016/j.otohns.2005.03.022
Dettman, S., Sadeghi-Barzalighi, A., Ambett, R., Dowell, R., Trotter, M., and Briggs, R. (2011). Cochlear implants in forty-eight children with cochlear and/or vestibular abnormality. Audiol. Neurotol. 16, 222–232. doi: 10.1159/000320608
Dhanasingh, A., Dietz, A., Jolly, C., and Roland, P. (2019). Human inner-ear malformation types captured in 3d. J. Int. Adv. Otol. 15, 77–82. doi: 10.5152/iao.2019.6246
Fayad, J. N., and Linthicum, F. H. (2006). Multichannel cochlear implants: relation of histopathology to performance. Laryngoscope 116, 1310–1320. doi: 10.1097/01.mlg.0000227176.09500.28
Jackler, R. K., Luxford, W. M., and House, W. F. (1987). Congenital malformations of the inner ear: a classification based on embryo genesis. Laryngoscope 97, 2–14. doi: 10.1002/lary.5540971301
Liao, S., (2018). E-3D Medical Software V17.80 (Central South University, Changsha C. e3d-med n.d. Available online at: http://www.e3d-med.com/
Ma, H., Han, P., Liang, B., Tian, Z., Lei, Z., Kong, W., et al. (2008). Multislice spiral computed tomography imaging in congenital inner ear malformations. J. Comput. Assist. Tomogr. 32, 146–150. doi: 10.1097/rct.0b013e318063c64a
McElveen, J. T., Carrasco, V. N., Miyamoto, R. T., and Linthicum, F. H. (1997). Cochlear implantation in common cavity malformations using a transmastoid labyrinthotomy approach. Laryngoscope 107, 1032–1036. doi: 10.1097/00005537-199708000-00005
Molter, D. W., Pate, B. R., and Mcelveen, J. T. (1993). Cochlear implantation in the congenitally malformed Ear. Otolaryngol. Neck Surg. 108, 174–177. doi: 10.1177/019459989310800212
Papsin, B. C. (2005). Cochlear implantation in children with anomalous cochleovestibular anatomy. Laryngoscope 115, 1–26. doi: 10.1097/00005537-200501001-200501001
Robbins, A. M., Renshaw, J. J., and Berry, S. W. (1991). Evaluating meaningful auditory integration in profoundly hearing-impaired children. Am. J. Otol. 12, 144–150.
Sennaroğlu, L., and Bajin, M. D. (2017). Classification and current management of inner ear malformations. Balkan Med. J. 34, 397–411. doi: 10.4274/balkanmedj.2017.0367
Sennaroglu, L., Atay, G., and Bajin, M. D. (2014). A new cochlear implant electrode with a “cork”-type stopper for inner ear malformations. Auris Nasus Larynx 41, 331–336. doi: 10.1016/j.anl.2013.12.011
Sennaroğlu, L., Sennaroğlu, G., Yücel, E., Bilginer, B., Atay, G., Bajin, M. D., et al. (2016). Long-term results of abi in children with severe inner ear malformations. Otol. Neurotol. 37, 865–872. doi: 10.1097/MAO.0000000000001050
Shi, Y., Li, Y., Gong, Y., Chen, B., and Chen, J. (2019). Cochlear implants for patients with inner ear malformation: experience in a cohort of 877 surgeries. Clin. Otolaryngol. 44, 702–706. doi: 10.1111/coa.13360
Skinner, M. W., Ketten, D. R., Holden, L. K., Harding, G. W., Smith, P. G., Gates, G. A., et al. (2002). CT-derived estimation of cochlear morphology and electrode array position in relation to word recognition in nucleus-22 recipients. JARO - J. Assoc. Res. Otolaryngol. 3, 332–350. doi: 10.1007/s101620020013
Toh, E. H., and Luxford, W. M. (2008). Cochlear and Brainstem Implantation. Neurosurg. Clin. N. Am. 19, 317–329. doi: 10.1016/j.nec.2008.02.014
Tucci, D. L., Telian, S. A., Zimmerman-Phillips, S., Zwolan, T. A., and Kileny, P. R. (1995). Cochlear implantation in patients with cochlear malformations. Arch. Otolaryngol. Neck Surg. 121, 833–838. doi: 10.1001/archotol.1995.01890080005001
Verbist, B. M., Skinner, M. W., Cohen, L. T., Leake, P. A., James, C., Boëx, C., et al. (2010). Consensus panel on a cochlear coordinate system applicable in histologic, physiologic, and radiologic studies of the human cochlea. Otol. Neurotol. 31, 722–730. doi: 10.1097/MAO.0b013e3181d279e0
Wei, X., Li, Y., Chen, B., Gong, Y., Fu, Q. J., Liu, T., et al. (2017). Predicting auditory outcomes from radiological imaging in cochlear implant patients with cochlear nerve deficiency. Otol. Neurotol. 38, 685–693. doi: 10.1097/MAO.0000000000001382
Wei, X., Li, Y., Fu, Q., Gong, Y., Chen, B., Chen, J., et al. (2018). Slotted labyrinthotomy approach with customized electrode for patients with common cavity deformity. Laryngoscope 128, 468–472. doi: 10.1002/lary.26627
Keywords: common cavity deformity, cochlear implantation, auditory development, speech development, 3D reconstruction
Citation: Zhang L, Chen B, Kong Y, Liau N, Wei X, Shi Y, Chen J, Yang M, Dhanasingh A and Li Y (2022) Analysis of Long-Term Cochlear Implantation Outcomes and Correlation With Imaging Characteristics in Patients With Common Cavity Deformity. Front. Neurosci. 16:857855. doi: 10.3389/fnins.2022.857855
Received: 19 January 2022; Accepted: 14 February 2022;
Published: 23 March 2022.
Edited by:
Fei Chen, Southern University of Science and Technology, ChinaReviewed by:
Mohan Kameswaran, Madras ENT Research Foundation (P) Ltd., IndiaCopyright © 2022 Zhang, Chen, Kong, Liau, Wei, Shi, Chen, Yang, Dhanasingh and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yongxin Li, ZW50bHl4QGNjbXUuZWR1LmNu
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.