
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Neurosci. , 22 April 2022
Sec. Neuroprosthetics
Volume 16 - 2022 | https://doi.org/10.3389/fnins.2022.854219
This article is part of the Research Topic Human Machine Interface-based Neuromodulation Solutions for Neurorehabilitation View all 19 articles
Background: Rehabilitation of post-stroke dysphagia is an urgent clinical problem, and repetitive transcranial magnetic stimulation (rTMS) has been widely used in the study of post-stroke function. However, there is no reliable evidence-based medicine to support the effect of rTMS on post-stroke dysphagia. This review aims to evaluate the effectiveness and safety of rTMS on post-stroke dysphagia.
Methods: English-language literature published before December 20, 2021, were searched in six electronic databases. Identified articles were screened, data were extracted, and the methodological quality of included trials was assessed. Meta-analysis was performed using RevMan 5.3 software. The GRADE method was used to assess the quality of the evidence.
Results: A total of 10 studies with 246 patients were included. Meta-analysis showed that rTMS significantly improved overall swallowing function (standardized mean difference [SMD]−0.76, 95% confidence interval (CI)−1.07 to−0.46, p < 0.0001, n = 206; moderate-quality evidence), Penetration Aspiration Scale (PAS) (mean difference [MD]−1.03, 95% CI−1.51 to−0.55, p < 0.0001, n = 161; low-quality evidence) and Barthel index scale (BI) (MD 23.86, 95% CI 12.73 to 34.99, p < 0.0001, n = 136; moderate-quality evidence). Subgroup analyses revealed that (1) rTMS targeting the affected hemisphere and targeting both hemispheres significantly enhanced overall swallowing function and reduced aspiration. (2) Low-frequency rTMS significantly enhanced overall swallowing function and reduced aspiration, and there was no significant difference between high-frequency rTMS and control group in reducing aspiration (p = 0.09). (3) There was no statistical difference in the dropout rate (low-quality evidence) and adverse effects (moderate-quality evidence) between the rTMS group and the control group.
Conclusion: rTMS improved overall swallowing function and activity of daily living ability and reduced aspiration in post-stroke patients with good acceptability and mild adverse effects.
Stroke, as a common cerebrovascular disease, is the primary cause of disability worldwide (Gorelick, 2019). About 19-81% of survivors after stroke are left with dysphagia, which is characterized by varing degree of eating disorders, choking cough, salivation and abnormal pronunciation (Martino et al., 2005; Suntrup et al., 2015). Dysphagia is associated with increased risk of malnutrition and pneumonia, and leads to prolonged hospital stay, poor prognosis and mortality (Park et al., 2017; Pandian et al., 2018; Alamer et al., 2020). Therefore, the rehabilitation of post-stroke dysphagia is still an urgent clinical problem.
Repetitive transcranial magnetic stimulation (rTMS), as a non-invasive neuromodulation technique, is an emerging choice for post-stroke dysphagia (Lefaucheur et al., 2020). In general, rTMS can be divided into two main treatment protocols according to the stimulation frequency: low frequency (≤ 1 Hz) and high frequency (> 1 Hz). Low frequency rTMS (LF-rTMS) inhibits cortical excitability, while high frequency rTMS (HF-rTMS) activates cortical excitability (Lin et al., 2019). It is now recognized that rTMS can inhibit maladaptive cortical plasticity, improve adaptive cortical activity, and promote neurological recovery after stroke (Kobayashi and Pascual-Leone, 2003). According to the latest evidence-based guidelines for rTMS, rTMS has been proved to show the efficacy of A grade in treatment of depression, neuropathic pain, and upper limb dysfunction after stroke (Lefaucheur et al., 2020).
In recent years, several meta-analyses (Yang et al., 2015, 2021; Liao et al., 2017; Lin et al., 2019; Cheng et al., 2021; Li et al., 2021) have investigated the effects of rTMS on post-stroke dysphagia, suggesting that rTMS may have beneficial effects on swallowing disorders. However, some of reviews focused on non-invasive brain stimulation (NIBS), including rTMS, transcranial Direct Current Stimulation (tDCS) and other kinds of stimulation, while few of reviews further analyzed the effects of stimulation site, frequency and stimulation time on dysphagia. A recent meta-analysis (Yang et al., 2021), partially affirming the effects of rTMS on post-stroke dysphagia, concluded in its subgroup analysis of intervention frequency that there was no statistically significant difference between either the high-frequency and low-frequency groups or the conventional training group, which may be related to incorrect data extraction and exclusion of some studies that met their inclusion criteria. A growing body of evidence supports the beneficial effects of transcranial magnetic stimulation on post-stroke dysphagia (Lefaucheur et al., 2020), but the relationship between transcranial magnetic stimulation and factors such as target, parameter settings, and treatment course remains to be further investigated. Therefore, this meta-analysis aims to provide the latest evidence on the effects of transcranial magnetic stimulation on post-stroke swallowing disorders.
This work adhered to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines (Ardern et al., 2021).
The following databases were searched to identify studies on the effect of rTMS on post-stroke dysphagia, published before December 20, 2021: PubMed, Cochrane Library, ScienceDirect, MEDLINE, and Web of Science for relevant studies. The English keywords used for the database searches were “stroke,” “transcranial magnetic stimulation,” “repetitive transcranial magnetic stimulation,” “TMS,” “rTMS,” “deglutition disorders,” and “dysphagia.” The reference lists of identified articles were checked for other potential studies.
Two review authors independently assessed the methodological quality of the included studies. We recorded and resolved any disagreements through discussions with a third reviewer.
Clinical studies that meet the following criteria were included:
(1) All patients with ischemic or hemorrhagic stroke displayed definitive radiographic evidence of relevant pathology on magnetic resonance imaging (MRI) or computed tomography (CT);
(2) All participants were identified as having dysphagia;
(3) No participants had swallowing disorders caused by other diseases;
(4) Randomized controlled trials compared rTMS with sham stimulation or other routine rehabilitation training.
If data were repeated or shared in multiple studies, the study that best met the above criteria were considered. All published or unpublished studies were investigated. If the information required for the analysis could not be obtained from the publication, the author was contacted to obtain the necessary details.
Two review authors independently assessed the methodological quality of the included studies. A third reviewer recorded and resolved any disagreements. Each RCT used Cochrane's collaborative tools to assess the risk of bias, including adequacy of sequence generation, concealment of allocation, blinding of participants and personnel, blinding of result evaluators, incomplete results' data, selective reporting, and other biases (Higgins et al., 2011; Corbett et al., 2014). The Grading of Recommendations Assessment, Development and Evaluation (GRADE) guidelines for systematic reviews were used to evaluate the quality of outcomes (Guyatt et al., 2008).
All searches and included studies were conducted by two independent reviewers. If there was any objection, a third reviewer made the final decision. The following data were extracted from the final included researches: basic study information (study authors, year of publication), participant characteristics (age, and sample size), rTMS parameters [stimulus site, true stimulus frequency, stimulus intensity (% of motor threshold (MT)), and treatment regimen], overall swallowing function and activity of daily living outcome measures, dropout rate, and adverse effects.
Outcome measures for the efficacy of therapy were as follows: (1) DD (Dysphagia Grade); (2) Functional Dysphagia Scale (FDS); (3) Videofluoroscopic Dysphagia Scale (VDS); (4) Penetration Aspiration Scale (PAS); (5) Barthel index scale (BI); (6) dropout rate; (7) adverse effects.
The DD is a four-level score for the swallowing function according to patients' clinical manifestations (Khedr and Abo-Elfetoh, 2010). The FDS is a scale quantifying dysphagia severity (Han et al., 2001). The VDS, with a sum of 100 points, is a reliable, objective, and quantifiable predictor of long-term dysphagia after stroke (Kim et al., 2014). The PAS is an 8 point multidimensional indicator of airway invasion that measures selected aspects such as penetration and inhalation, depth of invasion into the delivery airway, and whether substances entering the airway are expelled (Martin-Harris et al., 2005). The higher the score of the above 4 scales, the worse the swallowing function. If dysphagia outcomes were reported from multiple time points, those from immediately after the intervention were obtained for meta-analysis.
All statistical analysis used the RevMan 5.3 statistical software (The Nordic Cochrane Center, The Cochrane Collaboration, Copenhagen, Denmark), and the heterogeneity of different research results was tested by the overlap of confidence intervals and chi-square tests. When there was no heterogeneity in the test results, fixed-effect model was used for the meta-analysis, and when the test results were heterogeneous, the random-effect model was used. For enumeration data, the risk ratio (RR) and 95% confidence intervals (CIs) were used as the statistical tool for the efficacy analysis and the effect size, respectively. If substantial heterogeneity was detected (I2 > 50%), subgroup analysis or sensitivity analysis was conducted to determine the source of heterogeneity.
The study selection process is shown in Figure 1. A total of 206 potential relevant studies were screened from six English-language databases using a relevant search strategy. Of these relevant studies, 102 duplicates were removed and the remaining 104 studies were further evaluated for eligibility. An additional 48 articles were removed after screening the title and abstract. Finally, after reviewing the full text of the remaining 56 articles, 46 articles were excluded, and a total of 10 studies were included.
Table 1 shows the characteristics of the 10 studies included in this meta-analysis with a total of 246 participants (149 in the rTMS group and 109 in the control group). Participants all were identified dysphagia according to either the videofluoroscopic swallowing study (VFSS) or Fiberoptic endoscopic evaluation of swallowing (FEES). Four studies (Lim et al., 2014; Tarameshlu et al., 2019; Unluer et al., 2019; Cabib et al., 2020) used LF-rTMS; 4 studies (Khedr et al., 2009; Khedr and Abo-Elfetoh, 2010; Park et al., 2013, 2017) used HF-rTMS; and 2 studies (Kim et al., 2011; Du et al., 2016) compared the efficacy of LF-rTMS and HF-rTMS. rTMS stimulation sites included the ipsilesional hemisphere, the contralesional hemisphere, and the bilateral hemisphere. The interventions of control group included sham rTMS stimulation among 7 studies (Khedr et al., 2009; Khedr and Abo-Elfetoh, 2010; Kim et al., 2011; Park et al., 2013, 2017; Du et al., 2016; Cabib et al., 2020), and 3 studies (Lim et al., 2014; Tarameshlu et al., 2019; Unluer et al., 2019) with conventional therapy.
In terms of outcome measures, different dysphagia measurement tools were used to assess swallowing function within the same study or between studies. Overall swallowing function measures included DD [4 studies (Khedr et al., 2009; Khedr and Abo-Elfetoh, 2010; Du et al., 2016; Tarameshlu et al., 2019)], FDS [2 studies (Kim et al., 2011; Lim et al., 2014)], VDS [2 studies (Park et al., 2013, 2017)]. Aspiration was assessed by PAS [6 studies (Kim et al., 2011; Park et al., 2013, 2017; Lim et al., 2014; Unluer et al., 2019; Cabib et al., 2020)]. BI was used to assess activity of daily living.
In all included literature, some of articles designed two experimental groups based on parameters such as lesion site and stimulation frequency. According to this review, the two experimental groups did not interfere with each other in the same literature. Therefore, we treated each study in these three articles as a randomized controlled experiment. There was also one study that divided the patients into two randomized controlled trials based on the site of the disease, and we combined and merged the data. We selected 13 studies from 10 articles. Two researchers assessed the quality of the 13 included studies. Data completeness was assured in a large extent, but 4 studies (Park et al., 2013; Lim et al., 2014; Unluer et al., 2019; Cabib et al., 2020) had performance bias (complete blinding of subjects was not achieved) (Figure 2). The number of this meta-analysis included is very small, so we could not use funnel plots to assess publication bias. Therefore, publication bias could not be completely eliminated.
Ten studies involving a total of 206 patients with post-stroke dysphagia evaluated the effect of rTMS on overall swallowing function. Heterogeneity of included studies was low (I2 = 45%), and therefore a fixed-effect model was used for meta-analysis. The funnel plot revealed significant symmetry (Figure 3). The simulated results showed that the rTMS significantly improved overall swallowing function compared to the control group (standard mean difference [SMD]−0.76, 95% confidence interval (CI)−1.07 to−0.46, p < 0.0001) (Figure 4). According to the GRADE, the overall level of evidence for the effect of rTMS on global swallowing function was “Moderate” (Table 2).
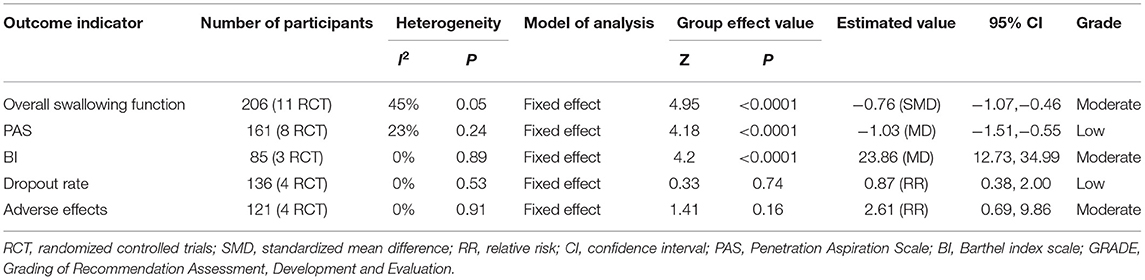
Table 2. GRADE quality of evidence assessment of individual outcome indicators for the efficacy of repetitive transcranial magnetic stimulation in the treatment of dysphagia.
Subgroup analyses were performed according to stimulus site (the ipsilesional hemisphere, the contralesional hemisphere, and the bilateral hemisphere). Subgroup analysis showed that the SMD for trials involving the “the ipsilesional hemisphere” stimulus was−0.74 (95% CI−1.69 to 0.20, p = 0.12) and for trials involving the “the contralesional hemisphere” stimulus was−0.59 (95% CI−1.14 to−0.05, p = 0.03). The mean effect size for trials involving “the bilateral hemisphere” stimulus was−1.15 (95% CI−1.87 to−0.43) (Figure 5). Stimulation of the bilateral hemisphere may produce better therapeutic effects on overall swallowing function. Subgroup analyses were performed according to stimulation frequency (LF-rTMS, HF-rTMS). Subgroup analysis showed a SMD of−0.70 (95% CI−1.33 to -−0.06) for the studies of HF-rTMS. The study of LF-rTMS showed a SMD of−0.86 (95% CI−1.16 to - 0.34). These results suggested that LF-rTMS treatment produced better effects on overall swallowing function than HF-rTMS treatment (Figure 6).
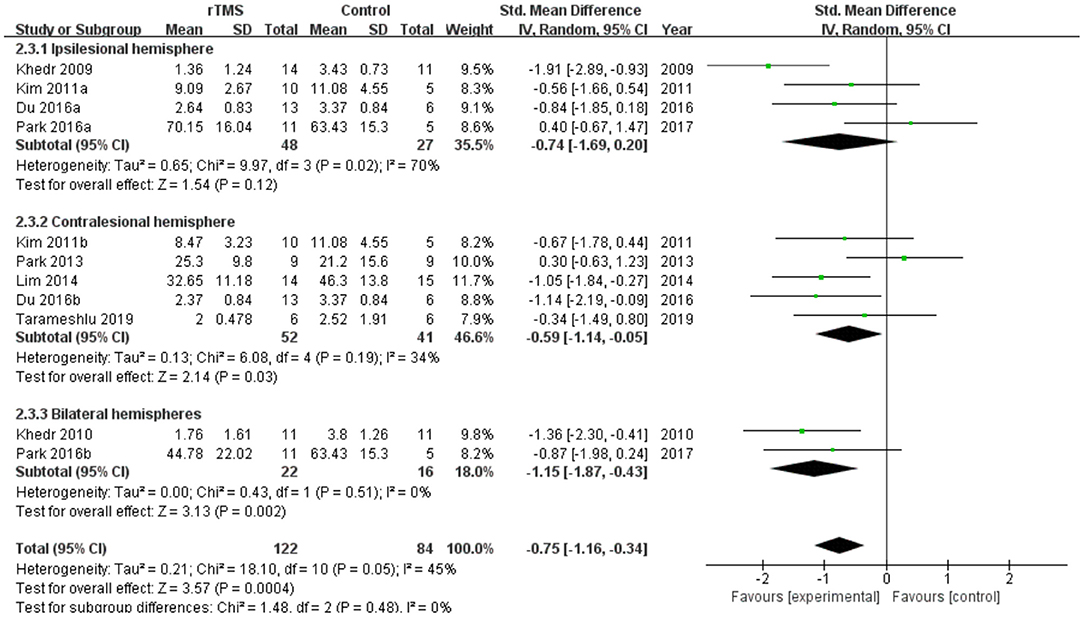
Figure 5. Forest plot for subgroup analysis for overall swallowing function: ipsilesional hemisphere vs. contralesional hemisphere vs. bilateral hemispheres.
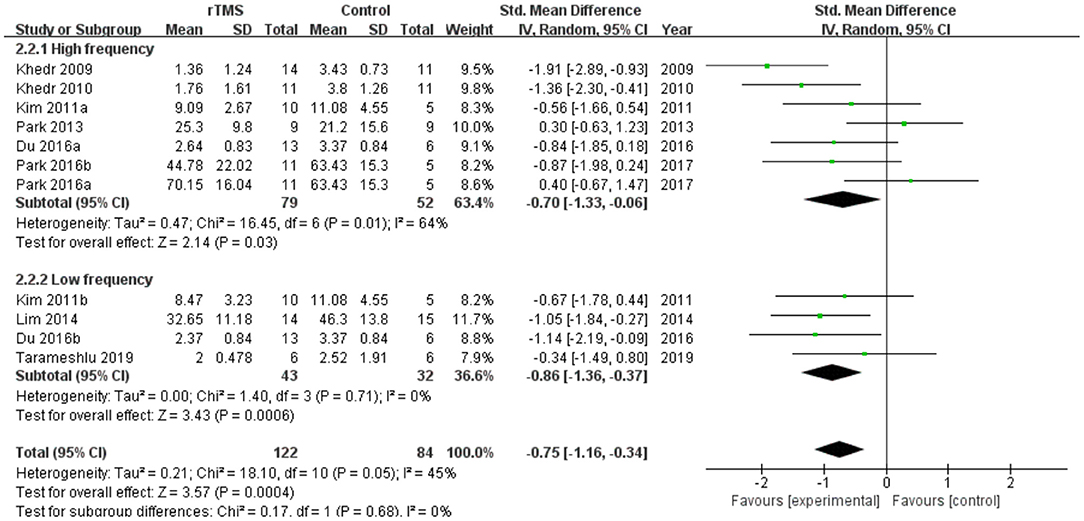
Figure 6. Forest plot for subgroup analysis for overall swallowing function: low frequency transcranial magnetic stimulation vs. high frequency transcranial magnetic stimulation.
Seven studies involving a total of 161 patients with post-stroke dysphagia evaluated the effect of rTMS on PAS. Heterogeneity of included studies was low (I2 = 23%), and therefore a fixed-effect model was used. The simulated results showed that the rTMS significantly reduced accidental aspiration compared to the control group (mean difference [MD]−1.03, 95% CI−1.51 to−0.55, p < 0.0001) (Figure 7). According to the GRADE, the overall level of evidence for the effect of rTMS on PAS was “Low” (Table 2).
Subgroup analyses were performed according to stimulus site (the ipsilesional hemisphere, the contralesional hemisphere, and the bilateral hemisphere). Subgroup analysis showed that the MD for trials involving the “the ipsilesional hemisphere” stimulus was−0.09 (95% CI−0.95 to 0.78, p = 0.85) and for trials involving the “the contralesional hemisphere” stimulus was−1.37 (95% CI−2.00 to−0.75, p < 0.0001). The MD for trials involving “the bilateral hemisphere” stimulus was−2.06 (95% CI−3.71 to−0.41) (Figure 8). Stimulation of the bilateral hemisphere may produce better therapeutic effects on overall swallowing function. Subgroup analyses were performed according to stimulation frequency (LF-rTMS, HF-rTMS). Subgroup analysis showed a MD of−0.60 (95% CI−1.31 to -−0.10) for the studies of HF-rTMS. The studies of LF-rTMS showed a SMD of−1.42 (95% CI−2.09 to - 0.75). These results suggest that LF-rTMS treatment produced better effects on overall swallowing function than HF-rTMS treatment (Figure 9).
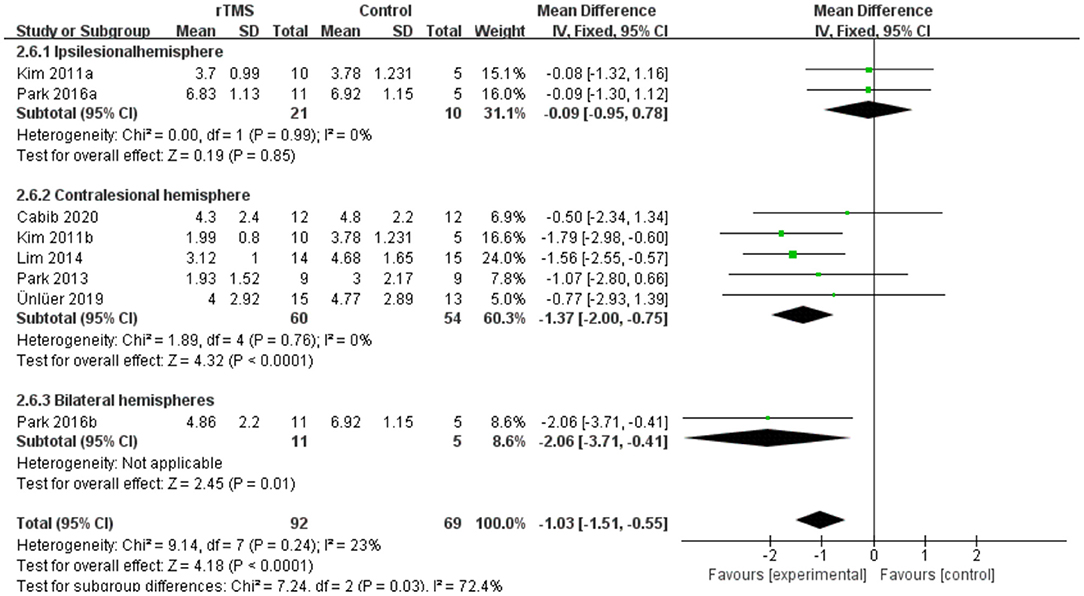
Figure 8. Forest plot for subgroup analysis for Penetration Aspiration Scale: ipsilesional hemisphere vs. contralesional hemisphere vs. bilateral hemispheres.
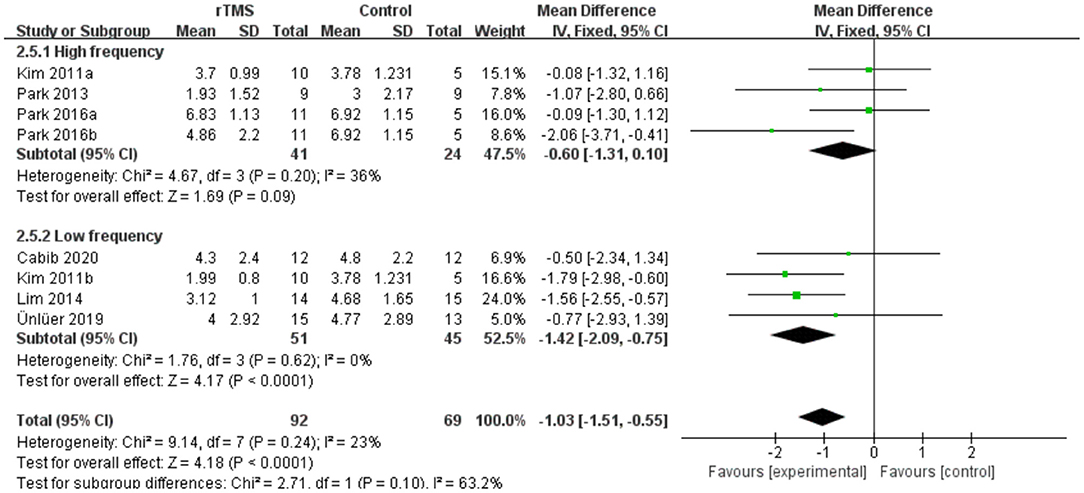
Figure 9. Forest plot for subgroup analysis for Penetration Aspiration Scale: low frequency Transcranial Magnetic Stimulation vs. high frequency Transcranial Magnetic Stimulation.
Four studies involving a total of 137 patients with post-stroke dysphagia evaluated the effect of rTMS on BI. Heterogeneity of included studies was low (I2 = 0%), and therefore a fixed-effect model was used for meta-analysis. The simulated results showed that the rTMS significantly improved activity of daily living compared to the control group (MD 23.86, 95% CI 12.73 to 34.99, p < 0.0001) (Figure 10). According to the GRADE, the overall level of evidence for the effect of rTMS on BI was “Moderate” (Table 2).
Four studies involving a total of 136 patients with post-stroke dysphagia evaluated the effect of rTMS on dropout rate. Heterogeneity of included studies was low (I2 = 0%), and therefore a fixed-effect model was used for meta-analysis. The results showed no differences in dropout rate between the rTMS group and the control group (RR 0.87, 95% CI 0.38 to 2.00, p = 0.74) (Figure 11). According to the GRADE, the overall level of evidence for the effect of rTMS on dropout rate was “Low” (Table 2).
No serious adverse reactions were reported in any of the included studies. Four studies reported minor adverse reactions. Seven of 67 patients in the rTMS group and 1 of 54 patients in the control group reported discomfort. Other adverse reactions included headache, dizziness, pain at the site of irritation, and tinnitus. There was no heterogeneity between studies (I2 = 0%). The results showed no differences in adverse effects between the rTMS group and the control group (RR 2.61, 95% CI 0.69 to 9.86, p = 0.16) (Figure 12). According to the GRADE, the overall level of evidence for the effect of rTMS on adverse effects was “Moderate” (Table 2).
This meta-analysis identified 10 studies including a total of 246 patients with post-stroke dysphagia, 149 of whom received 5 to 10 sessions of active rTMS and 109 of whom received sham rTMS or swallowing training. Overall, the results of our meta-analysis supported the benefits of rTMS on overall dysphagia function (moderate-quality evidence) and which reduced instances of aspiration (low-quality evidence) and improved activity of daily living (moderate-quality evidence) for patients with post-stroke dysphagia. rTMS was found to be safe and have no serious adverse effects reported.
Our meta-analysis suggested that rTMS improved swallowing function in post-stroke patients and the heterogeneity of all outcome indicators remained small (I2 <50%). The funnel plot was symmetrical, suggesting no publication bias in the included studies. The pooled results were generally consistent with previous reviews (Liao et al., 2017; Cheng et al., 2021; Wang et al., 2021), which reported a positive effect of rTMS on recovery from post-stroke dysphagia. The difference from the above studies was that we included only studies in which rTMS was compared with sham stimulation or conventional swallowing treatment, excluding the effect of other types such as NIBS on post-stroke dysphagia.
To reduce potential heterogeneity, we further performed subgroup analysis based on stimulation site and stimulation frequency. Stimulation location subgroup analysis showed that rTMS of the bilateral hemisphere and the contralesional hemisphere significantly improved swallowing function after stroke. In contrast, rTMS of the ipsilesional hemisphere produced lower effect values and the results of the meta-analysis suggested that stimulation of the ipsilesional hemisphere was ineffective, in agreement with the results of the meta-analysis by Liao et al. (2017) and Cheng et al. (2021). Momosaki et al. (2014) reported that 3 Hz rTMS of the bilateral pharyngeal motor cortex resulted in significant recovery on post-stroke dysphagia. Tarameshlu et al. (2019) and Unluer et al. (2019) found that rTMS was effective in improving post-stroke dysphagia and swallowing coordination after stimulation of the unaffected hemisphere. In a randomized controlled trial (Park et al., 2017), Park et al. showed no effect of high-frequency rTMS of the affected hemisphere on swallowing function. However, Du et al. (2016) observed a positive effect of rTMS of the affected hemisphere on swallowing disorders. Therefore, more studies are needed to further validate the effects of rTMS on the affected hemisphere.
Within-frequency subgroup analysis showed that LF-rTMS produced greater effect values than HF-rTMS, suggesting that LF-rTMS is more effective than HF-rTMS on swallowing disorders, which is consistent with the results of Cheng et al. (2021). Kim et al. (2011) conducted a RCT comparing LF-rTMS and HF-rTMS in improving post-stroke dysphagia and found that the effect of LF-rTMS was significant compared with HF-rTMS. In contrast, a meta-analysis by Liao et al. (2017) concluded that the effect size of the HF-rTMS subgroup was greater than that of the LF-rTMS subgroup. This may be related to its early publication and the inclusion of only six studies. Also, we found that the results of our subgroup analysis were not fully consistent with the results of some meta-analyses. Yang et al. (2021) found a simulated effect size SMD = 0.65 (95% CI = 0.04 - 1.26, p = 0.04) for rTMS, suggesting that rTMS treatment was superior to conventional treatment. However, subgroup analysis showed no statistical difference between LF-rTMS and HF-rTMS and the conventional training group. This is slightly different from our results, that rTMS showed a significant improvement in overall swallowing function in our pooled analysis, and was also effective in both groups in the subgroup analysis. This may be related to the fact that the review by Yang et al. missed some of the studies that met their inclusion criteria.
Recovery of impaired swallowing function after stroke is complex. Functional magnetic resonance imaging studies suggest that swallowing function may be associated with primary motor sensory cortex, insula, cingulate gyrus, prefrontal cortex, temporal lobe and occipital areas (Mihai et al., 2014, 2016). After stroke, if the injury involves the cortical brainstem tract, medulla oblongata reticular structure or nerve nucleus, the swallowing muscles will not work properly, thus affecting swallowing function (Wilmskoetter et al., 2020). The corticomedullary is the bridge between the brainstem and the swallowing cortex, and a study by Michou et al. confirmed that increased excitability of the corticomedullary was associated with improved swallowing safety (Mihai et al., 2014). Hamdy et al. shown the human swallowing system is bilaterally innervated and is asymmetric (Hamdy et al., 1996). The bilateral cerebral hemispheres maintain normal swallowing function by inhibiting homeostasis through the interaction of the corpus callosum (Lefaucheur et al., 2020). Hamdy et al. also suggested that reorganization of the contralateral pharyngeal cortex was associated with recovery of swallowing function, which demonstrates the role of intact hemispheric reorganization in the recovery of swallowing function after stroke (Hamdy et al., 1998; Fraser et al., 2002).
Therefore, different stimulation protocols will improve post-stroke swallowing disorders through different pathways. First, in unilateral cortical stimulation protocols, current mainstream studies are generally based on the interhemispheric inhibition model, such as Tarameshlu et al. (2019) and Khedr and Abo-Elfetoh (2010) included in our meta-analysis. This theory suggests that the damaged hemisphere decreases excitatory output after brain injury, while the unaffected hemisphere produces excessive inhibition on the affected hemisphere, resulting in various functional impairments (Alia et al., 2017). LF-rTMS stimulates the contralesional hemisphere to produce a long-term depression effect or HF-rTMS stimulates the ipsilesional hemisphere to produce a long-term potentiation effect, thus bringing the rebalance. The long term potentiation effect of HF-rTMS of the ipsilesional hemisphere can bring the imbalanced cortical excitability back to balance, thus improving the function (Lefaucheur et al., 2020). This theory has been widely applied to various types of NIBS, but it can only partially explain the results obtained from our subgroup analysis.
Again, in the compensatory model it was noted that the recovery of dysfunction after brain injury may be related to compensatory reorganization in the unaffected hemisphere, and stimulation of the unaffected hemisphere with HF-rTMS may facilitate the emergence of this compensation and contribute to the recovery of swallowing function (Hamdy et al., 1998). However, our results in the subgroup analysis of overall swallowing function showed that the SMD of HF-rTMS (-0.70, P = 0.03) was smaller than LF-rTMS (-0.86, P = 0.0006); the subgroup analysis in PAS showed no significant effect of HF-rTMS (P = 0.09). At the same time, some of the studies (Park et al., 2013, 2017) included in this review used this model and did not observe any significant improvement in swallowing disorders. Therefore, it's rational to suspect that the mechanism of recovery from dysphagia after stroke is more complex than the interhemispheric inhibition model or the compensatory model.
In addition to this, a bimodal balance-recovery model has recently been proposed to describe the process of neuroplastic changes after stroke. This model incorporates the concept of “structural reserve”; if the brain has extensive damage and low structural reserve, then input from the unaffected hemisphere will be critical to replace the lost function; and conversely, if the structural reserve is high, then neural stimulation based on the interhemispheric inhibition model may be more appropriate (Sankarasubramanian et al., 2017). This provides a possible explanation for our conclusion. We found that bilateral stimulation was more effective than unilateral stimulation in some studies, and we considered that the use of transcranial magnetic stimulation in both hemispheres could produce a significant swallowing recovery effect by promoting plasticity in both hemispheres. We have found similar results in rTMS to improve other types of post-stroke dysfunction. For example, Jiang et al. (2020) observed higher effect values for the bilateral hemisphere compared to unilateral hemisphere stimulation in a meta-analysis of rTMS improvement of cognitive dysfunction. However, few studies have done the subgroup analyse according to degree of injury because the recruited patients had different degrees of brain injury. Raw data were also very difficult to obtain, so it was difficult for us to analyze them in subgroups according to different levels of injury. Future studies should be conducted in further subgroups according to different injury levels and time of stroke onset to explore the development of individualized treatment plans for patients.
In activity of daily living ability, we found significant improvement in the rTMS group, which is consistent with the studies of Liu et al. (2021) and Sui et al. (2021) rTMS can further improve hand function and cognitive function after stroke, thus further improving patients' motor ability and activity of daily living ability (Lefaucheur et al., 2020; Sharma et al., 2020).
In terms of treatment acceptability, the results of this study suggest that rTMS treatment was well-tolerated and there was no significant difference in the dropout rate between the rTMS treatment group and the control group. The reasons for follow-up failure were not mainly related to rTMS treatment, and no serious adverse effects were reported in any of the included trials. On the other hand, adverse reactions associated with rTMS were rare and mild, although patients in the rTMS-treated group were more likely to experience adverse reactions than the control group. The most common were transient headache and dizziness.
Several limitations should be considered when interpreting the results of the current study. First, the small sample size (12–29) of the included studies may limit the statistical power to detect the effects of rTMS on swallowing function in patients with post-stroke dysphagia. Second, there was considerable heterogeneity in the stimulation parameters (frequency, intensity and pulse) in the included studies. Therefore, the optimal stimulation parameters for rTMS are not clear. Third, our paper only performed subgroup analyses for frequency and stimulation site, but there was heterogeneity in the results of some of the subgroup analyses. The efficacy of rTMS may also be influenced by other parameters, such as brain injury severity and time to stroke onset.
In conclusion, this meta-analysis study suggests that rTMS has a favorable effect on swallowing function in patients with post-stroke dysphagia. However, there are many parameters that can influence the efficacy, such as the frequency and the site of stimulation. Further research on the mechanism of rTMS and the setting of optimal parameters will be important for the development of this novel intervention in clinical practice.
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author/s.
Y-lX and SW were responsible for the literature screening and data extraction. SW, J-mJ, and Y-hX were responsible for risk of bias assessment. Y-lX and XC were responsible for statistical analysis and writing up the article. WQ and Y-xW were responsible for planning and guidance on this paper. All authors contributed to the article and approved the submitted version.
This work was supported by Sichuan Medical Research Project Plan [Q18038]; Research and Development Project of Affiliated Hospital of North Sichuan Medical College [2021ZD014] and China Nanchong City-School Cooperative Scientific Research Special Fund [19SXHZ0103].
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Alamer, A., Melese, H., and Nigussie, F. (2020). Effectiveness of neuromuscular electrical stimulation on post-stroke dysphagia: a systematic review of randomized controlled trials. Clin. Interv. Aging. 15, 1521–1531. doi: 10.2147/CIA.S262596
Alia, C., Spalletti, C., Lai, S., Panarese, A., Lamola, G., Bertolucci, F., et al. (2017). Neuroplastic changes following brain ischemia and their contribution to stroke recovery: novel approaches in neurorehabilitation. Front. Cell. Neurosci. 11, 76. doi: 10.3389/fncel.2017.00076
Ardern, C. L., Buttner, F., Andrade, R., Weir, A., Ashe, M. C., Holden, S., et al. (2021). Implementing the 27 PRISMA 2020 Statement items for systematic reviews in the sport and exercise medicine, musculoskeletal rehabilitation and sports science fields: the PERSiST (implementing Prisma in Exercise, Rehabilitation, Sport medicine and SporTs science) guidance. Br. J. Sports Med. 56:e103987. doi: 10.1136/bjsports-2021-103987
Cabib, C., Nascimento, W., Rofes, L., Arreola, V., Tomsen, N., Mundet, L., et al. (2020). Short-term neurophysiological effects of sensory pathway neurorehabilitation strategies on chronic poststroke oropharyngeal dysphagia. Neurogastroenterol. Motil. 32:e13887. doi: 10.1111/nmo.13887
Cheng, I., Sasegbon, A., and Hamdy, S. (2021). Effects of neurostimulation on poststroke dysphagia: a synthesis of current evidence from randomized controlled trials. Neuromodulation 24, 1388–1401. doi: 10.1111/ner.13327
Corbett, M. S., Higgins, J. P., and Woolacott, N. F. (2014). Assessing baseline imbalance in randomised trials: implications for the Cochrane risk of bias tool. Res. Synth. Methods 5, 79–85. doi: 10.1002/jrsm.1090
Du, J., Yang, F., Liu, L., Hu, J., Cai, B., Liu, W., et al. (2016). Repetitive transcranial magnetic stimulation for rehabilitation of poststroke dysphagia: A randomized, double-blind clinical trial. Clin. Neurophysiol. 127, 1907–1913. doi: 10.1016/j.clinph.2015.11.045
Fraser, C., Power, M., Hamdy, S., Rothwell, J., Hobday, D., Hollander, I., et al. (2002). Driving plasticity in human adult motor cortex is associated with improved motor function after brain injury. Neuron 34, 831–840. doi: 10.1016/s0896-6273(02)00705-5
Gorelick, P. B.. (2019). The global burden of stroke: persistent and disabling. Lancet Neurol. 18, 417–418. doi: 10.1016/S1474-4422(19)30030-4
Guyatt, G. H., Oxman, A. D., Vist, G. E., Kunz, R., Falck-Ytter, Y., Alonso-Coello, P., et al. (2008). GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ 336, 924–926. doi: 10.1136/bmj.39489.470347.AD
Hamdy, S., Aziz, Q., Rothwell, J. C., Power, M., Singh, K. D., Nicholson, D. A., et al. (1998). Recovery of swallowing after dysphagic stroke relates to functional reorganization in the intact motor cortex. Gastroenterology 115, 1104–1112. doi: 10.1016/s0016-5085(98)70081-2
Hamdy, S., Aziz, Q., Rothwell, J. C., Singh, K. D., Barlow, J., Hughes, D. G., et al. (1996). The cortical topography of human swallowing musculature in health and disease. Nat. Med. 2, 1217–1224. doi: 10.1038/nm1196-1217
Han, T. R., Paik, N. J., and Park, J. W. (2001). Quantifying swallowing function after stroke: A functional dysphagia scale based on videofluoroscopic studies. Arch. Phys. Med. Rehabil. 82, 677–682. doi: 10.1053/apmr.2001.21939
Higgins, J. P., Altman, D. G., Gotzsche, P. C., Juni, P., Moher, D., Oxman, A. D., et al. (2011). The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ. 343:d5928. doi: 10.1136/bmj.d5928
Jiang, L., Cui, H., Zhang, C., Cao, X., Gu, N., Zhu, Y., et al. (2020). Repetitive transcranial magnetic stimulation for improving cognitive function in patients with mild cognitive impairment: a systematic review. Front. Aging Neurosci. 12, 593000. doi: 10.3389/fnagi.2020.593000
Khedr, E. M., and Abo-Elfetoh, N. (2010). Therapeutic role of rTMS on recovery of dysphagia in patients with lateral medullary syndrome and brainstem infarction. J. Neurol. Neurosurg. Psychiatry. 81, 495–499. doi: 10.1136/jnnp.2009.188482
Khedr, E. M., Abo-Elfetoh, N., and Rothwell, J. C. (2009). Treatment of post-stroke dysphagia with repetitive transcranial magnetic stimulation. Acta Neurol. Scand. 119, 155–161. doi: 10.1111/j.1600-0404.2008.01093.x
Kim, J., Oh, B. M., Kim, J. Y., Lee, G. J., Lee, S. A., and Han, T. R. (2014). Validation of the videofluoroscopic dysphagia scale in various etiologies. Dysphagia 29, 438–443. doi: 10.1007/s00455-014-9524-y
Kim, L., Chun, M. H., Kim, B. R., and Lee, S. J. (2011). Effect of repetitive transcranial magnetic stimulation on patients with brain injury and Dysphagia. Ann. Rehabil. Med. 35, 765–771. doi: 10.5535/arm.2011.35.6.765
Kobayashi, M., and Pascual-Leone, A. (2003). Transcranial magnetic stimulation in neurology. Lancet Neurol. 2, 145–156. doi: 10.1016/s1474-4422(03)00321-1
Lefaucheur, J. P., Aleman, A., Baeken, C., Benninger, D. H., Brunelin, J., Di Lazzaro, V., et al. (2020). Evidence-based guidelines on the therapeutic use of repetitive transcranial magnetic stimulation (rTMS): An update (2014-2018). Clin. Neurophysiol. 131, 474–528. doi: 10.1016/j.clinph.2019.11.002
Li, L., Huang, H., Jia, Y., Yu, Y., Liu, Z., Shi, X., et al. (2021). Systematic review and network meta-analysis of noninvasive brain stimulation on dysphagia after stroke. Neural Plast. 2021:3831472. doi: 10.1155/2021/3831472
Liao, X., Xing, G., Guo, Z., Jin, Y., Tang, Q., He, B., et al. (2017). Repetitive transcranial magnetic stimulation as an alternative therapy for dysphagia after stroke: a systematic review and meta-analysis. Clin. Rehabil. 31, 289–298. doi: 10.1177/0269215516644771
Lim, K. B., Lee, H. J., Yoo, J., and Kwon, Y. G. (2014). Effect of low-frequency rTMS and NMES on subacute unilateral hemispheric stroke with dysphagia. Ann. Rehabil. Med. 38, 592–602. doi: 10.5535/arm.2014.38.5.592
Lin, Y., Jiang, W. J., Shan, P. Y., Lu, M., Wang, T., Li, R. H., et al. (2019). The role of repetitive transcranial magnetic stimulation (rTMS) in the treatment of cognitive impairment in patients with Alzheimer's disease: A systematic review and meta-analysis. J. Neurol. Sci. 398, 184–191. doi: 10.1016/j.jns.2019.01.038
Liu, Y., Li, H., Zhang, J., Zhao, Q. Q., Mei, H. N., and Ma, J. (2021). A meta-analysis: whether repetitive transcranial magnetic stimulation improves dysfunction caused by stroke with lower limb spasticity. Evid. Based Complement. Alternat. Med. 2021:7219293. doi: 10.1155/2021/7219293
Martin-Harris, B., Brodsky, M. B., Michel, Y., Ford, C. L., Walters, B., and Heffner, J. (2005). Breathing and swallowing dynamics across the adult lifespan. Arch. Otolaryngol. Head Neck Surg. 131, 762–770. doi: 10.1001/archotol.131.9.762
Martino, R., Foley, N., Bhogal, S., Diamant, N., Speechley, M., and Teasell, R. (2005). Dysphagia after stroke: incidence, diagnosis, and pulmonary complications. Stroke 36, 2756–2763. doi: 10.1161/01.STR.0000190056.76543.eb
Mihai, P. G., Otto, M., Domin, M., Platz, T., Hamdy, S., and Lotze, M. (2016). Brain imaging correlates of recovered swallowing after dysphagic stroke: A fMRI and DWI study. Neuroimage Clin. 12, 1013–1021. doi: 10.1016/j.nicl.2016.05.006
Mihai, P. G., Otto, M., Platz, T., Eickhoff, S. B., and Lotze, M. (2014). Sequential evolution of cortical activity and effective connectivity of swallowing using fMRI. Hum. Brain Mapp. 35, 5962–5973. doi: 10.1002/hbm.22597
Momosaki, R., Abo, M., and Kakuda, W. (2014). Bilateral repetitive transcranial magnetic stimulation combined with intensive swallowing rehabilitation for chronic stroke Dysphagia: a case series study. Case Rep. Neurol. 6, 60–67. doi: 10.1159/000360936
Pandian, J. D., Gall, S. L., Kate, M. P., Silva, G. S., Akinyemi, R. O., Ovbiagele, B. I., et al. (2018). Prevention of stroke: a global perspective. Lancet 392, 1269–1278. doi: 10.1016/S0140-6736(18)31269-8
Park, E., Kim, M. S., Chang, W. H., Oh, S. M., Kim, Y. K., Lee, A., et al. (2017). Effects of bilateral repetitive transcranial magnetic stimulation on post-stroke dysphagia. Brain Stimul. 10, 75–82. doi: 10.1016/j.brs.2016.08.005
Park, J. W., Oh, J. C., Lee, J. W., Yeo, J. S., and Ryu, K. H. (2013). The effect of 5Hz high-frequency rTMS over contralesional pharyngeal motor cortex in post-stroke oropharyngeal dysphagia: a randomized controlled study. Neurogastroenterol. Motil. 25, 324–e250. doi: 10.1111/nmo.12063
Sankarasubramanian, V., Machado, A. G., Conforto, A. B., Potter-Baker, K. A., Cunningham, D. A., Varnerin, N. M., et al. (2017). Inhibition versus facilitation of contralesional motor cortices in stroke: Deriving a model to tailor brain stimulation. Clin. Neurophysiol. 128, 892–902. doi: 10.1016/j.clinph.2017.03.030
Sharma, H., Vishnu, V. Y., Kumar, N., Sreenivas, V., Rajeswari, M. R., Bhatia, R., et al. (2020). Efficacy of low-frequency repetitive transcranial magnetic stimulation in ischemic stroke: a double-blind randomized controlled trial. Arch. Rehabil. Res. Clin. Transl. 2:100039. doi: 10.1016/j.arrct.2020.100039
Sui, Y. F., Tong, L. Q., Zhang, X. Y., Song, Z. H., and Guo, T. C. (2021). Effects of paired associated stimulation with different stimulation position on motor cortex excitability and upper limb motor function in patients with cerebral infarction. J. Clin. Neurosci. 90, 363–369. doi: 10.1016/j.jocn.2021.06.028
Suntrup, S., Kemmling, A., Warnecke, T., Hamacher, C., Oelenberg, S., Niederstadt, T., et al. (2015). The impact of lesion location on dysphagia incidence, pattern and complications in acute stroke. Part 1: dysphagia incidence, severity and aspiration. Eur. J. Neurol. 22, 832–838. doi: 10.1111/ene.12670
Tarameshlu, M., Ansari, N. N., Ghelichi, L., and Jalaei, S. (2019). The effect of repetitive transcranial magnetic stimulation combined with traditional dysphagia therapy on poststroke dysphagia: a pilot double-blinded randomized-controlled trial. Int. J. Rehabil. Res. 42, 133–138. doi: 10.1097/MRR.0000000000000336
Unluer, N. O., Temucin, C. M., Demir, N., Serel, A. S., and Karaduman, A. A. (2019). Effects of low-frequency repetitive transcranial magnetic stimulation on swallowing function and quality of life of post-stroke patients. Dysphagia. 34, 360–371. doi: 10.1007/s00455-018-09965-6
Wang, T., Dong, L., Cong, X., Luo, H., Li, W., Meng, P., et al. (2021). Comparative efficacy of non-invasive neurostimulation therapies for poststroke dysphagia: A systematic review and meta-analysis. Neurophysiol. Clin. 51, 493–506. doi: 10.1016/j.neucli.2021.02.006
Wilmskoetter, J., Daniels, S. K., and Miller, A. J. (2020). Cortical and subcortical control of swallowing-can we use information from lesion locations to improve diagnosis and treatment for patients with stroke? Am. J. Speech Lang. Pathol. 29, 1030–1043. doi: 10.1044/2019_AJSLP-19-00068
Yang, S. N., Pyun, S. B., Kim, H. J., Ahn, H. S., and Rhyu, B. J. (2015). Effectiveness of non-invasive brain stimulation in dysphagia subsequent to stroke: a systemic review and meta-analysis. Dysphagia. 30, 383–391. doi: 10.1007/s00455-015-9619-0
Keywords: deglutition disorders, transcranial magnetic stimulation, stroke, meta-analysis, systematic review
Citation: Xie Y-l, Wang S, Jia J-m, Xie Y-h, Chen X, Qing W and Wang Y-x (2022) Transcranial Magnetic Stimulation for Improving Dysphagia After Stroke: A Meta-Analysis of Randomized Controlled Trials. Front. Neurosci. 16:854219. doi: 10.3389/fnins.2022.854219
Received: 13 January 2022; Accepted: 21 March 2022;
Published: 22 April 2022.
Edited by:
Jinhua Zhang, Xi'an Jiaotong University, ChinaReviewed by:
Marcello Romano, Azienda Ospedaliera Ospedali Riuniti Villa Sofia Cervello, ItalyCopyright © 2022 Xie, Wang, Jia, Xie, Chen, Qing and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yin-xu Wang, MzQwODk2ODFAcXEuY29t; Wu Qing, Y2JrZjIwMTdAMTI2LmNvbQ==
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.