
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Neurosci., 10 August 2022
Sec. Sleep and Circadian Rhythms
Volume 16 - 2022 | https://doi.org/10.3389/fnins.2022.811771
This article is part of the Research TopicInsights in Sleep and Circadian Rhythms: 2021View all 8 articles
A commentary has been posted on this article:
Commentary: "Chronotype, circadian rhythm, and psychiatric disorders: Recent evidence and potential mechanisms"
The circadian rhythm is crucial for physiological and behavioral functions. Chronotype, which represents individual preferences for activity and performance, is associated with human health issues, particularly psychiatric disorders. This narrative review, which focuses on the relationship between chronotype and mental disorders, provides an insight into the potential mechanism. Recent evidence indicates that (1) the evening chronotype is a risk factor for depressive disorders and substance use disorders, whereas the morning chronotype is a protective factor. (2) Evening chronotype individuals with bipolar disorder tend to have more severe symptoms and comorbidities. (3) The evening chronotype is only related to anxiety symptoms. (4) The relationship between chronotype and schizophrenia remains unclear, despite increasing evidence on their link. (5) The evening chronotype is significantly associated with eating disorders, with the majority of studies have focused on binge eating disorders. Furthermore, the underlying mechanisms or influence factors are described in detail, including clock genes, brain characteristics, neuroendocrinology, the light/dark cycle, social factors, psychological factors, and sleep disorders. These findings provide the latest evidence on chronotypes and psychiatric disorders and serve as a valuable reference for researchers.
Circadian rhythms are driven by the internal biological clock, producing 24-h rhythm autonomously while being synchronized daily by environmental signals (zeitgebers). Chronotype reflects the individual variability in the phase of entrainment. In the modern world, social activities may not be consistent with personal preference for sleep–wake time, disrupting our normal circadian rhythm, known as circadian misalignment or social jet lag (SJL). Researchers have found that circadian misalignment may be associated with medical illnesses, like neurodevelopmental disorders, psychiatric disorders, neurodegenerative disorders, cardiac diseases, and cancers (Germain and Kupfer, 2008; Logan and McClung, 2019).
This narrative review aims to summarize recent studies on chronotype and examine its relationship with mental disorders. The structure is as follows. We first described the definition and relation of circadian rhythms, sleep regulation, chronotype, and SJL to clarify the potential mechanism and the relationship to human health. Then, we reviewed the differences between chronotypes and the relationship with psychiatric disorders. In the third section, we discussed the relationship between chronotype and psychiatric disorders in detail, including depressive disorders, bipolar disorders (BD), anxiety disorders, schizophrenia, substance use disorders, and eating disorders. Finally, we concluded with a summary of the current state of research, identifying gaps and providing suggestions regarding future research.
The word “circadian,” meaning a cycle across one day, can be deconstructed as “circa” (meaning “about”) and “dies or diem” (meaning “a day”) (Bauducco et al., 2020). The physiological parameters, behavioral performance, and cognitive functions of humans vary during the 24-h day (Croce et al., 2018). Circadian rhythms, which are defined as 24-h biological rhythms, are driven by endogenous oscillators (such as suprachiasmatic nuclei and pineal and peripheral oscillators), and exogenous factors (such as the light/dark cycle, social life, and the sleep-wake cycle) which can synchronize the biological clock. To be precise, those periodic exogenous factors, called “zeitgeber” (“time giver” in German), are the environmental time cues that are likely to synchronize/entrain circadian rhythms every day (Golombek and Rosenstein, 2010). The relationship through which the circadian oscillator synchronizes with zeitgebers in phase or period is called the entrainment. It is a necessary process since the period of the endogenous oscillators is close to but not exactly 24 h, which may result in a drift or desynchronization with the solar rhythm without being regularly reset by zeitgebers. The phase of entrainment depends on the intrinsic period of the circadian oscillator and the strength of the zeitgeber. The strength comprises the range of intensities and sensitivity of the circadian oscillator to the zeitgeber (Aschoff and Pohl, 1978).
The master endogenous oscillator is situated in the hypothalamic suprachiasmatic nuclei (SCN), which receives light signals through melanopsin-containing photoreceptors in the retinal ganglion cells (Logan and McClung, 2019). The SCN is an essential central pacemaker that produces and coordinates circadian rhythms in organisms and is known as the master clock or circadian oscillator in mammals. After the retinal ganglion cells are integrated, the external light signals project through the retinohypothalamic tract to the SCN via the optic chiasm (Etain et al., 2011; Jagannath et al., 2017). At the cellular-molecular level, the clock or oscillator is driven by a negative and transcriptional-translational feedback loop of clock genes (described in section “Clock genes and their polymorphisms play a role in phenotypes and are linked with psychiatric disorders”). The SCN is responsible for receiving and integrating information from the zeitgeber, regulating the functions of other brain areas and organs, and coordinating the body to maintain rhythms consistent with the external environment (Jagannath et al., 2017).
In humans, two intrinsic oscillators, namely the circadian oscillator (Process C) and the homeostatic oscillator (Process S), regulate two aspects of sleep (sleep duration and sleep time, respectively) (Gruber and Cassoff, 2014). In Process C, the circadian oscillator produces the waking drive, which increases during the daytime and reaches maximum intensity at the end of the day, and the sleep drive, which increases during the night and reaches a maximum at the end of the night (Taillard et al., 2021). This oscillator regulates sleep timing but not sleep duration. In Process S, the homeostatic oscillator generates homeostatic sleep pressure that increases during wakefulness and reduces during sleep. Regarding the mechanism of sleep pressure, the hypothesis is that the accumulation of neurotransmitters (such as adenosine) leads to neuronal activation during wakefulness (Dijk and von Schantz, 2005). This oscillator mainly regulates the depth and length of sleep. During a biological day, sleep pressure is counteracted by the drive of wakefulness to remain awake. Drowsiness occurs when the sleep pressure increases while the waking drive rapidly dissipates. After sleep onset, the sleep pressure begins to dissipate, but its effect is replaced by the increased circadian drive of sleep, which extends the sleep period. The dissipation of the sleep drive leads to the desire to wake up (Dijk and von Schantz, 2005).
In addition, orexin and orexin receptors play a crucial role in regulating sleep and wakefulness. The neurons in the lateral hypothalamic area (LHA) produce orexin A and B (regulators of feeding behavior), which can activate monoaminergic and cholinergic neurons in the hypothalamus and brain stem. These monoaminergic neurons drive arousal and wakefulness by producing norepinephrine, serotonin, dopamine, or histamine (Scammell et al., 2017). During the daytime, the SCN indirectly activates LHA, leading to these monoaminergic cells sending excitatory projections to the thalamus cortex and inhibiting the ventrolateral preoptic area (VLPO). During sleep, VLPO may be activated by sleep substances (e.g., adenosine) and inhibits monoaminergic cells and neurons in LHA to maintain sleep (Sakurai, 2007).
Chronotype, referred to as diurnal preference, is a part of circadian rhythmicity (Adan et al., 2012) and is typically defined as individual variation in the preferred timing of the sleep–wake cycle (Zavada et al., 2005). More precisely, chronotype reflects individual variability in the phase of entrainment and is associated with variations of physiological nature, such as the rhythm of body temperature and hormone secretion (Taylor and Hasler, 2018). Chronotype is evaluated using self-reported questionnaires, usually the Morningness-Eveningness Questionnaire (MEQ) (Horne and Ostberg, 1976), its reduced five-item version (rMEQ) (Adan and Almirall, 1991), and the Munich Chronotype Questionnaire (MCTQ) (Roenneberg et al., 2003). The Composite Scale of Morningness (CSM) (Smith et al., 1989), the Diurnal Type Scale (DTS), the Circadian Type Questionnaire (CTQ), and the Preference Scale (PS) can also be considered instruments for evaluating chronotype (Adan et al., 2012). Other relatively objective tools can evaluate chronotypes, including core body temperature, dim light melatonin onset (DLMO), a sleep diary (Carney et al., 2012), wrist accelerometry, and actigraphs (Kaufmann et al., 2018; Lyall et al., 2018). We have reviewed the three most commonly used questionnaires in the Supplementary material.
Recent studies commonly divide chronotypes into three (or five) types: morning chronotype (moderate morning chronotype and definite morning chronotype), evening chronotype (moderate evening chronotype and definite evening chronotype), and intermediate chronotype (Horne and Ostberg, 1976). Morning chronotype individuals, also known as early chronotypes or larks, prefer to be active in the morning and sleep and wake early. Evening chronotypes, also called late chronotypes or owls, prefer to be active in the evening and sleep and wake up late. The intermediate chronotype (neutral or neither type) has no preference for morning or evening. Morning chronotype individuals achieve peak physical and mental performance in the early part of the day after waking up. Conversely, evening chronotype individuals have the best mental and physical performance before sleeping (Song et al., 2018). Most research indicated that the circadian phase of the morning chronotype is advanced by two or three hours than the evening chronotype by measuring the core temperature or melatonin (Baehr et al., 2000; Lack et al., 2009; Taillard et al., 2011). In addition, evening chronotype is also related to larger daily sleep debt, a great need for sleep, and morning sleepiness (Taillard et al., 1999, 2004). Taillard et al. investigated the effect of chronotype on the sleep-wake cycle in a large and demographic matching sample of all ages (617 subjects between 17 and 80 years). This study has shown that evening chronotypes are related to greater subjective sleepiness but have no difference in sleep duration, which presents significant daily sleep debt during the week in the eveningness. In addition, evening chronotypes show more variable sleep–wake habits and greater caffeine consumption (Taillard et al., 1999).
There are two main hypotheses to explain the differences between chronotypes. The first is the difference in the intrinsic circadian period between chronotypes. Single mutations in clock genes may contribute to significant variation in the intrinsic circadian period (Gentry et al., 2021). The intrinsic period or the period of the endogenous oscillators is not exactly 24 h and will be entrained by zeitgeber every day. Hence, a short intrinsic period is related to a rapid biological clock resulting in phase advance or morning chronotype with time elapsing. Conversely, a long intrinsic period eventually leads to the evening chronotype (Taillard et al., 2021). The lengths of intrinsic period are 24.3 h in the evening chronotype to 24.1 h in the morning chronotype (Lack et al., 2009). These results demonstrate a specific relationship between the properties of the circadian oscillator (the intrinsic circadian period) and chronotype (variability in the circadian phase) (Duffy et al., 2001). Indeed, this hypothesis included two aspects that could affect the behavior difference between chronotypes: genetics and zeitgebers.
Furthermore, the kinetic differences in the accumulation of sleep pressure in process S are another possible mechanism. The evening chronotype has a slower sleep pressure accumulation when awake and a slower decrease during sleep, which leads to relative sleepiness rhythm delays (Taillard et al., 2003; Lack et al., 2009). The homeostatic oscillator is interactive with the circadian oscillator: the intensity of sleep pressure is negatively related to the amplitude of the circadian rhythm (Taillard et al., 2021). These kinetic differences between chronotypes lead to differences in sleep parameters, especially the strength of subjective sleepiness. Most researchers indicated higher subjective sleepiness in the evening chronotype than the morning (Taillard et al., 1999, 2011). Among young people, the evening chronotype tends to wake up later because they have higher subjective sleepiness (5–9 h) than the morning chronotype (Lack et al., 2009). Interestingly, there is no difference in daytime objective alertness and performance between chronotypes though the evening chronotypes have greater subjective sleepiness (Taillard et al., 2011).
The internal mechanism of chronotype includes the interaction between the circadian process (affected by genetics and zeitgebers) and the sleep homeostatic process (driven by the accumulation and dissipation of neurotransmitters). According to Roenneberg et al. (2019), the chronotype reflects a stable state of individuals but not a trait because it varies under different strengths of zeitgeber. Nonetheless, chronotypes have high stability among individuals in real-world situations (McHill et al., 2021). This stability is so strong that the chronotype will relapse once the intervention of light exposure stops (Zerbini et al., 2020). It is important to understand that the essence of chronotype is state or trait-like, although not a trait exactly.
The evening chronotype has been associated with physical and psychological health problems, when the internal chronotype and external environment desynchronize. Some mental disorders have been associated with evening chronotype, including MDD, BD, SAD, anxiety disorders, psychotic disorders, and addictive disorders (Jagannath et al., 2017; Taylor and Hasler, 2018). Evening chronotype was associated with depressive and anxious symptoms, habitual use of alcohol and tobacco, and an increased risk of suicidality (Gau et al., 2007; Gaspar-Barba et al., 2009). On the other hand, some mental disorders may influence the association between chronotypes and other illnesses or specific behaviors. Depression and anxiety fully mediated the relationship between evening chronotype and cyberbullying perpetration (Tosuntas et al., 2018). In terms of marital satisfaction, chronotype also has a predictive role, with the morning chronotype having higher marital satisfaction (Shareh and Eshaghi Sani, 2019). Chronotype has been associated with nervous system diseases such as dementia, fibromyalgia (Thapa et al., 2020; Turkoglu and Selvi, 2020), and epilepsy (Manni et al., 2016), or physiological factors such as adiposity (Cespedes Feliciano et al., 2019).
Since exogenous factors (e.g., school schedule, shift work, and jet lag) exist, there is a misalignment between activity and sleep time between life rhythmicity and the biological clock, usually known as circadian rhythm disruption or SJL. SJL is commonly calculated as the absolute value of the difference in the midpoint of sleep time between weekdays and weekends using MCTQ (Henderson et al., 2019). It is a measure to describe and quantify circadian rhythm disruption, which is described as an abnormal phase angle difference between two or more rhythms. Specifically, SJL quantifies the circadian misalignment of the biological and social clocks only (Wittmann et al., 2006). Disrupted circadian rhythmicity or SJL may be crucial in the development, outcome, and treatment of human health (Foster et al., 2013; Jagannath et al., 2017; Gold and Kinrys, 2019). A study of more than 90,000 participants indicated that circadian dysfunction is related to a range of mental disorders, such as major depressive disorder (MDD), BD, and seasonal affective disorder (SAD), as well as to poorer mental experiences, such as more loneliness, lower happiness, and reduced satisfaction (Lyall et al., 2018). Research using unpredictable chronic mild stress in a mice model with depression-like behaviors identified decreased amplitude of activity and body temperature rhythm (Logan et al., 2015). Association between SJL and a higher risk of depression was found in both shift and non-shift workers (Lee et al., 2017; Islam et al., 2020). In addition, SJL and evening chronotype are related to metabolic disorders in adolescent girls (Cespedes Feliciano et al., 2019).
The evening chronotypes show larger SJL because it accumulates greater sleep debt during the weekdays, which results in later and longer sleep during weekends as compensation (Roenneberg et al., 2019). Since the development of sociality (use of artificial light and alarm clocks), solar zeitgebers have decreased influence on human circadian rhythms, whereas social zeitgebers have become more significant (Borisenkov et al., 2019). Compared to other ages, teenagers and young adults show the largest SJL (Foster et al., 2013) since the school schedule is more adaptive to morning chronotype. In contrast, the proportion of evening chronotype in teenagers and young adults is large. The conclusion that evening chronotype is more likely to have circadian misalignment gives us a clue as to why evening chronotype is associated with psychological health in humans.
A series of studies showed that clock genes contribute to the association between chronotype and mental health (Taylor and Hasler, 2018). In humans, Brain and Muscle ARNT-like 1 (BMAL1) and Circadian Locomotor Output Cycles Kaput Gene (CLOCK) proteins form heterodimers, Basic Helix-Loop-Helix -Per-Arnt-sim (BHLH-PAS), through their PAS domain and bind to the E-box vis the BHLH domain, enhancing unit of the Period gene (PER1–3) and Cryptochrome (CRY1, 2) genes by driving their transcription (Bolsius et al., 2021). As PER and CRY proteins accumulate in the cytoplasm during a subjective day, the two heterodimers suppress the transcription mediated by BMAL1-CLOCK and ultimately inhibit the transcription of themselves (PER and CRY) (Daut and Fonken, 2019). This inhibition is relieved due to the ubiquitin-mediated degradation of those proteins. This time-delay cycle by transcription and accumulation/degradation of proteins transforms a negative-feedback homeostat into a self-sustained oscillator (approximately 24 h) (Gelder, 1998; Bolsius et al., 2021). Other regulatory genes, such as retinoic acid Receptor–related Orphan Receptors (RORs) and the REV-ERB proteins Nuclear Receptor subfamily 1, group D, member 1 or 2 (NR1D1 and NR1D2), activate or inhibit the transcription of BMAL1 respectively. RORα competes with NR1D1 in binding to the BMAL1 and induces its expression while NR1D1 represses its transcription (Huang et al., 2011).
The individual difference in chronotypes may be associated with the clock genes, although we still do not know the potential physiological or molecular mechanisms. So far, there is much evidence that clock gene polymorphisms are related to the chronotype, which includes Aryl Hydrocarbon Receptor Nuclear Translocator-Like Gene (ARNTL), CLOCK, PER, CRY, F-Box protein (FBXL), Regulator of G-protein Signaling (RGS), Adenylate kinase (AK), NR1D1, 2, D-site of albumin promoter Binding Protein (DBP), Basic Helix–Loop–Helix family gene member E40 (BHLHE40, 41), Timeless Gene (TIM), Nuclear Factor, Interleukin 3 regulated (NFIL3), and RORC. A recent GWAS in 697,828 individuals identified 351 loci related to chronotype, including many essential SCN clock genes, such as PER1, PER2, PER3, CRY, FBXL3, and ARNTL (Jones et al., 2019). Similar results suggested that PER2, RGS16, FBXL13, and AK5 were related to chronotypes (Jones et al., 2016; Kalmbach et al., 2017). RGS16 regulates cAMP signaling by synchronizing intercellular communication between pacemaker neurons in the SCN (Jones et al., 2016). In a GWAS on a larger population, NR1D2 rs4131403 was found to be related to chronotype (Maukonen et al., 2020). NFIL3 rs2482705 and RORC rs3828057 were inversely associated with evening chronotype (Kripke et al., 2014).
Furthermore, these polymorphisms of clock genes are also related to psychiatric disorders. Compared to healthy controls, the brain issues of participants with MDD inhibited abnormal circadian gene expression, including ARNTL, PER1-3, NR1D1, DBP, and BHLHE40, 41 (Li et al., 2013). In addition, genetic mechanisms of chronotype can influence seasonality in MDD and BD in an age-related manner (Ferrer et al., 2020). CRY1 was also correlated to MDD, ADHD (attention deficit hyperactivity disorder), insomnia, and anxiety (Onat et al., 2020). Women with the PER3 genotype have fewer depressive symptoms and more anxiety symptoms. Importantly, there is no association between chronotype and PER3 polymorphism (Silva A. et al., 2020). This conclusion may arise because the gender-specific effects of chronotype and depression were overlooked (Weiss et al., 2020). Other scholars also identified PER3 mutations associated with chronotype and anxiety (Liberman et al., 2017). Suzuki et al. (2017) tested BD patients and found that CLOCK rs1801260, associated with evening chronotype, is related to the discrepancy between subjective and objective severity of depressive symptoms. Similarly, the variant of the CLOCK gene showed an association with the efficacy of antidepressants in MDD patients (Ma et al., 2019). Moreover, ARNTL rs7107287 was related to depressive and stress symptoms and the negative impact of seasonality; TIM rs10876890 was related to hyperthymic temperament; and TIM rs2291738 was related to chronotype, which indicated that there were no associations between chronotype and affective dysfunction (Jankowski and Dmitrzak-Weglarz, 2017).
Other genes that were not the clock were also related to chronotype and psychiatric disorders. A study found that a candidate for Serotonin 2A receptor gene (5HT2A) receptor (HTR2A), which is located on chromosome 13q14-q21, was associated with mental (depression, schizophrenia) and behavioral (suicidal and aggressive behaviors) disorders and was associated with chronotype in a healthy population (Yeom et al., 2020). Additionally, GWAS of the UK Biobank also indicated HTR6 rs2050122 was related to chronotype. Some clock genes related to chronotype may modulate the association between chronotype and psychiatric disorders.
In summary, these polymorphisms of clock genes constituted the circadian oscillators are associated with chronotype and psychiatric disorders. However, the molecular and physiological mechanisms are unclear, particularly in how clock gene polymorphisms contribute to symptoms or characteristics of those disorders.
The structural and functional differences of the brain for chronotype may be the direct reason for mental health issues. Regarding the brain structure, compared to eveningness, morning chronotype is related to lower regional gray matter density in the precuneus, the left posterior parietal cortex, and their adjacent areas, and to higher regional gray matter density in the bilateral orbitofrontal cortex and the hypothalamic areas around the bilateral SCN. Moreover, the results suggested a relationship between morning chronotype and lower cognitive functions, higher competence in pro-social and self-disciplinary behavior, and less attentional and emotional problems in brain structure level (Takeuchi et al., 2015). A structural magnetic resonance imaging study indicated that although chronotype is not related to total hippocampal volume, there is an association between evening chronotype and localized atrophy in the mid-anterior region of the right hippocampus independent of age, gender, sleep quality, and mood, which was explained to prolonged exposure to glucocorticoids and stress (Horne and Norbury, 2018a). A recent MRI study suggested that the evening chronotype is associated with a greater volume of the left anterior occipital sulcus (Evans et al., 2021). This difference from the visual cortex may reflect the effect of chronotype on the input signal of the environment to the circadian system. A diffusion tensor imaging study suggested the existence of differences in white matter integrity in the frontal and temporal lobes, cingulate gyrus, and corpus callosum in the evening chronotype compared to the morning and intermediate chronotype (Rosenberg et al., 2014). The chronotype-specificity whiter matter characteristics in corpus callosum may be related to BDs, while frontal lobes play essential roles in substance use disorders.
The default mode network (DMN), which consists of the posterior cingulate cortex (PCC), precuneus, medial prefrontal cortex (mPFC), inferior parietal lobule (IPL), and bilateral temporal cortex (BTC), is vital in maintaining consciousness awakening, emotion processing, attentional control, and working memory. Regarding brain function, many studies reported the association between mental disorders and altered functional connectivity of the DMN. A study on the brain’s resting-state functional connectivity suggested that functional connectivity of the DMN is fundamentally different in evening and morning chronotype individuals during working days (Facer-Childs et al., 2019a). Tian et al. (2020) observed that those evening chronotype individuals with poor sleep quality had higher precuneus-mPFC connectivity, which fully mediated the effect of chronotype on sleep quality. In addition, the evening chronotypes exhibited less mPFC reactivity during reward anticipation and more ventral striatum reactivity during win outcomes than the morning chronotype, demonstrating reduced regulatory control and elevated reward sensitivity, which may contribute to alcohol abuse (Hasler et al., 2013). Indeed, mood disorders like depression and anxiety are commonly comorbid with substance abuse, which the common neurobiological process may explain. Compared with morning chronotypes, evening chronotypes showed a diurnal variation of positive affect in line with the diurnal activity of the affective neural circuitry (mPFC and striatum), potentially constituting a risk for mood disorders among evening chronotype individuals (Hasler et al., 2012). The evening chronotype is associated with increased sensitivity to negative emotional facial expressions in the bilateral amygdala and reduced dorsal anterior cingulate cortex (dACC)–amygdala functional connectivity, suggesting that the evening chronotype is related to impaired emotional regulation circuitry (Horne and Norbury, 2018b). On the other hand, specific circadian rhythm changes have been identified in the nucleus accumbens (NAcc) in a depression-like mice model induced by chronic stress (Logan et al., 2015). The clock genes of MDD patients had an abnormal expression in the dorsolateral prefrontal cortex (DLPFC), anterior cingulate cortex (ACG), hippocampus, amygdala, NAcc, and cerebellum (Li et al., 2013).
Taken together, a growing body of evidence suggested the neural difference between chronotypes on the level of structure (gray matter, white matter, visual cortex, and hippocampal) and function (DMN, reward circuits, and limbic system), which contributes to the differences in psychological performance and etiology of mental disorders.
Neurotransmitter switching may provide a core biological basis for chronotypes and chronotherapeutics (Wirz-Justice and Benedetti, 2020). Under normal circumstances, the onset of melatonin secretion occurs about 2 h before bedtime; core body temperature is the lowest overnight (2–3 h before waking); plasma cortisol concentrations reach their lowest point in the evening and peak shortly after waking in the morning (arousal). The temporal association between those physical parameters’ circadian rhythms and sleep timing is different (phase advanced, phase delayed, and internal desynchrony) (Hickie and Rogers, 2011). Melatonin, a pineal neurohormone, is activated by the circadian clock locked in the SCN and limited by light exposure and secretion following a 24-h cycle (inhibited by day and activated at night). Melatonin receptors are widely distributed in the brain, including in the SCN of the hypothalamus, substantia nigra, hippocampus, cerebellum ventral tegmental area, and the NAcc (Hickie and Rogers, 2011). Researchers have found specific differences in melatonin rhythms in individuals of different chronotypes. That is, those with evening chronotype have later melatonin rhythms with decreased mean levels and peaks (Lack et al., 2009; Razavi et al., 2019; McHill et al., 2021). Dim-light melatonin onset, which is a circadian phase marker, varies by almost two hours between chronotypes (Taillard et al., 2011) and shows chronotype-dependent effects on weekly and seasonal variation (both the DLMO and sleep time advanced one hour in summer than winter and delayed on weekdays compared to on weekends) (Zerbini et al., 2021).
In a sleep deprivation study, attenuated salivary melatonin secretion was identified in female MDD patients (Birchler-Pedross et al., 2011). Melatonin and other hormones may also play a role in the relationship between evening chronotype and obesity (Sun et al., 2020). In a sample of breast cancer patients, a negative correlation was found between melatonin levels and insomnia severity and depression severity (Zaki et al., 2020). Indeed, some neurotransmitters (dopamine, noradrenaline, serotonin, and tryptophan) involved in the etiology of psychiatric disorders participate directly in melatonin synthesis (Adan et al., 2012). The serotonergic system is associated with mood and circadian rhythm, and it seems to serve as a mediator between childhood trauma, circadian disruption, and mood disorder (Park, 2020). Serotonin plays adaptive roles in circadian rhythm, mood, anxiety, and cognitive functions (Bacque-Cazenave et al., 2020). Due to the bidirectional communication between serotonin and circadian rhythm and the influence of stress-induced changes in cortisol, the serotonergic system plays an essential role in intermediating the circadian regulation of vulnerability to MDD (Daut and Fonken, 2019). In terms of treatment, the evening chronotype reported poorer efficacy of SSRI medications (McGlashan et al., 2018). Cortisol, an endogenous hormone, has a circadian change and is regulated by negative feedback loops within the hypothalamic-pituitary-adrenal (HPA) axis (Girshkin et al., 2016). The disrupted HPA axis function is associated with psychiatric disorders such as MMD, BD, and schizophrenia (Wong et al., 2000; Menke, 2019). In healthy adolescents and adults, the morning chronotype showed higher levels of cortisol awakening response than the evening chronotype (Randler and Schaal, 2010; Correa et al., 2014). It seems that the earlier the chronotype, the higher the level of cortisol secretion (Minelli et al., 2021). However, some other researchers have found the opposite result of no significant difference in chronotype and cortisol levels when investigated in small and limited samples (Dockray and Steptoe, 2011; Toda et al., 2013).
The age-related changes in chronotype exist throughout the life span after controlling for demographic and socioeconomic factors. Age is a significant predictor of chronotype (Randler and Bilger, 2009; Roenneberg et al., 2019). Chronotype strongly depends on age (Holler et al., 2021), and it is well-known that the elderly are more likely to wake up and sleep early. In general, morning chronotype is predominant during babyhood (Simpkin et al., 2014). The evening chronotype is more prevalent among young adults (ages 25–34), while the morning chronotype has a greater proportion in the middle and old-age populations (Merikanto and Partonen, 2020). According to the MCTQ database from Roenneberg et al. (2004) children are early chronotypes, and the distribution of later chronotypes increases with the age of groups increasing from 10 to 20. After 20, chronotypes tend to be early (phase advance) until 70 (Roenneberg et al., 2004). Notably, results about age and chronotype measured by MEQ should be carefully considered since the inappropriate classification of the original version may not be suitable for the middle-aged population (Taillard et al., 2004). On the other hand, cohort effects or other confounders are needed since most research is cross-sectional.
The age-related intrinsic circadian period may explain the association between chronotype and age. The phase and amplitude of melatonin, core body temperature, and cortisol vary at different age stages. The intrinsic circadian period is greater (more than 24 h) (Carskadon et al., 1999). The phase of melatonin rhythm shows a similar trend among different age groups (Logan and McClung, 2019). Apart from melatonin, the sensitivity of zeitgeber may differ in various ages. For instance, deterioration of the eyes may weaken the effect of the light/dark cycle (Hood and Amir, 2017). In addition, based on the same data, the tendency of females to shift to a later chronotype seems to occur earlier than it does for boys around the age of 20, which indicates a gender effect in chronotype (Roenneberg et al., 2004).
Gender differences exist between psychiatric disorders and chronotype, respectively. Psychiatric disorders are characterized by marked gender differences. For example, females are more likely to develop MDD and show more severe symptoms, worse social function, and a higher risk of anxiety (Labonte et al., 2017; Hasbi et al., 2020). Although many studies have attempted to explore sex-specific effects on psychiatric disorders from various perspectives, such as socio-cultural factors (Salk et al., 2017), gene organization and expression (Labonte et al., 2017; Hasbi et al., 2020), neuro-function or neuro-immunity (Bekhbat and Neigh, 2018), the mechanisms remain unclear. However, chronotype is also characterized by sexual differences. Many studies exhibited that the morning chronotype represents a larger proportion among females, and the evening chronotype is more common among males (Tonetti et al., 2011; Adan et al., 2012; Borisenkov et al., 2012). However, the others suggested an opposite conclusion (Merikanto et al., 2012), or that there are no gender differences in the chronotypes (Zimmermann, 2011; Walsh et al., 2021). Duarte et al. (2014) investigated 14,650 volunteers and demonstrated that, compared to males, females were more morning-oriented before 30 years old while were more evening-oriented after the age of 45 years, and found no gender differences between the ages of 30–45 years. This result showed the gender differences in the plasticity of the circadian timekeeping system in men and women throughout ontogeny (Duarte et al., 2014). Furthermore, there are gender differences linking the chronotype and psychiatric disorders. A study of more than 5,000 non-shift-working adults indicated that evening chronotype was related to depression only in women, not men. The evening chronotype among females has some unfavorable aspects concerning school and work in young and old females (Fabbian et al., 2016). A potential explanation is the gender difference in melatonin, sex hormones, and behavioral and psychological factors (sleep quality, alcohol intake) (Kim et al., 2020). In addition, the circadian gene PER3 variable number of tandem repeats was independently related to chronotype only in males (Weiss et al., 2020). Taken together, both the chronotype and psychiatric disorders showed significant sex-specific effects, although the causes and mechanisms are unclear.
The light/dark cycle is the primary zeitgeber synchronizing circadian rhythm in a wide array of species (Czeisler et al., 2005) and is significantly related to circadian rhythmicity by regulating SCN (Jagannath et al., 2017; Wirz-Justice et al., 2021). The visual effects of light rely on photoreceptor cells (rod and cones) which coordinate the image-forming tasks and lead to vision. On the other hand, the non-visual light effects depend on intrinsically photosensitive retinal ganglion cells (ipRGCs) to regulate physiological function, behavior, and emotion, such as circadian rhythm, the pupillary light reflexes, sleep, and mood (Guido et al., 2020). The ipRGCs are a subset of retinal ganglion cells containing melanopsin (Opn4), a member of the G-protein coupled receptor family (Diaz et al., 2016). The light/dark cycle synchronizes circadian rhythm depending on the time and duration of daily light exposure, the intensity, and the spectral properties (Touitou et al., 2016). Light exposure generally advances the circadian phase in the morning, while in the evening, it delays it. The phase response curve (PRC) reflects the relationship between the time light exposure and phase shift of circadian rhythm (Minors et al., 1991). There are large differences in daily light exposure between chronotypes: the morning chronotypes have more minutes of daily bright light exposure (> 1000 lux) than the evening chronotypes (Goulet et al., 2007). Moreover, after a four-hour exposure to blue light, EEG results showed that individuals with morning chronotype had increased theta and low alpha spectral power in the afternoon compared to those exposed to polychromatic white light, indicating that morning chronotype individuals may be more sensitive to light (Siemiginowska and Iskra-Golec, 2020).
Artificial light weakens the strength of the natural light/dark cycle (depending on the difference between daytime and nighttime light intensity) and delays the circadian phase in almost all chronotypes except the definite morning chronotype (Roenneberg et al., 2003, 2019; Roenneberg and Merrow, 2016). Among adolescents, those living in an environment with more outdoor light at night have a stronger evening chronotype orientation than those not (Vollmer et al., 2012). Light exposure at night will disrupt circadian rhythm and lead to adverse consequences for human health (Bedrosian and Nelson, 2017). The animal model indicated that an SCN-independent pathway, which provides excitatory synaptic input to the neurons of the peri-habenular nucleus (PHb) of the dorsal thalamus through ipRGCs, mediated the regulation of mood (Fernandez et al., 2018). The higher correlated color temperature of light exposure is conducive to stimulating a more positive mood during daytime (Xiao et al., 2021). In addition, consecutive and acute night light exposure induced depression-like behavior in mice (Walker et al., 2020).
On the other hand, the light therapies based on a theory of circadian rhythm and sleep homeostasis are effective in depressive disorders. Particularly, bright light therapy is highly effective in both SAD and non-seasonal depression (Maruani and Geoffroy, 2019) and is more effective than dim red light in non-seasonal unipolar depression patients with evening chronotype (Chan J. W. et al., 2020).
In humans, social factors (such as shift work, alarm clocks, and regular eating) are one of the most important zeitgebers (only next to the light/dark cycle). The use of artificial light from electronic devices makes it possible for activity at night, which will also impact our normal circadian rhythm.
Shift work is a special working arrangement in which holiday, night, or other non-mainstream working times feature. Paramedics, firefighters, and police are occupations generally involved in shift work. Notably, it is important to examine chronotypes and the types of shift work separately when researching the influence of shift work on health. Juda et al. (2013) did elaborate work on 238 shift workers and indicated that the morning chronotypes have shorter sleep duration, higher SJL, and higher levels of sleep disturbance during night shifts (10 PM to 6 AM). On the contrary, the evening chronotypes show a similar pattern on morning shifts (6 AM to 2 PM). In addition, on evening-shift days, the evening chronotype has a longer sleep duration and better sleep quality than the morning chronotypes, while the reverse is true for morning-shift days (Juda et al., 2013). In other words, compared to the morning chronotypes, evening subjects are more adapted to night shifts while morning subjects are similar to morning shifts. This adaptability may be because the active/rest cycle of chronotypes is consistent with different shift schedules separately. However, there are various types of shift work in the real world. For instance, a rotating shift is a schedule of 2 days of morning duty followed by two days of night duty with four days of break among the rosters (Khan et al., 2020). The discrepancy between the shift schedule and chronotypes, or circadian misalignment, contributes to shift workers having more severe insomnia, excessive daytime sleepiness, and worse mood disturbances, such as depression and anxiety. A meta-analysis of longitudinal studies indicated that shift work is associated with worse mental health, specifically depressive symptoms. This conclusion is consistent with another meta-analysis showing that night shift work is related to an increased risk of depression (Lee et al., 2017; Torquati et al., 2019). The night shift workers are exposed to long-term artificial light at night, which may lead to chronic desynchronization of their circadian rhythm. In addition, a systematic investigation also demonstrated that female nurses with evening chronotype are more prone to anxiety, insomnia, and fatigue (Lopez-Soto et al., 2019). The study collected data from 10637 participants and demonstrated that shift workers with evening chronotype were more vulnerable to mood disorders manifesting as sleep problems or susceptibility to artificial light exposure (Cheng et al., 2021). A study of paramedics in Australia indicated that individuals with evening chronotype have worse mental health, poorer sleep quality, and lower well-being than those with morning chronotype (Khan et al., 2020). Evening chronotype was also associated with depressive moods in nurses and firefighters (Choi et al., 2020). Taken together, these above findings may demonstrate that, despite different chronotypes being more adapted to corresponding shift works, the evening chronotype showed worse adaptability in variably real-world shift works.
The relationship between excessive use of electronic devices and evening chronotype was confirmed in children, adolescents, and adults (Fossum et al., 2014; Eid et al., 2020; Przepiorka et al., 2021). Evening chronotype individuals also showed more frequent use of electronic devices than morning chronotypes (Akçay et al., 2021). The evening subjects are more novelty-seeking and open-minded than the morning (Adan et al., 2012). These personalities may be why they spend more time on electronic devices than the morning chronotypes, and the same for insomnia. Electronic devices in bed were correlated with both evening chronotype and insomnia (Fossum et al., 2014). In other words, those people with evening chronotype or insomnia have to use electronic devices to pass the time. On the other hand, the information from electronic devices (such as games, videos, and social media) is very attractive and may cause a later sleep time and reduced sleep duration by promoting physiological and psychological arousal (Cain and Gradisar, 2010). After controlling for chronotype, the relationship between electronic device use and shorter sleep duration and longer sleep latency remains but is weakened (Hisler et al., 2020). In addition, artificial light, mainly blue light, may impact the normal circadian rhythm and contribute to a phase delay of the circadian clock (Touitou et al., 2016). The non-visual effects of light may explain the association between electronic use and evening chronotype. Light from self-luminous devices may suppress melatonin and delay the sleep phase due. In particular, adolescents showed more sensitivity to light (Figueiro and Overington, 2016). Since the majority of studies are cross-sectional, it remains unclear whether the evening chronotypes use electronic devices because they need to pass time until feeling sleepy, or the light from these devices and the physiological and psychological arousal from the contents lead to a phase delay of circadian rhythm. Alternatively, these processes could work together in the real world. Nonetheless, the evening chronotypes who overuse electronic devices will accumulate sleep debt every workday, leading to SJL, which contributes to psychiatric disorders. Indeed, individuals with evening chronotype and frequent internet use were found to be more depressive (Przepiorka et al., 2021). Furthermore, we cannot ignore the influence of network information. A study of a massive data set of Twitter content in the United Kingdom indicated that positive words were more likely to be used during the day. In contrast, negative words (indicating sadness and anger) appeared at night more frequently (Dzogang et al., 2017).
Personality is a relatively stable predisposition and affects patterns of thinking, feeling, and acting (Friedman and Kern, 2014). The characteristics of personality in different chronotypes are important for understanding the relationship between chronotype and psychiatric disorders. According to the review by Adan et al. (2012), who summarized research evidence, the morning chronotypes are more conscientious, persistent, introverted, agreeable, and emotionally stable, while the evening chronotypes are more extraverted, impulsive, novelty-seeking, open-minded, and more likely to have psychoticism and a less adaptive emotional profile. Later researchers showed a similar conclusion. A meta-analysis investigated the relationship between chronotype and the Big Five factors of personality and indicated that evening chronotype is associated with extraversion and open-mindedness, while morning chronotype is strongly and positively related to conscientiousness and has a unique and negative association with neuroticism (Lipnevich et al., 2017). Similarly, a study composed of 1134 adolescents (age 10–14) demonstrated a large proportion of left-thinkers (prefer verbal and analytic strategies in processing information) among the morning chronotypes, while right-thinkers (more creative and intuitive) were among the evening chronotypes (Diaz-Morales and Escribano, 2013). Current evidence indicates that three personality traits (neuroticism, extraversion, and conscientiousness) are associated with psychiatric disorders such as depression and anxiety disorders. Higher neuroticism and lower extraversion predict a poorer course and response to treatment in depression. In addition, there is a strong negative relationship between conscientiousness and depression (Klein et al., 2011). Overall, the relationship between morning chronotype and neuroticism (negative) and conscientiousness (positive) may explain why morning chronotype is a protective factor of some mental disorders.
The emotion regulation strategy is critical in explaining the relationship between chronotype and psychiatric disorders. The association between evening chronotype and depression is mediated by a lower behavioral activation system (BAS) and lower positive affect (PA), and the evening chronotype is related to reduced reward responsiveness (Hasler et al., 2010). Diurnal variation exists in positive and negative affect, respectively (Dzogang et al., 2017), and the energetic arousal characteristic for chronotypes (Stolarski et al., 2016). After controlling for confounders, the evening chronotype showed impaired emotion regulation strategies (reduced cognitive reappraisal and increased expressive suppression), potentially increasing vulnerability to psychiatric disorders (Watts and Norbury, 2017). Researchers also found that reduced cognitive reappraisal and self-blame were associated with evening chronotype (Van den Berg et al., 2018). Increased expressive suppression was also correlated with poorer sleep quality (Latif et al., 2019). Evening chronotype was positively associated with perceived stress (You et al., 2020) and less use of the emotion regulation strategy, described as “putting [things] into perspective” (Henrich et al., 2021). A study of medical students indicated that evening chronotype individuals are less happy than others. This relationship between chronotype and happiness was significant after controlling for confounding variables, such as age and gender (Tan et al., 2020). Rumination is passive and repetitive thinking about negative emotions and their possible causes and consequences. It includes forced thinking and introspective thinking. Rumination was thought to be a unique cognitive predisposing factor of depression and was associated with evening chronotype by mediating the relationship between chronotype and depression (Antypa et al., 2017) and potentially aggravating the association between depression and cognition (Sun et al., 2014). A functional connectivity study observed that the evening chronotype was correlated with the precuneus-mPFC functional connectivity involved in self-referential processing (Tian et al., 2020). Rumination is also related to self-referential processing in evening chronotype and depression (Antypa et al., 2017), demonstrating a neural mechanism of the association between chronotype and mental diseases. Social support provided by family, friends, and teammates seems to be a potential mechanism by which social support is a partial mediator between evening chronotype and depressive symptoms (Wills et al., 2021). A recent study concluded that morning chronotypes have higher social support and mindfulness (Walsh et al., 2021). Compared to other chronotypes, evening chronotypes have conflicts in their social lives, potentially leading to low support and feelings of loneliness. One possible reason is that evening chronotypes may have sufficient social support but may be unaware or unwilling to recognize their support because of negative cognitive bias (Walsh et al., 2021). Overall, evening chronotype is highly linked with negative emotion strategies (such as reward responsiveness, cognitive reappraisal, and expressive suppression), rumination, and negative cognitive bias toward social support, which contributes to psychiatric disorders.
Cognitive functions reflect the brain’s characteristics, states, and interrelations of objective things, including sensation, memory, perception, attention, and execution. Executive function is the ability to flexibly integrate and coordinate different cognitive processes to achieve a specific goal and composes working memory, inhibition control, and cognitive flexibility. There is a synchrony effect of chronotype, showing better performance on cognitive function at their optimal times of day than at their non-optimal times (Hahn et al., 2012). In other words, the evening chronotypes do better in the evening while the morning chronotypes do better during the daytime. Previous research revealed that, despite the evening chronotypes showing higher intelligence and cognitive ability than the morning chronotypes, they have worse academic achievement (Preckel et al., 2011; Diaz-Morales and Escribano, 2013). The synchrony effect may explain it since the test time is not too late, usually at school. The evening chronotypes have higher correct answer rates than the morning chronotypes in the 2-back and 3-back tasks, which commonly test working memory. The synchrony effect of chronotype appears only at a higher complexity of the task (3-back) (Schmidt et al., 2015). However, current evidence is unable to clarify the relationship between chronotype and cognitive functions. The interplay between cognitive function and emotion regulation may be executed by the same set of neural mechanisms and may contribute to psychiatric disorders (Gruber and Cassoff, 2014). Cognitive impairments are not only symptoms of some psychiatric disorders but also the main factor of functional recovery. Evening chronotype and poor sleep quality contribute to cognitive impairments in MDD independently (Cabanel et al., 2019). Impaired response inhibition (considered a marker of vulnerability to BD and substance use disorders), high impulsivity, and disinhibition are more frequently associated with evening chronotype and maybe a core personality trait involved in circadian rhythm (Kang et al., 2015). In summary, the synchrony effect of chronotype on various cognition is confirmed. Evening chronotype seems to have vulnerabilities in cognition, which contributes to psychiatric disorders. Nonetheless, future works should prove these results since current evidence in cognitive tasks, experimental designs, samples, and measuring methods shows large differences.
As we described already, it is clear that chronotype is somehow connected with sleep disorders. The chronotypes showed different sleep problems. Morning chronotype is related to sleep phase-advance syndrome and difficulty in maintaining sleep. Simultaneously, evening chronotype is associated with difficulty in initiating sleep and morning sleepiness. Even in those who are considered good sleepers, this relationship exists (morningness to sleep phase-advance syndrome and eveningness to morning sleepiness) (Taillard et al., 2001). Chronotype was the strongest predictor of sleep disorders (Sun et al., 2019). People with evening chronotype have worse sleep quality and more severe insomnia, while being a morning chronotype is a protective factor (Salfi et al., 2021). In addition, evening chronotype was an independent predictor of mental health issues, such as MDD, BD, and anxiety disorders, and was positively associated with depressive symptoms. A large population study in Japan suggested that evening chronotype is related to an increased risk of depressive symptoms after adjusting for sleep factors (Kitamura et al., 2010). Simor’s conclusion supported this hypothesis after adjusting for age, gender, circadian misalignment, and sleep complaints (Simor et al., 2015). Chan J. W. et al. (2014) suggested that evening chronotype is an independent risk factor of non-remission in MDD.
On the other hand, sleep disorders or sleep phenotypes, such as insomnia, hypersomnia, and daytime sleepiness, have proven to be independently linked to mental disorders, especially depressive disorders (Wang et al., 2019). Indeed, sleep disorders and mood disorders are risk factors for each other. Recent studies have demonstrated that sleep disorders are associated with depression and anxiety (Cox and Olatunji, 2019; Dagnew et al., 2020; Furihata et al., 2020). In a survey of 8580 subjects aged 16–74 years, 83% of depressed patients have reported insomnia symptoms, and 41% of them were diagnosed with insomnia depending on DSM-IV criteria (Stewart et al., 2006). Inadequate sleep was associated with depressive symptoms in a study of US and Chinese adolescents (Yang et al., 2020). The GWAS of over one million individuals revealed the causal effects of insomnia on depression (Jansen et al., 2019). A study of healthy older adults indicated that older people with insomnia had reduced motivation and sensitivity to monetary reward, suggesting that a dysregulated reward system may become a mechanism whereby insomnia contributes to late-life depression (Boyle et al., 2020). Sleep disturbance is associated with reduced well-being in college students (Fischer et al., 2020). Insomnia severity, especially difficulty in initiating sleep but not sleep duration, is a predictor of first onset MDD (Correa et al., 2014). A prospective study showed that sleep irregularity was associated with depressive episodes in BD patients (Ng et al., 2016).
A large body of evidence demonstrated that poor sleep quality or sleep disorders mediate the association between evening chronotype and depressive symptoms in healthy subjects and patients with mood disorders (Van den Berg et al., 2018; Caruso et al., 2020; Li et al., 2020; Tian et al., 2020; Uzer and Yucens, 2020b; Lin et al., 2021; Zhou et al., 2021) or other adverse outcomes (Cox and Olatunji, 2019; Kandeger et al., 2019; Najem et al., 2020; Bradford et al., 2021). A study of older adults with depression indicated that the relationship between evening chronotype and depression is fully mediated by insomnia severity (Uzer and Yucens, 2020b). Clinical evaluation of patients with BD indicated that poor sleep quality mediated the association between evening chronotype and residual depressive symptoms (Caruso et al., 2020). A recent study revealed that insomnia partially mediates the effect of chronotype on depression and anxiety, completely mediating the association between chronotype and suicidal ideation (Bradford et al., 2021). The relationship between evening chronotype and psychological distress showed a mediation effect (Hou et al., 2020). Zhou et al. (2021) suggested that resilience could moderate the effects of chronotype and sleep quality on depressive symptoms and that enhanced resilience could effectively alleviate the symptoms of mood disorders. Evening chronotype was associated with high levels of problematic use of social media, more psychological distress (such as depression, higher suicide ideation, and lower PA), and daytime sleepiness. In addition, sleep quality and severe insomnia were significant mediators in these associations (Lin et al., 2021). Depressive symptoms improved after insomnia was cured in evening chronotype adolescents (Chan N. Y. et al., 2020).
However, there are opposite outcomes. The mediated effect of sleep problems between chronotype and mood disorders is unproven. A large cohort study indicated that the association between evening chronotype and MMD could not be fully explained by sleep problems, such as insomnia and inadequate sleep duration (Antypa et al., 2016). According to mediation analysis, hopelessness, defined as negative expectations for the future and core symptoms of MDD, mediates the relationship between evening chronotype and depressive symptoms, but not sleep quality (Uzer and Yucens, 2020a). Taken together, although there were conflicting results regarding the relationship between psychiatric disorders, chronotype, and sleep disorders, the general view is that evening chronotype is an independent risk factor for psychiatric disorders, especially depressive disorders, in which sleep disorders show a partially or fully mediating effect.
Depressive disorders are characterized by significant and persistent depressive symptoms as the primary clinical feature, often accompanied by the rhythmical fluctuation of mood and high morbidity, heavy burden, and great social attention (Mrazek et al., 2014; Malhi and Mann, 2018). MDD represents the classic condition in this group of disorders. It is characterized by discrete episodes of at least two weeks (although most episodes last considerably longer) involving clear-cut changes in affect, cognition, neurovegetative functions, and inter-episode remissions (Arlington and Publishing, 2013). Although many hypotheses have tried to explain the pathogenesis of depressive disorders, such as the monoamine depletion hypothesis, the neuroplasticity impairment hypothesis, the neuroendocrine disruption hypothesis, and the circadian rhythm disruption hypothesis, the potential mechanism remains unclear (Malhi and Mann, 2018; Lynch et al., 2020). Depression often shows diurnal mood variation (Morris et al., 2009), diurnal changes in hormone levels (Hickie and Rogers, 2011), and sleep disorders, such as early awakening, insomnia, and shallow sleep (Germain and Kupfer, 2008; Emens et al., 2009). Disruption of circadian rhythmicity is associated with the severity of depressive symptoms, the impairment of cognitive function, and clinical treatment, efficacy, and prognosis. The evening chronotype is often referred to as a risk factor for depression disorder, whereas the morning chronotype is a protective factor (Adan et al., 2012; Chan J. W. et al., 2014).
Many studies have indicated that the evening chronotype is significantly associated with depressive symptoms in non-clinical samples. Most studies have found that evening chronotype is consistently associated with depression and is an independent risk factor (Simor et al., 2015). This conclusion was made in studies on children, adolescents, and adults (Gau et al., 2007; Alvaro et al., 2014; Chan N. Y. et al., 2020; Eid et al., 2020; Weiss et al., 2020; Koo et al., 2021) and in different countries (Alvaro et al., 2014; Kivela et al., 2018; Walsh et al., 2021). Even excluding some effects of social factors like shift work, evening chronotype was found to be related to a higher incidence of depression (investigated in 1165 non-shift workers by Taillard et al., 2001). A recent meta-analysis of 43 studies demonstrated a reliable association between evening chronotype and depressive symptoms (Norbury, 2021). A large population-based study of 10,503 adults indicated that evening chronotypes are at higher risk of having depressive disorders, needing to take antidepressant medication, and experiencing depressive symptoms (Merikanto et al., 2015).
In addition, compared to non-psychiatric controls, participants with a high risk of psychosis and patients with depressive disorders exhibited a greater preference for the evening chronotype (Lunsford-Avery et al., 2021). There was also an association between evening chronotype and suicidality, which is firmly and fully mediated by depressive symptoms (Mokros et al., 2021). A study of 236 homogenous samples of young men suggested that more severe depressive symptoms were associated with evening chronotype, higher perceived stress, poorer sleep quality, and lower levels of morning salivary cortisol (Tonon et al., 2020). Mendelian randomization analysis indicated a protective relationship between morning diurnal preference (correspond to a 1-h earlier sleep midpoint) and a lower risk of MDD (Daghlas et al., 2021). However, unlike most studies, an epidemiological survey found that morning chronotype subjects have shorter sleep duration and were more likely to be patients with depressive disorder (Lemoine et al., 2013). This result is probably due to the lack of healthy subjects, the use of rMEQ (only six questions), the high age (average age of 51.64 years), and the large gender ratio gap (29.3% were males) of depressive subjects.
The evening chronotype was independently related to depressive disorders (Chan J. W. et al., 2014; Antypa et al., 2016; Au and Reece, 2017; Ferrer et al., 2020; Lunsford-Avery et al., 2021). A meta-analysis of 36 studies demonstrated that the evening chronotype is related to mood symptoms, such as MDD, BD, and SAD (Au and Reece, 2017). A case-control study of young persons suggested higher rates of evening chronotype in young individuals with depression and anxiety than in healthy controls (Fares et al., 2015). The evening chronotype was associated with the severity of the disease and a higher occurrence of suicidal thoughts (Gaspar-Barba et al., 2009). In a 1-year naturalistic follow-up study of 253 MDD patients, evening chronotypes have more severe depressive symptoms, higher incidence of suicide, and more suicidal ideation (Chan J. W. et al., 2014). A literature review shows that the evening chronotype was a critical characteristic of suicidal ideation and behavior (Rumble et al., 2018). Depressive patients with evening chronotypes have higher levels of negative affect on weekends (Bruckmann et al., 2020). A 7-year follow-up longitudinal study showed that, first, the earlier chronotype individuals experience milder depressive symptoms, and second, the changes in chronotype are consistent with changes of the severity of depressive but not of anxiety symptoms over time (Druiven et al., 2020). A genetic study in a large population demonstrated that morning chronotype was negatively related to depressive symptoms and MDD while positively correlated with subjective well-being (Jones et al., 2019). In short, the evening chronotype is a risk factor for depression, whereas the morning chronotype seems to be a protective factor. The relationship between chronotype and depression (or depressive symptoms) was examined in studies that used different samples, such as healthy individuals (children, adolescents, and adults) and patients with MDD. This relationship has clinical implications for improving prevention, diagnosis, and treatment.
As we described above, the hypothesis of circadian dysfunction is that the genetic vulnerabilities, zeitgeber, age, and sex, work together to contribute to depression in the evening chronotypes. The mutations of clock genes could induce variation in the intrinsic period but also affect hormone secretion such as glucocorticoids (Shi et al., 2016) and serotonin (Daut and Fonken, 2019). Depression commonly emerges in adolescents or young adults (Crouse et al., 2021), while the evening chronotype has a higher distribution in similar ages. The characteristics of age and gender in chronotype are consistent with a depressed population, which may be the result or phenomenon of the underlying mechanism that we do not yet know. From a psychological view, the evening chronotypes tend to have psychoticism and a less adaptive emotion profile in personality, in addition to being more associated with negative emotion strategies, rumination, and cognitive bias. These mental traits are potentially increasing vulnerability to depressive disorders. On the other hand, some researchers suggested that sleep disorders may contribute to mood disorders independently. However, evening chronotype is an independent risk factor for psychiatric disorders, especially depressive disorders, in which sleep disorders show a partially or fully mediating effect. Although it seems evident that evening chronotype is independently and positively related to depression as its risk factor, the physiopathology remains unclear. Nonetheless, bright light therapy is highly effective in treating depressive disorders, which suggests the hypothesis of circadian and sleep disruption show excellent prospects.
Bipolar disorders, including BD-I and BD-II, are mood disorders. BD-I is characterized by at least one manic episode, which may have been preceded by and may be followed by hypomanic or major depressive episodes. BD II is characterized by a clinical course of recurring mood episodes consisting of one or more major depressive episodes and at least one episode of hypomania (Arlington and Publishing, 2013). Circadian rhythm disruption, evening chronotype, abnormal hormone secretion, and vulnerability of the CLOCK gene have been documented in BD (Takaesu, 2018; Ferrer et al., 2020; Ozdogan et al., 2020). The association between evening chronotype and BD was confirmed in subjective (questionnaire) and objective (actigraph) chronotype measurements (Gershon et al., 2018; Kaufmann et al., 2018). A recent large cross-sectional study of 773 BD patients and 146 control subjects demonstrated evening chronotype as a discrete clinical subtype in BD patients who were younger and had an earlier age of onset, worse clinical symptoms (more depressive and manic episodes, higher rate of suicide attempts), more comorbid anxiety, and substance use disorder (Romo-Nava et al., 2020a). An 18-month prospective study among 80 BD patients (the most prevalent diagnosis was BD-I, n = 69) demonstrated that the evening chronotype was associated with more severe anxiety, more mood episodes, and being overweight, suggesting a poor prognosis for BD (Melo et al., 2020). Evening chronotype was relevant in increased depression, mania, and insomnia in patients with BD, and lithium is more effective in treating patients with morning chronotype (McCarthy et al., 2019). Simultaneously, a meta-analysis showed similar findings suggesting that chronotype may be a potential biomarker of lithium treatment in BD patients even though only five studies were included (Xu et al., 2021). For the comorbidity aspect, compared to other types of BD, individuals with evening chronotype have increased rates of anxiety, substance use disorders (Romo-Nava et al., 2020a), and disordered eating behavior (or more unhealthy dietary patterns) (Romo-Nava et al., 2020b). A prospective and naturalistic follow-up study indicated that sleep irregularity might be a risk for the onset of major depressive episodes in BD (Ng et al., 2016). Patients with BD have been reported to have less social rhythm regularity than healthy individuals, a factor potentially predicting affective episodes (Shen et al., 2008). Dysfunction of circadian rhythms provides a new and hopeful perspective for treating BD patients at the genetic level (Oliveira et al., 2018). However, disrupted circadian rhythm and evening chronotype are common in BD, but their influence on mood episodes is unclear (Melo et al., 2017).
Notably, other studies have discovered no significant association between chronotypes and the two BD subtypes (Lewis et al., 2020). This case-control study of 4,672 BD participants and 5,714 control subjects suggested that BD-I had a greater genetic liability to longer sleep duration as did BD-II to insomnia. Nevertheless, the variables of age and education, which show significant influence on chronotype, were not adjusted due to there being no control participants. In short, evening chronotype may be a high-risk subtype in BD because of the worse symptoms and more comorbidity, while current evidence is weak and shows unclear mechanisms. Genetics may be the most important prospect because of high hereditary susceptibility. Future studies need to focus on the relationship between chronotype and subtypes of BD.
Anxiety disorders include disorders that share the features of excessive fear, anxiety, and related behavioral disturbances. Fear is the emotional response to a real or perceived imminent threat, whereas anxiety is the anticipation of a future threat (Arlington and Publishing, 2013). The association between chronotype and anxiety has been found in many studies, and chronotype may be one of the contributors to anxiety processes (Cox and Olatunji, 2019). Some studies have shown a relationship between a high level of anxiety symptoms and morning chronotype (Haraden et al., 2019). Others indicated that anxiety is associated with evening chronotype (Gau et al., 2007; Passos et al., 2017; Walsh et al., 2021), and individuals with anxiety showed higher rates of evening chronotype than healthy individuals did (Fares et al., 2015; Walsh et al., 2021). A study with 3,160 healthy participants suggests that the evening chronotype shows more severe symptoms of anxiety, depression, and sleep disorders than other chronotypes (Walsh et al., 2021). A study of 64 patients with chronic primary insomnia indicated that the evening chronotype is related to higher trait, state, and pre-sleep state anxiety, while the proportion of female subjects was too high (n = 50) (Passos et al., 2017). In addition, other studies on university students concluded that the evening preference was related to poorer sleep quality, which can be explained by higher sleep latency, shorter sleep time, late onset of sleep, and higher trait and state anxiety than students with a morning preference (Silva V. M. et al., 2020). The possible explanation is that sleep factor is more related to anxiety symptoms than chronotype.
In fibromyalgia patients, the evening chronotype was found to be related to increased severity of anxiety symptoms and poorer sleep quality (Turkoglu and Selvi, 2020). Extreme sleep duration is associated with elevated anxiety symptoms (Zhou et al., 2020). However, other research has demonstrated that chronotype is not associated with anxiety. In a longitudinal study, a chronotype change was not relevant to severity of anxiety symptoms (Druiven et al., 2020). Alvaro et al. (2014) suggested that the evening chronotype during adolescence uniquely predicted depression and insomnia but not anxiety disorders. Considering the current evidence, although there is an exact link between chronotype and anxiety symptoms in a non-clinical population, the underlying mechanism and causality remain unclear. In particular, there are few studies of samples with clinically diagnosed anxiety disorders.
Schizophrenia is a multifactorial disorder defined by abnormalities in one or more of five domains, namely delusions, hallucinations, disorganized thinking or speech, grossly disorganized, or abnormal motor behavior, and negative symptoms (Arlington and Publishing, 2013). Circadian rhythm disruption may be a risk factor for schizophrenia by affecting the disorder onset and severity of symptoms (Delorme et al., 2020). Some previous reviews have suggested little evidence linking chronotype and schizophrenia (Ahn et al., 2008; Kivela et al., 2018; Taylor and Hasler, 2018). However, with the deepening of research interest in circadian rhythmicity in recent years, more and more evidence has emerged. A recent meta-analysis of five studies comprising more than 300 patients and over 600 healthy controls indicated that patients with schizophrenia are more evening-oriented than healthy individuals, independent of age, gender, and the type of questionnaire assessing chronotype (Linke and Jankowski, 2021). Genome-wide association studies (GWAS) demonstrated that the chronotype is associated with schizophrenia (Lane et al., 2016) and that people with the morning chronotype have a lower risk of schizophrenia and greater subjective well-being (Jones et al., 2019). A case-control study indicated that patients with schizophrenia are more prone to have evening chronotype than healthy participants are, but this correlation was not clarified (Kilicaslan, 2020). In summary, current evidence could not provide a clear relationship between chronotype and schizophrenia. More clinical case-control and longitudinal studies are needed in the future.
Substance use disorders are defined as the repeated use of one or more psychoactive substance to the extent that the user is periodically or chronically poisoned (Arlington and Publishing, 2013). Disrupted circadian rhythmicity may contribute to addiction or substance abuse. Growing evidence suggests that the evening chronotype is associated with substance abuse, especially alcohol and tobacco (FernÁNdez-Mendoza et al., 2010; Hasler et al., 2017; Borisenkov et al., 2018; Taylor et al., 2020). A research study involving 1,271 individuals in a Spanish university found that individuals with evening chronotype were frequent users of stimulants (chocolate, tobacco, cocaine, amphetamines, and ecstasy) and depressants (alcohol and cannabis) (FernÁNdez-Mendoza et al., 2010). A longitudinal study of adolescents showed that evening chronotype prospectively predicted increased alcohol and marijuana abuse (Hasler et al., 2017). Similarly, college students with evening chronotype reported a higher level of alcohol use disorders. However, the author failed to test the mediated effect of emotion regulation in chronotype and alcohol use disorder (Taylor et al., 2020). Alcohol abuse among evening chronotypes may be accompanied by altered neural responses to the reward circuit (Hasler et al., 2013). Notably, all this research above investigated non-clinical populations. Nonetheless, Kervran et al. (2015) collected and analyzed 333 subjects with an addiction who enrolled as outpatients and assessed them by clinical interview. The results demonstrated that evening chronotype is significantly related to cannabis addiction, non-substance addiction, poly-problematic addiction, and mood disorders, while not to the severity of addiction due to the lack of difference in severity scores in these clinical samples (Kervran et al., 2015). Food addiction (FA) is a concept whereby a preference for high-calorie or highly processed foods eventually leads to changes in behavior and physiology. Evening chronotype and depressive symptoms were associated with FA (Borisenkov et al., 2020). However, a cross-sectional study of 1,323 university students indicated that evening chronotype was indirectly related to FA, which is mediated by insomnia and impulsivity (Kandeger et al., 2019). Internet addiction was associated with evening chronotype, a trait that may foster internet addiction (Randler et al., 2013). The clock–specific therapies may improve the outcomes when treating substance use disorders (Logan et al., 2014; Gulick and Gamsby, 2018). In this regard, compared to the healthy population, a higher trend in morning chronotype was observed in patients with substance use disorders who were under treatment for more than three months (Antunez et al., 2016). It is helpful to improve circadian rhythm in the prevention and therapy of substance use disorders patients (Capella et al., 2018). Overall, the evening chronotype seems to be a risk factor in the onset and maintenance of substance use disorders, while the morning chronotype is considered a protection factor (Adan, 2012).
Disruption of circadian rhythms is rarely considered in the etiology of substance use disorders, although it may increase susceptibility to addiction. Similar to depressive disorders, prevalence of substance use disorders and chronotype show parallel traits. In other words, evening chronotype orientation and the prevalence are highest among adolescents and young adults (Gulick and Gamsby, 2018). This result may be due to the development of the prefrontal cortex and reward circuit, and patients with substance use disorders show aberrant expression of clock genes in reward regions (Falcon and McClung, 2009). As we described above, the evening chronotypes inhibited altered neural response to reward-related areas (mPFC and ventral striatum) compared to the morning chronotypes, which may lead to alcohol abuse (Hasler et al., 2013).
Eating disorders, which include rumination disorder, avoidant/restrictive food intake disorder, anorexia nervosa, bulimia nervosa, and binge eating disorder, are characterized by a persistent disturbance of eating or eating-related behavior resulting in altered consumption or absorption of food, significantly impairing physical health or psychosocial functioning (Arlington and Publishing, 2013). Evening chronotype was related to eating disorders, and evidence was found in children (Rodriguez-Cortes et al., 2022), adolescents (Fleig and Randler, 2009; Rodriguez-Cortes et al., 2022), adults (Kandeger et al., 2018; Sun et al., 2020), and BD patients (Romo-Nava et al., 2020b). A recent meta-analysis demonstrated that children and adolescents with an evening chronotype had a higher body mass index, consumed more junk food, or were more predisposed to suffer from FA and night eating syndrome (Rodriguez-Cortes et al., 2022). A scoping review demonstrated that evening chronotype was related to later mealtimes, breakfast skipping habits, higher energy intake, notably before bedtime, and an unhealthy diet structure (Mazri et al., 2019). Individuals with binge eating and night eating behavior were more likely to be evening chronotypes than morning chronotypes. In a study that included 1197 middle-aged men and women (mean age 48.2 years), the evening chronotype was found to be independently associated with obesity, and the potential mechanism of this association was influenced by unhealthy dietary habits or eating behavior disorders (Sun et al., 2020). A recent cohort study of US women indicated that evening chronotype contributed to a poorer habitual diet, partially mediating the association between evening chronotype and poorer cardiovascular health (Zuraikat et al., 2021). Gender differences could also be seen in eating behavior disorders. A cross-sectional study of 400 students from a Lebanese university suggested that females are more likely to engage in emotional eating than males, and males with lower chronotype scores are more prone to uncontrolled eating (Aoun et al., 2019). In general, there is a significant association between evening chronotype and eating disorders, though most research focused on binge eating disorders. In addition, gender differences are evident. The association between evening chronotype and eating behavior may be influenced by differences in personal worldviews regarding time perspectives (Guenther and Stolarski, 2019) or food craving traits (Teixeira et al., 2020). The personality of morning chronotypes, such as conscientiousness and persistence, may protect them from unhealthy diet habits. Moreover, irregular eating behaviors may delay the circadian phase and contribute to evening chronotype orientation.
The stability of circadian rhythm is an essential prerequisite for human health, and chronotype as a stable state of people has been associated with physical and psychiatric disorders. The review of recent research indicates that chronotype plays a vital role in depressive disorders, BD, anxiety disorders, substance use disorders, and eating disorders. The findings are as follows.
(1) Depression may be the most closely related condition to chronotype: the evening chronotype is a risk factor for depressive disorders, whereas the morning chronotype is a protective factor.
(2) Evening chronotype individuals with BD show more severe symptoms and comorbidity. Chronotype may be a potential biomarker of lithium treatment in BD.
(3) The evening chronotype is only related to anxiety symptoms due to the lack of evidence on research samples diagnosed with anxiety disorders.
(4) The association between chronotype and schizophrenia remains unclear, although new evidence has emerged.
(5) Evening chronotype individuals reported a higher rate of substance use disorders. The evening chronotype seems to be a risk factor in the onset and maintenance of substance use disorders, while the morning chronotype is considered a protective factor. It is beneficial for patients with substance use disorders to use chronotherapy to establish regular daily activities.
(6) The evening chronotype is significantly associated with eating disorders, though most studies focused on binge eating disorders.
Most importantly, although psychiatric disorders are classified depending on their particular syndrome, most patients in the real world have at least one comorbidity (such as depression, anxiety, and sleep disorders). This comorbidity is evident because some psychiatric disorders share common genetic and neural physiological processes. In summary, chronotype is controlled by genetics and zeitgebers, while it is deeply affected by age and gender. Two main oscillators regulate chronotype: the circadian systems and the homeostatic process. The polymorphisms of clock genes control the intrinsic circadian period and directly/indirectly affect the sleep process. After the expression of clock genes, the execution of related functions depends on the structure and function of the brain. In humans, the light/dark cycle mainly entrains circadian oscillator compared with social factors. Some studies in blind people supported this conclusion and demonstrated that blind patients show more disrupted circadian rhythms though they live with strong social activities (Sack et al., 2000; Golombek and Rosenstein, 2010), especially in those without light perception (Hartley et al., 2018).
The evening chronotype accumulates sleep debt and leads to SJL or circadian misalignment due to their different phases from zeitgeists (such as artificial light at night and social activities) with the development of industrialization. In other words, the conflict between modern social lifestyle and personal preference in sleep time may partly explain why chronotype is a risk factor for some psychiatric disorders. Chronotype represents a stable state but not a trait (Roenneberg et al., 2019). Chronotype changes under different entraining environments (Facer-Childs et al., 2019b), while it will relapse without intervention since its genetic basis determines the intrinsic circadian period. This nature of chronotype reflects the interaction between innate factors and environmental factors.
However, as the pathogenesis of most psychiatric disorders remains unclear, we could not clarify the mechanism and causal relationship between chronotype and psychiatric disorders. Current hypotheses stress the effect of aberrant neural circuit neuroendocrinology in the etiology of mental disorders. As described above, the two extreme chronotypes exhibit significant differences in gray and white matters at the structural level and their functional regions, such as DMN and rewarding circuits. Furthermore, the process of neuroendocrinology is deeply involved in circadian and homeostatic systems and participates in the etiology of psychiatric disorders. Together with neural development and hormone secretion, there are age-related changes and gender differences in both chronotype and psychiatric disorders. In addition, psychology and behavior play a significant role. The evening chronotypes are more likely to demonstrate negative personalities and poor emotion regulation than the morning chronotype. The evening chronotypes are less conscientious, less persistent, more impulsive, and more likely to have negative cognitive bias and rumination, which leads to worse performances despite their better intelligence and cognitive functions. In addition, these psychological and behavioral differences depend on the variation of the brain (Takeuchi et al., 2015). To our knowledge, what is notable is that sleep disorders share a similar physiological process with chronotype and are comorbid with many psychiatric disorders. In our opinion, sleep disorders show a mediating effect between chronotype and other mental disorders (Figure 1).
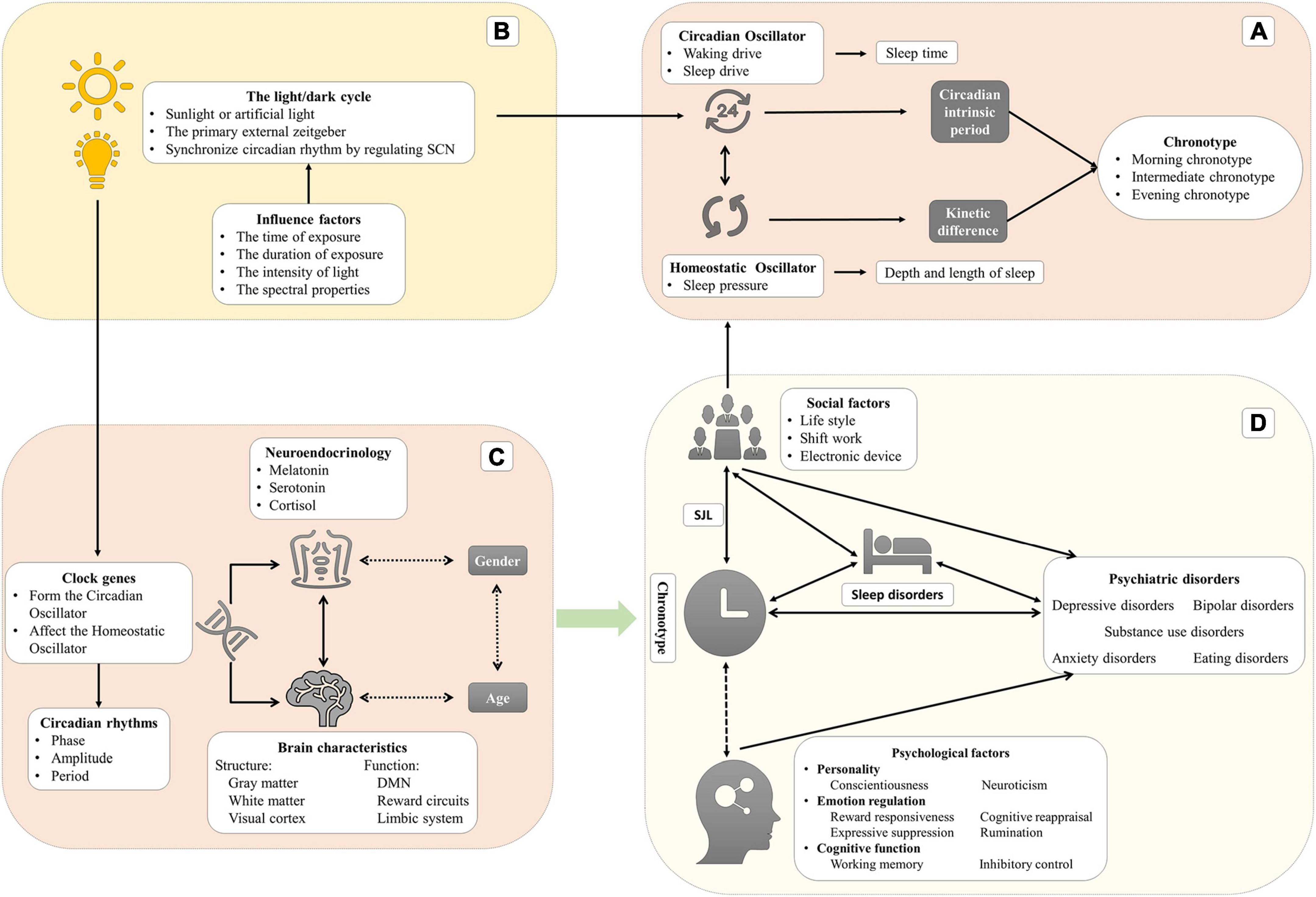
Figure 1. Putative potential mechanisms between chronotype and psychiatric disorders. (A) The two oscillators regulate sleep parameters and lead to chronotypes. (B) The light/dark cycle and its influence factors. (C) The physiological bases contribute to differences in chronotypes. (D) The potential mechanisms and relationship between chronotype and psychiatric disorders. The direction of the arrows indicates the possible causality. The solid and dotted lines correspond to the strength of the connection. SCN, suprachiasmatic nuclei; DMN, default mode network; SJL, social jet lag.
There are some limitations to the current research. First, most studies are cross-sectional. Therefore, there is a need for longitudinal and experimental research. Due to limitations in the study’s design, the causality between chronotype and psychiatric disorders is unclear. Little evidence was found on the neuroimaging and psychological mechanisms of chronotypes, notably concerning psychiatric disorders. Further investigation and improvement would be worthwhile. There is a need for an experimental design to examine the relationship between chronotype and mood disorders, in which chronotype is not feasibly manipulated or randomly assigned (Au and Reece, 2017). In a cortisol-induced depression-like behavior mice model, alterations in circadian rhythm preceded the onset of depression, demonstrating that circadian rhythm may cause or predict the onset of depression (Spulber et al., 2015). Moreover, a large population of GWAS demonstrated no evidence to suggest that poor mental health influences chronotype, providing a reference from a genetic perspective (Jones et al., 2019). Second, most studies used the MEQ or MCTQ to assess and classify chronotypes, but the self-reported data may have been biased. Few studies have objectively employed physiological measures of chronotype, such as wrist accelerometry, body temperature, and hormone measurement (Kivela et al., 2018), because objective measures are not feasible within a large population sample (Evans et al., 2021). In particular, there is a discrepancy between subjective and objective measures, which may obstruct the experimental results (Chung et al., 2020). The third is the variability of chronotypes. Aging, gender, and intervention can change chronotype, requiring future research to investigate these confounders. Fourth, some studies relied on non-clinical or student samples. Therefore, their findings were not applicable to clinical populations or other age groups.
Evening chronotype affects early diagnosis, treatment, and prognosis. Since MDD (Gau et al., 2007; Gaspar-Barba et al., 2009) and BD (Romo-Nava et al., 2020a) patients with evening chronotype have a high risk of suicide, it is vital not only to investigate the association between chronotype and suicide in psychiatric disorders but also to prevent, and to intervene early in, the onset of suicidal ideation. The development of precision medicine means that chronotype identification has a potential advantage in developing targeted and personalized treatments for psychiatric disorders, especially mood disorders (Adan et al., 2012; Gershon et al., 2018; Lynch et al., 2020). The effects of treatment of circadian dysfunction in psychiatric disorders with psychosocial, pharmacological, and light therapy are encouraging (Gold and Kinrys, 2019). Chronotype could also be a potential biomarker for lithium treatment in patients with BD (Xu et al., 2021). Some drugs that affect circadian rhythms, such as melatonin and its analogs, can potentially treat neuropsychiatric disorders (Hickie and Rogers, 2011).
HWZ wrote the original text, drew the Figure 1 and Table 1, and revised and assisted with editing the manuscript. HLZ helped generate ideas for the framework of the manuscript, conducted the linguistic sorting investigation, and assisted with editing the manuscript. RY provided editorial direction and assisted with revising the manuscript. ZY helped generate ideas for the framework of the manuscript and revised the manuscript. QL helped generate ideas for the framework of the manuscript and revised the manuscript. All authors contributed to and approved the final manuscript.
This work was supported by the National Natural Science Foundation of China (81871066); the Jiangsu Provincial Key Research and Development Program (BE2018609 and BE2019675), the Jiangsu Provincial Medical Innovation Team of the Project of Invigorating Health Care through Science, Technology and Education (CXTDC2016004), the Key Project supported by the Medical Science and Technology Development Foundation, Jiangsu Commission of Health (K2019011), and the Humanities and Social Sciences Research Fund Project of the Ministry of Education (17YJA740023).
We thank all the participants and their families.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnins.2022.811771/full#supplementary-material
Adan, A. (2012). A chronobiological approach to addiction. J. Subst. Use 18, 171–183. doi: 10.3109/14659891.2011.632060
Adan, A., and Almirall, H. (1991). Horne and Östberg morningness–eveningness questionnaire: a reduced scale. Person. Indiv. Diff. 1991, 241–253. doi: 10.1016/0191-8869(91)90110-W
Adan, A., Archer, S. N., Hidalgo, M. P., Milia, L. Di, Natale, V., and Randler, C. (2012). Circadian typology: a comprehensive review. Chronobiol. Int. 29, 1153–1175. doi: 10.3109/07420528.2012.719971
Ahn, Y. M., Chang, J., Joo, Y. H., Kim, S. C., Lee, K. Y., and Kim, Y. S. (2008). Chronotype distribution in bipolar I disorder and schizophrenia in a Korean sample. Bipolar Disord. 10, 271–275. doi: 10.1111/j.1399-5618.2007.00573.x
Akçay, B. D., Akçay, D., and Yetkin, S. (2021). The effects of mobile electronic devices use on the sleep states of university students. Alpha Psychiatry 22, 31–37.
Alvaro, P. K., Roberts, R. M., and Harris, J. K. (2014). The independent relationships between insomnia, depression, subtypes of anxiety, and chronotype during adolescence. Sleep Med. 15, 934–941. doi: 10.1016/j.sleep.2014.03.019
Antunez, J. M., Capella, M. D., Navarro, J. F., and Adan, A. (2016). Circadian rhythmicity in substance use disorder male patients with and without comorbid depression under ambulatory and therapeutic community treatment. Chronobiol. Int. 33, 1410–1421. doi: 10.1080/07420528.2016.1223092
Antypa, N., Verkuil, B., Molendijk, M., Schoevers, R., Penninx, B., and Van Der Does, W. (2017). Associations between chronotypes and psychological vulnerability factors of depression. Chronobiol. Int. 34, 1125–1135. doi: 10.1080/07420528.2017.1345932
Antypa, N., Vogelzangs, N., Meesters, Y., Schoevers, R., and Penninx, B. W. (2016). Chronotype associations with depression and anxiety disorders in a large cohort study. Depress Anxiety 33, 75–83. doi: 10.1002/da.22422
Aoun, C., Nassar, L., Soumi, S., El Osta, N., Papazian, T., and Rabbaa Khabbaz, L. (2019). The cognitive, behavioral, and emotional aspects of eating habits and association with impulsivity, chronotype, anxiety, and depression: a cross-sectional study. Front. Behav. Neurosci. 13:204. doi: 10.3389/fnbeh.2019.00204
Arlington, V., and Publishing, U. A. P. (2013). Diagnostic and statistical manual of mental disorders, 5th edition (DSM-5). Washington, D.C: American Psychiatric Association.
Aschoff, J., and Pohl, H. (1978). Phase relations between a circadian rhythm and its zeitgeber within the range of entrainment. Naturwissenschaften 65, 80–84. doi: 10.1007/BF00440545
Au, J., and Reece, J. (2017). The relationship between chronotype and depressive symptoms: a meta-analysis. J. Affect. Disord. 218, 93–104. doi: 10.1016/j.jad.2017.04.021
Bacque-Cazenave, J., Bharatiya, R., Barriere, G., Delbecque, J. P., Bouguiyoud, N., Di Giovanni, G., et al. (2020). Serotonin in animal cognition and behavior. Int. J. Mol. Sci. 21:5. doi: 10.3390/ijms21051649
Baehr, E. K., Revelle, W., and Eastman, C. I. (2000). Individual differences in the phase and amplitude of the human circadian temperature rhythm: with an emphasis on morningness-eveningness. J. Sleep Res. 9, 117–127. doi: 10.1046/j.1365-2869.2000.00196.x
Bauducco, S., Richardson, C., and Gradisar, M. (2020). Chronotype, circadian rhythms and mood. Curr. Opin. Psychol. 34, 77–83. doi: 10.1016/j.copsyc.2019.09.002
Bedrosian, T. A., and Nelson, R. J. (2017). Timing of light exposure affects mood and brain circuits. Transl. Psychiatry 7:e1017. doi: 10.1038/tp.2016.262
Bekhbat, M., and Neigh, G. N. (2018). Sex differences in the neuro-immune consequences of stress: Focus on depression and anxiety. Brain Behav. Immun. 67, 1–12. doi: 10.1016/j.bbi.2017.02.006
Birchler-Pedross, A., Frey, S., Chellappa, S. L., Gotz, T., Brunner, P., Knoblauch, V., et al. (2011). Higher frontal EEG synchronization in young women with major depression: a marker for increased homeostatic sleep pressure? Sleep 34, 1699–1706. doi: 10.5665/sleep.1440
Bolsius, Y. G., Zurbriggen, M. D., Kim, J. K., Kas, M. J., Meerlo, P., Aton, S. J., et al. (2021). The role of clock genes in sleep, stress and memory. Biochem. Pharmacol. 191:114493. doi: 10.1016/j.bcp.2021.114493
Borisenkov, M. F., Kosova, A. L., and Kasyanova, O. N. (2012). Impact of perinatal photoperiod on the chronotype of 11- to 18-year-olds in northern European Russia. Chronobiol. Int. 29, 305–310. doi: 10.3109/07420528.2011.653612
Borisenkov, M. F., Polugrudov, A. S., Paderin, N. M., and Bakutova, L. A. (2018). Young inhabitants of the North with late chronotype and social jetlag consume more high-calorie foods and alcohol. Biolog. Rhyth. Res. 50, 418–428. doi: 10.1080/09291016.2018.1455867
Borisenkov, M. F., Popov, S. V., Tserne, T. A., Bakutova, L. A., Pecherkina, A. A., Dorogina, O. I., et al. (2020). Food addiction and symptoms of depression among inhabitants of the european north of russia: associations with sleep characteristics and photoperiod. Eur. Eat. Disord. Rev. 28, 332–342. doi: 10.1002/erv.2728
Borisenkov, M. F., Vetosheva, V. I., Kuznetsova, Y. S., Khodyrev, G. N., Shikhova, A. V., Popov, S. V., et al. (2019). Chronotype, social jetlag, and time perspective. Chronobiol. Int. 36, 1772–1781. doi: 10.1080/07420528.2019.1683858
Boyle, C. C., Cho, J. H., Eisenberger, N. I., Olmstead, R. E., Piber, D., Sadeghi, N., et al. (2020). Motivation and sensitivity to monetary reward in late-life insomnia: moderating role of sex and the inflammatory marker CRP. Neuropsychopharmacology 45, 1664–1671. doi: 10.1038/s41386-020-0735-7
Bradford, D. R. R., Biello, S. M., and Russell, K. (2021). Insomnia symptoms mediate the association between eveningness and suicidal ideation, defeat, entrapment, and psychological distress in students. Chronobiol. Int. 2021, 1–12. doi: 10.1080/07420528.2021.1931274
Bruckmann, K. F., Hennig, J., Muller, M. J., Fockenberg, S., Schmidt, A. M., Cabanel, N., et al. (2020). Influence of chronotype on daily mood fluctuations: pilot study in patients with depression. BJPsych. Open 6:e17. doi: 10.1192/bjo.2019.103
Cabanel, N., Schmidt, A. M., Fockenberg, S., Bruckmann, K. F., Haag, A., Muller, M. J., et al. (2019). Evening preference and poor sleep independently affect attentional-executive functions in patients with depression. Psychiatry Res. 281:112533. doi: 10.1016/j.psychres.2019.112533
Cain, N., and Gradisar, M. (2010). Electronic media use and sleep in school-aged children and adolescents: a review. Sleep Med. 11, 735–742. doi: 10.1016/j.sleep.2010.02.006
Capella, M. D. M., Martinez-Nicolas, A., and Adan, A. (2018). Circadian rhythmic characteristics in men with substance use disorder under treatment. influence of age of onset of substance use and duration of abstinence. Front. Psychiatry 9:373. doi: 10.3389/fpsyt.2018.00373
Carney, C. E., Buysse, D. J., Ancoli-Israel, S., Edinger, J. D., Krystal, A. D., Lichstein, K. L., et al. (2012). The consensus sleep diary: standardizing prospective sleep self-monitoring. Sleep 35, 287–302. doi: 10.5665/sleep.1642
Carskadon, M. A., Labyak, S. E., Acebo, C., and Seifer, R. (1999). Intrinsic circadian period of adolescent humans measured in conditions of forced desynchrony. Neurosci. Lett. 260, 129–132. doi: 10.1016/s0304-3940(98)00971-9
Caruso, D., Meyrel, M., Krane-Gartiser, K., Benard, V., Benizri, C., Brochard, H., et al. (2020). Eveningness and poor sleep quality contribute to depressive residual symptoms and behavioral inhibition in patients with bipolar disorder. Chronobiol. Int. 37, 101–110. doi: 10.1080/07420528.2019.1685533
Cespedes Feliciano, E. M., Rifas-Shiman, S. L., Quante, M., Redline, S., Oken, E., and Taveras, E. M. (2019). Chronotype, social jet lag, and cardiometabolic risk factors in early adolescence. JAMA Pediatr. 2019:3089. doi: 10.1001/jamapediatrics.2019.3089
Chan, J. W., Lam, S. P., Li, S. X., Chau, S. W., Chan, S. Y., Chan, N. Y., et al. (2020). Adjunctive bright light treatment with gradual advance in unipolar major depressive disorder with evening chronotype - A randomized controlled trial. Psychol. Med. 2020, 1–10. doi: 10.1017/S0033291720003232
Chan, J. W., Lam, S. P., Li, S. X., Yu, M. W., Chan, N. Y., Zhang, J., et al. (2014). Eveningness and insomnia: independent risk factors of nonremission in major depressive disorder. Sleep 37, 911–917. doi: 10.5665/sleep.3658
Chan, N. Y., Zhang, J., Tsang, C. C., Li, A. M., Chan, J. W. Y., Wing, Y. K., et al. (2020). The associations of insomnia symptoms and chronotype with daytime sleepiness, mood symptoms and suicide risk in adolescents. Sleep Med. 74, 124–131. doi: 10.1016/j.sleep.2020.05.035
Cheng, W. J., Puttonen, S., Vanttola, P., Koskinen, A., Kivimaki, M., and Harma, M. (2021). Association of shift work with mood disorders and sleep problems according to chronotype: a 17-year cohort study. Chronobiol. Int. 38, 518–525. doi: 10.1080/07420528.2021.1885431
Choi, S. J., Song, P., Suh, S., Joo, E. Y., and Lee, S. I. (2020). Insomnia symptoms and mood disturbances in shift workers with different chronotypes and working schedules. J. Clin. Neurol. 16, 108–115. doi: 10.3988/jcn.2020.16.1.108
Chung, K. F., Poon, Y. P. Y., Ng, T. K., and Kan, C. K. (2020). Subjective-Objective Sleep Discrepancy in Schizophrenia. Behav. Sleep Med. 18, 653–667. doi: 10.1080/15402002.2019.1656077
Correa, A., Molina, E., and Sanabria, D. (2014). Effects of chronotype and time of day on the vigilance decrement during simulated driving. Accid. Anal. Prev. 67, 113–118. doi: 10.1016/j.aap.2014.02.020
Cox, R. C., and Olatunji, B. O. (2019). Differential associations between chronotype, anxiety, and negative affect: a structural equation modeling approach. J. Affect. Disord 257, 321–330. doi: 10.1016/j.jad.2019.07.012
Croce, P., Quercia, A., Costa, S., and Zappasodi, F. (2018). Circadian rhythms in fractal features of eeg signals. Front. Physiol. 9:1567. doi: 10.3389/fphys.2018.01567
Crouse, J. J., Carpenter, J. S., Song, Y. J. C., Hockey, S. J., Naismith, S. L., Grunstein, R. R., et al. (2021). Circadian rhythm sleep–wake disturbances and depression in young people: implications for prevention and early intervention. Lancet Psychiatry 8, 813–823. doi: 10.1016/s2215-0366(21)00034-1
Czeisler, C. A., Buxton, O. M., and Sbs, K. (2005). The human circadian timing system and sleep-wake regulation. Prin. Pract. Sleep Med. 375:94.
Daghlas, I., Lane, J., Saxena, R., and Vetter, C. J. J. P. (2021). Genetically proxied diurnal preference, sleep timing, and risk of major depressive disorder. JAMA Psychiatry 78, 903–910. doi: 10.1001/jamapsychiatry.2021.0959
Dagnew, B., Andualem, Z., and Dagne, H. (2020). Excessive daytime sleepiness and its predictors among medical and health science students of University of Gondar, Northwest Ethiopia: institution-based cross-sectional study. Health Qual. Life Outcomes 18:299. doi: 10.1186/s12955-020-01553-3
Daut, R. A., and Fonken, L. K. (2019). Circadian regulation of depression: a role for serotonin. Front. Neuroendocrinol. 54:100746. doi: 10.1016/j.yfrne.2019.04.003
Delorme, T. C., Srivastava, L. K., and Cermakian, N. (2020). Are circadian disturbances a core pathophysiological component of schizophrenia?. J. Biol. Rhythms 35, 325–339. doi: 10.1177/0748730420929448
Diaz, N. M., Morera, L. P., and Guido, M. E. (2016). Melanopsin and the non-visual photochemistry in the inner retina of vertebrates. Photochem. Photobiol. 92, 29–44. doi: 10.1111/php.12545
Diaz-Morales, J. F., and Escribano, C. (2013). Circadian preference and thinking styles: implications for school achievement. Chronobiol. Int. 30, 1231–1239. doi: 10.3109/07420528.2013.813854
Dijk, D. J., and von Schantz, M. (2005). Timing and consolidation of human sleep, wakefulness, and performance by a symphony of oscillators. J. Biol. Rhythms 20, 279–290. doi: 10.1177/0748730405278292
Dockray, S., and Steptoe, A. (2011). Chronotype and diurnal cortisol profile in working women: differences between work and leisure days. Psychoneuroendocrinology 36, 649–655. doi: 10.1016/j.psyneuen.2010.09.008
Druiven, S. J. M., Hovenkamp-Hermelink, J. H. M., Knapen, S. E., Kamphuis, J., Haarman, B. C. M., Penninx, B., et al. (2020). Stability of chronotype over a 7-year follow-up period and its association with severity of depressive and anxiety symptoms. Depress Anxiety 37, 466–474. doi: 10.1002/da.22995
Duarte, L. L., Menna-Barreto, L., Miguel, M. A., Louzada, F., Araujo, J., Alam, M., et al. (2014). Chronotype ontogeny related to gender. Braz. J. Med. Biol. Res. 47, 316–320. doi: 10.1590/1414-431x20143001
Duffy, J. F., Rimmer, D. W., and Czeisler, C. A. (2001). Association of intrinsic circadian period with morningness-eveningness, usual wake time, and circadian phase. Behav. Neurosci. 115, 895–899. doi: 10.1037//0735-7044.115.4.895
Dzogang, F., Lightman, S., and Cristianini, N. (2017). Circadian mood variations in Twitter content. Brain Neurosci. Adv. 1:2398212817744501. doi: 10.1177/2398212817744501
Eid, B., Bou Saleh, M., Melki, I., Torbey, P. H., Najem, J., Saber, M., et al. (2020). Evaluation of chronotype among children and associations with bmi, sleep, anxiety, and depression. Front. Neurol. 11:416. doi: 10.3389/fneur.2020.00416
Emens, J., Lewy, A., Kinzie, J. M., Arntz, D., and Rough, J. (2009). Circadian misalignment in major depressive disorder. Psychiatry Res. 168, 259–261. doi: 10.1016/j.psychres.2009.04.009
Etain, B., Milhiet, V., Bellivier, F., and Leboyer, M. (2011). Genetics of circadian rhythms and mood spectrum disorders. Eur. Neuropsychopharmacol. 21(Suppl. 4), S676–S682. doi: 10.1016/j.euroneuro.2011.07.007
Evans, S. L., Leocadio-Miguel, M. A., Taporoski, T. P., Gomez, L. M., Horimoto, A., Alkan, E., et al. (2021). Evening preference correlates with regional brain volumes in the anterior occipital lobe. Chronobiol. Int. 38, 1135–1142. doi: 10.1080/07420528.2021.1912077
Fabbian, F., Zucchi, B., De Giorgi, A., Tiseo, R., Boari, B., Salmi, R., et al. (2016). Chronotype, gender and general health. Chronobiol. Int. 33, 863–882. doi: 10.1080/07420528.2016.1176927
Facer-Childs, E. R., Campos, B. M., Middleton, B., Skene, D. J., and Bagshaw, A. P. (2019a). Circadian phenotype impacts the brain’s resting-state functional connectivity, attentional performance, and sleepiness. Sleep 42:5. doi: 10.1093/sleep/zsz033
Facer-Childs, E. R., Middleton, B., Skene, D. J., and Bagshaw, A. P. (2019b). Resetting the late timing of ‘night owls’ has a positive impact on mental health and performance. Sleep Med. 60, 236–247. doi: 10.1016/j.sleep.2019.05.001
Falcon, E., and McClung, C. A. (2009). A role for the circadian genes in drug addiction. Neuropharmacology 56(Suppl. 1), 91–96. doi: 10.1016/j.neuropharm.2008.06.054
Fares, S., Hermens, D. F., Naismith, S. L., White, D., Hickie, I. B., and Robillard, R. (2015). Clinical correlates of chronotypes in young persons with mental disorders. Chronobiol. Int. 32, 1183–1191. doi: 10.3109/07420528.2015.1078346
Fernandez, D. C., Fogerson, P. M., Lazzerini Ospri, L., Thomsen, M. B., Layne, R. M., Severin, D., et al. (2018). Light affects mood and learning through distinct retina-brain pathways. Cell 175, 71–84e18. doi: 10.1016/j.cell.2018.08.004
FernÁNdez-Mendoza, J., Ilioudi, C., Montes, M. I., Olavarrieta-Bernardino, S., Aguirre-Berrocal, A., De La Cruz-Troca, J. J., et al. (2010). Circadian preference, nighttime sleep and daytime functioning in young adulthood. Sleep Biol. Rhythms 8, 52–62. doi: 10.1111/j.1479-8425.2010.00430.x
Ferrer, A., Costas, J., Gratacos, M., Martinez-Amoros, E., Labad, J., Soriano-Mas, C., et al. (2020). Clock gene polygenic risk score and seasonality in major depressive disorder and bipolar disorder. Genes Brain Behav. 19:e12683. doi: 10.1111/gbb.12683
Figueiro, M., and Overington, D. (2016). Self-luminous devices and melatonin suppression in adolescents. Light. Res. Technol. 48, 966–975. doi: 10.1177/1477153515584979
Fischer, D., McHill, A. W., Sano, A., Picard, R. W., Barger, L. K., Czeisler, C. A., et al. (2020). Irregular sleep and event schedules are associated with poorer self-reported well-being in US college students. Sleep 43:6. doi: 10.1093/sleep/zsz300
Fleig, D., and Randler, C. (2009). Association between chronotype and diet in adolescents based on food logs. Eat Behav. 10, 115–118. doi: 10.1016/j.eatbeh.2009.03.002
Fossum, I. N., Nordnes, L. T., Storemark, S. S., Bjorvatn, B., and Pallesen, S. (2014). The association between use of electronic media in bed before going to sleep and insomnia symptoms, daytime sleepiness, morningness, and chronotype. Behav. Sleep Med. 12, 343–357. doi: 10.1080/15402002.2013.819468
Foster, R. G., Peirson, S. N., Wulff, K., Winnebeck, E., Vetter, C., and Roenneberg, T. (2013). Sleep and circadian rhythm disruption in social jetlag and mental illness. Prog. Mol. Biol. Transl. Sci. 119, 325–346. doi: 10.1016/B978-0-12-396971-2.00011-7
Friedman, H. S., and Kern, M. L. (2014). Personality, well-being, and health. Annu. Rev. Psychol. 65, 719–742. doi: 10.1146/annurev-psych-010213-115123
Furihata, R., Saitoh, K., Suzuki, M., Jike, M., Kaneita, Y., Ohida, T., et al. (2020). A composite measure of sleep health is associated with symptoms of depression among Japanese female hospital nurses. Compr. Psychiatry 97:152151. doi: 10.1016/j.comppsych.2019.152151
Gaspar-Barba, E., Calati, R., Cruz-Fuentes, C. S., Ontiveros-Uribe, M. P., Natale, V., De Ronchi, D., et al. (2009). Depressive symptomatology is influenced by chronotypes. J. Affect Disord. 119, 100–106. doi: 10.1016/j.jad.2009.02.021
Gau, S. S., Shang, C. Y., Merikangas, K. R., Chiu, Y. N., Soong, W. T., and Cheng, A. T. (2007). Association between morningness-eveningness and behavioral/emotional problems among adolescents. J. Biol. Rhythms 22, 268–274. doi: 10.1177/0748730406298447
Gentry, N. W., Ashbrook, L. H., Fu, Y. H., and Ptacek, L. J. (2021). Human circadian variations. J Clin Invest 131, 16. doi: 10.1172/JCI148282
Germain, A., and Kupfer, D. J. (2008). Circadian rhythm disturbances in depression. Hum. Psychopharmacol. 23, 571–585. doi: 10.1002/hup.964
Gershon, A., Kaufmann, C. N., Depp, C. A., Miller, S., Do, D., Zeitzer, J. M., et al. (2018). Subjective versus objective evening chronotypes in bipolar disorder. J. Affect. Disord. 225, 342–349. doi: 10.1016/j.jad.2017.08.055
Girshkin, L., O’Reilly, N., Quide, Y., Teroganova, N., Rowland, J. E., Schofield, P. R., et al. (2016). Diurnal cortisol variation and cortisol response to an MRI stressor in schizophrenia and bipolar disorder. Psychoneuroendocrinology 67, 61–69. doi: 10.1016/j.psyneuen.2016.01.021
Gold, A. K., and Kinrys, G. (2019). Treating circadian rhythm disruption in bipolar disorder. Curr. Psychiatry Rep. 21:14. doi: 10.1007/s11920-019-1001-8
Golombek, D. A., and Rosenstein, R. E. (2010). Physiology of circadian entrainment. Physiol. Rev. 90, 1063–1102. doi: 10.1152/physrev.00009.2009
Goulet, G., Mongrain, V., Desrosiers, C., Paquet, J., and Dumont, M. (2007). Daily light exposure in morning-type and evening-type individuals. J. Biol. Rhythms 22, 151–158. doi: 10.1177/0748730406297780
Gruber, R., and Cassoff, J. (2014). The interplay between sleep and emotion regulation: conceptual framework empirical evidence and future directions. Curr. Psychiatry Rep. 16:500. doi: 10.1007/s11920-014-0500-x
Guenther, K., and Stolarski, M. (2019). Linking morningness with healthy eating behaviors: the mediating role of time perspectives. Biol. Rhythm Res. 52, 1618–1630. doi: 10.1080/09291016.2019.1684795
Guido, M. E., Marchese, N. A., Rios, M. N., Morera, L. P., Diaz, N. M., Garbarino-Pico, E., et al. (2020). Non-visual opsins and novel photo-detectors in the vertebrate inner retina mediate light responses within the blue spectrum region. Cell Mol. Neurobiol. 2020:997. doi: 10.1007/s10571-020-00997-x
Gulick, D., and Gamsby, J. J. (2018). Racing the clock: the role of circadian rhythmicity in addiction across the lifespan. Pharmacol. Ther. 188, 124–139. doi: 10.1016/j.pharmthera.2018.03.003
Hahn, C., Cowell, J. M., Wiprzycka, U. J., Goldstein, D., Ralph, M., Hasher, L., et al. (2012). Circadian rhythms in executive function during the transition to adolescence: the effect of synchrony between chronotype and time of day. Dev. Sci. 15, 408–416. doi: 10.1111/j.1467-7687.2012.01137.x
Haraden, D. A., Mullin, B. C., and Hankin, B. L. (2019). Internalizing symptoms and chronotype in youth: a longitudinal assessment of anxiety, depression and tripartite model. Psychiatry Res. 272, 797–805. doi: 10.1016/j.psychres.2018.12.117
Hartley, S., Dauvilliers, Y., and Quera-Salva, M. A. (2018). Circadian rhythm disturbances in the blind. Curr. Neurol. Neurosci. Rep. 18:65. doi: 10.1007/s11910-018-0876-9
Hasbi, A., Nguyen, T., Rahal, H., Manduca, J. D., Miksys, S., Tyndale, R. F., et al. (2020). Sex difference in dopamine D1-D2 receptor complex expression and signaling affects depression- and anxiety-like behaviors. Biol. Sex Differ. 11:8. doi: 10.1186/s13293-020-00285-9
Hasler, B. P., Allen, J. J., Sbarra, D. A., Bootzin, R. R., and Bernert, R. A. (2010). Morningness-eveningness and depression: preliminary evidence for the role of the behavioral activation system and positive affect. Psychiatry Res. 176, 166–173. doi: 10.1016/j.psychres.2009.06.006
Hasler, B. P., Franzen, P. L., de Zambotti, M., Prouty, D., Brown, S. A., Tapert, S. F., et al. (2017). Eveningness and later sleep timing are associated with greater risk for alcohol and marijuana use in adolescence: initial findings from the national consortium on alcohol and neurodevelopment in adolescence study. Alcohol. Clin. Exp. Res. 41, 1154–1165. doi: 10.1111/acer.13401
Hasler, B. P., Germain, A., Nofzinger, E. A., Kupfer, D. J., Krafty, R. T., Rothenberger, S. D., et al. (2012). Chronotype and diurnal patterns of positive affect and affective neural circuitry in primary insomnia. J. Sleep Res. 21, 515–526. doi: 10.1111/j.1365-2869.2012.01002.x
Hasler, B. P., Sitnick, S. L., Shaw, D. S., and Forbes, E. E. (2013). An altered neural response to reward may contribute to alcohol problems among late adolescents with an evening chronotype. Psychiatry Res. 214, 357–364. doi: 10.1016/j.pscychresns.2013.08.005
Henderson, S. E. M., Brady, E. M., and Robertson, N. (2019). Associations between social jetlag and mental health in young people: a systematic review. Chronobiol. Int. 36, 1316–1333. doi: 10.1080/07420528.2019.1636813
Henrich, L. C., Antypa, N., and Van den Berg, J. F. (2021). Sleep quality in students: associations with psychological and lifestyle factors. Curr. Psychol. 2021:9. doi: 10.1007/s12144-021-01801-9
Hickie, I. B., and Rogers, N. L. (2011). Novel melatonin-based therapies: potential advances in the treatment of major depression. Lancet 378, 621–631. doi: 10.1016/s0140-6736(11)60095-0
Hisler, G., Twenge, J. M., and Krizan, Z. (2020). Associations between screen time and short sleep duration among adolescents varies by media type: evidence from a cohort study. Sleep Med. 66, 92–102. doi: 10.1016/j.sleep.2019.08.007
Holler, Y., Gudjonsdottir, B. E., Valgeirsdottir, S. K., and Heimisson, G. T. (2021). The effect of age and chronotype on seasonality, sleep problems, and mood. Psychiatry Res. 297:113722. doi: 10.1016/j.psychres.2021.113722
Hood, S., and Amir, S. (2017). The aging clock: circadian rhythms and later life. J. Clin. Invest. 127, 437–446. doi: 10.1172/JCI90328
Horne, C. M., and Norbury, R. (2018b). Late chronotype is associated with enhanced amygdala reactivity and reduced fronto-limbic functional connectivity to fearful versus happy facial expressions. Neuroimage 171, 355–363. doi: 10.1016/j.neuroimage.2018.01.025
Horne, C. M., and Norbury, R. (2018a). Exploring the effect of chronotype on hippocampal volume and shape: a combined approach. Chronobiol. Int. 35, 1027–1033. doi: 10.1080/07420528.2018.1455056
Horne, J., and Ostberg, O. J. I. J. O. C. (1976). A self-assessment questionnaire to determine morningness-eveningness in human circadian rhythms. Int. J. Chronobiol. 4, 97–110.
Hou, T., Zhang, F., Mao, X., and Deng, G. (2020). Chronotype and psychological distress among Chinese rural population: a moderated mediation model of sleep quality and age. PLoS One 15:e0241301. doi: 10.1371/journal.pone.0241301
Huang, W., Ramsey, K. M., Marcheva, B., and Bass, J. (2011). Circadian rhythms, sleep, and metabolism. J. Clin. Invest. 121, 2133–2141. doi: 10.1172/JCI46043
Islam, Z., Hu, H., Akter, S., Kuwahara, K., Kochi, T., Eguchi, M., et al. (2020). Social jetlag is associated with an increased likelihood of having depressive symptoms among the Japanese working population: the furukawa nutrition and health study. Sleep 43:1. doi: 10.1093/sleep/zsz204
Jagannath, A., Taylor, L., Wakaf, Z., Vasudevan, S. R., and Foster, R. G. (2017). The genetics of circadian rhythms, sleep and health. Hum. Mol. Genet. 26, R128–R138. doi: 10.1093/hmg/ddx240
Jankowski, K. S., and Dmitrzak-Weglarz, M. (2017). ARNTL, CLOCK and PER3 polymorphisms - links with chronotype and affective dimensions. Chronobiol. Int. 34, 1105–1113. doi: 10.1080/07420528.2017.1343341
Jansen, P. R., Watanabe, K., Stringer, S., Skene, N., Bryois, J., Hammerschlag, A. R., et al. (2019). Genome-wide analysis of insomnia in 1,331,010 individuals identifies new risk loci and functional pathways. Nat. Genet. 51, 394–403. doi: 10.1038/s41588-018-0333-3
Jones, S. E., Lane, J. M., Wood, A. R., van Hees, V. T., Tyrrell, J., Beaumont, R. N., et al. (2019). Genome-wide association analyses of chronotype in 697,828 individuals provides insights into circadian rhythms. Nat. Commun. 10:343. doi: 10.1038/s41467-018-08259-7
Jones, S. E., Tyrrell, J., Wood, A. R., Beaumont, R. N., Ruth, K. S., Tuke, M. A., et al. (2016). Genome-wide association analyses in 128,266 individuals identifies new morningness and sleep duration loci. PLoS Genet. 12:e1006125. doi: 10.1371/journal.pgen.1006125
Juda, M., Vetter, C., and Roenneberg, T. (2013). Chronotype modulates sleep duration, sleep quality, and social jet lag in shift-workers. J. Biol. Rhythms 28, 141–151. doi: 10.1177/0748730412475042
Kalmbach, D. A., Schneider, L. D., Cheung, J., Bertrsand, S. J., Kariharan, T., Pack, A. I., et al. (2017). Genetic basis of chronotype in humans: insights from three landmark GWAS. Sleep 40:2. doi: 10.1093/sleep/zsw048
Kandeger, A., Egilmez, U., Sayin, A. A., and Selvi, Y. (2018). The relationship between night eating symptoms and disordered eating attitudes via insomnia and chronotype differences. Psychiatry Res. 268, 354–357. doi: 10.1016/j.psychres.2018.08.003
Kandeger, A., Selvi, Y., and Tanyer, D. K. (2019). The effects of individual circadian rhythm differences on insomnia, impulsivity, and food addiction. Eat. Weight Disord. 24, 47–55. doi: 10.1007/s40519-018-0518-x
Kang, J. I., Park, C. I., Sohn, S. Y., Kim, H. W., Namkoong, K., and Kim, S. J. (2015). Circadian preference and trait impulsivity, sensation-seeking and response inhibition in healthy young adults. Chronobiol. Int. 32, 235–241. doi: 10.3109/07420528.2014.965313
Kaufmann, C. N., Gershon, A., Depp, C. A., Miller, S., Zeitzer, J. M., and Ketter, T. A. (2018). Daytime midpoint as a digital biomarker for chronotype in bipolar disorder. J. Affect Disord. 241, 586–591. doi: 10.1016/j.jad.2018.08.032
Kervran, C., Fatseas, M., Serre, F., Taillard, J., Beltran, V., Leboucher, J., et al. (2015). Association between morningness/eveningness, addiction severity and psychiatric disorders among individuals with addictions. Psychiatry Res. 229, 1024–1030. doi: 10.1016/j.psychres.2015.05.026
Khan, W. A. A., Conduit, R., Kennedy, G. A., and Jackson, M. L. (2020). The relationship between shift-work, sleep, and mental health among paramedics in Australia. Sleep Health 6, 330–337. doi: 10.1016/j.sleh.2019.12.002
Kilicaslan, E. E. (2020). Associations between neurological soft signs, chronotype and sleep quality in schizophrenia. Dusunen Adam 2020:91. doi: 10.14744/dajpns.2020.00091
Kim, K. M., Han, S. M., Heo, K., Kim, W. J., and Chu, M. K. (2020). Sex differences in the association between chronotype and risk of depression. Sci. Rep. 10:18512. doi: 10.1038/s41598-020-75724-z
Kitamura, S., Hida, A., Watanabe, M., Enomoto, M., Aritake-Okada, S., Moriguchi, Y., et al. (2010). Evening preference is related to the incidence of depressive states independent of sleep-wake conditions. Chronobiol. Int. 27, 1797–1812. doi: 10.3109/07420528.2010.516705
Kivela, L., Papadopoulos, M. R., and Antypa, N. (2018). Chronotype and Psychiatric Disorders. Curr. Sleep Med. Rep. 4, 94–103. doi: 10.1007/s40675-018-0113-8
Klein, D. N., Kotov, R., and Bufferd, S. J. (2011). Personality and depression: explanatory models and review of the evidence. Annu. Rev. Clin. Psychol. 7, 269–295. doi: 10.1146/annurev-clinpsy-032210-104540
Koo, D. L., Yang, K. I., Kim, J. H., Kim, D., Sunwoo, J. S., Hwangbo, Y., et al. (2021). Association between morningness-eveningness, sleep duration, weekend catch-up sleep and depression among Korean high-school students. J. Sleep Res. 30:e13063. doi: 10.1111/jsr.13063
Kripke, D. F., Klimecki, W. T., Nievergelt, C. M., Rex, K. M., Murray, S. S., Shekhtman, T., et al. (2014). Circadian polymorphisms in night owls, in bipolars, and in non-24-hour sleep cycles. Psychiatry Investig. 11, 345–362. doi: 10.4306/pi.2014.11.4.345
Labonte, B., Engmann, O., Purushothaman, I., Menard, C., Wang, J., Tan, C., et al. (2017). Sex-specific transcriptional signatures in human depression. Nat. Med. 23, 1102–1111. doi: 10.1038/nm.4386
Lack, L., Bailey, M., Lovato, N., and Wright, H. (2009). Chronotype differences in circadian rhythms of temperature, melatonin, and sleepiness as measured in a modified constant routine protocol. Nat. Sci. Sleep 1, 1–8.
Lane, J. M., Vlasac, I., Anderson, S. G., Kyle, S. D., Dixon, W. G., Bechtold, D. A., et al. (2016). Genome-wide association analysis identifies novel loci for chronotype in 100,420 individuals from the UK Biobank. Nat. Commun. 7:10889. doi: 10.1038/ncomms10889
Latif, I., Hughes, A. T. L., and Bendall, R. C. A. (2019). Positive and negative affect mediate the influences of a maladaptive emotion regulation strategy on sleep quality. Front. Psychiatry 10:628. doi: 10.3389/fpsyt.2019.00628
Lee, A., Myung, S. K., Cho, J. J., Jung, Y. J., Yoon, J. L., and Kim, M. Y. (2017). Night shift work and risk of depression: meta-analysis of observational studies. J. Korean Med. Sci. 32, 1091–1096. doi: 10.3346/jkms.2017.32.7.1091
Lemoine, P., Zawieja, P., and Ohayon, M. M. (2013). Associations between morningness/eveningness and psychopathology: an epidemiological survey in three in-patient psychiatric clinics. J. Psychiatr. Res. 47, 1095–1098. doi: 10.1016/j.jpsychires.2013.04.001
Lewis, K. J. S., Richards, A., Karlsson, R., Leonenko, G., Jones, S. E., Jones, H. J., et al. (2020). Comparison of genetic liability for sleep traits among individuals with bipolar disorder i or ii and control participants. JAMA Psychiatry 77, 303–310. doi: 10.1001/jamapsychiatry.2019.4079
Li, J. Z., Bunney, B. G., Meng, F., Hagenauer, M. H., Walsh, D. M., Vawter, M. P., et al. (2013). Circadian patterns of gene expression in the human brain and disruption in major depressive disorder. Proc. Natl. Acad. Sci. U S A 110, 9950–9955. doi: 10.1073/pnas.1305814110
Li, T., Xie, Y., Tao, S., Yang, Y., Xu, H., Zou, L., et al. (2020). Chronotype, sleep, and depressive symptoms among chinese college students: a cross-sectional study. Front. Neurol. 11:592825. doi: 10.3389/fneur.2020.592825
Liberman, A. R., Kwon, S. B., Vu, H. T., Filipowicz, A., Ay, A., and Ingram, K. K. (2017). Circadian clock model supports molecular link between per3 and human anxiety. Sci. Rep. 7:9893. doi: 10.1038/s41598-017-07957-4
Lin, C. Y., Imani, V., Griffiths, M. D., Brostrom, A., Nygardh, A., Demetrovics, Z., et al. (2021). Temporal associations between morningness/eveningness, problematic social media use, psychological distress and daytime sleepiness: mediated roles of sleep quality and insomnia among young adults. J. Sleep Res. 30:e13076. doi: 10.1111/jsr.13076
Linke, M., and Jankowski, K. S. (2021). Chronotype in individuals with schizophrenia: a meta-analysis. Schizophr. Res. 235, 74–79. doi: 10.1016/j.schres.2021.07.020
Lipnevich, A. A., Crede, M., Hahn, E., Spinath, F. M., Roberts, R. D., and Preckel, F. (2017). How distinctive are morningness and eveningness from the Big Five factors of personality? A meta-analytic investigation. J. Pers. Soc. Psychol. 112, 491–509. doi: 10.1037/pspp0000099
Logan, R. W., Edgar, N., Gillman, A. G., Hoffman, D., Zhu, X., and McClung, C. A. (2015). Chronic stress induces brain region-specific alterations of molecular rhythms that correlate with depression-like behavior in mice. Biol. Psychiatry 78, 249–258. doi: 10.1016/j.biopsych.2015.01.011
Logan, R. W., and McClung, C. A. (2019). Rhythms of life: circadian disruption and brain disorders across the lifespan. Nat. Rev. Neurosci. 20, 49–65. doi: 10.1038/s41583-018-0088-y
Logan, R. W., Williams, W. P. III, and McClung, C. A. (2014). Circadian rhythms and addiction: mechanistic insights and future directions. Behav. Neurosci. 128, 387–412. doi: 10.1037/a0036268
Lopez-Soto, P. J., Fabbian, F., Cappadona, R., Zucchi, B., Manfredini, F., Garcia-Arcos, A., et al. (2019). Chronotype, nursing activity, and gender: a systematic review. J. Adv. Nurs. 75, 734–748. doi: 10.1111/jan.13876
Lunsford-Avery, J. R., Pelletier-Baldelli, A., Korenic, S. A., Schiffman, J., Ellman, L. M., Jackson, L., et al. (2021). Eveningness chronotype preference among individuals at clinical high risk for psychosis. Schizophr. Res. 236, 3–8. doi: 10.1016/j.schres.2021.07.034
Lyall, L. M., Wyse, C. A., Graham, N., Ferguson, A., Lyall, D. M., Cullen, B., et al. (2018). Association of disrupted circadian rhythmicity with mood disorders, subjective wellbeing, and cognitive function: a cross-sectional study of 91105 participants from the UK Biobank. Lancet Psychiatry 5, 507–514. doi: 10.1016/S2215-0366(18)30139-1
Lynch, C. J., Gunning, F. M., and Liston, C. (2020). Causes and consequences of diagnostic heterogeneity in depression: paths to discovering novel biological depression subtypes. Biol. Psychiatry 88, 83–94. doi: 10.1016/j.biopsych.2020.01.012
Ma, H. Y., Liu, Z. F., Xu, Y. F., Hu, X. D., Sun, N., Li, X. R., et al. (2019). The association study of CLOCK gene polymorphisms with antidepressant effect in Chinese with major depressive disorder. Per. Med. 16, 115–122. doi: 10.2217/pme-2018-0123
Malhi, G. S., and Mann, J. J. (2018). Depression. Lancet 392, 2299–2312. doi: 10.1016/s0140-6736(18)31948-2
Manni, R., De Icco, R., Cremascoli, R., Ferrera, G., Furia, F., Zambrelli, E., et al. (2016). Circadian phase typing in idiopathic generalized epilepsy: dim light melatonin onset and patterns of melatonin secretion-semicurve findings in adult patients. Epilepsy Behav. 61, 132–137. doi: 10.1016/j.yebeh.2016.05.019
Maruani, J., and Geoffroy, P. A. (2019). Bright light as a personalized precision treatment of mood disorders. Front. Psychiatry 10:85. doi: 10.3389/fpsyt.2019.00085
Maukonen, M., Havulinna, A. S., Mannisto, S., Kanerva, N., Salomaa, V., and Partonen, T. (2020). Genetic associations of chronotype in the finnish general population. J. Biol. Rhythms. 35, 501–511. doi: 10.1177/0748730420935328
Mazri, F. H., Manaf, Z. A., Shahar, S., and Mat Ludin, A. F. (2019). The association between chronotype and dietary pattern among adults: a scoping review. Int. J. Environ. Res. Public Health 17:1. doi: 10.3390/ijerph17010068
McCarthy, M. J., Wei, H., Nievergelt, C. M., Stautland, A., Maihofer, A. X., Welsh, D. K., et al. (2019). Chronotype and cellular circadian rhythms predict the clinical response to lithium maintenance treatment in patients with bipolar disorder. Neuropsychopharmacology 44, 620–628. doi: 10.1038/s41386-018-0273-8
McGlashan, E. M., Drummond, S. P. A., and Cain, S. W. (2018). Evening types demonstrate reduced SSRI treatment efficacy. Chronobiol. Int. 35, 1175–1178. doi: 10.1080/07420528.2018.1458316
McHill, A. W., Sano, A., Hilditch, C. J., Barger, L. K., Czeisler, C. A., Picard, R., et al. (2021). Robust stability of melatonin circadian phase, sleep metrics, and chronotype across months in young adults living in real-world settings. J. Pineal. Res. 70:e12720. doi: 10.1111/jpi.12720
Melo, M. C., Garcia, R. F., Araujo, C. F., Luz, J. H., Bruin, P. F., and Bruin, V. M. (2020). Chronotype in bipolar disorder: an 18-month prospective study. Braz. J. Psychiatry 42, 68–71. doi: 10.1590/1516-4446-2019-0489
Melo, M. C. A., Abreu, R. L. C., Linhares Neto, V. B., de Bruin, P. F. C., and de Bruin, V. M. S. (2017). Chronotype and circadian rhythm in bipolar disorder: a systematic review. Sleep Med. Rev. 34, 46–58. doi: 10.1016/j.smrv.2016.06.007
Menke, A. (2019). is the hpa axis as target for depression outdated, or is there a new hope?. Front. Psychiatry 10:101. doi: 10.3389/fpsyt.2019.00101
Merikanto, I., Kronholm, E., Peltonen, M., Laatikainen, T., Lahti, T., and Partonen, T. (2012). Relation of chronotype to sleep complaints in the general finnish population. Chronobiol. Int. 29, 311–317. doi: 10.3109/07420528.2012.655870
Merikanto, I., Kronholm, E., Peltonen, M., Laatikainen, T., Vartiainen, E., and Partonen, T. (2015). Circadian preference links to depression in general adult population. J. Affect. Disord. 188, 143–148. doi: 10.1016/j.jad.2015.08.061
Merikanto, I., and Partonen, T. (2020). Increase in eveningness and insufficient sleep among adults in population-based cross-sections from 2007 to 2017. Sleep Med. 75, 368–379. doi: 10.1016/j.sleep.2020.07.046
Minelli, A., Di Palma, M., Rocchi, M. B. L., Ponzio, E., Barbadoro, P., Bracci, M., et al. (2021). Cortisol, chronotype, and coping styles as determinants of tolerance of nursing staff to rotating shift work. Chronobiol. Int. 38, 666–680. doi: 10.1080/07420528.2021.1887883
Minors, D. S., Waterhouse, J. M., and Wirz-Justice, A. (1991). A human phase-response curve to light. Neurosci. Lett. 133, 36–40. doi: 10.1016/0304-3940(91)90051-t
Mokros, L., Nowakowska-Domagala, K., Koprowicz, J., Witusik, A., and Pietras, T. (2021). The association between chronotype and suicidality among students of the medicine and psychology faculties - the mediating role of general mental health indices. Chronobiol. Int. 38, 509–517. doi: 10.1080/07420528.2020.1865393
Morris, D. W., Trivedi, M. H., Fava, M., Wisniewski, S. R., Balasubramani, G. K., Khan, A. Y., et al. (2009). Diurnal mood variation in outpatients with major depressive disorder. Depress Anxiety 26, 851–863. doi: 10.1002/da.20557
Mrazek, D. A., Hornberger, J. C., Altar, C. A., and Degtiar, I. (2014). A review of the clinical, economic, and societal burden of treatment-resistant depression: 1996-2013. Psychiatr. Serv. 65, 977–987. doi: 10.1176/appi.ps.201300059
Najem, J., Saber, M., Aoun, C., El Osta, N., Papazian, T., and Rabbaa Khabbaz, L. (2020). Prevalence of food addiction and association with stress, sleep quality and chronotype: a cross-sectional survey among university students. Clin. Nutr. 39, 533–539. doi: 10.1016/j.clnu.2019.02.038
Ng, T. H., Chung, K. F., Ng, T. K., Lee, C. T., and Chan, M. S. (2016). Correlates and prognostic relevance of sleep irregularity in inter-episode bipolar disorder. Compr. Psychiatry 69, 155–162. doi: 10.1016/j.comppsych.2016.05.016
Norbury, R. (2021). Diurnal preference and depressive symptomatology: a meta-analysis. Sci. Rep. 11:12003. doi: 10.1038/s41598-021-91205-3
Oliveira, T., Marinho, V., Carvalho, V., Magalhaes, F., Rocha, K., Ayres, C., et al. (2018). Genetic polymorphisms associated with circadian rhythm dysregulation provide new perspectives on bipolar disorder. Bipolar. Disord. 20, 515–522. doi: 10.1111/bdi.12624
Onat, O. E., Kars, M. E., Gul, S., Bilguvar, K., Wu, Y., Ozhan, A., et al. (2020). Human CRY1 variants associate with attention deficit/hyperactivity disorder. J. Clin. Invest. 130, 3885–3900. doi: 10.1172/JCI135500
Ozdogan, M. G., Aydin, E. F., Ustundag, M. F., Ceyhun, H. A., Oral, E., and Bakan, E. (2020). Homocysteine, chronotype and clinical course in bipolar disorder patients. Nord. J. Psychiatry 74, 340–345. doi: 10.1080/08039488.2019.1710250
Park, Y. M. (2020). Relationship between Auditory Evoked Potentials and Circadian Preference in Patients with Major Depressive Episodes. Brain Sci. 10:6. doi: 10.3390/brainsci10060370
Passos, G. S., Santana, M. G., Poyares, D., D’Aurea, C. V., Teixeira, A. A., Tufik, S., et al. (2017). Chronotype and anxiety are associated in patients with chronic primary insomnia. Braz. J. Psychiatry 39, 183–186. doi: 10.1590/1516-4446-2016-2007
Preckel, F., Lipnevich, A. A., Schneider, S., and Roberts, R. D. (2011). Chronotype, cognitive abilities, and academic achievement: a meta-analytic investigation. Learn. Indiv. Diff. 21, 483–492. doi: 10.1016/j.lindif.2011.07.003
Przepiorka, A., Blachnio, A., and Cudo, A. (2021). Relationships between morningness, Big Five personality traits, and problematic internet use in young adult university students: mediating role of depression. Chronobiol. Int. 38, 248–259. doi: 10.1080/07420528.2020.1851703
Randler, C., and Bilger, S. (2009). Associations among sleep, chronotype, parental monitoring, and pubertal development among German adolescents. J. Psychol. 143, 509–520. doi: 10.3200/JRL.143.5.509-520
Randler, C., Horzum, M. B., and Vollmer, C. (2013). Internet addiction and its relationship to chronotype and personality in a turkish university student sample. Soc. Sci. Comp. Rev. 32, 484–495. doi: 10.1177/0894439313511055
Randler, C., and Schaal, S. (2010). Morningness-eveningness, habitual sleep-wake variables and cortisol level. Biol. Psychol. 85, 14–18. doi: 10.1016/j.biopsycho.2010.04.006
Razavi, P., Devore, E. E., Bajaj, A., Lockley, S. W., Figueiro, M. G., Ricchiuti, V., et al. (2019). Shift work, chronotype, and melatonin rhythm in nurses. Cancer Epidemiol. Biomarkers Prev. 28, 1177–1186. doi: 10.1158/1055-9965.EPI-18-1018
Rodriguez-Cortes, F. J., Morales-Cane, I., Rodriguez-Munoz, P. M., Cappadona, R., Giorgi, A. De, Manfredini, R., et al. (2022). Individual circadian preference, eating disorders and obesity in children and adolescents: a dangerous liaison? a systematic review and a meta-analysis. Children 9:2. doi: 10.3390/children9020167
Roenneberg, T., Kuehnle, T., Pramstaller, P. P., Ricken, J., Havel, M., Guth, A., et al. (2004). A marker for the end of adolescence. Curr. Biol. 14, R1038–R1039. doi: 10.1016/j.cub.2004.11.039
Roenneberg, T., and Merrow, M. (2016). The circadian clock and human health. Curr. Biol. 26, R432–R443. doi: 10.1016/j.cub.2016.04.011
Roenneberg, T., Pilz, L. K., Zerbini, G., and Winnebeck, E. C. (2019). Chronotype and social jetlag: a (self-) critical review. Biology 8:3. doi: 10.3390/biology8030054
Roenneberg, T., Wirz-Justice, A., and Merrow, M. (2003). Life between clocks: daily temporal patterns of human chronotypes. J. Biol. Rhythms 18, 80–90. doi: 10.1177/0748730402239679
Romo-Nava, F., Blom, T. J., Cuellar-Barboza, A. B., Winham, S. J., Colby, C. L., Nunez, N. A., et al. (2020a). Evening chronotype as a discrete clinical subphenotype in bipolar disorder. J. Affect. Disord. 266, 556–562. doi: 10.1016/j.jad.2020.01.151
Romo-Nava, F., Blom, T. J., Guerdjikova, A., Winham, S. J., Cuellar-Barboza, A. B., Nunez, N. A., et al. (2020b). Evening chronotype, disordered eating behavior, and poor dietary habits in bipolar disorder. Acta Psychiatr. Scand. 142, 58–65. doi: 10.1111/acps.13179
Rosenberg, J., Maximov, M. II, Reske, F. G., and Shah, N. J. (2014). Early to bed, early to rise”: diffusion tensor imaging identifies chronotype-specificity. Neuroimage 84, 428–434. doi: 10.1016/j.neuroimage.2013.07.086
Rumble, M. E., Dickson, D., McCall, W. V., Krystal, A. D., Case, D., Rosenquist, P. B., et al. (2018). The relationship of person-specific eveningness chronotype, greater seasonality, and less rhythmicity to suicidal behavior: a literature review. J. Affect. Disord. 227, 721–730. doi: 10.1016/j.jad.2017.11.078
Sack, R. L., Brandes, R. W., Kendall, A. R., and Lewy, A. J. (2000). Entrainment of free-running circadian rhythms by melatonin in blind people. N. Engl. J. Med. 343, 1070–1077. doi: 10.1056/NEJM200010123431503
Sakurai, T. (2007). The neural circuit of orexin (hypocretin): maintaining sleep and wakefulness. Nat. Rev. Neurosci. 8, 171–181. doi: 10.1038/nrn2092
Salfi, F., D’Atri, A., Tempesta, D., and Ferrara, M. (2021). Sleeping under the waves: a longitudinal study across the contagion peaks of the COVID-19 pandemic in Italy. J. Sleep Res. 2021:e13313. doi: 10.1111/jsr.13313
Salk, R. H., Hyde, J. S., and Abramson, L. Y. (2017). Gender differences in depression in representative national samples: meta-analyses of diagnoses and symptoms. Psychol. Bull. 143, 783–822. doi: 10.1037/bul0000102
Scammell, T. E., Arrigoni, E., and Lipton, J. O. (2017). Neural Circuitry of Wakefulness and Sleep. Neuron 93, 747–765. doi: 10.1016/j.neuron.2017.01.014
Schmidt, C., Collette, F., Reichert, C. F., Maire, M., Vandewalle, G., Peigneux, P., et al. (2015). Pushing the limits: chronotype and time of day modulate working memory-dependent cerebral activity. Front. Neurol. 6:199. doi: 10.3389/fneur.2015.00199
Shareh, H., and Eshaghi Sani, M. (2019). Predictive role of morningness-eveningness personality, cognitive flexibility and cognitive emotion regulation in marital satisfaction in middle-aged women. Iran. J. Psychiatry Clin. Psychol. 2019, 384–399. doi: 10.32598/ijpcp.24.4.384
Shen, G. H., Alloy, L. B., Abramson, L. Y., and Sylvia, L. G. (2008). Social rhythm regularity and the onset of affective episodes in bipolar spectrum individuals. Bipolar. Disord. 10, 520–529. doi: 10.1111/j.1399-5618.2008.00583.x
Shi, S. Q., White, M. J., Borsetti, H. M., Pendergast, J. S., Hida, A., Ciarleglio, C. M., et al. (2016). Molecular analyses of circadian gene variants reveal sex-dependent links between depression and clocks. Transl. Psychiatry 6:e748. doi: 10.1038/tp.2016.9
Siemiginowska, P., and Iskra-Golec, I. (2020). Blue light effect on EEG activity - the role of exposure timing and chronotype. Light. Res. Technol. 52, 472–484. doi: 10.1177/1477153519876969
Silva, A., Dos Santos, M. J., Goes Gitai, D. L., de Miranda Coelho, J. A. P., and de Andrade, T. G. (2020). Depression and anxiety symptoms correlate with diurnal preference, sleep habits, and Per3 VNTR polymorphism (rs57875989) in a non-clinical sample. J. Affect. Disord. 277, 260–270. doi: 10.1016/j.jad.2020.07.138
Silva, V. M., Magalhaes, J. E. M., and Duarte, L. L. (2020). Quality of sleep and anxiety are related to circadian preference in university students. PLoS One 15:e0238514. doi: 10.1371/journal.pone.0238514
Simor, P., Zavecz, Z., Palosi, V., Torok, C., and Koteles, F. (2015). The influence of sleep complaints on the association between chronotype and negative emotionality in young adults. Chronobiol. Int. 32, 1–10. doi: 10.3109/07420528.2014.935786
Simpkin, C. T., Jenni, O. G., Carskadon, M. A., Wright, K. P. Jr., Akacem, L. D., Garlo, K. G., et al. (2014). Chronotype is associated with the timing of the circadian clock and sleep in toddlers. J. Sleep. Res. 23, 397–405. doi: 10.1111/jsr.12142
Smith, C. S., Reilly, C., and Midkiff, K. (1989). Evaluation of three circadian rhythm questionnaires with suggestions for an improved measure of morningness. J. Appl. Psychol. 74, 728–738. doi: 10.1037/0021-9010.74.5.728
Song, J., Feng, P., Zhao, X., Xu, W., Xiao, L., Zhou, J., et al. (2018). Chronotype regulates the neural basis of response inhibition during the daytime. Chronobiol. Int. 35, 208–218. doi: 10.1080/07420528.2017.1392550
Spulber, S., Conti, M., DuPont, C., Raciti, M., Bose, R., Onishchenko, N., et al. (2015). Alterations in circadian entrainment precede the onset of depression-like behavior that does not respond to fluoxetine. Transl Psychiatry 5, e603. doi: 10.1038/tp.2015.94
Stewart, R., Besset, A., Bebbington, P., Brugha, T., Lindesay, J., Jenkins, R., et al. (2006). Insomnia comorbidity and impact and hypnotic use by age group in a national survey population aged 16 to 74 years. Sleep 29, 1391–1397. doi: 10.1093/sleep/29.11.1391
Stolarski, M., Jankowski, K. S., Matthews, G., and Kawalerczyk, J. (2016). Wise “birds” follow their clock: the role of emotional intelligence and morningness-eveningness in diurnal regulation of mood. Chronobiol. Int. 33, 51–63. doi: 10.3109/07420528.2015.1115413
Sun, H., Tan, Q., Fan, G., and Tsui, Q. (2014). Different effects of rumination on depression: key role of hope. Int. J. Ment. Health Syst. 8:53. doi: 10.1186/1752-4458-8-53
Sun, J., Chen, M., Cai, W., Wang, Z., Wu, S., Sun, X., et al. (2019). Chronotype: implications for sleep quality in medical students. Chronobiol. Int. 36, 1115–1123. doi: 10.1080/07420528.2019.1619181
Sun, X., Gustat, J., Bertisch, S. M., Redline, S., and Bazzano, L. (2020). The association between sleep chronotype and obesity among black and white participants of the Bogalusa Heart Study. Chronobiol. Int. 37, 123–134. doi: 10.1080/07420528.2019.1689398
Suzuki, M., Dallaspezia, S., Locatelli, C., Lorenzi, C., Uchiyama, M., Colombo, C., et al. (2017). CLOCK gene variants associated with the discrepancy between subjective and objective severity in bipolar depression. J. Affect. Disord. 210, 14–18. doi: 10.1016/j.jad.2016.12.007
Taillard, J., Philip, P., and Bioulac, B. (1999). Morningness/eveningness and the need for sleep. J. Sleep Res. 8, 291–295. doi: 10.1046/j.1365-2869.1999.00176.x
Taillard, J., Philip, P., Chastang, J. F., and Bioulac, B. (2004). Validation of Horne and Ostberg morningness-eveningness questionnaire in a middle-aged population of French workers. J. Biol. Rhythms 19, 76–86. doi: 10.1177/0748730403259849
Taillard, J., Philip, P., Chastang, J. F., Diefenbach, K., and Bioulac, B. (2001). Is self-reported morbidity related to the circadian clock? J. Biol. Rhythms 16, 183–190. doi: 10.1177/074873001129001764
Taillard, J., Philip, P., Claustrat, B., Capelli, A., Coste, O., Chaumet, G., et al. (2011). Time course of neurobehavioral alertness during extended wakefulness in morning- and evening-type healthy sleepers. Chronobiol. Int. 28, 520–527. doi: 10.3109/07420528.2011.590623
Taillard, J., Philip, P., Coste, O., Sagaspe, P., and Bioulac, B. (2003). The circadian and homeostatic modulation of sleep pressure during wakefulness differs between morning and evening chronotypes. J. Sleep. Res. 2003:369. doi: 10.1046/j.0962-1105.2003.00369.x
Taillard, J., Sagaspe, P., Philip, P., and Bioulac, S. (2021). Sleep timing, chronotype and social jetlag: impact on cognitive abilities and psychiatric disorders. Biochem. Pharmacol. 191:114438. doi: 10.1016/j.bcp.2021.114438
Takaesu, Y. (2018). Circadian rhythm in bipolar disorder: a review of the literature. Psychiatry Clin. Neurosci. 72, 673–682. doi: 10.1111/pcn.12688
Takeuchi, H., Taki, Y., Sekiguchi, A., Nouchi, R., Kotozaki, Y., Nakagawa, S., et al. (2015). Regional gray matter density is associated with morningness–eveningness: evidence from voxel-based morphometry. Neuroimage 117, 294–304. doi: 10.1016/j.neuroimage.2015.05.037
Tan, M. N., Mevsim, V., Pozlu Cifci, M., Sayan, H., Ercan, A. E., Ergin, O. F., et al. (2020). Who is happier among preclinical medical students: the impact of chronotype preference. Chronobiol. Int. 37, 1163–1172. doi: 10.1080/07420528.2020.1761373
Taylor, B. J., Bowman, M. A., Brindle, A., Hasler, B. P., Roecklein, K. A., Krafty, R. T., et al. (2020). Evening chronotype, alcohol use disorder severity, and emotion regulation in college students. Chronobiol. Int. 37, 1725–1735. doi: 10.1080/07420528.2020.1800028
Taylor, B. J., and Hasler, B. P. (2018). Chronotype and mental health: recent advances. Curr. Psychiatry Rep. 20:59. doi: 10.1007/s11920-018-0925-8
Teixeira, G. P., Balieiro, L. C. T., Gontijo, C. A., Fahmy, W. M., Maia, Y. C. P., and Crispim, C. A. (2020). The association between chronotype, food craving and weight gain in pregnant women. J. Hum. Nutr. Diet. 33, 342–350. doi: 10.1111/jhn.12723
Thapa, N., Kim, B., Yang, J. G., Park, H. J., Jang, M., Son, H. E., et al. (2020). The relationship between chronotype, physical activity and the estimated risk of dementia in community-dwelling older adults. Int. J. Environ. Res. Public Health 2020:17. doi: 10.3390/ijerph17103701
Tian, Y., Chen, X., Xu, D., Yu, J., and Lei, X. (2020). Connectivity within the default mode network mediates the association between chronotype and sleep quality. J. Sleep Res. 29:e12948. doi: 10.1111/jsr.12948
Toda, M., Kawai, T., Takeo, K., Rokutan, K., and Morimoto, K. (2013). Associations between chronotype and salivary endocrinological stress markers. Endocr. Res. 38, 1–7. doi: 10.3109/07435800.2012.683225
Tonetti, L., Fabbri, M., Martoni, M., and Natale, V. (2011). Season of birth and sleep-timing preferences in adolescents. Chronobiol. Int. 28, 536–540. doi: 10.3109/07420528.2011.590261
Tonon, A. C., Carissimi, A., Schimitt, R. L., de Lima, L. S., Pereira, F. D. S., and Hidalgo, M. P. (2020). How do stress, sleep quality, and chronotype associate with clinically significant depressive symptoms? a study of young male military recruits in compulsory service. Braz. J. Psychiatry 42, 54–62. doi: 10.1590/1516-4446-2018-0286
Torquati, L., Mielke, G. I., Brown, W. J., Burton, N. W., and Kolbe-Alexander, T. L. (2019). Shift work and poor mental health: a meta-analysis of longitudinal studies. Am. J. Public Health 109, e13–e20. doi: 10.2105/AJPH.2019.305278
Tosuntas, S. B., Balta, S., Emirtekin, E., Kircaburun, K., and Griffiths, M. D. (2018). Adolescents’ eveningness chronotype and cyberbullying perpetration: the mediating role of depression-related aggression and anxiety-related aggression. Biolog. Rhyt. Res. 51, 40–50. doi: 10.1080/09291016.2018.1513132
Touitou, Y., Touitou, D., and Reinberg, A. (2016). Disruption of adolescents’ circadian clock: the vicious circle of media use, exposure to light at night, sleep loss and risk behaviors. J. Physiol. Paris 110(4 Pt B), 467–479. doi: 10.1016/j.jphysparis.2017.05.001
Turkoglu, G., and Selvi, Y. (2020). The relationship between chronotype, sleep disturbance, severity of fibromyalgia, and quality of life in patients with fibromyalgia. Chronobiol. Int. 37, 68–81. doi: 10.1080/07420528.2019.1684314
Uzer, A., and Yucens, B. (2020a). Chronotype and depressive symptoms in healthy subjects: the mediating role of hopelessness and subjective sleep quality. Chronobiol. Int. 37, 1173–1180. doi: 10.1080/07420528.2020.1775629
Uzer, A., and Yucens, B. (2020b). The effect of circadian preferences on insomnia severity and depressive symptoms via sleep hygiene in older adults with depression and healthy controls. Psychogeriatrics 20, 871–879. doi: 10.1111/psyg.12610
Van den Berg, J. F., Kivela, L., and Antypa, N. (2018). Chronotype and depressive symptoms in students: an investigation of possible mechanisms. Chronobiol. Int. 35, 1248–1261. doi: 10.1080/07420528.2018.1470531
Vollmer, C., Michel, U., and Randler, C. (2012). Outdoor light at night (LAN) is correlated with eveningness in adolescents. Chronobiol. Int. 29, 502–508. doi: 10.3109/07420528.2011.635232
Walker, W. H. Borniger, J. C., Gaudier-Diaz, M. M., Hecmarie Melendez-Fernandez, O., Pascoe, J. L., Courtney DeVries, A., et al. (2020). Acute exposure to low-level light at night is sufficient to induce neurological changes and depressive-like behavior. Mol. Psychiatry 25, 1080–1093. doi: 10.1038/s41380-019-0430-4
Walsh, N. A., Repa, L. M., and Garland, S. N. (2021). Mindful larks and lonely owls: The relationship between chronotype, mental health, sleep quality, and social support in young adults. J. Sleep Res. 2021:e13442. doi: 10.1111/jsr.13442
Wang, H., Lane, J. M., Jones, S. E., Dashti, H. S., Ollila, H. M., Wood, A. R., et al. (2019). Genome-wide association analysis of self-reported daytime sleepiness identifies 42 loci that suggest biological subtypes. Nat. Commun. 10:3503. doi: 10.1038/s41467-019-11456-7
Watts, A. L., and Norbury, R. (2017). Reduced effective emotion regulation in night owls. J. Biol. Rhythms 32, 369–375. doi: 10.1177/0748730417709111
Weiss, C., Woods, K., Filipowicz, A., and Ingram, K. K. (2020). Sleep quality, sleep structure, and per3 genotype mediate chronotype effects on depressive symptoms in young adults. Front. Psychol. 11:2028. doi: 10.3389/fpsyg.2020.02028
Wills, C., Ghani, S., Tubbs, A., Fernandez, F. X., Athey, A., Turner, R., et al. (2021). Chronotype and social support among student athletes: impact on depressive symptoms. Chronobiol. Int. 2021, 1–11. doi: 10.1080/07420528.2021.1927072
Wirz-Justice, A., and Benedetti, F. (2020). Perspectives in affective disorders: Clocks and sleep. Eur. J. Neurosci. 51, 346–365. doi: 10.1111/ejn.14362
Wirz-Justice, A., Skene, D. J., and Munch, M. (2021). The relevance of daylight for humans. Biochem. Pharmacol. 191:114304. doi: 10.1016/j.bcp.2020.114304
Wittmann, M., Dinich, J., Merrow, M., and Roenneberg, T. (2006). Social jetlag: misalignment of biological and social time. Chronobiol. Int. 23, 497–509. doi: 10.1080/07420520500545979
Wong, M. L., Kling, M. A., Munson, P. J., Listwak, S., Licinio, J., Prolo, P., et al. (2000). Pronounced and sustained central hypernoradrenergic function in major depression with melancholic features: relation to hypercortisolism and corticotropin-releasing hormone. Proc. Natl. Acad. Sci. U S A 97, 325–330. doi: 10.1073/pnas.97.1.325
Xiao, H., Cai, H., and Li, X. (2021). Non-visual effects of indoor light environment on humans: a review(). Physiol. Behav. 228:113195. doi: 10.1016/j.physbeh.2020.113195
Xu, N., Shinohara, K., Saunders, K. E. A., Geddes, J. R., and Cipriani, A. (2021). Effect of lithium on circadian rhythm in bipolar disorder: a systematic review and meta-analysis. Bipolar. Disord. 2021:13070. doi: 10.1111/bdi.13070
Yang, Y.-T., Kaplan, K. A., and Zeitzer, J. M. (2020). A comparison of sleep, depressive symptoms, and parental perceptions between U.S. and Taiwan adolescents with self-reported sleep problems. Sleep Adv. 2020: zaa004. doi: 10.1093/sleepadvances/zpaa004/5905468
Yeom, J. W., Jeong, S., Seo, J. Y., Jeon, S., and Lee, H. J. (2020). Association of the serotonin 2a receptor rs6311 polymorphism with diurnal preference in koreans. Psychiatry Investig. 17, 1137–1142. doi: 10.30773/pi.2020.0358
You, M., Laborde, S., Dosseville, F., Salinas, A., and Allen, M. S. (2020). Associations of chronotype, Big Five, and emotional competences with perceived stress in university students. Chronobiol. Int. 37, 1090–1098. doi: 10.1080/07420528.2020.1752705
Zaki, N. F., Sabri, Y. M., Farouk, O., Abdelfatah, A., Spence, D. W., Bahammam, A. S., et al. (2020). Depressive symptoms, sleep profiles and serum melatonin levels in a sample of breast cancer patients. Nat. Sci. Sleep 12, 135–149. doi: 10.2147/NSS.S206768
Zavada, A., Gordijn, M. C., Beersma, D. G., Daan, S., and Roenneberg, T. (2005). Comparison of the munich chronotype questionnaire with the horne-ostberg’s morningness-eveningness score. Chronobiol. Int. 22, 267–278. doi: 10.1081/cbi-200053536
Zerbini, G., Kantermann, T., and Merrow, M. (2020). Strategies to decrease social jetlag: reducing evening blue light advances sleep and melatonin. Eur. J. Neurosci. 51, 2355–2366. doi: 10.1111/ejn.14293
Zerbini, G., Winnebeck, E. C., and Merrow, M. (2021). Weekly, seasonal, and chronotype-dependent variation of dim-light melatonin onset. J. Pineal. Res. 70:e12723. doi: 10.1111/jpi.12723
Zhou, J., Hsiao, F. C., Shi, X., Yang, J., Huang, Y., Jiang, Y., et al. (2021). Chronotype and depressive symptoms: a moderated mediation model of sleep quality and resilience in the 1st-year college students. J. Clin. Psychol. 77, 340–355. doi: 10.1002/jclp.23037
Zhou, L., Zhang, H., Luo, Z., Liu, X., Yang, L., Hu, H., et al. (2020). Abnormal night sleep duration and inappropriate sleep initiation time are associated with elevated anxiety symptoms in chinese rural adults: the henan rural cohort. Psychiatry Res. 291:113232. doi: 10.1016/j.psychres.2020.113232
Zimmermann, L. K. (2011). Chronotype and the transition to college life. Chronobiol. Int. 28, 904–910. doi: 10.3109/07420528.2011.618959
Zuraikat, F. M., St-Onge, M. P., Makarem, N., Boege, H. L., Xi, H., and Aggarwal, B. (2021). Evening chronotype is associated with poorer habitual diet in us women, with dietary energy density mediating a relation of chronotype with cardiovascular health. J. Nutr. 151, 1150–1158. doi: 10.1093/jn/nxaa442
Keywords: chronotype, circadian rhythm, psychiatric disorders, depression, sleep disorder
Citation: Zou H, Zhou H, Yan R, Yao Z and Lu Q (2022) Chronotype, circadian rhythm, and psychiatric disorders: Recent evidence and potential mechanisms. Front. Neurosci. 16:811771. doi: 10.3389/fnins.2022.811771
Received: 09 November 2021; Accepted: 21 June 2022;
Published: 10 August 2022.
Edited by:
Ritchie Edward Brown, United States Department of Veterans Affairs, United StatesReviewed by:
Leandro Lourenção Duarte, Federal University of Recôncavo of Bahia, BrazilCopyright © 2022 Zou, Zhou, Yan, Yao and Lu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhijian Yao, emp5YW9Abmp1LmVkdS5jbg==; Qing Lu, bHVxQHNldS5lZHUuY24=
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.