- 1IFIBIO Houssay, Department of Physiology, School of Medicine, University of Buenos Aires, Buenos Aires, Argentina
- 2Department of Internal Medicine, Pulmonary and Sleep Medicine Service, Austral University Hospital, Buenos Aires, Argentina
Sleep spindles are thought to promote memory consolidation. Recently, we have shown that visuomotor adaptation (VMA) learning increases the density of spindles and promotes the coupling between spindles and slow oscillations, locally, with the level of spindle-SO synchrony predicting overnight memory retention. Yet, growing evidence suggests that the rhythmicity in spindle occurrence may also influence the stabilization of declarative and procedural memories. Here, we examined if VMA learning promotes the temporal organization of sleep spindles into trains. We found that VMA increased the proportion of spindles and spindle-SO couplings in trains. In agreement with our previous work, this modulation was observed over the contralateral hemisphere to the trained hand, and predicted overnight memory retention. Interestingly, spindles grouped in a cluster showed greater amplitude and duration than isolated spindles. The fact that these features increased as a function of train length, provides evidence supporting a biological advantage of this temporal arrangement. Our work opens the possibility that the periodicity of NREM oscillations may be relevant in the stabilization of procedural memories.
Introduction
The last decade has seen remarkable progress in the identification of the neural signatures of sleep-dependent consolidation. Overnight reactivation of hippocampal memories appears to depend on sleep spindles, slow oscillations (SO) and their precise synchrony (Maingret et al., 2016; Ladenbauer et al., 2017; Helfrich et al., 2018; Muehlroth et al., 2019; Navarro-Lobato and Genzel, 2019). Recently, we have shown that motor learning, specifically visuomotor adaptation (VMA), also promotes the coupling between spindles and SOs (Solano et al., 2021). The fact that the level of spindle-SO synchrony predicts overnight memory retention opens the possibility that common mechanisms operate in the stabilization of declarative and motor memories.
Sleep spindles are bursts of oscillatory thalamocortical activity thought to support the reactivation of newly formed memories (Rasch and Born, 2013; Staresina et al., 2015; Schönauer, 2018). Individual spindles may promote memory consolidation through the modulation of intrinsic features such as their amplitude (Barakat et al., 2012; Bergmann et al., 2012; Boutin et al., 2018b), duration (Morin et al., 2008; Laventure et al., 2016), power (Ngo et al., 2020) and density (Nishida and Walker, 2007; Barakat et al., 2011; Albouy et al., 2013; Solano et al., 2021). In the last few years, it has been argued that the rhythmicity in their occurrence may also be a relevant parameter influencing memory stabilization (Lecci et al., 2017; Antony et al., 2018; Boutin and Doyon, 2020). Specifically, during NREM sleep spindles tend to cluster into trains of two or more, separated by inter-spindle intervals (ISI) of 3 to 6 s (Evans and Richardson, 1995; Achermann and Borbély, 1997; Antony et al., 2018). This ISI is believed to reflect a refractory period. It has been proposed that recently acquired memories would be reinstated during a spindle oscillation and subsequently reprocessed during the following refractory period, thereby protecting memory reinstatement from potential interference (Antony et al., 2018, 2019). Boutin et al. (2018a,2019) and Boutin and Doyon (2020) have in fact shown that some metrics of spindle trains, such as the ISI, predict offline gains in motor sequence learning. Yet, whether clustering modulates spindle’s intrinsic features such as duration and/or amplitude remains unknown. This information may be key to unveil the physiological advantage of spindle grouping.
Here, we followed up on our previous findings (Solano et al., 2021), to examine if VMA learning promotes the temporal organization of sleep spindles into trains. We also explored if the spindle-SO coupling which, as we showed is modulated by VMA, is also influenced by spindle rhythmicity. Animal studies have shown that the cluster of spindles into trains may increase the recruitment and synchronization of a greater number of thalamic and cortical neurons involved in spindle generation (Contreras and Steriade, 1996), which from the EEG recording would manifest as an increment in spindle amplitude. Clustering may also strengthen cortico-thalamic connectivity and long-range synchronization (Bonjean et al., 2011), which would reveal as longer spindle duration. Thus, to shed light on the biological relevance of spindle clustering, we also quantified the relationship between their duration/amplitude and train length. We found that VMA increased the proportion of spindles in trains locally, over the contralateral hemisphere to the trained hand. Importantly, clustered spindles were longer and larger than isolated spindles, pointing to a physiological benefit of this temporal organization.
Materials and Methods
Participants
Ten healthy volunteers [5 females, age: (mean ± SD) 24.3 ± 3.1 years old] completed the whole study. All subjects were right-handed, and none of them declared neurological nor psychiatric disorders. Only subjects fulfilling the criteria for good sleep quality (Buysse et al., 1989; Johns, 1991) were included in the study.
All volunteers signed the informed consent approved by the Ethics Committee of the Hospital de Clínicas, University of Buenos Aires (approved on Nov, 24, 2015, and renewed every year), which complies with the Declaration of Helsinki in its latest version, and with the National Law on the Protection of Personal Data.
Experimental Paradigm and Procedure
Subjects performed a visuomotor adaptation task (VMA) consisting of moving a cursor that represented their hand, from a start point located in the center of a computer screen to one of 8 visual targets arranged concentrically, using a joystick. The latter was controlled with the thumb and index finger of the right hand, while vision of the hand was occluded. One cycle consisted of eight trials, whereas there were 11 cycles in a block.
Subjects were instructed to perform fast shooting movements through each of the eight targets presented pseudo-randomly within a cycle. There were three different types of trials throughout the study. During perturbed trials a clockwise 45-degree visual rotation was imposed to the cursor relative to the movement of the hand (ROT). During null, i.e., unperturbed trials (N) in which no visual rotation was applied, the movement of the cursor directly mapped onto the hand movement. Finally, during error-clamp (EC) trials, visual feedback was manipulated to provide fake “straight” paths to the target that mimicked those generated during correct trials. This was accomplished by projecting the actual movement to the straight line with some additional variability (mean error = 0°, SD = 10°). EC trials prevent further learning, and allow estimating memory retention based on the internal state of the motor system (Criscimagna-Hemminger and Shadmehr, 2008; Villalta et al., 2015).
Experimental Design
The experimental design of this study is fully described in a previous publication derived from the same dataset (Solano et al., 2021). Briefly, we conducted a within-subject experiment consisting of three sessions separated by 7 days each: (i) a familiarization session, (ii) a visuomotor adaptation session (VMA) in which a visual rotation (ROT) was applied, and (iii) a control session (CTL) in which subjects performed unperturbed, null trials (N). The order of the VMA and CTL sessions was counterbalanced, so that half of the volunteers performed the VMA condition first and the other half performed it the following week. In the VMA and CTL conditions, subjects performed the task before and after sleep on Day 1 and Day 2. On Day 1, they performed one block of N trials followed by six blocks of ROT/N trials, depending on the condition. On Day 2, they performed two cycles of EC trials to assess memory retention, followed by 6 blocks of ROT/N trials, depending on the condition, and three blocks of N trials. In each session, subjects went to bed ∼10 min after performing the task on Day 1 for a full night of sleep, and a polysomnographic (PSG) recording was obtained throughout the night.
Electroencephalogram Recording and Processing
For the PSG recording, eleven surface electroencephalogram (EEG) electrodes were placed over prefrontal, motor and parietal areas (FC1, FC2, FC5, FC6, C3, C4, P3, P4), and over the midline (Fz, Cz, Pz). Both mastoids were used as references. Electrooculography (EOG) and electromyography (EMG) signals were also obtained. All signals were acquired at 200 Hz.
EEG, EOG, and EMG signals were bandpass-filtered to facilitate sleep scoring (EEG: 0.5–30 Hz; EOG: 0.5–15 Hz; EMG: 20–99 Hz). All PSG recordings were sleep staged manually, according to standard criteria (Iber, 2004). Namely, 30-s epochs were classified as either Wake (W), Non Rapid Eye Movement (NREM1, NREM2, and NREM3), or Rapid Eye Movement (REM) stage.
Sleep spindles (10–16 Hz) and slow oscillations (0.5–1.25 Hz) were automatically identified from the EEG signal corresponding to the stages NREM2 and NREM3, using previously reported algorithms (see below).
Sleep Spindles Detection
The algorithm was based in the work of Ferrarelli et al. (2007) and Mölle et al. (2011). First, EEG signal for each channel and session was bandpass-filtered (10–16 Hz) before calculating the instantaneous amplitude (IA) and instantaneous frequency (IF) by applying the Hilbert Transform (Tort et al., 2010). The IA was used as a new time series and smoothed with a 350 ms moving average window. Next, those segments that exceeded an upper magnitude threshold (90th percentile of all IA points) were labeled as potential spindles. Spindles onset and offset were defined as the time points in which the signal dropped below a lower threshold (70th percentile of all IA points). Potential spindles with a duration between 0.5 and 3 s were labeled as true spindles. Finally, spindles were further classified into two types according to their frequency: slow spindles (<12 Hz) and fast spindles (≥12 Hz) (Mölle et al., 2011; Cox et al., 2017).
Detection of Slow Oscillations
The algorithm was based on that reported by Mölle et al. (2011) and Antony and Paller (2016). First, the EEG signal was bandpass-filtered (0.5–1.25 Hz). Then, we identified zero crossings and labeled them as positive-to-negative (PN) or negative-to-positive (NP). Those EEG segments between two NP zero crossings were considered slow oscillations if they lasted between 0.8 and 2 s. Next, we computed their peak-to-peak (P-P) amplitude. Finally, we determined the median of the P-P amplitudes for each channel, each subject and each session, and kept those SOs with a P-P amplitude greater than the median value (Mizrahi-Kliger et al., 2018).
We quantified a spindle-SO coupling if a spindle had its maximum peak-to-peak amplitude during the course of a SO.
Spindle Trains
In a recent publication, Boutin and Doyon (2020) defined a spindle train as a group of two or more locally occurring sleep spindles separated by a maximum inter-spindle interval (max_ISI) of 6 s. This criterion was based on the distribution of ISIs reported in previous studies (Evans and Richardson, 1995; Achermann and Borbély, 1997; Antony et al., 2018). Spindles occurring at longer ISIs than the max_ISI threshold were thus considered isolated spindles. In this approach the max_ISI is a fixed parameter invariant across channels and subjects. To examine if this criterion adjusted well to observed data, we contrasted it with that obtained from applying an adaptive method used in neurophysiology to identify bursts of action potentials (Kapucu et al., 2012). This approach, which allows to compute a max_ISI to identify spike bursts, is composed of the following steps, illustrated in Supplementary Figure 1. First, ISIs are quantified and the corresponding histogram is constructed. Second, a Cumulative Moving Average (CMA) curve is derived from the histogram according to the function:
where yi is the number of observations for each bin of the ISI histogram, i = 1,…,N, and N the total number of ISI bins. CMAI is the Ith value of the CMA curve, with I ≤ N. Then, based on the skewness of the CMA curve, a scaling parameter, α, is determined, which will then be used to identify the max_ISI. The max_ISI then corresponds to the ISI where the CMA curve falls from its maximum (CMAmax) to α*CMAmax. We applied this method on data from each channel and each subject, and then computed the median of the max_ISI for each sleep stage (NREM2 and NREM3) and each experimental session. This yielded four max_ISI values per subject. To optimize the ability of the algorithm to resolve one train from another, we kept the minimum max_ISI out of the four values.
Data Analysis
Behavior
Motor performance was measured based on the angle defined by the movement direction of the joystick and the line segment connecting the start point and target position (pointing angle). Trial-by-trial data were converted into cycle-by-cycle time series by computing the median pointing angle for each cycle of eight trials and each subject. To assess memory retention the pointing angle of the two EC cycles was expressed as a percentage of the pointing angle asymptote (median of the last block of learning), and then averaged.
Electroencephalographic Signal
Slow Oscillations and Spindle Measures
Given that some electrodes came off after the first 2 h of EEG recording, we focused the EEG analysis on the first sleep cycle.
The following measures were computed: spindle duration (msec), peak-to-peak amplitude (μV), inter-spindle interval (ISI), proportion of spindles in trains (number of spindles in trains/total number of spindles) and the proportion of spindle-SOs in trains (number of spindle-SO in trains/total number of spindle-SO).
To assess how these measures differed between VMA and CTL sessions we computed their relative difference according to the function ((VMA-CTL)/CTL*100) for each EEG channel and each subject (Huber et al., 2004, 2006; Solano et al., 2021). To illustrate the spatial distribution of the effects, we report the results in topographic maps (MNE-Python; Gramfort et al., 2013). Furthermore, given that our previous work has shown that VMA modulates NREM oscillations contralaterally to the trained hand (Solano et al., 2021), we contrasted the data pooled across the electrodes of the left hemisphere (LH: FC1, FC5, C3 and P3) against the data pooled across the electrodes of the right hemisphere (RH: FC2, FC6, C4 and P4). Given that the midline (Fz, Cz, Pz) may capture electrical activity from both hemispheres it was excluded from this analysis.
To investigate whether the intrinsic features of spindles were influenced by being temporally organized into trains, we examined the amplitude and duration of spindles in trains versus isolated spindles. We chose these measures because they reflect the size of neural populations active during a spindle and their level of synchronization, which are often modulated by learning (Morin et al., 2008; Barakat et al., 2012; Bergmann et al., 2012; Laventure et al., 2016; Boutin et al., 2018b). Moreover, to compare trains of different length, we focused on the features of the first and last spindle in a train. We hypothesized that if being part of a train was somewhat advantageous, then these features would be potentiated in clustered vs. isolated spindles, and this potentiation would become stronger as a function of train length. To this aim, we first calculated the median duration and amplitude of isolated spindles and that of the first and last spindle in a train, for each EEG channel and each subject, and then computed the mean duration and amplitude across channels for each subject. Finally, for each initial and final spindle in a train, we computed the percent difference in amplitude and duration relative to that of isolated spindles, and statistically contrasted them.
Statistical Analysis
The sample size of this study (n = 10) was determined based on a priori power analysis (simulation approach based on DeBruine and Barr, 2021, and Kumle et al., 2021) conducted on the work by Huber et al. (2004), in which they examined the impact of VMA on the power of delta oscillations (1–4 Hz).
Statistical analyses were carried out using R (v3.4.1; R Core Team, 2017). Statistical differences were assessed at the 95% level of confidence, and were carried out by fitting Linear Mixed Models (LMM, using the “lmer” function implemented in the “lme4” package in R, Bates et al., 2015). Data from each of the four electrodes that were pooled together from each hemisphere were considered replicates. The response variable was the relative difference between sessions ((VMA-CTL)/CTL*100). The fixed effects were the sleep stage (NREM2 and NREM3), the cerebral hemisphere (Left and Right) or the type of spindle (fast and slow), depending on the analysis. To assess the statistical significance of fixed effects and obtain p-values, we used F tests or t-tests with Kenward-Roger’s approximation of the degrees of freedom (Halekoh and Højsgaard, 2014).
A one sample t-test was used to compare the max_ISIs found by applying the CMA algorithm with the fixed 6 s threshold.
Finally, to statistically assess whether train length impacts on the duration and amplitude of spindles, we first conducted repeated-measure correlations using the “rmcorr” package in R (Bakdash and Marusich, 2017). The repeated-measure correlation coefficient, rRM, represents the strength of the linear association but accounting for the repeated observations for each subject. Next, we ran LMMs to assess whether the intrinsic features of spindles varied along the train, by comparing the first and last spindles. The response variable was the relative difference of the intrinsic features for the first and last spindle in a train relative to isolated spindles. The fixed effects were the position of the spindle (first or last) and the length of the train. The latter was included in the model as a covariate of no interest.
Results
Sleep architecture was similar across all three sessions for all computed measures (Supplementary Table 1).
Organization of Sleep Spindles Into Trains
After identifying individual sleep spindles, we computed the ISIs to explore their periodicity. Figure 1A shows the distribution of ISIs during NREM2 and NREM3. As previously shown, the distribution of ISIs is right-skewed and reveals that most spindles are separated by ∼3–6 s intervals. Based on this observation, Boutin and Doyon (2020) set a max_ISI of 6 s to determine if a group of spindles constitute a train. We compared this criterion to that obtained from applying the adaptive algorithm created by Kapucu et al. (2012). This approach yielded a max_ISI of 5.16 ± 0.5 s (mean ± SE). Given that the two criteria did not statistically differ (one sample t-test, t(9) = −1.7, p = 0.12), we used the fixed 6 s criterion for train quantification.
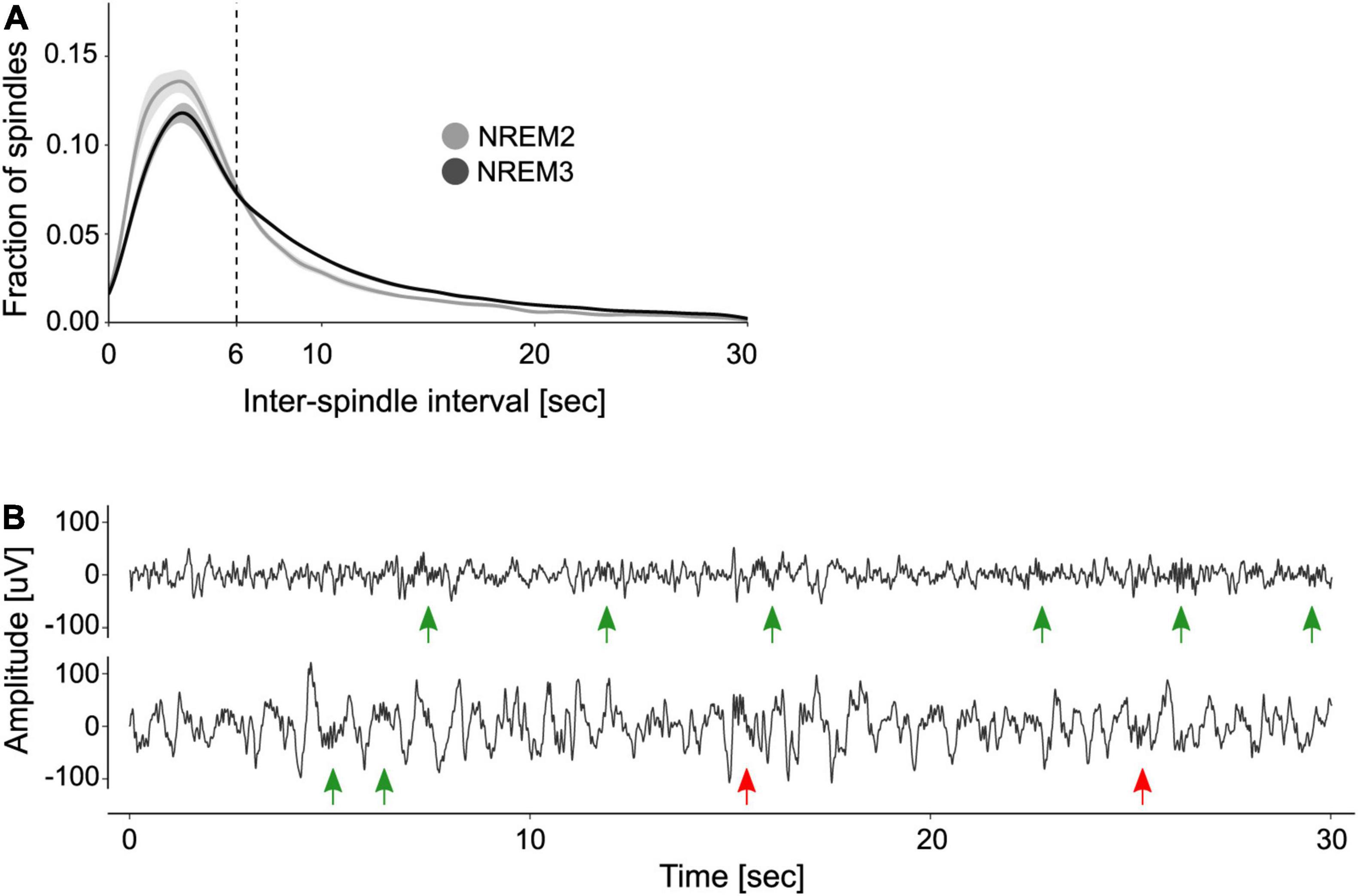
Figure 1. Organization of sleep spindles into trains. (A) Distribution of inter-spindle intervals. Shown are the mean ± SE of the distribution of inter-spindle intervals (ISIs) during NREM2 and NREM3. (B) Examples of spindle trains. Two 30 s segments of sleep EEG are depicted, one from NREM2 (top trace) and one from NREM3 (bottom trace). Green arrows indicate sleep spindles clustered into trains, whereas red arrows indicate isolated spindles. Note that according to the 6 s max_ISI criterion, there are two trains of three spindles on the top trace and one train of two spindles on the bottom trace.
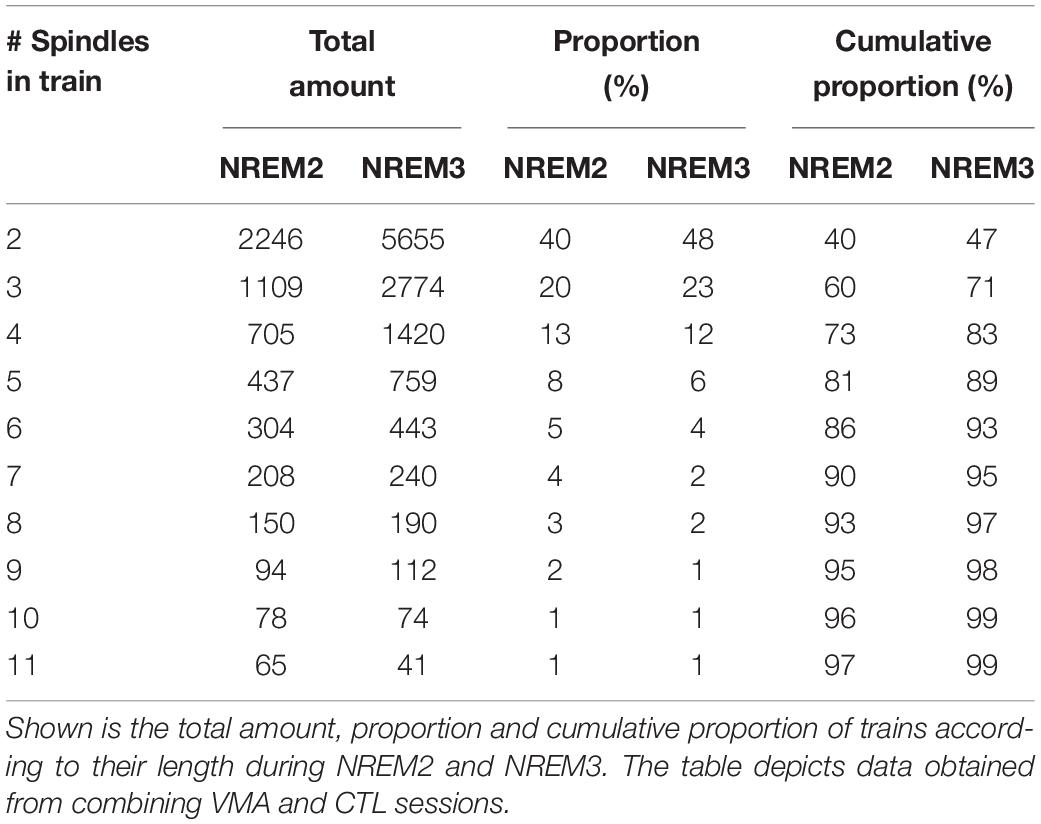
Figure 1B depicts two EEG traces from NREM2 and NREM3 that illustrate spindle trains (green arrows) and isolated spindles (red arrows). As detailed in Table 1, most trains contained between 2 and 3 spindles, whereas few trains may encompass seven spindles or more.
Visuomotor Adaptation Modulates the Clustering of Spindles Into Trains
To explore if the temporal clustering of spindles into trains was modulated by motor learning, we computed the proportion of spindles in trains relative to the total number of spindles during NREM2 and NREM3, for each EEG channel and each subject. Figure 2A depicts the topographic maps for NREM2 (top row) and NREM3 (bottom row). We found a learning-related increase in the proportion of spindles organized into trains over the contralateral hemisphere to the trained hand, specifically during NREM3 (relative change in proportion, mean ± SE: NREM2: LH = −1.3 ± 2.1 %, RH = −0.3 ± 1.9%; NREM3: LH = 5.7 ± 1.9%, RH = −0.2 ± 1.3; LMM stats, sleep stage by hemisphere interaction: F(1,121.9) = 4.59, p = 0.03; β = 5.94, SE = 2.77; t-test for Left hemisphere during NREM3 vs. zero: t(8.99) = 2.14, p = 0.03, β = 5.67, SE = 2.04). Interestingly, VMA increased the proportion of spindles in trains differentially for fast (≥12Hz) but not slow (<12 Hz) spindles during NREM3 (relative change in proportion, mean ± SE: fast spindles = 8.5 ± 2.1%, slow spindles = −3.4 ± 0.8%; LMM stats, main effect of spindle type: F(1,8.9) = 5.21, p = 0.04, β = 6.34, SE = 2.78).
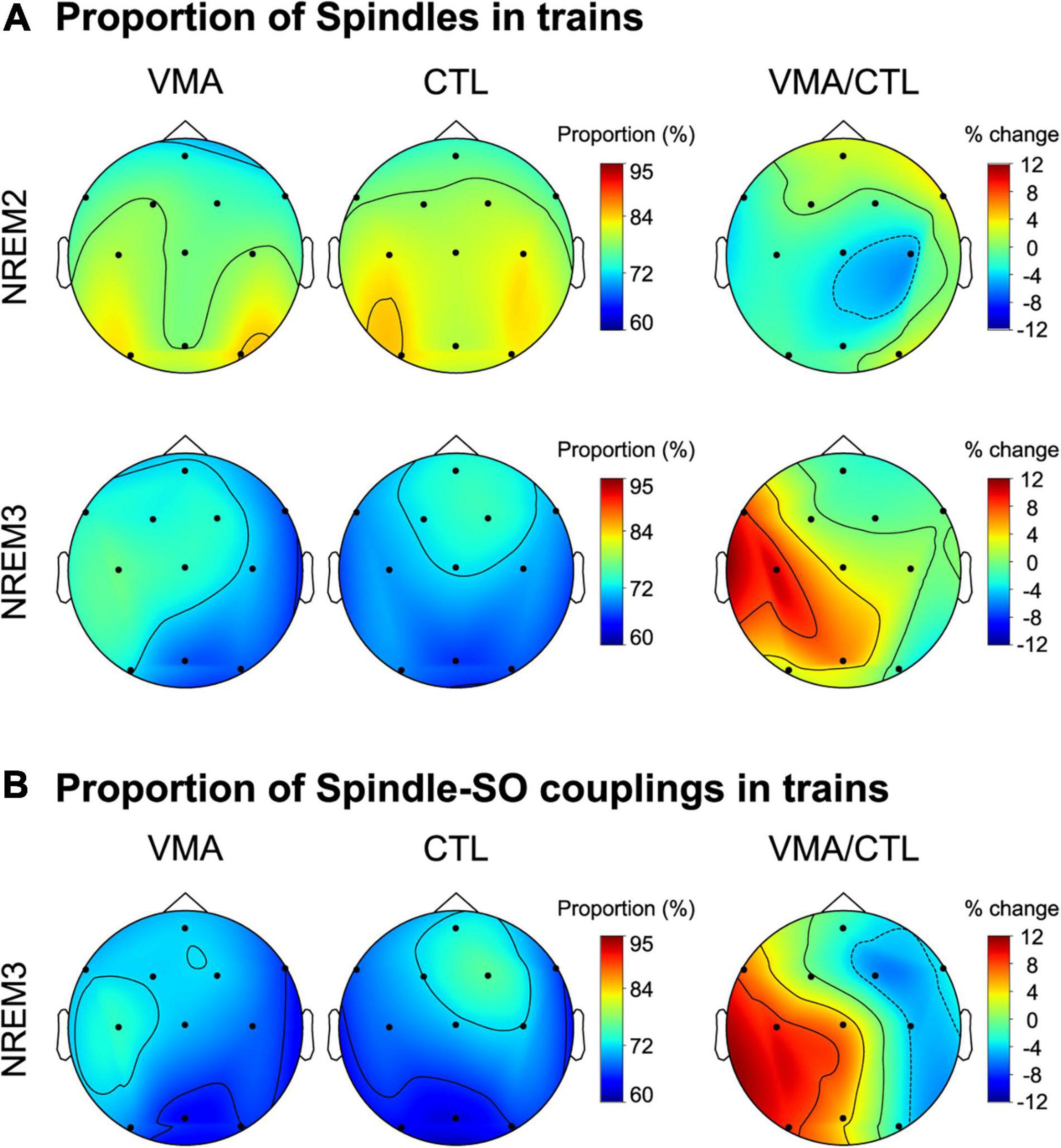
Figure 2. Local modulation of the proportion of spindles and spindle-SO couplings clustered into trains. (A) Visuomotor adaptation increases the proportion of spindles in trains. Shown are the topographic plots for the proportion of spindles in trains (number of spindles in trains relative to the total number of spindles) corresponding to the VMA session, the CTL session and their relative difference (VMA/CTL) computed according to the function ((VMA-CTL)/CTL*100), during NREM2 (top row) and NREM3 (bottom row). (B) Visuomotor adaptation increases the proportion of spindle-SO couplings clustered into trains. Shown are the topographic plots for the proportion of spindle-SO couplings in trains (number of spindle-SO couplings in trains relative to the total number of spindle-SO couplings) corresponding to the VMA session, the CTL session and their relative difference (VMA/CTL), during NREM3. Colorbars represent the proportion for the VMA and CTL conditions, and their percent change, respectively.
In order to examine whether this interhemispheric modulation was associated with long-term memory, we next correlated the interhemispheric difference in the proportion of spindles in trains with overnight memory retention. We found a significant correlation (Pearson correlation, r = 0.68, p = 0.045), suggesting that the temporal organization of spindles into trains may benefit motor memory stabilization during sleep. Critically, the density of sleep spindles was not related to long-term memory (r = −0.0034, p = 0.99), strengthening the specificity of our result.
Lastly, given that in our previous work we showed that VMA augmented the density of slow oscillations coupled with spindles, we examined if it also increased the proportion of spindle-SO couplings clustered into trains. As shown in Figure 2B, we found a learning-related interhemispheric modulation for this measure, driven by the contralateral hemisphere to the trained hand (LMM stats, main effect of hemisphere; F(1,8.95) = 8.95, p = 0.01, β = 5.1, SE = 1.7).
Train Length Modulates Intrinsic Parameters of the Constituting Spindles
So far, we have shown that VMA modulates the amount of spindles into trains, locally. But what may be the physiological benefit of this temporal organization? Sleep spindles may provide a time window favoring the reinstatement of recently acquired memories (Buzsáki, 2015; Antony et al., 2019), and thus, memory stabilization (Antony et al., 2018; Boutin and Doyon, 2020). Then, one possibility is that spindle grouping upregulates intrinsic features of the spindles, such as amplitude and/or duration, thereby increasing the instances of memory reinstatement.
To investigate whether the intrinsic features of spindles were influenced by being temporally organized in trains, we explored the percent difference in duration and amplitude between clustered spindles and isolated spindles (the raw values for these intrinsic features are depicted in Supplementary Table 2). Trains with 2 to 6 spindles were considered in the analysis to include 90% of the trains identified for each subject. Figure 3 illustrates the relative differences in duration (Figure 3A) and amplitude (Figure 3B), as a function of train length. The plots reveal that both intrinsic features increased as a function of train length, and reached greater values than those of isolated spindles. This positive relationship was statistically confirmed through repeated-measure correlations, and held both for the first and last spindle in a train (repeated measures correlations (rRM), Duration: First vs. Isolated spindle: rRM = 0.67, p < 0.001; Last vs. Isolated Spindle: rRM = 0.75, p < 0.001; Amplitude: First vs. Isolated spindle: rRM = 0.58, p < 0.001; Last vs. Isolated Spindle: rRM = 0.48, p < 0.001). To explore further whether spindle amplitude and duration varied along the train, we next compared the first and last spindles. A significant effect would speak in favor of an advantage of being part of a cluster. We found that spindle duration and amplitude were larger in magnitude for the last spindle than for the first spindle in a train (LMM stats, main effect of spindle position: Duration: F(1,9) = 7.31, p = 0.024, β = 3.86, SE = 0.51; Amplitude: F(1,9) = 5.31; p = 0.046, β = 1.73, SE = 0.34).
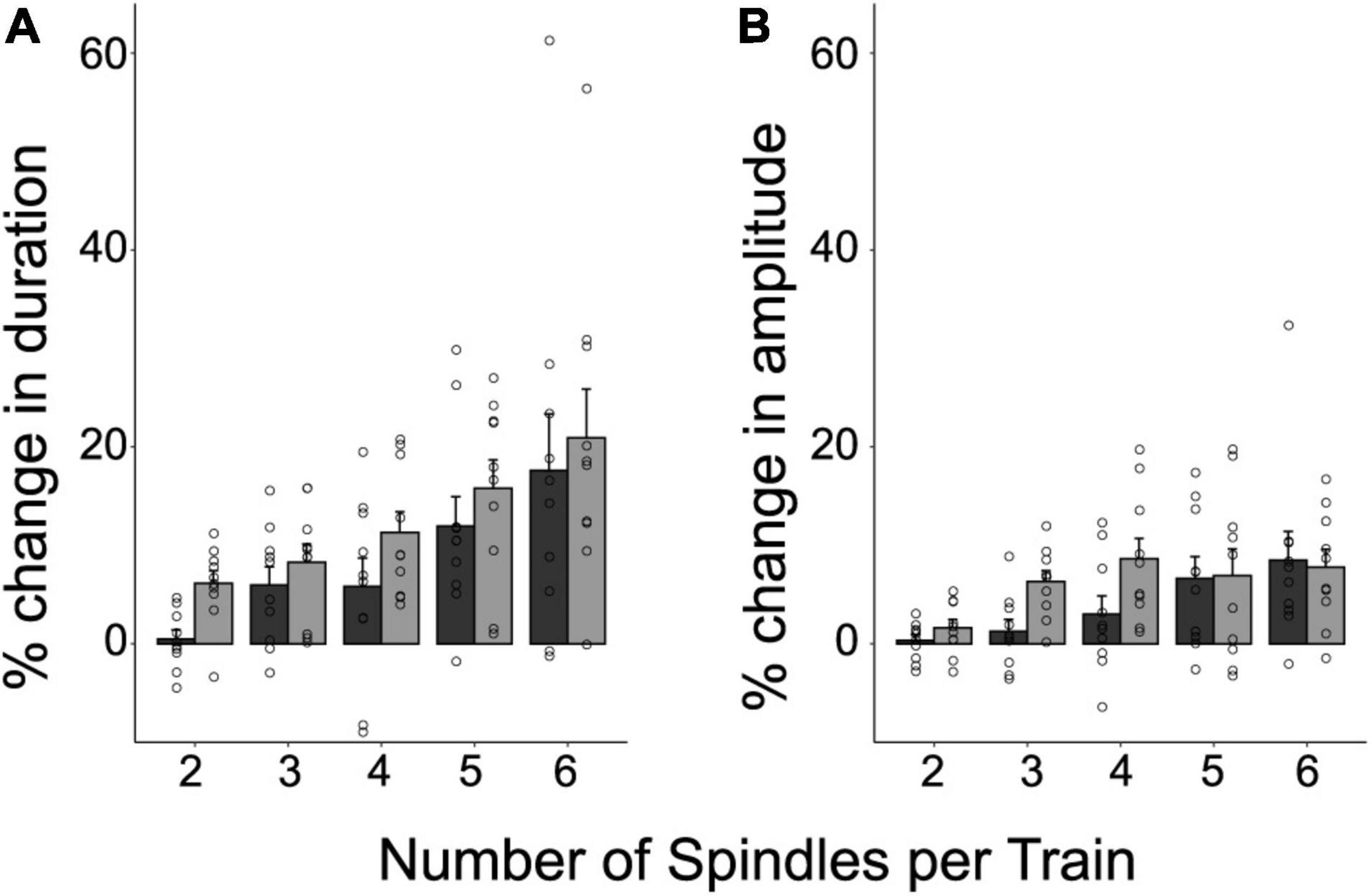
Figure 3. The temporal clustering of spindles modulates their intrinsic features. Shown are the individual values, and the mean ± SE of the percent difference in duration (A) and amplitude (B), for the first and last spindle in a train relative to isolated spindles. This was computed for 90% of trains containing between 2 and 6 spindles. Note that the last spindle in a train tends to be longer and larger than the first spindle (p < 0.05).
Discussion
Uncovering the neural signatures of procedural memory stabilization during sleep is of major relevance to neuroscience research. Recently we found that visuomotor adaptation learning is associated with an increment in the level of coupling between spindles and SOs locally, which predicts overnight memory retention (Solano et al., 2021). Here, we explored further whether VMA also promotes the temporal organization of sleep spindles into trains. We found that VMA increased the proportion of spindles in trains over the contralateral hemisphere of the trained hand; this interhemispheric modulation predicted overnight memory retention. Spindle clustering mostly involved fast spindles during NREM3 -not NREM2. VMA also increased the proportion of spindle-SO couplings organized into trains. The fact that intrinsic features of spindles such as their duration and amplitude augmented with train length points to a physiological advantage of this temporal organization.
Spindle trains are hypothesized to influence memory processing through the alternation between instances of memory reinstatement and refractory periods (Antony et al., 2018, 2019; Boutin and Doyon, 2020). To date, only a few studies have examined the relationship between spindle clustering and memory stabilization. On one hand, Lecci et al. (2017) and Antony et al. (2018) have shown that cluster periodicity (∼0.02 Hz) influences the recall of declarative memory during NREM2 and NREM3. On the other hand, Boutin et al. (2018a,2019) found that motor sequence learning (MSL) reduces the spindle ISI during NREM2, pointing to an increment in the level of clustering, and that this modulation predicted offline gains overnight. Our work contributes further to the understanding of the temporal arrangement of spindles in several ways. First, we show that a type of motor learning heavily dependent on an implicit component increases the clustering of both uncoupled and SO-coupled spindles. Second, this modulation took place specifically during NREM3. Third, the proportion of grouped spindles was augmented over the contralateral hemisphere, suggesting that the network active during learning may reactivate during sleep (Della-Maggiore et al., 2017; Klinzing et al., 2019). Finally, the interhemispheric modulation of spindle clustering predicted overnight memory retention, suggesting a behavioral relevance of this temporal arrangement. Contrary to some of the literature described above, train modulation was specifically observed during NREM3. This may respond to mechanistic differences inherent of the type of learning (declarative vs. non-declarative and VMA vs. MSL; Krakauer et al., 2019), and/or the experimental design. Alternatively, it may relate to the fact that we focused the analysis on the first sleep cycle; the temporal organization of spindles into trains may change as the duration of sleep stages vary through the night (Cowan et al., 2020).
Although trains may have a role in mnemonic processes, the physiological mechanisms through which spindle grouping modulates memory stabilization remain unexplored. Here we found that both spindle duration and amplitude increased in temporally clustered spindles as a function of train length. Longer spindles may reflect higher cortico-thalamic connectivity and coordination (Bonjean et al., 2011), whereas larger spindles may reflect a greater recruitment of thalamo-cortical neurons (Contreras and Steriade, 1996). We speculate that VMA may impact on long-term memory by promoting the development of longer and larger spindles, thereby facilitating cortico-thalamic synchronicity. Our work points to a physiological advantage in the temporal arrangement of spindles into clusters.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
The studies involving human participants were reviewed and approved by Ethics Committee of the Hospital de Clínicas, University of Buenos Aires. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
AS, VD-M, and LR designed the experiment. AS conducted the experiment and analyzed data. DP-C provided equipment, guidance and assistance with PSG studies. AS and VD-M wrote the manuscript. AS, VD-M, LR, and DP-C revised the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by the Argentinian Ministry of Defense (PIDDEF-2014-2017#17) and the Argentinian Agency for the promotion of Science and Technology (ANPCyT: PICT2019-02156; PICT2018-1150).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnins.2022.803387/full#supplementary-material
References
Achermann, P., and Borbély, A. A. (1997). Low-frequency (<1Hz) oscillations in the human sleep electroencephalogram. Neuroscience 81, 213–222. doi: 10.1016/s0306-4522(97)00186-3
Albouy, G., Fogel, S., Pottiez, H., Nguyen, V. A., Ray, L., Lungu, O., et al. (2013). Daytime Sleep Enhances Consolidation of the Spatial but Not Motoric Representation of Motor Sequence Memory. PLoS One 8:e52805. doi: 10.1371/journal.pone.0052805
Antony, J. W., and Paller, K. A. (2016). Using Oscillating Sounds to Manipulate Sleep Spindles. Sleep 40:zsw068. doi: 10.1093/sleep/zsw068
Antony, J. W., Piloto, L., Wang, M., Pacheco, P., Norman, K. A., and Paller, K. A. (2018). Sleep Spindle Refractoriness Segregates Periods of Memory Reactivation. Curr. Biol. 28, 1736–1743.e4. doi: 10.1016/j.cub.2018.04.020
Antony, J. W., Schönauer, M., Staresina, B. P., and Cairney, S. A. (2019). Sleep Spindles and Memory Reprocessing. Trends Neurosci. 42, 1–3. doi: 10.1016/j.tins.2018.09.012
Bakdash, J. Z., and Marusich, L. R. (2017). Repeated Measures Correlation. Front. Psychol. 8:456. doi: 10.3389/fpsyg.2017.00456
Barakat, M., Carrier, J., Debas, K., Lungu, O., Fogel, S., Vandewalle, G., et al. (2012). Sleep spindles predict neural and behavioral changes in motor sequence consolidation. Hum. Brain Mapp. 34, 2918–2928. doi: 10.1002/hbm.22116
Barakat, M., Doyon, J., Debas, K., Vandewalle, G., Morin, A., Poirier, G., et al. (2011). Fast and slow spindle involvement in the consolidation of a new motor sequence. Behav. Brain Res. 217, 117–121. doi: 10.1016/j.bbr.2010.10.019
Bates, D., Mächler, M., Bolker, B., and Walker, S. (2015). Fitting Linear Mixed-Effects Models Using lme4. J. Stat. Softw. 67, 1–48.
Bergmann, T. O., Mölle, M., Diedrichs, J., Born, J., and Siebner, H. R. (2012). Sleep spindle-related reactivation of category-specific cortical regions after learning face-scene associations. NeuroImage 59, 2733–2742. doi: 10.1016/j.neuroimage.2011.10.036
Bonjean, M., Baker, T., Lemieux, M., Timofeev, I., Sejnowski, T., and Bazhenov, M. (2011). Corticothalamic Feedback Controls Sleep Spindle Duration In Vivo. J. Neurosci. 31, 9124–9134. doi: 10.1523/jneurosci.0077-11.2011
Boutin, A., and Doyon, J. (2020). A sleep spindle framework for motor memory consolidation. Philos. Trans. R. Soc. B Biol. Sci. 375:20190232. doi: 10.1098/rstb.2019.0232
Boutin, A., Pinsard, B., Boré, A., Carrier, J., Fogel, S. M., and Doyon, J. (2018b). Transient synchronization of hippocampo-striato-thalamo-cortical networks during sleep spindle oscillations induces motor memory consolidation. NeuroImage 169, 419–430. doi: 10.1016/j.neuroimage.2017.12.066
Boutin, A., Gabitov, E., Pinsard, B., and Doyon, J. (2018a). “Clustered and temporally organised occurrence of NREM-stage2 sleep spindles mediates motor memory consolidation,” in 24th Congress of the European Sleep Research Society, (Basel, Switzerland. Hoboken, NJ: Wiley), 25–28.
Boutin, A., Gabitov, E., Pinsard, B., and Doyon, J. (2019). Hierarchical organization of sleep spindles mediates motor memory consolidation. J. Sport Exerc. Psychol. 41, S79–S80.
Buysse, D. J., Reynolds, C. F. III, Monk, T. H., Berman, S. R., and Kupfer, D. J. (1989). The Pittsburgh sleep quality index: a new instrument for psychiatric practice and research. Psychiatry Res. 28, 193–213.
Buzsáki, G. (2015). Hippocampal sharp wave-ripple: a cognitive biomarker for episodic memory and planning. Hippocampus 25, 1073–1188. doi: 10.1002/hipo.22488
Contreras, D., and Steriade, M. (1996). Spindle oscillation in cats: the role of corticothalamic feedback in a thalamically generated rhythm. J. Physiol. 490, 159–179. doi: 10.1113/jphysiol.1996.sp021133
Cowan, E., Liu, A., Henin, S., Kothare, S., Devinsky, O., and Davachi, L. (2020). Sleep Spindles Promote the Restructuring of Memory Representations in Ventromedial Prefrontal Cortex through Enhanced Hippocampal–Cortical Functional Connectivity. J. Neurosci. 40, 1909–1919. doi: 10.1523/jneurosci.1946-19.2020
Cox, R., Schapiro, A. C., Manoach, D. S., and Stickgold, R. (2017). Individual Differences in Frequency and Topography of Slow and Fast Sleep Spindles. Front. Hum. Neurosci. 11:433. doi: 10.3389/fnhum.2017.00433
Criscimagna-Hemminger, S. E., and Shadmehr, R. (2008). Consolidation Patterns of Human Motor Memory. J. Neurosci. 28, 9610–9618. doi: 10.1523/jneurosci.3071-08.2008
DeBruine, L. M., and Barr, D. J. (2021). Understanding mixed-effects models through data simulation. Adv. Methods Pract. Psychol. Sci. 4, 1–15.
Della-Maggiore, V., Villalta, J. I., Kovacevic, N., and McIntosh, A. R. (2017). Functional Evidence for Memory Stabilization in Sensorimotor Adaptation: a 24-h Resting-State fMRI Study. Cereb. Cortex 23, 1748–1757. doi: 10.1093/cercor/bhv289
Evans, B. M., and Richardson, N. E. (1995). Demonstration pf a 3–5s periodicity between the spindle bursts in NREM sleep in man. J. Sleep Res. 4, 196–197. doi: 10.1111/j.1365-2869.1995.tb00169.x
Ferrarelli, F., Huber, R., Peterson, M. J., Massimini, M., Murphy, M., Riedner, B. A., et al. (2007). Reduced Sleep Spindle Activity in Schizophrenia Patients. Am. J. Psychiatry 164, 483–492. doi: 10.1176/ajp.2007.164.3.483
Gramfort, A., Luessi, M., Larson, E., Engemann, D. A., Strohmeier, D., Brodbeck, C., et al. (2013). MEG and EEG data analysis with MNE-Python. Front. Neurosci. 7:267. doi: 10.3389/fnins.2013.00267
Halekoh, U., and Højsgaard, S. (2014). A Kenward-Roger Approximation and Parametric Bootstrap Methods for Tests in Linear Mixed Models - TheRPackagepbkrtest. J. Stat. Softw. 59, 1–32. doi: 10.18637/jss.v059.i09
Helfrich, R. F., Mander, B. A., Jagust, W. J., Knight, R. T., and Walker, M. P. (2018). Old Brains Come Uncoupled in Sleep: slow Wave-Spindle Synchrony, Brain Atrophy, and Forgetting. Neuron 97, 221–230.e4. doi: 10.1016/j.neuron.2017.11.020
Huber, R., Ghilardi, M. F., Massimini, M., Ferrarelli, F., Riedner, B. A., Peterson, M. J., et al. (2006). Arm immobilization causes cortical plastic changes and locally decreases sleep slow wave activity. Nat. Neurosci. 9, 1169–1176. doi: 10.1038/nn1758
Huber, R., Ghilardi, M. F., Massimini, M., and Tononi, G. (2004). Local sleep and learning. Nature 430, 78–81. doi: 10.1038/nature02663
Iber, C. (2004). Development of a New Manual for Characterizing Sleep. Sleep 27, 190–192. doi: 10.1093/sleep/27.2.190
Johns, M. W. (1991). A New Method for Measuring Daytime Sleepiness: the Epworth Sleepiness Scale. Sleep 14, 540–545. doi: 10.1093/sleep/14.6.540
Kapucu, F. E., Tanskanen, J. M. A., Mikkonen, J. E., Ylä-Outinen, L., Narkilahti, S., and Hyttinen, J. A. K. (2012). Burst analysis tool for developing neuronal networks exhibiting highly varying action potential dynamics. Front. Comput. Neurosci. 6:38. doi: 10.3389/fncom.2012.00038
Klinzing, J. G., Niethard, N., and Born, J. (2019). Mechanisms of systems memory consolidation during sleep. Nat. Neurosci. 22, 1598–1610. doi: 10.1038/s41593-019-0467-3
Krakauer, J. W., Hadjiosif, A. M., Xu, J., Wong, A. L., and Haith, A. M. (2019). Motor Learning. Compr. Physiol. 9, 613–663. doi: 10.1002/cphy.c170043
Kumle, L., Võ, M. L. H., and Draschkow, D. (2021). Estimating power in (generalized) linear mixed models: an open introduction and tutorial in R. Behav. Res. Methods 53, 2528–2543. doi: 10.3758/s13428-021-01546-0
Ladenbauer, J., Ladenbauer, J., Külzow, N., de Boor, R., Avramova, E., Grittner, U., et al. (2017). Promoting Sleep Oscillations and Their Functional Coupling by Transcranial Stimulation Enhances Memory Consolidation in Mild Cognitive Impairment. J. Neurosci. 37, 7111–7124. doi: 10.1523/jneurosci.0260-17.2017
Laventure, S., Fogel, S., Lungu, O., Albouy, G., Sévigny-Dupont, P., Vien, C., et al. (2016). NREM2 and Sleep Spindles Are Instrumental to the Consolidation of Motor Sequence Memories. PLoS Biol. 14:e1002429. doi: 10.1371/journal.pbio.1002429
Lecci, S., Fernandez, L. M. J., Weber, F. D., Cardis, R., Chatton, J.-Y., Born, J., et al. (2017). Coordinated infraslow neural and cardiac oscillations mark fragility and offline periods in mammalian sleep. Sci. Adv. 3:e1602026. doi: 10.1126/sciadv.1602026
Maingret, N., Girardeau, G., Todorova, R., Goutierre, M., and Zugaro, M. (2016). Hippocampo-cortical coupling mediates memory consolidation during sleep. Nat. Neurosci. 19, 959–964. doi: 10.1038/nn.4304
Mizrahi-Kliger, A. D., Kaplan, A., Israel, Z., and Bergman, H. (2018). Desynchronization of slow oscillations in the basal ganglia during natural sleep. Proc. Natl. Acad. Sci. U. S. A. 115, E4274–E4283. doi: 10.1073/pnas.1720795115
Mölle, M., Bergmann, T. O., Marshall, L., and Born, J. (2011). Fast and Slow Spindles during the Sleep Slow Oscillation: disparate Coalescence and Engagement in Memory Processing. Sleep 34, 1411–1421. doi: 10.5665/sleep.1290
Morin, A., Doyon, J., Dostie, V., Barakat, M., Tahar, A. H., Korman, M., et al. (2008). Motor sequence learning increases sleep spindles and fast frequencies in post-training sleep. Sleep 31, 1149–1156. doi: 10.5665/sleep/31.8.1149
Muehlroth, B. E., Sander, M. C., Fandakova, Y., Grandy, T. H., Rasch, B., Shing, Y. L., et al. (2019). Precise Slow Oscillation–Spindle Coupling Promotes Memory Consolidation in Younger and Older Adults. Sci. Rep. 9:1940. doi: 10.1038/s41598-018-36557-z
Navarro-Lobato, I., and Genzel, L. (2019). The up and down of sleep: from molecules to electrophysiology. Neurobiol. Learn. Mem. 160, 3–10. doi: 10.1016/j.nlm.2018.03.013
Ngo, H.-V., Fell, J., and Staresina, B. (2020). Sleep spindles mediate hippocampal-neocortical coupling during long-duration ripples. ELife 9:e57011. doi: 10.7554/elife.57011
Nishida, M., and Walker, M. P. (2007). Daytime Naps, Motor Memory Consolidation and Regionally Specific Sleep Spindles. PLoS One 2:e341. doi: 10.1371/journal.pone.0000341
R Core Team (2017). R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing.
Rasch, B., and Born, J. (2013). About Sleep’s Role in Memory. Physiol. Rev. 93, 681–766. doi: 10.1152/physrev.00032.2012
Schönauer, M. (2018). Sleep Spindles: timed for Memory Consolidation. Curr. Biol. 28, R656–R658. doi: 10.1016/j.cub.2018.03.046
Solano, A., Riquelme, L. A., Perez-Chada, D., and Della-Maggiore, V. (2021). Motor Learning Promotes the Coupling between Fast Spindles and Slow Oscillations Locally over the Contralateral Motor Network. Cereb. Cortex [Epub Online ahead of print]. doi: 10.1093/cercor/bhab360
Staresina, B. P., Bergmann, T. O., Bonnefond, M., van der Meij, R., Jensen, O., Deuker, L., et al. (2015). Hierarchical nesting of slow oscillations, spindles and ripples in the human hippocampus during sleep. Nat. Neurosci. 18, 1679–1686. doi: 10.1038/nn.4119
Tort, A. B. L., Komorowski, R., Eichenbaum, H., and Kopell, N. (2010). Measuring Phase-Amplitude Coupling Between Neuronal Oscillations of Different Frequencies. J. Neurophysiol. 104, 1195–1210.
Keywords: human, motor learning, sleep, spindle train, slow oscillation
Citation: Solano A, Riquelme LA, Perez-Chada D and Della-Maggiore V (2022) Visuomotor Adaptation Modulates the Clustering of Sleep Spindles Into Trains. Front. Neurosci. 16:803387. doi: 10.3389/fnins.2022.803387
Received: 27 October 2021; Accepted: 21 February 2022;
Published: 16 March 2022.
Edited by:
Elena Urrestarazu, University of Navarra Clinic, SpainReviewed by:
F. Javier Puertas, Catholic University of Valencia San Vicente Mártir, SpainDorothee Pöhlchen, Max Planck Society, Germany
Copyright © 2022 Solano, Riquelme, Perez-Chada and Della-Maggiore. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Valeria Della-Maggiore, dmRlbGxhbWFnZ2lvcmVAZm1lZC51YmEuYXI=
 Agustín Solano
Agustín Solano Luis A. Riquelme
Luis A. Riquelme Daniel Perez-Chada2
Daniel Perez-Chada2 Valeria Della-Maggiore
Valeria Della-Maggiore