
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Neurosci. , 22 December 2022
Sec. Translational Neuroscience
Volume 16 - 2022 | https://doi.org/10.3389/fnins.2022.1084362
Background: Hegu is the most commonly used acupoints for pain relief. Recently, several functional neuroimaging studies have been performed on acupuncture at Hegu in healthy volunteers, but these studies have yielded diverse findings. Therefore, there is an urgent need to understand the brain response characteristics of acupuncture at Hegu.
Methods: Neuroimaging studies on acupuncture at Hegu published before October 2022 were collected from PubMed, Web of Science, Google Scholar, Embase, and CNKI (China National Knowledge Infrastructure) databases, and were screened by strict inclusion and exclusion criteria. The extraction of brain coordinates was performed by two independent researchers, and the results were analyzed using activation likelihood estimation (ALE) analysis based on quantitative coordinates.
Results: In total, 338 studies were searched, of which 19 studies were included in the final analysis after a rigorous double-blind screening review. Activation likelihood estimation showed that postcentral gyrus in the left brain were activated, whereas the anterior cingulate in the left brain and superior temporal gyrus in the right brain were deactivated.
Conclusion: Acupuncture at Hegu in healthy volunteers did not reveal specific brain regions. This finding implies that organismal status of the study subjects may have an important impact on the effect of acupoints.
Systematic review registration: [https://www.crd.york.ac.uk], identifier [CRD42020197296].
Hegu is one of the most commonly used acupoints, located at the dorsal part of the hand and the midpoint of the radial side of the second metacarpal bone (Chae et al., 2007; Zhang et al., 2013). Numerous clinical studies have demonstrated the efficacy of Hegu in treatment of shoulder pain, carpal tunnel syndrome, toothache and other painful diseases. However, the underlying biological mechanism of action remains unclear, and further studies are necessary (Sheng and Chang, 1960; Wang et al., 2006; Napadow et al., 2007; Lu, 2011; Asadi et al., 2015).
In the absence of clinical conditions, healthy individuals are ideal subjects for determining brain activity during acupuncture stimulation (Huang et al., 2022). To date, neuroimaging techniques have been used to identify brain patterns that activate or deactivate during acupuncture at Hegu in healthy individuals. Research has found evidence that a large number of brain regions are involved, but due to a variety of discrepancies in research settings (e.g., study design, etc.), no consensus has been reached. For example, a previous study found that the analgesic effect of acupuncture at Hegu is related to the pregenual anterior cingulate and hippocampal (Claunch et al., 2012). However, Hegu has also been shown to evoke activation in the thalamus, basal ganglia and cerebellum, as well as in the left putamen (Gu et al., 2015). In addition, identifying brain regions that change activity when acupuncture Hegu helps to determine the effect of acupuncture on the central nervous in humans. Therefore, there is an urgent need to collate previous evidence and uncover the altered brain activity associated with acupuncture at Hegu.
Activation likelihood estimation (ALE) has developed as an important meta-analysis approach for synthesizing neuroimaging data (Tillisch et al., 2011). It has been widely used in the study of acute sleep deprivation, insomnia, and other disorders (Tahmasian et al., 2018a; Javaheripour et al., 2019). ALE can maximize consistency of locating information, among quantitative studies, and can also minimize heterogeneity of the analytical method. With the aid of ALE, this study aimed to perform a meta-analysis of the results of previous studies to obtain reliable conclusions about the functional changes in brain regions induced by acupuncture at Hegu, and to help elucidate the mechanisms of Hegu.
We searched PubMed, Web of Science, Google Scholar, Embase, and CNKI (China National Knowledge Infrastructure) database for relevant studies published up to October 2022. Our search contains the following search terms: (Hegu OR LI 4) AND (voxel-based morphometry OR morphometric OR VBM OR neuroimaging OR functional neuroimaging OR functional magnetic resonance imaging OR functional MRI OR fMRI OR positron emission tomography OR PET) AND (acupuncture therapy OR acupuncture OR electroacupuncture OR electro-acupuncture OR acupoint* OR meridian*). Studies that met the following criteria were included: (1) analyzed only on healthy volunteers with acupuncture at Hegu; (2) Acupuncture includes body acupuncture, manual acupuncture, warm acupuncture, ear acupuncture, plum blossom needling, fire needling and electrical acupuncture; (3) reported results were on whole-brain scans rather than area-of-interest scans; (4) reported results were in normalized spatial coordinates, including Montreal neurological institute (MNI) or Talairach coordinates; and (5) the study adopted a task design. Conversely, those that met the following criteria were excluded: (1) transcutaneous electrical nerve stimulation, acupressure and laser stimulation as acupuncture interventions; (2) conference papers, reviews and animal experiment studies; or (3) only reported individual subject rather than group data. Data extraction for the included studies was independently performed by two reviewers, based on a data collection list that included effective sample size, age and gender distribution, handiness, acupuncture method, acupuncture laterality, task paradigms, and reported peak coordinates (x, y, z) in the standard atlas (Talairach or MNI). A third reviewer double-checked the data when necessary to ensure consistency.
Activation likelihood estimation is a method that uses voxel coordinates to locate and analyze functional brain regions. This approach has been previously utilized to integrate reported coordinates from different studies (Turkeltaub et al., 2012; Tahmasian et al., 2018b). Through the method, consistency of spatial positions among various studies can be maximized while minimizing subjectivity of the analysis method. All coordinates published in Talairach space were transformed into MNI space, using the Ginger ALE software (BrainMap Ginger ALE 3.0.2; Research Imaging Center, University of Texas Health Science Center at San Antonio), while individual coordinates were modeled by a 3-D Gaussian probability distribution. Thereafter, we created a modeled activation map (MA map) after combining the probability distributions of all foci, then incorporated the MA maps to produce the final ALE map, which reflected the likelihood convergence of results across all studies (Eickhoff et al., 2012). The ALE map was assessed against null-distribution of random spatial association, using histogram integration, at a statistical significance of p < 0.05 family wise error in cluster level (cFWE) to correct for multiple comparisons (Eickhoff et al., 2017; Müller et al., 2018). Finally, we adopted the anatomical image overlay program Mango (Creators, Jack L. Lancaster and Michael J. Martinez)1 to illustrate the meta-analysis results using MNI coordinates.
The quality of the included studies was assessed using an 11-point checklist that has been used in previous meta-analyses, which focused on population demographic characteristics, scanner parameters, and methodological details (Shepherd et al., 2012; Du et al., 2014; Cheng et al., 2020). Two reviewers independently conducted the quality assessment of the study, and any disagreements were resolved by a third reviewer. The meta-analysis has been registered in the PROSPERO International Prospective Register of Systematic Reviews of the University of York (No. CRD42020197296).
In this meta-analysis, from 338 retrieved studies, 19 studies and 399 subjects (216 females) were eligible to be included in this meta-analysis (Figure 1). These 19 studies were all functional magnetic resonance imaging (fMRI), including 16 block designs and 3 non-repeat event-related design (Table 1). All included studies had a mean quality score of 9.34, out of a possible total of 11, indicating that they were of high quality.
The meta-analysis of the activation patterns of acupuncture at Hegu included 19 studies, 407 subjects and 307 foci (Figure 2A and Table 2). Analysis showed that acupuncture stimulation caused an activation pattern in the left postcentral gyrus.
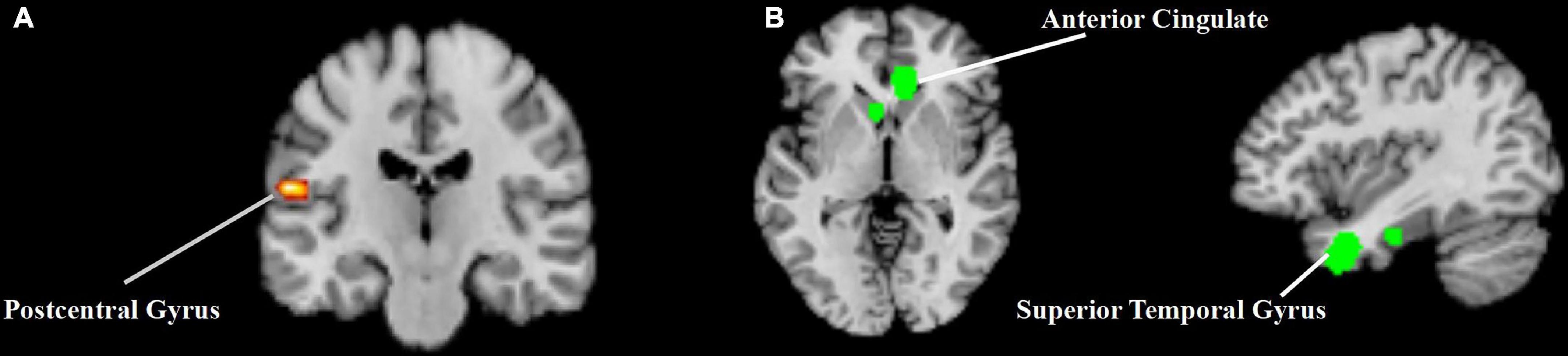
Figure 2. The results of the activation likelihood estimation (ALE) analysis from acupuncture at Hegu. (A) The activation likelihood estimation (ALE) analysis result of the activation patterns of acupuncture at Hegu. (B) The activation likelihood estimation (ALE) analysis result of the deactivation patterns of acupuncture at Hegu.
The meta-analysis of the deactivation patterns of acupuncture at Hegu included 11 studies, 301 subjects and 168 foci (Figure 2B and Table 2). Analysis showed that acupuncture stimulation caused occurrence of common deactivation patterns across various brain regions, such as right anterior cingulate and left superior temporal gyrus.
Since at least 17 studies were required to achieve 80% power for moderate effects (Eickhoff et al., 2016), separate subgroup analyses were not performed for the different experimental designs.
To the best of our knowledge, this study used a voxel-based meta-analysis to provide the first report of brain response characteristics during acupuncture at Hegu. The results showed that acupuncture at Hegu caused functional activation or deactivation across several non-specific brain regions, including the sensorimotor network and the limbic system.
In this study, postcentral gyrus showed significant signal increases. The results revealed that acupuncture at Hegu activated the sensorimotor network, which receives sensory information from the periphery and is critical in bodily sensation and in generating appropriate motor responses, and is the main brain network responsible for pain perception (Mayer et al., 2015). Since acupuncture is an invasive form of mechanical stimulation, when the needle penetrates the body, the acupuncture signal is received by the pain receptors, integrated through the thalamus, and transmitted to postcentral gyrus. The postcentral gyrus serves as the primary somatosensory cortex responsible for receiving nociceptive and proprioceptive sensations from the contralateral body, processing sensory information from the somatosensory areas and participating in the discrimination of the level, location and duration of painful stimuli (Zhang et al., 2014). Although not entirely consistent, the present results and the findings of Chae et al. (2013) confirm that mechanical stimulation caused by the therapeutic tool during needling activates sensorimotor network, which is caused by acupuncture itself. Consistent with previous studies (Hui et al., 2000), this study also found evidence that acupuncture at Hegu triggers extensive deactivation of the cerebral cortex, including the anterior cingulate cortex (ACC) and superior temporal gyrus that overlap with the limbic system (Catani et al., 2013; Fulford, 2015). Furthermore, the limbic system has been shown to play an important role in the acupuncture effects of multiple acupoints (Fang et al., 2009). For example, Zusanli (ST 36) also deactivates brain regions such as the middle superior frontal gyrus (Xiang et al., 2019) ang ACC (Claunch et al., 2012). Apparently, no specific brain regions were observed in the present study during acupuncture at Hegu.
Several previous reviews have explored the modulatory effects of acupuncture on the brain. A systematic review based on acupuncture at Zusanli in healthy volunteers showed that brain regions such as superior temporal gyrus, insula, and postcentral gyrus were activated after acupuncture, which is similar to our findings, suggesting similar brain responses at different acupoints (Huang et al., 2022). However, Dhond et al. (2007) analyzed the functional changes of the brain under various pain states, and proposed that certain limbic brain networks may play a specific therapeutic role in the process of acupuncture analgesia. Consistent with Huang et al.’s (2012) study, the presence of this difference suggests that patients and healthy volunteers have different brain responses to acupuncture stimulation. According to traditional Chinese medicine theory, acupoints are considered a dynamic functional area, which can reflect the internal condition of the body (Tan et al., 2019). Under physiological conditions, acupoints are silent and their functions are not manifested or obvious, but under pathological conditions, acupoints are activated and their functional effects can be manifested (Zhu, 2019). Several studies have shown that acupuncture at the same acupoints has significant differences in brain function changes under different organismal states. For example, a study found that compared with healthy subjects, acupuncture in patients with low back pain have increased functional connectivity in brain regions such as the thalamus, brainstem, and insula (Ye et al., 2011). Cho et al. (2013) also proposed that brain signal activations during the same acupuncture were different between the healthy and the stroke patients. All of these brain imaging studies confirmed that functional status is an essential impact factor for cerebral responses to acupuncture stimulation, and specific brain regions are difficult to obtain through healthy subjects. In addition, the application of acupuncture in clinical practice is specific to the pathological rather than the physiological state. Therefore, disease is a better way to study the effects of acupoints.
Task state design is a unique and diverse design approach that analyses activated brain regions by presenting subjects with specific stimuli during scanning. To improve the homogeneity of this meta-analysis, we only included studies with a task state design to improve and this helped in identifying brain patterns associated with acupuncture. In addition, we chose a more rigorous FWE algorithm for brain imaging meta-analysis to make the results more reliable (Eickhoff et al., 2016). However, this study has limitations that need to be addressed. Since the sample sizes of such studies are usually small, we proposed stricter inclusion and exclusion criteria, resulting in a limited sample size for the final meta-analysis. In addition, due to the large number of identical brain regions, we reported the results only for the peak with the largest ALE value.
Acupuncture at Hegu in healthy volunteers resulted in activation or deactivation of the sensorimotor network and the limbic system, did not reveal specific brain regions. The action of acupoints is dynamic and the functional status appears to be an essential impact factor for cerebral responses to acupuncture stimulation. Future studies should focus on the brain’s response to acupuncture treatment under different diseases.
ZG contributed to the study conception and design, conceived the data analysis strategy, collated and analyzed the data, and drafted the manuscript. MC and JZ acquired the data. ZG, MC, JZ, and LJ discussed, read, and revised the manuscript. All authors approved the publication of this manuscript.
This work was supported by the National Natural Science Foundation of China (No. 8207152138). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
We thank Dr. Yin Tao for assistance and suggestions.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnins.2022.1084362/full#supplementary-material
Asadi, N., Maharlouei, N., Khalili, A., Darabi, Y., Davoodi, S., Raeisi Shahraki, H., et al. (2015). Effects of LI-4 and SP-6 acupuncture on labor pain, cortisol level and duration of labor. J. Acupunct. Meridian Stud. 8, 249–254. doi: 10.1016/j.jams.2015.08.003
Asghar, A. U., Green, G., Lythgoe, M. F., Lewith, G., and MacPherson, H. (2010). Acupuncture needling sensation: The neural correlates of deqi using fMRI. Brain Res. 1315, 111–118. doi: 10.1016/j.brainres.2009.12.019
Catani, M., Dell’acqua, F., and Thiebaut de Schotten, M. (2013). A revised limbic system model for memory, emotion and behaviour. Neurosci. Biobehav. Rev. 37, 1724–1737. doi: 10.1016/j.neubiorev.2013.07.001
Chae, Y., Chang, D. S., Lee, S. H., Jung, W. M., Lee, I. S., Jackson, S., et al. (2013). Inserting needles into the body: A meta-analysis of brain activity associated with acupuncture needle stimulation. J. Pain 14, 215–222. doi: 10.1016/j.jpain.2012.11.011
Chae, Y., Lee, I. S., Jung, W. M., Park, K., Park, H. J., and Wallraven, C. (2015). Psychophysical and neurophysiological responses to acupuncture stimulation to incorporated rubber hand. Neurosci. Lett. 591, 48–52. doi: 10.1016/j.neulet.2015.02.025
Chae, Y., Park, H. J., Hahm, D. H., Hong, M., Ha, E., Park, H. K., et al. (2007). fMRI review on brain responses to acupuncture: The limitations and possibilities in traditional Korean acupuncture. Neurol. Res. 29(Suppl. 1) S42–S48. doi: 10.1179/016164107x172284
Chen, F. Y., Shen, Z. W., Guan, J. T., Xiao, Y. Y., Du, L., and Wu, R. H. (2011). Observation of the relation between brain activity and de qi sensation with manual acupuncture at LI4 (Hegu). Chin. J. Magn. Reson. Imaging 2, 112–117.
Chen, Y. Y. (2018). Multi-modality fMRI study on the correlation between negative BOLD responses and GABA/Glx elicited by needling. Beijing: China Academy of Chinese Medical Sciences.
Cheng, S., Xu, G., Zhou, J., Qu, Y., Li, Z., He, Z., et al. (2020). A multimodal meta-analysis of structural and functional changes in the brain of tinnitus. Front. Hum. Neurosci. 14:28. doi: 10.3389/fnhum.2020.00028
Cho, S. Y., Kim, M., Sun, J. J., Jahng, G. H., Kim, H. J., Park, S. U., et al. (2013). A comparison of brain activity between healthy subjects and stroke patients on fMRI by acupuncture stimulation. Chin. J. Integr. Med. 19, 269–276. doi: 10.1007/s11655-013-1436-4
Claunch, J. D., Chan, S. T., Nixon, E. E., Qiu, W. Q., Sporko, T., Dunn, J. P., et al. (2012). Commonality and specificity of acupuncture action at three acupoints as evidenced by FMRI. Am. J. Chin. Med. 40, 695–712. doi: 10.1142/s0192415x12500528
Dhond, R. P., Kettner, N., and Napadow, V. (2007). Do the neural correlates of acupuncture and placebo effects differ? Pain 128, 8–12. doi: 10.1016/j.pain.2007.01.001
Du, M., Liu, J., Chen, Z., Huang, X., Li, J., Kuang, W., et al. (2014). Brain grey matter volume alterations in late-life depression. J. Psychiatry Neurosci. 39, 397–406. doi: 10.1503/jpn.130275
Eickhoff, S. B., Bzdok, D., Laird, A. R., Kurth, F., and Fox, P. T. (2012). Activation likelihood estimation meta-analysis revisited. Neuroimage 59, 2349–2361. doi: 10.1016/j.neuroimage.2011.09.017
Eickhoff, S. B., Laird, A. R., Fox, P. M., Lancaster, J. L., and Fox, P. T. (2017). Implementation errors in the GingerALE Software: Description and recommendations. Hum. Brain Mapp. 38, 7–11. doi: 10.1002/hbm.23342
Eickhoff, S. B., Nichols, T. E., Laird, A. R., Hoffstaedter, F., Amunts, K., Fox, P. T., et al. (2016). Behavior, sensitivity, and power of activation likelihood estimation characterized by massive empirical simulation. Neuroimage 137, 70–85. doi: 10.1016/j.neuroimage.2016.04.072
Fang, J., Jin, Z., Wang, Y., Li, K., Kong, J., Nixon, E. E., et al. (2009). The salient characteristics of the central effects of acupuncture needling: Limbic-paralimbic-neocortical network modulation. Hum. Brain Mapp. 30, 1196–1206. doi: 10.1002/hbm.20583
Fulford, A. J. (2015). Endogenous nociceptin system involvement in stress responses and anxiety behavior. Vitam. Horm. 97, 267–293. doi: 10.1016/bs.vh.2014.12.012
Gu, W., Jiang, W., He, J., Liu, S., and Wang, Z. (2015). Blockade of the brachial plexus abolishes activation of specific brain regions by electroacupuncture at LI4: A functional MRI study. Acupunct. Med. 33, 457–464. doi: 10.1136/acupmed-2015-010901
Hou, J. W., Huang, W. H., Wang, Q., Feng, Q. W., Pu, Y. L., and Gao, J. H. (2002). Functional MRI studies of acupuncture analgesia modulating within the human brain. Chin. J. Radiol. 3, 14–18.
Huang, H., Yue, X., Huang, X., Long, W., Kang, S., Rao, Y., et al. (2022). Brain activities responding to acupuncture at ST36 (zusanli) in healthy subjects: A systematic review and meta-analysis of task-based fMRI studies. Front. Neurol. 13:930753. doi: 10.3389/fneur.2022.930753
Huang, W., Pach, D., Napadow, V., Park, K., Long, X., Neumann, J., et al. (2012). Characterizing acupuncture stimuli using brain imaging with FMRI–a systematic review and meta-analysis of the literature. PLoS One 7:e32960. doi: 10.1371/journal.pone.0032960
Hui, K. K., Liu, J., Makris, N., Gollub, R. L., Chen, A. J., Moore, C. I., et al. (2000). Acupuncture modulates the limbic system and subcortical gray structures of the human brain: Evidence from fMRI studies in normal subjects. Hum. Brain Mapp. 9, 13–25. doi: 10.1002/(sici)1097-019320009:1<13::aid-hbm2<3.0.co;2-f
Javaheripour, N., Shahdipour, N., Noori, K., Zarei, M., Camilleri, J. A., Laird, A. R., et al. (2019). Functional brain alterations in acute sleep deprivation: An activation likelihood estimation meta-analysis. Sleep Med. Rev. 46, 64–73. doi: 10.1016/j.smrv.2019.03.008
Kong, J., Ma, L., Gollub, R. L., Wei, J., Yang, X., Li, D., et al. (2002). A pilot study of functional magnetic resonance imaging of the brain during manual and electroacupuncture stimulation of acupuncture point (LI-4 Hegu) in normal subjects reveals differential brain activation between methods. J. Altern. Complement. Med. 8, 411–419. doi: 10.1089/107555302760253603
Li, K., Shan, B. C., Liu, H., Wang, W., Xu, J. Y., Lu, N., et al. (2005). Functional magnetic resonance imaging of brain by acupuncture at Hegu. Chin. J. Med. Imaging Technol. 9, 1329–1331.
Li, K., Shan, B., Xu, J., Liu, H., Wang, W., Zhi, L., et al. (2006). Changes in FMRI in the human brain related to different durations of manual acupuncture needling. J. Altern. Complement. Med. 12, 615–623. doi: 10.1089/acm.2006.12.615
Liu, Y. (2014). A study based on fMRI on the central mechanism of specific correlation between Hegu point and the orofacial part doctorate. Jinan: Shandong University of Chinese Medicine.
Lu, F. X. (2011). [Acupuncture at Hegu (LI 4) combined with manual reduction for intractable shoulder dislocation]. Zhongguo Zhen Jiu 31, 599–600.
MacPherson, H., Green, G., Nevado, A., Lythgoe, M. F., Lewith, G., Devlin, R., et al. (2008). Brain imaging of acupuncture: Comparing superficial with deep needling. Neurosci. Lett. 434, 144–149. doi: 10.1016/j.neulet.2008.01.058
Mayer, E. A., Labus, J. S., Tillisch, K., Cole, S. W., and Baldi, P. (2015). Towards a systems view of IBS. Nat. Rev. Gastroenterol. Hepatol. 12, 592–605. doi: 10.1038/nrgastro.2015.121
Müller, V. I., Cieslik, E. C., Laird, A. R., Fox, P. T., Radua, J., Mataix-Cols, D., et al. (2018). Ten simple rules for neuroimaging meta-analysis. Neurosci. Biobehav. Rev. 84, 151–161. doi: 10.1016/j.neubiorev.2017.11.012
Napadow, V., Kettner, N., Liu, J., Li, M., Kwong, K. K., Vangel, M., et al. (2007). Hypothalamus and amygdala response to acupuncture stimuli in carpal tunnel syndrome. Pain 130, 254–266. doi: 10.1016/j.pain.2006.12.003
Sheng, L. C., and Chang, T. H. (1960). Electroacupuncture anesthesia in oral surgery: A preliminary report. Chin. Med. J. 80, 97–99.
Shepherd, A. M., Matheson, S. L., Laurens, K. R., Carr, V. J., and Green, M. J. (2012). Systematic meta-analysis of insula volume in schizophrenia. Biol. Psychiatry 72, 775–784. doi: 10.1016/j.biopsych.2012.04.020
Tahmasian, M., Noori, K., Samea, F., Zarei, M., Spiegelhalder, K., Eickhoff, S. B., et al. (2018a). A lack of consistent brain alterations in insomnia disorder: An activation likelihood estimation meta-analysis. Sleep Med. Rev. 42, 111–118. doi: 10.1016/j.smrv.2018.07.004
Tahmasian, M., Zarei, M., Noori, K., Khazaie, H., Samea, F., Spiegelhalder, K., et al. (2018b). Reply to Hua Liu, HaiCun Shi and PingLei Pan: Coordinate based meta-analyses in a medium sized literature: Considerations, limitations and road ahead. Sleep Med. Rev. 42, 236–238. doi: 10.1016/j.smrv.2018.08.004
Tan, H., Tumilty, S., Chapple, C., Liu, L., McDonough, S., Yin, H., et al. (2019). Understanding acupoint sensitization: A narrative review on phenomena. Potential Mechanism, and Clinical Application. Evid. Based Complement. Alternat. Med. 2019:6064358. doi: 10.1155/2019/6064358
Tang, J. S., Jiang, Y., Zang, J. T., and Weng, G. S. (2009). Functional magnetic resonance imaging analysis of the nerve center mechanism of treatment of orofacial diseases by using Hegu. J. Tradit. Chin. Med. 40, 56–57.
Tillisch, K., Mayer, E. A., and Labus, J. S. (2011). Quantitative meta-analysis identifies brain regions activated during rectal distension in irritable bowel syndrome. Gastroenterology 140, 91–100. doi: 10.1053/j.gastro.2010.07.053
Tong, J. S. (2008). The research of pain mechanism and acupuncture anesthesia based on EEG and fMRI. Zhejiang: Zhejiang University.
Turkeltaub, P. E., Eickhoff, S. B., Laird, A. R., Fox, M., Wiener, M., and Fox, P. (2012). Minimizing within-experiment and within-group effects in activation likelihood estimation meta-analyses. Hum. Brain Mapp. 33, 1–13. doi: 10.1002/hbm.21186
Wang, B., Liu, J. Y., Han, Y., Zhang, N., Ren, X. Q., Zhai, G. R., et al. (2006). [Study on effect of electroacupuncture at Hegu (LI 4) on the uterotonic time in parturients of uterus inertia]. Zhongguo Zhen Jiu 26, 843–846.
Wang, L., Xu, C., Zhu, Y., Li, C., and Yang, J. (2015). [Effects of acupuncture at left and right Hegu (LI 4) for cerebral function laterality]. Zhongguo Zhen Jiu 35, 806–811.
Wang, T. (2017). Study on the central mechanism of fMRI in the treatment of refractory peripheral facial paralysis by acupuncture. Hefei: Anhui University of Chinese Medicine.
Wang, W., Liu, L., Zhi, X., Huang, J. B., Liu, D. X., Wang, H., et al. (2007). Study on the regulatory effect of electro-acupuncture on hegu point (LI4) in cerebral response with functional magnetic resonance imaging. Chin. J. Integr. Med. 13, 10–16. doi: 10.1007/s11655-007-0010-3
Xiang, A. F., Liu, H., Liu, S., and Shen, X. Y. (2019). [Brain regions responding to acupuncture stimulation of Zusanli (ST36) in healthy subjects analyzed on the basis of spontaneous brain activity]. Zhen Ci Yan Jiu 44, 66–70. doi: 10.13702/j.1000-0607.180138
Yang, J., Li, C. F., Xu, C. S., Zang, Q. P., Chen, D. X., Wang, L. Y., et al. (2014). An fMRI study on needling in Hegu(LI4) and Houxi(SI3) of adult healthy volunteers. World Chin. Med. 9, 1575–1580.
Ye, Y. S., Liu, B., and Chen, Z. G. (2011). Resting-state-functional magnetic resonance imaging technology applied to a balancing acupuncture treatment for central mechanisms. Chin. J. Tissue Eng. Res. 15, 8998–9002.
Zhang, J., Wang, X., and Lü, R. (2013). Analgesic effect of acupuncture at hegu (LI 4) on transvaginal oocyte retrieval with ultrasonography. J. Tradit. Chin. Med. 33, 294–297. doi: 10.1016/s0254-6272(13)60167-3
Zhang, R., Zou, Y. Q., Huang, S. Q., Chen, Z. G., Liang, B. L., Li, Y., et al. (2007). MRI cerebral function in aging following acupuncture at Hegu, Zusanli, Neiguana and Sayinjiao points. Chin. J. Tissue Eng. Res. 22, 4271–4274.
Zhang, S. S., Wu, W., Liu, Z. P., Huang, G. Z., Guo, S. G., and Yang, J. M. (2014). Altered regional homogeneity in experimentally induced low back pain: A resting-state fMRI study. J. Neuroeng. Rehabil. 11:115. doi: 10.1186/1743-0003-11-115
Keywords: acupuncture, Hegu, LI 4, ALE, neuroimaging
Citation: Gao Z, Cui M, Zhang J and Ji L (2022) Activation likelihood estimation identifies brain regions activated during puncturing at Hegu in healthy volunteers: A meta-analysis. Front. Neurosci. 16:1084362. doi: 10.3389/fnins.2022.1084362
Received: 30 October 2022; Accepted: 29 November 2022;
Published: 22 December 2022.
Edited by:
Liang-Xiao Ma, Beijing University of Chinese Medicine, ChinaReviewed by:
Anfeng Xiang, China Three Gorges University, ChinaCopyright © 2022 Gao, Cui, Zhang and Ji. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Laixi Ji, dHlqaWxhaXhpQDEyNi5jb20=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.