
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Neurosci., 08 December 2022
Sec. Neurodegeneration
Volume 16 - 2022 | https://doi.org/10.3389/fnins.2022.1060556
The rapid aging of populations around the world has become an unprecedented challenge. Aging is associated with cognitive impairment, including dementia and mild cognitive impairment. Successful drug development for improving or maintaining cognition in the elderly is critically important. Although 4 drugs for improving cognition in Alzheimer’s disease have been approved, a variety of potential drugs targeting age-related cognitive impairment are still in development. In addition, non-pharmacological interventions, including cognition-oriented treatments, non-invasive brain stimulation physical exercise, and lifestyle-related interventions, have also been suggested as cognitive enhancers in the last decade. In this paper, we reviewed the recent evidence of pharmacological and non-pharmacological interventions aimed at improving or maintaining cognition in the elderly.
The world’s population is aging. The population aged 65 and above reached 398 million in 2020, about 9.3% in the total population (United Nations, 2019), and will reach 2 billion by 2050 (Clegg et al., 2013). Increased age is associated with cognitive impairment, including dementia and mild cognitive impairment (MCI). Dementia is characterized by the decline of memory, thinking, perception, language, decision-making, planning, and reasoning (Power et al., 2019). Globally, almost 50 million population are dementia, and the number will increase to 152 million by 2050 (World Health Organization, 2019). Dementia increases with age and is about 5.0% for people between 71 and 79 years old, 24.2% for people between 80 and 89 years old, and 34.7% for people aged 90 years or older (Plassman et al., 2007). Among dementia, Alzheimer’s disease (AD) is the most common type (World Health Organization, 2019). MCI is a stage between normal cognitive aging and dementia (Power et al., 2019), and it is about 10–20% in the population aged 65 years and above according to different diagnostic criteria, and it increases with age (Langa and Levine, 2014). MCI is assumed to be a precursor of dementia; MCI individuals have higher risk of dementia, and about 14.9% MCI elderly will be dementia in 2 years (Morley, 2018; Petersen et al., 2018).
Dementia could cause disability and dependency and results in a huge economic and social burden (Wimo et al., 2017). Therefore, there is a growing need to identify the mechanisms of aging-related cognitive impairment with a perspective on potential targets and explore interventions that can improve or maintain cognition and delay the progression of dementia. In the current study, we reviewed mechanisms of aging and cognitive impairment and summarized evidence of both pharmacological and non-pharmacological interventions and hoped to provide evidence for future studies.
As reported previously (Satoh et al., 2017), aging will result in the decline of cognitive function and alterations in brain structure. It leads to brain atrophy, especially in the hippocampus and prefrontal cortex (Satoh et al., 2017). Brain atrophy is related to age-related neuronal loss, reduced neurogenesis, and reduction in dendritic branching and dendritic spines. Aging also affects synapse density and synaptic function, leading a reduced synaptic transmission and plasticity (Bettio et al., 2017). Numerous factors contribute to structural and functional changes. Aging leads to intracellular accumulation of ROS, causing damage to cellular macromolecules and mitochondria (Castelli et al., 2019). Aging also induces microglia and astrocyte activation, leading to excessive neuroinflammation and consequent neuronal damage (Clarke et al., 2018; Edler et al., 2021). Amyloid-β deposition and tau aggregation have also been found in the aging brain (Zhang et al., 2021). Neurotrophins are associated with neurogenesis, neuronal survival, and synaptic plasticity (Sardar et al., 2021). Disruption of brain-derived neurotrophic factor (BDNF) and vascular endothelial-derived growth factor (VEGF) signaling in the aging brain was also associated with cognitive deficits (Bettio et al., 2017). Another factor to consider about the aging brain and impaired cognition is the decreased level of neurotransmitters, such as dopamine and serotonin (Peters, 2006). Aging contributes to cognitive decline, resulting in healthy individuals to neurodegenerative diseases, particularly AD. An increasing number of studies suggest normal aging and neurodegenerative disorders share similar functional, structural, and cellular changes. However, the structural brain changes are much more significant in neurodegenerative disorders (Yankner et al., 2008; Bettio et al., 2017).
Successful drug development for improving or maintaining cognition in seniors is critically important. Although many novel targets are being explored for improving cognition in the past two decades, there are only several drugs approved to improve cognition in AD and, no drug has been approved for cognitive protection in MCI patients (Fink et al., 2018; Petersen et al., 2018). Here, we introduced the clinical evidence on the approved drugs and promising drug targets (Table 1).
Acetylcholine is associated with memory, learning, attention, and other essential aspects of cognition (Hampel et al., 2018). Aging and AD are associated with cholinergic neuron loss (Terry and Buccafusco, 2003; Haam and Yakel, 2017). Cholinesterase inhibitors (ChEIs) block acetylcholinesterase, thereby delaying the breakdown of acetylcholine and enhancing cholinergic neurotransmission. ChEIs, including donepezil, galantamine, and rivastigmine, have been approved for treating AD (Oh and Rabins, 2019). Donepezil is a non-competitive acetylcholinesterase inhibitor that was approved in 1996. Donepezil administration for 12 or 24 weeks has a small beneficial improvement on cognition in AD. The beneficial effects of 10 or 23 mg daily are slightly larger than 5 mg daily. However, the rates of withdrawal and adverse events are higher with higher doses (Birks and Harvey, 2018). Rivastigmine can inhibit both acetylcholinesterase and butyrylcholinesterase and was approved in 2000. Rivastigmine could also improve cognitive function in AD (Birks and Grimley Evans, 2015). Galantamine is a competitive inhibitor of acetylcholinesterase. Administration with 16–40 mg galantamine daily for 8–28 weeks can significantly enhance cognitive function (Jiang et al., 2015). Although there are slight variations in the mode of actions, there are no differences in their efficacy for cognitive improvement in AD (Birks, 2006). A network meta-analysis assessed the efficacy of cholinesterase inhibitor for vascular dementia and other vascular cognitive impairments, donepezil, and galantamine were found to slightly improve cognition, although the effects were not clinically significant. The evidence for rivastigmine is less certain (Battle et al., 2021). A meta-analysis evaluating the clinical benefit of ChEIs for MCI has been published in 2019 by Matsunaga et al. (2019). That study included 14 randomized controlled trials (RCTs) with 5,278 subjects, where 6 studies involved donepezil, 4 involved galantamine, and 4 involved rivastigmine. The results found that ChEIs did not improve cognitive function in MCI adults, however, ChEIs significantly slowed down the progression of dementia. As there are higher incidences of discontinuation due to adverse events, including nausea, vomiting, abnormal dreams, diarrhea, dizziness, bradyarrhythmia, syncope, and weight loss, ChEIs are not recommended for improving MCI (Matsunaga et al., 2019; Oh and Rabins, 2019). New versions of ChEIs such as octohydroaminoacridine and AD-35 are in development (Cummings et al., 2021).
Besides cholinesterase inhibitors, memantine, a low-affinity NMDA receptor antagonist, has also been approved for treating AD (Huang and Mucke, 2012). In a Cochrane review, the clinical benefit of memantine was found in moderate-to-severe AD. However, there was no clinical benefit of memantine for mild AD. Whether a long duration of memantine administration is beneficial for mild AD needs to be assessed. There is limited evidence of memantine for MCI and other causes of dementia (Ferris et al., 2007; McShane et al., 2019). Currently, SAGE-718, another NMDA receptor positive allosteric modulator, is in Phase II clinical trial (Cummings et al., 2021).
Diabetes is another risk factor for dementia, and diabetes increase a 1.5–2 times higher risk of dementia (Areosa Sastre et al., 2017). Association between aging, dementia with disruption of insulin receptor signaling has been reported, insulin resistance has also been proposed as a mechanism for cognitive impairment. Restoration of insulin signaling in the brain can be a potential way to improve cognition (Boccardi et al., 2019; Erichsen et al., 2021).
Intranasal administration of insulin can increase insulin levels in the central nervous system (de la Monte, 2013; Erichsen et al., 2021). Previous studies demonstrated that intranasal insulin had potentially beneficial effects on cognitive functions (Reger et al., 2006, 2008a,b). Acute intranasal insulin (20 IU) treatment facilitates verbal memory in the elderly with MCI or AD, and these effects were stronger for patients without the APOE-epsilon4 allele (Reger et al., 2006, 2008a,b). Craft et al. (2012) reported chronic intranasal insulin therapy could preserve general cognition and improve delayed memory in MCI and AD patients. These findings indicate the potential beneficial effect of insulin on cognitive decline.
Metformin is a first-line antihyperglycemic drug and works by increasing insulin sensitivity in peripheral tissues and suppressing hepatic gluconeogenesis (Boccardi et al., 2019). Although a meta-analysis of 6 cohort studies has shown metformin might reduce the incidence of dementia in diabetic patients (Campbell et al., 2018), the evidence of metformin usage in non-diabetic adults is still limited. In a pilot randomized controlled trial with 80 MCI adults, metformin was found to increase verbal memory. Metformin was tolerated by 92.5% of participants, and no serious adverse events occurred (Luchsinger et al., 2016). Another study found that metformin administration for 8 weeks was associated with improved executive functioning in patients with MCI or mild dementia (Koenig et al., 2017), which revealed the potential beneficial effect of metformin in the elderly.
Cerebrolysin is a mixture of neuropeptide and amino acids produced by the enzymatic breakdown of pig’s brain tissue, which acts as endogenous neurotrophic factors (Cui et al., 2019). Currently, cerebrolysin is used as the treatment for dementia in Europe and Asia (Cui et al., 2019; Gavrilova and Alvarez, 2021). In a meta-analysis, cerebrolysin was suggested to have beneficial effects on cognitive function in mild-to-moderate AD (Gauthier et al., 2015). The beneficial effect of cerebrolysin on cognitive function was also found in vascular dementia (Guekht et al., 2011). Alvarez et al. (2011) compared cerebrolysin and donepezil, and found that cerebrolysin was as effective as donepezil in cognitive improvement, and the combinational therapy had better cognitive performance than single-drug treatment. In addition, whether cerebrolysin administration has a beneficial effect on cognitive function in cognitive healthy aging adults and in patients with MCI is still inconclusive, and whether cerebrolysin administration can delay the progression of dementia also needs to be further analyzed.
Drug development for AD has been challenging for the last two decades, although a variety of potential drug targets have been identified (Cummings, 2021; van Bokhoven et al., 2021). No cognitive enhancing agent for AD has been recently approved for cognitive improvement in AD (Cummings, 2021; van Bokhoven et al., 2021). Table 2 shows the 13 drugs in clinical trials for the treatment of cognitive. Among them, one drug is in phase I trial, 6 drugs are in phase II trial, and 6 drugs are in phase III trial (Cummings et al., 2021).
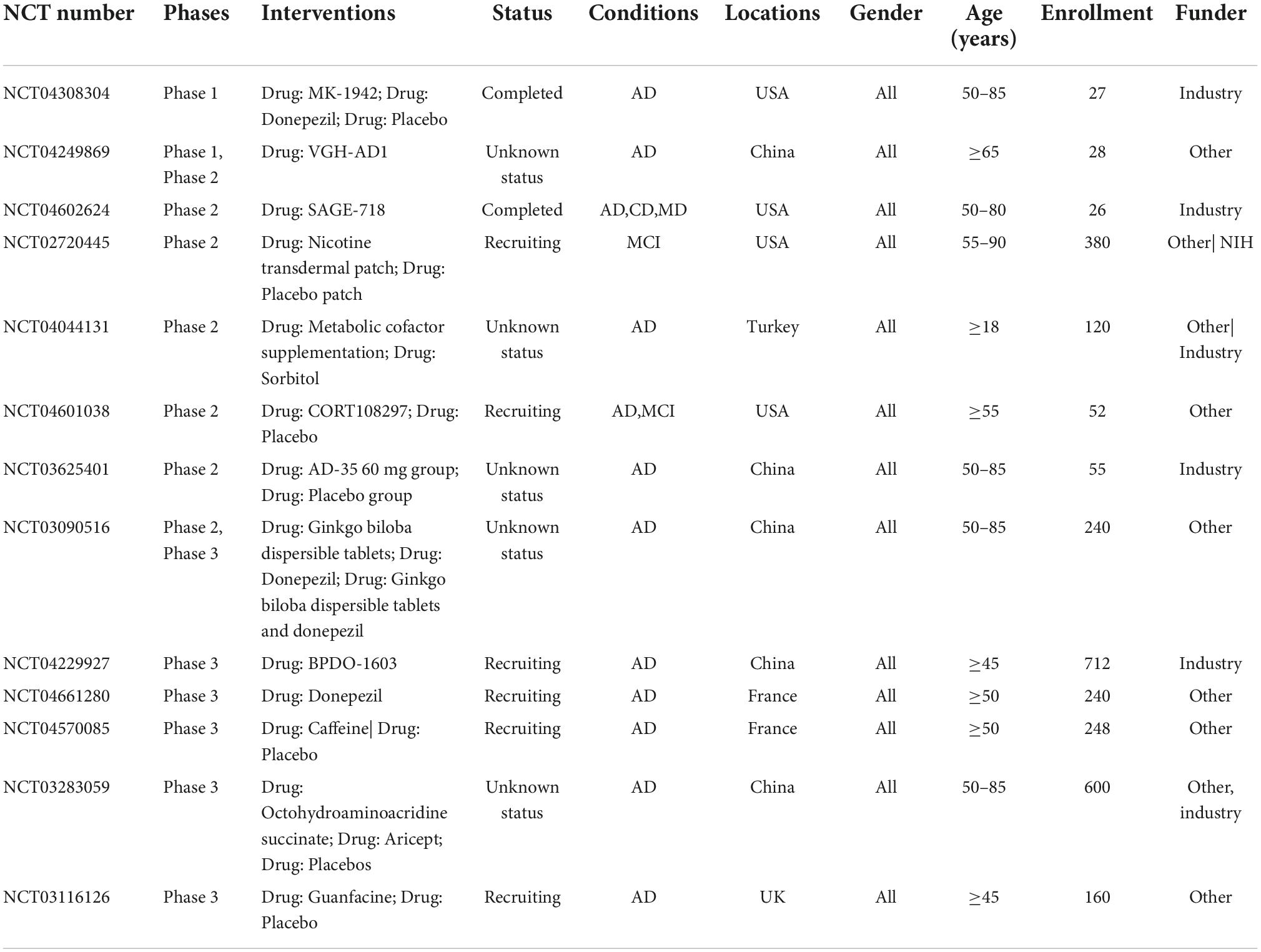
Table 2. Clinical trials target cognitive enhancement for Alzheimer’s disease (AD) patients in 2021 in Clinicaltrials.gov.
Phosphodiesterases (PDEs) are a superfamily (including 11 isoforms) that can catalyze second messengers cAMP and cGMP which have important roles in learning and memory (Wu et al., 2018). Almost all PDE isoforms are mostly expressed in the brain, especially in learning and memory regions (Wu et al., 2018). PDEs inhibitors showed remarkable cognitive enhancement in preclinical studies (Bruno et al., 2011; Peters et al., 2014; Zhang et al., 2018), and some PDE inhibitors have been tested in clinical trials. Most showed good safety and tolerability in phase I trial, and over 10 are in phase II/III/IV trials. Vinpocetine is a classical PDE1 inhibitor and was discovered about 40 years ago (Prickaerts et al., 2017). Though vinpocetine was found effect in improving learning and memory in preclinical study, vinpocetine didn’t show beneficial effect on cognition in AD patients (Szatmari and Whitehouse, 2003). Cilostazol is a selective PDE3 inhibitor. Cilostazol coadministration with donepezil or galantamine ameliorated cognitive decline efficiently in patients with moderate AD (Arai and Takahashi, 2009; Hishikawa et al., 2017). A cohort study recruiting 9148 participants found cilostazol treatment reduced the risk of developing dementia (Tai et al., 2017). Roflumilast is a selective PDE4 Inhibitor. Roflumilast has completed the phase II trial as a cognition enhancer in healthy adults (Van Duinen et al., 2018). PF-04447943 is a selective PDE9A inhibitor. Although PF-04447943 was safe and well-tolerated, PF-04447943 administration did not affect cognition when compared to the placebo (Schwam et al., 2014).
Besides ChEIs, mAChRs agonists and nAChRs agonists are being tested as the other two promising drug targets for cognitive improvement in AD. Xanomeline, the first generation mAChRs agonist, has been demonstrated to improve cognition in a phase III trial. However, due to the relatively low M1 receptor selectivity, xanomeline can lead to cholinergic adverse effects such as sweating, salivation, and gastrointestinal disturbances because of activating peripheral M2 and M3 mAChRs (Scarpa et al., 2020). Unfortunately, later developed highly selective M1 mAChRs agonists, such as PF-06767832, MK-7622, and PF-06764427, lead to cholinergic toxicity and behavioral convulsions (Davoren et al., 2016; Voss et al., 2018). nAChR agonists are less developed than mAChR agonists. There are several nAChR agonists developed for the cognitive improvement of AD patients in the past two decades, such as encenicline (EVP-6124) and TC-1734 (AZD-3480). Encenicline is well tolerated at single doses in healthy volunteers. However, it was later suspended in the phase III trial due to serious gastrointestinal adverse effects in elderly patients (Hoskin et al., 2019). TC-1734 is a selective α4β2 nAChR agonist (Dunbar et al., 2011). A randomized placebo-controlled trial investigated its effects on cognition. Compared to the placebo group, patients in TC-1734 (50 mg) groups showed superior performance on attention and episodic memory (Dunbar et al., 2011). In addition, a phase II trial analyzed its effect on cognitive function in MCI adults (Cummings et al., 2021). mAChRs agonist and nAChRs agonist could be promising treatments. However, there is still a long way to go before advancing them into the market (Verma et al., 2018).
Decreased release of dopamine and decreased expression of dopamine receptors are associated with age-related cognitive decline (Volkow et al., 2000). There is evidence of the involvement of dopamine in AD. Restoration of dopamine transmission can improve learning and memory of AD (Guzmán-Ramos et al., 2012; Martorana and Koch, 2014). In a trial of 60 participants, dopaminergic agonist piribedil administration improves global cognitive function in MCI patients (Nagaraja and Jayashree, 2001). Koch et al. (2020) investigated the effect of the dopaminergic agonist rotigotine on cognitive functions in mild to moderate AD, and found rotigotine did not affect global cognition; however, it could improve cognition associated with the frontal lobe. Monoamine oxidase B (MAO-B) inhibition could increase the availability of dopamine (Koch et al., 2020). In a phase II trial, Matthews et al. evaluated the potential benefit of rasagiline (a selective MAO-B inhibitor) in mild to moderate AD with 50 participants. The results showed rasagiline could improve brain metabolism as measured by fluorodeoxyglucose–positron emission tomography. However, it did not affect globe cognitive function (Matthews et al., 2021). Larger sample size trials are needed to evaluate the effect of dopaminergic stimulation on cognitive impairment.
There were also drugs enhancing cognition in health adults in elderly, potential drugs including substances acting on neurotransmitters, hormones, transduction systems, and brain perfusion and metabolism (Milić et al., 2021). Stimulants such as amphetamine and methylphenidate were reported to improve executive function and memory in healthy adult (Smith and Farah, 2011; Ilieva et al., 2015). Modafinil is an FDA-approved eugeroic that could preserve alertness under conditions of sleep deprivation, through increases cortical catecholamine levels. Most studies found modafinil could enhances executive function, attention and learning and memory (Battleday and Brem, 2015; Farah, 2015). However, their cognitive enhancing effect and safety on elder adults are still needed investigation. What’s more, these drugs are often misused and have abuse potential, which should be paid with more caution (Buccafusco, 2009; Milić et al., 2021).
A growing number of studies show that non-pharmacological interventions can enhance cognition in the last decade (Gavelin et al., 2020; Sikkes et al., 2021). Non-pharmacological interventions covered a diverse range of intervention categories, including cognition-oriented treatments, non-invasive brain stimulation physical exercise, and lifestyle-related interventions (Table 3). Different clinical stages of cognitive impairment, from MCI to dementia, could all benefit from non-pharmacological treatments. Most non-pharmacological treatments have few adverse effects and can be combined with pharmacological treatments (Sikkes et al., 2021).
Cognition-oriented interventions, such as cognitive training, cognitive stimulation, and cognitive rehabilitation, are approaches for the prevention and treatment of cognitive decline in the elderly (Gavelin et al., 2020). They are in high availability, high accessibility, and low implementation costs. Cognitive training consists of repeated practices on standardized tasks aimed at improving or maintaining certain aspects of cognitive functions (Bahar-Fuchs et al., 2019). The difficulty of training tasks should be adjusted according to participants’ performance, which is particularly feasible when using computerized cognitive training. Cognitive stimulation involves non-specific engagement in activities for improving cognitive status (Bahar-Fuchs et al., 2019). Cognitive rehabilitation could achieve or preserve optimal levels of functioning in daily life (Gavelin et al., 2020). Cognitive training has also been reported to have a small to moderate effect on global cognition and a moderate effect on verbal semantic fluency for mild to moderate dementia (Bahar-Fuchs et al., 2019). Gates et al. evaluated the effect of computerized cognitive training through a meta-analysis. However, no conclusion could be drawn on whether computerized cognitive training had a beneficial effect on cognitive function as the evidence was of low quality, and most of the results were imprecise (Gates et al., 2019). Hu et al. included both MCI and dementia patients with computerized cognitive training in a systematic review in 2021. They included 12 studies and found computerized cognitive training could improve general cognition, especially memory. Subgroup analysis found computerized cognitive training on cognition for dementia was almost double for MCI (Hu et al., 2021). Cognitive stimulation was also associated with improved cognitive function, self-reported quality of life, and communication and social interactions in dementia people (Woods et al., 2012). Although cognition-oriented treatments showed a positive effect on cognitive function, high-quality with larger sample size trials are needed, and further studies should be performed to address the potential benefits of longer-term interventions and their clinical significance.
As for non-invasive brain stimulation, transcranial electrical stimulation (TES) and transcranial magnetic stimulation (TMS) are the main techniques. Both techniques are safe and can be well tolerated without sedation or anesthesia (Brunoni et al., 2019). They both work by modulating synaptic efficacy and neural circuit and have been used in clinical practice.
TES applicant a low-intensity (1–2 mA) electric current to the brain via two electrodes (anode and cathode), and transcranial direct current stimulation (tDCS) is the most studied. The effects of tDCS are determined by the electrical current direction. Anodal tDCS increases neuronal activities by depolarizing the resting potential, while the cathodal tDCS inhibits neuronal activities by hyperpolarizing the resting potential (Grimaldi et al., 2020). Cai et al. evaluated the effects of tDCS on cognition within mild to moderate AD patients through a meta-analysis. The results revealed that tDCS could enhance cognitive function; in addition, only a single session of tDCS was effective, repeated sessions of tDCS were not effective, and lower current density (0.06 mA/cm2) but not higher current density (0.08 mA/cm2) enhanced cognition (Cai et al., 2019). Recently, Chu et al. analyzed the cognitive effects of TES on AD and MCI. After a 1-month follow-up, cathodal tDCS revealed larger therapeutic responses than anodal tDCS on general cognitive function. Subgroup analysis only found patients with AD, but not MCI, significantly responded to cathodal tDCS (Chu et al., 2021).
Transcranial magnetic stimulation uses a magnetic field to induce action potentials. The effects of TMS are determined by stimulation frequency. When the frequency is equal to or below 1 Hz, neural excitability is decreased. When the frequency is between 5 and 20 Hz, neural excitability is increased (Cespón et al., 2018). TMS can use different stimulation patterns, including single-pulse TMS (sTMS), double (or paired) pulse TMS (dTMS), and repetitive TMS (rTMS). sTMS consists of the discharge of single pulses interleaved by at least 4 s periods off-stimulation, dTMS consists of the discharge of a test stimulus preceded by a conditioning stimulus, rTMS refers to more than two pulses delivered within a time interval of 2 s or less (Valero-Cabré et al., 2017). rTMS has been widely investigated in depression, and it has been approved by FDA for medication-resistant depression (Iriarte and George, 2018). In recent years, rTMS has been considered as a promising intervention for cognitive improvement (Iriarte and George, 2018). Two systematic reviews reported high frequency rTMS might show a moderate effect on cognition in AD and MCI patients (Cheng et al., 2018; Xie et al., 2021). However, the conclusion was limited by the small sample size of included studies. Larger RCTs and additional research are needed to identify the effect of TMS in the elderly with cognitive impairment.
Nutrition is an important factor that contributes to healthy aging. Adopting a healthier diet may be beneficial to cognition (Jennings et al., 2020). World Health Organization (2019) has advocated a healthy diet to reduce the risk of cognitive decline and/or dementia. Some, but not conclusive, evidence suggests that certain nutrients are protective of brain health in the elderly, including long-chain omega-3 fatty acids, vitamin B, vitamin D, selenium and etc. (Scarmeas et al., 2018). Dietary patterns were also suggested to be protective for brain health in elderly (Scarmeas et al., 2018; Power et al., 2019; Flanagan et al., 2020). The Mediterranean diet was the most extensively studied dietary pattern (Chen et al., 2019). It involves a high intake of vegetables, fruits, legumes, olive oil, whole grains, fish, low to moderate intake of dairy products, alcohol, and restrictions on red meat (Power et al., 2019). High adherence to the Mediterranean diet is associated with better global cognition and memory has been reported by meta-analysis (Coelho-Júnior et al., 2021). However, whether it could reduce the risk of developing MCI or dementia is still conflicted (Coelho-Júnior et al., 2021; García-Casares et al., 2021). The ketogenic diet was another specific diet, which might provide treatment benefits for AD patients. However, the current studies might be limited by small sample size, short-terms effects, and future studies should be further performed (Hersant and Grossberg, 2022). The dietary intervention could be considered alongside other individualized interventions to improve cognition in elderly adults.
Interaction between gut microbes and the brain has received considerable attention in the past decade (Martin et al., 2018; Willyard, 2021). Gut microbiota is found to be associated with emotion, cognition, and social behavior (Sarkar et al., 2018). Probiotic intervention works by delivering specific strains of bacteria that increase the diversity and number of beneficial microbes, thereby altering the gut microbiota (Eastwood et al., 2021). Lv et al. evaluated the probiotics on cognition by meta-analysis, and they found that probiotic supplementations improved cognitive function. Subgroup analyses further found the enhanced effect existed only in people with impaired cognition. Furthermore, a single strain was more effective than multiple strains (Lv et al., 2021). Thus, probiotics have been suggested as an effective and accessible cognitive therapy; however, more randomized controlled clinical trials are needed for this conclusion.
Emerging evidence indicates exercise not only promotes physical health but also contributes to the preservation of cognition function. The mechanisms account for the neuroprotective effects of exercise on the brain include evaluated neurotrophic factor levels, increased synaptogenesis, improved vascularization, decreased systemic inflammation, and reduced abnormal protein deposition (Kirk-Sanchez and McGough, 2014). Several meta-analyses analyzed the effects of exercise on cognition, focused predominantly on aerobic exercise (Jia et al., 2019; Sanders et al., 2019; López-Ortiz et al., 2021). Angevaren et al. (2008) performed a Cochrane review in older people without known cognitive impairment. They found that aerobic exercise increased cognitive capacity, including motor function, cognitive speed, and visual attention. Another meta-analysis reported aerobic exercise attenuated the cognitive decline in MCI and dementia people, and found that working memory decline was significantly attenuated, and the effects on other domains of cognitive functions were unclear. Moderate to high-intensity aerobic exercise had a better effect on cognition (Law et al., 2020). Another meta-analysis examined the dose-response relationship and found shorter sessions and higher frequencies of exercise could generate a better cognitive effect (Sanders et al., 2019).
Dancing intervention is another strategy, because it requires physical, cognitive, and social abilities, and thus been analyzed in many studies. In a recent meta-analysis in MCI populations, the results showed that dance had a small to moderate effect on cognitive function, such as attention, immediate and delayed recall, global cognition, and visuospatial ability (Chan et al., 2020). Another meta-analysis involving both healthy and MCI old adults also found dance enhanced global cognitive function and executive function (Hewston et al., 2021). The positive effect of dance intervention on cognitive function in adults with AD was also confirmed in a systematic review (Ruiz-Muelle and López-Rodríguez, 2019). Thus, dance has been suggested as an adjunct therapy for cognitive decline in the aging population.
As cognitive impairment is a complex, multifactorial disorder, multi-domain interventions have been suggested as a new strategy (Kivipelto et al., 2018). In the last decades, 3 large clinical trials with multi-domain interventions (FINGER, MAPT, and PreDIVA) have been reported. In the FINGER study, diet, exercise, cognitive training, and vascular risk monitoring were used to improve cognitive function in elderly people at risk for cognitive impairment (Ngandu et al., 2015). In the preDIVA study, a multi-domain intervention targeted vascular risk factors of smoking, unhealthy diet, physical inactivity, overweight, hypertension, dyslipidemia, and diabetes over 12 years was used. However, it did not reduce dementia risk in older people (Hoevenaar-Blom et al., 2021). In the MAPT trial, multi-domain intervention and Omega-3 PUFA supplementation were involved, and the results did not find significant effects on cognitive function (Andrieu et al., 2017). Despite these trials, several meta-analyses also analyzed multi-domain interventions on cognitive impairment. A Cochrane review found a small improvement in cognitive function with multi-domain interventions. However, whether multi-domain interventions could decrease dementia incidences was uncertain (Hafdi et al., 2021). Gavelin et al. (2021) reported that combined physical and cognitive training had a small beneficial effect on overall cognitive function in elder adults. Nutrition combined with physical exercise interventions could also improve global cognitive function in the elderly population (Liu et al., 2021).
In conclusion, various pharmacological (cholinesterase inhibitors, memantine, antidiabetic agents, probiotics, cerebrolysin) and non-pharmacological interventions (cognition-oriented treatments, non-invasive brain stimulation physical exercise, and lifestyle-related interventions) have been proposed for cognitive impairment in older people. Although a variety of new drug targets has been identified for cognition enhancement in older adults, the new drug is still in development. The existing potential drug targets should be further exploited, and discovering new drug targets could be a solution to the lack of effective drugs. Most non-pharmacological interventions showed a small to moderate beneficial effect on cognitive function in cognitive impairment old people. Thus, combinations of pharmacological and non-pharmacological interventions or combinations of different types of non-pharmacological interventions may be more efficient in improving or preserving cognition.
YZ designed and edited the review. LC searched the data and drafted the review. JJ searched the data. All authors contributed to the article and approved the submitted version.
This study was partly supported by the National Natural Science Foundation of China (No: 82001130), the Post Doctor Research Project of West China Hospital of Sichuan University (No: 19HXBH071), the China Postdoctoral Science Foundation (No: 2021M692274), and the Postdoctoral Research Project of Sichuan University (No: 2021SCU12001).
We thank American Journal Experts for improving the grammar and readability.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Alvarez, X. A., Cacabelos, R., Sampedro, C., Couceiro, V., Aleixandre, M., Vargas, M., et al. (2011). Combination treatment in Alzheimer’s disease: Results of a randomized, controlled trial with cerebrolysin and donepezil. Curr. Alzheimer Res. 8, 583–591. doi: 10.2174/156720511796391863
Andrieu, S., Guyonnet, S., Coley, N., Cantet, C., Bonnefoy, M., Bordes, S., et al. (2017). Effect of long-term omega 3 polyunsaturated fatty acid supplementation with or without multidomain intervention on cognitive function in elderly adults with memory complaints (MAPT): A randomised, placebo-controlled trial. Lancet Neurol. 16, 377–389. doi: 10.1016/S1474-4422(17)30040-6
Angevaren, M., Aufdemkampe, G., Verhaar, H. J., Aleman, A., and Vanhees, L. (2008). Physical activity and enhanced fitness to improve cognitive function in older people without known cognitive impairment. Cochrane Database Syst. Rev. Cd005381. doi: 10.1002/14651858.CD005381.pub3
Arai, H., and Takahashi, T. (2009). A combination therapy of donepezil and cilostazol for patients with moderate Alzheimer disease: Pilot follow-up study. Am. J. Geriatr. Psychiatry 17, 353–354. doi: 10.1097/JGP.0b013e31819431ea
Areosa Sastre, A., Vernooij, R. W., González-Colaço Harmand, M., and Martínez, G. (2017). Effect of the treatment of Type 2 diabetes mellitus on the development of cognitive impairment and dementia. Cochrane Database Syst. Rev. 6:Cd003804. doi: 10.1002/14651858.CD003804.pub2
Bahar-Fuchs, A., Martyr, A., Goh, A. M., Sabates, J., and Clare, L. (2019). Cognitive training for people with mild to moderate dementia. Cochrane Database Syst. Rev. 3:Cd013069. doi: 10.1002/14651858.CD013069.pub2
Battle, C. E., Abdul-Rahim, A. H., Shenkin, S. D., Hewitt, J., and Quinn, T. J. (2021). Cholinesterase inhibitors for vascular dementia and other vascular cognitive impairments: A network meta-analysis. Cochrane Database Syst. Rev. 2:Cd013306. doi: 10.1002/14651858.CD013306.pub2
Battleday, R. M., and Brem, A. K. (2015). Modafinil for cognitive neuroenhancement in healthy non-sleep-deprived subjects: A systematic review. Eur. Neuropsychopharmacol. 25, 1865–1881. doi: 10.1016/j.euroneuro.2015.07.028
Bettio, L. E. B., Rajendran, L., and Gil-Mohapel, J. (2017). The effects of aging in the hippocampus and cognitive decline. Neurosci. Biobehav. Rev. 79, 66–86. doi: 10.1016/j.neubiorev.2017.04.030
Birks, J. (2006). Cholinesterase inhibitors for Alzheimer’s disease. Cochrane Database Syst. Rev. 2006:Cd005593. doi: 10.1002/14651858.CD005593
Birks, J. S., and Grimley Evans, J. (2015). Rivastigmine for Alzheimer’s disease. Cochrane Database Syst. Rev. Cd001191. doi: 10.1002/14651858.CD001191.pub3
Birks, J. S., and Harvey, R. J. (2018). Donepezil for dementia due to Alzheimer’s disease. Cochrane Database Syst. Rev. 6:Cd001190. doi: 10.1002/14651858.CD001190.pub3
Boccardi, V., Murasecco, I., and Mecocci, P. (2019). Diabetes drugs in the fight against Alzheimer’s disease. Ageing Res Rev. 54:100936. doi: 10.1016/j.arr.2019.100936
Bruno, O., Fedele, E., Prickaerts, J., Parker, L. A., Canepa, E., Brullo, C., et al. (2011). GEBR-7b, a novel PDE4D selective inhibitor that improves memory in rodents at non-emetic doses. Br. J. Pharmacol. 164, 2054–2063. doi: 10.1111/j.1476-5381.2011.01524.x
Brunoni, A. R., Sampaio-Junior, B., Moffa, A. H., Aparício, L. V., Gordon, P., Klein, I., et al. (2019). Noninvasive brain stimulation in psychiatric disorders: A primer. Braz. J. Psychiatry 41, 70–81. doi: 10.1590/1516-4446-2017-0018
Buccafusco, J. J. (2009). Emerging cognitive enhancing drugs. Expert. Opin. Emerg. Drugs 14, 577–589. doi: 10.1517/14728210903257796
Cai, M., Guo, Z., Xing, G., Peng, H., Zhou, L., Chen, H., et al. (2019). Transcranial direct current stimulation improves cognitive function in mild to moderate Alzheimer disease: A meta-analysis. Alzheimer Dis. Assoc. Disord. 33, 170–178. doi: 10.1097/WAD.0000000000000304
Campbell, J. M., Stephenson, M. D., de Courten, B., Chapman, I., Bellman, S. M., and Aromataris, E. (2018). Metformin use associated with reduced risk of dementia in patients with diabetes: A systematic review and meta-analysis. J. Alzheimers Dis. 65, 1225–1236. doi: 10.3233/JAD-180263
Castelli, V., Benedetti, E., Antonosante, A., Catanesi, M., Pitari, G., Ippoliti, R., et al. (2019). Neuronal cells rearrangement during aging and neurodegenerative disease: Metabolism, oxidative stress and organelles dynamic. Front. Mol. Neurosci. 12:132. doi: 10.3389/fnmol.2019.00132
Cespón, J., Miniussi, C., and Pellicciari, M. C. (2018). Interventional programmes to improve cognition during healthy and pathological ageing: Cortical modulations and evidence for brain plasticity. Ageing Res. Rev. 43, 81–98. doi: 10.1016/j.arr.2018.03.001
Chan, J. S. Y., Wu, J., Deng, K., and Yan, J. H. (2020). The effectiveness of dance interventions on cognition in patients with mild cognitive impairment: A meta-analysis of randomized controlled trials. Neurosci. Biobehav. Rev. 118, 80–88. doi: 10.1016/j.neubiorev.2020.07.017
Chen, X., Maguire, B., Brodaty, H., and O’Leary, F. (2019). Dietary patterns and cognitive health in older adults: A systematic review. J. Alzheimers Dis. 67, 583–619. doi: 10.3233/JAD-180468
Cheng, C. P. W., Wong, C. S. M., Lee, K. K., Chan, A. P. K., Yeung, J. W. F., and Chan, W. C. (2018). Effects of repetitive transcranial magnetic stimulation on improvement of cognition in elderly patients with cognitive impairment: A systematic review and meta-analysis. Int. J. Geriatr. Psychiatry 33, e1–e13. doi: 10.1002/gps.4726
Chu, C. S., Li, C. T., Brunoni, A. R., Yang, F. C., Tseng, P. T., Tu, Y. K., et al. (2021). Cognitive effects and acceptability of non-invasive brain stimulation on Alzheimer’s disease and mild cognitive impairment: A component network meta-analysis. J. Neurol. Neurosurg. Psychiatry 92, 195–203. doi: 10.1136/jnnp-2020-323870
Clarke, L. E., Liddelow, S. A., Chakraborty, C., Münch, A. E., Heiman, M., and Barres, B. A. (2018). Normal aging induces A1-like astrocyte reactivity. Proc. Natl. Acad. Sci. U.S.A. 115, E1896–E1905. doi: 10.1073/pnas.1800165115
Clegg, A., Young, J., Iliffe, S., Rikkert, M. O., and Rockwood, K. (2013). Frailty in elderly people. Lancet 381, 752–762. doi: 10.1016/S0140-6736(12)62167-9
Coelho-Júnior, H. J., Trichopoulou, A., and Panza, F. (2021). Cross-sectional and longitudinal associations between adherence to Mediterranean diet with physical performance and cognitive function in older adults: A systematic review and meta-analysis. Ageing Res Rev. 70:101395. doi: 10.1016/j.arr.2021.101395
Craft, S., Baker, L. D., Montine, T. J., Minoshima, S., Watson, G. S., Claxton, A., et al. (2012). Intranasal insulin therapy for Alzheimer disease and amnestic mild cognitive impairment: A pilot clinical trial. Arch. Neurol. 69, 29–38. doi: 10.1001/archneurol.2011.233
Cui, S., Chen, N., Yang, M., Guo, J., Zhou, M., Zhu, C., et al. (2019). Cerebrolysin for vascular dementia. Cochrane Database Syst. Rev. 2019:CD008900. doi: 10.1002/14651858.CD008900.pub3
Cummings, J. (2021). New approaches to symptomatic treatments for Alzheimer’s disease. Mol. Neurodegener. 16:2. doi: 10.1186/s13024-021-00424-9
Cummings, J., Lee, G., Zhong, K., Fonseca, J., and Taghva, K. (2021). Alzheimer’s disease drug development pipeline: 2021. Alzheimers Dement. 7:e12179. doi: 10.1002/trc2.12179
Davoren, J. E., Lee, C. W., Garnsey, M., Brodney, M. A., Cordes, J., Dlugolenski, K., et al. (2016). Discovery of the Potent and Selective M1 PAM-Agonist N-[(3R,4S)-3-Hydroxytetrahydro-2H-pyran-4-yl]-5-methyl-4-[4-(1,3-thiazol-4-yl)benzyl]pyridine-2-carboxamide (PF-06767832): Evaluation of efficacy and cholinergic side effects. J. Med. Chem. 59, 6313–6328. doi: 10.1021/acs.jmedchem.6b00544
de la Monte, S. M. (2013). Intranasal insulin therapy for cognitive impairment and neurodegeneration: Current state of the art. Expert. Opin. Drug Deliv. 10, 1699–1709. doi: 10.1517/17425247.2013.856877
Dunbar, G. C., Kuchibhatla, R. V., and Lee, G. (2011). A randomized double-blind study comparing 25 and 50 mg TC-1734 (AZD3480) with placebo, in older subjects with age-associated memory impairment. J. Psychopharmacol. 25, 1020–1029. doi: 10.1177/0269881110367727
Eastwood, J., Walton, G., Van Hemert, S., Williams, C., and Lamport, D. (2021). The effect of probiotics on cognitive function across the human lifespan: A systematic review. Neurosci. Biobehav. Rev. 128, 311–327. doi: 10.1016/j.neubiorev.2021.06.032
Edler, M. K., Mhatre-Winters, I., and Richardson, J. R. (2021). Microglia in aging and Alzheimer’s disease: A comparative species review. Cells 10:1138. doi: 10.3390/cells10051138
Erichsen, J. M., Calva, C. B., Reagan, L. P., and Fadel, J. R. (2021). Intranasal insulin and orexins to treat age-related cognitive decline. Physiol. Behav. 234:113370. doi: 10.1016/j.physbeh.2021.113370
Farah, M. J. (2015). NEUROSCIENCE. The unknowns of cognitive enhancement. Science 350, 379–380. doi: 10.1126/science.aad5893
Ferris, S., Schneider, L., Farmer, M., Kay, G., and Crook, T. (2007). A double-blind, placebo-controlled trial of memantine in age-associated memory impairment (memantine in AAMI). Int. J. Geriatr. Psychiatry 22, 448–455. doi: 10.1002/gps.1711
Fink, H. A., Jutkowitz, E., McCarten, J. R., Hemmy, L. S., Butler, M., Davila, H., et al. (2018). Pharmacologic interventions to prevent cognitive decline, mild cognitive impairment, and clinical Alzheimer-type dementia: A systematic review. Ann. Intern. Med. 168, 39–51. doi: 10.7326/M17-1529
Flanagan, E., Lamport, D., Brennan, L., Burnet, P., Calabrese, V., Cunnane, S. C., et al. (2020). Nutrition and the ageing brain: Moving towards clinical applications. Ageing Res. Rev. 62, 101079. doi: 10.1016/j.arr.2020.101079
García-Casares, N., Gallego Fuentes, P., Barbancho, M., López-Gigosos, R., García-Rodríguez, A., and Gutiérrez-Bedmar, M. (2021). Alzheimer’s disease, mild cognitive impairment and mediterranean diet. A systematic review and dose-response meta-analysis. J. Clin. Med. 10:4642. doi: 10.3390/jcm10204642
Gates, N. J., Vernooij, R. W., Di Nisio, M., Karim, S., March, E., Martínez, G., et al. (2019). Computerised cognitive training for preventing dementia in people with mild cognitive impairment. Cochrane Database Syst. Rev. 3:Cd012279. doi: 10.1002/14651858.CD012279.pub2
Gauthier, S., Proaño, J. V., Jia, J., Froelich, L., Vester, J. C., and Doppler, E. (2015). Cerebrolysin in mild-to-moderate Alzheimer’s disease: A meta-analysis of randomized controlled clinical trials. Dement. Geriatr. Cogn. Disord. 39, 332–347. doi: 10.1159/000377672
Gavelin, H. M., Dong, C., Minkov, R., Bahar-Fuchs, A., Ellis, K. A., Lautenschlager, N. T., et al. (2021). Combined physical and cognitive training for older adults with and without cognitive impairment: A systematic review and network meta-analysis of randomized controlled trials. Ageing Res. Rev. 66:101232. doi: 10.1016/j.arr.2020.101232
Gavelin, H. M., Lampit, A., Hallock, H., Sabatés, J., and Bahar-Fuchs, A. (2020). Cognition-Oriented treatments for older adults: A systematic overview of systematic reviews. Neuropsychol. Rev. 30, 167–193. doi: 10.1007/s11065-020-09434-8
Gavrilova, S. I., and Alvarez, A. (2021). Cerebrolysin in the therapy of mild cognitive impairment and dementia due to Alzheimer’s disease: 30 years of clinical use. Med. Res. Rev. 41, 2775–2803. doi: 10.1002/med.21722
Grimaldi, D., Papalambros, N. A., Zee, P. C., and Malkani, R. G. (2020). Neurostimulation techniques to enhance sleep and improve cognition in aging. Neurobiol. Dis. 141:104865. doi: 10.1016/j.nbd.2020.104865
Guekht, A. B., Moessler, H., Novak, P. H., and Gusev, E. I. (2011). Cerebrolysin in vascular dementia: Improvement of clinical outcome in a randomized, double-blind, placebo-controlled multicenter trial. J. Stroke Cerebrovasc. Dis. 20, 310–318. doi: 10.1016/j.jstrokecerebrovasdis.2010.01.012
Guzmán-Ramos, K., Moreno-Castilla, P., Castro-Cruz, M., McGaugh, J. L., Martínez-Coria, H., LaFerla, F. M., et al. (2012). Restoration of dopamine release deficits during object recognition memory acquisition attenuates cognitive impairment in a triple transgenic mice model of Alzheimer’s disease. Learn. Mem. 19, 453–460. doi: 10.1101/lm.026070.112
Haam, J., and Yakel, J. L. (2017). Cholinergic modulation of the hippocampal region and memory function. J. Neurochem. 142(Suppl. 2), 111–121. doi: 10.1111/jnc.14052
Hafdi, M., Hoevenaar-Blom, M. P., and Richard, E. (2021). Multi-domain interventions for the prevention of dementia and cognitive decline. Cochrane Database Syst. Rev. 11:Cd013572. doi: 10.1002/14651858.CD013572.pub2
Hampel, H., Mesulam, M. M., Cuello, A. C., Farlow, M. R., Giacobini, E., Grossberg, G. T., et al. (2018). The cholinergic system in the pathophysiology and treatment of Alzheimer’s disease. Brain. 141, 1917–1933. doi: 10.1093/brain/awy132
Hersant, H., and Grossberg, G. (2022). The ketogenic diet and Alzheimer’s disease. J. Nutr. Health Aging. 26, 606–614. doi: 10.1007/s12603-022-1807-7
Hewston, P., Kennedy, C. C., Borhan, S., Merom, D., Santaguida, P., Ioannidis, G., et al. (2021). Effects of dance on cognitive function in older adults: A systematic review and meta-analysis. Age Ageing 50, 1084–1092. doi: 10.1093/ageing/afaa270
Hishikawa, N., Fukui, Y., Sato, K., Ohta, Y., Yamashita, T., and Abe, K. (2017). Comprehensive effects of galantamine and cilostazol combination therapy on patients with Alzheimer’s disease with asymptomatic lacunar infarction. Geriatr. Gerontol. Int. 17, 1384–1391. doi: 10.1111/ggi.12870
Hoevenaar-Blom, M. P., Richard, E., Moll van Charante, E. P., van Wanrooij, L. L., Busschers, W. B., van Dalen, J. W., et al. (2021). Targeting vascular risk factors to reduce dementia incidence in old age: Extended follow-up of the prevention of dementia by intensive vascular care (preDIVA) randomized clinical trial. JAMA Neurol. 78, 1527–1528. doi: 10.1001/jamaneurol.2021.3542
Hoskin, J. L., Al-Hasan, Y., and Sabbagh, M. N. (2019). Nicotinic acetylcholine receptor agonists for the treatment of Alzheimer’s dementia: An update. Nicotine Tob. Res. 21, 370–376. doi: 10.1093/ntr/nty116
Hu, M., Wu, X., Shu, X., Hu, H., Chen, Q., Peng, L., et al. (2021). Effects of computerised cognitive training on cognitive impairment: A meta-analysis. J. Neurol. 268, 1680–1688. doi: 10.1007/s00415-019-09522-7
Huang, Y., and Mucke, L. (2012). Alzheimer mechanisms and therapeutic strategies. Cell 148, 1204–1222. doi: 10.1016/j.cell.2012.02.040
Ilieva, I. P., Hook, C. J., and Farah, M. J. (2015). Prescription stimulants’ effects on healthy inhibitory control, working memory, and episodic memory: A meta-analysis. J. Cogn. Neurosci. 27, 1069–1089. doi: 10.1162/jocn_a_00776
Iriarte, I. G., and George, M. S. (2018). Transcranial magnetic stimulation (TMS) in the elderly. Curr. Psychiatry Rep. 20:6. doi: 10.1007/s11920-018-0866-2
Jennings, A., Cunnane, S. C., and Minihane, A. M. (2020). Can nutrition support healthy cognitive ageing and reduce dementia risk? BMJ 369:m2269. doi: 10.1136/bmj.m2269
Jia, R. X., Liang, J. H., Xu, Y., and Wang, Y. Q. (2019). Effects of physical activity and exercise on the cognitive function of patients with Alzheimer disease: A meta-analysis. BMC Geriatr. 19:181. doi: 10.1186/s12877-019-1175-2
Jiang, D., Yang, X., Li, M., Wang, Y., and Wang, Y. (2015). Efficacy and safety of galantamine treatment for patients with Alzheimer’s disease: A meta-analysis of randomized controlled trials. J. Neural Transm. 122, 1157–1166. doi: 10.1007/s00702-014-1358-0
Kirk-Sanchez, N. J., and McGough, E. L. (2014). Physical exercise and cognitive performance in the elderly: Current perspectives. Clin. Interv. Aging 9, 51–62. doi: 10.2147/CIA.S39506
Kivipelto, M., Mangialasche, F., and Ngandu, T. (2018). Lifestyle interventions to prevent cognitive impairment, dementia and Alzheimer disease. Nat. Rev. Neurol. 14, 653–666. doi: 10.1038/s41582-018-0070-3
Koch, G., Motta, C., Bonnì, S., Pellicciari, M. C., Picazio, S., Casula, E. P., et al. (2020). Effect of Rotigotine vs placebo on cognitive functions among patients with mild to moderate Alzheimer disease: A randomized clinical trial. JAMA Netw. Open 3:e2010372. doi: 10.1001/jamanetworkopen.2020.10372
Koenig, A. M., Mechanic-Hamilton, D., Xie, S. X., Combs, M. F., Cappola, A. R., Xie, L., et al. (2017). Effects of the insulin sensitizer metformin in Alzheimer Disease: Pilot data from a randomized placebo-controlled crossover study. Alzheimer Dis. Assoc. Disord. 31, 107–113. doi: 10.1097/WAD.0000000000000202
Langa, K. M., and Levine, D. A. (2014). The diagnosis and management of mild cognitive impairment: A clinical review. JAMA 312, 2551–2561. doi: 10.1001/jama.2014.13806
Law, C. K., Lam, F. M., Chung, R. C., and Pang, M. Y. (2020). Physical exercise attenuates cognitive decline and reduces behavioural problems in people with mild cognitive impairment and dementia: A systematic review. J. Physiother. 66, 9–18. doi: 10.1016/j.jphys.2019.11.014
Liu, T., Li, N., Hou, Z., Liu, L., Gao, L., Wang, L., et al. (2021). Nutrition and exercise interventions could ameliorate age-related cognitive decline: A meta-analysis of randomized controlled trials. Aging Clin. Exp. Res. 33, 1799–1809. doi: 10.1007/s40520-020-01730-w
López-Ortiz, S., Valenzuela, P. L., Seisdedos, M. M., Morales, J. S., Vega, T., Castillo-García, A., et al. (2021). Exercise interventions in Alzheimer’s disease: A systematic review and meta-analysis of randomized controlled trials. Ageing Res. Rev. 72:101479. doi: 10.1016/j.arr.2021.101479
Luchsinger, J. A., Perez, T., Chang, H., Mehta, P., Steffener, J., Pradabhan, G., et al. (2016). Metformin in amnestic mild cognitive impairment: Results of a pilot randomized placebo controlled clinical trial. J. Alzheimers Dis. 51, 501–514. doi: 10.3233/JAD-150493
Lv, T., Ye, M., Luo, F., Hu, B., Wang, A., Chen, J., et al. (2021). Probiotics treatment improves cognitive impairment in patients and animals: A systematic review and meta-analysis. Neurosci. Biobehav. Rev. 120, 159–172. doi: 10.1016/j.neubiorev.2020.10.027
Martin, C. R., Osadchiy, V., Kalani, A., and Mayer, E. A. (2018). The brain-gut-microbiome axis. Cell. Mol. Gastroenterol. Hepatol. 6, 133–148. doi: 10.1016/j.jcmgh.2018.04.003
Martorana, A., and Koch, G. (2014). Is dopamine involved in Alzheimer’s disease? Front. Aging Neurosci. 6:252. doi: 10.3389/fnagi.2014.00252
Matsunaga, S., Fujishiro, H., and Takechi, H. (2019). Efficacy and safety of cholinesterase inhibitors for mild cognitive impairment: A systematic review and meta-analysis. J. Alzheimers Dis. 71, 513–523. doi: 10.3233/JAD-190546
Matthews, D. C., Ritter, A., Thomas, R. G., Andrews, R. D., Lukic, A. S., Revta, C., et al. (2021). Rasagiline effects on glucose metabolism, cognition, and tau in Alzheimer’s dementia. Alzheimers Dement. 7:e12106.
McShane, R., Westby, M. J., Roberts, E., Minakaran, N., Schneider, L., Farrimond, L. E., et al. (2019). Memantine for dementia. Cochrane Database Syst. Rev. 3:Cd003154. doi: 10.1002/14651858.CD003154.pub6
Milić, J., Zeković, J., Stankić, D., Henèić, B., Janèić, J., and Samardžić, J. (2021). “Chapter 33–Cognition-enhancing drugs and applications to aging,” in Assessments, Treatments and Modeling in Aging and Neurological Disease, eds C. R. Martin, V. R. Preedy, and R. Rajendram (Cambridge, MA: Academic Press), 367–378. doi: 10.1016/B978-0-12-818000-6.00033-0
Morley, J. E. (2018). An overview of cognitive impairment. Clin. Geriatr. Med. 34, 505–513. doi: 10.1016/j.cger.2018.06.003
Nagaraja, D., and Jayashree, S. (2001). Randomized study of the dopamine receptor agonist piribedil in the treatment of mild cognitive impairment. Am. J. Psychiatry 158, 1517–1519. doi: 10.1176/appi.ajp.158.9.1517
Ngandu, T., Lehtisalo, J., Solomon, A., Levälahti, E., Ahtiluoto, S., Antikainen, R., et al. (2015). A 2 year multidomain intervention of diet, exercise, cognitive training, and vascular risk monitoring versus control to prevent cognitive decline in at-risk elderly people (FINGER): A randomised controlled trial. Lancet 385, 2255–2263. doi: 10.1016/S0140-6736(15)60461-5
Oh, E. S., and Rabins, P. V. (2019). Dementia. Ann. Intern. Med. 171, Itc33–Itc48. doi: 10.7326/AITC201909030
Peters, M., Bletsch, M., Stanley, J., Wheeler, D., Scott, R., and Tully, T. (2014). The PDE4 inhibitor HT-0712 improves hippocampus-dependent memory in aged mice. Neuropsychopharmacology 39, 2938–2948. doi: 10.1038/npp.2014.154
Peters, R. (2006). Ageing and the brain. Postgraduate Med. J. 82, 84–88. doi: 10.1136/pgmj.2005.036665
Petersen, R. C., Lopez, O., Armstrong, M. J., Getchius, T. S. D., Ganguli, M., Gloss, D., et al. (2018). Practice guideline update summary: Mild cognitive impairment: Report of the guideline development, dissemination, and implementation subcommittee of the American Academy of Neurology. Neurology 90, 126–135. doi: 10.1212/WNL.0000000000004826
Plassman, B. L., Langa, K. M., Fisher, G. G., Heeringa, S. G., Weir, D. R., Ofstedal, M. B., et al. (2007). Prevalence of dementia in the United States: The aging, demographics, and memory study. Neuroepidemiology 29, 125–132. doi: 10.1159/000109998
Power, R., Prado-Cabrero, A., Mulcahy, R., Howard, A., and Nolan, J. M. (2019). The role of nutrition for the aging population: Implications for cognition and Alzheimer’s disease. Annu. Rev. Food Sci. Technol. 10, 619–639. doi: 10.1146/annurev-food-030216-030125
Prickaerts, J., Heckman, P. R. A., and Blokland, A. (2017). Investigational phosphodiesterase inhibitors in phase I and phase II clinical trials for Alzheimer’s disease. Expert. Opin. Investig. Drugs 26, 1033–1048. doi: 10.1080/13543784.2017.1364360
Reger, M. A., Watson, G. S., Frey, W. H. II, Baker, L. D., Cholerton, B., Keeling, M. L., et al. (2006). Effects of intranasal insulin on cognition in memory-impaired older adults: Modulation by APOE genotype. Neurobiol. Aging 27, 451–458. doi: 10.1016/j.neurobiolaging.2005.03.016
Reger, M. A., Watson, G. S., Green, P. S., Baker, L. D., Cholerton, B., Fishel, M. A., et al. (2008a). Intranasal insulin administration dose-dependently modulates verbal memory and plasma amyloid-beta in memory-impaired older adults. J. Alzheimers Dis. 13, 323–331. doi: 10.3233/JAD-2008-13309
Reger, M. A., Watson, G. S., Green, P. S., Wilkinson, C. W., Baker, L. D., Cholerton, B., et al. (2008b). Intranasal insulin improves cognition and modulates beta-amyloid in early AD. Neurology 70, 440–448. doi: 10.1212/01.WNL.0000265401.62434.36
Ruiz-Muelle, A., and López-Rodríguez, M. M. (2019). Dance for people with Alzheimer’s disease: A systematic review. Curr. Alzheimer Res. 16, 919–933. doi: 10.2174/1567205016666190725151614
Sanders, L. M. J., Hortobágyi, T., la Bastide-van Gemert, S., van der Zee, E. A., and van Heuvelen, M. J. G. (2019). Dose-response relationship between exercise and cognitive function in older adults with and without cognitive impairment: A systematic review and meta-analysis. PLoS One 14:e0210036. doi: 10.1371/journal.pone.0210036
Sardar, R., Zandieh, Z., Namjoo, Z., Soleimani, M., Shirazi, R., and Hami, J. (2021). Laterality and sex differences in the expression of brain-derived neurotrophic factor in developing rat hippocampus. Metab. Brain Dis. 36, 133–144. doi: 10.1007/s11011-020-00620-4
Sarkar, A., Harty, S., Lehto, S. M., Moeller, A. H., Dinan, T. G., Dunbar, R. I. M., et al. (2018). The microbiome in psychology and cognitive neuroscience. Trends Cogn. Sci. 22, 611–636. doi: 10.1016/j.tics.2018.04.006
Satoh, A., Imai, S. I., and Guarente, L. (2017). The brain, sirtuins, and ageing. Nat. Rev. Neurosci. 18, 362–374. doi: 10.1038/nrn.2017.42
Scarmeas, N., Anastasiou, C. A., and Yannakoulia, M. (2018). Nutrition and prevention of cognitive impairment. Lancet Neurol. 17, 1006–1015. doi: 10.1016/S1474-4422(18)30338-7
Scarpa, M., Hesse, S., and Bradley, S. J. (2020). M1 muscarinic acetylcholine receptors: A therapeutic strategy for symptomatic and disease-modifying effects in Alzheimer’s disease? Adv. Pharmacol. 88, 277–310. doi: 10.1016/bs.apha.2019.12.003
Schwam, E. M., Nicholas, T., Chew, R., Billing, C. B., Davidson, W., Ambrose, D., et al. (2014). A multicenter, double-blind, placebo-controlled trial of the PDE9A inhibitor, PF-04447943, in Alzheimer’s disease. Curr. Alzheimer Res. 11, 413–421. doi: 10.2174/1567205011666140505100858
Sikkes, S. A. M., Tang, Y., Jutten, R. J., Wesselman, L. M. P., Turkstra, L. S., Brodaty, H., et al. (2021). Toward a theory-based specification of non-pharmacological treatments in aging and dementia: Focused reviews and methodological recommendations. Alzheimers Dement. 17, 255–270. doi: 10.1002/alz.12188
Smith, M. E., and Farah, M. J. (2011). Are prescription stimulants “smart pills”? The epidemiology and cognitive neuroscience of prescription stimulant use by normal healthy individuals. Psychol. Bull. 137, 717–741. doi: 10.1037/a0023825
Szatmari, S. Z., and Whitehouse, P. J. (2003). Vinpocetine for cognitive impairment and dementia. Cochrane Database Syst. Rev. 2003:CD003119. doi: 10.1002/14651858.CD003119
Tai, S. Y., Chien, C. Y., Chang, Y. H., and Yang, Y. H. (2017). Cilostazol use is associated with reduced risk of dementia: A nationwide cohort study. Neurotherapeutics 14, 784–791. doi: 10.1007/s13311-017-0512-4
Terry, A. V. Jr., and Buccafusco, J. J. (2003). The cholinergic hypothesis of age and Alzheimer’s disease-related cognitive deficits: Recent challenges and their implications for novel drug development. J. Pharmacol. Exp. Ther. 306, 821–827. doi: 10.1124/jpet.102.041616
United Nations, (2019). World population prospects: 2019 revision. Available online at: https://data.worldbank.org/indicator/sp.pop.65up.to.zs (accessed March 22, 2022).
Valero-Cabré, A., Amengual, J. L., Stengel, C., Pascual-Leone, A., and Coubard, O. A. (2017). Transcranial magnetic stimulation in basic and clinical neuroscience: A comprehensive review of fundamental principles and novel insights. Neurosci. Biobehav. Rev. 83, 381–404. doi: 10.1016/j.neubiorev.2017.10.006
van Bokhoven, P., de Wilde, A., Vermunt, L., Leferink, P. S., Heetveld, S., Cummings, J., et al. (2021). The Alzheimer’s disease drug development landscape. Alzheimers Res. Ther. 13:186. doi: 10.1186/s13195-021-00927-z
Van Duinen, M. A., Sambeth, A., Heckman, P. R. A., Smit, S., Tsai, M., Lahu, G., et al. (2018). Acute administration of roflumilast enhances immediate recall of verbal word memory in healthy young adults. Neuropharmacology 131, 31–38. doi: 10.1016/j.neuropharm.2017.12.019
Verma, S., Kumar, A., Tripathi, T., and Kumar, A. (2018). Muscarinic and nicotinic acetylcholine receptor agonists: Current scenario in Alzheimer’s disease therapy. J. Pharm. Pharmacol. 70, 985–993. doi: 10.1111/jphp.12919
Volkow, N. D., Logan, J., Fowler, J. S., Wang, G. J., Gur, R. C., Wong, C., et al. (2000). Association between age-related decline in brain dopamine activity and impairment in frontal and cingulate metabolism. Am. J. Psychiatry 157, 75–80. doi: 10.1176/ajp.157.1.75
Voss, T., Li, J., Cummings, J., Farlow, M., Assaid, C., Froman, S., et al. (2018). Randomized, controlled, proof-of-concept trial of MK-7622 in Alzheimer’s disease. Alzheimers Dement. 4, 173–181. doi: 10.1016/j.trci.2018.03.004
Willyard, C. (2021). How gut microbes could drive brain disorders. Nature 590, 22–25. doi: 10.1038/d41586-021-00260-3
Wimo, A., Guerchet, M., Ali, G. C., Wu, Y. T., Prina, A. M., Winblad, B., et al. (2017). The worldwide costs of dementia 2015 and comparisons with 2010. Alzheimers Dement. 13, 1–7. doi: 10.1016/j.jalz.2016.07.150
Woods, B., Aguirre, E., Spector, A. E., and Orrell, M. (2012). Cognitive stimulation to improve cognitive functioning in people with dementia. Cochrane Database Syst. Rev. Cd005562. doi: 10.1002/14651858.CD005562.pub2
World Health Organization (2019). Risk reduction of cognitive decline and dementia. Geneva: World Health Organization.
Wu, Y., Li, Z., Huang, Y. Y., Wu, D., and Luo, H. B. (2018). Novel phosphodiesterase inhibitors for cognitive improvement in Alzheimer’s disease. J. Med. Chem. 61, 5467–5483. doi: 10.1021/acs.jmedchem.7b01370
Xie, Y., Li, Y., Nie, L., Zhang, W., Ke, Z., and Ku, Y. (2021). Cognitive enhancement of repetitive transcranial magnetic stimulation in patients with mild cognitive impairment and early Alzheimer’s disease: A systematic review and meta-analysis. Front. Cell. Dev. Biol. 9:734046. doi: 10.3389/fcell.2021.734046
Yankner, B. A., Lu, T., and Loerch, P. (2008). The aging brain. Annu. Rev. Pathol. 3, 41–66. doi: 10.1146/annurev.pathmechdis.2.010506.092044
Zhang, K., Mizuma, H., Zhang, X., Takahashi, K., Jin, C., Song, F., et al. (2021). PET imaging of neural activity, β-amyloid, and tau in normal brain aging. Eur. J. Nucl. Med. Mol. Imaging 48, 3859–3871. doi: 10.1007/s00259-021-05230-5
Keywords: aging, Alzheimer’s disease, dementia, mild cognitive impairment, pharmacological, non-pharmacological, intervention
Citation: Chen L, Jiao J and Zhang Y (2022) Therapeutic approaches for improving cognitive function in the aging brain. Front. Neurosci. 16:1060556. doi: 10.3389/fnins.2022.1060556
Received: 03 October 2022; Accepted: 28 November 2022;
Published: 08 December 2022.
Edited by:
Gang Chen, The First Affiliated Hospital of Soochow University, ChinaReviewed by:
Janko Samardzic, Faculty of Medicine, University of Belgrade, SerbiaCopyright © 2022 Chen, Jiao and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yonggang Zhang, amVibV96aGFuZ0B5YWhvby5jb20=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.