- 1Key Laboratory of Hunan Province for Integrated Traditional Chinese and Western Medicine on Prevention and Treatment of Cardio-Cerebral Diseases, Hunan University of Chinese Medicine, Changsha, China
- 2Hunan University of Chinese Medicine, Changsha, China
Background: Scholars have long understood that gastrointestinal microorganisms are intimately related to human disorders. The literature on research involving the gut microbiome and neuroscience is emerging. This study exposed the connections between gut microbiota and neuroscience methodically and intuitively using bibliometrics and visualization. This study’s objectives were to summarize the knowledge structure and identify emerging trends and potential hotspots in this field.
Materials and methods: On October 18, 2022, a literature search was conducted utilizing the Web of Science Core Collection (WoSCC) database for studies on gut microbiota and neuroscience studies from 2002 to 2022 (August 20, 2022). VOSviewer and CiteSpace V software was used to conduct the bibliometrics and visualization analysis.
Results: From 2002 to 2022 (August 20, 2022), 2,275 publications in the WoSCC database satisfied the criteria. The annual volume of publications has rapidly emerged in recent years (2016–2022). The most productive nation (n = 732, 32.18%) and the hub of inter-country cooperation (links: 38) were the United States. University College Cork had the most research papers published in this area, followed by McMaster University and Harvard Medical School. Cryan JF, Dinan TG, and Clarke G were key researchers with considerable academic influence. The journals with the most publications are “Neurogastroenterology and Motility” and “Brain Behavior and Immunity.” The most cited article and co-cited reference was Cryan JF’s 2012 article on the impact of gut microbiota on the brain and behavior. The current research hotspot includes gastrointestinal microbiome, inflammation, gut-brain axis, Parkinson’s disease (PD), and Alzheimer’s disease (AD). The research focus would be on the “gastrointestinal microbiome, inflammation: a link between obesity, insulin resistance, and cognition” and “the role of two important theories of the gut-brain axis and microbial-gut-brain axis in diseases.” Burst detection analysis showed that schizophrenia, pathology, and psychiatric disorder may continue to be the research frontiers.
Conclusion: Research on “gastrointestinal microbiome, inflammation: a link between obesity, insulin resistance, and cognition” and “the role of two important theories of the gut-brain axis and microbial-gut-brain axis in diseases” will continue to be the hotspot. Schizophrenia and psychiatric disorder will be the key research diseases in the field of gut microbiota and neuroscience, and pathology is the key research content, which is worthy of scholars’ attention.
Introduction
Gut microbiota represents a complex and dynamic microbial community in the gastrointestinal tract (Quercia et al., 2014). Since the dawn of humanity, these microbes have developed niche ecosystems tailored to particular habitats and adapted to the physiological requirements of their hosts (Lloyd-Price et al., 2016). The term “neuroscience” refers to studies in non-clinical fields like neurobiology and neurochemistry as well as clinical specialties like neurology, neurosurgery, neuropsychiatry, and psychology (Adolphs, 2015). The brain and gastrointestinal tract communicate in both directions through the hypothalamus-pituitary-adrenal (HPA) and microbiota-gut-brain (MGB) axes (Cenit et al., 2017; Wiley et al., 2017). We now understand that certain neurological and neuropsychiatric conditions, including Alzheimer’s disease (AD), Parkinson’s disease (PD), depression and anxiety, and autism spectrum disorder (ASD), may be significantly influenced by the gastrointestinal microbiome. This influence is also bidirectional, as shown by the comorbidities of certain neural pathologies and intestinal dysbiosis [such as irritable bowel syndrome (IBS), insulin-resistance, inflammatory bowel disease, or alterations in the ratio of bacterial species of the microbiota itself detected in patients with depression) (Wu, 2012). Physiologically, microbiota affects amygdala maturation in mammals, and baseline neuronal activity in the amygdala is altered in germ-free animals, leading to neurodevelopmental disorders (Stilling et al., 2015). Among the factors associated with the field of neuroscience pathology, the gut microbiota can affect a broad range of host neuroscience disorders by interacting with the host through immune, metabolic, neural, and endocrine pathways (Afzal et al., 2020). In recent decades, the relationships between the gastrointestinal microbiome and neuroscience in animals and humans have been widely studied. For instance, many probiotic strains known as “psychobiotics,” such as Bifidobacteria, Lactobacillus helveticus had a positive impact on cognition (Savignac et al., 2015; Ni et al., 2019), Saccharomyces boulardii (Tao et al., 2021) and Lactobacillus rhamnosus (Chivero et al., 2021), as well as multi-species probiotics, have been shown to improve neurological diseases by regulating intestinal flora (Ma T. et al., 2021; Rajanala et al., 2021). Multiple studies have presented evidence that nervous system disease leads to microbiome changes or microbiota changes lead to neuroinflammation that can trigger neurological diseases and indicate a causal relationship between them (Yang et al., 2020; Farooq et al., 2022). Many human studies have also shown that gut microbiota is closely related to the development of neurological diseases. Such as specific bacterial taxa commonly associated with mental disorders, including lower levels of bacterial genera that produce short-chain fatty acids (e.g., butyrate), higher levels of lactic acid-producing bacteria, and higher levels of bacteria associated with glutamate and GABA metabolism (Anonymous, 2022). Additionally, some clinical trials have been conducted to explore the feasibility of probiotics for the treatment of neurological diseases. Such as probiotics can improve cognitive function in patients with Alzheimer’s disease and major depression (Agahi et al., 2018; Rudzki et al., 2019).
The bibliometric analysis is one method for measuring the development of an area of study (Flay, 1986; Milat et al., 2011), and it may be applied to explore, analyze, and summarize the formation of knowledge’s structure in the spatiotemporal dimensions. This approach, which has been used in a variety of domestic and international fields, offers a diverse perspective that traditional literature reviews and systematic reviews cannot (Chen et al., 2014; Cesarano et al., 2021; Ma S. et al., 2021; Pan et al., 2021; Pasin and Pasin, 2021; Shi et al., 2021; Wu et al., 2021a; Fu et al., 2022). Bibliometric analysis has been widely used in the field of microbial science, such as microbiome and COVID-19 (Xavier-Santos et al., 2022), gut microbiota and host immune response (Ni et al., 2022), gut microbiota and atherosclerosis (Wang Y. et al., 2022), microbiome-gut-brain axis (Wang H. et al., 2022), gut microbiota and heart failure (Mu et al., 2022), gut microbiome and cancer (Zyoud et al., 2022), the intestinal microbiome and inflammatory bowel disease (Xu et al., 2022), gut microbiota and Parkinson’s disease (Cabanillas-Lazo et al., 2022), and gut microbiota and depression (Zhu et al., 2021).
The link between gut microbiota and neuroscience has not yet been subjected to a bibliometric study. However, the volume of research on gut microbiota and neuroscience has exploded, making it challenging to uncover specific data and make more accurate predictions. Therefore, bibliometrics and visualization were employed in this study to intuitively and methodically disclose the connections between gut microbiota and neuroscience, the objectives were to summarize the knowledge structure and identify emerging trends and potential hotspots in this field.
Materials and methods
Data collection
This study utilized the Web of Science Core Collection (WoSCC) database as its data source. The WoSCC database, one of the most comprehensive, systematic, and authoritative databases, has been extensively used by a substantial number of academics for scientometric analysis and visualization of scientific literature (Chen et al., 2020; Devos and Menard, 2020; Wu et al., 2021b; Zhang et al., 2021).
‘‘Gastrointestinal microbiomes’’ was searched in the Medical subject headings (MeSH) of PubMed to obtain Gastrointestinal microbiome-related terms. Then these terms were used to do searches on WoSCC, the search strategy was as follows: ‘‘Topic: [(gastrointestinal microbiomes) OR (microbiome, gastrointestinal) OR (gut microbiome) OR (gut microbiomes) OR (microbiome, gut) OR (gut microbiota) OR (gut microbiotas) OR (microbiota, gut) OR (gastrointestinal flora) OR (flora, gastrointestinal) OR (gut flora) OR (flora, gut) OR (gastrointestinal microbiota) OR (gastrointestinal microbiotas) OR (microbiota, gastrointestinal) OR (gastrointestinal microbial community) OR (gastrointestinal microbial communities) OR (microbial community, gastrointestinal) OR (gastrointestinal microflora) OR (microflora, gastrointestinal) OR (gastric microbiome) OR (microbiome, gastric) OR (gastric microbiomes) OR (intestinal microbiome) OR (intestinal microbiomes) OR (microbiome, intestinal) OR (intestinal microbiota) OR (intestinal microbiotas) OR (intestinal microflora) OR (microflora, intestinal) OR (intestinal flora) OR (flora, intestinal) OR (enteric bacteria) OR (bacteria, enteric)] AND Language: (English) AND Document Type:(Article OR Review) AND Web of Science Categories: (Neurosciences) AND Publication Date: (2002-01-01 to 2022-08-20)’’ (Searchable link)1. “Full Record and Cited References” of the catalogue are exported in “Plain Text” format and were named “download *.txt.” The search was completed on 18 October 2022, a total of 2,275 articles were chosen and then used to perform a bibliometric analysis.
Data analysis and visualization
Excel 2016 was used to import and analyze data related to the number of articles published each year on the gastrointestinal microbiome and neuroscience. Use “thesaurus_terms.txt” in VOSviewer 1.6.17 to create thesaurus files, such as merged “ischemic-stroke” and “ischemic stroke,” “gut microbiome” and “gastrointestinal microbiomes,” “gut-brain-axis” and “gut-brain axis,” “Chinese acad sci” and “univ Chinese acad sci” in term analysis. We utilized VOSviewer 1.6.17 to locate the top prolific journals, institutions, countries/regions, citations of documents, co-cited references, terms, and related knowledge maps.
CiteSpace V (Version 6.1 R2)2 was used to detect a citation-burst analysis of WoS-generated keywords. Parameters of CiteSpace were set as follows: time slicing (2002–2022), years per slice (1 year), term source (keywords), node type (keywords), selection criteria (top 50), burstness (γ 1.0).
Results
Annual growth trend
There were 2,275 articles on the gastrointestinal microbiome and neuroscience in the WOSCC from 2002 to 2022 (August 20, 2022), including 1,433 original research articles and 842 reviews. The annual publication is exhibited in Figure 1. The growth of the publications showed three stages. The first stage was from 2002 to 2009, the second stage was from 2010 to 2015, and the third stage was from 2016 to 2022. The overall trend of articles and reviews was consistent with annual publications, but the number of articles was higher than that of reviews.
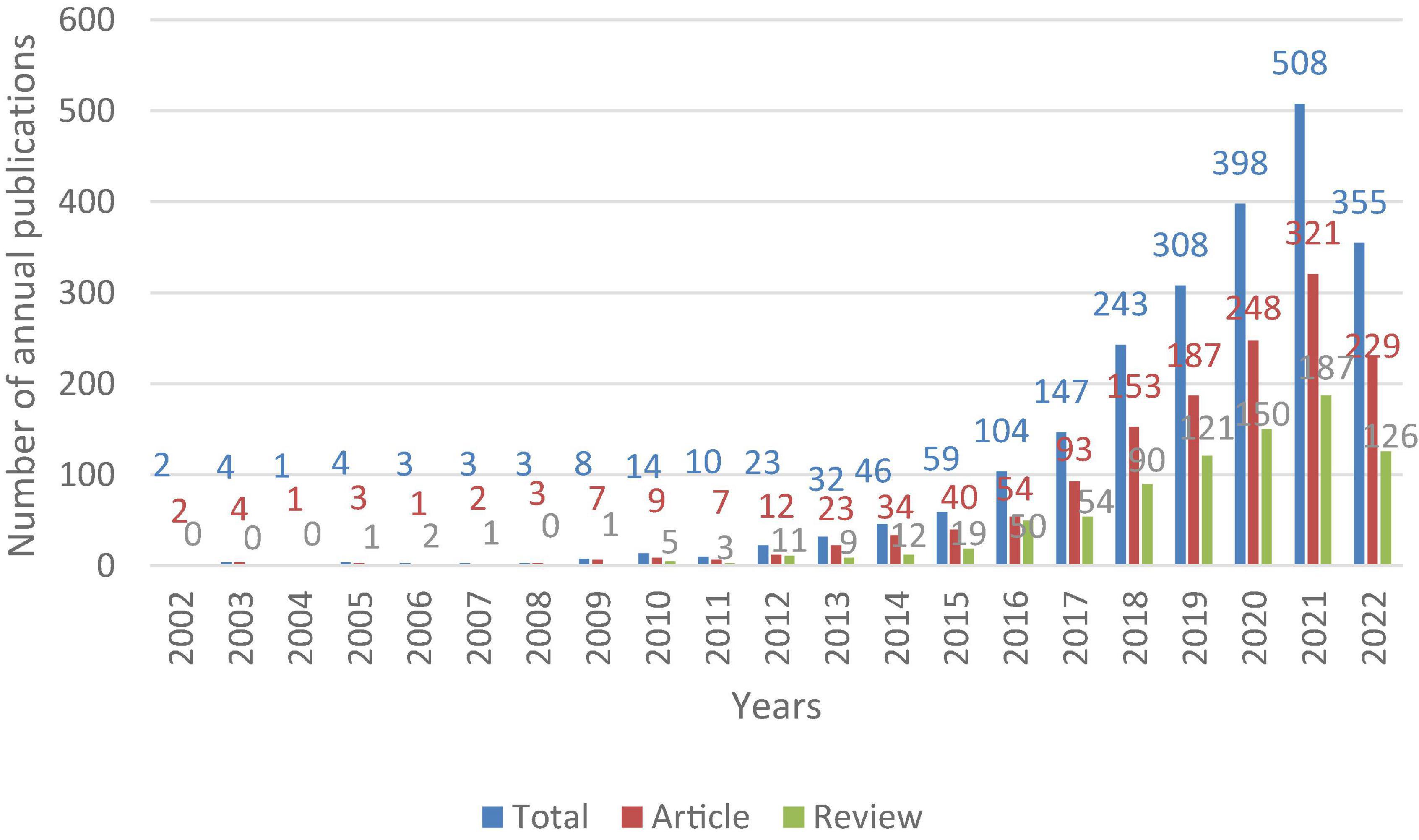
Figure 1. Trends in the number of publications for research in the gastrointestinal microbiome and neuroscience from 2002 to 2022.
Top active countries/Regions
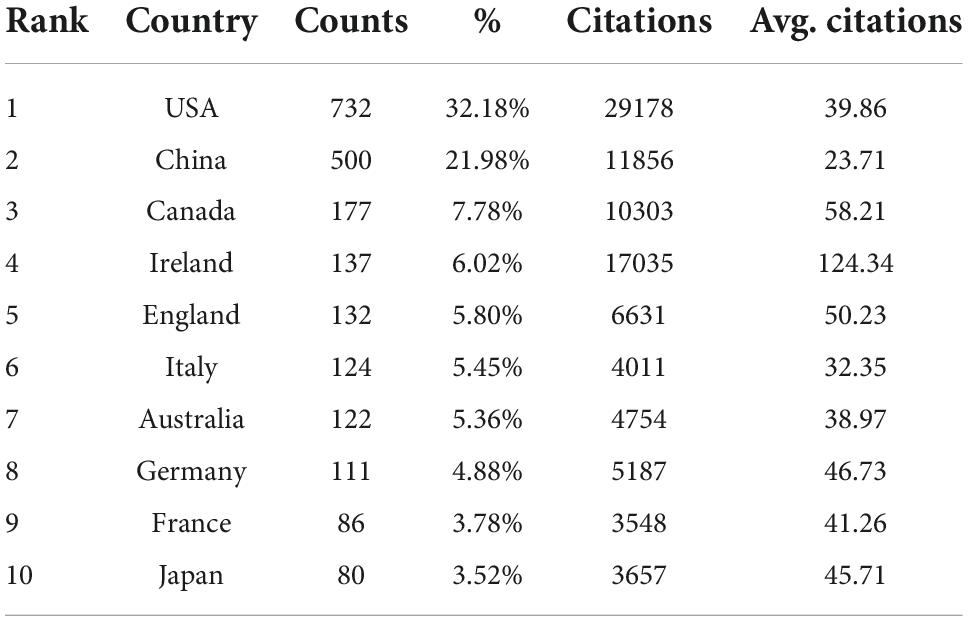
A total of 2,618 institutions from 83 countries/regions contributed to 2,275 publications. The United States produced the most publications (732, 32.18%), then China (500, 21.98%), Canada (177, 7.78%), Ireland (137, 6.02%), and England (132, 5.80%) (Table 1). Figure 2A shows that the number of publications in a country is positively correlated with its gross domestic product (GDP). Figure 2B is a cooperation network of countries/regions to study the gastrointestinal microbiome and neuroscience. This map showed 41 countries/regions with a minimum of 10 publications. The most cooperation with other countries/regions is the United States (links: 38), followed by England (links: 35), Australia (links: 32), Germany (links: 31), and Canada (links: 31).
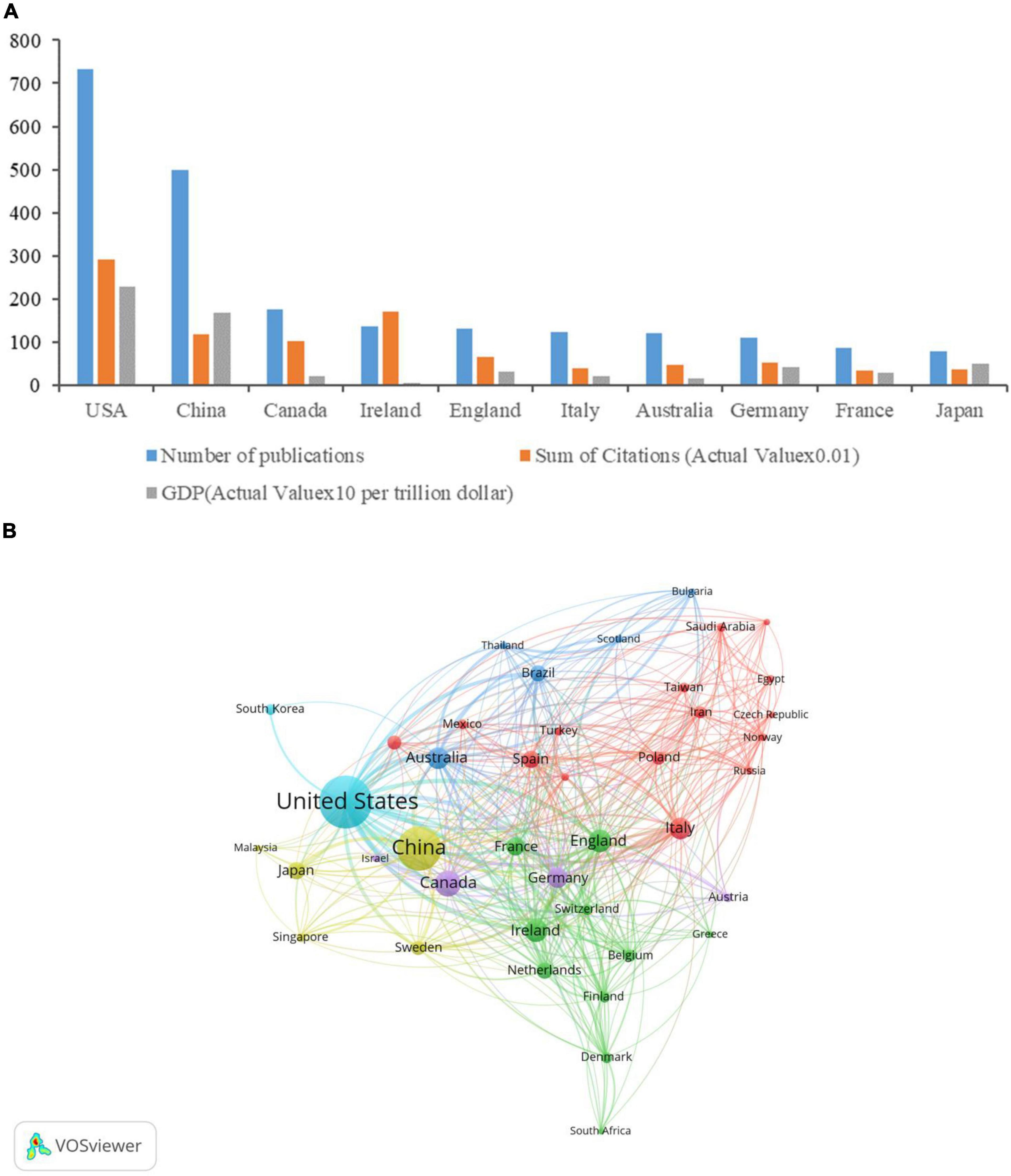
Figure 2. Contributions of different countries/regions to research on gastrointestinal microbiome and neuroscience. (A) Number of publications, sum of citations (Actual Value × 0.01), and GDP (Actual Value × 10 per trillion dollar) in the top 10 active countries/regions. (B) Cooperation network of countries/regions to study the gastrointestinal microbiome and neuroscience. Map of countries/regions cooperation showed 41 countries/regions with a minimum of 10 publications.
Top active institutions
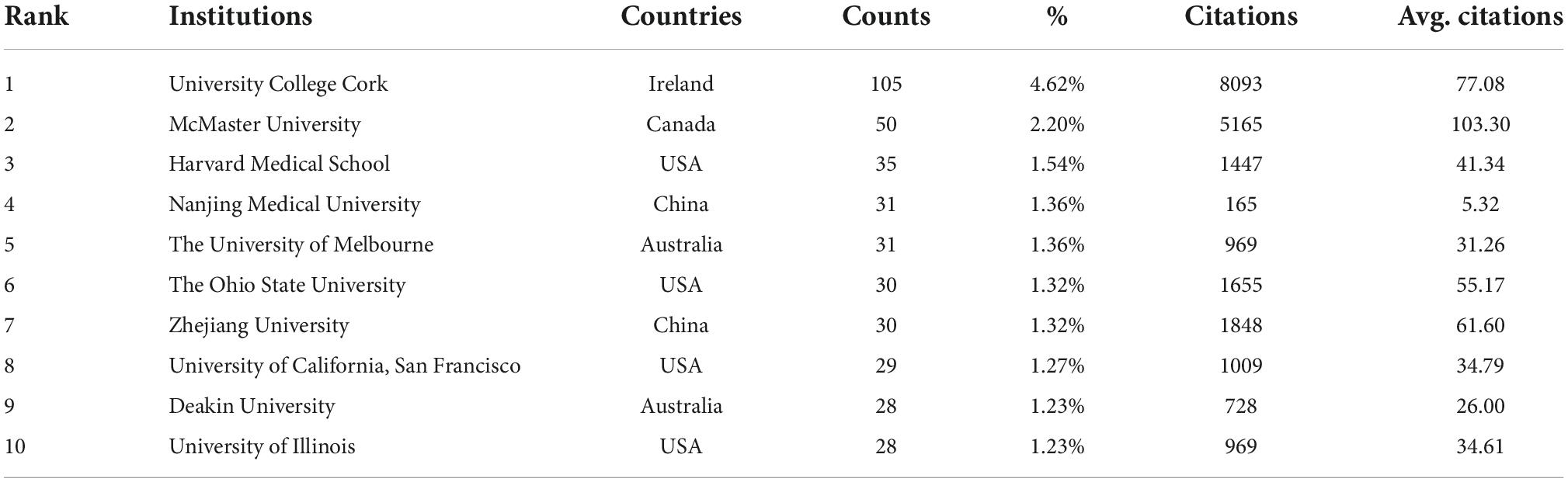
The top 10 institutions were from the USA (4/10), Australia (2/10), China (2/10), Ireland (1/10), and Canada (1/10) (Table 2). University College Cork (105, 4.62%) published the most papers, followed by McMaster University (50, 2.20%), Harvard Medical School (35, 1.54%), Nanjing Medical University (31, 1.36%), and the University of Melbourne (31, 1.36%) (Table 2). A collaboration network of institutions to research the gastrointestinal microbiome and neuroscience is shown in Figure 3. This map shows 87 institutions with a minimum of 10 publications. The Harvard Medical School has the most collaborations with other institutions (links: 31), followed by University College Cork (links:30), Deakin University (links:25), Massachusetts General Hospital (links: 21), and McMaster University (links: 21).
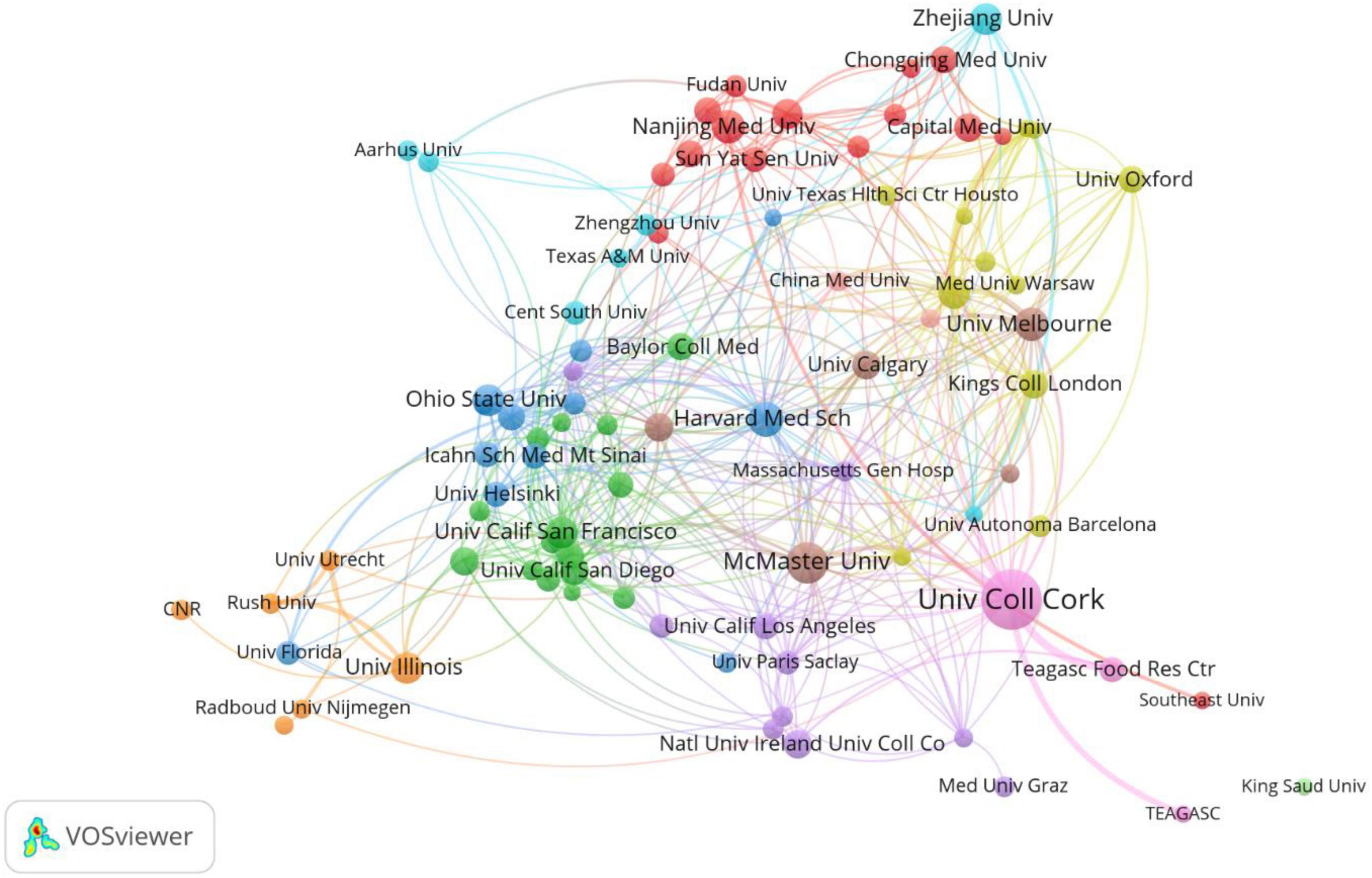
Figure 3. Cooperation network of institutions to study the gastrointestinal microbiome and neuroscience. Map of institutions’ cooperation showed 87 institutions with a minimum of 10 publications.
Authors and co-cited authors
A total of 11,617 authors were involved in gastrointestinal microbiome research in the field of neuroscience. Cryan JF published the most papers (n = 100), followed by Dinan TG (n = 79), Clarke G (n = 38), Stanton C (n = 23), and O’Mahony SM (n = 19) (Table 3). The authors (n = 52) who published at least eight papers (T ≥ 8) belong to the core authors in this field and they were included to build the network map of core authors (Figure 4). The same color represented the same cluster.
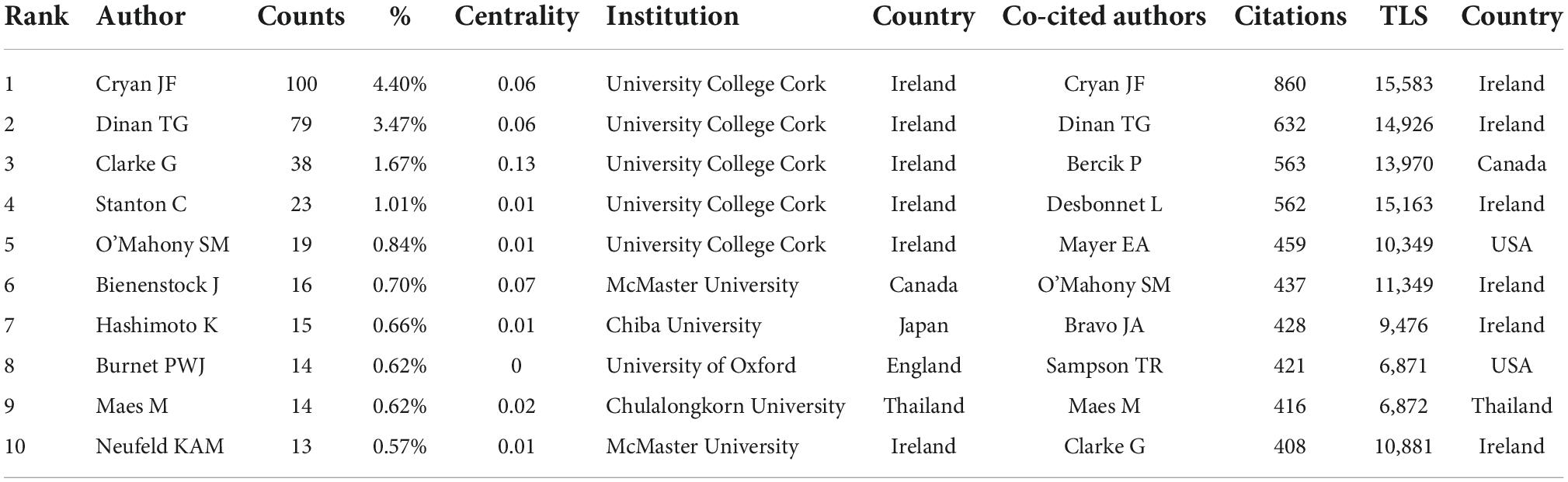
Table 3. Top 10 productive and most co-cited authors on gastrointestinal microbiome and neuroscience research.
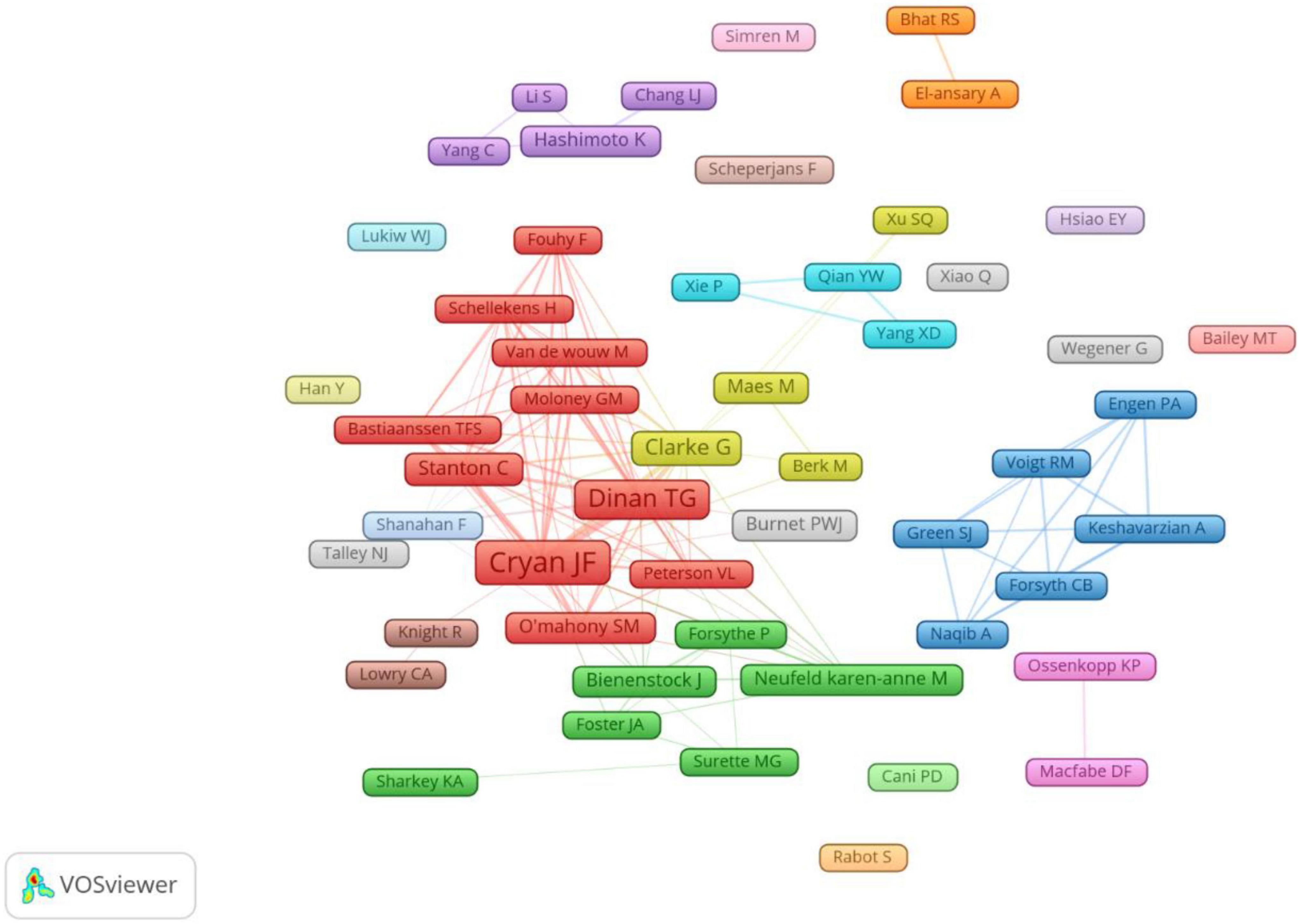
Figure 4. The co-occurrence map of authors on gastrointestinal microbiome and neuroscience research (T ≥ 8).
Co-cited authors are authors who have been co-cited together in a range of publications (42). Among 75,329 co-cited authors, 119 were co-cited over 100. Cryan JF (n = 860) ranked first, followed by Dinan TG (n = 632), Bercik P (n = 563), Desbonnet L (n = 562), Mayer EA (n = 459). The remaining five top authors were co-cited from 408 to 437 (Table 3). The distribution of countries shows that the authors with high co-citation frequencies are mainly from Ireland and the United States. The authors (n = 119) with co-citations of at least 100 (T ≥ 100) were used to make the density map (Figure 5), this type of knowledge map could present the high-frequency co-cited authors clearly.
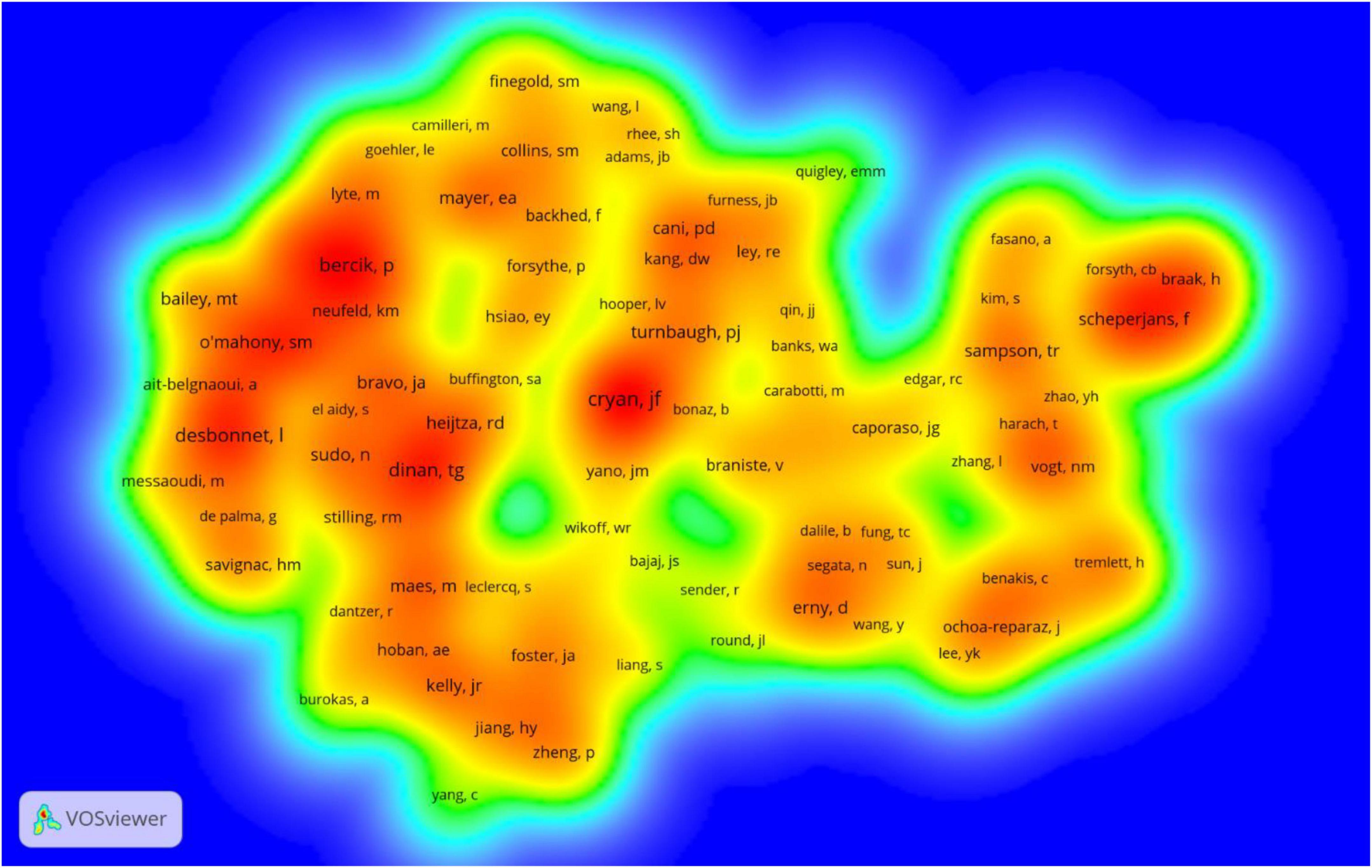
Figure 5. The density map of co-cited authors on gastrointestinal microbiome and neuroscience research (≥100).
Top active journals
To discover the most active journal research in gastrointestinal microbiome and neuroscience, we conducted citation journal analyses using VOSviewer. The results showed that 2,275 papers were published in 214 academic journals. Neurogastroenterology and Motility published the most papers (158, 6.95%), followed by Brain Behavior and Immunity (147, 6.46%), Frontiers in Neuroscience (127, 5.58%), Frontiers in Aging Neuroscience (72, 3.16%), and Journal of Alzheimer’s Disease (67, 2.95%) (Table 4). Among the top10 journals, five had an Impact Factor (IF) of more than five, and three were in the Q1 JCR division (Table 4).
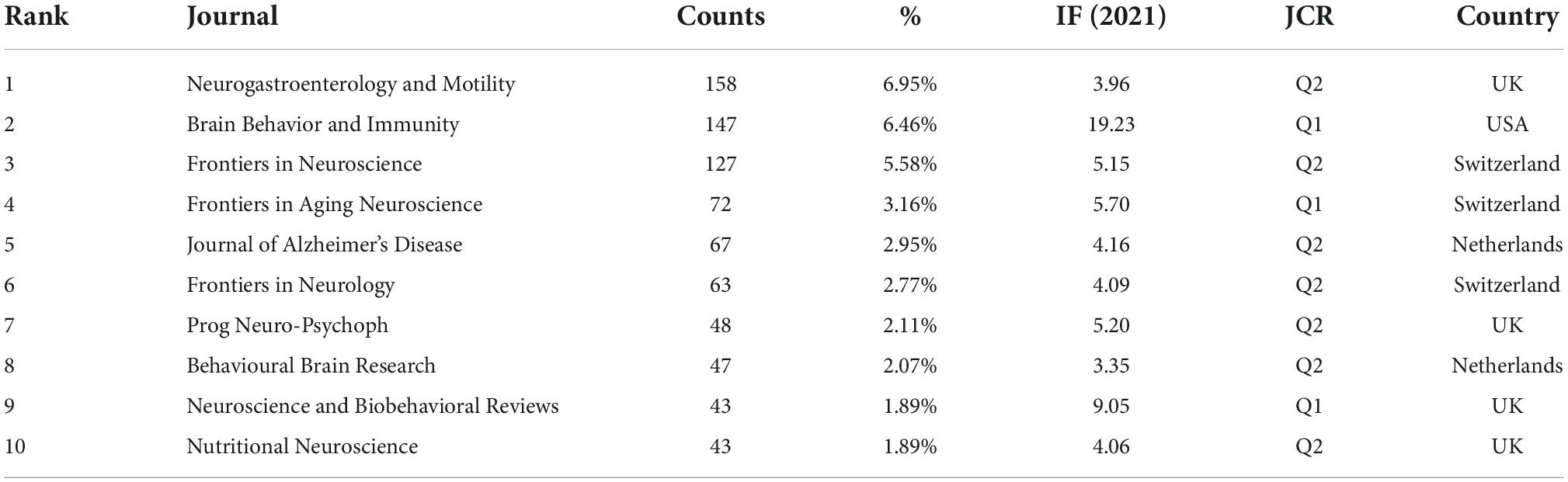
Table 4. The top 10 journals publishing articles in the gastrointestinal microbiome and neuroscience.
Top cited publications and co-cited references
Tables 5, 6 display the top 10 gastrointestinal microbiome-related cited articles and co-cited references in the discipline of neuroscience. The most often cited article on research in gastrointestinal microbiome and neuroscience was Cryan JF’s 2012 article on the impact of gut microbiota on the brain and behavior (Table 5). Four of the documents appeared on both lists, which were, respectively completed by the team of Cryan JF, Erny D, Sudo N, and Clarke G (Sudo et al., 2004; Cryan and Dinan, 2012; Clarke et al., 2013; Erny et al., 2015). The statistics imply that the articles on research in gastrointestinal microbiome and neuroscience by Cryan JF, Erny D, Sudo N, and Clarke G have been a significant contributor to the growth of the subject.
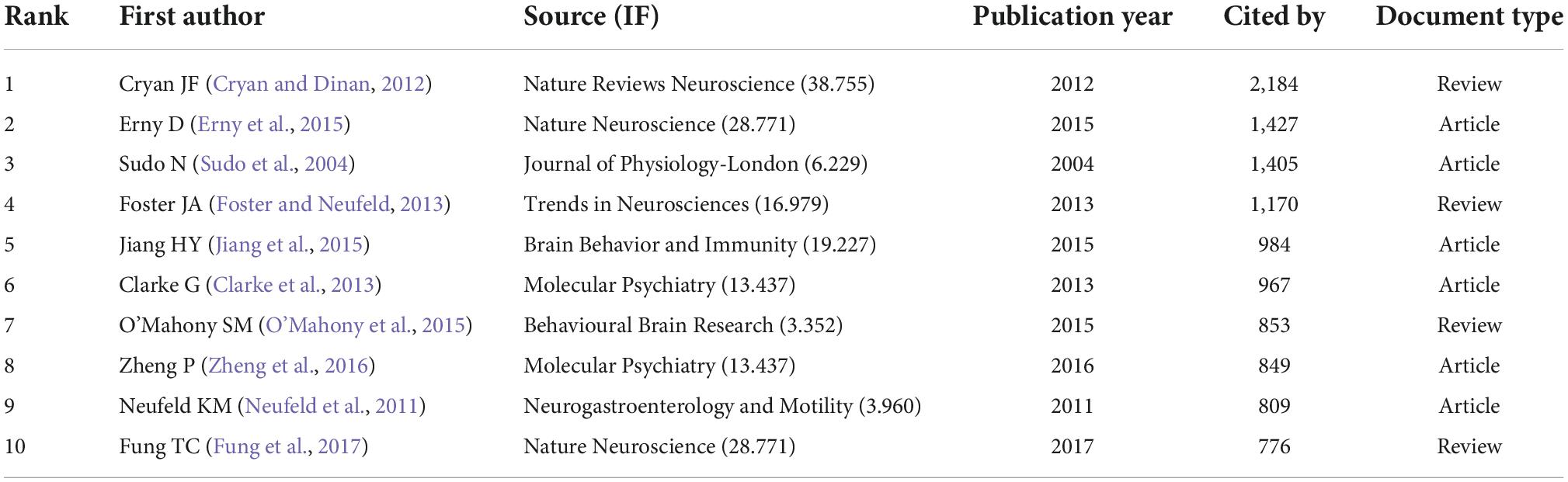
Table 5. Top 10 highly cited publications on research in gastrointestinal microbiome and neuroscience.
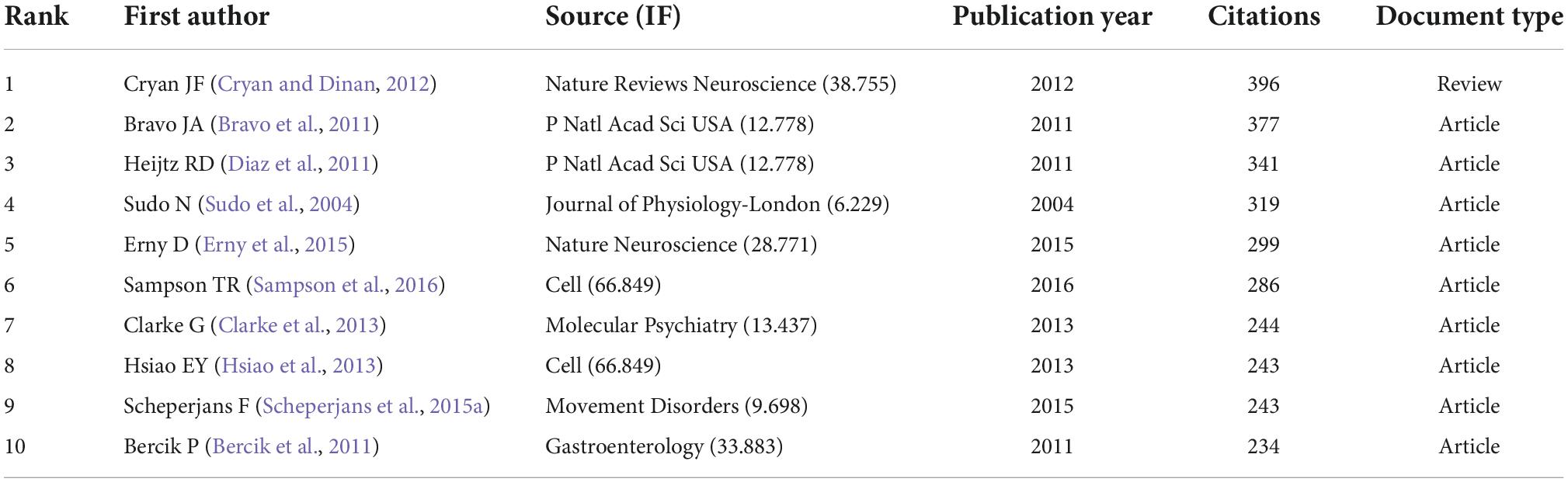
Table 6. Top 10 highly co-cited references on research in gastrointestinal microbiome and neuroscience.
Analysis of keywords
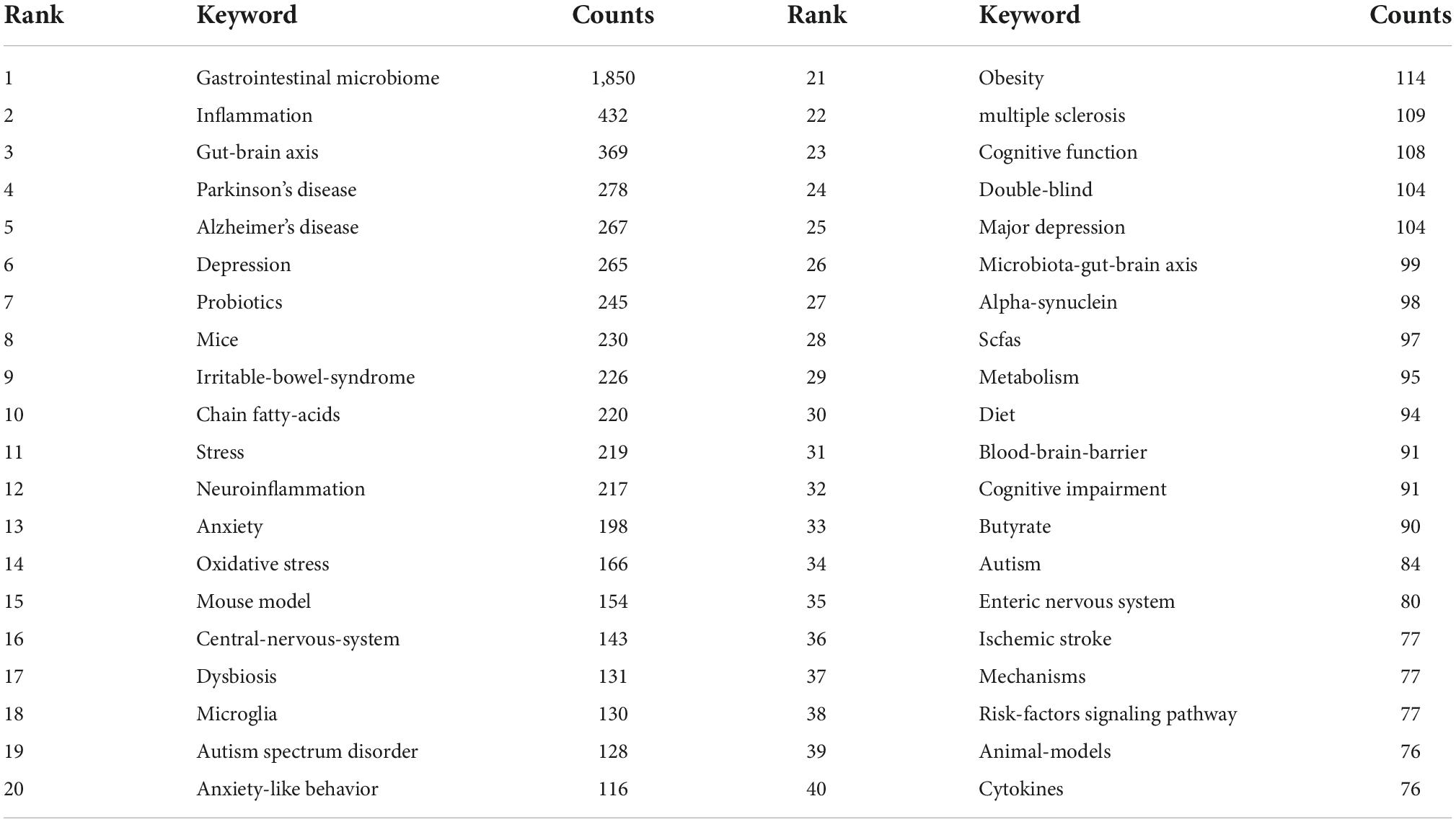
A map of keyword co-occurrence reflects research hotspots. Co-occurrence and cluster analysis keywords were presented using VOSviewer (c). A total of 7,900 terms were extracted from keywords of all the 2,275 publications. High-frequency keywords (more than 40 times) were the subject of a clustering study. As shown in Figure 6, there were 91 nodes and 3,396 links in the network map, the size of each node indicates the occurrence of the keyword. As we can see from Table 7, the gastrointestinal microbiome was the highest frequency term with 1,850 co-occurrences, followed by inflammation, gut-brain axis, Parkinson’s disease, and Alzheimer’s disease. Five clusters were shown in different colors, and nodes with common attributes were classified into a color-coded cluster, represented by red, green, blue, yellow, and purple.
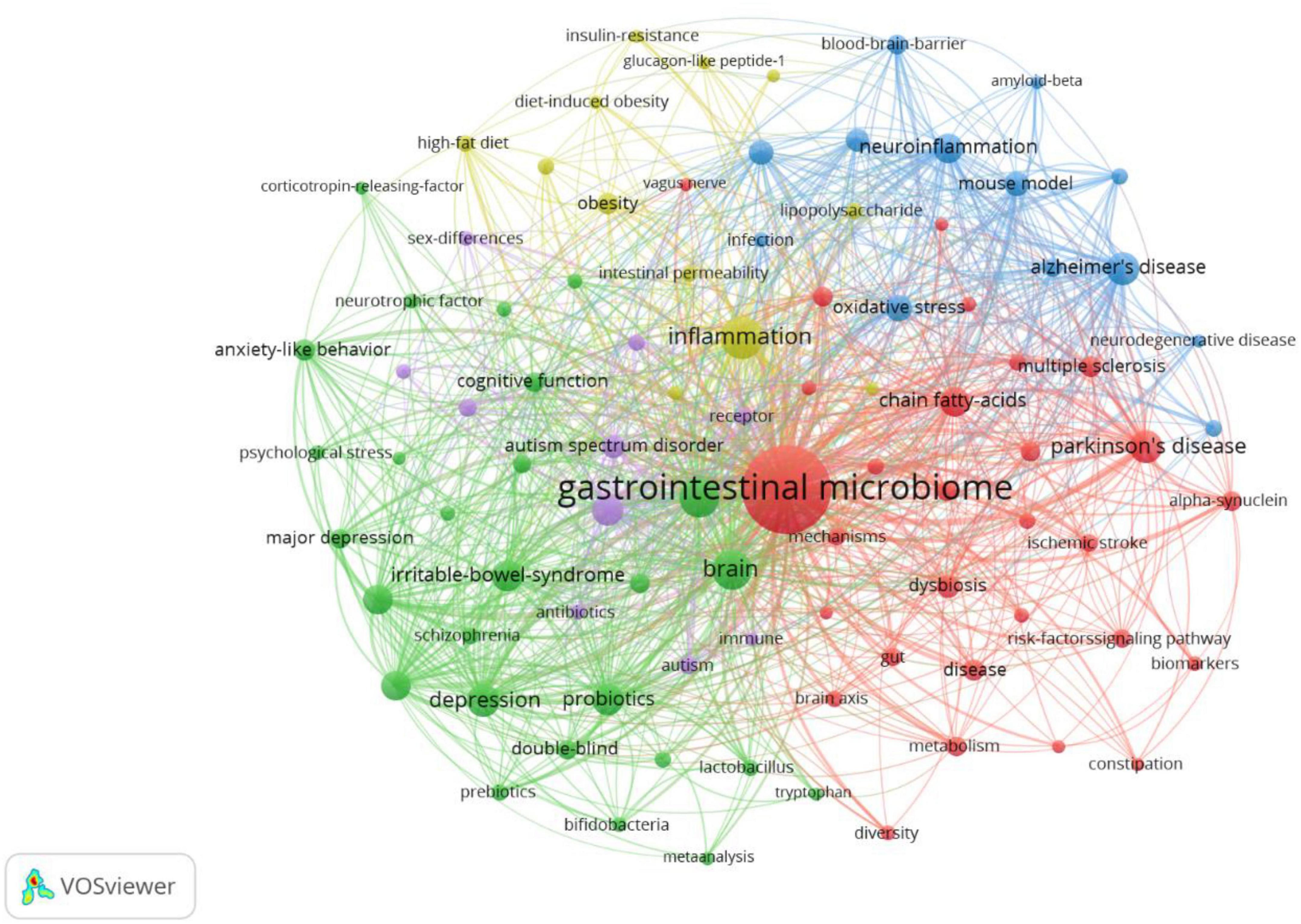
Figure 6. Co-occurrence and clustering of keywords in title/abstract fields of publications related to gastrointestinal microbiome and neuroscience. Map of keywords clustering showed 91 keywords with a minimum of 40 occurrences and divided into five clusters. Notes: The size of node and word reflects the co-occurrence frequencies, the link indicates the co-occurrence relationship, and nodes with the same color denote the same cluster.
In Figure 7, VOSviewer could mark keywords included in the overlay visualization map with different colors based on their average appearance year. The color blue stood for the keywords that appeared on the time course far earlier than those in yellow and red. As can be seen from Figure 7, large numbers of research hotspots related to gastrointestinal microbiomes and neuroscience have emerged in recent years, which indicates that the field is evolving at a tremendously fast pace.
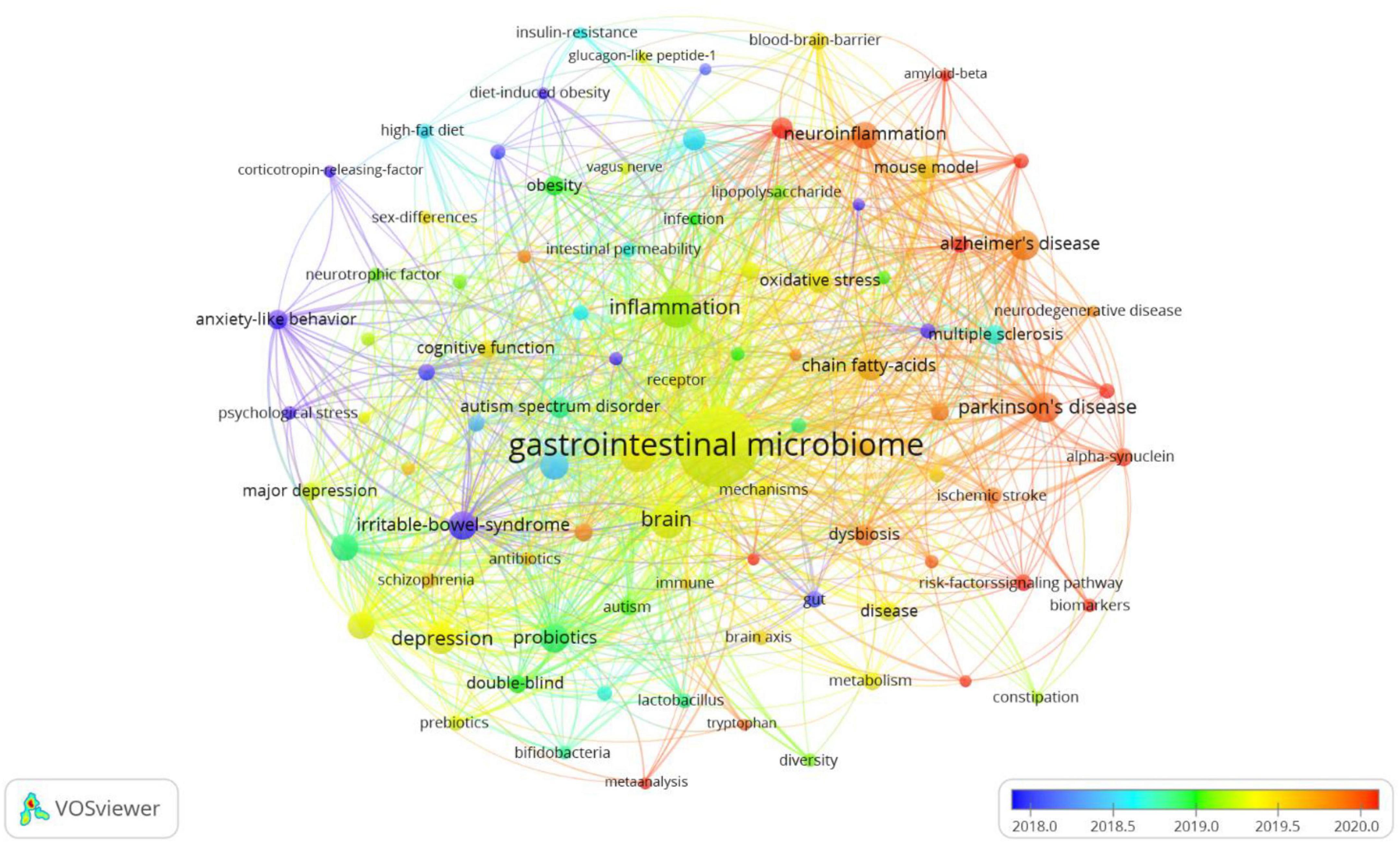
Figure 7. The overlay visualization map of keywords in title/abstract fields of publications related to gastrointestinal microbiome and neuroscience. Different colors were applied for each keyword based on their average appearance time in the overlay visualization map. Blue color represented the keywords that appeared relatively earlier than those in yellow and red upon time course.
Analysis of bursts
Top 35 keywords with the strongest citation bursts
Burstiness of Keywords CiteSpace was employed to carry out burst keyword detection (Figure 8). Keyword burstiness can represent new academic trends, foretell future avenues for frontier study, and highlight prospective hotspots in a discipline. The burst detection is represented as a red segment on the blue timeline, which denotes the start year, end year, and duration of the burst. The timeline is shown as a blue line. We were especially interested in terms that have research relevance among the top 35 keywords with the highest burst intensity. These terms represented the research trends in the fields of gut microbiome and neuroscience (Figure 8). The duration of “bacterial translocation” outbreaks is the longest (2003–2018). Irritable bowel syndrome had the highest burst intensity from 2002 to 2022 (32.54), followed by gastrointestinal microbiome (20.72) and anxiety-like behavior (17.41). The top 35 keyword outbreak cycles with the most number of occurrences covered the entire period from 2002 to 2022. Notably, the burst of schizophrenia, pathology, and psychiatric disorder is still ongoing.
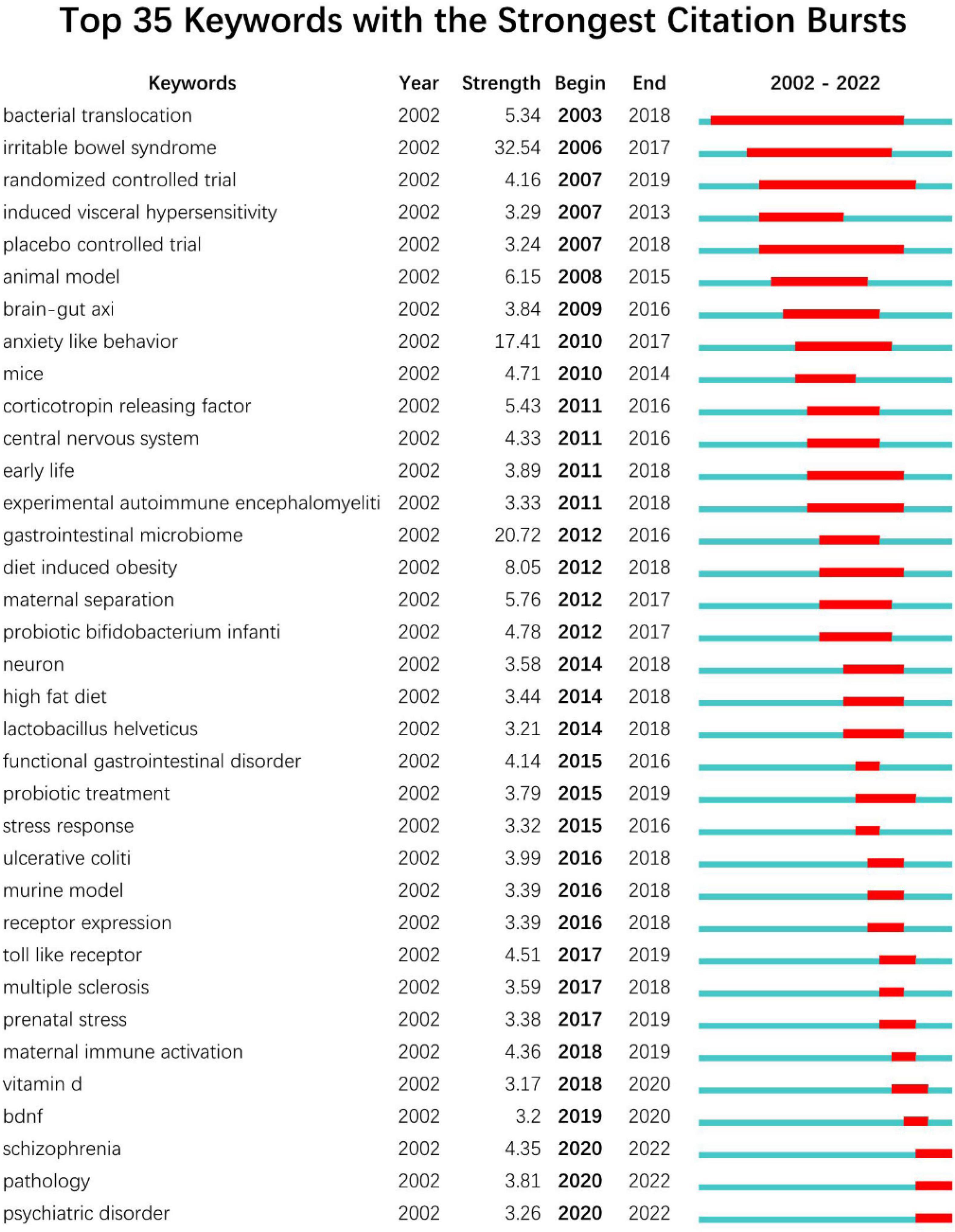
Figure 8. Top 35 Keywords with the Strongest Citation Bursts (sorted by the beginning year of burst) of the research in gastrointestinal microbiome and neuroscience during 2002–2022.
Discussion
This bibliometric article analyzes publications research in gastrointestinal microbiome and neuroscience using visual analysis software. 2,275 publications from the WoSCC database in total were analyzed. The first stage (2002–2009) was the flat period of the development of this field, with less than 10 papers published annually. The second stage (2010–2015) was a slow growth period, the number of related publications showed an overall increasing annual trend, the number of published articles increased by dozens per year, and the annual number of published papers was less than 100. The third stage (2016–2022) was the rapid development stage, and the annual number of published papers was more than 100, accounting for more than 91% of the total number of included studies. The volume of literature will continue to grow in the future, and the research in this field will still attract the attention of scholars in the next few years.
Knowledge structure of global
Countries/Regions
The United States was the leading contributor in this field with 732 publications, followed by China, Canada, Ireland, and England, which accounted for more than 73% of the total number of articles included in the study. GDP is an important factor in the number of publications of a country. The output of publications in the United States and China is far ahead, which is closely related to the top ranking of GDP (Figure 2A). One could presume that significant financial investments, such as the Human Microbiome Project (HMP) started by the NIH in 2007 and a remarkable research project on the gut microbiota-brain axis in 2013, affected research output on gut microbiota and neuroscience (Turnbaugh et al., 2007; Proctor, 2011; Human Microbiome Project Consortium, 2012, Robles-Alonso and Guarner, 2014). In addition, numerous experts in microbiology and neuroscience from Ireland, China, the United States, and other countries have studied the characteristics of the gastrointestinal microbiota in health and neuroscience disease states, the connection between pathological function and neuroscience disease, and other topics. These professionals, who serve as a foundation for the creation and publication of papers in this area, include Cryan JF, Dinan TG, Clarke G, Stanton C, O’Mahony SM, Bastiaanssen TFS, Yang C, Xie P, Bailey MT, Green SJ, Keshavarzian A, and Forsyth CB (Clarke et al., 2013; Dinan et al., 2014; Mayer et al., 2014; Stilling et al., 2015; Tarr et al., 2015; Dodiya et al., 2020; Joers et al., 2020; Li et al., 2020; van de Wouw et al., 2020; Frausto et al., 2021; Zheng et al., 2021; Connell et al., 2022; Teng et al., 2022). It is worth noting that the citations of articles published in the United States rank first, followed by Ireland and China. To some extent, the number of citations is related to the quality of the literature, but also to paying service, the time of publication of the paper, and the research hotspots or trend changes. In addition, it can be seen from the cooperation Network of countries/regions (Figure 2B) that compared with high-output countries such as the United States, England, and Australia, China has less cooperation with other countries in this field. To improve China’s influence in this field, it is necessary to improve the quality of literature and strengthen the exchange and cooperation with other high-output countries in this field.
Institutions
A total of 2,618 institutions around the world have been studying the gastrointestinal microbiome in neuroscience. We can see from Table 2, University College Cork was the most productive and influential institution located in Ireland, and the top 10 institutions active in research of gastrointestinal microbiome and neuroscience came from the top 10 nations or regions. The top 10 institutions in this sector helped the United States, Australia, China, Ireland, and Canada overtake other countries as the most active ones in this field. Notably, as seen in Figure 3, whereas some of these institutions have worked closely together, some have not. Therefore, it is strongly advised that nations and institutions with similar research topics broaden and deepen their cooperation and work together to advance the development and prosperity of this field.
Authors
Our results demonstrated that Cryan JF was the most productive author and the highest number of co-cited authors, who reviews the field of gut microbiota and neuroscience and discusses many theories (O’Mahony et al., 2009; Cryan and Dinan, 2012; Dinan and Cryan, 2012; Clarke et al., 2013; Mayer et al., 2014). The team represented by Cryan JF is active in this field and has more research results on the relationship between gastrointestinal microbiota and neuroscience, which plays an important guiding role. We also noticed that the top three frequently productive authors (Cryan JF, Dinan TG, and Clarke G) are all from the University College Cork (Table 3) and had a high centrality, suggesting that the University College Cork is an important institution in the research field. The co-occurrence map of authors (Figure 4) manifested there were active collaborations among authors in the same cluster, such as Cryan JF, Dinan TG, and O’Mahony SM, Clarke G, and Maes M, etc. Close cooperation was also observed among clusters, such as Dinan TG and Clarke G, Dinan TG and Neufeld KAM, Clarke G and Neufeld KAM, etc. However, some active authors on gastrointestinal microbiomes and neuroscience still lack collaboration with other scholars, such as the authors Hsiao EY, Bailey MT, and Han Y, etc., who have not yet formed a stable collaborative team.
Journals
Most relevant studies were published in the journals (Q1/Q2 journals) with world-class influence, such as Brain Behavior and Immunity, Frontiers in Aging Neuroscience, and Neuroscience and Biobehavioral Reviews (Table 4). These results suggested that the link between gastrointestinal microbiota and neuroscience has attracted the attention of numerous scholars, and its research difficulties and value have also been recognized by scholars. All the top 10 journals are in the discipline of neuroscience and are established in the UK, Switzerland, Netherlands, and USA. When researchers produce and read studies on gastrointestinal microbiota and neuroscience, the top 10 journals may be given preference.
Publications and references
The publications and references analysis has revealed the important references in the field of gastrointestinal microbiota and neuroscience in the past 20 years. The cited publications and co-cited references listed in Tables 5, 6 would provide an important reference for the study in this field. The findings revealed that multi-seed subjects closely related to research hotspots were highlighted in the top 10 highly cited publications and co-cited references. Four articles appear together in both lists (Tables 5, 6). The article with the most citations was on the impact of the gut microbiota on the brain and behavior by Cryan and Dinan (2012), which was published in Nature Reviews Neuroscience in 2012 and has a total of 2,184 citations. This research examines how the gut microbiota interacts with the central nervous system (CNS) via neural, endocrine, and immunological pathways, possibly affecting brain activity and behavior and influencing the regulation of anxiety, mood, cognition, and pain. The second article was published by Erny et al. (2015) from Nature Neuroscience in 2015. This study showed that the diverse microbiota partly restores microglia impairment while the host bacteria play a critical role in regulating microglia maturation and function. Research by Sudo et al. (2004) from the Journal of Physiology-London is the third most cited article. According to this study, the postnatal development of the hypothalamic-pituitary-adrenal (HPA) stress response in mice can be influenced by commensal bacteria. The fourth article came from Molecular Psychiatry and was produced by Clarke et al. (2013). This study showed that the absence of normal gut microbiota might seriously disturb 5-hydroxytryptamine neurotransmission in the central nervous system (CNS). The top 10 frequently cited publications and co-cited references may be given priority when researchers read and refer to articles on the gastrointestinal microbiome and neuroscience.
Research focus and trends of global
According to the co-occurring keyword analysis, we identified some of the most important hotspots in this field over the past two decades, including the gastrointestinal microbiome, inflammation, gut-brain axis, Parkinson’s disease, and Alzheimer’s disease. The most widely studied diseases in this field are Parkinson’s disease and Alzheimer’s disease. Reichmann (2011) speculates that bacteria may be responsible for the development of PD, it may spread out from the enteric nervous system of the gut via the vagal nerve up to the brain. Since 2015, “Parkinso’s disease” has become the new top hotspot in this field. Gut microbiome composition is significantly associated with Parkinson’s disease (Scheperjans et al., 2015b). Kumari et al. (2020) studied the salivary metabolic profiling of saliva were studied in patients with PD (n = 76) and healthy controls (HC, n = 37) were analyzed and differentiated PD from HC. It is found that patients with PD might be characterized by metabolic imbalances like gut microflora system, energy metabolites, and neurotransmitters (Kumari et al., 2020). The ratio of Firmicutes to Bacteroidetes increased and bacterial diversity decreased in the gut of PD mice treated with rotenone (Yang et al., 2018). The abundance of Prevotella in PD patients is decreased, which affects the gastrointestinal dysfunction of PD patients (Mertsalmi et al., 2017). AD is the most common neurodegenerative disease. Priming of the innate immune system by the microbiota may enhance the inflammatory response to cerebral amyloids (such as amyloid-beta and alpha-synuclein), leading to neuro-system-related diseases such as Parkinson’s disease and Alzheimer’s disease (Friedland, 2015). Probiotic supplementation (Lactobacillus acidophilus, Lactobacillus bifidobacterium, and Lactobacillus fermentans) improves cognitive function and metabolic status in patients with Alzheimer’s disease (Akbari et al., 2016). Moreover, fecal microbiota transplant (FMT) technology, an ancient administration route traced back to fourth-century China (Zhang et al., 2012), is receiving increasing attention. Accumulating studies suggest that FMT has potential therapeutic effects on neuropsychiatric areas-related disorders, owing to the increase in microbiota diversity (Kang et al., 2021; Wang et al., 2021).
Cluster analysis is a statistical method for dividing the subjects of a particular field into several groups, which can reflect the research focus in the field (van Eck and Waltman, 2010; Xu et al., 2022). Our clustering analysis of keywords identified five focus areas of the research in gastrointestinal microbiome and neuroscience utilizing VOSviewer. Cluster 1 (red nodes) was the larger cluster with 29 co-occurrence terms: gastrointestinal microbiome, Parkinson’s disease, chain fatty acids, multiple sclerosis, short-chain fatty acids, alpha-synuclein, metabolism, ischemic stroke, mechanisms, enteric nervous system, nervous system, brain axis, metabolites, fecal microbiota transplantation, metabolomics, vagus nerve, etc. The topic of Cluster 1 is the mechanism of the gastrointestinal microbiome in Parkinson’s disease, multiple sclerosis, and ischemic stroke. Cluster 2 (green nodes) is primarily concerned with the effect of probiotics (e.g., lactobacillus, bifidobacteria) on depression, anxiety, major depression, schizophrenia, etc., and the “gut-brain axis” and “microbiota-gut-brain axis” are two important theories that are involved. This cluster includes 27 terms, such as gut-brain axis, depression, probiotics, anxiety, anxiety-like behavior, major depression, cognitive function, double-blind, microbiota-gut-brain axis, lactobacillus, schizophrenia, neurotrophic factor, bifidobacteria, psychological stress, etc. Cluster 3 (blue nodes) focuses on the mechanism of neurodegenerative diseases, such as Alzheimer’s disease and dementia, which contains 13 terms: Alzheimer’s disease, neuroinflammation, oxidative stress, mouse model, central-nervous-system, microglia, blood-brain-barrier, cognitive impairment, dementia, neurodegenerative disease, amyloid-beta, etc. Cluster 4 (yellow nodes) is mainly related to “gastrointestinal microbiome, inflammation: a link between obesity, insulin resistance, and cognition” with 12 terms: inflammation, obesity, high-fat diet, intestinal permeability, lipopolysaccharide, TNF-alpha, bacterial translocation, insulin-resistance, glucagon-like peptide-1, etc. The topic of Cluster 5 (purple nodes), which includes the keywords mice, autism spectrum disorder, autism, animal models, immune system, sex differences, and more, is “the function of the gut microbiome in autism spectrum disorder and autism.” Despite the increasing recognition of these hotspots, as these research directions develop, further innovations and breakthroughs may be hindered. Therefore, in the coming years, it will be important to focus more on the frontier research topics that the burst keyword analysis has shown.
The “burst keywords” can be categorized into three phases based on when they started and ended. Studies in this field initially concentrated on controlled trials involving bacterial translocation and irritable bowel syndrome from 2003 to 2007 (O’Mahony et al., 2009; Dinan and Cryan, 2012; Kennedy et al., 2012; Park et al., 2013; Garate et al., 2014; Brzozowski et al., 2016; Pigrau et al., 2016; Azpiroz et al., 2017; Morris et al., 2017; Sundman et al., 2017; Lin et al., 2018; Roomruangwong et al., 2018, 2019). The second stage, which lasted from 2008 to 2014, concentrated on animal trials involving anxiety, the central nervous system, diet-induced obesity, experimental autoimmune encephalomyelitis, and the brain-gut axis (Ochoa-Reparaz et al., 2011; Foster and Neufeld, 2013; Winek et al., 2016; Scott et al., 2017; Vaughn et al., 2017). The research flora focused on lactobacillus helveticus and probiotic bifidobacterium, while the modeling method concentrated on the high-fat diet (McKernan et al., 2010; Ohland et al., 2013; Ait-Belgnaoui et al., 2014; Kang et al., 2014; Mayer et al., 2014; Liang et al., 2015; Magnusson et al., 2015; Guillemot-Legris et al., 2016; Baker et al., 2017; Li et al., 2018; Schachter et al., 2018; Hassan et al., 2019; Beraldi et al., 2020). After 2015, the main diseases studied were functional gastrointestinal disorders, stress response, ulcerative colitis, prenatal stress, schizophrenia, and mental disorders (Daulatzai, 2015; Schmidt et al., 2015; Davis et al., 2016; Evrensel and Ceylan, 2016; Gur et al., 2017, 2019; Abautret-Daly et al., 2018; Jasarevic et al., 2018; Ju et al., 2020; Bioque et al., 2021; Luo et al., 2021; Yanguas-Casas et al., 2021; Urbonaite et al., 2022). Brain-derived neurotrophic factors and Toll-like receptors were the key research factors (Zeraati et al., 2019; Haghighat et al., 2021). Pathological experiments were the primary study methodology, while probiotic therapy was the primary research topic (Akbari et al., 2016; Kozyrev et al., 2020; Bannerman et al., 2022; Pochakom et al., 2022). Notably, including “schizophrenia” (burst strength 4.35), “pathology” (burst strength 3.81), and “psychiatric disorder” (burst strength 3.26), which are the current research frontiers in this field and are currently within the burst period.
Conclusion
In this study, 2,275 original studies from 2002 to 2022 (August 20, 2022) related to the research in gastrointestinal microbiome and neuroscience were downloaded from the WoSCC database and analyzed using VOSviewer to generate knowledge maps. The number of articles on the research in gastrointestinal microbiome and neuroscience has increased rapidly in recent years. The United States and China have made the most notable contributions to the research in gastrointestinal microbiomes and neuroscience. Research on “gastrointestinal microbiome, inflammation: the link between obesity, insulin-resistance and cognition” and “the role of two important theories of the gut-brain axis and microbial-gut-brain axis in diseases in neuroscience” will continue to be the hotspot. Further studies on the mechanisms linking gastrointestinal microbiome and neuroscience will promote neuroscience disease therapy.
Data availability statement
The original contributions presented in this study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
DL and YD conceived the study and performed critical revision of the manuscript. JY designed the study, performed statistical analyses, and drafted the manuscript. YC, YL, LP, and ZL performed the article retrieval, data interpretation, and provided supervision. All authors read and approved the final manuscript for publication.
Funding
This work was supported by the Key Research and Development Project of Education Department of Hunan Province (No. 21A0228), Hunan Provincial Administration of Traditional Chinese Medicine (No. E2022010), Innovation Team of Department of Science and Technology of Hunan Province (No. 2020RC4050), and Scientific Research Project of Hunan Education Department (No. 20A365).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Footnotes
- ^ https://www.webofscience.com/wos/woscc/summary/156f3a2d-3623-4356-9a8a-55d8dbeeecc8-56b9ffef/relevance/1
- ^ https://sourceforge.net/
References
Abautret-Daly, A., Dempsey, E., Parra-Blanco, A., Medina, C., and Harkin, A. (2018). Gut-brain actions underlying comorbid anxiety and depression associated with inflammatory bowel disease. Acta Neuropsychiatr. 30, 275–296. doi: 10.1017/neu.2017.3
Adolphs, R. (2015). The unsolved problems of neuroscience. Trends Cogn. Sci. 19, 173–175. doi: 10.1016/j.tics.2015.01.007
Afzal, M., Mazhar, S. F., Sana, S., Naeem, M., Rasool, M. H., Saqalein, M., et al. (2020). Neurological and cognitive significance of probiotics: A holy grail deciding individual personality. Future Microbiol. 15, 1059–1074. doi: 10.2217/fmb-2019-0143
Agahi, A., Hamidi, G. A., Daneshvar, R., Hamdieh, M., Soheili, M., Alinaghipour, A., et al. (2018). Does severity of Alzheimer’s disease contribute to its responsiveness to modifying gut microbiota? A double blind clinical trial. Front. Neurol. 9:662. doi: 10.3389/fneur.2018.00662
Ait-Belgnaoui, A., Colom, A., Braniste, V., Ramalho, L., Marrot, A., Cartier, C., et al. (2014). Probiotic gut effect prevents the chronic psychological stress-induced brain activity abnormality in mice. Neurogastroent. Motil. 26, 510–520. doi: 10.1111/nmo.12295
Akbari, E., Asemi, Z., Kakhaki, R. D., Bahmani, F., Kouchaki, E., Tamtaji, O. R., et al. (2016). Effect of probiotic supplementation on cognitive function and metabolic status in Alzheimer’s disease: A randomized, double-blind and controlled trial. Front. Aging Neurosci. 8:256. doi: 10.3389/fnagi.2016.00256
Anonymous. (2022). A systematic review of gut microbiota composition in observational studies of major depressive disorder, bipolar disorder and schizophrenia. Mol. Psychiatr. 27, 1920–1935. doi: 10.1038/s41380-022-01456-3
Azpiroz, F., Dubray, C., Bernalier-Donadille, A., Cardot, J., Accarino, A., Serra, J., et al. (2017). Effects of scFOS on the composition of fecal microbiota and anxiety in patients with irritable bowel syndrome: A randomized, double blind, placebo controlled study. Neurogastroent. Motil. 29, e12911. doi: 10.1111/nmo.12911
Baker, K. D., Loughman, A., Spencer, S. J., and Reichelt, A. C. (2017). The impact of obesity and hypercaloric diet consumption on anxiety and emotional behavior across the lifespan. Neurosci. Biobehav. Rev. 83, 173–182. doi: 10.1016/j.neubiorev.2017.10.014
Bannerman, C. A., Douchant, K., Segal, J. P., Knezic, M., Mack, A. E., Lundell-Creagh, C., et al. (2022). Spinal cord injury in mice affects central and peripheral pathology in a severity-dependent manner. Pain 163, 1172–1185. doi: 10.1097/j.pain.0000000000002471
Beraldi, E. J., Borges, S. C., de Almeida, F. L. A., Dos Santos, A., Saad, M. J. A., and Buttow, N. C. (2020). Colonic neuronal loss and delayed motility induced by high-fat diet occur independently of changes in the major groups of microbiota in Swiss mice. Neurogastroent. Motil. 32:e137452. doi: 10.1111/nmo.13745
Bercik, P., Denou, E., Collins, J., Jackson, W., Lu, J., Jury, J., et al. (2011). The intestinal microbiota affect central levels of brain-derived neurotropic factor and behavior in mice. Gastroenterology 141, 599–609, 609.e1–3. doi: 10.1053/j.gastro.2011.04.052
Bioque, M., Gonzalez-Rodriguez, A., Garcia-Rizo, C., Cobo, J., Antonio Monreal, J., Usall, J., et al. (2021). Targeting the microbiome-gut-brain axis for improving cognition in schizophrenia and major mood disorders: A narrative review. Prog. Neuro Psychoph. 105:110130. doi: 10.1016/j.pnpbp.2020.110130
Bravo, J. A., Forsythe, P., Chew, M. V., Escaravage, E., Savignac, H. M., Dinan, T. G., et al. (2011). Ingestion of Lactobacillus strain regulates emotional behavior and central GABA receptor expression in a mouse via the vagus nerve. Proc. Natl. Acad. Sci. U.S.A. 108, 16050–16055. doi: 10.1073/pnas.1102999108
Brzozowski, B., Mazur-Bialy, A., Pajdo, R., Kwiecien, S., Bilski, J., Zwolinska-Wcislo, M., et al. (2016). Mechanisms by which stress affects the experimental and clinical inflammatory bowel disease (IBD): Role of brain-gut axis. Curr. Neuropharmacol. 14, 892–900. doi: 10.2174/1570159X14666160404124127
Cabanillas-Lazo, M., Quispe-Vicuna, C., Barja-Ore, J., Fernandez-Giusti, A., Munive-Degregori, A., Retamozo-Siancas, Y., et al. (2022). A 10-Year bibliometric analysis of global research on gut microbiota and Parkinson’s disease: Characteristics, impact, and trends. Biomed Res. Int. 2022:4144781. doi: 10.1155/2022/4144781
Cenit, M. C., Sanz, Y., and Codoner-Franch, P. (2017). Influence of gut microbiota on neuropsychiatric disorders. World J. Gastroenterol. 23, 5486–5498. doi: 10.3748/wjg.v23.i30.5486
Cesarano, C., Aulicino, G., Cerrano, C., Ponti, M., and Puce, S. (2021). Scientific knowledge on marine beach litter: A bibliometric analysis. Mar. Pollut. Bull. 173(Pt B):113102. doi: 10.1016/j.marpolbul.2021.113102
Chen, C., Dubin, R., and Kim, M. C. (2014). Emerging trends and new developments in regenerative medicine: A scientometric update (2000 - 2014). Expert Opin. Biol. Ther. 14, 1295–1317. doi: 10.1517/14712598.2014.920813
Chen, C., Lou, Y., Li, X. Y., Lv, Z. T., Zhang, L. Q., and Mao, W. (2020). Mapping current research and identifying hotspots on mesenchymal stem cells in cardiovascular disease. Stem. Cell Res. Ther. 11:498. doi: 10.1186/s13287-020-02009-7
Chivero, E. T., Sil, S., Singh, S., Thangaraj, A., Gordon, L., Evah-Nzoughe, G. B., et al. (2021). Protective Role of Lactobacillus rhamnosus probiotic in reversing cocaine-induced oxidative stress, glial activation and locomotion in mice. J. Neuroimmune Pharm. 1–14. doi: 10.1007/s11481-021-10020-9
Clarke, G., Grenham, S., Scully, P., Fitzgerald, P., Moloney, R. D., Shanahan, F., et al. (2013). The microbiome-gut-brain axis during early life regulates the hippocampal serotonergic system in a sex-dependent manner. Mol. Psychiatr. 18, 666–673. doi: 10.1038/mp.2012.77
Connell, E., Le Gall, G., Pontifex, M. G., Sami, S., Cryan, J. F., Clarke, G., et al. (2022). Microbial-derived metabolites as a risk factor of age-related cognitive decline and dementia. Mol. Psychiatr. 17:43. doi: 10.1186/s13024-022-00548-6
Cryan, J. F., and Dinan, T. G. (2012). Mind-altering microorganisms: The impact of the gut microbiota on brain and behaviour. Nat. Rev. Neurosci. 13, 701–712. doi: 10.1038/nrn3346
Daulatzai, M. A. (2015). Non-celiac gluten sensitivity triggers gut dysbiosis, neuroinflammation, gut-brain axis dysfunction, and vulnerability for dementia. CNS Neurol. Disord. Drug Targets 14, 110–131.
Davis, D. J., Bryda, E. C., Gillespie, C. H., and Ericsson, A. C. (2016). Microbial modulation of behavior and stress responses in zebrafish larvae. Behav. Brain Res. 311, 219–227. doi: 10.1016/j.bbr.2016.05.040
Devos, P., and Menard, J. (2020). Trends in worldwide research in hypertension over the period 1999-2018: A bibliometric study. Hypertension 76, 1649–1655. doi: 10.1161/HYPERTENSIONAHA.120.15711
Diaz, H. R., Wang, S., Anuar, F., Qian, Y., Bjorkholm, B., Samuelsson, A., et al. (2011). Normal gut microbiota modulates brain development and behavior. Proc. Natl. Acad. Sci. U.S.A. 108, 3047–3052. doi: 10.1073/pnas.1010529108
Dinan, T. G., and Cryan, J. F. (2012). Regulation of the stress response by the gut microbiota: Implications for psychoneuroendocrinology. Psychoneuroendocrinology 37, 1369–1378. doi: 10.1016/j.psyneuen.2012.03.007
Dinan, T. G., Borre, Y. E., and Cryan, J. F. (2014). Genomics of schizophrenia: Time to consider the gut microbiome? Mol. Psychiatr. 19, 1252–1257. doi: 10.1038/mp.2014.93
Dodiya, H. B., Forsyth, C. B., Voigt, R. M., Engen, P. A., Patel, J., Shaikh, M., et al. (2020). Chronic stress-induced gut dysfunction exacerbates Parkinson’s disease phenotype and pathology in a rotenone-induced mouse model of Parkinson’s disease. Neurobiol. Dis. 135:104352. doi: 10.1016/j.nbd.2018.12.012
Erny, D., de Angelis, A. L. H., Jaitin, D., Wieghofer, P., Staszewski, O., David, E., et al. (2015). Host microbiota constantly control maturation and function of microglia in the CNS. Nat. Neurosci. 18:965. doi: 10.1038/nn.4030
Evrensel, A., and Ceylan, M. E. (2016). Fecal microbiota transplantation and its usage in neuropsychiatric disorders. Clin. Psychopharmacol. Neurosci. 14, 231–237. doi: 10.9758/cpn.2016.14.3.231
Farooq, R. K., Alamoudi, W., Alhibshi, A., Rehman, S., Sharma, A. R., and Abdulla, F. A. (2022). Varied composition and underlying mechanisms of gut microbiome in neuroinflammation. Microorganisms 10:705. doi: 10.3390/microorganisms10040705
Flay, B. R. (1986). Efficacy and effectiveness trials (and other phases of research) in the development of health promotion programs. Prev. Med. 15, 451–474. doi: 10.1016/0091-7435(86)90024-1
Foster, J. A., and Neufeld, K. M. (2013). Gut-brain: How the microbiome influences anxiety and depression. Trends Neurosci. 36, 305–312. doi: 10.1016/j.tins.2013.01.005
Frausto, D. M., Forsyth, C. B., Keshavarzian, A., and Voigt, R. M. (2021). Dietary regulation of gut-brain axis in Alzheimer’s disease: Importance of microbiota metabolites. Front. Neurosci. 15:736814. doi: 10.3389/fnins.2021.736814
Friedland, R. P. (2015). Mechanisms of molecular mimicry involving the microbiota in neurodegeneration. J. Alzheimers Dis. 45, 349–362. doi: 10.3233/JAD-142841
Fu, R., Xu, H., Lai, Y., Sun, X., Zhu, Z., Zang, H., et al. (2022). A VOSviewer-based bibliometric analysis of prescription refills. Front. Med (Lausanne). 9:856420. doi: 10.3389/fmed.2022.856420
Fung, T. C., Olson, C. A., and Hsiao, E. Y. (2017). Interactions between the microbiota, immune and nervous systems in health and disease. Nat. Neurosci. 20, 145–155. doi: 10.1038/nn.4476
Garate, I., Garcia-Bueno, B., Munoz Madrigal, J. L., Caso, J. R., Alou, L., Luisa Gomez-Lus, M., et al. (2014). Toll-like 4 receptor inhibitor TAK-242 decreases neuroinflammation in rat brain frontal cortex after stress. J. Neuroinflamm. 11:8. doi: 10.1186/1742-2094-11-8
Guillemot-Legris, O., Masquelier, J., Everard, A., Cani, P. D., Alhouayek, M., and Muccioli, G. G. (2016). High-fat diet feeding differentially affects the development of inflammation in the central nervous system. J. Neuroinflamm. 13:206. doi: 10.1186/s12974-016-0666-8
Gur, T. L., Palkar, A. V., Rajasekera, T., Allen, J., Niraula, A., Godbout, J., et al. (2019). Prenatal stress disrupts social behavior, cortical neurobiology and commensal microbes in adult male offspring. Behav. Brain Res. 359, 886–894. doi: 10.1016/j.bbr.2018.06.025
Gur, T. L., Shay, L., Palkar, A. V., Fisher, S., Varaljay, V. A., Dowd, S., et al. (2017). Prenatal stress affects placental cytokines and neurotrophins, commensal microbes, and anxiety-like behavior in adult female offspring. Brain Behav. Immun. 64, 50–58. doi: 10.1016/j.bbi.2016.12.021
Haghighat, N., Rajabi, S., and Mohammadshahi, M. (2021). Effect of synbiotic and probiotic supplementation on serum brain-derived neurotrophic factor level, depression and anxiety symptoms in hemodialysis patients: A randomized, double-blinded, clinical trial. Nutr. Neurosci. 24, 490–499. doi: 10.1080/1028415X.2019.1646975
Hassan, A. M., Mancano, G., Kashofer, K., Froehlich, E. E., Matak, A., Mayerhofer, R., et al. (2019). High-fat diet induces depression-like behaviour in mice associated with changes in microbiome, neuropeptide Y, and brain metabolome. Nutr. Neurosci. 22, 877–893. doi: 10.1080/1028415X.2018.1465713
Hsiao, E. Y., McBride, S. W., Hsien, S., Sharon, G., Hyde, E. R., McCue, T., et al. (2013). Microbiota modulate behavioral and physiological abnormalities associated with neurodevelopmental disorders. Cell 155, 1451–1463. doi: 10.1016/j.cell.2013.11.024
Human Microbiome Project Consortium. (2012). A framework for human microbiome research. Nature 486, 215–221. doi: 10.1038/nature11209
Jasarevic, E., Howard, C. D., Morrison, K., Misic, A., Weinkopff, T., Scott, P., et al. (2018). The maternal vaginal microbiome partially mediates the effects of prenatal stress on offspring gut and hypothalamus. Nat. Neurosci. 21:1061. doi: 10.1038/s41593-018-0182-5
Jiang, H., Ling, Z., Zhang, Y., Mao, H., Ma, Z., Yin, Y., et al. (2015). Altered fecal microbiota composition in patients with major depressive disorder. Brain Behav. Immun. 48, 186–194. doi: 10.1016/j.bbi.2015.03.016
Joers, V., Masilamoni, G., Kempf, D., Weiss, A. R., Rotterman, T. M., Murray, B., et al. (2020). Microglia, inflammation and gut microbiota responses in a progressive monkey model of Parkinson’s disease: A case series. Neurobiol. Dis. 144:105027. doi: 10.1016/j.nbd.2020.105027
Ju, P., Ding, W., Chen, J., Cheng, Y., Yang, B., Huang, L., et al. (2020). The protective effects of Mogroside V and its metabolite 11-oxo-mogrol of intestinal microbiota against MK801-induced neuronal damages. Psychopharmacology 237, 1011–1026. doi: 10.1007/s00213-019-05431-9
Kang, S. S., Kurti, A., Fair, D. A., and Fryer, J. D. (2014). Dietary intervention rescues maternal obesity induced behavior deficits and neuroinflammation in offspring. J. Neuroinflamm. 11:156. doi: 10.1186/s12974-014-0156-9
Kang, Y., Kang, X., Zhang, H., Liu, Q., Yang, H., and Fan, W. (2021). Gut microbiota and Parkinson’s disease: Implications for faecal microbiota transplantation therapy. ASN Neuro 13:17590914211016217.
Kennedy, P. J., Clarke, G., Quigley, E. M. M., Groeger, J. A., Dinan, T. G., and Cryan, J. F. (2012). Gut memories: Towards a cognitive neurobiology of irritable bowel syndrome. Neurosci. Biobehav. Rev. 36, 310–340. doi: 10.1016/j.neubiorev.2011.07.001
Kozyrev, N., Albers, S., Yang, J., Prado, V. F., Prado, M. A. M., Fonseca, G. J., et al. (2020). Infiltrating hematogenous macrophages aggregate around beta-amyloid plaques in an age- and sex-dependent manner in a mouse model of Alzheimer disease. J. Neuropath. Exp. Neur. 79, 1147–1162. doi: 10.1093/jnen/nlaa093
Kumari, S., Goyal, V., Kumaran, S. S., Dwivedi, S. N., Srivastava, A., and Jagannathan, N. R. (2020). Quantitative metabolomics of saliva using proton NMR spectroscopy in patients with Parkinson’s disease and healthy controls. Neurol. Sci. 41, 1201–1210. doi: 10.1007/s10072-019-04143-4
Li, N., Wang, Q., Wang, Y., Sun, A., Lin, Y., Jin, Y., et al. (2018). Oral probiotics ameliorate the behavioral deficits induced by chronic mild stress in mice via the gut microbiota-inflammation axis. Front. Behav. Neurosci. 12:266. doi: 10.3389/fnbeh.2018.00266
Li, S., Hua, D., Wang, Q., Yang, L., Wang, X., Luo, A., et al. (2020). The Role of bacteria and its derived metabolites in chronic pain and depression: Recent findings and research progress. Int. J. Neuropsychoph. 23, 26–41. doi: 10.1093/ijnp/pyz061
Liang, S., Wang, T., Hu, X., Luo, J., Li, W., Wu, X., et al. (2015). administration of Lactobacillus helveticus ns8 improves behavioral, cognitive, and biochemical aberrations caused by chronic restraint stress. Neuroscience 310, 561–577. doi: 10.1016/j.neuroscience.2015.09.033
Lin, S. S., Zhang, R. Q., Shen, L., Xu, X. J., Li, K., Bazhin, A. V., et al. (2018). Alterations in the gut barrier and involvement of Toll-like receptor 4 in murine postoperative ileus. Neurogastroent. Motil. 30:e13286. doi: 10.1111/nmo.13286
Lloyd-Price, J., Abu-Ali, G., and Huttenhower, C. (2016). The healthy human microbiome. Gen. Med. 8:51. doi: 10.1186/s13073-016-0307-y
Luo, C., Wang, X., Huang, H., Mao, X., Zhou, H., and Liu, Z. (2021). Coadministration of metformin prevents olanzapine-induced metabolic dysfunction and regulates the gut-liver axis in rats. Psychopharmacology 238, 239–248. doi: 10.1007/s00213-020-05677-8
Ma, S., Yan, J., Chen, L., Zhu, Y., Chen, K., Zheng, C., et al. (2021). A bibliometric and visualized analysis of cardiac regeneration over a 20-year period. Front. Cardiovasc. Med. 8:789503. doi: 10.3389/fcvm.2021.789503
Ma, T., Jin, H., Kwok, L., Sun, Z., Liong, M., and Zhang, H. (2021). Probiotic consumption relieved human stress and anxiety symptoms possibly via modulating the neuroactive potential of the gut microbiota. Neurobiol. Stress 14:100294. doi: 10.1016/j.ynstr.2021.100294
Magnusson, K. R., Hauck, L., Jeffrey, B. M., Elias, V., Humphrey, A., Nath, R., et al. (2015). Relationships between diet-related changes in the gut microbiome and cognitive flexibility. Neuroscience 300, 128–140. doi: 10.1016/j.neuroscience.2015.05.016
Mayer, E. A., Knight, R., Mazmanian, S. K., Cryan, J. F., and Tillisch, K. (2014). Gut microbes and the brain: Paradigm shift in neuroscience. J. Neurosci. 34, 15490–15496. doi: 10.1523/JNEUROSCI.3299-14.2014
McKernan, D. P., Fitzgerald, P., Dinan, T. G., and Cryan, J. F. (2010). The probiotic Bifidobacterium infantis 35624 displays visceral antinociceptive effects in the rat. Neurogastroent. Motil. 22:1029. doi: 10.1111/j.1365-2982.2010.01520.x
Mertsalmi, T. H., Aho, V. T. E., Pereira, P. A. B., Paulin, L., Pekkonen, E., Auvinen, P., et al. (2017). More than constipation - bowel symptoms in Parkinson’s disease and their connection to gut microbiota. Eur. J. Neurol. 24, 1375–1383. doi: 10.1111/ene.13398
Milat, A. J., Bauman, A. E., Redman, S., and Curac, N. (2011). Public health research outputs from efficacy to dissemination: A bibliometric analysis. BMC Public Health. 11:934. doi: 10.1186/1471-2458-11-934
Morris, G., Berk, M., Carvalho, A., Caso, J. R., Sanz, Y., Walder, K., et al. (2017). The Role of the microbial metabolites including tryptophan catabolites and short chain fatty acids in the pathophysiology of immune-inflammatory and neuroimmune disease. Mol. Neurobiol. 54, 4432–4451. doi: 10.1007/s12035-016-0004-2
Mu, F., Tang, M., Guan, Y., Lin, R., Zhao, M., Zhao, J., et al. (2022). Knowledge Mapping of the links between the gut microbiota and heart failure: A scientometric investigation (2006-2021). Front. Cardiovascu. Med. 9:882660. doi: 10.3389/fcvm.2022.882660
Neufeld, K. M., Kang, N., Bienenstock, J., and Foster, J. A. (2011). Reduced anxiety-like behavior and central neurochemical change in germ-free mice. Neurogastroent. Motil. 23, 255–64,e119. doi: 10.1111/j.1365-2982.2010.01620.x
Ni, Y., Yang, X., Zheng, L., Wang, Z., Wu, L., Jiang, J., et al. (2019). Lactobacillus and Bifidobacterium improves physiological function and cognitive ability in aged mice by the regulation of gut microbiota. Mol. Nutr. Food Res. 63:e1900603. doi: 10.1002/mnfr.201900603
Ni, Z., Wang, S., Li, Y., Zhou, L., Zhai, D., Xia, D., et al. (2022). Mapping trends and hotspot regarding gut microbiota and host immune response: A bibliometric analysis of global research (2011-2021). Front. Microbiol. 13:932197. doi: 10.3389/fmicb.2022.932197
Ochoa-Reparaz, J., Mielcarz, D. W., Begum-Haque, S., and Kasper, L. H. (2011). Gut, bugs, and brain: Role of commensal bacteria in the control of central nervous system disease. Ann. Neurol. 69, 240–247. doi: 10.1002/ana.22344
Ohland, C. L., Kish, L., Bell, H., Thiesen, A., Hotte, N., Pankiv, E., et al. (2013). Effects of Lactobacillus helveticus on murine behavior are dependent on diet and genotype and correlate with alterations in the gut microbiome. psychoneuroendocrinology 38, 1738–1747. doi: 10.1016/j.psyneuen.2013.02.008
O’Mahony, S. M., Clarke, G., Borre, Y. E., Dinan, T. G., and Cryan, J. F. (2015). Serotonin, tryptophan metabolism and the brain-gut-microbiome axis. Behav. Brain Res. 277, 32–48. doi: 10.1016/j.bbr.2014.07.027
O’Mahony, S. M., Marchesi, J. R., Scully, P., Codling, C., Ceolho, A., Quigley, E. M. M., et al. (2009). Early life stress alters behavior, immunity, and microbiota in rats: Implications for irritable bowel syndrome and psychiatric illnesses. Biol. Psychiat. 65, 263–267. doi: 10.1016/j.biopsych.2008.06.026
Pan, C., Jiang, N., Cao, B., and Dong, C. (2021). Global trends and performances of Mediterranean diet: A bibliometric analysis in CiteSpace. Medicine (Baltimore) 100:e27175. doi: 10.1097/MD.0000000000027175
Park, A. J., Collins, J., Blennerhassett, P. A., Ghia, J. E., Verdu, E. F., Bercik, P., et al. (2013). Altered colonic function and microbiota profile in a mouse model of chronic depression. Neurogastroent. Motil. 25, 733–e575. doi: 10.1111/nmo.12153
Pasin, O., and Pasin, T. (2021). A bibliometric analysis of rheumatology and COVID-19 researches. Clin. Rheumatol. 40, 4735–4740. doi: 10.1007/s10067-021-05844-y
Pigrau, M., Rodino-Janeiro, B. K., Casado-Bedmar, M., Lobo, B., Vicario, M., Santos, J., et al. (2016). The joint power of sex and stress to modulate brain-gut-microbiota axis and intestinal barrier homeostasis: Implications for irritable bowel syndrome. Neurogastroent. Motil. 28, 463–486. doi: 10.1111/nmo.12717
Pochakom, A., Mu, C., Rho, J. M., Tompkins, T. A., Mayengbam, S., and Shearer, J. (2022). Selective probiotic treatment positively modulates the microbiota-gut-brain axis in the BTBR Mouse model of autism. Brain Sci. 12:781. doi: 10.3390/brainsci12060781
Proctor, L. M. (2011). The human microbiome project in 2011 and beyond. Cell Host Microbe 10, 287–291. doi: 10.1016/j.chom.2011.10.001
Quercia, S., Candela, M., Giuliani, C., Turroni, S., Luiselli, D., Rampelli, S., et al. (2014). From lifetime to evolution: Timescales of human gut microbiota adaptation. Front. Microbiol. 5:587. doi: 10.3389/fmicb.2014.00587
Rajanala, K., Kumar, N., and Chamallamudi, M. R. (2021). Modulation of gut-brain axis by probiotics: A promising anti-depressant approach. Curr. Neuropharmacol. 19, 990–1006. doi: 10.2174/1570159X19666201215142520
Reichmann, H. (2011). View point: Etiology in Parkinson’s disease. Dual hit or spreading intoxication. J. Neurol. Sci. 310, 9–11. doi: 10.1016/j.jns.2011.04.016
Robles-Alonso, V., and Guarner, F. (2014). From basic to applied research: Lessons from the human microbiome projects. J. Clin. Gastroenterol. 48(Suppl. 1) S3–S4. doi: 10.1097/MCG.0000000000000242
Roomruangwong, C., Anderson, G., Berk, M., Stoyanov, D., Carvalho, A. F., and Maes, M. (2018). A neuro-immune, neuro-oxidative and neuro-nitrosative model of prenatal and postpartum depression. Prog. Neuro Psychoph. 81, 262–274. doi: 10.1016/j.pnpbp.2017.09.015
Roomruangwong, C., Carvalho, A. F., Geffard, M., and Maes, M. (2019). The menstrual cycle may not be limited to the endometrium but also may impact gut permeability. Acta Neuropsychiatr. 31, 294–304. doi: 10.1017/neu.2019.30
Rudzki, L., Ostrowska, L., Pawlak, D., Malus, A., Pawlak, K., Waszkiewicz, N., et al. (2019). Probiotic Lactobacillus Plantarum 299v decreases kynurenine concentration and improves cognitive functions in patients with major depression: A double-blind, randomized, placebo controlled study. Psychoneuroendocrinology 100, 213–222. doi: 10.1016/j.psyneuen.2018.10.010
Sampson, T. R., Debelius, J. W., Thron, T., Janssen, S., Shastri, G. G., Ilhan, Z. E., et al. (2016). Gut microbiota regulate motor deficits and neuroinflammation in a model of Parkinson’s disease. Cell 167, 1469–1480. doi: 10.1016/j.cell.2016.11.018
Savignac, H. M., Tramullas, M., Kiely, B., Dinan, T. G., and Cryan, J. F. (2015). Bifidobacteria modulate cognitive processes in an anxious mouse strain. Behav. Brain Res. 287, 59–72. doi: 10.1016/j.bbr.2015.02.044
Schachter, J., Martel, J., Lin, C., Chang, C., Wu, T., Lu, C., et al. (2018). Effects of obesity on depression: A role for inflammation and the gut microbiota. Brain Behav. Immun. 69, 1–8. doi: 10.1016/j.bbi.2017.08.026
Scheperjans, F., Aho, V., Pereira, P. A., Koskinen, K., Paulin, L., Pekkonen, E., et al. (2015a). Gut microbiota are related to Parkinson’s disease and clinical phenotype. Mov. Disord. 30, 350–358. doi: 10.1002/mds.26069
Scheperjans, F., Pekkonen, E., Kaakkola, S., and Auvinen, P. (2015b). Linking smoking, coffee, urate, and Parkinson’s disease - A role for gut microbiota? J. Parkinson. Dis. 5, 255–262. doi: 10.3233/JPD-150557
Schmidt, K., Cowen, P. J., Harmer, C. J., Tzortzis, G., Errington, S., and Burnet, P. W. J. (2015). Prebiotic intake reduces the waking cortisol response and alters emotional bias in healthy volunteers. Psychopharmacology 232, 1793–1801. doi: 10.1007/s00213-014-3810-0
Scott, K. A., Ida, M., Peterson, V. L., Prenderville, J. A., Moloney, G. M., Izumo, T., et al. (2017). Revisiting Metchnikoff: Age-related alterations in microbiota-gut-brain axis in the mouse. Brain Behav. Immun. 65, 20–32. doi: 10.1016/j.bbi.2017.02.004
Shi, Y., Wei, W., Li, L., Wei, Q., Jiang, F., Xia, G., et al. (2021). The global status of research in breast cancer liver metastasis: A bibliometric and visualized analysis. Bioengineered 12, 12246–12262. doi: 10.1080/21655979.2021.2006552
Stilling, R. M., Ryan, F. J., Hoban, A. E., Shanahan, F., Clarke, G., Claesson, M. J., et al. (2015). Microbes & neurodevelopment - Absence of microbiota during early life increases activity-related transcriptional pathways in the amygdala. Brain Behav. Immun. 50, 209–220. doi: 10.1016/j.bbi.2015.07.009
Sudo, N., Chida, Y., Aiba, Y., Sonoda, J., Oyama, N., Yu, X. N., et al. (2004). Postnatal microbial colonization programs the hypothalamic-pituitary-adrenal system for stress response in mice. J. Physiol. 558, 263–275. doi: 10.1113/jphysiol.2004.063388
Sundman, M. H., Chen, N., Subbian, V., and Chou, Y. (2017). The bidirectional gut-brain-microbiota axis as a potential nexus between traumatic brain injury, inflammation, and disease. Brain Behav. Immun. 66, 31–44. doi: 10.1016/j.bbi.2017.05.009
Tao, D., Zhong, T., Pang, W., and Li, X. (2021). Saccharomyces boulardii improves the behaviour and emotions of spastic cerebral palsy rats through the gut-brain axis pathway. BMC Neurosci. 22:76. doi: 10.1186/s12868-021-00679-4
Tarr, A. J., Galley, J. D., Fisher, S. E., Chichlowski, M., Berg, B. M., and Bailey, M. T. (2015). The prebiotics 3’Sialyllactose and 6’Sialyllactose diminish stressor-induced anxiety-like behavior and colonic microbiota alterations: Evidence for effects on the gut-brain axis. Brain Behav. Immun. 50, 166–177. doi: 10.1016/j.bbi.2015.06.025
Teng, T., Clarke, G., Maes, M., Jiang, Y., Wang, J., Li, X., et al. (2022). Biogeography of the large intestinal mucosal and luminal microbiome in cynomolgus macaques with depressive-like behavior. Mol. Psychiatr. 27, 1059–1067. doi: 10.1038/s41380-021-01366-w
Turnbaugh, P. J., Ley, R. E., Hamady, M., Fraser-Liggett, C. M., Knight, R., and Gordon, J. I. (2007). The human microbiome project. Nature 449, 804–810. doi: 10.1038/nature06244
Urbonaite, G., Knyzeliene, A., Bunn, F. S., Smalskys, A., and Neniskyte, U. (2022). The impact of maternal high-fat diet on offspring neurodevelopment. Front. Neurosci. 16:909762. doi: 10.3389/fnins.2022.909762
van de Wouw, M., Lyte, J. M., Boehme, M., Sichetti, M., Moloney, G., Goodson, M. S., et al. (2020). The role of the microbiota in acute stress-induced myeloid immune cell trafficking. Brain Behav. Immun. 84, 209–217. doi: 10.1016/j.bbi.2019.12.003
van Eck, N. J., and Waltman, L. (2010). Software survey: VOSviewer, a computer program for bibliometric mapping. Scientometrics 84, 523–538. doi: 10.1007/s11192-009-0146-3
Vaughn, A. C., Cooper, E. M., DiLorenzo, P. M., O’Loughlin, L. J., Konkel, M. E., Peters, J. H., et al. (2017). Energy-dense diet triggers changes in gut microbiota, reorganization of gut-brain vagal communication and increases body fat accumulation. Acta Neurobiol. Exp. 77, 18–30. doi: 10.21307/ane-2017-033
Wang, H., Long, T., You, J., Li, P., and Xu, Q. (2022). bibliometric visualization analysis of microbiome-gut-brain axis from 2004 to 2020. Med. Sci. Monitor. 28:e936037. doi: 10.12659/MSM.936037
Wang, H., Yang, F., Zhang, S., Xin, R., and Sun, Y. (2021). Genetic and environmental factors in Alzheimer’s and Parkinson’s diseases and promising therapeutic intervention via fecal microbiota transplantation. NPJ Parkinsons Dis. 7:70. doi: 10.1038/s41531-021-00213-7
Wang, Y., Li, D., Jia, Z., Hui, J., Xin, Q., Zhou, Q., et al. (2022). A bibliometric analysis of research on the links between gut microbiota and atherosclerosis. Front. Cardiovascu. Med. 9:941607. doi: 10.3389/fcvm.2022.941607
Wiley, N. C., Dinan, T. G., Ross, R. P., Stanton, C., Clarke, G., and Cryan, J. F. (2017). The microbiota-gut-brain axis as a key regulator of neural function and the stress response: Implications for human and animal health. J. Anim. Sci. 95, 3225–3246. doi: 10.2527/jas.2016.1256
Winek, K., Dirnagl, U., and Meisel, A. (2016). The gut microbiome as therapeutic target in central nervous system diseases: Implications for stroke. Neurotherapeutics 13, 762–774. doi: 10.1007/s13311-016-0475-x
Wu, H., Cheng, K., Guo, Q., Yang, W., Tong, L., Wang, Y., et al. (2021a). Mapping knowledge structure and themes trends of osteoporosis in rheumatoid arthritis: A bibliometric analysis. Front. Med. (Lausanne) 8:787228. doi: 10.3389/fmed.2021.787228
Wu, H., Wang, Y., Tong, L., Yan, H., and Sun, Z. (2021b). Global research trends of ferroptosis: A rapidly evolving field with enormous potential. Front. Cell Dev. Biol. 9:646311. doi: 10.3389/fcell.2021.646311
Wu, J. C. (2012). Psychological co-morbidity in functional gastrointestinal disorders: Epidemiology, mechanisms and management. J. Neurogastroenterol. Motil. 18, 13–18. doi: 10.5056/jnm.2012.18.1.13
Xavier-Santos, D., Padilha, M., Fabiano, G. A., Vinderola, G., Gomes, C. A., Sivieri, K., et al. (2022). Evidences and perspectives of the use of probiotics, prebiotics, synbiotics, and postbiotics as adjuvants for prevention and treatment of COVID-19: A bibliometric analysis and systematic review. Trends Food Sci. Technol. 120, 174–192. doi: 10.1016/j.tifs.2021.12.033
Xu, P., Lv, T., Dong, S., Cui, Z., Luo, X., Jia, B., et al. (2022). Association between intestinal microbiome and inflammatory bowel disease: Insights from bibliometric analysis. Comput. Struct. Biotechnol. J. 20, 1716–1725. doi: 10.1016/j.csbj.2022.04.006
Yang, X., Qian, Y., Xu, S., Song, Y., and Xiao, Q. (2018). Longitudinal analysis of fecal microbiome and pathologic processes in a rotenone induced mice model of Parkinson’s disease. Front. Aging Neurosci. 9:441. doi: 10.3389/fnagi.2017.00441
Yang, Z., Li, J., Gui, X., Shi, X., Bao, Z., Han, H., et al. (2020). Updated review of research on the gut microbiota and their relation to depression in animals and human beings. Mol. Psychiatry 25, 2759–2772. doi: 10.1038/s41380-020-0729-1
Yanguas-Casas, N., Torres, C., Crespo-Castrillo, A., Diaz-Pacheco, S., Healy, K., Stanton, C., et al. (2021). High-fat diet alters stress behavior, inflammatory parameters and gut microbiota in Tg APP mice in a sex-specific manner. Neurobiol. Dis. 159:105495. doi: 10.1016/j.nbd.2021.105495
Zeraati, M., Enayati, M., Kafami, L., Shahidi, S. H., and Salari, A. (2019). Gut microbiota depletion from early adolescence alters adult immunological and neurobehavioral responses in a mouse model of multiple sclerosis. Neuropharmacology 157:107685. doi: 10.1016/j.neuropharm.2019.107685
Zhang, F., Luo, W., Shi, Y., Fan, Z., and Ji, G. (2012). Should we standardize the 1,700-year-old fecal microbiota transplantation? Am. J. Gastroenterol. 107, 1755–1756. doi: 10.1038/ajg.2012.251
Zhang, J., Song, L., Xu, L., Fan, Y., Wang, T., Tian, W., et al. (2021). Knowledge domain and emerging trends in ferroptosis research: A bibliometric and knowledge-map analysis. Front. Oncol. 11:686726. doi: 10.3389/fonc.2021.686726
Zheng, P., Wu, J., Zhang, H., Perry, S. W., Yin, B., Tan, X., et al. (2021). The gut microbiome modulates gut-brain axis glycerophospholipid metabolism in a region-specific manner in a nonhuman primate model of depression. Mol. Psychiatr. 26, 2380–2392. doi: 10.1038/s41380-020-0744-2
Zheng, P., Zeng, B., Zhou, C., Liu, M., Fang, Z., Xu, X., et al. (2016). Gut microbiome remodeling induces depressive-like behaviors through a pathway mediated by the host’s metabolism. Mol. Psychiatr. 21, 786–796. doi: 10.1038/mp.2016.44
Zhu, X., Hu, J., Deng, S., Tan, Y., Qiu, C., Zhang, M., et al. (2021). Bibliometric and visual analysis of research on the links between the gut microbiota and depression from 1999 to 2019. Front. Psychiatry 11:587670. doi: 10.3389/fpsyt.2020.587670
Keywords: gastrointestinal microbiome, neuroscience, bibliometric, visualization analysis, WoSCC, research trends, hotspots
Citation: Yang J, Deng Y, Cai Y, Liu Y, Peng L, Luo Z and Li D (2022) Mapping trends and hotspot regarding gastrointestinal microbiome and neuroscience: A bibliometric analysis of global research (2002–2022). Front. Neurosci. 16:1048565. doi: 10.3389/fnins.2022.1048565
Received: 19 September 2022; Accepted: 28 October 2022;
Published: 17 November 2022.
Edited by:
Silvia Turroni, University of Bologna, ItalyReviewed by:
Jan Pieter Konsman, Centre National de la Recherche Scientifique (CNRS), FranceKarolina Skonieczna-Żydecka, Pomeranian Medical University, Poland
Copyright © 2022 Yang, Deng, Cai, Liu, Peng, Luo and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Dingxiang Li, bGR4bHp5QGhvdG1haWwuY29t
 Jingjing Yang
Jingjing Yang Yihui Deng
Yihui Deng Yuzhe Cai1
Yuzhe Cai1