- 1Xiyuan Hospital, China Academy of Chinese Medical Sciences, Beijing, China
- 2Graduate School, China Academy of Chinese Medical Sciences, Beijing, China
- 3National Clinical Research Center for Chinese Medicine Cardiology, Beijing, China
- 4Institute of Geriatrics of China Academy of Chinese Medical Sciences, Beijing, China
- 5Wangjing Hospital, China Academy of Chinese Medical Sciences, Beijing, China
Background: With dementia significantly increasing hospitalization and disability rates, worldwide aging of the population presents major challenges to public health. The majority of cases of cognitive dysfunction among the elderly, however, are characterized by an identifiable, preventable and treatable vascular component. As such, increased study of preventative methods in the context of dementia is warranted. Traditional Chinese medicine compounds have been reported to be neuroprotective and improve cognitive function via a variety of mechanisms. Shen Ma Yi Zhi granule (SMYZG) is one such collection of compounds that has been proven clinically effective. Pharmacological mechanisms of action, pharmacokinetics and clinical applications of SMYZG have been previously studied using a variety of vascular dementia animal models. SMYZG activates and regulates four main signaling pathways relevant to vascular dementia including the AMPK/PPARα/PGC-1α/UCP2, Nrf2/HO-1, HIF-1/VEGF/Notch, and VEGF/Flk-1/p8 MAPK pathways. Furthermore, SMYZG influences anti-inflammatory and anti-oxidant stress responses, reverses demyelination of brain white matter and vascular endothelium, regulates pericyte function and normalizes mitochondrial metabolism. Neuroprotective effects of SMYZG, as well as those promoting regeneration of vascular endothelium, have also been reported in studies of rat models of vascular dementia. Future research concerning SMYG is warranted for development of vascular dementia preventative management strategies.
Introduction
Cognitive impairment significantly impairs thought, communication, comprehension, and memory formation processes. Vascular risk factors are among the leading etiologies of cognitive impairment among the elderly. Therapies capable of effectively delaying, preventing or treating cognitive decline, however, remain to be developed for clinical use. Recently, a number of studies have reported success in treating Alzheimer’s disease and vascular dementia with traditional Chinese medicine compounds as demonstrated using behavioral tests, histopathological examinations and indexes relevant to neurotransmitter catabolism. Importantly, these studies underscored the excellent potential that traditional Chinese medicine compounds have in future clinical use (Lecordier et al., 2021).
Shen Ma Yi Zhi granule (SMYZG), a Chinese herbal prescription, was demonstrated effective in treating vascular disease (Chang Surui, 2020). This compound consists of ginseng (Panax Ginseng C.A. Mey), Gastrodia elata (Gastrodia elata Bl), Euonymus alatus [Euonymus alatus (Thunb.) Sieb], and Ligustici (Ligusticum chuanxiong Hort). Pharmaceutical, pharmacodynamic and toxicological have been finished to determine the pharmaceutical extractions routing. In vivo studies have similarly been conducted (Li, 2017; Kun et al., 2020; Sun et al., 2021a,b; Lijuan et al., 2022). Importantly, aqueous extracts of SMYZG were reported to not only be neuroprotective but also beneficially affect learning and memory, hippocampal structure, central cholinergic system function as well as suppress the inflammatory and oxidative stress responses based on 2-VO and MID models; the primary active functional components were reported to be ginsenosides, gastrodin, ferulic acid, and quercetin (Nan-Nan et al., 2019). Toxicological studies revealed no negative effects on organs or critical biological parameters. Furthermore, SMYZG was awarded a National Invention Patent (Meixia, 2020) and recorded in the national registry of Chinese medicine preparations (No. Z20200005000).
Chemistry of Shen Ma Yi Zhi granule
Preparation processes
Shen Ma Yi Zhi granule is composed of ginseng (Panax Ginseng C.A. Mey), Gastrodia elata (Gastrodiaelata Bl), Euonymus alatus [Euonymus alatus (Thunb.) Sieb], and Ligustici (Ligusticum chuanxiong Hort) in a respective 3:3:3:2 ratio. All components meet the standards set forth by the Chinese Pharmacopoeia Commission (2015). Constituent weighting is achieved using the analytic hierarchy process (AHP) method. Orthogonal and single factor analyses were applied for optimization of extraction and purification technology used to prepare SMYZG by measuring yield rates of extraction and transition probability of gastrodin, p-hydroxybenzylalcohol, and ferulic acid. Briefly, Gastrodia elata, Euonymus alatus, and Ligustici slices are mixed, with water added to the mixture three times for 1 h each time. Ten times the amount of water per compound is added the first time while eight times the amount of water is added the second and third times. Ginseng slices are soaked in water for compound extraction twice and at 2 h per time; 12 times the amount of water is added to the ginseng the first time and 10 times the second. Mixing the extracts together in the Drug Manufacturing Room of Xiyuan Hospital, Chinese Academy of Chinese Medical Sciences, produces a crude drug extract of 2.44 g (Figure 1).
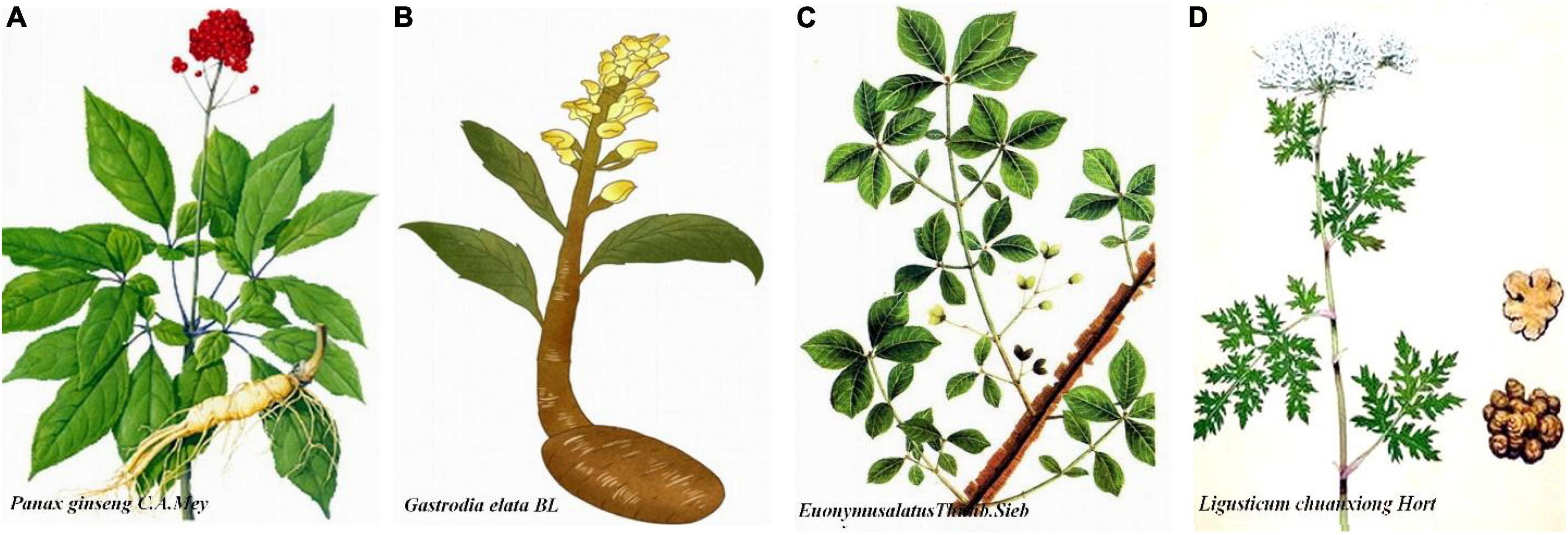
Figure 1. Detailing of herbal Shen Ma Yi Zhi granule (SMYZG) components. (A) Panax Ginseng C.A. Mey; (B) Gastrodia elata Bl; (C) Euonymus alatus (Thunb.) Sieb; (D) Ligusticum chuanxiong Hort. Sources for the images within this figure: 699pic.com and Chinese Pharmacopoeia.
Chemical components of Shen Ma Yi Zhi granule
Ginseng (Panax Ginseng C.A. Mey), a root of Panax (Araliaceae), contains panaxosides A, B, C, D, E, and F, volatile oil, Ginseng ene, vitamin B1, vitamin B2, nicotinic acid, niacinamide, pantothenic acid, choline, maltase, invertase, esterase, and a variety of amino acids (Jian and Shao-wa, 2021). Gastrodia elata (Gastrodiaelata Bl), i.e., dried Gastrodia elata plant content, contains compounds such as Gastrodia elata glucoside and Gastrodia elata ether glucoside (Wei et al., 2021). Euonymus alatus [Euonymus alatus (Thunb.) Sieb], the twig outgrowths of celastraceae plants, contains compounds such as stigmast-4-en-3-one, quercetin, β-sitosterol, dehydrodicatechin, aromadendrin, d-catechin, 4 β-sitosterone, alatamine, and wilfordine (Lei et al., 2015; Rui-xi et al., 2015; Yanxiu et al., 2021). Ligustici (Ligusticum chuanxiong Hort), i.e., dried Ligusticum plant content, contains compounds such as tetramethylpyrazine, perlolyrine, ligustilide, wallichilide, senkyunolide, vanillic acid, caffeic acid, protocatechuic acid, and ferulic acid (Jiangang et al., 2019; Li Qian, 2020; Zhonghui et al., 2020).
Importantly, there are four major clinically effective components of SMYZG, including b-D-glucopyranoside (3b, 12b)-3,12-dihydroxydammar-24-en-20-yl, 4 -(hydroxymethyl)phenyl-β-D-glucopyranoside, 4H-1-benzopyran-4-one, 2-(3,4-dihydroxyphenyl)-3,5,7-trihydroxy-flavone, 2–propenoic acid and 3-(4-hydroxy-3-methoxyphenyl). Molecular structures are detailed in Figure 2.
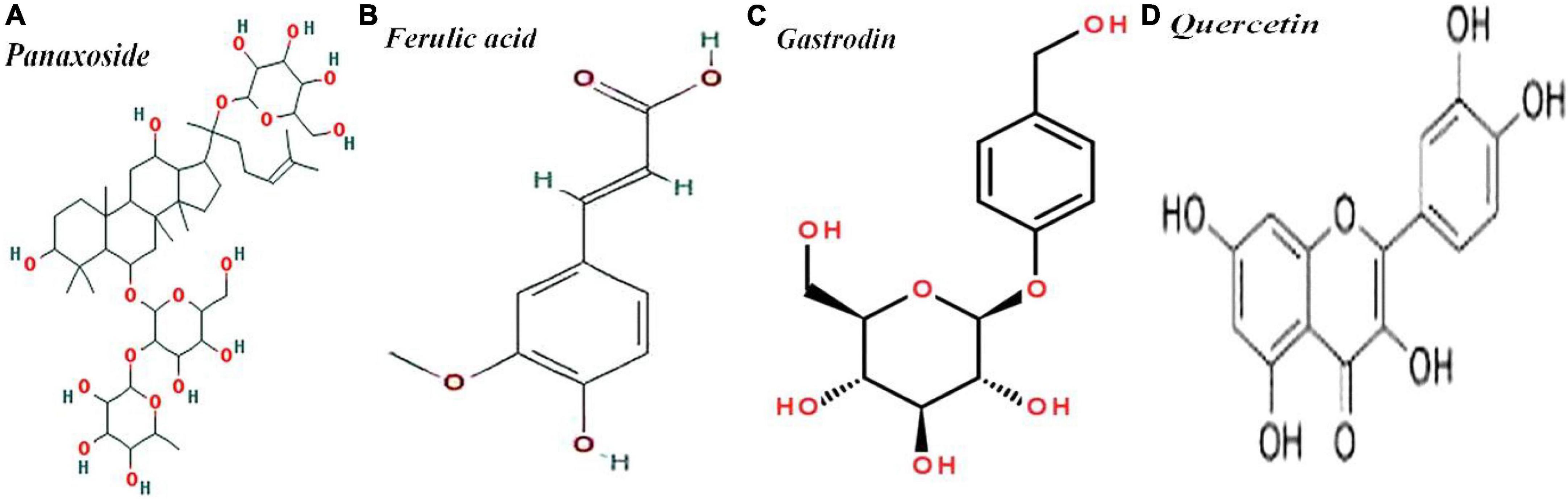
Figure 2. Molecular structures of (A) panaxoside/C36H62O8, (B) ferulic acid/C10H10O4, (C) gastrodin/C13H18O7, and (D) Quercetin/C15H10O7.
High-performance liquid chromatography parameters of SMYZG, shown in Figure 3, are as follows: chromatographic column, Tnature C18 column (4.6 mm × 250 mm, 5 min); drug elution times: 0–5 min, 0–14%; 6–10 min, 14–19%; 11–15 min, 19–20%; 16–20 min, 20–24%; 21–25 min, 20–24%. Mobile phase: methanol (A) approximately 0.1%; acetum (B) approximately 0.1%. Flow rate: 0.25 ml/min. UV detection wavelength: 321 nm. Injection volume: 5 μl (Figure 3).
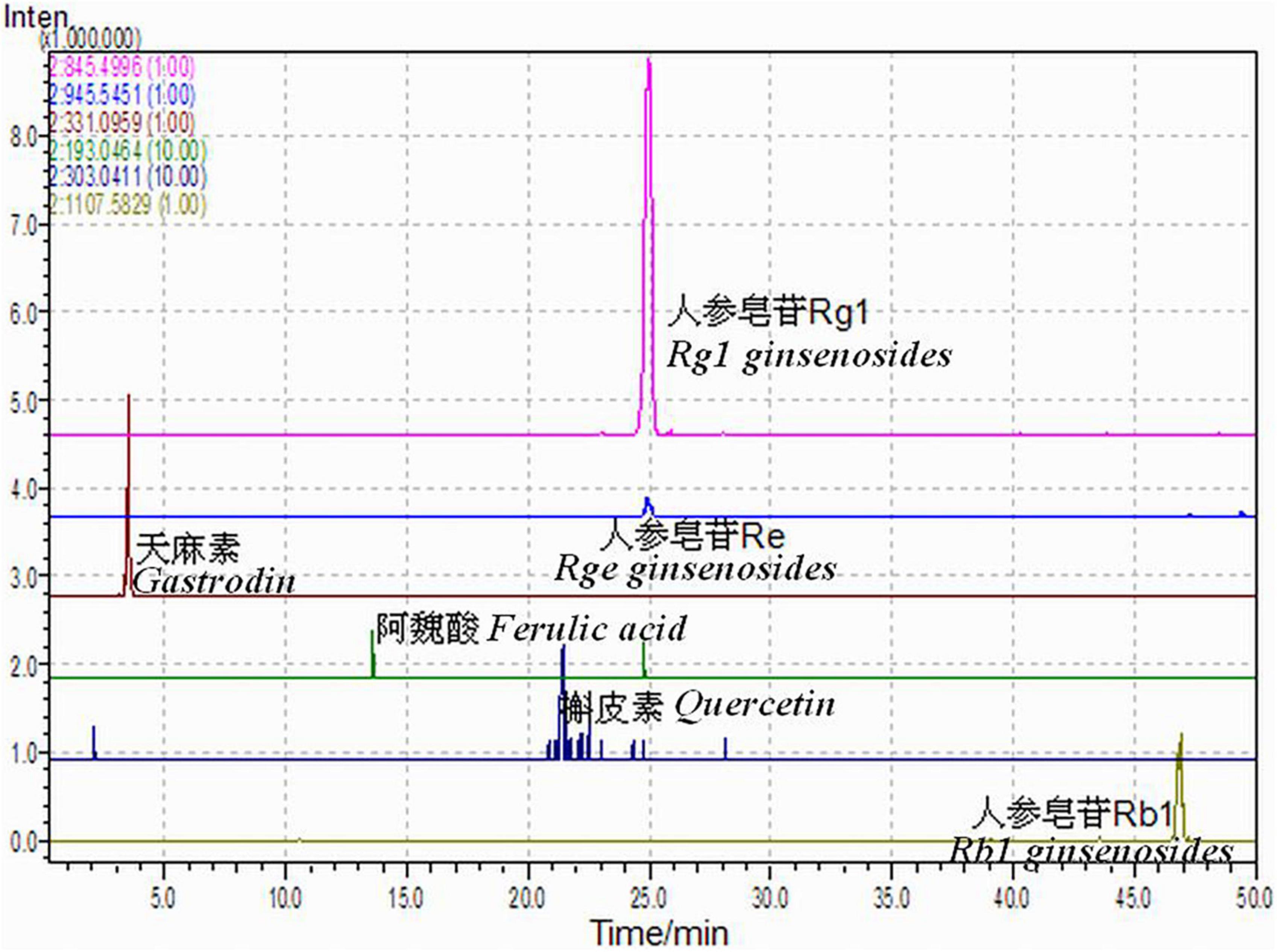
Figure 3. Shen Ma Yi Zhi granule (SMYZG) sample chromatogram absorption peaks and HPLC retention times.
Clinical studies concerning Shen Ma Yi Zhi granule
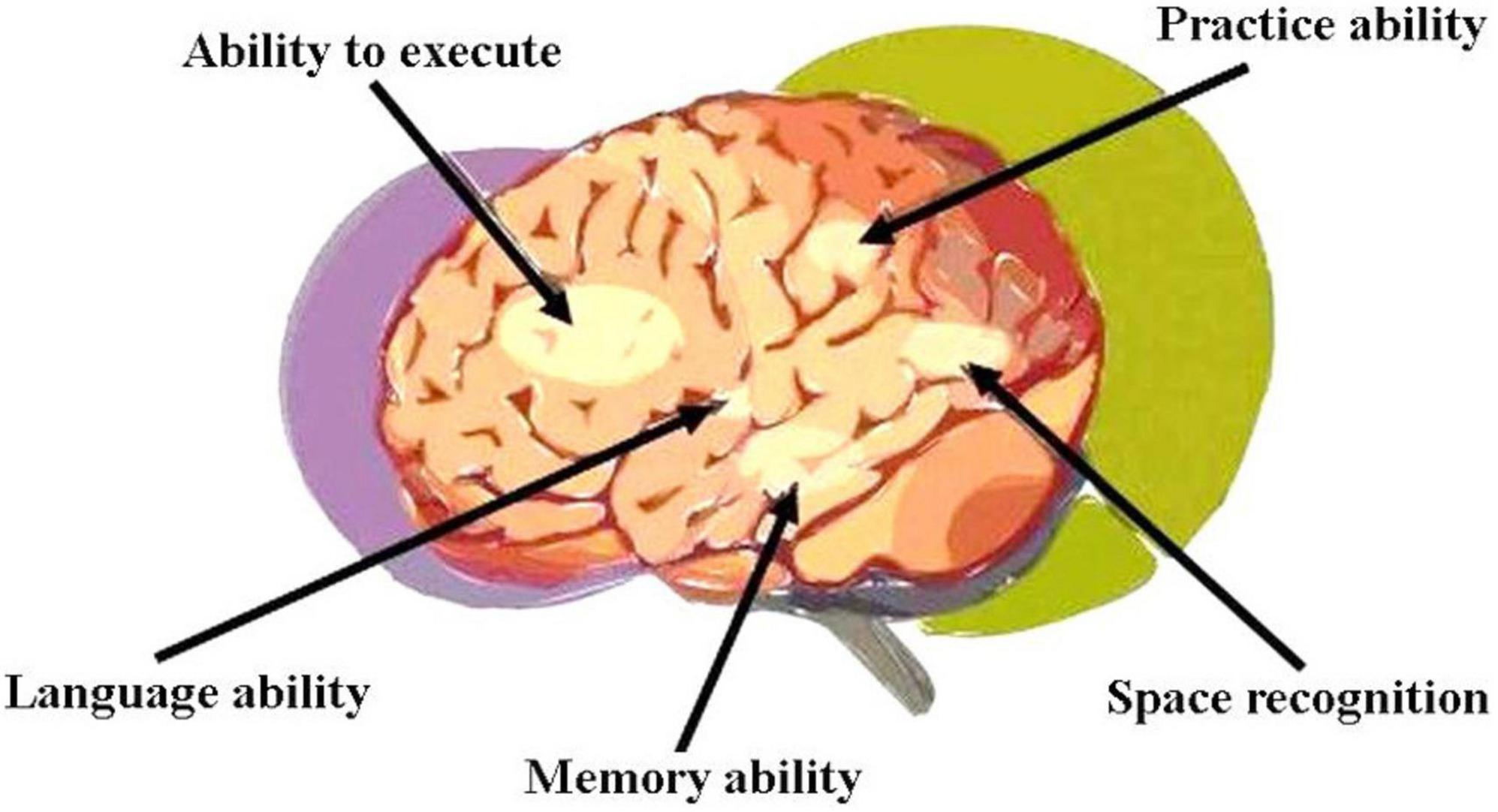
Vascular dementia (VaD), the second commonest form of dementia after Alzheimer’s disease (Huang et al., 2016), is primarily characterized by poor athletic performance, executive functioning, information processing, concentration, and memory (O’Brien et al., 2003; Hachinski et al., 2006; Moorhouse and Rockwood, 2008). Diagnosis is not always straightforward as a number of non-specific signs and wide variety of risk factors are often present among older patients. In contrast to Alzheimer’s, VaD generally manifests more acutely with executive functioning gradually declining and memory impairment fluctuating (Hachinski et al., 2006; Moorhouse and Rockwood, 2008). Functional regions (vascular control areas) involved in cortical dementia are shown in Figure 4.
Wu et al. (2017) recruited 60 mild and moderate VaD patients previously diagnosed according to Chinese guidelines for diagnosis and treatment of vascular cognitive impairment (Cognitive Impairment Committee NBCM, 2019), then observed the hemorheological indexes and tested their cognitive functioning. Mini-Mental State Exam (MMSE) scores of patients treated with SMYZG were found to have markedly improved; as such, SMYZG treatment was concluded to effectively improve cognition among this patient population. And it increased erythrocyte deformability, inhibited platelet function, reduced blood viscosity and improved the blood rheology.
One randomized controlled trial (Zhang et al., 2020) evaluating the clinical effectiveness of treating VaD patients with SMYZG in combination with Ginkgo biloba tablets revealed that this regimen increases MMSE scores, decreases Alzheimer’s Disease Assessment Scale-Cognitive Subscale (ADAS-Cog) scores, improves patient memory, attenuates mood fluctuations and improves ADL capacities. Furthermore, these compounds were noted to increase blood levels of both nitric oxide (NO) and vascular endothelial growth factor (VEGF) and thus improve endothelial function. In experimental group, the level of neuronspecific enolase (NSE) decreased and the level of brain-derived neurotrophic factor (BNDF) increased indicated that SMYZ had a certain clinical effect in nourishing nerves and repairing damaged nerves.
Model animal experiments
Selection of suitable animal models is key in successful experimental design and is also critical to result accuracy and objectivity. At least 10 methods of creating VaD animal models have been reported and include procedures such as vessel occlusion (VO), lacunar infarct embolization (MID) and photochemical induction (Lin et al., 2014). Studying multiple VaD animal models is therefore helpful in evaluating pharmacological mechanisms more precisely.
2-VO rat model data
The 2-VO rat model leads to chronic cerebral hypoperfusion and subsequent development of secondary cerebrovascular pathophysiological alterations, critical risk factors for VaD pathogenesis (Zhang et al., 2018). Advantages of this model include lower levels of injury inflicted on animals and rapid operating time. This model simulates hypoperfused (Tomimoto et al., 2003; Farkas et al., 2007) and hypoxic (Hu et al., 2017) states of the human brain, especially in structures related to recognition such as the hippocampus and cerebral cortex. These brain regions are more easily affected by oxidative stress, with resultant structural neuronal and cholinergic system damage significantly impairing learning, memory, and behavior (Luo et al., 2019; Zhu et al., 2019; Fan et al., 2020). Relevant mechanisms of these pathological processes are closely related with those of chronic cerebral ischemia (CCI) (Cheng et al., 2019).
Frequency of Morris water maze platform searching, frequency of original platform crossing and percentage of time spent swimming in the quadrant of the original platform location among rats in SMYZG treatment groups increased significantly. These data underscore that SMYZG improves learning and memory function among 2-VO rats. Importantly, SMYZG repairs 2-VO rat cortical damage by improving the loose arrangement of pyramidal cells, reducing axonotmesis and neuronal shrinkage, protecting the morphology and structure of neuronal mitochondria, as well as increasing the number of neuronal mitochondrial and surrounding microvasculature density.
MID rat model data
Multiple cerebral infarction, also known as white matter disease, is most commonly caused by systemic disease or brain lesions (Kalaria, 2016). Cerebral small vessel disease (CSVD) is related to the occurrence and development of MID and is one of the most common causes of VaD. MID patients with cognitive impairment frequently exhibit significant small vessel ischemic changes along with areas of cerebral infarction (Jiang et al., 2021). Many studies have utilized sludged blood or kelp microgelation (KMG) to construct MID rat models (Qun and Yongjun, 2015). Successful model construction is demonstrated by confirmation of multiple deep lacunar infarctions. Model rats exhibit obvious post-operative cognitive disorders (Qun and Yongjun, 2015).
Zhou et al. (Hao, 2020) utilized KMG for MID rat model construction. Among SMYZG treatment group rats, escape latency and swim distance in the platform quadrant were significantly shortened; the frequency of original platform crossing and percentage of swimming time, as well as percentage of swimming distance in the original platform quadrant were all significantly increased. Wen et al. reported that SMYZG ameliorates neuronal pathogenesis, shortens distances between pyramidal cells, increases Nissl body quantities, attenuates mitochondrial dysfunction and improves endoplasmic reticular function in the MID rat model (Li, 2017).
APOE–/– mice data
The Apolipoprotein E knock-out (ApoE–/–) mouse model is widely used to study atherosclerosis. ApoE–/– mice possess risk factors such as cerebral arteriosclerosis and lipometabolic disturbances. Blood-brain barrier disruptions and synaptic injury are among the pathological characteristics seen in this mouse model, as in VaD. Masliah et al. (1997) reported that 3-month-old ApoE–/– mice exhibit poorer learning and memory functionality as compared to control mice. Other studies similarly reported ApoE–/– mice to manifest neuropathologic changes resulting in worsened memory formation, among others. Along with worsened defense against oxidative damage in the ApoE–/– mouse brain, antioxidant enzyme levels decrease (Shea et al., 2002) and the hippocampus is significantly affected (Charles et al., 2000).
Screening of proteins expressed in vascular endothelium involved in brain lacunar infarcts (Wang et al., 2018) revealed pathologic changes in cerebral vascular endothelial cell permeability and vasodilation among ApoE–/– mice. Proteomics and network pharmacology suggests that SMYZG improves cognition via regulation of eNOS and CAV1 expression (Qiong et al., 2019).
Mechanistic studies concerning prevention of vascular dementia by Shen Ma Yi Zhi granule
Vascular dementia is commonly caused by hypoxic-ischemic brain damage via a number of mechanisms. Multiple infarcts can occur in the setting of numerous cardiovascular and cerebrovascular diseases as well as thrombotic conditions.
Effect on cholinergic levels
Central cholinergic system dysfunction induces hippocampal neuronal loss, decreased choline acetyltransferase and acetyl cholinesterase activity, and decreased muscarinic and nicotinic receptor density. Resultant neuronal damage manifests with memory and learning disorders (Jiamou et al., 2020; Ying et al., 2022).
The phenolic compounds erulic acid and alkaloid ligustrazine found in Ligustici are not metabolized and freely permeate the blood-brain barrier to reach the hippocampus (Mancuso and Santangelo, 2014; Koh, 2015; Qian and Zengchun, 2019). Like acetyl cholinesterase inhibitors, these compounds increase the amount of acetylcholine and exert neuroprotective effects (Yunfeng and Huixia, 2011). Gastrodin extract treatment was reported to shorten mouse escape time and platform search distance, as well as increase the amount of cerebral acetylcholine and alleviate dysmnesia induced by scopolamine (Chunni et al., 2014). Ginseng (Ying et al., 2014; Md et al., 2018), and in particular ginsenosides, play important roles in protecting against oxidative stress and regulating cholinergic signaling. Most notably, ginsenoside Rb1 was reported to increase hippocampal antioxidant levels (Rui-xi et al., 2015; Zhu et al., 2019). Quercetin extracted from Euonymus alatus was similarly reported to increase acetylcholinesterase levels (Rui-xi et al., 2015; Sharma et al., 2021; Poonam et al., 2022).
Effect on levels of inflammatory mediators
Neuroinflammation promotes VaD pathogenesis and imbalances in proinflammatory and antinflammatory factor secretion is relevant to involved pathomechanisms. A number of inflammatory markers have attracted attention as potential novel biomarkers due to changes in their levels early in VaD pathology. The inflammatory cascade is activated (Roux et al., 2001; Wyss-Coray and Mucke, 2002) by TNF-α, a monokine mainly produced by monocytes and macrophages. Belkhelfa et al. (2018) reported levels of TNF-α and IL-1β in the VaD hippocampus to be significantly higher as compared to those of control individuals. Their interaction with TLR-4, a receptor distributed mainly on the microglial cell surface, results in proinflammatory factor activation and neurodegeneration. Importantly, SMYZG decreases levels of IL-1β and TNF-α in VaD model rats.
Ferulic acid, a component of Ligustici, likely functions via ERK signaling (Guifang et al., 2021; Yuanyuan et al., 2021) to repress microglial activation and neuroinflammation. Ginsenosides (Bo et al., 2019) decrease levels of TLR3 and TRIF mRNA, resulting in activation of the TLR3/TRIF pathway and attenuation of inflammation in VaD rats. Gastrodia elata (Huan et al., 2017) ameliorates oxidative stress and inflammation and promotes humoral immunity, while quercetin (Poonam et al., 2022) decreases IL-6, IL-10, TNF-α, and acetylcholinesterase levels in VaD rats, attenuating endothelial dysfunction associated with hypertension.
Studies based on 2-VO rats suggest (Sun et al., 2020; Lijuan et al., 2022) that the level of 1L-1β and TNF-α increased in the model group, while decreased in SMYZ groups and had dose-effect relationship. Indicated that SMYZ can improve the learning and memory ability of rats with vascular cognitive disorder by inhibiting inflammatory response and improving oxidative stress state.
Effect on antioxidative stress system
The oxidative stress response increases the level of reactive oxygen species depletes antioxidative compound stores and negatively impacts neuronal survival (Agdeppa et al., 2003), thus damaging synaptic activity in the area of involvement. Alterations in subsequent neurotransmission result in cognitive impairment (Tönnies and Trushina, 2017).
Ginsenosides are known to exhibit antioxidative properties (Dongmin et al., 2020); the ginsenoside Rg1 was reported to reduce oxidative injury and attenuate cognitive impairment (Chen et al., 2018). In addition, this compound was reported to exert positive effects in the setting of cerebral ischemia-reperfusion injury (Chu et al., 2019). Ferulic acid is neuroprotective primarily via mechanisms that decrease intracellular oxidative stress (Jianliang et al., 2015; Ling et al., 2019). Both LT and aqueous extract of Ligustici decrease malondialdehyde (MDA) levels in hypoxic neurons and increase superoxide dismutase activity. Gastrodia extract passes through the blood-brain barrier and exerts effects directly on brain tissue to normalize hemangiectasis and increase blood flow to the ischemic area (Chunyan et al., 2009), thereby lessening neuronal injury. This extract also reduces cellular calcium overload and decreases toxic effects of excitatory amino acids to produce an anti-apoptotic effect (Yunlin, 2006; Qihai et al., 2011; Aili, 2018). Debin and Shaofen (2006) reported that flavonoids prevent MDA generation via decreasing levels of H2O2; quercetin (Wei Si-Can and Tian-lai, 2020) was found to decrease MDA levels and increase superoxide dismutase activity.
Studies based on 2-VO rats suggested (Yu Cao, 2019; Sun et al., 2020) that the level of SOD, GSH, and GSH-Px decreased significantly, the level of MDA increased in the model group. While the level of SOD, GSH, and GSH-Px increased, the level of MDA decreased in SMYZ groups. Indicated that SMYZ can depress activation and proliferation of glial cells in hippocampal CA1 region, and improve mitochondrial ultrastructure.
Effect on cerebral white matter myelination
Cerebral white matter lesions are the most common pathological marker of VaD (Prins and Scheltens, 2015; Alber et al., 2019), manifesting in myelin discontinuity (Hill and Grutzendler, 2019). Levels of myelin basic protein, a membrane protein found on the serosal myelin surface, is significantly related to the severity of myelinoclasis. It also serves as an important indicator of demyelinating disease severity and remyelination (Choi et al., 2016; Nasrabady et al., 2018). White matter lesions occur due to a number of etiologies such as hypoxia, oxidative stress and the resultant inflammatory response, blood-brain barrier permeability and neurovascular unit disorder (Li Zehui, 2020).
The ginsenoside Rb1 was previously reported to improve symptoms of neurologic impairment by increasing lacunar infarct density in areas of infarction via the promotion of angiogenesis-related factor expression, such as VEGF and Ang-2 (Xiao et al., 2019). This compound was also reported to promote cortical neuronal stem cell proliferation and differentiation toward neuroglia–like cells (Wang et al., 2009). In vitro studies of LT (Qin et al., 2018) revealed that phthalide compounds such as ligustilide and senkyunolide A facilitate the penetration by certain medications of the blood-brain barrier. Ligustilide protects damaged nerves by attenuating cerebral ischemia-reperfusion injury via antioxidant and anti-apoptotic activity (Peng et al., 2007; Li et al., 2017). Studies on models of corneal endothelial injury (Guofeng et al., 2012) revealed that LT protects the endothelia via amelioration of cell damage by inhibiting expression of COX-2 and NF-κB proteins, as well as decreasing MAPK phosphorylation. Phenolic compounds found in Gastrodia were reported to exert neuroprotective effects by decreasing hippocampal NO content and NOS activity, reducing nNOS and iNOS expression and promoting eNOS expression (Xiaohua et al., 2011; Table 1).
Signaling pathways relevant to vascular dementia treatment by Shen Ma Yi Zhi granule
Mechanistic studies of VaD have investigated apolipoprotein, tau and lipid metabolism, as well as immune and oxidative stress responses. Although amyloid-ß is currently considered to be the initiator of pathological changes in the setting of Alzheimer’s disease, genetic susceptibility factors mediating reactions to amyloid-ß such as metabolic processes, immunity and lysosomal function are of vital importance in the pathogenesis of VaD and Alzheimer’s.
Effect on mitochondrial metabolism
Chronic cerebral hypoperfusion refers to cerebral low-flow ischemia and hypoxic conditions due to angiostenosis. Chronic cerebral hypoperfusion is itself a risk factor for cognitive impairment and patients suffering cognitive decline commonly suffer significant cerebral hypoperfusion (Cao et al., 2016; Kalaria, 2016; Kazumata et al., 2019), and vice versa (Jessica et al., 2017).
Gastrodin was reported to increase neuronal oxygen metabolism, neuronal levels of ATP, promote neuronal glucose uptake and utilization, enhance memory and decrease lactate generation in the setting of ischemia (Meikang et al., 2010). The ginsenoside Re was reported to regenerate damaged neurons and improve cognition in rats via normalization of mitochondrial physiology (Wang et al., 2009).
Shen Ma Yi Zhi granule improves neuronal mitochondrial metabolism in 2-VO rats via the AMPK/PPARα/PGC-1α/UCP2 pathway (Sun et al., 2021b). Levels of AMPK, PPARα, PGC-1α, and ATP5A protein and mRNA were reported to be increased in the setting of SMYZG treatment, while levels of UCP2 gene expression were reported to be decreased (Figure 5).

Figure 5. Signaling pathways of Shen Ma Yi Zhi granule (SMYZG) in the context of vascular dementia (VaD) treatment. Sources for the images within this figure: 699pic.com and Chinese Pharmacopoeia.
Effect on anti-oxidative stress and ant-inflammatory signaling
Oxidative stress and inflammation directly damage cells (Beydoun et al., 2022). Bioactive products in the setting of oxidative stress initiate the inflammatory response (Wang et al., 2007) and are known to significantly aggravate cerebral injury. Astrocytes supply neuronal energy (Elkabes et al., 1996; Nakajima et al., 2001; Changhai and Rui, 2007; Gardiner et al., 2009), improve neuronal function, and promote memory generation and consolidation via secretion of BNDF (Allen et al., 2013). The hippocampal inflammatory response is the main pathologic characteristic of VaD (Stefaniak and O’Brien, 2016; Price et al., 2018). Nrf2 and HO-1, key signal molecules of the Nrf2/HO-1 pathway, exert anti-oxidative and ant-inflammatory effects (Wang et al., 2015).
The ginsenoside Rb1 improves learning and memory via attenuation of the hippocampal inflammatory response in a rat model of cerebral ischemia-reperfusion (Wang et al., 2009). Senkyunolide I reduces cerebral inflammation caused by oxidative stress and glucose deprivation as well as reoxygenation. Flavonoids and total steroids found in Euonymus alatus were found to possess signaling capabilities relevant to maintenance of oxygen free radical levels (Debin and Shaofen, 2006). Studies on VaD rats revealed that quercetin (Wei Si-Can and Tian-lai, 2020) administration resulted in increased neuronal lactate dehydrogenase activity and ATP levels as well as inhibition of cerebral NOS activity. Neuronal NO and lactate dehydrogenase levels as well as serum ET-1 levels were reported to be decreased, while serum levels of calcitonin gene-related peptide increased, thus promoting physiological neuronal metabolism and functioning. SMYZG (Kun et al., 2020; Wei Si-Can and Tian-lai, 2020) improves cognitive function and exerts neuroprotective effects by promoting the proliferation of reactive astrocyte-type cells via upregulation of the Nrf2/HO1 pathway.
Effect on the neurovascular unit
The neurovascular unit consists of the blood-brain barrier, neurons and glial cells including astrocytes, oligodendrocytes microglia. The neurovascular unit is a fairly novel concept that has attracted interest for consideration in research of stroke treatment (Muoio et al., 2014). The neurovascular unit together with pericytes as well as the corneal epithelial cells are critically important in the pathogenesis of ischemic cerebrovascular and hemorrhagic cerebral vascular diseases (Ozgur et al., 2017).
Effect on pericyte signal
Pericytes play critical roles in areas of lacunar infarction (Mark, 2009). Pericytes function to regulate blood-brain barrier permeability, clearing cellular components (Ferland-McCollough et al., 2017), maintaining homeostasis of cerebral vascular and protecting the central nervous system.
Surgeries (Sun et al., 2021a) can cause vascular injury and angiogenic instability via downregulation of Ang1 and PDGFR-β protein expression in corneal epithelial cells of brains among MID rats. SMYZG was reported to promote Ang1 and Ang1 mRNA expression in pericytes, increase neuronal PDGFR-β and PDGFR-β mRNA levels, and increase NG2 and NG2 mRNA levels. These results underscore that SMYZG protects pericytes and improves neurovascular homeostasis.
Effect on angiogenesis signaling
Vascular endothelial growth factor, astrocytes, Ang-1, Notch, and pericytes were found to synergistically interact to stimulate regeneration and maturation of blood vessels (Li, 2017; Kun et al., 2020). The hypoxia inducible factor-1 (HIF-1)-VEGF and Notch pathways are two main signaling pathways in angiogenesis. HIF-1 is one transcription factor relevant to intracellular oxygen homeostasis that is evoked by hypoxia. VEGF, another neuroprotective factor increased in neuronal tissue in the setting of cerebrovascular trauma, decreases blood-brain barrier permeability and promotes angiogenesis (Lian Jin et al., 2011). The Notch gene plays a key role in HIF-1/VEGF/Notch signaling and thus promotes blood vessel regeneration (Luttun et al., 2002; Yin, 2016).
Effect on endothelial cells and synaptic plasticity in the context of lacunar infarction
Endothelial cells of the blood-brain barrier are important early in the pathogenesis of VaD. Injury of proteins involved in neural synaptic plasticity is also important in cognitive impairment. Endothelial cells regulate neuronal activation via secretion of compounds such as VEGF, BDNF, and NO. Similarly, endothelial cells regulate synaptic plasticity via VEGF/Flk-1/p8 MAPK signaling.
Studies (Lian Jin et al., 2011; Li, 2017; Xuemei et al., 2019) of VaD rat models reported that SMYZG promotes expression of P38MAPK, NMDAR1, PSD-95, VEGF, and FLK-1 mRNA and protein in rat neuronal tissue, promotes expression of choline acetyltransferase, inhibits MMP9 expression. SMYZG was reported to improve cognition via inhibition of neuroglial cell activation and proliferation in the CA1 region. SMYZG also improves endothelial cellular function, activates VEGF/Flk-1/P38MAPK signaling and improves synaptic plasticity (Table 2).
Conclusion and perspectives
Vascular dementia (Gorelick et al., 2011) is a common, highly heterogeneous condition that manifests due to stroke, neurovascular trauma or cerebrovascular disease. The four major subtypes of VaD that can be further subclassified include MID, post-stroke dementia, subcortical ischemic vascular dementia and mixed dementia (Skrobot et al., 2016a,b, 2018; Hao et al., 2021). VaD is the second commonest dementia after Alzheimer’s, and although Alzheimer’s is characterized by episodic disturbances in memory, both conditions are clinically similar and challenging to distinguish (Laukka et al., 2004). Interestingly, VaD and Alzheimer’s were reported to commonly coexist in patients suffering cognitive impairment (Schneider et al., 2005; Sonnen et al., 2007; Launer et al., 2008; Wu, 2019). And end-stage VaD patients share the similar pathological basis with AD patients (Wu, 2019). As interest in VaD research increases, particular focus on treatment improvement, pathological mechanism elucidation and conduction of prospective drug development studies is warranted (Cognitive Impairment Committee NBCM, 2019; Hao et al., 2021).
Chronic cerebral hypoperfusion is the main etiological mechanism of VaD (Cao et al., 2016; Jessica et al., 2017; Kazumata et al., 2019), with half of VaD patients suffering subcortical white matter infarction (Kalaria, 2016). However, white matter lesions are considered to also be an etiology for chronic cerebral hypoperfusion and a pathologic characteristic common in both Alzheimer’s and VaD (Figure 6).
Figure 6. Possible pathomechanism of vascular dementia (VaD). Sources for the images within this figure: 699pic.com and Chinese Pharmacopoeia.
The concept of the neurovascular unit underscores the relationship between VaD and other cerebrovascular diseases. Investigation of interactions among neurons, glial cells and the cerebral vasculature should be encouraged from a holistic perspective. A variety of active compounds compose SMYZG such as ferulic acid, LT, ginsenoside Rg1, ginsenoside Rb1, gastrodin, and quercetin, as well as a number of flavonoids. Treatment targets have been reported to include dementia-associated proteins (e.g., beta-secretase, PS-1), apoptosis-associated factors (e.g., TLR3, TRIF), vascular endothelium- -associated factors (i.e., VEGF), components of cholinergic signaling (e.g., acetylcholinesterase, choline), inflammatory factors (e.g., IL-6, IL-10, NF-α) and factors important in oxidative stress (e.g., MDA, SOD, GSH, NO, iNOS). To further clarify effects of SMYZG in the clinical setting, multi-center randomized controlled trials with large sample sizes are required. Clinical monitoring of SMYZG blood levels as well as investigation of pharmacokinetic properties, are also warranted.
This article reviewed previously published data from studies using different animal models relevant to the pharmacological mechanism, pharmacokinetics and clinical applications of SMYZG, as well as the pathophysiological characteristics of VaD. Important processes ameliorated by SMYZG treatment include neuronal oxidative stress, cerebral white matter demyelination, pericyte function, neuronal mitochondrial metabolism, cerebral endothelial function and neuroinflammation. Relevant signaling pathways include the AMPK/PPARα/PGC-1α/UCP2, Nrf2/HO-1, HIF-1/VEGF/Notch, and VEGF/Flk-1/p8 MAPK pathways.
In this manuscript, we collected around 10 years reports for SMYZ of VaD prevention and treatment. For future applications, more approaches need to be designed in further studies along with the development of proteomics, metabonomics, transcriptomics, network pharmacology, and interdisciplinary research. To identify active ingredients based on the sense of system biology. To evaluate pharmaceutical and pharmacological effects of active ingredients based on metabonomics. To research complex components of herbal medicines by establishing combination-activity relationship (CAR) according to the relevance of herbal combination and bioactivity based on systems modeling (Liu et al., 2016; Liu, 2017). Various metabolites will be produced during the whole process after oral administration of SMYZ to form pharmaco-metabonomics (Xiao, 2014; National Medical Products Administration, 2020). We shall test active components in plasma, prototype components, gut bacteria and metabolites with approaches like nuclear magnetic resonance (NMR), gas chromatography-mass spectrometry (GC-MC) and ultra performance liquid chromatography-tandem mass spectrometry (UPLC-MS/MS). This methods are conducted by modern equipments that are characterized by high resolution, high-throughput and high sensitivity. Using of all above mentioned technologies is consistent with concept of holism of Traditional Chinese Medicine (TCM).
Chinese medicines are complicated chemical systems based on systematic chemical separation, biological expression, separation and preparation of herb components. For further study, our research group will put more efforts on figuring out the compatibility regularity and integrating multi-components. The basic pharmacology, mechanism of action and the relationship between active components and clinical efficacy of the drug must be determined including toxicologic effects. Upgrade the preparation technique and make new traditional Chinese medicines with controllable quality, low toxic and clearly mechanisms.
Author contributions
J-GL and HL conceived the topic and helped to draft the manuscript. J-GL and S-RC wrote the manuscript together. M-XL, D-DS, and L-JZ participated in the research. All authors contributed to the article and approved the submitted version.
Funding
We gratefully acknowledge the financial support from National Natural Science Foundation of China (Grant no. 81403266). Our research gain support as key project of basic research of “Major new drug development” projects of Ministry of Science and Technology, PRC (Grant no. 2019ZX09301-114), “Innovation Research Projects” of G20 engineering of Beijing Municipal Science and Technology Commission (Grant no. Z171100001717016).
Acknowledgments
We gratefully thank for pharmaceutical technology support from Rui Zheng of Xiyuan Hospital of China Academy of Chinese Medical Sciences. We thank for pharmaceutical analysis support from Yun Wei of State Key Laboratory of chemical resource engineering of Beijing University of Chemical Technology. We also thank for Sir Yuyang Liu to assist in reviewing and giving suggestions for revising this manuscript.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Agdeppa, E. D., Kepe, V., Liu, J., Small, G. W., Huang, S. C., Petric, A., et al. (2003). 2-Dialkylamino-6-acylmalononitrile substituted naphthalenes (DDNP analogs): Novel diagnostic and therapeutic tools in Alzheimer’s disease. Mol. Imaging Biol. 5, 404–417. doi: 10.1016/j.mibio.2003.09.010
Aili, W. (2018). Analysis of clinical and pharmacological effects of ligustrazine on cerebral infarction. Electron. J. Clin. Med. Lit. 5, 163–166.
Alber, J., Alladi, S., Bae, H., Barton, D. A., Beckett, L. A., Bell, J. M., et al. (2019). White matter hyperintensities in vascular contributions to cognitive impairment and dementia (VCID): Knowledge gaps and opportunities. Alzheimers Dement. 5, 107–117. doi: 10.1016/j.trci.2019.02.001
Allen, S. J., Watson, J. J., Shoemark, D. K., Barua, N. U., and Patel, N. K. (2013). GDNF, NGF and BDNF as therapeutic options for neurodegeneration. Pharmacol. Therap. 138, 155–175. doi: 10.1016/j.pharmthera.2013.01.004
Belkhelfa, M., Beder, N., Mouhoub, D., Amri, M., Hayet, R., Tighilt, N., et al. (2018). The involvement of neuroinflammation and necroptosis in the hippocampus during vascular dementia. J. Neuroimmunol. 320, 48–57. doi: 10.1016/j.jneuroim.2018.04.004
Beydoun, M. A., Beydoun, H. A., Fanelli-Kuczmarski, M. T., Weiss, J., Hossain, S., Canas, J. A., et al. (2022). Association of serum antioxidant vitamins and carotenoids with incident Alzheimer disease and all-cause dementia among US adults. Neurology 98, e2150–e2162. doi: 10.1212/WNL.0000000000200289
Bo, L., Xiaolei, H., and Chunyuan, Z. (2019). Research progress on anti-inflammatory effect and molecular mechanism of ginsenosides. Chin. J. Pharm. 54, 253–258.
Cao, Y., Liang, L., Xu, J., Wu, J., Yan, Y., Lin, P., et al. (2016). The effect of Scutellaria baicalensis stem-leaf flavonoids on spatial learning and memory in chronic cerebral ischemia-induced vascular dementia of rats. Acta Biochim. Biophys. Sin. (Shanghai) 48, 437–446. doi: 10.1093/abbs/gmw024
Chang Surui, L. J. L. M. (2020). Experimental study on acute toxicity of ShenmaYizhi decoction. Chin. J. integr. Med. Cardio Cereb. Vasc. Dis. 18, 424–426.
Charles, R., Pascale, K., Diana, A., Lussier-Cacan, S., Theroux, L., Christen, Y., et al. (2000). Impact of apoE deficiency on oxidative insults and antioxidant levels in the brain. Mol. Brain Res. 86, 76–83. doi: 10.1016/S0169-328X(00)00268-0
Chen, L., Yao, H., Chen, X., Wang, Z., Xiang, Y., Xia, J., et al. (2018). Ginsenoside Rg1 decreases oxidative stress and down-regulates Akt/mTOR signalling to attenuate cognitive impairment in mice and senescence of neural stem cells induced by D-galactose. Neurochem. Res. 43, 430–440. doi: 10.1007/s11064-017-2438-y
Cheng, C. S., Jiangang, L., Meixia, L., Hao, L., and Zenggang, L. (2019). Exploration of pathological mechanism of vascular dementia induced by chronic cerebral hypoperfusion and production of several common animal models. Acta Neuropharmacol. 9, 13–17.
Chinese Pharmacopoeia Commission (2015). Pharmacopoeia of the people’ s republic of China. Beijing: China Medical Science and Technology Press, 28–29.
Choi, B. R., Kim, D. H., and Back, D. B. (2016). Characterization of white matter injury in a rat model of chronic cerebral hypoperfusion. Stroke 47, 542–547. doi: 10.1161/STROKEAHA.115.011679
Chu, S. F., Zhang, Z., Zhou, X., He, W. B., Chen, C., Luo, P., et al. (2019). Ginsenoside Rg1 protects against ischemic/reperfusion-induced neuronal injury through miR-144/Nrf2/ARE pathway. Acta Pharmacol. Sin. 40, 13–25. doi: 10.1038/s41401-018-0154-z
Chunni, H., Fangyan, H., Ye, T., and Xiaohua, D. (2014). Effects of Gastrodia elata extract on cholinergic system in rats with memory acquisition disorder. China Pharm. 23.
Chunyan, Z., Yuping, L., Maoxu, L., and Ruilian, W. (2009). Effect of gastrodin on vascular dementia. J. Emerg. Tradit. Chin. Med. 18, 1220–1221.
Cognitive Impairment Committee NBCM (2019). 2019 Guidelines for the Diagnosis and Treatment of Vascular cognitive Impairment in China. Chin. Med. J. Pek. 99, 2737–2744.
Debin, H., and Shaofen, Y. (2006). The effect of three extracts of podophylla SPP. On oxygen free radical. J. Hubei Univ. Natl. 2006, 4–6.
Dongmin, C., Qinxiao, G., Yali, L., and Shumei, W. (2020). Serum metabolomics study of ginsenosides in the treatment of ischemic stroke based on 1 H-NMR. China J. Chin. Mater. Med. 45, 1142–1148.
Elkabes, S., DiCicco-Bloom, E. M., and Black, I. B. (1996). Brain microglia/macrophages express neurotrophins that selectively regulate microglial proliferation and function. J. Neurosci. 16, 2508–2521. doi: 10.1523/JNEUROSCI.16-08-02508.1996
Fan, W., Huang, Y., Zheng, H., Li, S., Li, Z., Yuan, L., et al. (2020). Ginsenosides for the treatment of metabolic syndrome and cardiovascular diseases: Pharmacology and mechanisms. Biomed. Pharmacother. 132:110915. doi: 10.1016/j.biopha.2020.110915
Farkas, E., Luiten, P. G. M., and Bari, F. (2007). Permanent, bilateral common carotid artery occlusion in the rat: A model for chronic cerebral hypoperfusion-related neurodegenerative diseases. Brain Res. Rev. 54, 162–180. doi: 10.1016/j.brainresrev.2007.01.003
Ferland-McCollough, D., Slater, S., Richard, J., Reni, C., and Mangialardi, G. (2017). Pericytes, an overlooked player in vascular pathobiology. Pharmacol. Therap. 171, 30–42. doi: 10.1016/j.pharmthera.2016.11.008
Gardiner, J., Barton, D., Overall, R., and Marc, J. (2009). Neurotrophic support and oxidative stress: Converging effects in the normal and diseased nervous system. Neuroscientist 15, 47–61. doi: 10.1177/1073858408325269
Gorelick, P. B., Scuteri, A., Black, S. E., Decarli, C., Greenberg, S. M., Iadecola, C., et al. (2011). Vascular contributions to cognitive impairment and dementia: A statement for healthcare professionals from the American heart association/American stroke association. Stroke 42, 2672–2713. doi: 10.1161/STR.0b013e3182299496
Guifang, L., Congshu, H., and Guihua, Z. (2021). Ferulic acid inhibits radiation-induced microglial inflammation through NLRP3 inflammasome. Pharmacol. Clin. Chin. Mater. Med. 37, 76–80.
Guofeng, W., Feng, L., Xia, Z., and Yang, C. (2012). Effects of ligustrazine on endothelial cell inflammation induced by oxidized low density lipoprotein. Chin. J. Hypertens. 20, 347–351.
Hachinski, V., Iadecola, C., Petersen, R. C., Breteler, M. M., Nyenhuis, D. L., Black, S. E., et al. (2006). National institute of neurological disorders and stroke-canadian stroke network vascular cognitive impairment harmonization standards. Stroke 37, 2220–2241. doi: 10.1161/01.STR.0000237236.88823.47
Hao, S., Dong, S., and Junjian, Z. (2021). Interpretation of ‘the diagnosis and treatment guideline of VaD of China’ (2019). Chin. J. Clin. 49, 655–657.
Hao, Z. L. L. N. (2020). Influence of Shen Ma Yizhi decoction on learning memory, inflammatory factors and antioxidant effects in vascular cognitive impaired rats induced by multiple cerebral infarction. Tradit. Chin. Drug Res. Clin. Pharmacol. 31, 294–299.
Hill, R. A., and Grutzendler, J. (2019). Uncovering the biology of myelin with optical imaging of the live brain. Glia 67, 2008–2019. doi: 10.1002/glia.23635
Hu, C., Lau, A. J., Wang, R., and Chang, T. K. H. (2017). Comparative analysis of ginsenosides in human glucocorticoid receptor binding, transactivation, and transrepression. Eur. J. Pharmacol. 815, 501–511. doi: 10.1016/j.ejphar.2017.10.019
Huan, W., Dayan, Z., Wei, W., Gao, J., Rao, C., Peng, C., et al. (2017). Effects of gastrodin on the expression of NF-kB inflammatory cascade in glucose and oxygen deprivation redonor cortical neurons. Chin. J. Exp. Tradit. Med. Formulae 23, 104–111.
Huang, Y., Chen, J., Yuan, J., and Shanquan, Z. (2016). Cognitive variations among vascular dementia subtypes caused by small-, large-, or mixed-vessel disease. Arch. Med. Sci. 12, 747–753. doi: 10.5114/aoms.2016.60962
Jessica, D., Akihiro, K., Yoshiki, H., Ihara, M., Kalaria, R. N., and Horsburgh, K. (2017). Chronic cerebral hypoperfusion: A key mechanism leading to vascular cognitive impairment and dementia. Closing the translational gap between rodent models and human vascular cognitive impairment and dementia. Clin. Sci. (Lond) 131, 2451–2468. doi: 10.1042/CS20160727
Jiamou, R., Yumei, G., Changxue, W., Yi, L., and Fan, W. (2020). Expression of cholinesterase and cholinacetylase in rats with vascular cognitive dysfunction. Chin. J. Gerontol. 40, 2396–2401.
Jian, G., and Shao-wa, L. (2021). Research progress in chemical constituents and pharmacological action of Renshen (Ginseng). Guid. J. Tradit. Chin. Med. Pharm. 27, 127–130.
Jiang, Y., Müller, K., Khan, M. A., Assmann, J. C., Lampe, J., Kilau, K., et al. (2021). Cerebral angiogenesis ameliorates pathological disorders in Nemo-deficient mice with small-vessel disease. J. Cereb. Blood Flow. Metab. 41, 219–235. doi: 10.1177/0271678X20910522
Jiangang, L., Dawu, Z., Yuyang, L., Jiatao, F., Jie, L., Dazhuo, S., et al. (2019). Separation of extracts from chuanxiong rhizoma and the pharmacological effects on myocardium of ischemia/reperfusion injury in rats. Chin. J. Mordern Appl. Pharm. 36, 2369–2375.
Jianliang, W., Minmin, S., Shuixin, Y., Xiang, W., and Zengchun, M. (2015). Inhibition of microglial inflammatory response by ferulic acid. Chin. Pharmacol. Bull. 31, 97–102.
Kalaria, R. N. (2016). Neuropathological diagnosis of vascular cognitive impairment and vascular dementia with implications for Alzheimer’s disease. Acta Neuropathol. 131, 659–685. doi: 10.1007/s00401-016-1571-z
Kazumata, K., Tokairin, K., Sugiyama, T., Ito, M., Uchino, H., Osanai, T., et al. (2019). Association of cognitive function with cerebral blood flow in children with moyamoya disease. J. Neurosurg. Pediatr. 25, 1–7. doi: 10.3171/2019.7.PEDS19312
Koh, P. (2015). Ferulic acid attenuates the down-regulation of MEK/ERK/p90RSK signaling pathway in focal cerebral ischemic injury. Neurosci. Lett. 588, 18–23. doi: 10.1016/j.neulet.2014.12.047
Kun, L., Hui, P., Yu, C., Lina, M., and Hao, L. (2020). Effects of Shenma Yizhi prescription on Nrf2/HO-1 pathway and microglia in rats with vascular dementia. Chin. J. Inf. TCM 27, 38–42.
Laukka, E. J., Jones, S., Small, B. J., Fratiglioni, L., and Bäckman, L. (2004). Similar patterns of cognitive deficits in the preclinical phases of vascular dementia and Alzheimer’s disease. J. Int. Neuropsychol. Soc. 10, 382–391. doi: 10.1017/S1355617704103068
Launer, L. J., Petrovitch, H., Ross, G. W., Markesbery, W., and White, L. R. (2008). AD brain pathology: Vascular origins? Results from the HAAS autopsy study. Neurobiol. Aging 29, 1587–1590. doi: 10.1016/j.neurobiolaging.2007.03.008
Lecordier, S., Manrique-Castano, D., El Moghrabi, Y., and ElAli, A. (2021). Neurovascular alterations in vascular dementia: Emphasis on risk factors. Front. Aging Neurosci. 13:727590. doi: 10.3389/fnagi.2021.727590
Lei, Z., Yan, Z., Xian-sheng, Y., Zhang, J., Zhang, W. K., and Li, P. (2015). Chemical constituents from twigs of Euonymus alatus. China J. Chin. Mater. Med. 40, 2612–2616.
Li Qian, W. X. (2020). New progress in research on chemical constituents and pharmacological action of Ligusticum chuanxiong Hort. Chem. Eng. 34, 62–64.
Li Zehui, C. Y. L. J. (2020). Effects of Shenma Yizhi prescription on cognitive function and white matter damage of vascular dementia rats with multiple cerebral infarction. World Chin. Med. 15, 1120–1123.
Li, J., Yu, J., Ma, H., Yang, N., Li, L., Zheng, D. D., et al. (2017). Intranasal pretreatment with Z-ligustilide, the main volatile component of rhizoma chuanxiong, confers prophylaxis against cerebral ischemia via Nrf2 and HSP70 signaling pathways. J. Agric. Food Chem. 65, 1533–1542. doi: 10.1021/acs.jafc.6b04979
Li, K. (2017). Effects of Shenma Yizhi prescription on hippocampal morphology and oxidative stress in vascular dementia model rats. Beijing Tradit. Chin. Med. 36, 397–400.
Lian Jin, H., Pennant, W. A., Hyung Lee, M., Su, S., Ah Kim, H., Lu Liu, M., et al. (2011). Neural stem cells modified by a hypoxia-inducible VEGF gene expression system improve cell viability under hypoxic conditions and spinal cord injury. Spine 36, 857–864. doi: 10.1097/BRS.0b013e3181e7f34b
Lijuan, Z., Meixia, L., Jiangang, L., Chengcheng, S., and Nannan, L. (2022). Research ideas and methods of traditional Chinese medicine compound in the treatment of vascular dementia development and research on the prevention and treatment of vascular dementia with Shenma Yizhi decoction. Modern Tradit. Chin. Med. Mater. Med. World Sci. Technol. 24, 217–233.
Lin, W., Xiaomei, M., and Haitao, Z. (2014). Advances in animal models of vascular dementia. J. Guangxi Univ. Chin. Med. 17, 93–96.
Ling, L., Zheng, X., and Youcai, W. (2019). Protective effect of ferulic acid on oxidative stress injury of H9c2 cardiomyocytes induced by advanced glycation end products. Chin. J. Clin. Pharmacol. 35, 1446–1448.
Liu, C. X., Chen, S. L., and Xiao, X. H. (2016). Quality marker of traditional Chinese medicine (Q-Marker): A new concept for quality control of traditional Chinese medicine products. Chin. Tradit. Herb. Drugs 47, 1443–1457.
Liu, X. (2017). Construction of TCM quality traceability system based on TCM quality markers. Chin. Tradit. Herb. Drugs 48, 3669–3676.
Luo, C., Xu, X., Wei, X., Feng, W., Huang, H., Liu, H., et al. (2019). Natural medicines for the treatment of fatigue: Bioactive components, pharmacology, and mechanisms. Pharmacol. Res. 148:104409. doi: 10.1016/j.phrs.2019.104409
Luttun, A., Carmeliet, G., and Carmeliet, P. (2002). Vascular progenitors: From biology to treatment. Trends Cardiovasc. Med. 12, 88–96. doi: 10.1016/S1050-1738(01)00152-9
Mancuso, C., and Santangelo, R. (2014). Ferulic acid: Pharmacological and toxicological aspects. Food Chem. Toxicol. 65, 185–195. doi: 10.1016/j.fct.2013.12.024
Mark, F. (2009). Pericyte signaling in the neurovascular unit. Stroke 40(Suppl. 3), S13–S15. doi: 10.1161/STROKEAHA.108.533117
Masliah, E., Samuel, W., Veinbergs, I., Mallory, M., Mante, M., and Saitoh, T. (1997). Neurodegeneration and cognitive impairment in apoE-deficient mice is ameliorated by infusion of recombinant apoE. Brain Res. 751, 307–314. doi: 10.1016/S0006-8993(96)01420-5
Md, J., Ezazul, H. M., Joonsoo, K., Cho, D. Y., Kim, I. S., and Choi, D. K. (2018). Active ginseng components in cognitive impairment: Therapeutic potential and prospects for delivery and clinical study. Oncotarget 9, 33601–33620. doi: 10.18632/oncotarget.26035
Meikang, W., Chunmei, M., and Xiangdang, W. (2010). Effect of gastrodin injection on cognitive dysfunction in vascular dementia. Chin. J. Med. 45, 58–59.
Meixia, L. (2020). New TCM compounds of preventing VaD- researching and development of shenmayizhi. Beijing: CNKI.
Moorhouse, P., and Rockwood, K. (2008). Vascular cognitive impairment: Current concepts and clinical developments. Lancet Neurol. 7, 246–255. doi: 10.1016/S1474-4422(08)70040-1
Muoio, V., Persson, P. B., and Sendeski, M. M. (2014). The neurovascular unit-concept review. Acta Physiol. (Oxf) 4, 790–798. doi: 10.1111/apha.12250
Nakajima, K., Honda, S., Tohyama, Y., Imai, Y., Kohsaka, S., and Kurihara, T. (2001). Neurotrophin secretion from cultured microglia. J. Neurosci. Res. 65, 322–331. doi: 10.1002/jnr.1157
Nan-Nan, L., Jian-Gang, L., and Rui, Z. (2019). Comparativestudy on pharmacological effects of Shenma Yizhi formula processed with three kinds of preparation techniques. Beijing J. Tradit. Chin. Med. 38, 427–432.
Nasrabady, S. E., Rizvi, B., Goldman, J. E., and Brickman, A. M. (2018). White matter changes in Alzheimer’s disease: A focus on myelin and oligodendrocytes. Acta Neuropathol. Commun. 6:22. doi: 10.1186/s40478-018-0515-3
National Medical Products Administration (2020). Circular of the center for drug control of the state food and drug administration on issuing the technical guidelines for the study of biological effects of traditional Chinese medicine (Trial) (No. 50 of 2020). Beijing: National Medical Products Administration.
O’Brien, J. T., Erkinjuntti, T., Reisberg, B., Roman, G., Sawada, T., Pantoni, L., et al. (2003). Vascular cognitive impairment. Lancet Neurol. 2, 89–98. doi: 10.1016/S1474-4422(03)00305-3
Ozgur, O., Ihsan, S., and Yasemin, G. (2017). The role of pericytes in neurovascular unit: Emphasis on stroke. Curr. Drug Targets 18, 1386–1391. doi: 10.2174/1389450117666160613104523
Peng, H., Du, J., Zhang, G., Kuang, X., Liu, Y. X., Qian, Z. M., et al. (2007). Neuroprotective effect of Z-ligustilide against permanent focal ischemic damage in rats. Biol. Pharm. Bull. 30, 309–312. doi: 10.1248/bpb.30.309
Poonam, S., Nikita, G., Shalini, J., Sharma, B. M., Singh, B., Kharkwal, H., et al. (2022). Salubrious effects of ulinastatin and quercetin alone or in combination in endothelial dysfunction and vascular dementia. Pharmacol. Rep. 74, 481–492. doi: 10.1007/s43440-022-00364-1
Price, B. R., Norris, C. M., Sompol, P., and Wilcock, D. M. (2018). An emerging role of astrocytes in vascular contributions to cognitive impairment and dementia. J. Neurochem. 144, 644–650. doi: 10.1111/jnc.14273
Prins, N. D., and Scheltens, P. (2015). White matter hyperintensities, cognitive impairment and dementia: An update. Nat. Rev. Neurol. 11, 157–165. doi: 10.1038/nrneurol.2015.10
Qian, H., and Zengchun, M. (2019). Research progress of ferulic acid in the treatment of Alzheimer’s disease. MIL Med. 43, 230–235.
Qihai, G., Jingshan, S., Danli, Y., Bin, H., and Xiaolong, X. (2011). Pharmacological action and mechanism of gastrodin in central nervous system. Chin. J. N. Drugs Clin. Med. 30, 176–179.
Qin, Z., Yu, T., Peng-Yi, H., Liu, D., Zhang, D., Yue, P., et al. (2018). The influence and mechanism of ligustilide, senkyunolide I, and senkyunolide A on echinacoside transport through MDCK-MDR1 cells as blood-brain barrier in vitro model. Phytother. Res. 32, 426–435. doi: 10.1002/ptr.5985
Qiong, W., Yu, C., Meixia, L., Liu, F., Brantner, A. H., Yang, Y., et al. (2019). Traditional Chinese medicine Shenmayizhi decoction ameliorates memory and cognitive impairment induced by scopolamine via preventing hippocampal cholinergic dysfunction in rats. Neuropsychiatr. Dis. Treat. 15, 3167–3176. doi: 10.2147/NDT.S214976
Qun, W., and Yongjun, W. (2015). An animal model of vascular dementia in rodents. Chin. J. Stroke 10, 279–283.
Roux, P. P., Bhakar, A. L., Kennedy, T. E., and Barker, P. A. (2001). The p75 neurotrophin receptor activates Akt (protein kinase B) through a phosphatidylinositol 3-kinase-dependent pathway. J. Biol. Chem. 276, 23097–23104. doi: 10.1074/jbc.M011520200
Rui-xi, S., Jing, P., Jian, G., Maoting, W., Heqing, H., and Ling, L. (2015). Mordern research on pharmacological action of Euonymus alatus (Thunb.) Sieb. Glob. Tradit. Chin. Med. 8, 245–249.
Schneider, J. A., Bienias, J. L., Wilson, R. S., Berry-Kravis, E., Evans, D. A., and Bennett, D. A. (2005). The apolipoprotein E epsilon4 allele increases the odds of chronic cerebral infarction [corrected] detected at autopsy in older persons. Stroke 36, 954–959. doi: 10.1161/01.STR.0000160747.27470.2a
Sharma, P., Aggarwal, K., Awasthi, R., Kulkarni, G. T., and Sharma, B. (2021). Behavioral and biochemical investigations to explore the efficacy of quercetin and folacin in experimental diabetes induced vascular endothelium dysfunction and associated dementia in rats. J. Basic Clin. Physiol. Pharmacol. doi: 10.1515/jbcpp-2020-0159
Shea, T. B., Rogers, E., Ashline, D., Ortiz, D., and Sheu, M. S. (2002). Apolipoprotein E deficiency promotes increased oxidative stress and compensatory increases in antioxidants in brain tissue. Free Radic. Biol. Med. 33, 1115–1120. doi: 10.1016/S0891-5849(02)01001-8
Skrobot, O. A., Attems, J., Esiri, M., Hortobágyi, T., Ironside, J. W., Kalaria, R. N., et al. (2016a). Vascular cognitive impairment neuropathology guidelines (VCING): The contribution of cerebrovascular pathology to cognitive impairment. Brain 139, 2957–2969. doi: 10.1093/brain/aww214
Skrobot, O. A., O’Brien, J., Black, S., Chen, C., DeCarli, C., Erkinjuntti, T., et al. (2016b). The vascular impairment of cognition classification consensus study. Alzheimers Dement. 13, 624–633.
Skrobot, O. A., Black, S. E., Chen, C., DeCarli, C., Erkinjuntti, T., Ford, G. A., et al. (2018). Progress toward standardized diagnosis of vascular cognitive impairment: Guidelines from the vascular impairment of cognition classification consensus study. Alzheimers Dement. 14, 280–292. doi: 10.1016/j.jalz.2017.09.007
Sonnen, J. A., Larson, E. B., Crane, P. K., Haneuse, S., Li, G., Schellenberg, G. D., et al. (2007). Pathological correlates of dementia in a longitudinal, population-based sample of aging. Ann. Neurol. 62, 406–413. doi: 10.1002/ana.21208
Stefaniak, J., and O’Brien, J. (2016). Imaging of neuroinflammation in dementia: A review. J. Neurol. Neurosurg. Psychiatry 87, 21–28.
Sun, C. C., Liu, J., Liu, M., Luo, Z. G., and Li, H. (2020). Intervention of Shenma Yizhi prescription on learning and memory in rats with vascular cognitive impairment induced by chronic cerebral ischemia. Chin. J. Exp. Tradit. Med. Formulae 26, 153–159.
Sun, C., Liu, J., Li, N., Liu, M., Luo, Z., and Li, H. (2021a). Traditional Chinese medicine Shenmayizhi decoction ameliorates memory and cognitive impairment induced by multiple cerebral infarctions. Evid Based Compl. Alternat. Med. 2021:6648455. doi: 10.1155/2021/6648455
Sun, C., Liu, M., Liu, J., Zhang, T., Zhang, L., Li, H., et al. (2021b). ShenmaYizhi decoction improves the mitochondrial structure in the brain and ameliorates cognitive impairment in VCI rats via the AMPK/UCP2 signaling pathway. Neuropsychiatr. Dis. Treat. 17, 1937–1951. doi: 10.2147/NDT.S302355
Tomimoto, H., Ihara, M., Wakita, H., Ohtani, R., Lin, J. X., Akiguchi, I., et al. (2003). Chronic cerebral hypoperfusion induces white matter lesions and loss of oligodendroglia with DNA fragmentation in the rat. Acta Neuropathol. 106, 527–534. doi: 10.1007/s00401-003-0749-3
Tönnies, E., and Trushina, E. (2017). Oxidative stress, synaptic dysfunction, and Alzheimer’s disease. J. Alzheimers Dis. 57, 1105–1121. doi: 10.3233/JAD-161088
Wang, F., Cao, Y., Ma, L., Pei, H., Rausch, W. D., and Li, H. (2018). Dysfunction of cerebrovascular endothelial cells: Prelude to vascular dementia. Front. Aging Neurosci. 10:376. doi: 10.3389/fnagi.2018.00376
Wang, Q., Tang, X. N., and Yenari, M. A. (2007). The inflammatory response in stroke. J. Neuroimmunol. 184, 53–68. doi: 10.1016/j.jneuroim.2006.11.014
Wang, X. R., Shi, G. X., Yang, J. W., Yan, C. Q., Lin, L. T., Du, S. Q., et al. (2015). Acupuncture ameliorates cognitive impairment and hippocampus neuronal loss in experimental vascular dementia through Nrf2-mediated antioxidant response. Free Radic. Biol. Med. 89, 1077–1084. doi: 10.1016/j.freeradbiomed.2015.10.426
Wang, Y., Chen, J., Chu, S., Wang, Y. S., Wang, X. Y., Chen, N. H., et al. (2009). Improvement of memory in mice and increase of hippocampal excitability in rats by ginsenoside Rg1’ s metabolites ginsenoside Rh1 and protopanaxatriol. J. Pharmacol. Sci. 109, 504–510. doi: 10.1254/jphs.08060FP
Wei Si-Can, L. I. N., and Tian-lai, H. L. S. Z. (2020). Quercetin activates mitochondrial autophagy via PINK1/parkin pathway to alleviate cerebral ischemia-reperfusion injury in rats. Chin. J. Pathophysiol. 36, 2251–2257.
Wei, L., Linhua, D., Dongli, Q., and Yingqiang, Z. (2021). Overview of the pharmacological effects of Gastrodia and its active ingredients. Pharmacol. Clin. Chin. Mater. Med. 37, 240–244.
Wei, Z., Yingqiang, Z., Jianguo, Z., Tian, L., and Juan, W. (2019). The influence of Tianm Gouteng decoction on the VEGF, TNF-α and EPCs mobilization in spontaneously hypertensive rats. Chin. J. Integr. Med. Cardio Cerebrovasc. Dis. 17, 2425–2429.
Wu, J. (2019). Comparison of pathological mechanisms and clinical studies between Alzheimer’s disease and vascular dementia. Zhejiang Med. J. 41, 1227–1231.
Wu, Q., Fang, L., and Jiangang, L. (2017). Effect of Shenma Yizhi decoction cognitive function hemorheological statein mildand moderate vascular dementia. Chin. J. Integr. Med. Cardio Cerebrovasc. Dis. 15, 2381–2385.
Wyss-Coray, T., and Mucke, L. (2002). Inflammation in neurodegenerative disease–a double-edged sword. Neuron 35, 419–432. doi: 10.1016/S0896-6273(02)00794-8
Xiao, X. H. (2014). Research and application of biological evaluation in quality standardization of traditional Chinese medicine. World Sci. Technol. Modern. Tradit. Chin. Med. 16, 514–518.
Xiao, Z., Jinzhi, Z., Zhenzhen, L., and Yan, L. (2019). Effect and mechanism of ginsenoside Rb1 on angiogenesis in rats with cerebral infarction. Med. Pharm. J. Chin. Peoples Liberat. Army 31, 10–15.
Xiaohua, D., Rong, D., Xiufang, L., Weigang, D., and Shengyou, W. (2011). Effects of gastrodiol on nitric oxide and nitric oxide synthase in hippocampus of cerebral ischemia model rats. Chin. J. Geriatr. Cardio Cerebrovasc. Dis. 13, 653–655.
Xuemei, D., Yu, C., and Yang, Y. (2019). Effects of Shenma Yizhi decoction on the cognitive function and hippocampal oxidative stress in rats with alcohol associated dementia. Chin. J. Integr. Med Cardio Cerebrovasc. Dis. 24, 3922–3928.
Yanxiu, G., Xi, S., Yi, M., Lin, L., Zhilai, Z., Ling, J., et al. (2021). Advances in the chemical constituents and pharmacological activities of the Euonymus alatus. Chin. J. Mordern Appl. Pharm. 38, 2305–2316.
Yin, C. (2016). Role of vascular endothelial growth factor in angiogenesis after ischemic brain injury West China. J. Pharm. 31, 103–107.
Ying, X., Eamonn, E., Jurgen, F., Pinsker, D., Thomas, P., Latter, M., et al. (2022). Reduced cortical cholinergic innervation measured using [18F]-FEOBV PET imaging correlates with cognitive decline in mild cognitive impairment. Neuroimage 34:102992. doi: 10.1016/j.nicl.2022.102992
Ying, Z., Li, L., Guang-Yu, L., Liu, J. X., and Li, T. (2014). [Pharmacokinetics and brain distribution of ginsenosides after administration of sailuotong]. Zhongguo Zhong Yao Za 39, 316–321.
Yu Cao, X. D. Z. L. (2019). The influence of ShenmaYizhi decoction hippocampal cognitive function in rats with bilateral carotid artery ligation. Chin. J. Integr. Med. Cardio Cerebrovasc. Dis. 17, 1151–1155.
Yu, C., Xuemei, D., Zehui, L., Kun, L., Meixia, L., Hui, P., et al. (2019). The influence of Shenma Yizhi decoction on hippocampal cognitive function in rats with bilateral carotid artery ligation. Chin. J. Integr. Tradit. West. Med. Cardio Cerebrovasc. Dis. 17, 1151–1155.
Yuanyuan, W., Hao, Z., and Yuhuan, S. (2021). Mechanism of ferulic acid alleviating hepatic fibrosis in rats by inhibiting MAPK and NF-κB/IκBα signaling pathways. Cent. South Pharm. 19, 2489–2495.
Yunfeng, J., and Huixia, L. (2011). Effects of ligusticum chuanxiong alkaloid on SOD activity, NO, NOS and MDA contents in rat brain tissue. Chin. Tradit. Med. modern Distance Educ. 9, 212–213.
Yunlin, Z. (2006). Treatment of senile vascular dementia with integrated traditional Chinese and Western medicine. Chin. J. Clin. 2006, 2–4.
Zhang, H., Cao, Y., Pei, H., Wang, H., Ma, L., Wang, Z., et al. (2020). Shenmayizhi formula combined with ginkgo extract tablets for the treatment of vascular dementia: A randomized, double-blind, controlled trial. Evid. Based Compl. Alternat. Med. 2020:8312347. doi: 10.1155/2020/8312347
Zhang, Y., Liu, J., and Yang, B. (2018). Ginkgo biloba extract inhibits astrocytic lipocalin-2 expression and alleviates neuroinflammatory injury via the JAK2/STAT3 pathway after ischemic brain stroke. Front. Pharmacol. 9:518. doi: 10.3389/fphar.2018.00518
Zhonghui, P., Min, D., Cheng, P., and Liang, X. (2020). Research progress on substance basis and pharmacological action of alkaloids in Ligusticum chuanxiong. China Pharm. 31, 1020–1024.
Keywords: Shen Ma Yi Zhi, VaD, traditional compounds, animal models, signal pathway
Citation: Chang S-r, Liu J-g, Li H, Liu M-x, Shi D-d and Zhou L-j (2022) Pharmaceutical and pharmacological studies of Shen Ma Yi Zhi granule for prevention of vascular dementia: A review. Front. Neurosci. 16:1044572. doi: 10.3389/fnins.2022.1044572
Received: 14 September 2022; Accepted: 04 November 2022;
Published: 24 November 2022.
Edited by:
Jianxun Liu, China Academy of Chinese Medical Sciences, ChinaReviewed by:
Zequn Yin, Hefei University of Technology, ChinaShuang Zhang, Hefei University of Technology, China
Copyright © 2022 Chang, Liu, Li, Liu, Shi and Zhou. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jian-gang Liu, bGl1amlhbmdhbmcyMDAyQHNpbmEuY29t; Hao Li, eHlocGxpaGFvMTk2NUAxMjYuY29t
 Su-rui Chang
Su-rui Chang Jian-gang Liu
Jian-gang Liu Hao Li
Hao Li Mei-xia Liu
Mei-xia Liu Dan-dan Shi1,4
Dan-dan Shi1,4