- 1Faculty of Medicine and Health, Kids Neuroscience Centre, The Children's Hospital at Westmead, University of Sydney, Westmead, NSW, Australia
- 2Faculty of Medicine and Health, Clinical School, The Children's Hospital at Westmead, University of Sydney, Westmead, NSW, Australia
- 3Faculty of Medicine and Health, Sydney Medical School, University of Sydney, Camperdown, NSW, Australia
Mental health and neurodevelopmental disorders are extremely common across the lifespan and are characterized by a complicated range of symptoms that affect wellbeing. There are relatively few drugs available that target disease mechanisms for any of these disorders. Instead, therapeutics are focused on symptoms and syndromes, largely driven by neurotransmitter hypotheses, such as serotonin or dopamine hypotheses of depression. Emerging evidence suggests that maternal inflammation during pregnancy plays a key role in neurodevelopmental disorders, and inflammation can influence mental health expression across the lifespan. It is now recognized that commonly used psychiatric drugs (anti-depressants, anti-psychotics, and mood stabilizers) have anti-inflammatory properties. In this review, we bring together the human evidence regarding the anti-inflammatory mechanisms for these main classes of psychiatric drugs across a broad range of mental health disorders. All three classes of drugs showed evidence of decreasing levels of pro-inflammatory cytokines, particularly IL-6 and TNF-α, while increasing the levels of the anti-inflammatory cytokine, IL-10. Some studies also showed evidence of reduced inflammatory signaling via nuclear factor- (NF-)κB and signal transducer and activator of transcription (STAT) pathways. As researchers, clinicians, and patients become increasingly aware of the role of inflammation in brain health, it is reassuring that these psychiatric drugs may also abrogate this inflammation, in addition to their effects on neurotransmission. Further studies are required to determine whether inflammation is a driver of disease pathogenesis, and therefore should be a therapeutic target in future clinical trials.
1. Introduction
Mental health disorders are extremely common across the lifespan, affecting many people from childhood through to old age. Neurodevelopmental disorders, such as autism spectrum disorder (ASD), attention deficit hyperactivity disorder (ADHD), Tourette syndrome (TS), and obsessive-compulsive disorder (OCD) affect 10% of all children and are a leading cause of disability globally (Global Research on Developmental Disabilities Collaborators, 2018). Neurodevelopmental disorders often co-occur with mental health disorders, namely major depressive disorder (MDD) and anxiety, resulting in a complicated range of symptoms that can affect wellbeing (Salazar et al., 2015; Hansen et al., 2018). Adolescents are particularly vulnerable to mental health disorders, often associated with environmental stress, trauma, and substance abuse (Shanahan et al., 2008; Merikangas et al., 2010; Schulte and Hser, 2014), leading to poorer life outcomes in adulthood (Gibb et al., 2010). Major depression, bipolar disease, and schizophrenia are common in adulthood, and are considered some of the costliest disorders to humanity. People with dementia are also at high risk of developing co-existing psychiatric and behavioral disturbances, requiring supportive care (Kales et al., 2005, 2015). Collectively, neurodevelopmental and mental health disorders reduce quality of life for a significant proportion of the population and place an enormous economic burden on society (Greenberg et al., 2015; Leigh and Du, 2015).
Although genetic contributions are manifest in mental health disorders (Cross-Disorder Group of the Psychiatric Genomics Consortium, 2019), highly penetrant genetic variations are uncommon. Instead, a combination of genetic vulnerability and environmental factors is the more commonly accepted model of disease (Tsuang et al., 2004; Caspi and Moffitt, 2006). Despite intensive research efforts, there are relatively few drugs available that target disease mechanisms for any of these disorders (Wong et al., 2010). Instead, therapeutics are driven by symptoms and syndromes, often characterized by Diagnostic and Statistical Manual of Mental Disorders (DSM) or International Classification of Diseases (ICD) criteria.
Several serendipitous findings of the mid-20th century gave rise to the field of modern neuropharmacology and revolutionized the way in which specific symptoms of an array of disorders were managed. Following observations of the CNS-modulating effects of chlorpromazine as an anesthetic, it was supplied to a small group of psychiatrists who trialed the drug in schizophrenic and manic patients (Shen, 1999). Symptomatic relief and functional improvement in patients, paired with a global decrease of psychiatric inpatient admissions, rapidly confirmed chlorpromazine as a drug of profound clinical significance. Following the success of chlorpromazine, molecular modification of compounds with similar chemical structures gained momentum. Imipramine, developed as a weak anti-histamine with mild anti-cholinergic effects, proved ineffective in schizophrenia, however early researchers recognized the potential mood modifying effects of the drug and it was released for clinical use within a year of its first publication (Pereira and Hiroaki-Sato, 2018). Parallel to these studies, the clinical effects of iproniazid were also being investigated, with the observation this drug greatly stimulated the CNS initially being listed as a side effect, before the potential of this stimulation was realized in the context of depression (Pereira and Hiroaki-Sato, 2018).
The studies of the early 1950s and 1960s laid the groundwork for the development of many distinct classes of drugs in neuropsychology, as well as neurotransmitter-specific hypotheses of pathogenesis related to serotonin, dopamine, glutamate, noradrenaline, GABA (γ-aminobutyric acid), and acetylcholine (Wong et al., 2010). For example, the serotonin hypothesis in emotional disorders, such as anxiety, depression, and OCD, has supported the development of serotonergic drugs in these syndromes, such as selective serotonin reuptake inhibitors (SSRIs), which are now the first line medication of choice for emotional disorders (Vaswani et al., 2003). Despite this, the evidence for serotonergic dysfunction in emotional disorders lacks definitive acceptance (Nordquist and Oreland, 2010; Gardner and Boles, 2011). Likewise, in psychosis, dopaminergic and glutamate hypotheses are supported by multiple lines of evidence, but the origins of these neurotransmitter dysfunctions are unclear (Stahl, 2018). The main classes of psychiatric drugs, namely anti-depressants, anti-psychotics, and mood stabilizers are used in both children and adults to treat a wide range of neurodevelopmental and mental health problems. It is now widely accepted that inflammation plays a role in many of these health problems, and many studies have documented the therapeutic effects of anti-inflammatory drugs in psychiatric disorders (Miller and Raison, 2015; Müller, 2019; Fitton et al., 2022).
2. The role of inflammation across the lifespan
In parallel to this literature, there is emerging evidence to support the role of inflammation in neurodevelopmental and mental health disorders. The immune system plays a critical role in both health and disease across the lifespan (Bilbo and Schwarz, 2009; Furman et al., 2019). Immune dysregulation has been reported in all major syndromes, including ASD, ADHD, OCD, TS/tics, MDD, anxiety, schizophrenia, and psychosis (Ashwood et al., 2011; Mitchell and Goldstein, 2014; Young et al., 2014; Masi et al., 2015; Radhakrishnan et al., 2017). Many environmental factors associated with mental health disorders are known to be pro-inflammatory, such as stress (Rohleder, 2019) and chronic disease (Pawelec et al., 2014). Furthermore, the maternal immune activation hypothesis proposes a link between maternal inflammation during pregnancy and increased risk of neurodevelopmental and neuropsychiatric disorders in offspring (Han et al., 2021a). Evidence shows that pro-inflammatory disorders affecting the mother during pregnancy, such as autoimmunity, infection, asthma, obesity, and gestational diabetes are associated with increased risk of ASD, ADHD, and TS in offspring (Han et al., 2021b). Ongoing pro-inflammatory factors such as stress, obesity, diet, exercise, smoking, pollution, and chronic disease continue to play a role in adulthood and may have causal or exacerbating influences (Furman et al., 2019). Therefore, inflammation resulting from environmental risk factors (e.g., stress, trauma, chronic disease) may play a role in expression of disease throughout the lifespan, from pre-conception, pregnancy, childhood, and into adulthood.
The main psychiatric drugs commonly used in neurodevelopmental and mental health disorders (in both children and adults), such as anti-depressants, anti-psychotics, and mood stabilizers, were designed for their neurotransmitter effects. However, it is now recognized that these drugs have multiple mechanisms of action, including anti-inflammatory effects. Animal models have been developed to study the effects of inflammation on behavior, such as the lipopolysaccharide (LPS) challenge or the interleukin (IL)-1β-induced model of depression in mice. These models have shown that many psychiatric drugs have anti-inflammatory characteristics (reviewed thoroughly by Song and Wang, 2011 and Ma et al., 2017). However, debate continues as to the translational validity of these animal studies to human disease (Barroca et al., 2022).
Here, we have reviewed the human evidence regarding the anti-inflammatory properties of commonly used psychiatric drugs. We present this literature in a narrative review, divided by major drug subgroups, starting with SSRIs, then anti-psychotics and mood stabilizers, followed by other remaining psychiatric drugs. There have been several systematic reviews or meta-analyses (discussed further in subsequent sections) that focus on classes of drugs in certain disorders with specific outcome measures; for example, the effect of anti-psychotics on peripheral cytokine levels in schizophrenia (Tourjman et al., 2013). Therefore, we have not conducted a systematic review. Rather, aim to address a gap in the literature by drawing together the human evidence of anti-inflammatory mechanisms for the main classes of psychiatric drugs across a broad range of mental health disorders.
3. Overview of the investigation of anti-inflammatory mechanisms of psychiatric drugs
Tables 1–6 capture some of the existing investigation into the effects of psychiatric drugs on immune function in humans. The methods that have been used to examine these anti-inflammatory properties are disparate, and we separate them into two main types of methodology: in vivo peripheral immune studies and ex vivo/in vitro cell culture studies. The in vivo peripheral immune studies focus primarily on measuring peripheral cytokine levels in the blood as markers of inflammation. These studies compare cytokine levels between patients with psychiatric disorders and controls at baseline, as well as between patient cohorts before and after treatment. The pro-inflammatory cytokines most commonly measured to investigate these effects are IL-1β, IL-6, interferon (IFN) species, and tumor necrosis factor (TNF)-α, while IL-4 and IL-10 are the most commonly measured anti-inflammatory cytokines. The ex vivo/in vitro cell culture studies involve isolating and culturing immune cells from patients with psychiatric disease or healthy controls and examining the effects of drugs through functional analyses of cell signaling pathways, or cytokine production.
3.1. Selective serotonin reuptake inhibitors
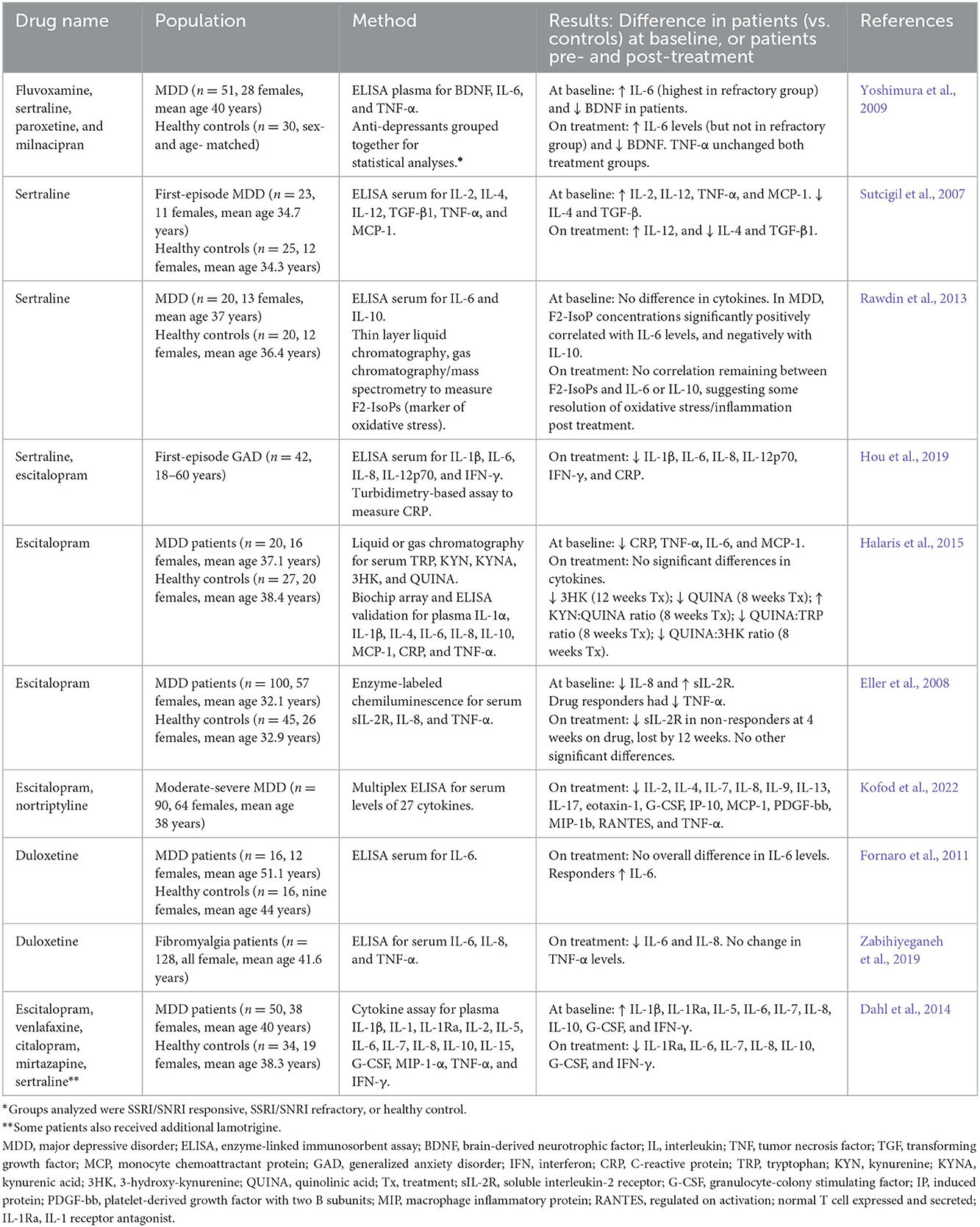
SSRIs are common first-line therapeutics for a variety of emotional and psychiatric conditions, such as MDD, OCD, and anxiety disorders. More recently, another class of drug with additional actions on the noradrenaline system, termed SNRIs (serotonin-norepinephrine reuptake inhibitors), have been developed. SNRIs are now also common first-line anti-depressant therapeutics with effects in anxiety, depression, and OCD, but there is also some evidence of utility in pain syndromes and fibromyalgia (Zabihiyeganeh et al., 2019). When first developed, SSRIs revolutionized treatment of mood disorders by targeting monoaminergic systems, but there is increasing evidence that SSRIs and SNRIs can also modulate inflammation and immune activation, as summarized in Table 1. Notably, these studies have been performed solely in adults (rather than children), and largely in the context of depression, with a small number in anxiety and pain disorders. Most of these studies compared serum or plasma cytokines (single cytokines or panels of cytokines) at baseline in patients with controls, and then compared cytokines on treatment compared to baseline.
The main theme is that SSRIs/SNRIs can reduce expression of pro-inflammatory cytokines, particularly IL-6 and TNF-α (Table 1). As well as cytokines, metabolites in the kynurenine pathway can provide valuable insight into cellular function and inflammation: briefly, kynurenine is increased in inflammation, kynurenic acid (KYNA) is an anti-inflammatory and neuroprotective metabolite, and conversely quinolinic acid (QUINA) is a pro-inflammatory and neurotoxic metabolite produced within this pathway. An elegant study from Halaris et al. (2015) in patients with MDD showed treatment with the SSRI escitalopram ameliorated neurotoxicity by increasing the ratio of neuroprotective KYNA to QUINA and decreasing the ratio of QUINA to tryptophan. A study from Borsini et al. (2017) builds on these findings (Table 2) and showed incubation with the SSRI sertraline in vitro also reduces levels of QUINA and other enzymes in the kynurenine pathway.
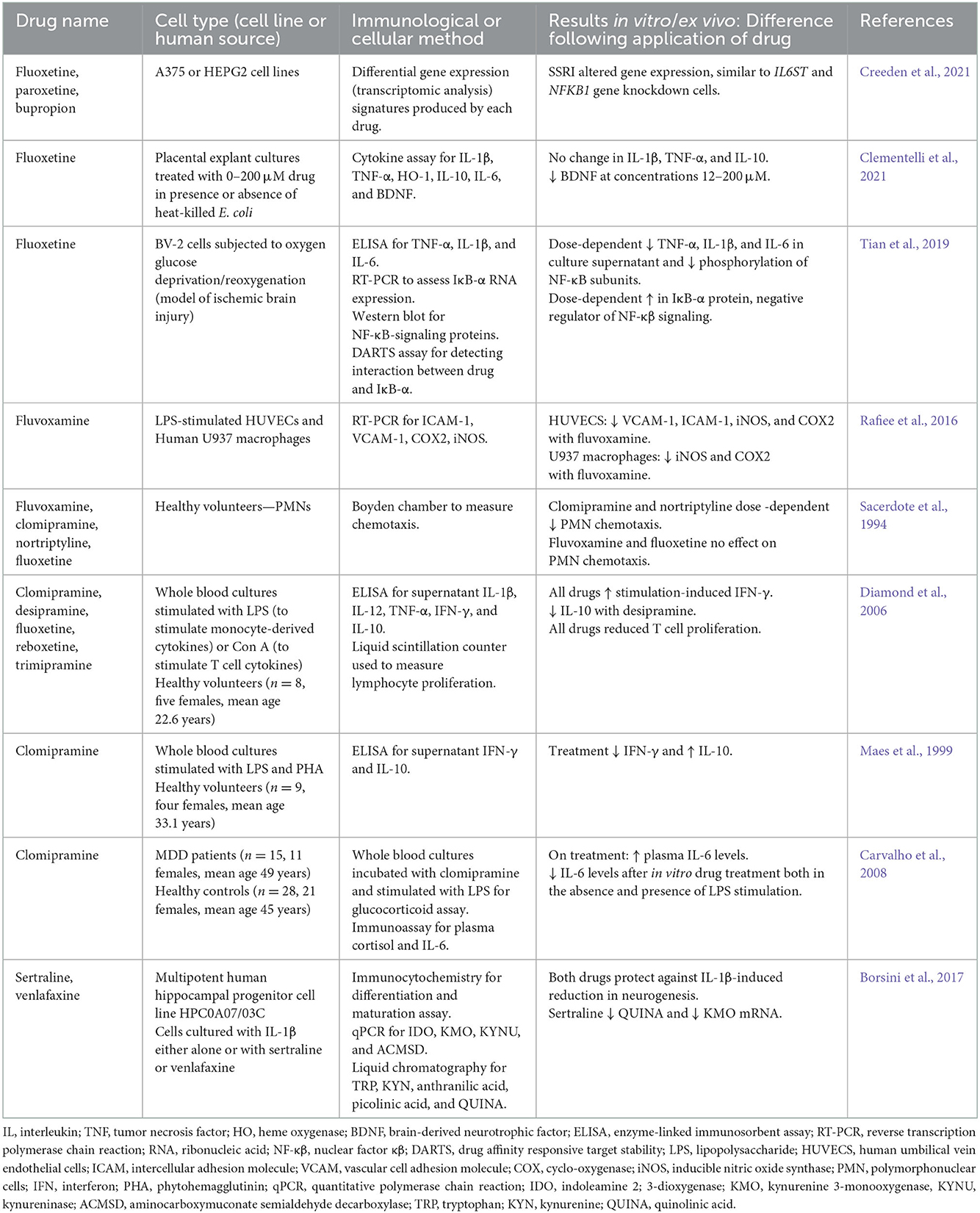
Table 2. Anti-inflammatory effects of SSRIs and/or SNRIs using in vitro or ex vivo human cells in culture or stimulation assays.
Similarly, many in vitro studies have investigated inflammatory cytokine expression following SSRI/SNRI treatment (Table 2). In various in vitro models of inflammation, SSRIs have been shown to reduce inflammatory cytokines such as IL-6, TNF-α, IFN-γ, and IL-1, while often increasing levels of the anti-inflammatory IL-10. A study by Creeden et al. (2021) also demonstrated the ability of SSRIs to disrupt nuclear factor- (NF-)κB signaling, in turn reducing transcriptional activation of IL-6 and ameliorating the “cytokine storm” often seen with acute inflammation (see Figure 1 for schematic of common signaling pathways). Likewise, in an in vitro model of ischemic brain injury it was shown that, while decreasing inflammatory cytokine levels, the SSRI fluoxetine also decreased levels of NF-κB subunits through dose-dependent upregulation of the protein IκB, a negative regulator of the NF-κB signaling pathway (Tian et al., 2019) (Figure 1). SSRIs are also able to inhibit expression of inflammatory genes related to adhesion molecules ICAM-1 and VCAM-1 (intracellular cell adhesion molecule-1 and vascular cell adhesion molecule-1, respectively), which are usually upregulated on vascular endothelium and leukocytes during inflammatory events. Furthermore, SSRIs/SNRIs also reduce expression of inflammatory mediators COX2 and iNOS (cyclo-oxygenase 2 and inducible nitric oxide synthase, respectively) (Rafiee et al., 2016). Additionally, a small number of studies have shown SSRIs can reduce polymorphonuclear chemotaxis (Sacerdote et al., 1994) and reduce T cell proliferation (Diamond et al., 2006). Systematic reviews and meta-analyses have also confirmed the modulatory effects of SSRIs and SNRIs in inflammatory contexts. One meta-analysis concluded that peripheral levels of IL-6, TNF-α, and IL-10 were decreased following SSRI therapy (Köhler et al., 2017), with another similarly showing reductions in plasma TNF-α but not IL-6 (Almeida et al., 2020); this second meta-analysis used stringent inclusion criteria, and the authors acknowledge that very few studies met their inclusion criteria which may have contributed to this conflicting result.

Figure 1. Common signaling pathways. TLRs are activated by various stimuli, commonly recognizing viral and bacterial products, or host-derived endogenous compounds (i.e., DAMPs or PAMPs, respectively). Triggering of TLRs can activate multiple pathways which produce complexes that activate the IKK structure (comprising catalytic IKKα and IKKβ, and regulatory IKKγ subunits). NF-κB is produced and either translocates to the nucleus or is negatively regulated by phosphorylation of IκB. In the nucleus, NF-κB influences the expression of many inflammatory genes, in turn affecting production of cytokines, chemokines, adhesion molecules, and costimulatory molecules. Activation of the JAK/STAT pathway via cytokine receptors also contributes to NF-κB signaling and alterations in the expression of inflammatory genes. JAK, Janus kinase; STAT, signal transducer and activator of transcription; TLR, toll-like receptor; DAMP, damage-associated molecular patterns; PAMP, pathogen-associated molecular pattern; MyD88, myeloid differentiation primary response 88; IKK, inhibitor of κB kinase; IRAK, interleukin receptor-associated kinase; TRAF, tumor necrosis factor receptor-associated factor; IκB, inhibitor of nuclear factor κB; NF-κB, nuclear factor κB; TNF, tumor necrosis factor; IL, interleukin; CCL, CC motif chemokine ligand;; CXCL, CXC motif chemokine ligand; ICAM, intercellular adhesion molecule; VCAM, vascular cell adhesion molecule; CD, cluster of differentiation. Figure adapted from “TLR Signaling Pathway”, by BioRender.com (2015). Retrieved from: https://app.biorender.com/biorender-templates.
3.2. Anti-psychotics
Anti-psychotics, such as risperidone, olanzapine, and aripiprazole, are primarily used to treat schizophrenia and psychosis (Gardner, 2005); however, there is first-level evidence of their effect in treating behavioral disturbance in people with ASD (Aman et al., 2008; Posey et al., 2008). Anti-psychotics have many mechanisms of action, although their primary proposed action is to alter dopaminergic neurotransmission in the brain (Horacek et al., 2006). Clozapine, a major pharmacological agent in managing treatment-resistant schizophrenia, is distinct in its mechanism of action, antagonizing dopamine receptors while also binding with high affinity to several serotonin receptors, histamine receptors, adrenergic receptors, and muscarinic receptors (Gammon et al., 2021). Studies investigating the effects of anti-psychotics on inflammation have largely been conducted in adults with schizophrenia or psychosis (Table 3). Most of these studies measured the effect of anti-psychotic treatment on serum cytokine/chemokine levels using enzyme-linked immunosorbent assays (ELISAs) and other cytokine assays. At baseline, patients with schizophrenia and psychosis had higher levels of inflammatory cytokines, particularly IL-6 and TNF-α, compared to controls (Song et al., 2014; Noto et al., 2015). Treatment with anti-psychotics reduced these inflammatory cytokines (Song et al., 2014; Noto et al., 2015; Sobiś et al., 2015; Juncal-Ruiz et al., 2018) and, in some cases, increased the levels of anti-inflammatory cytokines, such as IL-10 (Sobiś et al., 2015). A meta-analysis of 23 studies investigating the effect of anti-psychotic treatment on peripheral cytokine levels in schizophrenia found reduced levels of IL-1β and IFN-γ, and increased plasma levels of soluble IL-2 receptor after treatment (Tourjman et al., 2013), similar to other studies shown in Table 3. Additionally, a meta-analysis of first-episode psychosis cohorts found that anti-psychotic treatment decreased peripheral levels of pro-inflammatory cytokines IL-1β, IL-6, IFN-γ, and TNF-α (Marcinowicz et al., 2021). The levels of anti-inflammatory IL-4 and IL-10 were also decreased following anti-psychotic treatment in this study, which contradicts some studies shown in Table 3.
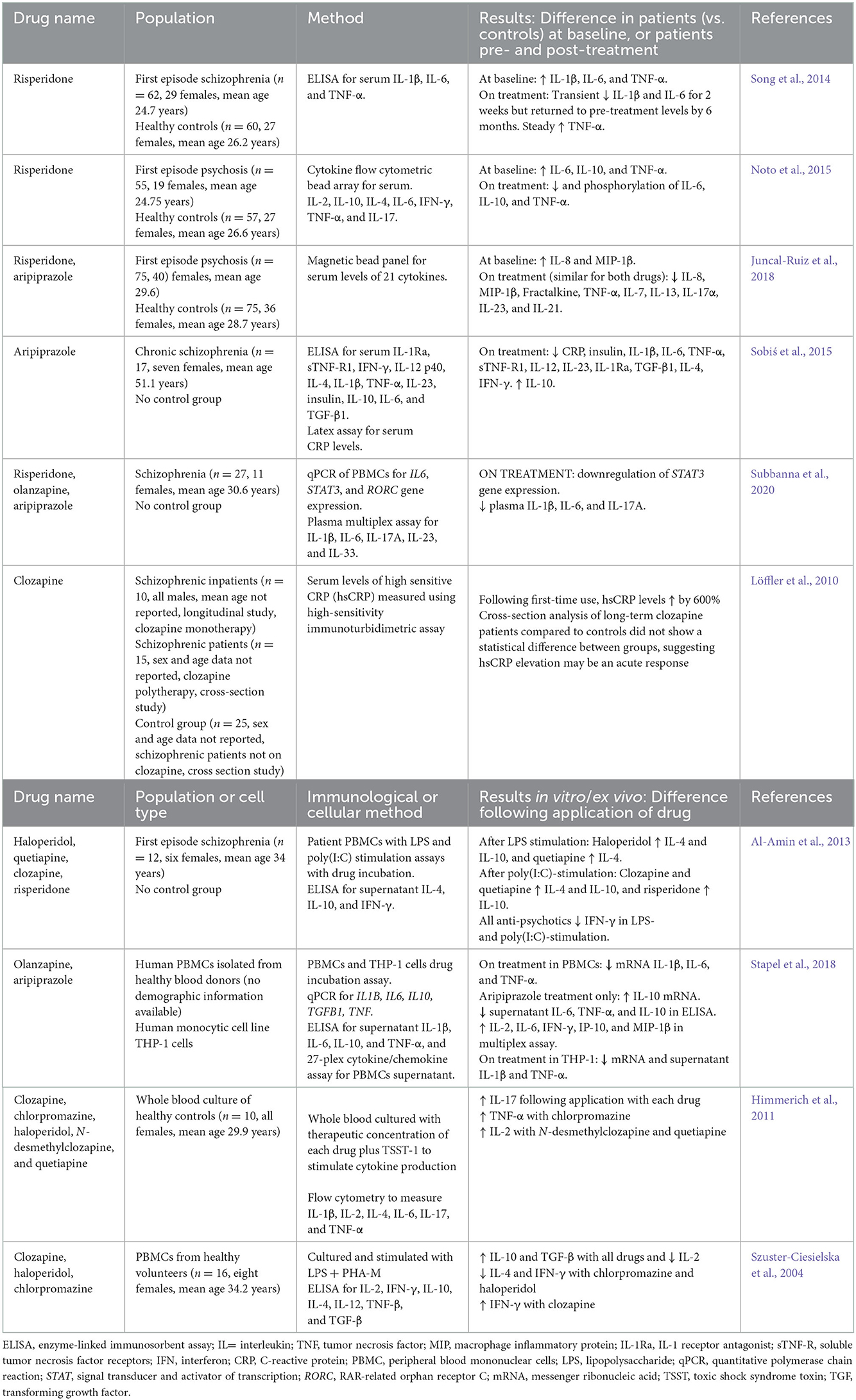
Table 3. Anti-inflammatory effects of anti-psychotics in human studies and in studies using in vitro or ex vivo human cells in culture or stimulation assays.
Several studies also used peripheral blood mononuclear cells (PBMCs) from patients with schizophrenia or healthy volunteers to conduct in vitro stimulation and signaling assays. Patient PBMCs showed decreases in IFN-γ and increases in IL-4 and IL-10 in cell culture supernatant after incubation with anti-psychotics (Al-Amin et al., 2013). One study also showed downregulation of STAT3 gene expression, indicating that anti-psychotics reduced inflammatory cell signaling via the signal transducer and activator of transcription (STAT) pathways (Subbanna et al., 2020) (Figure 1). PBMCs from healthy volunteers showed decreases in IL-6 and TNF-α and increases in IL-10 when incubated with anti-psychotics in vitro, similar to the serum cytokine studies in patient cohorts (Stapel et al., 2018).
3.3. Mood stabilizers
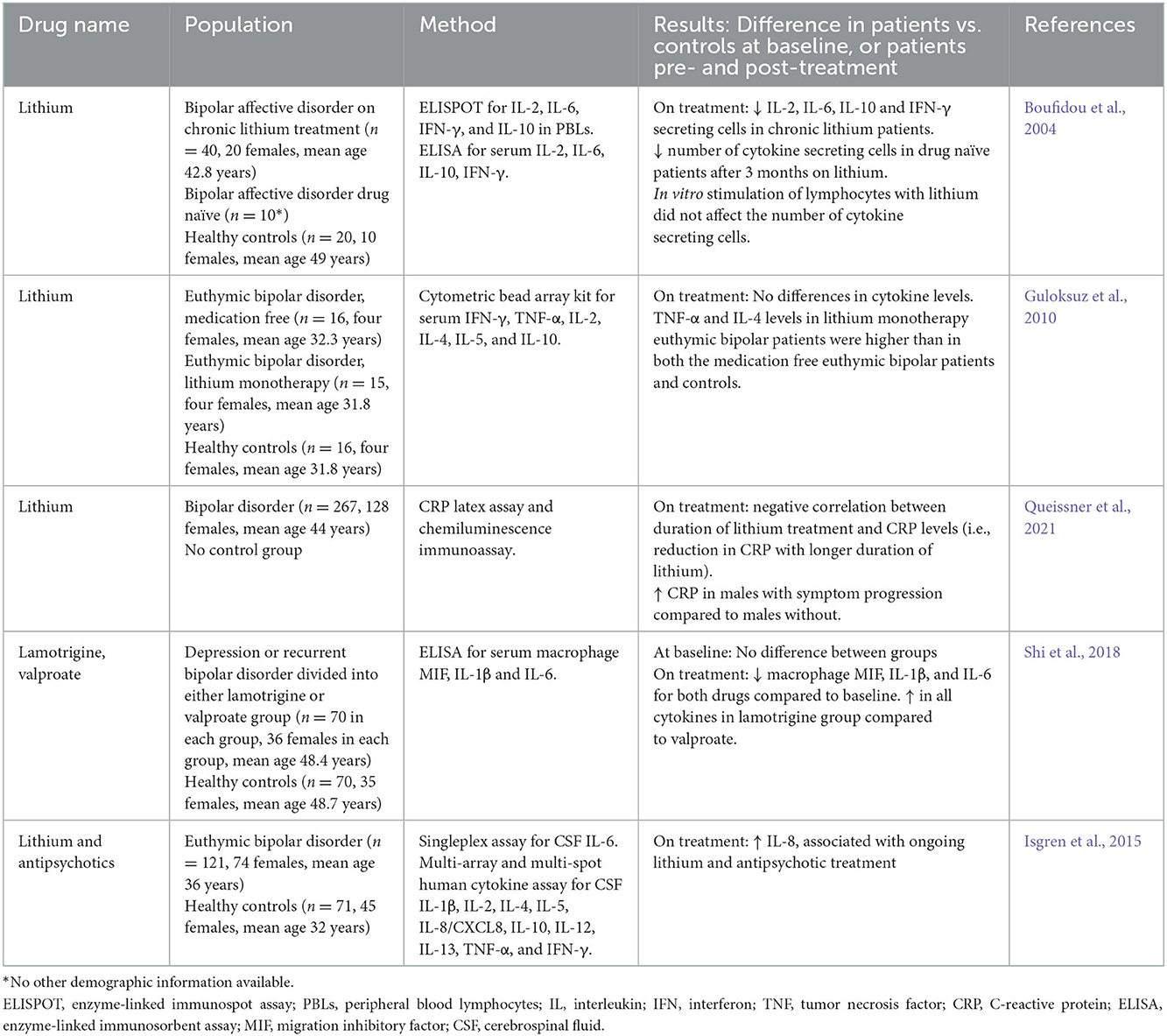
Mood stabilizers, such as lithium, valproate, and lamotrigine, are commonly used to treat bipolar disorder and psychosis, with some evidence to support their use in ASD (Aman et al., 2008; Canitano, 2015). These drugs have complex mechanisms of action, all affecting neurotransmission in the brain (Rapoport et al., 2009; Chiu et al., 2013). The anti-inflammatory effects of commonly used mood stabilizers have primarily been investigated in adults with bipolar disorder, using ELISAs and other assays to measure peripheral cytokine/chemokine levels. Meta-analyses have shown higher levels of pro-inflammatory and anti-inflammatory cytokines, TNF-α, IL-1β, IL-6, IL-4, and IL-10 in patients with bipolar disorder compared to controls at baseline (Modabbernia et al., 2013). The studies reviewed in Table 4 found that lithium and lamotrigine decreased the levels of IL-6, IL-10, IFN-γ, and IL-1β, as well as C-reactive protein (CRP) (Boufidou et al., 2004; Shi et al., 2018; Queissner et al., 2021). Conversely, increased levels of TNF-α and IL-4 were found in euthymic bipolar patients on lithium monotherapy (Guloksuz et al., 2010). In general, mood stabilizing drugs have been shown to normalize elevated peripheral pro-inflammatory cytokine levels in bipolar disorder (van den Ameele et al., 2016). In cerebrospinal fluid (CSF), levels of 1L-8 were found to be increased in patients with euthymic bipolar disorder compared to controls, and this was positively associated with ongoing lithium and antipsychotic treatment (Isgren et al., 2015). Ex vivo studies (Table 5) using peripheral blood monocytes from patients with bipolar disorder have also shown that lithium reduces expression of inflammatory genes (IL-6, TNF, CXCL2) (Padmos et al., 2008) and decreases IL-6 and IL-1β production (Knijff et al., 2007). Similarly, in cultures from healthy volunteers, lithium reduced IL-6, IL-1β, IL-2, TNF-α, and IFN-γ, and increased IL-4, IL-10, and IL-22 (Rapaport and Manji, 2001; Himmerich et al., 2013). In human microglia cultures, lithium reduced IFN-γ and STAT1/STAT3 signaling (Göttert et al., 2022). Valproic acid had similar effects on cytokines as lithium, and also reduced differentiation of T helper (Th) 17 cells and dendritic cells, chemotaxis migration of dendritic cells, lymphocyte proliferation, NF-κB activation, and nuclear levels of IFN regulatory factors (Nencioni et al., 2007; Leu et al., 2017) (Figure 1).

Table 5. Anti-inflammatory effects of mood stabilizers using in vitro or ex vivo human cells in culture or stimulation assays.
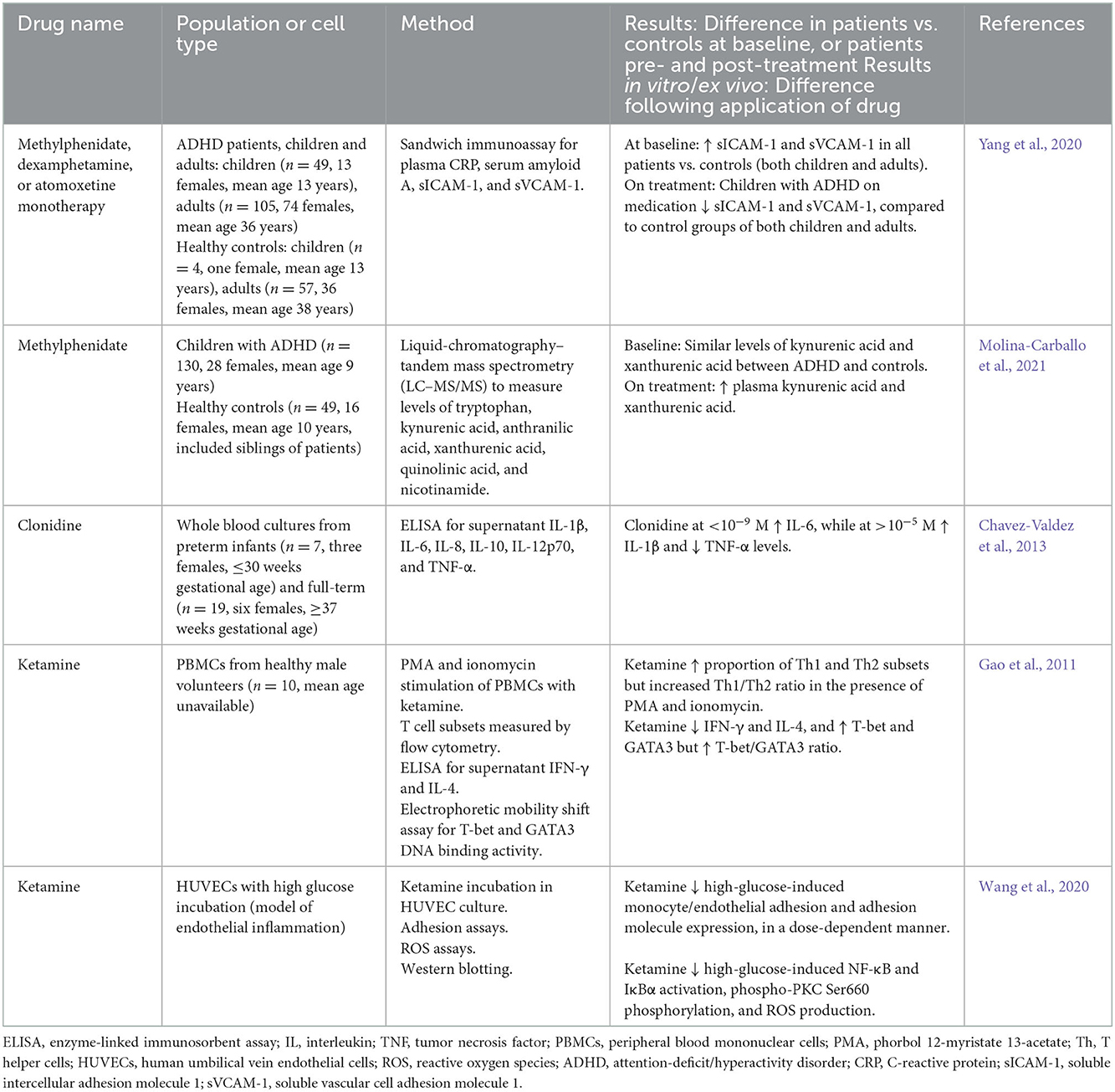
Table 6. Anti-inflammatory effects of other drugs in human studies and in studies using in vitro or ex vivo human cells in culture or stimulation assays.
3.4. Other psychiatric drugs
There is a smaller literature regarding the anti-inflammatory effects of other psychiatric drugs such as stimulants, α-agonists, and glutamatergic drugs. Studies investigating the anti-inflammatory effects of common ADHD drugs (e.g., methylphenidate, dexamphetamine, or atomoxetine) in humans are limited. One study found that these drugs increased levels of soluble ICAM-1, and soluble VCAM-1 (sICAM-1 and sVCAM-1, respectively) in children with ADHD (Yang et al., 2020), contrasting with the published effects of SSRIs on adhesion molecules. Methylphenidate has also been shown to increase plasma KYNA and xanthurenic acid in children with ADHD (Molina-Carballo et al., 2021). As previously mentioned, KYNA is anti-inflammatory, and this increase is similar to the effect of SSRIs shown by Halaris et al. (2015). Clonidine is an α2 adrenergic agonist and primarily used as an anti-hypertensive agent; however, it is also effective in treating ADHD and behavioral disturbances in neurodevelopmental disorders (Ming et al., 2008; Kollins et al., 2011). Studies investigating the biological effects of clonidine in humans in the context of ADHD are limited. In an in vitro study of whole blood cultured from infants, incubation with clonidine increased the levels of pro-inflammatory cytokine IL-1β but reduced TNF-α, similar to the effects of lithium.
Ketamine is a non-competitive antagonist at the N-methyl-D-aspartate (NMDA) receptor and commonly used as an analgesic in pain management (Visser and Schug, 2006). Ketamine has been shown to have dopaminergic and anti-depressant effects and is sometimes used to treat complex neuropsychiatric symptoms, with an emerging role in depression (Kokkinou et al., 2018). Human studies examining the anti-inflammatory effects of ketamine are very limited. In PBMCs from healthy males, ketamine decreased the proportion of Th1 and Th2 cell subsets but increased the Th1/Th2 ratio (Gao et al., 2011). Ketamine also decreased expression of transcription factors T-bet and GATA3, thereby preventing Th1 and Th2 cell differentiation. Subsequent cytokine production was also reduced, demonstrated by decreased levels of IFN-γ (Th1-produced cytokine) and IL-4 (Th2-produced cytokine) (Gao et al., 2011). In Human Umbilical Vein Endothelial Cells (HUVECs), ketamine reduced high-glucose-induced monocyte/endothelial adhesion, while also reducing NF-κB activation and reactive oxygen species (ROS) production (Wang et al., 2020).
4. Discussion
Our review highlights that commonly used psychiatric drugs, namely anti-depressants, anti-psychotics, and mood stabilizers, have demonstrated anti-inflammatory effects in humans. In general, there is evidence to suggest that all drug classes reduce peripheral levels of pro-inflammatory cytokines (particularly, IL-6 and TNF-α) and decrease inflammatory pathway signaling (e.g., NF-κB, STAT). However, the literature to date is limited in scope, both clinically and biologically. Most studies have been conducted in adult cohorts, mostly in depression, psychosis, and bipolar disorder. Although these drugs are commonly used in children to treat neurodevelopmental and neuropsychiatric symptoms, there is a lack of pediatric studies in this context. Disorders with relatively strong support for the role of inflammation, such as ASD (Meltzer and Van de Water, 2017) and post-traumatic stress disorder (Passos et al., 2015), have had limited investigation into the anti-inflammatory effects of psychiatric drugs. Though they are used very commonly, there is particularly limited human evidence regarding ADHD drugs (e.g., methylphenidate, dexamphetamine, or atomoxetine); however, they have shown anti-inflammatory effects in animal studies (Aga-Mizrachi et al., 2014; Yssel et al., 2018).
In addition, the methodology used to demonstrate anti-inflammatory effects is restricted: most studies only examine the levels of cytokines in peripheral blood before and after treatment, and in vitro and ex vivo studies likewise tend to examine cytokines or cytokine signaling. While cytokine and chemokine measures are commonly used as clinical indicators of inflammation, it is important to note that the stability of these measures can be affected by many factors, including sample collection issues, duration and method of storage, time of collection, fasting, physical activity and stress. Cytokine levels can vary substantially between serum, plasma, whole blood, and CSF. Additionally, the method of analysis, for example ELISAs compared to multiplex assays, can also influence the quantification results (Leng et al., 2008; Zhou et al., 2010; Liu et al., 2021). As there are no standardized, gold-standard guidelines on how to reliably quantify cytokines, results regarding cytokine data should be interpreted and compared with caution. Studies of the CNS would also be of immense value as this field moves forward. Studies have begun to investigate markers of inflammation in CSF in mental health disorders, yet studies measuring CSF following use of a therapeutic drug are limited. A study by Miller et al. (2017) showed that despite pregnant women taking SSRIs having significantly reduced levels of IFN-γ and IL-8 in peripheral blood compared to pregnant women not using SSRIs, no significant differences were observed in CSF. A meta-analysis of markers of central inflammation in MDD patients determined CSF levels of IL-6 and TNF-α were higher in MDD patients compared to controls, and there was no significant correlation between abnormalities in CSF and those seen in peripheral blood (Enache et al., 2019), further complicating understanding the mechanisms underlying these conditions and how pharmacological agents may interact. The literature on inflammatory metabolism (e.g., kynurenine pathway), and epigenetic regulation of immune cells, is very limited, but provides opportunities for future studies.
Another remaining key issue is whether inflammation is relevant to all patients with mental health disorders, or only in specific subgroups. Resolving this is paramount to allow targeted therapeutics to be developed and implemented clinically in the future. Studies targeting pro-inflammatory cytokines, such as TNF-α, have exemplified this problem (Raison et al., 2013). In a study of refractory depression, infliximab, a TNF-α inhibitor, did not have generalizable benefit in the treatment of refractory depression, but did result in clinical benefits in patients with elevated CRP at baseline. This study supports the “immune subgroup” hypothesis in mental health disorders, emphasizing the need for patient subgrouping and individualized therapies. As well, as mentioned previously, the neurotransmitter hypotheses relating to many mental health disorders are not conclusive. While there is significant evidence supporting neurotransmitter dysfunction in these contexts, the origins of these dysfunctions are ambiguous, and it is unclear whether neurotransmitter imbalance in mental health disorders is primary or secondary to other processes.
A final question, and one not addressed by the current literature, is the issue of whether inflammation has a causal pathway in the generation or perpetuation of mental health disorders, as opposed to an “association” only. None of the human studies we have reviewed here showed causation between inflammatory states and mental health symptoms, only association. While animal models have convincingly shown (neuro)inflammation causes and exacerbates mental health disorders, and ameliorating inflammation produces symptomatic benefits, this degree of evidence is largely absent in humans, to date (Hayley, 2011; Song and Wang, 2011; Harvey and Boksa, 2012; Gumusoglu and Stevens, 2019). Hence, even though there is evidence that commonly used psychiatric drugs have anti-inflammatory properties, there is no evidence in human patients of clinical benefit being a direct result of reduced inflammation. Similarly, there are minimal comparative studies between anti-inflammatory properties of drugs between classes (e.g., comparing SSRIs/SNRIs with anti-psychotics), and no studies exploring augmentative properties (i.e., additive anti-inflammatory effects) of multiple psychiatric drug classes in patients.
In summary, we have presented emerging evidence for the anti-inflammatory properties of commonly used psychiatric drugs. As researchers, clinicians, and patients and their families become increasingly aware of the role of inflammation in brain health, there should be a degree of comfort in the idea that drugs used to treat common mental health symptoms may also abrogate this inflammation.
Author contributions
SP and BK: conceptual design, literature review, and primary writing and editing of manuscript. RD: conceptual design and primary writing and editing of manuscript. All authors contributed to the article and approved the submitted version.
Funding
This work was funded by the National Health and Medical Research Council (NHMRC) Australia Investigator Grant APP1193648 (RD) and the Petre Foundation (RD).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Aga-Mizrachi, S., Cymerblit-Sabba, A., Gurman, O., Balan, A., Shwam, G., Deshe, R., et al. (2014). Methylphenidate and desipramine combined treatment improves PTSD symptomatology in a rat model. Transl. Psychiatry 4, e447. doi: 10.1038/tp.2014.82
Al-Amin, M., Uddin, M. M. N., and Reza, H. M. (2013). Effects of antipsychotics on the inflammatory response system of patients with schizophrenia in peripheral blood mononuclear cell cultures. Clin. Psychopharmacol. Neurosci. 11, 144–151. doi: 10.9758/cpn.2013.11.3.144
Almeida, I. B., Gomes, I. A., Shanmugam, S., de Moura, T. R., Magalhães, L. S., de Aquino, L. A. G., et al. (2020). Inflammatory modulation of fluoxetine use in patients with depression: a systematic review and meta-analysis. Cytokine 131, 155100. doi: 10.1016/j.cyto.2020.155100
Aman, M. G., Farmer, C. A., Hollway, J., and Arnold, L. E. (2008). Treatment of inattention, overactivity, and impulsiveness in autism spectrum disorders. Child Adolesc. Psychiatr. Clin. N. Am. 17, 713–738. doi: 10.1016/j.chc.2008.06.009
Ashwood, P., Krakowiak, P., Hertz-Picciotto, I., Hansen, R., Pessah, I. N., and Van de Water, J. (2011). Associations of impaired behaviors with elevated plasma chemokines in autism spectrum disorders. J. Neuroimmunol. 232, 196–199. doi: 10.1016/j.jneuroim.2010.10.025
Barroca, N. C. B., Della Santa, G., Suchecki, D., García-Cairasco, N., Umeoka, E., and de Henrique, L. (2022). Challenges in the use of animal models and perspectives for a translational view of stress and psychopathologies. Neurosci. Biobehav. Rev. 140, 104771. doi: 10.1016/j.neubiorev.2022.104771
Bilbo, S. D., and Schwarz, J. M. (2009). Early-life programming of later-life brain and behavior: a critical role for the immune system. Front. Behav. Neurosci. 3, 14. doi: 10.3389/neuro.08.014.2009
Borsini, A., Alboni, S., Horowitz, M. A., Tojo, L. M., Cannazza, G., Su, K. P., et al. (2017). Rescue of IL-1β-induced reduction of human neurogenesis by omega-3 fatty acids and antidepressants. Brain Behav. Immun. 65, 230–238. doi: 10.1016/j.bbi.2017.05.006
Boufidou, F., Nikolaou, C., Alevizos, B., Liappas, I. A., and Christodoulou, G. N. (2004). Cytokine production in bipolar affective disorder patients under lithium treatment. J. Affect. Disord. 82, 309–313. doi: 10.1016/j.jad.2004.01.007
Canitano, R. (2015). Mood stabilizers in children and adolescents with autism spectrum disorders. Clin. Neuropharmacol. 38, 177–182. doi: 10.1097/WNF.0000000000000096
Carvalho, L. A., Juruena, M. F., Papadopoulos, A. S., Poon, L., Kerwin, R., Cleare, A. J., et al. (2008). Clomipramine in vitro reduces glucocorticoid receptor function in healthy subjects but not in patients with major depression. Neuropsychopharmacology 33, 3182–3189. doi: 10.1038/npp.2008.44
Caspi, A., and Moffitt, T. E. (2006). Gene–environment interactions in psychiatry: joining forces with neuroscience. Nat. Rev. Neurosci. 7, 583–590. doi: 10.1038/nrn1925
Chavez-Valdez, R., Kovell, L., Ahlawat, R., McLemore, G. L., Wills-Karp, M., and Gauda, E. B. (2013). Opioids and clonidine modulate cytokine production and opioid receptor expression in neonatal immune cells. J. Perinatol. 33, 374–382. doi: 10.1038/jp.2012.124
Chiu, C.-T., Wang, Z., Hunsberger, J. G., and Chuang, D.-M. (2013). Therapeutic potential of mood stabilizers lithium and valproic acid: beyond bipolar disorder. Pharmacol. Rev. 65, 105–142. doi: 10.1124/pr.111.005512
Clementelli, C., Arita, Y., Ahmed, S., Pijush, D. B., Jeong Park, H., Levenson, A. S., et al. (2021). Short communication: ex-vivo effects of fluoxetine on production of biomarkers for inflammation and neurodevelopment by the placenta. Placenta 107, 46–50. doi: 10.1016/j.placenta.2021.02.018
Creeden, J. F., Imami, A. S., Eby, H. M., Gillman, C., Becker, K. N., Reigle, J., et al. (2021). Fluoxetine as an anti-inflammatory therapy in SARS-CoV-2 infection. Biomed. Pharmacother. 138, 111437. doi: 10.1016/j.biopha.2021.111437
Cross-Disorder Group of the Psychiatric Genomics Consortium (2019). Genomic relationships, novel loci, and pleiotropic mechanisms across eight psychiatric disorders. Cell 179, 1469–1482.e1411. doi: 10.1016/j.cell.2019.11.020
Dahl, J., Ormstad, H., Aass, H. C. D., Malt, U. F., Bendz, L. T., Sandvik, L., et al. (2014). The plasma levels of various cytokines are increased during ongoing depression and are reduced to normal levels after recovery. Psychoneuroendocrinology 45, 77–86. doi: 10.1016/j.psyneuen.2014.03.019
Diamond, M., Kelly, J. P., and Connor, T. J. (2006). Antidepressants suppress production of the Th1 cytokine interferon-γ, independent of monoamine transporter blockade. Eur. Neuropsychopharmacol. 16, 481–490. doi: 10.1016/j.euroneuro.2005.11.011
Eller, T., Vasar, V., Shlik, J., and Maron, E. (2008). Pro-inflammatory cytokines and treatment response to escitaloprsam in major depressive disorder. Progr. Neuropsychopharmacol. Biol. Psychiatry 32, 445–450. doi: 10.1016/j.pnpbp.2007.09.015
Enache, D., Pariante, C. M., and Mondelli, V. (2019). Markers of central inflammation in major depressive disorder: a systematic review and meta-analysis of studies examining cerebrospinal fluid, positron emission tomography and post-mortem brain tissue. Brain Behav. Immun. 81, 24–40. doi: 10.1016/j.bbi.2019.06.015
Fitton, R., Sweetman, J., Heseltine-Carp, W., and van der Feltz-Cornelis, C. (2022). Anti-inflammatory medications for the treatment of mental disorders: a scoping review. Brain Behav. Immunity Health 26, 100518. doi: 10.1016/j.bbih.2022.100518
Fornaro, M., Martino, M., Battaglia, F., Colicchio, S., and Perugi, G. (2011). Increase in IL-6 levels among major depressive disorder patients after a 6-week treatment with duloxetine 60 mg/day: a preliminary observation. Neuropsychiatr. Dis. Treat. 7, 51–56. doi: 10.2147/NDT.S16382
Furman, D., Campisi, J., Verdin, E., Carrera-Bastos, P., Targ, S., Franceschi, C., et al. (2019). Chronic inflammation in the etiology of disease across the life span. Nat. Med. 25, 1822–1832. doi: 10.1038/s41591-019-0675-0
Gammon, D., Cheng, C., Volkovinskaia, A., Baker, G. B., and Dursun, S. M. (2021). Clozapine: why is it so uniquely effective in the treatment of a range of neuropsychiatric disorders? Biomolecules 11, 1030. doi: 10.3390/biom11071030
Gao, M., Jin, W., Qian, Y., Ji, L., Feng, G., and Sun, J. (2011). Effect of N-methyl-D-aspartate receptor antagonist on T helper cell differentiation induced by phorbol-myristate-acetate and ionomycin. Cytokine 56, 458–465. doi: 10.1016/j.cyto.2011.06.022
Gardner, A., and Boles, R. G. (2011). Beyond the serotonin hypothesis: mitochondria, inflammation and neurodegeneration in major depression and affective spectrum disorders. Prog. Neuropsychopharmacol. Biol. Psychiatry 35, 730–743. doi: 10.1016/j.pnpbp.2010.07.030
Gardner, D. M. (2005). Modern antipsychotic drugs: a critical overview. Can. Med. Assoc. J. 172, 1703–1711. doi: 10.1503/cmaj.1041064
Gibb, S. J., Fergusson, D. M., and Horwood, L. J. (2010). Burden of psychiatric disorder in young adulthood and life outcomes at age 30. Br. J. Psychiatry 197, 122–127. doi: 10.1192/bjp.bp.109.076570
Global Research on Developmental Disabilities Collaborators (2018). Developmental disabilities among children younger than 5 years in 195 countries and territories, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Global Health 6, e1100–e1121. doi: 10.1016/S2214-109X(18)30309-7
Göttert, R., Fidzinski, P., Kraus, L., Schneider, U. C., Holtkamp, M., Endres, M., et al. (2022). Lithium inhibits tryptophan catabolism via the inflammation-induced kynurenine pathway in human microglia. Glia 70, 558–571. doi: 10.1002/glia.24123
Greenberg, P. E., Fournier, A.-A., Sisitsky, T., Pike, C. T., and Kessler, R. C. (2015). The economic burden of adults with major depressive disorder in the United States (2005 and 2010). J. Clin. Psychiatry 76, 155–162. doi: 10.4088/JCP.14m09298
Guloksuz, S., Aktas Cetin, E., Cetin, T., Deniz, G., Oral, E. T., and Nutt, D. J. (2010). Cytokine levels in euthymic bipolar patients. J. Affect. Disord. 126, 458–462. doi: 10.1016/j.jad.2010.04.027
Gumusoglu, S. B., and Stevens, H. E. (2019). Maternal inflammation and neurodevelopmental programming: a review of preclinical outcomes and implications for translational psychiatry. Biol. Psychiatry 85, 107–121. doi: 10.1016/j.biopsych.2018.08.008
Halaris, A., Myint, A. M., Savant, V., Meresh, E., Lim, E., Guillemin, G., et al. (2015). Does escitalopram reduce neurotoxicity in major depression? J. Psychiatr. Res. 66–67, 118–126. doi: 10.1016/j.jpsychires.2015.04.026
Han, V. X., Patel, S., Jones, H. F., and Dale, R. C. (2021a). Maternal immune activation and neuroinflammation in human neurodevelopmental disorders. Nat. Rev. Neurol. 17, 564–579. doi: 10.1038/s41582-021-00530-8
Han, V. X., Patel, S., Jones, H. F., Nielsen, T. C., Mohammad, S. S., Hofer, M. J., et al. (2021b). Maternal acute and chronic inflammation in pregnancy is associated with common neurodevelopmental disorders: a systematic review. Transl. Psychiatry 11, 71. doi: 10.1038/s41398-021-01198-w
Hansen, B. H., Oerbeck, B., Skirbekk, B., Petrovski, B. E., and Kristensen, H. (2018). Neurodevelopmental disorders: prevalence and comorbidity in children referred to mental health services. Nord. J. Psychiatry 72, 285–291. doi: 10.1080/08039488.2018.1444087
Harvey, L., and Boksa, P. (2012). Prenatal and postnatal animal models of immune activation: relevance to a range of neurodevelopmental disorders. Dev. Neurobiol. 72, 1335–1348. doi: 10.1002/dneu.22043
Hayley, S. (2011). Toward an anti-inflammatory strategy for depression. Front. Behav. Neurosci. 5, 19. doi: 10.3389/fnbeh.2011.00019
Himmerich, H., Bartsch, S., Hamer, H., Mergl, R., Schönherr, J., Petersein, C., et al. (2013). Impact of mood stabilizers and antiepileptic drugs on cytokine production in-vitro. J. Psychiatr. Res. 47, 1751–1759. doi: 10.1016/j.jpsychires.2013.07.026
Himmerich, H., Schönherr, J., Fulda, S., Sheldrick, A. J., Bauer, K., and Sack, U. (2011). Impact of antipsychotics on cytokine production in-vitro. J. Psychiatr. Res. 45, 1358–1365. doi: 10.1016/j.jpsychires.2011.04.009
Horacek, J., Bubenikova-Valesova, V., Kopecek, M., Palenicek, T., Dockery, C., Mohr, P., et al. (2006). Mechanism of action of atypical antipsychotic drugs and the neurobiology of schizophrenia. CNS Drugs 20, 389–409. doi: 10.2165/00023210-200620050-00004
Hou, R., Ye, G., Liu, Y., Chen, X., Pan, M., Zhu, F., et al. (2019). Effects of SSRIs on peripheral inflammatory cytokines in patients with generalized anxiety disorder. Brain Behav. Immun. 81, 105–110. doi: 10.1016/j.bbi.2019.06.001
Ichiyama, T., Okada, K., Lipton, J. M., Matsubara, T., Hayashi, T., and Furukawa, S. (2000). Sodium valproate inhibits production of TNF-α and IL-6 and activation of NF-κB. Brain Res. 857, 246–251. doi: 10.1016/S0006-8993(99)02439-7
Isgren, A., Jakobsson, J., Pålsson, E., Ekman, C. J., Johansson, A. G. M., Sellgren, C., et al. (2015). Increased cerebrospinal fluid interleukin-8 in bipolar disorder patients associated with lithium and antipsychotic treatment. Brain Behav. Immun. 43, 198–204. doi: 10.1016/j.bbi.2014.10.001
Juncal-Ruiz, M., Riesco-Dávila, L., Ortiz-García de la Foz, V., Martínez-Garcia, O., Ramírez-Bonilla, M., Ocejo-Viñals, J. G., et al. (2018). Comparison of the anti-inflammatory effect of aripiprazole and risperidone in 75 drug-naïve first episode psychosis individuals: a 3 months randomized study. Schizophr. Res. 202, 226–233. doi: 10.1016/j.schres.2018.06.039
Kales, H. C., Chen, P., Blow, F. C., Welsh, D. E., and Mellow, A. M. (2005). Rates of clinical depression diagnosis, functional impairment, and nursing home placement in coexisting dementia and depression. Am. J. Geriatr. Psychiatry 13, 441–449. doi: 10.1097/00019442-200506000-00002
Kales, H. C., Gitlin, L. N., and Lyketsos, C. G. (2015). Assessment and management of behavioral and psychological symptoms of dementia. BMJ 350, h369. doi: 10.1136/bmj.h369
Knijff, E. M., Nadine Breunis, M., Kupka, R. W., De Wit, H. J., Ruwhof, C., Akkerhuis, G. W., et al. (2007). An imbalance in the production of IL-1β and IL-6 by monocytes of bipolar patients: restoration by lithium treatment. Bipolar Disord. 9, 743–753. doi: 10.1111/j.1399-5618.2007.00444.x
Kofod, J., Elfving, B., Nielsen, E. H., Mors, O., and Köhler-Forsberg, O. (2022). Depression and inflammation: correlation between changes in inflammatory markers with antidepressant response and long-term prognosis. Eur. Neuropsychopharmacol. 54, 116–125. doi: 10.1016/j.euroneuro.2021.09.006
Köhler, C. A., Freitas, T. H., Stubbs, B., Maes, M., Solmi, M., Veronese, N., et al. (2017). Peripheral alterations in cytokine and chemokine levels after antidepressant drug treatment for major depressive disorder: systematic review and meta-analysis. Mol. Neurobiol. 55, 4195–4206. doi: 10.1007/s12035-017-0632-1
Kokkinou, M., Ashok, A. H., and Howes, O. D. (2018). The effects of ketamine on dopaminergic function: meta-analysis and review of the implications for neuropsychiatric disorders. Mol. Psychiatry 23, 59–69. doi: 10.1038/mp.2017.190
Kollins, S. H., Jain, R., Brams, M., Segal, S., Findling, R. L., Wigal, S. B., et al. (2011). Clonidine extended-release tablets as add-on therapy to psychostimulants in children and adolescents with ADHD. Pediatrics 127, e1406–e1413. doi: 10.1542/peds.2010-1260
Leigh, J. P., and Du, J. (2015). Brief report: forecasting the economic burden of autism in 2015 and 2025 in the United States. J. Autism Dev. Disord. 45, 4135–4139. doi: 10.1007/s10803-015-2521-7
Leng, S. X., McElhaney, J. E., Walston, J. D., Xie, D., Fedarko, N. S., and Kuchel, G. A. (2008). ELISA and multiplex technologies for cytokine measurement in inflammation and aging research. J. Gerontol. Ser. A 63, 879–884. doi: 10.1093/gerona/63.8.879
Leu, S.-J., Yang, Y.-Y., Liu, H.-C., Cheng, C.-Y., Wu, Y.-C., Huang, M.-C., et al. (2017). Valproic acid and lithium meditate anti-inflammatory effects by differentially modulating dendritic cell differentiation and function. J. Cell. Physiol. 232, 1176–1186. doi: 10.1002/jcp.25604
Liu, C., Chu, D., Kalantar-Zadeh, K., George, J., Young, H. A., and Liu, G. (2021). Cytokines: from clinical significance to quantification. Adv. Sci. 8, 2004433. doi: 10.1002/advs.202004433
Löffler, S., Löffler-Ensgraber, M., Fehsel, K., and Klimke, A. (2010). Clozapine therapy raises serum concentrations of high sensitive C-reactive protein in schizophrenic patients. Int. Clin. Psychopharmacol. 25, 101–106. doi: 10.1097/YIC.0b013e32833643fd
Ma, L., Demin, K. A., Kolesnikova, T. O., Kharsko, S. L., Zhu, X., Yuan, X., et al. (2017). Animal inflammation-based models of depression and their application to drug discovery. Exp. Opin. Drug Discov. 12, 995–1009. doi: 10.1080/17460441.2017.1362385
Maes, M., Song, C., Lin, A.-H., Bonaccorso, S., Kenis, G., De Jongh, R., et al. (1999). Negative immunoregulatory effects of antidepressants: inhibition of interferon-γ and stimulation of interleukin-10 secretion. Neuropsychopharmacology 20, 370–379. doi: 10.1016/S0893-133X(98)00088-8
Marcinowicz, P., Wiedłocha, M., Zborowska, N., Debowska, W., Podwalski, P., Misiak, B., et al. (2021). A meta-analysis of the influence of antipsychotics on cytokines levels in first episode psychosis. J. Clin. Med. 10, 2488. doi: 10.3390/jcm10112488
Masi, A., Quintana, D. S., Glozier, N., Lloyd, A. R., Hickie, I. B., and Guastella, A. J. (2015). Cytokine aberrations in autism spectrum disorder: a systematic review and meta-analysis. Mol. Psychiatry 20, 440–446. doi: 10.1038/mp.2014.59
Meltzer, A., and Van de Water, J. (2017). The role of the immune system in autism spectrum disorder. Neuropsychopharmacology 42, 284–298. doi: 10.1038/npp.2016.158
Merikangas, K. R., He, J.-P., Burstein, M., Swanson, S. A., Avenevoli, S., Cui, L., et al. (2010). Lifetime prevalence of mental disorders in U.S. adolescents: results from the National Comorbidity Survey Replication–Adolescent Supplement (NCS-A). J. Am. Acad. Child Adolesc. Psychiatry 49, 980–989. doi: 10.1016/j.jaac.2010.05.017
Miller, A. H., and Raison, C. L. (2015). Are anti-inflammatory therapies viable treatments for psychiatric disorders?: Where the rubber meets the road. JAMA Psychiatry 72, 527–528. doi: 10.1001/jamapsychiatry.2015.22
Miller, E. S., Sakowicz, A., Roy, A., Clayberger, C., Sullivan, J. T., Grobman, W. A., et al. (2017). The association of SSRIs with inflammatory cytokines in maternal cerebrospinal fluid, peripheral blood and unbilical cord blood. Am. J. Obstet. Gynecol. 216, S404–S405. doi: 10.1016/j.ajog.2016.11.424
Ming, X., Gordon, E., Kang, N., and Wagner, G. C. (2008). Use of clonidine in children with autism spectrum disorders. Brain Dev. 30, 454–460. doi: 10.1016/j.braindev.2007.12.007
Mitchell, R. H., and Goldstein, B. I. (2014). Inflammation in children and adolescents with neuropsychiatric disorders: a systematic review. J. Am. Acad. Child Adolesc. Psychiatry 53, 274–296. doi: 10.1016/j.jaac.2013.11.013
Modabbernia, A., Taslimi, S., Brietzke, E., and Ashrafi, M. (2013). Cytokine alterations in bipolar disorder: a meta-analysis of 30 studies. Biol. Psychiatry 74, 15–25. doi: 10.1016/j.biopsych.2013.01.007
Molina-Carballo, A., Cubero-Millán, I., Fernández-López, L., Checa-Ros, A., Machado-Casas, I., Jerez-Calero, A., et al. (2021). Methylphenidate ameliorates the homeostatic balance between levels of kynurenines in ADHD children. Psychiatry Res. 303, 114060. doi: 10.1016/j.psychres.2021.114060
Müller, N. (2019). COX-2 inhibitors, aspirin, and other potential anti-inflammatory treatments for psychiatric disorders. Front. Psychiatry 10, 375. doi: 10.3389/fpsyt.2019.00375
Nencioni, A., Beck, J., Werth, D., Grünebach, F., Patrone, F., Ballestrero, A., et al. (2007). Histone deacetylase inhibitors affect dendritic cell differentiation and immunogenicity. Clin. Cancer Res. 13, 3933–3941. doi: 10.1158/1078-0432.CCR-06-2903
Nordquist, N., and Oreland, L. (2010). Serotonin, genetic variability, behaviour, and psychiatric disorders - a review. Ups. J. Med. Sci. 115, 2–10. doi: 10.3109/03009730903573246
Noto, C., Ota, V. K., Gouvea, E. S., Rizzo, L. B., Spindola, L. M. N., Honda, P. H. S., et al. (2015). Effects of risperidone on cytokine profile in drug-naive first-episode psychosis. Int. J. Neuropsychopharmacol. 18, pyu042-pyu042. doi: 10.1093/ijnp/pyu042
Padmos, R. C., Hillegers, M. H. J., Knijff, E. M., Vonk, R., Bouvy, A., Staal, F. J. T., et al. (2008). A discriminating messenger RNA signature for bipolar disorder formed by an aberrant expression of inflammatory genes in monocytes. Arch. Gen. Psychiatry 65, 395. doi: 10.1001/archpsyc.65.4.395
Passos, I. C., Vasconcelos-Moreno, M. P., Costa, L. G., Kunz, M., Brietzke, E., Quevedo, J., et al. (2015). Inflammatory markers in post-traumatic stress disorder: a systematic review, meta-analysis, and meta-regression. Lancet Psychiatry 2, 1002–1012. doi: 10.1016/S2215-0366(15)00309-0
Pawelec, G., Goldeck, D., and Derhovanessian, E. (2014). Inflammation, ageing and chronic disease. Curr. Opin. Immunol. 29, 23–28. doi: 10.1016/j.coi.2014.03.007
Pereira, V. S., and Hiroaki-Sato, V. A. (2018). A brief history of antidepressant drug development: from tricyclics to beyond ketamine. Acta Neuropsychiatr. 30, 307–322. doi: 10.1017/neu.2017.39
Posey, D. J., Stigler, K. A., Erickson, C. A., and McDougle, C. J. (2008). Antipsychotics in the treatment of autism. J. Clin. Investig. 118, 6–14. doi: 10.1172/JCI32483
Queissner, R., Lenger, M., Birner, A., Dalkner, N., Fellendorf, F., Bengesser, S., et al. (2021). The association between anti-inflammatory effects of long-term lithium treatment and illness course in bipolar disorder. J. Affect. Disord. 281, 228–234. doi: 10.1016/j.jad.2020.11.063
Radhakrishnan, R., Kaser, M., and Guloksuz, S. (2017). The link between the immune system, environment, and psychosis. Schizophr. Bull. 43, 693–697. doi: 10.1093/schbul/sbx057
Rafiee, L., Hajhashemi, V., and Javanmard, S. H. (2016). Fluvoxamine inhibits some inflammatory genes expression in LPS/stimulated human endothelial cells, U937 macrophages, and carrageenan-induced paw edema in rat. Iran. J. Basic Med. Sci. 19, 977–984.
Raison, C. L., Rutherford, R. E., Woolwine, B. J., Shuo, C., Schettler, P., Drake, D. F., et al. (2013). A randomized controlled trial of the tumor necrosis factor antagonist infliximab for treatment-resistant depression. JAMA Psychiatry 70, 31. doi: 10.1001/2013.jamapsychiatry.4
Rapaport, M. H., and Manji, H. K. (2001). The effects of lithium on ex vivo cytokine production. Biol. Psychiatry 50, 217–224. doi: 10.1016/S0006-3223(01)01144-1
Rapoport, S. I., Basselin, M., Kim, H.-W., and Rao, J. S. (2009). Bipolar disorder and mechanisms of action of mood stabilizers. Brain Res. Rev. 61, 185–209. doi: 10.1016/j.brainresrev.2009.06.003
Rawdin, B. J., Mellon, S. H., Dhabhar, F. S., Epel, E. S., Puterman, E., Su, Y., et al. (2013). Dysregulated relationship of inflammation and oxidative stress in major depression. Brain Behav. Immun. 31, 143–152. doi: 10.1016/j.bbi.2012.11.011
Rohleder, N. (2019). Stress and inflammation – The need to address the gap in the transition between acute and chronic stress effects. Psychoneuroendocrinology 105, 164–171. doi: 10.1016/j.psyneuen.2019.02.021
Sacerdote, P., Bianchi, M., and Panerai, A. E. (1994). Chlorimipramine and nortriptyline but not fluoxetine and fluvoxamine inhibit human polymorphonuclear cell chemotaxis in vitro. Gen. Pharmacol. Vasc. Syst. 25, 409–412. doi: 10.1016/0306-3623(94)90188-0
Salazar, F., Baird, G., Chandler, S., Tseng, E., O'Sullivan, T., Howlin, P., et al. (2015). Co-occurring psychiatric disorders in preschool and elementary school-aged children with autism spectrum disorder. J. Autism Dev. Disord. 45, 2283–2294. doi: 10.1007/s10803-015-2361-5
Schulte, M. T., and Hser, Y.-I. (2014). Substance use and associated health conditions throughout the lifespan. Public Health Rev. 35. doi: 10.1007/BF03391702
Shanahan, L., Copeland, W., Jane Costello, E., and Angold, A. (2008). Specificity of putative psychosocial risk factors for psychiatric disorders in children and adolescents. J. Child Psychol. Psychiatry 49, 34–42. doi: 10.1111/j.1469-7610.2007.01822.x
Shen, W. W. (1999). A history of antipsychotic drug development. Compr. Psychiatry 40, 407–414. doi: 10.1016/S0010-440X(99)90082-2
Shi, S., Li, L., Song, L., and Wang, X. (2018). Effect of lamotrigine on cognitive function and serum inflammatory factors in patients with depression of recurrent bipolar disorder. Pak. J. Pharm. Sci. 31, 2775–2778.
Sobiś, J., Rykaczewska-Czerwińska, M., Swietochowska, E., and Gorczyca, P. (2015). Therapeutic effect of aripiprazole in chronic schizophrenia is accompanied by anti-inflammatory activity. Pharmacol. Rep. 67, 353–359. doi: 10.1016/j.pharep.2014.09.007
Song, C., and Wang, H. (2011). Cytokines mediated inflammation and decreased neurogenesis in animal models of depression. Progr. Neuropsychopharmacol. Biol. Psychiatry 35, 760–768. doi: 10.1016/j.pnpbp.2010.06.020
Song, X., Fan, X., Li, X., Zhang, W., Gao, J., Zhao, J., et al. (2014). Changes in pro-inflammatory cytokines and body weight during 6-month risperidone treatment in drug naïve, first-episode schizophrenia. Psychopharmacology 231, 319–325. doi: 10.1007/s00213-013-3382-4
Stahl, S. M. (2018). Beyond the dopamine hypothesis of schizophrenia to three neural networks of psychosis: dopamine, serotonin, and glutamate. CNS Spectr. 23, 187–191. doi: 10.1017/S1092852918001013
Stapel, B., Sieve, I., Falk, C. S., Bleich, S., Hilfiker-Kleiner, D., and Kahl, K. G. (2018). Second generation atypical antipsychotics olanzapine and aripiprazole reduce expression and secretion of inflammatory cytokines in human immune cells. J. Psychiatr. Res. 105, 95–102. doi: 10.1016/j.jpsychires.2018.08.017
Subbanna, M., Shivakumar, V., Venugopal, D., Narayanaswamy, J. C., Berk, M., Varambally, S., et al. (2020). Impact of antipsychotic medication on IL-6/ STAT3 signaling axis in peripheral blood mononuclear cells of drug-naive schizophrenia patients. Psychiatry Clin. Neurosci. 74, 64–69. doi: 10.1111/pcn.12938
Sutcigil, L., Oktenli, C., Musabak, U., Bozkurt, A., Cansever, A., Uzun, O., et al. (2007). Pro- and anti-inflammatory cytokine balance in major depression: effect of sertraline therapy. Clin. Dev. Immunol. 2007, 076396. doi: 10.1155/2007/76396
Szuster-Ciesielska, A., Slotwinska, M., Stachura, A., Marmurowska-Michalowska, H., and Kandefer-Szerszen, M. (2004). Neuroleptics modulate cytokine and reactive oxygen species production in blood leukocytes of healthy volunteers. Arch. Immunol. Ther. Exp. 52, 59–67.
Tian, M., Yang, M., Li, Z., Wang, Y., Chen, W., Yang, L., et al. (2019). Fluoxetine suppresses inflammatory reaction in microglia under OGD/R challenge via modulation of NF-κB signaling. Biosci. Rep. 39. doi: 10.1042/BSR20181584
Tourjman, V., Kouassi, É., Koué, M.-È., Rocchetti, M., Fortin-Fournier, S., Fusar-Poli, P., et al. (2013). Antipsychotics' effects on blood levels of cytokines in schizophrenia: a meta-analysis. Schizophr. Res. 151, 43–47. doi: 10.1016/j.schres.2013.10.011
Tsuang, M. T., Bar, J. L., Stone, W. S., and Faraone, S. V. (2004). Gene-environment interactions in mental disorders. World Psychiatry 3, 73–83.
van den Ameele, S., van Diermen, L., Staels, W., Coppens, V., Dumont, G., Sabbe, B., et al. (2016). The effect of mood-stabilizing drugs on cytokine levels in bipolar disorder: a systematic review. J. Affect. Disord. 203, 364–373. doi: 10.1016/j.jad.2016.06.016
Vaswani, M., Linda, F. K., and Ramesh, S. (2003). Role of selective serotonin reuptake inhibitors in psychiatric disorders: a comprehensive review. Progr. Neuropsychopharmacol. Biol. Psychiatry 27, 85–102. doi: 10.1016/S0278-5846(02)00338-X
Visser, E., and Schug, S. A. (2006). The role of ketamine in pain management. Biomed. Pharmacother. 60, 341–348. doi: 10.1016/j.biopha.2006.06.021
Wang, T., Zhu, H., Hou, Y., Duan, W., Meng, F., and Liu, Y. (2020). Ketamine attenuates high-glucose-mediated endothelial inflammation in human umbilical vein endothelial cells. Can. J. Physiol. Pharmacol. 98, 156–161. doi: 10.1139/cjpp-2019-0185
Wong, E. H., Yocca, F., Smith, M. A., and Lee, C. M. (2010). Challenges and opportunities for drug discovery in psychiatric disorders: the drug hunters' perspective. Int. J. Neuropsychopharmacol. 13, 1269–1284. doi: 10.1017/S1461145710000866
Yang, L. L., Stiernborg, M., Skott, E., Söderström, Å., Giacobini, M., and Lavebratt, C. (2020). Proinflammatory mediators and their associations with medication and comorbid traits in children and adults with ADHD. Eur. Neuropsychopharmacol. 41, 118–131. doi: 10.1016/j.euroneuro.2020.10.005
Yoshimura, R., Hori, H., Ikenouchi-Sugita, A., Umene-Nakano, W., Ueda, N., and Nakamura, J. (2009). Higher plasma interleukin-6 (IL-6) level is associated with SSRI- or SNRI-refractory depression. Progr. Neuropsychopharmacol. Biol. Psychiatry 33, 722–726. doi: 10.1016/j.pnpbp.2009.03.020
Young, J. J., Bruno, D., and Pomara, N. (2014). A review of the relationship between proinflammatory cytokines and major depressive disorder. J. Affect. Disord. 169, 15–20. doi: 10.1016/j.jad.2014.07.032
Yssel, J. D., O'Neill, E., Nolan, Y. M., Connor, T. J., and Harkin, A. (2018). Treatment with the noradrenaline re-uptake inhibitor atomoxetine alone and in combination with the α2-adrenoceptor antagonist idazoxan attenuates loss of dopamine and associated motor deficits in the LPS inflammatory rat model of Parkinson's disease. Brain Behav. Immun. 69, 456–469. doi: 10.1016/j.bbi.2018.01.004
Zabihiyeganeh, M., Afshar, S. V., Kadijani, A. A., Jafari, D., Bagheriferd, A., Janbozorgi, M., et al. (2019). The effect of cognitive behavioral therapy on the circulating proinflammatory cytokines of fibromyalgia patients: A pilot controlled clinical trial. Gen. Hosp. Psychiatr. 57, 23–28. doi: 10.1016/j.genhosppsych.2019.01.003
Keywords: inflammation, anti-depressants, anti-psychotics, mood stabilizers, mental health
Citation: Patel S, Keating BA and Dale RC (2023) Anti-inflammatory properties of commonly used psychiatric drugs. Front. Neurosci. 16:1039379. doi: 10.3389/fnins.2022.1039379
Received: 08 September 2022; Accepted: 06 December 2022;
Published: 10 January 2023.
Edited by:
Yasir Ahmed Syed, Cardiff University, United KingdomReviewed by:
Belinda Lennox, University of Oxford, United KingdomWang-Tso Lee, National Taiwan University Hospital, Taiwan
Copyright © 2023 Patel, Keating and Dale. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Russell C. Dale,  cnVzc2VsbC5kYWxlQGhlYWx0aC5uc3cuZ292LmF1
cnVzc2VsbC5kYWxlQGhlYWx0aC5uc3cuZ292LmF1
†These authors have contributed equally to this work and share first authorship
 Shrujna Patel1,2,3†
Shrujna Patel1,2,3† Russell C. Dale
Russell C. Dale