
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Neurosci., 05 December 2022
Sec. Translational Neuroscience
Volume 16 - 2022 | https://doi.org/10.3389/fnins.2022.1035061
This article is part of the Research TopicAdvances in Theranostics of CNS Injuries and Diseases: From Basic Research to Clinical PracticeView all 10 articles
Background: Antihypertensive therapy in the acute phase of intracerebral hemorrhage (ICH) can reduce hematoma expansion. Numerous studies have demonstrated that blood pressure variability secondary to antihypertensive therapy has adverse effects on neurological outcomes, but the conclusions are diverse, and the mechanism of this occurrence is unknown. The aim of this research was to analyze the impact of blood pressure variability after antihypertensive treatment on the prognosis of patients with acute ICH, along with the possible mechanism.
Materials and methods: A total of 120 patients within 20 h of onset of ICH were divided into a good prognosis group (mRS ≤ 2 points) and a poor prognosis group (mRS ≥ 3 points) according to their 90-day mRS scores. The basic patient information, NIHSS score, GCS score, mRS score at 90 days after admission, head CT examination at admission and 24 h and CTP examination at 24 h were collected from some patients. The blood pressure values of patients were collected within 24 h, and multiple blood pressure variation (BPV) parameters within 1 and 24 h were calculated.
Results: (1) After excluding confounding factors such as age, whether the hematoma ruptured into the ventricle, confounding signs, amount of bleeding, edema around the hematoma, NIHSS on admission, operation or non-operation, and 24-h hematoma increment, the fourth quartile systolic blood pressure (SBP) maximum and minimum difference within 1 h [OR: 5.069, CI (1.036–24.813) P = 0.045] and coefficient of continuous variation (SV) within 24 h [OR: 2.912 CI (1.818–71.728) P = 0.009] were still independent factors affecting the 90-day mRS in ICH patients. (2) There was a negative correlation between SBP SV and CBF in terms of the difference between the contralateral side and the perihematomal region at 24 h (Rs = −0.692, P = 0.013).
Conclusion: Blood pressure variability after antihypertensive therapy in acute ICH is one of the influencing factors for 90-day mRS in patients. A 1-h dramatic drop in SBP and 24-h SBP SV may affect the long-term prognosis of patients by reducing whole cerebral perfusion.
Elevated blood pressure in the acute phase of cerebral hemorrhage (ICH) patients is not only the cause of bleeding but also an important clinical manifestation (Fischer et al., 2014). High blood pressure in the hyperacute phase is the main factor causing rebleeding (Murthy et al., 2016; Qureshi and Qureshi, 2018). A large number of clinical studies have confirmed that controlling the increase in blood pressure in patients with ICH in the acute phase can effectively reduce hematoma expansion and prevent the deterioration of neurological deficit symptoms and the occurrence of adverse outcomes (Yamaguchi et al., 2018; Qureshi et al., 2020). Clinical guidelines (Chinese Society of Neurology et al., 2019) domestically and overseas recommend rapid intensive antihypertensive therapy in the acute phase as level A evidence. However, antihypertensive treatment does not provide the additional benefit of preventing hematoma expansion (Qureshi et al., 2020; Moullaali et al., 2022). Several studies have shown that blood pressure variability (BPV) after antihypertensive treatment is one of the risk factors for poor prognosis of patients (Manning et al., 2014; Chung et al., 2018; Qureshi et al., 2020), but the targets and outcomes of the studies are inconsistent, and the mechanism is still unclear.
Thus, we investigated the clinical data of hospitalized patients experiencing ICH within the past 2 years to observe whether the BPV caused by acute antihypertensive treatment affects the 90 mRS of patients and to explore the possible mechanism.
This was a prospective study. Written approval was obtained from the Medical Ethics Committee of our hospital. In addition to the diagnostic criteria set by the 2019 Chinese Guidelines for the Diagnosis and Treatment of Intracerebral Hemorrhage as the inclusion criteria, patients also met the following conditions: age > 18 years old, onset < 24 h, SBP > 140 mmHg, neurological deficit symptoms, and imaging examination suggesting intracerebral hemorrhage. Exclusion criteria were intracerebral hemorrhage patients with onset > 24 h or admission SBP < 140 mmHg, subarachnoid hemorrhage, arteriovenous malformation, cerebrovascular amyloidosis, anticoagulant drugs, and secondary intracerebral hemorrhage due to vasculitis. Patients with severe illness and short-term death were also excluded.
Patients with cerebral hemorrhage were given intravenous antihypertensive therapy with urapidil and maintained for 24 h after admission. The goal was to reduce the 1-h systolic blood pressure as much as possible to 120–140 mmHg or 20% lower than that at admission. Blood pressure measurements were recorded during the first 24 h and every 5 min from admission until 30 min after admission, every 10 min from 30 min to 1 h after admission, and every hour from 1 to 24 h after admission, for a total of 34 times. The blood pressure variability index (BPV) was calculated. Basic clinical data were collected, and mRS scores were followed up for 3 months. It was defined good prognosis group (mRS ≤ 2 points) and a poor prognosis group (mRS ≥ 3 points).
A GE Light VCT 64-slice spiral CT scanner and an AW4.5 independent workstation were used as imaging equipment. Head CT at admission and 24 h after admission was completed, and simultaneous CT perfusion (CTP) examination was performed in some patients. Hematoma volume was measured using the formula of Taguda and calculated in ml. The final result was calculated as the mean of the measured values of two attending physicians. The increase in hematoma at 24 h (△ICH, ml) was calculated as the volume of hematoma at the 24-h reexamination (ml) − the volume of hematoma at admission (ml).
The selection of regions of interest for head CTP included 3–4 regions of interest (ROIs) selected 1 cm along the edge of the hematoma and the contralateral mirror region. The perfusion parameters cerebral blood flow (CBF) and cerebral blood volume (CBV) were obtained.
Microsoft Office Professional Plus 2013 was used to access all the data of the input after completion for BPV parameters, including the average (Mean), difference (Max − Min), standard deviation (SD), coefficient of variation (CV), and continuous variation (SV).
Statistical Product and Service Solutions (SPSS) 24.0 statistical packages were used for data analysis. In univariate analysis, an independent sample t-test was used to compare the continuous variables with normal distribution between groups. The non-parametric Mann–Whitney U test was used for comparisons between groups if the distribution was not normal. Categorical variables were compared between groups using the chi-square test. Variables that were significantly different in univariate analysis were further included in multivariate binary logistic regression analysis to assess the association between the 90-day mRS score and BPV. In the comparison of continuous variables between the cerebral perfusion value and BPV, Pearson correlation analysis was used for those with a normal distribution. If the data did not conform to a normal distribution, Spearman correlation analysis was used. Statistical differences were observed at P < 0.05.
A total of 120 patients were enrolled in this study, including 71 patients in the good prognosis group and 49 patients in the poor prognosis group. The results showed (Table 1) that age, rupture into the ventricle, confounding sign, treatment method, admission NIHSS score, admission GCS score, hematoma volume, and edema range at admission, and 24-h △ICH were all different between the two groups and were related factors affecting the prognosis.
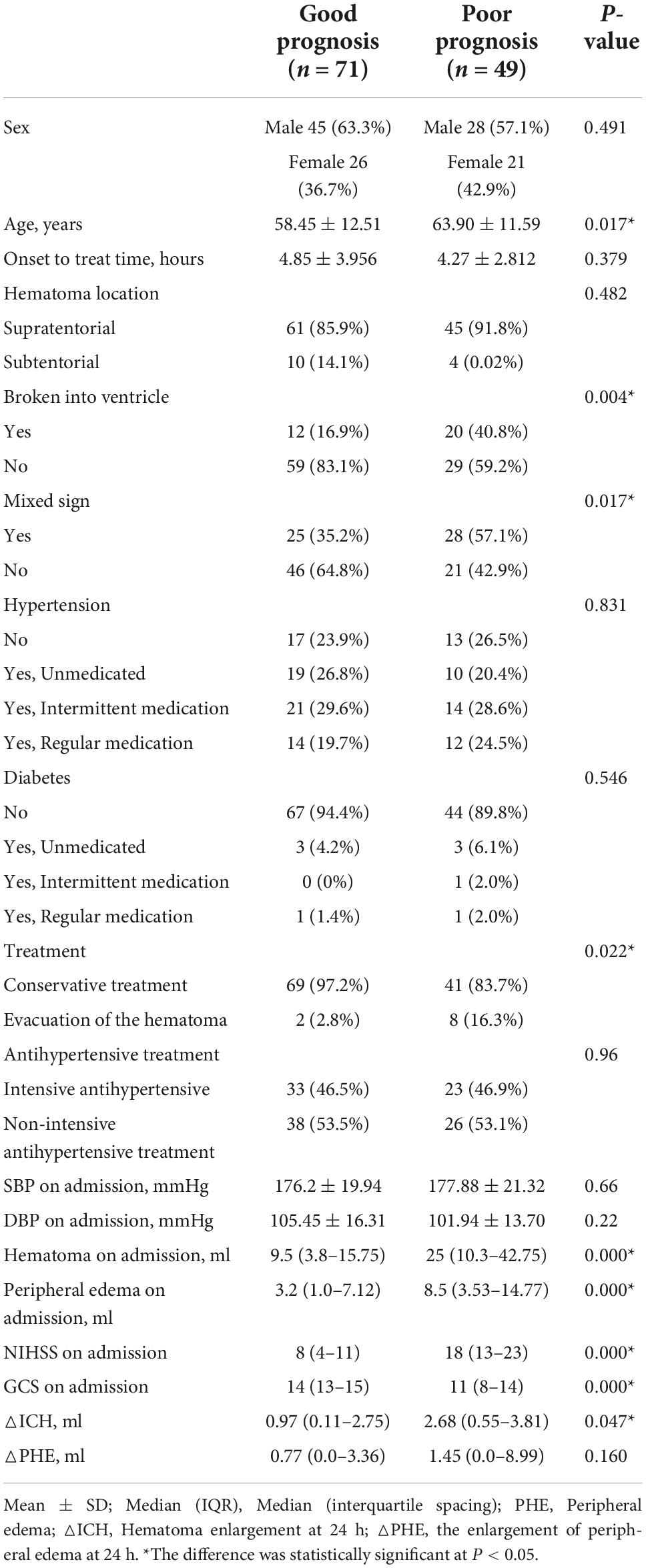
Table 1. Univariate analysis of the baseline characteristics of subjects with intracerebral hemorrhage in the good and poor prognosis groups.
The effect of BPV at different time periods (within 1 and 24 h) on clinical outcomes was evaluated. The results showed (Table 2) that the SBP max-min, SD and CV of the two time periods and 24-h SBP SV were different between the good prognosis group and the poor prognosis group (P < 0.05). The 1-h SBP SV (P < 0.1) was included in the multivariate analysis.
There was no significant difference in diastolic blood pressure variation parameters between the two groups (P > 0.05, data not listed).
Because there was no statistical difference in the above parameters obtained according to Table 2 in the multivariate analysis, the parameters of BPV with statistically significant differences were divided into four groups (q1–q4) according to the quartile as the boundary value. The R × C chi-square test was used to compare the incidence of poor prognosis in each group. The correlation between BPV and poor prognosis was analyzed by quartile group comparison. The results showed that 1 h SBP Max-Min (χ2 = 11.746, P = 0.008) 24 h SBP Max-Min (χ2 = 11.908, P = 0.008), 1 h SBP SD (χ2 = 9.21, P = 0.027), 24 h SBP SV (χ2 = 8.658, P = 0.034), suggesting that the above variables were correlated with prognosis (Figures 1–4 below for details).
Multivariate binary logistic regression analysis was used to evaluate the effect of BPV parameters in different time periods and different intervals on the poor prognosis of neurological function in patients with acute intracerebral hemorrhage. The results showed that the 1-h Max-Min and 24-h SBP SV q4 regions were significantly associated with 90-day neurological outcomes (Table 3).
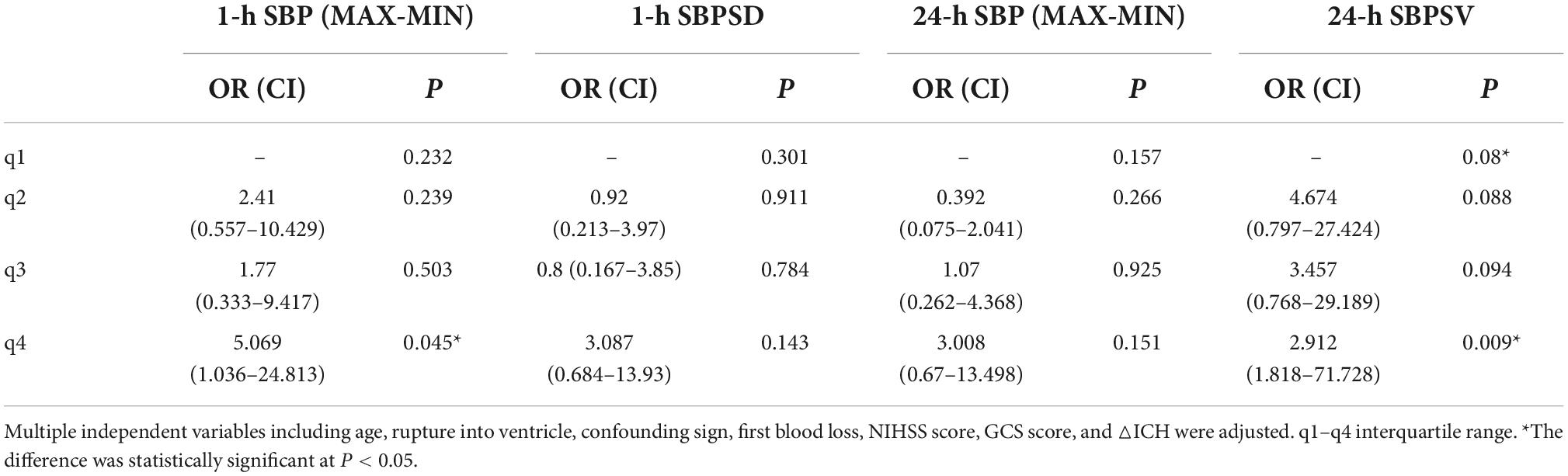
Table 3. Multivariate logistic regression analysis of the prediction of different interval BPV parameters for poor prognosis.
Twenty-two patients were randomly selected to undergo a 24-h CT (Figure 5) scan and simultaneous perfusion (CTP) examination. CTP (Figures 6–8) showed a significant reduction in perfusion in the core area and periphery of the hematoma, as well as a significant reduction in CBF and CBV.

Figure 7. The ROI and CBF values of the hematoma marginal area, cortical area, and corresponding mirror area.
The Spearman correlation analysis of blood pressure variation parameters and cerebral perfusion parameters at each time period showed that the difference in CBF between the perihematoma and contralateral mirror area (contralateral to affected-side blood flow) was negatively correlated with 24 h SBP SV, while there was no statistically significant difference in the correlation analysis of CBF and CBV around the hematoma or CBF and CBV in the mirror area (Table 4).
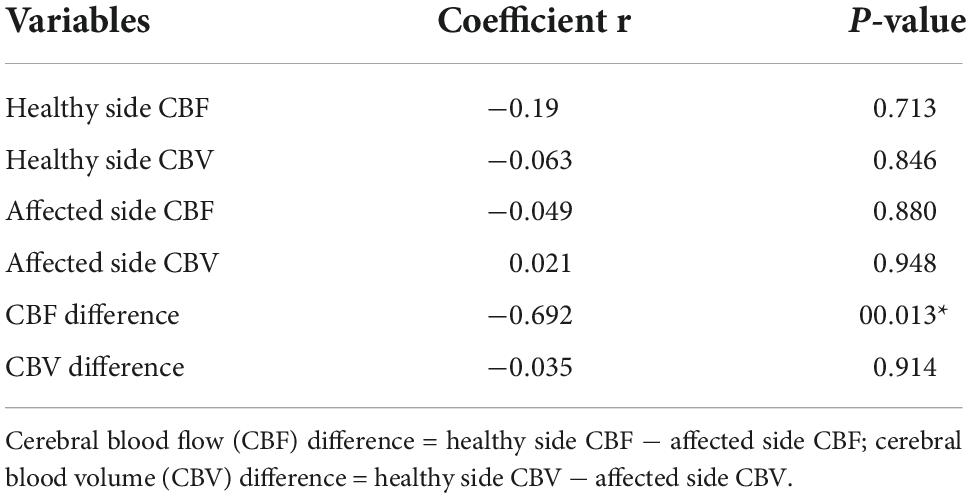
Table 4. Spearman correlation analysis of the relationship between the 24-h SBP SV and the CBF difference and CBV difference.
A 43-year-old male presented with 3 h of numbness and immobility in the right limb. Head CT (Figure 5) showed cerebral hemorrhage in the left basal ganglia area. CTP (Figure 6) showed the hematoma area; CTP (Figure 7) showed the ROI and CBF values of the hematoma marginal area, cortical area, and corresponding mirror area; CTP (Figure 8) showed the CBV values in each ROI area.
In the past 10 years, a number of large-scale international clinical studies have confirmed that early intensive antihypertensive therapy can effectively control hematoma expansion and bring more obvious blood pressure fluctuations (blood pressure variability, BPV), which may have adverse effects on patients with ICH.
Chung et al. (2018) analyzed the Fast-Mag Trial (Field Administration of Stroke Therapy-Magnesium), a study of prehospital care. The patients with obvious blood pressure variability in the hyperacute phase (0–6 h) and acute phase (0–24 h) had a poor prognosis rate (mRS 3–6 scores) of 69.9%. Among them, SD, CV, and SV were all related to poor prognosis in the acute phase and hyperacute phase. The OR of the highest quintile was 3.73, the OR of the coefficient of variation was 4.78, and the OR of the SV was 3.39. Conclusion: blood pressure variability in the acute phase of ICH in the prehospital emergency room is an independent risk factor for poor prognosis, and it is also one of the targets of clinical treatment. Qureshi et al. (2020) retrospectively analyzed a clinical multicenter blood pressure control study from 2011 to 2015. The results showed that 228 patients with ICH whose initial blood pressure was equal to or greater than 220 mmHg were treated with intensive blood pressure control or standard blood pressure control. The proportion of neurological deterioration within 24 h in the intensive treatment group was higher than that in the non-intensive treatment group (15.5 vs. 6.8%; RR 2.28 [95% CI, 1.03–5.07]; P = 0.04). There was no significant difference in mortality or severe disability rate between the two groups (39.0 vs. 38.4%; RR 1.02 [95% CI, 0.73–1.78]; P = 0.92). This suggests that early and rapid lowering of blood pressure may be harmful. Manning et al. (2014) conducted a retrospective analysis of the INTERACT2 study, which showed that the maximum systolic blood pressure was in the hyperacute phase (24 h), and SD was a marker of death in the acute phase (2–7 days) and poor prognosis at 90 days (OR = 1.41, P = 0.016; OR = 1.57, P = 0.012). It is suggested that smooth and sustained control of blood pressure is needed in the ultra early stage. Liu W. et al. (2021) included 7 randomized controlled prospective studies with 5,201 participants in their meta-analysis. Increased SBP variability was associated with an increased risk of poor functional outcomes: The results are SD (OR: 1.38, P = 0.001), CV (OR: 1.98, P = 0.017), SV (OR: 1.30, P = 0.006), and the effect of SBP variability on the risk of poor functional outcomes in ICH patients was affected by country, study design, mean age, stroke type, outcome definition, and study quality.
The aim of our study was to observe the 90-day prognosis of patients with ICH after antihypertensive treatment. The results showed that after excluding other factors that clearly affect the prognosis of ICH, the top quartile of SBP 1-h max-min and 24-h SBP SV was still an independent risk factor for ICH patients with a poor prognosis at 90 days. We hypothesized that BPV affects clinical outcomes through the following mechanisms: 1. Destroy perihematomal perfusion. 2. Change the microcirculation of the whole brain.
Whether early lowering of blood pressure results in lower early cerebral perfusion remains controversial. On the basis of INTERACT1 and ATACH experiments, studies such as ICH ADAPT suggest that there is no significant correlation between the magnitude of blood pressure reduction and the relative cerebral blood flow around the hematoma (Powers et al., 2001). Moreover, the SBP in a small number of ICH patients is controlled at the target value within 24 h. The blood flow of the brain tissue around the hematoma can be maintained by autoregulation (Gould et al., 2014; Tanaka et al., 2014; Tamm et al., 2016). With the progress of INTERACT2 and ATACH2 experiments, Gould et al. (2014) demonstrated that the blood perfusion around the hematoma, perihematoma tissue area and watershed tissue area of early ICH (<2 h) was below the ischemic threshold by CTP, but there was no significant correlation between intensive hypotension and hypoperfusion area. In Our study, CTP of some patients was performed at the same time as reexamination of head CT 24 h after admission, and decreased CBF and CBV around the hematoma was found. Table 3 showed that only the 1-h SBP Max-Min in the fourth quartile was associated with poor outcome, suggesting that the amplitude of blood pressure reduction is a factor of functional recovery. Kuang et al. (2015) showed the perihematomal ischemic injury in acute supratentorial large hematoma. Also found a synchronous decreased CBF and CBV around hematoma suggesting microcirculation compression. As a result, the autoregulation ability of cerebral perfusion in this region was weakened or disappeared. The sharp short-term drop in blood pressure again causes perihematoma hypoperfusion to aggravate neurological injury.
In addition, the above studies were limited to the hyperacute phase. Regarding sustained blood pressure reduction, a recent study by Morotti et al. (2022) showed that cerebral perfusion around the hematoma gradually decreased with the development of the disease, and 7-day CBF < 20 ml/100 g/min was shown to be an independent predictor of poor functional outcome (OR: 2.45, 95% CI 1.08-5-54, p = 0.032).
To further explore the relationship between BPV and cerebral perfusion, we performed correlation analysis on the related variation parameters affecting the poor prognosis of patients and ultimately showed that there was a negative correlation between 24-h SBP SV and the CBF difference between the healthy side and the affected side in patients with ICH; that is, the larger the 24-h SBP SV was, the smaller the CBF difference value. It is speculated that the reason for this phenomenon is that the cerebral perfusion around the hematoma loses its autoregulation ability early due to compression by the hematoma and edema and is less affected by blood pressure fluctuations.
Previous studies (Rickards and Tzeng, 2014; Kario, 2016) have demonstrated that cerebral hemodynamic variables (blood pressure and cerebral blood flow) have physiologically protective characteristics and can cause extensive damage to organ function. These apparently different outcomes may lie in the time scale of hemodynamic variability; short time-scale variability appears to be brain protective, whereas medium- and long-term fluctuations are associated with primary and secondary end-organ dysfunction. Blood pressure fluctuations can damage vascular endothelium and blood-brain barrier, increase small vessel resistance, and reduce brain cell metabolism (Liu N. et al., 2021) resulting in decreased CBF. The change is systemic and as to healthy tissue is particularly significant compared to impaired blood circulation around the hematoma.
There was no significant difference in cerebral perfusion parameters between healthy brain areas and surrounding hematoma, which may be related to the small sample size or the fact that the difference did not reach statistical significance. Unfortunately, although 1-h SBP max-min is a poor prognostic factor in ICH patients, its role in cerebral perfusion is not clear due to the short time from admission to hospitalization.
The 1-h quartile difference in SBP and 24-h SBP SV are independent risk factors for poor prognosis in patients with intracerebral hemorrhage. Excessive hypotension and secondary continuous blood pressure fluctuations cause secondary brain injury through whole-brain perfusion and then aggravate the neurological impairment of patients with cerebral hemorrhage.
The original contributions presented in this study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
The studies involving human participants were reviewed and approved by the Ethics Committee of Affiliated Hospital of North Sichuan Medical College 2020ER194-1. The patients/participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
XS: project design, declaration, experiment coordinating and organization, translation, and revision. XJ: data collection, analysis, and draft. QM, QY, and TC: clinical cases. GJ: project administration. All authors contributed to the article and approved the submitted version.
The work was supported by the Education Department of Sichuan Province (No. 16ZA0243).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Chinese Society of Neurology., Chinese Stroke Society, and Neurovascular Intervention Group of Chinese Society of Neurology (2019). Chinese guidelines for the diagnosis and treatment of cerebral hemorrhage. Chin J. Neurol. 52, 994–1005.
Chung, P. W., Kim, J. T., Sanossian, N., Starkmann, S., Hamilton, S., Gornbein, J., et al. (2018). Association Between Hyperacute Stage Blood Pressure Variability and Outcome in Patients With Spontaneous Intracerebral Hemorrhage. Stroke 49, 348–354. doi: 10.1161/STROKEAHA.117.017701
Fischer, U., Cooney, M. T., Bull, L. M., Silver, L. E., Chalmers, J., Anderson, C. S., et al. (2014). Acute post-stroke blood pressure relative to premorbid levels in intracerebral haemorrhage versus major ischaemic stroke: A population-based study. Lancet Neurol. 13, 374–384. doi: 10.1016/S1474-4422(14)70031-6
Gould, B., McCourt, R., Gioia, L. C., Kate, M., Hill, M. D., Asdaghi, N., et al. (2014). Acute Blood Pressure Reduction in Patients With Intracerebral Hemorrhage Does Not Result in Borderzone Region Hypoperfusion. Stroke 45, 2894–2899. doi: 10.1161/STROKEAHA.114.005614
Kario, K. (2016). Systemic Hemodynamic Atherothrombotic Syndrome and Resonance Hypothesis of Blood Pressure Variability: Triggering Cardiovascular Events. Korean Circ. 46, 456–467.
Kuang, Y., Chen, W., Zheng, K., Fu, J., Hu, Z., Yang, Y., et al. (2015). CT perfusion imaging evaluation on hemodynamic changes of acute spontaneous intracerebral hemorrhage surrounding tissues. Zhonghua Yi Xue Za Zhi 95, 3514–3518.
Liu, N., Xue, Y., Tang, J., Zhang, M., Ren, X., Fu, J. H., et al. (2021). Research progress of impaired cerebral hemodynamics in cerebral small vessel disease. Chin. J. Contemp. Neurol. Neurosurg. 21, 25–29.
Liu, W., Zhuang, X., and Zhang, L. (2021). Prognostic Value of Blood Pressure Variability for Patients With Acute or Subacute Intracerebral Hemorrhage: A Meta-Analysis of Prospective Studies. Front. Neurol. 11:6065–6094. doi: 10.3389/fneur.2021.606594
Manning, L., Hirakawa, Y., Arima, H., Wang, X., Chalmers, J., Wang, J., et al. (2014). Blood pressure variability and outcome after acute intracerebral haemorrhage: A post-hoc analysis of INTERACT2, a randomised controlled trial. Lancet Neurol. 13, 364–373. doi: 10.1016/S1474-4422(14)70018-3
Morotti, A., Busto, G., Boulouis, G., Scola, E., Bernardoni, A., Fiorenza, A., et al. (2022). Delayed perihematomal hypoperfusion is associated with poor outcome in intracerebral haemorrhage. Eur. J. Clin. Invest. 52:e13696. doi: 10.1111/eci.13696
Moullaali, T. J., Wang, X., Sandset, E. C., Woodhouse, L. J., Law, Z. K., Arima, H., et al. (2022). Early lowering of blood pressure after acute intracerebral haemorrhage: A systematic review and meta-analysis of individual patient data. J. Neurol. Neurosurg. Psychiatry 93, 6–13.
Murthy, S. B., Urday, S., Beslow, L. A., Dawson, J., Lees, K., Kimberly, W. T., et al. (2016). Rate of perihaematomal oedema expansion is associated with poor clinical outcomes in intracerebral haemorrhage. J. Neurol. Neurosurg. Psychiatry 87, 1169–1173. doi: 10.1136/jnnp-2016-313653
Powers, W. J., Zazulia, A. R., Videen, T. O., Adams, R. E., Yundt, K. D., Aiyagari, V., et al. (2001). Autoregulation of cerebral blood flow surrounding acute (6 to 22 hours) intracerebral hemorrhage. Neurology 57, 18–24. doi: 10.1212/wnl.57.1.18
Qureshi, A. I., Huang, W., Lobanova, I., Barsan, W. G., Hanley, D. F., Hsu, C. Y., et al. (2020). Outcomes of Intensive Systolic Blood Pressure Reduction in Patients With Intracerebral Hemorrhage and Excessively High Initial Systolic Blood Pressure: Post Hoc Analysis of a Randomized Clinical Trial. JAMA Neurol. 77, 1355–1365. doi: 10.1001/jamaneurol.2020.3075
Qureshi, A. I., and Qureshi, M. H. (2018). Acute hypertensive response in patients with intracerebral hemorrhage pathophysiology and treatment(Review). J. Cereb. Blood Flow Metab. 38, 1551–1563.
Rickards, C. A., and Tzeng, Y. C. (2014). Arterial pressure and cerebral blood flow variability: Friend or foe? A review. Front. Physiol. 5:120. doi: 10.3389/fphys.2014.00120
Tamm, A. S., McCourt, R., Gould, B., Kate, M., Kosior, J. C., Jeerakathil, T., et al. (2016). Cerebral Perfusion Pressure is Maintained in Acute Intracerebral Hemorrhage: A CT Perfusion Study. AJNR Am. J. Neuroradiol. 37, 244–251. doi: 10.3174/ajnr.A4532
Tanaka, E., Koga, M., Kobayashi, J., Kario, K., Kamiyama, K., Furui, E., et al. (2014). Blood pressure variability on antihypertensive therapy in acute intracerebral hemorrhage: The Stroke Acute Management with Urgent Risk-factor Assessment and Improvement-intracerebral hemorrhage study. Stroke 45, 2275–2279. doi: 10.1161/STROKEAHA.114.005420
Yamaguchi, Y., Koga, M., Sato, S., Yamagami, H., Todo, K., Okuda, S., et al. (2018). Early Achievement of Blood Pressure Lowering and Hematoma Growth in Acute Intracerebral Hemorrhage: Stroke Acute Management with Urgent Risk-Factor Assessment and Improvement-Intracerebral Hemorrhage Study. Cerebrovasc. Dis. 46, 116–122. doi: 10.1159/000492728
Keywords: cerebral hemorrhage, antihypertensive treatment, blood pressure variability, prognosis, brain CT perfusion
Citation: Sun X, Jv X, Mi Q, Yang Q, Chen T and Jiang G (2022) The effect of blood pressure variability on the prognosis of patients with acute cerebral hemorrhage: Possible mechanism. Front. Neurosci. 16:1035061. doi: 10.3389/fnins.2022.1035061
Received: 02 September 2022; Accepted: 07 November 2022;
Published: 05 December 2022.
Edited by:
Bo Li, Sun Yat-sen Memorial Hospital, Sun Yat-sen University, ChinaReviewed by:
Yunzhong Cheng, Beijing Chao-Yang Hospital, Capital Medical University, ChinaCopyright © 2022 Sun, Jv, Mi, Yang, Chen and Jiang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiangrong Sun, c3VueGlhbmdyb25nMTk3NDA3QDE2My5jb20=
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.