- Department of Critical Care Medicine, Xianyang Central Hospital, Xianyang, China
Objectives: Ischemic stroke (IS) is the major cause of death and disability. While previous studies confirmed that CYP11B1 is closely associated with IS, the present study aimed to analyze the impact of CYP11B1 gene polymorphisms on the IS susceptibility.
Methods: The present study genotyped six single nucleotide polymorphisms (SNPs) (including rs4736312, rs5017238, rs5301, rs5283, rs6410, and rs4534) of CYP11B1 in peripheral blood samples from IS and control populations. Logistic regression analysis was used to analyze the association between the SNPs and IS risk. The multifactor dimensionality reduction (MDR) method was used to determine the roles of SNP–SNP interactions in IS.
Results: The present study showed that rs5283 was associated with an increased susceptibility to IS [odds ratio (OR) 1.81, p = 0.012]. On the contrary, rs6410 had a protective influence on IS risk (OR 0.56, p = 0.020). Stratified analyses indicated that rs5283 could enhance the risk of IS in subjects aged >63 years (OR 2.41, p = 0.011), of female gender (OR 3.31, p = 0.001), that do not smoke (OR 1.64, p = 0.005), and with hypertension (OR 2.07, p = 0.003). Whereas, rs6410 was related to a lower susceptibility to IS in subjects aged >63 years (OR 0.43, p = 0.032), of female gender (OR 0.30, p = 0.006), do not smoke (OR 0.42, p = 0.017), and with hypertension (OR 0.52, p = 0.022). Besides, rs4736312 reduced the IS susceptibility in non-smokers (OR 0.69, p = 0.031). Rs4534 had a risk-decreasing impact on IS in non-drinking (OR 0.54, p = 0.016). Moreover, the results of the MDR analysis corroborate that the best prediction model for IS was rs5283.
Conclusion: This study revealed that CYP11B1 gene polymorphisms strongly correlated with IS in the Chinese Han population.
Introduction
Stroke, a common cerebrovascular disease, is the second leading cause of death and the third leading cause of disability and death combined worldwide (Feigin et al., 2021). Ischemic stroke (IS) is the most common type of stroke, accounting for 87% of all stroke cases (Krishnamurthi et al., 2013). In China, stroke is the major cause of death among adults and accounts for a huge burden on medical resources (Wang W. et al., 2017). Stroke-related pain and complications have a phenomenal impact on the patient's quality of life (QoL). At the same time, such complications bring untold misery and burden to the patient's family besides imposing a huge burden on the society at large. Bearing in mind the enormity of treatment to be given to the afflicted patient, effective prevention and therapeutic strategies are warranted and are the need of the hour. Stroke is a multifactorial and complex neurological disorder, including in its fold and scope a host of conventional risk factors, genetic factors, and their interactions. The traditional risk factors for stroke are age, gender, smoking, hypertension, diabetes, obesity, dyslipidemia, etc. (Johnson et al., 2016; Zhang et al., 2019; Zhuo et al., 2020). Previous researches showed that there is a close link between genetic factors and the occurrence of IS (Chauhan and Debette, 2016; Georgakis et al., 2019). Epidemiological studies revealed that gene polymorphisms may be involved in the pathophysiological processes of IS and thereby play a major role in triggering pathophysiological changes occurring in IS (Gao et al., 2019). Moreover, recent studies identified many genetic susceptibility variants which have contributed to the risk of the onset of stroke, such as ACE (Goyal et al., 2021), ADH1B (Lin et al., 2021), MTHFR, MTR (Mialovytska and Nebor, 2021), IL-10 (Rui et al., 2020), CYP2J2 (Wang S. Y. et al., 2017), and CYP2C8 (Yi et al., 2017).
Cytochrome P450 family 11 subfamily B member 1 (CYP11B1 gene) is located on chromosome 8q24.3 and encodes the steroid 11 β-hydroxylase, which influences the synthesis of aldosterone and activates cellular pathways to promote hypertension and cardiovascular disease (Hussain and Awan, 2018). CYP11B1 genetic variants are involved in the occurrence and progression of important clinical abnormalities such as late-life depression (Ancelin et al., 2021), Cushing's syndrome (Valassi et al., 2017), hypertensive patients (Hussain et al., 2020), autism (Deng et al., 2016), and coronary heart disease (Huang et al., 2022). The CYP11B1 and CYP11B2 genes share 90–95% sequence identity in their non-coding and coding regions. CYP11B2 gene polymorphisms were found to be associated with the occurrence of IS (Munshi et al., 2010; Yan and Wang, 2012). Taking all these facts together, we speculated that CYP11B1 gene polymorphisms may have an important role in the development of IS. However, the role of CYP11B1 gene polymorphism in IS has not yet been reported.
In this study, we carried out a case-control study (that included 550 IS patients and 550 normal populations) to explore the correlation between CYP11B1 gene polymorphisms and IS susceptibility in the Chinese Han population. Our study provides a new biomarker for the prevention and diagnosis of IS.
Materials and methods
The present study was approved by the Ethics Committee of the Xianyang Central Hospital. We explained to each participant the purpose of undertaking this study and also obtained an informed consent from the concerned participants before the commencement of the study. In this study, we randomly recruited 550 patients with IS and age- and sex-matched 550 healthy populations from the Xianyang Central Hospital during the same period. The inclusion criteria for the study required only those patients who were newly diagnosed as having IS and who should have been confirmed to have suffered IS by two experienced neurologists based on the test records of clinical examination, magnetic resonance imaging (MRI), cerebral scanner, and/ or computed tomography (CT) in accordance with the diagnostic guidelines for stroke (Liberman et al., 2016). Patients who possessed any of the exclusion criteria were ruled out from participation. The exclusion criteria for the cases were as follows: (1) patients with genetic disease; (2) patients with a family history of stroke; (3) patients with any type of cancer, including brain tumor; and (4) patients with neurological, cardiogenic, and autoimmune diseases. The control group included a healthy population who had undergone physical examination. The inclusion criteria stipulated for the controls were as follows: (1) controls matched to cases for age and gender and (2) controls with no family history of brain and neurological diseases. The basic characteristics of all participants were fetched from medical records and a standardized demographic questionnaire that included the participant's health details such as age, gender, smoking status, alcohol intake, hypertension status, total cholesterol, triglycerides, high-density lipoprotein cholesterol (HDL-c), and low-density lipoprotein cholesterol (LDL-c).
SNP selection and genotyping
The detailed steps that had to be undertaken for the selection of CYP11B1 single nucleotide polymorphisms (SNPs) were as follows: (1) We obtained the physical position of the CYP11B1 gene on the chromosome 8:142872356-142879846 through the human Ensembl GRCh37 database (http://asia.ensembl.org/Homo_sapiens/Info/Index). In the VCF to PED Converter window (http://grch37.ensembl.org/Homo_sapiens/Tools/VcftoPed), we entered the gene location, selected the Chinese Han population in Beijing (CHB) population, and downloaded the PED and info file for the SNPs of CYP11B1. We obtained 31 SNPs within CYP11B1 from the database. (2) Then, we used Haploview software for quality control [minor allele frequency (MAF) > 5%, min genotype > 75%, r2 <0.8, and Hardy–Weinberg equilibrium (HWE) > 0.05] to select the tag-SNP. (3) The call rate of each SNP was >95%. Other SNPs in the CYP11B1 gene did not meet the above standards. Finally, six SNPs (including rs4736312, rs5017238, rs5301, rs5283, rs6410, and rs4534) that met the above standards were selected for investigation and further study. Genomic DNA from the peripheral blood samples was extracted using the DNA kit. The primers for polymerase chain reaction (PCR) amplification were designed by the Agena Design software. The six SNPs were detected by the Agena MassARRAY iPLEX platform following the manufacturer's protocols. In addition, the genotyping data were analyzed by the Agena Bioscience Typer software.
Bioinformatics analysis
Haploreg (Version 4.1) online software was used to predict the possible functions of the six SNPs(https://pubs.broadinstitute.org/mammals/haploreg/haploreg.php).
Statistical analyses
In this study, statistical tests were analyzed by SPSS software (version 22.0), with a two-tailed test. Student's t-test was performed to detect the statistical differences in age, total cholesterol, triglycerides, HDL-c, and LDL-c between the case and control groups, respectively. Pearson's chi-squared (χ2) test was used to analyze the statistical differences in gender, smoking status, and alcohol intake. Fisher's exact test was carried out to calculate the HWE to detect the allele frequencies in normal controls. The association of CYP11B1 gene polymorphisms with IS susceptibility was evaluated by a logistic regression analysis under allele, codominant, dominant, recessive, and log-additive models. The Benjamini and Hochberg's false discovery rate (FDR) method was used to correct for multiple comparisons. Besides, the positive findings about the correlations between SNPs and IS risk were verified with the false-positive report probability (FPRP) analysis. Moreover, the MDR method was used to determine the influence of interactions among SNPs on IS susceptibility.
Results
Basic characteristics of the study population
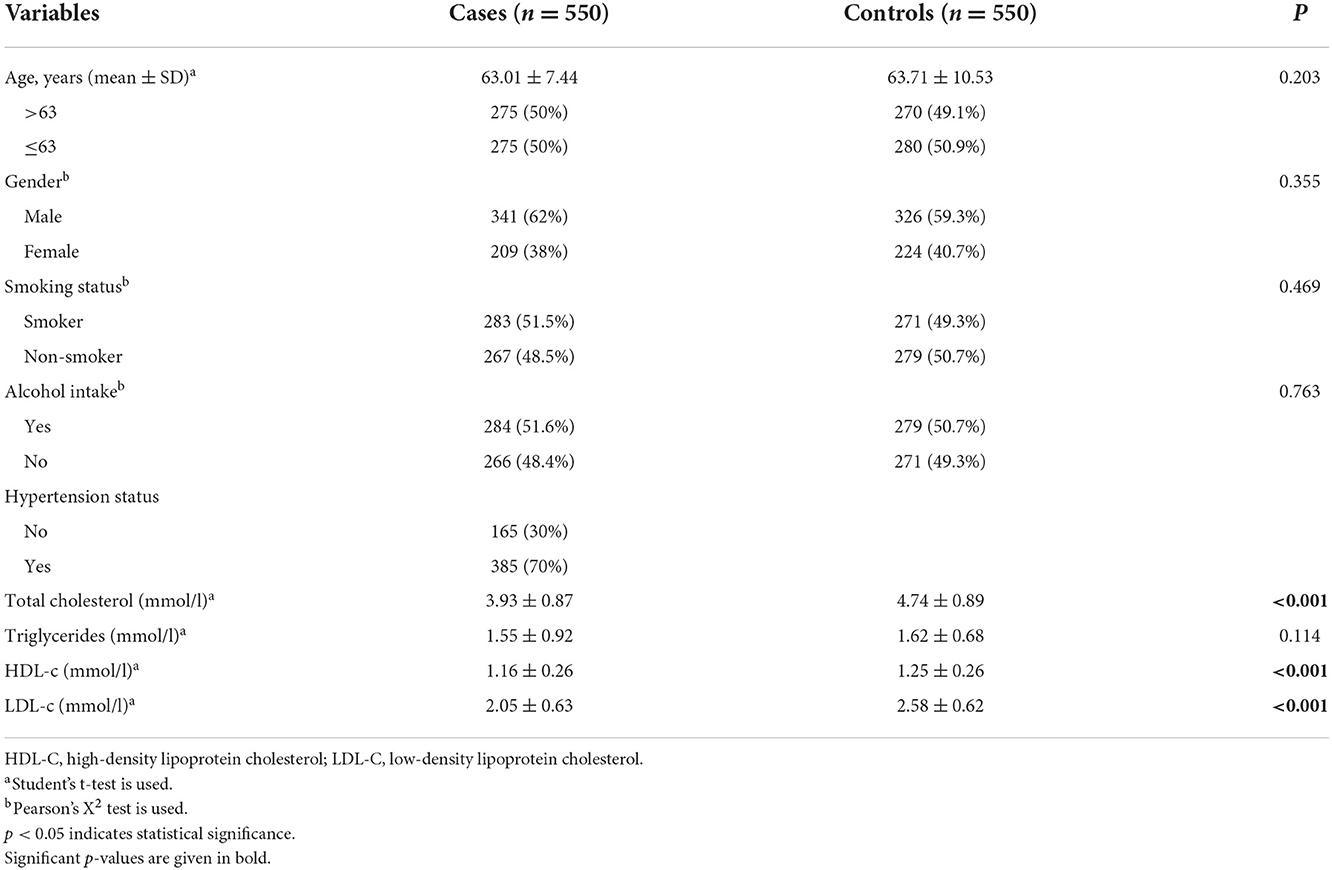
As shown in Table 1, our study involved 550 patients with IS (341 men and 209 women) and 550 healthy subjects (326 men and 224 women). The mean age was 63.01 ± 7.44 years for the cases and 63.71 ± 10.53 years for the controls. The concentrations of total cholesterol, HDL-c, and LDL-c in patients with IS were significantly lower than those in the control group (all p < 0.001). In terms of age, gender, smoking status, alcohol intake, and triglyceride level, there was no significant difference between the two groups (p = 0.203, p = 0.355, p = 0.469, p = 0.763, and p = 0.114, respectively).
The impact of CYP11B1 gene polymorphisms on ischemic stroke susceptibility
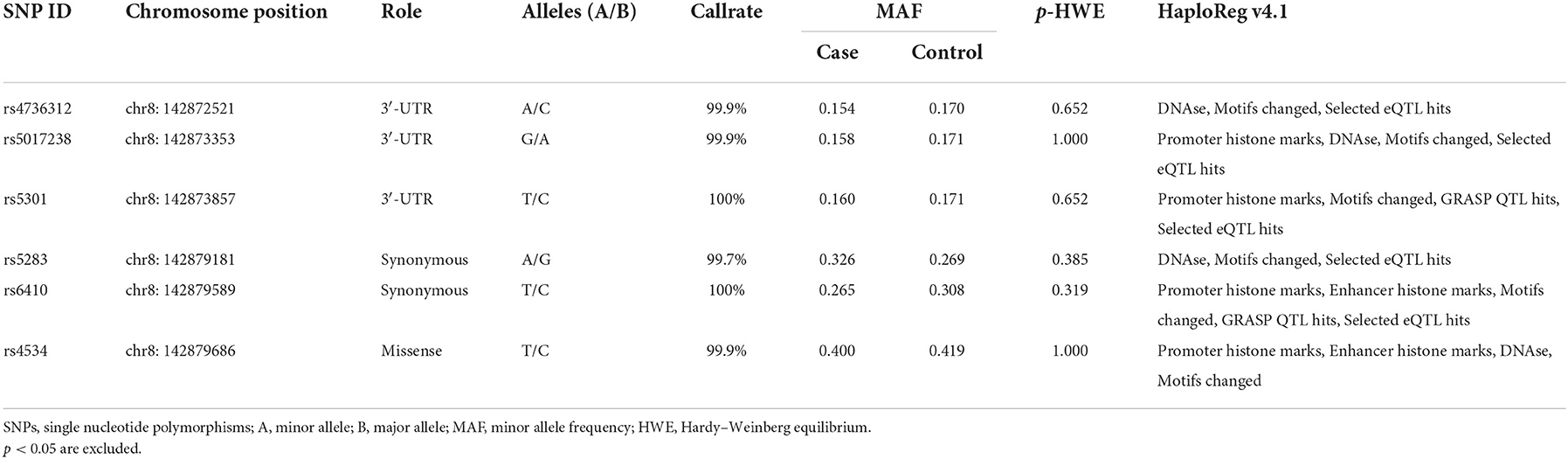
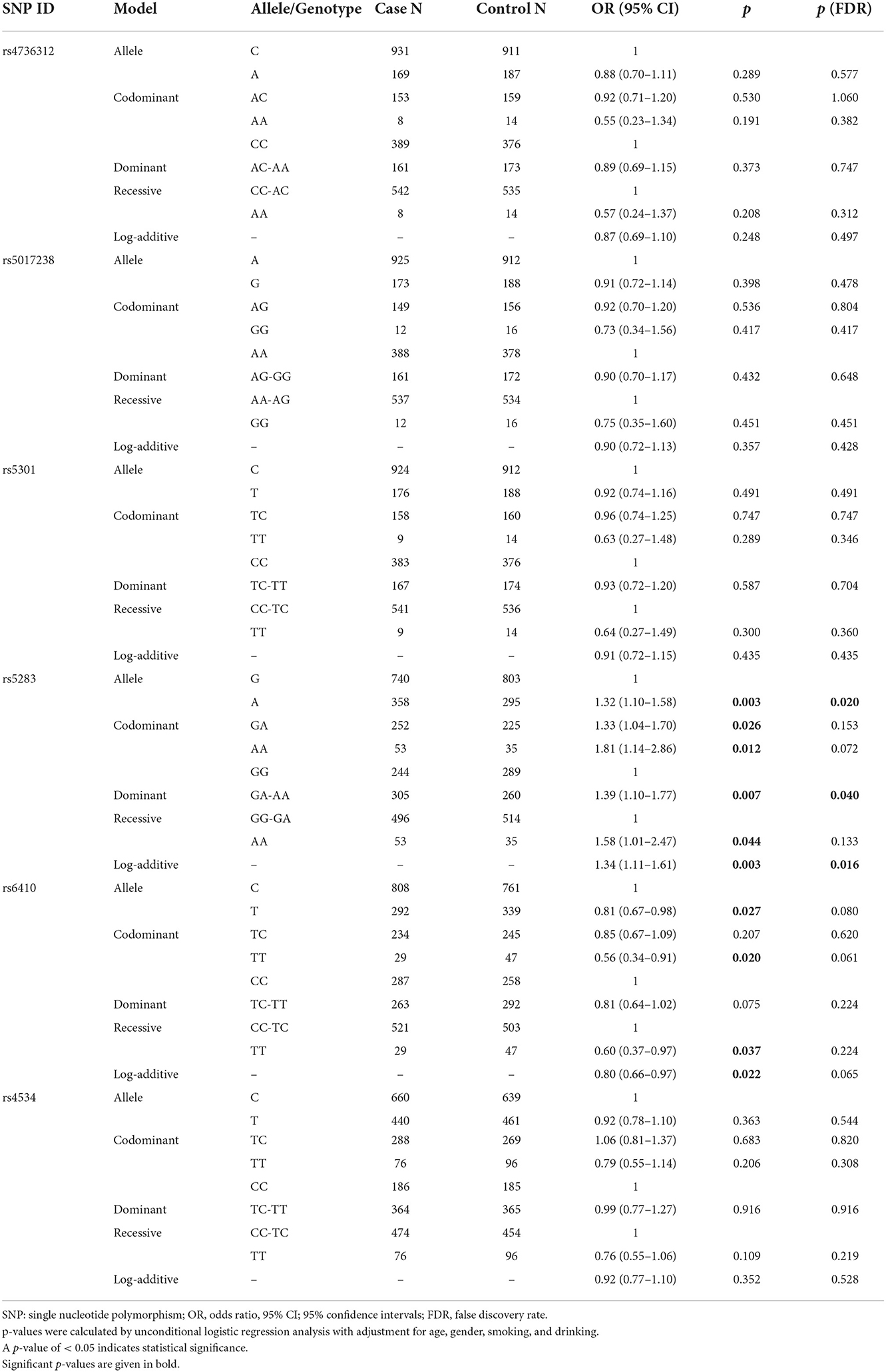
We successfully investigated six SNPs (rs4736312, rs5017238, rs5301, rs5283, rs6410, and rs4534), and the allele frequency distribution and the potential function of the SNPs are listed in Table 2. The allele frequencies for each SNP in the controls were assigned in accordance with the HWE (all p > 0.05). The association of CYP11B1 polymorphisms with IS is listed in Table 3. Rs5283 was significantly associated with an increased susceptibility to IS in allele [OR 1.32, p = 0.003, p (FDR) = 0.020], codominant [GA vs. GG, OR 1.33, p = 0.026, p (FDR) = 0.153; AA vs. GG, OR 1.81, p = 0.012, p (FDR) = 0.072], dominant [OR 1.39, p = 0.007, p (FDR) = 0.040], recessive [OR 1.58, p = 0.044, p (FDR) = 0.133], and log-additive models [OR 1.34, p = 0.003, p (FDR) = 0.016]. Whereas, rs6410 had a risk-decreasing influence on IS in allele [OR 0.81, p = 0.027, p (FDR) = 0.080], codominant [TT vs. CC, OR 0.56, p = 0.020, p (FDR) = 0.061], recessive [OR 0.60, p = 0.037, p (FDR) = 0.224], and log-additive models [OR 0.80, p = 0.022, p (FDR) = 0.065].
CYP11B1 SNPs associated with risk factors for ischemic stroke
We further analyzed the impact of CYP11B1 SNPs on risk factors (gender, age, alcohol intake, smoking, and hypertension) for patients with IS. As shown in Table 4, age-stratified analysis indicated that rs5283 was related to an increased risk of IS in people aged >63 years [allele: OR 1.38, p = 0.015, p (FDR) = 0.089; codominant: GA vs. GG, OR 1.58, p = 0.018, p (FDR) = 0.108, and AA vs. GG, OR 2.41, p = 0.011, p (FDR) = 0.032; dominant: OR 1.69, p = 0.005, p (FDR) = 0.028; recessive: OR 1.93, p = 0.047, p (FDR) = 0.282; log-additive: OR 1.56, p = 0.002, p (FDR) = 0.013]. Rs6410 was related to a lower risk in ischemic stroke aged >63 years under codominant [TT vs. CC, OR 0.43, p = 0.032, p (FDR) = 0.194] and log-additive models [OR 0.72, p = 0.031, p (FDR) = 0.094]. When stratified by gender, it was found that rs5283 showed an enhanced susceptibility to IS in women [allele: OR 1.87, p < 0.001, p (FDR) < 0.001; codominant: GA vs. GG, OR 2.24, p < 0.001, p (FDR) = 0.001, and AA vs. GG, OR 3.31, p = 0.001, p (FDR) = 0.009; dominant: OR 2.39, p < 0.001, p (FDR) <0.001; recessive: OR 2.25, p = 0.024, p (FDR) = 0.073; log-additive: OR 1.99, p < 0.001, p (FDR) < 0.001]. However, rs6410 [allele: OR 0.64, p = 0.003, p (FDR) = 0.009; codominant: TC vs. CC, OR 0.67, p = 0.045, p (FDR) = 0.136, and TT vs. CC, OR 0.30, p = 0.006, p (FDR) = 0.017; dominant: OR 0.60, p = 0.009, p (FDR) = 0.028; recessive: OR 0.36, p = 0.017, p (FDR) = 0.103; log-additive: OR 0.61, p = 0.002, p (FDR) = 0.006] and rs4534 [recessive: OR 0.58, p = 0.046, p (FDR) = 0.093] had a protective impact on the risk of IS in women.

Table 4. Correlation of CYP11B1 polymorphisms and ischemic stroke susceptibility stratified by age and gender.
After stratified by smoking and alcohol intake (Table 5), we found that rs4736312 [allele: OR 0.72, p = 0.045, p (FDR) = 0.091; log-additive: OR 0.69, p = 0.031, p (FDR) = 0.063] and rs6410 [OR 0.74, p = 0.027, p (FDR) = 0.082; codominant: OR 0.42, p = 0.017, p (FDR) = 0.104; recessive: OR 0.46, p = 0.029, p (FDR) = 0.172; log-additive: OR 0.73, p = 0.024, p (FDR) = 0.073] could decrease the risk of patients that do not smoke. In addition, rs5283 [allele: OR 1.40, p = 0.012, p (FDR) = 0.070; codominant: GA vs. GG, OR 1.62, p = 0.008, p (FDR) = 0.051; dominant: OR 1.64, p = 0.005, p (FDR) = 0.029; log-additive: OR 1.45, p = 0.008, p (FDR) = 0.047] could increase the susceptibility of IS in patients that do not smoke. Rs4534 was related to decreased susceptibility in patients that do not consume alcohol under codominant [TT vs. CC, OR 0.57, p = 0.041, p (FDR) = 0.245] and recessive models [OR 0.54, p = 0.016, p (FDR) = 1.977]. We further evaluated the correlations between SNPs and IS complicated with hypertension. As summarized in Table 6, rs5283 significantly increased the risk of IS complicated with hypertension in allele [OR 1.34, p = 0.004, p (FDR) = 0.026], codominant [AA vs. GG, OR 2.07, p = 0.003, p (FDR) = 0.020], dominant [OR 1.35, p = 0.024, p (FDR) = 0.143], recessive [OR 1.87, p = 0.009, p (FDR) = 0.233], and log-additive models [OR 1.35, p = 0.004, p (FDR) = 0.022]. Rs6410 had a lower susceptibility to IS complicated with hypertension [allele: OR 0.80, p = 0.031, p (FDR) = 0.094, codominant: OR 0.52, p = 0.022, p (FDR) = 0.067, recessive: OR 0.56, p = 0.039, p (FDR) = 0.875, and log-additive: OR 0.78, p = 0.025, p (FDR) = 0.074].
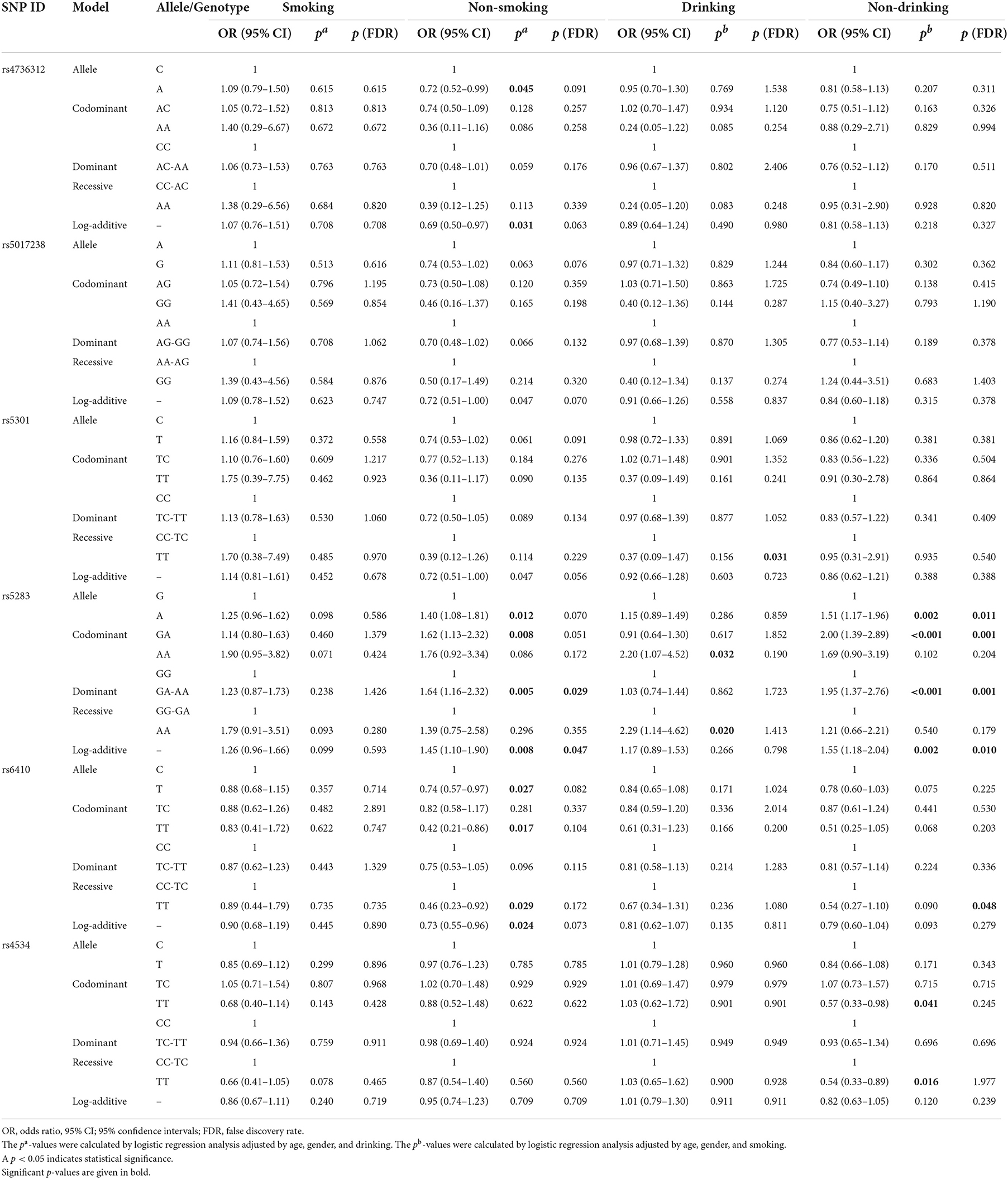
Table 5. Correlation of CYP11B1 polymorphisms and ischemic stroke susceptibility stratified by smoking and drinking.
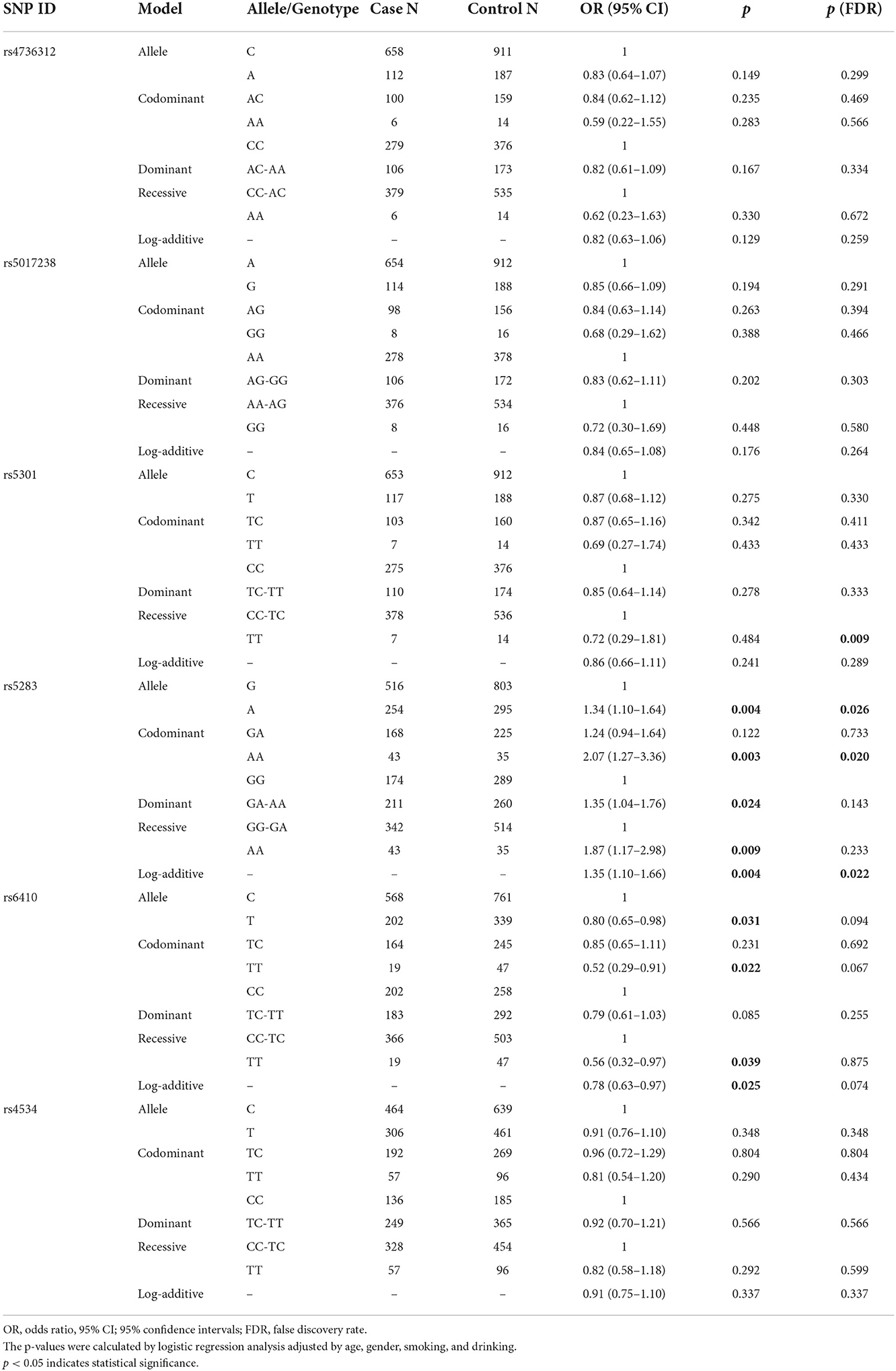
Table 6. The relationship between CYP11B1 polymorphisms and the risk of ischemic stroke complicated with hypertension.
FRPR results
We performed the FPRP analysis to verify the positive data in the study. As shown in Supplementary Table S1, it was found that the associations between CYP11B1 gene polymorphism and IS in the total group and subgroup, almost all of them, were significant (FPRP < 0.2).
SNP–SNP interactions influenced ischemic stroke risk
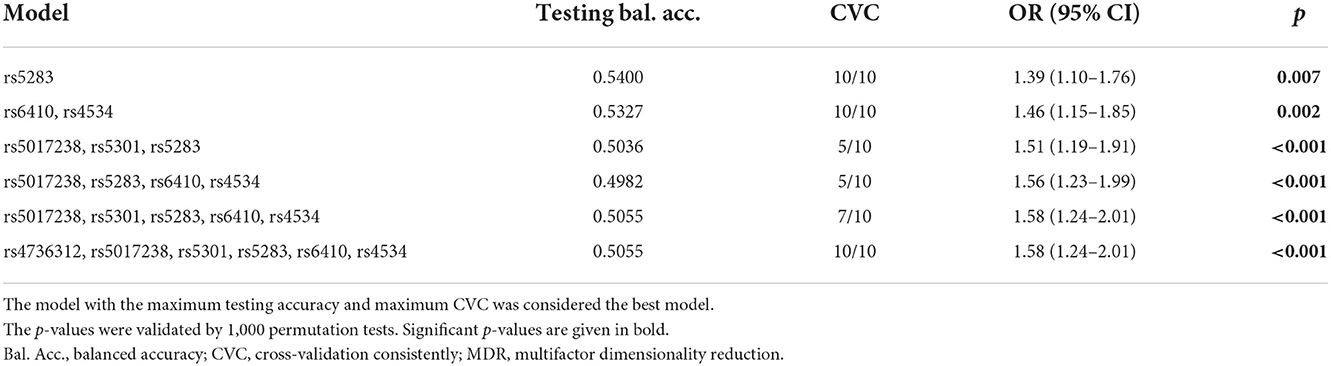
The MDR method was used to analyze the correlation between SNP–SNP interactions and IS. As presented in Table 7, rs5283 was the best predictive model for IS (OR 1.39, p = 0.007), with the highest testing accuracy (0.5400) and perfect cross-validation consistently (CVC) (10/10). The interaction map showed that rs4534 and rs6410 had a positive synergistic interaction (0.05%), and the interaction map with negative percent entropy indicated the redundancy or independence of each pairwise combination of SNPs (Figure 1).
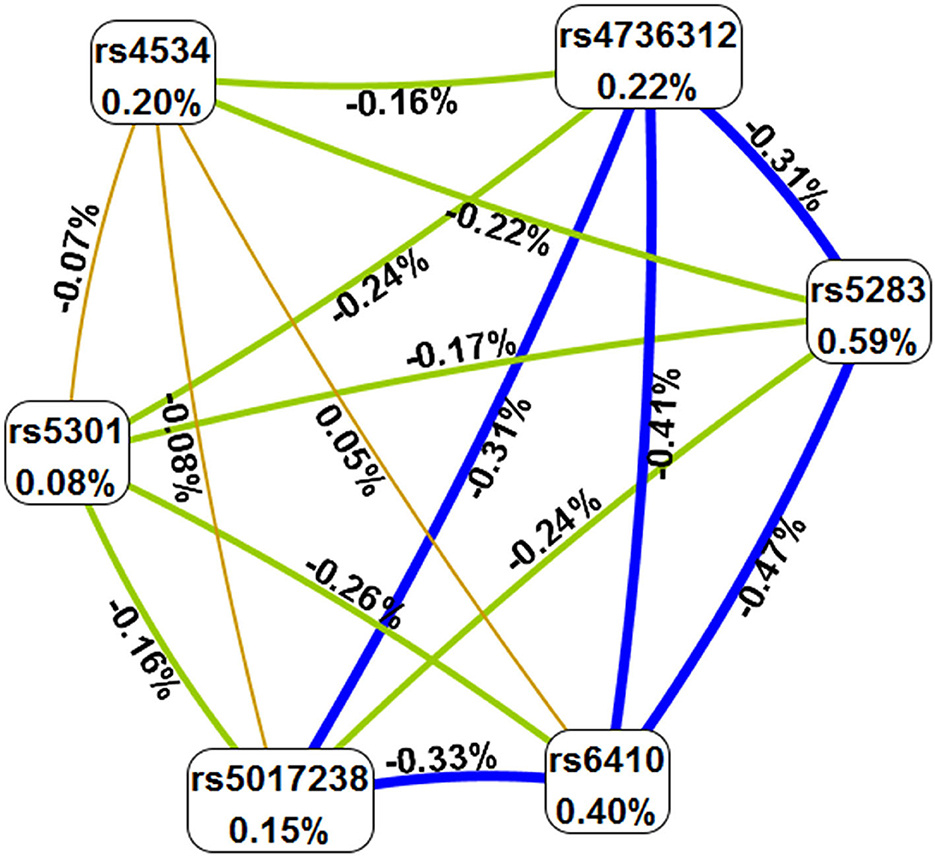
Figure 1. The SNP–SNP interaction map. Values in nodes represent the information gains of the individual attributes (main effects). Values between nodes are information gains of each pair of attributes (interaction effects). Orange with positive percent entropy indicates a strong synergistic interaction. Light range, blue, and green with negative percent entropy indicate redundancy or independence.
Discussion
We studied the impact of CYP11B1 SNPs on the IS risk. We found that rs5283 and rs6410 were closely related to the risk of IS. To the best of our knowledge, this study is the first of its kind to reveal an association between the CYP11B1 gene polymorphisms and the risk of stroke in the Han population.
Rs5283, rs6410, rs4736312, and rs4534 are located on the second exon, the first exon, the 3′ UTR, and the first exon of the CYP11B1 gene, respectively. Our study showed that rs5283 increases the risk of IS significantly, whereas rs6410 played a protective role in IS. However, Zhang et al. (2010) reported that rs6410 was associated with an increased risk of primary hyperaldosteronism. This difference may be caused by the type of disease. The incidence of stroke is proportional to age, with ~75% of stroke occurring in patients above 64 years (Mackay et al., 2004). The average age of the participants was 63 years; thus, we stratified the age group by 63 years. We found that rs5283 was associated with an increased susceptibility to IS in people aged >63 years. On the contrary, rs6410 decreased susceptibility to IS in people aged >63 years. Yang et al. (2020) reported that rs6068816 enhanced the IS risk in people aged >64 years. Cai et al. (2020) showed that rs4646 could increase the IS risk in people aged >64 years. Besides, rs2074633 and rs28688791 enhanced the risk of stroke in people aged <60 years (Wang et al., 2019). Taking various points from the above, we speculate that the association of CYP11B1 gene polymorphisms with IS susceptibility may rely on age. In addition, we observed that rs5283 and rs6410 were closely related to IS risk in women. Some studies showed that SNPs were related to IS susceptibility but influenced by gender (Xu et al., 2017; Gu et al., 2018; Yuan et al., 2021). Besides, sex differences are very important to influence the occurrence of IS (Bushnell et al., 2018). Thus, we guess that CYP11B1 genetic variants impact on the risk of IS relying on gender. Smoking and hypertension are risk factors for IS. We also analyzed the correlation between CYP11B1 polymorphisms and IS risk stratified by smoking and hypertension. We observed that rs5283 could enhance the risk of IS in patients that do not smoke and those with hypertension. Rs6410 was related to decreased susceptibility to IS risk in patients that do not smoke and those with hypertension. In addition, rs473631 polymorphism reduced IS risk in patients that do not smoke. Similar to our results, Aysun et al. revealed that genetic variants determine IS risk influenced by hypertension and smoking (Türkanoglu Özçelik et al., 2018). Tu, Yang, and Diakite showed that SNPs were related to the susceptibility of IS with hypertension (Diakite et al., 2016; Tu et al., 2020; Yang et al., 2020). Cheng et al. (2017) showed that the interactions between SNPs and smoking turned out to be significant in IS. Based on the above, we concluded that gene polymorphisms together with age, gender, smoking, and hypertension are very significant risk factors for IS.
Function prediction found that rs5283 and rs4736312 were related to the regulation of deoxyribonuclease (DNAse), motifs changed, and selected expression quantitative trait loci (eQTL) hits. Rs6410 influences the regulation of promoter histone marks, enhancer histone marks, motifs changed, GRASP (Genome-Wide Repository of Associations Between SNPs and Phenotypes) QTL hits, and selected eQTL hits. Besides, rs4534 contributed to the regulation of promoter histone marks, DNAse, motifs changed, and enhancer histone marks, which have given that these SNPs had some molecular functions in IS. Studies showed that SNPs participate in the occurrence of human diseases by regulating the expression of the gene (Alvarez-Madrazo et al., 2013; Song et al., 2020). We estimate that CYP11B1 gene polymorphism may affect the occurrence of the disease by regulating its expression, and molecular experiments have to be carried out to verify it.
There are a few disadvantages in our study. First, SNPs in CYP11B1 may influence the occurrence of IS by regulating the expression of CYP11B1, but we have not detected a similar trait in the current study, so, this aspect merits future investigation. Second, the functional experiments of CYP11B1 gene polymorphisms in patients with IS are still lacking in clarity and require further investigation and an in-depth study. Despite these limitations, our study is the first to explore the roles of CYP11B1 gene polymorphisms in patients with IS.
Conclusion
In summary, our study provided evidence that CYP11B1 gene polymorphisms influence IS susceptibility in the Chinese Han population, which has given a new biomarker for the diagnosis and prevention of IS.
Data availability statement
The original contributions presented in the study are publicly available. This data can be found here: https://doi.org/10.5281/zenodo.7344449.
Ethics statement
The studies involving human participants were reviewed and approved by Xianyang Central Hospital. The patients/participants provided their written informed consent to participate in this study.
Author contributions
YD conceived, designed the experiments, and revised the manuscript. GL performed the experiment, analyzed the data, and wrote the manuscript. Both authors read and approved the final manuscript.
Acknowledgments
The authors thank all participants and volunteers in this study.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnins.2022.1030551/full#supplementary-material
References
Alvarez-Madrazo, S., Mackenzie, S. M., Davies, E., Fraser, R., Lee, W. K., Brown, M., et al. (2013). Common polymorphisms in the CYP11B1 and CYP11B2 genes: evidence for a digenic influence on hypertension. Hypertension 61, 232–239. doi: 10.1161/HYPERTENSIONAHA.112.200741
Ancelin, M. L., Norton, J., Ritchie, K., Chaudieu, I., and Ryan, J. (2021). 11β-Hydroxylase (CYP11B1) gene variants and new-onset depression in later life. J. Psychiat. Neurosci. 46, E147–e153. doi: 10.1503/jpn.190177
Bushnell, C. D., Chaturvedi, S., Gage, K. R., Herson, P. S., Hurn, P. D., Jiménez, M. C., et al. (2018). Sex differences in stroke: challenges and opportunities. J. Cereb. Blood Flow Metab. 38, 2179–2191. doi: 10.1177/0271678X18793324
Cai, Q., Zheng, J., Bai, M., He, X., Wang, L., He, Y., et al. (2020). Genetic variations of CYP19A1 gene and stroke susceptibility: a case-control study in the Chinese Han population. J. Cardiovasc. Pharmacol. 75, 344–350. doi: 10.1097/FJC.0000000000000793
Chauhan, G., and Debette, S. (2016). Genetic risk factors for ischemic and hemorrhagic stroke. Curr. Cardiol. Rep. 18, 124. doi: 10.1007/s11886-016-0804-z
Cheng, Q., Li, Y. K., Lu, F., Yin, L., Wang, Y. Z., Wei, W., et al. (2017). Interactions between ACYP2 genetic polymorphisms and environment factors with susceptibility to ischemic stroke in a Han Chinese population. Oncotarget 8, 97913–97919. doi: 10.18632/oncotarget.18430
Deng, H. Z., You, C., Xing, Y., Chen, K. Y., and Zou, X. B. (2016). A family-based association study of CYP11A1 and CYP11B1 gene polymorphisms with autism in Chinese trios. J. Child Neurol. 31, 733–737. doi: 10.1177/0883073815620672
Diakite, B., Hamzi, K., Hmimech, W., and Nadifi, S. (2016). Genetic polymorphisms of T-1131C APOA5 and ALOX5AP SG13S114 with the susceptibility of ischaemic stroke in Morocco. J. Genet. 95, 303–309. doi: 10.1007/s12041-016-0635-0
Feigin, V. L., Stark, B. A., Johnson, C. O., Roth, G. A., Bisignano, C., Abady, G. G., et al. (2021). Global, regional, and national burden of stroke and its risk factors, 1990-2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet Neurol. 20, 795–820. doi: 10.1016/S1474-4422(21)00252-0
Gao, N., Guo, T., Luo, H., Tu, G., Niu, F., Yan, M., et al. (2019). Association of the MMP-9 polymorphism and ischemic stroke risk in southern Chinese Han population. BMC Neurol. 19, 67. doi: 10.1186/s12883-019-1285-7
Georgakis, M. K., Gill, D., Rannikmäe, K., Traylor, M., Anderson, C. D., Lee, J. M., et al. (2019). Genetically determined levels of circulating cytokines and risk of stroke. Circulation 139, 256–268. doi: 10.1161/CIRCULATIONAHA.118.035905
Goyal, A., Saluja, A., Saraswathy, K. N., Bansal, P., and Dhamija, R. K. (2021). Role of ACE polymorphism in acute ischemic stroke. Neurol. India 69, 1217–1221. doi: 10.1016/j.jns.2021.118676
Gu, L., Huang, J., Li, J., Huang, S., Li, M., Gong, L., et al. (2018). Association of CALM1 rs3179089 polymorphism with ischemic stroke in Chinese Han population. Neuromol. Med. 20, 271–279. doi: 10.1007/s12017-018-8492-z
Huang, X., Cheng, Y., and Wang, N. (2022). Genetic variants in CYP11B1 influence the susceptibility to coronary heart disease. BMC Med. Genom. 15, 158. doi: 10.1186/s12920-022-01307-8
Hussain, M., and Awan, F. R. (2018). Hypertension regulating angiotensin peptides in the pathobiology of cardiovascular disease. Clin. Exp. Hypertens. 40, 344–352. doi: 10.1080/10641963.2017.1377218
Hussain, M., Bilal, A., and Awan, F. R. (2020). Pharmacogenetic study of ACE, AGT, CYP11B1, CYP11B2 and eNOS gene variants in hypertensive patients from Faisalabad, Pakistan. JPMA 70, 624–629. doi: 10.5455/JPMA.6666
Johnson, W., Onuma, O., Owolabi, M., and Sachdev, S. (2016). Stroke: a global response is needed. Bull. World Health Organ. 94, 634–634a. doi: 10.2471/BLT.16.181636
Krishnamurthi, R. V., Feigin, V. L., Forouzanfar, M. H., Mensah, G. A., Connor, M., Bennett, D. A., et al. (2013). Global and regional burden of first-ever ischaemic and haemorrhagic stroke during 1990–2010: findings from the Global Burden of Disease Study 2010. Lancet 1, e259–e281. doi: 10.1016/S2214-109X(13)70089-5
Liberman, A. L., Kamel, H., Mullen, M. T., and Mess,é, S. R. (2016). International classification of diseases, ninth revision (ICD-9) diagnosis codes can identify cerebral venous thrombosis in hospitalized adults. Neurohospitalist 6, 147–150. doi: 10.1177/1941874416648198
Lin, C. H., Nfor, O. N., Ho, C. C., Hsu, S. Y., Tantoh, D. M., Liaw, Y. C., et al. (2021). Association of ADH1B polymorphism and alcohol consumption with increased risk of intracerebral hemorrhagic stroke. J. Transl. Med. 19, 227. doi: 10.1186/s12967-021-02904-4
Mackay, J., Mensah, G. A., Mendis, S., and Greenlund, K. (2004). The Atlas of Heart Disease and Stroke. Geneva: WHO, Myriad Editions Ltd. 112.
Mialovytska, O., and Nebor, Y. (2021). Analysis of relationship between polymorphism of MTHFR (C677T), MTHFR (A1298C), MTR (A2756G) genes in the development of ischemic stroke in young patients. Georgian Med. News 319, 87–92.
Munshi, A., Sharma, V., Kaul, S., Rajeshwar, K., Babu, M. S., Shafi, G., et al. (2010). Association of the−344C/T aldosterone synthase (CYP11B2) gene variant with hypertension and stroke. J. Neurol. Sci. 296, 34–38. doi: 10.1016/j.jns.2010.06.013
Rui, X. D., Sha, Y. Q., Wen, S., Sun, Q. Y., Hu, J. M., Yan, F. F., et al. (2020). Serum level of IL-10 and IL-10-1082G/A polymorphism are associated with the risk of ischemic stroke: a meta-analysis. J. Biol. Regul. Homeost. Agents 34, 1445–1449. doi: 10.21203/rs.2.21934/v1
Song, J., Hao, L., Wei, W., Yang, R., Wang, C., Geng, H., et al. (2020). A SNP in the 3'UTR of the porcine IGF-1 gene interacts with miR-new14 to affect IGF-1 expression, proliferation and apoptosis of PK-15 cells. Domest. Anim. Endocrinol. 72, 106430. doi: 10.1016/j.domaniend.2019.106430
Tu, Q., Yan, L., Wang, C., Han, A., Qin, Y., Cui, L., et al. (2020). Associations between aquaglyceroporin gene polymorphisms and risk of stroke among patients with hypertension. BioMed Res. Int. 2020, 9358290. doi: 10.1155/2020/9358290
Türkanoglu Özçelik, A., Öner, T., Can Demirdögen, B., Bek, V. S., and Demirkaya, S. (2018). O. Adali. Genetic polymorphisms of vitamin D3 metabolizing CYP24A1 and CYP2R1 enzymes in Turkish patients with ischemic stroke. Neurol. Res. 40, 364–371. doi: 10.1080/01616412.2018.1446281
Valassi, E., Aulinas, A., Glad, C. A., Johannsson, G., Ragnarsson, O., Webb, S. M., et al. (2017). A polymorphism in the CYP17A1 gene influences the therapeutic response to steroidogenesis inhibitors in Cushing's syndrome. Clin. Endocrinol. 87, 433–439. doi: 10.1111/cen.13414
Wang, M., Gu, M., Li, Z., Sun, B., Cheng, X., Dai, Z., et al. (2019). HDAC9 polymorphisms predict susceptibility, severity, and short-term outcome of large artery atherosclerotic stroke in chinese population. J. Mol. Neurosci. 67, 165–171. doi: 10.1007/s12031-018-1221-0
Wang, S. Y., Xing, P. F., Zhang, C. Y., and Deng, B. Q. (2017). Association of CYP2J2 gene polymorphisms with ischemic stroke and stroke subtypes in Chinese population. Medicine 96, e6266. doi: 10.1097/MD.0000000000006266
Wang, W., Jiang, B., Sun, H., Ru, X., Sun, D., Wang, L., et al. (2017). Prevalence, incidence, and mortality of stroke in China: results from a nationwide population-based survey of 480 687 adults. Circulation 135, 759–771. doi: 10.1161/CIRCULATIONAHA.116.025250
Xu, Z., Li, Y., Huang, X., Shen, W., Bai, J., Shen, C., et al. (2017). ESR2 genetic variants and combined oral contraceptive use associated with the risk of stroke. Arch. Med. Res. 48, 203–211. doi: 10.1016/j.arcmed.2017.03.015
Yan, G., and Wang, Y. (2012). Association of CYP11B2 gene polymorphism with ischemic stroke in the north Chinese Han population. Neurol. India 60, 504–509. doi: 10.4103/0028-3886.103196
Yang, W., Ma, F., Wang, L., He, X., Zhang, H., Zheng, J., et al. (2020). The association analysis between CYP24A1 genetic polymorphisms and the risk of ischemic stroke in Chinese Han population. Brain Behav. 10, e01503. doi: 10.1002/brb3.1503
Yi, X., Lin, J., Wang, Y., Zhou, J., and Zhou, Q. (2017). Interaction among CYP2C8, GPIIIa and P2Y12 variants increase susceptibility to ischemic stroke in Chinese population. Oncotarget 8, 70811–70820. doi: 10.18632/oncotarget.19991
Yuan, H., Fan, P., Yao, L., Lv, Y., Wei, H., Zheng, J., et al. (2021). Contribution of WNT2B genetic variants to ischemic stroke occurrence in a Chinese Han population. J. Cardiovasc. Pharmacol. 78:e128–e135. doi: 10.1097/FJC.0000000000001032
Zhang, G. X., Wang, B. J., Ouyang, J. Z., Deng, X. Y., Ma, X., Li, H. Z., et al. (2010). Polymorphisms in CYP11B2 and CYP11B1 genes associated with primary hyperaldosteronism. Hypertension Res. 33, 478–484. doi: 10.1038/hr.2010.21
Zhang, S., Zhang, W., and Zhou, G. (2019). Extended risk factors for stroke prevention. J. Natl. Med. Assoc. 111, 447–456. doi: 10.1016/j.jnma.2019.02.004
Keywords: ischemic stroke, susceptibility, CYP11B1, gene polymorphisms, case-control study
Citation: Liu G and Duan Y (2022) CYP11B1 gene polymorphisms and susceptibility to ischemic stroke in a Chinese Han population. Front. Neurosci. 16:1030551. doi: 10.3389/fnins.2022.1030551
Received: 08 September 2022; Accepted: 08 November 2022;
Published: 01 December 2022.
Edited by:
Jun Zhang, Texas Tech University Health Sciences Center, United StatesReviewed by:
Yonggang Hao, Sir Run Run Shaw Hospital, ChinaYipeng Ding, Hainan General Hospital, China
Copyright © 2022 Liu and Duan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ying Duan, ZHVhbnlpbmc3MkAxNjMuY29t
 Gaowen Liu
Gaowen Liu Ying Duan
Ying Duan