- 1Department of Neurobiology and Behavior, University of California, Irvine, Irvine, CA, United States
- 2Center for the Neurobiology of Learning and Memory, Institute for Memory Impairments and Neurological Disorders, University of California, Irvine, Irvine, CA, United States
The loss of olfactory stimulation correlates well with at least 68 widely differing neurological disorders, including depression, and we raise the possibility that this relationship may be causal. That is, it seems possible that olfactory loss makes the brain vulnerable to expressing the symptoms of these neurological disorders, while daily olfactory enrichment may decrease the risk of expressing these symptoms. This situation resembles the cognitive reserve that is thought to protect people with Alzheimer’s neuropathology from expressing the functional deficit in memory through the cumulative effect of intellectual stimulation. These relationships also resemble the functional response of animal models of human neurological disorders to environmental enrichment, wherein the animals continue to have the induced neuropathology, but do not express the symptoms as they do in a standard environment with restricted sensorimotor stimulation.
Introduction
Epidemiology of depression
Neurological disorders are the greatest cause of disability and the second greatest cause of deaths around the world (GBD 2015 Neurology Collaborators, 2016). One in two women and one in three men will develop dementia, stroke, or Parkinson’s disease during their lifetime (Licher et al., 2019). Depression is characterized by feelings of sadness and hopelessness, and is the leading neurological disorder in disability-adjusted life years, (i.e., the sum of years lost due to premature mortality plus the years with a disability). Around 350 million people are affected by depression around the world (Lopez and Murray, 1998; GBD 2015 Neurology Collaborators, 2016). The lifetime risk of depression in the U.S. is about 20% (Hasin et al., 2018), and in other affluent countries, 40% of women and 30% of men have been found to have this disorder before they are 65 years old (Kruijshaar et al., 2005), making it a major medical issue. The existing pharmaceutical treatments for this serious emotional problem range from somewhat effective to largely ineffective (Rush et al., 2006; Ioannidis, 2008; Cipriani et al., 2018). Moreover, the drugs come with a wide range of side effects, including changes in bodyweight, sleep pattern, and libido (Ramic et al., 2020).
Drug treatment for depression
While most of the drugs used to treat depression are specific serotonin reuptake inhibitors (SSRIs), it is unlikely that the efficacy of increasing this or other monoamines improves the emotional status of people with depression through the mechanism of treating a monoamine deficiency (Boku et al., 2018). Rather, it is more likely that the effects of these drugs are mediated by their ability to increase levels of brain-derived neurotropic factor (BDNF). Increased BDNF has a salutary effect on keeping hippocampal neurons alive under conditions of chronic stress, which is often involved in triggering a depressive episode (Björkholm and Monteggia, 2016; Tafet and Nemeroff, 2016). It is the neural repopulation of this brain area that seems to be critical in recovering from the disorder (Boku et al., 2018).
Two new antidepressant drugs have been approved recently by the FDA and they use very different mechanisms of action to improve depression symptoms. Ketamine in a racemic mixture of R-ketamine and S-ketamine has been in use as an anesthetic, is administered as an infusion (i.v.), and also has antidepressant action when used in lower doses and acts rapidly compared to other antidepressants (Covvey et al., 2012). Esketamine (S-ketamine) alone, administered as a nasal spray, has also been shown to reduce depression symptoms, also with a rapid onset of action (Dean et al., 2021; Doty et al., 2021). However, for both drugs, there is very low−certainty evidence provided for their efficacious use (Dean et al., 2021; Doty et al., 2021). Both versions of ketamine seem to have their mechanism of action as an antagonist on the NMDA glutamate receptor.
Another drug with a more convenient, oral method of administration also has been approved recently for use in treatment-resistant major depression and it also seems to have a rapid action to suppress depression symptoms. Called Auvelity, it contains dextromethorphan, which is an anti-tussive that is a non-competitive antagonist for the NMDA receptor and to the sigma opioid receptor, and bupropion, an antidepressant on its own that increases synaptic norepinephrine and dopamine by decreasing their reuptake (Stahl et al., 2004; Iosifescu et al., 2022).
Electrical stimulation as a treatment for depression
Only 40–60% of patients with major depressive disorder are successfully treated with pharmaceuticals (Rush et al., 2006; Malhi et al., 2020). One alternative for drug treatment-resistant patients is the use of electrical stimulation. Electrical brain stimulation in the form of electroconvulsive therapy can be an effective treatment for depression and has the advantage of being fast-acting, compared to the weeks required before SSRIs become effective (Li et al., 2021). The rapidity with which this treatment works allows it to be used by people who are severely depressed and who are considering suicide. In this method of brain activation, electrical stimulation is delivered to the skull and a grand mal seizure is induced in the patient. This approach is often successful, with 50–60% of drug treatment-resistant patients coming into remission with this therapy (Mutz et al., 2019). One of the adverse side effects, however, include the loss of recent memories (Espinoza and Kellner, 2022).
Transcranial stimulation as a treatment for depression
Transcranial magnetic stimulation uses electromagnetic stimulation to treat major depressive disorder by activating the dorsolateral prefrontal cortex. Compared to electrical stimulation, a benefit of this method is greater spatial and temporal control, without provoking seizures (Rizvi and Khan, 2019). While different systems and protocols produce different outcomes, a typical finding is that after 5 sessions per week for 4 weeks, followed by a continuation phase of twice-weekly treatment for an additional 12 weeks, the remission rates for drug treatment-resistant patients were 32.6% for transcranial magnetic therapy vs. 14.6% for sham treatment (Perera et al., 2016). Although moderately successful for some drug treatment-resistant patients, it requires multiple medical visits which can be inconvenient, expensive, and time-consuming.
Deep brain stimulation as a treatment for depression
Another treatment method is deep brain stimulation (DBS), which involves implanting electrodes in the brain and using electrical stimulation to regulate brain activity. DBS has been used in a variety of brain regions to treat drug treatment-resistant depression and has had some level of success (Dandekar et al., 2018; Aibar-Durán et al., 2022). However, this treatment incurs high costs and lacks consistent positive outcomes.
Animal models of depression
Ideally, the treatment for depression should be highly effective for all levels of the disorder, inexpensive, effortless, and without significant side effects. A different approach has been developed using animal models of depression, which mimic both the etiology of depression and the human-like depression symptoms (McKinney and Bunney, 1969). Indeed, depression-like symptoms resembling that seen in the human response to chronic stress can be induced in rats and mice following chronic isolation, pain, learned helplessness, social defeat, minor stressors, maternal deprivation, striatal intracerebral hemorrhage, forced swimming, restraint, olfactory bulb removal, and tail suspension (Kelly et al., 1997; Veena et al., 2009; Richter et al., 2013; Gong et al., 2018; Seong et al., 2018; Hao et al., 2019; Wang et al., 2019; Sparling et al., 2020; Borba et al., 2021; Cordner et al., 2021; Ramírez-Rodríguez et al., 2021; Réus et al., 2021; Taheri Zadeh et al., 2021; Kimura et al., 2022). Moreover, the therapies used to treat humans with depression are also effective in reversing induced depression in lab animals, including antidepressants, electroconvulsive seizure therapy, and deep brain stimulation (Jeannotte et al., 2009; Falowski et al., 2011; Tizabi et al., 2012; Belujon and Grace, 2014; Song and Kim, 2021).
Environmental enrichment
Environmental enrichment improves cognitive ability, emotional status, and the human-like symptoms of many neurological disorders in lab animals
This phenomenon was initially described by Hebb (1947), who found neurobehavioral improvements in rats that he kept uncaged at his home, compared to the rats kept in small box cages in the lab. Other researchers then systematized the experimental paradigm to keep the enriched rats in a large cage with other conspecifics, an exercise wheel, and multiple children’s toys that were changed on a regular basis (Rosenzweig et al., 1962). Control rats kept in standard cages either had a single conspecific or no conspecifics, and no additional features. Compared to controls, the brains of the enriched rats were much larger and more complex and had higher levels of neurotransmitters and trophic factors. Enriched rats also exhibited improved cognitive ability, emotional status, and motor ability. A large body of work has followed this landmark study, and impressively, in animal models of many other human neurological disorders, environmental enrichment greatly ameliorated or completely eliminated symptoms of these and other disorders: autism, stroke, seizures, traumatic brain injury, ADHD, prenatal alcohol syndrome, lead exposure, multiple sclerosis, addiction, schizophrenia, epilepsy, Alzheimer’s Huntington’s disease, Parkinson’s disease, Down syndrome, fragile X syndrome, Rett syndrome, and Potocki-Lupski syndrome (Nithianantharajah and Hannan, 2009; Hannan, 2014).
In rodents, environmental enrichment improves the neural and cognitive loss normally experienced with aging
A loss of hippocampal neurons is involved in the gradual memory loss experienced with normal aging, and a highly specific form of environmental enrichment can reverse such neural and cognitive changes. Specifically, exposing mice to olfactory enrichment, which involved the daily exposure to multiple diverse odorants, restored the hippocampal neural population and improved cognition in older mice. Veyrac et al. (2009) showed that in mice, olfactory enrichment alone both improved memory and increased brain neurogenesis. They further showed that novelty was the critical element in this kind of stimulation, as exposure to multiple odorants individually resulted in these changes, while exposure to odorant mixtures did not. Rusznák et al. (2018) also showed that exposure to various essential oils alone for 30 min/day over 3 months induced neurogenesis in both the olfactory bulb and the hippocampus, and the neuronal number in those regions increased to a level comparable to what they found in mice housed in an enriched environment. Finally, Zhang et al. (2022) found that olfactory enrichment for 21 days, initiated prior to anesthesia/laparotomy surgery, normalized post-anesthesia/surgery olfactory and cognitive functioning, which were diminished in animals not receiving the enrichment.
Environmental enrichment improves depression symptoms in animal models
Animal models of depression show improvement in behavioral and biochemical depression-like symptoms following environmental enrichment (Ilin and Richter-Levin, 2009; Veena et al., 2009; Richter et al., 2013; Gong et al., 2018; Seong et al., 2018; Hao et al., 2019; Wang et al., 2019; Brenes et al., 2020; Moradi-Kor et al., 2020; Sparling et al., 2020; Borba et al., 2021; Cordner et al., 2021; Huang et al., 2021; Ramírez-Rodríguez et al., 2021; Taheri Zadeh et al., 2021; Kimura et al., 2022). For example, Jha et al. (2011) found that environmental enrichment treatment in a mouse model of depression not only reversed depression-like behavior, it also restored the reductions in neurogenesis and BDNF levels in the hippocampus.
Olfactory system connectivity is ideally situated to improve cognition and depression in humans
The olfactory system is the only sensory system that has direct projections to the brain’s cognitive and emotional control areas—the hippocampal-amygdala complex (Haberly and Price, 1977; Zhou et al., 2019; Noto et al., 2021), while the other sensory systems have indirect connections via the thalamus. The unique access of the olfactory system to the cognitive and emotional systems may allow highly specific activation of these systems that could improve cognitive and emotional symptoms.
Loss of olfactory ability induces deterioration of cognitive brain areas
Olfactory deterioration occurs just before the deterioration of cognitive ability in older adults (Doty et al., 1984; Schaie et al., 2004; Sanna et al., 2021). In addition, the loss or compromise of the olfactory system results in a significant volume loss of both gray matter and white matter in human brains (Bitter et al., 2010a,b; Segura et al., 2013; Yao et al., 2014; Kollndorfer et al., 2015). Even a chronic stuffed nose resulted in the deterioration of gray matter density in the adult brain (Han et al., 2017). Olfactory dysfunction also predicts cognitive dysfunction in human adults (Choi et al., 2018). Moreover, a degradation of olfactory ability predicts an elevated risk of mild cognitive impairment (MCI) and it also predicts which individuals with MCI will develop Alzheimer’s disease (Peters et al., 2003; Adams et al., 2018).
Increasing olfactory stimulation restores olfactory function
Olfactory enrichment has been shown to improve olfactory function in humans who have experienced olfactory loss due to a variety of problems, such as post-infectious olfactory dysfunction, head trauma, Parkinson’s, and aging (Hummel et al., 2009; Haehner et al., 2013; Konstantinidis et al., 2013; Damm et al., 2014; Geißler et al., 2014; Patel et al., 2017).
Environmental enrichment, including olfactory enrichment, improves autism symptoms
Environmental enrichment using olfactory enrichment as a central component reduces the symptoms of autism significantly within 6 months in 42% of the children, compared to 7% in standard care, improves IQ by 8 points, compared to no improvement in standard care, improves communication by over 200%, compared to 8% in standard care, and reverses the diagnosis of 21% of the enriched children with classic autism, compared to 0% in standard care (Woo and Leon, 2013; Woo et al., 2015).
This autism treatment is now available with an online algorithm that individualizes the enrichment exercises according to the child’s current symptoms. When we looked at the outcomes of over 1,000 children along the entire autism spectrum (Aronoff et al., 2016), we found that these children did even better than those in the original clinical trials, with a large effect size of 1.85. Not only did the core symptoms of autism improve, but the co-morbid symptoms, also improved: sensory processing, self-awareness, sleeping, communication, eating, motor skills, learning, memory, anxiety, attention and mood, including depression symptoms.
Olfaction is associated with depression
Olfactory impairment and depression
Many studies have shown that olfactory impairment is associated with depression in human adults (Atanasova et al., 2008; Croy et al., 2014; Kohli et al., 2016; Croy and Hummel, 2017; Taalman et al., 2017; Rochet et al., 2018; Rottstaedt et al., 2018; Wegener et al., 2018; Pabel et al., 2020; Qazi et al., 2020; Wang et al., 2020; Athanassi et al., 2021; Kim and Bae, 2022; Liu et al., 2022; Sabiniewicz et al., 2022). Moreover, olfactory dysfunction precedes and predicts the development of depression in older adults (Eliyan et al., 2021). Additionally, individuals with congenital anosmia have elevated Beck Depression Inventory scores relative to nornosmic controls (Croy et al., 2012) and higher depression scores are observed in people with either congenital anosmia or acquired anosmia relative to nornosmic controls (Lemogne et al., 2015; Kohli et al., 2016).
In a longitudinal study, adults who had olfactory dysfunction were more likely to develop depression symptoms after 5–10 years than adults who had no olfactory loss at baseline. On the other hand, those adults who had depression symptoms at baseline were not more likely to develop olfactory dysfunction. These data resemble those in which olfactory dysfunction occurs well before the motor or cognitive problems emerge in Parkinson’s and Alzheimer’s diseases (Sohrabi et al., 2012; Devanand, 2016; Fullard et al., 2017). Moreover, the severity of the olfactory dysfunction predicts the severity of depression (Kohli et al., 2016) and the size of the olfactory bulb predicts both the severity of depression and the probability of therapeutic success (Negoias et al., 2016). It is also the case that in lab animals, removal of the olfactory bulb and the consequent loss of olfaction induces a depression-like state that has been used as a model of the disorder (Kelly et al., 1997), the symptoms of which can be ameliorated by anti-depressant drugs (Jarosik et al., 2007).
Can olfactory enrichment also help to treat depression?
Olfactory enrichment modifies brain structures and improves cognitive and emotional status. Al Aïn et al. (2019) investigated the effects of 6 weeks of olfactory enrichment in healthy young adults and found that the enrichment led to increased cortical thickness in the inferior frontal gyrus, the bilateral fusiform gyrus and the entorhinal cortex compared to visual-training controls. Gellrich et al. (2017) found that olfactory enrichment of people with olfactory deficiencies increased gray matter volume in the hippocampus and the thalamus, changes that could underly the ability of olfactory enrichment to improve cognitive and emotional status in older adults. Han et al. (2021) found that individuals using olfactory enrichment with 4 odorants for 7 months had improved odor identification and larger gray matter volume in the bilateral cerebellum, bilateral thalamus, precentral gyrus, gyrus rectus, and medial orbitofrontal cortex.
Olfactory enrichment initiated after the onset of neurological symptom also can ameliorate those symptoms. Haehner et al. (2013) showed that patients with Parkinson’s disease improved their verbal fluency after olfactory enrichment. Wegener et al. (2018) provided olfactory enrichment to older adults using 4 essential-oil odorants twice a day for 5 months. Controls solved daily Sudoku puzzles during that time. The olfactory-enriched group had a significant improvement of olfactory function, improved verbal function, and decreased depression symptoms.
Cha et al. (2022) exposed 34 older adults with dementia (but with a Mini-Mental State Examination score of at least 10) to 40 odorants twice a day for 15 days. The control group consisted of 31 individuals with dementia that received no such stimulation; there were no initial differences between groups. Their results were remarkable, as the olfactory-enriched group showed highly significant improvements in memory, olfactory identification, depression symptoms, attention, and language skills. In detail, they found that olfactory-enriched individuals had an improvement in olfactory identification, while controls did not, as assessed by the Olfactory Identification Test (p < 0.001). The Verbal Fluency Test to assess semantic memory also showed significant improvements for the enriched group relative to the controls (p = 0.001). Similarly, the Boston Naming Test to assess language function revealed a significant improvement in the enriched subjects relative to controls (p = 0.001). The Word-List Memory Test to assess attention and working memory, the Word-List Recall Test to assess verbal memory encoding, and the Word List Recognition Test to assess verbal memory retrieval all showed similar remarkable improvements in the enriched group relative to the control group (p < 0.001, p = 0.031, and p < 0.001, respectively). Finally, the Geriatric Depression Scale showed a reduction in depression symptoms in enriched patients, but not in controls (p < 0.001).
Taken all together, olfactory environmental enrichment may enable the brain to compensate for the neuropathology and restore normal functioning, consistent with the idea that olfactory enrichment could be a formidable and facile treatment option for depression.
Olfactory loss increases neurological risk
Olfactory loss and the risk of neurological disorders
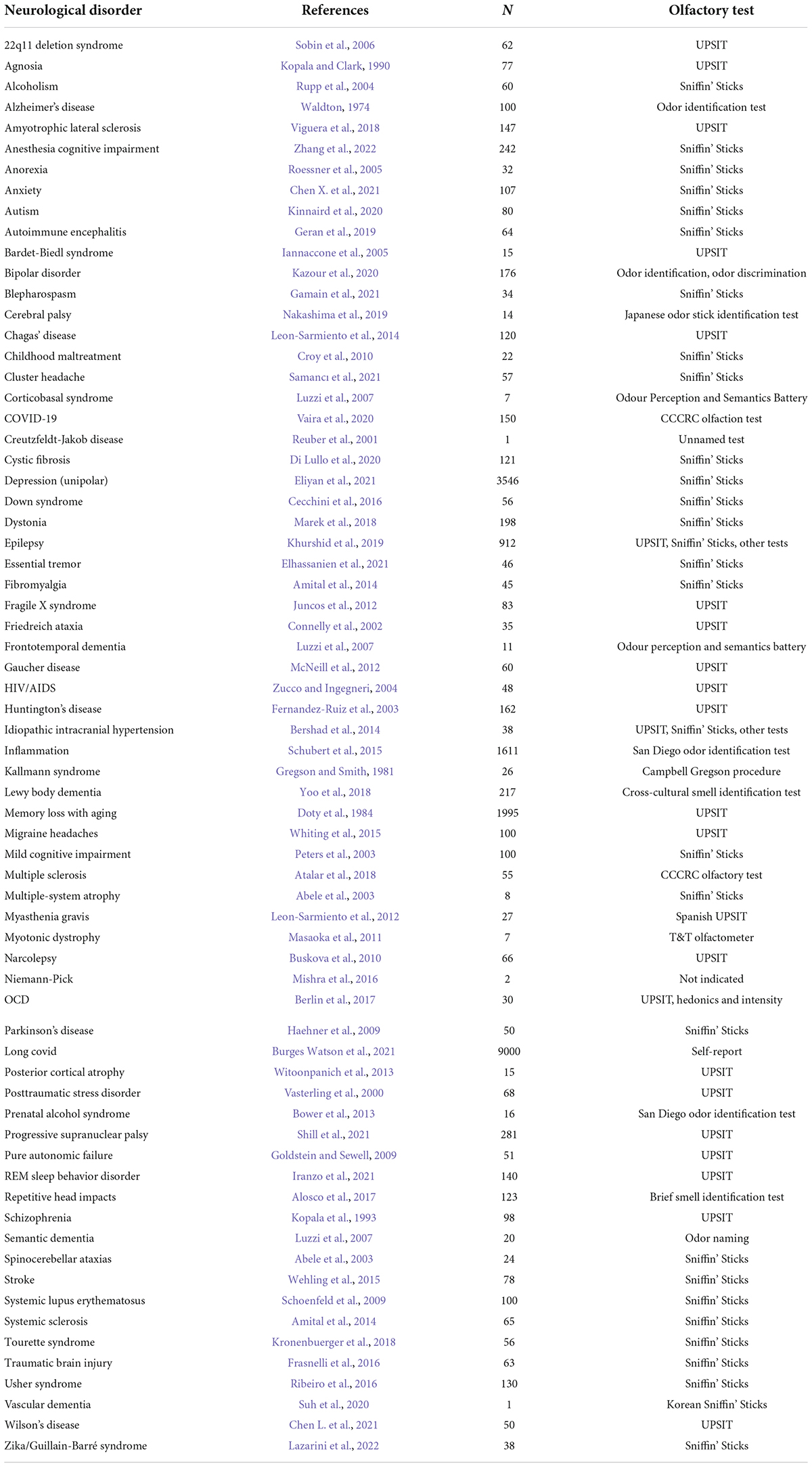
Olfactory loss accompanies 68 neurological disorders of which we are aware (Table 1). There are also other medical disorders that are accompanied by olfactory loss, such as diabetes, hypertension, cardiovascular disease, cancer, and obesity; disorders that may well have a neurological component (Bartoshuk, 1990; Liu et al., 2018; Zhang et al., 2019; Roh et al., 2021; Faour et al., 2022).
While we have drawn attention to the remarkable number of neurological disorders that are correlated with olfactory loss, we don’t know in each case whether these associations are causal, and in most cases, we don’t even know if the olfactory loss comes before or after the onset of the other neurological symptoms. Moreover, we don’t know if there are common mechanisms underlying the olfactory or other neurological symptoms. At the same time, the fact that olfactory enrichment can ameliorate both the olfactory loss and the neurological symptoms of the various disorders with which it has been tested raises the possibility that there is a causal mechanism in which the loss of olfaction increases the risk of expressing the symptoms characteristic of the various disorders. The large number of correlations adds to the weight of evidence suggesting a causal, rather than simply a correlational relationship in some or many of these relationships.
How can environmental enrichment improve outcomes for neurological disorders including depression?
While environmental enrichment can relieve the symptoms of various neurological disorders that have been imposed on lab animals, that experience does not mend all the damage in the brain, but rather, the brain seems to compensate for the damage under enriched conditions by making a neural work-around that normalizes the cognition and emotions without directly fixing the underlying problem.
Discussion
The need for olfactory stimulation
The loss of olfaction in each of these neurological disorders may be due to a common disruption to normal neural mechanisms. But given the very large number of very different neurological disorders with very different etiologies, that possibility seems unlikely. Another possibility is that the olfactory loss precedes the neurological symptoms, which is the case for Alzheimer’s disease, Parkinson’s disease, the cognitive loss that can accompany long COVID, and depression (Walker et al., 2021; Zamponi et al., 2021). Similarly, olfactory loss may precede all the disorders listed above and if olfactory loss precedes the cognitive, emotional, and motor symptoms, perhaps the olfactory loss plays a role in increasing the risk of developing these functional problems. On the other hand, olfactory enrichment may allow the brain to construct workarounds to normalize the cognitive, emotional, and motor issues, as seen in the animal models of human neurological disorders after environmental enrichment.
Indeed, adequate olfactory stimulation may confer a brain/cognitive reserve, that allows the human brain to compensate for neuropathology and avoid experiencing the symptoms typically associated with neurological disorders (Stern et al., 2019). This phenomenon has been reported for Alzheimer’s disease, where some individuals don’t experience the cognitive loss despite the high level of neuropathology (Nilsson and Lövdén, 2018; Montine et al., 2019; Stern et al., 2020). The ability to avoid memory problems may be due to previous intellectual stimulation (Hertzog et al., 2008; Cooper et al., 2017).
It may be that humans in the affluent world are chronically deprived of the high levels of olfactory stimulation that was present when humans evolved. When people have a brain that is chronically deprived of sufficient olfactory stimulation, it may allow the symptoms of neurological disorders to emerge, just as lab animals display the neurological symptoms when they are deprived of sufficient environmental stimulation, but do not show these symptoms when they are living in an enriched environment. In both humans and rodents, the neurological problem remains, but the enriched brain can compensate for that damage and veil the symptoms. Increased vulnerability of older adults to such disorders therefore may be the reason that poor olfactory ability predicts all-cause mortality in humans after middle age, with anosmic individuals experiencing a fourfold increase in mortality risk compared to those with normal olfactory ability (Wilson et al., 2011; Gopinath et al., 2012; Pinto et al., 2014; Devanand et al., 2015; Ekström et al., 2017; Schubert et al., 2017; Fuller-Thomson and Fuller-Thomson, 2019; Kamath and Leff, 2019; Liu et al., 2019; Choi et al., 2021; Pinto, 2021; Xiao et al., 2021; Pang et al., 2022). Ongoing olfactory enrichment may therefore be a critical environmental component needed to prevent the symptoms of neurological disorders, including depression, from being expressed over the course of a lifetime.
Data availability statement
The original contributions presented in this study are included in the article/supplementary material, further inquiries can be directed to the corresponding author/s.
Author contributions
Both authors listed have made a substantial, direct, and intellectual contribution to the work, and approved it for publication.
Funding
This work was funded by the Susan Samueli Integrative Health Institute for the preparation of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Abele, M., Riet, A., Hummel, T., Klockgether, T., and Wüllner, U. (2003). Olfactory dysfunction in cerebellar ataxia and multiple system atrophy. J. Neurol. 250, 1453–1455. doi: 10.1007/s00415-003-0248-4
Adams, D. R., Kern, D. W., Wroblewski, K. E., McClintock, M. K., Dale, W., and Pinto, J. M. (2018). Olfactory dysfunction predicts subsequent dementia in older U.S. adults. J. Am. Geriat. Soc. 66, 140–144. doi: 10.1111/jgs.15048
Aibar-Durán, J. Á, Rodríguez Rodríguez, R., de Diego Adeliño, F. J., Portella, M. J., Álvarez-Holzapfel, M. J., Martín Blanco, A., et al. (2022). Long-term results of deep brain stimulation for treatment-resistant depression: Outcome analysis and correlation with lead position and electrical parameters. Neurosurgery 90, 72–80. doi: 10.1227/NEU.0000000000001739
Al Aïn, S., Poupon, D., Hétu, S., Mercier, N., Steffener, J., and Frasnelli, J. (2019). Smell training improves olfactory function and alters brain structure. Neuroimage 189, 45–54. doi: 10.1016/j.neuroimage.2019.01.008
Alosco, M. L., Jarnagin, J., Tripodis, Y., Platt, M., Martin, B., Chaisson, C. E., et al. (2017). Olfactory function and associated clinical correlates in former national football league players. J. Neurotrauma 34, 772–780. doi: 10.1089/neu.2016.4536
Amital, H., Agmon-Levin, N., Shoenfeld, N., Arnson, Y., Amital, D., Langevitz, P., et al. (2014). Olfactory impairment in patients with the fibromyalgia syndrome and systemic sclerosis. Immunol. Res. 60, 201–207. doi: 10.1007/s12026-014-8573-5
Aronoff, E., Hillyer, R., and Leon, M. (2016). Environmental enrichment therapy for autism: Outcomes with increased access. Neural Plast. 2016:2734915. doi: 10.1155/2016/2734915
Atalar, A. Ç, Erdal, Y., Tekin, B., Yıldız, M., Akdoğan, Ö, and Emre, U. (2018). Olfactory dysfunction in multiple sclerosis. Mult. Scler. Relat. Disord. 21, 92–96. doi: 10.1016/j.msard.2018.02.032
Atanasova, B., Graux, J., El Hage, W., Hommet, C., Camus, V., and Belzung, C. (2008). Olfaction: A potential cognitive marker of psychiatric disorders. Neurosci. Biobehav. Rev. 32, 1315–1325. doi: 10.1016/j.neubiorev.2008.05.003
Athanassi, A., Dorado Doncel, R., Bath, K. G., and Mandairon, N. (2021). Relationship between depression and olfactory sensory function: A review. Chem. Senses 46:bjab044. doi: 10.1093/chemse/bjab044
Belujon, P., and Grace, A. A. (2014). Restoring mood balance in depression: Ketamine reverses deficit in dopamine-dependent synaptic plasticity. Biol. Psychiatry 76, 927–936. doi: 10.1016/j.biopsych.2014.04.014
Berlin, H. A., Stern, E. R., Ng, J., Zhang, S., Rosenthal, D., Turetzky, R., et al. (2017). Altered olfactory processing and increased insula activity in patients with obsessive-compulsive disorder: An fMRI study. Psychiatry Res. Neuroimaging 262, 15–24. doi: 10.1016/j.pscychresns.2017.01.012
Bershad, E. M., Urfy, M. Z., Calvillo, E., Tang, R., Cajavilca, C., Lee, A. G., et al. (2014). Marked olfactory impairment in idiopathic intracranial hypertension. J. Neurol. Neurosurg. Psychiatry 85, 959–964. doi: 10.1136/jnnp-2013-307232
Bitter, T., Bruderle, J., Gudziol, H., Burmeister, H. P., Gaser, C., and Guntinas-Lichius, O. (2010a). Gray and white matter reduction in hyposmic subjects – a voxel-based morphometry study. Brain Res. 1347, 42–47. doi: 10.1016/j.brainres.2010.06.003
Bitter, T., Gudziol, H., Burmeister, H. P., Mentzel, H.-J., Guntinas-Lichius, O., and Gaser, C. (2010b). Anosmia leads to a loss of gray matter in cortical brain areas. Chem. Senses 35, 407–415. doi: 10.1093/chemse/bjq028
Björkholm, C., and Monteggia, L. M. (2016). BDNF – a key transducer of antidepressant effects. Neuropharmacology 102, 72–79. doi: 10.1016/j.neuropharm.2015.10.034
Boku, S., Nakagawa, S., Toda, H., and Hishimoto, A. (2018). Neural basis of major depressive disorder: Beyond monoamine hypothesis. Psychiatry Clin. Neurosci. 72, 3–12. doi: 10.1111/pcn.12604
Borba, L. A., Broseghini, L. D. R., Manosso, L. M., de Moura, A. B., Botelho, M. E. M., Arent, C. O., et al. (2021). Environmental enrichment improves lifelong persistent behavioral and epigenetic changes induced by early-life stress. J. Psychiatr. Res. 138, 107–116. doi: 10.1016/j.jpsychires.2021.04.008
Bower, E., Szajer, J., Mattson, S. N., Riley, E. P., and Murphy, C. (2013). Impaired odor identification in children with histories of heavy prenatal alcohol exposure. Alcohol 47, 275–278. doi: 10.1016/j.alcohol.2013.03.002
Brenes, J. C., Fornaguera, J., and Sequeira-Cordero, A. (2020). Environmental enrichment and physical exercise attenuate the depressive-like effects induced by social isolation stress in rats. Front. Pharmacol. 11:804. doi: 10.3389/fphar.2020.00804
Burges Watson, D. L., Campbell, M., Hopkins, C., Smith, B., Kelly, C., and Deary, V. (2021). Altered smell and taste: Anosmia, parosmia and the impact of long Covid-19. PLoS One 16:e0256998. doi: 10.1371/journal.pone.0256998
Buskova, J., Klaschka, J., Sonka, K., and Nevsimalova, S. (2010). Olfactory dysfunction in narcolepsy with and without cataplexy. Sleep Med. 11, 558–561. doi: 10.1016/j.sleep.2010.01.009
Cecchini, M. P., Viviani, D., Sandri, M., Hähner, A., Hummel, T., and Zancanaro, C. (2016). Olfaction in people with down syndrome: A comprehensive assessment across four decades of age. PLoS One 11:e0146486. doi: 10.1371/journal.pone.0146486
Cha, H., Kim, S., Kim, H., Kim, G., and Kwon, K. Y. (2022). Effect of intensive olfactory training for cognitive function in patients with dementia. Geriatr. Gerontol. Int. 22, 5–11. doi: 10.1111/ggi.14287
Chen, L., Wang, X., Doty, R. L., Cao, S., Yang, J., Sun, F., et al. (2021). Olfactory impairment in wilson’s disease. Brain Behav. 11:e02022. doi: 10.1002/brb3.2022
Chen, X., Guo, W., Yu, L., Luo, D., Xie, L., and Xu, J. (2021). Association between anxious symptom severity and olfactory impairment in young adults with generalized anxiety disorder: A case-control study. Neuropsychiatr. Dis. Treat. 17, 2877–2883. doi: 10.2147/NDT.S314857
Choi, J. S., Hur, K., Chow, M., Shen, J., and Wrobel, B. (2018). Olfactory dysfunction and cognition among older adults in the United States. Int. Forum Allergy Rhinol. 8, 648–654. doi: 10.1002/alr.22078
Choi, J. S., Jang, S. S., Kim, J., Hur, K., Ference, E., and Wrobel, B. (2021). Association between olfactory dysfunction and mortality in US adults. JAMA Otolaryngol. Head Neck Surg. 147, 49–55. doi: 10.1001/jamaoto.2020.3502
Cipriani, A., Furukawa, T. A., Salanti, G., Chaimani, A., Atkinson, L. Z., Ogawa, Y., et al. (2018). Comparative efficacy and acceptability of 21 antidepressant drugs for the acute treatment of adults with major depressive disorder: A systematic review and network meta-analysis. Lancet 391, 1357–1366. doi: 10.1016/S0140-6736(17)32802-7
Connelly, T., Farmer, J. M., Lynch, D. R., and Doty, R. L. (2002). Olfactory dysfunction in degenerative ataxias. J. Neurol. Neurosurg. Psychiatry 74, 1435–1437. doi: 10.1136/jnnp.74.10.1435
Cooper, C., Fox, N., Gitlin, L. N., Howard, R., Kales, H. C., Larson, E. B., et al. (2017). Dementia prevention, intervention, and care. Lancet 390, 2673–2734. doi: 10.1016/S0140-6736(17)31363-6
Cordner, Z. A., Marshall-Thomas, I., Boersma, G. J., Lee, R. S., Potash, J. B., and Tamashiro, K. L. K. (2021). Fluoxetine and environmental enrichment similarly reverse chronic social stress-related depression- and anxiety-like behavior, but have differential effects on amygdala gene expression. Neurobiol. Stress 15:100392. doi: 10.1016/j.ynstr.2021.100392
Covvey, J. R., Crawford, A. N., and Lowe, D. K. (2012). Intravenous ketamine for treatment-resistant major depressive disorder. Ann. Pharmacother. 46, 117–123. doi: 10.1345/aph.1Q371
Croy, I., and Hummel, T. (2017). Olfaction as a marker for depression. J. Neurol. 264, 631–638. doi: 10.1007/s00415-016-8227-8
Croy, I., Negoias, S., Novakova, L., Landis, B. N., and Hummel, T. (2012). Learning about the functions of the olfactory system from people without a sense of smell. PLoS One 73:e33365. doi: 10.1371/journal.pone.0033365
Croy, I., Schellong, J., Gerber, J., Joraschky, P., Iannilli, E., and Hummel, T. (2010). Women with a history of childhood maltreatment exhibit more activation in association areas following non-traumatic olfactory stimuli: A fMRI study. PLoS One 5:e9362. doi: 10.1371/journal.pone.0009362
Croy, I., Symmank, A., Schellong, J., Hummel, C., Gerber, J., Joraschky, P., et al. (2014). Olfaction as a marker for depression in humans. J. Affect. Disord. 160, 80–86. doi: 10.1016/j.jad.2013.12.026
Damm, M., Pikart, L. K., Reimann, H., Burkert, S., Göktas, Ö, Haxel, B., et al. (2014). Olfactory training is helpful in postinfectious olfactory loss: A randomized, controlled, multicenter study. Laryngoscope 124, 826–831. doi: 10.1002/lary.24340
Dandekar, M. P., Fenoy, A. J., Carvalho, A. F., Soares, J. C., and Quevedo, J. (2018). Deep brain stimulation for treatment-resistant depression: An integrative review of preclinical and clinical findings and translational implications. Mol. Psychiatry 23, 1094–1112. doi: 10.1038/mp.2018.2
Dean, R. L., Hurducas, C., Hawton, K., Spyridi, S., Cowen, P. J., Hollingsworth, S., et al. (2021). Ketamine and other glutamate receptor modulators for depression in adults with unipolar major depressive disorder. Cochrane Database Syst. Rev. 9:CD011612. doi: 10.1002/14651858.CD011612.pub3
Devanand, D. P. (2016). Olfactory identification deficits, cognitive decline, and dementia in older adults. Am. J. Geriatr. Psychiatry 24, 1151–1157. doi: 10.1016/j.jagp.2016.08.010
Devanand, D. P., Lee, S., Manly, J., Andrews, H., Schupf, N., Masurkar, A., et al. (2015). Olfactory identification deficits and increased mortality in the community. Ann. Neurol. 78, 401–411. doi: 10.1002/ana.24447
Di Lullo, A. M., Iacotucci, P., Comegna, M., Amato, F., Dolce, P., Castaldo, G., et al. (2020). Cystic fibrosis: The sense of smell. Am. J. Rhinol. Allergy 34, 35–42. doi: 10.1177/1945892419870450
Doty, R. L., Popova, V., Wylie, C., Fedgchin, M., Daly, E., Janik, A., et al. (2021). Effect of esketamine nasal spray on olfactory function and nasal tolerability in patients with treatment-resistant depression: Results from four multicenter, randomized, double-blind, placebo-controlled, phase III studies. CNS Drugs 35, 781–794. doi: 10.1007/s40263-021-00826-9
Doty, R. L., Shaman, P., Applebaum, S. L., Giberson, R., Siksorski, L., and Rosenberg, L. (1984). Smell identification ability: Changes with age. Science 226, 1441–1443. doi: 10.1126/science.6505700
Ekström, I., Sjölund, S., Nordin, S., Nordin Adolfsson, A., Adolfsson, R., Nilsson, L. G., et al. (2017). Smell loss predicts mortality risk regardless of dementia conversion. J. Am. Geriatr. Soc. 65, 1238–1243. doi: 10.1111/jgs.14770
Elhassanien, M. E. M., Bahnasy, W. S., El-Heneedy, Y. A. E., Kishk, A. M., Tomoum, M. O., Ramadan, K. M., et al. (2021). Olfactory dysfunction in essential tremor versus tremor dominant parkinson disease. Clin. Neurol. Neurosurg. 200:106352. doi: 10.1016/j.clineuro.2020.106352
Eliyan, Y., Wroblewski, K. E., McClintock, M. K., and Pinto, J. M. (2021). Olfactory dysfunction predicts the development of depression in older US adults. Chem. Senses 46:bjaa075. doi: 10.1093/chemse/bjaa075
Espinoza, R. T., and Kellner, C. H. (2022). Electroconvulsive therapy. N. Engl. J. Med. 17, 667–672. doi: 10.1056/NEJMra2034954
Falowski, S. M., Sharan, A., Reyes, B. A., Sikkema, C., Szot, P., and Van Bockstaele, E. J. (2011). An evaluation of neuroplasticity and behavior after deep brain stimulation of the nucleus accumbens in an animal model of depression. Neurosurgery 69, 1281–1290. doi: 10.1227/NEU.0b013e3182237346
Faour, M., Magnan, C., Gurden, H., and Martin, C. (2022). Olfaction in the context of obesity and diabetes: Insights from animal models to humans. Neuropharmacology 206:108923. doi: 10.1016/j.neuropharm.2021.108923
Fernandez-Ruiz, J., Diaz, R., Hall-Haro, C., Vergara, P., Fiorentini, A., Nunez, L., et al. (2003). Olfactory dysfunction in hereditary ataxia and basal ganglia disorders. Neuroreport 14, 1339–1341. doi: 10.1097/00001756-200307180-00011
Frasnelli, J., Laguë-Beauvais, M., LeBlanc, J., Alturki, A. Y., Champoux, M. C., Couturier, C., et al. (2016). Olfactory function in acute traumatic brain injury. Clin. Neurol. Neurosurg. 140, 68–72. doi: 10.1016/j.clineuro.2015.11.013
Fullard, M. E., Morley, J. F., and Duda, J. E. (2017). Olfactory dysfunction as an early biomarker in parkinson’s disease. Neurosci. Bull. 33, 515–525. doi: 10.1007/s12264-017-0170-x
Fuller-Thomson, E. R., and Fuller-Thomson, E. G. (2019). Relationship between poor olfaction and mortality. Ann. Intern. Med. 171, 525–526. doi: 10.7326/L19-0467
Gamain, J., Herr, T., Fleischmann, R., Stenner, A., Vollmer, M., Willert, C., et al. (2021). Smell and taste in idiopathic blepharospasm. J. Neural Transm. 128, 1215–1224. doi: 10.1007/s00702-021-02366-4
GBD 2015 Neurology Collaborators (2016). Global, regional, and national disability-adjusted life-years (DALYs) for 315 diseases and injuries and healthy life expectancy (HALE), 1990–2015: A systematic analysis for the global burden of disease study 2015. Lancet 388, 1603–1658.
Geißler, K., Reimann, H., Gudziol, H., Bitter, T., and Guntinas-Lichius, O. (2014). Olfactory training for patients with olfactory loss after upper respiratory tract infections. Eur. Arch. Otorhinolaryngol. 271, 1557–1562. doi: 10.1007/s00405-013-2747-y
Gellrich, J., Han, P., Manesse, C., Betz, A., Junghanns, A., Raue, C., et al. (2017). Brain volume changes in hyposmic patients before and after olfactory training. Laryngoscope 128, 1531–1536. doi: 10.1002/lary.27045
Geran, R., Uecker, F. C., Prüss, H., Haeusler, K. G., Paul, F., Ruprecht, K., et al. (2019). Olfactory and gustatory dysfunction in patients with autoimmune encephalitis. Front. Neurol. 10:480. doi: 10.3389/fneur.2019.00480
Goldstein, D. S., and Sewell, L. (2009). Olfactory dysfunction in pure autonomic failure: Implications for the pathogenesis of Lewy body diseases. Parkinsonism Relat. Disord. 1, 516–520. doi: 10.1016/j.parkreldis.2008.12.009
Gong, X., Chen, Y., Chang, J., Huang, Y., Cai, M., and Zhang, M. (2018). Environmental enrichment reduces adolescent anxiety- and depression-like behaviors of rats subjected to infant nerve injury. J. Neuroinflammation 15:262. doi: 10.1186/s12974-018-1301-7
Gopinath, B., Sue, C. M., Kifley, A., and Mitchell, P. (2012). The association between olfactory impairment and total mortality in older adults. J. Gerontol. Ser. A 67, 204–209. doi: 10.1093/gerona/glr165
Gregson, R. A., and Smith, D. A. (1981). The clinical assessment of olfaction: Differential diagnoses including kallman’s syndrome. J. Psychosom. Res. 25, 165–174. doi: 10.1016/0022-3999(81)90029-5
Haberly, L. B., and Price, J. L. (1977). The axonal projection patterns of the mitral and tufted cells of the olfactory bulb in the rat. Brain Res. 129, 152–157. doi: 10.1016/0006-8993(77)90978-7
Haehner, A., Hummel, T., and Reichmann, H. (2009). Olfactory dysfunction as a diagnostic marker for parkinson’s disease. Expert Rev. Neurother. 9, 1773–1779. doi: 10.1586/ern.09.115
Haehner, A., Tosch, C., Wolz, M., Klingelhoefer, L., Fauser, M., Storch, A., et al. (2013). Olfactory training in patients with parkinson’s disease. PLoS One 8:e61680. doi: 10.1371/journal.pone.0061680
Han, P., Musch, M., Abolmaali, N., and Hummel, T. (2021). Improved odor identification ability and increased regional gray matter volume after olfactory training in patients with idiopathic olfactory loss. Iperception 12:20416695211005811. doi: 10.1177/20416695211005811
Han, P., Whitcroft, K. L., Fischer, J., Gerber, J., Cuevas, M., Andrews, P., et al. (2017). Olfactory brain gray matter volume reduction in patients with chronic rhinosinusitis. Int. Forum Allergy Rhinol. 7, 551–556. doi: 10.1002/alr.21922
Hannan, A. J. (2014). Environmental enrichment and brain repair: Harnessing the therapeutic effects of cognitive stimulation and physical activity to enhance experience-dependent plasticity. Neuropathol. Appl. Neurobiol. 40, 13–25. doi: 10.1111/nan.12102
Hao, Y., Ge, H., Sun, M., and Gao, Y. (2019). Selecting an appropriate animal model of depression. Int. J. Mol. Sci. 20:4827. doi: 10.3390/ijms20194827
Hasin, D. S., Sarvet, A. L., Meyers, J. L., Saha, T. D., Ruan, W. J., Stohl, M., et al. (2018). Epidemiology of adult DSM-5 major depressive disorder and its specifiers in the United States. JAMA Psychiatry 75, 336–346. doi: 10.1001/jamapsychiatry.2017.4602
Hebb, D. (1947). The effects of early experience on problem solving at maturity. Am. Psychol. 2, 306–307.
Hertzog, C., Kramer, A. F., Wilson, R. S., and Lindenberger, U. (2008). Enrichment effects on adult cognitive development: Can the functional capacity of older adults be preserved and enhanced? Psychol. Sci. Public Interest 9, 1–65. doi: 10.1111/j.1539-6053.2009.01034.x
Huang, H., Wang, Q., Guan, X., Zhang, X., Zhang, Y., Cao, J., et al. (2021). Effects of enriched environment on depression and anxiety-like behavior induced by early life stress: A comparison between different periods. Behav. Brain Res. 411:113389. doi: 10.1016/j.bbr.2021.113389
Hummel, T., Rissom, K., Reden, J., Hähner, A., Weidenbecher, M., and Hüttenbrink, K.-B. (2009). Effects of olfactory training in patients with olfactory loss. Laryngoscope 119, 496–499. doi: 10.1002/lary.20101
Iannaccone, A., Mykytyn, K., Persico, A. M., Searby, C. C., Baldi, A., Jablonski, M. M., et al. (2005). Clinical evidence of decreased olfaction in bardet-biedl syndrome caused by a deletion in the BBS4 gene. Am. J. Med. Genet. A 132, 343–346. doi: 10.1002/ajmg.a.30512
Ilin, Y., and Richter-Levin, G. (2009). Enriched environment experience overcomes learning deficits and depressive-like behavior induced by juvenile stress. PLoS One 4:e4329. doi: 10.1371/journal.pone.0004329
Ioannidis, J. P. (2008). Effectiveness of antidepressants: An evidence myth constructed from a thousand randomized trials? Philos. Ethics Humanit. Med. 3:14. doi: 10.1186/1747-5341-3-14
Iosifescu, D. V., Jones, A., O’Gorman, C., Streicher, C., Feliz, S., Fava, M., et al. (2022). Efficacy and safety of AXS-05 (Dextromethorphan-bupropion) in patients with major depressive disorder: A phase 3 randomized clinical trial (GEMINI). J. Clin. Psychiatry 83:21m14345. doi: 10.4088/JCP.21m14345
Iranzo, A., Marrero-González, P., Serradell, M., Gaig, C., Santamaria, J., and Vilaseca, I. (2021). Significance of hyposmia in isolated REM sleep behavior disorder. J. Neurol. 268, 963–966. doi: 10.1007/s00415-020-10229-3
Jarosik, J., Legutko, B., Unsicker, K., and von Bohlen Und Halbach, O. (2007). Antidepressant-mediated reversal of abnormal behavior and neurodegeneration in mice following olfactory bulbectomy. Exp. Neurol. 204, 20–28. doi: 10.1016/j.expneurol.2006.09.008
Jeannotte, A. M., McCarthy, J. G., Redei, E. E., and Sidhu, A. (2009). Desipramine modulation of alpha-, gamma-synuclein, and the norepinephrine transporter in an animal model of depression. Neuropsychopharmacology 34, 987–998. doi: 10.1038/npp.2008.146
Jha, S., Dong, B., and Sakata, K. (2011). Enriched environment treatment reverses depression-like behavior and restores reduced hippocampal neurogenesis and protein levels of brain-derived neurotrophic factor in mice lacking its expression through promoter IV. Transl. Psychiatry 1:e40. doi: 10.1038/tp.2011.33
Juncos, J. L., Lazarus, J. T., Rohr, J., Allen, E. G., Shubeck, L., Hamilton, D., et al. (2012). Olfactory dysfunction in fragile X tremor ataxia syndrome. Mov. Disord. 27, 1556–1559. doi: 10.1002/mds.25043
Kamath, V., and Leff, B. (2019). Mortality risk in older adults: What the nose knows. Ann. Intern. Med. 170, 722–723. doi: 10.7326/M19-1013
Kazour, F., Richa, S., Char, C. A., Atanasova, B., and El-Hage, W. (2020). Olfactory memory in depression: State and trait differences between bipolar and unipolar disorders. Brain Sci. 10:189. doi: 10.3390/brainsci10030189
Kelly, J. P., Wrynn, A. S., and Leonard, B. E. (1997). The olfactory bulbectomized rat as a model of depression: An update. Pharmacol. Ther. 74, 299–316. doi: 10.1016/S0163-7258(97)00004-1
Khurshid, K., Crow, A. J. D., Rupert, P. E., Minniti, N. L., Carswell, M. A., Mechanic-Hamilton, D. J., et al. (2019). A quantitative meta-analysis of olfactory dysfunction in epilepsy. Neuropsychol. Rev. 29, 328–337. doi: 10.1007/s11065-019-09406-7
Kim, B. Y., and Bae, J. H. (2022). Olfactory function and depression: A meta-analysis. Ear Nose Throat J. 31:1455613211056553. [Epub ahead of print]. doi: 10.1177/01455613211056553
Kimura, L. F., Novaes, L. S., Picolo, G., Munhoz, C. D., Cheung, C. W., and Camarini, R. (2022). How environmental enrichment balances out neuroinflammation in chronic pain and comorbid depression and anxiety disorders. Br. J. Pharmacol. 179, 1640–1660. doi: 10.1111/bph.15584
Kinnaird, E., Stewart, C., and Tchanturia, K. (2020). The relationship of autistic traits to taste and olfactory processing in anorexia nervosa. Mol. Autism 11:25. doi: 10.1186/s13229-020-00331-8
Kohli, P., Soler, Z. M., Nguyen, S. A., Muus, J. S., and Schlosser, R. J. (2016). The association between olfaction and depression: A systematic review. Chem. Senses 41, 479–486. doi: 10.1093/chemse/bjw061
Kollndorfer, K., Jakab, A., Mueller, C., Trattnig, S., and Schopf, V. (2015). Effects of chronic peripheral olfactory loss on functional brain networks. Neuroscience 310, 589–599. doi: 10.1016/j.neuroscience.2015.09.045
Konstantinidis, I., Tsakiropoulou, E., Bekiaridou, P., Kazantzidou, C., and Constantinidis, J. (2013). Use of olfactory training in post-traumatic and postinfectious olfactory dysfunction. Laryngoscope 123, E85–E90. doi: 10.1002/lary.24390
Kopala, L. C., Clark, C., and Hurwitz, T. (1993). Olfactory deficits in neuroleptic naive patients with schizophrenia. Schizophr. Res. 8, 245–250. doi: 10.1016/0920-9964(93)90022-B
Kopala, L., and Clark, C. (1990). Implications of olfactory agnosia for understanding sex differences in schizophrenia. Schizophr. Bull. 16, 255–261. doi: 10.1093/schbul/16.2.255
Kronenbuerger, M., Belenghi, P., Ilgner, J., Freiherr, J., Hummel, T., and Neuner, I. (2018). Olfactory functioning in adults with tourette syndrome. PLoS One 13:e0197598. doi: 10.1371/journal.pone.0197598
Kruijshaar, M. E., Barendregt, J., Vos, T., de Graaf, R., Spijker, J., and Andrews, G. (2005). Lifetime prevalence estimates of major depression: An indirect estimation method and a quantification of recall bias. Eur. J. Epidemiol. 20, 103–111. doi: 10.1007/s10654-004-1009-0
Lazarini, F., Lannuzel, A., Cabié, A., Michel, V., Madec, Y., Chaumont, H., et al. (2022). Olfactory outcomes in zika virus-associated guillain-barré syndrome. Eur. J. Neurol. [Epub ahead of print]. doi: 10.1111/ene.15444
Lemogne, C., Smadja, J., El-H, Z., Soudry, Y., Robin, M., Berthoz, S., et al. (2015). Congenital anosmia and emotion recognition: A case-control study. Neuropsychologia 72, 52–58. doi: 10.1016/j.neuropsychologia.2015.04.028
Leon-Sarmiento, F. E., Bayona, E. A., Bayona-Prieto, J., Osman, A., and Doty, R. L. (2012). Profound olfactory dysfunction in myasthenia gravis. PLoS One 7:e45544. doi: 10.1371/journal.pone.0045544
Leon-Sarmiento, F. E., Bayona, E. A., Rizzo-Sierra, C. V., Garavito, A., Campos, M. F., and Doty, R. (2014). Olfactory dysfunction in chagas’ disease. Neurology 82(Suppl. 10). [Epub ahead of print].
Li, Z., Ruan, M., Chen, J., and Fang, Y. (2021). Major depressive disorder: Advances in neuroscience research and translational applications. Neurosci. Bull. 37, 863–880. doi: 10.1007/s12264-021-00638-3
Licher, S., Darweesh, S. K. L., Wolters, F. J., Fani, L., Heshmatollah, A., Mutlu, U., et al. (2019). Lifetime risk of common neurological diseases in the elderly population. J. Neurol. Neurosurg. Psychiatry 90, 148–156. doi: 10.1136/jnnp-2018-318650
Liu, B., Luo, Z., and Chen, H. (2019). Relationship between poor olfaction and mortality. Ann. Intern. Med. 171:526. doi: 10.7326/L19-0468
Liu, D. T., Prem, B., Sharma, G., Kaiser, J., Besser, G., and Mueller, C. A. (2022). Depression symptoms and olfactory-related quality of life. Laryngoscope [Epub ahead of print]. doi: 10.1002/lary.30122
Liu, Y. H., Huang, Z., Vaidya, A., Li, J., Curhan, G. C., Wu, S., et al. (2018). A longitudinal study of altered taste and smell perception and change in blood pressure. Nutr. Metab. Cardiovasc. Dis. 28, 877–883. doi: 10.1016/j.numecd.2018.05.002
Lopez, A. D., and Murray, C. C. (1998). The global burden of disease, 1990-2020. Nat. Med. 4, 1241–1243. doi: 10.1038/3218
Luzzi, S., Snowden, J. S., Neary, D., Coccia, M., Provinciali, L., and Lambon Ralph, M. A. (2007). Distinct patterns of olfactory impairment in alzheimer’s disease, semantic dementia, frontotemporal dementia, and corticobasal degeneration. Neuropsychologia 45, 1823–1831. doi: 10.1016/j.neuropsychologia.2006.12.008
Malhi, G. S., Bell, E., Singh, A. B., Bassett, D., Berk, M., Boyce, P., et al. (2020). The 2020 royal Australian and New Zealand college of psychiatrists clinical practice guidelines for mood disorders: Major depression summary. Bipolar Disord. 22, 788–804. doi: 10.1111/bdi.13035
Marek, M., Linnepe, S., Klein, C., Hummel, T., and Paus, S. (2018). High prevalence of olfactory dysfunction in cervical dystonia. Parkinsonism Relat. Disord. 53, 33–36. doi: 10.1016/j.parkreldis.2018.04.028
Masaoka, Y., Kawamura, M., Takeda, A., Kobayakawa, M., Kuroda, T., Kasai, H., et al. (2011). Impairment of odor recognition and odor-induced emotions in type 1 myotonic dystrophy. Neurosci. Lett. 503, 163–166. doi: 10.1016/j.neulet.2011.08.006
McKinney, W. T., and Bunney, W. E. (1969). Animal model of depression. I. Review of evidence: Implications for research. Arch. Gen. Psychiatry 21, 240–248. doi: 10.1001/archpsyc.1969.01740200112015
McNeill, A., Duran, R., Proukakis, C., Bras, J., Hughes, D., Mehta, A., et al. (2012). Hyposmia and cognitive impairment in gaucher disease patients and carriers. Mov. Disord. 27, 526–532. doi: 10.1002/mds.24945
Mishra, S., Karan, K., Nag, D., and Sengupta, P. (2016). Adult onset niemann–pick type C disease: Two different presentations. Neurol. India 64, 1044–1047. doi: 10.4103/0028-3886.190242
Montine, T. J., Cholerton, B. A., Corrada, M. M., Edland, S. D., Flanagan, M. E., Hemmy, L. S., et al. (2019). Concepts for brain aging: Resistance, resilience, reserve, and compensation. Alzheimers Res. Ther. 11:22. doi: 10.1186/s13195-019-0479-y
Moradi-Kor, N., Dadkhah, M., Ghanbari, A., Rashidipour, H., Bandegi, A. R., Barati, M., et al. (2020). Protective effects of spirulina platensis, voluntary exercise and environmental interventions against adolescent stress-induced anxiety and depressive-like symptoms, oxidative stress and alterations of BDNF and 5HT-3 receptors of the prefrontal cortex in female rats. Neuropsychiatr. Dis. Treat. 16, 1777–1794. doi: 10.2147/NDT.S247599
Mutz, J., Vipulananthan, V., Carter, B., Hurlemann, R., Fu, C. H. Y., and Young, A. H. (2019). Comparative efficacy and acceptability of non-surgical brain stimulation for the acute treatment of major depressive episodes in adults: Systematic review and network meta-analysis. BMJ 364:l1079. doi: 10.1136/bmj.l1079
Nakashima, T., Katayama, N., Sugiura, S., Teranishi, M., Suzuki, H., Hirabayashi, M., et al. (2019). Olfactory function in persons with cerebral palsy. J. Policy Pract. Intellect. Disabil. 16, 217–222. doi: 10.1111/jppi.12284
Negoias, S., Hummel, T., Symmank, A., Schellong, J., Joraschky, P., and Croy, I. (2016). Olfactory bulb volume predicts therapeutic outcome in major depression disorder. Brain Imaging Behav. 10, 367–372. doi: 10.1007/s11682-015-9400-x
Nilsson, J., and Lövdén, M. (2018). Naming is not explaining: Future directions for the “cognitive reserve” and “brain maintenance” theories. Alzheimers Res. Ther. 10:34. doi: 10.1186/s13195-018-0365-z
Nithianantharajah, J., and Hannan, A. J. (2009). The neurobiology of brain and cognitive reserve: Mental and physical activity as modulators of brain disorders. Prog. Neurobiol. 89, 369–382. doi: 10.1016/j.pneurobio.2009.10.001
Noto, T., Zhou, G., Yang, Q., Lane, G., and Zelano, C. (2021). Human primary olfactory amygdala subregions form distinct functional networks, suggesting distinct olfactory functions. Front. Syst. Neurosci. 15:752320. doi: 10.3389/fnsys.2021.752320
Pabel, L. D., Murr, J., Weidner, K., Hummel, T., and Croy, I. (2020). Null effect of olfactory training with patients suffering from depressive disorders – an exploratory randomized controlled clinical trial. Front. Psychiatry 11:593. doi: 10.3389/fpsyt.2020.00593
Pang, N. Y., Song, H. J. J. M. D., Tan, B. K. J., Tan, J. X., Chen, A. S. R., See, A., et al. (2022). Association of olfactory impairment with all-cause mortality: A systematic review and meta-analysis. JAMA Otolaryngol. Head Neck Surg. 148, 436–445. doi: 10.1001/jamaoto.2022.0263
Patel, Z. M., Wise, S. K., and Del Gaudio, J. M. (2017). Randomized controlled trial demonstrating cost-effective method of olfactory training in clinical practice: Essential oils at uncontrolled concentration. Laryngoscope Invest. Otolaryngol. 2, 53–56. doi: 10.1002/lio2.62
Perera, T., George, M. S., Grammer, G., Janicak, P. G., Pascual-Leone, A., and Wirecki, T. S. (2016). The clinical TMS society consensus review and treatment recommendations for TMS therapy for major depressive disorder. Brain Stimul. 9, 336–346. doi: 10.1016/j.brs.2016.03.010
Peters, J. M., Hummel, T., Kratzsch, T., Lötsch, J., Skarke, C., and Frölich, L. (2003). Olfactory function in mild cognitive impairment and alzheimer’s disease: An investigation using psychophysical and electrophysiological techniques. Am. J. Psychiatry 160, 1995–2002. doi: 10.1176/appi.ajp.160.11.1995
Pinto, J. M. (2021). The specter of olfactory impairment: Lessons about mortality in older US adults. JAMA Otolaryngol. Head Neck Surg. 147, 56–57. doi: 10.1001/jamaoto.2020.3745
Pinto, J. M., Wroblewski, K. E., Kern, D. W., Schumm, L. P., and McClintock, M. K. (2014). Olfactory dysfunction predicts 5-year mortality in older adults. PLoS One 9:e107541. doi: 10.1371/journal.pone.0107541
Qazi, J. J., Wilson, J. H., Payne, S. C., and Mattos, J. L. (2020). Association between smell, taste, and depression in nationally representative sample of older adults in the United States. Am. J. Rhinol. Allergy 34, 369–374. doi: 10.1177/1945892419897217
Ramic, E., Prasko, S., Gavran, L., and Spahic, E. (2020). Assessment of the antidepressant side effects occurrence in patients treated in primary care. Mater. Sociomed. 32, 131–134. doi: 10.5455/msm.2020.32.131-134
Ramírez-Rodríguez, G. B., Vega-Rivera, N. M., Meneses-San Juan, D., Ortiz-López, L., Estrada-Camarena, E. M., and Flores-Ramos, M. (2021). Short daily exposure to environmental enrichment, fluoxetine, or their combination reverses deterioration of the coat and anhedonia behaviors with differential effects on hippocampal neurogenesis in chronically stressed mice. Int. J. Mol. Sci. 22:10976. doi: 10.3390/ijms222010976
Reuber, M., Al-Din, As, Baborie, A., and Chakrabarty, A. (2001). New variant creutzfeldt-jakob disease presenting with loss of taste and smell. J. Neurol. Neurosurg. Psychiatry 71, 412–413. doi: 10.1136/jnnp.71.3.412
Réus, G. Z., Abitante, M. S., Manosso, L. M., de Moura, A. B., Borba, L. A., Botelho, M. E. M., et al. (2021). Environmental enrichment rescues oxidative stress and behavioral impairments induced by maternal care deprivation: Sex- and developmental-dependent differences. Mol. Neurobiol. [Epub ahead of print]. doi: 10.1007/s12035-021-02588-3
Ribeiro, J. C., Oliveiros, B., Pereira, P., António, N., Hummel, T., Paiva, A., et al. (2016). Accelerated age-related olfactory decline among type 1 usher patients. Sci. Rep. 6:28309. doi: 10.1038/srep28309
Richter, S. H., Zeuch, B., Riva, M. A., Gass, P., and Vollmayr, B. (2013). Environmental enrichment ameliorates depressive-like symptoms in young rats bred for learned helplessness. Behav. Brain Res. 252, 287–292. doi: 10.1016/j.bbr.2013.06.021
Rizvi, S., and Khan, A. M. (2019). Use of transcranial magnetic stimulation for depression. Cureus 11:e4736. doi: 10.7759/cureus.4736
Rochet, M., El-Hage, W., Richa, S., Kazour, F., and Atanasova, B. (2018). Depression, olfaction, and quality of life: A mutual relationship. Brain Sci. 8:80. doi: 10.3390/brainsci8050080
Roessner, V., Bleich, S., Banaschewski, T., and Rothenberger, A. (2005). Olfactory deficits in anorexia nervosa. Eur. Arch. Psychiatry Clin. Neurosci. 255, 6–9. doi: 10.1007/s00406-004-0525-y
Roh, D., Lee, D. H., Kim, S. W., Kim, S. W., Kim, B. G., Kim, D. H., et al. (2021). The association between olfactory dysfunction and cardiovascular disease and its risk factors in middle-aged and older adults. Sci. Rep. 11:1248. doi: 10.1038/s41598-020-80943-5
Rosenzweig, M. R., Krech, D., Bennett, E. L., and Diamond, M. C. (1962). Effects of environmental complexity and training on brain chemistry and anatomy: A replication and extension. J. Comp. Physiol. Psychol. 55, 429–437. doi: 10.1037/h0041137
Rottstaedt, F., Weidner, K., Strauß, T., Schellong, J., Kitzler, H., Wolff-Stephan, S., et al. (2018). Size matters – The olfactory bulb as a marker for depression. J. Affect. Disord. 229, 193–198. doi: 10.1016/j.jad.2017.12.047
Rupp, C. I., Fleischhacker, W. W., Hausmann, A., Mair, D., Hinterhuber, H., and Kurz, M. (2004). Olfactory functioning in patients with alcohol dependence: Impairments in odor judgements. Alcohol 39, 514–519. doi: 10.1093/alcalc/agh100
Rush, A. J., Trivedi, M. H., Wisniewski, S. R., Nierenberg, A. A., Stewart, J. W., Warden, D., et al. (2006). Acute and longer-term outcomes in depressed outpatients requiring one or several treatment steps: A STAR/D report. Am. J. Psychiatry 163, 1905–1917. doi: 10.1176/ajp.2006.163.11.1905
Rusznák, Z., Sengul, G., Paxinos, G., Kim, W. S., and Fu, Y. (2018). Odor enrichment increases hippocampal neuron numbers in mouse. Exp. Neurobiol. 27, 94–102. doi: 10.5607/en.2018.27.2.94
Sabiniewicz, A., Hoffmann, L., Haehner, A., and Hummel, T. (2022). Symptoms of depression change with olfactory function. Sci. Rep. 12:5656. doi: 10.1038/s41598-022-09650-7
Samancı, B., Şahin, E., Şen, C., Samancı, Y., Sezgin, M., Emekli, S., et al. (2021). Olfactory dysfunction in patients with cluster headache. Eur. Arch. Otorhinolaryngol. 278, 4361–4365. doi: 10.1007/s00405-021-06738-0
Sanna, F., Loy, F., Piras, R., Moat, A., and Masala, C. (2021). Age-related cognitive decline and the olfactory identification deficit are associated to increased level of depression. Front. Neurosci. 15:599593. doi: 10.3389/fnins.2021.599593
Schaie, W. K., Willis, S. L., and Caskie, G. I. L. (2004). The seattle longitudinal study: Relationship between personality and cognition aging. Neuropsychol. Cog. 11, 304–324. doi: 10.1080/13825580490511134
Schoenfeld, N., Agmon-Levin, N., Flitman-Katzevman, I., Paran, D., Katz, B. S., Kivity, S., et al. (2009). The sense of smell in systemic lupus erythematosus. Arthritis Rheum. 60, 1484–1487. doi: 10.1002/art.24491
Schubert, C. R., Cruickshanks, K. J., Fischer, M. E., Klein, B. E., Klein, R., and Pinto, A. A. (2015). Inflammatory and vascular markers and olfactory impairment in older adults. Age Ageing 44, 878–882. doi: 10.1093/ageing/afv075
Schubert, C. R., Fischer, M. E., Pinto, A. A., Klein, B. E. K., Klein, R., Tweed, T. S., et al. (2017). Sensory impairments and risk of mortality in older adults. J. Gerontol. A Biol. Sci. Med. Sci. 72, 710–715.
Segura, B., Baggio, H. C., Solanaa, E., Palacios, E. M., Vendrell, P., Bargalló, N., et al. (2013). Neuroanatomical correlates of olfactory loss in normal aged subjects. Behav. Brain Res. 246, 148–153. doi: 10.1016/j.bbr.2013.02.025
Seong, H. H., Park, J. M., and Kim, Y. J. (2018). Antidepressive effects of environmental enrichment in chronic stress-induced depression in rats. Biol. Res. Nurs. 20, 40–48. doi: 10.1177/1099800417730400
Shill, H. A., Zhang, N., Driver-Dunckley, E., Mehta, S., Adler, C. H., and Beach, T. G. (2021). Olfaction in neuropathologically defined progressive supranuclear palsy. Mov. Disord. 36, 1700–1704. doi: 10.1002/mds.28568
Sobin, C., Kiley-Brabeck, K., Dale, K., Monk, S. H., Khuri, J., and Karayiorgou, M. (2006). Olfactory disorder in children with 22q11 deletion syndrome. Pediatrics 118, e697–e703. doi: 10.1542/peds.2005-3114
Sohrabi, H. R., Bates, K. A., Weinborn, M. G., Johnston, A. N., Bahramian, A., Taddei, K., et al. (2012). Olfactory discrimination predicts cognitive decline among community-dwelling older adults. Transl. Psychiatry 2:e118. doi: 10.1038/tp.2012.43
Song, J., and Kim, Y. K. (2021). Animal models for the study of depressive disorder. CNS Neurosci. Ther. 27, 633–642. doi: 10.1111/cns.13622
Sparling, J. E., Barbeau, K., Boileau, K., and Konkle, A. T. M. (2020). Environmental enrichment and its influence on rodent offspring and maternal behaviours, a scoping style review of indices of depression and anxiety. Pharmacol. Biochem. Behav. 197:172997. doi: 10.1016/j.pbb.2020.172997
Stahl, S. M., Pradko, J. F., Haight, B. R., Modell, J. G., Rockett, C. B., and Learned-Coughlin, S. (2004). A review of the neuropharmacology of bupropion, a dual norepinephrine and dopamine reuptake inhibitor. Prim. Care Companion J. Clin. Psychiatry 6, 159–166. doi: 10.4088/PCC.v06n0403
Stern, Y., Arenaza-Urquijo, E. M., Bartrés-Faz, D., Belleville, S., Cantilon, M., Chetelat, G., et al. (2020). Whitepaper: Defining and investigating cognitive reserve, brain reserve, and brain maintenance. Alzheimers Dement. 16, 1305–1311. doi: 10.1016/j.jalz.2018.07.219
Stern, Y., Barnes, C. A., Grady, C., Jones, R. N., and Raz, N. (2019). Brain reserve, cognitive reserve, compensation, and maintenance: Operationalization, validity, and mechanisms of cognitive resilience. Neurobiol. Aging 83, 124–129. doi: 10.1016/j.neurobiolaging.2019.03.022
Suh, K. D., Kim, S. M., Han, D. H., Min, H. J., and Kim, K. S. (2020). Olfactory function test for early diagnosis of vascular dementia. Korean J. Fam. Med. 41, 202–204. doi: 10.4082/kjfm.18.0202
Taalman, H., Wallace, C., and Milev, R. (2017). Olfactory functioning and depression: A systematic review. Front. Psychiatry 8:190. doi: 10.3389/fpsyt.2017.00190
Tafet, G. E., and Nemeroff, C. B. (2016). The links between stress and depression: Psychoneuroendocrinological, genetic, and environmental interactions. J. Neuropsychiatry Clin. Neurosci. 28, 77–88. doi: 10.1176/appi.neuropsych.15030053
Taheri Zadeh, Z., Rahmani, S., Alidadi, F., Joushi, S., and Esmaeilpour, K. (2021). Depression, anxiety and other cognitive consequences of social isolation: Drug and non-drug treatments. Int. J. Clin. Pract. 75:e14949. doi: 10.1111/ijcp.14949
Tizabi, Y., Bhatti, B. H., Manaye, K. F., Das, J. R., and Akinfiresoye, L. (2012). Antidepressant-like effects of low ketamine dose is associated with increased hippocampal AMPA/NMDA receptor density ratio in female wistar-kyoto rats. Neuroscience 213, 72–80. doi: 10.1016/j.neuroscience.2012.03.052
Vaira, L. A., Hopkins, C., Petrocelli, M., Lechien, J. R., Chiesa-Estomba, C. M., Salzano, G., et al. (2020). Smell and taste recovery in coronavirus disease 2019 patients: A 60-day objective and prospective study. J. Laryngol. Otol. 134, 703–709. doi: 10.1017/S0022215120001826
Vasterling, J. J., Brailey, K., and Sutker, P. B. (2000). Olfactory identification in combat-related posttraumatic stress disorder. J. Trauma. Stress 13, 241–253. doi: 10.1023/A:1007754611030
Veena, J., Srikumar, B. N., Raju, T. R., and Shankaranarayana Rao, B. S. (2009). Exposure to enriched environment restores the survival and differentiation of newborn cells in the hippocampus and ameliorates depressive symptoms in chronically stressed rats. Neurosci. Lett. 455, 178–182. doi: 10.1016/j.neulet.2009.03.059
Veyrac, A., Sacquet, J., Nguyen, V., Marien, M., Jourdan, F., and Didier, A. (2009). Novelty determines the effects of olfactory enrichment on memory and neurogenesis through noradrenergic mechanisms. Neuropsychopharmacology 34, 786–795. doi: 10.1038/npp.2008.191
Viguera, C., Wang, J., Mosmiller, E., Cerezo, A., and Maragakis, N. J. (2018). Olfactory dysfunction in amyotrophic lateral sclerosis. Ann. Clin. Transl. Neurol. 5, 976–981. doi: 10.1002/acn3.594
Waldton, S. (1974). Clinical observations of impaired cranial nerve function in senile dementia. Acta Psychiatr. Scand. 50, 539–547. doi: 10.1111/j.1600-0447.1974.tb09714.x
Walker, I. M., Fullard, M. E., Morley, J. F., and Duda, J. E. (2021). Olfaction as an early marker of parkinson’s disease and alzheimer’s disease. Handb. Clin. Neurol. 182, 317–329. doi: 10.1016/B978-0-12-819973-2.00030-7
Wang, F., Wu, X., Gao, J., Li, Y., Zhu, Y., and Fang, Y. (2020). The relationship of olfactory function and clinical traits in major depressive disorder. Behav. Brain Res. 386:112594. doi: 10.1016/j.bbr.2020.112594
Wang, X. M., Zhang, G. F., Jia, M., Xie, Z. M., Yang, J. J., Shen, J. C., et al. (2019). Environmental enrichment improves pain sensitivity, depression-like phenotype, and memory deficit in mice with neuropathic pain: Role of NPAS4. Psychopharmacology 236, 1999–2014. doi: 10.1007/s00213-019-5187-6
Wegener, B. A., Croy, I., Hähner, A., and Hummel, T. (2018). Olfactory training with older people. Int. J. Geriatr. Psychiatry 33, 212–220. doi: 10.1002/gps.4725
Wehling, E., Naess, H., Wollschlaeger, D., Hofstad, H., Bramerson, A., Bende, M., et al. (2015). Olfactory dysfunction in chronic stroke patients. BMC Neurol. 15:199. doi: 10.1186/s12883-015-0463-5
Whiting, A. C., Marmura, M. J., Hegarty, S. E., and Keith, S. W. (2015). Olfactory acuity in chronic migraine: A cross-sectional study. Headache 55, 71–75. doi: 10.1111/head.12462
Wilson, R. S., Yu, L., and Bennett, D. A. (2011). Odor identification and mortality in old age. Chem. Senses 36, 63–67. doi: 10.1093/chemse/bjq098
Witoonpanich, P., Cash, D. M., Shakespeare, T. J., Yong, K. X., Nicholas, J. M., Omar, R., et al. (2013). Olfactory impairment in posterior cortical atrophy. J. Neurol. Neurosurg. Psychiatry 84, 588–590. doi: 10.1136/jnnp-2012-304497
Woo, C. C., and Leon, M. (2013). Environmental enrichment as an effective treatment for autism: A randomized controlled trial. Behav. Neurosci. 127, 487–497. doi: 10.1037/a0033010
Woo, C. C., Donnelly, J. H., Steinberg-Epstein, R., and Leon, M. (2015). Environmental enrichment as a therapy for autism: A clinical trial replication and extension. Behav. Neurosci. 129, 412–422. doi: 10.1037/bne0000068
Xiao, Z., Zhao, Q., Liang, X., Wu, W., Cao, Y., and Ding, D. (2021). Poor odor identification predicts mortality risk in older adults without neurodegenerative diseases: The Shanghai aging study. J. Am. Med. Dir. Assoc. 22, 2218–2219. doi: 10.1016/j.jamda.2021.05.026
Yao, L., Pinto, J. M., Yi, X., Li, L., Peng, P., and Wei, Y. (2014). Gray matter volume reduction of olfactory cortices in patients with idiopathic olfactory loss. Chem. Senses 39, 755–760. doi: 10.1093/chemse/bju047
Yoo, H. S., Jeon, S., Chung, S. J., Yun, M., Lee, P. H., Sohn, Y. H., et al. (2018). Olfactory dysfunction in alzheimer’s disease- and lewy body-related cognitive impairment. Alzheimers Dement. 14, 1243–1252. doi: 10.1016/j.jalz.2018.05.010
Zamponi, H. P., Juarez-Aguaysol, L., Kukoc, G., Dominguez, M. E., Pini, B., Padilla, E. G., et al. (2021). Olfactory dysfunction and chronic cognitive impairment following SARS-CoV-2 infection in a sample of older adults from the Andes mountains of Argentina. Alzheimers Dement. 17:e057897. doi: 10.1002/alz.057897
Zhang, C., Han, Y., Liu, X., Tan, H., Dong, Y., Zhang, Y., et al. (2022). Odor enrichment attenuates the anesthesia/surgery-induced cognitive impairment. Ann. Surg. [Epub ahead of print]. doi: 10.1097/SLA.0000000000005599
Zhang, Z., Zhang, B., Wang, X., Zhang, X., Yang, Q. X., Qing, Z., et al. (2019). Olfactory dysfunction mediates adiposity in cognitive impairment of type 2 diabetes: Insights from clinical and functional neuroimaging studies. Diabetes Care 42, 1274–1283. doi: 10.2337/dc18-2584
Zhou, G., Lane, G., Cooper, S. L., Kahnt, T., and Zelano, C. (2019). Characterizing functional pathways of the human olfactory system. eLife 8:e47177. doi: 10.7554/eLife.47177
Keywords: olfaction, depression, brain reserve, neurological disorders, brain stimulation
Citation: Leon M and Woo CC (2022) Olfactory loss is a predisposing factor for depression, while olfactory enrichment is an effective treatment for depression. Front. Neurosci. 16:1013363. doi: 10.3389/fnins.2022.1013363
Received: 06 August 2022; Accepted: 08 September 2022;
Published: 28 September 2022.
Edited by:
Fabrizio Sanna, University of Cagliari, ItalyReviewed by:
Bertold Renner, TU Dresden, GermanyZoltan Rusznak, Australian Catholic University, Australia
Copyright © 2022 Leon and Woo. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Michael Leon, bWxlb25AdWNpLmVkdQ==
†These authors have contributed equally to this work
 Michael Leon
Michael Leon Cynthia C. Woo1†
Cynthia C. Woo1†