
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Neurosci., 12 October 2021
Sec. Gut-Brain Axis
Volume 15 - 2021 | https://doi.org/10.3389/fnins.2021.753915
This article is part of the Research TopicGut biodiversity and its influence in brain healthView all 6 articles
Brain physiological functions or pathological dysfunctions do surely depend on the activity of both neuronal and non-neuronal populations. Nevertheless, over the last decades, compelling and fast accumulating evidence showed that the brain is not alone. Indeed, the so-called “gut brain,” composed of the microbial populations living in the gut, forms a symbiotic superorganism weighing as the human brain and strongly communicating with the latter via the gut–brain axis. The gut brain does exert a control on brain (dys)functions and it will eventually become a promising valuable therapeutic target for a number of brain pathologies. In the present review, we will first describe the role of gut microbiota in normal brain physiology from neurodevelopment till adulthood, and thereafter we will discuss evidence from the literature showing how gut microbiota alterations are a signature in a number of brain pathologies ranging from neurodevelopmental to neurodegenerative disorders, and how pre/probiotic supplement interventions aimed to correct the altered dysbiosis in pathological conditions may represent a valuable future therapeutic strategy.
The brain is the organ generating and controlling any behavior of an individual. It is composed of billions of different neuronal and non-neuronal cell populations interconnected by extremely complex structural networks. The activity of these networks generates a plethora of lower and higher brain functions, ranging from simple motor reflexes in invertebrates and complex motor actions in non-human primates to sensory perceptions and higher cognitive functions in humans (for example, attention and decision-making). Importantly, if a normal (healthy) structural and functional brain is surely the means to achieve normal brain functions in normal physiological conditions, it is also clearly true that defects causing structural and/or functional alterations in the brain are responsible for brain dysfunctions in pathological conditions. Given the extreme complexity of the human brain, it does not come as a surprise that most of the human pathologies described so far are related to the brain.
Notably, the brain is not alone. Indeed, from the first pioneering studies on the microbial ecology back to more than three decades ago (Savage, 1977), the following extensive scientific research in the domain of the so-called microbiome (or biome) shed light on and revealed the essential contribution of microbial communities populating and living within the human body into the general physiological functions and health of an individual (Liang et al., 2018). Over 98% of our body’s microbes are located within the gastrointestinal (GI) tract. The microorganisms specifically living and residing in the gut, referred to as gut microbiota, represent a dynamic population of microbes forming a symbiotic superorganism (approximately 1014 cells) containing 100 times the number of genes of the human genome and weighing approximately the same as the human brain. The gut microbiota communicates with the brain via the gut–brain axis. The gut brain has recently become a major player both in regulating normal brain functions in normal physiological conditions and in contributing in being a risk factor/causative for neuropathological conditions (Ma et al., 2019). In this review, after a general outline of the microbiome profiles in the brain in physiological (health) and pathological conditions (disease) spanning the whole ontogeny (from development to adulthood), we will propose how pre/probiotics supplementation to people with neurological disorders will eventually provide in the near future a new therapeutic tool aiming to either decrease the probability that a healthy brain will shift into pathology or to slow down an existing neuropathology (see Figure 1 for an overview).
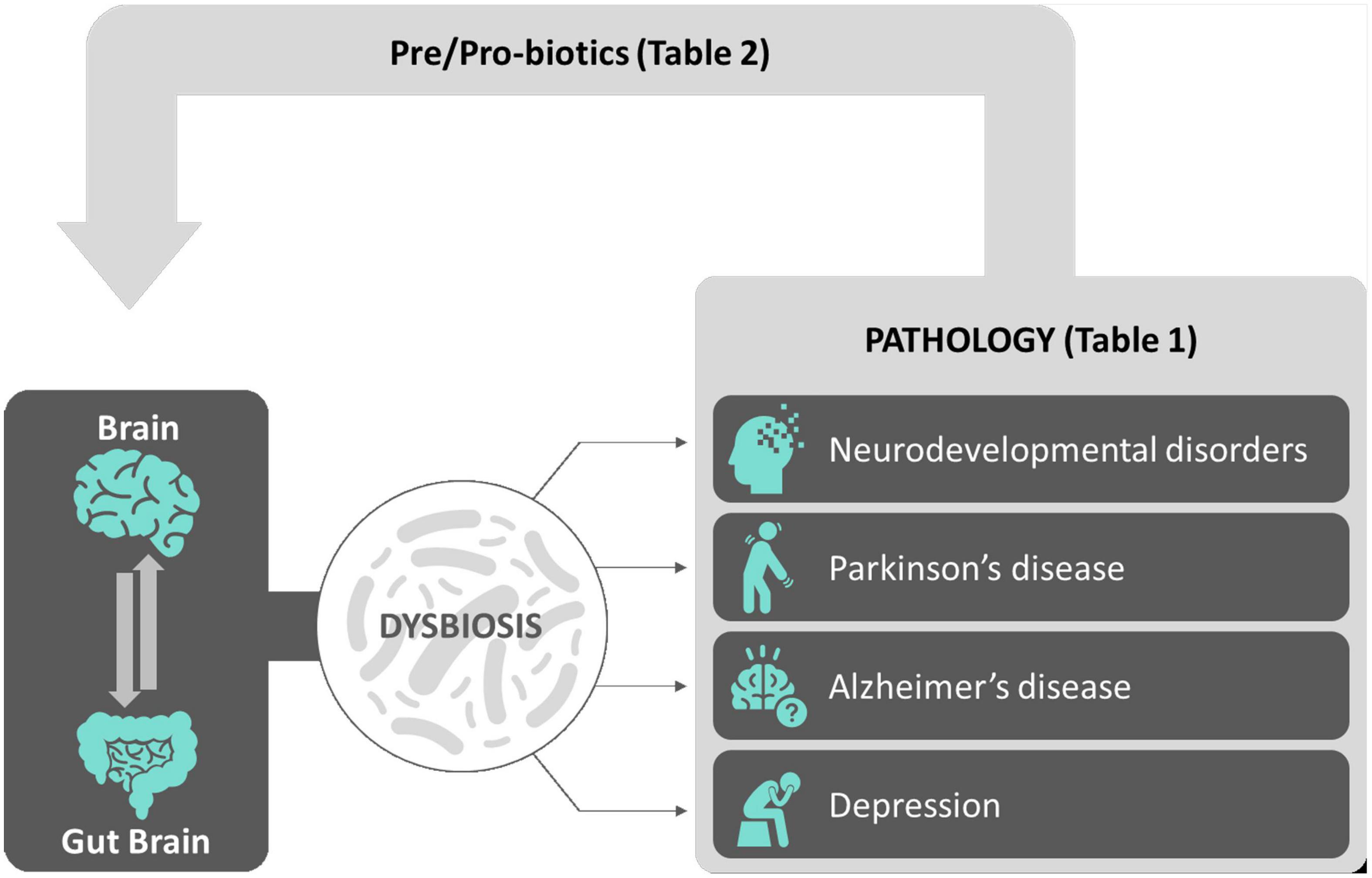
Figure 1. Sketch depicting an overview of the review. The complex interactions between the brain and the gut–brain (gut microbiota) contribute to the physiological balance achieved during normal brain functions. Alterations of gut microbiota microflora leading to gut dysbiosis have been correlated to a number of brain conditions including neurodevelopmental disorders, neurodegenerative diseases, and depression, described both in animal models and in mankind (see Table 1 for a summary). Among possible effective new therapeutic avenues, pre/probiotic supplements aimed to correct gut microflora dysbiosis and to reinstate neurophysiological balance in neuropathological background had been shown so far to be effective in a number of clinical trials (see Table 2 for a summary).
Our world is home to a plethora of microbial species ranging from archaea to viruses, from fungi to parasites (protozoa and worms) that form complex microbial communities called microbiota. These communities interact with each other, cross act within an ecological niche, and form a complex of interconnected networks that communicate, cross-feed, recombine, co-evolve, and cooperate not only with each other, but also with the animal species that host them (Layeghifard et al., 2017). As humans, we are not an exception; in fact, our body is also home to communities of microorganisms formed by thousands of microbial species. For adaptive and host co-evolution strategies, the microbial community of each area of the human body is unique in its composition and it presents different microbial ecology niches (Lloyd-Price et al., 2016). These microbial communities can be beneficial to the human host (Mao et al., 2013; Knights et al., 2014) and have indubitably implications in both health and disease (Maynard et al., 2012). Already from the early infant life, the microbial colonization of the infant gut (who inherits the own microbiota from the mother at birth) is essential for the early postnatal development of the innate immune system (Arboleya et al., 2012; Gomez de Aguero et al., 2016). In fact, the intestinal microbiota profile of preterm infants showed higher levels of facultative anaerobic microorganisms and reduced levels of strict anaerobes such as Bifidobacterium, Bacteroides, and Atopobium compared with that of full-term, vaginally delivered, breast-fed infants. Later in life, these microbial communities can significantly affect human health and even modulate the clinical outcomes of infections (Liang et al., 2018). Vice versa, alteration/perturbations/imbalance in the microbial community composition (dysbiosis) can lead to unfavorable responses and/or pathological conditions in the host, including modifications in relevant behaviors. After birth, the microbial flora changes depending on the dietary habits and environment effects (Koh et al., 2016; Wahlstrom et al., 2016).
The human gut is home to a variety of bacteria, archaea, fungi (mostly yeasts), microbial eukaryotes (usually Blastocystis and a variety of pathogenic and non-pathogenic taxa), and viruses/phages. This collection of microbes is called the gut microbiota, and their genes are complexly called the microbiome (Turnbaugh et al., 2007). The general composition of gut microbial community included the following five phyla: Bacteroidetes, Firmicutes, Actinobacteria, Proteobacteria, and Verrucomicrobia (The Human Microbiome Project Consortium, 2012). In the healthy gut, the anaerobic Bacteroidetes and Firmicutes contribute to more than 90% of the total bacterial species, and their ratio changes across different individuals mostly because of (i) differences in individual (host) genomes and (ii) environmental factors (antibiotic use, lifestyle, hygiene, and diet) (The Human Microbiome Project Consortium, 2012). Dysbiosis of the human gut microbiome is associated with a wide range of pathologies, including obesity (Perry et al., 2016), diabetes (Gulden et al., 2015), diarrhea (Pop et al., 2014), and irritable bowel syndrome (IBS) (Jalanka-Tuovinen et al., 2014). The concept of microbial dysbiosis also includes the microbiome bacteriophage components that are implicated in a wide range of physiological (health) and pathological conditions (Manrique et al., 2017). Bacteriophages, also called phages, parasite their host, the bacteria, and do play an important regulator role in the host–microbiome interactions through horizontal gene transfer and antagonistic coevolution (Scanlan, 2017; Duerkop, 2018).
Nevertheless, the gut microbiota is not an isolated community that simply lives in the gut and within the host, but it does profoundly communicate with the other organs (even distant ones) by means of microbial signals transmitted across the intestinal epithelium and via different pathways, for example, (i) the trimethylamine (TMA)/trimethylamine N-oxide (TMAO), (ii) the short chain fatty acids (SCFAs), and (iii) the primary and secondary bile acid (BAs) pathways (Pluznick et al., 2013). Gut–brain communication occurs also via the vagus nerve. Microbiota-derived molecules act either by functionally interacting with other endocrine hormones (i.e., ghrelin, leptin, glucagon-like peptide 1, and peptide YY) or by stimulating the parasympathetic nervous system, thereby affecting glucose homeostasis and other metabolic processes linked to the production of microbiota-generated metabolites promoting metabolic benefits, as, for example, in promoting body weight and glucose control (demonstrated in animal models) (De Vadder et al., 2014). Despite the presence of the brain–blood barrier (BBB), these molecules can reach the brain thanks to the brain–gut–microbiota axis: a bidirectional communication system enabling gut microbes to communicate with the brain and the brain with the gut, and it can have a profound effect on brain physiological state (Rhee et al., 2009). The mechanisms of signal transmission are complex and include neural, endocrine, immune, and metabolic pathways (Grenham et al., 2011; El Aidy et al., 2015). This ability of the microbes to influence brain-related behaviors suggests that they induce the release of host immune factors (immune-mediated, i.e., cytokines and inflammatory mediators) targeting both the central nervous system (CNS) and the enteric nervous system (ENS) (Wood, 2004). Moreover, bacteria can themselves synthetize and regulate the level of many neurotransmitters in the brain including major (GABA, glutamate) and neuromodulatory ones (serotonin, dopamine, and norepinephrine) that are known to play pivotal roles in social behavior (Liu et al., 2017; Strandwitz et al., 2019). For example, most of the serotonin in the body is produced in the gut by enterochromaffin cells under the influence of the microbiome (Yano et al., 2015), and Bifidobacterium infantis has been shown in animal models to elevate the plasma levels of tryptophan (the precursor of the serotonin synthesis), thus influencing those brain systems involved in mood disorders (Desbonnet et al., 2010). In this review, we will specifically focus on the relation between the gut microbiota and the brain.
The ability of pathogens to profoundly influence and affect relevant brain behaviors of their hosts has been described a long time ago in the literature. In rodents, for example, Toxoplasma gondii parasitic infection ultimately results in a dramatic (and fatal) decrease in anxiety-like behavior in the infected rodents that no longer show fear of feline predators (Berdoy et al., 2000). Not only (external) pathogens that cause infections and alter animal behavior, but also microbial species residing within the gut can have profound effects, revealing the recently engendered use of the term “mind-altering bugs” that in fact stresses how variations and changes in the composition of the gut microbiota can influence normal brain physiology and behavior, and also contribute to diseases ranging from inflammation to obesity to the regulation of anxiety, mood, cognition, and pain (Cryan and Dinan, 2012). For instance, the gut microbiota can influence social interactions by acting on the nutritional behavior of individual animals (Pasquaretta et al., 2018). Low oral doses of Campylobacter jejuni, a bacterium within the gut, induce anxiety-like behavior in mice through a vagal-mediated pathway in the absence of any immune activation (Goehler et al., 2005). In humans, people with inflammatory bowel diseases (characterized by altered microbial diversity) show alteration in anxiety and depression behaviors (Blanchard et al., 1990).
Moreover, the host microbiota constantly control maturation and function of macrophages in the brain (Erny et al., 2015), the microglia cells critically involved in brain diseases.
The gut microbiota serves thereby as a proper “microbial organ” (i) being in continuous communication with the neurophysiological system of the host and (ii) acting as a driver of homeostasis and disease (Lyte, 2010). Thus, if an unhealthy gut might play a pivotal role in an unhealthy brain and contribute to neurodegenerative diseases (Spielman et al., 2018), consequently the modulation of the gut microbiota may be a tractable strategy for the development of new therapeutic strategies for complex neurological disorders (Cryan and Dinan, 2012). Table 1 summarizes gut microbiota alterations in animal models and in mankind across brain disorder and diseases.
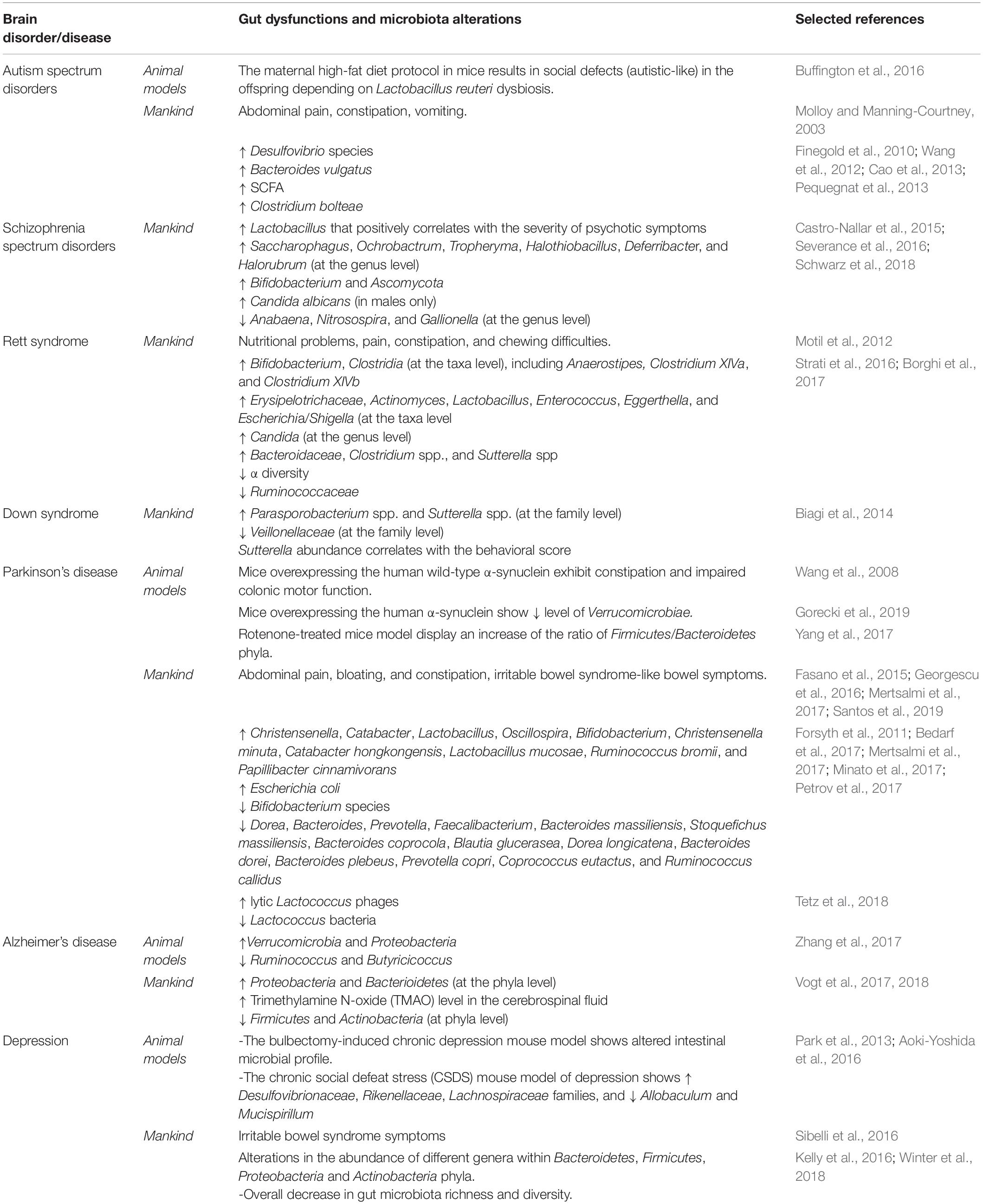
Table 1. Summary of gut microbiota alterations in animal models and in mankind across brain disorders and diseases.
The adult brain is the result of complex neurodevelopmental processes involving genetic, molecular, and environmental factors. Early during brain development, neural progenitor cells undergo cell division, and once committed to a neural fate, they migrate and differentiate into their final location in the brain making contact with their final targets. During postnatal life, experience refines the immature neuronal network during time windows of plasticity, called critical periods, when experience provides essential input for the proper refinement and final maturation of the adult brain functions. These time windows are “critical,” because if an incorrect experience occurs, it will permanently affect the structure and function of the brain (Deidda et al., 2014, 2021). So far, critical periods for the maturation of sensory systems and language productions (among others) have been described (Berardi et al., 2000; Hensch, 2004; Erzurumlu and Gaspar, 2012; Levelt and Hubener, 2012). Physiological brain development will result in normal adult brain functions; on the other hand, defects in brain development during (mal)developmental critical periods result in neurodevelopmental disorders [including autism, Rett syndrome, Down syndrome (DS), and schizophrenia] that feature main cognitive impairments (Deidda et al., 2014, 2021).
Little is still known about the role of the gut microbiome in normal brain development and to which extent it is mainly involved in neurodevelopmental disorders, but it should not come as a surprise that microbes do affect the developing brain, since the nervous system developed and had evolutionary evolved in the presence of signals from the microbes. Surely, the gut microbiota is essential for normal brain development and maturation of normal brain functions into adulthood as demonstrated in animals with absent microbiota throughout life (germ-free) that show alterations in cognition, social behavior, and stress response (Cryan and Dinan, 2012). The fact that some of these defects can be corrected by an early exposure to the microbiota (Clarke et al., 2013) suggests that there is a “critical period” for the effects on the brain to take place upon microbe exposure. Taking an example from the invertebrate animal kingdom, the development of the light-emitting organ of the Hawaiian bobtail squid critically depends on the entry of the bacterium Vibrio fischeri inside the organ during a critical period during development, namely, within minutes from hatching (Altura et al., 2013).
Microbiota impact on brain development starts already in utero and continues in the postnatal life. Prenatally (in utero), although the womb is considered to be sterile, the maternal microbiota can influence fetal brain development by means of metabolites reaching the fetus (Gomez de Aguero et al., 2016; Li et al., 2020). Eventually, the indirect effects of the maternal microbiota via its metabolites may prepare the fetus for the direct exposure to microbes at delivery. Studies performed in germ-free animals showed that maternal microbiota influences the in utero development of (i) the BBB as fetus gestating in pregnant germ-free mice have reduced expression of endothelial tight junction proteins and increased BBB permeability (Braniste et al., 2014); (ii) brain’s innate immune system as microglia cells are more numerous and with more branches in germ-free embryos than in controls (Thion et al., 2018); (iii) thalamo-cortical axon growth (Vuong et al., 2020); and (iv) sympathetic nerve projections to the heart (this control occurs via a mechanism dependent on SCFAs) (Kimura et al., 2020). Thus, maternal microbe-dependent metabolites affect offspring neural development already in utero.
Soon after birth, by means of the first swallow, the mammalian fetus is in contact with a world populated by microbes coming both from the mother and from the environment; next, by means of the first breast-feeding (and daily after birth) the newborn will ingest millions of bacteria contained in the breast milk (Le Doare et al., 2018). These microbes rapidly colonize the newborn in all body sites and reach the gut within a few hours where the system is already in place to convey information from gut microbiota to the brain. In fact, the connections from the vagus nerve to the duodenum and the distal small intestine already occurred in utero: in mice at embryonic days E14 and E16, respectively (Ratcliffe et al., 2011). In the mouse, the first microbes to colonize the guts are Streptococcus 1 day after birth, followed by Lactobacillus (required for fermenting milk lactose) by day 3 (Pantoja-Feliciano et al., 2013); this balance will shift around weaning to a more stable microbial community more dominated by Bacteroides. Within the 12–14 h from birth, the microbiota controls neuronal cell death (apoptosis) (Castillo-Ruiz et al., 2018), a process that massively occurs early during the first postnatal life and that sculpts the brain; the control on apoptosis was not seen prenatally, suggesting that the direct exposure to the microbes is essential. In humans, a similar sequence of gut microbe colonization occurs, but by the 1st week of life Bifidobacterium became more dominant (Fanaro et al., 2003).
If a normal brain development results in normal brain functions and behavior, defective brain developments have been associated with a number of neurodevelopmental disorders, including autism spectrum disorders (ASDs), Rett syndrome, Down syndrome (DS), and schizophrenia spectrum disorders (SSDs) (Di Cristo, 2007; Deidda et al., 2014, 2021). ASD is a heterogeneous neurodevelopmental disorder characterized by deficits in social communication, social interaction, and restricted/repetitive behavioral patterns. In humans, GI symptoms represent a common comorbidity in ASD (Molloy and Manning-Courtney, 2003) and the gut microbiota profile is altered in children with ASD (Cao et al., 2013) showing higher levels of Desulfovibrio species and Bacteroides vulgatus, higher levels of SCFA (Finegold et al., 2010; Wang et al., 2012), and higher levels of Clostridium bolteae (Pequegnat et al., 2013) compared to controls. In animal models, Buffington et al. (2016) reported that maternal high-fat diet in mice (resulting in maternal obesity during pregnancy) induced a shift in microbial composition that negatively impacts offspring social behavior (associated with increased risk of ASD). Interestingly, both social deficits and gut microbiota dysbiosis could be prevented by co-housing with offspring of mothers housed on a regular diet. Moreover, the selective re-introduction of Lactobacillus reuteri restored social deficits (Buffington et al., 2016), suggesting that probiotic treatment might be an effective strategy to ameliorate specific social behavioral deficits associated with neurodevelopmental disorders. Since ASD is associated with defect in cortical development (heterotopia) (Piven et al., 1990; Guerrini and Dobyns, 2014; Moffat et al., 2015), Kim et al. (2017) found out that maternal gut bacteria promote neurodevelopmental abnormalities via activation of the immune response in the offspring (Kim et al., 2017), possibly suggesting that some gut microbiota profiles might promote neurodevelopmental disorders.
Rett syndrome is an X chromosome-linked dominant neurodevelopmental disorder (the most prevalent in females) sharing features with ASD. Ninety-five percent of Rett syndrome cases are associated with pathogenic variants in the MECP2 (Methyl CpG Binding Protein 2) gene, encoding a chromatin-associated protein (Amir et al., 1999; Boggio et al., 2010). Main neurological symptoms include impaired language production and stereotypic hand movements. Most girls with Rett syndrome also experience epileptic seizures (Jian et al., 2006). Gastrointestinal and nutritional problems are also a common condition throughout life including pain, constipation, and chewing difficulties (Motil et al., 2012). The studies investigating gut microbiota dysfunctions in Rett syndrome are very limited with only few so far, but still clearly indicating gut microbiota dysfunctions. In the first study (Strati et al., 2016), the authors showed dysbiosis in terms of relative abundances of both the bacterial and fungal component of the gut microbiota. In particular, gut microbiota in the Rett syndrome cohort was dominated by microbial taxa belonging to Bifidobacterium, Clostridia (including Anaerostipes, Clostridium XIVa, and Clostridium XIVb) as well as Erysipelotrichaceae, Actinomyces, Lactobacillus, Enterococcus, Eggerthella, Escherichia/Shigella, and the fungal genus Candida. Importantly, and contrary to what was previously expected, the alterations of the gut microbiota did not depend on the constipation status. In the second study (Borghi et al., 2017), the authors found (i) a lower α diversity; (ii) an enrichment in Bacteroidaceae, Clostridium spp., and Sutterella spp.; and (iii) a slight depletion in Ruminococcaceae in girls and women with Rett syndrome.
Down syndrome (DS) is the most frequent cause of intellectual disability; it is caused by the trisomy of human chromosome 21 and it presents multiple GABA dysfunctions in animal models and humans (Bittles et al., 2007; Dierssen, 2012; Deidda et al., 2015; Contestabile et al., 2017). One study had investigated the DS gut microbial community in adult (Biagi et al., 2014). Although DS gut microbiota did not differ from healthy control in the relative abundance of the dominant microbial families (i.e., Ruminococcaceae, Lachnospiraceae, Clostridiales, Bifidobacteriaceae, and Bacteroidaceae), Parasporobacterium spp. and Sutterella spp. were increased, whereas Veillonellaceae was reduced. Interestingly, Sutterella abundance was positively correlated with the aberrant behavior score (Biagi et al., 2014).
Schizophrenia spectrum disorder (SSD) is a heterogeneous neurodevelopmental disorder characterized by positive symptoms (namely, symptoms that involve any change in normal behavior; they arise with the pathology, for example, delusions and hallucinations), negative symptoms (involving an impairment in normal behavior; for example lack of interest in social interactions, affective flattening, and avolition), and cognitive symptoms. Psychotic symptoms usually manifest clinically during the adolescent period. The gut microbiota was proposed to have an impact on social cognition affecting SSD (Dinan et al., 2014) and several clinical trials investigated microbiota profile differences between people with SSD and controls. Schwarz et al. (2018) investigated the gut microbiota in people with first episode psychosis (FEP) and found an increase in Lactobacillus, Saccharophagus, Ochrobactrum, Tropheryma, Halothiobacillus, Deferribacter, and Halorubrum (at the genus level), and a decrease in Anabaena, Nitrosospira, and Gallionella in comparison to healthy controls. The number of Lactobacillus bacteria correlated positively with severity of psychotic symptoms (Schwarz et al., 2018). Castro-Nallar et al. (2015) analyzed the oropharyngeal microbiome finding an increase in the numbers of Lactobacillus, Bifidobacterium, and Ascomycota in people with SSD (Castro-Nallar et al., 2015). When analyzing the gut fungal composition (the mycobiome), Severance et al. (2016) found an increase in Candida albicans specifically in male with SSD, not in females (Severance et al., 2016) calling for possible probiotic treatment interventions.
Altogether, the findings reviewed so far clearly show how the gut microbiota plays a role in normal brain development (already in utero), and how neurodevelopmental disorders are characterized by major gut microbiota dysbiosis. So far, despite the fact that these findings suggest that the link between neurodevelopmental disorders and gut microbiota is correlative rather than causal, probiotic strategies aimed to reinstate physiological microflora balance might be a promising new path to be undertaken for alleviating both GI and eventually core symptoms in people with neurodevelopmental disorders.
Parkinson’s disease is the second most common neurodegenerative disorder of aging and it affects more than 6 million people in the world (Disease et al., 2016). It results primarily from the death of dopaminergic neurons in the substantia nigra pars compacta, a basal ganglia structure located in the midbrain and involved in the control of movements (Moore et al., 2005). The neuropathological hallmark in Parkinson’s disease is the presence of Lewy bodies, namely, cytoplasmic inclusions of α-synuclein aggregates in the neurons of the substantia nigra pars compacta (Okazaki et al., 1961; Polymeropoulos et al., 1997) whose soluble oligomeric conformations is thought to mediate the toxic effects in the pathology (Miller et al., 2021).
People with Parkinson’s disease display a major impairment in their motor behavior showing characteristic symptoms including tremor at rest, rigidity, and slowness in starting the movement (bradykinesia) (Antony et al., 2013). Moreover, non-motor symptoms become common in the advanced stages of the disease, and they include cognitive impairment (dementia), psychosis, apathy, restlessness, impulse control disorders, sleep disorders, and associated comorbidities (depression and anxiety) (Stacy, 2011; Antony et al., 2013; Pfeiffer, 2016). Moreover, olfactory dysfunctions (Haehner et al., 2009; Fullard et al., 2017), pain, and sensory disturbances (Chen Y. et al., 2015; Tseng and Lin, 2017) are common. These non-motor symptoms have, in particular, been considered valuable for the early diagnosis of Parkinson’s disease during the so-called “premotor phase” of the disease because they can occur years before the onset of the motor symptoms (Chen H. et al., 2015; Rizzo et al., 2016).
Among the pre-motor events, Braak et al. (2003) previously postulated that lesions in the ENS that occurred at a very early stage of the disease could represent a route of entry for a putative exogenous “unknown pathogen” that passes across the gastric mucosa and then is retrogradely transported up the vagus nerve to the medulla to initiate the pathological processes leading to Parkinson’s (Braak et al., 2003). Following this interesting hypothesis, hard and solid data are still missing and evidence in the literature show contradicting results. For example, one study in humans showed no statistically significant association between T. gondii infection and idiopathic Parkinson’s disease (Celik et al., 2010), while another one in mice showed a significant increase in dopamine metabolism in neural cells (Prandovszky et al., 2011). A very recent study challenged Braak’s hypothesis revealing α-synuclein aggregates in the vagus nerve and in the stomach of people with Parkinson’s disease, but not in normal elderly subjects, thereby supporting initiation of α-synuclein pathology in the brain and is thereby against the “Body-First” hypothesis (Beach et al., 2021).
Independently whether the latter hypothesis would be confirmed or not, nowadays, it is widely accepted that among the non-motor symptoms, the GI ones are common among people with Parkinson’s disease (Fasano et al., 2015; Santos et al., 2019) who report abdominal pain, bloating, and constipation (Georgescu et al., 2016) even before being diagnosed with the disease (Abbott et al., 2001). Moreover, IBS-like bowel symptoms have been reported and, importantly, connected with a disruption of the gut microbiota linked to a lower abundance of Prevotella species in patient fecal samples (Mertsalmi et al., 2017). Lower Bifidobacterium species counts were also found (Minato et al., 2017).
Plenty of evidence in the literature indicates gut microbiome dysfunctions in people with Parkinson’s disease (Hamano et al., 1993; Clairembault et al., 2015; Hasegawa et al., 2015; Keshavarzian et al., 2015; Klingelhoefer and Reichmann, 2015; Mulak and Bonaz, 2015; Unger et al., 2016; Bedarf et al., 2017; Hill-Burns et al., 2017; Parashar and Udayabanu, 2017; Scheperjans et al., 2018; Sun and Shen, 2018; Breen et al., 2019; Santos et al., 2019) with changes in the microbiome composition of the upper (oral) GI tract occurring at the early stage of the disease (Mihaila et al., 2019), suggesting that probiotics/psychobiotics and microbiota transplantation might be beneficial (Dinan and Cryan, 2015). Apart from GI disorders, changes in the gut bacterial abundances of microbes (such as Prevotellaceae and Enterobacteriaceae) (Scheperjans et al., 2015), activation in the brain of microglial cells by short-chain fatty acids (SCFA), and intestinal flora metabolites have been reported (Erny et al., 2015).
Aggregates of α-synuclein were described in the ENS of people with Parkinson’s disease (Barrenschee et al., 2017), and recent evidence obtained in animal models (rat and Caenorhabditis elegans) showed that alterations in the gut microbiome may in fact induce accumulation of α-synuclein aggregates in the ENS (Chen et al., 2016). In a very interesting study, Sampson et al. (2016) demonstrated that the gut microbiota might play a sufficient role for the onset of the disease. The authors demonstrated in mice overexpressing α-synuclein that (i) the presence of gut microbiota is necessary to promote Parkinson-like pathological alterations, and that (ii) transplantation of fecal samples derived from people with Parkinson’s disease into normal mice resulted in impaired motor function, suggesting that microbes in the gut may play a pivotal role in the onset of Parkinson’s disease (Sampson et al., 2016). Moreover, mice overexpressing the human wild-type α-synuclein exhibit constipation and impaired colonic motor function (Wang et al., 2008).
The microbiota pattern in people with Parkinson’s disease can trigger local inflammation followed by aggregation of α-synuclein and generation of Lewy bodies exhibiting thereby a pro-inflammatory profile; the changes in the microbiota content includes 9 genera and 15 species of microorganisms: (i) a reduced content of Dorea, Bacteroides, Prevotella, Faecalibacterium, Bacteroides massiliensis, Stoquefichus massiliensis, Bacteroides coprocola, Blautia glucerasea, Dorea longicatena, Bacteroides dorei, Bacteroides plebeus, Prevotella copri, Coprococcus eutactus, and Ruminococcus callidus, and (ii) an increased content of Christensenella, Catabacter, Lactobacillus, Oscillospira, Bifidobacterium, Christensenella minuta, Catabacter hongkongensis, Lactobacillus mucosae, Ruminococcus bromii, and Papillibacter cinnamivorans (Bedarf et al., 2017; Petrov et al., 2017). The resulting increased intestinal permeability (gut leakiness) to proinflammatory bacterial products in the intestine correlated with the increased intestinal mucosa staining for Escherichia coli bacteria, nitrotyrosine, and α-synuclein, markers in early phases of Parkinson’s disease (Forsyth et al., 2011), giving strength to the view that the intestine might be an early site of Parkinson’s disease in response to an environmental toxin or pathogen. Also, bacterial amyloids may favor pro-inflammatory environment in the intestine (Miraglia and Colla, 2019). In rats, lipopolysaccharide (LPS) intranigral injection induces inflammatory reaction that damages the nigrostriatal dopaminergic system, indicating that this event may be indeed implicated in the neurodegeneration processes (Castano et al., 1998). Gorecki et al. (2019) further explored the interaction between the gut microbiota and α-synuclein aggregation investigating the effects of LPS in mice overexpressing the human α-synuclein. By using 16S ribosomal RNA sequencing, the authors first found out that the relative abundance of mucin-degrading Verrucomicrobiae and LPS-producing Gammaproteobacteria were greater in fecal samples from people with Parkinson’s disease, while in mice overexpressing the human α-synuclein Verrucomicrobiae were reduced. Then, they investigated the effect of LPS on intestinal barrier function in vitro and found out that LPS exposure reduced and altered the distribution of the cell membrane tight junctions. Moreover, in vivo LPS administration resulted in the emergence of early motor manifestations in mice overexpressing the human α-synuclein, supporting the concept that proinflammatory gut microbiome environment might be a trigger for Parkinson pathogenesis (Gorecki et al., 2019). Despite being extensively described in Parkinson’s disease, the neuropathological role of inflammation is common among other neurodegenerative diseases too (Cappellano et al., 2013).
In studies performed in rodents, gut microbiota transplants from a mouse model of Parkinson’s disease induced motor impairment and striatal neurotransmitter decrease into normal mice and fecal microbiota transplantation (FMT) reduced the activation of microglia and astrocytes in the substantia nigra, reduced gut microbial dysbiosis, decreased fecal SCFAs, alleviated physical impairment, and increased striatal dopamine and serotonin content in a mouse model of Parkinson’s disease (Sun et al., 2018).
As far as phage-related dysbiosis in Parkinson’s disease is concerned, evidence in the literature suggests that phages might be implicated in the misfolding of the α-synuclein (Tetz and Tetz, 2018) and that the lytic bacteriophages in the gut might contribute to the onset of the pathology. In fact, the lytic Lactococcus phages are more abundant in people with Parkinson’s disease than in healthy individuals, and it is associated with a strong reduction in Lactococcus bacteria (Tetz et al., 2018); since Lactococcus bacteria play an important role in producing the neurotransmitter dopamine (Strandwitz et al., 2019), and do regulate the permeability of the gut (Darby et al., 2019) [dopamine and gut permeability are two factors linked to the early signs of Parkinson’s Disease in the gut (Houser and Tansey, 2017)] The depletion of Lactococcus bacteria caused by the high numbers of strictly lytic phages in people with Parkinson’s disease might play a role in triggering α-synuclein misfolding (Braak et al., 2003; Asano et al., 2012; Scheperjans, 2016).
Mitochondrial dysfunctions (including impairment in the mitochondrial electron transport chain, alterations in the mitochondrial morphology, and mutations in mitochondrial DNA) also play a role in the pathophysiology of Parkinson’s disease occurring early in the pathogenesis (both in the sporadic and monogenic forms of the disease) and leading to impairments in energy production, generation of reactive oxygen species, and induction of stress-induced apoptosis (Subramaniam and Chesselet, 2013). Consequently, mitochondria represent a valuable target for neuroprotective interventions.
Despite the fact that gut microbiota dysfunctions in Parkinson’s disease are well documented, it is unclear whether the changes in the intestinal microflora are either a cause or an effect of the disease. The study led by Yang et al. (2017) showed that it might be a cause of the pathology. In fact, in a rotenone-treated mouse model, alteration of fecal microbiota compositions (an overall decrease in bacteria diversity and changes of microbiota composition with an increase in the ratio of Firmicutes/Bacteroidetes phyla) precedes the onset of α-synuclein pathology (Yang et al., 2017). In humans, the intestinal flora changes as the pathology progresses, and these changes do correlate with the clinical symptoms of the disease (Li et al., 2017).
Taken together, gut microbiota shows profound dysbiosis in Parkinson’s disease, as shown in humans and in animal models, and by correcting this dysbiosis would open new therapeutic avenues for the disease.
Alzheimer’s disease is the most common form of dementia in the elderly, with main medical and social problems in economically developed countries connected to a significant increase in human life span. It is characterized by an irreversible neurodegeneration impacting learning and memory functions, ultimately leading to disability (Wimo et al., 2017). The prevalence rate of the disease as well as the degree of cognitive impairment are influenced by factors like older age, low education level, and poor health conditions (de Souza-Talarico et al., 2016). During the progression of the disease, neuronal death leads to progressive impairments in synaptic function. Neurodegeneration also strikes catecholaminergic neurons located in the locus coeruleus (LC) that provides dense innervations to the thalamus and amygdala, and sparse innervations of the neocortex, hippocampus, cerebellum, and spinal cord. Similarly to Parkinson’s disease where α-synuclein forms aggregates, Alzheimer’s disease neuropathology is characterized by deposits of extracellular amyloid-β (Aβ) peptide and intraneuronal neurofibrillary changes of hyper-phosphorylated protein tau (Braak and Braak, 1991; Tiraboschi et al., 2004). Aβ peptides are produced by proteolytic cleavage of the transmembrane amyloid precursor protein (APP), whereas tau is a brain-specific and axon-enriched protein normally associated with cytoskeleton microtubules. Upon phosphorylation, tau loses its affinity with the microtubules and destabilizes the neuronal cytoskeleton leading to synaptic neuronal deficiency, disruption of Ca2+ homeostasis and ultimately neuronal death via apoptosis (Yankner et al., 1990; Hardy and Higgins, 1992). Despite the extensive preclinical/clinical research and pharmacological development, the precise mechanisms underlying Alzheimer’s disease are unclear, and the current therapeutic approaches targeting Aβ deposits are able to only provide modest symptom relief (Bachurin et al., 2018). Apart from genetic factors, oxidative stress represents one of the mechanisms where the pathological intra-/extracellular deposits mediate the neurodegenerative process, including an inadequate antioxidant system, high oxygen consumption, excitotoxic amino acids, and high iron content, altogether promoting the production of reactive oxygen (ROS) and nitrogen species (RNS) (Kim et al., 2015).
Similar to Braak’s hypothesis for Parkinson’s disease where an external pathogen would cause neuroinflammation [described earlier in the text (Braak et al., 2003)], pathogen-induced inflammation might also play a role in Alzheimer’s disease (Wyss-Coray and Rogers, 2012; Cappellano et al., 2013); for example T. gondii, which is an obligate intracellular parasitic protozoan (phylum Apicomplexa) that uses a variety of mammals as intermediate hosts (including humans), with the cat as the final one, causes the infectious disease toxoplasmosis (da Silva and Langoni, 2009). It has been described that it can induce immune response in the host and inflammation in the CNS, alteration in the levels of neurotransmitters, and direct infections of neuronal and astrocytic cells (in rat primary cell culture) (Luder et al., 1999). For these reasons, T. gondii can be considered a possible etiologic risk factor for Alzheimer’s disease (Nayeri et al., 2021).
Apart from T. gondii, a number of other infectious agents may be involved in Alzheimer’s disease including viral [i.e., Herpes simplex virus-1 (HSV-1) (Itzhaki et al., 1997); Herpes simplex virus type 2 (HHV-2) (Kristen et al., 2015); cytomegalovirus (CMV) (Renvoize et al., 1979; Renvoize and Hambling, 1984); hepatitis C virus (HCV) (Chiu et al., 2014)], fungal [i.e., Candida famata, C. albicans, Candida glabrata, and Syncephalastrum racemosum (Pisa et al., 2015a,b)], and bacterial [i.e., Borrelia burgdorferi (MacDonald and Miranda, 1987); Chlamydia pneumonia (Paradowski et al., 2007; Lim et al., 2014); Treponema pallidum (Riviere et al., 2002); and Helicobacter pylori (Kountouras et al., 2009)].
It is surprising to consider that the intracellular Aβ production and deposition in Alzheimer’s disease could be a consequence (rather than the cause) set in motion to counteract and protect from the pathogen invasion in the CNS. Indeed, several types of spirochetes have been associated to dementia, cortical atrophy, and biological hallmarks of the disease; neurons infected by HSV-1 showed Aβ and hyperphosphorylated tau accumulation, and Aβ displayed anti-microbial properties capable of inducing pore formation, thus justifying their infection-mediated accumulation (Ashraf et al., 2019).
As far as microbial communities residing in the gut are concerned, recent studies have implicated the gut microbiome as another etiological factor involved in the pathogenesis of Alzheimer’s disease both in humans and in animal models (Harach et al., 2017; Vogt et al., 2017; Zhang et al., 2017; Spielman et al., 2018; Ashraf et al., 2019; Kowalski and Mulak, 2019; Li et al., 2019). In humans, the composition of the intestinal microbiome differs between people with Alzheimer’s disease and healthy controls, including a decreased numbers of Firmicutes and Actinobacteria and an increase in the number of bacteria belonging to the Proteobacteria and Bacterioidetes phyla (Vogt et al., 2017). Interestingly, Gammaproteobacteria, Enterobacteriales, and Enterobacteriaceae of phylum Proteobacteria that are known to induce inflammation (“pro-inflammatory bacteria”) increased steadily from healthy controls to mild cognitive impairment and dementia stage (Vogt et al., 2017). Moreover, microbiota-derived metabolic molecule trimethylamine N-oxide (TMAO, a small molecule produced by the metabolism of dietary choline) were detected in the cerebrospinal fluid of people with Alzheimer’s disease and associated with biomarkers of Alzheimer’s pathology (i.e., phosphorylated tau and phosphorylated tau/Aβ42) (Vogt et al., 2018). Interestingly, the microbiota alterations might arise earlier than Alzheimer’s clinical symptoms as suggested by a study that shows similar alterations in gut microbiota composition between people with mild cognitive impairment and Alzheimer’s disease (Li et al., 2019); thus, it can be considered as an early presymptomatic event as already suggested for Parkinson’s disease. In animal models, (i) in the Aβ precursor protein (APP) transgenic mouse model, APP-mutant germ-free mice displayed decreased cerebral Aβ amyloid pathology in comparison to APP mice in control conditions, and anti-Aβ effects could be blocked by reconstruction of these germ-free APP mice with microbiota derived from conventional mice (Harach et al., 2017); (ii) long-term broad-spectrum antibiotic treatment reduces Aβ deposition and improves the neuropathological phenotype in the APPSWE/PS1DeltaE9 mouse model (Minter et al., 2016); (iii) fecal samples derived from another model of the disease showed dramatic elevations in Verrucomicrobia and Proteobacteria, as well as significant reductions of Ruminococcus and Butyricicoccus, suggesting a pathological relevant microbiota composition and diversity (Zhang et al., 2017). Activated microglia also contribute to the pathology of AD by inhibiting Aβ clearance and increasing Aβ deposition (Cai et al., 2014).
Taken together, the evidence reviewed here clearly indicates that specific species of gut microbiota activate Aβ signaling pathways and contribute to the pathogenesis of Alzheimer’s disease. Consequently, modification of the gut microbiota composition by probiotic supplementation may create new preventive and therapeutic options for interventions in people with Alzheimer’s disease.
Depression is a common and heterogeneous disorder responsible for significant disability worldwide, and it is characterized by a state of low mood often accompanied with loss of interest in activities that the individual normally perceives as pleasurable. Its severe form, major depression, is classified as a mood disorder. Depression is a leading cause of disability worldwide, it has greater negative effects on people health (Moussavi et al., 2007), and it carries a similar risk for mortality akin to smoking, blood pressure, and alcohol intake (Mykletun et al., 2009). It is an associated comorbidity in epilepsy (Stafford-Clark, 1954; Harden, 2002) and in major neurodegenerative disorders, and its etiology includes metabolic (Jokela et al., 2014), neuroendocrine (Stetler and Miller, 2011), and neuroimmune factors (Dowlati et al., 2010).
As for the other brain diseases discussed so far (Parkinson’s and Alzheimer’s diseases) although depression is primarily a disease of the brain, the brain does not exist in isolation, but it is embedded within the overall physiology of the body, including the gut (Valles-Colomer et al., 2019). If the gut microbiota of adult healthy individuals is primarily dominated (90%) by Bacteroidetes and Firmicutes phyla, the gut microbiota of people with major depressive disorder displays a significant alteration in the abundance of different genera within Bacteroidetes, Firmicutes, Proteobacteria, and Actinobacteria phyla (Winter et al., 2018). In order to explore the relation between depression and microbiota, Kelly et al. (2016) investigated in humans and animal models whether changes in the gut microbiota composition and function might mediate the dysregulation of the neuroimmune and neuroendocrine pathways, both involved in depression. The authors assessed the cytokines, salivary cortisol and plasma LPS binding protein, and collected fecal samples from both groups to determine the microbiota profiles; they found that clinical depression was associated with decreased gut microbiota richness and diversity. Next, they obtained fecal microbiota samples derived from either people with major depression or controls, and performed FMT by means of oral gavage in a microbiota-deficient rat model. Strikingly, FMT from people with depression induced behavioral and physiological features characteristic of depression, including anhedonia and anxiety-like behaviors (Kelly et al., 2016).
Depression often coexists with IBS (Sibelli et al., 2016), which is characterized by alterations in gut function, and data obtained from animal studies show that the gut microbiota may affect the neurobiological features of depression. Park et al. (2013) exploited the bilateral olfactory bulbectomy in mice, known to induce depression-like behavior, to investigate whether it would result in changes in the microbiota composition: the bulbectomy-induced chronic depression resulted in an altered intestinal microbial profile together with an increase in colon motility, c-Fos activity, and serotonin levels (Park et al., 2013). Since depression is a common symptom in people with obesity (Bruce-Keller et al., 2009), Bruce-Keller et al. (2015) investigated whether the transplanted microbiome from obese mice (that show depression-like behavior) into non-obese control mice would result in depressive-like behavior. Before the transplantation, control mice were fully depleted of their own microbiome. Obese-derived microbiome re-colonization in control non-obese mice resulted in a disruption in exploratory, cognitive, and stereotypical behavior in the absence of significant differences in body weight (Bruce-Keller et al., 2015).
Depressive-like behaviors can also be induced in mice by means of stress models, for example, that of the chronic social defeat stress (CSDS) paradigm where mice are repeatedly subjected to social defeat by a larger and more aggressive mouse. Stressed mice developed depressive-like behaviors and showed changes in microbial diversity with an increase in Desulfovibrionaceae, Rikenellaceae, and Lachnospiraceae families and a decrease in Allobaculum and Mucispirillum (Aoki-Yoshida et al., 2016).
This evidence suggests that the gut microbiota may play a causal role in the development of depression and may be considered a valuable target in the treatment/prevention of this disorder.
The concept that gut microbiota affects not only the gut, but also the brain, and that brain–gut communication dysfunctions might play a role in the pathogenesis of neurological psychiatric disorders brought general interest in the search for new therapeutic solutions to tackle these pathologies. In particular, the use of pre- or probiotics (live microbes) aimed to correct the dysbiosis and/or microbiota defects and ameliorate the pathophysiological condition came into the spotlight recently.
When thinking about strategies to control/restrict uncontrolled/pathogenic gut microbial populations, the antimicrobial therapy (aimed to target directly the microbes) surely comes to mind. The antimicrobial therapy indeed firstly came into the spotlight, but it lost scientific and therapeutic interest over recent decades because of both the lack of development of new anti-bacterial agents and the widespread antimicrobial resistance, the latter alone being considered to be a great challenge to the health of the entire population because it results in 700,000 deaths every year, and, if not tackled properly with innovative strategies, “would lead to 10 million deaths by 2050, exceeding the toll of cancer” (Gorski et al., 2016). Consequently (and necessarily), the antimicrobial strategy was overcome by an alternative phage therapy that exploits the use of bacteria-specific viruses (phages) (Hyman and Abedon, 2010) to combat populations of uncontrolled or pathogenic bacteria (Chan et al., 2013; Gorski et al., 2016), for example, against multi-drug resistance Staphylococcus aureus (Gupta and Prasad, 2011). Generally speaking, phage therapy has multiple strengths in comparison to chemical antibiotics (used in the antimicrobial therapy), and it has limitations as well. Among the advantages, phages are effective bactericidal agents thereby limiting the viability of infected bacteria, contrarily to certain antibiotics that, being bacteriostatic rather than bactericidal, might still allow the evolution toward antibiotic resistance (Stratton, 2003). Also, the narrow host range (Hyman and Abedon, 2010) limits per se the number of bacterial types that could achieve phage resistance. Moreover, phages are easily discovered (often from sewage or waste materials); they are basically non-toxic, being composed of nucleic acids and proteins (Abedon and Thomas-Abedon, 2010), and they specifically increase in number where the bacterial host is located (“auto dosing”) (Carlton, 1999). Again, thanks to their specific host selectivity (Hyman and Abedon, 2010), phages exert a minimal disruption of normal flora (Skurnik et al., 2007). Finally, since phages amplify themselves, they can be applied with a single dose, already achieving active therapy (Abedon and Thomas-Abedon, 2010). The single dose (or less frequent doses) consequently increases the safety and reduces the environmental impact of phage therapy (Abedon and Thomas-Abedon, 2010). Phage therapy comes with disadvantages and limitations. Indeed, not all phages meet the criteria for being good therapeutics (Abedon and Thomas-Abedon, 2010), thereby limiting the bacterial strains that can be targeted. Moreover, they can interact with the immune system and release bacterial components (including toxins) while killing bacteria, resulting in harmful immune responses. In addition, the medical establishment is mostly unfamiliar with phages and few phage product passed regulatory standards (Kutter et al., 2010). Finally, the general perception by the general public of phages as “viruses” might be misinterpreted and limits its exploitation in therapy being connected to viral pathogens that lead to human diseases. Although the advantages of phage therapy looked promising, so far it has been exploited more for the phages’ ability to directly reverse the aggregation of misfolded proteins in the brain [for example, in Alzheimer’s of Parkinson’s diseases (Messing, 2016)] rather than to target and correct gut dysbiosis related to brain pathologies. For example, the phage therapy might contribute to future therapeutic intervention in Parkinson’s disease, which should need to be confirmed in further studies through targeted approaches to manipulate the phagobiota. This will be based on significant alterations in the representation of certain bacteriophages in the phagobiota of people with Parkinson’s disease (Tetz et al., 2018). Nevertheless, so far, the phage therapy had not been directly exploited to modulate gut microbiota in order to ameliorate neurological conditions.
Beyond the antimicrobial and phage therapies, the FMT, which involves the transplantation of functional microbiota from healthy individuals into the GI tract of people suffering from a certain disease in order to “reconstruct” the healthy microbial composition and improve the clinical symptoms, surely represents a new therapeutic approach from the gut to the brain, and it has been investigated in a number of clinical trials (both concluded and ongoing) in multiple conditions, such as hepatic encephalopathy, ASD, Parkinson’s disease, multiple sclerosis, Alzheimer’s disease, and epilepsy [see the reviews (Evrensel and Ceylan, 2016; Xu et al., 2021)].
Studies performed in animal models showed that FMT is effective is transferring/restoring disease-associated features: (i) FMT using fecal transplants derived from normal mice into a mouse model of Parkinson’s disease reduced Parkinson-related signatures, namely, microglia and astrocyte activation in the substantia nigra, gut microbial dysbiosis, and decreased fecal SCFAs, and it alleviated physical impairment and increased striatal dopamine and serotonin content (Sun et al., 2018); (ii) FMT using fecal transplants obtained from people with major depression into normal mice induced behavioral and physiological features characteristic of depression, including anhedonia and anxiety-like behaviors (Kelly et al., 2016); (iii) FMT from normal mice to the Fmr1 KO mice (an animal model of Fragile X syndrome) ameliorated the autistic-like behaviors, especially memory deficits and social withdrawal (Goo et al., 2020).
In humans, three rounds of FMT were effective in improving (i) constipation and motor symptoms in a person with Parkinson’s disease (the improvement of motor symptoms lasted only for 2 months) (Huang et al., 2019), and (ii) neurological and GI symptoms in a 17-year-old patient with epilepsy complicated with Crohn’s disease (He et al., 2017). In children with ASD, FMT showed to be effective not only in reducing GI disorders (Zebrowska et al., 2021), but also in alleviating neurological symptoms. In fact, FMT performed in children with ASD improved the autistic behavioral symptoms together with an improvement of bacterial diversity toward a physiological state (Kang et al., 2019). The positive effects persisted for 8 weeks after the end of FMT. Another independent clinical trial showed the positive effect of FMT in ameliorating autistic-like symptoms (Zhao et al., 2019).
In particular, so far, FMT proved to be effective in ameliorating neurological symptoms in patients that underwent fecal transplantation firstly because of infections caused by C. difficile (Carlucci et al., 2016; Evrensel and Ceylan, 2016; Zhang et al., 2018; Vendrik et al., 2020; Xu et al., 2021). In a very recent case report, Park et al. (2021) explored the FMT strategy in a patient with Alzheimer’s disease. The patient was diagnosed with severe C. difficile infection and, given the failure of the antibiotics treatment, she underwent two rounds of FMT; stools were derived from a healthy donor. Following the transplantation and the eradication of the pathogen, the patient showed improvements in GI symptoms and, importantly, a slight improvement in the cognitive functions in terms of short-term memory, semantic skills, attention, and non-verbal learning, together with a marked improvement in mood and expressive affection (Park et al., 2021). A second study showed remission of C. difficile infection and improvement in cognitive performance in a patient with Alzheimer’s disease who underwent FMT derived from stools from his wife (Hazan, 2020). The latter two studies in humans confirmed the results obtained in a previous study obtained in a mouse model of Alzheimer’s disease that showed improved cognitive function after FMT derived from normal control (Sun et al., 2019). Altogether, these studies in humans and in an animal model gave insights into how FMT could represent a valuable tool to ameliorate both GI and cognitive symptoms in Alzheimer’s disease. Two studies examined the effect of FMT in a total of four patients with multiple sclerosis and diagnosed with C. difficile infection (Borody et al., 2011; Makkawi et al., 2018). FMT resolved C. difficile infection and constipation together with a progressive improvement in neurological symptoms. Some cases reported slow resolution of leg paresthesia and regaining the ability to walk long distances unassisted.
Despite the fact that FMT showed remarkable therapeutic features, it transfers hundreds of strains and it could thereby potentially be problematic because donors could transfer opportunistic pathogens or infections to recipients. Different from FMT, the use of probiotics, which only supplements some bacterial strains, has been shown to affect the functionality of the CNS by means of beneficial interactions with the commensal microbial gut community and by modulation of gut-derived inflammation (Wang et al., 2016). For example, since pro-inflammatory events triggered by LPS might be implicated in neurodegenerative processes [like that one of the nigrostriatal dopaminergic system in rodents (Castano et al., 1998)], studies challenged whether the administration of probiotics can attenuate LPS effects. In a first study, Rodes et al. (2013) investigated the effects of probiotics Lactobacillus and Bifidobacterium on gut-derived LPS and inflammatory cytokine concentrations using human colonic microbiota model. After 14 days of administration with either B. bifidum, L. rhamnosus, B. longum, and B. longum subsp. Infantis, LPS concentrations were reduced. B. longum subsp. infantis showed higher ability to reduce TNF-α concentrations and to increase IL-4 concentrations (Rodes et al., 2013). In a successive study, Musa et al. (2017) investigated in mice whether lactic acid bacteria (either Lactobacillus fermentum LAB9 or L. casei LABPC) fermented cow’s milk exhibited neuroprotective properties against the LPS-induced inflammatory effects. The authors found out that oral administration of fermented cow’s milk containing lactic acid bacteria attenuated memory deficits (assessed via the Morris Water Maze) in mice treated with LPS. Moreover, the treatment increased antioxidants and reduced pro-inflammatory cytokines (Musa et al., 2017). These studies showed potential applications of probiotics in the field of biotherapy. Here, it follows an extensive overview of the microbiota-targeted strategies in brain disorders using pre- and probiotics. Table 2 summarizes pre/probiotic effects in animal models and in clinical trials in people with neurological disorders.
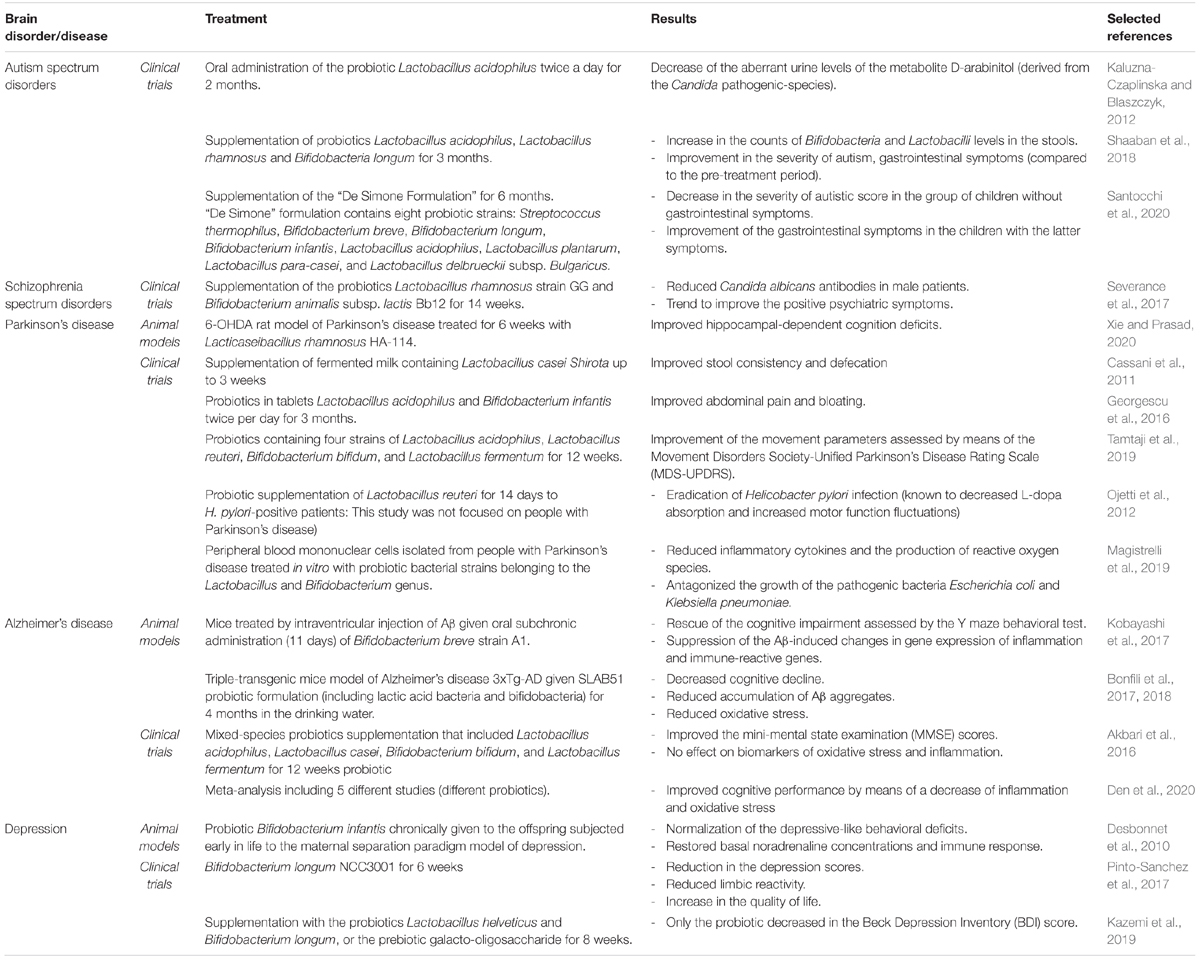
Table 2. Pre/probiotic effects in animal models and in clinical trials in people with neurological disorders.
As far as studies on normal neurodevelopment are concerned, a two-center randomized placebo-controlled trial by Slykerman et al. (2018) aimed to determine whether probiotic supplementation early in life improves childhood cognition outcomes (intelligence, executive function, attention, depression, and anxiety) later in life (at 11 years of age). The authors investigated the effect of early probiotic by giving Lactobacillus rhamnosus, Bifidobacteria animalis, and Bifidobacterium lactis HN019 to mothers 35 weeks pregnant until 6 months and to their children from birth to 2 years. Overall, there were no significant differences in the neurocognitive outcomes (Slykerman et al., 2018). Even if it was unsuccessful, the main and most relevant finding of this study was that probiotics, even if given early in life during critical brain development, did not have a negative impact in normal brain function outcomes later in life.
Clinical studies exploited the use of probiotic in ameliorating the core symptoms of children with ASD. Two clinical trials reported improved GI symptoms (lower abdominal pain) by introducing prebiotics in the diet of children with ASD (Grimaldi et al., 2018; Sanctuary et al., 2019). Among the first clinical trials using probiotics, Kaluzna-Czaplinska and Blaszczyk (2012) investigated whether the probiotic Lactobacillus acidophilus could control the level of D-arabinitol (DA), a metabolite of most pathogenic Candida species found to be elevated in urine of autistic patients (despite the fact that there are data that endogenous DA might be derived also from the brain and not entirely attributable to Candida). The probiotic was given orally twice a day for 2 months, and it did interestingly decreased the DA level in the urine (Kaluzna-Czaplinska and Blaszczyk, 2012). In another study, Tomova et al. (2015) showed that a probiotic mixture of three strains of Lactobacillus, two strains of Bifidobacteria, and one strain of Streptococcus reduced levels of Bifidobacteria and Lactobacillus reduced levels of TNFα in the stools (Tomova et al., 2015). Shaaban et al. (2018) evaluated the efficacy and tolerability of probiotics L. acidophilus, Lactobacillus rhamnosus, and Bifidobacteria longum in an Egyptian cohort of children with ASD (trial number UMIN000026157). After 3 months of treatment, the stool from autistic children showed increases in the counts of Bifidobacteria and Lactobacilli levels, and a significant improvement in the severity of autism and GI symptoms, compared to the baseline (Shaaban et al., 2018). Santocchi et al. (2020) performed a double-blind randomized, placebo-controlled trial (NCT02708901) to determine the effects of supplementation with probiotics on specific GI symptoms, core ASD deficits, and cognitive and language development. The probiotic supplementation (De Simone Formulation) was a patented mixture (approved for use in children; marketed as Vivomixx® in EU, Visbiome® in United States) containing eight probiotic strains: Streptococcus thermophilus, Bifidobacterium breve, Bifidobacterium longum, B. infantis, L. acidophilus, Lactobacillus plantarum, Lactobacillus para-casei, and Lactobacillus delbrueckii subsp. bulgaricus. After 6 months of supplementation with the probiotics mixture, there was no effect when pooling all the data together, but differences were detected when grouping autistic children presenting or not presenting GI symptoms: in the group of children without GI symptoms, the authors reported a decrease in the severity of ASD score, while in the group with GI symptoms, the authors reported an improvement of the latter symptoms (Santocchi et al., 2020). Although the reviewed clinical trials were not standardized for the probiotics used (multiple different strains and concentrations were used) and varied for the duration of treatments, they opened up new and interesting non-invasive therapeutic avenues for ameliorating both the GI and the core symptoms in children with ASD.
As far as probiotic treatment in SSDs is concerned, given the increase in the abundance of gut C. albicans in male patients seen by Severance et al. (2016), the same group performed a randomized, double-blind, placebo-controlled trial (NCT01242371) over a 14-week period to test whether the manipulation of the intestinal microbiome by probiotics could ameliorate yeast imbalances, associated gut discomfort, and psychiatric symptoms. The probiotic formulation contained Lactobacillus rhamnosus strain GG and Bifidobacterium animalis subsp. lactis Bb12. The treatment reduced C. albicans antibodies in males, but not in females, and it showed a trend to improve the positive psychiatric symptoms (Severance et al., 2017) showing the effectiveness of probiotic treatment in restoring fungal gut imbalance and relieving SSD symptoms. Earlier, Dickerson et al. (2014) used the same probiotics formulation over a 14-week treatment trial finding no effect in reducing symptom severity, but the treatment did ameliorate bowel difficulty (Dickerson et al., 2014).
As discussed earlier, Parkinson’s disease is a cognitive and movement disorder characterized by the progressive degeneration of dopaminergic neurons in substantia nigra pars compacta resulting in dopamine deficiency in the striatum, and gut microbiota dysfunctions play a role in the pathogenesis of the disease, providing new targets for therapeutic interventions by means of the use of probiotics. Initial studies showed that (i) the use probiotics in the form of fermented milk containing Lactobacillus casei shirota improved stool consistency and defecation in people with Parkinson’s disease (Cassani et al., 2011), and that (ii) probiotics in tablets with L. acidophilus and B. infantis improved abdominal pain and bloating (Georgescu et al., 2016). The latter studies were followed by a randomized placebo-controlled trial showing that the intake of fermented milk containing fibers and different strains of probiotics relieved constipation in people with Parkinson’s disease (Barichella et al., 2016). Despite the fact that it is known that probiotics can increase gut motility (Waller et al., 2011) and constipation (Dimidi et al., 2017) in normal adults, it is currently unknown how they improve the GI symptoms in people with Parkinson’s disease. One randomized, double-blind, placebo-controlled clinical trial (IRCT2017082434497N4) (Tamtaji et al., 2019) assessed the impacts of probiotic supplementation on movement and metabolic parameters (assessed by means of The Movement Disorders Society-Unified Parkinson’s Disease Rating Scale, MDS-UPDRS) in individuals with Parkinson’s disease. The tested probiotics, capsules containing four strains (L. acidophilus, L. reuteri, Bifidobacterium bifidum, and L. fermentum), had useful impact on the MDS-UPDRS.
Infections caused by H. pylori decreased L-dopa absorption and increased motor function fluctuations in people with Parkinson’s disease (Pierantozzi et al., 2001). Since it has been shown that these effects are reversed by eradicating the pathogen (Pierantozzi et al., 2006; Hashim et al., 2014), two studies, one in wild type (normal) mice using probiotic supplementation of B. bifidum CECT 7366 (Chenoll et al., 2011) and one in humans using L. reuteri (Ojetti et al., 2012) (respectively), contribute to the eradication of the pathogen.
Since oxidative stress and peripheral immune activation do play a role in Parkinson pathogenesis, Magistrelli et al. (2019) used an in vitro approach to explore the effects of probiotic bacterial strains belonging to the Lactobacillus and Bifidobacterium genus on the peripheral blood mononuclear cells isolated from people with Parkinson’s disease and healthy controls, and found out that probiotics decreased pro-inflammatory cytokines and pathogenic bacterial overgrowth. First, the authors showed that all the strains used in the study did not carry the tyrosine decarboxylase genes, known to reduce the level of l-3,4-dihydroxyphenylalanine (levodopa) in people with Parkinson’s disease under treatment. Moreover, they found out that all probiotics tested reduced inflammatory cytokines and the production of reactive oxygen species (ROS) in both groups, with L. salivarius LS01 and L. acidophilus found to reduce pro-inflammatory and increase the anti-inflammatory cytokines with a higher extent in cells derived from people with Parkinson’s disease than from healthy controls (Magistrelli et al., 2019). Furthermore, most of the probiotics antagonized the growth of the pathogenic bacteria E. coli and Klebsiella pneumoniae (Magistrelli et al., 2019).
As far as probiotic effect on cognitive impairment is concerned, Xie and Prasad (2020) exploit the neurotoxin (6-hydroxydopamine, 6-OHDA) rat model of Parkinson’s disease to explore the impact of a probiotic treatment (Lacticaseibacillus rhamnosus HA-114) on anxiety and memory. After 6-OHDA-lesion of the striatum, (i) the authors assessed anxiety-like behavior using the elevated plus maze, and (ii) cognition was assessed for both non-hippocampal- and hippocampal-dependent memory using the novel object recognition and novel place recognition behavioral tests. Despite the fact that the probiotic L. rhamnosus had no impact on anxiety-like behavior induced by 6-OHDA-lesion, it did selectively improve hippocampal-dependent cognition deficits (Xie and Prasad, 2020) pointing to the potential of probiotics as adjunctive treatment for non-motor symptoms in Parkinson’s disease. However, while certain probiotic strains were shown to be effective for the treatment of cognitive symptoms of Alzheimer’s disease both in animal models (Bonfili et al., 2017, 2018) and in humans (Akbari et al., 2016), it is still unknown whether these therapies may be effective for alleviated cognitive symptoms also in people with Parkinson’s disease.
As for Parkinson’s disease, the finding that the gut microbiota is altered in animal models and people with Alzheimer’s disease raises the possibility of the use of probiotics, which could be an effective therapeutic strategy for managing this neurodegenerative process and to ameliorate the cognitive pathological feature. Kobayashi et al. (2017) investigated the effects of oral subchronic (11 days) administration of B. breve strain A1 on the behavior and the physiological processes using a mouse model of the disease, namely, mice treated by intraventricular injection of Aβ. The authors found out that the treatment reversed the cognitive impairment assessed by the Y maze behavioral test and reduced the latency time in the passive avoidance test, thus indicating that the probiotic prevented the cognitive dysfunctions induced by Aβ. Moreover, the authors showed that B. breve strain A1 suppressed the Aβ-induced changes in gene expression of inflammation and immune-reactive genes in the hippocampus, and bacteria-derived metabolite acetate partially ameliorated the cognitive decline observed in the murine model (Kobayashi et al., 2017). In another work, Bonfili et al. (2017) assessed whether a modulation of the microbiota may induce positive effects on the neuronal pathways known to be able to slow down the progression of the pathology. In order to achieve this, the authors assessed whether treatment with the SLAB51 probiotic formulation (including lactic acid bacteria and bifidobacteria) could prevent the onset and affect the progression of the pathology in the triple-transgenic mouse model of Alzheimer’s disease, named 3xTg-AD. The SLAB51 probiotic formulation is known to affect the composition of gut microbiota and its metabolites by influencing plasma concentration of inflammatory cytokines and key metabolic hormones that were considered therapeutic targets in neurodegeneration. The mice that received the SLAB51 formulation showed (i) partial restoration of two impaired neuronal proteolytic pathways, namely, the ubiquitin proteasome system and autophagy; (ii) decreased cognitive decline, due to a reduction in brain damage; and (iii) reduced accumulation of Aβ aggregates (Bonfili et al., 2017). In a successive study, the same research group demonstrated that the SLAB51 formulation exerted a neuroprotective effect by reducing oxidative stress via the Sirtuin-1 (SIRT1) pathway, a NAD + -dependent protein deacetylase pathway (Bonfili et al., 2018). Thus, this research group demonstrated that a modulation of the microbiota by means of probiotics can slow down the progression of the disease.
As far as the effect of probiotics in people with Alzheimer’s disease is concerned, Akbari et al. (2016) performed a randomized, double-blind, and controlled clinical trial (IRCT201511305623N60) among 60 people with Alzheimer’s disease to assess the effects of a 12-week probiotic supplementation on cognitive function and metabolic status. The authors used a mixed-species product that included L. acidophilus, L. casei, B. bifidum, and L. fermentum. The oral chronic treatment improved the mini-mental state examination (MMSE) scores, while it had no effect on other biomarkers of oxidative stress and inflammation (Akbari et al., 2016). A recent meta-analysis including five studies involving 297 subjects indicated that probiotics improved cognitive performance in people with Alzheimer’s disease or mild cognitive impairment via decreasing inflammation and oxidative stress (Den et al., 2020).
Altogether, these studies in rodent models and humans clearly show the potential use of probiotic supplementation in preventing, ameliorating, and slowing down the cognitive deficits of Alzheimer’s disease.
As far as depression is concerned, a study performed by Desbonnet et al. (2010) investigated the effect of the probiotic B. infantis in a very established model of depression, the maternal separation paradigm, where separation of the rat pups from the mother results in depression-like behaviors (Tractenberg et al., 2016). The authors applied the maternal separation protocol to the pups during the early postnatal life, and later in life, adults were chronically treated with either Bifidobacteria probiotic or citalopram (an commonly used antidepressant) and their depression-like behavior was assessed by means of the forced swim test (FST; a test centered on the rat response to the threat of drowning; rats with a depression-like behavior swim less than those not showing a depressive-like behavior). In control animals, as expected, maternal separation reduced swim behavior and increased immobility in the FST; moreover, it decreased noradrenaline (NA) content in the brain, and enhanced peripheral interleukin (IL)-6 release. Surprisingly, Bifidobacteria probiotic chronic treatment normalized the depressive-like behavioral deficits and restored basal NA concentrations and also the immune response, suggesting that probiotics (Bifidobacteria in this case) may have therapeutic applications in mood disorders (Desbonnet et al., 2010).
A randomized double-blind placebo-controlled clinical trial (NCT01276626) examined the effect of the B. longum NCC3001 in adults with IBS and also mild to moderate anxiety and/or depression (Pinto-Sanchez et al., 2017). The authors found out that after 6 weeks of probiotics treatment, there was a reduction in the depression scores (but not anxiety) together with an increase in the quality of life in comparison with the placebo. The improvement in depression was associated with changes in brain activation patterns assessed by means of functional magnetic resonance imaging (fMRI) that indicate that the probiotic reduces limbic reactivity. In another randomized double-blind placebo-controlled clinical trial, Kazemi et al. (2019) compared the effect of supplementation with the probiotics Lactobacillus helveticus and B. longum, or the prebiotic galacto-oligosaccharide on people with major depressive disorder. Eight weeks of probiotics supplementation resulted in a significant decrease in the Beck Depression Inventory (BDI) score in comparison, while the prebiotic supplementation had no effect in comparison to the placebo group (Kazemi et al., 2019). The reviewed studies performed in humans highlighted the potential therapeutic effect of probiotic in people with depression.
In conclusion, the gut brain plays an important role in both the physiology and the pathology of the brain, as highlighted here in brain conditions spanning from neurodevelopmental disorders to Alzheimer’s and Parkinson’s diseases and depression. Even if their precise mechanism of action required further investigations, probiotic supplement treatments are valuable tools with beneficial future therapeutic avenues for neurological conditions. Many data recently emerged on dysbiotic states connected to neuropathological conditions, but still more clinical data are needed to demonstrate that correcting the dysbiotic state would restore physiological brain functions. Probiotics still come with limitations because the field is still poorly regulated by law and they are investigated in clinical trials as a “food supplement” rather than as a “drug.” Moreover, as shown in this review, there are many promising bacterial families that need to be addressed in clinical trials, but often they are not translated in probiotics simply because of the limited choice of bacteria available as probiotics. Probiotic supplementation occurs via oral intake, although few studies are available about the effective gut colonization of the bacterial strains present in probiotics. Nevertheless, different probiotic formulations guarantee microbe survival in their transit through the acidic pH of the stomach to reach the gut. In the near future, thanks to the continuing research on gut brain–brain interactions in physiological and pathological conditions together with the increment of available microbial strains suitable as probiotic, specific gut dysbiosis will be specifically tackled by targeted interventions in order to equilibrate/compensate the dysbiotic state.
Both authors conceptualized, wrote, read, and revised the manuscript.
This study was supported by the BioArte Ltd.
MB is the CEO of BioArte. GD is a consultant for BioArte.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbott, R. D., Petrovitch, H., White, L. R., Masaki, K. H., Tanner, C. M., Curb, J. D., et al. (2001). Frequency of bowel movements and the future risk of Parkinson’s disease. Neurology 57, 456–462. doi: 10.1212/wnl.57.3.456
Abedon, S. T., and Thomas-Abedon, C. (2010). Phage therapy pharmacology. Curr. Pharm. Biotechnol. 11, 28–47. doi: 10.2174/138920110790725410
Akbari, E., Asemi, Z., Daneshvar Kakhaki, R., Bahmani, F., Kouchaki, E., Tamtaji, O. R., et al. (2016). Effect of probiotic supplementation on cognitive function and metabolic status in Alzheimer’s disease: a randomized, double-blind and controlled trial. Front. Aging Neurosci. 8:256. doi: 10.3389/fnagi.2016.00256
Altura, M. A., Heath-Heckman, E. A., Gillette, A., Kremer, N., Krachler, A. M., Brennan, C., et al. (2013). The first engagement of partners in the Euprymna scolopes-vibrio fischeri symbiosis is a two-step process initiated by a few environmental symbiont cells. Environ. Microbiol. 15, 2937–2950.
Amir, R. E., Van Den Veyver, I. B., Wan, M., Tran, C. Q., Francke, U., and Zoghbi, H. Y. (1999). Rett syndrome is caused by mutations in X-linked MECP2, encoding methyl-CpG-binding protein 2. Nat. Genet. 23, 185–188. doi: 10.1038/13810
Antony, P. M., Diederich, N. J., Kruger, R., and Balling, R. (2013). The hallmarks of Parkinson’s disease. FEBS J. 280, 5981–5993.
Aoki-Yoshida, A., Aoki, R., Moriya, N., Goto, T., Kubota, Y., Toyoda, A., et al. (2016). Omics studies of the murine intestinal ecosystem exposed to subchronic and mild social defeat stress. J. Proteome Res. 15, 3126–3138. doi: 10.1021/acs.jproteome.6b00262
Arboleya, S., Binetti, A., Salazar, N., Fernandez, N., Solis, G., Hernandez-Barranco, A., et al. (2012). Establishment and development of intestinal microbiota in preterm neonates. FEMS Microbiol. Ecol. 79, 763–772. doi: 10.1111/j.1574-6941.2011.01261.x
Asano, Y., Hiramoto, T., Nishino, R., Aiba, Y., Kimura, T., Yoshihara, K., et al. (2012). Critical role of gut microbiota in the production of biologically active, free catecholamines in the gut lumen of mice. Am. J. Physiol. Gastrointest. Liver Physiol. 303, G1288–G1295.
Ashraf, G. M., Tarasov, V. V., Makhmutovsmall, A., Chubarev, V. N., Avila-Rodriguez, M., Bachurin, S. O., et al. (2019). The possibility of an infectious etiology of Alzheimer disease. Mol. Neurobiol. 56, 4479–4491. doi: 10.1007/s12035-018-1388-y
Bachurin, S. O., Gavrilova, S. I., Samsonova, A., Barreto, G. E., and Aliev, G. (2018). Mild cognitive impairment due to Alzheimer disease: contemporary approaches to diagnostics and pharmacological intervention. Pharmacol. Res. 129, 216–226. doi: 10.1016/j.phrs.2017.11.021
Barichella, M., Pacchetti, C., Bolliri, C., Cassani, E., Iorio, L., Pusani, C., et al. (2016). Probiotics and prebiotic fiber for constipation associated with Parkinson disease: an RCT. Neurology 87, 1274–1280. doi: 10.1212/wnl.0000000000003127
Barrenschee, M., Zorenkov, D., Bottner, M., Lange, C., Cossais, F., Scharf, A. B., et al. (2017). Distinct pattern of enteric phospho-alpha-synuclein aggregates and gene expression profiles in patients with Parkinson’s disease. Acta Neuropathol. Commun. 5:1.
Beach, T. G., Adler, C. H., Sue, L. I., Shill, H. A., Driver-Dunckley, E., Mehta, S. H., et al. (2021). Vagus nerve and stomach synucleinopathy in Parkinson’s disease, incidental lewy body disease, and normal elderly subjects: evidence against the “Body-First” Hypothesis. J. Parkinsons Dis. 12, 1–11.
Bedarf, J. R., Hildebrand, F., Coelho, L. P., Sunagawa, S., Bahram, M., Goeser, F., et al. (2017). Functional implications of microbial and viral gut metagenome changes in early stage L-DOPA-naive Parkinson’s disease patients. Genome Med. 9:39.
Berardi, N., Pizzorusso, T., and Maffei, L. (2000). Critical periods during sensory development. Curr. Opin. Neurobiol. 10, 138–145. doi: 10.1016/s0959-4388(99)00047-1
Berdoy, M., Webster, J. P., and Macdonald, D. W. (2000). Fatal attraction in rats infected with Toxoplasma gondii. Proc. Biol. Sci. 267, 1591–1594.
Biagi, E., Candela, M., Centanni, M., Consolandi, C., Rampelli, S., Turroni, S., et al. (2014). Gut microbiome in Down syndrome. PLoS One 9:e112023. doi: 10.1371/journal.pone.0112023
Bittles, A. H., Bower, C., Hussain, R., and Glasson, E. J. (2007). The four ages of Down syndrome. Eur. J. Public Health 17, 221–225. doi: 10.1093/eurpub/ckl103
Blanchard, E. B., Scharff, L., Schwarz, S. P., Suls, J. M., and Barlow, D. H. (1990). The role of anxiety and depression in the irritable bowel syndrome. Behav. Res. Ther. 28, 401–405.
Boggio, E. M., Lonetti, G., Pizzorusso, T., and Giustetto, M. (2010). Synaptic determinants of rett syndrome. Front. Synaptic Neurosci. 2:28. doi: 10.3389/fnsyn.2010.00028
Bonfili, L., Cecarini, V., Berardi, S., Scarpona, S., Suchodolski, J. S., Nasuti, C., et al. (2017). Microbiota modulation counteracts Alzheimer’s disease progression influencing neuronal proteolysis and gut hormones plasma levels. Sci. Rep. 7:2426.
Bonfili, L., Cecarini, V., Cuccioloni, M., Angeletti, M., Berardi, S., Scarpona, S., et al. (2018). SLAB51 probiotic formulation activates SIRT1 pathway promoting antioxidant and neuroprotective effects in an AD mouse model. Mol. Neurobiol. 55, 7987–8000. doi: 10.1007/s12035-018-0973-4
Borghi, E., Borgo, F., Severgnini, M., Savini, M. N., Casiraghi, M. C., and Vignoli, A. (2017). Rett syndrome: a focus on gut microbiota. Int. J. Mol. Sci. 18:344. doi: 10.3390/ijms18020344
Borody, T. L. S., Campbell, J., Torres, M., and Nowak, A. (2011). Fecal microbiota transplantation (FMT) in multiple sclerosis (MS). Am. J. Gastroenterol. 106:S352.
Braak, H., and Braak, E. (1991). Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol. 82, 239–259. doi: 10.1007/bf00308809
Braak, H., Rub, U., Gai, W. P., and Del Tredici, K. (2003). Idiopathic Parkinson’s disease: possible routes by which vulnerable neuronal types may be subject to neuroinvasion by an unknown pathogen. J. Neural Transm. (Vienna) 110, 517–536. doi: 10.1007/s00702-002-0808-2
Braniste, V., Al-Asmakh, M., Kowal, C., Anuar, F., Abbaspour, A., Toth, M., et al. (2014). The gut microbiota influences blood-brain barrier permeability in mice. Sci. Transl. Med. 6:263ra158.
Breen, D. P., Halliday, G. M., and Lang, A. E. (2019). Gut-brain axis and the spread of alpha-synuclein pathology: vagal highway or dead end? Mov. Disord. 34, 307–316. doi: 10.1002/mds.27556
Bruce-Keller, A. J., Keller, J. N., and Morrison, C. D. (2009). Obesity and vulnerability of the CNS. Biochim. Biophys. Acta 1792, 395–400.
Bruce-Keller, A. J., Salbaum, J. M., Luo, M., Blanchard, E. T., Taylor, C. M., Welsh, D. A., et al. (2015). Obese-type gut microbiota induce neurobehavioral changes in the absence of obesity. Biol. Psychiatry 77, 607–615. doi: 10.1016/j.biopsych.2014.07.012
Buffington, S. A., Di Prisco, G. V., Auchtung, T. A., Ajami, N. J., Petrosino, J. F., and Costa-Mattioli, M. (2016). Microbial reconstitution reverses maternal diet-induced social and synaptic deficits in offspring. Cell 165, 1762–1775. doi: 10.1016/j.cell.2016.06.001
Cai, Z., Hussain, M. D., and Yan, L. J. (2014). Microglia, neuroinflammation, and beta-amyloid protein in Alzheimer’s disease. Int. J. Neurosci. 124, 307–321. doi: 10.3109/00207454.2013.833510
Cao, X., Lin, P., Jiang, P., and Li, C. (2013). Characteristics of the gastrointestinal microbiome in children with autism spectrum disorder: a systematic review. Shanghai Arch. Psychiatry 25, 342–353.
Cappellano, G., Carecchio, M., Fleetwood, T., Magistrelli, L., Cantello, R., Dianzani, U., et al. (2013). Immunity and inflammation in neurodegenerative diseases. Am. J. Neurodegener. Dis. 2, 89–107.
Carlton, R. M. (1999). Phage therapy: past history and future prospects. Arch. Immunol. Ther. Exp. (Warsz) 47, 267–274.
Carlucci, C., Petrof, E. O., and Allen-Vercoe, E. (2016). Fecal microbiota-based therapeutics for recurrent clostridium difficile infection, ulcerative colitis and obesity. EBioMedicine 13, 37–45. doi: 10.1016/j.ebiom.2016.09.029
Cassani, E., Privitera, G., Pezzoli, G., Pusani, C., Madio, C., Iorio, L., et al. (2011). Use of probiotics for the treatment of constipation in Parkinson’s disease patients. Minerva Gastroenterol. Dietol. 57, 117–121.
Castano, A., Herrera, A. J., Cano, J., and Machado, A. (1998). Lipopolysaccharide intranigral injection induces inflammatory reaction and damage in nigrostriatal dopaminergic system. J. Neurochem. 70, 1584–1592. doi: 10.1046/j.1471-4159.1998.70041584.x
Castillo-Ruiz, A., Mosley, M., George, A. J., Mussaji, L. F., Fullerton, E. F., Ruszkowski, E. M., et al. (2018). The microbiota influences cell death and microglial colonization in the perinatal mouse brain. Brain Behav. Immun. 67, 218–229. doi: 10.1016/j.bbi.2017.08.027
Castro-Nallar, E., Bendall, M. L., Perez-Losada, M., Sabuncyan, S., Severance, E. G., Dickerson, F. B., et al. (2015). Composition, taxonomy and functional diversity of the oropharynx microbiome in individuals with schizophrenia and controls. PeerJ 3:e1140. doi: 10.7717/peerj.1140
Celik, T., Kamisli, O., Babur, C., Cevik, M. O., Oztuna, D., and Altinayar, S. (2010). Is there a relationship between Toxoplasma gondii infection and idiopathic Parkinson’s disease? Scand. J. Infect. Dis. 42, 604–608. doi: 10.3109/00365541003716500
Chan, B. K., Abedon, S. T., and Loc-Carrillo, C. (2013). Phage cocktails and the future of phage therapy. Future Microbiol. 8, 769–783. doi: 10.2217/fmb.13.47
Chen, H., Zhao, E. J., Zhang, W., Lu, Y., Liu, R., Huang, X., et al. (2015). Meta-analyses on prevalence of selected Parkinson’s nonmotor symptoms before and after diagnosis. Transl. Neurodegener. 4:1.
Chen, S. G., Stribinskis, V., Rane, M. J., Demuth, D. R., Gozal, E., Roberts, A. M., et al. (2016). Exposure to the functional bacterial amyloid protein curli enhances alpha-synuclein aggregation in aged fischer 344 rats and Caenorhabditis elegans. Sci. Rep. 6:34477.
Chen, Y., Mao, C. J., Li, S. J., Wang, F., Chen, J., Zhang, H. J., et al. (2015). Quantitative and fiber-selective evaluation of pain and sensory dysfunction in patients with Parkinson’s disease. Parkinsonism Relat. Disord. 21, 361–365. doi: 10.1016/j.parkreldis.2015.01.008
Chenoll, E., Casinos, B., Bataller, E., Astals, P., Echevarria, J., Iglesias, J. R., et al. (2011). Novel probiotic Bifidobacterium bifidum CECT 7366 strain active against the pathogenic bacterium Helicobacter pylori. Appl. Environ. Microbiol. 77, 1335–1343. doi: 10.1128/aem.01820-10
Chiu, W. C., Tsan, Y. T., Tsai, S. L., Chang, C. J., Wang, J. D., Chen, P. C., et al. (2014). Hepatitis C viral infection and the risk of dementia. Eur. J. Neurol. 21:1068-e59.
Clairembault, T., Leclair-Visonneau, L., Neunlist, M., and Derkinderen, P. (2015). Enteric glial cells: new players in Parkinson’s disease? Mov. Disord. 30, 494–498. doi: 10.1002/mds.25979
Clarke, G., Grenham, S., Scully, P., Fitzgerald, P., Moloney, R. D., Shanahan, F., et al. (2013). The microbiome-gut-brain axis during early life regulates the hippocampal serotonergic system in a sex-dependent manner. Mol. Psychiatry 18, 666–673. doi: 10.1038/mp.2012.77
Contestabile, A., Magara, S., and Cancedda, L. (2017). The GABAergic hypothesis for cognitive disabilities in down syndrome. Front. Cell. Neurosci. 11:54. doi: 10.3389/fncel.2017.00054
Cryan, J. F., and Dinan, T. G. (2012). Mind-altering microorganisms: the impact of the gut microbiota on brain and behaviour. Nat. Rev. Neurosci. 13, 701–712. doi: 10.1038/nrn3346
da Silva, R. C., and Langoni, H. (2009). Toxoplasma gondii: host-parasite interaction and behavior manipulation. Parasitol. Res. 105, 893–898. doi: 10.1007/s00436-009-1526-6
Darby, T. M., Owens, J. A., Saeedi, B. J., Luo, L., Matthews, J. D., Robinson, B. S., et al. (2019). Lactococcus Lactis Subsp. cremoris is an efficacious beneficial bacterium that limits tissue injury in the Intestine. iScience 12, 356–367. doi: 10.1016/j.isci.2019.01.030
de Souza-Talarico, J. N., De Carvalho, A. P., Brucki, S. M., Nitrini, R., and Ferretti-Rebustini, R. E. (2016). Dementia and cognitive impairment prevalence and associated factors in indigenous populations: a systematic review. Alzheimer Dis. Assoc. Disord. 30, 281–287. doi: 10.1097/wad.0000000000000140
De Vadder, F., Kovatcheva-Datchary, P., Goncalves, D., Vinera, J., Zitoun, C., Duchampt, A., et al. (2014). Microbiota-generated metabolites promote metabolic benefits via gut-brain neural circuits. Cell 156, 84–96. doi: 10.1016/j.cell.2013.12.016
Deidda, G., Bozarth, I. F., and Cancedda, L. (2014). Modulation of GABAergic transmission in development and neurodevelopmental disorders: investigating physiology and pathology to gain therapeutic perspectives. Front. Cell Neurosci. 8:119. doi: 10.3389/fncel.2014.00119
Deidda, G., Parrini, M., Naskar, S., Bozarth, I. F., Contestabile, A., and Cancedda, L. (2015). Reversing excitatory GABAAR signaling restores synaptic plasticity and memory in a mouse model of Down syndrome. Nat. Med. 21, 318–326. doi: 10.1038/nm.3827
Deidda, G., Pierucci, M., Crunelli, V., and Di Giovanni, G. (2021). 5-HT/GABA interaction in neurodevelopment and plasticity. Prog. Brain Res. 259, 287–317. doi: 10.1016/bs.pbr.2021.01.009
Den, H., Dong, X., Chen, M., and Zou, Z. (2020). Efficacy of probiotics on cognition, and biomarkers of inflammation and oxidative stress in adults with Alzheimer’s disease or mild cognitive impairment - a meta-analysis of randomized controlled trials. Aging (Albany NY) 12, 4010–4039. doi: 10.18632/aging.102810
Desbonnet, L., Garrett, L., Clarke, G., Kiely, B., Cryan, J. F., and Dinan, T. G. (2010). Effects of the probiotic Bifidobacterium infantis in the maternal separation model of depression. Neuroscience 170, 1179–1188. doi: 10.1016/j.neuroscience.2010.08.005
Di Cristo, G. (2007). Development of cortical GABAergic circuits and its implications for neurodevelopmental disorders. Clin. Genet. 72, 1–8. doi: 10.1111/j.1399-0004.2007.00822.x
Dickerson, F. B., Stallings, C., Origoni, A., Katsafanas, E., Savage, C. L., Schweinfurth, L. A., et al. (2014). Effect of probiotic supplementation on schizophrenia symptoms and association with gastrointestinal functioning: a randomized, placebo-controlled trial. Prim. Care Companion CNS Disord. 16:PCC.13m01579.
Dierssen, M. (2012). Down syndrome: the brain in trisomic mode. Nat. Rev. Neurosci. 13, 844–858. doi: 10.1038/nrn3314
Dimidi, E., Christodoulides, S., Scott, S. M., and Whelan, K. (2017). Mechanisms of action of probiotics and the gastrointestinal microbiota on gut motility and constipation. Adv. Nutr. 8, 484–494. doi: 10.3945/an.116.014407
Dinan, T. G., and Cryan, J. F. (2015). The impact of gut microbiota on brain and behaviour: implications for psychiatry. Curr. Opin. Clin. Nutr. Metab. Care 18, 552–558. doi: 10.1097/mco.0000000000000221
Dinan, T. G., Borre, Y. E., and Cryan, J. F. (2014). Genomics of schizophrenia: time to consider the gut microbiome? Mol. Psychiatry 19, 1252–1257. doi: 10.1038/mp.2014.93
Disease, G. B. D., Injury, I., and Prevalence, C. (2016). Global, regional, and national incidence, prevalence, and years lived with disability for 310 diseases and injuries, 1990-2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet 388, 1545–1602.
Dowlati, Y., Herrmann, N., Swardfager, W., Liu, H., Sham, L., Reim, E. K., et al. (2010). A meta-analysis of cytokines in major depression. Biol. Psychiatry 67, 446–457. doi: 10.1016/j.biopsych.2009.09.033
Duerkop, B. A. (2018). Bacteriophages shift the focus of the mammalian microbiota. PLoS Pathog. 14:e1007310. doi: 10.1371/journal.ppat.1007310
El Aidy, S., Dinan, T. G., and Cryan, J. F. (2015). Gut microbiota: the conductor in the orchestra of immune-neuroendocrine communication. Clin. Ther. 37, 954–967. doi: 10.1016/j.clinthera.2015.03.002
Erny, D., Hrabe De Angelis, A. L., Jaitin, D., Wieghofer, P., Staszewski, O., David, E., et al. (2015). Host microbiota constantly control maturation and function of microglia in the CNS. Nat. Neurosci. 18, 965–977. doi: 10.1038/nn.4030
Erzurumlu, R. S., and Gaspar, P. (2012). Development and critical period plasticity of the barrel cortex. Eur. J. Neurosci. 35, 1540–1553. doi: 10.1111/j.1460-9568.2012.08075.x
Evrensel, A., and Ceylan, M. E. (2016). Fecal microbiota transplantation and its usage in neuropsychiatric disorders. Clin. Psychopharmacol. Neurosci. 14, 231–237. doi: 10.9758/cpn.2016.14.3.231
Fanaro, S., Chierici, R., Guerrini, P., and Vigi, V. (2003). Intestinal microflora in early infancy: composition and development. Acta Paediatr. Suppl. 91, 48–55. doi: 10.1111/j.1651-2227.2003.tb00646.x
Fasano, A., Visanji, N. P., Liu, L. W., Lang, A. E., and Pfeiffer, R. F. (2015). Gastrointestinal dysfunction in Parkinson’s disease. Lancet Neurol. 14, 625–639.
Finegold, S. M., Dowd, S. E., Gontcharova, V., Liu, C., Henley, K. E., Wolcott, R. D., et al. (2010). Pyrosequencing study of fecal microflora of autistic and control children. Anaerobe 16, 444–453. doi: 10.1016/j.anaerobe.2010.06.008
Forsyth, C. B., Shannon, K. M., Kordower, J. H., Voigt, R. M., Shaikh, M., Jaglin, J. A., et al. (2011). Increased intestinal permeability correlates with sigmoid mucosa alpha-synuclein staining and endotoxin exposure markers in early Parkinson’s disease. PLoS One 6:e28032. doi: 10.1371/journal.pone.0028032
Fullard, M. E., Morley, J. F., and Duda, J. E. (2017). Olfactory dysfunction as an early biomarker in Parkinson’s disease. Neurosci. Bull. 33, 515–525. doi: 10.1007/s12264-017-0170-x
Georgescu, D., Ancusa, O. E., Georgescu, L. A., Ionita, I., and Reisz, D. (2016). Nonmotor gastrointestinal disorders in older patients with Parkinson’s disease: is there hope? Clin. Interv. Aging 11, 1601–1608. doi: 10.2147/cia.s106284
Goehler, L. E., Gaykema, R. P., Opitz, N., Reddaway, R., Badr, N., and Lyte, M. (2005). Activation in vagal afferents and central autonomic pathways: early responses to intestinal infection with Campylobacter jejuni. Brain Behav. Immun. 19, 334–344. doi: 10.1016/j.bbi.2004.09.002
Gomez de Aguero, M., Ganal-Vonarburg, S. C., Fuhrer, T., Rupp, S., Uchimura, Y., Li, H., et al. (2016). The maternal microbiota drives early postnatal innate immune development. Science 351, 1296–1302. doi: 10.1126/science.aad2571
Goo, N., Bae, H. J., Park, K., Kim, J., Jeong, Y., Cai, M., et al. (2020). The effect of fecal microbiota transplantation on autistic-like behaviors in Fmr1 KO mice. Life Sci. 262:118497. doi: 10.1016/j.lfs.2020.118497
Gorecki, A. M., Preskey, L., Bakeberg, M. C., Kenna, J. E., Gildenhuys, C., Macdougall, G., et al. (2019). Altered gut microbiome in Parkinson’s disease and the influence of lipopolysaccharide in a human alpha-synuclein over-expressing mouse model. Front. Neurosci. 13:839. doi: 10.3389/fnins.2019.00839
Gorski, A., Miedzybrodzki, R., Weber-Dabrowska, B., Fortuna, W., Letkiewicz, S., Rogoz, P., et al. (2016). Phage therapy: combating infections with potential for evolving from merely a treatment for complications to targeting diseases. Front. Microbiol. 7:1515. doi: 10.3389/fmicb.2016.01515
Grenham, S., Clarke, G., Cryan, J. F., and Dinan, T. G. (2011). Brain-gut-microbe communication in health and disease. Front. Physiol. 2:94. doi: 10.3389/fphys.2011.00094
Grimaldi, R., Gibson, G. R., Vulevic, J., Giallourou, N., Castro-Mejia, J. L., Hansen, L. H., et al. (2018). A prebiotic intervention study in children with autism spectrum disorders (ASDs). Microbiome 6:133.
Guerrini, R., and Dobyns, W. B. (2014). Malformations of cortical development: clinical features and genetic causes. Lancet Neurol. 13, 710–726. doi: 10.1016/s1474-4422(14)70040-7
Gulden, E., Wong, F. S., and Wen, L. (2015). The gut microbiota and Type 1 diabetes. Clin. Immunol. 159, 143–153.
Gupta, R., and Prasad, Y. (2011). Efficacy of polyvalent bacteriophage P-27/HP to control multidrug resistant Staphylococcus aureus associated with human infections. Curr. Microbiol. 62, 255–260. doi: 10.1007/s00284-010-9699-x
Haehner, A., Hummel, T., and Reichmann, H. (2009). Olfactory dysfunction as a diagnostic marker for Parkinson’s disease. Expert Rev. Neurother. 9, 1773–1779. doi: 10.1586/ern.09.115
Hamano, D. M., Helm, K. S., and Papin, P. J. (1993). Computer-assisted management of liquid radioactive waste at the University of California, San Diego. Health Phys. 64, 192–194. doi: 10.1097/00004032-199302000-00011
Harach, T., Marungruang, N., Duthilleul, N., Cheatham, V., Mc Coy, K. D., Frisoni, G., et al. (2017). Reduction of abeta amyloid pathology in APPPS1 transgenic mice in the absence of gut microbiota. Sci. Rep. 7:41802.
Harden, C. L. (2002). The co-morbidity of depression and epilepsy: epidemiology, etiology, and treatment. Neurology 59, S48–S55.
Hardy, J. A., and Higgins, G. A. (1992). Alzheimer’s disease: the amyloid cascade hypothesis. Science 256, 184–185.
Hasegawa, S., Goto, S., Tsuji, H., Okuno, T., Asahara, T., Nomoto, K., et al. (2015). Intestinal dysbiosis and lowered serum lipopolysaccharide-binding protein in Parkinson’s disease. PLoS One 10:e0142164. doi: 10.1371/journal.pone.0142164
Hashim, H., Azmin, S., Razlan, H., Yahya, N. W., Tan, H. J., Manaf, M. R., et al. (2014). Eradication of Helicobacter pylori infection improves levodopa action, clinical symptoms and quality of life in patients with Parkinson’s disease. PLoS One 9:e112330. doi: 10.1371/journal.pone.0112330
Hazan, S. (2020). Rapid improvement in Alzheimer’s disease symptoms following fecal microbiota transplantation: a case report. J. Int. Med. Res. 48:300060520925930.
He, Z., Cui, B. T., Zhang, T., Li, P., Long, C. Y., Ji, G. Z., et al. (2017). Fecal microbiota transplantation cured epilepsy in a case with Crohn’s disease: the first report. World J. Gastroenterol. 23, 3565–3568. doi: 10.3748/wjg.v23.i19.3565
Hensch, T. K. (2004). Critical period regulation. Annu. Rev. Neurosci. 27, 549–579. doi: 10.1146/annurev.neuro.27.070203.144327
Hill-Burns, E. M., Debelius, J. W., Morton, J. T., Wissemann, W. T., Lewis, M. R., Wallen, Z. D., et al. (2017). Parkinson’s disease and Parkinson’s disease medications have distinct signatures of the gut microbiome. Mov. Disord. 32, 739–749. doi: 10.1002/mds.26942
Houser, M. C., and Tansey, M. G. (2017). The gut-brain axis: is intestinal inflammation a silent driver of Parkinson’s disease pathogenesis? NPJ Parkinsons Dis. 3:3.
Huang, H., Xu, H., Luo, Q., He, J., Li, M., Chen, H., et al. (2019). Fecal microbiota transplantation to treat Parkinson’s disease with constipation: a case report. Medicine (Baltimore) 98:e16163. doi: 10.1097/md.0000000000016163
Hyman, P., and Abedon, S. T. (2010). Bacteriophage host range and bacterial resistance. Adv. Appl. Microbiol. 70, 217–248. doi: 10.1016/s0065-2164(10)70007-1
Itzhaki, R. F., Lin, W. R., Shang, D., Wilcock, G. K., Faragher, B., and Jamieson, G. A. (1997). Herpes simplex virus type 1 in brain and risk of Alzheimer’s disease. Lancet 349, 241–244. doi: 10.1016/s0140-6736(96)10149-5
Jalanka-Tuovinen, J., Salojarvi, J., Salonen, A., Immonen, O., Garsed, K., Kelly, F. M., et al. (2014). Faecal microbiota composition and host-microbe cross-talk following gastroenteritis and in postinfectious irritable bowel syndrome. Gut 63, 1737–1745. doi: 10.1136/gutjnl-2013-305994
Jian, L., Nagarajan, L., De Klerk, N., Ravine, D., Bower, C., Anderson, A., et al. (2006). Predictors of seizure onset in Rett syndrome. J. Pediatr. 149, 542–547. doi: 10.1016/j.jpeds.2006.06.015
Jokela, M., Hamer, M., Singh-Manoux, A., Batty, G. D., and Kivimaki, M. (2014). Association of metabolically healthy obesity with depressive symptoms: pooled analysis of eight studies. Mol. Psychiatry 19, 910–914. doi: 10.1038/mp.2013.162
Kaluzna-Czaplinska, J., and Blaszczyk, S. (2012). The level of arabinitol in autistic children after probiotic therapy. Nutrition 28, 124–126. doi: 10.1016/j.nut.2011.08.002
Kang, D. W., Adams, J. B., Coleman, D. M., Pollard, E. L., Maldonado, J., Mcdonough-Means, S., et al. (2019). Long-term benefit of microbiota transfer therapy on autism symptoms and gut microbiota. Sci. Rep. 9:5821.
Kazemi, A., Noorbala, A. A., Azam, K., Eskandari, M. H., and Djafarian, K. (2019). Effect of probiotic and prebiotic vs placebo on psychological outcomes in patients with major depressive disorder: a randomized clinical trial. Clin. Nutr. 38, 522–528. doi: 10.1016/j.clnu.2018.04.010
Kelly, J. R., Borre, Y., O’ Brien, C., Patterson, E., El Aidy, S., Deane, J., et al. (2016). Transferring the blues: depression-associated gut microbiota induces neurobehavioural changes in the rat. J. Psychiatr. Res. 82, 109–118. doi: 10.1016/j.jpsychires.2016.07.019
Keshavarzian, A., Green, S. J., Engen, P. A., Voigt, R. M., Naqib, A., Forsyth, C. B., et al. (2015). Colonic bacterial composition in Parkinson’s disease. Mov. Disord. 30, 1351–1360. doi: 10.1002/mds.26307
Kim, G. H., Kim, J. E., Rhie, S. J., and Yoon, S. (2015). The role of oxidative stress in neurodegenerative diseases. Exp. Neurobiol. 24, 325–340.
Kim, S., Kim, H., Yim, Y. S., Ha, S., Atarashi, K., Tan, T. G., et al. (2017). Maternal gut bacteria promote neurodevelopmental abnormalities in mouse offspring. Nature 549, 528–532. doi: 10.1038/nature23910
Kimura, I., Miyamoto, J., Ohue-Kitano, R., Watanabe, K., Yamada, T., Onuki, M., et al. (2020). Maternal gut microbiota in pregnancy influences offspring metabolic phenotype in mice. Science 367:eaaw8429. doi: 10.1126/science.aaw8429
Klingelhoefer, L., and Reichmann, H. (2015). Pathogenesis of Parkinson disease–the gut-brain axis and environmental factors. Nat. Rev. Neurol. 11, 625–636. doi: 10.1038/nrneurol.2015.197
Knights, D., Silverberg, M. S., Weersma, R. K., Gevers, D., Dijkstra, G., Huang, H., et al. (2014). Complex host genetics influence the microbiome in inflammatory bowel disease. Genome Med. 6:107.
Kobayashi, Y., Sugahara, H., Shimada, K., Mitsuyama, E., Kuhara, T., Yasuoka, A., et al. (2017). Therapeutic potential of Bifidobacterium breve strain A1 for preventing cognitive impairment in Alzheimer’s disease. Sci. Rep. 7:13510.
Koh, A., De Vadder, F., Kovatcheva-Datchary, P., and Backhed, F. (2016). From dietary fiber to host physiology: short-chain fatty acids as key bacterial metabolites. Cell 165, 1332–1345. doi: 10.1016/j.cell.2016.05.041
Kountouras, J., Boziki, M., Gavalas, E., Zavos, C., Deretzi, G., Grigoriadis, N., et al. (2009). Increased cerebrospinal fluid Helicobacter pylori antibody in Alzheimer’s disease. Int. J. Neurosci. 119, 765–777. doi: 10.1080/00207450902782083
Kowalski, K., and Mulak, A. (2019). Brain-gut-microbiota axis in Alzheimer’s disease. J. Neurogastroenterol. Motil. 25, 48–60.
Kristen, H., Santana, S., Sastre, I., Recuero, M., Bullido, M. J., and Aldudo, J. (2015). Herpes simplex virus type 2 infection induces AD-like neurodegeneration markers in human neuroblastoma cells. Neurobiol. Aging 36, 2737–2747. doi: 10.1016/j.neurobiolaging.2015.06.014
Kutter, E., De Vos, D., Gvasalia, G., Alavidze, Z., Gogokhia, L., Kuhl, S., et al. (2010). Phage therapy in clinical practice: treatment of human infections. Curr. Pharm. Biotechnol. 11, 69–86. doi: 10.2174/138920110790725401
Layeghifard, M., Hwang, D. M., and Guttman, D. S. (2017). Disentangling interactions in the microbiome: a network perspective. Trends Microbiol. 25, 217–228. doi: 10.1016/j.tim.2016.11.008
Le Doare, K., Holder, B., Bassett, A., and Pannaraj, P. S. (2018). Mother’s milk: a purposeful contribution to the development of the infant microbiota and immunity. Front. Immunol. 9:361. doi: 10.3389/fimmu.2018.00361
Levelt, C. N., and Hubener, M. (2012). Critical-period plasticity in the visual cortex. Annu. Rev. Neurosci. 35, 309–330.
Li, B., He, Y., Ma, J., Huang, P., Du, J., Cao, L., et al. (2019). Mild cognitive impairment has similar alterations as Alzheimer’s disease in gut microbiota. Alzheimers Dement. 15, 1357–1366. doi: 10.1016/j.jalz.2019.07.002
Li, W., Wu, X., Hu, X., Wang, T., Liang, S., Duan, Y., et al. (2017). Structural changes of gut microbiota in Parkinson’s disease and its correlation with clinical features. Sci. China Life Sci. 60, 1223–1233. doi: 10.1007/s11427-016-9001-4
Li, Y., Toothaker, J. M., Ben-Simon, S., Ozeri, L., Schweitzer, R., Mccourt, B. T., et al. (2020). In utero human intestine harbors unique metabolome, including bacterial metabolites. JCI Insight 5:e138751.
Liang, D., Leung, R. K., Guan, W., and Au, W. W. (2018). Involvement of gut microbiome in human health and disease: brief overview, knowledge gaps and research opportunities. Gut Pathog. 10:3.
Lim, C., Hammond, C. J., Hingley, S. T., and Balin, B. J. (2014). Chlamydia pneumoniae infection of monocytes in vitro stimulates innate and adaptive immune responses relevant to those in Alzheimer’s disease. J. Neuroinflammation 11:217.
Liu, R., Hong, J., Xu, X., Feng, Q., Zhang, D., Gu, Y., et al. (2017). Gut microbiome and serum metabolome alterations in obesity and after weight-loss intervention. Nat. Med. 23, 859–868.
Lloyd-Price, J., Abu-Ali, G., and Huttenhower, C. (2016). The healthy human microbiome. Genome Med. 8:51.
Luder, C. G., Giraldo-Velasquez, M., Sendtner, M., and Gross, U. (1999). Toxoplasma gondii in primary rat CNS cells: differential contribution of neurons, astrocytes, and microglial cells for the intracerebral development and stage differentiation. Exp. Parasitol. 93, 23–32. doi: 10.1006/expr.1999.4421
Lyte, M. (2010). The microbial organ in the gut as a driver of homeostasis and disease. Med. Hypotheses 74, 634–638. doi: 10.1016/j.mehy.2009.10.025
Ma, Q., Xing, C., Long, W., Wang, H. Y., Liu, Q., and Wang, R. F. (2019). Impact of microbiota on central nervous system and neurological diseases: the gut-brain axis. J. Neuroinflammation 16:53.
MacDonald, A. B., and Miranda, J. M. (1987). Concurrent neocortical borreliosis and Alzheimer’s disease. Hum. Pathol. 18, 759–761. doi: 10.1016/s0046-8177(87)80252-6
Magistrelli, L., Amoruso, A., Mogna, L., Graziano, T., Cantello, R., Pane, M., et al. (2019). Probiotics may have beneficial effects in Parkinson’s disease: in vitro evidence. Front. Immunol. 10:969. doi: 10.3389/fimmu.2019.00969
Makkawi, S., Camara-Lemarroy, C., and Metz, L. (2018). Fecal microbiota transplantation associated with 10 years of stability in a patient with SPMS. Neurol. Neuroimmunol. Neuroinflamm. 5:e459. doi: 10.1212/nxi.0000000000000459
Manrique, P., Dills, M., and Young, M. J. (2017). The human gut phage community and its implications for health and disease. Viruses 9:141. doi: 10.3390/v9060141
Mao, Y. K., Kasper, D. L., Wang, B., Forsythe, P., Bienenstock, J., and Kunze, W. A. (2013). Bacteroides fragilis polysaccharide A is necessary and sufficient for acute activation of intestinal sensory neurons. Nat. Commun. 4:1465.
Maynard, C. L., Elson, C. O., Hatton, R. D., and Weaver, C. T. (2012). Reciprocal interactions of the intestinal microbiota and immune system. Nature 489, 231–241. doi: 10.1038/nature11551
Mertsalmi, T. H., Aho, V. T. E., Pereira, P. A. B., Paulin, L., Pekkonen, E., Auvinen, P., et al. (2017). More than constipation – bowel symptoms in Parkinson’s disease and their connection to gut microbiota. Eur. J. Neurol. 24, 1375–1383. doi: 10.1111/ene.13398
Messing, J. (2016). Phage M13 for the treatment of Alzheimer and Parkinson disease. Gene 583, 85–89. doi: 10.1016/j.gene.2016.02.005
Mihaila, D., Donegan, J., Barns, S., Larocca, D., Du, Q., Zheng, D., et al. (2019). The oral microbiome of early stage Parkinson’s disease and its relationship with functional measures of motor and non-motor function. PLoS One 14:e0218252. doi: 10.1371/journal.pone.0218252
Miller, K. M., Mercado, N. M., and Sortwell, C. E. (2021). Synucleinopathy-associated pathogenesis in Parkinson’s disease and the potential for brain-derived neurotrophic factor. NPJ Parkinsons Dis. 7:35.
Minato, T., Maeda, T., Fujisawa, Y., Tsuji, H., Nomoto, K., Ohno, K., et al. (2017). Progression of Parkinson’s disease is associated with gut dysbiosis: two-year follow-up study. PLoS One 12:e0187307. doi: 10.1371/journal.pone.0187307
Minter, M. R., Zhang, C., Leone, V., Ringus, D. L., Zhang, X., Oyler-Castrillo, P., et al. (2016). Antibiotic-induced perturbations in gut microbial diversity influences neuro-inflammation and amyloidosis in a murine model of Alzheimer’s disease. Sci. Rep. 6:30028.
Miraglia, F., and Colla, E. (2019). Microbiome, Parkinson’s disease and molecular mimicry. Cells 8:222. doi: 10.3390/cells8030222
Moffat, J. J., Ka, M., Jung, E. M., and Kim, W. Y. (2015). Genes and brain malformations associated with abnormal neuron positioning. Mol. Brain 8:72.
Molloy, C. A., and Manning-Courtney, P. (2003). Prevalence of chronic gastrointestinal symptoms in children with autism and autistic spectrum disorders. Autism 7, 165–171. doi: 10.1177/1362361303007002004
Moore, D. J., West, A. B., Dawson, V. L., and Dawson, T. M. (2005). Molecular pathophysiology of Parkinson’s disease. Annu. Rev. Neurosci. 28, 57–87.
Motil, K. J., Caeg, E., Barrish, J. O., Geerts, S., Lane, J. B., Percy, A. K., et al. (2012). Gastrointestinal and nutritional problems occur frequently throughout life in girls and women with Rett syndrome. J. Pediatr. Gastroenterol. Nutr. 55, 292–298. doi: 10.1097/mpg.0b013e31824b6159
Moussavi, S., Chatterji, S., Verdes, E., Tandon, A., Patel, V., and Ustun, B. (2007). Depression, chronic diseases, and decrements in health: results from the World Health Surveys. Lancet 370, 851–858. doi: 10.1016/s0140-6736(07)61415-9
Mulak, A., and Bonaz, B. (2015). Brain-gut-microbiota axis in Parkinson’s disease. World J. Gastroenterol. 21, 10609–10620.
Musa, N. H., Mani, V., Lim, S. M., Vidyadaran, S., Abdul Majeed, A. B., and Ramasamy, K. (2017). Lactobacilli-fermented cow’s milk attenuated lipopolysaccharide-induced neuroinflammation and memory impairment in vitro and in vivo. J. Dairy Res. 84, 488–495. doi: 10.1017/s0022029917000620
Mykletun, A., Bjerkeset, O., Overland, S., Prince, M., Dewey, M., and Stewart, R. (2009). Levels of anxiety and depression as predictors of mortality: the HUNT study. Br. J. Psychiatry 195, 118–125. doi: 10.1192/bjp.bp.108.054866
Nayeri, T., Sarvi, S., Sharif, M., and Daryani, A. (2021). Toxoplasma gondii: a possible etiologic agent for Alzheimer’s disease. Heliyon 7:e07151. doi: 10.1016/j.heliyon.2021.e07151
Ojetti, V., Bruno, G., Ainora, M. E., Gigante, G., Rizzo, G., Roccarina, D., et al. (2012). Impact of Lactobacillus reuteri supplementation on anti-Helicobacter pylori levofloxacin-based second-line therapy. Gastroenterol. Res. Pract. 2012:740381.
Okazaki, H., Lipkin, L. E., and Aronson, S. M. (1961). Diffuse intracytoplasmic ganglionic inclusions (Lewy type) associated with progressive dementia and quadriparesis in flexion. J. Neuropathol. Exp. Neurol. 20, 237–244. doi: 10.1097/00005072-196104000-00007
Pantoja-Feliciano, I. G., Clemente, J. C., Costello, E. K., Perez, M. E., Blaser, M. J., Knight, R., et al. (2013). Biphasic assembly of the murine intestinal microbiota during early development. ISME J. 7, 1112–1115. doi: 10.1038/ismej.2013.15
Paradowski, B., Jaremko, M., Dobosz, T., Leszek, J., and Noga, L. (2007). Evaluation of CSF-Chlamydia pneumoniae, CSF-tau, and CSF-Abeta42 in Alzheimer’s disease and vascular dementia. J. Neurol. 254, 154–159. doi: 10.1007/s00415-006-0298-5
Parashar, A., and Udayabanu, M. (2017). Gut microbiota: implications in Parkinson’s disease. Parkinsonism Relat. Disord. 38, 1–7. doi: 10.1016/b978-0-12-821039-0.00009-5
Park, A. J., Collins, J., Blennerhassett, P. A., Ghia, J. E., Verdu, E. F., Bercik, P., et al. (2013). Altered colonic function and microbiota profile in a mouse model of chronic depression. Neurogastroenterol. Motil. 25, 733–e575.
Park, S. H., Lee, J. H., Shin, J., Kim, J. S., Cha, B., Lee, S., et al. (2021). Cognitive function improvement after fecal microbiota transplantation in Alzheimer’s dementia patient: a case report. Curr. Med. Res. Opin. 1–6. doi: 10.1080/03007995.2021.1957807
Pasquaretta, C., Gomez-Moracho, T., Heeb, P., and Lihoreau, M. (2018). Exploring interactions between the gut microbiota and social behavior through nutrition. Genes (Basel) 9:534. doi: 10.3390/genes9110534
Pequegnat, B., Sagermann, M., Valliani, M., Toh, M., Chow, H., Allen-Vercoe, E., et al. (2013). A vaccine and diagnostic target for Clostridium bolteae, an autism-associated bacterium. Vaccine 31, 2787–2790. doi: 10.1016/j.vaccine.2013.04.018
Perry, R. J., Peng, L., Barry, N. A., Cline, G. W., Zhang, D., Cardone, R. L., et al. (2016). Acetate mediates a microbiome-brain-beta-cell axis to promote metabolic syndrome. Nature 534, 213–217. doi: 10.1038/nature18309
Petrov, V. A., Saltykova, I. V., Zhukova, I. A., Alifirova, V. M., Zhukova, N. G., Dorofeeva, Y. B., et al. (2017). Analysis of gut microbiota in patients with Parkinson’s disease. Bull. Exp. Biol. Med. 162, 734–737.
Pfeiffer, R. F. (2016). Non-motor symptoms in Parkinson’s disease. Parkinsonism Relat. Disord. 22(Suppl. 1), S119–S122.
Pierantozzi, M., Pietroiusti, A., Brusa, L., Galati, S., Stefani, A., Lunardi, G., et al. (2006). Helicobacter pylori eradication and l-dopa absorption in patients with PD and motor fluctuations. Neurology 66, 1824–1829. doi: 10.1212/01.wnl.0000221672.01272.ba
Pierantozzi, M., Pietroiusti, A., Sancesario, G., Lunardi, G., Fedele, E., Giacomini, P., et al. (2001). Reduced L-dopa absorption and increased clinical fluctuations in Helicobacter pylori-infected Parkinson’s disease patients. Neurol. Sci. 22, 89–91. doi: 10.1007/s100720170061
Pinto-Sanchez, M. I., Hall, G. B., Ghajar, K., Nardelli, A., Bolino, C., Lau, J. T., et al. (2017). Probiotic Bifidobacterium longum NCC3001 reduces depression scores and alters brain activity: a pilot study in patients with irritable bowel syndrome. Gastroenterology 153, 448–459.e8.
Pisa, D., Alonso, R., Juarranz, A., Rabano, A., and Carrasco, L. (2015a). Direct visualization of fungal infection in brains from patients with Alzheimer’s disease. J. Alzheimers Dis. 43, 613–624. doi: 10.3233/jad-141386
Pisa, D., Alonso, R., Rabano, A., Rodal, I., and Carrasco, L. (2015b). Different brain regions are infected with fungi in Alzheimer’s disease. Sci. Rep. 5:15015.
Piven, J., Berthier, M. L., Starkstein, S. E., Nehme, E., Pearlson, G., and Folstein, S. (1990). Magnetic resonance imaging evidence for a defect of cerebral cortical development in autism. Am. J. Psychiatry 147, 734–739. doi: 10.1176/ajp.147.6.734
Pluznick, J. L., Protzko, R. J., Gevorgyan, H., Peterlin, Z., Sipos, A., Han, J., et al. (2013). Olfactory receptor responding to gut microbiota-derived signals plays a role in renin secretion and blood pressure regulation. Proc. Natl. Acad. Sci. U.S.A. 110, 4410–4415. doi: 10.1073/pnas.1215927110
Polymeropoulos, M. H., Lavedan, C., Leroy, E., Ide, S. E., Dehejia, A., Dutra, A., et al. (1997). Mutation in the alpha-synuclein gene identified in families with Parkinson’s disease. Science 276, 2045–2047. doi: 10.1126/science.276.5321.2045
Pop, M., Walker, A. W., Paulson, J., Lindsay, B., Antonio, M., Hossain, M. A., et al. (2014). Diarrhea in young children from low-income countries leads to large-scale alterations in intestinal microbiota composition. Genome Biol. 15:R76.
Prandovszky, E., Gaskell, E., Martin, H., Dubey, J. P., Webster, J. P., and Mcconkey, G. A. (2011). The neurotropic parasite Toxoplasma gondii increases dopamine metabolism. PLoS One 6:e23866. doi: 10.1371/journal.pone.0023866
Ratcliffe, E. M., Fan, L., Mohammed, T. J., Anderson, M., Chalazonitis, A., and Gershon, M. D. (2011). Enteric neurons synthesize netrins and are essential for the development of the vagal sensory innervation of the fetal gut. Dev. Neurobiol. 71, 362–373. doi: 10.1002/dneu.20869
Renvoize, E. B., and Hambling, M. H. (1984). Cytomegalovirus infection and Alzheimer’s disease. Age Ageing 13, 205–209.
Renvoize, E. B., Hambling, M. H., Pepper, M. D., and Rajah, S. M. (1979). Possible association of Alzheimer’s disease with HLA-BW15 and cytomegalovirus infection. Lancet 1:1238. doi: 10.1016/s0140-6736(79)91914-7
Rhee, S. H., Pothoulakis, C., and Mayer, E. A. (2009). Principles and clinical implications of the brain-gut-enteric microbiota axis. Nat. Rev. Gastroenterol. Hepatol. 6, 306–314. doi: 10.1038/nrgastro.2009.35
Riviere, G. R., Riviere, K. H., and Smith, K. S. (2002). Molecular and immunological evidence of oral Treponema in the human brain and their association with Alzheimer’s disease. Oral Microbiol. Immunol. 17, 113–118. doi: 10.1046/j.0902-0055.2001.00100.x
Rizzo, G., Copetti, M., Arcuti, S., Martino, D., Fontana, A., and Logroscino, G. (2016). Accuracy of clinical diagnosis of Parkinson disease: a systematic review and meta-analysis. Neurology 86, 566–576. doi: 10.1212/wnl.0000000000002350
Rodes, L., Khan, A., Paul, A., Coussa-Charley, M., Marinescu, D., Tomaro-Duchesneau, C., et al. (2013). Effect of probiotics Lactobacillus and Bifidobacterium on gut-derived lipopolysaccharides and inflammatory cytokines: an in vitro study using a human colonic microbiota model. J. Microbiol. Biotechnol. 23, 518–526. doi: 10.4014/jmb.1205.05018
Sampson, T. R., Debelius, J. W., Thron, T., Janssen, S., Shastri, G. G., Ilhan, Z. E., et al. (2016). Gut microbiota regulate motor deficits and neuroinflammation in a model of Parkinson’s disease. Cell 167, 1469–1480.e12.
Sanctuary, M. R., Kain, J. N., Chen, S. Y., Kalanetra, K., Lemay, D. G., Rose, D. R., et al. (2019). Pilot study of probiotic/colostrum supplementation on gut function in children with autism and gastrointestinal symptoms. PLoS One 14:e0210064. doi: 10.1371/journal.pone.0210064
Santocchi, E., Guiducci, L., Prosperi, M., Calderoni, S., Gaggini, M., Apicella, F., et al. (2020). Effects of probiotic supplementation on gastrointestinal, sensory and core symptoms in autism spectrum disorders: a randomized controlled trial. Front. Psychiatry 11:550593. doi: 10.3389/fpsyt.2020.550593
Santos, S. F., De Oliveira, H. L., Yamada, E. S., Neves, B. C., and Pereira, A. Jr. (2019). The gut and Parkinson’s disease-a bidirectional pathway. Front. Neurol. 10:574. doi: 10.3389/fneur.2019.00574
Savage, D. C. (1977). Microbial ecology of the gastrointestinal tract. Annu. Rev. Microbiol. 31, 107–133.
Scanlan, P. D. (2017). Bacteria-bacteriophage coevolution in the human gut: implications for microbial diversity and functionality. Trends Microbiol. 25, 614–623. doi: 10.1016/j.tim.2017.02.012
Scheperjans, F. (2016). Gut microbiota, 1013 new pieces in the Parkinson’s disease puzzle. Curr. Opin. Neurol. 29, 773–780. doi: 10.1097/wco.0000000000000389
Scheperjans, F., Aho, V., Pereira, P. A., Koskinen, K., Paulin, L., Pekkonen, E., et al. (2015). Gut microbiota are related to Parkinson’s disease and clinical phenotype. Mov. Disord. 30, 350–358.
Scheperjans, F., Derkinderen, P., and Borghammer, P. (2018). The gut and Parkinson’s disease: hype or hope? J. Parkinsons Dis. 8, S31–S39.
Schwarz, E., Maukonen, J., Hyytiainen, T., Kieseppa, T., Oresic, M., Sabunciyan, S., et al. (2018). Analysis of microbiota in first episode psychosis identifies preliminary associations with symptom severity and treatment response. Schizophr. Res. 192, 398–403. doi: 10.1016/j.schres.2017.04.017
Severance, E. G., Gressitt, K. L., Stallings, C. R., Katsafanas, E., Schweinfurth, L. A., Savage, C. L., et al. (2016). Candida albicans exposures, sex specificity and cognitive deficits in schizophrenia and bipolar disorder. NPJ Schizophr. 2:16018.
Severance, E. G., Gressitt, K. L., Stallings, C. R., Katsafanas, E., Schweinfurth, L. A., Savage, C. L. G., et al. (2017). Probiotic normalization of Candida albicans in schizophrenia: a randomized, placebo-controlled, longitudinal pilot study. Brain Behav. Immun. 62, 41–45. doi: 10.1016/j.bbi.2016.11.019
Shaaban, S. Y., El Gendy, Y. G., Mehanna, N. S., El-Senousy, W. M., El-Feki, H. S. A., Saad, K., et al. (2018). The role of probiotics in children with autism spectrum disorder: a prospective, open-label study. Nutr. Neurosci. 21, 676–681. doi: 10.1080/1028415x.2017.1347746
Sibelli, A., Chalder, T., Everitt, H., Workman, P., Windgassen, S., and Moss-Morris, R. (2016). A systematic review with meta-analysis of the role of anxiety and depression in irritable bowel syndrome onset. Psychol. Med. 46, 3065–3080. doi: 10.1017/s0033291716001987
Skurnik, M., Pajunen, M., and Kiljunen, S. (2007). Biotechnological challenges of phage therapy. Biotechnol. Lett. 29, 995–1003. doi: 10.1007/s10529-007-9346-1
Slykerman, R. F., Kang, J., Van Zyl, N., Barthow, C., Wickens, K., Stanley, T., et al. (2018). Effect of early probiotic supplementation on childhood cognition, behaviour and mood a randomised, placebo-controlled trial. Acta Paediatr. 107, 2172–2178. doi: 10.1111/apa.14590
Spielman, L. J., Gibson, D. L., and Klegeris, A. (2018). Unhealthy gut, unhealthy brain: the role of the intestinal microbiota in neurodegenerative diseases. Neurochem. Int. 120, 149–163. doi: 10.1016/j.neuint.2018.08.005
Stafford-Clark, D. (1954). Epilepsy and depression: implications of empirical therapy. Guys Hosp. Rep. 103, 306–316.
Stetler, C., and Miller, G. E. (2011). Depression and hypothalamic-pituitary-adrenal activation: a quantitative summary of four decades of research. Psychosom. Med. 73, 114–126. doi: 10.1097/psy.0b013e31820ad12b
Strandwitz, P., Kim, K. H., Terekhova, D., Liu, J. K., Sharma, A., Levering, J., et al. (2019). GABA-modulating bacteria of the human gut microbiota. Nat. Microbiol. 4, 396–403. doi: 10.1038/s41564-018-0307-3
Strati, F., Cavalieri, D., Albanese, D., De Felice, C., Donati, C., Hayek, J., et al. (2016). Altered gut microbiota in Rett syndrome. Microbiome 4:41.
Stratton, C. W. (2003). Dead bugs don’t mutate: susceptibility issues in the emergence of bacterial resistance. Emerg. Infect. Dis. 9, 10–16. doi: 10.3201/eid0901.020172
Subramaniam, S. R., and Chesselet, M. F. (2013). Mitochondrial dysfunction and oxidative stress in Parkinson’ disease. Prog. Neurobiol. 106-107, 17–32.
Sun, J., Xu, J., Ling, Y., Wang, F., Gong, T., Yang, C., et al. (2019). Fecal microbiota transplantation alleviated Alzheimer’s disease-like pathogenesis in APP/PS1 transgenic mice. Transl. Psychiatry 9:189.
Sun, M. F., and Shen, Y. Q. (2018). Dysbiosis of gut microbiota and microbial metabolites in Parkinson’s Disease. Ageing Res. Rev. 45, 53–61. doi: 10.1016/j.arr.2018.04.004
Sun, M. F., Zhu, Y. L., Zhou, Z. L., Jia, X. B., Xu, Y. D., Yang, Q., et al. (2018). Neuroprotective effects of fecal microbiota transplantation on MPTP-induced Parkinson’s disease mice: gut microbiota, glial reaction and TLR4/TNF-alpha signaling pathway. Brain Behav. Immun. 70, 48–60.
Tamtaji, O. R., Taghizadeh, M., Daneshvar Kakhaki, R., Kouchaki, E., Bahmani, F., Borzabadi, S., et al. (2019). Clinical and metabolic response to probiotic administration in people with Parkinson’s disease: a randomized, double-blind, placebo-controlled trial. Clin. Nutr. 38, 1031–1035. doi: 10.1016/j.clnu.2018.05.018
Tetz, G., and Tetz, V. (2018). Bacteriophages as new human viral pathogens. Microorganisms 6:54. doi: 10.3390/microorganisms6020054
Tetz, G., Brown, S. M., Hao, Y., and Tetz, V. (2018). Parkinson’s disease and bacteriophages as its overlooked contributors. Sci. Rep. 8:10812.
The Human Microbiome Project Consortium (2012). Structure, function and diversity of the healthy human microbiome. Nature 486, 207–214. doi: 10.1038/nature11234
Thion, M. S., Low, D., Silvin, A., Chen, J., Grisel, P., Schulte-Schrepping, J., et al. (2018). Microbiome Influences prenatal and adult microglia in a sex-specific manner. Cell 172, 500–516.e16.
Tiraboschi, P., Hansen, L. A., Thal, L. J., and Corey-Bloom, J. (2004). The importance of neuritic plaques and tangles to the development and evolution of AD. Neurology 62, 1984–1989. doi: 10.1212/01.wnl.0000129697.01779.0a
Tomova, A., Husarova, V., Lakatosova, S., Bakos, J., Vlkova, B., Babinska, K., et al. (2015). Gastrointestinal microbiota in children with autism in Slovakia. Physiol. Behav. 138, 179–187. doi: 10.1016/j.physbeh.2014.10.033
Tractenberg, S. G., Levandowski, M. L., De Azeredo, L. A., Orso, R., Roithmann, L. G., Hoffmann, E. S., et al. (2016). An overview of maternal separation effects on behavioural outcomes in mice: evidence from a four-stage methodological systematic review. Neurosci. Biobehav. Rev. 68, 489–503. doi: 10.1016/j.neubiorev.2016.06.021
Tseng, M. T., and Lin, C. H. (2017). Pain in early-stage Parkinson’s disease: implications from clinical features to pathophysiology mechanisms. J. Formos. Med. Assoc. 116, 571–581. doi: 10.1016/j.jfma.2017.04.024
Turnbaugh, P. J., Ley, R. E., Hamady, M., Fraser-Liggett, C. M., Knight, R., and Gordon, J. I. (2007). The human microbiome project. Nature 449, 804–810.
Unger, M. M., Spiegel, J., Dillmann, K. U., Grundmann, D., Philippeit, H., Burmann, J., et al. (2016). Short chain fatty acids and gut microbiota differ between patients with Parkinson’s disease and age-matched controls. Parkinsonism Relat. Disord. 32, 66–72. doi: 10.1016/j.parkreldis.2016.08.019
Valles-Colomer, M., Falony, G., Darzi, Y., Tigchelaar, E. F., Wang, J., Tito, R. Y., et al. (2019). The neuroactive potential of the human gut microbiota in quality of life and depression. Nat. Microbiol. 4, 623–632. doi: 10.1038/s41564-018-0337-x
Vendrik, K. E. W., Ooijevaar, R. E., De Jong, P. R. C., Laman, J. D., Van Oosten, B. W., Van Hilten, J. J., et al. (2020). Fecal microbiota transplantation in neurological disorders. Front. Cell Infect. Microbiol. 10:98. doi: 10.3389/fcimb.2020.00098
Vogt, N. M., Kerby, R. L., Dill-Mcfarland, K. A., Harding, S. J., Merluzzi, A. P., Johnson, S. C., et al. (2017). Gut microbiome alterations in Alzheimer’s disease. Sci. Rep. 7:13537.
Vogt, N. M., Romano, K. A., Darst, B. F., Engelman, C. D., Johnson, S. C., Carlsson, C. M., et al. (2018). The gut microbiota-derived metabolite trimethylamine N-oxide is elevated in Alzheimer’s disease. Alzheimers Res. Ther. 10:124.
Vuong, H. E., Pronovost, G. N., Williams, D. W., Coley, E. J. L., Siegler, E. L., Qiu, A., et al. (2020). The maternal microbiome modulates fetal neurodevelopment in mice. Nature 586, 281–286. doi: 10.1038/s41586-020-2745-3
Wahlstrom, A., Sayin, S. I., Marschall, H. U., and Backhed, F. (2016). Intestinal crosstalk between bile acids and microbiota and its impact on host metabolism. Cell Metab. 24, 41–50. doi: 10.1016/j.cmet.2016.05.005
Waller, P. A., Gopal, P. K., Leyer, G. J., Ouwehand, A. C., Reifer, C., Stewart, M. E., et al. (2011). Dose-response effect of Bifidobacterium lactis HN019 on whole gut transit time and functional gastrointestinal symptoms in adults. Scand. J. Gastroenterol. 46, 1057–1064. doi: 10.3109/00365521.2011.584895
Wang, H., Lee, I. S., Braun, C., and Enck, P. (2016). Effect of probiotics on central nervous system functions in animals and humans: a systematic review. J. Neurogastroenterol. Motil. 22, 589–605. doi: 10.5056/jnm16018
Wang, L., Christophersen, C. T., Sorich, M. J., Gerber, J. P., Angley, M. T., and Conlon, M. A. (2012). Elevated fecal short chain fatty acid and ammonia concentrations in children with autism spectrum disorder. Dig. Dis. Sci. 57, 2096–2102. doi: 10.1007/s10620-012-2167-7
Wang, L., Fleming, S. M., Chesselet, M. F., and Tache, Y. (2008). Abnormal colonic motility in mice overexpressing human wild-type alpha-synuclein. Neuroreport 19, 873–876. doi: 10.1097/wnr.0b013e3282ffda5e
Wimo, A., Guerchet, M., Ali, G. C., Wu, Y. T., Prina, A. M., Winblad, B., et al. (2017). The worldwide costs of dementia 2015 and comparisons with 2010. Alzheimers Dement. 13, 1–7. doi: 10.1016/j.jalz.2016.07.150
Winter, G., Hart, R. A., Charlesworth, R. P. G., and Sharpley, C. F. (2018). Gut microbiome and depression: what we know and what we need to know. Rev. Neurosci. 29, 629–643. doi: 10.1515/revneuro-2017-0072
Wood, J. D. (2004). Enteric neuroimmunophysiology and pathophysiology. Gastroenterology 127, 635–657.
Wyss-Coray, T., and Rogers, J. (2012). Inflammation in Alzheimer disease-a brief review of the basic science and clinical literature. Cold Spring Harb. Perspect. Med. 2:a006346. doi: 10.1101/cshperspect.a006346
Xie, C., and Prasad, A. A. (2020). Probiotics treatment improves hippocampal dependent cognition in a rodent model of Parkinson’s disease. Microorganisms 8:1661. doi: 10.3390/microorganisms8111661
Xu, H. M., Huang, H. L., Zhou, Y. L., Zhao, H. L., Xu, J., Shou, D. W., et al. (2021). Fecal microbiota transplantation: a new therapeutic attempt from the gut to the brain. Gastroenterol. Res. Pract. 2021:6699268.
Yang, X., Qian, Y., Xu, S., Song, Y., and Xiao, Q. (2017). Longitudinal analysis of fecal microbiome and pathologic processes in a rotenone induced mice model of Parkinson’s disease. Front. Aging Neurosci. 9:441. doi: 10.3389/fnagi.2017.00441
Yankner, B. A., Duffy, L. K., and Kirschner, D. A. (1990). Neurotrophic and neurotoxic effects of amyloid beta protein: reversal by tachykinin neuropeptides. Science 250, 279–282. doi: 10.1126/science.2218531
Yano, J. M., Yu, K., Donaldson, G. P., Shastri, G. G., Ann, P., Ma, L., et al. (2015). Indigenous bacteria from the gut microbiota regulate host serotonin biosynthesis. Cell 161, 264–276. doi: 10.1016/j.cell.2015.02.047
Zebrowska, P., Laczmanska, I., and Laczmanski, L. (2021). Future directions in reducing gastrointestinal disorders in children with ASD using fecal microbiota transplantation. Front. Cell Infect. Microbiol. 11:630052. doi: 10.3389/fcimb.2021.630052
Zhang, F., Cui, B., He, X., Nie, Y., Wu, K., Fan, D., et al. (2018). Microbiota transplantation: concept, methodology and strategy for its modernization. Protein Cell 9, 462–473. doi: 10.1007/s13238-018-0541-8
Zhang, L., Wang, Y., Xiayu, X., Shi, C., Chen, W., Song, N., et al. (2017). Altered gut microbiota in a mouse model of Alzheimer’s disease. J. Alzheimers Dis. 60, 1241–1257.
Keywords: brain disorders, neurodevelopmental disorders, neurodegenerative diseases, microbiota, gut-brain, gut dysbiosis, prebiotics, probiotics
Citation: Deidda G and Biazzo M (2021) Gut and Brain: Investigating Physiological and Pathological Interactions Between Microbiota and Brain to Gain New Therapeutic Avenues for Brain Diseases. Front. Neurosci. 15:753915. doi: 10.3389/fnins.2021.753915
Received: 05 August 2021; Accepted: 16 September 2021;
Published: 12 October 2021.
Edited by:
Silvia Turroni, University of Bologna, ItalyReviewed by:
Kiran Veer Sandhu, University College Cork, IrelandCopyright © 2021 Deidda and Biazzo. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Gabriele Deidda, Z2FicmllbGUuZGVpZGRhQHVtLmVkdS5tdA==; Z2FicmllbGVkZWlkZGFAaG90bWFpbC5pdA==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.