- Acupuncture and Tuina School, Acupuncture and Brain Science Research Center, Chengdu University of Traditional Chinese Medicine, Chengdu, China
Objectives: This study was conducted in order to investigate the study design and main outcomes of acupuncture neuroimaging studies on low back pain (LBP).
Methods: Neuroimaging studies of acupuncture on LBP were collected from three English databases such as PubMed and four Chinese databases such as China National Knowledge Infrastructure (CNKI) from inception to December 31, 2020. Study selection, data extraction, and assessment of risk of bias were performed independently by two investigators. The quality of studies was appraised with the Cochrane's risk of bias tools. Information on basic information, methodology, and brain imaging data were extracted.
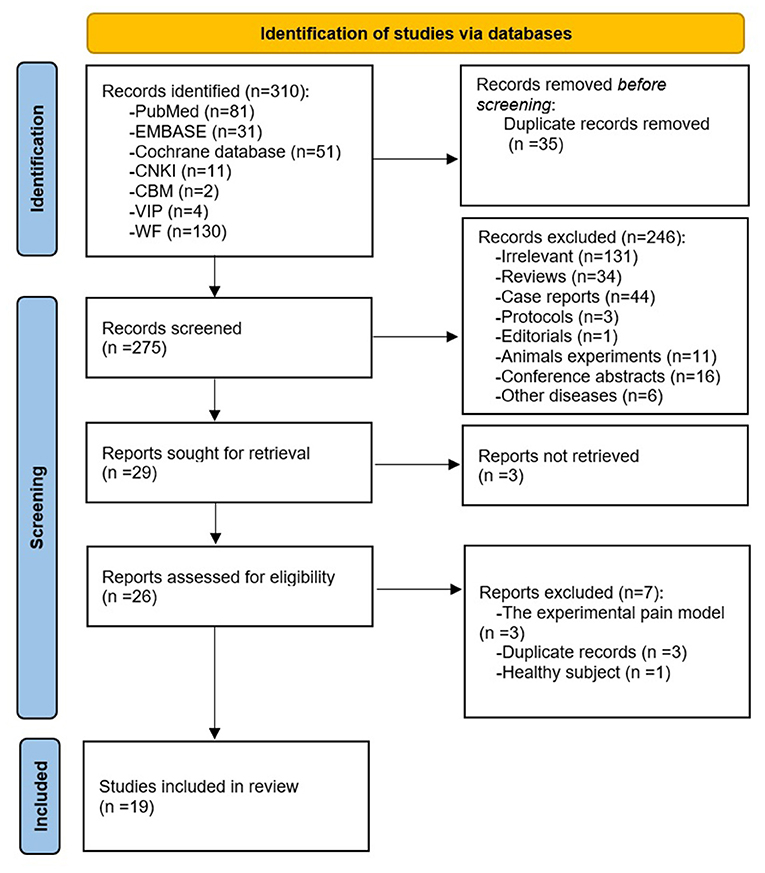
Results: The literature search returned 310 potentially eligible studies and 19 articles met inclusion criteria; 78.9% of studies chose manual acupuncture as the intervention, 89.5% of studies evaluated functional changes elicited by acupuncture, and 68.4% of studies used resting-state fMRI as imaging condition. The most frequently reported acupuncture-induced brain alterations of LBP patients were in the prefrontal cortex, insula, cerebellum, primary somatosensory cortex, and anterior cingulate cortex. There was a significant correlation between improved clinical outcomes and changes in the brain.
Conclusions: The results suggested that improving abnormal structure and functional activities in the brain of the LBP patient is an important mechanism of acupuncture treatment for LBP. The brain regions involved in acupuncture analgesia for LBP were mainly located in the pain matrix, default mode network (DMN), salience network (SN), and descending pain modulatory system (DPMS). However, it was difficult to draw a generalized conclusion due to the heterogeneity of study designs. Further well-designed multimodal neuroimaging studies investigating the mechanism of acupuncture on LBP are warranted.
Introduction
Low back pain (LBP) is a common disorder defined as the pain between the lower rib margins and the buttock creases (Kreiner et al., 2020). The lifetime and mean point prevalence of LBP were around 40 and 20%, respectively (Hoy et al., 2012; Calvo-Muñoz et al., 2013). LBP is the leading cause of disability that accounts for around 60.1 million years lived with disability (YLD) globally, and affects people of all ages (GBD 2016 Disease Injury Incidence Prevalence Collaborators, 2017). As an effective and safe therapy, acupuncture has been widely used for LBP. Many systematic reviews indicate that acupuncture is effective in relieving pain and improving function in LBP patients (Xu et al., 2013; Chou et al., 2017; Fuentes et al., 2020; Su et al., 2021). Since the first acupuncture neuroimaging study for LBP published in 2007 (Ji et al., 2007), there has been a surge of interest in investigating the therapeutic mechanism that underpins acupuncture treatment for LBP with neuroimaging technologies.
In the past 14 years, around 20 acupuncture neuroimaging studies for LBP have been published. These studies provide visualized and real-time evidence for understanding the mechanism of acupuncture treatment for LBP. However, the methodology issues of these studies greatly differed from each other. For example, some studies selected manual acupuncture (MA) as acupuncture modality, while others used electroacupuncture (EA). Some studies were performed with resting-state fMRI (rs-fMRI), while others with the task-state fMRI (ts-fMRI). Some focused on the functional changes elicited by acupuncture, while others centered on the structural alterations resulting from acupuncture. Therefore, this review aimed to (1) analyze the methodology issues of the published acupuncture neuroimaging studies on LBP, (2) summarize the core brain regions involved in acupuncture analgesia for LBP, and (3) provide references for future studies and the application of the neuroimaging results in the clinic.
Methods
Literature Search
The following seven databases were searched from inception to December 31, 2020: PubMed, EMBASE, Cochrane database, China National Knowledge Infrastructure (CNKI), Chinese Biomedical Literature (CBM), Chongqing VIP Database (VIP, Chinese Database), and Wanfang Database (WF, Chinese Database). The language was restricted to English or Chinese. The keywords were a combination of “low back pain,” “acupuncture,” and “neuroimaging technologies.” The search strategy for each of the electronic databases queried is shown in Supplementary Table 1. Additional studies were found by screening references of included articles and relevant reviews.
Eligibility Criteria
Studies were eligible for inclusion if they (1) were peer-reviewed original research conducted in LBP patients, (2) applied acupuncture as the intervention, and (3) used structural and functional magnetic resonance imaging (sMRI, fMRI), positron emission tomography (PET), electroencephalography (EEG), and functional near-infrared spectroscopy (fNIRS), etc.
We excluded the experimental pain model, conference abstracts, case reports, protocols, animal study, monograph, and reviews.
Study Selection, Data Extraction, and Assessment of Risk of Bias
Study selection, data extraction, and assessment of risk of bias were performed independently by two investigators (XD and YQ). Two Cochrane's risk of bias tools (Sterne et al., 2016, 2019) were utilized for evaluating the risk of bias of included randomized controlled trials (RCTs) and non-RCTs, respectively. Discrepancies were addressed by discussion or consulting a third party (PM).
For the eligible studies, we retrieved the following: (1) basic information: first author's name, year of publication, country, and diagnosis; (2) methodology: characteristics and sample size of participants, study design, acupuncture intervention (treatment courses, acupoints, manipulation modality, and needle sensation), imaging modality, and analysis methods; and (3) brain imaging data.
Synthesis of Results
There is a great difference in the methodology issues of the included studies, and a meta-analysis was considered inappropriate. A descriptive analysis was presented to summarize (1) the brain alterations of LBP patients, (2) acupuncture-induced brain alterations, (3) brain functional alterations induced by MA and EA, and (4) acupuncture-related brain activities in resting-state and task-state fMRI.
Results
Study Selection and Description
The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) flow diagram (Page et al., 2021) of literature search and screening process is shown in Figure 1. A total of 310 results were yielded from the databases. After deduplication and screening phases, 19 studies (Ji et al., 2007; Junhai et al., 2008; Yongsong et al., 2011; Li et al., 2012, 2014; Guoqiang et al., 2014; Jialiang et al., 2014; Tao et al., 2015; Lin and Xianmo, 2017; Yijun et al., 2017; Makary et al., 2018; Lee et al., 2019; Tu et al., 2019; Xiang et al., 2019; Yan et al., 2019; Kim et al., 2020; Lan et al., 2020; Liu et al., 2020; Yu et al., 2020) between 2007 and 2020 were incorporated in this review (Table 1). Among the articles, 14 studies were performed in China (Ji et al., 2007; Junhai et al., 2008; Yongsong et al., 2011; Li et al., 2012, 2014; Guoqiang et al., 2014; Jialiang et al., 2014; Tao et al., 2015; Lin and Xianmo, 2017; Yijun et al., 2017; Xiang et al., 2019; Yan et al., 2019; Lan et al., 2020; Liu et al., 2020), three were conducted in the USA (Tu et al., 2019; Kim et al., 2020; Yu et al., 2020), and two were conducted in South Korea (Makary et al., 2018; Lee et al., 2019). Five kinds of diseases were involved in these studies: chronic low back pain (Li et al., 2014; Tu et al., 2019; Xiang et al., 2019; Yan et al., 2019; Kim et al., 2020; Yu et al., 2020), non-specific low back pain (Makary et al., 2018; Lee et al., 2019), lumbar disc herniation (Yongsong et al., 2011; Guoqiang et al., 2014; Jialiang et al., 2014; Tao et al., 2015; Lin and Xianmo, 2017; Yijun et al., 2017; Lan et al., 2020), sciatica (Ji et al., 2007; Li et al., 2012; Liu et al., 2020), and low back and leg pain (Junhai et al., 2008).
Details of Methodology
Participants
A total of 613 LBP patients and 120 healthy controls (HC) were investigated. Six studies (Yongsong et al., 2011; Li et al., 2012, 2014; Yan et al., 2019; Kim et al., 2020; Liu et al., 2020) compared LBP patients and HC, while the remaining 13 studies (Ji et al., 2007; Junhai et al., 2008; Guoqiang et al., 2014; Jialiang et al., 2014; Tao et al., 2015; Lin and Xianmo, 2017; Yijun et al., 2017; Makary et al., 2018; Lee et al., 2019; Tu et al., 2019; Xiang et al., 2019; Lan et al., 2020; Yu et al., 2020) only recruited patients. Thirteen studies included LBP patients between 18 and 85 years of age, and the mean age of patients in the other six studies (in which the age range was not reported) ranged from 25.7 to 56.4 years. Eighteen studies described the gender of the patients (289 female and 280 male). One study (Lan et al., 2020) did not mention the gender of the patients. In the six studies that compared LBP patients and HC, the maximum and minimum sample sizes of LBP/HC were 23/50 and 10/10 per group, respectively. The average sample size of LBP/HC was 16/20 per group. In the 13 studies that only recruited patients, the maximum and minimum sample sizes were 50 and 7 per group, respectively. The average sample size of LBP was 20 per group.
Study Design
Nine RCTs and 10 non-RCTs were involved in these studies. The control groups in RCTs mainly included sham acupuncture (Makary et al., 2018; Lee et al., 2019; Tu et al., 2019; Kim et al., 2020; Lan et al., 2020; Yu et al., 2020), different acupoints (Jialiang et al., 2014; Tao et al., 2015; Lin and Xianmo, 2017), and usual care (Kim et al., 2020). Among the studies that compared verum acupuncture with sham acupuncture, the sham acupuncture methods included (1) phantom acupuncture (watched a recorded video clip of a verum stimulation to avoid any somatosensory afference) (Makary et al., 2018; Lee et al., 2019), (2) Streitberger placebo acupuncture needle at sham acupoints (Tu et al., 2019; Yu et al., 2020), (3) Streitberger placebo acupuncture needle at acupoints (Kim et al., 2020), and (4) mock laser acupuncture (Kim et al., 2020). Among the studies that compared different acupoints, LBP patients were randomly assigned into two different acupoints groups (balanced acupuncture/body acupuncture, yaosanzhen acupuncture/body acupuncture) (Jialiang et al., 2014; Tao et al., 2015; Lin and Xianmo, 2017).
Details of Interventions
Fifteen studies used MA, while four trials used EA. Thirteen studies focused on the instant effect of acupuncture, and the duration of stimulation was from 3 to 30 min (Ji et al., 2007; Junhai et al., 2008; Yongsong et al., 2011; Guoqiang et al., 2014; Jialiang et al., 2014; Li et al., 2014; Tao et al., 2015; Lin and Xianmo, 2017; Yijun et al., 2017; Makary et al., 2018; Lee et al., 2019; Xiang et al., 2019; Yu et al., 2020). Six studies focused on the cumulative effect of acupuncture, and the treatment courses were from 1 to 8 weeks (Li et al., 2012; Tu et al., 2019; Yan et al., 2019; Kim et al., 2020; Lan et al., 2020; Liu et al., 2020). Four studies chose single acupoint (Yongsong et al., 2011; Guoqiang et al., 2014; Yijun et al., 2017; Xiang et al., 2019), two studies used two acupoints (Ji et al., 2007; Junhai et al., 2008), nine studies applied a combination of three or more acupoints (Jialiang et al., 2014; Li et al., 2014; Tao et al., 2015; Lin and Xianmo, 2017; Makary et al., 2018; Lee et al., 2019; Tu et al., 2019; Kim et al., 2020; Yu et al., 2020), and four chose acupoints by syndrome differentiation (Li et al., 2012; Yan et al., 2019; Lan et al., 2020; Liu et al., 2020). The most frequently used acupoints were ashi points, BL23, BL40, and BL25 (Supplementary Figure 1). Eleven studies emphasized the needle sensation (Deqi) during acupuncture stimulation (Junhai et al., 2008; Yongsong et al., 2011; Li et al., 2012, 2014; Yijun et al., 2017; Makary et al., 2018; Lee et al., 2019; Tu et al., 2019; Lan et al., 2020; Liu et al., 2020; Yu et al., 2020).
Imaging Condition and Analysis
Magnetic resonance imaging (MRI) was applied in all studies to measure neuronal activity and brain structure in LBP patients treated by acupuncture.
Two studies evaluated structure changes. One study employed the single modality of diffusion tensor imaging (DTI) to investigate fractional anisotropy (FA) and axial diffusivity (AD) (Lan et al., 2020). One study combined DTI and voxel-based morphology to explore both gray matter volume (GMV) and FA (Kim et al., 2020).
Seventeen studies evaluated functional changes. One study combined resting and task-state fMRI (Yijun et al., 2017). Five studies used ts-fMRI (Ji et al., 2007; Junhai et al., 2008; Guoqiang et al., 2014; Yijun et al., 2017; Makary et al., 2018). Three studies were performed with a block design (Li et al., 2014; Sterne et al., 2016, 2019). Two studies were performed with event-related experimental paradigm (Guoqiang et al., 2014; Yijun et al., 2017). The needle is stimulated continuously for a duration from 45 s to 2 min during two to five blocks.
Thirteen studies (Yongsong et al., 2011; Li et al., 2012, 2014; Jialiang et al., 2014; Tao et al., 2015; Lin and Xianmo, 2017; Yijun et al., 2017; Lee et al., 2019; Tu et al., 2019; Xiang et al., 2019; Yan et al., 2019; Liu et al., 2020; Yu et al., 2020) used rs-fMRI to evaluate regional homogeneity (ReHo) (five studies) (Jialiang et al., 2014; Tao et al., 2015; Lin and Xianmo, 2017; Yan et al., 2019; Liu et al., 2020), functional connectivity (FC) (four studies) (Yongsong et al., 2011; Tu et al., 2019; Liu et al., 2020; Yu et al., 2020), independent component analysis (ICA) (three studies) (Li et al., 2012, 2014; Lee et al., 2019), and amplitude of low-frequency fluctuations/fractional amplitude of low-frequency fluctuations (ALFF/fALFF) (one study) (Xiang et al., 2019), one of which used machine learning approaches to predict acupuncture treatment responses (Tu et al., 2019).
Results of Risk-of-Bias Assessments
The results of risk-of-bias assessments are presented in Supplementary Tables 2, 3.
Of the nine RCTs, two studies were rated as “low” overall risks of bias (Tu et al., 2019; Yu et al., 2020), whereas seven studies were rated as “some concerns” of overall risks of bias due to concerns regarding the randomization process (Jialiang et al., 2014; Tao et al., 2015; Lin and Xianmo, 2017; Makary et al., 2018; Lee et al., 2019; Kim et al., 2020; Lan et al., 2020) and missing outcome data (Jialiang et al., 2014; Tao et al., 2015; Lin and Xianmo, 2017).
Of the 10 non-RCTs, nine studies were rated as “low” overall risks of bias (Tu et al., 2019; Yu et al., 2020). However, one study was rated as “no information” of overall risks of bias due to concerns regarding the missing outcome data (Guoqiang et al., 2014).
Brain Imaging Data
Brain imaging data of frequently reported brain regions are summarized in Table 2.
Brain Alterations of LBP Patients
Five studies reported the functional and structural alterations of LBP patients. Compared with HC, LBP patients mainly showed alterations in PFC (four studies) (Li et al., 2012, 2014; Yan et al., 2019; Liu et al., 2020), ACC (three studies) (Li et al., 2012, 2014; Liu et al., 2020), precuneus (three studies) (Li et al., 2012, 2014; Yan et al., 2019), insula (two studies) (Yan et al., 2019; Liu et al., 2020), and the primary somatosensory cortex (SI) (two studies) (Yan et al., 2019; Kim et al., 2020).
Correlation analyses showed a positive correlation between posterior cingulate cortex (PCC)–right inferior parietal lobule FC and the duration of sciatica (Liu et al., 2020) (Figure 2A).
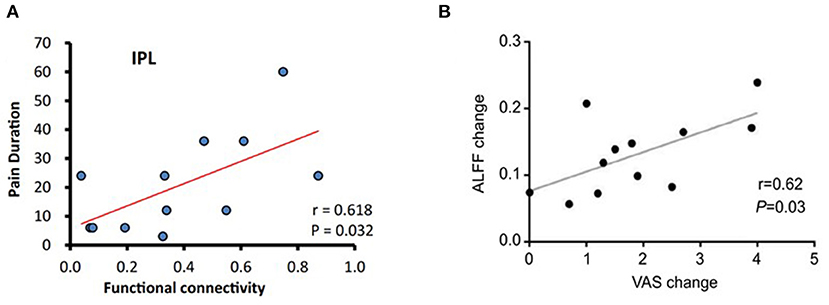
Figure 2. Correlation analysis in some studies (Xiang et al., 2019; Liu et al., 2020). (A) The PCC-seeded FC was positively correlated with pain duration in the right inferior parietal lobule at baseline in LBP patients. (B) There was a significant correlation between ALFF change in the left insular and VAS change after acupuncture treatment. ALFF, amplitude of low-frequency fluctuation; FC, functional connectivity; LBP, low back pain; PCC, posterior cingulate cortex; VAS, visual analog scale/score.
Acupuncture-Related Brain Alterations in LBP Patients
The most frequently reported acupuncture-related brain functional alterations in LBP patients were in PFC (12 studies) (Junhai et al., 2008; Yongsong et al., 2011; Li et al., 2012, 2014; Guoqiang et al., 2014; Jialiang et al., 2014; Lin and Xianmo, 2017; Makary et al., 2018; Lee et al., 2019; Tu et al., 2019; Yan et al., 2019; Liu et al., 2020), insula (nine studies) (Ji et al., 2007; Junhai et al., 2008; Yongsong et al., 2011; Guoqiang et al., 2014; Makary et al., 2018; Lee et al., 2019; Tu et al., 2019; Xiang et al., 2019; Liu et al., 2020), cerebellum (nine studies) (Ji et al., 2007; Junhai et al., 2008; Yongsong et al., 2011; Guoqiang et al., 2014; Jialiang et al., 2014; Tao et al., 2015; Lin and Xianmo, 2017; Yijun et al., 2017; Liu et al., 2020), SI (eight studies) (Ji et al., 2007; Junhai et al., 2008; Yongsong et al., 2011; Jialiang et al., 2014; Tao et al., 2015; Lin and Xianmo, 2017; Makary et al., 2018; Yan et al., 2019), and ACC (seven studies) (Ji et al., 2007; Junhai et al., 2008; Yongsong et al., 2011; Li et al., 2012, 2014; Makary et al., 2018; Liu et al., 2020).
Two studies found corresponding results regarding regional structural changes in LBP patients after acupuncture treatment. Following a 4-week course of acupuncture, GVM was reduced and white matter FA was increased in the SI–back, and the changes were associated with improvements in tactile acuity over the back (Kim et al., 2020; Lan et al., 2020).
One study found that pretreatment FC between the medial prefrontal cortex (mPFC) and other brain regions can predict treatment responsiveness of acupuncture on LBP patients (Tu et al., 2019). In addition, baseline periaqueductal gray (PAG)–amygdala FC can predict bothersomeness reduction after acupuncture treatments (Yu et al., 2020). There was a significant correlation between mean ALFF change in the left insula, FC of the PFC–insula, and the decreased pain (Lee et al., 2019; Xiang et al., 2019) (Figure 2B).
MA- and EA-Related Brain Alterations in LBP Patients
Of the 15 studies that used MA, the most frequently reported EA-related brain alterations in LBP patients were in the PFC (nine studies) (Yongsong et al., 2011; Guoqiang et al., 2014; Jialiang et al., 2014; Li et al., 2014; Lin and Xianmo, 2017; Makary et al., 2018; Lee et al., 2019; Tu et al., 2019; Liu et al., 2020), cerebellum (seven studies) (Yongsong et al., 2011; Guoqiang et al., 2014; Jialiang et al., 2014; Tao et al., 2015; Lin and Xianmo, 2017; Yijun et al., 2017; Liu et al., 2020), insula (seven studies) (Yongsong et al., 2011; Guoqiang et al., 2014; Makary et al., 2018; Lee et al., 2019; Tu et al., 2019; Xiang et al., 2019; Liu et al., 2020), SI (five studies) (Yongsong et al., 2011; Jialiang et al., 2014; Tao et al., 2015; Lin and Xianmo, 2017; Makary et al., 2018), and ACC (four studies) (Yongsong et al., 2011; Li et al., 2014; Makary et al., 2018; Liu et al., 2020).
Of the four studies that used EA, the most frequently reported EA-related brain alterations in LBP patients were in the PFC (four studies) (Ji et al., 2007; Junhai et al., 2008; Li et al., 2012; Yan et al., 2019), ACC (three studies) (Ji et al., 2007; Junhai et al., 2008; Li et al., 2012), superior temporal gyrus (STG) (three studies) (Ji et al., 2007; Junhai et al., 2008; Yan et al., 2019), SI (three studies) (Ji et al., 2007; Junhai et al., 2008; Yan et al., 2019), and insula (two studies) (Ji et al., 2007; Junhai et al., 2008).
Acupuncture-Related Brain Activities in Resting-State and Task-State fMRI
Of the 13 studies that used resting-state fMRI, the most frequently reported acupuncture-related brain alterations of LBP patients were in the PFC (nine studies) (Yongsong et al., 2011; Li et al., 2012, 2014; Jialiang et al., 2014; Lin and Xianmo, 2017; Lee et al., 2019; Tu et al., 2019; Yan et al., 2019; Liu et al., 2020), cerebellum (five studies) (Yongsong et al., 2011; Jialiang et al., 2014; Tao et al., 2015; Lin and Xianmo, 2017; Liu et al., 2020), insula (five studies) (Yongsong et al., 2011; Lee et al., 2019; Tu et al., 2019; Xiang et al., 2019; Liu et al., 2020), SI (five studies) (Yongsong et al., 2011; Jialiang et al., 2014; Tao et al., 2015; Lin and Xianmo, 2017; Yan et al., 2019), and ACC (four studies) (Yongsong et al., 2011; Li et al., 2012, 2014; Liu et al., 2020).
Of the five studies that used task-state fMRI, the most frequently reported acupuncture-related brain alterations of LBP patients were in the cerebellum (four studies) (Ji et al., 2007; Junhai et al., 2008; Guoqiang et al., 2014; Yijun et al., 2017), insula (four studies) (Ji et al., 2007; Junhai et al., 2008; Guoqiang et al., 2014; Makary et al., 2018), SI (three studies) (Ji et al., 2007; Junhai et al., 2008; Makary et al., 2018), secondary somatosensory cortex (SII) (three studies) (Ji et al., 2007; Junhai et al., 2008; Makary et al., 2018), PFC (three studies) (Junhai et al., 2008; Guoqiang et al., 2014; Makary et al., 2018), and ACC (three studies) (Ji et al., 2007; Junhai et al., 2008; Makary et al., 2018).
Correlation analyses between resting-state brain activity and behavior measurements showed that increased FC in default mode network (DMN) and PAG–amygdala were associated with decreased pain scores in LBP patients after acupuncture (Li et al., 2014; Yu et al., 2020). Moreover, there were significant correlations between the task-state fMRI signal in the left insula, STG, right supramarginal gyrus, SI, and VAS (Makary et al., 2018).
Discussion
Nineteen articles that used MRI to investigate the central mechanism of acupuncture were enrolled in this review. In the past 14 years, using neuroimaging technologies to explore the central mechanism of acupuncture for treating LBP has attracted increasing attention. This review aims to analyze the methodology issues and study results by the systematic review of 19 neuroimaging papers on acupuncture for LBP so as to provide reference to deeply understand the current status and approaches for future studies.
Functional and Structural Abnormalities in the Brain of LBP Patients
In this review, five studies reported that LBP patients showed cerebral functional and structural alterations compared with HC. The structural alterations mainly include the elevated GVM in the SI and reduced FA in SI–back regions. The functional changes usually manifest as reduced functional connectivities of DMN; higher ReHo values in the bilateral inferior temporal gyrus, left STG, and left superior parietal gyrus; and lower ReHo values in bilateral postcentral gyrus, bilateral superior frontal gyrus, and right supplementary motor area. Furthermore, a systematic review on neuroimaging studies of LBP patients indicated that brain regions such as the ACC, insula, mPFC, and cerebellum were involved in the central pathological changes of LBP patients (Kregel et al., 2015). These studies mapped the functional and structural alterations in LBP patients and provided the potential target for exploring the central responses to acupuncture stimulation in LBP patients.
Acupuncture-Induced Brain Alterations in LBP Patients
For LBP patients, acupuncture can elicit widely cerebral responses. These brain regions included the PFC (middle frontal gyrus and superior frontal gyrus), precentral gyrus, ACC, PCC, insula, thalamus, postcentral gyrus, putamen, precuneus, angular gyrus, parahippocampus, PAG, rostral ventral medulla (RVM), posterior inferior parietal lobe, and cerebellum regions and were mainly distributed in the “pain matrix,” DMN, salience network (SN), and descending pain modulatory system (DPMS) (Figure 3). Previous studies have reported the abnormal connectivity in DMN and SN in pain disorders (Zhao et al., 2017; Tu et al., 2020). In this review, several studies found that acupuncture can positively regulate the function of DMN and SN (Li et al., 2012, 2014; Lee et al., 2019; Xiang et al., 2019; Yan et al., 2019). Multiple neuroimaging studies suggested that modulating the activity of DMN is an important mechanism of acupuncture therapy (Deng et al., 2016; Fu et al., 2017; Sun et al., 2021) and regulating the cerebral function of the “pain matrix” is the common characteristic of acupuncture analgesia (Zhao et al., 2014; Shen et al., 2019). Thus, improving abnormal structural and functional activities in the brain of the LBP patient is an important mechanism of acupuncture treatment for LBP. In addition, abundant evidence suggests that modulating the DPMS, comprising the PAG and RVM, is one of the mechanisms of acupuncture analgesia (Chen et al., 2015; Li et al., 2016).
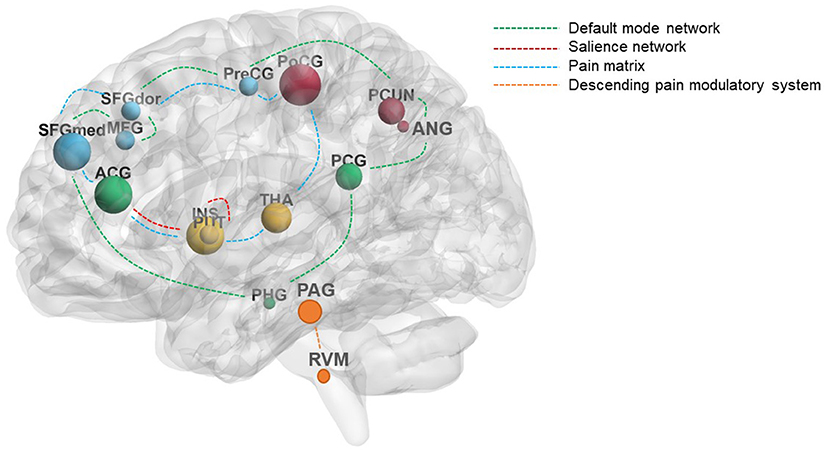
Figure 3. The main reported brain regions induced by acupuncture in LBP patients. The size of the nodes represents the frequency of the brain regions; the different colors of the dashed lines represent the different modulation pathways of acupuncture. The green dashed lines mainly represent the default mode network, the red dashed lines mainly represent the salience network, the blue dashed lines mainly represent the pain matrix, and the yellow dashed lines mainly represent the descending pain modulatory system. ACG, cingulate gyrus, anterior part; ANG, angular gyrus; INS, insula; LBP, low back pain; MFG, middle frontal gyrus; PAG, periaqueductal gray; PCG, cingulate gyrus, posterior part; PCUN, precuneus; PHG, parahippocampus; PoCG, postcentral gyrus; PreCG, precentral gyrus; PUT, putamen; RVM, rostral ventral medulla; SFGdor, superior frontal gyrus, dorsolateral; SFGmed, superior frontal gyrus, medial; THA, thalamus.
Among dozens of brain regions which responded to acupuncture for treating LBP, the PFC, insula, cerebellum, SI, and ACC were the main reported brain regions. The PFC and ACC are not only the main nodes of the “pain matrix” but also the key regions in DMN. They participate in pain modulatory, pain anticipation, affective, and cognitive processing (Tracey and Mantyh, 2007; Qin et al., 2008). It is interesting that Makary et al. reported that activation in ACC was unique to verum acupuncture and that activation in the PFC was observed in the sham acupuncture (Makary et al., 2018) and significantly correlated with the belief in acupuncture effectiveness score (Makary et al., 2018). Actually, the PFC is believed to be linked with expectancy-related modulation of pain processing (Casey, 1999). As a key region in the pain matrix and SN, the insula plays an important role in the cognitive and affective perception of pain (Kong et al., 2006), manifests a significant activation in chronic pain (Ihara et al., 2019) and experimental pain (Peyron and Fauchon, 2019), and is widely involved in acupuncture analgesia (Chae et al., 2013). In this review, nine studies identified the participation of insula in acupuncture for treating LBP. The cerebellum plays an important role in sensorimotor and vestibular control and also participates in cognition, autonomic, and emotional control (Schmahmann, 2019). Hui et al. reported that activation of the cerebellum elicited by pain occurred during the acupuncture stimulations (Hui et al., 2005). The SI cortex is a major component in the pain matrix participating in pain localization and discrimination. Kim et al. found that 4 weeks of acupuncture treatment for LBP can normalize the anatomic alterations of both gray matter (GM) and white matter (WM) in SI including decreasing the GVM and increasing the FA and AD. A similar structural modulation in SI was reported in a neuroimaging study on acupuncture for treating carpal tunnel syndrome (Maeda et al., 2017).
Generally, the majority of acupuncture-neuroimaging studies focus on the functional changes resulting from acupuncture stimulation, while a few studies centered on the structural alteration induced by acupuncture intervention. Among the 19 studies included in this review, only two studies reported the improvements in altered GM/WM of LBP patients. This phenomenon of emphasizing the function over structure in acupuncture-neuroimaging studies is closely related to the characteristic that acupuncture is good at regulating functional abnormalities. The few studies on acupuncture influencing the brain structure provide valuable evidence for the promotion of structural plasticity in the central nervous system (CNS) by acupuncture. Future studies could pay more attention to the structural plasticity in the CNS induced by acupuncture and take the accumulation effect of long-term acupuncture treatment and the cerebral structural changes in physiological period into consideration.
The Brain Functional Alterations Induced by MA and EA
MA and EA are the two main modalities in acupuncture clinic practice. The advantages of MA are traditional and convenient, while the strong point of EA is quantifiable. Some studies hold that EA was more effective than MA in analgesia (Kong et al., 2002), while some studies hold that MA produced a better-sustained effect than EA (Schliessbach et al., 2011). In this review, among the 17 studies on acupuncture regulating the cerebral function in LBP patients, 13 studies selected MA as acupuncture intervention and four studies used EA. Either MA or EA could elicit the cerebral activity changes in the PFC, insula, SI, and ACC. These regions all belong to the “pain matrix” and the high-frequency brain regions in acupuncture-neuroimaging for pain. It is a pity that there was no published study which investigated the similarities and differences in cerebral responses between MA and EA in LBP patients. Some studies on healthy subjects have shown that both EA and MA could activate the PFC and insula (Kong et al., 2002; Jiang et al., 2013), EA produces more widespread fMRI signal changes than MA (Napadow et al., 2005), and EA can produce more activation and less deactivation than MA (Li et al., 2003; Schliessbach et al., 2011). These studies on healthy subjects suggest that different brain mechanisms may be recruited during MA and EA (Li et al., 2003).
Acupuncture-Induced Brain Alterations in Resting-State and Task-State fMRI
Resting state and task state are the two main study designs in fMRI. The advantage of ts-fMRI is that it can directly reflect the effects of an explicit task in the brain, while the lack of tasks makes rs-fMRI quite simple in experimental design and easy to cooperate with patients. In this review, 13 studies used resting-state fMRI, and five studies used task-state fMRI. The common brain regions that were induced by acupuncture in resting-state and task-state fMRI were the PFC, insula, SI, SII, brainstem, and ACC. Generally, ts-fMRI is used to evaluate the instant effect of acupuncture analgesia, and rs-fMRI is applied to evaluate the cumulative effect of acupuncture. Notably, the angular gyrus was altered when evaluating the cumulative effect of acupuncture, but not when evaluating the instant effect. The angular gyrus is located in the anterolateral region of parietal lobe and is an important part of the DMN. Liu et al. (2021) found that there was a significantly greater ReHo value increase in the angular gyrus after the 12th acupuncture sessions compared with after the first acupuncture session in migraine patients, which indicated that the cumulative effect of acupuncture is more extensive and significant than the instant effect. This review showed that in the neuroimaging studies of acupuncture treatment for LBP, researchers not only pay attention to the instant effect of acupuncture but also pay attention to the cumulative effect of acupuncture.
In this review, five out of 13 rs-fMRI studies used ReHo as analytical approach. As an example of functional segregation, Reho has the advantages of simplicity, stability, and repeatability. However, since the brain is an integrated network rather than an isolated cluster, a functional integration approach is more popular nowadays. Therefore, future studies combining functional segregation with functional integration approach will help to better clarify the mechanism of acupuncture treatment for LBP.
Conclusion
The neuroimaging studies associated with acupuncture treatment on LBP have been widely conducted. These studies covered the functional and structural changes elicited by acupuncture stimulation, included both EA and MA, and took the resting state and task state into consideration of the study design. The brain regions involved in acupuncture analgesia for LBP were mainly located in the pain matrix, DMN, SN, and DPMS, especially in the PFC, insula, cerebellum, SI, and ACC. However, acupuncture neuroimaging studies for LBP were only performed with MRI. In the future, the combination of multiple imaging technologies might be a new approach to deeply investigate the central mechanism of acupuncture for LBP.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Author Contributions
FZ designed the study. XD and YQ performed the paper search, paper selection, and data extraction. QW, PM, RS, LL, TY, YL, and QX discussed the results and wrote the paper. All authors have read and approved the publication of the final manuscript.
Funding
This work was supported by grants from the National Natural Science Foundation of China (Grant No. 81973960) and Sichuan Province Scientific and Technological Innovation Team for Youths (Grant No. 2019JDTD0011).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnins.2021.730322/full#supplementary-material
References
Calvo-Muñoz, I., Gómez-Conesa, A., and Sánchez-Meca, J. (2013). Prevalence of low back pain in children and adolescents: a meta-analysis. BMC Pediatr. 13:14. doi: 10.1186/1471-2431-13-14
Casey, K. L. (1999). Forebrain mechanisms of nociception and pain: analysis through imaging. Proc. Natl. Acad. Sci. U. S. A. 96, 7668–7674. doi: 10.1073/pnas.96.14.7668
Chae, Y., Chang, D. S., Lee, S. H., Jung, W. M., Lee, I. S., Jackson, S., et al. (2013). Inserting needles into the body: a meta-analysis of brain activity associated with acupuncture needle stimulation. J. Pain 14, 215–222. doi: 10.1016/j.jpain.2012.11.011
Chen, X., Spaeth, R. B., Freeman, S. G., Scarborough, D. M., Hashmi, J. A., Wey, H. Y., et al. (2015). The modulation effect of longitudinal acupuncture on resting state functional connectivity in knee osteoarthritis patients. Mol. Pain. 11:67. doi: 10.1186/s12990-015-0071-9
Chou, R., Deyo, R., Friedly, J., Skelly, A., Hashimoto, R., Weimer, M., et al. (2017). Nonpharmacologic therapies for low back pain: a systematic review for an American college of physicians clinical practice guideline. Ann. Intern. Med. 166, 493–505. doi: 10.7326/M16-2459
Deng, D., Liao, H., Duan, G., Liu, Y., He, Q., Liu, H., et al. (2016). Modulation of the default mode network in first-episode, drug-naïve major depressive disorder via acupuncture at Baihui (GV20) acupoint. Front. Hum. Neurosci. 10:230. doi: 10.3389/fnhum.2016.00230
Fu, C. H., Li, K. S., Ning, Y. Z., Tan, Z. J., Zhang, Y., Liu, H. W., et al. (2017). Altered effective connectivity of resting state networks by acupuncture stimulation in stroke patients with left hemiplegia: a multivariate granger analysis. Medicine (Baltimore) 96:e8897. doi: 10.1097/MD.0000000000008897
Fuentes, R. C., Organ, B., Creech, J., Broszko, C. M., and Nashelsky, J. (2020). Acupuncture for low back pain. Can. Fam. Phys. 66, 186–187.
GBD 2016 Disease and Injury Incidence and Prevalence Collaborators (2017). Global, regional, and national incidence, prevalence, and years lived with disability for 328 diseases and injuries for 195 countries, 1990-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet 390, 1211–1259. doi: 10.1016/S0140-6736(17)32154-2
Guoqiang, Y., Xiaoling, L., Feng, W., Yingjie, X., Danna, C., Guijun, Z., et al. (2014). Effects of acupuncture at Zulinqi (GB41) on patients with lumbocrural pain: an fMRI study (Chinese Version). Chin. J. Gerontol. 19:5443–5446. doi: 10.3969/j.issn.1005-9202.2014.19.054
Hoy, D., Bain, C., Williams, G., March, L., Brooks, P., Blyth, F., et al. (2012). A systematic review of the global prevalence of low back pain. Arthritis Rheum. 64, 2028–2037. doi: 10.1002/art.34347
Hui, K. K., Liu, J., Marina, O., Napadow, V., Haselgrove, C., Kwong, K. K., et al. (2005). The integrated response of the human cerebro-cerebellar and limbic systems to acupuncture stimulation at ST 36 as evidenced by fMRI. Neuroimage 27, 479–496. doi: 10.1016/j.neuroimage.2005.04.037
Ihara, N., Wakaizumi, K., Nishimura, D., Kato, J., Yamada, T., Suzuki, T., et al. (2019). Aberrant resting-state functional connectivity of the dorsolateral prefrontal cortex to the anterior insula and its association with fear avoidance belief in chronic neck pain patients. PLoS ONE 14:e0221023. doi: 10.1371/journal.pone.0221023
Ji, L., Junhai, Z., and Jingcheng, D. (2007). Ji, L., Junhai, Z., and Jingcheng, D. (2007). Influence of acupuncture analgesia on cerebral function imaging in sciatica patients (Chinese Version). Shanghai J. Acupunct. Moxibust. 26, 3–6. doi: 10.3969/j.issn.1005-0957.2007.08.002
Jialiang, C., Guoqing, L., Jun, C., Xian, L., Xiaofan, L., and Yitai, L. (2014). The resting state fMRI study of different acupuncture analgesia therapies(Chinese version). Chin. J. Gerontol. 11, 2977–2979. doi: 10.3969/j.issn.1005-9202.2014.11.032
Jiang, Y., Wang, H., Liu, Z., Dong, Y., Dong, Y., Xiang, X., et al. (2013). Manipulation of and sustained effects on the human brain induced by different modalities of acupuncture: an fMRI study. PLoS ONE 8:e66815. doi: 10.1371/journal.pone.0066815
Junhai, Z., Xiaoyuan, F., and Ke, L. (2008). A primary functional MRI study of electroacupuncture modulate the sympathetic activities (Chinese Version). Chinese Comput. Med. Imaging 14. doi: 10.3969/j.issn.1006-5741.2008.02.002
Kim, H., Mawla, I., Lee, J., Gerber, J., Walker, K., Kim, J., et al. (2020). Reduced tactile acuity in chronic low back pain is linked with structural neuroplasticity in primary somatosensory cortex and is modulated by acupuncture therapy. Neuroimage 217:116899. doi: 10.1016/j.neuroimage.2020.116899
Kong, J., Ma, L., Gollub, R. L., Wei, J., Yang, X., Li, D., et al. (2002). A pilot study of functional magnetic resonance imaging of the brain during manual and electroacupuncture stimulation of acupuncture point (LI-4 Hegu) in normal subjects reveals differential brain activation between methods. J. Altern. Complement. Med. 8, 411–419. doi: 10.1089/107555302760253603
Kong, J., White, N. S., Kwong, K. K., Vangel, M. G., Rosman, I. S., Gracely, R. H., et al. (2006). Using fMRI to dissociate sensory encoding from cognitive evaluation of heat pain intensity. Hum. Brain Mapp. 27, 715–721. doi: 10.1002/hbm.20213
Kregel, J., Meeus, M., Malfliet, A., Dolphens, M., Danneels, L., Nijs, J., et al. (2015). Structural and functional brain abnormalities in chronic low back pain: a systematic review. Semin Arthritis Rheum. 45, 229–237. doi: 10.1016/j.semarthrit.2015.05.002
Kreiner, D. S., Matz, P., Bono, C. M., Cho, C. H., Easa, J. E., Ghiselli, G., et al. (2020). Guideline summary review: an evidence-based clinical guideline for the diagnosis and treatment of low back pain. Spine J. 20, 998–1024. doi: 10.1016/j.spinee.2020.04.006
Lan, G., Bopei, Z., Fuxia, Y., Zhongxian, P., and Shaoqing, Q. (2020). Brain functional mechanism of analgesic effect of Acupuncture on L5 nerve root pain based on functional magnetic resonance imaging (Chinese Version). J. Imaging Res. Med. Appl. 4, 112–114.
Lee, J., Eun, S., Kim, J., Lee, J. H., and Park, K. (2019). Differential influence of acupuncture somatosensory and cognitive/affective components on functional brain connectivity and pain reduction during low back pain state. Front. Neurosci. 13:11. doi: 10.3389/fnins.2019.01062
Li, G., Cheung, R. T., Ma, Q. Y., and Yang, E. S. (2003). Visual cortical activations on fMRI upon stimulation of the vision-implicated acupoints. Neuroreport. 14, 669–673. doi: 10.1097/00001756-200304150-00002
Li, J., Dong, J. C., and Yue, J. J. (2012). Effects of acupuncture on default mode network images of chronic sciatica patients in the resting network state (Chinese Version). Zhongguo Zhong Xi Yi Jie He Za Zhi 32, 1624–1627.
Li, J., Zhang, J. H., Yi, T., Tang, W. J., Wang, S. W., and Dong, J. C. (2014). Acupuncture treatment of chronic low back pain reverses an abnormal brain default mode network in correlation with clinical pain relief. Acupunct. Med. 32, 102–108. doi: 10.1136/acupmed-2013-010423
Li, Z., Liu, M., Lan, L., Zeng, F., Makris, N., Liang, Y., et al. (2016). Altered periaqueductal gray resting state functional connectivity in migraine and the modulation effect of treatment. Sci Rep. 6:20298. doi: 10.1038/srep20298
Lin, X., and Xianmo, W. (2017). Effects of Yaosanzhen acupuncture on Patients with lumbar intervertebral disc herniation: an resting state fMRI study (Chinese Version). J. Yangtze Univ, 14, 1–3. doi: 10.3969/j.issn.1673-1409.2017.08.002
Liu, C. H., Yeh, T. C., Kung, Y. Y., Tseng, H. P., Yang, C. J., Hong, T. Y., et al. (2020). Changes in resting-state functional connectivity in nonacute sciatica with acupuncture modulation: a preliminary study. Brain Behav. 10:12. doi: 10.1002/brb3.1494
Liu, S., Luo, S., Yan, T., Ma, W., Wei, X., Chen, Y., et al. (2021). differential modulating effect of acupuncture in patients with migraine without aura: a resting functional magnetic resonance study. Front. Neurol. 12:864. doi: 10.3389/fneur.2021.680896
Maeda, Y., Kim, H., Kettner, N., Kim, J., Cina, S., Malatesta, C., et al. (2017). Rewiring the primary somatosensory cortex in carpal tunnel syndrome with acupuncture. Brain 140, 914–927. doi: 10.1093/brain/awx015
Makary, M. M., Lee, J., Lee, E., Eun, S., Kim, J., Jahng, G. H., et al. (2018). Phantom acupuncture induces placebo credibility and vicarious sensations: a parallel fMRI study of low back pain patients. Sci Rep. 8:22. doi: 10.1038/s41598-017-18870-1
Napadow, V., Makris, N., Liu, J., Kettner, N. W., Kwong, K. K., and Hui, K. K. (2005). Effects of electroacupuncture versus manual acupuncture on the human brain as measured by fMRI. Hum. Brain Mapp. 24, 193–205. doi: 10.1002/hbm.20081
Page, M. J., McKenzie, J. E., Bossuyt, P. M., Boutron, I., Hoffmann, T. C., Mulrow, C. D., et al. (2021). The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 372:n71.
Peyron, R., and Fauchon, C. (2019). Functional imaging of pain. Rev Neurol (Paris) 175, 38–45. doi: 10.1016/j.neurol.2018.08.006
Qin, W., Tian, J., Bai, L., Pan, X., Yang, L., Chen, P., et al. (2008). FMRI connectivity analysis of acupuncture effects on an amygdala-associated brain network. Mol. Pain. 4:55. doi: 10.1186/1744-8069-4-55
Schliessbach, J., van der Klift, E., Arendt-Nielsen, L., Curatolo, M., and Streitberger, K. (2011). The effect of brief electrical and manual acupuncture stimulation on mechanical experimental pain. Pain Med. 12, 268–275. doi: 10.1111/j.1526-4637.2010.01051.x
Schmahmann, J. D. (2019). The cerebellum and cognition. Neurosci. Lett. 688, 62–75. doi: 10.1016/j.neulet.2018.07.005
Shen, Z., Yu, S., Wang, M., She, T., Yang, Y., Wang, Y., et al. (2019). Abnormal amygdala resting-state functional connectivity in primary dysmenorrhea. Neuroreport 30, 363–368. doi: 10.1097/WNR.0000000000001208
Sterne, J. A., Hernán, M. A., Reeves, B. C., Savović, J., Berkman, N. D., Viswanathan, M., et al. (2016). ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ 355:i4919. doi: 10.1136/bmj.i4919
Sterne, J. A. C., Savović, J., Page, M. J., Elbers, R. G., Blencowe, N. S., Boutron, I., et al. (2019). RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ 366:l4898. doi: 10.1136/bmj.l4898
Su, X., Qian, H., Chen, B., Fan, W., Xu, D., Tang, C., et al. (2021). Acupuncture for acute low back pain: a systematic review and meta-analysis. Ann. Palliat. Med. 10:3924–3936. doi: 10.21037/apm-20-1998
Sun, R., He, Z., Ma, P., Yin, S., Yin, T., Liu, X., et al. (2021). The participation of basolateral amygdala in the efficacy of acupuncture with deqi treating for functional dyspepsia. Brain Imaging Behav. 15, 216–230. doi: 10.1007/s11682-019-00249-7
Tao, G., Sheng, X., Wei, C., and Ming, L. (2015). Changes of different analgesic acupuncture treatments for regional homogeneity of brain activity in resting state by functional magnetic resonance imaging and the similarities and differences between their mechanisms (Chinese Version). J. Clin. Acupunct. Moxibust. 31, 25–27. doi: 10.3969/j.issn.1005-0779.2015.08.010
Tracey, I., and Mantyh, P. W. (2007). The cerebral signature for pain perception and its modulation. Neuron 55, 377–391. doi: 10.1016/j.neuron.2007.07.012
Tu, Y., Fu, Z., Mao, C., Falahpour, M., Gollub, R. L., Park, J., et al. (2020). Distinct thalamocortical network dynamics are associated with the pathophysiology of chronic low back pain. Nat. Commun. 11:3948. doi: 10.1038/s41467-020-17788-z
Tu, Y., Ortiz, A., Gollub, R. L., Cao, J., Gerber, J., Lang, C., et al. (2019). Multivariate resting-state functional connectivity predicts responses to real and sham acupuncture treatment in chronic low back pain. NeuroImage Clin. 23:101885. doi: 10.1016/j.nicl.2019.101885
Xiang, A. F., Yu, Y., Jia, X. Z., Ma, H. B., Liu, H., Zhang, Y., et al. (2019). The low-frequency BOLD signal oscillation response in the insular associated to immediate analgesia of ankle acupuncture in patients with chronic low back pain. J. Pain Res. 12, 841–850. doi: 10.2147/JPR.S189390
Xu, M., Yan, S., Yin, X., Li, X., Gao, S., Han, R., et al. (2013). Acupuncture for chronic low back pain in long-term follow-up: a meta-analysis of 13 randomized controlled trials. Am. J. Chin. Med. 41, 1–19. doi: 10.1142/S0192415X13500018
Yan, Z., Weijun, T., Songwei, W., Jianhua, H., and Ji, L. (2019). Objective evaluation on brain network imaging of“ treating same disease with different methods” effect of acupuncture for chronic low back pain (cLBP) (Chinese Version). Fudan Univ. J. Med. Sci. 46, 167–173.
Yijun, L., Yong, Y., Bin, Z., Fei, S., Xiaoyan, C., and Youlong, Z. (2017). Central mechanism of analgesic effect of Huaisanzhen on L5 nerve root pain based on functional magnetic resonance imaging (Chinese Version). J. Beijing Univ. Tradit. Chinese Med. 40, 259–264.
Yongsong, Y., Bo, L., and Zhiguang, C. (2011). Resting state-functional magnetic resonance imaging technology applied to a balancing acupuncture treatment for central mechanisms (Chinese Version). J. Clin. Rehabil. Tissue Eng. Res. 15, 8998–9002. doi: 10.3969/j.issn.1673-8225.2011.48.017
Yu, S., Ortiz, A., Gollub, R. L., Wilson, G., Gerber, J., Park, J., et al. (2020). Acupuncture treatment modulates the connectivity of key regions of the descending pain modulation and reward systems in patients with chronic low back pain. J. Clin. Med. 9:1719. doi: 10.3390/jcm9061719
Zhao, L., Liu, J., Zhang, F., Dong, X., Peng, Y., Qin, W., et al. (2014). Effects of long-term acupuncture treatment on resting-state brain activity in migraine patients: a randomized controlled trial on active acupoints and inactive acupoints. PLoS ONE 9:e99538. doi: 10.1371/journal.pone.0099538
Keywords: acupuncture, neuroimaging, fMRI, low back pain, systematic review
Citation: Wen Q, Ma P, Dong X, Sun R, Lan L, Yin T, Qu Y, Liu Y, Xiao Q and Zeng F (2021) Neuroimaging Studies of Acupuncture on Low Back Pain: A Systematic Review. Front. Neurosci. 15:730322. doi: 10.3389/fnins.2021.730322
Received: 24 June 2021; Accepted: 16 August 2021;
Published: 20 September 2021.
Edited by:
Man Li, Huazhong University of Science and Technology, ChinaReviewed by:
Yan Bai, Henan Provincial People's Hospital, ChinaLing-Li Zeng, National University of Defense Technology, China
Sheng-Feng Lu, Nanjing University of Chinese Medicine, China
Copyright © 2021 Wen, Ma, Dong, Sun, Lan, Yin, Qu, Liu, Xiao and Zeng. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Fang Zeng, emVuZ19mYW5nQDEyNi5jb20=
†These authors have contributed equally to this work and share first authorship
 Qiao Wen
Qiao Wen Peihong Ma
Peihong Ma Xiaohui Dong†
Xiaohui Dong† Lei Lan
Lei Lan Tao Yin
Tao Yin Fang Zeng
Fang Zeng