- 1Integrative Brain Imaging Center, National Center of Neurology and Psychiatry, Tokyo, Japan
- 2Department of Psychiatry, Center Hospital, National Center of Neurology and Psychiatry, Tokyo, Japan
- 3Department of Drug Dependence Research, National Institute of Mental Health, National Center of Neurology and Psychiatry, Tokyo, Japan
- 4Department of Radiology, Center Hospital, National Center of Neurology and Psychiatry, Tokyo, Japan
- 5Cyclotron and Drug Discovery Research Center, Southern TOHOKU Research Institute for Neuroscience, Koriyama, Japan
- 6Department of Biofunctional Imaging, Fukushima Medical University, Fukushima, Japan
Introduction: Altered dopaminergic neurotransmission, especially in the functioning of dopamine D2-type receptors, is considered central to the etiology of a variety of neuropsychiatric disorders. In particular, individuals with substance use disorders have been consistently observed to exhibit lower D2-type receptor availability (quantified as binding potential; BPND) using positron emission tomography (PET). Upregulation of D2-type receptor density thus may therefore provide a therapeutic effect for substance use disorders. Importantly, in vitro studies reveal that D2 receptors coexist with adenosine 2A (A2A) receptors to form the highest density of heteromers in the whole striatum, and there is a functional interaction between these two receptors. As such, blockade of A2A receptor’s function may prevent D2 receptor downregulation, yet no study has currently examined this hypothesis in humans.
Methods and Analysis: This double-blind, randomized controlled trial aims to evaluate the effect of the A2A receptor antagonist istradefylline (compared to placebo) on both dopamine D2-type receptor availability in the human brain and on neuropsychological measurements of impulsivity. It is hypothesized that istradefylline will both increase striatal D2-type BPND and improve control of impulsivity more than placebo. Forty healthy participants, aged 20–65 with no history of psychiatric or neurological disorders, will be recruited and randomized into two groups and will undergo [11C]raclopride PET, once before and once after administration of either 40 mg/day istradefylline or placebo for 2 weeks. Neuropsychological measurements will be administered on the same days of the PET scans.
Ethics and Dissemination: The study protocol was approved by the Certified Review Boards (CRB) of National Center of Neurology and Psychiatry (CR18-011) and prospectively registered with the Japan Registry of Clinical Trials (jRCTs031180131; https://jrct.niph.go.jp/latest-detail/jRCTs031180131). The findings of this study will be disseminated through peer reviewed scientific journals and conferences.
Clinical Trial Registration: www.ClinicalTrials.gov, identifier jRCTs031180131.
Highlights
Strengths and limitations of this study:
- This double-blind randomized controlled trial is the first study to test the effects of an adenosine 2A (A2A) receptor antagonist on dopamine D2-type receptors in the living human brain.
- Both positron emission tomography with a ligand with high affinity for D2-type receptors and neuropsychological measurements will be administered both pre- and post-intervention.
- Istradefylline, the only prescription selective A2A receptor antagonist, will be used as a pharmacological intervention for 2 weeks.
- The double-blind design enables rigorous evaluation of the efficacy of A2A receptor antagonist on D2-type receptors.
Introduction
Alterations in dopaminergic neurotransmission has been shown to play an important role in a variety of neuropsychiatric disorders such as schizophrenia (Howes and Kapur, 2009; McCutcheon et al., 2019), major depressive disorders (Belujon and Grace, 2017), Parkinson syndrome (Fahn, 2008), attention deficit/hyperactivity disorder (ADHD) (Tripp and Wickens, 2009; Kollins and Adcock, 2014), eating disorders (Wang et al., 2011; Frank, 2019), and substance use disorders (Solinas et al., 2019). With regards to the latter group of disorders, studies using positron emission tomography (PET) have repeatedly shown that compared to non-drug using healthy controls, participants who abuse drugs exhibit lower striatal dopamine D2-type receptor availability (quantified as binding potential; BPND) (see reviews Volkow et al., 2009; Ashok et al., 2017; London, 2020). Moreover, in methamphetamine users, lower striatal dopamine D2-type BPND is associated both with greater impulsivity (Lee et al., 2009; Ghahremani et al., 2012; Moeller et al., 2018) and with greater drug-seeking behaviors as determined by the choosing of methamphetamine-related images in a choice task (Moeller et al., 2018).
On the basis of these findings, enhancing dopaminergic signaling through dopamine D2-type receptors may provide a therapeutic approach for substance use disorders. However, to date, no dopamine D2-type receptor agonist has provided a therapeutic effect for any addictive disorder (Verrico et al., 2013; Blum et al., 2014). Importantly, this lack of an effect may be due to the fact that such medications only stimulate D2-type receptors but do not recover D2-type receptor’s function; upregulation of D2-type receptor density may therefore provide a therapeutic effect for addictive disorders.
Adenosine 2A (A2A) receptors and dopamine D2-type receptors are both G protein-coupled receptors (GPCRs), and are mostly expressed on the dendritic spines of striatopallidal GABAergic neurons where they constitute the highest density of A2A receptor-D2 receptor heteromers in the whole striatum. As such, there is a strong functional interaction between these two receptors in this region (Ferre et al., 2016). For instance, selective A2A receptor antagonism suppresses D2-type receptor internalization from the membrane into the cell body of cultivated rat cells (Huang et al., 2013). On this basis, A2A receptor antagonism could prevent D2-type receptor internalization and may therefore induce upregulation of these receptors in the human living brain.
Istradefylline, a structural analog of caffeine, is a selective antagonist at the A2A receptors and is used as an add-on treatment to levodopa for Parkinson’s disease. Because its long-term safety and high tolerability has been proved (Kondo and Mizuno, 2015), istradefylline has been used for this purpose since 2013 in Japan, and since 2019 in the United States. Specifically, istradefylline partially treats the negative effects of Parkinsonism by reducing “off” periods, which are defined as those periods in which the effects of levodopa are wearing off (Tao and Liang, 2015). Although istradefylline’s selective A2A receptor antagonism may result in upregulation of D2 receptors, no study has yet examined this potential effect in the living human brain.
The main research aim of this study will be to determine whether medium-term (i.e., 2-week) administration of istradefylline can produce upregulation of dopamine D2 receptors in humans (Aim 1). It is hypothesized that participants who receive 2-week administration of istradefylline, as compared to placebo, will exhibit increased dopamine D2-type receptor BPND as indicated by a significant group (istradefylline vs. placebo) × time (pre- vs. post-intervention) interaction (Hypothesis 1). The secondary aims will be to assess the effects of istradefylline on cognitive measures (Aim 2a), and to evaluate the extent to which any effects on cognition are related to changes in BPND (Aim 2b). Therefore, the secondary hypotheses are that istradefylline administration would improve cognitive performance more than placebo administration (as indicated by a significant group × time interaction; Hypothesis 2a), and that any effects of istradefylline administration on D2-type receptor BPND will be associated with such effects on cognitive performance (Hypothesis 2b).
Methods and Analysis
Research Design
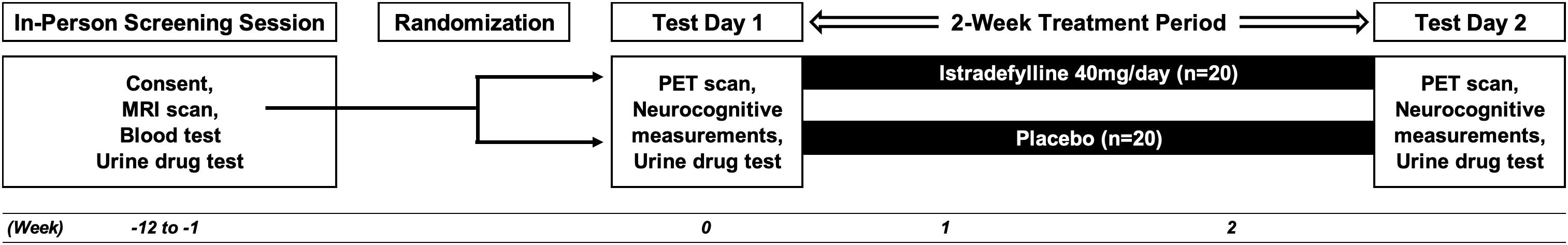
The study is a 2-week, double-blind, placebo-controlled, randomized controlled trial that aims to determine the potential effect of istradefylline, a selective A2A receptor antagonist, on dopamine D2-type BPND within the striatum. Participants will be randomized into two groups in a between-subjects design, one in which participants will receive (40 mg/day) of istradefylline, and the other in which they will receive a placebo (see Figure 1).
Participants
Forty participants, all aged 20–65 years old with no history of psychiatric or neurological disorders will be recruited for this study. Inclusion criteria are: being male, being able to comprehend Japanese and being of any race or ethnicity. Exclusion criteria include the following: smoking at least one cigarette or electronic cigarette within the past 1 year; lifetime history or current possibility of psychiatric disorder (confirmed using the Mini International Neuropsychiatric Interview; M.I.N.I.); intracranial lesion(s) as observed from the structural magnetic resonance image (MRI; see below); impairment of liver and/or renal function; HIV seropositive; positive urine-sample test for illegal drug; past history of suicidal ideation or attempt; claustrophobia or aichmophobia; metals inside the body incompatible with MRI; use of psychotropic drugs which could have an impact on dopamine D2-type BPND; use of drugs that interact with CYP3A4, a major metabolizing enzyme of istradefylline; caffeine consumption greater than 400 mg/day (e.g., more than five cups of coffee per day). Female participants will not be enrolled into this current study because of the reported influence of the menstrual cycle on D2-type BPND (Wong et al., 1988; Munro et al., 2006; Czoty et al., 2009). Because the main aim of this study is to determine whether A2A receptor blockade upregulates dopamine D2 receptor in humans rather than to determine the clinical utility of such blockade in a generalized sample of participants, we decided to enroll only male participants in order to eliminate the potential confounding factor of the menstrual cycle.
Patient and Public Involvement
Participants were not involved in the recruitment and conduct of the study. Moreover, participants will not be involved in deciding which data and results to report. However, members of the research team may listen to input from participants during the process of deciding what to report; regardless, the final decision as to which findings to report will be made only by the scientists.
Screening Visit
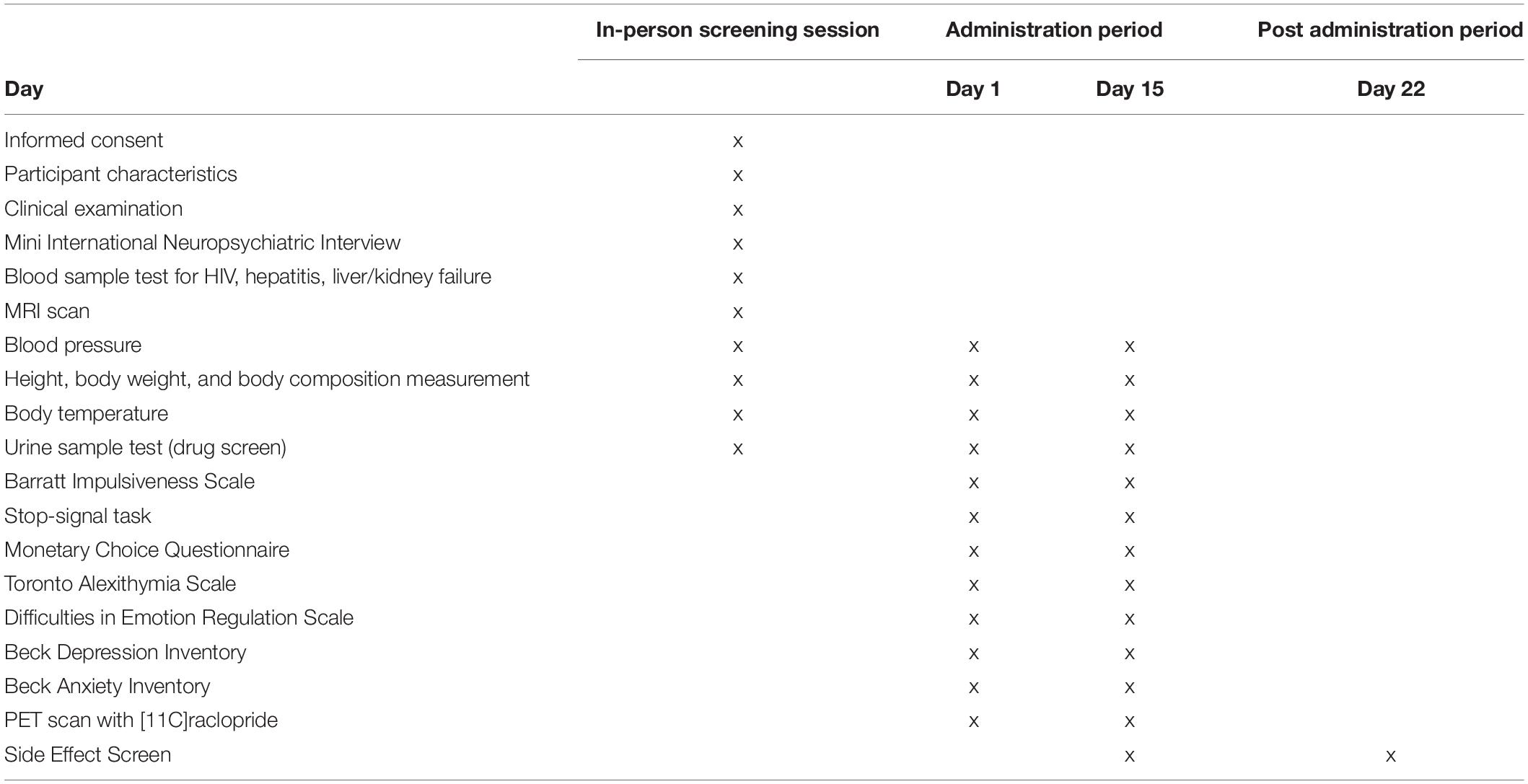
All aspects of this study will take place at the National Center of Neurology and Psychiatry (NCNP) in Kodaira, Tokyo, Japan. The study procedure has been reviewed and approved by the Certified Review Boards (CRBs) of NCNP. Study participants will be recruited through online methods and via print advertisement flyers distributed to public locations in Kodaira, including community centers, libraries, city halls, and schools. Potential participants will contact the research team by email or phone to be briefly screened over the phone to roughly determine eligibility; those who meet inclusion/exclusion criteria will then be invited to come into the office for further screening. At the initial in-person screening visit, participants will be given a full description of the study procedure and will be asked to read and sign the CRB-approved informed consent forms with the PI. After signing the consent form, a comprehensive baseline assessment including M.I.N.I., urine drug test, blood test and MRI scan (see below for details) will be performed for rigorous screening (see Table 1). The study physician will determine medical eligibility for the study via examination of all data acquired at the screening visit. Ineligible individuals are referred for clinical follow-up.
Study Medication
Participants will receive either istradefylline (40 mg/day) or placebo for 2 weeks in a double-blind, between-subjects design. This dose (40 mg/day) was selected because it is the maximum dose approved in clinical practice. Both istradefylline (brand name Nourianz®; Kyowa Kirin, Inc., Tokyo, Japan), and placebo have been encapsulated in sufficiently opaque and colored gelatin capsules (DBcaps®, Lonza, Basel, Switzerland) so as not to reveal contents inside or disclose any possible difference between the two capsule types. Active medication capsules consist of two 20 mg Nourianz® tablets and placebo capsules consist of two Satisfake® tablets (METGREEN, Co., Ltd., Tokyo, Japan). All study capsules contain dextrose as the inert filling agent and have been brought to proper packing level in color-matched, opaque, identically sized capsules. Participants will be instructed on Day 1 to take each capsule in the morning between 6 and 8 AM (i.e., once daily) to minimize a potential risk of drug-induced insomnia (Kondo and Mizuno, 2015). As such, participants will start taking their first capsule on the following morning, and will take their final capsule on the morning of Day 15. Participants will receive email notification from the investigator team every morning to check adherence and potential side effect.
Random Allocation
Participants will be randomly assigned in a 1:1 manner using a stratified block-randomization procedure with age as the stratification factor. The allocation sequence has been computer-generated and will be carried out by a study statistician who is not involved in any other study procedures in order to preserve the double-blind design. A blinded stratification list, including subject ID and age will be used to assign participants a group based on the stratification factor (i.e., active or placebo). A study pharmacist in charge will match this information to the unblinded stratification list and fills the prescription. The pharmacist is the only person who will have access to the unblinded stratification list.
Adverse Event Reporting
An annual summary of adverse events will be submitted to the Japan Registry of Clinical Trials (jRCTs) and the CRB at NCNP. In the event that significant medical problems occur, or in the case that an investigator believes that any aspects of the study may be causing harm to participants’ health, the blinding will be unlocked and medical treatment will be provided. Significant medical problems are defined as any fatal event, any immediately life-threatening event, any permanent or substantially disabling event, any event that requires or prolongs inpatient hospitalization, any congenital anomaly, or any unexpected adverse drug experiences that have not previously been observed. The PI will promptly report all severe adverse events to the director of NCNP, the CRB of NCNP, the Pharmaceuticals and Medical Devices Agency, and the Ministry of Health, Labor and Welfare (where appropriate). A decision to unlock the blinding will be determined by the PI given the severity of the medical problem and its relevance to study medication.
Data Monitoring
An independent review board at the Department of Clinical Epidemiology, Translational Medical Center at NCNP will perform regular monitoring over the course of the study. They will oversee the case report forms to verify that all study procedures are in compliance with approved study protocol, and to provide monitoring reports to the PI.
Neuroimaging
Magnetic Resonance Image Scan
Structural MRI scans of the brain will be acquired for co-registration with PET images and definition of volumes-of-interest (VOIs). A T1-weighted scan will be acquired using a whole-brain magnetization-prepared rapid acquisition with gradient echo (MPRAGE) (TR = 1900 ms, TE = 4.38 ms, flip angle = 15, field of view = 250 × 250 × 165, 165 slices, thickness = 1 mm).
Positron Emission Tomography Scan
Positron emission tomography scans with [11C]raclopride, a radiotracer with selective affinity for dopamine D2-type receptors (Kohler et al., 1985) will be acquired. PET scans with [11C]raclopride will be acquired; importantly, the test-retest reliability of the scan has been shown to be good (Alakurtti et al., 2015). Participants will undergo 2 PET scan; one scan pre-intervention and a second scan 6 h after the final drug administration, both of which will begin at 1 PM local time. PET data were acquired using a Biograph TruePoint 6 PET/CT (Siemens Healthineers, Erlangen, Germany), which has a resolution full-width at half-maximum (FWHM) of 4.1 mm, and an axial field of view of 162 mm in the 3D mode. Participants will be placed in the supine position, with their head secured with a plastic band to avoid movement during the scan. After a low-dose CT scan was performed for attenuation correction, emission data will be collected for 60 min after a bolus injection of 10 mCi (±10%; range of 9–11 mCi) [11C]raclopride, which has a specific activity of > 1 Ci/μmol.
Data Processing
Using the 3-dimention ordered subset expectation maximization (3D-OSEM) algorithm (Lee et al., 2014), the PET data will be reconstructed into twelve 20-s frames, sixteen 60-s frames, and ten 240-s frames (Kubota et al., 2017). FSL MCFLIRT (FMRIB Centre, Dept. Clinical Neurology, University of Oxford) will be used for motion correction (Jenkinson et al., 2002). The images will then be co-registered to the MPRAGE image using a 6-parameter, rigid-body spatial transformation (FSL FLIRT).
VOIs will be derived from individual MPRAGE images using FSL FIRST (Patenaude et al., 2011). Caudate and putamen VOIs will be combined to constitute a single striatum VOI. The cerebellum will be used as the reference region (Hall et al., 1994). A cerebellum VOI, including the hemispheres but not the vermis, will be manually created in standard space (MNI152 template) and transformed into native space with FSL FNIRT.
Time-activity data within VOIs will be extracted from PET images and imported into PMOD Kinetic Modeling (PKIN) (PMOD Technologies Ltd., Zurich). The simplified reference tissue model (SRTM), will be used to calculate BPND. This method will be used because its accuracy for [11C]raclopride scans has been fully validated (Farde et al., 1989; Lammertsma and Hume, 1996; Lammertsma et al., 1996), and because this method eliminates the need for measuring the arterial input function which in turn avoids the potential risks associated with arterial cannulation. BPND will be calculated with time–activity curves from VOIs as follows: CT(t) = R1CR(t) + (k2 – R1k2/(1 + BPND))CR(t) ∗ exp(−k2t/(1 + BPND)) where CT(t) is the total radioactivity concentration in the striatum VOI measured by PET, R1 is the ratio of K1–K1’ (K1, influx rate constant for the striatum; K1’, influx rate constant for the cerebellum), CR(t) is the radioactivity concentration in the reference region (cerebellum), and ∗ denotes the convolution integral. The parameters R1, k2, and BPND in this model are estimated by a non-linear curve-fitting procedure.
Neuropsychological Measurements
The following measurements will be administered starting at 10 AM on each day of PET scan (i.e., 3 h before the scan) (see Table 1).
Barratt Impulsiveness Scale
This 30-item self-report questionnaire assesses impulsive personality traits (Patton et al., 1995). A form of this measure that is sensitive to changes in impulsivity over time (Ghahremani et al., 2013) will be used.
Stop-Signal Task
On “go” trials, a series of visual stimuli are presented to prompt pressing a left or right corresponding key. On “stop” trials (i.e., 25% of all trials), a tone (i.e., a “stop-signal”), will be presented after a short delay after the “go” stimulus (this delay is termed the stop-signal delay; SSD); this “stop-signal” will indicate to the participant that they should withhold responding. The SSD is adjusted on a trial-by-trial basis according to performance. The primary dependent variable, stop-signal reaction time, is the mean difference between the SSD and the reaction time to “go” stimuli, and indicates how quickly the participant can inhibit an ongoing motor response.
Monetary Choice Questionnaire
Participants must select between one of two hypothetical options in 27 questions to receive a certain amount of money immediately or a larger amount later. The discrepancies between the monetary amounts and duration of the delay are varied across questions. A hyperbolic statistical function is fit to the data to estimate the individual’s point of indifference between the options. The primary dependent variable is the indifference point, or total k value, determined from the participant’s selections across the task. Scores are typically adjusted with the natural log to compensate for skew (Kirby et al., 1999).
Toronto Alexithymia Scale-20
Emotional self-awareness will be assessed using the Toronto Alexithymia Scale-20 (TAS-20; Bagby et al., 1994), a widely used questionnaire that consists of three subscales that provide scores for corresponding factors. Two of the factors assess emotional self-awareness: Factor 1: Difficulty Identifying Feelings; and Factor 2: Difficulty Describing Feelings. Factor 3 assesses Externally-Oriented Thinking. TAS-20 total score (the sum of the three subscale scores) is interpreted as follows: 0–51: no alexithymia; 52–60: possible alexithymia; 61 and above: alexithymia (Taylor et al., 1999).
Difficulties in Emotion Regulation Scale
Difficulties in Emotion Regulation Scale (DERS) will be administered to assess an emotion dysregulation (Gratz and Roemer, 2004). This 36-item questionnaire is divided into six-distinct subscales: Non-acceptance of Emotional Responses (NON-ACCEPT); Difficulties Engaging in Goal-Directed Behavior (GOALS); Impulse Control Difficulties (IMPULSE); Lack of Emotional Awareness (AWARE); Limited Access to Emotion Regulation Strategies (STRATEGIES); and Lack of Emotional Clarity (CLARITY). Scores on each subscale can be summed to create a total score which can range from 30 to 180; higher scores represent more difficulties in emotion regulation.
Beck Depression Inventory and Beck Anxiety Inventory
The Beck Depression Inventory (BDI) (Beck et al., 1996) and Beck Anxiety Inventory (BAI) (Beck et al., 1988) are self-report inventories to verify depressive and anxiety symptoms respectively. They will be employed to verify exclusion criteria and monitor potential adverse event related to drug administration.
Data Analytic Plan
Power Considerations
A sample size was selected based on a power analysis that was performed previously. Eighteen subjects per group is assumed to be necessary to provide sufficient power (β > 0.8) to detect interaction effects as small as f = 0.2, assuming a within-subject correlation coefficient of 0.8 based on previous study (Alakurtti et al., 2015) for the repeated measures and two-sided significance of α = 0.05. Taking into account the potential drop out from the study of roughly 10% of all recruited participants, we set a sample size of N = 40. A previous study that aimed to show D2-type receptor upregulation in humans after completion of an exercise intervention revealed a group-by-time interaction on the dependent variable to the magnitude of f = 0.28 (Robertson et al., 2016). Thus, the current study appears to be powered to detect a significant effect in our main aim.
Statistical Analysis
We will use graphical and numerical summaries to screen the data for outliers and violations of model assumptions. For Aim 1, the hypothesis will be tested by evaluating the effects of treatment group (istradefylline vs. placebo), time (pre- vs. post-intervention), and the group-by-time interaction on D2-type BPND (which will be the dependent variable) using a repeated measures ANCOVA. In post hoc tests, the effect of time will be tested using paired t-tests in each group to identify the sign of any interaction effect. For Aim 2a, the hypothesis will be tested by using the same above-defined ANCOVA model but with cognitive measures as the dependent variable instead of D2-type BPND. For aim 2b, partial correlation analysis will be performed to evaluate the potential associations between change in BPND and change in cognitive measures. These statistical analyses will be conducted using SPSS IBM 19 (IBM, Armonk, NY, United States).
Discussion
A variety of psychiatric and neurological disorders are characterized by impaired dopaminergic neurotransmission through dopamine D2-type receptor. In particular, lower striatal D2-type BPND has been repeatedly observed in participants with substance use disorders when compared with healthy controls (Volkow et al., 2009; Ashok et al., 2017; London, 2020). Importantly, because such observations were made from cross-sectional studies, it may be that these findings could reflect innate biological characteristics of individuals with substance use disorders that precede the initiation of substance use. However, given that most addictive substances have been shown to induce phasic dopamine release in the human brain (Nutt et al., 2015), and because agonism of GPCRs initiates processes that are involved in the subsequent desensitization of these receptors that is associated with their downregulation (Ferguson, 2001; Tabor et al., 2017), it seems likely that accumulative substance use contributes to D2-receptor downregulation (Volkow et al., 2004). Further, in vitro studies using cultured cells derived from human neuroblastoma indicate that concurrent agonist stimulation of A2A receptors and D2 receptors induces coaggregation and co-internalization of those receptors into cell bodies (Hillion et al., 2002; Bartlett et al., 2005). Therefore, A2A receptor antagonists may help to upregulate D2 receptors by preventing such receptor internalization.
A human longitudinal PET study using the same PET ligand as we will use in this study has shown that striatal D2-type receptor availability increases after a single administration of caffeine. Because caffeine is an agonist of not only the A2A receptor subtype, but also of other subtypes of adenosine receptors; it may be that this increase in D2-type receptor availability is a consequence of controlling D2 receptor internalization by A2A receptors (Volkow et al., 2015). To date, this is the only study that has demonstrated the potential allosteric effect between A2A receptors and D2 receptors in the living human brain. However, since caffeine has an antagonistic action on any subtype of adenosine receptors (Ribeiro and Sebastiao, 2010), the results of Volkow et al. (2015) do not necessarily indicate that an A2A receptor antagonist could induce D2-type receptor upregulation. In addition, although the study design employed by Volkow et al. (2015) was a simple pre- vs. post-comparison, it would be prudent to employ a randomized control trial design to eliminate any possible evaluation biases.
Thus, the proposed study will monitor changes in striatal D2-type receptor BPND due to administration of istradefylline, a selective A2AR antagonist using a double-blind, randomized, placebo-controlled design. Importantly, our study design cannot distinguish between acute and chronic effects because the interval between the last drug administration and a final (i.e., second) scan is only 6 h. However, positive findings, positive findings from this study would have wide-ranging significance, especially for the potential treatment of a variety of neuropsychiatric disorders that are characterized by dysfunctional dopamine D2-type receptors, for many of which no pharmacological intervention has been proven effective.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
The studies involving human participants were reviewed and approved by Certified Review Boards of the National Center of Neurology and Psychiatry. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
All authors made a significant contribution to the conception and design of the trial protocol. KO is the PI of the study and made major contributions to the design of this trial, development of the original trial protocol and drafting of the initial manuscript.
Funding
This research was funded by following research grants and fellowships: KAKENHI Grants-in-Aid for Scientific Research – Research Activity Start-up (Grant Number: 19K21217); the Research Fellowship by Japan Research Foundation for Clinical Pharmacology (Grant Number: N/A); the Research Fellowship by Astellas Foundation for Research on Metabolic Disorders (Grant Number: N/A); and the Naito Foundation Research Grant (Grant Number: N/A).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
The authors are grateful to the following contributors: Mitsuru Syakadou, Kaori Takeda, Yumi Saito, Atsushi Yokoyama, Masato Ogura, Midori Kusama, Kenji Hatano, Kazunori Oih, Ichiko Iizuka, Atsuko Asano, Takami Ishizuka, Minako Yamagishi, Komei Shimokawa, and Hirofumi Komaki.
References
Alakurtti, K., Johansson, J. J., Joutsa, J., Laine, M., Bäckman, L., Nyberg, L., et al. (2015). Long-term test-retest reliability of striatal and extrastriatal dopamine D2/3 receptor binding: study with [(11)C]raclopride and high-resolution PET. J. Cereb. Blood Flow Metab. 35, 1199–1205. doi: 10.1038/jcbfm.2015.53
Ashok, A. H., Mizuno, Y., Volkow, N. D., and Howes, O. D. (2017). Association of stimulant use with dopaminergic alterations in users of cocaine, amphetamine, or methamphetamine: a systematic review and meta-analysis. JAMA Psychiatry 74, 511–519.
Bagby, R. M., Parker, J. D., and Taylor, G. J. (1994). The twenty-item Toronto alexithymia scale–I. Item selection and cross-validation of the factor structure. J. Psychosom. Res. 38, 23–32. doi: 10.1016/0022-3999(94)90005-1
Bartlett, S. E., Enquist, J., Hopf, F. W., Lee, J. H., Gladher, F., Kharazia, V., et al. (2005). Dopamine responsiveness is regulated by targeted sorting of D2 receptors. Proc. Natl. Acad. Sci. U.S.A. 102, 11521–11526.
Beck, A. T., Epstein, N., Brown, G., and Steer, R. A. (1988). An inventory for measuring clinical anxiety: psychometric properties. J. Consult. Clin. Psychol. 56, 893–897.
Beck, A. T., Steer, R. A., Ball, R., and Ranieri, W. (1996). Comparison of beck depression inventories -IA and -II in psychiatric outpatients. J. Pers. Assess. 67, 588–597. doi: 10.1207/s15327752jpa6703_13
Belujon, P., and Grace, A. A. (2017). Dopamine system dysregulation in major depressive disorders. Int. J. Neuropsychopharmacol. 20, 1036–1046.
Blum, K., Thanos, P. K., and Gold, M. S. (2014). Dopamine and glucose, obesity, and reward deficiency syndrome. Front. Psychol. 5:919.
Czoty, P. W., Riddick, N. V., Gage, H. D., Sandridge, M., Nader, S. H., Garg, S., et al. (2009). Effect of menstrual cycle phase on dopamine D2 receptor availability in female cynomolgus monkeys. Neuropsychopharmacology 34, 548–554. doi: 10.1038/npp.2008.3
Fahn, S. (2008). The history of dopamine and levodopa in the treatment of Parkinson’s disease. Mov. Disord. 23(Suppl. 3), S497–S508.
Farde, L., Eriksson, L., Blomquist, G., and Halldin, C. (1989). Kinetic analysis of central [11C]raclopride binding to D2-dopamine receptors studied by PET–a comparison to the equilibrium analysis. J. Cereb. Blood Flow Metab. 9, 696–708. doi: 10.1038/jcbfm.1989.98
Ferguson, S. S. (2001). Evolving concepts in G protein-coupled receptor endocytosis: the role in receptor desensitization and signaling. Pharmacol. Rev. 53, 1–24.
Ferre, S., Bonaventura, J., Tomasi, D., Navarro, G., Moreno, E., Cortes, A., et al. (2016). Allosteric mechanisms within the adenosine A2A-dopamine D2 receptor heterotetramer. Neuropharmacology 104, 154–160.
Ghahremani, D. G., Lee, B., Robertson, C. L., Tabibnia, G., Morgan, A. T., De Shetler, N., et al. (2012). Striatal dopamine D(2)/D(3) receptors mediate response inhibition and related activity in frontostriatal neural circuitry in humans. J. Neurosci. 32, 7316–7324. doi: 10.1523/JNEUROSCI.4284-11.2012
Ghahremani, D. G., Oh, E. Y., Dean, A. C., Mouzakis, K., Wilson, K. D., and London, E. D. (2013). Effects of the youth empowerment seminar on impulsive behavior in adolescents. J. Adolesc. Health 53, 139–141. doi: 10.1016/j.jadohealth.2013.02.010
Gratz, K., and Roemer, L. (2004). Multidimensional assessment of emotion regulation and dysregulation: development, factor structure, and initial validation of the difficulties in emotion regulation scale. J. Psychopathol. Behav. Assess. 26, 41–54. doi: 10.1177/0145445514566504
Hall, H., Sedvall, G., Magnusson, O., Kopp, J., Halldin, C., and Farde, L. (1994). Distribution of D1-and D2-dopamine receptors, and dopamine and its metabolites in the human brain. Neuropsychopharmacology 11, 245–256. doi: 10.1038/sj.npp.1380111
Hillion, J., Canals, M., Torvinen, M., Casado, V., Scott, R., Terasmaa, A., et al. (2002). Coaggregation, cointernalization, and codesensitization of adenosine A2A receptors and dopamine D2 receptors. J. Biol. Chem. 277, 18091–18097.
Howes, O. D., and Kapur, S. (2009). The dopamine hypothesis of schizophrenia: version III–the final common pathway. Schizophr. Bull. 35, 549–562. doi: 10.1093/schbul/sbp006
Huang, L., Wu, D. D., Zhang, L., and Feng, L. Y. (2013). Modulation of A(2)a receptor antagonist on D(2) receptor internalization and ERK phosphorylation. Acta Pharmacol. Sin. 34, 1292–1300.
Jenkinson, M., Bannister, P., Brady, M., and Smith, S. (2002). Improved optimization for the robust and accurate linear registration and motion correction of brain images. Neuroimage 17, 825–841. doi: 10.1016/s1053-8119(02)91132-8
Kirby, K. N., Petry, N. M., and Bickel, W. K. (1999). Heroin addicts have higher discount rates for delayed rewards than non-drug-using controls. J. Exp. Psychol. Gen. 128, 78–87.
Kohler, C., Hall, H., Ogren, S. O., and Gawell, L. (1985). Specific in vitro and in vivo binding of 3H-raclopride. A potent substituted benzamide drug with high affinity for dopamine D-2 receptors in the rat brain. Biochem. Pharmacol. 34, 2251–2259. doi: 10.1016/0006-2952(85)90778-6
Kollins, S. H., and Adcock, R. A. (2014). ADHD, altered dopamine neurotransmission, and disrupted reinforcement processes: implications for smoking and nicotine dependence. Prog. Neuro Psychopharmacol. Biol. Psychiatry 52, 70–78. doi: 10.1016/j.pnpbp.2014.02.002
Kondo, T., and Mizuno, Y. (2015). A long-term study of istradefylline safety and efficacy in patients with Parkinson disease. Clin. Neuropharmacol. 38, 41–46. doi: 10.1097/WNF.0000000000000073
Kubota, M., Nagashima, T., Takano, H., Kodaka, F., Fujiwara, H., Takahata, K., et al. (2017). Affinity states of striatal dopamine D2 receptors in antipsychotic-free patients with schizophrenia. Int. J. Neuropsychopharmacol. 20, 928–935. doi: 10.1093/ijnp/pyx063
Lammertsma, A. A., and Hume, S. P. (1996). Simplified reference tissue model for PET receptor studies. NeuroImage 4, 153–158.
Lammertsma, A. A., Bench, C. J., Hume, S. P., Osman, S., Gunn, K., Brooks, D. J., et al. (1996). Comparison of methods for analysis of clinical [11C]raclopride studies. J. Cereb. Blood Flow Metab. 16, 42–52.
Lee, B., London, E. D., Poldrack, R. A., Farahi, J., Nacca, A., Monterosso, J. R., et al. (2009). Striatal dopamine D2/D3 receptor availability is reduced in methamphetamine dependence and is linked to impulsivity. J. Neurosci. 29, 14734–14740. doi: 10.1523/JNEUROSCI.3765-09.2009
Lee, Y. S., Kim, J. S., Kim, K. M., Kang, J. H., Lim, S. M., and Kim, H. J. (2014). Performance measurement of PSF modeling reconstruction (True X) on Siemens biograph truepoint TrueV PET/CT. Ann. Nucl. Med. 28, 340–348. doi: 10.1007/s12149-014-0815-z
London, E. D. (2020). Human brain imaging links dopaminergic systems to impulsivity. Curr. Top. Behav. Neurosci. 47, 53–71.
McCutcheon, R. A., Abi-Dargham, A., and Howes, O. D. (2019). Schizophrenia, dopamine and the striatum: from biology to symptoms. Trends Neurosci. 42, 205–220.
Moeller, S. J., Okita, K., Robertson, C. L., Ballard, M. E., Konova, A. B., Goldstein, R. Z., et al. (2018). Low striatal dopamine D2-type receptor availability is linked to simulated drug choice in methamphetamine users. Neuropsychopharmacology 43, 751–760. doi: 10.1038/npp.2017.138
Munro, C. A., Mccaul, M. E., Wong, D. F., Oswald, L. M., Zhou, Y., Brasic, J., et al. (2006). Sex differences in striatal dopamine release in healthy adults. Biol. Psychiatry 59, 966–974.
Nutt, D. J., Lingford-Hughes, A., Erritzoe, D., and Stokes, P. R. (2015). The dopamine theory of addiction: 40 years of highs and lows. Nat. Rev. Neurosci. 16, 305–312. doi: 10.1038/nrn3939
Patenaude, B., Smith, S. M., Kennedy, D. N., and Jenkinson, M. (2011). A Bayesian model of shape and appearance for subcortical brain segmentation. NeuroImage 56, 907–922.
Patton, J. H., Stanford, M. S., and Barratt, E. S. (1995). Factor structure of the Barratt impulsiveness scale. J. Clin. Psychol. 51, 768–774.
Ribeiro, J. A., and Sebastiao, A. M. (2010). Caffeine and adenosine. J. Alzheimers Dis. 20(Suppl. 1), S3–S15.
Robertson, C. L., Ishibashi, K., Chudzynski, J., Mooney, L. J., Rawson, R. A., Dolezal, B. A., et al. (2016). Effect of exercise training on striatal dopamine D2/D3 receptors in methamphetamine users during behavioral treatment. Neuropsychopharmacology 41, 1629–1636. doi: 10.1038/npp.2015.331
Solinas, M., Belujon, P., Fernagut, P. O., Jaber, M., and Thiriet, N. (2019). Dopamine and addiction: what have we learned from 40 years of research. J. Neural Transm. 126, 481–516.
Tabor, A., Möller, D., Hübner, H., Kornhuber, J., and Gmeiner, P. (2017). Visualization of ligand-induced dopamine D2S and D2L receptor internalization by TIRF microscopy. Sci. Rep. 7:10894. doi: 10.1038/s41598-017-11436-1
Tao, Y., and Liang, G. (2015). Efficacy of adenosine A2A receptor antagonist istradefylline as augmentation for Parkinson’s disease: a meta-analysis of randomized controlled trials. Cell Biochem. Biophys. 71, 57–62. doi: 10.1007/s12013-014-0162-7
Taylor, G. J., Bagby, R. M., and Parker, J. D. A. (1999). Disorders of Affect Regulation: Alexithymia in Medical and Psychiatric Illness. Cambridge: Cambridge University Press.
Verrico, C. D., Haile, C. N., Newton, T. F., Kosten, T. R., and De La Garza, R. II (2013). Pharmacotherapeutics for substance-use disorders: a focus on dopaminergic medications. Expert Opin. Investig. Drugs 22, 1549–1568.
Volkow, N. D., Fowler, J. S., Wang, G. J., and Swanson, J. M. (2004). Dopamine in drug abuse and addiction: results from imaging studies and treatment implications. Mol. Psychiatry 9, 557–569.
Volkow, N. D., Fowler, J. S., Wang, G. J., Baler, R., and Telang, F. (2009). Imaging Dopamine’s role in drug abuse and addiction. Neuropharmacology 56(Suppl. 1), 3–8.
Volkow, N. D., Wang, G. J., Logan, J., Alexoff, D., Fowler, J. S., Thanos, P. K., et al. (2015). Caffeine increases striatal dopamine D2/D3 receptor availability in the human brain. Transl. Psychiatry 5:e549. doi: 10.1038/tp.2015.46
Wang, G. J., Geliebter, A., Volkow, N. D., Telang, F. W., Logan, J., Jayne, M. C., et al. (2011). Enhanced striatal dopamine release during food stimulation in binge eating disorder. Obesity 19, 1601–1608. doi: 10.1038/oby.2011.27
Keywords: dopamine D2 receptors, adenosine 2A receptor, positron emission tomography, striatum, randomized control study
Citation: Okita K, Kato K, Shigemoto Y, Sato N, Matsumoto T and Matsuda H (2021) Effects of an Adenosine A2A Receptor Antagonist on Striatal Dopamine D2-Type Receptor Availability: A Randomized Control Study Using Positron Emission Tomography. Front. Neurosci. 15:729153. doi: 10.3389/fnins.2021.729153
Received: 24 June 2021; Accepted: 18 August 2021;
Published: 13 September 2021.
Edited by:
Francisco Ciruela, University of Barcelona, SpainReviewed by:
Kristoffer Sahlholm, Umeå University, SwedenJordi Bonaventura, National Institute on Drug Abuse (NIDA), United States
Copyright © 2021 Okita, Kato, Shigemoto, Sato, Matsumoto and Matsuda. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Kyoji Okita, a29raXRhQG5jbnAuZ28uanA=
 Kyoji Okita
Kyoji Okita Koichi Kato1
Koichi Kato1 Hiroshi Matsuda
Hiroshi Matsuda