
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Neurosci. , 23 August 2021
Sec. Neuroprosthetics
Volume 15 - 2021 | https://doi.org/10.3389/fnins.2021.722231
This article is part of the Research Topic Human Machine Interface-based Neuromodulation Solutions for Neurorehabilitation View all 19 articles
 Guiyuan Cai1,2†
Guiyuan Cai1,2† Manfeng Wu1,2†
Manfeng Wu1,2† Qian Ding1,3†
Qian Ding1,3† Tuo Lin1,3
Tuo Lin1,3 Wanqi Li1,3
Wanqi Li1,3 Yinghua Jing1,3
Yinghua Jing1,3 Hongying Chen1,2
Hongying Chen1,2 Huiting Cai4
Huiting Cai4 Tifei Yuan4,5,6*
Tifei Yuan4,5,6* Guangqing Xu7*
Guangqing Xu7* Yue Lan1,3*
Yue Lan1,3*Transcranial magnetic stimulation (TMS) has a wide range of clinical applications, and there is growing interest in neural oscillations and corticospinal excitability determined by TMS. Previous studies have shown that corticospinal excitability is influenced by fluctuations of brain oscillations in the sensorimotor region, but it is unclear whether brain network activity modulates corticospinal excitability. Here, we addressed this question by recording electroencephalography (EEG) and TMS measurements in 32 healthy individuals. The resting motor threshold (RMT) and active motor threshold (AMT) were determined as markers of corticospinal excitability. The least absolute shrinkage and selection operator (LASSO) was used to identify significant EEG metrics and then correlation analysis was performed. The analysis revealed that alpha2 power in the sensorimotor region was inversely correlated with RMT and AMT. Innovatively, graph theory was used to construct a brain network, and the relationship between the brain network and corticospinal excitability was explored. It was found that the global efficiency in the theta band was positively correlated with RMT. Additionally, the global efficiency in the alpha2 band was negatively correlated with RMT and AMT. These findings indicated that corticospinal excitability can be modulated by the power spectrum in sensorimotor regions and the global efficiency of functional networks. EEG network analysis can provide a useful supplement for studying the association between EEG oscillations and corticospinal excitability.
Transcranial Magnetic Stimulation (TMS) induces an electric field through a time-varying magnetic field, resulting in induced electric currents and changing the action potential of nerve cells in the cerebral cortex, thus affecting blood circulation, metabolism, and nerve excitability in the brain (Wanalee et al., 2015). As an effective non-invasive nerve stimulation technology, TMS is widely used in clinical practice and can be used to improve motor function in stroke patients, improve individual cognitive function, and reduce depression (Myczkowski et al., 2018; Chen et al., 2019).
Neuron oscillatory activity plays an important role in the cortical response to TMS. To clarify the interaction between neural activity and TMS, researchers explored the association between electroencephalography (EEG) and corticospinal excitability, defined as the cortical output in response to TMS. It was found that the fluctuation of EEG oscillations selectively affects corticospinal excitability (Berger et al., 2014; Bergmann et al., 2019; Ogata et al., 2019). Although EEG oscillations were assumed to regulate cortical excitability, the findings in previous studies were inconsistent. Some studies reported a correlation between corticospinal excitability and pre-stimulation alpha oscillation power (Sauseng et al., 2009) as well as beta oscillation power (Maki and Ilmoniemi, 2010; Hussain et al., 2019b) while others found no correlations between various EEG frequencies (Iscan et al., 2016). There are contradictions in these studies. Sauseng et al. (2009) found that the amplitude of motor evoked potentials (MEPs) is negatively correlated with the pre-stimulation alpha oscillation power, while Ogata et al. (2019) reported the opposite results. These conflicting results have evoked the need for further investigation of the relationship between EEG oscillations and corticospinal excitability to obtain reliable results.
Interestingly, previous studies focused on how EEG oscillations in sensorimotor regions modulated corticospinal excitability, while ignoring the effects of brain global network activity. Early neuroscience research focused on the function of single brain regions, while modern approaches tend to use complex network methods to analyze the structure and dynamic behavior of neural networks. Brain regions such as the frontal and parietal lobes regulate corticospinal excitability (Cattaneo and Barchiesi, 2011). A previous study showed that corticospinal excitability was regulated by the attention network, providing another perspective for understanding the association between corticospinal excitability and brain networks (Avenanti et al., 2018). A recent study indicated that TMS efficacy was modulated by the functional state of the target brain network (Schiena et al., 2020), suggesting that researchers should pay more attention to the impact of brain networks on corticospinal excitability rather than a single brain region.
In the field of brain networks, techniques for the construction and analysis of brain networks are still evolving. As a branch of scientific computing, graph theory involves the construction of a network by defining a series of nodes and connecting edges. This model fits the pattern of brain activity, which makes it a great tool for brain functional segmentation and integration (Sporns, 2018). The global efficiency and the clustering coefficient can be calculated using graph theory to measure the features of the brain network. Graph theory analysis of EEG has been gradually applied to describe neural electrophysiological activity (Rubinov and Sporns, 2010). A previous study showed that adjusting brain excitability through transcranial direct current stimulation can change the small-world propensity in brain networks (Vecchio et al., 2018), suggesting that graph theory can be a useful approach to study the interactions between brain networks and neural regulation technology.
For the purpose of this study, we aimed to explore the association between corticospinal excitability and EEG oscillations. The resting motor threshold (RMT) and active motor threshold (AMT) were identified as markers of corticospinal excitability according to previous study (Stefanou et al., 2020). Innovatively, we used graph theory analysis methods to construct brain networks to explore the relationship between the network properties and corticospinal excitability. Considering the factors mentioned above, we assumed that corticospinal excitability depends not only on the neural activity of the motor region, but also on the functional activity of the brain network. Clarifying the mechanism by which oscillating brain activity modulates corticospinal excitability will help elucidate how TMS works, which is conducive to enhancing the effect of TMS by stimulating the brain based on the current neural state.
A total of 32 right-handed individuals (mean age: 21.53 ± 1.46; 7 men), all college students, were included in this study. The participants did not take psychotropic drugs and had no history of central nervous system diseases or head trauma. Individuals with contraindications to TMS were excluded (Rossi et al., 2021). Before the experiment, the participants were required to get enough sleep to maintain a good mental state during the experiment. All participants were informed of the purpose and content of the experiment and signed informed consent forms before the experiment. This study was approved by the Ethics Committee of Guangzhou First People’s Hospital.
Resting-state EEG were recorded before application of TMS. The EEG data collection was performed in the electrophysiological laboratory and the room was quiet during EEG recording. The participants sat in a comfortable chair and their resting EEG was recorded for 10 min while their eyes were closed. They were required to remain awake throughout the recording. EEG data were recorded by using 128-channel HydroCel Geodesic Sensor Net (Electrical Geodesics, Inc., Eugene, OR, United States), and the Cz electrodes were used as the online reference. The impedances of all electrodes were kept below 10 kΩ by input impedance amplifiers (Geodesic EEG system 400). The signal was amplified at a sampling rate of 2,048 Hz and filtered through a 0.1–100 Hz band-pass filter. Data were processed offline after continuous EEG acquisition.
Electroencephalography processing was conducted with Matlab R2013a (The MathWorks, Natick, MA, United States) and eeglab12.0.1 After reducing the sampling rate to 1,000 Hz, the data were filtered through 0.1–40 Hz with a finite impulse response (FIR) filter. Continuous data were segmented into 2 s for each epoch. Bad data were defined when the amplitude exceeded ± 100 μV. Independent components analysis (ICA) was conducted to eliminate electro-oculograms after removing bad epochs and interpolating electrodes with high noise.
To obtain spectrum information for the EEG data, Fast Fourier Transform (FFT) was used to decompose the data. We calculated the power of the four frequency bands: delta (1–4 Hz), theta (4–8 Hz), alpha1 (8–10 Hz), alpha2 (10–13 Hz), beta1 (13–20 Hz), and beta2 (20–30 Hz). In accordance with previous research, data collected from the cluster of EEG electrodes around C4 were averaged to represent the activity of the sensorimotor region (Bayram et al., 2015).
As for functional connectivity, the phase lag index (PLI) was applied to characterize the connections between the electrode pairs because it can eliminate the volumetric conduction effect. Band-pass filtering was performed on the electrode signal, and Hilbert Transform was performed on the filtered electrical signal to extract the phase at each time point. Afterward, the PLI of each electrode pair in the six frequency bands was calculated. PLI was calculated using the following formula (Su et al., 2017).
The range of the PLI value was 0–1; 0 indicated that the two signals did not have a linear dependence in this frequency band, while 1 indicated complete synchronization.
In this study, the GRETNA toolbox2 was used for graph theoretical network analysis (Wang et al., 2015). The undirected weighted network was set up using electrical poles as nodes and the PLI value as the edge weight (de Waal et al., 2014). Based on previous studies, the clustering coefficient and efficiency, which are the most commonly used metrics, were selected to characterize the complex networks (Zomorrodi et al., 2019). Since there was no definite method for selecting a single threshold, referring to previous studies, we integrated the metrics over the entire threshold range to obtain the area under the curve (AUC) to characterize the brain network (Wang et al., 2015; Yan et al., 2017).
The clustering coefficient is defined as the ratio of the actual number of edges between a given node and its neighbors and the total number of possible edges between these nodes, and is used to measure the tightness between a node and its neighbor nodes in a network.
The clustering coefficient of the whole network is the average of all nodes in the network.
Efficiency refers to the reciprocal of the harmonic average distance between all nodes in the network. The efficiency of a node is used to measure the information transmission capacity of the given node in the network.
The global efficiency is the average of all nodes in the network.
For the formula and interpretation of these metrics, see Rubinov and Sporns (2010). The clustering coefficient and node efficiency of the motor cortex are the average values from the electrodes in the motor region.
Resting motor threshold and AMT was recorded immediately after EEG acquisition. Stimulation was applied using a figure-eight coil connected to the NS5000 Magnetic Stimulator (YIRUIDE Medical Co., Wuhan, China) with a maximum magnetic field intensity of 2.5 T. The participants assumed a sitting position with the body relaxed. The coil was placed in the projection of the primary motor cortex on the body surface of the right hemisphere, tangent to the scalp with the handle points facing backward and 45° away from the midline. This orientation induced a posterolateral to anteromedial current in the brain that preferentially activated the cortical-spinal system through horizontal cortical–cortical connections (Premoli et al., 2014). A single TMS pulse was applied to the M1 region, and MEPs from the left first digital interosseous (FDI) muscle were recorded with surface electromyography. The resting motion threshold was determined using the relative frequency method, defined as the minimum intensity that was sufficient for the MEPs to reach an amplitude > 50 μV in at least five out of ten of the subsequent stimuli. AMT was determined during muscle contraction (approximately 20% of maximum muscle strength) and was defined as the minimum intensity able to elicit MEPs (peak amplitude > 200 μV) in 50% of the subsequent stimuli. Furthermore, we used the neuro-navigation system (Visor2, ANT Neuro, Hengelo, Netherlands) to record the FDI hot spots to ensure that the coil would not deviate from the stimulus target throughout the experiment.
To confirm the relationship between spontaneous EEG oscillations and corticospinal excitability, the least absolute shrinkage and selection operator (LASSO) was used to identify significant features and correlation analysis was conducted for these features. The basic idea of LASSO is that it compresses the coefficient of variables by adding penalty functions to the model and eliminates variables with a regression coefficient of 0 to facilitate the selection of variables (Li et al., 2017). The R 4.0.5 software3 and glmnet package (Friedman et al., 2010) were used to perform LASSO. The parameter Lambda (λ) was tuned by 10-fold cross-validation based on the minimum criteria. To obtain the best fitting effect, the model with minimum λ was chosen. Correlations between significant features extracted by LASSO and corticospinal excitability were then evaluated. Statistical analysis was conducted using SPSS 25.0 (SPSS Inc., Chicago, IL, United States). The Shapiro-Wilk normality test was performed on all variables, and Pearson correlation analysis was used for variables with normal distribution; otherwise, Spearman correlation analysis was applied. p < 0.05 was considered statistically significant.
The range of RMT was from 21 to 73% MSO and the mean value was 46.25% MSO [standard deviation (SD) = 13.579]. AMT ranged from 12 to 57% MSO and the mean value was 32.31% MSO (SD = 11.183). The descriptive results for other variables are shown in Table 1.
Least absolute shrinkage and selection operator was used to construct the regression model for EEG parameters to predict RMT, and the regression model with the minimum λ value was selected because it had the best prediction. The minimum λ was 1.548, and eight potential predictors were identified, including alpha2 and beta1 oscillations power in the sensorimotor region, nodal efficiency in the alpha1 and alpha2 bands, global efficiency in the delta, theta, and alpha2 bands, and global clustering coefficient in the theta band. In the model used to predict AMT, the minimum λ was 1.844. Alpha2 oscillations power in the sensorimotor region, nodal efficiency in the theta and alpha1 bands, global efficiency in the theta and alpha2 bands, and global clustering coefficient in the beta2 band were selected as predictors. All significant factors were included in the subsequent correlation analysis. See Figure 1.
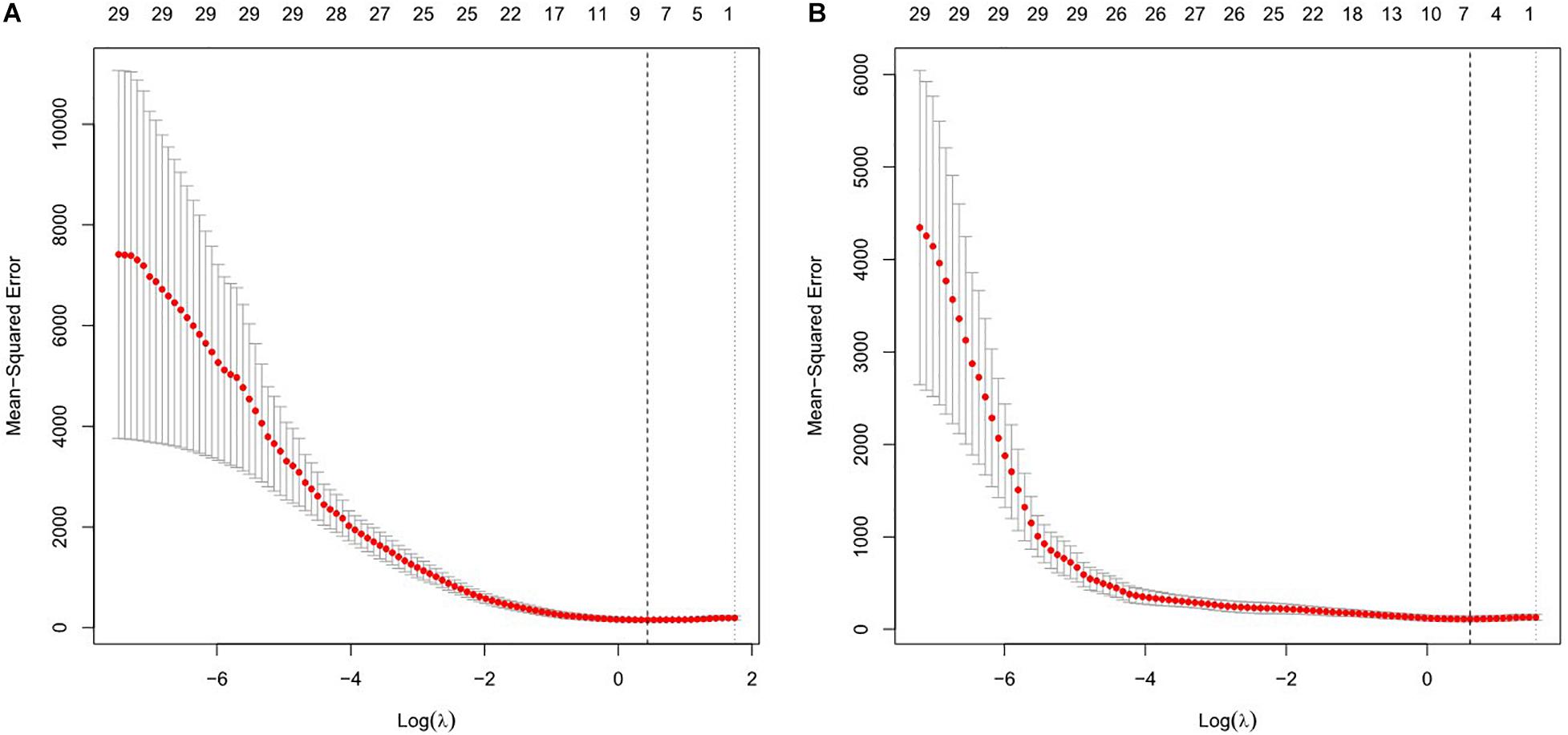
Figure 1. Features selection using the least absolute shrinkage and selection operator (LASSO) regression model for resting motor threshold (RMT) (A) and active motor threshold (AMT) (B). The horizontal axis plotted value of log λ and the vertical axis plotted mean squared error. The dotted vertical line was plotted at the optimal λ values based on minimum criteria.
Correlation analysis showed that the power of alpha2 oscillations in the sensorimotor region was negatively correlated with RMT (ρ = −0.376, p = 0.034). Similarly, alpha2 power in the sensorimotor region showed an inverse correlation with AMT (ρ = −0.432, p = 0.014). The strength of the correlation between beta1 and RMT did not reach a statistically significant level (ρ = −0.328, p = 0.066), similar to that between beta1 and AMT (ρ = −0.314, p = 0.080). See Figure 2.
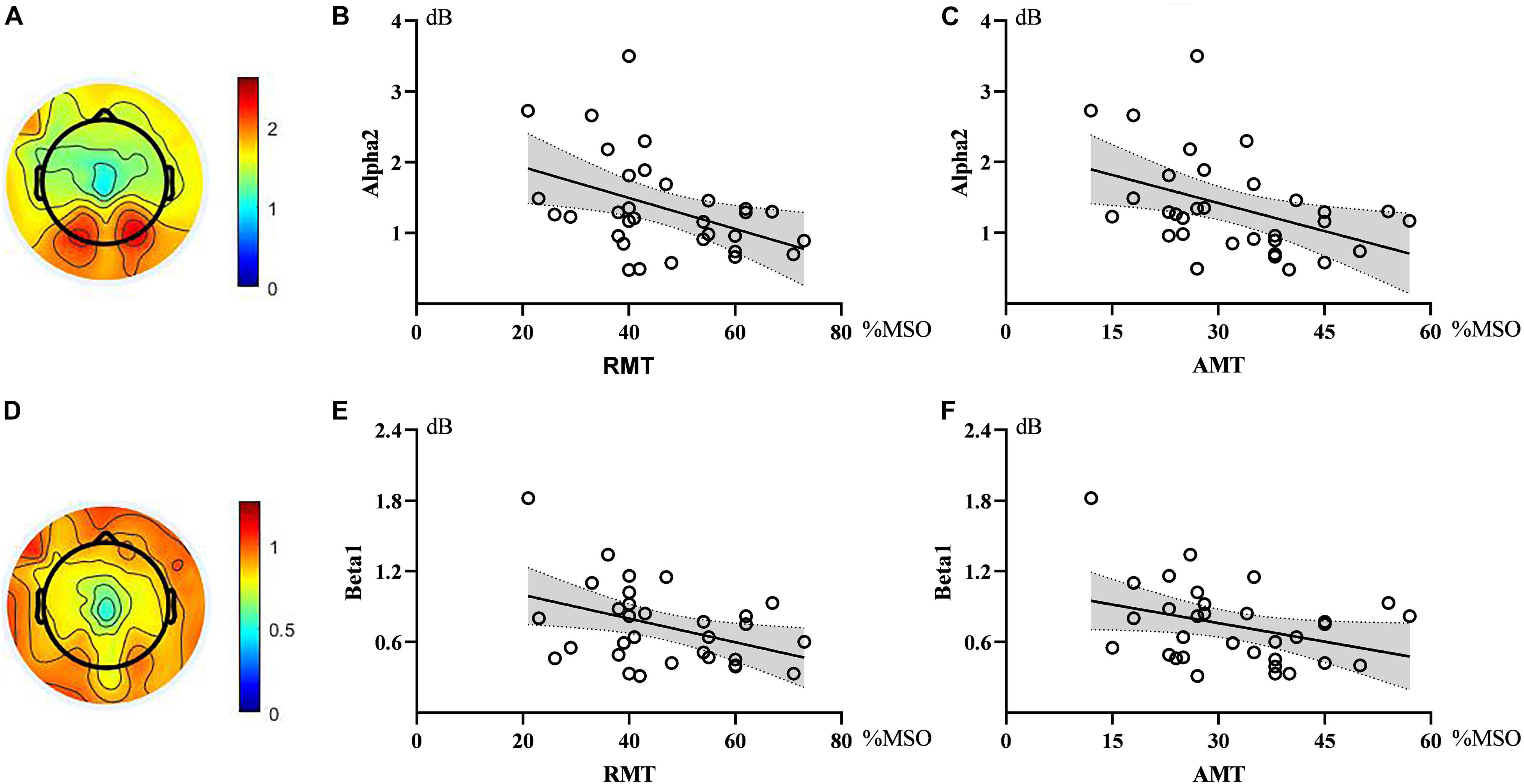
Figure 2. Topographic maps and scatter diagrams. Topographic maps showed the power of alpha2 oscillations and beta1 oscillations. The color bar represents the value of power (A,D). Scatter diagrams showed the correlation between electroencephalography (EEG) oscillations and motor threshold (B,C,E,F). There were significant negative correlations between alpha2 and RMT as well as AMT.
When exploring the relationship between the nodal metrics and RMT, we found that RMT was negatively correlated with nodal efficiency in the alpha2 band, but the correlation did not reach statistical significance (r = −0.347, p = 0.051). Nodal efficiency in the theta band had no correlation with RMT (r = 0.023, p = 0.900) and AMT (r = 0.336, p = 0.060) and there was no correlation between nodal efficiency in the alpha1 band and RMT (r = −0.247, p = 0.173) or AMT (r = −0.210, p = 0.249). See Figure 3.
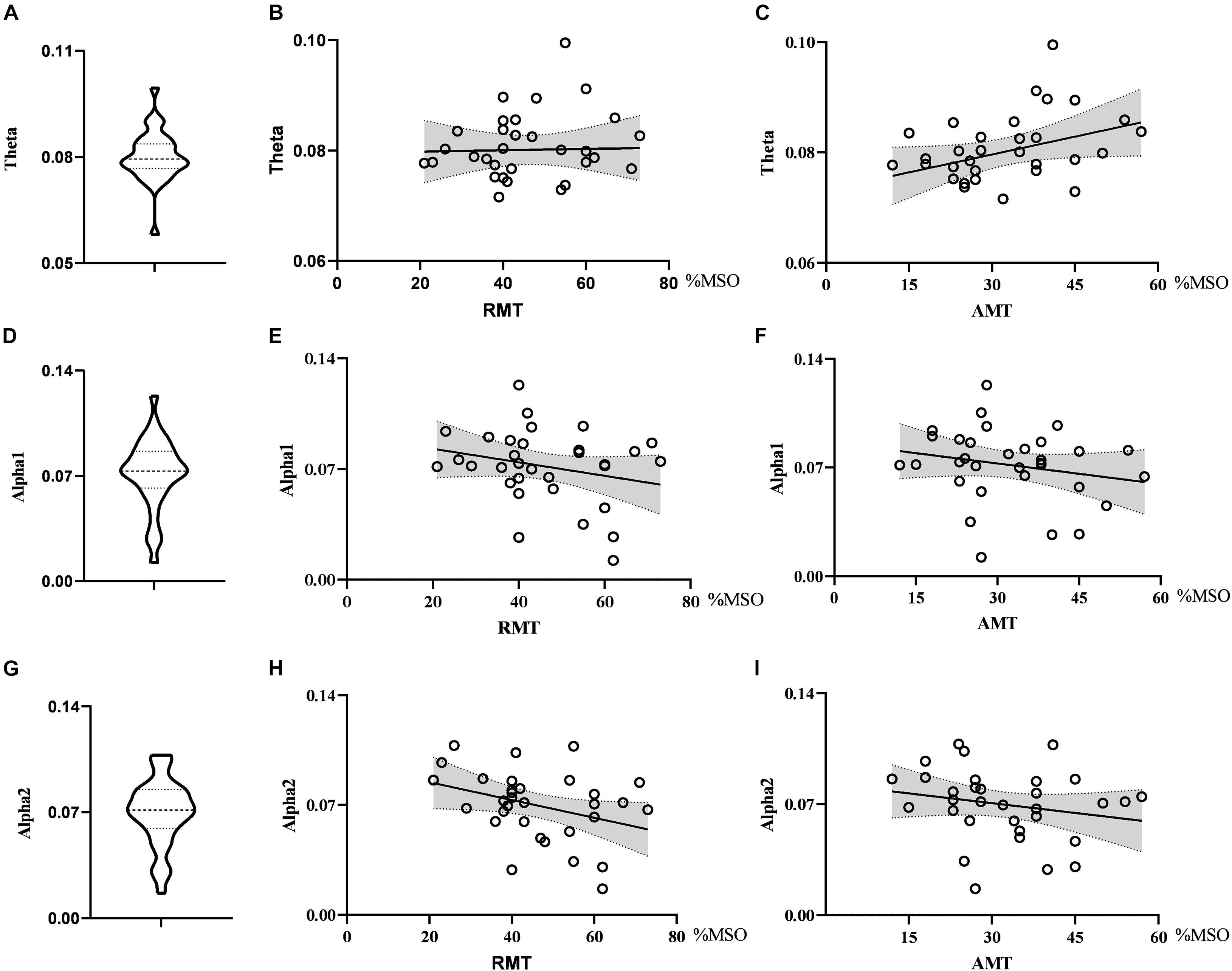
Figure 3. Violin diagrams and scatter diagrams. Violin diagrams showed the distribution of nodal efficiency in theta, alpha1, and alpha2 band (A,D,G). Scatter diagrams showed the correlation between nodal efficiency and motor threshold (B,C,E,F,H,I).
As for global metrics, we found that the global efficiency in the theta band was positively correlated with RMT (r = 0.374, p = 0.035), while the global efficiency in the alpha2 band was negatively correlated with RMT (ρ = −0.363, p = 0.041), showing the opposite trend. Similarly, the global efficiency in the alpha2 band was negatively correlated with the AMT (ρ = −0.427, p = 0.015) and the correlation strength between the global efficiency in the theta band and AMT did not reach the statistically significant level (r = 0.291, p = 0.106). There was no correlation between global efficiency in the delta band and RMT (r = 0.074, p = 0.688) as well as AMT (r = −0.281, p = 0.119). Further, the global clustering coefficient in the theta and beta2 band had no significant correlation with RMT or AMT. See Figures 4, 5.
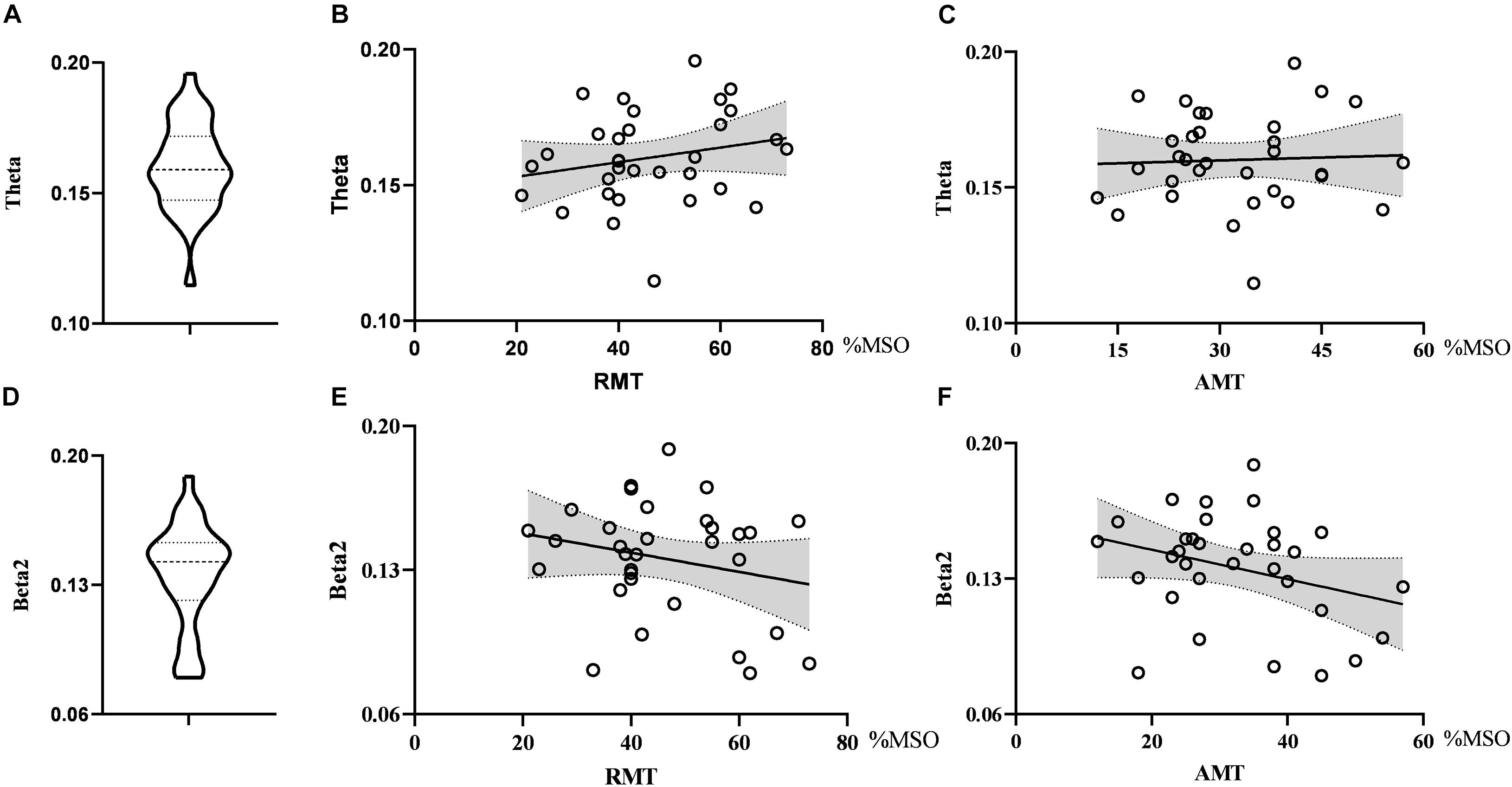
Figure 4. Violin diagrams and scatter diagrams. Violin diagrams showed the distribution of clustering coefficient in theta and beta2 band (A,D). Scatter diagrams showed the correlation between clustering coefficient and motor threshold (B,C,E,F).
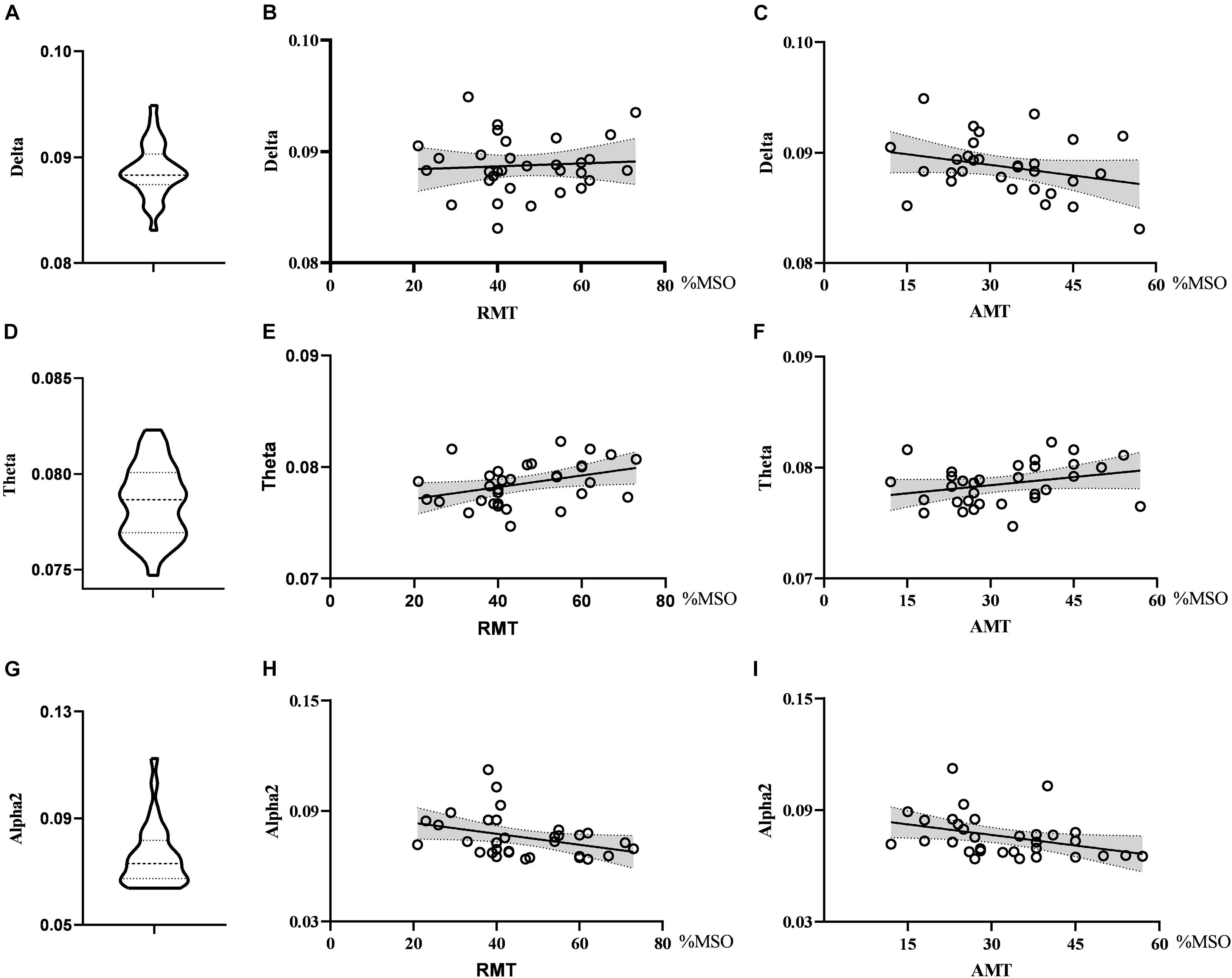
Figure 5. Violin diagrams and scatter diagrams. Violin diagrams showed the distribution of global efficiency in delta, theta, and alpha2 band (A,D,G). Scatter diagrams showed the correlation between global efficiency and motor threshold (B,C,E,F,H,I). There was significant positive correlation between global efficiency in theta and RMT. And there were significant negative correlations between global efficiency in alpha2 and RMT as well as AMT.
In this study, we explored the relationship between EEG oscillations and corticospinal excitability. Analysis revealed that the alpha2 power in the sensorimotor region showed an inverse correlation with RMT and AMT. Innovatively, we explored the relationship between brain activity and corticospinal excitability from the perspective of the functional network and found that the global efficiency in the theta band was positively correlated with RMT. Additionally, the global efficiency in the alpha2 band was negatively correlated with RMT and AMT. These findings indicated that the power spectrum in sensorimotor regions and the global efficiency of functional networks modulate corticospinal excitability, which provides an important basis for understanding the interactions between neural electrical activity and TMS.
The spontaneous oscillation of EEG reflects the rhythmic changes in the membrane potential of neurons and thus reflects the current excitatory–inhibitory balance of underlying neuronal cell assemblies (Klimesch et al., 2007; Jensen and Mazaheri, 2010; Schulz et al., 2014). Each EEG band is associated with different cognitive and behavioral functions. Low-frequency EEG oscillations are associated with responsible motivation, emotion, and reward processing, while high-frequency EEG oscillations may reflect cognitive processes such as attention control, memory encoding, and recognition (MacLean et al., 2012; Sandler et al., 2016; De Pascalis et al., 2020). Alpha oscillations are the most significant phenomenon in human EEG recordings, and their occurrence and function are some of the basic research topics in neuroscience. At first, researchers believed that the alpha activity reflected the idle state of the brain, but the current leading interpretation of alpha oscillations is that alpha oscillations causally determine excitability and modulate signal processing instead of passively respond to stimulus. Converging evidence suggests that alpha oscillations are related to the cyclic regulation of neuronal excitability and can affect the response of neurons to sensory stimuli (Palva and Palva, 2011). Currently, researchers recognize that the alpha oscillations contain at least two sub-components. The first is lower alpha or alpha1, which is endogenous and independent of any internal or external stimuli. The second is upper alpha or alpha2, mainly exists in the sensorimotor cortex, and is related to the function of the sensorimotor system (Vecchio et al., 2018).
In this study, RMT and AMT decreased when the alpha2 oscillation power increased, indicating an inverse correlation between alpha2 oscillations and corticospinal excitability. A number of studies support the view that the alpha rhythm reflects cortical excitability. Previous studies have shown that central alpha oscillations (mu rhythms) are associated with the resting state of the primary sensory and motor cortex by correlating the rhythm strength with fMRI blood signals (Ritter et al., 2009). Alpha oscillations in the central region are suppressed during movement and return to the baseline when the movement ends (Hao et al., 2019). According to the pulsed inhibition hypothesis, alpha oscillations are associated with the underlying localized global suppression of neuronal activity in cortical circuits, with high alpha power representing the suppressed state and low alpha power representing the excited state (Palva and Palva, 2011; Schulz et al., 2014). Since RMT and AMT are inversely associated with cortical excitability, similar to alpha oscillations, there should be a positive correlation between RMT, AMT, and alpha oscillations. However, in this study, alpha2 oscillations were negatively correlated with corticospinal excitability, which seems controversial. A recent study explored the relationship between pre-stimulus EEG oscillations and MEPs, and the results showed that high alpha and low beta power before stimulation could lead to high MEP amplitudes (Ogata et al., 2019). In a real-time TMS-EEG study, researchers explored the relationship between the phase of the alpha oscillations in the sensory motor area and MEPs. They found that the MEP amplitude increased during the high alpha power, indicating that the alpha oscillations could promote corticospinal excitability (Bergmann et al., 2019). These results were consistent with our findings. The discrepancies between studies may be caused by different experimental designs. Previous studies proved the negative correlation between alpha oscillations and cortical excitability mainly by exploring the changes of alpha rhythm during motor tasks, such as voluntary movement and motor imagery, whereas our study recorded EEG and motor threshold at rest and the task state would contribute to the relationship between EEG oscillations and corticospinal excitability. Our results suggested that alpha oscillations can modulate the cortical response to TMS.
The relationship between beta oscillations and cortical excitability has been studied using different modalities. Generally, beta oscillations in the motor cortex decrease in amplitude during movement and increase in amplitude when movement stops (Darch et al., 2020). Hussain et al. (2019a) found that the beta rhythm power before TMS stimulation can be used to predict the amplitude of MEPs. The stronger the beta rhythm power is, the larger the MEP amplitude will be, indicating that the beta rhythm is positively correlated with corticospinal excitability. These findings may be because beta activity reflects the activity of the II/III interlayer neurons, which could regulate the excitability of the spinal cord by activating descending corticospinal neurons (Hussain et al., 2019b). In this study, beta1 oscillation was a significant feature in the regression model, and correlation analysis showed no significant correlation between beta oscillations and corticospinal excitability. This may be due to the small sample size. Nevertheless, the results of this study are valuable, because previous studies focused on how alpha oscillations regulate corticospinal excitability and beta oscillations were used to be ignored. Future studies can further explore the relationship between beta oscillations and corticospinal excitability.
With the advancement of neuroscience, the network connection pattern formed by the cooperation of multiple brain regions has been identified as the physiological foundation for information processing in the brain. The small-world network is the most commonly studied complex network which has both a large clustering coefficient and a small path length (Bassett and Bullmore, 2006). It examines the brain’s functional connectivity architecture, focusing on the brain’s ability to integrate and transmit information between different regions. Efficiency is an important metric to measure the ability of network information exchange, and its contribution to cortical excitability has attracted the attention of researchers. Blain-Moraes et al. (2017) investigated the neural activity of subjects under general anesthesia and found that while the subjects were unconscious, the alpha network efficiency decreased and the alpha network clustering coefficient increased significantly. This showed that the efficiency of the alpha network is positively correlated with cortical excitability. Previous studies have shown that the efficiency of the alpha network increased during exercise, possibly due to increased metabolism and cortical arousal during exercise (Tamburro et al., 2020). High global efficiency corresponds to fast information transmission. Our study showed that RMT decreased with the elevation of alpha network efficiency, indicating that brain activity increased with the enhancement of the alpha network information processing capacity, making it easier to respond to TMS.
Theta oscillations are often considered to be related to cognitive functions such as memory and emotion (Wang et al., 2018), and a few studies show that the theta rhythm contributes to the movement process. Popovych et al. (2016) reported that a significant phase-locking effect in the delta-theta frequency band could be observed in the M1 area when individuals completed an exercise task, suggesting that the theta oscillations were related to movement and indirectly showing that the theta rhythm contributes to the excitability of the motor cortex. Storti et al. (2016) used graph theory to analyze the brain network characteristics of individuals during movement and found that the betweenness in the theta band of the motor cortex increased significantly, providing a new perspective for exploring the theta network and the excitability of the motor cortex. Interestingly, in this study, the modulating effects of the global efficiency of the theta band and alpha band on RMT were in the opposite direction, which may indicate that different rhythm networks regulate cortical excitability in different directions and coordinate with each other to regulate brain activities.
In this study, the LASSO regression showed that the clustering coefficient in the theta and beta2 bands was the predictor of the motion threshold, but the correlation analysis results indicated that there was no correlation. This may result from the differences of statistical methods. Correlation analysis is univariate analysis, while LASSO is a multivariate analysis method and the results would be affected by the interaction between variables. The combination of clustering coefficients and other parameters can provide more comprehensive information about corticospinal excitability.
The strength of this study is that it explored the relationship between TMS and neural oscillation from a network perspective using graph theory analysis. In the application of graph theory, a network is constructed by defining a series of nodes and connecting edges and the model can fit the pattern of brain activity, which makes it a great tool for brain functional segmentation and integration. In this study, graph theory was used to construct a brain network, and the relationship between neural oscillatory activities and TMS was explained from the perspective of the global network, which can help to explore the electrophysiological mechanism of TMS.
There are some limitations to this study. Firstly, we studied the association between EEG oscillation and TMS in non-dominant hemisphere, and the generalizability of the results to dominant hemisphere can be explored in future study. Besides, EEG were recorded before application of TMS in this study. Future study can record them simultaneously to provide stronger evidences. We chose the EEG spectrum and graph theory to analyze the relationship between EEG oscillation and cortical excitability because these are the classic metrics used to describe the characteristics of EEG. In future studies, other metrics such as entropy and complexity which quantifies the non-linear dynamic characteristics of EEG and reflect cortical functional state can be selected to explore the relationship between EEG oscillation and corticospinal excitability comprehensively.
In conclusion, we found that the alpha2 power in the sensorimotor region showed an inverse correlation with RMT and AMT. And the global efficiency in the theta band was positively correlated with RMT. Additionally, the global efficiency in the alpha2 band was negatively correlated with RMT and AMT. It is crucial to understand the mechanisms of TMS from the perspective of neurophysiology. The findings of this study indicate that the effect of TMS on the cortex is dependent on the activity of local neurons and global network activity, which provides an important basis for uncovering the regulatory effect of TMS on neural electrical activity. The network analysis of EEG can provide a useful supplement for studying the brain response to TMS.
The raw data supporting the conclusion of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by the Ethics Committee of Guangzhou First People’s Hospital. The patients/participants provided their written informed consent to participate in this study.
YL, GX, and TY: study design. QD, GC, MW, HCh, and HCa: data acquisition and processing. TL: statistical analysis. GC and MW: drafting of the manuscript. QD: critical revision of the manuscript. YJ and WL: technical assistance. All authors contributed to the article and approved the submitted version.
This work was supported by the National Science Foundation of China [Grant Numbers 81772438 (YL), 81974357 (YL), 82072548 (GX), and 81802227 (TL)]; the Guangzhou Municipal Science and Technology Program [Grant Number 201803010083 (YL)]; the Fundamental Research Funds for the Central University [Grant Number 2018PY03 (YL)]; National Key R&D Program of China [Grant Number 2017YFB1303200 (YL)]; Guangdong Basic and Applied Basic Research Foundation [Grant Number 2020A1515110761 (QD)]; and Guangzhou Postdoctoral Science Foundation (QD).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
We sincerely thank all the subjects for their support and cooperation in our experiment.
Avenanti, A., Wright, D. J., Wood, G., Franklin, Z. C., Marshall, B., Riach, M., et al. (2018). Directing visual attention during action observation modulates corticospinal excitability. PLoS One 13:e0190165. doi: 10.1371/journal.pone.0190165
Bassett, D. S., and Bullmore, E. (2006). Small-world brain networks. Neuroscientist 12, 512–523. doi: 10.1177/1073858406293182
Bayram, M. B., Siemionow, V., and Yue, G. H. (2015). Weakening of corticomuscular signal coupling during voluntary motor action in aging. J. Gerontol. 70, 1037–1043. doi: 10.1093/gerona/glv014
Berger, B., Minarik, T., Liuzzi, G., Hummel, F. C., and Sauseng, P. (2014). EEG oscillatory phase-dependent markers of corticospinal excitability in the resting brain. Biomed. Res. Int. 2014:936096.
Bergmann, T. O., Lieb, A., Zrenner, C., and Ziemann, U. (2019). Pulsed facilitation of corticospinal excitability by the sensorimotor mu-alpha rhythm. J. Neurosci. 39, 10034–10043. doi: 10.1523/jneurosci.1730-19.2019
Blain-Moraes, S., Tarnal, V., Vanini, G., Bel-Behar, T., Janke, E., Picton, P., et al. (2017). Network efficiency and posterior alpha patterns are markers of recovery from general anesthesia: a high-density electroencephalography study in healthy volunteers. Front. Hum. Neurosci. 11:328. doi: 10.3389/fnhum.2017.00328
Cattaneo, L., and Barchiesi, G. (2011). Transcranial magnetic mapping of the short-latency modulations of corticospinal activity from the ipsilateral hemisphere during rest. Front. Neural Circuits 5:14. doi: 10.3389/fncir.2011.00014
Chen, Y.-J., Huang, Y.-Z., Chen, C.-Y., Chen, C.-L., Chen, H.-C., Wu, C.-Y., et al. (2019). Intermittent theta burst stimulation enhances upper limb motor function in patients with chronic stroke: a pilot randomized controlled trial. BMC Neurol. 19:69. doi: 10.1186/s12883-019-1302-x
Darch, H. T., Cerminara, N. L., Gilchrist, I. D., and Apps, R. (2020). Pre-movement changes in sensorimotor beta oscillations predict motor adaptation drive. Sci. Rep. 10:17946.
De Pascalis, V., Vecchio, A., and Cirillo, G. (2020). Resting anxiety increases EEG delta–beta correlation: relationships with the reinforcement sensitivity theory personality traits. Pers. Indiv. Differ. 156:109796. doi: 10.1016/j.paid.2019.109796
de Waal, H., Stam, C. J., Lansbergen, M. M., Wieggers, R. L., Kamphuis, P. J., Scheltens, P., et al. (2014). The effect of souvenaid on functional brain network organisation in patients with mild Alzheimer’s disease: a randomised controlled study. PLoS One 9:e86558. doi: 10.1371/journal.pone.0086558
Friedman, J., Hastie, T., and Tibshirani, R. (2010). Regularization paths for generalized linear models via coordinate descent. J. Stat. Softw. 33, 1–22.
Hao, J., Feng, W., Zhang, L., and Liao, Y. (2019). The post-movement beta rebound and motor-related Mu suppression in children. J. Motor Behav. 52, 590–600. doi: 10.1080/00222895.2019.1662762
Hussain, S. J., Claudino, L., Bonstrup, M., Norato, G., Cruciani, G., Thompson, R., et al. (2019a). Sensorimotor oscillatory phase-power interaction gates resting human corticospinal output. Cereb. Cortex 29, 3766–3777. doi: 10.1093/cercor/bhy255
Hussain, S. J., Cohen, L. G., and Bonstrup, M. (2019b). Beta rhythm events predict corticospinal motor output. Sci. Rep. 9:18305.
Iscan, Z., Nazarova, M., Fedele, T., Blagovechtchenski, E., and Nikulin, V. V. (2016). Pre-stimulus alpha oscillations and inter-subject variability of motor evoked potentials in single- and paired-pulse TMS paradigms. Front. Hum. Neurosci. 10:504. doi: 10.3389/fnhum.2016.00504
Jensen, O., and Mazaheri, A. (2010). Shaping functional architecture by oscillatory alpha activity: gating by inhibition. Front. Hum. Neurosci. 4:186. doi: 10.3389/fnhum.2010.00186
Klimesch, W., Sauseng, P., and Hanslmayr, S. (2007). EEG alpha oscillations: the inhibition-timing hypothesis. Brain Res. Rev. 53, 63–88. doi: 10.1016/j.brainresrev.2006.06.003
Li, J., Wang, J., Chen, Y., Yang, L., and Chen, S. (2017). A prognostic 4-gene expression signature for squamous cell lung carcinoma. J. Cell Physiol. 232, 3702–3713. doi: 10.1002/jcp.25846
MacLean, M. H., Arnell, K. M., and Cote, K. A. (2012). Resting EEG in alpha and beta bands predicts individual differences in attentional blink magnitude. Brain Cogn. 78, 218–229.
Maki, H., and Ilmoniemi, R. J. (2010). EEG oscillations and magnetically evoked motor potentials reflect motor system excitability in overlapping neuronal populations. Clin. Neurophysiol. 121, 492–501. doi: 10.1016/j.clinph.2009.11.078
Myczkowski, M. L., Fernandes, A., Moreno, M., Valiengo, L., Lafer, B., Moreno, R. A., et al. (2018). Cognitive outcomes of TMS treatment in bipolar depression: Safety data from a randomized controlled trial. J. Affect. Disord. 235, 20–26.
Ogata, K., Nakazono, H., Uehara, T., and Tobimatsu, S. (2019). Prestimulus cortical EEG oscillations can predict the excitability of the primary motor cortex. Brain Stimul. 12, 1508–1516. doi: 10.1016/j.brs.2019.06.013
Palva, S., and Palva, J. M. (2011). Functional roles of alpha-band phase synchronization in local and large-scale cortical networks. Front. Psychol. 2:204. doi: 10.3389/fpsyg.2011.00204
Popovych, S., Rosjat, N., Toth, T. I., Wang, B. A., Liu, L., Abdollahi, R. O., et al. (2016). Movement-related phase locking in the delta-theta frequency band. Neuroimage 139, 439–449. doi: 10.1016/j.neuroimage.2016.06.052
Premoli, I., Castellanos, N., Rivolta, D., Belardinelli, P., Bajo, R., Zipser, C., et al. (2014). TMS-EEG signatures of GABAergic neurotransmission in the human cortex. J. Neurosci. 34, 5603–5612. doi: 10.1523/jneurosci.5089-13.2014
Ritter, P., Moosmann, M., and Villringer, A. (2009). Rolandic alpha and beta EEG rhythms’ strengths are inversely related to fMRI-BOLD signal in primary somatosensory and motor cortex. Hum. Brain Mapp. 30, 1168–1187. doi: 10.1002/hbm.20585
Rossi, S., Antal, A., Bestmann, S., Bikson, M., Brewer, C., Brockmoller, J., et al. (2021). Safety and recommendations for TMS use in healthy subjects and patient populations, with updates on training, ethical and regulatory issues: expert guidelines. Clin. Neurophysiol. 132, 269–306. doi: 10.1016/j.clinph.2020.10.003
Rubinov, M., and Sporns, O. (2010). Complex network measures of brain connectivity: uses and interpretations. Neuroimage 52, 1059–1069. doi: 10.1016/j.neuroimage.2009.10.003
Sandler, H., Tamm, S., Fendel, U., Rose, M., Klapp, B. F., and Bosel, R. (2016). Positive emotional experience: induced by vibroacoustic stimulation using a body monochord in patients with psychosomatic disorders: is associated with an increase in EEG-theta and a decrease in EEG-alpha power. Brain Topogr. 29, 524–538. doi: 10.1007/s10548-016-0480-8
Sauseng, P., Klimesch, W., Gerloff, C., and Hummel, F. C. (2009). Spontaneous locally restricted EEG alpha activity determines cortical excitability in the motor cortex. Neuropsychologia 47, 284–288. doi: 10.1016/j.neuropsychologia.2008.07.021
Schiena, G., Maggioni, E., Pozzoli, S., and Brambilla, P. (2020). Transcranial magnetic stimulation in major depressive disorder: response modulation and state dependency. J. Affect. Disord. 266, 793–801. doi: 10.1016/j.jad.2020.02.006
Schulz, H., Ubelacker, T., Keil, J., Muller, N., and Weisz, N. (2014). Now I am ready-now i am not: the influence of pre-TMS oscillations and corticomuscular coherence on motor-evoked potentials. Cereb. Cortex 24, 1708–1719. doi: 10.1093/cercor/bht024
Sporns, O. (2018). Graph theory methods: applications in brain networks. Dial. Clin. Neurosci. 20, 111–121. doi: 10.31887/dcns.2018.20.2/osporns
Stefanou, M. I., Galevska, D., Zrenner, C., Ziemann, U., and Nieminen, J. O. (2020). Interhemispheric symmetry of micro-rhythm phase-dependency of corticospinal excitability. Sci. Rep. 10:7853.
Storti, S. F., Formaggio, E., Manganotti, P., and Menegaz, G. (2016). Brain network connectivity and topological analysis during voluntary arm movements. Clin. EEG Neurosci. 47, 276–290. doi: 10.1177/1550059415598905
Su, S., Yu, D., Cheng, J., Chen, Y., Zhang, X., Guan, Y., et al. (2017). Decreased global network efficiency in young male smoker: an EEG study during the resting state. Front. Psychol. 8:1605. doi: 10.3389/fpsyg.2017.01605
Tamburro, G., Di Fronso, S., Robazza, C., Bertollo, M., and Comani, S. (2020). Modulation of brain functional connectivity and efficiency during an endurance cycling task: a source-level EEG and graph theory approach. Front. Hum. Neurosci. 14:243. doi: 10.3389/fnhum.2020.00243
Vecchio, F., Di Iorio, R., Miraglia, F., Granata, G., Romanello, R., Bramanti, P., et al. (2018). Transcranial direct current stimulation generates a transient increase of small-world in brain connectivity: an EEG graph theoretical analysis. Exp. Brain Res. 236, 1117–1127. doi: 10.1007/s00221-018-5200-z
Wanalee, K., Rose, K., and Alexandra, L. V. (2015). Basic principles of transcranial magnetic stimulation (TMS) and repetitive TMS (rTMS). Ann. Phys. Rehabil. Med. 58, 208–213. doi: 10.1016/j.rehab.2015.05.005
Wang, B., Li, P., Li, D., Niu, Y., Yan, T., Li, T., et al. (2018). Increased functional brain network efficiency during audiovisual temporal asynchrony integration task in aging. Front. Aging Neurosci. 10:316. doi: 10.3389/fnagi.2018.00316
Wang, J., Wang, X., Xia, M., Liao, X., Evans, A., and He, Y. (2015). GRETNA: a graph theoretical network analysis toolbox for imaging connectomics. Front. Hum. Neurosci. 9:386. doi: 10.3389/fnhum.2015.00386
Yan, T., Wang, W., Liu, T., Chen, D., Wang, C., Li, Y., et al. (2017). Increased local connectivity of brain functional networks during facial processing in schizophrenia: evidence from EEG data. Oncotarget 8, 107312–107322. doi: 10.18632/oncotarget.20598
Keywords: electroencephalography, transcranial magnetic stimulation, corticospinal excitability, network, power spectrum
Citation: Cai G, Wu M, Ding Q, Lin T, Li W, Jing Y, Chen H, Cai H, Yuan T, Xu G and Lan Y (2021) The Corticospinal Excitability Can Be Predicted by Spontaneous Electroencephalography Oscillations. Front. Neurosci. 15:722231. doi: 10.3389/fnins.2021.722231
Received: 08 June 2021; Accepted: 31 July 2021;
Published: 23 August 2021.
Edited by:
Jing Wang, Xi’an Jiaotong University, ChinaReviewed by:
Bin Hu, University of Calgary, CanadaCopyright © 2021 Cai, Wu, Ding, Lin, Li, Jing, Chen, Cai, Yuan, Xu and Lan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Tifei Yuan, eXRmMDcwN0AxMjYuY29t; Guangqing Xu, Z3VhbmdjaGluZ3hAMTYzLmNvbQ==; Yue Lan, Ymx1ZW1vb25pbmdAMTYzLmNvbQ==
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.