
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
METHODS article
Front. Neurosci., 15 July 2021
Sec. Brain Imaging Methods
Volume 15 - 2021 | https://doi.org/10.3389/fnins.2021.682812
Background: Longitudinal brain MRI monitoring in neurodegeneration potentially provides substantial insights into the temporal dynamics of the underlying biological process, but is time- and cost-intensive and may be a burden to patients with disabling neurological diseases. Thus, the conceptualization of follow-up time-intervals in longitudinal MRI studies is an essential challenge and substantial for the results. The objective of this work is to discuss the association of time-intervals and the results of longitudinal trends in the frequently used design of one baseline and two follow-up scans.
Methods: Different analytical approaches for calculating the linear trend of longitudinal parameters were studied in simulations including their performance of dealing with outliers; these simulations were based on the longitudinal striatum atrophy in MRI data of Huntington’s disease patients, detected by atlas-based volumetry (ABV).
Results: For the design of one baseline and two follow-up visits, the simulations with outliers revealed optimum results for identical time-intervals between baseline and follow-up scans. However, identical time-intervals between the three acquisitions lead to the paradox that, depending on the fit method, the first follow-up scan results do not influence the final results of a linear trend analysis.
Conclusions: This theoretical study analyses how the design of longitudinal imaging studies with one baseline and two follow-up visits influences the results. Suggestions for the analysis of longitudinal trends are provided.
There is a constantly growing number of longitudinal neuroimaging studies in neurological and particularly neurodegenerative diseases over the last 15 years. Longitudinal brain MRI studies in neurology are time- and cost-intensive and may be a burden to patients with disabling diseases. Thus, the schedule of longitudinal time-intervals is an essential aspect in the conceptualization of longitudinal MRI studies and may be substantial for the validity of the results. Compared to cross-sectional data, the longitudinal design can provide increased statistical power by reducing the confounding effect of between-subject variability (Thompson et al., 2011). Furthermore, longitudinal studies provide unique insights into the temporal dynamics of the underlying biological process (Sabuncu et al., 2011; Jack et al., 2012); a serial assessment can be the only way to unambiguously characterize the effect of interest in a randomized experiment such as a pharmacological clinical trial (Davis et al., 2005; Dickerson and Sperling, 2005).
In the domain of clinical neuroimaging, the design of longitudinal studies with respect to the schedule of the follow-ups is not widely discussed and in practice is subject to the limitations imposed by participants as well as financial considerations of the study. However, there are approaches to longitudinal study design from study targets such as aging processes (Newman, 2010). As the strategic planning of a longitudinal study includes dimensions such as the hypothesized true variance in change, indicator reliability, the number and spacing of measurement occasions, total study time, and sample size, the main search goal is to select a research design that best addresses the guiding questions and hypotheses of the planned study while heeding applicable external conditions and constraints, including time, money, feasibility, and ethical considerations. Brandmaier et al. (2015) proposed the Longitudinal Interactive Front End Study Planner (LIFESPAN) framework to generate a set of alternative models with equal statistical power to detect hypothesized effects and delineates trade-off relations among relevant parameters.
In longitudinal MRI studies of neurodegenerative diseases, due to a balance between costs and timeline and the validity of the results, a common concept is to plan with one baseline and two follow-up studies with (almost) identical time intervals, i.e., bisectioning the observation time period. Examples for such a study design can be found in amyotrophic lateral sclerosis (ALS) (Cardenas-Blanco et al., 2016; Kassubek et al., 2018; Kalra et al., 2020; Steinbach et al., 2021; Tahedl et al., 2021), in Huntington’s disease (Tabrizi et al., 2009; Hobbs et al., 2015; Müller et al., 2019). This study design usually allows for a linear fit to obtain longitudinal trends of resulting parameters in the respective imaging domain. Here, we theoretically investigated how the fit methods of linear regression analysis and the choice of time intervals influence the final results for longitudinal trends of a given (arbitrary) parameter of interest. We expected the results of this investigation to facilitate the scheduling of acquisition time points for upcoming studies and to guide in calculating linear trends for already ongoing studies with three measurement time points and identical intervals.
Several fit methods for calculating the linear trend of longitudinal parameters were tested analytically and in simulations, with special respect on their performance to deal with the effect of outliers.
A linear trend analysis of N data points (ti; yi) follows:
with inclination I and intercept b.
We specifically investigated the frequently used design of three acquisition time points (see e.g., Cardenas-Blanco et al., 2016; Tahedl et al., 2021), i.e., time point 1 at baseline and time points 2 and 3 at follow-up 1 and 2, respectively.
Figure 1 schematically illustrates different trend analysis approaches for the fit of three data points for identical time intervals, i.e., bisectioning the observation time period. The data point at t2 was offset by an arbitrary amount from the modeled linear trend to simulate a disturbance (e.g., a measurement affected by motion artifacts). Possible trend analysis approaches are listed below, following (Rencher and Schaalje, 2007):
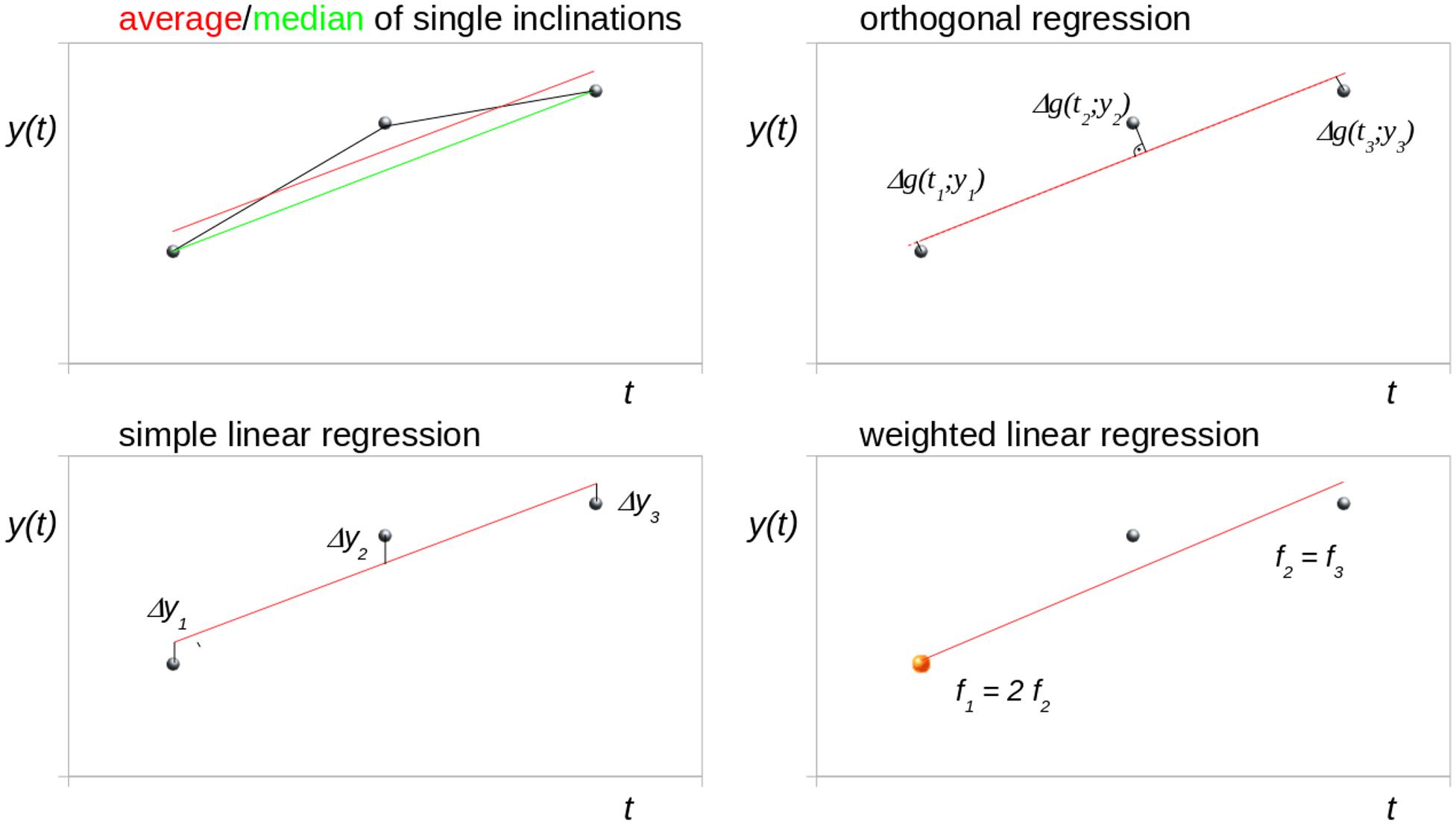
Figure 1. Schematic illustration of different trend analysis approaches for the fit of three data points for identical time intervals (bisectioning the observation time). The central data point was shifted to simulate a disturbance. Δg denotes the orthogonal distance to the regression line.
The linear longitudinal trend of an arbitrary parameter can be calculated from the arithmetic average of the single inclinations between two time points (I12, I23, I13). For three time points (t1, t2, t3), the mean inclination Tavg is calculated as
As for any arithmetic average, the values to be averaged should be normally distributed.
In the case of non-normal distribution, however, the median value can be used instead of the arithmetic average to estimate the overall linear trend:
An advanced approach to estimate the overall trend by fitting a straight line using a least-squares approach is presented in Press et al. (1992a). In this approach, the sum of squared differences di2 of y-values to the regression line is minimized:
The general formula for calculating the overall trend then reads as follows:
where ui and vi denote the differences to the mean in time and the mean in y-values, respectively:
The terms composed of ui and vi can be abbreviated to:
This prepares subsequent Eq. 8 in the orthogonal regression approach.
For three time points, the trend TlinReg is calculated as:
If the measurement errors are normally distributed, then this fitting function will give maximum likelihood parameter estimations of the overall linear trend; if the errors are not normally distributed, then the estimations are not maximum likelihood, but may still be useful in a practical sense (Press et al., 1992a).
In the orthogonal regression, the squared orthogonal differences of data points to the fitted line are minimized; Eq. 4 changes to
and Eq. 5, thus, changes to
The difference to TlinReg is that this approach minimizes not only the distances of yi but also the distances of ti to the regression line.
If the variance and/or the quality of the measurements is not equal, this knowledge can be incorporated into the regression via least squares weighting. For each data point, the squared difference (Eq. 4) is multiplied by a weighting factor fi prior to minimization:
In general, the respective weights are the reciprocal variance of the measurements so that values with low variance are given higher weights and values with higher variance are given lower weights.
There are generally three potential sources of variability which influence the correlation structure in longitudinal data, i.e., (1) between-subject variation, (2) inherent within-subject biological change, and (3) measurement error (Fitzmaurice et al., 2011). Complex fit models for harmonizing longitudinal imaging data based on empirical Bayesian methods (combining batches – ComBat) (Beer et al., 2020), conditional growth models (Tahedl et al., 2021) or mixed effect models (Cardenas-Blanco et al., 2016) are used for analysis to take these influences into account. The linear mixed effects model for longitudinal data (Bernal-Rusiel et al., 2013) follows:
where yi is the vector of longitudinal measurements for subject i, xi is the subject design matrix for the fixed effects (including clinical group, etc.), β is a vector of unknown fixed effects regression coefficients, ti is the design matrix for the random effects (e.g., scan time), ui is a vector of random effects, and ei is a vector of measurement errors, with constraints for the expectations
The linear mixed effects model has a randomly varying intercept (β1 + u11) and slope (β2 + u21):
where yij is the j-th measurement from subject i. The model of Eq. (13) allows each individual’s measurements to have a unique linear mean trend. A major advantage of this approach in detecting longitudinal group differences is that the subjects in the study are not required to have a common set of measurement time points (Bernal-Rusiel et al., 2013), i.e., time points could differ or even be missing. As a source of variation possibly influencing the longitudinal linear trend, between-subject variation does not play a role in the approach of the current study with three measurement time points from only one subject. Assuming that aberrations because of, for example, noisy measurements due to patient movements, have a much larger share in the change of the linear trend than inherent within-subject biological change, linear mixed effect models would not develop their full power in answering the question about the timing of only three measurements. In the simplified approach of the current study where only one data set (for one subject) with three timepoints was discussed, Eq. 13 reduces to
i.e., from three equations only β1 and β2 can be determined. Simplified linear mixed effects modeling was then realized by the application of a downhill simplex fitting routine (Press et al., 1992b).
For the concept of three measurement time points (one baseline and two follow-up scans) which is frequently used for longitudinal MRI studies in neurodegenerative diseases, a simulation with outliers/aberrations was performed, i.e., the effect of an artificial additive offset value at time points t2 and t3 was analyzed. The ensuing deviation of trend results compared to the original value of the overall trend (without any additive offset) was calculated.
In neurodegenerative diseases, at baseline (inclusion time) most participants are motivated and in a comparatively good physical condition. Thus, the time interval to follow-up is usually planned depending on the clinical evaluation of the disease progression, the estimation of the trend of a given parameter under observation, and the corresponding power calculations for the study. The reason for a second follow-up is often the interest in long-term observations and/or the intention to stabilize the trend results by another measurement; the time limitation here is the total duration of the planned study. However, during the course of the study, often the physical condition and also the motivation of the patients are worsening, leading to potentially reduced quality of scans at second follow-up.
In order to use concrete realistic numeric values we performed the data simulation based on the longitudinal striatum atrophy in MRI data of patients with Huntington’s disease; the underlying volumetric results were determined by atlas-based volumetry (ABV) of structural MRI data (Müller et al., 2019). ABV is an objective, investigator- independent technique with low intra-scanner variability to determine the volumes of intracranial compartments and cerebral substructures from the MRI data of individual subjects (Huppertz et al., 2010). Nevertheless, the results concerning time-interval planning and fit approach can be directly transferred to any MRI-based parameter in neurodegenerative diseases.
According to the results of the above-mentioned study in HD patients (Müller et al., 2019), a study period of 15 months and a monthly volume decrease of the striatum of 0.2% (in relation to its size at baseline) were assumed for our simulation. Numerical aberrations at time points t2 and t3 were simulated by a constant offset value of +0.3% (in relation to the striatum size at baseline, i.e., 8.3 cm3). The total study time (baseline to follow-up 2) was kept constant at 15 months in our simulation, but the follow-up 1 time was varied from 0 to 15 months and the aforementioned offset was applied.
(i) at t2 (follow-up 1) – simulating the situation of a high quality measurement at t3 and a measurement at t2 of limited quality.
(ii) at t3 (follow-up 2) – simulating the situation of a high quality measurement at t2 and a measurement at t3 of limited quality (corresponding to less patient motivation or worse patient condition).
For identical time intervals, i.e., bisectioning the observation time period yields
For identical time intervals, Eq. 2 simplifies to
That means that identical time-intervals have the effect that data points at t2 (follow-up 1) do not influence the overall trend.
It is an intrinsic property for the median calculation Eq. 3 that the median is also independent of (t2,y2).
For identical time intervals , Eq. 7 simplifies to
Thus, in all three cases (average or median of inclinations, simple regression), identical time-intervals have the effect that data points at t2 (follow-up 1) do not influence the overall trend for either calculation method based on a linear model.
In general, the calculation of the slope for a linear regression simplifies for identical time intervals to:
The other two approaches, i.e., orthogonal regression and weighted linear regression, theoretically take time point 2 (i.e., follow-up scan 1) into account when calculating the trend, but each have their own limitations.
For the case of three acquisitions with two identical time intervals, i.e., bisectioning the observation time period, the orthogonal regression theoretically incorporates the data point at t2 (follow-up scan 1). However, the variety between orthogonal regression and simple linear regression depends on the magnitude of the trend, i.e., the smaller the trend, the smaller is the variety between orthogonal regression and simple linear regression (both are equal for an inclination of 0). Given that the orthogonal regression is based on a minimization of t- and y-distances, however, a minimization of distances of y-values is usually preferred for a scenario of defined t- and measured y-values rather than a minimization of t-values (that way supporting the conditions of longitudinal MRI studies where the time points are exactly defined).
The weighting of the different measurement points would, depending on the specific weighting factors, lead to consideration of the second time point, but would be a challenge, as the weighting has to be based on an independent parameter, e.g., a measurement quality control prior to the regression calculation. However, the weighted linear regression approach could be an option for specific conditions, e.g., when it is expected that the first measurement (when the patients are usually in a better condition compared to measurements at a later disease stage) will be of better quality than subsequent measurements. In this case, the weighting could be for example f1 = 1.5, and f2 = f3 = 0.75 for the three visits, i.e., data points. However, the weighting is depending on the experimental design and needs a justification by the rater (based on data quality, patient condition, etc.). The justification of the weighting factors is a critical item. In order to use weighted linear regression, longitudinal studies could be planned in a way that the baseline results are stabilized by e.g., repeated scans at the baseline visit [in general, averaging within-session scans will improve between-session reliability (Lau and Goodyear, 2007)].
In the following, the scenario of section “Simulations With Outliers and Time-Interval Dependency” was simulated, i.e., a striatal atrophy in Huntington’s disease with a linear volume loss of −0.2%/month:
(i) For the situation of a high quality measurement at t3 and a measurement at t2 of reduced quality, a follow-up 1 interval of 7.5 months (bisectioned observation period [t1; t3]) leads to a minimum of deviation of trend results from original values for Tavg as well as for TlinReg (as the artificial offset due to a low quality measurement at t2 is less or not considered); Tmedian provides no deviation at all for any time-position of t2 (Figure 2A). TorthReg, TlinReg, and TfitApproach reveal an almost equal course; TorthReg and TlinReg are almost equal due to a comparatively low trend value (in the limit of trend = 0, TorthReg and TlinReg would be equal). It has to be noted that especially the results for orthogonal regression TorthReg highly depend on the amplitude of the trend. The position of the minimum of TweightReg is depending on the weighting factors.
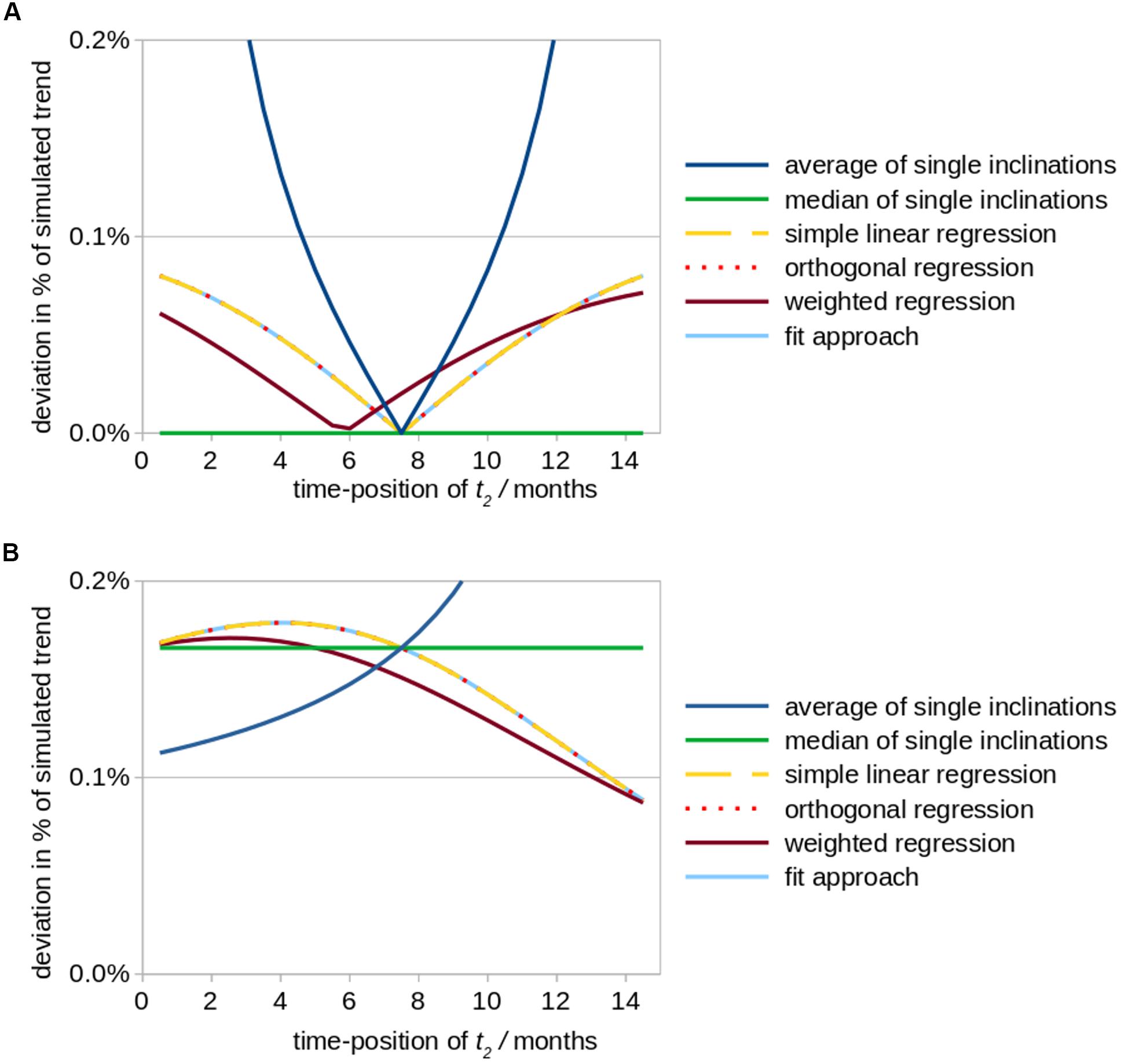
Figure 2. Simulated effects of a modified positioning of time point t2 (follow up 1) on calculations of striatal atrophy in Huntington patients. According to the underlying real world study (Müller et al., 2019), the total observation period amounted to 15 months and a monthly volume decrease of the striatum of 0.2% (in relation to its size at baseline) was assumed as original value of the overall linear trend. The position of time point t2 was varied between a position just behind baseline up to almost 15 months, while the total study duration of 15 months was kept constant. Aberrational data values at time point t2 (results shown in subfigure (A) and time point t3 (B) were simulated by an arbitrary artificial offset value of +0.3% (in relation to the striatum size at baseline, i.e., 8.3 cm3). The subfigures demonstrate the ensuing deviations (in percent of the original linear trend results) for different fit approaches. (A) Simulated aberration at t2 (follow-up 1). (B) Simulated aberration at t3 (follow-up 2).
The results described above (i.e., with minimum deviations when time point 2 is exactly in the middle of the observation period) fits to the intuitive planning of identical time intervals and results from the fact that for identical time intervals the value of the first follow-up at time point 2 has no influence on the resulting trend (see section “Approaches Which Incorporate Timepoint 2 in the Trend Calculation”), even if aberrations occur. Out of the three fit approaches Tavg, TlinReg, and Tmedian, Tavg is most sensitive to disturbances (aberrations), especially for non-equal time intervals. An alternative is to switch to a non-parametric calculation by using Tmedian.
(ii) For the situation of a high quality measurement at t2 and a measurement at t3 of limited quality, similar deviations independent of the fit approach are observed. Only for a small interval between the two follow-up scans (t2 → t3), in Tavg higher deviations could be observed (Figure 2B). TorthReg, TlinReg, and TfitApproach reveal an almost equal course and (together with Tweighted) reach a minimum deviation for (t2 → t3). Tmedian shows intermediate deviations.
Statistically, timing of the middle visit reveals that the deviation is minimized when follow-up 1 is shifted in time toward the time point of follow-up 2 (Figure 2). However, such a schedule might rather be realized for controls than for patients given that long-term observation periods in neurodegenerative diseases may be difficult due to a worsening of the physical and/or mental condition of the included patients with the consequences of decreased data quality and even dropouts.
In study design external conditions, and constraints, including time, budget, and feasibility, should be taken into account rather than searching for appropriate analysis tools with data in hand. With appropriate time interval planning, linear mixed effect models (Bernal-Rusiel et al., 2013) could be applied for data analysis as well as the further linear regression models (Rencher and Schaalje, 2007). The current theoretical study has been set up to induce a framework when longitudinal brain MRI studies in neurodegenerative diseases are planned for the specific but frequent case of one baseline and two follow-up scans. It is discussed which is the optimum model to be used as regression line for longitudinal trends. For the situation of one baseline and two follow-up MRI scans, the simulation of outliers on the one hand side reveals as optimal solution a simple schedule of identical time-intervals between baseline and follow-up scans which is the intuitive situation without any previous simulations. On the other hand, identical time-intervals constitute the paradox that follow-up 1 scan data do not influence the final trend results for either calculation method based on a linear model. This calls for the question if these follow-up 1 scans are indeed useful when the corresponding outcome parameters do not (or only partially) influence the trend results.
Thus, based on this theoretical framework, we provide a proposal how the time intervals in a longitudinal MRI study in neurodegenerative diseases could be conceptualized:
(1) We propose the time point for follow-up 1 to be shifted in time toward the time point of follow-up 2, that way considering that long-term observation periods in neurodegenerative diseases may be difficult due to a worsening of the physical and/or mental condition of the included patients. By this choice, it would be assured that follow-up 1 (t2) measurements influence the trend results and potentially stabilize them, which is particularly helpful if the last measurement [follow-up 2 (t3)] is of limited quality.
(2) We propose to avoid bisectioning the total observation period (baseline to follow-up 2) by follow-up 1. If bisectioning is unavoidable, the rater should be aware that the choice of the fit approach can significantly influence the trend results. An additional aspect is that it could be an alternative in the study schedule to skip the “median scans” (i.e., the follow-up 1 measurement in case of three visits) and to use the resources for a repeated follow-up 2 measurement.
(3) As a general consideration, it is recommended to perform a quality control of the imaging data prior to the fit procedure. Depending on the scan quality, it might be an option to discard single visit measurements (due to limited data quality) and to perform the fit with only two time points (instead of three time points) rather than attempting to increase the results quality by variation of the fit model. That way, it might be better to re-analyze a whole study with a higher quality requirement (on the data) than varying the fit approach at the analysis level.
Generally, it can be held that, in a given longitudinal MRI study with an even number of scans, the situation cannot occur that one scan does not influence the trend results for any time-interval planning. If, on the other hand, a study is planned with an odd number of scans and equal time-intervals, the “median” scans mostly do not influence the trend results.
(4) In neurodegenerative diseases, most participants are motivated and in a comparatively good physical condition at baseline. Thus, the time interval to follow-up is usually scheduled depending on the clinical evaluation of the disease progression and also the patients’ predicted physical condition (among further constraints). During the course of the study, the patients’ physical condition and their ability to comply with the requirements of neuroimaging are usually worsening, leading to missing data and (more important) to potentially reduced quality of scans at follow-up acquisitions. The latter effect leads to a variability of noise across subjects especially between controls and patients in longitudinal group comparisons which could be addressed by the application of a mixed effect model (if all recorded data passed quality control – see item 3).
Although concepts for longitudinal trend analysis are an issue of well-established research (Rencher and Schaalje, 2007), the technical complexity of modeling longitudinal data remains a topic of discussion and is, at least in certain aspects, incompletely understood and/or appreciated (Bernal-Rusiel et al., 2013). The application of linear regression methods in longitudinal neuroimaging studies has to be thoroughly considered and in general, the applicability of linear mixed effect models (Rencher and Schaalje, 2007) should be an analysis option under consideration, especially for the analysis of ex post facto data when the time intervals were already fixed (and equal). However, when a study with one baseline and two follow-up scans is conceptualized, a bisectioning of the observation time should be avoided in order to provide any analysis method the full power of accuracy. Advanced models such as least absolute shrinkage and selection operator (Lasso) (Tibshirani, 1996) which is a regression analysis method that performs both variable selection and regularization in order to enhance the prediction accuracy are generally oversized for solving linear regression of three data points. In addition to sophisticated models for harmonizing longitudinal multi-scanner imaging data (Beer et al., 2020), statistical analysis of longitudinal data with linear mixed effect models (Bernal-Rusiel et al., 2013), and normative estimates of longitudinal data (Fotenos et al., 2005), the current theoretical study has been set up to induce a framework when longitudinal brain MRI studies in neurodegenerative diseases are planned for the specific but frequent case of one baseline and two follow-up scans.
The key message of this theoretical study is that for the specific but frequent case of one baseline and two follow-up scans by bisectioning the total observation period (baseline to follow-up 2), follow-up 1 measurements do not influence the trend results or only in a weakened form. That way, we recommend to shift the follow-up 1 measurement toward the end of the study so that a bisectioning of the total time interval is avoided.
H-PM: study concept and design, simulations and interpretation of data, and drafting of manuscript. AB, GL, and H-JH: interpretation of data and critical revision of manuscript for intellectual content. JK: study concept and design, interpretation of data, and critical revision of manuscript for intellectual content. All authors contributed to the article and approved the submitted version.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Beer, J. C., Tustison, N. J., Cook, P. A., Davatzikos, C., Sheline, Y. I., Shinohara, R. T., et al. (2020). Longitudinal ComBat: a method for harmonizing longitudinal multi-scanner imaging data. Neuroimage 220:117129. doi: 10.1016/j.neuroimage.2020.117129
Bernal-Rusiel, J. L., Greve, D. N., Reuter, M., Fischl, B., Sabuncu, M. R., and Alzheimer’s Disease Neuroimaging Initiative (2013). Statistical analysis of longitudinal neuroimage data with Linear Mixed Effects models. Neuroimage 66, 249–260. doi: 10.1016/j.neuroimage.2012.10.065
Brandmaier, A. M., von Oertzen, T., Ghisletta, P., Hertzog, C., and Lindenberger, U. (2015). LIFESPAN: a tool for the computer-aided design of longitudinal studies. Front. Psychol. 6:272. doi: 10.3389/fpsyg.2015.00272
Cardenas-Blanco, A., Machts, J., Acosta-Cabronero, J., Kaufmann, J., Abdulla, S., Kollewe, K., et al. (2016). Structural and diffusion imaging versus clinical assessment to monitor amyotrophic lateral sclerosis. Neuroimage Clin. 11, 408–414. doi: 10.1016/j.nicl.2016.03.011
Davis, C. E., Jeste, D. V., and Eyler, L. T. (2005). Review of longitudinal functional neuroimaging studies of drug treatments in patients with schizophrenia. Schizophr. Res. 78, 45–60. doi: 10.1016/j.schres.2005.05.009
Dickerson, B. C., and Sperling, R. A. (2005). Neuroimaging biomarkers for clinical trials of disease-modifying therapies in Alzheimer’s disease. NeuroRx. 2, 348–360. doi: 10.1602/neurorx.2.2.348
Fitzmaurice, G. M., Laird, N. M., and Ware, J. H. (2011). Applied Longitudinal Analysis. Wiley: John Wiley & Sons.
Fotenos, A. F., Snyder, A. Z., Girton, L. E., Morris, J. C., and Buckner, R. L. (2005). Normative estimates of cross-sectional and longitudinal brain volume decline in aging and AD. Neurology 64, 1032–1039. doi: 10.1212/01.wnl.0000154530.72969.11
Hobbs, N. Z., Farmer, R. E., Rees, E. M., Cole, J. H., Haider, S., Malone, I. B., et al. (2015). Short-interval observational data to inform clinical trial design in Huntington’s disease. J. Neurol. Neurosurg. Psychiatry 86, 1291–1298. doi: 10.1136/jnnp-2014-309768
Huppertz, H. J., Kröll-Seger, J., Klöppel, S., Ganz, R. E., and Kassubek, J. (2010). Intra- and interscanner variability of automated voxel-based volumetry based on a 3D probabilistic atlas of human cerebral structures. Neuroimage 49, 2216–2224. doi: 10.1016/j.neuroimage.2009.10.066
Jack, C. R., Vemuri, P., Wiste, H. J., Weigand, S. D., Lesnick, T. G., Lowe, V., et al. (2012). Shapes of the trajectories of 5 major biomarkers of Alzheimer disease. Arch. Neurol. 69, 856–867.
Kalra, S., Müller, H. P., Ishaque, A., Zinman, L., Korngut, L., Genge, A., et al. (2020). A prospective harmonized multicenter DTI study of cerebral white matter degeneration in ALS. Neurology 95, e943–e952.
Kassubek, J., Müller, H. P., Del Tredici, K., Lulé, D., Gorges, M., Braak, H., et al. (2018). Imaging the pathoanatomy of amyotrophic lateral sclerosis in vivo: targeting a propagation-based biological marker. J. Neurol. Neurosurg. Psychiatry 89, 374–381. doi: 10.1136/jnnp-2017-316365
Lau, R. W., and Goodyear, B. G. (2007). Minimum detectable change in water diffusion using 3-T magnetic resonance imaging. Neuroimage 36, 491–496. doi: 10.1016/j.neuroimage.2007.03.015
Müller, H. P., Huppertz, H. J., Dreyhaupt, J., Ludolph, A. C., Tabrizi, S. J., Roos, R. A. C., et al. (2019). Combined cerebral atrophy score in Huntington’s disease based on atlas-based MRI volumetry: sample size calculations for clinical trials. Parkinsonism Relat. Disord. 63, 179–184. doi: 10.1016/j.parkreldis.2019.02.004
Newman, A. B. (2010). An overview of the design, implementation, and analyses of longitudinal studies on aging. J. Am. Geriatr. Soc. 58, S287–S291.
Press, W. H., Flannery, B. P., Teukolsky, S. A., and Vetterling, W. T. (1992a). Numerical Recipes In C: The Art Of Scientific Computing. Chap. 15. New York: Cambridge University Press, 2, 661–666.
Press, W. H., Flannery, B. P., Teukolsky, S. A., and Vetterling, W. T. (1992b). Numerical Recipes In C: The Art Of Scientific Computing. Chap. 10. New York: Cambridge University Press, 4, 408–412.
Rencher, A. C., and Schaalje, G. B. (2007). Linear Models In Statistics. New Jersey: Wiley-Interscience.
Sabuncu, M. R., Desikan, R. S., Sepulcre, J., Yeo, B. T., Liu, H., Schmansky, N. J., et al. (2011). The dynamics of cortical and hippocampal atrophy in Alzheimer disease. Arch. Neurol. 68, 1040–1048. doi: 10.1001/archneurol.2011.167
Steinbach, R., Gaur, N., Roediger, A., Ilse, B., Witte, O. W., Grosskreutz, J., et al. (2021). Disease aggressiveness signatures of amyotrophic lateral sclerosis in white matter tracts revealed by the D50 disease progression model. Hum. Brain Mapp. 42, 737–752. doi: 10.1002/hbm.25258
Tabrizi, S. J., Langbehn, D. R., Leavitt, B. R., Roos, R. A., Durr, A., Craufurd, D., et al. (2009). Biological and clinical manifestations of Huntington’s disease in the longitudinal TRACK-HD study: cross-sectional analysis of baseline data. Lancet Neurol. 8, 791–801. doi: 10.1016/s1474-4422(09)70170-x
Tahedl, M., Chipika, R. H., Lope, J., Li Hi Shing, S., Hardiman, O., and Bede, P. (2021). Cortical progression patterns in individual ALS patients across multiple timepoints: a mosaic-based approach for clinical use. J. Neurol. 268, 1913–1926. doi: 10.1007/s00415-020-10368-7
Thompson, W. K., Hallmayer, J., O’Hara, R., and Neuroimaging Initiative (2011). Design considerations for characterizing psychiatric trajectories across the lifespan: application to effects of APOE-ε4 on cerebral cortical thickness in Alzheimer’s disease. Am. J. Psychiatry 168, 894–903. doi: 10.1176/appi.ajp.2011.10111690
Keywords: magnetic resonance imaging, longitudinal study, time-interval, linear fit, regression analysis
Citation: Müller H-P, Behler A, Landwehrmeyer GB, Huppertz H-J and Kassubek J (2021) How to Arrange Follow-Up Time-Intervals for Longitudinal Brain MRI Studies in Neurodegenerative Diseases. Front. Neurosci. 15:682812. doi: 10.3389/fnins.2021.682812
Received: 19 March 2021; Accepted: 08 June 2021;
Published: 15 July 2021.
Edited by:
Kevin C. Chan, New York University, United StatesReviewed by:
Robert Fekete, New York Medical College, United StatesCopyright © 2021 Müller, Behler, Landwehrmeyer, Huppertz and Kassubek. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hans-Peter Müller, aGFucy1wZXRlci5tdWVsbGVyQHVuaS11bG0uZGU=
†These authors share senior authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.