- 1Iverson Health Innovation Research Institute, Swinburne University of Technology, Melbourne, VIC, Australia
- 2Department of Mental Health, St Vincent's Hospital Melbourne, Fitzroy, VIC, Australia
- 3Department of Psychiatry, University of Melbourne, Melbourne, VIC, Australia
- 4Centre for Mental Health, Swinburne University of Technology, Melbourne, VIC, Australia
- 5Department of Mental Health, Austin Health, Melbourne, VIC, Australia
- 6Library Service, St Vincent's Hospital Melbourne, Fitzroy, VIC, Australia
Background: Autonomic nervous system (ANS) dysfunction has been suggested to contribute to the high prevalence of cardiovascular complications in individuals with anorexia nervosa (AN), yet has not been thoroughly investigated. The current review aimed to synthesize the evidence of basal ANS function in individuals with a current diagnosis of AN and those with a previous diagnosis who had achieved weight restoration, as compared to controls.
Methods: A systematic review of nine databases was conducted and studies that were published in a peer-review journal, in English, that included at least one assessment of ANS function in individuals with a current or previous diagnosis of AN were selected. Forty-six studies were included with a total of 811 participants with a current diagnosis of AN and 123 participants with a previous diagnosis of AN.
Results: ANS function was assessed through heart rate variability (n = 27), orthostatic challenge, blood pressure variability or baroreflex sensitivity (n = 11), adrenergic activity (n = 14), skin conductance level (n = 4), and pupillometry (n = 1). Individuals with AN demonstrated increased parasympathetic activity and decreased sympathetic activity, suggestive of autonomic dysregulation. Following weight restoration, autonomic function trended toward, or was equivalent to, control levels.
Discussion: Autonomic dysregulation is indicated through a range of assessments in individuals with AN. Future investigations should utilize a variety of assessments together in order to conclusively establish the nature of autonomic dysfunction in AN, and following extended weight restoration. Moreover, investigation into the co-occurrence of ANS function and cardiovascular risk is required.
Introduction
Anorexia Nervosa (AN) is an eating disorder characterized by restriction of food intake, an intense fear of gaining weight and a distorted self-perception of body image (American Psychiatric Association, 2013). AN has been recognized as an increasingly prevalent psychiatric condition among young people in Western societies, with the incidence also increasing in a variety of racial and ethnic groups (Nakai et al., 2016), mostly in women (Hoek, 2006). AN has a typical onset in adolescence (Hoek and Van Hoeken, 2003) and has an estimated lifetime prevalence of 1.7% in the general population (Smink et al., 2014). The etiology and pathophysiology of AN are complex, involving biological, psychological, and sociocultural development and maintenance factors (Phillipou et al., 2019). The chronic nature of AN is evidenced by a 50% relapse rate (Pike, 1998), with learned maladaptive behaviors becoming deeply entrenched and difficult to alter (Steinglass and Walsh, 2016).
The energy deprivation and malnutrition associated with AN places immense pressure on the cardiovascular system, with up to 80% of patients suffering from cardiovascular complications (Spaulding-Barclay et al., 2016). These include structural, conduction, and hemodynamic abnormalities (Sachs et al., 2016; Giovinazzo et al., 2019; Smythe et al., 2020), and are a major contributor to the high mortality rate in AN (Nakai et al., 2016), which is approximately six times that of the general population (Papadopoulos et al., 2009; Arcelus et al., 2011). Cardiovascular problems occur not only during the starvation state of AN; there are also specific cardiac complications that arise during the process of re-feeding, such as arrhythmia, tachycardia, and congestive heart failure (Casiero and Frishman, 2006; Vignaud et al., 2010). Despite the profound psychological and physical burdens that accompany AN, the underlying physiological mechanisms behind the cardiovascular complications of the illness remain poorly understood. It has been suggested that disturbances in cardiac autonomic regulation may contribute to the increased cardiovascular complications and mortality in AN (Mazurak et al., 2011a).
The autonomic nervous system (ANS) provides the link between the cardiovascular system and the central nervous system, and is responsible for the regulation of internal bodily processes in response to physiological and environmental changes (Palma and Benarroch, 2014). The ANS is a dynamic regulatory function that involves interpretation of sensory feedback from the organs by higher brain areas, including the brainstem and hypothalamus, in order to adapt the output of the ANS to adjust the physiological state of the body (Porges, 2007; Buijs et al., 2013). Through the regulation of heart rate (HR), blood pressure (BP), and rate of respiration among other visceral activities, the ANS maintains cardiovascular homeostasis via the opposing inputs of its two branches; the sympathetic (SNS) and parasympathetic (PNS) nervous systems (Gordan et al., 2015). Activation of the SNS results in increased arousal, such as increased HR and blood vessel constriction through the release of noradrenaline (NE), whereas the PNS (or vagal nerve) acts in opposition to decrease HR and BP. Evaluation of the ANS can be derived from various techniques including hemodynamic, biochemical and neurophysiological assessments with each presenting its own limitations (Grassi and Esler, 1999). Therefore, multiple assessments of autonomic function should be undertaken together in order to provide an overview of neural function; some of which are briefly detailed below.
Hemodynamic assessments can provide insight into the autonomic regulation of blood flow. Sinus bradycardia (Yahalom et al., 2013) and low BP levels (Sachs et al., 2016) are commonly observed in individuals with AN and are suggestive of abnormalities in autonomic regulation of HR and BP. The majority of previous investigations into autonomic function in individuals with AN have assessed heartrate variability [HRV; the beat-to-beat variation in HR (Task Force of The European Society of Cardiology The North American Society of Pacing Electrophysiology, 1996; Billman, 2011)] as an estimation of autonomic cardiac regulation, with inconclusive findings (see Mazurak et al., 2011a for a review). While the review by Mazurak et al. (2011a) found the majority of studies that investigated HRV in AN reported parasympathetic dominance, some reported sympathetic dominance, while others found no difference in comparison to controls; this led the authors to suggest that HRV may not be suitable for the assessment of ANS in AN (Mazurak et al., 2011a). Another hemodynamic assessment of autonomic function is the orthostatic stress test, which provides a window into autonomic regulation through the baroreceptor reflex control of BP and HR (Grassi and Esler, 1999; Westerhof et al., 2006). Conditions related to orthostatic intolerance, such as orthostatic hypotension, syncope and postural orthostatic tachycardia syndrome (POTS) represent autonomic failure and have also been reported in AN (Sachs et al., 2016).
Biochemical assessment of plasma NE levels can provide an index of sympathetic neural function that have been shown to vary according to weight (Lambert et al., 2007). However, circulating NE represents only a fraction of the amount secreted from nerve terminals and is dependent on secretion, clearance and re-uptake processes (Esler et al., 1990), therefore this method provides a “confounded” index of systematic sympathetic activation (Grassi et al., 2015). Measurement of the NE metabolite, 3-methyl-4-hydroxyphenylglycol (MHPG) is another common biochemical assessment that is undertaken to further inform regional NE synthesis, release and re-uptake (Grassi and Esler, 1999).
In addition to regional NE spillover, the other “preferred” assessment for sympathetic nervous system evaluation, is the neurophysiological technique of “microneurography” (Grassi et al., 2015). Microneurography provides a direct continuous recording of muscle sympathetic nerve activity (MSNA) to give a measure of central nervous system sympathetic neural outflow to the skeletal muscles (Grassi and Esler, 1999), including blood vessels. Increased sympathetic neural drive, as assessed by microneurography, is associated with increased cardiovascular risk (Kaye et al., 1995; Grassi, 2006), yet microneurography remains less commonly used due to its semi-invasive nature.
It is beyond the scope of the current review to provide an overview of all assessments of ANS function; previous thorough reviews have been conducted (Grassi and Esler, 1999; Tjalf and Timo, 2019). To our knowledge, there has been no prior systematic review of autonomic function in individuals with AN. Moreover, most studies have primarily assessed function in individuals in the acute state of AN and it is less clear whether any abnormalities persist after weight restoration. In order to advance the knowledge of ANS function in AN, the current systematic review aims to synthesize studies investigating resting-state ANS function in individuals with AN, including those who have achieved weight restoration, as compared to healthy controls. Given the important clinical implications of abnormalities in autonomic cardiovascular control, a greater understanding of any abnormalities in ANS function in individuals with AN, and following weight restoration, is crucial.
Materials and Methods
Search Strategy
This systematic review was carried out in accordance with the Preferred Reporting Items for Systematic Review and Meta-Analyses (PRISMA) (Supplementary Material) (Moher et al., 2010) and was registered with the International Prospective Register of Systematic Reviews (PROSPERO identifier CRD42020177195). Studies were identified through systematic searches of nine databases: Ovid MEDLINE(R) ALL 1946 to November 03, 2020; Embase 1974 to 2020 November 03 (Ovid); Ovid Emcare 1995 to 2020 Week 44; APA PsycInfo 1806 to October Week 4 2020 (Ovid); Ovid Nursing Database 1946 to October Week 4 2020; CINAHL (EBSCOhost); Health Collection, Humanities & Social Sciences Collection (Informit); Cochrane Library and Clinicaltrials.gov. Search strategies were developed by a medical librarian, HW, in consultation with the review team. Strategies combined the general concepts of anorexia nervosa AND autonomic nervous system using a combination of subject headings and textwords relevant to each database. Results were limited to English language, but no date limits were applied. Animal studies were excluded. An initial strategy was developed for Medline and then adapted for other databases (Appendix 1 in Supplementary Material). All searches were updated on 5 November 2020. Reference lists of included studies were screened for additional publications.
Study Selection
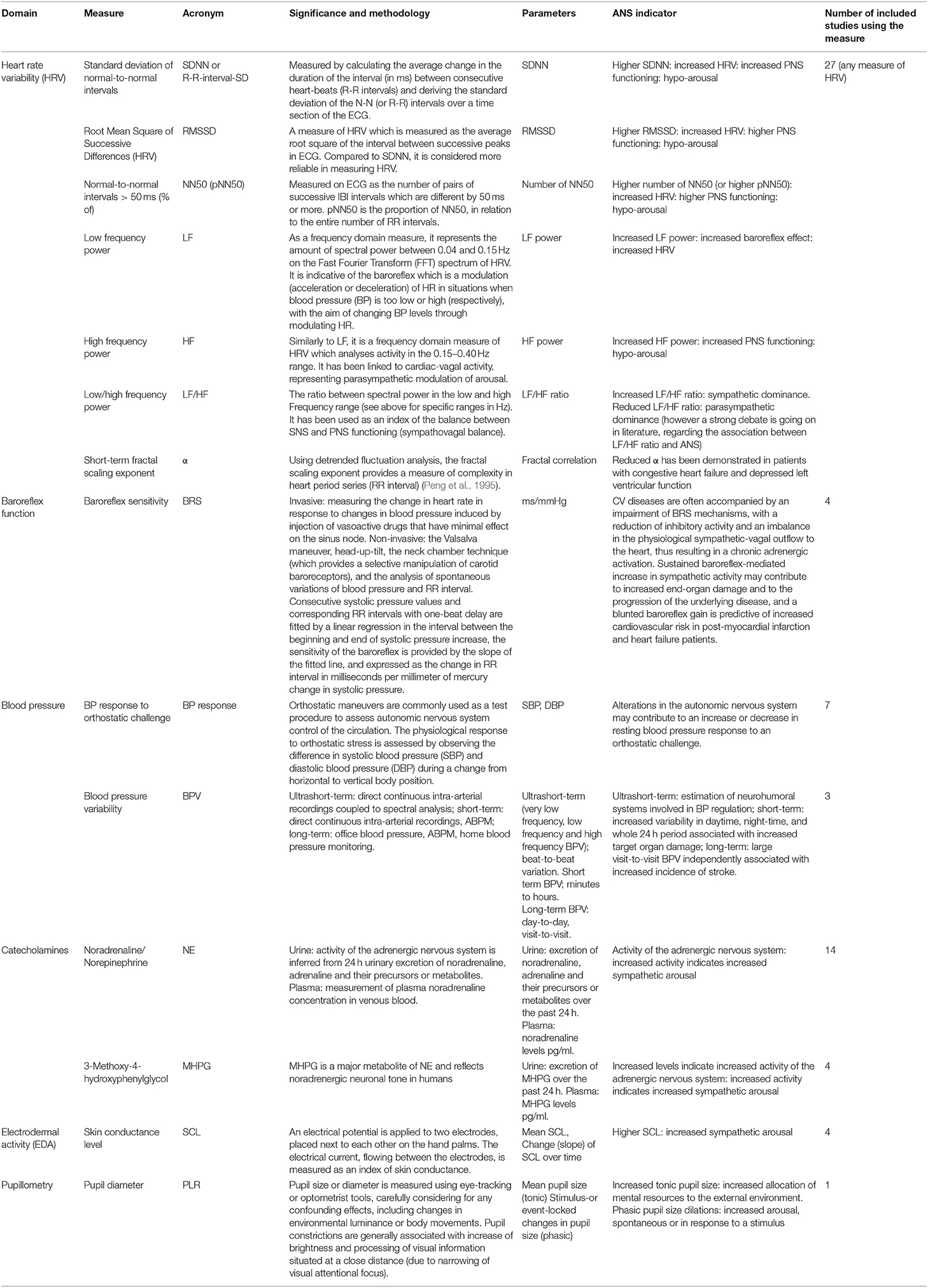
Search results were exported to Endnote bibliographic management software, duplicates removed, and the remainder uploaded to Covidence systematic review software (www.covidence.org) by HW. Two authors (Z.J., E.L.) independently screened records on title and abstract and then full text against the following exclusion criteria: primary condition not AN, no diagnostic criteria referenced, no control group, no basal ANS assessment outcome, protocol paper, review article, dissertation, conference abstract, case series/study. A third reviewer (N.E.) resolved any conflicts. Studies that included at least one of the ANS measures in basal conditions listed in Table 1 were included (see Table 1 for a summary of ANS outcomes, description of assessment and relationship to ANS functioning). A meta-analysis was not performed as there were too few similarities between study methods and measures.
Data Extraction
Two reviewers (Z.J. and E.L.) independently extracted data and consensus was confirmed by a third reviewer (N.E. or A.P.) Extracted data included information on study characteristics and basal ANS assessment and outcomes.
Risk of Bias/Quality Assessment
The risk of bias among included studies was assessed independently by two authors (Z.J. and D.C.) using a modified version of the Newcastle-Ottawa Quality Assessment Scale (NOS; see Appendix 2 in Supplementary Material) for cohort/case-control studies, in which a high score indicates a low risk of bias (Wells et al., 2006). Studies were assessed on three domains; participant selection, comparability and outcome assessment and were classified as at low, moderate, or high risk of bias. The risk of bias was not used as an exclusion criterion in the selection of studies to provide a complete overview of available data.
HRV Risk of Bias/Quality Assessment
Given the large number of included studies that assessed HRV, we used a modified version of a previously published measure of study quality in studies of HRV in functional somatic disorders to specifically evaluate quality of HRV methods (Tak et al., 2009). We modified the tool to incorporate the items listed in the Guidelines for Reporting Articles on Psychiatry and Heart rate variability (GRAPH) criteria (Quintana et al., 2016) to provide a more comprehensive assessment of HRV quality and risk of bias (see Appendix 3 in Supplementary Material). We assessed three general domains: appropriate selection of participants, appropriate quantification collection of HRV and appropriate control for confounding factors. Potential scores ranged from 0 to 22.
Results
The literature search yielded 2,126 unique citations. The full text of 105 citations were examined and, of these, 46 articles met our inclusion criteria (see Figure 1).
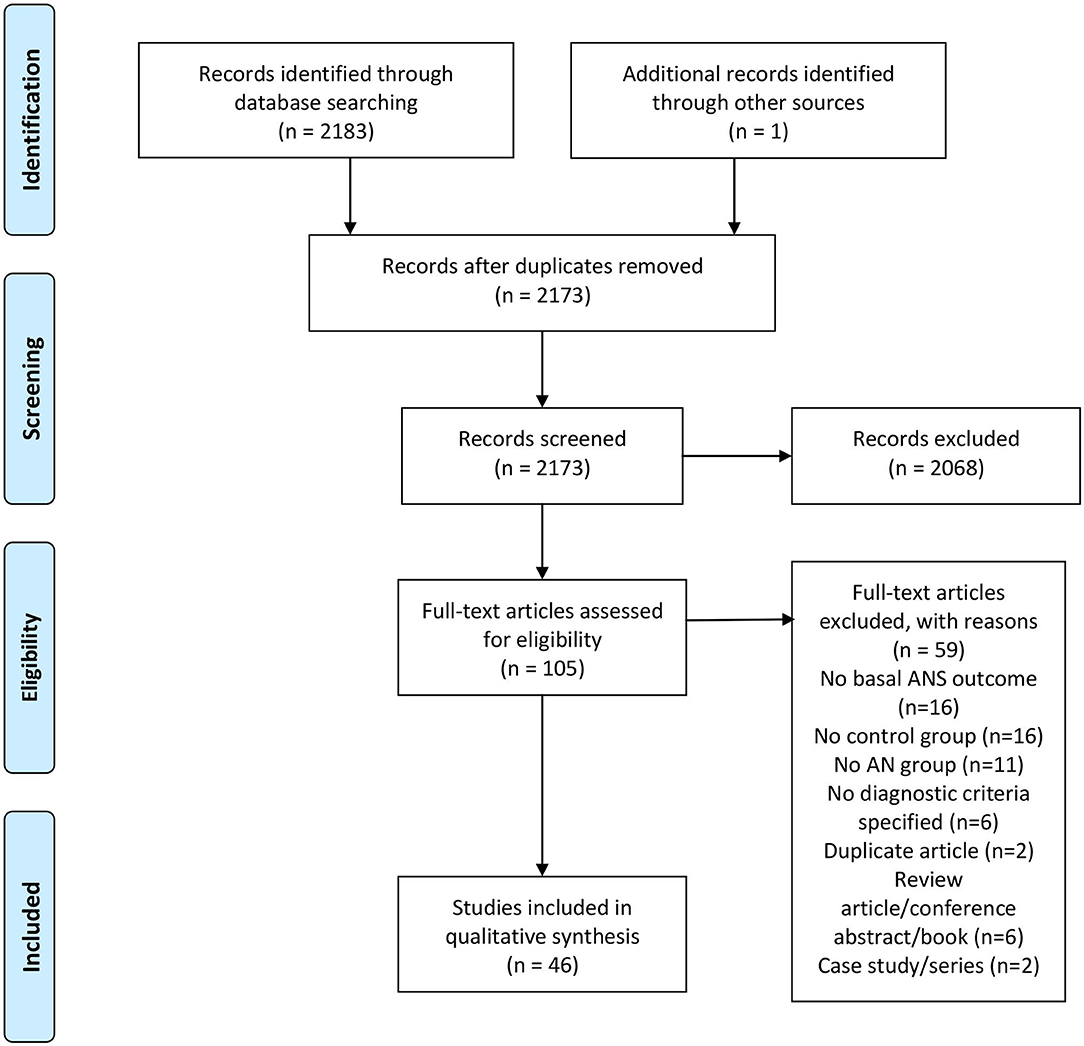
Figure 1. PRISMA flowchart. From Moher et al. (2009).
Study Characteristics
Characteristics of the included studies for qualitative synthesis are shown in Table 2. All included studies utilized cross-sectional study design; 39 assessed participants at a single time point and seven included assessments at multiple time points after weight restoration (Gross et al., 1979; Riederer et al., 1982; Lesem et al., 1989; Kaye et al., 1990; Kreipe et al., 1994; Bar et al., 2006; Lachish et al., 2009). The 46 studies included assessments of 811 participants with a current diagnosis of AN (757 female, 11 male, 43 not specified), 123 participants with a previous diagnosis of AN who were at various stages of treatment and weight restoration (AN-WR; 100 female, 2 male, 21 not specified) and 867 control participants (834 female, 20 male, 13 not specified). Sample sizes ranged from 7 to 89 participants with a current diagnosis of AN, 4–18 weight-restored participants, and 8–39 controls. One study did not specify the sample size of their control group (Lechin et al., 2010), four studies did not specify the sex of the AN participants (Kaye et al., 1990; Pirke et al., 1992; Rommel et al., 2015; Palomba et al., 2017) and three studies did not specify the sex of the AN-WR participants (Riederer et al., 1982; Kaye et al., 1990; Pirke et al., 1992). The average duration of illness ranged from 8 months to 10 years and the duration of weight restoration ranged from 2 weeks to 3 years.
Study Quality Assessment
The NOS scores of the included studies ranged from 3 to 10. Among the 46 included studies, two were at high risk of bias (4.3%), 17 were at moderate risk (37.0%), and 27 were at low risk (58.7%) (see Table 2 for classification or detailed assessment in Appendix 4 in Supplementary Material). The HRV quality summary score is listed in Table 3 (see Appendix 5 in Supplementary Material for a detailed assessment).
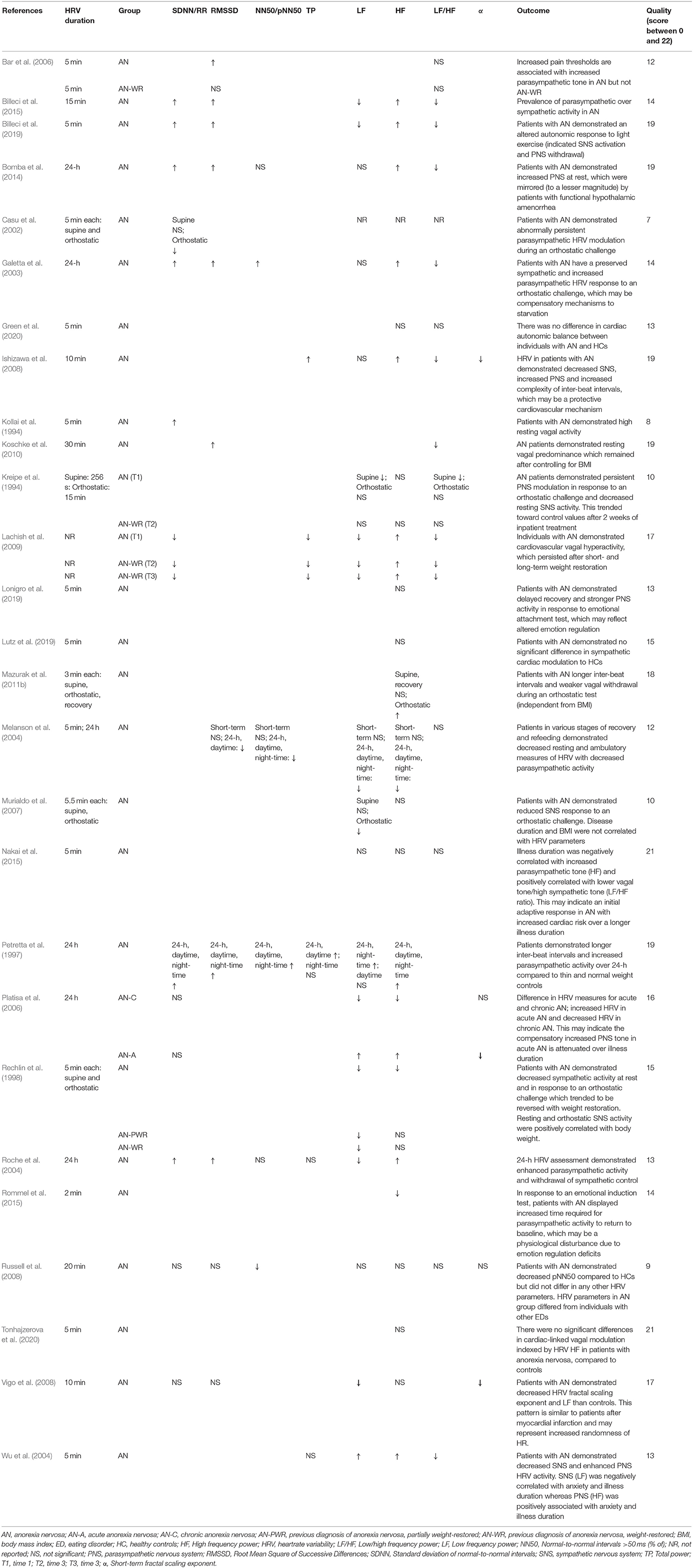
Heartrate Variability
Resting HRV was an outcome measure in 27 articles (Kollai et al., 1994; Kreipe et al., 1994; Petretta et al., 1997; Rechlin et al., 1998; Casu et al., 2002; Galetta et al., 2003; Melanson et al., 2004; Roche et al., 2004; Wu et al., 2004; Bar et al., 2006; Platisa et al., 2006; Murialdo et al., 2007; Ishizawa et al., 2008; Russell et al., 2008; Vigo et al., 2008; Lachish et al., 2009; Koschke et al., 2010; Mazurak et al., 2011b; Bomba et al., 2014; Billeci et al., 2015, 2019; Nakai et al., 2015; Rommel et al., 2015; Lonigro et al., 2019; Lutz et al., 2019; Green et al., 2020; Tonhajzerova et al., 2020) (see Table 3). Studies used various durations of HRV assessment; 21 reported HRV outcomes from short-term recordings (Kollai et al., 1994; Kreipe et al., 1994; Rechlin et al., 1998; Casu et al., 2002; Wu et al., 2004; Bar et al., 2006; Murialdo et al., 2007; Ishizawa et al., 2008; Russell et al., 2008; Vigo et al., 2008; Lachish et al., 2009; Koschke et al., 2010; Mazurak et al., 2011b; Billeci et al., 2015, 2019; Nakai et al., 2015; Rommel et al., 2015; Lonigro et al., 2019; Lutz et al., 2019; Green et al., 2020; Tonhajzerova et al., 2020), five used ambulatory HRV recordings taken over 24 h (Petretta et al., 1997; Galetta et al., 2003; Roche et al., 2004; Platisa et al., 2006; Bomba et al., 2014) and one study reported both (Melanson et al., 2004). Regarding the HRV measures reported, 15 of the included studies reported on time domain HRV (Kollai et al., 1994; Petretta et al., 1997; Casu et al., 2002; Galetta et al., 2003; Melanson et al., 2004; Roche et al., 2004; Bar et al., 2006; Platisa et al., 2006; Russell et al., 2008; Vigo et al., 2008; Lachish et al., 2009; Koschke et al., 2010; Bomba et al., 2014; Billeci et al., 2015, 2019), 16 reported low frequency (LF) (Kreipe et al., 1994; Petretta et al., 1997; Rechlin et al., 1998; Casu et al., 2002; Galetta et al., 2003; Melanson et al., 2004; Roche et al., 2004; Wu et al., 2004; Platisa et al., 2006; Murialdo et al., 2007; Ishizawa et al., 2008; Lachish et al., 2009; Bomba et al., 2014; Billeci et al., 2015, 2019; Nakai et al., 2015), 22 reported high frequency (HF) (Kreipe et al., 1994; Petretta et al., 1997; Rechlin et al., 1998; Casu et al., 2002; Galetta et al., 2003; Melanson et al., 2004; Roche et al., 2004; Wu et al., 2004; Platisa et al., 2006; Murialdo et al., 2007; Ishizawa et al., 2008; Lachish et al., 2009; Mazurak et al., 2011b; Bomba et al., 2014; Billeci et al., 2015, 2019; Nakai et al., 2015; Rommel et al., 2015; Lonigro et al., 2019; Lutz et al., 2019; Green et al., 2020; Tonhajzerova et al., 2020) and 14 studies reported on calculated ratios of LF to HF (LF/HF) (Kreipe et al., 1994; Casu et al., 2002; Galetta et al., 2003; Melanson et al., 2004; Wu et al., 2004; Bar et al., 2006; Ishizawa et al., 2008; Lachish et al., 2009; Koschke et al., 2010; Bomba et al., 2014; Billeci et al., 2015, 2019; Nakai et al., 2015; Green et al., 2020). Four studies used detrended fluctuation analysis (DFA) to calculate the scaling exponent (α) of HRV (Platisa et al., 2006; Ishizawa et al., 2008; Russell et al., 2008; Vigo et al., 2008).
(i) Current AN
Basal time domain HRV in individuals with a current diagnosis of AN was reported in 15 studies. Of the ten studies that assessed short-term time domain HRV, individuals with AN had increased HRV in five studies (Kollai et al., 1994; Bar et al., 2006; Koschke et al., 2010; Billeci et al., 2015, 2019), decreased in two studies (Russell et al., 2008; Lachish et al., 2009) and unchanged in three studies (Casu et al., 2002; Melanson et al., 2004; Vigo et al., 2008), as compared to controls. Of the six studies that assessed time domain HRV using ambulatory recordings, four reported increased HRV (Petretta et al., 1997; Galetta et al., 2003; Roche et al., 2004; Bomba et al., 2014), one reported decreased HRV (Melanson et al., 2004) and one reported unchanged HRV (Platisa et al., 2006). Two studies demonstrated increased time domain HRV, as compared to lean controls (Petretta et al., 1997; Galetta et al., 2003).
Frequency domain HRV was assessed in 20 short-term recordings and 6 ambulatory recordings. Of the 16 studies that reported resting LF, seven reported decreased LF (Kreipe et al., 1994; Rechlin et al., 1998; Roche et al., 2004; Vigo et al., 2008; Lachish et al., 2009; Billeci et al., 2015, 2019), two reported increased LF (Petretta et al., 1997; Wu et al., 2004) and six reported unchanged LF (Galetta et al., 2003; Murialdo et al., 2007; Ishizawa et al., 2008; Russell et al., 2008; Bomba et al., 2014; Nakai et al., 2015), compared to controls. One study reported decreased LF HRV in acute AN (average illness duration of 12 months) and increased LF in chronic AN (average illness duration of 36 months) (Platisa et al., 2006). Of the 22 studies that reported resting HF in individuals with a current diagnosis of AN, nine reported increased HF (Petretta et al., 1997; Galetta et al., 2003; Roche et al., 2004; Wu et al., 2004; Ishizawa et al., 2008; Lachish et al., 2009; Bomba et al., 2014; Billeci et al., 2015, 2019), two reported decreased HF (Rechlin et al., 1998; Roche et al., 2004) and ten reported unchanged HF (Kreipe et al., 1994; Murialdo et al., 2007; Russell et al., 2008; Vigo et al., 2008; Mazurak et al., 2011b; Nakai et al., 2015; Lonigro et al., 2019; Lutz et al., 2019; Green et al., 2020; Tonhajzerova et al., 2020), as compared to controls. Platisa et al. (2006) found increased HF in acute AN and decreased HF in chronic AN, while Melanson et al. (2004) assessed both short-term and ambulatory HRV, reporting no difference between groups in short-term recording of LF or HF, yet ambulatory results demonstrated both decreased LF and HF in individuals with AN, compared to controls. Of the 14 studies that reported a calculated LF/HF ratio, nine reported decreased LF/HF ratio (Kreipe et al., 1994; Galetta et al., 2003; Wu et al., 2004; Ishizawa et al., 2008; Lachish et al., 2009; Koschke et al., 2010; Bomba et al., 2014; Billeci et al., 2015, 2019) and five reported unchanged LF/HF ratios (Melanson et al., 2004; Bar et al., 2006; Russell et al., 2008; Nakai et al., 2015; Green et al., 2020), as compared to controls.
Of the four studies that reported non-linear assessments of HRV and reported the scaling exponent (α), two found decreased α (Ishizawa et al., 2008; Vigo et al., 2008) and one reported no difference in α compared to controls (Russell et al., 2008). Platisa et al. (2006) again highlighted differences according to duration of AN, reporting decreased α in those with a shorter illness duration and no difference to controls in those with an extended illness duration.
Overall, three studies indicated differences in HRV modulation according to duration of illness. A shorter illness duration was demonstrated by increased parasympathetic modulation which was attenuated over time in two studies (Platisa et al., 2006; Nakai et al., 2015). However, Wu et al. (2004) found a negative correlation between enhanced SNS activity and illness duration and a positive correlation between PNS activity and illness duration.
(ii) Weight-Restored AN
Four studies reported on HRV in individuals with a previous diagnosis of AN who were in varying stages of weight restoration. Two reported time domain HRV; one reported decreased HRV (no change from the current AN group) as compared to controls (Lachish et al., 2009) and the other reported no difference between AN-WR and controls (Bar et al., 2006). Three reported LF HRV in AN-WR; two reported maintenance of decreased LF (Rechlin et al., 1998; Lachish et al., 2009) and one reported no difference in LF (Kreipe et al., 1994) between AN-WR and controls. The same three studies also recorded HF in AN-WR; one reported maintenance of high HF in AN-WR (Lachish et al., 2009) and two reported no difference in HF (Kreipe et al., 1994; Rechlin et al., 1998) between AN-WR and controls. Three studies calculated the LF/HF ratio; one reported sustained low LF/HF after weight restoration (Lachish et al., 2009) and two reported no difference in LF/HF between AN-WR and controls (Kreipe et al., 1994; Bar et al., 2006).
Orthostatic Response, Blood Pressure Variability, and Baroreflex Sensitivity
Eleven studies reported the response to an orthostatic challenge, blood pressure variability (BPV), or baroreflex sensitivity (BRS) as an outcome measure (Gross et al., 1979; Lesem et al., 1989; Van Binsbergen et al., 1991; Kollai et al., 1994; Kreipe et al., 1994; Casu et al., 2002; Murialdo et al., 2007; Ishizawa et al., 2008; Lechin et al., 2010; Takimoto et al., 2014; Tonhajzerova et al., 2020) (see Table 4).
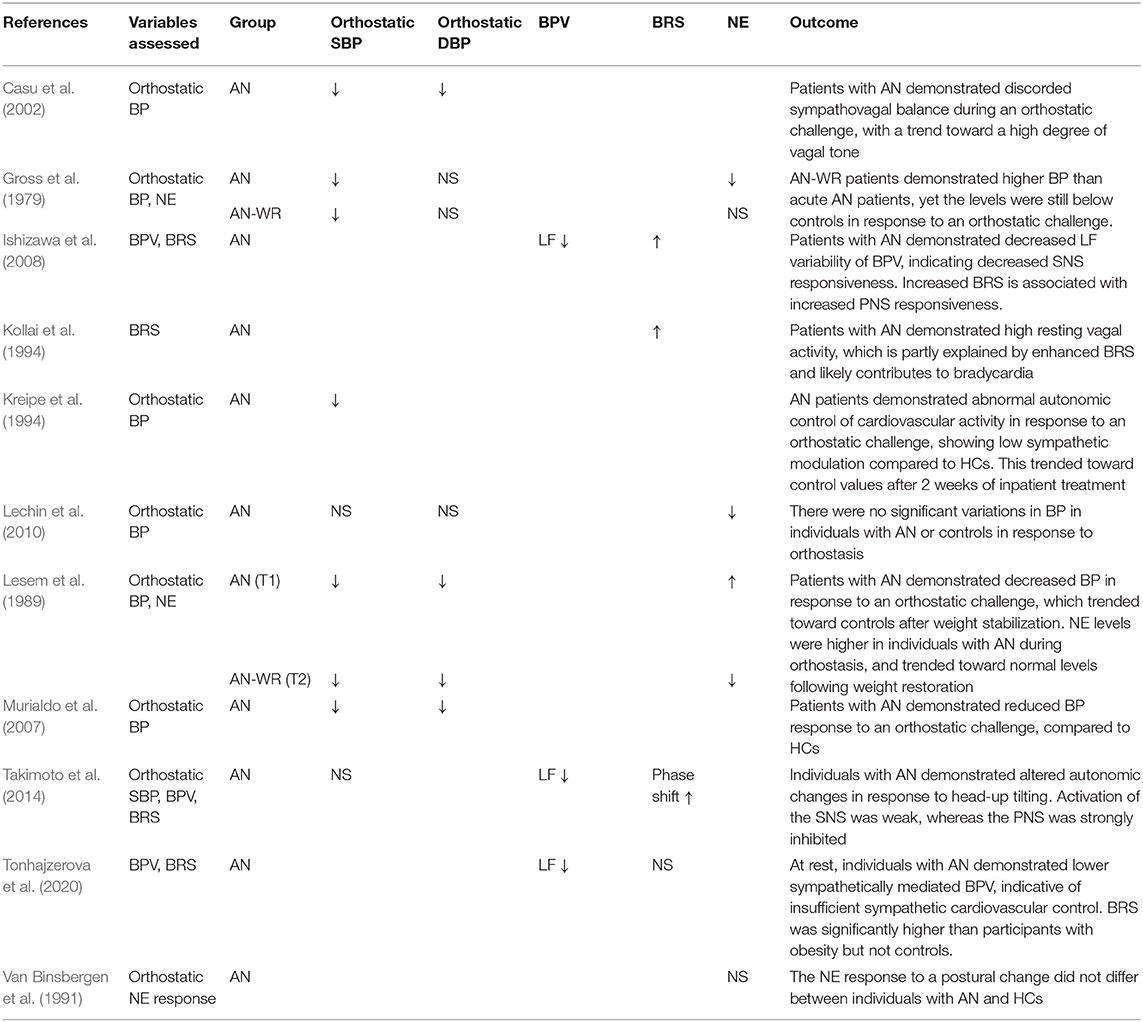
Table 4. Orthostatic response, blood pressure variability and baroreflex sensitivity, as compared to controls.
(i) Current AN
Six studies assessed BP response to an orthostatic challenge in individuals with a current diagnosis of AN; five reported decreased systolic BP (SBP) and/or diastolic BP (DBP) (Gross et al., 1979; Lesem et al., 1989; Kreipe et al., 1994; Casu et al., 2002; Murialdo et al., 2007) and one did not directly compare the response to controls (Lechin et al., 2010). Four studies investigated NE levels in response to an orthostatic challenge; two found a decreased response (Gross et al., 1979; Lechin et al., 2010), one an increased response (Lesem et al., 1989), and one found no difference (Van Binsbergen et al., 1991), as compared to controls.
Three studies reported increased BRS in individuals with a current diagnosis of AN (Kollai et al., 1994; Ishizawa et al., 2008; Takimoto et al., 2014) but Tonhajzerova et al. (2020) reported no difference in BRS to controls. All three studies that assessed BPV in individuals with AN reported decreased LF variability of BP (Ishizawa et al., 2008; Takimoto et al., 2014; Tonhajzerova et al., 2020).
(ii) Weight-Restored AN
Two studies assessed BP response to an orthostatic challenge in AN-WR groups, with both reporting maintenance of decreased BP response (Gross et al., 1979; Lesem et al., 1989). However, both reports of NE response to an orthostatic challenge were no different from controls (Gross et al., 1979), or trended toward control levels (Lesem et al., 1989) following weight restoration.
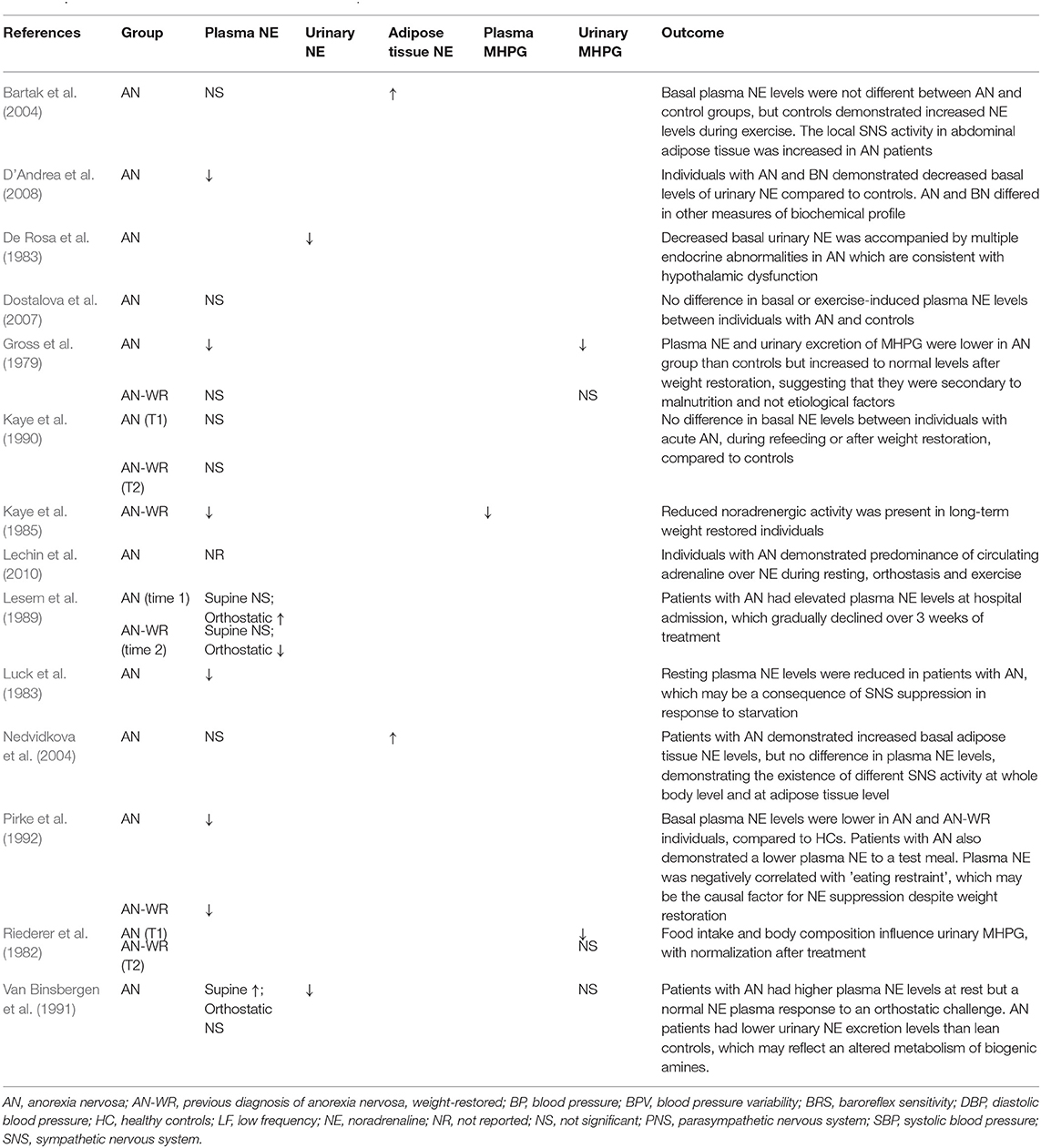
Adrenergic Assessment
Fourteen studies reported basal NE or MHPG as an outcome measure (Gross et al., 1979; Riederer et al., 1982; De Rosa et al., 1983; Luck et al., 1983; Kaye et al., 1985, 1990; Lesem et al., 1989; Van Binsbergen et al., 1991; Pirke et al., 1992; Bartak et al., 2004; Nedvidkova et al., 2004; Dostalova et al., 2007; D'Andrea et al., 2008; Lechin et al., 2010) (see Table 5).
(i) Current AN
Thirteen studies reported basal NE or MHPG levels in individuals with a current diagnosis of AN. Of these studies, 11 reported basal plasma NE levels; four reported decreased plasma NE (Gross et al., 1979; Luck et al., 1983; Pirke et al., 1992; D'Andrea et al., 2008), one reported increased plasma NE (Van Binsbergen et al., 1991) and six reported no difference in basal plasma NE (Lesem et al., 1989; Kaye et al., 1990; Bartak et al., 2004; Nedvidkova et al., 2004; Dostalova et al., 2007; Lechin et al., 2010), as compared to controls. Lechin et al. (2010) proposed that individuals with AN present with adrenal sympathetic overactivity, as evidenced by the low NE: adrenaline plasma ratio, yet did not directly compare NE levels to controls. Beta-adrenergic receptor activity was assessed by Kaye et al. (1990) who found an erratic response to increasing doses of isoproterenol in individuals with AN, as compared with controls, proposing that altered regulation of presynaptic adrenoreceptors may account for the discrepancy in assessments of NE levels across studies.
Two studies assessed adipose tissue levels of NE and both reported increased NE (Bartak et al., 2004; Nedvidkova et al., 2004). Two studies assessed urinary NE levels and both found decreased urinary NE, as compared to normal weight controls (De Rosa et al., 1983; Van Binsbergen et al., 1991) and lean controls (Van Binsbergen et al., 1991), despite one also reporting increased plasma NE levels (Van Binsbergen et al., 1991). Three studies assessed urinary excretion levels of MHPG; in two, MHPG levels were decreased in individuals with AN (Gross et al., 1979; Riederer et al., 1982) and in the third, there was no difference to controls (Van Binsbergen et al., 1991).
(ii) Weight-Restored AN
Six studies reported basal NE or MHPG levels in individuals with a previous diagnosis of AN (Gross et al., 1979; Riederer et al., 1982; Kaye et al., 1985, 1990; Lesem et al., 1989; Pirke et al., 1992). Five studies reported plasma NE levels; two of which reported decreased NE (Kaye et al., 1985; Pirke et al., 1992) and three reported no difference to controls (Gross et al., 1979; Lesem et al., 1989; Kaye et al., 1990). Two studies reported urinary MHPG levels and both found them to be comparable to control levels (Gross et al., 1979; Riederer et al., 1982) whereas one study assessed plasma MHPG, which was decreased in AN-WR participants (Kaye et al., 1985).
Skin Conductance Level and Pupil Response
Four studies reported skin conductance level (SCL) as an outcome measure in individuals with a current diagnosis of AN (see Table 6); two reported decreased SCL (Abell et al., 1987; Palomba et al., 2017) and two reported no difference in SCL compared to controls (Calloway et al., 1983; Léonard et al., 1998).
The only study that assessed pupil response (PLR) found decreased PLR response in individuals with a current diagnosis of AN, which did not persist after weight restoration (Bar et al., 2006).
Discussion
The current review provides the first synthesis of investigations into ANS function in individuals with AN and those who have a previous diagnosis and have achieved weight restoration. The assessment of ANS function across modalities is discussed below.
Heartrate Variability
The majority of studies that assessed HRV in the time domain demonstrated increased beat-to-beat variability in HR in individuals with a current diagnosis of AN, consistent with a recent review (Peyser et al., 2020). Moreover, increased time domain HRV parameters were demonstrated in patients with AN when compared to lean controls (Petretta et al., 1997; Galetta et al., 2003). The studies that reported decreased time domain HRV presented some methodological limitations. One did not specify duration of AN and stated that participants had recently started various antidepressant and antipsychotic agents (Russell et al., 2008), which have been associated with decreased HRV (Licht et al., 2010), another did not report the length of HRV assessment (Lachish et al., 2009) and the third reported results from a small sample size of six patients (Melanson et al., 2004). Following weight restoration, one reported no difference to controls and the other reported decreased HRV, yet did not report the HRV assessment length (Lachish et al., 2009). Therefore, based on the current review results, beat-to-beat variability in HR is increased in the acute state of AN, which does not continue following weight restoration.
Assessment of HRV in the frequency domain, specifically in the LF and HF frequency bands, trended toward increased HF and decreased LF which was reflected in a trend toward decreased LF/HF ratios in patients with a current diagnosis of AN. Assessment of HRV in the frequency domain in WR participants primarily suggested normalization of HRV, with either no difference or levels trending toward controls. Akin to HRV assessed in the time domain, the acute state of AN is marked by increased parasympathetic activity and decreased sympathetic activity in the frequency domain, which appears to normalize following weight restoration.
Non-linear analysis of HRV was also assessed to provide a measure of complexity (α), or randomness, in heart period series that has been demonstrated to be reduced in individuals with congestive heart failure (Peng et al., 1995) and a prognostic indicator of cardiac mortality (Huikuri et al., 2000). Decreased α values were demonstrated in individuals with a current diagnosis of AN (Ishizawa et al., 2008; Vigo et al., 2008) and in those with a shorter duration of AN (termed “acute”) (Platisa et al., 2006), reflective of HRV patterns seen in patients with heart failure, which was postulated to be a mechanism of cardiac autonomic dysfunction and sudden death in AN (Vigo et al., 2008).
While the majority of studies indicated concordant results in HRV assessment, discrepancies are likely to be due in part to the duration of AN, the potential for comorbid conditions to impact HRV and the assessment methodology. The impact of chronicity (or duration of AN) was repeatedly highlighted as a distinguishing feature of HRV profile HRV (Platisa et al., 2006; Nakai et al., 2015). It was suggested that the HRV profile was so distinct between initial and chronic stages of illness that it could be used to distinguish between phases of illness, whereby initial starvation is typified by increased parasympathetic activity (increased HF) and an extended duration of illness was characterized by increased sympathetic activity (LF) (Petretta et al., 1997; Melanson et al., 2004; Roche et al., 2004; Platisa et al., 2006; Nakai et al., 2015). A single study found contrasting results (a positive correlation between increased illness duration and HF but a negative correlation between duration and LF), yet did not specify illness duration, therefore potential extrapolation is uncertain (Wu et al., 2004). A tentative conclusion may be that the relative increase or decrease in HF and LF is dependent on duration of AN. However, further investigation is required to confirm this hypothesis.
In addition to duration of illness, another potential influence on HRV that must be taken into account is the potential impact of comorbid psychiatric conditions on HRV parameters (Shinba et al., 2008). Anxiety and stress have been demonstrated to increase sympathetic activity (Lucini et al., 2002) and evoke cardiac vagal withdrawal, a physiological response thought to be related to the hypersensitivity engendered in anxiety disorders (for a review on the topic, see Friedman, 2007). Similarly, decreased HRV has frequently been associated with depression (independent from cardiovascular disease) (Musselman et al., 1998; Kemp et al., 2010) and antidepressant use (Licht et al., 2010; Michael and Kaur, 2021). Given that the majority of studies did not specify comorbid psychiatric conditions or psychoactive medication use, the impact of these in the current review cannot be ascertained. There is a wide literature on the influence of psychological state on HRV (Thayer et al., 2012), with common reference to Porges' polyvagal theory which stipulates that HRV is associated with experience and expression of social and emotional behavior (Porges, 2007). Given the high rate of comorbid psychiatric disorders in individuals with AN (O'Brien and Vincent, 2003), it may be difficult to extrapolate reliably, the influence of AN alone on HRV.
Further consideration must be applied when considering the HRV assessment methodology. Assessments of HRV in the current review were derived from both ambulatory recordings and short-term recordings of varying length. While HRV analyses of different lengths of time are generally closely correlated (Costa et al., 1994), results between short-term and ambulatory recordings can differ (Li et al., 2019) and should not be compared (Task Force of The European Society of Cardiology The North American Society of Pacing Electrophysiology, 1996). Indeed, the only study that assessed both short-term and ambulatory HRV in the current review reported no difference in short-term HRV but decreased HRV over long-term recordings (Melanson et al., 2004).
A separate consideration is concern over whether HRV is a reflection of the autonomic state of the entire body or the regulation of the sinoatrial node alone (Hayano and Yuda, 2019). The use of HRV as a sole index of ANS activity is potentially problematic given that frequency domain analysis of HRV reportedly over-simplifies the non-linear interactions between the SNS and PNS (Billman, 2013). While HRV provides some insight into vagal activity, it has the disadvantage of giving a poor indication of sympathetic activity (Esler and Lambert, 2003; Billman, 2013). Indeed, LF heart rate spectral power (often interpreted as sympathetic activity) has been demonstrated as unrelated to direct assessments of sympathetic activity, such as NE spillover, MSNA (Kingwell et al., 1994), and cardiac sympathetic innervation quantified by positron emission tomographic neuroimaging (Rahman et al., 2011). Moreover, in the current review, 17 out of the 25 studies that assessed HRV did not use any other method to assess autonomic function in individuals with AN, a limitation underscored by Ishizawa et al. (2008) and Takimoto et al. (2014).
Overall, the assessments of HRV indicated alterations in autonomic regulation of heart rate in AN characterized by increased heart rate variance and increased vagal activity. While persistent sympathetic excitation and depressed vagal activity are associated with ventricular arrhythmias and sudden cardiac death (Task Force of The European Society of Cardiology The North American Society of Pacing Electrophysiology, 1996), the implications of persistent vagal activation and autonomic dysregulation remain unclear. However, there have been indications of increased parasympathetic activity and autonomic dysregulation at the onset of acute myocardial infarction (Webb et al., 1972), with the suggestion that autonomic dysregulation is a risk factor for sudden cardiac death in individuals with amyotrophic lateral sclerosis (Asai et al., 2007). Therefore, it remains to be determined whether consistent elevation of HRV and increased vagal modulation of cardiac control represent cardiovascular risk for individuals with AN.
Orthostatic Response, Blood Pressure Variability, and Baroreflex Sensitivity
Assessment of the physiological response to an orthostatic challenge can provide powerful insight into cardiac autonomic regulation. During a head-up tilt, the resultant peripheral venous pooling and decreased cardiac output triggers stimulation of aortic, carotid and cardiopulmonary baroreceptors, resulting in increased sympathetic outflow and inhibition of parasympathetic activity in healthy individuals (Ramírez-Marrero et al., 2007).
Observations that assessed the change in BP from a supine to upright position were limited; while BP response to orthostasis was blunted in individuals with AN in one study (Casu et al., 2002), it did not differ from controls in others (Lechin et al., 2010; Takimoto et al., 2014). Multiple studies compared absolute BP levels between AN and HC groups during an orthostatic challenge; a methodology which is limited in providing an indication of autonomic regulation given that BP is principally decreased in individuals with AN. However, assessments of HRV, BPV and adrenergic response to orthostasis revealed that individuals with AN failed to exhibit an increased sympathetic response to a head-up tilt. While a normal response is demonstrated by a decrease in the HF and increase in LF components of HRV and BPV, these reflex mechanisms were not seen in individuals with AN (Casu et al., 2002; Murialdo et al., 2007; Takimoto et al., 2014). Furthermore, individuals with AN did not demonstrate increased adrenergic outflow during a change in position (Gross et al., 1979; Lechin et al., 2010), yet were comparable to controls after weight restoration (Gross et al., 1979; Lesem et al., 1989).
While at rest, individuals with AN demonstrated decreased variability in BP and increased baroreflex sensitivity, further suggesting increased parasympathetic control over the heart. Together, these assessments of orthostatic response, BPV and BRS in individuals with AN demonstrate an abnormal regulation of the cardiovascular system through a failure to activate a sympathetic response and inhibit parasympathetic activity. Altered orthostatic regulation suggests that individuals with AN are at risk of a range of conditions associated with altered orthostatic regulation, such as syncope, orthostatic hypertension, and POTS (Grubb, 2005), many of which have indeed been reported in AN. Following weight-restoration, responses trended toward those of controls, reflective of the suggestion that resolution of a normal orthostatic response can determine medical stability and readiness for discharge following treatment (Shamim et al., 2003).
Adrenergic Assessment
While many of the studies that assessed static adrenergic activity in the current review found no difference in plasma NE levels between individuals with AN and controls, there was a trend toward decreased plasma NE or MHPG levels. Decreased NE was interpreted as a chronic adaptation to malnutrition by some authors (Riederer et al., 1982; Dostalova et al., 2007), which contributed to hypothalamic dysfunction during the acute state of AN (Gross et al., 1979; De Rosa et al., 1983). Another interpretation suggested that NE levels varied over the course of treatment according to stress levels and psychological (as opposed to physical) stabilization (Lesem et al., 1989). Moreover, altered regulation of presynaptic beta-adrenoreceptors was reported, suggesting that altered noradrenergic receptor function may also be present in individuals with AN (Kaye et al., 1990).
Similarly, urinary excretion of NE and MHPG was decreased in individuals with AN compared to both normal weight (Gross et al., 1979; De Rosa et al., 1983) and lean controls (Van Binsbergen et al., 1991), which increased following treatment (Gross et al., 1979; Riederer et al., 1982). While MHPG is the major metabolite of NE in the brain, urinary MHPG is predominantly the product of peripheral SNS, rather than central nervous system NE metabolism. Given that urinary catecholamine excretion is dependent on renal function (Esler et al., 1988), which has previously been shown to be impaired in individuals with AN (Stheneur et al., 2014), interpretation of decreased urinary excretion of NE and MHPG in AN is constrained.
In contrast, assessment of NE levels in adipose tissue revealed localized elevated levels of sympathetic activity in individuals with AN, compared to controls (Bartak et al., 2004; Nedvidkova et al., 2004), despite no difference in overall plasma NE (Bartak et al., 2004). Given that local adipose tissue sympathetic activity is not a reflection of overall whole body sympathetic activity (Patel et al., 2002), an increase in localized sympathetic activity within adipose tissue was suggested to be a protective mechanism to protect fat stores from further depletion through downregulation of lipolysis (Bartak et al., 2004), a process supported by prolonged fasting models (Migliorini et al., 1997).
Each assessment of adrenergic activity in individuals with a current diagnosis of AN, and after weight restoration, provided an alternate assessment of NE presence and metabolism. Given that circulating NE levels represent a small proportion of NE secreted from nerve terminals (Grassi and Esler, 1999), it is difficult to surmise a conclusive interpretation of sympathetic activity from these results. However, there was a trend toward decreased NE levels in individuals with a current diagnosis of AN, which normalized after weight restoration.
Skin Conductance Level and Pupillary Response
In comparison to alternate measurements of autonomic function, SCL and PLR were less commonly assessed. Notwithstanding this, reduced sympathetic activation in SCL (Abell et al., 1987; Palomba et al., 2017) and altered SCL responses between AN subtype (Calloway et al., 1983) were reported. All assessments of SCL were conducted on the palms, of which are prone to emotional sweating (Vetrugno et al., 2003). Indeed, alterations to SCL in AN were observed to be correlated with psychological factors (including anxiety and metacognitive dysfunction) (Léonard et al., 1998; Palomba et al., 2017). Given that sympathetic skin response has been demonstrated to be emotionally activated (Cheshire et al., 2020), the use of SCL to provide insight into thermoregulatory autonomic function is therefore limited.
The only study that investigated PLR found decreased sympathetic and increased parasympathetic pupil response in individuals with AN, yet only in the acute state, which normalized following weight restoration (Bar et al., 2006). Given that only a single investigation has been conducted into PLR, which identified changes in autonomic nervous system activity in individuals with AN, further investigations of this non-invasive parameter should be undertaken in future studies.
Limitations
The purpose of the current review was to synthesize the evidence of ANS function associated with AN. Several methodological factors must be taken into account when comparing the assessments of ANS function in the current review. Given the serious nature and medical instability associated with AN, many studies utilized small sample sizes, which no doubt contributed to the lack of consistency among results in individual studies. Moreover, the studies investigating individuals with a previous diagnosis of AN included varied durations of weight restoration, precluding the ability to draw a succinct conclusion. Many studies did not detail or compare differences between restrictive and binge eating-purging subtypes of AN, therefore any differences related to specific AN behaviors cannot be determined by the current review. Future investigations into ANS function after a prolonged period of weight restoration would allow a better understanding of the impact of AN in any long-term alterations to ANS function. Similarly, delineation of AN subtype and assessment of comorbid psychiatric diagnoses in future assessments could reveal any differences in autonomic function according to subtype and comorbidities.
Implications and Conclusion
The current review provides a synthesis of the evidence to date assessing resting autonomic function in individuals with AN, and after weight restoration. It is indicated that individuals with AN demonstrate autonomic dysregulation characterized by decreased sympathetic activity and increased parasympathetic activity as well as increased complexity of the ANS through a variety of assessment methodologies. Given the ease and convenience of HRV assessment, it is tempting to use the measure as a sole assessment of autonomic function. However, the demonstrated impact that both illness duration and psychiatric comorbidities can have on HRV infer that assessment of autonomic activity should be established via additional accompanying measures. While the duration of weight restoration in the current review was widely varied, the majority of studies to date indicated that autonomic regulation tended to normalize after weight restoration. Moreover, there has been no assessment of SNS activity in individuals with AN to date using either microneurographic measurement of muscle sympathetic nerve activity or assessment of organ-specific NE spillover; the two “preferred” assessments of human adrenergic function (Grassi and Esler, 1999).
The underlying mechanisms that contribute to the abnormalities in ANS function in acute AN remain speculative. It has been proposed that the parasympathetic dominance seen in AN is an adaptive physiological response to conserve energy in response to malnutrition (Buchhorn et al., 2016; Sachs et al., 2016; Kalla et al., 2017). However, it remains unclear whether energy preservation alone is underlying the changes in ANS function, given that the three studies that included lean control groups did not find a linear relationship between BMI and ANS function. Specifically, HRV and NE excretion in patients with AN were significantly different than both normal-weight and lean controls, who satisfied the weight, but not psychological, criterion for AN (Van Binsbergen et al., 1991; Petretta et al., 1997; Galetta et al., 2003). There is growing evidence of an intrinsic connection between the brain and the heart, including interplay between frontal-vagal (brain-heart) and depression networks (Iseger et al., 2020), that purportedly contributes to cardiovascular disease (Makovac et al., 2017). Given the demonstrated dysregulation of other neural regulatory systems in AN [including dopaminergic and serotonergic systems, which are thought to contribute to both physiological and psychological traits seen in AN (Kaye et al., 2005; Fladung et al., 2010)], there may be central dysregulation of ANS networks in AN, yet this remains putative.
The implications of the current review are that increased vagal activity is likely to underlie the widespread bradycardia in individuals with AN. Moreover, inhibited SNS activation during orthostasis would result in insufficient blood flow to organs and contribute to episodes of syncope. Less clear are the implications of the increased autonomic complexity demonstrated by HRV and BRS parameters. While cardiovascular disease is commonly associated with sympathetic overactivity (Malpas, 2010), the consequences of sustained parasympathetic overactivity and autonomic dysregulation are yet to be determined. It remains to be ascertained whether the autonomic dysregulation indicated in individuals with AN contributes to the widespread cardiovascular complications.
This review has demonstrated that autonomic dysregulation is indicated in individuals with AN, yet there have been no thorough assessments of autonomic function utilizing multiple methodologies. Due to the variability in both methodology and quality of assessments to date, conclusions drawn from these data should be interpreted with caution. Furthermore, in order to determine the association between autonomic dysregulation and widespread cardiovascular complications in AN conclusively, future investigations should employ a variety of assessments of autonomic function in conjunction with markers of cardiovascular risk. It will also be important to assess the impact of comorbid psychiatric conditions and duration of illness in order to conclusively establish the nature of autonomic (dys)function in AN. Similarly, future investigations in individuals with an extended duration of weight restoration are still required. Determination of autonomic function through a variety of assessment methodologies in individuals with a current, and previous, diagnosis of AN alongside assessments of cardiovascular risk will aid in determining the contributing factors to cardiovascular complications. This will allow clinicians to identify individuals at risk and aid in the prevention, treatment and development of interventions to reduce the inadvertent mortality rate of AN.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
Author Contributions
ZJ conceived the project and drafted the manuscript. HW conducted the search. ZJ, EL, and NE conducted the study selection. ZJ, NE, AP, DC, and EL conducted the data extraction and risk of bias. All authors designed and approved the protocol, reviewed, and approved.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnins.2021.682208/full#supplementary-material
References
Abell, T. L., Malagelada, J. R., Lucas, A. R., Brown, M. L., Camilleri, M., Go, V. L., et al. (1987). Gastric electromechanical and neurohormonal function in anorexia nervosa. Gastroenterology 93, 958–965. doi: 10.1016/0016-5085(87)90557-9
American Psychiatric Association (2013). Diagnostic and Statistical Manual of Mental Disorders (DSM-5®). American Psychiatric Pub. doi: 10.1176/appi.books.9780890425596
Arcelus, J., Mitchell, A. J., Wales, J., and Nielsen, S. (2011). Mortality rates in patients with anorexia nervosa and other eating disorders: a meta-analysis of 36 studies. Arch. Gen. Psychiatry 68, 724–731. doi: 10.1001/archgenpsychiatry.2011.74
Asai, H., Hirano, M., Udaka, F., Shimada, K., Oda, M., Kubori, T., et al. (2007). Sympathetic disturbances increase risk of sudden cardiac arrest in sporadic ALS. J. Neurol. Sci. 254, 78–83. doi: 10.1016/j.jns.2007.01.007
Bar, K. J., Boettger, S., Wagner, G., Wilsdorf, C., Gerhard, U. J., Boettger, M. K., et al. (2006). Changes of pain perception, autonomic function, and endocrine parameters during treatment of anorectic adolescents. J. Am. Acad. Child Adolesc. Psychiatry 45, 1068–1076. doi: 10.1097/01.chi.0000227876.19909.48
Bartak, V., Vybiral, S., Papezova, H., Dostalova, I., Pacak, K., and Nedvidkova, J. (2004). Basal and exercise-induced sympathetic nervous activity and lipolysis in adipose tissue of patients with anorexia nervosa. Eur. J. Clin. Invest. 34, 371–377. doi: 10.1111/j.1365-2362.2004.01344.x
Billeci, L., Tartarisco, G., Brunori, E., Crifaci, G., Scardigli, S., Balocchi, R., et al. (2015). The role of wearable sensors and wireless technologies for the assessment of heart rate variability in anorexia nervosa. Eat. Weight Disord. 20, 23–31. doi: 10.1007/s40519-014-0135-2
Billeci, L., Tonacci, A., Brunori, E., Raso, R., Calderoni, S., Maestro, S., et al. (2019). Autonomic nervous system response during light physical activity in adolescents with anorexia nervosa measured by wearable devices. Sensors 19:2820. doi: 10.3390/s19122820
Billman, G. E. (2011). Heart rate variability–a historical perspective. Front. Physiol. 2:86. doi: 10.3389/fphys.2011.00086
Billman, G. E. (2013). The LF/HF ratio does not accurately measure cardiac sympatho-vagal balance. Front. Physiol. 4:26. doi: 10.3389/fphys.2013.00026
Bomba, M., Corbetta, F., Gambera, A., Nicosia, F., Bonini, L., Neri, F., et al. (2014). Heart rate variability in adolescents with functional hypothalamic amenorrhea and anorexia nervosa. Psychiatry Res. 215, 406–409. doi: 10.1016/j.psychres.2013.11.012
Buchhorn, R., Hauk, F., Meint, S., and Willaschek, C. (2016). The impact of nutrition on the autonomic nervous system. Int. J. Food Nutr. Sci. 3, 1–16. doi: 10.15436/2377-0619.16.942
Buijs, R. M., Escobar, C., and Swaab, D. F. (2013). “Chapter 15 - The circadian system and the balance of the autonomic nervous system,” in Handbook of Clinical Neurology, eds R. M. Buijs and D. F. Swaab), (Elsevier), 173–191.
Calloway, P., Fonagy, P., and Wakeling, A. (1983). Autonomic arousal in eating disorders: further evidence for the clinical subdivision of anorexia nervosa. Br. J. Psychiatry 142, 38–42. doi: 10.1192/bjp.142.1.38
Casiero, D., and Frishman, W. H. (2006). Cardiovascular complications of eating disorders. Cardiol. Rev. 14, 227–231. doi: 10.1097/01.crd.0000216745.96062.7c
Casu, M., Patrone, V., Gianelli, M. V., Marchegiani, A., Ragni, G., Murialdo, G., et al. (2002). Spectral analysis of R-R interval variability by short-term recording in anorexia nervosa. Eat. Weight Disord. 7, 239–243. doi: 10.1007/BF03327462
Cheshire, W. P., Freeman, R., Gibbons, C. H., Cortelli, P., Wennin, G. K., Hilz, M. J., et al. (2020). Electrodiagnostic assessment of the autonomic nervous system: a consensus statement endorsed by the American Autonomic Society and the International Federation of Clinical Neurophysiology. Clin. Neurophysiol. 132, 666–682. doi: 10.1016/j.clinph.2020.11.024
Costa, O., Lago, P., Rocha, A. P., Carvalho, M. J., Freitas, A., Freitas, J., et al. (1994). Heart rate variability in 24-hour holter recordings: comparative study between short-and long-term time-and frequency-domain analyses. J. Electrocardiol. 27, 251–254. doi: 10.1016/S0022-0736(94)80009-X
D'Andrea, G., Ostuzzi, R., Bolner, A., Francesconi, F., Musco, F., D'onofrio, F., et al. (2008). Study of tyrosine metabolism in eating disorders. Possible correlation with migraine. Neurol. Sci. 29, S88–S92. doi: 10.1007/s10072-008-0895-4
De Rosa, G., Corsello, S. M., De Rosa, E., Della Casa, S., Ruffilli, M. P., Grasso, P., et al. (1983). Endocrine study of anorexia nervosa. Exp. Clin. Endocrinol. 82, 160–172. doi: 10.1055/s-0029-1210272
Dostalova, I., Bartak, V., Papezova, H., and Nedvidkova, J. (2007). The effect of short-term exercise on plasma leptin levels in patients with anorexia nervosa. Metabolism 56, 497–503. doi: 10.1016/j.metabol.2006.11.008
Esler, M., Jennings, G., Korner, P., Willett, I., Dudley, F., Hasking, G., et al. (1988). Assessment of human sympathetic nervous system activity from measurements of norepinephrine turnover. Hypertension 11, 3–20. doi: 10.1161/01.HYP.11.1.3
Esler, M., Jennings, G., Lambert, G., Meredith, I., Horne, M., and Eisenhofer, G. (1990). Overflow of catecholamine neurotransmitters to the circulation: source, fate, and functions. Physiol. Rev. 70, 963–985. doi: 10.1152/physrev.1990.70.4.963
Esler, M., and Lambert, E. (2003). Reduced HRV and baroreflex sensitivity as universally applicable cardiovascular “risk factors”; waiting for the bubble to burst. Clin. Auton. Res. 13, 170–172. doi: 10.1007/s10286-003-0101-y
Feighner, J. P., Robins, E., Guze, S. B., Woodruff, R. A., Winokur, G., and Munoz, R. (1972). Diagnostic criteria for use in psychiatric research. Arch. Gen. Psychiatry 26, 57–63. doi: 10.1001/archpsyc.1972.01750190059011
Fladung, A.-K., Grön, G., Grammer, K., Herrnberger, B., Schilly, E., Grasteit, S., et al. (2010). A neural signature of anorexia nervosa in the ventral striatal reward system. Am. J. Psychiatry 167, 206–212. doi: 10.1176/appi.ajp.2009.09010071
Friedman, B. H. (2007). An autonomic flexibility–neurovisceral integration model of anxiety and cardiac vagal tone. Biol. Psychol. 74, 185–199. doi: 10.1016/j.biopsycho.2005.08.009
Galetta, F., Franzoni, F., Prattichizzo, F., Rolla, M., Santoro, G., and Pentimone, F. (2003). Heart rate variability and left ventricular diastolic function in anorexia nervosa. J. Adolesc. Health 32, 416–421. doi: 10.1016/S1054-139X(03)00048-X
Giovinazzo, S., Sukkar, S., Rosa, G., Zappi, A., Bezante, G., Balbi, M., et al. (2019). Anorexia nervosa and heart disease: a systematic review. Eat. Weight Disord. 24, 199–207. doi: 10.1007/s40519-018-0567-1
Gordan, R., Gwathmey, J. K., and Xie, L. H. (2015). Autonomic and endocrine control of cardiovascular function. World J. Cardiol. 7:204. doi: 10.4330/wjc.v7.i4.204
Grassi, G. (2006). Sympathetic overdrive and cardiovascular risk in the metabolic syndrome. Hypertens. Res. 29, 839–847. doi: 10.1291/hypres.29.839
Grassi, G., and Esler, M. (1999). How to assess sympathetic activity in humans. J. Hypertens. 17, 719–734. doi: 10.1097/00004872-199917060-00001
Grassi, G., Mark, A., and Esler, M. (2015). The sympathetic nervous system alterations in human hypertension. Circ. Res. 116, 976–990. doi: 10.1161/CIRCRESAHA.116.303604
Green, M., Miles, L., Sage, E., Smith, J., Carlson, G., Hogan, K., et al. (2020). Cardiac biomarkers of disordered eating as a function of diagnostic subtypes. Eat. Behav. 39:101425. doi: 10.1016/j.eatbeh.2020.101425
Gross, H. A., Lake, C. R., Ebert, M. H., Ziegler, M. G., and Kopin, I. J. (1979). Catecholamine metabolism in primary anorexia nervosa. J. Clin. Endocrinol. Metab. 49, 805–809. doi: 10.1210/jcem-49-6-805
Grubb, B. P. (2005). Neurocardiogenic syncope and related disorders of orthostatic intolerance. Circulation 111, 2997–3006. doi: 10.1161/CIRCULATIONAHA.104.482018
Halmi, K. A., Goldberg, S. C., Eckert, E., Casper, R., and Davis, J. M. (1977). “Pretreatment evaluation in anorexia nervosa,” in Anorexia Nervosa ed R. A. Vigersky (New York, NY: Raven Press), 43–54.
Hayano, J., and Yuda, E. (2019). Pitfalls of assessment of autonomic function by heart rate variability. J. Physiol. Anthropol. 38, 1–8. doi: 10.1186/s40101-019-0193-2
Hoek, H. W. (2006). Incidence, prevalence and mortality of anorexia nervosa and other eating disorders. Curr. Opin. Psychiatry 19, 389–394. doi: 10.1097/01.yco.0000228759.95237.78
Hoek, H. W., and Van Hoeken, D. (2003). Review of the prevalence and incidence of eating disorders. Int. J. Eating Disord. 34, 383–396. doi: 10.1002/eat.10222
Huikuri, H. V., MäKikallio, T. H., Peng, C. K., Goldberger, A. L., Hintze, U., and Møller, M. (2000). Fractal correlation properties of RR interval dynamics and mortality in patients with depressed left ventricular function after an acute myocardial infarction. Circulation 101, 47–53. doi: 10.1161/01.CIR.101.1.47
Iseger, T. A., Van Bueren, N. E., Kenemans, J. L., Gevirtz, R., and Arns, M. (2020). A frontal-vagal network theory for major depressive disorder: implications for optimizing neuromodulation techniques. Brain Stimul. 13, 1–9. doi: 10.1016/j.brs.2019.10.006
Ishizawa, T., Yoshiuchi, K., Takimoto, Y., Yamamoto, Y., and Akabayashi, A. (2008). Heart rate and blood pressure variability and baroreflex sensitivity in patients with anorexia nervosa. Psychosom. Med. 70, 695–700. doi: 10.1097/PSY.0b013e31817bb090
Kalla, A., Krishnamoorthy, P., Gopalakrishnan, A., Garg, J., Patel, N. C., and Figueredo, V. M. (2017). Gender and age differences in cardiovascular complications in anorexia nervosa patients. Int. J. Cardiol. 227, 55–57. doi: 10.1016/j.ijcard.2016.11.209
Kaye, D. M., Lefkovits, J., Jennings, G. L., Bergin, P., Broughton, A., and Esler, M. D. (1995). Adverse consequences of high sympathetic nervous activity in the failing human heart. J. Am. Coll. Cardiol. 26, 1257–1263. doi: 10.1016/0735-1097(95)00332-0
Kaye, W. H., Frank, G. K., Bailer, U. F., Henry, S. E., Meltzer, C. C., Price, J. C., et al. (2005). Serotonin alterations in anorexia and bulimia nervosa: new insights from imaging studies. Physiol. Behav. 85, 73–81. doi: 10.1016/j.physbeh.2005.04.013
Kaye, W. H., George, D. T., Gwirtsman, H. E., Jimerson, D. C., Goldstein, D. S., Ebert, M. H., et al. (1990). Isoproterenol infusion test in anorexia nervosa: assessment of pre- and post-beta-noradrenergic receptor activity. Psychopharmacol. Bull. 26, 355–359.
Kaye, W. H., Jimerson, D. C., Lake, C. R., and Ebert, M. H. (1985). Altered norepinephrine metabolism following long-term weight recovery in patients with anorexia nervosa. Psychiatry Res. 14, 333–342. doi: 10.1016/0165-1781(85)90101-5
Kemp, A. H., Quintana, D. S., Gray, M. A., Felmingham, K. L., Brown, K., and Gatt, J. M. (2010). Impact of depression and antidepressant treatment on heart rate variability: a review and meta-analysis. Biol. Psychiatry 67, 1067–1074. doi: 10.1016/j.biopsych.2009.12.012
Kingwell, B. A., Thompson, J. M., Kaye, D. M., Mcpherson, G., Jennings, G. L., and Esler, M. D. (1994). Heart rate spectral analysis, cardiac norepinephrine spillover, and muscle sympathetic nerve activity during human sympathetic nervous activation and failure. Circulation 90, 234–240. doi: 10.1161/01.CIR.90.1.234
Kollai, M., Bonyhay, I., Jokkel, G., and Szonyi, L. (1994). Cardiac vagal hyperactivity in adolescent anorexia nervosa. Eur. Heart J. 15, 1113–1118. doi: 10.1093/oxfordjournals.eurheartj.a060636
Koschke, M., Boettger, M. K., Macholdt, C., Schulz, S., Yeragani, V. K., Voss, A., et al. (2010). Increased QT variability in patients with anorexia nervosa–an indicator for increased cardiac mortality? Int. J. Eat. Disord. 43, 743–750. doi: 10.1002/eat.20765
Kreipe, R. E., Goldstein, B., Deking, D. E., Tipton, R., and Kempski, M. H. (1994). Heart rate power spectrum analysis of autonomic dysfunction in adolescents with anorexia nervosa. Int. J. Eat. Disord. 16, 159–165. doi: 10.1002/1098-108X(199409)16:2<159::AID-EAT2260160207>3.0.CO;2-H
Lachish, M., Stein, D., Kaplan, Z., Matar, M., Faigin, M., Korsunski, I., et al. (2009). Irreversibility of cardiac autonomic dysfunction in female adolescents diagnosed with anorexia nervosa after short- and long-term weight gain. World J. Biol. Psychiatry 10, 503–511. doi: 10.1080/15622970902980770
Lambert, E., Straznicky, N., Schlaich, M., Esler, M., Dawood, T., Hotchkin, E., et al. (2007). Differing pattern of sympathoexcitation in normal-weight and obesity-related hypertension. Hypertension 50, 862–868. doi: 10.1161/HYPERTENSIONAHA.107.094649
Lechin, F., Van Der Dijs, B., Pardey-Maldonado, B., Rivera, J. E., Baez, S., and Lechin, M. E. (2010). Anorexia nervosa depends on adrenal sympathetic hyperactivity: opposite neuroautonomic profile of hyperinsulinism syndrome. Diabetes Metab. Syndr. Obes. 3, 311–317. doi: 10.2147/DMSO.S10744
Léonard, T., Pepinà, C., Bond, A., and Treasure, J. (1998). Assessment of test-meal induced autonomic arousal in anorexic, bulimic and control females. Eur. Eat. Disord. Rev. 6, 188–200. doi: 10.1002/(SICI)1099-0968(199809)6:3<188::AID-ERV227>3.0.CO;2-G
Lesem, M. D., George, D. T., Kaye, W. H., Goldstein, D. S., and Jimerson, D. C. (1989). State-related changes in norepinephrine regulation in anorexia nervosa. Biol. Psychiatry 25, 509–512. doi: 10.1016/0006-3223(89)90208-4
Li, K., Rüdiger, H., and Ziemssen, T. (2019). Spectral analysis of heart rate variability: time window matters. Front. Neurol. 10:545. doi: 10.3389/fneur.2019.00545
Licht, C. M., De Geus, E. J., Van Dyck, R., and Penninx, B. W. (2010). Longitudinal evidence for unfavorable effects of antidepressants on heart rate variability. Biol. Psychiatry 68, 861–868. doi: 10.1016/j.biopsych.2010.06.032
Lonigro, A., Pallini, S., Zanna, V., Marech, L., Rosa, M., Criscuolo, M., et al. (2019). Autonomic response to the adult attachment projective in anorexia nervosa. Eat. Weight Disord. 25, 1799–1804. doi: 10.1007/s40519-019-00792-8
Lucini, D., Norbiato, G., Clerici, M., and Pagani, M. (2002). Hemodynamic and autonomic adjustments to real life stress conditions in humans. Hypertension 39, 184–188. doi: 10.1161/hy0102.100784
Luck, P., Mikhailidis, D. P., Dashwood, M. R., Barradas, M. A., Sever, P. S., Dandona, P., et al. (1983). Platelet hyperaggregability and increased alpha-adrenoceptor density in anorexia nervosa. J. Clin. Endocrinol. Metab. 57, 911–914. doi: 10.1210/jcem-57-5-911
Lutz, A. P. C., Schulz, A., Voderholzer, U., Koch, S., Van Dyck, Z., and Vögele, C. (2019). Enhanced cortical processing of cardio-afferent signals in anorexia nervosa. Clin. Neurophysiol. 130, 1620–1627. doi: 10.1016/j.clinph.2019.06.009
Makovac, E., Thayer, J. F., and Ottaviani, C. (2017). A meta-analysis of non-invasive brain stimulation and autonomic functioning: implications for brain-heart pathways to cardiovascular disease. Neurosci. Biobehav. Rev. 74, 330–341. doi: 10.1016/j.neubiorev.2016.05.001
Malpas, S. C. (2010). Sympathetic nervous system overactivity and its role in the development of cardiovascular disease. Physiol. Rev. 90, 513–557. doi: 10.1152/physrev.00007.2009
Mazurak, N., Enck, P., Muth, E., Teufel, M., and Zipfel, S. (2011a). Heart rate variability as a measure of cardiac autonomic function in anorexia nervosa: a review of the literature. Eur. Eat. Disord. Rev. 19, 87–99. doi: 10.1002/erv.1081
Mazurak, N., Stein, J., Kipphan, S., Muth, E. R., Teufel, M., Zipfel, S., et al. (2011b). Heart rate variability in anorexia nervosa and the irritable bowel syndrome. Neurogastroenterol. Motil. 23, e470–478. doi: 10.1111/j.1365-2982.2011.01785.x
Melanson, E. L., Donahoo, W. T., Krantz, M. J., Poirier, P., and Mehler, P. S. (2004). Resting and ambulatory heart rate variability in chronic anorexia nervosa. Am. J. Cardiol. 94, 1217–1220. doi: 10.1016/j.amjcard.2004.07.103
Michael, J. A., and Kaur, M. (2021). The heart-brain connection in depression: can it inform a personalised approach for repetitive transcranial magnetic stimulation (rTMS) treatment? Neurosci. Biobehav. Rev. 127, 136–143. doi: 10.1016/j.neubiorev.2021.04.016
Migliorini, R. H., Garofalo, M., and Kettelhut, I. C. (1997). Increased sympathetic activity in rat white adipose tissue during prolonged fasting. Am. J. Physiol. 272, R656–R661. doi: 10.1152/ajpregu.1997.272.2.R656
Moher, D., Liberati, A., Tetzlaff, J., and Altman, D. G. (2010). Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Int. J. Surg. 8, 336–341. doi: 10.1016/j.ijsu.2010.02.007
Moher D. Liberati A. Tetzlaff J. Altman D. G. The PRISMA Group (2009). Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 6:e1000097. doi: 10.1371/journal.pmed.1000097
Murialdo, G., Casu, M., Falchero, M., Brugnolo, A., Patrone, V., Cerro, P. F., et al. (2007). Alterations in the autonomic control of heart rate variability in patients with anorexia or bulimia nervosa: correlations between sympathovagal activity, clinical features, and leptin levels. J. Endocrinol. Invest. 30, 356–362. doi: 10.1007/BF03346310
Musselman, D. L., Evans, D. L., and Nemeroff, C. B. (1998). The relationship of depression to cardiovascular disease: epidemiology, biology, and treatment. Arch. Gen. Psychiatry 55, 580–592. doi: 10.1001/archpsyc.55.7.580
Nakai, Y., Fujita, M., Nin, K., Noma, S., and Teramukai, S. (2015). Relationship between duration of illness and cardiac autonomic nervous activity in anorexia nervosa. Biopsychosoc. Med. 9:12. doi: 10.1186/s13030-015-0032-6
Nakai, Y., Noma, S., Fukusima, M., Taniguchi, A., and Teramukai, S. (2016). Serum lipid levels in patients with eating disorders. Intern. Med. 55, 1853–1857. doi: 10.2169/internalmedicine.55.5632
Nedvidkova, J., Dostalova, I., Bartak, V., Papezov, H., and Pacak, K. (2004). Increased subcutaneous abdominal tissue norepinephrine levels in patients with anorexia nervosa: an in vivo microdialysis study. Physiol. Res. 53, 409–413.
O'Brien, K. M., and Vincent, N. K. (2003). Psychiatric comorbidity in anorexia and bulimia nervosa: nature, prevalence, and causal relationships. Clin. Psychol. Rev. 23, 57–74. doi: 10.1016/S0272-7358(02)00201-5
Palma, J. A., and Benarroch, E. E. (2014). Neural control of the heart: recent concepts and clinical correlations. Neurology 83, 261–271. doi: 10.1212/WNL.0000000000000605
Palomba, D., Venturini, M., Rausa, M., Contin, S. A., Penolazzi, B., Schumann, R., et al. (2017). Reduced sympathetic activity and dysfunctional metacognition in patients with anorexia nervosa: a preliminary study. J. Evid. Based Psychother. 17:1. doi: 10.24193/jebp.2017.1.1
Papadopoulos, F. C., Ekbom, A., Brandt, L., and Ekselius, L. (2009). Excess mortality, causes of death and prognostic factors in anorexia nervosa. Br. J. Psychiatry 194, 10–17. doi: 10.1192/bjp.bp.108.054742
Patel, J., Coppack, S., Goldstein, D., Miles, J., and Eisenhofer, G. (2002). Norepinephrine spillover from human adipose tissue before and after a 72-hour fast. J. Clin. Endocrinol. Metab. 87, 3373–3377. doi: 10.1210/jcem.87.7.8695
Peng, C. K., Havlin, S., Stanley, H. E., and Goldberger, A. L. (1995). Quantification of scaling exponents and crossover phenomena in nonstationary heartbeat time series. Chaos 5, 82–87. doi: 10.1063/1.166141
Petretta, M., Bonaduce, D., Scalfi, L., De Filippo, E., Marciano, F., Migaux, M. L., et al. (1997). Heart rate variability as a measure of autonomic nervous system function in anorexia nervosa. Clin. Cardiol. 20, 219–224. doi: 10.1002/clc.4960200307
Peyser, D., Scolnick, B., Hildebrandt, T., and Taylor, J. A. (2020). Heart rate variability as a biomarker for anorexia nervosa: a review. Eur. Eat. Disord. Rev. 29, 20–31. doi: 10.1002/erv.2791
Phillipou, A., Music, S., and Lee Rossell, S. (2019). A biopsychosocial proposal to progress the field of anorexia nervosa. Aust. N. Z. J. Psychiatry 53, 1145–1147. doi: 10.1177/0004867419849487
Pike, K. M. (1998). Long-term course of anorexia nervosa: response, relapse, remission, and recovery. Clin. Psychol. Rev. 18, 447–475. doi: 10.1016/S0272-7358(98)00014-2
Pirke, K. M., Kellner, M., Philipp, E., Laessle, R., Krieg, J. C., and Fichter, M. M. (1992). Plasma norepinephrine after a standardized test meal in acute and remitted patients with anorexia nervosa and in healthy controls. Biol. Psychiatry 31, 1074–1077. doi: 10.1016/0006-3223(92)90102-6
Platisa, M. M., Nestorovic, Z., Damjanovic, S., and Gal, V. (2006). Linear and non-linear heart rate variability measures in chronic and acute phase of anorexia nervosa. Clin. Physiol. Funct. Imaging 26, 54–60. doi: 10.1111/j.1475-097X.2005.00653.x
Porges, S. W. (2007). The polyvagal perspective. Biol. Psychol. 74, 116–143. doi: 10.1016/j.biopsycho.2006.06.009
Quintana, D., Alvares, G. A., and Heathers, J. (2016). Guidelines for Reporting Articles on Psychiatry and Heart rate variability (GRAPH): recommendations to advance research communication. Transl. Psychiatry 6:e803. doi: 10.1038/tp.2016.73
Rahman, F., Pechnik, S., Gross, D., Sewell, L., and Goldstein, D. S. (2011). Low frequency power of heart rate variability reflects baroreflex function, not cardiac sympathetic innervation. Clin. Auton. Res. 21, 133–141. doi: 10.1007/s10286-010-0098-y
Ramírez-Marrero, F. A., Charkoudian, N., Zhong, L., Hesse, C., and Eisenach, J. H. (2007). Balance between sympathetic response to head-up tilt and cardiac vagal factors in healthy humans. Clin. Auton. Res. 17, 227–230. doi: 10.1007/s10286-007-0427-y
Rechlin, T., Weis, M., Ott, C., Bleichner, F., and Joraschky, P. (1998). Alterations of autonomic cardiac control in anorexia nervosa. Biol. Psychiatry 43, 358–363. doi: 10.1016/S0006-3223(97)00026-7
Riederer, P., Toifl, K., and Kruzik, P. (1982). Excretion of biogenic amine metabolites in anorexia nervosa. Clin. Chim. Acta 123, 27–32. doi: 10.1016/0009-8981(82)90109-7
Roche, F., Estour, B., Kadem, M., Millot, L., Pichot, V., Duverney, D., et al. (2004). Alteration of the QT rate dependence in anorexia nervosa. Pacing Clin. Electrophysiol. 27, 1099–1104. doi: 10.1111/j.1540-8159.2004.00591.x
Rommel, D., Nandrino, J. L., De Jonckheere, J., Swierczek, M., Dodin, V., and Logier, R. (2015). Maintenance of parasympathetic inhibition following emotional induction in patients with restrictive type anorexia nervosa. Psychiatry Res. 225, 651–657. doi: 10.1016/j.psychres.2014.11.030
Russell, G. F. (1970). Anorexia nervosa: its identity as an illness and its treatment. Mod. Trends Psychol. Med. 2, 131–164.
Russell, J., Hijazi, S., Edington, L., Spence, I., and Jelinek, H. F. (2008). Cardiovascular complications and sudden death associated with eating disorders. Internet J. Cardiovasc. Res. 7.
Sachs, K. V., Harnke, B., Mehler, P. S., and Krantz, M. J. (2016). Cardiovascular complications of anorexia nervosa: a systematic review. Int. J. Eat. Disord. 49, 238–248. doi: 10.1002/eat.22481
Shamim, T., Golden, N. H., Arden, M., Filiberto, L., and Shenker, I. R. (2003). Resolution of vital sign instability: an objective measure of medical stability in anorexia nervosa. J. Adolesc. Health 32, 73–77. doi: 10.1016/S1054-139X(02)00533-5
Shinba, T., Kariya, N., Matsui, Y., Ozawa, N., Matsuda, Y., and Yamamoto, K. I. (2008). Decrease in heart rate variability response to task is related to anxiety and depressiveness in normal subjects. Psychiatry Clin. Neurosci. 62, 603–609. doi: 10.1111/j.1440-1819.2008.01855.x
Smink, F. R., Van Hoeken, D., Oldehinkel, A. J., and Hoek, H. W. (2014). Prevalence and severity of DSM-5 eating disorders in a community cohort of adolescents. Int. J. Eat. Disord. 47, 610–619. doi: 10.1002/eat.22316
Smythe, J., Colebourn, C., Prisco, L., Petrinic, T., and Leeson, P. (2020). Cardiac abnormalities identified with echocardiography in anorexia nervosa: systematic review and meta-analysis. Br. J. Psychiatry. 1–10. doi: 10.1192/bjp.2020.1
Spaulding-Barclay, M. A., Stern, J., and Mehler, P. S. (2016). Cardiac changes in anorexia nervosa. Cardiol. Young 26, 623–628. doi: 10.1017/S104795111500267X
Steinglass, J. E., and Walsh, B. T. (2016). Neurobiological model of the persistence of anorexia nervosa. J. Eat. Disord. 4:19. doi: 10.1186/s40337-016-0106-2
Stheneur, C., Bergeron, S., and Lapeyraque, A. L. (2014). Renal complications in anorexia nervosa. Eat. Weight Disord. 19, 455–460. doi: 10.1007/s40519-014-0138-z
Tak, L. M., Riese, H., De Bock, G. H., Manoharan, A., Kok, I. C., and Rosmalen, J. G. M. (2009). As good as it gets? A meta-analysis and systematic review of methodological quality of heart rate variability studies in functional somatic disorders. Biol. Psychol. 82, 101–110. doi: 10.1016/j.biopsycho.2009.05.002
Takimoto, Y., Yoshiuchi, K., Ishizawa, T., Yamamoto, Y., and Akabayashi, A. (2014). Autonomic dysfunction responses to head-up tilt in anorexia nervosa. Clin. Auton. Res. 24, 175–181. doi: 10.1007/s10286-014-0250-1
Task Force of The European Society of Cardiology and The North American Society of Pacing and Electrophysiology (1996). Heart rate variability. Standards of measurement, physiological interpretation, and clinical use. Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Eur. Heart J. 17, 354–381.
Thayer, J. F., Åhs, F., Fredrikson, M., Sollers Iii, J. J., and Wager, T. D. (2012). A meta-analysis of heart rate variability and neuroimaging studies: implications for heart rate variability as a marker of stress and health. Neurosci. Biobehav. Rev. 36, 747–756. doi: 10.1016/j.neubiorev.2011.11.009
Tjalf, Z., and Timo, S. (2019). The investigation of the cardiovascular and sudomotor autonomic nervous system—a review. Front. Neurol. 10:53. doi: 10.3389/fneur.2019.00053
Tonhajzerova, I., Mestanikova, A., Jurko, A. Jr., Grendar, M., Langer, P., Ondrejka, I., et al. (2020). Arterial stiffness and haemodynamic regulation in adolescent anorexia nervosa versus obesity. Appl. Physiol. Nutr. Metab. 45, 81–90. doi: 10.1139/apnm-2018-0867
Van Binsbergen, C. J., Odink, J., Van Der Beek, E. J., Westenberg, H. M., and Bennink, H. J. (1991). Biogenic amines in anorexia nervosa: circadian rhythm in urinary excretion and influence of posture and physical task load on plasma catecholamines. Psychosom. Med. 53, 440–452. doi: 10.1097/00006842-199107000-00009
Vetrugno, R., Liguori, R., Cortelli, P., and Montagna, P. (2003). Sympathetic skin response. Clin. Auton. Res. 13, 256–270. doi: 10.1007/s10286-003-0107-5
Vignaud, M., Constantin, J. M., Ruivard, M., Villemeyre-Plane, M., Futier, E., Bazin, J. E., et al. (2010). Refeeding syndrome influences outcome of anorexia nervosa patients in intensive care unit: an observational study. Crit. Care 14:R172. doi: 10.1186/cc9274
Vigo, D. E., Castro, M. N., Dorpinghaus, A., Weidema, H., Cardinali, D. P., Siri, L. N., et al. (2008). Nonlinear analysis of heart rate variability in patients with eating disorders. World J. Biol. Psychiatry 9, 183–189. doi: 10.1080/15622970701261604
Webb, S., Adgey, A., and Pantridge, J. (1972). Autonomic disturbance at onset of acute myocardial infarction. Br. Med. J. 3, 89–92. doi: 10.1136/bmj.3.5818.89
Wells, G. A., Shea, B., O'connell, D., Peterson, J., Welch, V., Losos, M., et al. (2006). The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Nonrandomised Studies in Meta-Analyses. Available online at: http://www.ohri.ca/programs/clinical_epidemiology/nos_manual.pdf
Westerhof, B. E., Gisolf, J., Karemaker, J. M., Wesseling, K. H., Secher, N. H., and Van Lieshout, J. J. (2006). Time course analysis of baroreflex sensitivity during postural stress. Am. J. Physiol. Heart Circ. Physiol. 291, H2864–H2874. doi: 10.1152/ajpheart.01024.2005
Wu, Y., Nozaki, T., Inamitsu, T., and Kubo, C. (2004). Physical and psychological factors influencing heart rate variability in anorexia nervosa. Eat. Weight Disord. 9, 296–299. doi: 10.1007/BF03325085
Keywords: anorexia nervosa, eating disorders, autonomic nervous system, sympathetic nervous system, parasympathetic nervous system, heartrate variability, noradrenaline, orthostatic response
Citation: Jenkins ZM, Eikelis N, Phillipou A, Castle DJ, Wilding HE and Lambert EA (2021) Autonomic Nervous System Function in Anorexia Nervosa: A Systematic Review. Front. Neurosci. 15:682208. doi: 10.3389/fnins.2021.682208
Received: 18 March 2021; Accepted: 18 May 2021;
Published: 28 June 2021.
Edited by:
Valdir Andrade Braga, Federal University of Paraíba, BrazilReviewed by:
Paola Sandroni, Mayo Clinic, United StatesMichal Javorka, Comenius University, Slovakia
Copyright © 2021 Jenkins, Eikelis, Phillipou, Castle, Wilding and Lambert. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zoe M. Jenkins, em9lLmplbmtpbnNAc3ZoYS5vcmcuYXU=
 Zoe M. Jenkins
Zoe M. Jenkins Nina Eikelis1
Nina Eikelis1 Andrea Phillipou
Andrea Phillipou David J. Castle
David J. Castle Helen E. Wilding
Helen E. Wilding Elisabeth A. Lambert
Elisabeth A. Lambert