
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Neurosci. , 26 January 2021
Sec. Neuroendocrine Science
Volume 14 - 2020 | https://doi.org/10.3389/fnins.2020.629003
Stress can increase the release of corticotropin-releasing factor (CRF) in the hypothalamus, resulting in attenuation of gastric motor functions. In contrast, central neuropeptide Y (NPY) can reduce the biological actions of CRF, and in turn weaken stress responses. Although electroacupuncture (EA) at stomach 36 (ST-36) has been shown to have anti-stress effects, its mechanism has not yet been investigated. The effect of EA at ST-36 on the hypothalamus-pituitary-adrenal (HPA) axis and gastrointestinal motility in chronic complicated stress (CCS) conditions have not been studied and the inhibitory mechanism of NPY on CRF through the gamma-aminobutyric acid (GABA)A receptor need to be further investigated. A CCS rat model was set up, EA at ST-36 was applied to the bilateral hind limbs every day prior to the stress loading. Further, a GABAA receptor antagonist was intracerebroventricularly (ICV) injected daily. Central CRF and NPY expression levels were studied, serum corticosterone and NPY concentrations were analyzed, and gastric motor functions were assessed. CCS rats showed significantly elevated CRF expression and corticosterone levels, which resulted in inhibited gastric motor functions. EA at ST-36 significantly increased central NPY mRNA expression and reduced central CRF mRNA expression as well as the plasma corticosterone level, helping to restore gastric motor function. However, ICV administration of the GABAA receptor antagonist significantly abolished these effects. EA at ST-36 upregulates the hypothalamic NPY system. NPY may, through the GABAA receptor, significantly antagonize the overexpressed central CRF and attenuate the HPA axis activities in CCS conditions, exerting influences and helping to restore gastric motor function.
The pathogeneses of functional gastrointestinal disorders (FGIDs), such as functional dyspepsia (FD), are highly related to stress in humans (Levy et al., 2006; Talley et al., 2015). Gastrointestinal (GI) dysmotility might develop as a result of the accumulation of repeated or continuous stress in some individuals.
Corticotropin-releasing factor (CRF) in the central nervous system plays a significant role in mediation of stress-induced GI dysmotility (Lenz et al., 1988; Tache and Bonaz, 2007). Acute restraint stress (ARS) inhibits gastric motility via sympathetic pathways and central CRF type 2 (CRF2) receptors (Nakade et al., 2005). In the chronic complicated stress (CCS) rat model, delayed gastric emptying (GE) and attenuated gastric contraction were also observed when rats received different types of stressors for many days, with increased CRF messenger RNA (mRNA) expression in the paraventricular nucleus (PVN) of the hypothalamus, which resulted in increased hypothalamus-pituitary-adrenal (HPA) axis activities (Zheng et al., 2010). Although peripheral CRF receptor antagonists have been developed, the efficiency of the antagonists to treat stress-induced GI dysmotility remains to be further investigated (Kormos and Gaszner, 2013; Bali et al., 2014).
Acupuncture has widely been used to treat numerous diseases for over 3,000 years across Asia and has most recently gained popularity as an alternative or complementary treatment in Western cultures (Campbell, 2002; Hurtak, 2002). According to the World Health Organization (2003), acupuncture is useful as adjunct therapy in more than 50 disorders. In particular, acupuncture and electroacupuncture (EA) at acupoint Zusanli, stomach 36 (ST-36) has been used in patients for a variety of conditions including GI diseases (Hurtak, 2002). ST-36 is one of the most commonly used acupoints for FGIDs, including FD and irritable bowel syndrome (IBS) (Chan et al., 1997; Diehl, 1999; Fireman et al., 2001). Studies have shown that EA at ST-36 can attenuate the HPA axis activity and sympathetic nervous system (SNS) pathway, and in turn reduce the stress responses in patients and rats (Middlekauff et al., 2002; Yang et al., 2002; Eshkevari et al., 2013; Zhou et al., 2017). Interestingly, recent studies also found that EA can increases hypothalamic NPY expression and reduce the stress responses in chronic stress conditions (Lee et al., 2009; Sun et al., 2015). Neuropeptide Y (NPY), belongs to the pancreatic polypeptide family, a 36 amino acids peptide, which is expressed at high levels within the mammalian nervous system (Adrian et al., 1983; Allen et al., 1983). NPY is also synthesized and released from peripheral sympathetic neurons (Heilig and Thorsell, 2002; Heilig, 2004). Several studies have shown that NPY is involved in stress related disorders, such as depression, anxiety, and post-traumatic stress disorder (PTSD) (Rasmusson et al., 2010; Sabban et al., 2016).
Neuropeptide Y inhibits the biological actions of CRF and is involved in the termination of the stress and anxiety response (Morales-Medina et al., 2010). Endogenous NPY is potently anxiolytic, acting as a buffer that promotes behavioral adaptation to cope with stress (Primeaux et al., 2005; Reichmann and Holzer, 2016). The actions of NPY are mediated through at least five G protein coupled receptors (Dumont et al., 1998). The anxiolytic effect of NPY is mediated primarily through the Y1 receptor (Morales-Medina et al., 2010; Reichmann and Holzer, 2016). Our recent study also showed that the central NPY via the Y1 receptor plays an important role in mediating the adaptation mechanism against repeated restraint stress in rats (Yang et al., 2018).
Furthermore, in the study of the inhibition mechanism of NPY on CRF, it was found that central NPY could regulate the excitability of the central nuclei of the amygdala (CeA) through the gamma-aminobutyric acid (GABA)A receptor and improves the adaptability of organisms to stress responses (Molosh et al., 2013). In addition, NPY and GABA were co-expressed in the arcuate nucleus of the hypothalamus (ARC) neurons and projected to PVN (Muroya et al., 2005). The regulation of NPY on the feeding function is also mediated by the GABAA receptor (Pu et al., 1999). Therefore, it is necessary to further explore the regulatory and inhibitory mechanism of NPY on CRF through the GABAA receptor under chronic stress.
The present study aimed to evaluate, using a CCS rat model, whether EA at ST-36 could up-regulate NPY system activity, and in turn attenuate the HPA axis activity in CCS conditions. It was also evaluated whether their effects help to restore GI dysmotility. The inhibitory effect of NPY on CRF through the GABAA receptor system was further explored, to clarify the mechanisms of stimulatory effects of EA on GI dysmotility.
All research procedures were carried out in accordance with the guidelines for the ethical review of laboratory animal welfare People’s Republic of China National Standard GB/T 35892-2018 and approved by the Animal Care and Use Committee of China Medical University. Efforts were made to minimize animal suffering and the number of animals used.
Male Sprague-Dawley rats (adult, 260–300 g, obtained from the laboratory animal center of China Medical University) were housed in individual cages under conditions (22–24°C, 12:12 h light/dark cycle) with ad libitum access to food and water. All experiments were started at 9 AM each day. The approval reference number is CMU2019225.
The rats (n = 6–8) received different types of stressors for 7 consecutive days, as previously reported (Zheng et al., 2010; Yang et al., 2019). The stress paradigms used were fasten restraint stress (FRS), force swimming stress (FSS), cold restraint stress (CRS), and water avoidance stress (WAS). The specific conditions for each type of stress are as follows:
(1) FRS: rats were placed on a wooden plate with their trunks wrapped in a confining harness for 90 min. For the control group (n = 6–8), the rats were housed in original individual cages for 90 min, but were given limited access to food and water.
(2) FSS: rats were placed individually in a plastic tank (52 × 37 × 20 cm) filled with room temperature (RT) water to a depth of 15 cm for 20 min. The depth of the water forced the animal to swim or float without their hindlimbs touching the bottom of the tank. Control rats were placed individually in a waterless container tank for 20 min.
(3) CRS: rats were kept restrained at 4°C for 45 min. Control rats were kept at RT for 45 min.
(4) WAS: rats were placed on a platform (6 × 8 cm) in the middle of a plastic container (50 × 30 × 20 cm) filled with RT water to 1 cm below the height of the platform for 60 min. Control rats were placed on the same platform in a waterless container for 60 min.
Rats were exposed to different stressors each day for 7 days:
(1) Day 1: FRS [90 min, ante meridiem (AM)], FSS [20 min, post meridiem (PM)]; Day 2: CRS (45 min, AM); Day 3: FRS (90 min, AM), WAS (60 min); Day 4: CRS (45 min, AM); Day 5: FRS (90 min, AM), FSS (20 min, PM); Day 6: CRS (45 min, AM); Day 7: FRS (90 min, AM).
The electro-acupuncture (EA) ST-36 in a rat is located at the proximal one-fifth of the craniolateral surface of the leg distal to the head of the tibia, in a depression between the muscles of the cranial tibia and long digital extensor, bilaterally. These points were identified using rat mapping for acupuncture, as previously reported (Iwa et al., 2006). To avoid the spontaneous removal of inserted electrodes from the rat body, thin stainless steel needles 34G (0.22 mm) and 1 inch (25 mm) (Millennia, Shanghai, China) were bent in a hook shape, as previously reported (Iwa et al., 2006; Yoshimoto et al., 2012). Bilaterally inserted needles at the hind limbs were connected to an EA machine (ITO-ES320, Japan) and stimulated by electricity (10 Hz, 3 V, 0.5 ms) for 30 min. EA was performed every day prior to the stress loading.
The sham group received non-acupuncture map points EA treatment on the back of the animal (EA at back), 2 cm lateral to the tail region, which was then connected to the EA machine and stimulated by electricity for 30 min, every day prior to the stress loading.
To exclude the influence of anesthesia and restraint conditions, rats were allowed to move freely in the cage during the EA procedure, as reported previously (Iwa et al., 2006; Yoshimoto et al., 2012). The performance of the rats was monitored, and the wires connecting with the inserted needles were fixed on the back by tape to prevent the researchers from being bitten.
The experimental rats were euthanized immediately by pentobarbital sodium (Sigma-Aldrich, St. Louis, MO, United States, 200 mg/kg intraperitoneal injection) after FRS, at the 7th day of CCS loading.
At the time of death, trunk blood was collected immediately via a cardiac puncture, and then the samples were allowed to clot in tubes and were centrifuged at 4°C for 10 min at 3,000 rpm to separate the serum. The serum fraction was stored at −80°C for further analysis. Corticosterone concentrations were measured by enzyme-linked immunosorbent assay (ELISA) using a corticosterone ELISA kit (Cat# ADI-900-097, Enzo Life Sciences, Plymouth meeting, PA, United States, detection level 32–20,000 pg/ml, sensitivity 27.0 pg/ml), as previously reported (Kinn Rød et al., 2017). NPY concentrations were measured by NPY EIA kit (Cat# EK-049-03, Phoenix Pharmaceuticals, Belmont, CA, United States, detection level 0.09–1.43 ng/ml, sensitivity 0.09 ng/ml), as previously reported (Serova et al., 2013; Yang et al., 2018). All procedures were carried out according to the manufacturer’s instructions. The experiments were run in triplicate and the results represent their average values.
The rat brain tissue micropunching technique was applied to acquire hypothalamus tissue samples from specific regions with micro-punchers. Briefly, after stress loading, the experimental rats were euthanized, and the brains were removed immediately and cut into 450 μm coronal sections. Punches were collected from the left and right PVN (1.8 mm caudal to bregma; 0.4 mm lateral to midline; 7.6 mm ventral to the brain surface), as previously reported (Zheng et al., 2010; Yang et al., 2018). All coordinates were based on the rat brain atlas and hypothalamic images previously reported (Paxinos and Watson, 1997).
Samples were stored at −80°C until use. Total RNA was extracted from the brain tissues using Trizol (Invitrogen, Carlsbad, CA, United States), and trace DNA contamination was removed by DNase digestion (Promega, Madison, WI, United States). Complementary DNA was synthesized from 3 μg total RNA by use of Superscript III reverse transcriptase (Invitrogen, Carlsbad, CA, United States).
The following primers were designed to amplify rat CRF: sense primer 5′-CCAGGGCAGAGCAGTTAGCT-3′, antisense primer 5′-CAAGCGCAACATTTCATTTCC-3′. The following primers were designed to amplify rat NPY: sense primer 5′-CAGAGGCGCCCAGAGCAG-3′, antisense primer 5′-CAGCCCCATTCGTTTGTTACC-3′. For an internal control, the following primers were designed to amplify rat β–actin: sense primer 5′-TGGCACCACACCTTCTACAATGAG-3′, antisense primer 5′-GGGTCATCTTTTCACGGTTGG-3′, as previously reported (Zheng et al., 2010; Yang et al., 2018).
Quantitative polymerase chain reaction (PCR) was performed using SYBR Premix Ex Taq (TaKaRa Biotech, Dalian, China). Amplification reactions were performed using the ABI 7500 Real-time PCR instrument (Applied Biosystems, San Mateo, CA, United States). Initial template denaturation was performed for 30 s at 95°C. The cycle profiles were programmed as follows: 5 s at 95°C (denaturation), 20 s at 60°C (annealing), and 15 s at 72°C (extension). Forty-five cycles of the profile were run, and the final cooling step was continued for 30 s at 40°C. Quantitative measurement of each mRNA sample was achieved by establishing a linear amplification curve from serial dilutions of each plasmid containing the amplicon sequence. Amplicon size and specificity were confirmed by melting curve analysis. The relative amount of each mRNA was normalized by the amount of β-actin mRNA, as previously reported (Zheng et al., 2010; Yang et al., 2018).
The rats were anesthetized with isoflurane (5% for induction; 2% for maintenance in pure oxygen gas; RWD life science, Shenzhen, China), and placed in a stereotaxic apparatus (RWD life science, Shenzhen, China). After the skin and muscles of the head were dissected, a 24-gage plastic sterile cannula (Plastics One, United States) was implanted into the right lateral ventricle (1.5 mm caudal, 2 mm lateral from the bregma; 6 mm ventral from the skull surface), as previously reported (Zheng et al., 2010; Yang et al., 2018). The cannula was fixed with cement (Kyowa, Tokyo, Japan) and acrylic resin (Shofu, San Marcos, CA, United States). A stainless-steel obturator (Plastics One, Roanoke, VA, United States), was inserted to maintain cannula patency. After cannulation, the rats were allowed to recover for 1 week.
To investigate whether the GABAA receptor is involved in NPY mediated restoration following CCS, Bicuculline Methiodide (BMI, Sigma Aldrich, MO, United States) (100 ng/5 μl, ICV) was injected daily, 15 min prior to the stress loading. Saline (5 μl, ICV) was injected daily for the controls. It has been shown that ICV administration of BMI (100 ng) was effective in the antagonization of GABAA receptor subtypes in rats, as previously reported (Li et al., 2006; Bülbül et al., 2011).
At the end of the experiment, the implantation site of the ICV-cannula was confirmed by the presence of Evans blue (Sigma-Aldrich, St. Louis, MO, United States, 5%; 1 μl) after injection via the cannula, as previously reported (Zheng et al., 2010; Yang et al., 2018).
Rats were anesthetized with isoflurane (2%). Strain gage transducers were implanted on the antrum to record gastric contractions. All wires were tunneled subcutaneously to exit at the back of the rat’s neck and were protected by a protective jacket (Star Medical, Tokyo, Japan). The abdominal wall was closed, and the rats were housed individually with access to a standard diet and tap water. After 7 days, the rats completely recovered from the surgery, including their body weight and daily food intake. Rats on a fixed-feeding schedule (food administered 22-6 PM daily) were monitored from 8 AM to 4 PM for gastric motility, as previously reported (Zheng et al., 2010; Yang et al., 2018): The wires from the transducers were connected to a recording system (Power Lab 8SP; AD Instruments, Colorado Springs, CO, United States). Gastric contractions were monitored before, during, and after restraint stress. Quantification of gastric motility was studied by the calculating motility index (MI).
MI was equivalent to the area under the curve of the motility recording.
The MI was defined as MI log e (sum of amplitudes × total number of contraction waves + 1) which is equivalent to the area under the contractility recording curve and the baseline. MI was calculated using a computer-assisted system (Power Lab; AD Instruments, Colorado Springs, CO, United States), as previously reported (Zheng et al., 2010; Yang et al., 2018).
Rats were fasted for 24 h prior to the measurement of GE. Preweighed standard rodent pellets (1.6 g) were given, and the rats that did not consume 1.6 g of food within 10 min were excluded from the study. After feeding, in the control group, rats were put back into their original cages for 90 min, but with limited access to food and water. The rats were then euthanized by pentobarbital sodium. Immediately after finishing the feeding, the rats in the stress group were subjected to restraint stress for 90 min. The experimental rats were then euthanized as mentioned above. The stomach was surgically isolated and removed. The gastric contents were recovered from the stomach, dried, and weighed. Solid GE was calculated according to the following formula, as previously described (Zheng et al., 2010; Yang et al., 2018):
To study the effects of EA at ST-36 in the CCS rat model, EA was performed every day prior to the stress loading. Non-acupuncture map points with EA treatment on the back of the animal (EA at back) made up the sham group, and NS (non-stressed) rats were the controls. Central CRF and NPY mRNA expression (in PVN) were analyzed, serum corticosterone and NPY concentrations were measured, gastric motor function was evaluated by a motility recording system, and GE was measured.
The inhibitory mechanism of NPY on CRF via the GABAA receptor under the CCS condition was studied. In ICV cannulated rats, the GABAA receptor antagonist BMI was injected daily, 15 min prior to EA at ST-36. Non-acupuncture map points on the back of the rats (EA at back), made up the sham group. The rats were then subjected to the CCS stress loading, and in NS rats, saline (5 μl, ICV) was injected daily as a control. Central CRF and NPY mRNA expression (in PVN) were analyzed, serum corticosterone and NPY concentrations were measured, gastric motor function was evaluated by motility recording system, and GE was measured.
An analysis was performed using SPSS 20.0 statistical software (SPSS Inc., Chicago, IL, United States). Results were shown as mean ± standard error (SEM). Statistical analyses were performed using a two-way classification ANOVA with Tukey’s post hoc tests to determine the significant interaction between different stress groups and drug treatment. Differences with P < 0.05 were considered statistically significant.
In the NS groups, CRF mRNA expression in the PVN showed very low levels. Both the sham group (EA at back) and EA at ST-36 did not change the CRF mRNA expression significantly. In the CCS groups, CRF mRNA expression increased significantly compared to that of NS rats (n = 6). The sham group (EA at back) did not change the CRF mRNA expression significantly, however, the EA at ST-36 significantly decreased the CRF mRNA expression compared to that of the CCS non-treated group (n = 6, P < 0.05, Figure 1A).
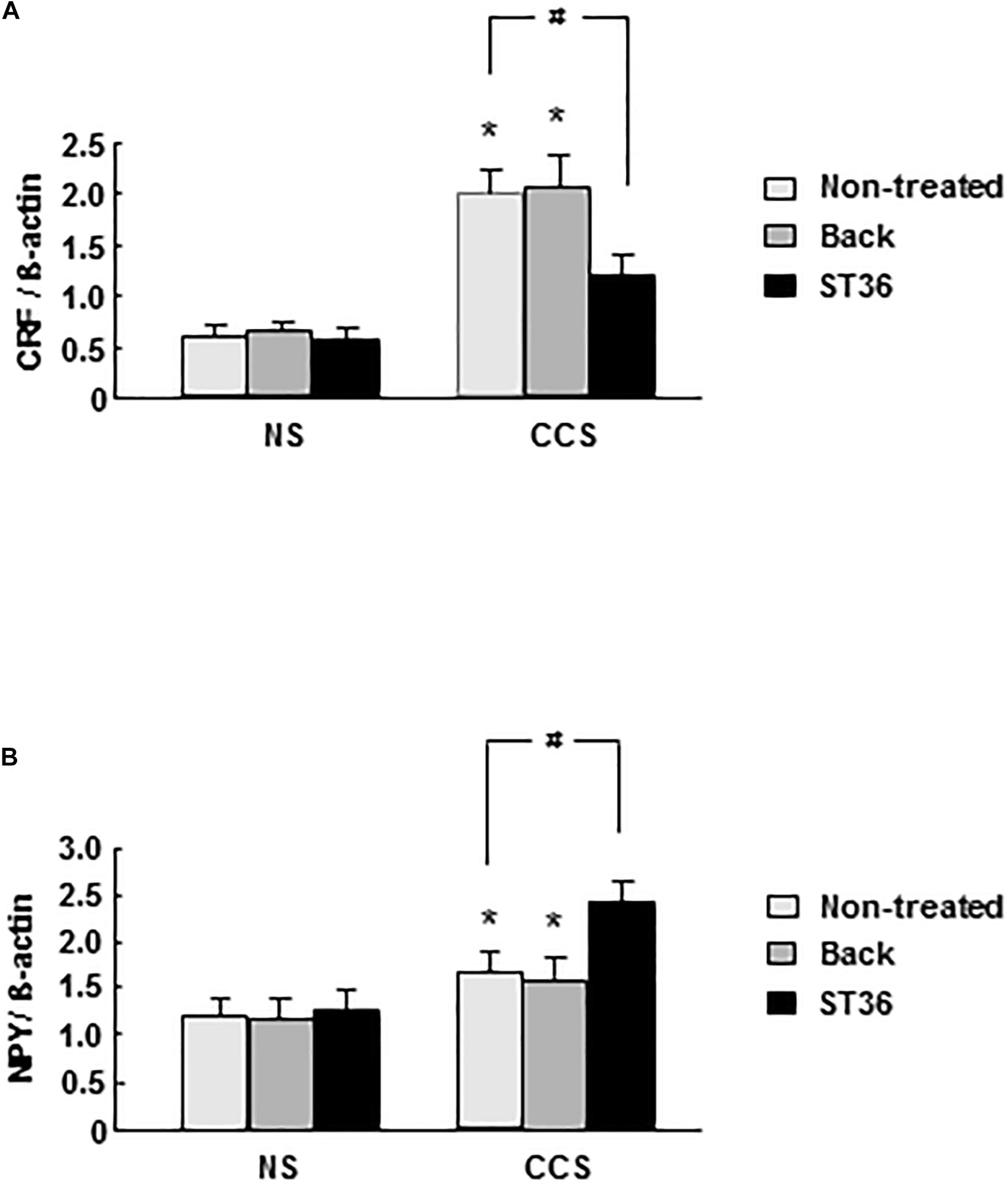
Figure 1. Effects of EA on central CRF (A) and NPY (B) mRNA expression in response to CCS. (A) In the non-stressed (NS) groups, both EA at back and at ST-36 had no effect on the CRF mRNA expression. In the CCS groups, CRF mRNA expression increased significantly (non-treated). EA at back did not change the CRF mRNA expression significantly, however, the EA at ST-36 significantly decreased the CRF mRNA expression. (B) In the NS groups, both EA at back and at ST-36 had no effect on the NPY mRNA expression. In the CCS groups, NPY mRNA expression increased significantly. EA at back did not change the CRF mRNA expression significantly, however, the EA at ST-36 further significantly increased the NPY mRNA expression. The mRNA expression was standardized with the ratio of internal control of β-actin (n = 6, *P < 0.05 compared with NS non-treated group, #P < 0.05 compared with CCS non-treated group).
In the NS groups, NPY mRNA expression in the PVN showed low levels. Both the sham group (EA at back) and EA at ST-36 did not change the NPY mRNA expression significantly. In the CCS groups, NPY mRNA expression increased significantly compared to that of NS rats (n = 6). The sham group (EA at back) did not change the NPY mRNA expression significantly, however, the EA at ST-36 further significantly increased the NPY mRNA expression compared to that of the CCS non-treated group (n = 6, P < 0.05, Figure 1B).
In the NS groups, serum corticosterone concentration showed low levels (63.6 ± 6.6 ng/ml, n = 6). Both the sham group (EA at back) and EA at ST-36 did not change the corticosterone level significantly (66.5 ± 7.3 and 62.8 ± 7.5 ng/ml, respectively, n = 6). In the CCS groups, serum corticosterone concentration significantly increased to 135.4 ± 13.6 ng/ml, compared to that of NS rats (n = 6, P < 0.025). The sham group (EA at back) did not change the corticosterone level significantly (143.3 ± 12.5 ng/ml, n = 6). However, the EA at ST-36 significantly decreased the corticosterone level to 89.7 ± 9.6 ng/ml, compared to that of CCS non-treated rats (n = 6, P < 0.05, Figure 2A).
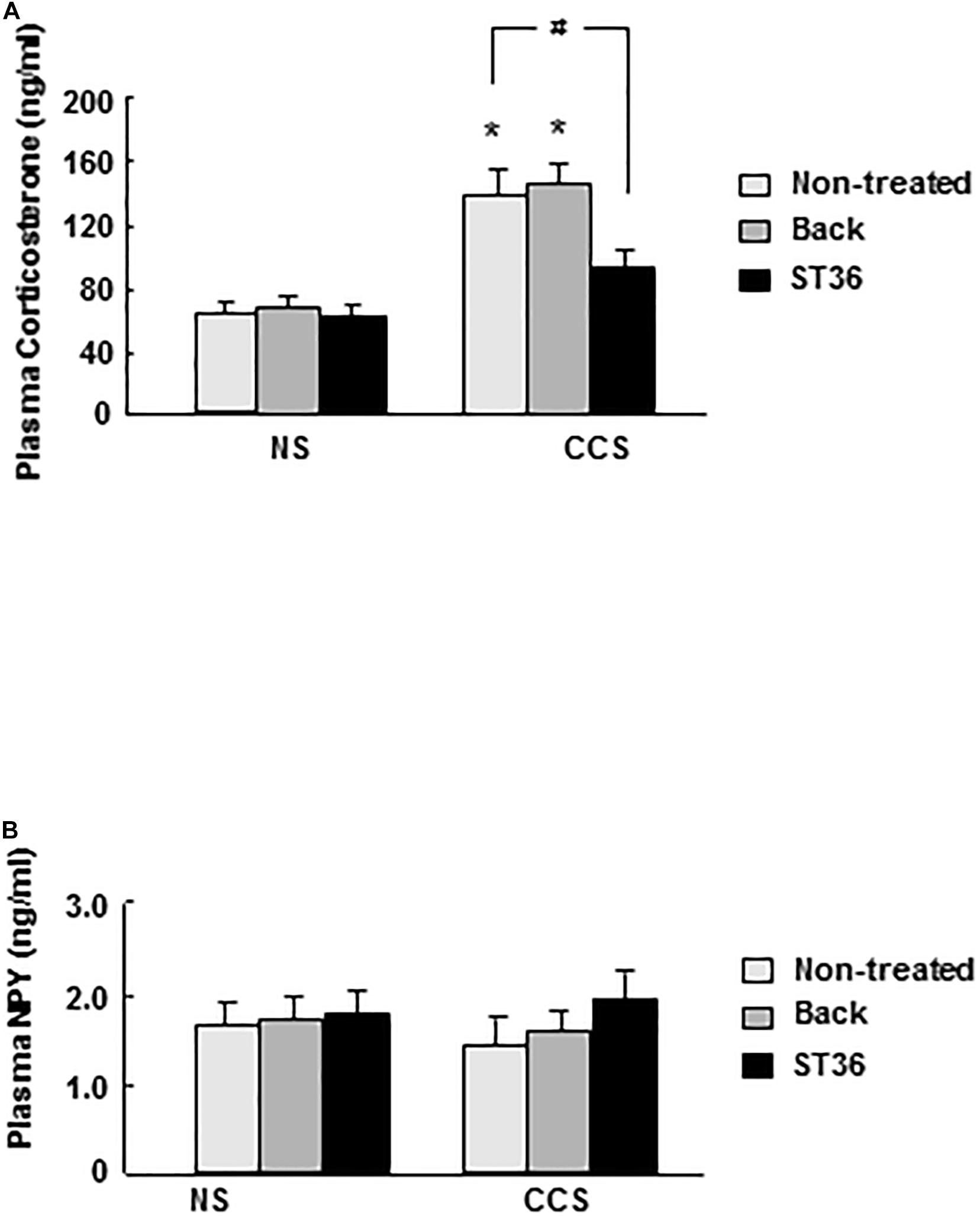
Figure 2. Effects of EA on serum corticosterone (A) and NPY (B) levels in response to CCS. (A) In the non-stressed (NS) groups, both EA at back and at ST-36 had no effect on the corticosterone level. In the CCS groups, corticosterone level increased significantly (non-treated). EA at back did not change the corticosterone level significantly, however, EA at ST-36 significantly decreased the corticosterone level. (B) In the non-stressed (NS) groups, both EA at back and at ST-36 had no effect on the NPY level. In the CCS groups, NPY level decreased, but not shown significantly changed (non-treated). Both EA at back and EA at ST-36 also did not change the NPY level significantly (n = 6, *P < 0.05 compared with NS non-treated group, #P < 0.05 compared with CCS non-treated group).
In the NS groups, serum NPY concentration showed basal levels (1.73 ± 0.13 ng/ml, n = 6). Both the sham group (EA at back) and EA at ST-36 did not change the NPY level significantly (1.78 ± 0.13 and 1.83 ± 0.15 ng, respectively, n = 6). In the CCS groups, serum NPY level decreased to 1.43 ± 0.18 ng/ml but did not show significant change (n = 6). The sham group (EA at back) did not change the serum NPY level significantly (1.69 ± 0.11 ng/ml, n = 6). The EA at ST-36 also did not change the serum NPY level significantly (1.99 ± 0.23 ng/ml, n = 6) compared to that of the CCS non-treated group (n = 6, P < 0.05, Figure 2B).
In the NS groups, regular gastric phase III-like contractions were observed in fixed-fed rats. Both the sham group (EA at back) and EA at ST-36 had no effects on the amplitude and frequency of gastric phase III-like contractions (data not shown). In the CCS groups, restraint stress abolished gastric phase III-like contractions. The sham group (EA at back) had no effects on the amplitude and frequency of gastric phase III-like contractions. However, the EA at ST-36 helped to partially restore the gastric phase III-like contractions in the CCS groups (Figure 3A).
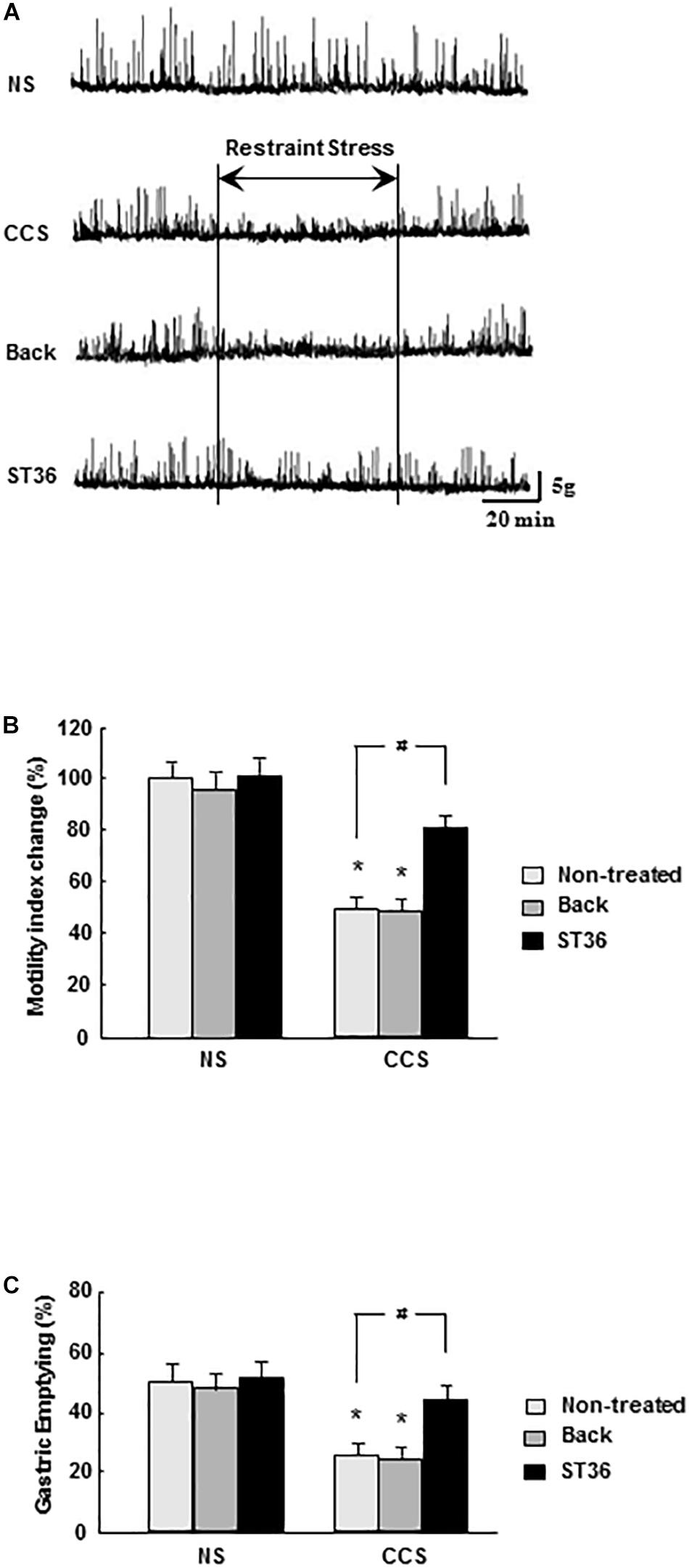
Figure 3. Effects of EA on gastric motility (A), gastric MI changes (B), and GE (C) in response to CCS. (A) The gastric phase III-like contractions in the non-stressed groups (NS). EA at back and EA at ST-36 had no effect on the gastric contractions (data not shown). CCS abolished gastric phase III-like contractions in the non-treated group. EA at back had no effects on the gastric contractions. However, the EA at ST-36 helped to partially restore the gastric phase III-like contractions. (B) In the NS group, EA at back and EA at ST-36 did not significantly alter the gastric MI change. In the CCS groups, gastric MI change was significantly decreased (non-treated). EA at back did not alter the gastric MI change significantly, however, the EA at ST-36 significantly increased the MI change. (C) In the NS groups, EA at back and EA at ST-36 did not significantly change the GE. In the CCS groups, the GE was significantly decreased. EA at back did not change the GE significantly, however, the EA at ST-36 significantly increased the GE (n = 6, *P < 0.05 compared with NS non-treated group, #P < 0.05 compared with CCS non-treated group).
Each recorded experiment was individually repeated at least three times and similar results were obtained (n = 4).
In the NS groups, the gastric MI change was 100 ± 8% (n = 6). Both the sham group (EA at back) and EA at ST-36 did not alter the gastric MI change significantly (96 ± 8% and 102 ± 6%, respectively, n = 6). In the CCS groups, the gastric MI change was significantly decreased to 51 ± 5% (P < 0.05, n = 6). The sham group (EA at back) did not significantly change the gastric MI change (49 ± 5%, n = 6). However, the EA at ST-36 significantly helped to restore the gastric MI change to 80 ± 5% (P < 0.05, n = 6, Figure 3B).
In the NS groups, the GE was 51.4 ± 2.9% (n = 6). Both the sham group (EA at back) and EA at ST-36 did not alter the GE significantly (48.6 ± 2.9% and 53.4 ± 3.5%, respectively, n = 6). In the CCS groups, the GE was significantly decreased to 25.4 ± 2.2% (P < 0.05, n = 6). The sham group (EA at back) did not significantly change GE (24.7 ± 2.5%, n = 6), however, the EA at ST-36 significantly increased the GE to 45.9 ± 2.8% (P < 0.05, n = 6, Figure 3C).
In the NS groups, CRF mRNA expression showed low levels (EA at back, and saline 5 μl ICV administered as a control), however, the combination of EA at back or ST-36 with ICV administered saline or BMI did not change the CRF mRNA expression significantly (n = 6). In the CCS conditions, the CRF mRNA expression in the sham group (EA at back, and saline ICV administered) was significantly elevated. ICV administered BMI (100 ng/5 μl, 15 min prior to stress the loading for 7 consecutive days) did not change the CRF mRNA expression significantly (n = 6). However, in the EA at ST-36 groups, the decreased CRF mRNA expression was significantly increased by ICV administered BMI (n = 6, P < 0.05, saline 5 μl ICV injected as a control; Figure 4A).
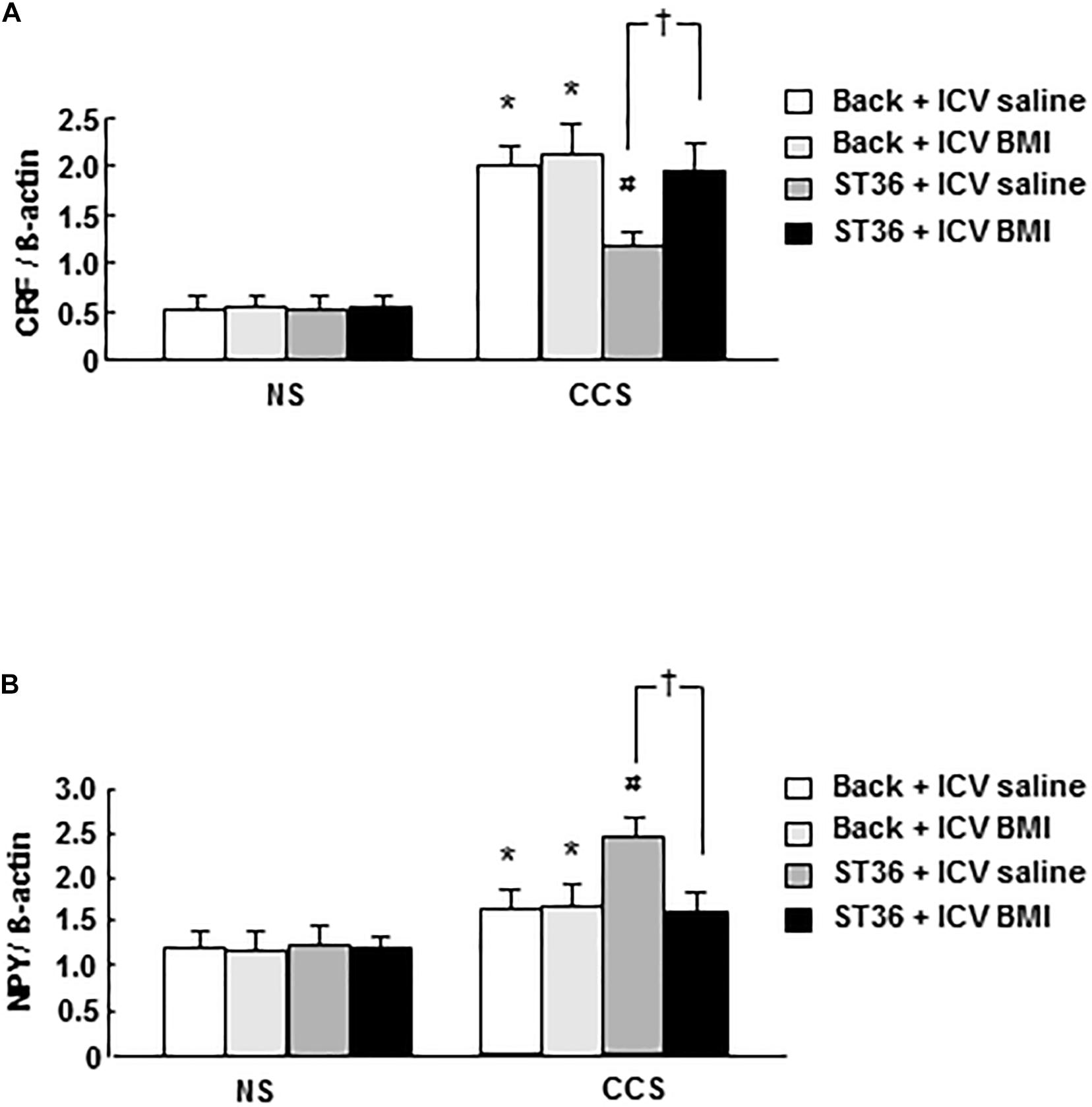
Figure 4. Effects of EA at ST-36 and GABAA receptor antagonist on central CRF mRNA expression (A) and NPY mRNA expression (B) in response to CCS. (A) In the NS groups, CRF mRNA expression showed low level, EA at back (with ICV administered saline or BMI) did not change the CRF mRNA expression significantly. However, EA at ST-36 (with ICV administered saline or BMI) also did not change the CRF mRNA expression significantly. In the CCS conditions, CCS highly elevated the CRF mRNA expression in the sham group (EA at back, and saline ICV administered). In the EA at back groups, ICV administered BMI did not change the CRF mRNA expression significantly. However, in the EA at ST-36 groups, the reduced CRF mRNA expression was significantly increased by ICV administered BMI. (B) In the NS groups, NPY mRNA expression showed low level, EA at back (with ICV administered saline or BMI) did not change the NPY mRNA expression significantly. However, EA at ST-36 (with ICV administered saline or BMI) also did not change the NPY mRNA expression significantly. In the CCS conditions, CCS elevated the NPY mRNA expression in the sham group (EA at back, and saline ICV administered). In the EA at back groups, ICV administered BMI did not change the NPY mRNA expression significantly. However, in the EA at ST-36 groups, the further significantly increased NPY mRNA expression (saline ICV administered), was significantly inhibited by ICV administered BMI (n = 6, *P < 0.05 compared with NS non-treated group, #P < 0.05 compared with CCS non-treated group, †P < 0.05 compared with CCS ST36 + ICV saline group).
In the NS groups, NPY mRNA expression showed low level (EA at back, and saline 5 μl ICV administered as a control), however, the combination of EA at back or ST-36 with ICV administered saline or BMI did not change the NPY mRNA expression significantly (n = 6). In the CCS conditions, the NPY mRNA expression increased significantly in the sham group (EA at back, and saline ICV administered). ICV administered BMI (EA at back) did not change the NPY mRNA expression significantly (n = 6). However, in the EA at ST-36 groups, the further significantly increased NPY mRNA expression (saline ICV administered), was significantly inhibited by ICV administered BMI (n = 6, P < 0.05, saline 5 μl ICV injected as a control; Figure 4B).
In the NS groups, the serum corticosterone concentration showed low levels (64.2 ± 7.0 ng/ml, EA at back, and saline 5 μl ICV administered as a control), however, the combination of EA at back or ST-36 with ICV administered saline or BMI did not change the corticosterone concentration significantly (62.4 ± 8.1, 63.9 ± 6.9, and 65.6 ± 6.7, respectively, n = 6). In the CCS conditions, the serum corticosterone concentration in the sham group (EA at back, and saline ICV administered) was significantly elevated (139.9 ± 10.4 ng/ml, n = 6). ICV administered GABAA receptor antagonist BMI did not change the serum corticosterone concentration significantly (143.8 ± 12.4 ng/ml, n = 6, saline 5 μl ICV injected as a control). However, in the EA at ST-36 groups, the decreased serum corticosterone level was significantly increased by ICV administered BMI (from 98.9 ± 9.7 to 137.7 ± 10.2 ng/ml, n = 6, P < 0.05, saline 5 μl ICV injected as a control; Figure 5A).
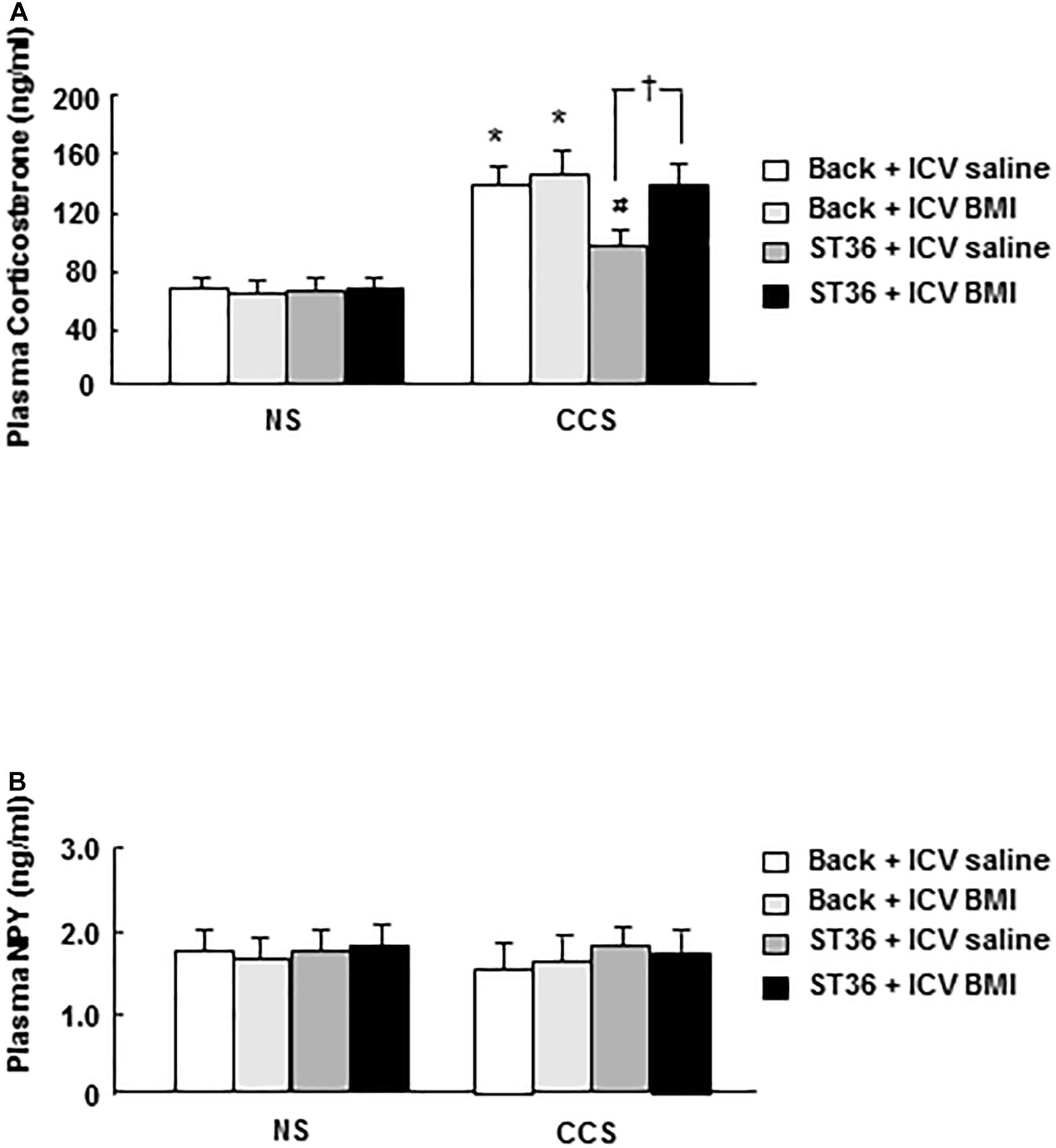
Figure 5. Effects of EA at ST-36 and GABAA receptor antagonist on serum corticosterone (A) and NPY (B) levels in response to CCS. (A) In the NS groups, serum corticosterone concentration showed low level, EA at back (with ICV administered saline or BMI) did not change the corticosterone level significantly. However, EA at ST-36 (with ICV administered saline or BMI) also did not change the corticosterone level significantly. In the CCS conditions, CCS highly elevated the serum corticosterone level in the sham group (EA at back, and saline ICV administered). In the EA at back groups, ICV administered BMI did not change the corticosterone level significantly. However, in the EA at ST-36 groups, the significantly decreased corticosterone level was completely antagonized by ICV administered BMI. (B) In the NS groups, serum NPY concentration showed low level, EA at back (with ICV administered saline or BMI) did not change the NPY level significantly. However, EA at ST-36 (with ICV administered saline or BMI) also did not change the NPY level significantly. In the CCS conditions, the serum NPY level in the sham group did not show significant change. In the EA at back groups, ICV administered BMI did not change the NPY level significantly. However, EA at ST-36 (with ICV administered saline or BMI) also did not change the NPY level significantly (n = 6, *P < 0.05 compared with NS non-treated group, #P < 0.05 compared with CCS non-treated group, †P < 0.05 compared with CCS ST36 + ICV saline group).
In the NS groups, the serum NPY concentration showed low levels (1.79 ± 0.14 ng/ml, EA at back, and saline 5 μl ICV administered as a control), however, the combination of EA at back or ST-36 with ICV administered saline or BMI did not change the NPY concentration significantly (1.75 ± 0.13, 1.80 ± 0.12, and 1.83 ± 0.15 ng/ml, respectively, n = 6). In the CCS conditions, the serum NPY level in the sham group decreased to 1.60 ± 0.14 ng/ml but did not show a significant change (n = 6). However, the combination of EA at back or ST-36 with ICV administered saline or BMI did not change the NPY concentration significantly (1.65 ± 0.18, 1.86 ± 0.15, and 1.79 ± 0.13 ng/ml, respectively, n = 6, saline 5 μl ICV injected as a control; Figure 5B).
In the NS groups, regular gastric phase III-like contractions were observed in fixed-fed rats. The combination of EA at back or ST-36 with ICV administered saline or BMI had no effects on the amplitude and frequency of gastric phase III-like contractions (data not shown). In the CCS condition, restraint stress abolished gastric phase III-like contractions in the sham group (EA at back, and saline ICV administered). ICV administered GABAA receptor antagonist BMI (100 ng/5 μl, 15 min prior to stress the loading for 7 consecutive days) did not change the gastric phase III-like contractions noticeably. However, in the EA at ST-36 groups, the partially restored gastric phase III-like contractions was completely abolished by ICV administered BMI. Each recorded experiment was individually repeated at least three times and similar results were obtained (n = 4; Figure 6A).
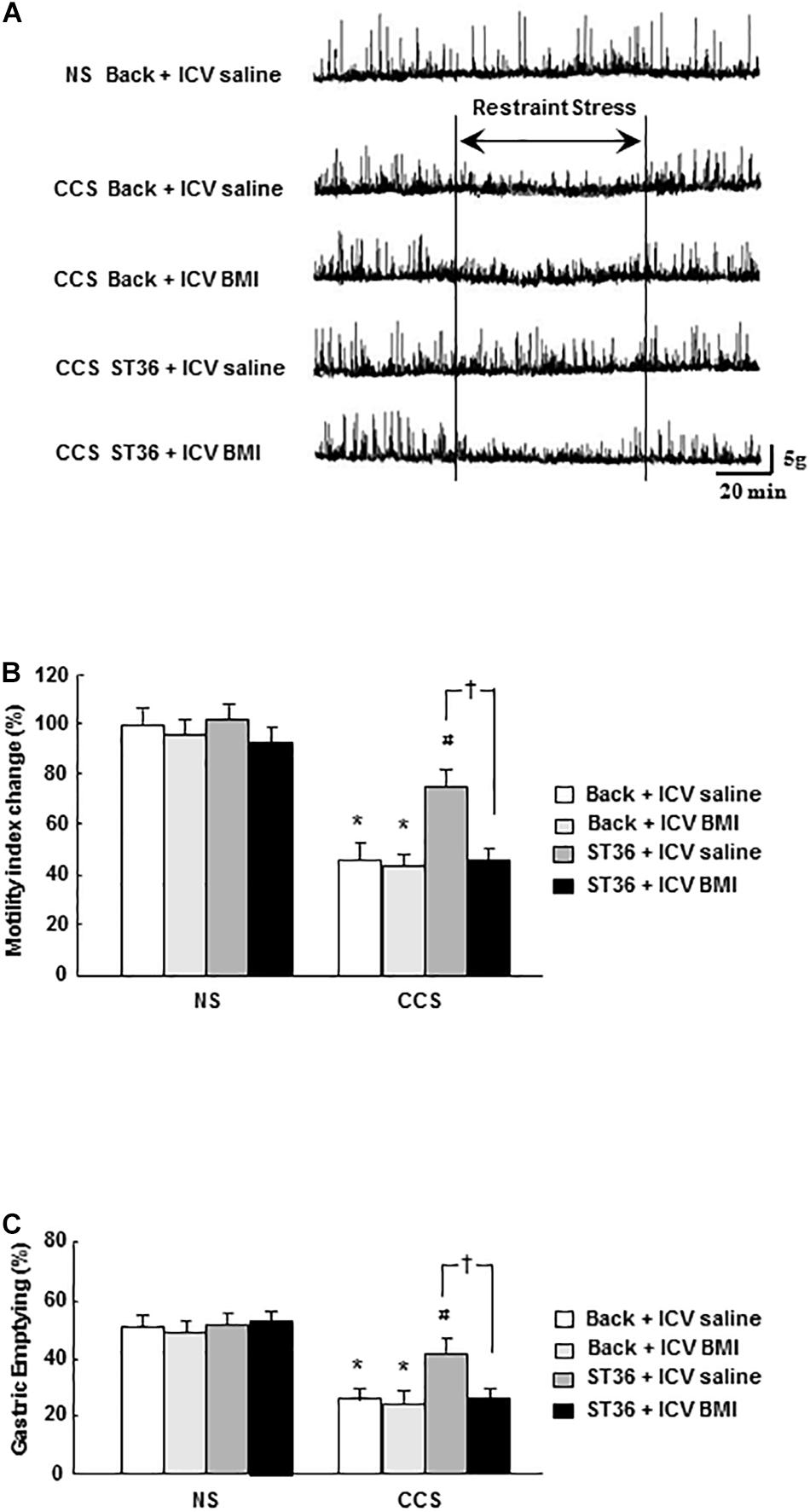
Figure 6. Effects of EA at ST-36 and GABAA receptor antagonist on gastric motility (A), gastric MI change (B), and GE (C) in response to CCS. (A) The gastric phase III-like contractions in the non-stressed groups (NS). EA at back (with ICV administered saline or BMI) and EA at ST-36 (with ICV administered saline or BMI) both had no effects on gastric contractions (data not shown). In the CCS conditions, CCS abolished gastric phase III-like contractions in the sham group (EA at back, and saline ICV administered). ICV injection of BMI also had no effect on the gastric contractions. However, in the EA at ST-36 groups, the partially restored gastric contraction was completely abolished by ICV administered of BMI. (B) In the NS groups, the gastric MI change showed high value. EA at back (with ICV administered saline or BMI) had no effects on the gastric MI change. However, EA at ST-36 (with ICV administered saline or BMI) also had no impacts on the gastric MI change. In the CCS conditions, CCS significantly decreased the gastric MI change in the sham group (EA at back, and saline ICV administered). ICV injection of BMI also had no effect on the gastric MI change. However, in the EA at ST-36 groups, the significantly increased gastric MI change was completely antagonized by ICV administered BMI. (C) In the NS groups, the GE showed high value. EA at back (with ICV administered saline or BMI) had no effects on the GE. However, EA at ST-36 (with ICV administered saline or BMI) also had no impacts on the GE. In the CCS conditions, CCS significantly decreased the GE in the sham group (EA at back, and saline ICV administered). ICV injection of BMI also had no effect on the GE. However, in the EA at ST-36 groups, the significantly increased GE was completely antagonized by ICV administered BMI (n = 6, *P < 0.05 compared with NS non-treated group, #P < 0.05 compared with CCS non-treated group, †P < 0.05 compared with CCS ST36 + ICV saline group).
In the NS groups, the gastric MI change is 100 ± 7% (EA at back, and saline 5 μl ICV administered as a control, n = 6). The combination of EA at back or ST-36 with ICV administered saline or BMI did not alter the gastric MI change significantly (97 ± 8, 102 ± 6, and 96 ± 7%, respectively, n = 6). In the CCS groups, in the sham group (EA at back, and saline ICV administered), the gastric MI change was decreased to 48 ± 5% (n = 6). ICV administered GABAA receptor antagonist BMI did not significantly alter the gastric MI change (46 ± 4%, n = 6). However, in the EA at ST-36 groups, the partially restored gastric MI change was significantly decreased by ICV administered BMI (from 78 ± 7 to 48 ± 5%, saline 5 μl ICV injected as a control; n = 6, P < 0.05, Figure 6B).
In the NS groups, the GE was 50.9 ± 3.2% (EA at back, and saline 5 μl ICV administered as a control, n = 6). The combination of EA at back or ST-36 with ICV administered saline or BMI did not change the GE significantly (48.4 ± 2.8, 51.1 ± 3.1, and 52.3 ± 2.7%, respectively, n = 6). In the CCS groups, in the sham group (EA at back, and saline ICV administered), the GE was decreased to 25.4 ± 2.2% (n = 6). ICV administered GABAA receptor antagonist BMI did not significantly alter the gastric MI change (24.2 ± 2.1%, n = 6). However, in the EA at ST-36 groups, the partially restored GE was significantly decreased by ICV administered BMI (from 43.1 ± 3.8 to 25.2 ± 2.4%, saline 5 μl ICV injected as a control; n = 6, P < 0.05, Figure 6C).
Restraint stress has been used frequently as a psychogenic and physical stress model in rodents. ARS stimulates the central CRF release and plays an important role in influencing GI motor function (Lenz et al., 1988; Tache and Bonaz, 2007). In contrast to ARS, repeated experiences with the same stressor (chronic repeated restraint stress, CRRS) produce the habituation or diminution of behavioral responses. Our previous studies also shown that in the CRRS condition, the elevated central CRF expression and serum corticosterone level, caused by ARS, was attenuated on day 5 and 7 of consecutive stress loading. ARS-induced GI dysmotility, such as delayed GE or impaired gastric phase III-like contractions, gradually returned to normal levels (Zheng et al., 2009; Yang et al., 2018). As mentioned above, stress adaptation is likely to be an important mechanism in maintaining optimal physiological and psychological functions in the face of repeated stress.
However, in the present study, we set up a CCS model, and found that attenuated gastric motility and delayed GE were also observed when rats received different types of stressors for 7 days, with highly elevated CRF mRNA expression in the PVN of the hypothalamus, resulting in activation of the HPA axis. These results are consistent with previous studies (Zheng et al., 2010; Yang et al., 2019).
Previous studies have shown that EA at ST36, can attenuate stress responses acting through not only HPA axis activity but also by stimulating parasympathetic activity and inhibiting the SNS pathway (Middlekauff et al., 2002; Yang et al., 2002; Eshkevari et al., 2013), further resulting in the restoration of impaired gastric motility (Iwa et al., 2006; Zhou et al., 2017). Recent studies also found that EA can increase hypothalamic NPY expression and reduce the stress responses in chronic stress conditions (Lee et al., 2009; Sun et al., 2015).
Neuropeptide Y is one of the most widely expressed neuropeptides in the central nervous system, and its wide distribution suggests its involvement in numerous physiological processes (Heilig and Thorsell, 2002; Heilig, 2004). Endogenous NPY has anxiolytic properties (Hirsch and Zukowska, 2012; Reichmann and Holzer, 2016). NPY might counteract the biological actions of CRF, interact with the HPA axis, and be involved in moderating and improving the body’s ability to cope with stress (Heilig, 2004).
Neuropeptide Y expression in the brain under stress conditions has been well studied, however, the magnitude and the direction of stress-induced NPY alterations heavily depend on the type and duration of the stress. For example, central NPY mRNA expression is increased after foot shock stress (Kas et al., 2005; de Lange et al., 2008), but was unaltered by mild stress loading comprising acute water avoidance and acute air puff stress (Ishiguchi et al., 2001; Hassan et al., 2014). However, in ARS, under a moderate degree of stimulus, the NPY mRNA expression was still debatable: some studies showed increased central NPY mRNA expression (Conrad and McEwen, 2000; Sweerts et al., 2001), and unaltered or decreased results were also reported (Thorsell et al., 1998, 1999). In studies of chronic stress, central NPY expression is usually upregulated in response to repeated stress loading (Sweerts et al., 2001; McDougall et al., 2005). Thus, as mentioned above, central NPY usually reacts to acute stress more than a moderate degree of stress or chronic stress. In our present study, where rats received different types of stressors for 7–9 days, NPY mRNA expression increased, and EA at ST36 further up-regulated central NPY and made an adequate termination of the stress responses. These results support and extend the above findings.
Quantitative measurement of CRF and NPY mRNA expression in the PVN of the hypothalamus, is a very effective method, which has been applied in our previous and recent studies (Zheng et al., 2010; Yang et al., 2018, 2019). Efforts were also made to minimize the number of animals used in the current study, and further investigation is needed to examine the protein expression of NPY and CRF in the PVN of the hypothalamus.
In the peripheral nervous system, NPY is found in three main pools: sympathetic nerves, platelets, and the adrenal medulla. The circulating plasma NPY levels are in the low range under resting conditions; however, in many stress conditions, the release of NPY is dependent on the intensity and duration of stress, as well the pattern of sympathetic nerve activation (Hirsch and Zukowska, 2012).
Norepinephrine (NE) is the primary sympathetic neurotransmitter, whereas NPY as a co-transmitter is also released during stress, but the proportions of these two transmitters vary depending on the type of stress. The release of NE is the mildest acute stress; however, NPY requires a more prolonged and/or intense stimulation of sympathetic nerves (Zukowska-Grojec, 1995; Abe et al., 2010).
Our recent study also found that, in a FRS condition, which is a moderate stress stimulus, the serum NPY levels did not increase significantly in response to ARS but were significantly increased at repeated restraint stress (Yang et al., 2018). However, in our present study of the CCS condition, serum NPY levels decreased lower than 1.43 ± 0.18 ng/ml but did not show significant change. This supports the theory that in a higher intensity chronic stress condition, the NPY system will be attenuated and limited, and that central NPY failed to play an important role to produce an adaptation (Zukowska-Grojec, 1995; Hirsch and Zukowska, 2012). Our present study found that EA at ST36 increased the NPY mRNA expression and decreased CRF mRNA expression at the PVN following CCS, but in the peripheral, EA at ST36 did not change the serum NPY level significantly. Our present results support and extend the recent findings that EA at ST36 probably does not act through the peripheral NPY system but acts through central regulation of the CRF and attenuates the HPA axis (Eshkevari et al., 2015). Further studies are needed to clarify the mechanism that may be involved in the peripheral NPY system.
In experiment 2 of our current study, a further study based on the results of the experiment 1 was performed, to show whether the inhibitory mechanism of NPY on CRF via the GABAA receptor under CCS condition, in the ICV cannulated CCS rat model, in the GABAA receptor antagonist BMI was ICV injected. The sham group was the non-acupuncture map points on the back of the rats (EA at back) as a control, so the non-treated group was not included in experiment 2.
The significance of NPY in stress adaptation seems to be receptor dependent. NPY shows strong affinity for the Y1, Y2, and Y5 receptors (Dumont et al., 1998; Kask et al., 2002; Reichmann and Holzer, 2016). Many studies have demonstrated that the anxiolytic behavioral effect of NPY is mediated primarily through post-synaptic Y1 receptors (Kask et al., 2002; Heilig, 2004). Our previous study also found that central NPY via the Y1 receptor plays an important role in mediating the adaptation mechanism against chronic stress (Yang et al., 2018).
However, in the study of the inhibition mechanism of NPY on CRF, it was found that central NPY could regulate the excitability of CeA through the GABAA receptor and improved the adaptability of organism to stress responses (Molosh et al., 2013). In addition, NPY and GABA were co-expressed in ARC neurons of the hypothalamus and projected to PVN (Muroya et al., 2005). The regulation of NPY on feeding function is also mediated by the GABAA receptor (Pu et al., 1999). GABA is the major inhibitory amino acid transmitter of the mammalian central nervous system. GABA exerts its effects through GABAA and GABAB receptors. GABA-projecting neurons into the PVN are known to inhibit CRF expression via the GABAA receptors (Bülbül et al., 2011). Released corticosterone in response to acute stress, inhibits CRF release via a feedback mechanism, which is mediated via GABAA receptors in the PVN (Cullinan et al., 2008). It has been suggested that the GABAergic system is also involved in the inhibitory mechanism of intranasal administration of NPY on CRF mRNA expression, in chronic stress conditions.
Our present study found that ICV administered GABAA receptor antagonist BMI (100 ng, 15 min prior to the stress loading for 7 consecutive days) did not significantly change the CRF mRNA expression, the serum corticosterone level, and the gastric motility in CCS condition. However, in the EA groups, the decreased CRF mRNA expression and the serum corticosterone level were significantly antagonized by ICV administered BMI, while the restored gastric contraction and GE was inhibited by BMI. Thus, in a higher intensity chronic stress condition, an EA stimulated up-regulated NPY system was expected in the termination of the stress responses of the CCS condition.
A recent study also found that ICV administered BMI 100 ng (200 pmol) was effective in antagonization of the GABAA receptor subtypes in an acute and chronic stress condition, but not in non-stressed rats (Bülbül et al., 2011). In the current study, we also found that ICV administered BMI 100 ng significantly abolished the effects induced by EA, suggesting that the GABAergic system is also involved in the inhibitory mechanism of the up-regulated NPY system induced by EA, in chronic stress conditions. But in non-stressed conditions, administration of BMI did not show significant changes. Our present study agrees with the above report.
However, in non-stressed conditions, the effects of central administration of BMI are still controversial. Previous studies showed that ICV administered BMI (50–200 pmol) significantly increased the central sympathetic neurons and peripheral nerve activities in rats (Li et al., 2006). Conversely, studies also found that central microinjection of BMI (20 pmol) significantly increased the activities of the DMV (dorsal motor nucleus of the vagus) neurons and vagal pathways, and as a consequence produced an increase in gastric motility (Herman et al., 2009). The different results of these studies, may be due to the position, timing, and even the doses of central injection, and further investigation is needed clarify this issue.
In conclusion, our current study showed that attenuated gastric motor function induced by CCS was significantly restored by EA at ST-36 in rats. EA at ST-36 increased the central NPY mRNA expression but not the peripheral NPY level, through central regulation of CRF, and in turn decreased HPA axis activity. The stimulated effect of EA on attenuated gastric motor function was antagonized by ICV injection of the GABAA receptor antagonist. These suggest that EA may act on NPY neurons at the hypothalamus, via GABAA receptor resulting in reduced CRF expression and restoration of gastric dysmotility following CCS.
Our study may contribute to a better understanding of the mechanism and the treatment strategies in GI dysmotility of stress in daily life. GI dysmotility induced by non-habituating stress may be treatable by EA at ST-36, because of its stimulatory effects on the NPY system, which may be a new approach for treatment of stress-induced GI motility disorders.
All datasets generated for this study are included in the article/supplementary material.
The animal study was reviewed and approved by the Animal Care and Use Committee of China Medical University.
YY, HY, RB, and BS performed the experiment. WS and XZ were involved in the study supervision and critical revision of the manuscript. JZ designed the experiment, analyzed the data, and wrote the manuscript. All authors contributed to the article and approved the submitted version.
This work was supported by the Natural Science Foundation of Liaoning Province of China (No. 2013021084) and the Scientific Research Foundation for the Returned Overseas Chinese Scholars, State Education Ministry (No. 2013693) (Grant to JZ).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Abe, K., Kuo, L., and Zukowska, Z. (2010). Neuropeptide Y is a mediator of chronic vascular and metabolic maladaptations to stress and hypernutrition. Exp. Biol. Med. (Maywood) 235, 1179–1184. doi: 10.1258/ebm.2010.009136
Adrian, T. E., Allen, J. M., Bloom, S. R., Ghatei, M. A., Rossor, M. N., Roberts, G. W., et al. (1983). Neuropeptide Y distribution in human brain. Nature 306, 584–586. doi: 10.1038/306584a0
Allen, Y. S., Adrian, T. E., Allen, J. M., Tatemoto, K., Crow, T. J., Bloom, S. R., et al. (1983). Neuropeptide Y distribution in the rat brain. Science 221, 877–879. doi: 10.1126/science.6136091
Bali, A., Singh, N., and Jaggi, A. S. (2014). Neuropeptides as therapeutic targets to combat stress-associated behavioral and neuroendocrinological effects. CNS Neurol. Disord. Drug Targets 13, 347–368. doi: 10.2174/1871527313666140314163920
Bülbül, M., Babygirija, R., Cerjak, D., Yoshimoto, S., Ludwig, K., and Takahashi, T. (2011). Hypothalamic oxytocin attenuates CRF expression via GABA(A) receptors in rats. Brain Res. 1387, 39–45. doi: 10.1016/j.brainres.2011.02.091
Campbell, A. (2002). The origins of acupuncture. Acupunct. Med. 20:141. doi: 10.1136/aim.20.2-3.141-a
Chan, J., Carr, I., and Mayberry, J. F. (1997). The role of acupuncture in the treatment of irritable bowel syndrome: a pilot study. Hepatogastroenterology 44, 1328–1330.
Conrad, C. D., and McEwen, B. S. (2000). Acute stress increases neuropeptide Y mRNA within the arcuate nucleus and hilus of the dentate gyrus. Brain Res. Mol. Brain Res. 79, 102–109. doi: 10.1016/s0169-328x(00)00105-4
Cullinan, W. E., Ziegler, D. R., and Herman, J. P. (2008). Functional role of local GABAergic influences on the HPA axis. Brain Struct. Funct. 213, 63–72. doi: 10.1007/s00429-008-0192-2
de Lange, R. P., Wiegant, V. M., and Stam, R. (2008). Altered neuropeptide Y and neurokinin messenger RNA expression and receptor binding in stress-sensitised rats. Brain Res. 1212, 35–47. doi: 10.1016/j.brainres.2008.03.018
Diehl, D. L. (1999). Acupuncture for gastrointestinal and hepatobiliary disorders. J. Altern Complement Med. 5, 27–45. doi: 10.1089/acm.1999.5.27
Dumont, Y., Jacques, D., Bouchard, P., and Quirion, R. (1998). Species differences in the expression and distribution of the neuropeptide Y Y1, Y2, Y4, and Y5 receptors in rodents, guinea pig, and primates brains. J. Comp. Neurol. 402, 372–384. doi: 10.1002/(sici)1096-9861(19981221)402:3<372::aid-cne6>3.0.co;2-2
Eshkevari, L., Mulroney, S. E., Egan, R., and Lao, L. (2015). Effects of acupuncture, RU-486 on the hypothalamic-pituitary-adrenal axis in chronically stressed adult male rats. Endocrinology 156, 3649–3660. doi: 10.1210/EN.2015-1018
Eshkevari, L., Permaul, E., and Mulroney, S. E. (2013). Acupuncture blocks cold stress-induced increases in the hypothalamus-pituitary-adrenal axis in the rat. J. Endocrinol. 217, 95–104. doi: 10.1530/JOE-12-0404
Fireman, Z., Segal, A., Kopelman, Y., Sternberg, A., and Carasso, R. (2001). Acupuncture treatment for irritable bowel syndrome. A double-blind controlled study. Digestion 64, 100–103. doi: 10.1159/000048847
Hassan, A. M., Jain, P., Reichmann, F., Mayerhofer, R., Farzi, A., Schuligoi, R., et al. (2014). Repeated predictable stress causes resilience against colitis-induced behavioral changes in mice. Front. Behav. Neurosci. 8:386. doi: 10.3389/fnbeh.2014.00386
Heilig, M. (2004). The NPY system in stress, anxiety and depression. Neuropeptides 38, 213–224. doi: 10.1016/j.npep.2004.05.002
Heilig, M., and Thorsell, A. (2002). Brain neuropeptide Y (NPY) in stress and alcohol dependence. Rev. Neurosci. 13, 85–94. doi: 10.1515/revneuro.2002.13.1.85
Herman, M. A., Cruz, M. T., Sahibzada, N., Verbalis, J., and Gillis, R. A. (2009). GABA signaling in the nucleus tractus solitarius sets the level of activity in dorsal motor nucleus of the vagus cholinergic neurons in the vagovagal circuit. Am. J. Physiol. Gastrointest. Liver Physiol. 296, G101–G111. doi: 10.1152/ajpgi.90504.2008
Hirsch, D., and Zukowska, Z. (2012). NPY and stress 30 years later: the peripheral view. Cell Mol. Neurobiol. 32, 645–659. doi: 10.1007/s10571-011-9793-z
Hurtak, J. J. (2002). An overview of acupuncture medicine. J. Altern Complement Med. 8, 535–538. doi: 10.1089/107555302320825020
Ishiguchi, T., Amano, T., Matsubayashi, H., Tada, H., Fujita, M., and Takahashi, T. (2001). Centrally administered neuropeptide Y delays gastric emptying via Y2 receptors in rats. Am. J. Physiol. Regul. Integr. Comp. Physiol. 281, 1522–1530. doi: 10.1152/ajpregu.2001.281.5.R1522
Iwa, M., Nakade, Y., Pappas, T. N., and Takahashi, T. (2006). Electroacupuncture elicits dual effects: stimulation of delayed gastric emptying and inhibition of accelerated colonic transit induced by restraint stress in rats. Dig. Dis. Sci. 51, 1493–1500. doi: 10.1007/s10620-006-9083-7
Kas, M. J., Bruijnzeel, A. W., Haanstra, J. R., Wiegant, V. M., and Adan, R. A. (2005). Differential regulation of agouti-related protein and neuropeptide Y in hypothalamic neurons following a stressful event. J. Mol. Endocrinol. 35, 159–164. doi: 10.1677/jme.1.01819
Kask, A., Harro, J., von Horsten, S., Redrobe, J. P., Dumont, Y., and Quirion, R. (2002). The neurocircuitry and receptor subtypes mediating anxiolytic-like effects of neuropeptide Y. Neurosci. Biobehav. Rev. 26, 259–283. doi: 10.1016/s0149-7634(01)00066-5
Kinn Rød, A. M., Harkestad, N., Jellestad, F. K., and Murison, R. (2017). Comparison of commercial ELISA assays for quantification of corticosterone in serum. Sci. Rep. 7:6748. doi: 10.1038/s41598-017-06006-4
Kormos, V., and Gaszner, B. (2013). Role of neuropeptides in anxiety, stress, and depression: from animals to humans. Neuropeptides 47, 401–419. doi: 10.1016/j.npep.2013.10.014
Lee, B., Shim, I., Lee, H. J., Yang, Y., and Hahm, D. H. (2009). Effects of acupuncture on chronic corticosterone-induced depression-like behavior and expression of neuropeptide Y in the rats. Neurosci. Lett. 453, 151–156. doi: 10.1016/j.neulet.2009.01.076
Lenz, H. J., Raedler, A., Greten, H., Vale, W. W., and Rivier, J. E. (1988). Stress-induced gastrointestinal secretory and motor responses in rats are mediated by endogenous corticotropin-releasing factor. Gastroenterology 95, 1510–1517. doi: 10.1016/s0016-5085(88)80070-2
Levy, R. L., Olden, K. W., Naliboff, B. D., Bradley, L. A., Francisconi, C., Drossman, D. A., et al. (2006). Psychosocial aspects of the functional gastrointestinal disorders. Gastroenterology 130, 1447–1458. doi: 10.1053/j.gastro.2005.11.057
Li, Y. F., Jackson, K. L., Stern, J. E., Rabeler, B., and Patel, K. P. (2006). Interaction between glutamate and GABA systems in the integration of sympathetic outflow by the paraventricular nucleus of the hypothalamus. Am. J. Physiol. Heart Circ. Physiol. 291, H2847–H2856. doi: 10.1152/ajpheart.00625.2005
McDougall, S. J., Widdop, R. E., and Lawrence, A. J. (2005). Differential gene expression in WKY and SHR brain following acute and chronic air-puff stress. Brain Res. Mol. Brain Res. 133, 329–336. doi: 10.1016/j.molbrainres.2004.10.010
Middlekauff, H. R., Hui, K., Yu, J. L., Hamilton, M. A., Fonarow, G. C., Moriguchi, J., et al. (2002). Acupuncture inhibits sympathetic activation during mental stress in advanced heart failure patients. J. Cardiac Fail. 8, 399–406. doi: 10.1054/jcaf.2002.129656
Molosh, A. I., Sajdyk, T. J., Truitt, W. A., Zhu, W., Oxford, G. S., and Shekhar, A. (2013). NPY Y1 receptors differentially modulate GABAA and NMDA receptors via divergent signal-transduction pathways to reduce excitability of amygdala neurons. Neuropsychopharmacology 38, 1352–1364. doi: 10.1038/npp.2013.33
Morales-Medina, J. C., Dumont, Y., and Quirion, R. (2010). A possible role of neuropeptide Y in depression and stress. Brain Res. 1314, 194–205. doi: 10.1016/j.brainres.2009.09.077
Muroya, S., Funahashi, H., Uramura, K., Shioda, S., and Yada, T. (2005). Gamma aminobutyric acid regulates glucosensitive neuropeptide Y neurons in arcuate nucleus via A/B receptors. Neuroreport 16, 897–901. doi: 10.1097/00001756-200506210-00005
Nakade, Y., Tsuchida, D., Fukuda, H., Iwa, M., Pappas, T. N., and Takahashi, T. (2005). Restraint stress delays solid gastric emptying via a central CRF and peripheral sympathetic neuron in rats. Am. J. Physiol. Regul. Integr. Comp. Physiol. 288, R427–R432. doi: 10.1152/ajpregu.00499.2004
Paxinos, G., and Watson, C. (1997). The Rat Brain in Stereotaxic Coordinates, 3rd Edn. San Francisco, CA: Academic, 1997.
Primeaux, S. D., Wilson, S. P., Cusick, M. C., York, D. A., and Wilson, M. A. (2005). Effects of altered amygdalar neuropeptide Y expression on anxiety-related behaviors. Neuropsychopharmacology 30, 1589–1597. doi: 10.1038/sj.npp.1300705
Pu, S., Jain, M. R., Horvath, T. L., Diano, S., Kalra, P. S., and Kalra, S. P. (1999). Interactions between neuropeptide Y and gamma-aminobutyric acid in stimulation of feeding: a morphological and pharmacological analysis. Endocrinology 140, 933–940. doi: 10.1210/endo.140.2.6495
Rasmusson, A. M., Schnurr, P. P., Zukowska, Z., Scioli, E., and Forman, D. E. (2010). Adaptation to extreme stress: posttraumatic stress disorder, neuropeptide Y and metabolic syndrome. Exp. Biol. Med. (Maywood) 235, 1150–1162. doi: 10.1258/ebm.2010.009334
Reichmann, F., and Holzer, P. (2016). Neuropeptide Y. A stressful review. Neuropeptides 55, 99–109. doi: 10.1016/j.npep.2015.09.008
Sabban, E. L., Alaluf, L. G., and Serova, L. I. (2016). Potential of neuropeptide Y for preventing or treating post-traumatic stress disorder. Neuropeptides 56, 19–24. doi: 10.1016/j.npep.2015.11.00
Serova, L. I., Tillinger, A., Alaluf, L. G., Laukova, M., Keegan, K., and Sabban, E. L. (2013). Single intranasal neuropeptide Y infusion attenuates development of PTSD-like symptoms to traumatic stress in rats. Neuroscience 236, 298–312. doi: 10.1016/j.neuroscience.2013.01.040
Sun, J., Wu, X., Meng, Y., Cheng, J., Ning, H., Peng, Y., et al. (2015). Electro-acupuncture decreases 5-HT, CGRP and increases NPY in the brain-gut axis in two rat models of Diarrhea-predominant irritable bowel syndrome(D-IBS). BMC Complement Altern Med. 15:340. doi: 10.1186/s12906-015-0863-5
Sweerts, B. W., Jarrott, B., and Lawrence, A. J. (2001). The effect of acute and chronic restraint on the central expression of prepro-neuropeptide Y mRNA in normotensive and hypertensive rats. J. Neuroendocrinol. 13, 608–617. doi: 10.1046/j.1365-2826.2001.00674.x
Tache, Y., and Bonaz, B. (2007). Corticotropin-releasing factor receptors and stress-related alterations of gut motor function. J. Clin. Invest. 117, 33–40. doi: 10.1172/JCI30085
Talley, N. J., Holtmann, G., and Walker, M. M. (2015). Therapeutic strategies for functional dyspepsia and irritable bowel syndrome based on pathophysiology. J. Gastroenterol. 50, 601–613. doi: 10.1007/s00535-015-1076-x
Thorsell, A., Carlsson, K., Ekman, R., and Heilig, M. (1999). Behavioral and endocrine adaptation, and up-regulation of NPY expression in rat amygdale following repeated restraint stress. Neuroreport 10, 3003–3007. doi: 10.1097/00001756-199909290-00024
Thorsell, A., Svensson, P., Wiklund, L., Sommer, W., Ekman, R., and Heilig, M. (1998). Suppressed neuropeptide Y (NPY) mRNA in rat amygdala following restraint stress. Regul. Pept. 7, 247–254. doi: 10.1016/s0167-0115(98)00075-5
World Health Organization (2003). Acupuncture: Review and Analysis Reports on Controlled Clinical Trials. WHO Library Cataloguing-in-Publication Data. Geneva: WHO, 1–87.
Yang, C. H., Lee, B. B., Jung, H. S., Shim, I., Roh, P. U., and Golden, G. T. (2002). Effect of electroacupuncture on response to immobilization stress. Pharmacol. Biochem. Behav. 72, 847–855. doi: 10.1016/s0091-3057(02)00769-4
Yang, Y., Babygirija, R., Zheng, J., Shi, B., Sun, W., Zheng, X., et al. (2018). Central neuropeptide Y plays an important role in mediating the adaptation mechanism against chronic stress in male rats. Endocrinology 159, 1525–1536. doi: 10.1210/en.2018-00045
Yang, Y., Yu, H., Babygirija, R., Shi, B., Sun, W., Zheng, X., et al. (2019). Intranasal administration of oxytocin attenuates stress responses following chronic complicated stress in rats. J. Neurogastroenterol. Motil. 25, 611–622. doi: 10.5056/jnm19065
Yoshimoto, S., Babygirija, R., Dobner, A., Ludwig, K., and Takahashi, T. (2012). Anti-stress effects of transcutaneous electrical nerve stimulation (TENS) on colonic motility in rats. Dig. Dis. Sci. 57, 1213–1221. doi: 10.1007/s10620-012-2040-8
Zheng, J., Babygirija, R., Bülbül, M., Cerjak, D., Ludwig, K., and Takahashi, T. (2010). Hypothalamic oxytocin mediates adaptation mechanism against chronic stress in rats. Am. J. Physiol. Gastrointest Liver Physiol. 299, G946–G953. doi: 10.1152/ajpgi.00483.2009
Zheng, J., Dobner, A., Babygirija, R., Ludwig, K., and Takahashi, T. (2009). Effects of repeated restraint stress on gastric motility in rats. Am. J. Physiol. Regul. Integr. Comp. Physiol. 296, R1358–R1365. doi: 10.1152/ajpregu.90928.2008
Zhou, J., Li, S., Wang, Y., Foreman, R. D., Yin, J., Zhang, S., et al. (2017). Inhibitory effects and mechanisms of electroacupuncture via chronically implanted electrodes on stress-induced gastric hypersensitivity in rats with neonatal treatment of iodoacetamide. Neuromodulation 20, 767–773. doi: 10.1111/ner.12602
Keywords: stress, functional dyspepsia, electroacupuncture, neuropeptide Y, gamma-aminobutyric acid A receptor
Citation: Yang Y, Yu H, Babygirija R, Shi B, Sun W, Zheng X and Zheng J (2021) Electro-Acupuncture Attenuates Chronic Stress Responses via Up-Regulated Central NPY and GABAA Receptors in Rats. Front. Neurosci. 14:629003. doi: 10.3389/fnins.2020.629003
Received: 13 November 2020; Accepted: 29 December 2020;
Published: 26 January 2021.
Edited by:
Fumihiko Maekawa, National Institute for Environmental Studies (NIES), JapanReviewed by:
Chunhui Bao, Shanghai University of Traditional Chinese Medicine, ChinaCopyright © 2021 Yang, Yu, Babygirija, Shi, Sun, Zheng and Zheng. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jun Zheng, emhlbmdfY211QDE2My5jb20=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.