
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Neurosci., 19 November 2020
Sec. Brain Imaging Methods
Volume 14 - 2020 | https://doi.org/10.3389/fnins.2020.589050
 Lan Deng1†
Lan Deng1† Yun-Dong Zhang2*
Yun-Dong Zhang2* Jian-Wen Ji2†
Jian-Wen Ji2† Wen-Song Yang1,3
Wen-Song Yang1,3 Xiao Wei4
Xiao Wei4 Yi-Qing Shen1,3
Yi-Qing Shen1,3 Rui Li1
Rui Li1 Shu-Qiang Zhang1
Shu-Qiang Zhang1 Xin-Ni Lv1
Xin-Ni Lv1 Xin-Hui Li1,3
Xin-Hui Li1,3 Zhou-Ping Tang5
Zhou-Ping Tang5 Guo-Feng Wu6
Guo-Feng Wu6 Li-Bo Zhao7,8
Li-Bo Zhao7,8 Peng Xie1,3,7 and Qi Li1,3,7*
Peng Xie1,3,7 and Qi Li1,3,7*Objective: To investigate the relationship between hematoma ventricle distance (HVD) and clinical outcome in patients with intracerebral hemorrhage (ICH).
Methods: We prospectively enrolled consecutive patients with ICH in a tertiary academic hospital between July 2011 and April 2018. We retrospectively reviewed images for all patients receiving a computed tomography (CT) within 6 h after onset of symptoms and at least one follow-up CT scan within 36 h. The minimum distance of hematoma border to nearest ventricle was measured as HVD. Youden index was used to evaluate the cutoff of HVD predicting functional outcome. Logistic regression model was used to assess the HVD data and clinical poor outcome (modified Rankin Scale 4–6) at 90 days.
Results: A total of 325 patients were included in our final analysis. The median HVD was 2.4 mm (interquartile range, 0–5.7 mm), and 119 (36.6%) patients had poor functional outcome at 3 months. After adjusting for age, admission Glasgow coma scale, intraventricular hemorrhage, baseline ICH volume, admission systolic blood pressure, blood glucose, hematoma expansion, withdrawal of care, and hypertension, HVD ≤ 2.5 mm was associated with increased odds of clinical poor outcome [odd ratio, 3.59, (95%CI = 1.72–7.50); p = 0.001] in multivariable logistic regression analysis.
Conclusion: Hematoma ventricle distance allows physicians to quickly select and stratify patients in clinical trials and thereby serve as a novel and useful addition to predict ICH prognosis.
Intracerebral hemorrhage (ICH) is the most severe and devastating stroke subtype, with an up to 40% mortality at 30 days (van Asch et al., 2010; Krishnamurthi et al., 2014; Poon et al., 2014). Only 12–39% of ICH survivors achieve long-term functional independence (Krishnamurthi et al., 2014; An et al., 2017). Baseline hematoma volume (Broderick et al., 1993), infratentorial location (Delcourt et al., 2017), and the presence of intraventricular hemorrhage (Witsch et al., 2015; IVH) are independent predictors of functional outcome. Among them, the hematoma volume is the most powerful predictor of poor functional outcome. Several imaging markers such as computed tomographic (CT) angiography spot sign and non-contrast CT markers are also predictive of outcome (Sporns et al., 2018).
The location of hematoma also plays a key role in predicting outcome. Recent studies identified several affected anatomic regions that were associated with functional outcomes (Eslami et al., 2019). It is reported hematomas that involve the posterior limb of internal capsule or thalamus increases the risks of death or disability and of disability alone. However, few studies focused on specific brain structures and functional outcome. We hypothesized that hematomas located in the deep brain structures or close to the ventricles are more likely to have worse functional outcome than superficially located hematomas. We aim to derive a location-specific outcome prediction model to enhance the clinicians’ ability to prognosticate functional outcome after ICH. We hypothesized that patients may have poor outcome if the hematoma disrupts deep brain structures. Ventricles are located in the deep brain area, and the distance from the hematoma border to the ventricle may reflect the extent of involvement of deep brain structures.
Therefore, we derived a simple CT-based measurement of hematoma ventricle distance (HVD) to reflect the distance of hematoma to ventricles. The aim of our study was to investigate the association between HVD and functional outcome.
We prospectively enrolled consecutive patients with spontaneous non-lobar ICH in a tertiary academic hospital between July 2011 and April 2018. Briefly, we included patients with age > 18 years old, who received a CT within 6 h after onset of symptoms and at least one follow-up CT scan within 36 h. The main exclusion criteria were (1) primary IVH; (2) anticoagulants-associated bleeding; (3) secondary ICH due to trauma or tumor; (4) lobar hemorrhage; (5) secondary ICH due to rupture of arteriovenous malformation or intracranial aneurysm; and (6) surgery prior to follow-up CT.
Enrollment and data collection have been previously described (Li et al., 2019, 2020). In brief, the baseline clinical data included demographic data, hypertension, medication use, admission blood pressure, the National Institute of Health Stroke Scale (NIHSS) score, Glasgow Coma Scale (GCS) score, and pre-morbid modified Rankin Scale (mRS). End point was assessed using mRS score at 90 days. Poor outcome was defined as a mRS score of 4 to 6 (Eslami et al., 2019). Written informed consent was obtained from patients or legal representatives before enrollment. Approval for this study was obtained from The Ethics Committee of The First Affiliated Hospital of Chongqing Medical University.
All CT examinations were preformed following standard protocol as described before (Li et al., 2017, 2020). CT scans with 5 mm thick slices of axial view were stored in DICOM format. Trained neurologists (blinded to clinical data and outcome) evaluated the baseline CT scans. Hematoma expansion (HE) was defined as absolute growth of >6 ml or relative growth of >33% at follow-up CT scan (Poli et al., 2020). Hematoma location was evaluated on baseline CT and classified as lobar and non-lobar ICH. Lobar hemorrhage was defined as involvement of cortex or cortical–subcortical junction hematoma. Hematomas that are located at thalamus, basal ganglia, internal capsule, deep periventricular white matter, brain stem, or cerebellum was defined as non-lobar hemorrhage (Morotti et al., 2020). HVD was defined as minimum distance of hematoma border to nearest ventricle (Figure 1). HVD was measured independently by two trained researchers who were blinded to the clinical profiles of the patients, and HVD was measured using RadiAnt DICOM Viewer (Version 2020.2; Medixant Corporation, Poznań, Poland). The HVD was 0 if the hematoma reaches the border of ventricles with and without IVH extension (Figure 1D). We have operationally defined deeply located ICH as hematomas with HVD ≤ 2.5 mm.
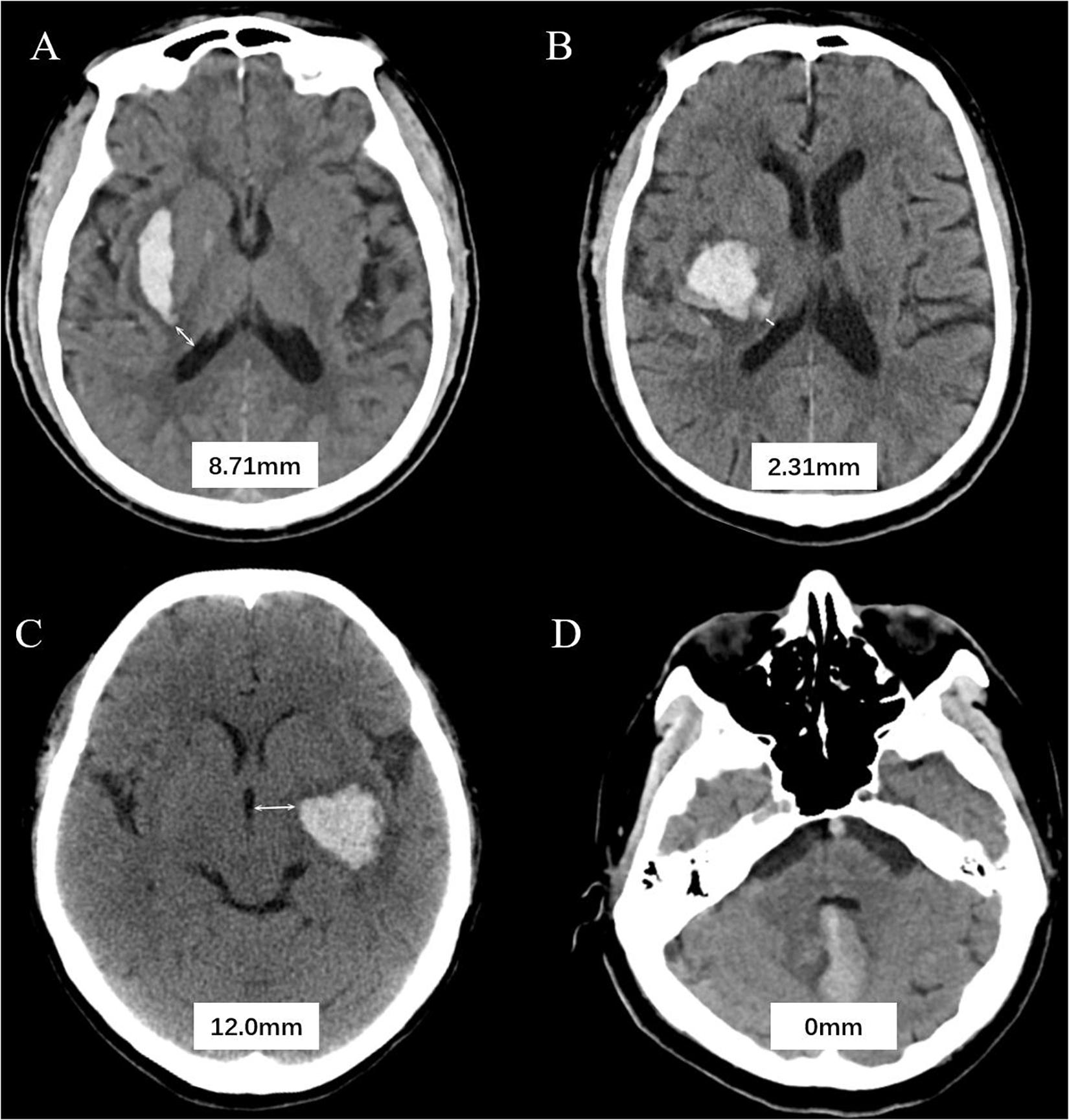
Figure 1. Graphic representation of the hematoma ventricle distance (HVD) on computed tomography. (A) A Baseline CT scan reveals a basal ganglia hematoma with HVD of 8.71 mm. (B) In patient B, the HVD is 2.31 mm. (C) A admission CT shows the distance between hematoma and the third ventricle is 2.0 mm. (D) Cerebellar hematoma reaches the border of ventricles without IVH extension.
All Statistical analyses were performed using SPSS software (Version 23.0; IBM Corporation, Armonk, NY, United States). Continuous variables were presented by mean [standard deviation (SD)] or median [interquartile range (IQR)], and categorical variables were summarized using number (percentage). Intergroup differences were assessed using Fisher exact test, Student’s t test, or the Mann–Whitney U test as appropriate. Intra-class correlation coefficient (ICC) was used to determine the interobserver agreement. Receiver operating characteristic curves were used to determine HVD cutoffs and associated sensitivity and specificity in predicting 3-month poor outcome. The Youden Index combining sensitivity and specificity was used for classification of dichotomous outcome to identify the cutoff of HVD. Then, the identified cutoffs were validated by univariable logistic regression model and multivariable logistic regression models. Since some of the variables are related to each other (e.g., HVD and neuroimaging variables), variance inflation factor (VIF) was used to calculate the collinearity, and a predictor of VIF > 3 was considered as an indicative collinearity. Multivariable logistic regression model was built using covariates with p < 0.1 in univariable logistic regression. We conducted a stepwise forward multivariable logistic regression model to assess whether deeply located ICH (HVD ≤ 2.5 mm), age, hypertension history, admission diastolic blood pressure, baseline hematoma volume, HE, blood glucose, withdrawal of care, and presence of IVH were associated with poor clinical outcome at 90 days. All selected variables were presented with odds ratio (OR), 95% confidence interval (CI), and p value. The p value of score test was displayed in non-selected variables, and no interaction terms were shown. We ascertained the calibration of the model using likelihood ratio and Hosmer and Lemeshow goodness-of-fit test. A p value < 0.05 was considered for statistical significance.
Among 470 spontaneous ICH, 325 patients met the eligibility criteria and were included in our study. The flow diagram of patient selection is illustrated in Figure 2. The study population included 209 (64.3%) men, and IVH extension was observed in 104 of 325 (32.0%) patients on the initial CT scan. Mean (±SD) ICH volume was 16.5 ± 14.0 ml. The ICH lesions were in the basal ganglia (n = 213, 65.5%), thalamus (n = 84, 25.9%), brainstem (n = 11, 3.4%), and cerebellum (n = 17, 5.2%). A total of 119 participants (36.6%) with poor clinical outcome had worse clinical severity scores (GCS and NIHSS, both p < 0.001), larger baseline hematoma volumes (p < 0.001), more IVH (p < 0.001), and more likely to be older (p = 0.008). The detailed intergroup differences between participants with poor clinical outcome and those without are shown in Table 1.
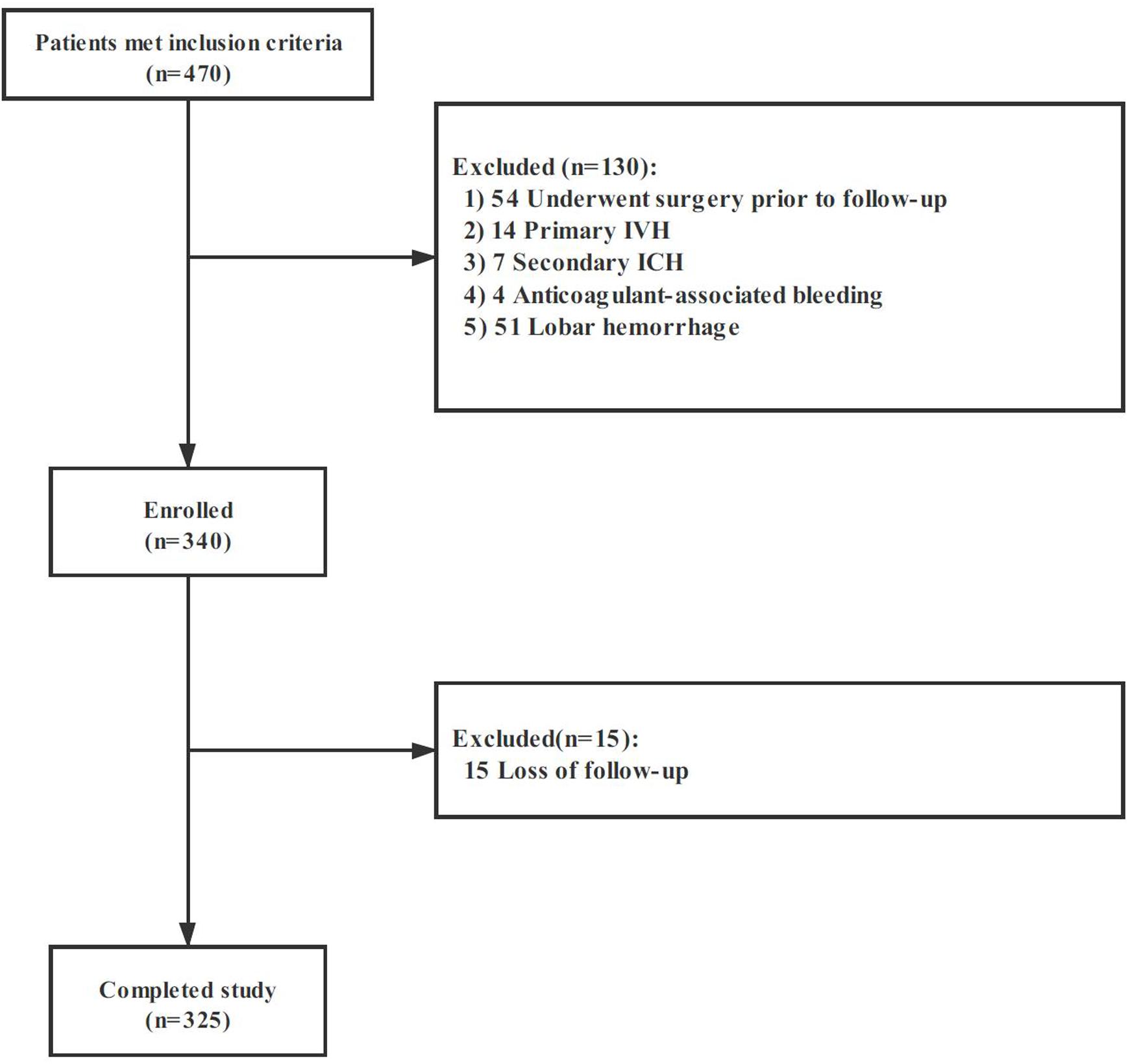
Figure 2. Cohort selection flowchart. Abbreviation: IVH, intraventricular hemorrhage; ICH, intracerebral hemorrhage.
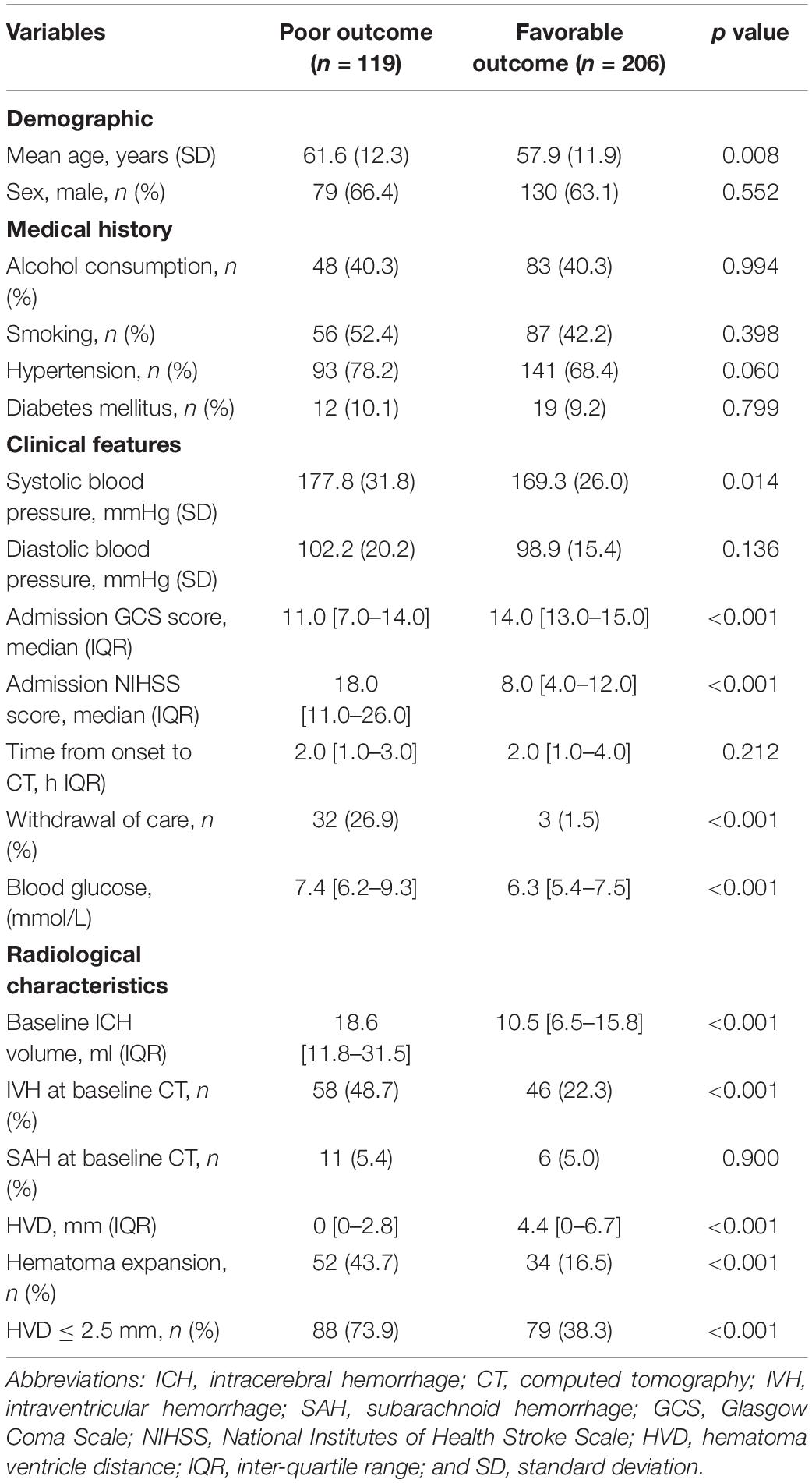
Table 1. Comparison of baseline demographic, clinical, and radiological characteristics between patients with and without poor outcome.
Excellent interobserver agreement [ICC = 0.882 (95%CI, 0.798–0.935), p < 0.001] and intrarater agreement [ICC = 0.941 (95%CI, 0.926–0.953), p < 0.001] were obtained for measurement of HVD. Overall, the median HVD was 2.4 (IQR, 0–5.7) mm. Patients with poor clinical outcome were more likely to have deeply located ICH (median 0 mm vs. 4.4 mm, p < 0.001, Table 1). We identified an optimal cutoff value of 2.47 mm for predicting poor outcome, and 2.5 mm was operationally chosen as clinically useful cutoff value. On baseline CT, deeply located ICH (HVD ≤ 2.5 mm) was observed in 167 (51.4%) participants. Among those, 104 out of 167 (62.3%) had concurrent IVH. In patients with deeply located ICH (HVD ≤ 2.5 mm), these were located in basal ganglia (n = 71, 42.5%), thalamus (n = 76, 45.5%), brainstem (n = 7, 4.2%), and cerebellum (n = 13, 7.8%). Patients with deeply located ICH (HVD ≤ 2.5 mm) were more likely to have higher NIHSS score (p < 0.001), lower GCS score (p < 0.001) and poor outcome (p < 0.001; Table 2).
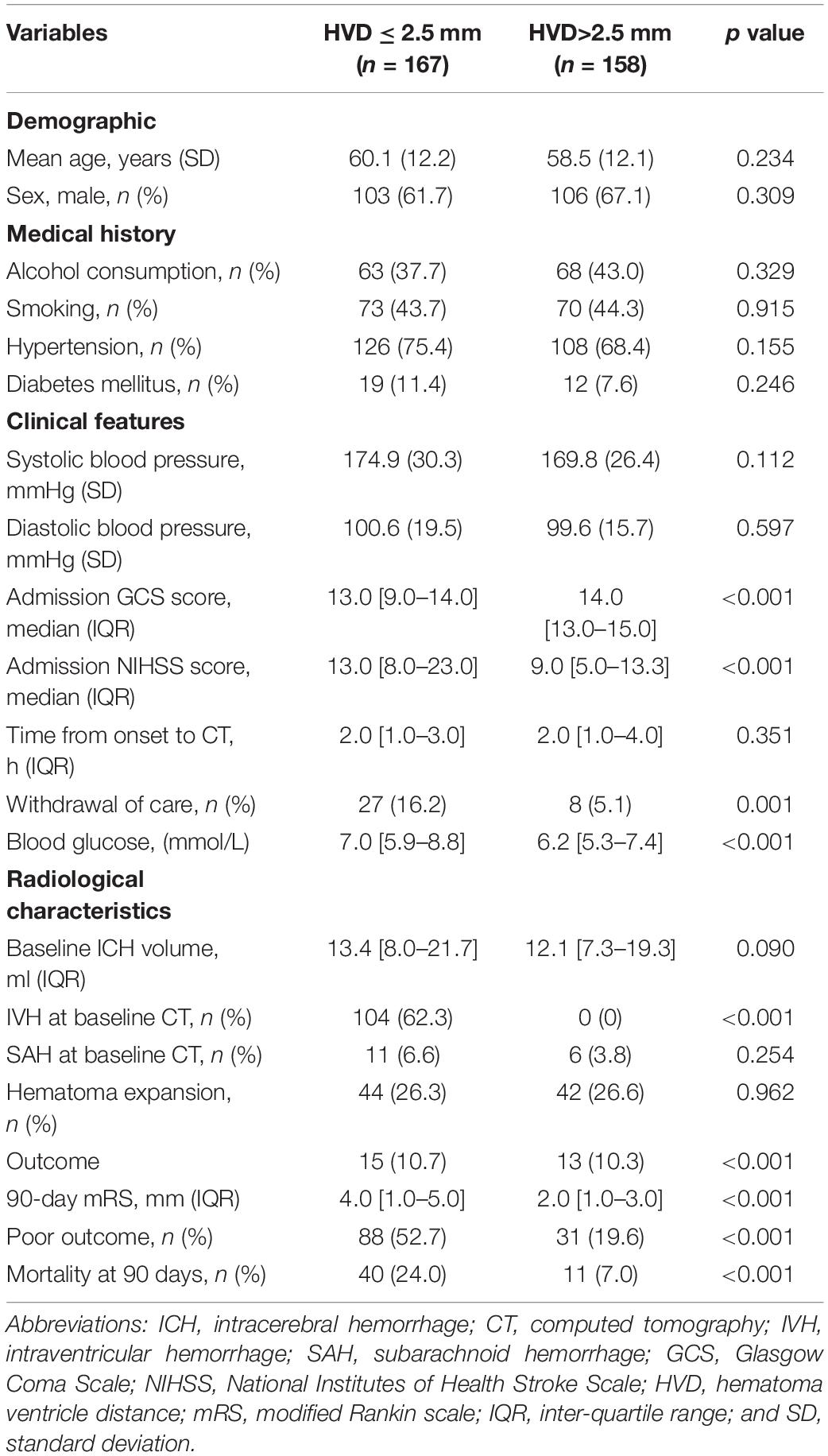
Table 2. Comparison of baseline demographic, clinical, and radiological characteristics between patients with hematoma ventricular distance shorter than 2.5 mm and those without.
The relationship between HVD and 90-day mRS score is shown in Figure 3. A total of 119 (36.6%) had poor functional outcome (mRS = 4–6) at 3-month follow-up. Patients with deeply located ICH (HVD ≤ 2.5 mm) were associated with poor functional outcome [OR, 3.59 (95%CI = 1.72–7.50); p = 0.001].
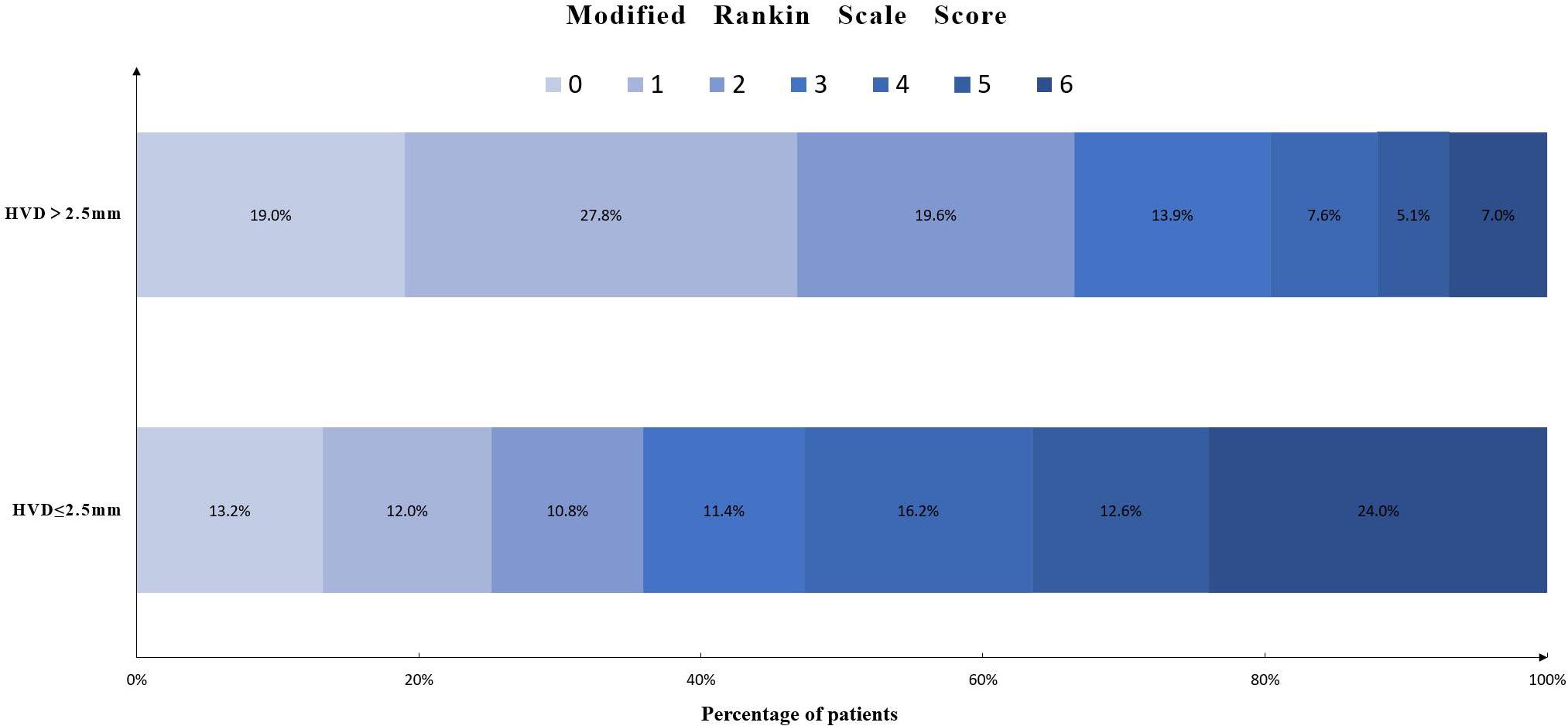
Figure 3. Distribution of modified Rankin scale score in patients with HVD ≤ 2.5 mm or HVD > 2.5 mm. Abbreviation: HVD, hematoma ventricle distance.
Univariate logistic regression showed that older age, higher systolic blood pressure, lower GCS score, higher NIHSS score, larger baseline hematoma volume, higher blood glucose, hematoma expansion, withdrawal of care, presence of IVH as well as the HVD increased the odds of poor clinical outcome (Table 3). In multivariable logistic regression analysis, HVD ≤ 2.5 mm remained an independent predictor of functional independence [OR, 3.59 (95%CI = 1.72–7.50); p = 0.001; Table 3] after adjusting for age, admission GCS score, systolic blood pressure, hypertension, baseline ICH volume, blood glucose, hematoma expansion, withdrawal of care, and IVH. This model was well built (likelihood ratio test, p < 0.001), and Hosmer and Lemeshow goodness-of-fit test showed good calibration (χ2 = 2.4; p = 0.967).
In the receiver operating characteristic analysis for the poor clinical outcome (mRS 3–6, without cerebellar/brainstem hemorrhage), the area under the curve of deeply located ICH (HVD ≤ 2.5 mm), HVD, and IVH were 0.69, 0.67, and 0.63, respectively. The sensitivity, specificity, positive predictive value, and negative predictive value of HVD ≤ 2.5 mm for predicting poor clinical outcome at 3 months were 65.5, 66.4, 66.0, and 66.0%, respectively.
In our study, we demonstrated that the distance between ventricle and hematoma on CT was independently associated with poor clinical outcome in patients with ICH. Furthermore, HVD seemed to be a better predictor of functional outcome as compared with baseline IVH. Our study provided evidence that deeply located ICHs (HVD ≤ 2.5 mm) were associated with poor functional outcome in patients with ICH. Since CT is widely available in almost all clinical settings, our finding may allow physicians to quickly select and stratify patients in clinical trials.
Non-contrast CT is a widely available diagnostic method of choice in patients with ICH. In our study, we have proposed a novel parameter called “hematoma ventricle distance” that represents the anatomic location of hematoma to ventricles. Previous studies suggested that deep and lobar ICHs may have different pathophysiological features (Hanley, 2009; Falcone et al., 2013). Basal ganglia or thalamic hemorrhages are more likely to damage deep brain structure than lobar hemorrhages. Non-lobar hemorrhages are more likely caused by hypertension, while lobar hemorrhages are mostly cerebral amyloid angiopathy related (Matsukawa et al., 2012; Falcone et al., 2013). Therefore, lobar hemorrhages were excluded. We have shown that the parameter is easy to measure with good inter-rater agreement, and the HVD is easy to identify on the baseline CT scan in comparison to other prognostic parameters. To our knowledge, this is the first study that demonstrated the predictive value of hematoma-to-ventricle distance with clinical outcome. Further, we investigated the quantitative threshold of HVD in predicting favorable and poor clinical outcome and found that a value of 2.5 mm could be optimal. Multivariable logistic regression analysis showed deeply located ICH (HVD ≤ 2.5 mm), GCS score, baseline ICH volume, HE, and withdrawal of care were independent predictors of functional independence.
The location of hematoma and functional outcome has been explored in several studies (Arboix et al., 2002; Delcourt et al., 2017; Eslami et al., 2019). A larger study of 2,066 patients from Intensive Blood Pressure Reduction in Acute Cerebral Haemorrhage Trial 2 (INTERACT 2) suggested that posterior limb of internal capsule, thalamus, and infratentorial involvement were associated with poor outcome (Lee et al., 2015; Delcourt et al., 2017). A recent secondary analysis of 467 ICH patients from Clot Lysis: Evaluating Accelerated Resolution of Intraventricular Hemorrhage III trial (CLEAR III) showed thalamic hemorrhage globus pallidus/putamen and posterior limb internal capsule had the associations with worse clinical outcome (Eslami et al., 2019). Another set of 1345 patients from Ethnic/Racial Variations of ICH (ERICH) study also reported poor outcome for thalamic hemorrhage (Leasure et al., 2018). These studies investigated the relationship between the specific anatomic hematoma location and clinical outcome, and some studies indicated the hematomas that are close to ventricles are more likely to have poor outcome (Feng et al., 2020). However, they did not further confirm it. We investigated the distance between hematoma margin and ventricle system, and it suggested that deeply located ICH (HVD ≤ 2.5 mm) represented a subtly different neuroimaging feature.
We also found a significant proportion of patients with HVD ≤ 2.5 mm had concurrent ICH. Recent studies suggested that IVH was a powerful predictor of poor clinical outcome, and reported 30-day mortality rate for patients with IVH was nearly 5 times higher than those without IVH (Tuhrim et al., 1999; Steiner et al., 2006; Mayer et al., 2008; Hanley, 2009; Hinson et al., 2010). In our study, IVH was an independent predictor of poor outcome in univariate analysis. We did not find IVH to be an independent predictor of poor clinical outcome in multivariate model (p = 0.139). However, HVD ≤ 2.5 mm remains an independent predictor of poor outcome in the multivariable logistic regression model, suggesting that HVD ≤ 2.5 mm is a more robust outcome parameter than IVH presence. We also demonstrated that HVD ≤ 2.5 mm has relatively high sensitivity for predicting poor outcome. Therefore, it might be used as an easy-to-use imaging marker for screening of patients who were likely to have poor outcome. However, future studies are needed to explore whether these patients are potential candidates for surgical or interventions.
The exact underlying mechanism of HVD and outcome remains unknown. A possible explanation is that hematomas that are close to ventricles are more likely to have short HVD. Recent studies suggested that structural lesions in the periventricular areas may cause clinical symptoms because of disruption of fiber tracts (Hanley, 2009; Eslami et al., 2019; Hurford et al., 2019). The clinical severity of the deficit is dependent on the anatomic location of such lesions and the functional integrity of affected fiber bundles. Since the periventricular area is located deep within the brain where white matter tracts are abundant, damage to this area is highly likely to have severe neurological deficits. Therefore, future studies employing diffuse tensor imaging of white matter tract damages are needed to validate this hypothesis.
There are several limitations in our study. First, our study is a retrospective analysis of a single-center cohort. Second, the sample size was relatively small, and it was not externally validated. Third, we only included patients with presumed hypertensive ICH. Whether our findings are generalizable to patients with lobar ICH is unclear. Fourth, computed tomography angiography (CTA) spot sign was not compared with HVD to see if alone or in combination they could give us better predictive power.
In summary, we demonstrated HVD ≤ 2.5 mm was associated with poor outcome independent of other established predictors. HVD seems to be an easy-to-use parameter for prognostic stratification in clinical settings, which could help guide the use of clinical resources by identifying patients who might benefit from intensive care.
The datasets presented in this article are not readily available because the datasets generated for this study are available on request to the corresponding author. Requests to access the datasets should be directed to QL cWlsaV9tZEAxMjYuY29t.
The studies involving human participants were reviewed and approved by The Ethics Committee of The First Affiliated Hospital of Chongqing Medical University. The patients/participants provided their written informed consent to participate in this study.
QL and LD: study concept and design. QL, RL, L-BZ, Y-DZ, J-WJ, W-SY, Y-QS, LD, X-NL, XW, and S-QZ: acquisition of data. LD: statistical analysis. Analysis and interpretation of data: all authors. LD: drafting of the manuscript. QL, Y-DZ, J-WJ, G-FW, Z-PT, and PX: critical revision of the manuscript for important intellectual content. QL: obtained funding. QL, Y-DZ, and PX: study supervision.
This study was supported by grants from the National Key R&D Program of China (No. 2018YFC1312200 and No.2018YFC1312203) and Chongqing High-end Young Investigator Project (No. 2019GDRC005).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
An, S. J., Kim, T. J., and Yoon, B. W. (2017). Epidemiology, risk factors, and clinical features of intracerebral hemorrhage: an update. J. Stroke 19, 3–10. doi: 10.5853/jos.2016.00864
Arboix, A., Comes, E., García-Eroles, L., Massons, J., Oliveres, M., Balcells, M., et al. (2002). Site of bleeding and early outcome in primary intracerebral hemorrhage. Acta Neurol. Scand. 105, 282–288. doi: 10.1034/j.1600-0404.2002.1o170.x
Broderick, J. P., Brott, T. G., Duldner, J. E., Tomsick, T., and Huster, G. (1993). Volume of intracerebral hemorrhage. A powerful and easy-to-use predictor of 30-day mortality. Stroke 24, 987–993. doi: 10.1161/01.str.24.7.987
Delcourt, C., Sato, S., Zhang, S., Sandset, E. C., Zheng, D., Chen, X., et al. (2017). Intracerebral hemorrhage location and outcome among INTERACT2 participants. Neurology 88, 1408–1414. doi: 10.1212/WNL.0000000000003771
Eslami, V., Tahsili-Fahadan, P., Rivera-Lara, L., Gandhi, D., Ali, H., Parry-Jones, A., et al. (2019). Influence of intracerebral hemorrhage location on outcomes in patients with severe intraventricular hemorrhage. Stroke 50, 1688–1695. doi: 10.1161/STROKEAHA.118.024187
Falcone, G. J., Biffi, A., Brouwers, H. B., Anderson, C. D., Battey, T. W., Ayres, A., et al. (2013). Predictors of hematoma volume in deep and lobar supratentorial intracerebral hemorrhage. JAMA Neurol. 70, 988–994. doi: 10.1001/jamaneurol.2013.98
Feng, H., Wang, X., Wang, W., and Zhao, X. (2020). Association between non-high-density lipoprotein cholesterol and 3-month prognosis in patients with spontaneous intracerebral hemorrhage. Front. Neurol. 11:920. doi: 10.3389/fneur.2020.00920
Hanley, D. F. (2009). Intraventricular hemorrhage: severity factor and treatment target in spontaneous intracerebral hemorrhage. Stroke 40, 1533–1538. doi: 10.1161/STROKEAHA.108.535419
Hinson, H. E., Hanley, D. F., and Ziai, W. C. (2010). Management of intraventricular hemorrhage. Curr. Neurol. Neurosci. Rep. 10, 73–82. doi: 10.1007/s11910-010-0086-6
Hurford, R., Vail, A., Heal, C., Ziai, W. C., Dawson, J., Murthy, S. B., et al. (2019). Oedema extension distance in intracerebral haemorrhage: association with baseline characteristics and long-term outcome. Eur. Stroke J. 4, 263–270. doi: 10.1177/2396987319848203
Krishnamurthi, R. V., Moran, A. E., Forouzanfar, M. H., Bennett, D. A., Mensah, G. A., Lawes, C. M., et al. (2014). The global burden of hemorrhagic stroke: a summary of findings from the GBD 2010 study. Glob. Heart 9, 101–106. doi: 10.1016/j.gheart.2014.01.003
Leasure, A. C., Sheth, K. N., Comeau, M., Aldridge, C., Vashkevich, A., Rosand, J., et al. (2018). Characterization of deep, supratentorial intracerebral hemorrhage in the Ethnic/Racial Variations of ICH Study [abstract P6.322]. Neurology 90(Suppl.):366.
Lee, S. H., Park, K. J., Kang, S. H., Jung, Y. G., Park, J. Y., and Park, D. H. (2015). Prognostic factors of clinical outcomes in patients with spontaneous thalamic hemorrhage. Med. Sci. Monit. 21, 2638–2646. doi: 10.12659/MSM.894132
Li, Q., Li, R., Zhao, L. B., Yang, X. M., Yang, W. S., Deng, L., et al. (2020). Intraventricular hemorrhage growth: definition, prevalence and association with hematoma expansion and prognosis [published online ahead of print, 2020 Mar 26]. Neurocrit. Care [Epub ahead of print]. doi: 10.1007/s12028-020-00958-8
Li, Q., Liu, Q. J., Yang, W. S., Wang, X. C., Zhao, L. B., Xiong, X., et al. (2017). Island sign: an imaging predictor for early hematoma expansion and poor outcome in patients with intracerebral hemorrhage. Stroke 48, 3019–3025. doi: 10.1161/STROKEAHA.117.017985
Li, Q., Shen, Y. Q., Xie, X. F., Xue, M. Z., Cao, D., Yang, W. S., et al. (2019). Expansion-prone hematoma: defining a population at high risk of hematoma growth and poor outcome. Neurocrit. Care 30, 601–608. doi: 10.1007/s12028-018-0644-3
Matsukawa, H., Shinoda, M., Fujii, M., Takahashi, O., Yamamoto, D., Murakata, A., et al. (2012). Factors associated with lobar vs. non-lobar intracerebral hemorrhage. Acta Neurol. Scand. 126, 116–121. doi: 10.1111/j.1600-0404.2011.01615.x
Mayer, S. A., Brun, N. C., Begtrup, K., Broderick, J., Davis, S., Diringer, M. N., et al. (2008). Efficacy and safety of recombinant activated factor vii for acute intracerebral hemorrhage. N. Engl. J. Med. 358, 2127–2137. doi: 10.1056/NEJMoa0707534
Morotti, A., Poli, L., Leuci, E., Mazzacane, F., Costa, P., De Giuli, V., et al. (2020). Subarachnoid extension predicts lobar intracerebral hemorrhage expansion. Stroke 51, 1470–1476. doi: 10.1161/STROKEAHA.119.028338
Poli, L., Leuci, E., Costa, P., De Giuli, V., Caria, F., Candeloro, E., et al. (2020). Validation and comparison of noncontrast CT scores to predict intracerebral hemorrhage expansion. Neurocrit. Care 32, 804–811. doi: 10.1007/s12028-019-00797-2
Poon, M. T., Fonville, A. F., and Al-Shahi Salman, R. (2014). Long-term prognosis after intracerebral haemorrhage: systematic review and meta-analysis. J. Neurol. Neurosurg. Psychiatry 85, 660–667. doi: 10.1136/jnnp-2013-306476
Sporns, P. B., Kemmling, A., Schwake, M., Minnerup, J., Nawabi, J., Broocks, G., et al. (2018). Triage of 5 noncontrast computed tomography markers and spot sign for outcome prediction after intracerebral hemorrhage. Stroke 49, 2317–2322. doi: 10.1161/STROKEAHA.118.021625
Steiner, T., Diringer, M. N., Schneider, D., Mayer, S. A., Begtrup, K., Broderick, J., et al. (2006). Dynamics of intraventricular hemorrhage in patients with spontaneous intracerebral hemorrhage: risk factors, clinical impact, and effect of hemostatic therapy with recombinant activated factor VII. Neurosurgery 59, 767–773. doi: 10.1227/01.NEU.0000232837.34992.32
Tuhrim, S., Horowitz, D. R., Sacher, M., and Godbold, J. H. (1999). Volume of ventricular blood is an important determinant of outcome in supratentorial intracerebral hemorrhage. Crit. Care Med. 27:617621. doi: 10.1097/00003246-199903000-00045
van Asch, C. J., Luitse, M. J., van der Tweel, I., Algra, A., and Klijn, C. J. (2010). Incidence, case fatality, and functional outcome of intracerebral haemorrhage over time, according to age, sex, and ethnic origin: a systematic review and meta-analysis. Lancet Neurol. 9, 167–176. doi: 10.1016/S1474-4422(09)70340-0
Keywords: intracerebral haemorrhage, hematoma ventricle distance, outcome, neuroimaging, stroke
Citation: Deng L, Zhang Y-D, Ji J-W, Yang W-S, Wei X, Shen Y-Q, Li R, Zhang S-Q, Lv X-N, Li X-H, Tang Z-P, Wu G-F, Zhao L-B, Xie P and Li Q (2020) Hematoma Ventricle Distance on Computed Tomography Predicts Poor Outcome in Intracerebral Hemorrhage. Front. Neurosci. 14:589050. doi: 10.3389/fnins.2020.589050
Received: 30 July 2020; Accepted: 19 October 2020;
Published: 19 November 2020.
Edited by:
Anand Joshi, University of Southern California, Los Angeles, United StatesReviewed by:
Craig S. Anderson, University of New South Wales, AustraliaCopyright © 2020 Deng, Zhang, Ji, Yang, Wei, Shen, Li, Zhang, Lv, Li, Tang, Wu, Zhao, Xie and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Qi Li, cWlsaV9tZEAxMjYuY29t; Yun-Dong Zhang, emhhbmdoeDY4QHNpbmEuY29t
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.