
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Neurosci. , 12 February 2020
Sec. Neuropharmacology
Volume 14 - 2020 | https://doi.org/10.3389/fnins.2020.00101
The roles of the hypothalamus and particularly the lateral hypothalamus (LH) in the regulation of inflammation and pain have been widely studied. The LH consists of a parasympathetic area that has connections with all the major parts of the brain. It controls the autonomic nervous system (ANS), regulates feeding behavior and wakeful cycles, and is a part of the reward system. In addition, it contains different types of neurons, most importantly the orexin neurons. These neurons, though few in number, perform critical functions such as inhibiting pain transmission and interfering with the reward system, feeding behavior and the hypothalamic pituitary axis (HPA). Recent evidence has identified a new role for orexin neurons in the modulation of pain transmission associated with several inflammatory diseases, including rheumatoid arthritis and ulcerative colitis. Here, we review recent findings on the various physiological functions of the LH with special emphasis on the orexin/receptor system and its role in mediating inflammatory pain.
The functions of the lateral hypothalamus (LH) in the regulation of vital body functions have become a popular research topic. Important among these functions is that of the regulation of inflammatory pain. The LH is also a site for integration of autonomic and endocrine responses, and a crucial regulator of pituitary function and homeostatic balance (Ferguson and Samson, 2003; Timper and Bruning, 2017). It is divided into three rostro-caudal zones – the anterior LH (aLH), the tuberal LH (tLH), and the posterior LH (pLH) (Berthoud and Munzberg, 2011). The aLH is continuous rostrally with the lateral preoptic area, and extends caudally to the level of the rostral pole of the venteromedial nucleus (VMN). While the tLH is coextensive with the VMN, the pLH follows the tuberal division at the level of the mamillary complex (Bernardis and Bellinger, 1993).
The LH plays a key role in regulating autonomic functions and relays information to all major parts of the brain including the major hypothalamic nuclei (Seoane-Collazo et al., 2015; Stuber and Wise, 2016). Converging evidence from functional, structural, and behavioral studies confirmed the importance of this region not only in regulating metabolism and feeding behavior, but also in serving as a motivation-cognition interface (Stuber and Wise, 2016; Petrovich, 2018). Interestingly, neurons in the LH are the largest in the hypothalamus and are topographically well organized (Bernardis and Bellinger, 1993). Chief among them are the orexin neurons that project widely to the neuraxis and undertake many important functions. A growing body of evidence suggests that orexin neurons play a key role in regulating wakefulness (Eggermann et al., 2003), sleep (Inutsuka and Yamanaka, 2013), food intake (Barson and Leibowitz, 2017), autonomic and endocrine functions (Grimaldi et al., 2014), reward-related behaviors (Espana, 2012) and pain-related behaviors (Inutsuka et al., 2016). However, despite significant progress in research, a more refined understanding of the detailed functions of orexin neurons of the LH in inflammatory pain is needed. This review provides a closer look on the functions and anatomy of LH neurons with an emphasis on the role of the orexin system in pain transmission and inflammatory disorders, and discusses avenues for future research.
The LH is involved in numerous functions, including sleep, arousal, and the regulation of the autonomic nervous system (ANS) (Gerashchenko and Shiromani, 2004; Szymusiak and McGinty, 2008; Seoane-Collazo et al., 2015). More importantly, it is considered to be a key regulatory center in feeding, hence its description as a “feeding center” (Petrovich, 2018).
LH neurons control feeding, blood pressure, heart rate, water intake and sodium excretion largely through the activation of adrenergic receptors (Shiraishi, 1991; Saad et al., 2000; Mendonca et al., 2018). In addition, they receive inhibitory noradrenergic input from the locus coeruleus, which helps prevent excessive activity in the arousal pathway during the waking cycle (Breton-Provencher and Sur, 2019). Also, beta (β)-adrenoceptors activation by noradrenaline in the LH appears to be involved in the suppression of feeding behavior (Leibowitz, 1970). On the other hand, activation of alpha 1 (α1)-adrenoceptors of the LH has been linked to behavioral activation and exploration, despite the insignificant number of these receptors in the LH (Lin et al., 2008).
The LH also plays an important role in the brain reward system. This was demonstrated by studies using intracranial self-stimulation in rodents showing that animals will willingly perform an operant response to receive rewarding pulses of electrical stimulation within the LH (Fakhoury et al., 2016; Ide et al., 2017). The rewarding effect of LH self-simulation is largely influenced by the dopamine and opioid systems as alterations in these systems were shown to either suppress or disrupt the self-stimulation behavior (Koob et al., 1978; Ide et al., 2017). The LH, through GABA neurons, also plays an important role in learning to respond to cues that predict the delivery of a reward (Sharpe et al., 2017). In addition, GABA neurons of the LH highly project to the ventral tegmental area (VTA) (Sharpe et al., 2017), a center rich in dopamine neurons that is known to be crucial for learning, reward processes and feeding behavior (Hommel et al., 2006; Ranaldi, 2014).
Recently, many reports have implicated the LH in the regulation of inflammatory pain (Holden and Pizzi, 2008; Jeong and Holden, 2009a; Wardach et al., 2016). For instance, studies have shown that stimulation of the LH produces analgesic and anti-nociceptive effects in an animal model of inflammatory pain (Jeong and Holden, 2009a), and that this effect is largely due to the activation of α-adrenoceptors in the dorsal horn of the spinal cord (Aimone and Gebhart, 1987; Jeong and Holden, 2009a), and to the involvement of lateral hypothalamic orexin neurons (Inutsuka et al., 2016). The specific role of the LH and orexin neurons in the regulation of pain and inflammation is discussed in details in subsequent sections.
Lateral hypothalamus neurons are composed of many overlapping neuronal populations that play distinct functions in the central nervous system (CNS) (Figure 1A). Studies over the past few decades have largely focused on the functions of GABAergic neurons of the LH and their projections in reward and feeding behavior (Suyama and Yada, 2018). Evidence suggests that these neurons encode information necessary for associating specific cues with reward delivery. In experiments employing optogenetics, a highly specific technique that involves the use of light to activate or inhibit neurons, inhibition of LH GABA neurons was shown to reduce responding to a cue predicting a food reward, indicating that these neurons encode information pertaining to reward prediction (Sharpe et al., 2017). On the other hand, optogenetic inhibition of LH GABA neurons that project to the VTA increased responding to the food reward-paired cue, suggesting that these neurons may play a role in relaying reward-predictive information to other neuronal structures (Sharpe et al., 2017). Interestingly, a study evaluating the role of LH GABA neurons projecting to the VTA showed that optogenetic activation of this pathway can either induce a feeding or rewarding effect depending on the frequency of the stimulation used (Barbano et al., 2016). Last but not least, a recent study by Giardino et al. (2018) found that the bed nucleus of the stria terminalis sends two non-overlapping GABAergic projections to the LH that express several neuropeptides including corticotropin-releasing factor (CRF) and cholecystokinin.
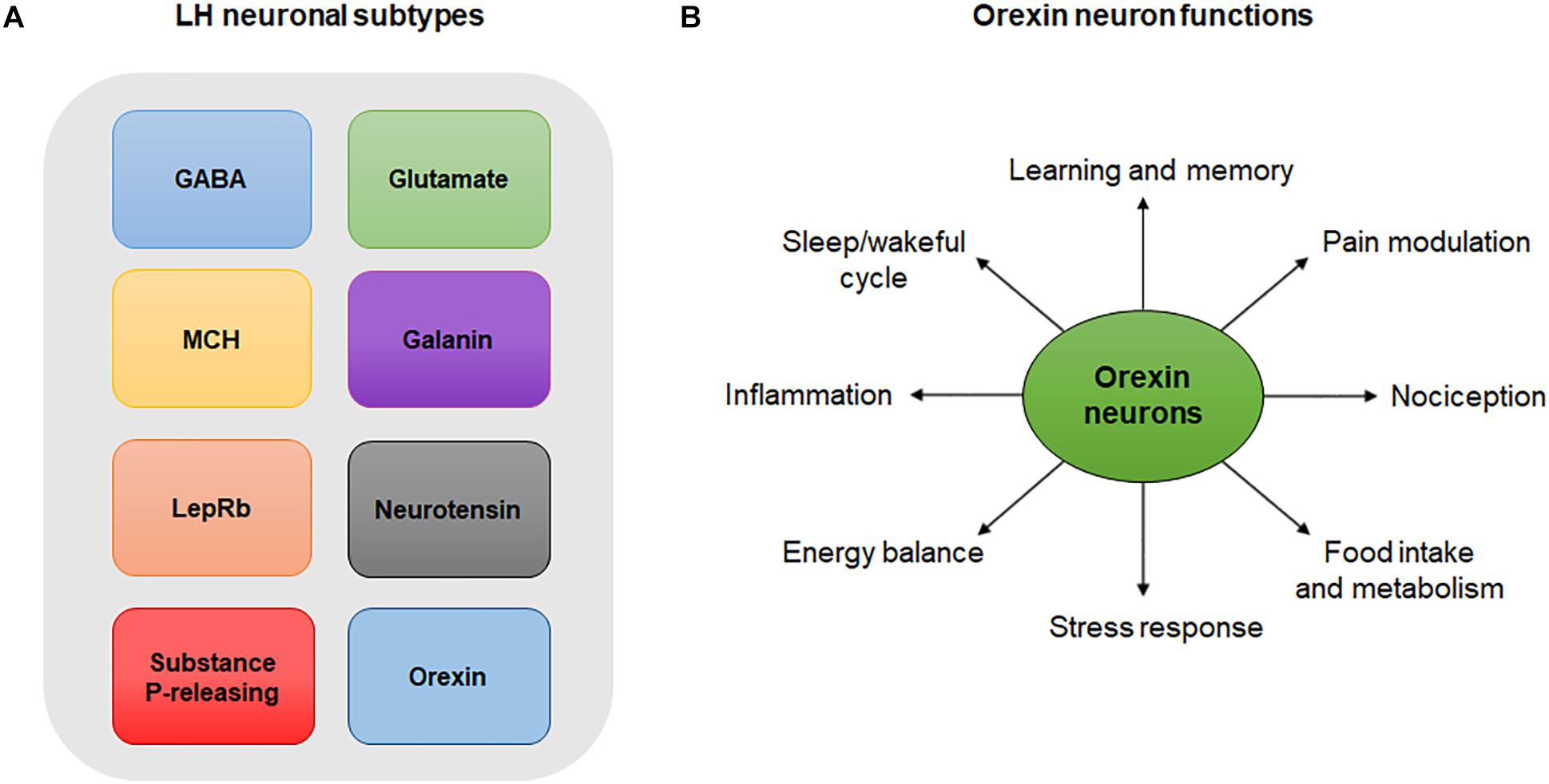
Figure 1. (A) LH neuronal subtypes. Simplistic diagram showing existing neuronal populations in the LH. Neuronal populations in the LH include, but are not limited to, GABA neurons, glutamate neurons, MCH-expressing neurons, galanin-expressing neurons, LepRb-expressing neurons, neurotensin-releasing neurons, substance P-releasing neurons and orexin neurons. The degree to which these neuronal populations overlap is not represented in this diagram. (B) Orexin neuron functions. Orexin neurons are involved in numerous physiological and behavioral processes including sleep/wakeful cycles, learning, memory, pain, nociception, food intake, metabolism, stress, energy balance and inflammation.
Glutamate neurons of the LH mediate important physiological processes in the CNS. Studies have shown that LH glutamate neurons produce behavioral functions opposite to those of LH GABA neurons. In mice, optogenetic activation of putative glutamate neurons of the LH suppressed feeding and produced aversion-related phenotypes (Jennings et al., 2013), while the opposite effect was observed following optogenetic or chemogenetic activation of LH GABA neurons (Jennings et al., 2015). The opposite functions of LH glutamate and GABA neurons in feeding and reward-related processes are mainly explained by differences in their projection pattern. Indeed, glutamate neurons of the LH send dense projections to the lateral habenula (LHb), a region involved in processing aversive stimuli (Stamatakis et al., 2016), in contrast to LH GABA neurons, whose projections mainly target the VTA (Barbano et al., 2016). Besides its role in the regulation of feeding and aversion-related behaviors, LH glutamate neurons have been implicated in compulsive (Mangieri et al., 2018) and hyperkinetic (Schneeberger et al., 2018) behaviors.
Neurons expressing melanin-concentrating hormone (MCH) are also widely present in the LH. Functional and anatomical studies showed that MCH neurons co-express the vesicular glutamate transporter 2 (VGLUT2), indicating a glutamatergic identity (Schneeberger et al., 2018), and project to several regions of the CNS ranging from the cortex to the spinal cord (Bittencourt and Elias, 1998). MCH is an orexigenic hypothalamic peptide that exerts inhibitory effects on lateral hypothalamic neurons (Gao, 2009). Although the mechanism of action of MCH is yet to be fully determined, its inhibitory effect on LH neurons is largely mediated by the attenuation of excitatory glutamate transmission presynaptically (Gao, 2009). MCH was also shown to depress synaptic activity of LH GABA neurons, suggesting a substantial level of complexity in its modulation of LH neuron activity (Gao and van den Pol, 2001). Mounting evidence suggests that MCH neurons directly regulate feeding behavior. Indeed, both administration of MCH (Qu et al., 1996; Clegg et al., 2003) and activation of MCH receptors (Shearman et al., 2003) increases food intake and facilitates body weight gain in rodents. Conversely, genetic knockout of the MCH gene (Shimada et al., 1998) or pharmacological blockade of MCH receptors (Shearman et al., 2003) leads to substantial decreases in food intake in mice. In addition, through their direct projections to gonadotropin-releasing hormone (GnRH) synthesizing neurons, MCH neurons convey critical homeostatic signals to the reproductive axis, and contribute considerably to the functional connection between the regulation of food intake and reproduction (Gao and van den Pol, 2001; Skrapits et al., 2015).
Another type of neuron found in the LH is the galanin-containing neuron (Qualls-Creekmore et al., 2017). These neurons represent a GABAergic subpopulation of LH neurons with a distinct molecular phenotype and projection pattern. Unlike GABAergic neurons of the LH, which project to the VTA, galanin neurons of the LH lack direct VTA innervation (Qualls-Creekmore et al., 2017). Instead, galanin neurons of the LH strongly innervate the locus coeruleus (Laque et al., 2015), a site involved in the control of arousal (Gonzalez and Aston-Jones, 2006; Sara and Bouret, 2012) and reward processing (Bouret and Richmond, 2015; Hofmeister and Sterpenich, 2015). Galanin is a 29 amino acid neuropeptide widely distributed in the brain (Skofitsch and Jacobowitz, 1986; Gentleman et al., 1989; Perez et al., 2001) that acts as an inhibitor of synaptic transmission in the hypothalamus (Kinney et al., 1998). Galanin also acts in the hypothalamus to produce behavioral hyperalgesia through activation of two descending pronociceptive pathways; one that involves the medullary dorsal reticular nucleus, and another one that involves serotonin neurons acting on the spinal cord (Amorim et al., 2015). Galanin has also been reported to promote feeding behavior. Indeed, chemogenetic activation of LH galanin neurons (Qualls-Creekmore et al., 2017) or central injection of galanin (Kyrkouli et al., 1990) enhances food-seeking behavior, while targeted knockout of the galanin gene (Adams et al., 2008) or the galanin receptor (Zorrilla et al., 2007) reduces dietary fat intake.
Another neuronal population of the LH involved in the regulation of feeding behaviors is the leptin-receptor (LepRb) expressing neuron. LepRb-expressing neurons are widely expressed in the brain, but are particular enriched within the hypothalamus and the brainstem (Elmquist et al., 1998). In mice, leptin acts on LepRb-expressing neurons of the LH to decrease feeding and body weight (Leinninger et al., 2009). LepRb-expressing neurons of the LH also innervate the VTA, and leptin action on these neurons increases VTA dopamine neuron activity, suggesting a link between the anorexic effect of leptin and the mesolimbic DA system (Leinninger et al., 2009). Interestingly, LepRb-expressing neurons in the LH are thought to be GABAergic (Leinninger et al., 2009), and a subpopulation of these neurons in the LH was shown to co-express the inhibitory acting neuropeptide galanin (Laque et al., 2013), suggesting that the anorexic effect of leptin is likely due to its interaction with other neuropeptidergic receptors in the LH.
Substance P and neurotensin-releasing neurons are also found in the LH (Ljungdahl et al., 1978; Yamano et al., 1986). Substance P is a member of the tachykinin neuropeptide family and is associated with multiple physiological processes including wound healing, neurogenic inflammation and tissue homeostasis (Suvas, 2017). In the LH, substance P-containing neurons have been proposed to exert antinociceptive functions by activating spinally projecting serotonin neurons in the rostral ventromedial medulla (RVM) (Holden and Pizzi, 2008). These cells activate spinally projecting serotonin neurons either through direct contact, or indirectly through the innervation of interneurons in the RVM, thereby altering nociceptive responses in the dorsal horn of the spinal cord (Holden and Pizzi, 2008).
On the other hand, neurotensin neurons of the LH are involved in the regulation of the sleep/wake cycle (Gerashchenko and Shiromani, 2004) and are implicated in feeding behavior (Woodworth et al., 2017) and reward processes (Kempadoo et al., 2013). Studies exploring the role of neurotensin in reward and feeding have indicated that this neuropeptide promotes reward by enhancing glutamate transmission in the mesolimbic dopaminergic system (Kempadoo et al., 2013) and promotes weight loss by suppressing the increased appetitive drive through activation of the G-protein-coupled neurotensin receptor-1 (Woodworth et al., 2017). Finally, neurotensin neurons of the LH have been implicated in a number of other physiological processes, including hyperthermia and energy balance, though the central mechanisms by which these processes are mediated remain to be fully elucidated (Brown et al., 2018; Naganuma et al., 2019).
By being an extensively researched population of cells in the past recent years, orexinergic neurons, and especially those of the LH, have been shown to have different roles in inflammatory pain and in the balance of psychological functions (Figure 1B). Orexinergic neurons synthesize two neuropeptides (Orexin A and B) from the precursor prepro-orexin (Chieffi et al., 2017). Furthermore, inhibition of orexin neurons by local GABAergic neurons of the LH is thought to disrupt the sleep cycle (Ferrari et al., 2018). On the other hand, inhibition of orexin neurons through activation of acetylcholine and dynorphin promotes wakefulness (Ferrari et al., 2018). The anatomy and distribution of orexin neurons and peptides as well as their functions in pain and inflammation is discussed in details in later sections.
Other neuronal populations in the LH not mentioned above include neurons that express cocaine- and amphetamine-regulated transcript, thyrotropin-releasing hormone, encephalin, urocortin-3 and corticotropin-releasing hormone (for review see Bonnavion et al., 2016; Tyree and de Lecea, 2017).
Inflammation is a biological response of the immune system that is triggered when the tissue is altered by any form of injury. It is characterized by the release of cytokines and by a noticeable change in the number of white blood cells (Zhang and An, 2007; Stiegel et al., 2016). Cytokines relay important inflammatory signals that initiate and maintain the inflammatory response along the cell signaling pathway (Zhang and An, 2007). This inflammatory response can ultimately alter the corresponding body organ function and induce a number of inflammatory-associated diseases (Chen L. et al., 2018; Jeon and Kim, 2018). Numerous studies indicate that the LH plays a significant role in the regulation of inflammatory pain. In particular, findings suggest that stimulation of the LH produces analgesic and anti-nociceptive effects in models of inflammatory pain (Jeong and Holden, 2009a; Jahangirvand et al., 2016; Wardach et al., 2016). Consistently, lateral hypothalamic nuclei were shown to receive dense nociceptive inputs from the spinothalamic tract, which conveys information related to pain (Dado et al., 1994).
In addition to its role in the regulation of inflammatory pain, the LH has been shown to regulate stress response though its functional connection with the HPA axis (Bonnavion et al., 2015; Mokhtarpour et al., 2016). The HPA axis is a complex set of neuronal connections that relay the hypothalamus to the pituitary gland, and the pituitary gland to the adrenal gland above the kidney (Herman et al., 2016). It mainly responds bi-directionally, either stimulating or inhibiting the secretion of certain hormones. This system is mainly activated when subject to a stressful event. It also maintains body balance at several levels, including metabolic, immune and endocrine. Any alteration to the HPA axis leads to stress response alterations (Kyrou and Tsigos, 2009). Hypophysiotropic neurons, which are found in the paraventricular nucleus (PVN), synthesize and secrete CRF. Stress responses stimulate the release of CRF into the hypophysial portal vessels, which in turn stimulate the anterior pituitary gland to release adrenocorticotropic hormone (ACTH). This hormone then acts on the adrenal cortex, its main target, which will stimulate the zona fasciculata to synthesize and secrete glucocorticoids (Gallo-Payet et al., 2017). Glucocorticoids are responsible for regulating physiological changes in the body and activating the HPA axis through intracellular receptors (Smith and Vale, 2006).
Numerous studies indicate that the HPA axis is highly influenced by inflammation and stress responses (Stephens and Wand, 2012; Chen et al., 2017). Activation of the HPA axis occurs through the corticotropin releasing hormone (CRH), which binds to CRH1 and CRH2 receptors, whose activation regulate several behavioral and physiological processes including anxiety and sleep (Reul and Holsboer, 2002). Activation of CRH receptors also lead to the excitation of orexin neurons (Winsky-Sommerer et al., 2004). These orexin neurons interfere with the arousal stress response states, and play a role in the modulation of the HPA axis (Messina et al., 2016). In particular, they mediate stress responses by regulating the release of CRH and by mediating the release of corticosterone and ACTH (Mokhtarpour et al., 2016; Grafe et al., 2017). On the other hand, the excitation of orexin and the release of corticosterone in the LH during stress responses is suppressed by leptin, a satiety hormone which acts through a network of leptin-sensitive GABA neurons within the LH (Bonnavion et al., 2015). Orexin, leptin and GABA neurons of the LH all play a crucial role in balancing the HPA axis, either directly or indirectly by acting on intermediate structures (Bonnavion et al., 2015).
Altogether, the findings mentioned above indicate that the LH, in conjunction with the HPA axis, functions to coordinate inflammation and stress responses in the CNS. In the following sections, a particular emphasis will be given to the anatomy and distribution of LH orexin neurons and their role in pain and inflammation.
Orexin, also known as hypocretin, is a neuropeptide secreted by orexin neurons in the lateral hypothalamic area. They are two types of orexin; orexin A (also referred to as hypocretin-1) and orexin B (also referred to as hypocretin-2). These neuropeptides originate from the same precursor known as prepro-orexin (Chieffi et al., 2017). Orexin-A is a 33-amino-acid peptide while orexin-B is a 28-amino-acid peptide (Chieffi et al., 2017). Both orexin A and orexin B bind to the G-protein coupled receptors orexin receptor 1 (OX-1) and 2 (OX-2) (also known as hypocretin receptors type 1 and 2) (Smart and Jerman, 2002). Orexin A binds to both OX-1 and OX-2 with the same affinity while orexin B has a higher affinity for OX-2 over OX-1 receptors (Sakurai et al., 1998).
In the CNS, orexin is co-localized with other transmitters, some of which include dynorphin (Chou et al., 2001), glutamate (Abrahamson et al., 2001; Torrealba et al., 2003), galanin (Barson et al., 2011) and prolactin (Risold et al., 1999). Experiments employing in situ hybridization and immunohistochemical techniques indicate that orexin neurons in the LH mostly express the vesicular glutamate transporters, VGLUT1 and VGLUT2, suggesting that they are glutamatergic (Rosin et al., 2003).
Orexin neurons project their axons to most parts in the brain and spinal cord, especially to areas that are involved in the modulation of pain (Watanabe et al., 2005). In addition, orexin neurons in the LH send projections to multiple sites related to arousal including the serotonergic dorsal raphe (Brown et al., 2002). Orexin neurons also project to the tuberomammillary nucleus (TMN) (Torrealba et al., 2003), a center involved in the control of arousal, learning and memory (Huston et al., 1997; Sakai et al., 2010). Pre-synaptically, orexin increases the release of glutamate and GABA in the hypothalamus, while post-synaptically, it increases Ca2+ levels, thus leading to the depolarization, hence activation, of TMN neurons by glutamatergic orexin terminals (Torrealba et al., 2003).
Last but not least, orexin neurons have been shown to directly interact with neuropeptide Y (NPY), a peptide that plays a role in the regulation of feeding behavior, metabolism and energy balance (Beck, 2006). This neuropeptide is primarily synthesized by neurons in the arcuate nucleus (ARC) and is present in different areas of the brain including the cortex, hippocampus, hindbrain and hypothalamus (Beck, 2006). Through its heavy projections to the ARC (Guan et al., 2001), orexin neurons interact with NPY to regulate numerous physiological processes and behaviors including food intake and Ca2+ signaling (Yamanaka et al., 2000; Muroya et al., 2004).
The distribution of OX-1 and OX-2 receptors has been established in different species including rats and mice. Studies employing in situ hybridization, immunohistochemistry and quantitative reverse transcription–polymerase chain reaction in rodents found that these receptors are widely distributed throughout the brain and spinal cord (Trivedi et al., 1998; Hervieu et al., 2001). Although some overlap exist in the distribution pattern of OX-1 and OX-2 receptors, these receptors are differentially expressed in the CNS (Trivedi et al., 1998; Marcus et al., 2001).
OX-1 receptors are primarily expressed in the ventromedial hypothalamic nucleus, prefrontal and infralimbic cortex, hippocampus, paraventricular thalamic nucleus, dorsal raphe, and locus coeruleus (Trivedi et al., 1998; Hervieu et al., 2001; Marcus et al., 2001), and to a lesser extent in the medial preoptic area, lateroanterior and dorsomedial hypothalamic nuclei, lateral mammillary nucleus and posterior hypothalamic area (Trivedi et al., 1998). They are also found in the periaqueductal gray and dorsal root ganglia, which suggests a role in the regulation of pain (Hervieu et al., 2001; Ho et al., 2011), and in the spinal cord, which suggests a role in the regulation of the parasympathetic and sympathetic system (Hervieu et al., 2001). On the other hand, OX-2 receptors are predominantly expressed in the TMN, PVN, cerebral cortex, NAc, subthalamic and paraventricular thalamic nuclei, septal nuclei, raphe nuclei, and anterior pretectal nucleus, and to a lesser extent in the ventromedial/dorsomedial hypothalamic nuclei and the posterior and lateral hypothalamic areas (Trivedi et al., 1998; Marcus et al., 2001).
Interestingly, a study examining the expression of OX-1 and OX-2 receptors mRNA with in situ hybridization in rats and mice found some species-specific differences (Ikeno and Yan, 2018). For instance, OX-1 receptors are expressed in the caudate putamen and ventral TMN in rats only, while they are detected in the bed nucleus of the stria terminalis, medial division, posteromedial part in mice only (Ikeno and Yan, 2018). On the other hand, OX-2 receptors show similar pattern of expression between the two species, though they are more widely expressed in the ventral TMN of rats compared to mice (Ikeno and Yan, 2018). This differential distribution of orexin receptors is consistent with the proposed multifaceted roles of orexin in regulating homeostasis and other functions in the CNS.
Orexin A and B neuropeptides, as demonstrated in the literature, are also widely expressed in different regions of the brain and spinal cord. Findings from immunohistochemical and radioimmunoassay techniques indicate that orexin A fibers are found throughout the hypothalamus, septum, thalamus, locus coeruleus and spinal cord, and in the paraventricular and supraoptic nucleus (Cutler et al., 1999; Date et al., 2000; Bingham et al., 2001; Nixon and Smale, 2007). In addition, orexin A fibers colocalize with substance P positive afferents of dorsal root ganglia neurons, which further strengthens its confirmed role in the regulation of pain (Colas et al., 2014). On the other hand, orexin B fibers are distributed sparsely in the hypothalamus and the spinal cord (Cutler et al., 1999; Date et al., 2000), but are absent in the paraventricular and supraoptic nucleus (Nixon and Smale, 2007). Interestingly, a study investigating the distribution of orexin A and orexin B in the brain of nocturnal and diurnal rodents found striking differences among species, in particular in the lateral mammillary nucleus, ventromedial hypothalamic nucleus and flocculus (Nixon and Smale, 2007).
Orexin neurons play multifaceted functions in the CNS. Not only do they play a role in the regulation of sleep/wakeful cycle (Inutsuka and Yamanaka, 2013), feeding behavior (Barson and Leibowitz, 2017), endocrine system (Taylor and Samson, 2003), stress response (Sargin, 2019), energy homeostasis (Sakurai, 2003) and rewarding behavior (Espana, 2012), they also play a role in the regulation of cognitive functions including learning and memory (Telegdy and Adamik, 2002; Akbari et al., 2007; Sharf et al., 2010; Aitta-Aho et al., 2016; Mavanji et al., 2017). More importantly, orexin neurons have been shown to play a crucial role in the modulation of pain transmission (Razavi and Hosseinzadeh, 2017).
First of all, orexin neurons project to areas involved in pain and sensation. Indeed, studies employing in situ hybridization and immunohistochemical techniques have shown that orexin receptors are present in high amounts in the NAc, dorsal root ganglia and spinal cord (Trivedi et al., 1998; Hervieu et al., 2001). All of these areas are known to modulate pain response (Thomas Cheng, 2010; Chang et al., 2014; Guha and Shamji, 2016), suggesting that orexin neurons are highly involved in pain regulation and nociceptive perception. The presence of heavy projections from orexin neurons of the lateral hypothalamic area to the dorsal horn of the spinal cord strongly implicates orexin neurons in nociceptive processing (van den Pol, 1999). In addition, localization of the fibers of orexin-containing neurons in the hypothalamus, locus coeruleus, thalamus and periaqueductal gray is consistent with their crucial roles in sensory processing (Marcus and Elmquist, 2006).
In animal models of inflammatory pain, administration of orexin receptor antagonists in the NAc or VTA was shown to decrease the LH-induced antinociceptive effect, indicating a role of orexin neurons projecting to the mesolimbic system in the modulation of inflammatory pain (Sadeghi et al., 2013; Ezzatpanah et al., 2016; Jahangirvand et al., 2016). Recent studies have also highlighted the dual integrative role of orexin neurons in nociceptive perception and analgesic regulation (Inutsuka et al., 2016). Evidently, orexin modulates pain perception at both spinal and supra-spinal levels (Razavi and Hosseinzadeh, 2017). Indeed, experiments in rodents showed that orexin-A neurons that project to the dorsal horn of the spinal cord mediates the antinociceptive effect of posterior hypothalamic stimulation (Jeong and Holden, 2009b), and that activation of orexin-1 receptor in the spinal cord suppresses pain responses in rodents (Yamamoto et al., 2002, 2003b; Kajiyama et al., 2005). At the supraspinal level, orexin was shown to act on several sites, including the periaqueductal gray (PAG) (Chen Y.H. et al., 2018) and the RVM (Azhdari-Zarmehri et al., 2014), to modulate pain perception.
In support of the notion that the orexin system plays a role in pain modulation, intrathecal administration of orexin A or B (Azhdari Zarmehri et al., 2011; Ghasemi et al., 2015; Azhdari-Zarmehri et al., 2018) and pharmacogenetic activation of orexin neurons (Inutsuka et al., 2016) was shown to produce anti-nociceptive and analgesic effects in experimental animals, respectively. Evidence also indicates that intrathecal or intracerebroventricular injection of orexin-A produces anti-mechanical allodynic effect in a rat model of neuropathic pain (Yamamoto et al., 2003a). It is important to note, however, that the anti-nociceptive effects of orexin A are more remarkable than those of orexin B, indicating that orexin-1 receptors are more involved than orexin-2 receptors in the regulation of nociceptive transmission (Yamamoto et al., 2002; Mobarakeh et al., 2005). In addition, the anti-nociceptive effects of orexin A were shown to be suppressed by administration of either dopamine (Okumura et al., 2015) or adenosine (Mobarakeh et al., 2005; Okumura et al., 2016) receptor antagonists, suggesting a possible involvement of the adenosine and dopamine pathways in orexin-induced antinociceptive actions. Accordingly, specific ablation of orexin neurons in mice was shown to increase pain perception generated by mechanical, thermal and chemical noxious stimuli (Inutsuka et al., 2016). Conversely, pharmacogenetic activation of orexin neurons was shown to induce analgesia in experimental animals (Inutsuka et al., 2016). Studies have also demonstrated the involvement of the endocannabinoid signaling in the analgesic and antinociceptive effects of orexin neurons (Ho et al., 2011; Lee et al., 2016).
Overall, stimulation of orexin neurons in the hypothalamus by peripheral inflammation and stressful conditions produces analgesic effects by activating descending inhibitory pathways. This analgesic effect is largely mediated by descending pathways from the hypothalamus to the dorsal horn of the spinal cord through the release of oxytocin, a neuropeptide elaborated by the hypothalamic paraventricular and supraoptic nuclei (Eliava et al., 2016). Release of oxytocin onto sensory spinal cord neurons in an animal model of inflammatory pain was previously shown to suppress nociception and promote analgesia (Eliava et al., 2016). One important supraspinal site of orexin pain modulation is the PAG (Razavi and Hosseinzadeh, 2017). Also, orexin neurons of the hypothalamus control nociceptive processing by receiving inputs from the dorsal horn of the spinal cord and from the adenosine and dopamine pathways, and through its interaction with the endocannabinoid system.
Owing to its important role in the regulation of inflammatory pain, it is not surprising that the orexin system has been implicated in the underlying mechanisms of a number of inflammatory disorders including rheumatoid arthritis and ulcerative colitis. Rheumatoid arthritis is a chronic inflammatory disease that primarily affects the lining of the synovial joints, with symptoms ranging from pain and stiffness to muscle weakness and weight loss (Combe, 2009). In a rat model of rheumatoid arthritis, intravenous administration of orexin A was shown to reduce pain sensation as well as the serum level of nerve growth factor (NGF), a major mediator of inflammatory and neuropathic pain (Mohamed and El-Hadidy, 2014); effects that are likely attributed to the activation of OX-1 receptors (Yamamoto and Shono, 2007). Accordingly, the expression of OX-1 receptors was found to be decreased in rheumatoid arthritis, indicating that these receptors may constitute a critical target for the treatment of this disease (Sun et al., 2018). Besides its role in arthritis, the orexin system was shown to be involved in the pathophysiology of ulcerative colitis. Ulcerative colitis is a chronic type of inflammatory bowel disease that causes damage in the mucosa and the superficial submucosa of the colon and the rectum resulting in inflammation of the large intestine (Fakhoury et al., 2014). Immunostaining of OX-1 receptors in human colonic mucosa show that OX-1 receptors are present in the inflamed mucosa of patients with ulcerative colitis, but absent in normal colon (Messal et al., 2018). In addition, intraperitoneal administration of orexin A in mouse models of colitis was shown to result in significant reductions in symptoms of ulcerative colitis, including colon length reduction and weight loss, and led to a marked reduction in the level of pro-inflammatory cytokines including IFN-gamma, IL-6, and TNF alpha (Messal et al., 2018). Thus, OX-1 receptors, along with orexin A, induce an anti-inflammatory effect by inhibiting the production of pro-inflammatory cytokines, thereby protecting the epithelium from inflammatory damage (Messal et al., 2018).
In addition, the orexin system has been involved in a number of other diseases where inflammation constitutes a key pathological feature; these include Multiple sclerosis (MS), Huntington’s disease (HD), Parkinson’s disease (PD), and Alzheimer’s disease (AD). In particular, findings indicate that patients with MS have significantly lower serum (Gencer et al., 2019) and cerebrospinal fluid (CSF) (Kato et al., 2003) levels of orexin A compared to normal values, suggesting that the orexin system could be an interesting target for MS treatment. Consistently, peripheral administration of orexin A in mice undergoing experimental autoimmune encephalomyelitis (EAE), a well-established model of MS, was shown to induce anti-inflammatory and neuroprotective effects, suggesting that it might constitute a potential therapeutic approach in MS (Becquet et al., 2019). On the other hand, in R6/2 mice, a well-established mouse model of HD, Petersen et al. (2005) reported a dramatic atrophy of orexin neurons in the LH and a significant decrease in the CSF level of orexin A (Petersen et al., 2005). A decrease in orexin-immunopositive neurons was also found in the hypothalamus of five HD patients (Petersen et al., 2005). Paradoxically, a more recent clinical report found no differences between the CSF levels of orexin A in patients with HD compared to healthy individuals (Baumann et al., 2006). Such discrepancy in the results could be attributed to differences in the method employed to quantify changes in the orexin system. In patients with PD, studies showed a significant decrease in the number of orexin neurons (Fronczek et al., 2007) and the level of orexin in ventricular CSF (Drouot et al., 2003). However, these results are inconsistent with reports indicating that CSF orexin level are not disturbed in PD (Ripley et al., 2001; Overeem et al., 2002), suggesting a complex role of the orexin system in the underlying pathophysiology of PD. Finally, studies concerned with the role of the orexin system in AD showed that CSF levels of orexin are elevated in AD patients compared to control individuals (Wennstrom et al., 2012; Liguori et al., 2016, 2017). On the contrary, another report showed that the number of orexin A-immunoreactive neurons and the concentration of orexin A in the CSF is markedly reduced in AD patients (Fronczek et al., 2012), suggesting some degree of complexity in the relationship between the orexin system and AD. Clearly more work is needed to better understand the role of the orexin system in the underlying pathophysiology of AD and other disorders characterized by aberrant inflammatory response.
The neuronal network of the LH has been implicated in many vital physiological functions in the body such as feeding behavior, reward systems, sleep/wakefulness cycle, stress regulation and inhibition of inflammatory pain. Integrated in this network are orexin neurons; though few in numbers, they play a big part in controlling several aspects of bodily functions because of their extensive projections in the CNS and the abundance of orexin receptors in peripheral organs. The role of these neurons in mediating inflammatory processes and regulating pain perception has been highlighted in several studies, however, more experiments are needed to better understand the signaling mechanisms underlying their anti-inflammatory and anti-nociceptive effects. Future work should also focus on better understanding the role of the orexin system in inflammatory disorders. The multifunctional role of orexin receptors makes them a credible therapeutic target for disorders characterized by inflammatory pain and could ultimately be used in clinical practice to help patients cope with their symptoms.
MF and IS contributed to the initial design of the review and wrote the first draft of the manuscript. WN and GM wrote sections of the manuscript. All authors contributed to manuscript revision, read, and approved the submitted version.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Abrahamson, E. E., Leak, R. K., and Moore, R. Y. (2001). The suprachiasmatic nucleus projects to posterior hypothalamic arousal systems. Neuroreport 12, 435–440. doi: 10.1097/00001756-200102120-00048
Adams, A. C., Clapham, J. C., Wynick, D., and Speakman, J. R. (2008). Feeding behaviour in galanin knockout mice supports a role of galanin in fat intake and preference. J. Neuroendocrinol. 20, 199–206. doi: 10.1111/j.1365-2826.2007.01638.x
Aimone, L. D., and Gebhart, G. F. (1987). Spinal monoamine mediation of stimulation-produced antinociception from the lateral hypothalamus. Brain Res. 403, 290–300. doi: 10.1016/0006-8993(87)90066-7
Aitta-Aho, T., Pappa, E., Burdakov, D., and Apergis-Schoute, J. (2016). Cellular activation of hypothalamic hypocretin/orexin neurons facilitates short-term spatial memory in mice. Neurobiol. Learn. Mem. 136, 183–188. doi: 10.1016/j.nlm.2016.10.005
Akbari, E., Naghdi, N., and Motamedi, F. (2007). The selective orexin 1 receptor antagonist SB-334867-A impairs acquisition and consolidation but not retrieval of spatial memory in Morris water maze. Peptides 28, 650–656. doi: 10.1016/j.peptides.2006.11.002
Amorim, D., Viisanen, H., Wei, H., Almeida, A., Pertovaara, A., and Pinto-Ribeiro, F. (2015). Galanin-mediated behavioural hyperalgesia from the dorsomedial nucleus of the hypothalamus involves two independent descending pronociceptive pathways. PLoS One 10:e0142919. doi: 10.1371/journal.pone.0142919
Azhdari-Zarmehri, H., Ghasemi, E., Heidari-Oranjaghi, N., and Sadegh, M. (2018). Analgesic tolerance induced by repeated morphine injections induces cross-tolerance to the analgesic effect of orexin-A in rats. Neuroreport 29, 224–228. doi: 10.1097/WNR.0000000000000964
Azhdari-Zarmehri, H., Semnanian, S., Fathollahi, Y., and Pakdel, F. G. (2014). Tail flick modification of orexin-a induced changes of electrophysiological parameters in the rostral ventromedial medulla. Cell J. 16, 131–140.
Azhdari Zarmehri, H., Semnanian, S., Fathollahi, Y., Erami, E., Khakpay, R., Azizi, H., et al. (2011). Intra-periaqueductal gray matter microinjection of orexin-A decreases formalin-induced nociceptive behaviors in adult male rats. J. Pain 12, 280–287. doi: 10.1016/j.jpain.2010.09.006
Barbano, M. F., Wang, H. L., Morales, M., and Wise, R. A. (2016). Feeding and reward are differentially induced by activating GABAergic lateral hypothalamic projections to VTA. J. Neurosci. 36, 2975–2985. doi: 10.1523/JNEUROSCI.3799-15.2016
Barson, J. R., Chang, G. Q., Poon, K., Morganstern, I., and Leibowitz, S. F. (2011). Galanin and the orexin 2 receptor as possible regulators of enkephalin in the paraventricular nucleus of the hypothalamus: relation to dietary fat. Neuroscience 193, 10–20. doi: 10.1016/j.neuroscience.2011.07.057
Barson, J. R., and Leibowitz, S. F. (2017). Orexin/hypocretin system: role in food and drug overconsumption. Int. Rev. Neurobiol. 136, 199–237. doi: 10.1016/bs.irn.2017.06.006
Baumann, C. R., Hersberger, M., and Bassetti, C. L. (2006). Hypocretin-1 (orexin A) levels are normal in Huntington’s disease. J. Neurol. 253, 1232–1233. doi: 10.1007/s00415-006-0146-7
Beck, B. (2006). Neuropeptide Y in normal eating and in genetic and dietary-induced obesity. Philos. Trans. R. Soc. Lond. B Biol. Sci. 361, 1159–1185. doi: 10.1098/rstb.2006.1855
Becquet, L., Abad, C., Leclercq, M., Miel, C., Jean, L., Riou, G., et al. (2019). Systemic administration of orexin A ameliorates established experimental autoimmune encephalomyelitis by diminishing neuroinflammation. J. Neuroinflamm 16:64. doi: 10.1186/s12974-019-1447-y
Bernardis, L. L., and Bellinger, L. L. (1993). The lateral hypothalamic area revisited: neuroanatomy, body weight regulation, neuroendocrinology and metabolism. Neurosci. Biobehav. Rev. 17, 141–193. doi: 10.1016/s0149-7634(05)80149-6
Berthoud, H. R., and Munzberg, H. (2011). The lateral hypothalamus as integrator of metabolic and environmental needs: from electrical self-stimulation to opto-genetics. Physiol. Behav. 104, 29–39. doi: 10.1016/j.physbeh.2011.04.051
Bingham, S., Davey, P. T., Babbs, A. J., Irving, E. A., Sammons, M. J., Wyles, M., et al. (2001). Orexin-A, an hypothalamic peptide with analgesic properties. Pain 92, 81–90. doi: 10.1016/s0304-3959(00)00470-x
Bittencourt, J. C., and Elias, C. F. (1998). Melanin-concentrating hormone and neuropeptide EI projections from the lateral hypothalamic area and zona incerta to the medial septal nucleus and spinal cord: a study using multiple neuronal tracers. Brain Res. 805, 1–19. doi: 10.1016/s0006-8993(98)00598-8
Bonnavion, P., Jackson, A. C., Carter, M. E., and de Lecea, L. (2015). Antagonistic interplay between hypocretin and leptin in the lateral hypothalamus regulates stress responses. Nat. Commun. 6:6266. doi: 10.1038/ncomms7266
Bonnavion, P., Mickelsen, L. E., Fujita, A., de Lecea, L., and Jackson, A. C. (2016). Hubs and spokes of the lateral hypothalamus: cell types, circuits and behaviour. J. Physiol. 594, 6443–6462. doi: 10.1113/JP271946
Bouret, S., and Richmond, B. J. (2015). Sensitivity of locus ceruleus neurons to reward value for goal-directed actions. J. Neurosci. 35, 4005–4014. doi: 10.1523/JNEUROSCI.4553-14.2015
Breton-Provencher, V., and Sur, M. (2019). Active control of arousal by a locus coeruleus GABAergic circuit. Nat. Neurosci. 22, 218–228. doi: 10.1038/s41593-018-0305-z
Brown, J., Sagante, A., Mayer, T., Wright, A., Bugescu, R., Fuller, P. M., et al. (2018). Lateral hypothalamic area neurotensin neurons are required for control of orexin neurons and energy balance. Endocrinology 159, 3158–3176. doi: 10.1210/en.2018-0031
Brown, R. E., Sergeeva, O. A., Eriksson, K. S., and Haas, H. L. (2002). Convergent excitation of dorsal raphe serotonin neurons by multiple arousal systems (orexin/hypocretin, histamine and noradrenaline). J. Neurosci. 22, 8850–8859. doi: 10.1523/jneurosci.22-20-08850.2002
Chang, P. C., Pollema-Mays, S. L., Centeno, M. V., Procissi, D., Contini, M., Baria, A. T., et al. (2014). Role of nucleus accumbens in neuropathic pain: linked multi-scale evidence in the rat transitioning to neuropathic pain. Pain 155, 1128–1139. doi: 10.1016/j.pain.2014.02.019
Chen, L., Deng, H., Cui, H., Fang, J., Zuo, Z., Deng, J., et al. (2018). Inflammatory responses and inflammation-associated diseases in organs. Oncotarget 9, 7204–7218. doi: 10.18632/oncotarget.23208
Chen, X., Gianferante, D., Hanlin, L., Fiksdal, A., Breines, J. G., Thoma, M. V., et al. (2017). HPA-axis and inflammatory reactivity to acute stress is related with basal HPA-axis activity. Psychoneuroendocrinology 78, 168–176. doi: 10.1016/j.psyneuen.2017.01.035
Chen, Y. H., Lee, H. J., Lee, M. T., Wu, Y. T., Lee, Y. H., Hwang, L. L., et al. (2018). Median nerve stimulation induces analgesia via orexin-initiated endocannabinoid disinhibition in the periaqueductal gray. Proc. Natl. Acad. Sci. U.S.A. 115, E10720–E10729. doi: 10.1073/pnas.1807991115
Chieffi, S., Carotenuto, M., Monda, V., Valenzano, A., Villano, I., Precenzano, F., et al. (2017). Orexin System: the key for a healthy life. Front. Physiol. 8:357. doi: 10.3389/fphys.2017.00357
Chou, T. C., Lee, C. E., Lu, J., Elmquist, J. K., Hara, J., Willie, J. T., et al. (2001). Orexin (hypocretin) neurons contain dynorphin. J. Neurosci. 21:RC168.
Clegg, D. J., Air, E. L., Benoit, S. C., Sakai, R. S., Seeley, R. J., and Woods, S. C. (2003). Intraventricular melanin-concentrating hormone stimulates water intake independent of food intake. Am. J. Physiol. Regul. Integr. Comp. Physiol. 284, R494–R499. doi: 10.1152/ajpregu.00399.2002
Colas, D., Manca, A., Delcroix, J. D., and Mourrain, P. (2014). Orexin A and orexin receptor 1 axonal traffic in dorsal roots at the CNS/PNS interface. Front. Neurosci. 8:20. doi: 10.3389/fnins.2014.00020
Combe, B. (2009). Progression in early rheumatoid arthritis. Best Pract. Res. Clin. Rheumatol. 23, 59–69. doi: 10.1016/j.berh.2008.11.006
Cutler, D. J., Morris, R., Sheridhar, V., Wattam, T. A., Holmes, S., Patel, S., et al. (1999). Differential distribution of orexin-A and orexin-B immunoreactivity in the rat brain and spinal cord. Peptides 20, 1455–1470. doi: 10.1016/s0196-9781(99)00157-6
Dado, R. J., Katter, J. T., and Giesler, G. J. (1994). Spinothalamic and spinohypothalamic tract neurons in the cervical enlargement of rats. I. Locations of antidromically identified axons in the thalamus and hypothalamus. J. Neurophysiol. 71, 959–980. doi: 10.1152/jn.1994.71.3.959
Date, Y., Mondal, M. S., Matsukura, S., and Nakazato, M. (2000). Distribution of orexin-A and orexin-B (hypocretins) in the rat spinal cord. Neurosci. Lett. 288, 87–90. doi: 10.1016/s0304-3940(00)01195-1192
Drouot, X., Moutereau, S., Nguyen, J. P., Lefaucheur, J. P., Creange, A., Remy, P., et al. (2003). Low levels of ventricular CSF orexin/hypocretin in advanced PD. Neurology 61, 540–543. doi: 10.1212/01.wnl.0000078194.53210.48
Eggermann, E., Bayer, L., Serafin, M., Saint-Mleux, B., Bernheim, L., Machard, D., et al. (2003). The wake-promoting hypocretin-orexin neurons are in an intrinsic state of membrane depolarization. J. Neurosci. 23, 1557–1562. doi: 10.1523/jneurosci.23-05-01557.2003
Eliava, M., Melchior, M., Knobloch-Bollmann, H. S., Wahis, J., da Silva Gouveia, M., et al. (2016). A new population of parvocellular oxytocin neurons controlling magnocellular neuron activity and inflammatory pain processing. Neuro 89, 1291–1304. doi: 10.1016/j.neuron.2016.01.041
Elmquist, J. K., Bjorbaek, C., Ahima, R. S., Flier, J. S., and Saper, C. B. (1998). Distributions of leptin receptor mRNA isoforms in the rat brain. J. Comp. Neurol. 395, 535–547. doi: 10.1002/(sici)1096-9861(19980615)395:4<535::aid-cne9>3.0.co;2-2
Espana, R. A. (2012). Hypocretin/orexin involvement in reward and reinforcement. Vitam. Horm. 89, 185–208. doi: 10.1016/B978-0-12-394623-2.00010-X
Ezzatpanah, S., Babapour, V., and Haghparast, A. (2016). Differential contribution of orexin receptors within the ventral tegmental area to modulation of persistent inflammatory pain. Eur. J. Pain 20, 1090–1101. doi: 10.1002/ejp.833
Fakhoury, M., Negrulj, R., Mooranian, A., and Al-Salami, H. (2014). Inflammatory bowel disease: clinical aspects and treatments. J. Inflamm. Res. 7, 113–120. doi: 10.2147/JIR.S65979
Fakhoury, M., Rompre, P. P., and Boye, S. M. (2016). Role of the dorsal diencephalic conduction system in the brain reward circuitry. Behav. Brain Res. 296, 431–441. doi: 10.1016/j.bbr.2015.10.038
Ferguson, A. V., and Samson, W. K. (2003). The orexin/hypocretin system: a critical regulator of neuroendocrine and autonomic function. Front. Neuroendocrinol. 24, 141–150. doi: 10.1016/S0091-3022(03)00028-1
Ferrari, L. L., Park, D., Zhu, L., Palmer, M. R., Broadhurst, R. Y., and Arrigoni, E. (2018). Regulation of lateral hypothalamic orexin activity by local GABAergic neurons. J. Neurosci. 38, 1588–1599. doi: 10.1523/JNEUROSCI.1925-17.2017
Fronczek, R., Overeem, S., Lee, S. Y., Hegeman, I. M., van Pelt, J., van Duinen, S. G., et al. (2007). Hypocretin (orexin) loss in Parkinson’s disease. Brain 130(Pt 6), 1577–1585. doi: 10.1093/brain/awm090
Fronczek, R., van Geest, S., Frolich, M., Overeem, S., Roelandse, F. W., Lammers, G. J., et al. (2012). Hypocretin (orexin) loss in Alzheimer’s disease. Neurobiol. Aging 33, 1642–1650. doi: 10.1016/j.neurobiolaging.2011.03.014
Gallo-Payet, N., Martinez, A., and Lacroix, A. (2017). Editorial: ACTH action in the adrenal cortex: from molecular biology to pathophysiology. Front. Endocrinol. 8:101. doi: 10.3389/fendo.2017.00101
Gao, X. B. (2009). Electrophysiological effects of MCH on neurons in the hypothalamus. Peptides 30, 2025–2030. doi: 10.1016/j.peptides.2009.05.006
Gao, X. B., and van den Pol, A. N. (2001). Melanin concentrating hormone depresses synaptic activity of glutamate and GABA neurons from rat lateral hypothalamus. J. Physiol. 533(Pt 1), 237–252. doi: 10.1111/j.1469-7793.2001.0237b.x
Gencer, M., Akbayir, E., Sen, M., Arsoy, E., Yilmaz, V., Bulut, N., et al. (2019). Serum orexin-A levels are associated with disease progression and motor impairment in multiple sclerosis. Neurol. Sci. 40, 1067–1070. doi: 10.1007/s10072-019-3708-z
Gentleman, S. M., Falkai, P., Bogerts, B., Herrero, M. T., Polak, J. M., and Roberts, G. W. (1989). Distribution of galanin-like immunoreactivity in the human brain. Brain Res. 505, 311–315. doi: 10.1016/0006-8993(89)91458-3
Gerashchenko, D., and Shiromani, P. J. (2004). Different neuronal phenotypes in the lateral hypothalamus and their role in sleep and wakefulness. Mol. Neurobiol. 29, 41–59. doi: 10.1385/MN:29:1,41
Ghasemi, E., Heidari-Oranjaghi, N., Azhdari-Zarmehri, H., and Sadegh, M. (2015). Repeated injections of orexin-A developed behavioral tolerance to its analgesic effects in rats. Iran J. Basic Med. Sci. 18, 1183–1188.
Giardino, W., Eban-Rothschild, A., Christoffel, D., Li, S. B., Malenka, R., and Lecea, L. (2018). Parallel circuits from the bed nuclei of stria terminalis to the lateral hypothalamus drive opposing emotional states. Nat. Neurosci. 21, 1084–1095. doi: 10.1038/s41593-018-0198-x
Gonzalez, M. M., and Aston-Jones, G. (2006). Circadian regulation of arousal: role of the noradrenergic locus coeruleus system and light exposure. Sleep 29, 1327–1336. doi: 10.1093/sleep/29.10.1327
Grafe, L. A., Eacret, D., Luz, S., Gotter, A. L., Renger, J. J., Winrow, C. J., et al. (2017). Orexin 2 receptor regulation of the hypothalamic-pituitary-adrenal (HPA) response to acute and repeated stress. Neuroscience 348, 313–323. doi: 10.1016/j.neuroscience.2017.02.038
Grimaldi, D., Silvani, A., Benarroch, E. E., and Cortelli, P. (2014). Orexin/hypocretin system and autonomic control: new insights and clinical correlations. Neurology 82, 271–278. doi: 10.1212/WNL.0000000000000045
Guan, J. L., Saotome, T., Wang, Q. P., Funahashi, H., Hori, T., Tanaka, S., et al. (2001). Orexinergic innervation of POMC-containing neurons in the rat arcuate nucleus. Neuroreport 12, 547–551. doi: 10.1097/00001756-200103050-00023
Guha, D., and Shamji, M. F. (2016). The dorsal root ganglion in the pathogenesis of chronic neuropathic pain. Neurosurgery 63(Suppl. 1), 118–126. doi: 10.1227/NEU.0000000000001255
Herman, J. P., McKlveen, J. M., Ghosal, S., Kopp, B., Wulsin, A., Makinson, R., et al. (2016). Regulation of the hypothalamic-pituitary-adrenocortical stress response. Compr. Physiol. 6, 603–621. doi: 10.1002/cphy.c150015
Hervieu, G. J., Cluderay, J. E., Harrison, D. C., Roberts, J. C., and Leslie, R. A. (2001). Gene expression and protein distribution of the orexin-1 receptor in the rat brain and spinal cord. Neuroscience 103, 777–797. doi: 10.1016/s0306-4522(01)00033-31
Ho, Y. C., Lee, H. J., Tung, L. W., Liao, Y. Y., Fu, S. Y., Teng, S. F., et al. (2011). Activation of orexin 1 receptors in the periaqueductal gray of male rats leads to antinociception via retrograde endocannabinoid (2-arachidonoylglycerol)-induced disinhibition. J. Neurosci. 31, 14600–14610. doi: 10.1523/JNEUROSCI.2671-11.2011
Hofmeister, J., and Sterpenich, V. (2015). A role for the locus ceruleus in reward processing: encoding behavioral energy required for goal-directed actions. J. Neurosci. 35, 10387–10389. doi: 10.1523/JNEUROSCI.1734-15.2015
Holden, J. E., and Pizzi, J. A. (2008). Lateral hypothalamic-induced antinociception may be mediated by a substance P connection with the rostral ventromedial medulla. Brain Res. 1214, 40–49. doi: 10.1016/j.brainres.2008.03.051
Hommel, J. D., Trinko, R., Sears, R. M., Georgescu, D., Liu, Z. W., Gao, X. B., et al. (2006). Leptin receptor signaling in midbrain dopamine neurons regulates feeding. Neuron 51, 801–810. doi: 10.1016/j.neuron.2006.08.023
Huston, J. P., Wagner, U., and Hasenohrl, R. U. (1997). The tuberomammillary nucleus projections in the control of learning, memory and reinforcement processes: evidence for an inhibitory role. Behav. Brain Res. 83, 97–105. doi: 10.1016/s0166-4328(97)86052-4
Ide, S., Takahashi, T., Takamatsu, Y., Uhl, G. R., Niki, H., Sora, I., et al. (2017). Distinct roles of opioid and dopamine systems in lateral hypothalamic intracranial self-stimulation. Int. J. Neuropsychopharmacol. 20, 403–409. doi: 10.1093/ijnp/pyw113
Ikeno, T., and Yan, L. (2018). A comparison of the orexin receptor distribution in the brain between diurnal Nile grass rats (Arvicanthis niloticus) and nocturnal mice (Mus musculus). Brain Res. 1690, 89–95. doi: 10.1016/j.brainres.2018.04.002
Inutsuka, A., and Yamanaka, A. (2013). The physiological role of orexin/hypocretin neurons in the regulation of sleep/wakefulness and neuroendocrine functions. Front. Endocrinol. 4:18. doi: 10.3389/fendo.2013.00018
Inutsuka, A., Yamashita, A., Chowdhury, S., Nakai, J., Ohkura, M., Taguchi, T., et al. (2016). The integrative role of orexin/hypocretin neurons in nociceptive perception and analgesic regulation. Sci. Rep. 6:29480. doi: 10.1038/srep29480
Jahangirvand, M., Yazdi, F., Moradi, M., and Haghparast, A. (2016). Intra-accumbal Orexin-1 receptors are involved in antinociception induced by stimulation of the lateral hypothalamus in the formalin test as an animal model of persistent inflammatory pain. Iran J. Pharm. Res. 15, 851–859.
Jennings, J. H., Rizzi, G., Stamatakis, A. M., Ung, R. L., and Stuber, G. D. (2013). The inhibitory circuit architecture of the lateral hypothalamus orchestrates feeding. Science 341, 1517–1521. doi: 10.1126/science.1241812
Jennings, J. H., Ung, R. L., Resendez, S. L., Stamatakis, A. M., Taylor, J. G., Huang, J., et al. (2015). Visualizing hypothalamic network dynamics for appetitive and consummatory behaviors. Cell 160, 516–527. doi: 10.1016/j.cell.2014.12.026
Jeon, S. W., and Kim, Y. K. (2018). The role of neuroinflammation and neurovascular dysfunction in major depressive disorder. J. Inflamm. Res. 11, 179–192. doi: 10.2147/JIR.S141033
Jeong, Y., and Holden, J. E. (2009a). Lateral hypothalamic-induced alpha-adrenoceptor modulation occurs in a model of inflammatory pain in rats. Biol. Res. Nurs. 10, 331–339. doi: 10.1177/1099800408325053
Jeong, Y., and Holden, J. E. (2009b). The role of spinal orexin-1 receptors in posterior hypothalamic modulation of neuropathic pain. Neuroscience 159, 1414–1421. doi: 10.1016/j.neuroscience.2009.02.006
Kajiyama, S., Kawamoto, M., Shiraishi, S., Gaus, S., Matsunaga, A., Suyama, H., et al. (2005). Spinal orexin-1 receptors mediate anti-hyperalgesic effects of intrathecally-administered orexins in diabetic neuropathic pain model rats. Brain Res. 1044, 76–86. doi: 10.1016/j.brainres.2005.03.007
Kato, T., Kanbayashi, T., Yamamoto, K., Nakano, T., Shimizu, T., Hashimoto, T., et al. (2003). Hypersomnia and low CSF hypocretin-1 (orexin-A) concentration in a patient with multiple sclerosis showing bilateral hypothalamic lesions. Intern. Med. 42, 743–745. doi: 10.2169/internalmedicine.42.743
Kempadoo, K. A., Tourino, C., Cho, S. L., Magnani, F., Leinninger, G. M., Stuber, G. D., et al. (2013). Hypothalamic neurotensin projections promote reward by enhancing glutamate transmission in the VTA. J. Neurosci. 33, 7618–7626. doi: 10.1523/JNEUROSCI.2588-12.2013
Kinney, G. A., Emmerson, P. J., and Miller, R. J. (1998). Galanin receptor-mediated inhibition of glutamate release in the arcuate nucleus of the hypothalamus. J. Neurosci. 18, 3489–3500. doi: 10.1523/jneurosci.18-10-03489.1998
Koob, G. F., Fray, P. J., and Iversen, S. D. (1978). Self-stimulation at the lateral hypothalamus and locus coeruleus after specific unilateral lesions of the dopamine system. Brain Res. 146, 123–140. doi: 10.1016/0006-8993(78)90222-6
Kyrkouli, S. E., Stanley, B. G., Seirafi, R. D., and Leibowitz, S. F. (1990). Stimulation of feeding by galanin: anatomical localization and behavioral specificity of this peptide’s effects in the brain. Peptides 11, 995–1001. doi: 10.1016/0196-9781(90)90023-x
Kyrou, I., and Tsigos, C. (2009). Stress hormones: physiological stress and regulation of metabolism. Curr. Opin. Pharmacol. 9, 787–793. doi: 10.1016/j.coph.2009.08.007
Laque, A., Yu, S., Qualls-Creekmore, E., Gettys, S., Schwartzenburg, C., Bui, K., et al. (2015). Leptin modulates nutrient reward via inhibitory galanin action on orexin neurons. Mol. Metab. 4, 706–717. doi: 10.1016/j.molmet.2015.07.002
Laque, A., Zhang, Y., Gettys, S., Nguyen, T. A., Bui, K., Morrison, C. D., et al. (2013). Leptin receptor neurons in the mouse hypothalamus are colocalized with the neuropeptide galanin and mediate anorexigenic leptin action. Am. J. Physiol. Endocrinol. Metab. 304, E999–E1011. doi: 10.1152/ajpendo.00643.2012
Lee, H. J., Chang, L. Y., Ho, Y. C., Teng, S. F., Hwang, L. L., Mackie, K., et al. (2016). Stress induces analgesia via orexin 1 receptor-initiated endocannabinoid/CB1 signaling in the mouse periaqueductal gray. Neuropharmacology 105, 577–586. doi: 10.1016/j.neuropharm.2016.02.018
Leibowitz, S. F. (1970). Reciprocal hunger-regulating circuits involving alpha- and beta-adrenergic receptors located, respectively, in the ventromedial and lateral hypothalamus. Proc. Natl. Acad. Sci. U.S.A. 67, 1063–1070. doi: 10.1073/pnas.67.2.1063
Leinninger, G. M., Jo, Y. H., Leshan, R. L., Louis, G. W., Yang, H., Barrera, J. G., et al. (2009). Leptin acts via leptin receptor-expressing lateral hypothalamic neurons to modulate the mesolimbic dopamine system and suppress feeding. Cell Metab. 10, 89–98. doi: 10.1016/j.cmet.2009.06.011
Liguori, C., Chiaravalloti, A., Nuccetelli, M., Izzi, F., Sancesario, G., Cimini, A., et al. (2017). Hypothalamic dysfunction is related to sleep impairment and CSF biomarkers in Alzheimer’s disease. J. Neurol. 264, 2215–2223. doi: 10.1007/s00415-017-8613-x
Liguori, C., Nuccetelli, M., Izzi, F., Sancesario, G., Romigi, A., Martorana, A., et al. (2016). Rapid eye movement sleep disruption and sleep fragmentation are associated with increased orexin-A cerebrospinal-fluid levels in mild cognitive impairment due to Alzheimer’s disease. Neurobiol. Aging 40, 120–126. doi: 10.1016/j.neurobiolaging.2016.01.007
Lin, Y., Quartermain, D., Dunn, A. J., Weinshenker, D., and Stone, E. A. (2008). Possible dopaminergic stimulation of locus coeruleus alpha1-adrenoceptors involved in behavioral activation. Synapse 62, 516–523. doi: 10.1002/syn.20517
Ljungdahl, A., Hokfelt, T., and Nilsson, G. (1978). Distribution of substance P-like immunoreactivity in the central nervous system of the rat–I. Cell bodies and nerve terminals. Neuroscience 3, 861–943. doi: 10.1016/0306-4522(78)90116-90111
Mangieri, L. R., Lu, Y., Xu, Y., Cassidy, R. M., Xu, Y., Arenkiel, B. R., et al. (2018). A neural basis for antagonistic control of feeding and compulsive behaviors. Nat. Commun. 9:52. doi: 10.1038/s41467-017-02534-9
Marcus, J. N., Aschkenasi, C. J., Lee, C. E., Chemelli, R. M., Saper, C. B., Yanagisawa, M., et al. (2001). Differential expression of orexin receptors 1 and 2 in the rat brain. J. Comp. Neurol. 435, 6–25. doi: 10.1002/cne.1190
Marcus, J. N., and Elmquist, J. K. (2006). “Orexin projections and localization of orexin receptors,” in The Orexin/Hypocretin System, eds S. Nishino, and T. Sakurai, (Totowa, NJ: Humana Press).
Mavanji, V., Butterick, T. A., Duffy, C. M., Nixon, J. P., Billington, C. J., and Kotz, C. M. (2017). Orexin/hypocretin treatment restores hippocampal-dependent memory in orexin-deficient mice. Neurobiol. Learn. Mem. 146, 21–30. doi: 10.1016/j.nlm.2017.10.014
Mendonca, M. M., Santana, J. S., da Cruz, K. R., Ianzer, D., Ghedini, P. C., Nalivaiko, E., et al. (2018). Involvement of GABAergic and adrenergic neurotransmissions on paraventricular nucleus of hypothalamus in the control of cardiac function. Front. Physiol. 9:670. doi: 10.3389/fphys.2018.00670
Messal, N., Fernandez, N., Dayot, S., Gratio, V., Nicole, P., Prochasson, C., et al. (2018). Ectopic expression of OX1R in ulcerative colitis mediates anti-inflammatory effect of orexin-A. Biochim. Biophys. Acta Mol. Basis Dis. 1864, 3618–3628. doi: 10.1016/j.bbadis.2018.08.023
Messina, A., De Fusco, C., Monda, V., Esposito, M., Moscatelli, F., Valenzano, A., et al. (2016). Role of the orexin system on the hypothalamus-pituitary-thyroid axis. Front. Neural Circ. 10:66. doi: 10.3389/fncir.2016.00066
Mobarakeh, J. I., Takahashi, K., Sakurada, S., Nishino, S., Watanabe, H., Kato, M., et al. (2005). Enhanced antinociception by intracerebroventricularly and intrathecally-administered orexin A and B (hypocretin-1 and -2) in mice. Peptides 26, 767–777. doi: 10.1016/j.peptides.2005.01.001
Mohamed, A. R., and El-Hadidy, W. F. (2014). Effect of orexin-A (hypocretin-1) on hyperalgesic and cachectic manifestations of experimentally induced rheumatoid arthritis in rats. Can. J. Physiol. Pharmacol. 92, 813–820. doi: 10.1139/cjpp-2014-0258
Mokhtarpour, M., Elahdadi Salmani, M., Lashkarbolouki, T., Abrari, K., and Goudarzi, I. (2016). Lateral hypothalamus orexinergic system modulates the stress effect on pentylenetetrazol induced seizures through corticotropin releasing hormone receptor type 1. Neuropharmacology 110(Pt A), 15–24. doi: 10.1016/j.neuropharm.2016.07.005
Muroya, S., Funahashi, H., Yamanaka, A., Kohno, D., Uramura, K., Nambu, T., et al. (2004). Orexins (hypocretins) directly interact with neuropeptide Y, POMC and glucose-responsive neurons to regulate Ca 2+ signaling in a reciprocal manner to leptin: orexigenic neuronal pathways in the mediobasal hypothalamus. Eur. J. Neurosci. 19, 1524–1534. doi: 10.1111/j.1460-9568.2004.03255.x
Naganuma, F., Kroeger, D., Bandaru, S. S., Absi, G., Madara, J. C., and Vetrivelan, R. (2019). Lateral hypothalamic neurotensin neurons promote arousal and hyperthermia. PLoS Biol. 17:e3000172. doi: 10.1371/journal.pbio.3000172
Nixon, J. P., and Smale, L. (2007). A comparative analysis of the distribution of immunoreactive orexin A and B in the brains of nocturnal and diurnal rodents. Behav. Brain Funct. 3:28. doi: 10.1186/1744-9081-3-28
Okumura, T., Nozu, T., Kumei, S., Takakusaki, K., Miyagishi, S., and Ohhira, M. (2015). Involvement of the dopaminergic system in the central orexin-induced antinociceptive action against colonic distension in conscious rats. Neurosci. Lett. 605, 34–38. doi: 10.1016/j.neulet.2015.08.013
Okumura, T., Nozu, T., Kumei, S., Takakusaki, K., Miyagishi, S., and Ohhira, M. (2016). Adenosine A1 receptors mediate the intracisternal injection of orexin-induced antinociceptive action against colonic distension in conscious rats. J. Neurol. Sci. 362, 106–110. doi: 10.1016/j.jns.2016.01.031
Overeem, S., van Hilten, J. J., Ripley, B., Mignot, E., Nishino, S., and Lammers, G. J. (2002). Normal hypocretin-1 levels in Parkinson’s disease patients with excessive daytime sleepiness. Neurology 58, 498–499. doi: 10.1212/wnl.58.3.498
Perez, S. E., Wynick, D., Steiner, R. A., and Mufson, E. J. (2001). Distribution of galaninergic immunoreactivity in the brain of the mouse. J. Comp. Neurol. 434, 158–185. doi: 10.1002/cne.1171
Petersen, A., Gil, J., Maat-Schieman, M. L., Bjorkqvist, M., Tanila, H., Araujo, I. M., et al. (2005). Orexin loss in Huntington’s disease. Hum. Mol. Genet. 14, 39–47. doi: 10.1093/hmg/ddi004
Petrovich, G. D. (2018). Lateral hypothalamus as a motivation-cognition interface in the control of feeding behavior. Front. Syst. Neurosci. 12:14. doi: 10.3389/fnsys.2018.00014
Qu, D., Ludwig, D. S., Gammeltoft, S., Piper, M., Pelleymounter, M. A., Cullen, M. J., et al. (1996). A role for melanin-concentrating hormone in the central regulation of feeding behaviour. Nature 380, 243–247. doi: 10.1038/380243a0
Qualls-Creekmore, E., Yu, S., Francois, M., Hoang, J., Huesing, C., Bruce-Keller, A., et al. (2017). Galanin-expressing GABA neurons in the lateral hypothalamus modulate food reward and noncompulsive locomotion. J. Neurosci. 37, 6053–6065. doi: 10.1523/JNEUROSCI.0155-17.2017
Ranaldi, R. (2014). Dopamine and reward seeking: the role of ventral tegmental area. Rev. Neurosci. 25, 621–630. doi: 10.1515/revneuro-2014-0091
Razavi, B. M., and Hosseinzadeh, H. (2017). A review of the role of orexin system in pain modulation. Biomed. Pharmacother. 90, 187–193. doi: 10.1016/j.biopha.2017.03.053
Reul, J. M., and Holsboer, F. (2002). On the role of corticotropin-releasing hormone receptors in anxiety and depression. Dialog. Clin. Neurosci. 4, 31–46.
Ripley, B., Overeem, S., Fujiki, N., Nevsimalova, S., Uchino, M., Yesavage, J., et al. (2001). CSF hypocretin/orexin levels in narcolepsy and other neurological conditions. Neurology 57, 2253–2258. doi: 10.1212/wnl.57.12.2253
Risold, P. Y., Griffond, B., Kilduff, T. S., Sutcliffe, J. G., and Fellmann, D. (1999). Preprohypocretin (orexin) and prolactin-like immunoreactivity are coexpressed by neurons of the rat lateral hypothalamic area. Neurosci. Lett. 259, 153–156. doi: 10.1016/s0304-3940(98)00906-909
Rosin, D. L., Weston, M. C., Sevigny, C. P., Stornetta, R. L., and Guyenet, P. G. (2003). Hypothalamic orexin (hypocretin) neurons express vesicular glutamate transporters VGLUT1 or VGLUT2. J. Comp. Neurol. 465, 593–603. doi: 10.1002/cne.10860
Saad, W. A., Guarda, I. F., Ferreira, A. C., de Arruda Camargo, L. A., Neto, A. F., and dos Santos, T. A. (2000). Participation of alpha-1 and alpha-2 adrenoceptors of the lateral hypothalamic area in water intake, and renal sodium, potassium and urinary volume excretion induced by central administration of angiotensin II. Brain Res. Bull. 52, 491–497. doi: 10.1016/s0361-9230(00)00285-289
Sadeghi, S., Reisi, Z., Azhdari-Zarmehri, H., and Haghparast, A. (2013). Involvement of orexin-1 receptors in the ventral tegmental area and the nucleus accumbens in antinociception induced by lateral hypothalamus stimulation in rats. Pharmacol. Biochem. Behav. 105, 193–198. doi: 10.1016/j.pbb.2013.02.011
Sakai, K., Takahashi, K., Anaclet, C., and Lin, J. S. (2010). Sleep-waking discharge of ventral tuberomammillary neurons in wild-type and histidine decarboxylase knock-out mice. Front. Behav. Neurosci. 4:53. doi: 10.3389/fnbeh.2010.00053
Sakurai, T. (2003). Orexin: a link between energy homeostasis and adaptive behaviour. Curr. Opin. Clin. Nutr. Metab. Care 6, 353–360. doi: 10.1097/01.mco.0000078995.96795.91
Sakurai, T., Amemiya, A., Ishii, M., Matsuzaki, I., Chemelli, R. M., Tanaka, H., et al. (1998). Orexins and orexin receptors: a family of hypothalamic neuropeptides and G protein-coupled receptors that regulate feeding behavior. Cell 92:696. doi: 10.1016/s0092-8674(02)09256-5
Sara, S. J., and Bouret, S. (2012). Orienting and reorienting: the locus coeruleus mediates cognition through arousal. Neuron 76, 130–141. doi: 10.1016/j.neuron.2012.09.011
Sargin, D. (2019). The role of the orexin system in stress response. Neuropharmacology 154, 68–78. doi: 10.1016/j.neuropharm.2018.09.034
Schneeberger, M., Tan, K., Nectow, A. R., Parolari, L., Caglar, C., Azevedo, E., et al. (2018). Functional analysis reveals differential effects of glutamate and MCH neuropeptide in MCH neurons. Mol. Metab. 13, 83–89. doi: 10.1016/j.molmet.2018.05.001
Seoane-Collazo, P., Ferno, J., Gonzalez, F., Dieguez, C., Leis, R., Nogueiras, R., et al. (2015). Hypothalamic-autonomic control of energy homeostasis. Endocrine 50, 276–291. doi: 10.1007/s12020-015-0658-y
Sharf, R., Sarhan, M., Brayton, C. E., Guarnieri, D. J., Taylor, J. R., and DiLeone, R. J. (2010). Orexin signaling via the orexin 1 receptor mediates operant responding for food reinforcement. Biol. Psychiatry 67, 753–760. doi: 10.1016/j.biopsych.2009.12.035
Sharpe, M. J., Marchant, N. J., Whitaker, L. R., Richie, C. T., Zhang, Y. J., Campbell, E. J., et al. (2017). Lateral hypothalamic GABAergic neurons encode reward predictions that are relayed to the ventral tegmental area to regulate learning. Curr. Biol. 27, 2089–2100.e5. doi: 10.1016/j.cub.2017.06.024
Shearman, L. P., Camacho, R. E., Sloan Stribling, D., Zhou, D., Bednarek, M. A., Hreniuk, D. L., et al. (2003). Chronic MCH-1 receptor modulation alters appetite, body weight and adiposity in rats. Eur. J. Pharmacol. 475, 37–47. doi: 10.1016/s0014-2999(03)02146-2140
Shimada, M., Tritos, N. A., Lowell, B. B., Flier, J. S., and Maratos-Flier, E. (1998). Mice lacking melanin-concentrating hormone are hypophagic and lean. Nature 396, 670–674. doi: 10.1038/25341
Shiraishi, T. (1991). Noradrenergic neurons modulate lateral hypothalamic chemical and electrical stimulation-induced feeding by sated rats. Brain Res. Bull. 27, 347–351. doi: 10.1016/0361-9230(91)90123-2
Skofitsch, G., and Jacobowitz, D. M. (1986). Quantitative distribution of galanin-like immunoreactivity in the rat central nervous system. Peptides 7, 609–613. doi: 10.1016/0196-9781(86)90035-5
Skrapits, K., Kanti, V., Savanyu, Z., Maurnyi, C., Szenci, O., Horvath, A., et al. (2015). Lateral hypothalamic orexin and melanin-concentrating hormone neurons provide direct input to gonadotropin-releasing hormone neurons in the human. Front. Cell Neurosci. 9:348. doi: 10.3389/fncel.2015.00348
Smart, D., and Jerman, J. (2002). The physiology and pharmacology of the orexins. Pharmacol. Ther. 94, 51–61. doi: 10.1016/s0163-7258(02)00171-7
Smith, S. M., and Vale, W. W. (2006). The role of the hypothalamic-pituitary-adrenal axis in neuroendocrine responses to stress. Dialog. Clin. Neurosci. 8, 383–395.
Stamatakis, A. M., Van Swieten, M., Basiri, M. L., Blair, G. A., Kantak, P., and Stuber, G. D. (2016). Lateral hypothalamic area glutamatergic neurons and their projections to the lateral habenula regulate feeding and reward. J. Neurosci. 36, 302–311. doi: 10.1523/JNEUROSCI.1202-15.2016
Stephens, M. A., and Wand, G. (2012). Stress and the HPA axis: role of glucocorticoids in alcohol dependence. Alcohol. Res. 34, 468–483.
Stiegel, M. A., Pleil, J. D., Sobus, J. R., and Madden, M. C. (2016). Inflammatory cytokines and white blood cell counts response to environmental levels of diesel exhaust and ozone inhalation exposures. PLoS One 11:e0152458. doi: 10.1371/journal.pone.0152458
Stuber, G. D., and Wise, R. A. (2016). Lateral hypothalamic circuits for feeding and reward. Nat Neurosci 19, 198–205. doi: 10.1038/nn.4220
Sun, M., Wang, W., Li, Q., Yuan, T., and Weng, W. (2018). Orexin a may suppress inflammatory response in fibroblast-like synoviocytes. Biomed. Pharmacother. 107, 763–768. doi: 10.1016/j.biopha.2018.07.159
Suvas, S. (2017). Role of substance P Neuropeptide in inflammation, wound healing, and tissue homeostasis. J. Immunol. 199, 1543–1552. doi: 10.4049/jimmunol.1601751
Suyama, S., and Yada, T. (2018). New insight into GABAergic neurons in the hypothalamic feeding regulation. J. Physiol. Sci. 68, 717–722. doi: 10.1007/s12576-018-0622-8
Szymusiak, R., and McGinty, D. (2008). Hypothalamic regulation of sleep and arousal. Ann. N. Y. Acad. Sci. 1129, 275–286. doi: 10.1196/annals.1417.027
Taylor, M. M., and Samson, W. K. (2003). The other side of the orexins: endocrine and metabolic actions. Am. J. Physiol. Endocrinol. Metab. 284, E13–E17. doi: 10.1152/ajpendo.00359.2002
Telegdy, G., and Adamik, A. (2002). The action of orexin A on passive avoidance learning. Involvement of transmitters. Regul. Pept. 104, 105–110. doi: 10.1016/s0167-0115(01)00341-x
Thomas Cheng, H. (2010). Spinal cord mechanisms of chronic pain and clinical implications. Curr. Pain Headache Rep. 14, 213–220. doi: 10.1007/s11916-010-0111-0
Timper, K., and Bruning, J. C. (2017). Hypothalamic circuits regulating appetite and energy homeostasis: pathways to obesity. Dis. Model. Mech. 10, 679–689. doi: 10.1242/dmm.026609
Torrealba, F., Yanagisawa, M., and Saper, C. B. (2003). Colocalization of orexin a and glutamate immunoreactivity in axon terminals in the tuberomammillary nucleus in rats. Neuroscience 119, 1033–1044. doi: 10.1016/s0306-4522(03)00238-0
Trivedi, P., Yu, H., MacNeil, D. J., Van der Ploeg, L. H., and Guan, X. M. (1998). Distribution of orexin receptor mRNA in the rat brain. FEBS Lett. 438, 71–75. doi: 10.1016/s0014-5793(98)01266-6
Tyree, S. M., and de Lecea, L. (2017). Lateral hypothalamic control of the ventral tegmental area: reward evaluation and the driving of motivated behavior. Front. Syst. Neurosci. 11:50. doi: 10.3389/fnsys.2017.00050
van den Pol, A. N. (1999). Hypothalamic hypocretin (orexin): robust innervation of the spinal cord. J. Neurosci. 19, 3171–3182. doi: 10.1523/jneurosci.19-08-03171.1999
Wardach, J., Wagner, M., Jeong, Y., and Holden, J. E. (2016). Lateral hypothalamic stimulation reduces hyperalgesia through spinally descending orexin-a neurons in neuropathic pain. West J. Nurs. Res. 38, 292–307. doi: 10.1177/0193945915610083
Watanabe, S., Kuwaki, T., Yanagisawa, M., Fukuda, Y., and Shimoyama, M. (2005). Persistent pain and stress activate pain-inhibitory orexin pathways. Neuroreport 16, 5–8. doi: 10.1097/00001756-200501190-00002
Wennstrom, M., Londos, E., Minthon, L., and Nielsen, H. M. (2012). Altered CSF orexin and alpha-synuclein levels in dementia patients. J. Alzheimers Dis. 29, 125–132. doi: 10.3233/JAD-2012-111655
Winsky-Sommerer, R., Yamanaka, A., Diano, S., Borok, E., Roberts, A. J., Sakurai, T., et al. (2004). Interaction between the corticotropin-releasing factor system and hypocretins (orexins): a novel circuit mediating stress response. J. Neurosci. 24, 11439–11448. doi: 10.1523/JNEUROSCI.3459-04.2004
Woodworth, H. L., Beekly, B. G., Batchelor, H. M., Bugescu, R., Perez-Bonilla, P., Schroeder, L. E., et al. (2017). Lateral hypothalamic neurotensin neurons orchestrate dual weight loss behaviors via distinct mechanisms. Cell Rep. 21, 3116–3128. doi: 10.1016/j.celrep.2017.11.068
Yamamoto, T., Nozaki-Taguchi, N., and Chiba, T. (2002). Analgesic effect of intrathecally administered orexin-A in the rat formalin test and in the rat hot plate test. Br. J. Pharmacol. 137, 170–176. doi: 10.1038/sj.bjp.0704851
Yamamoto, T., Saito, O., Shono, K., Aoe, T., and Chiba, T. (2003a). Anti-mechanical allodynic effect of intrathecal and intracerebroventricular injection of orexin-A in the rat neuropathic pain model. Neurosci. Lett. 347, 183–186. doi: 10.1016/s0304-3940(03)00716-x
Yamamoto, T., Saito, O., Shono, K., and Hirasawa, S. (2003b). Activation of spinal orexin-1 receptor produces anti-allodynic effect in the rat carrageenan test. Eur. J. Pharmacol. 481, 175–180. doi: 10.1016/j.ejphar.2003.09.022
Yamanaka, A., Kunii, K., Nambu, T., Tsujino, N., Sakai, A., Matsuzaki, I., et al. (2000). Orexin-induced food intake involves neuropeptide Y pathway. Brain Res. 859, 404–409. doi: 10.1016/s0006-8993(00)02043-6
Yamano, M., Inagaki, S., Kito, S., and Tohyama, M. (1986). A substance P-containing pathway from the hypothalamic ventromedial nucleus to the medial preoptic area of the rat: an immunohistochemical analysis. Neuroscience 18, 395–402. doi: 10.1016/0306-4522(86)90161-2
Zhang, J. M., and An, J. (2007). Cytokines, inflammation, and pain. Int. Anesthesiol. Clin. 45, 27–37. doi: 10.1097/AIA.0b013e318034194e
Keywords: hypocretin, lateral hypothalamus, inflammation, orexin, pain
Citation: Fakhoury M, Salman I, Najjar W, Merhej G and Lawand N (2020) The Lateral Hypothalamus: An Uncharted Territory for Processing Peripheral Neurogenic Inflammation. Front. Neurosci. 14:101. doi: 10.3389/fnins.2020.00101
Received: 30 September 2019; Accepted: 24 January 2020;
Published: 12 February 2020.
Edited by:
Andrew C. McCreary, Uniqure B.V., NetherlandsReviewed by:
Charles Evans Wood, University of Florida, United StatesCopyright © 2020 Fakhoury, Salman, Najjar, Merhej and Lawand. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Marc Fakhoury, bWYxMTlAYXViLmVkdS5sYg==; Nada Lawand, bmwwOEBhdWIuZWR1Lmxi
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.