
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
PERSPECTIVE article
Front. Neurosci. , 31 January 2020
Sec. Neurodegeneration
Volume 14 - 2020 | https://doi.org/10.3389/fnins.2020.00047
 Frances Theunissen1,2
Frances Theunissen1,2 Loren L. Flynn1,3,4
Loren L. Flynn1,3,4 Ryan S. Anderton1,3,5
Ryan S. Anderton1,3,5 Frank Mastaglia1,3
Frank Mastaglia1,3 Julia Pytte1,2
Julia Pytte1,2 Leanne Jiang1,6
Leanne Jiang1,6 Stuart Hodgetts1,2
Stuart Hodgetts1,2 Daniel K. Burns7
Daniel K. Burns7 Ann Saunders7
Ann Saunders7 Sue Fletcher3,4
Sue Fletcher3,4 Steve D. Wilton1,3,4
Steve D. Wilton1,3,4 Patrick Anthony Akkari1,3,4*
Patrick Anthony Akkari1,3,4*The underlying genetic and molecular mechanisms that drive amyotrophic lateral sclerosis (ALS) remain poorly understood. Structural variants within the genome can play a significant role in neurodegenerative disease risk, such as the repeat expansion in C9orf72 and the tri-nucleotide repeat in ATXN2, both of which are associated with familial and sporadic ALS. Many such structural variants reside in uncharacterized regions of the human genome, and have been under studied. Therefore, characterization of structural variants located in and around genes associated with ALS could provide insight into disease pathogenesis, and lead to the discovery of highly informative genetic tools for stratification in clinical trials. Such genomic variants may provide a deeper understanding of how gene expression can affect disease etiology, disease severity and trajectory, patient response to treatment, and may hold the key to understanding the genetics of sporadic ALS. This article outlines the current understanding of amyotrophic lateral sclerosis genetics and how structural variations may underpin some of the missing heritability of this disease.
Amyotrophic lateral sclerosis (ALS) is a devastating progressive neurodegenerative disorder characterized by the loss of lower and upper motor neurons, resulting in paralysis of the limb, bulbar and respiratory muscles, and is typically fatal within 3–5 years from disease onset (Robberecht and Philips, 2013). The disease incidence is ∼2 per 100,000 population and it is projected that rates of ALS will increase from ∼222,000 worldwide in 2015 to ∼376,000 in 2040 (Arthur et al., 2016). In addition to the growing financial burden of this disease, there is a significant burden on the patients’ families and carers. As such, it is critical to improve our understanding of how genetic mechanisms may contribute to the pathogenesis of this devastating disease. Investigation of short structural variations (SVs) in known ALS genes has multiple potential objectives, and may help to uncover some of the missing heritability in sporadic ALS. Characterization of short SVs may inform the discovery of novel disease mechanisms and therapeutic targets, and be useful for stratification of patient subgroups in clinical trials.
ALS is a heterogeneous disease, with variable clinical presentation between patients, and is characterized by progressive motor deficits that evolve over weeks or months, eventually affecting most voluntary muscles in the body (Talman et al., 2016). The heterogeneity of clinical presentation and diverse rates of progression make the disease challenging to diagnose, and there is currently no definitive diagnostic test. As a result, it is usually characterized on the basis of the site and pattern of onset, and the degree of involvement of upper and lower motor neurons, and can be classified into the following categories: (i) progressive bulbar palsy (difficulty swallowing and speech disturbances); (ii) limb-onset ALS; (iii) progressive muscular atrophy (involving only lower motor neurons); and (iv) upper motor neuron predominant ALS (Kinsley and Siddique, 2015). Most commonly, individuals will present with asymmetrical focal weakness of the extremities (poor hand grip, foot drop, stumbling) or bulbar characteristics (dysarthria and dysphagia). Other typical symptoms include muscle fasciculation, cramps and hyperreflexia in regions of atrophy, without accompanying sensory disturbances (Kinsley and Siddique, 2015). Importantly, the different clinical phenotypes exhibit differing rates of progression, with the bulbar-onset form having the most rapid progression and shortest survival time (Okamoto et al., 1993). In addition, there is considerable variability between cases within the same diagnostic category. Given the degree of heterogeneity, it remains to be determined whether the different clinical phenotypes all represent variations of the same disease, or whether there is also heterogeneity in the underlying genetic and molecular disease determinants. At present, there is a lack of specific genetic or other biomarkers for the different disease subtypes, or indicators of disease trajectory in well-documented patient cohorts.
Approximately 10% of ALS cases are classed as familial (fALS), while the remainder, with no prior family involvement, are classified as having the sporadic form of the disease (sALS) (Renton et al., 2014). Since the landmark discovery of the first mutation in the superoxide dismutase 1 gene (SOD1) in fALS in the early 1990s (Rosen et al., 1993), there has since been significant progress in understanding of the genetics of the familial disease, with approximately 70% of the genetic mutations that contribute to fALS having been identified (Cook and Petrucelli, 2019). However the genetic underpinnings of sporadic ALS (sALS) remain a formidable challenge (Al-Chalabi et al., 2012; Renton et al., 2014). Comparatively, few mutations have been described for sALS, despite the application of high throughput genetic analysis methods (Nguyen et al., 2018). These methodologies have failed to identify disease-associated genetic variations in the majority of sALS patients, highlighting the complexity and genetic heterogeneity contributing to this disease phenotype. Approximately 10% of sALS cases can be explained by mutations in 25 known ALS-linked genes, with the remaining 90% of cases as yet having an undetermined genetic contributor (Andersen and Al-Chalabi, 2011; Renton et al., 2014; Dharmadasa et al., 2017). At a glance these data may imply that the genetic contributions to sALS are minor, however, heritability estimates and twin studies suggest a genetic contribution of up to 65% (Al-Chalabi et al., 2010; Al-Chalabi and Visscher, 2014). It is therefore likely that additional genetic contributors to sALS risk remain to be identified. The situation may be analogous to that for Alzheimer’s disease where the APOE ε4 (Apolipoprotein E) risk allele has a frequency of only 14% and does not fully explain the Alzheimer’s disease age-of-onset risk (Corder et al., 1993; Roses et al., 2016). However, after discovery of the structural variant (rs10524523) in the neighboring TOMM40 (Translocase of outer mitochondrial membrane 40) gene, age of onset risk could now be assessed for >90% of the at risk population (Roses et al., 2010).
Currently there are only two therapeutics available for the treatment of ALS, Riluzole (approved in Australia, United States, and Europe) and Edaravone (approved in Japan, South Korea, and United States) (Rothstein, 2017) that impact excitotoxicity (Doble, 1996) or anti-oxidant pathways (Rothstein, 2017), respectively and may slow disease progression for a relatively short period of time. However, these drugs are only effective in some patients and there is currently no way to determine those most likely to respond to the drugs. For the patients that do show a response, life is only prolonged by approximately 3 months. Further understanding of ALS pathogenesis should inform the development of more effective therapies and help identify patients likely to respond to specific treatments.
Genome wide association studies analysing single nucleotide polymorphisms (SNP) and whole exome sequencing studies have provided a wealth of information relating to common variants associated with a range of diseases. Despite this, such approaches have generally identified genes that are either inherited in fALS, those that are weakly associated with sALS, or mutations associated with rare forms of the disease (Renton et al., 2014). Some of the ALS genes identified by these techniques include UNC13A, C9orf72, DPP6, ELP3, KIFAP3, TBK1, CHCHD10, TUBA4A, CCNF, MATR3, NEK1, C21orf2, ANXA11, and TIA1 (see Table 1; Andersen and Al-Chalabi, 2011; Nguyen et al., 2018). A major issue with utilizing these technologies is that by testing thousands of SNPs across the genome, low effect size associations are generated for numerous gene regions, inevitably leading to very high thresholds of significance for potentially weak genetic effects (Roses, 2016). This can lead to false positive associations or a lack of reproducibility between different populations that have rarely translated into tools for clinical trial patient stratifications, with the exception of UNC13A (see below). The amount of information these techniques can provide regarding complex disease and the functional outcomes of SNPs are limited. Particularly, these methods have not been able to account for the variation in age-of-onset and progression in ALS patients (Al-Chalabi and Hardiman, 2013), and fail to explain the missing heritability of the disease (Al-Chalabi et al., 2017; Mejzini et al., 2019). Whole genome sequencing can examine the entirety of the genome to better capture larger scale variations, as opposed to single nucleotide changes, however, these methods come with their own technical limitations; high throughput short-read sequencing technologies are unable to accurately capture these regions due to amplification stutter, and misaligning of the short-read sequences, often misrepresenting their true variability (Cameron et al., 2019). Recently, it was demonstrated that variable areas of the genome can camouflage each other, particularly where short-read DNA sequences map equally well to different loci, resulting in poor SV characterization (Ebbert et al., 2019). To date, these techniques have only accounted for the genetic cause of approximately 10% of sALS cases (Cook and Petrucelli, 2019). Therefore, it is essential to rethink the approach and acknowledge the limitations of these technologies when interrogating the genome. Specifically, there are vast regions of genetic variability yet to be uncovered in non-coding regions, which might have significant implications in the context of complex disease.
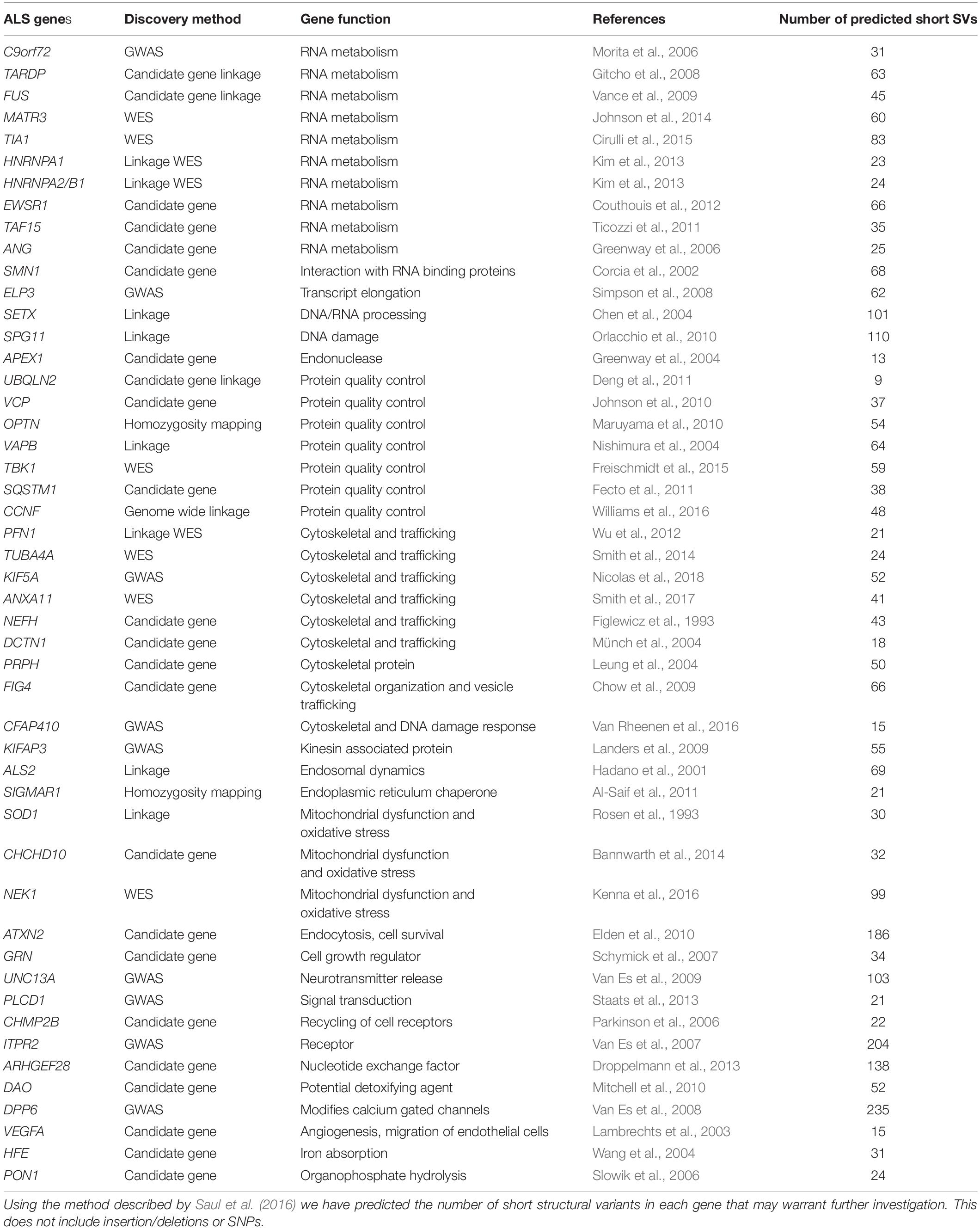
Table 1. This table lists published genes that have been associated with ALS and highlights the discovery method for each gene as well as the gene function.
Structural variants (SVs) are defined as insertions, deletions, inversions and microsatellites that can be repeated hundreds of times. SVs predominantly occur in non-coding regions of the genome and often do not change the composition of the mature protein (Roses et al., 2016). Despite this, changes in the size and composition of SVs can have a significant impact on the regulatory elements that modulate gene expression (Chiang et al., 2017). Therefore, SVs can potentially provide a deeper understanding of how gene expression in complex genetic disease can affect disease etiology, duration, progression and patient outcomes (Feuk et al., 2006). SVs have been implicated in many complex diseases including retinitis pigmentosa (MSR1) (Rose et al., 2016), Alzheimer’s (TOMM40) (Lyall et al., 2013), frontotemporal dementia (C9orf72) (DeJesus-Hernandez et al., 2011; Renton et al., 2011), and other neurodegenerative diseases (Beck et al., 2013).
The ability of SVs to alter gene expression is likely dependent on their location within and around the gene or intergenic region, with their effects occurring via several mechanisms including, influencing the binding of regulatory elements that determine transcription, mRNA splicing and processing, genome folding and higher order structure, and translation (Roses et al., 2016). This may differentiate mechanisms of disease pathogenesis, including risk of disease, risk for a specific phenotype, symptom presentation, disease course and response to treatment, between individuals (Figure 1A). Due to the variable nature, as well as the repeat structure of SVs, many remain poorly characterized by analysis platforms such as next generation sequencing (Cameron et al., 2019; Ebbert et al., 2019).
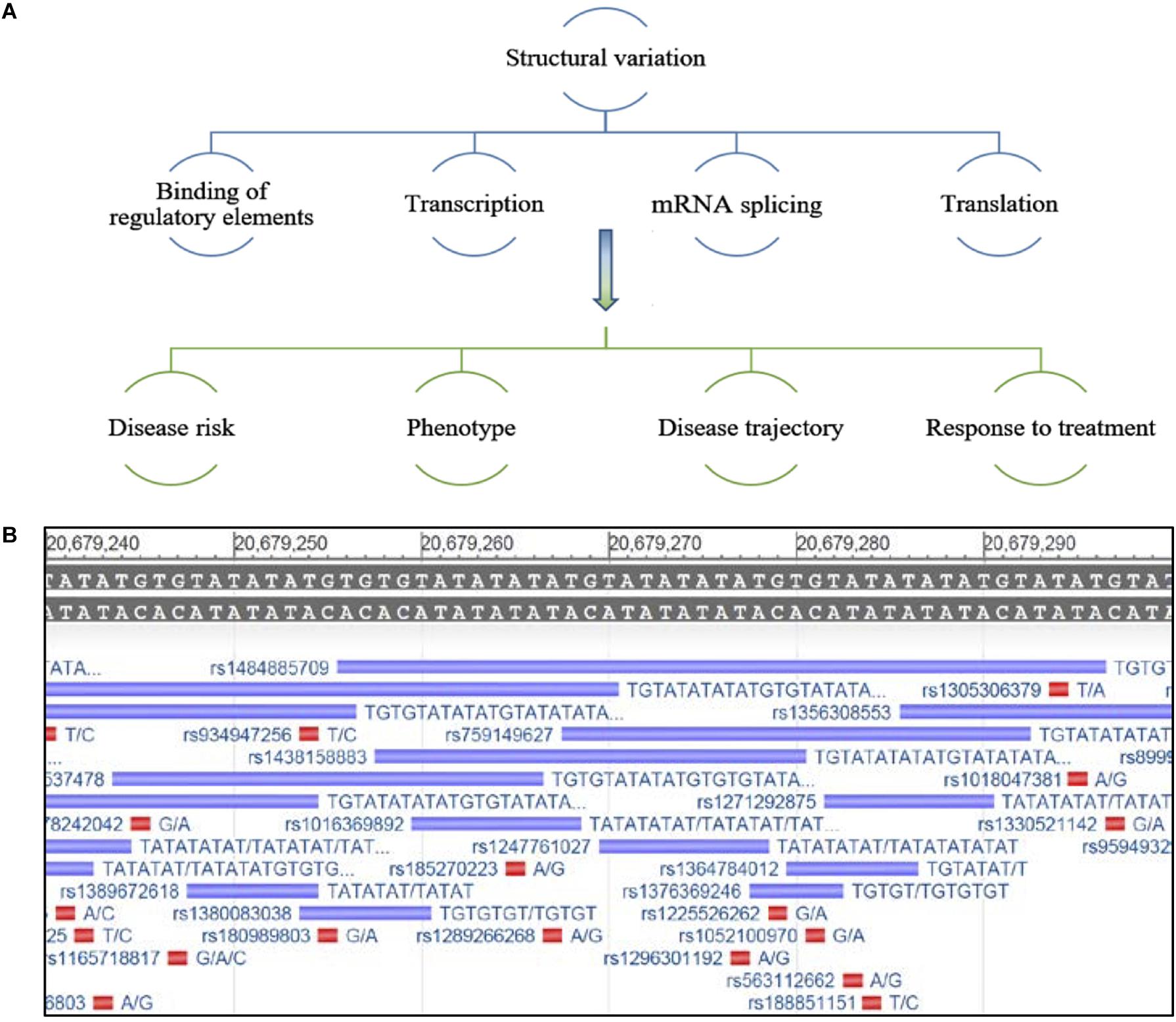
Figure 1. (A) Schematic representing potential regulation of gene expression by structural variants and possible effects on disease characteristics. (B) Example of an under-characterized gene from the NCBI database GRch38.p13 primary assembly, a region that has been repeatedly mapped but lacks consensus around the nature of this repeat sequence and its true variability. The reference sequence is located at the top of the image, the purple bars depict a sequencing entry with variable size and its associated rs number and red bars represent a SNP entry (Gene [Internet]. Bethesda (MD): National Library of Medicine (US), National Center for Biotechnology Information; 2004 – [cited 2019 September 24]. Available from: https://www.ncbi.nlm.nih.gov/gene/).
Historically, research on SVs has focused on genomic elements that are larger in size (>1 kb) and much easier to capture by high throughput techniques, such as copy number variations, transposable elements, larger insertion/deletions, translocations, and duplications (Sebat et al., 2004; Feuk et al., 2006; Huang et al., 2010; Alkan et al., 2011; Sudmant et al., 2015); yet other small variable regions of the genome remain under characterized and are more difficult to capture with short-read sequencing techniques (Figure 1B; Chaisson et al., 2019; Ebbert et al., 2019). Recently, a comprehensive review highlighted the strengths and weakness of various sequencing methods and bioinformatics tools used for SV calling, and the difficulties in capturing smaller novel variations (Mahmoud et al., 2019). In addition, comparing the accuracy of SV characterization between methodologies when different in silico data sets are often utilized remains a challenge (Mahmoud et al., 2019). Furthermore, how these methods translate and generalize to patient samples is unclear, making it critical to establish standard procedures and bench-marks for the interpretation of SV data. There is a growing need to utilize SV information in the clinical setting to inform variation in patient phenotypes. Therefore we and others believe, important genes that may be critical to understanding the variation between patients that cannot be fully explained by SNPs or other mutations, should be reinvestigated for short SVs. Unrecognized or under characterized SVs could influence the expression of these genes, thereby contributing to the risk of ALS.
Recent studies investigating the transcriptome of the spinal cord anterior horn have identified significantly different RNA profiles between ALS patients and controls, for a multitude of gene pathways (D’Erchia et al., 2017). Since SVs can exhibit a range of regulatory effects that can impact levels of gene expression and potentially the phenotype, it is essential that these regions are also properly characterized. For example, a microsatellite repeat element in the promotor region of PRPF31 (precursor mRNA-processing factor 31) results in some mutation carriers developing retinitis pigmentosa, whilst others remain asymptomatic (Rose et al., 2016). The length of this SV was shown to impact the penetrance of the mutation by suppressing transcription of this region by 50–115-fold, resulting in haploinsufficiency (Rose et al., 2016). Such disease mechanisms also warrant investigation in ALS.
An example of an SV that results in ALS pathogenesis is the repeat expansion in the C9orf72 gene. The protein encoded by this gene is thought to play a role in endosomal membrane trafficking and autophagy (Farg et al., 2014). The SV region of C9orf72 is a hexanucleotide repeat located in intron 1, GGGGCC that is usually repeated up to 30 times in healthy individuals. Expansion of this repeat to hundreds or thousands of repeated segments is a recognized cause of fALS, frontotemporal dementia, and occasionally also sALS (Mis et al., 2017). The DNA encoding this repeat is transcribed bi-directionally, resulting in nuclear RNA inclusions, and is thought to promote gain of function toxicity (Ly and Miller, 2018; Staats et al., 2019). Other potential mechanisms include C9orf72 loss of function (Shi et al., 2018; Staats et al., 2019), or proteotoxicity (Gitler and Tsuiji, 2016). In particular, patients with expanded repeats have a more severe phenotype, are predominantly bulbar onset, exhibit an earlier age of disease onset, cognitive and behavioral impairment and reduced survival (Byrne et al., 2012; Cooper-Knock et al., 2014). In addition, microsatellite repeats are subjected to an unconventional mechanism called repeat associated non-ATG (RAN) translation (Zu et al., 2011), whereby proteins can be coded by the additional two reading frames (GCA and AGC) resulting in up to six dipeptide proteins (Cleary and Ranum, 2014, 2017). The accumulation of these dipeptide proteins is seen in the central nervous system of individuals with ALS and contributes to a multitude of mechanisms that can induce protein-mediated toxicity (Balendra and Isaacs, 2018). One of the current strategies employed in the development of therapeutics for C9orf72 is to target the SV region with antisense oligonucleotides to induce transcript degradation by RNase H enzymatic cleavage, preventing the build-up of toxic C9orf72 transcript and protein. Other strategies focus on modulating the expression of transcription factors specifically involved in transcribing expanded repeats (Ly and Miller, 2018). Recently, it was shown that small ribosomal subunit protein (RPS25) plays a direct role in RAN translation, and decreasing its levels through RNA interface mediated reduction prolonged the lifespan of Drosophila with the expanded repeat (Yamada et al., 2019). In addition, antisense oligonucleotide targeting of RPS25 enhanced the survival of C9orf72 derived motor neurons reducing poly-GR and poly-PR foci (Yamada et al., 2019). The recent identification of a RAN translation regulator is a big step forward in demonstrating the functional implications of SV repeats in disease, and indicates antisense oligonucleotide or small molecules could be a viable therapeutic option for targeting RPS25 for patients with C9orf72 (Hutten and Dormann, 2019). However, further research is needed to understand the regulation of expanded repeats and determine the relative contribution of repeat RNA and dipeptide repeat proteins to patient phenotype and cellular toxicity that promotes neurodegeneration in ALS.
Another SV contributing to ALS pathogenesis occurs in the gene encoding ataxin-2 (ATXN2). ATXN2 contains a CAG repeat, initially found to be associated with a class of neuromuscular and neurological disorders, known as polyglutamine disorders, caused by the expansion of the microsatellite repeat within the coding sequence (Al-Chalabi et al., 2012; Lattante et al., 2014). The ATXN2 protein is involved in endocytosis and modulates mTOR signals, critical to maintaining cell growth and survival, thereby modifying translation and mitochondrial function (Carmo-Silva et al., 2017). The N-terminal of this protein contains a polyglutamine tract that generally consists of 14–31 residues that when expanded, can carry up to 200 residues in the pathogenic state (Sproviero et al., 2017). Long expanded repeats were initially found to cause spinocerebellar ataxia 2 and subsequently, intermediate length repeats were shown to increase the risk of developing ALS (Daoud et al., 2011; Van Damme et al., 2011), with one study demonstrating that ALS risk increases exponentially with repeat length until the cut-off for developing spinocerebellar ataxia is reached (Sproviero et al., 2017). The polyglutamine disease causing mechanism differs between conditions and may include a loss of function resulting from hyper-methylation (Jin and Warren, 2000), a toxic gain of function through RAN translation (Scoles et al., 2015), protein misfolding and aggregation (Kayatekin et al., 2014), and in ALS, increasing TDP-43 toxicity (Elden et al., 2010). Longer repeats were expected to result in increased toxic TDP-43 build-up, resulting in increased risk of disease, an earlier age of onset and faster progression, however, this is not necessarily seen in patients (Al-Chalabi and Hardiman, 2013). The possibility of oligogenic inheritance is the likely explanation, where multiple risk factors from polymorphic structural variants are required to act together over time, with environmental stressors, to cause the development of ALS (Al-Chalabi et al., 2014), accounting for the particular variability seen in age-of-onset and disease progression. A better understanding of these variable regions of the genome and how they might work together to cumulatively increase disease risk, resulting in motor neuron dysfunction and susceptibility to neuronal degeneration, is imperative.
Over the past 20 years, more than 50 controlled trials of putative ALS therapeutics, testing 60 molecules have failed to demonstrate clinical efficacy (Petrov et al., 2017). Transgenic SOD1 mice have been used for the majority of ALS pre-clinical drug development studies; however, these models do not translate well to human disease (Mitsumoto et al., 2014). Alternative approaches need to be used to evaluate the potential efficacy of compounds. Identification and utilization of genetic markers, such as SVs informative for ALS, could be incorporated into clinical trial design to reduce the participant heterogeneity (Van Eijk et al., 2019).
Poor understanding of the pathogenic mechanisms of ALS neuro-degeneration remains a barrier to the development of novel therapeutic approaches. Moreover, there are few biomarkers that allow patient stratification according to disease mechanism (Agah et al., 2018; Khalil et al., 2018; Mitsumoto and Saito, 2018; Vejux et al., 2018). As a result, efficacy can only be evaluated by clinical measures during clinical trials (Mitsumoto et al., 2014; Mitsumoto and Saito, 2018). Clearly, there is a critical unmet need to establish well-characterized molecular biomarkers that can be used as therapeutic targets, or to inform on the validity of certain treatment approaches. As ALS is a complex, heterogenous disorder with a varied age of onset and expression, it is likely that no single therapeutic will be effective for all patients. Therefore, we must develop strategies to identify patient subgroups and develop compounds to address the specific molecular defect.
Establishing molecular targets and markers for ALS could lead to improved patient stratification for clinical trials, to enable treatment effects to be identified within specific patient sub-groups. An example of the success of this approach is provided by clinical research with lithium carbonate in ALS patients. After a pilot study demonstrated attenuation of disease progression in a small number of ALS patients treated with lithium carbonate (Fornai et al., 2008), a number of follow up clinical studies have failed to replicate the finding (Aggarwal et al., 2010; Chiò et al., 2010; Miller et al., 2011; Verstraete et al., 2012; UKMND-LiCALS Study Group, 2013). In a meta-analysis of three trials that failed to show a significant effect of lithium treatment in ALS, Van Eijk et al. (2017) retrospectively demonstrated that lithium-treated patients who carried the UNC13A (C/C) genotype had a slower disease trajectory and showed a 70% improvement in 12 month survival, whilst carriers of the same genotype receiving no treatment had significantly reduced survival trajectories (Van Eijk et al., 2017). This survival benefit was only evident once the patients were stratified by their genotype, as the heterogeneous trial cohort originally masked the identification of any therapeutic benefit for a subgroup. In a more recent report Van Eijk et al. (2019) demonstrated that different genotypes including the repeat expansion C9orf72 can interact with both primary and secondary endpoints of clinical trials. Interestingly, in this report C9orf72 carriers did not have reduced survival, however, they did exhibit an accelerated monthly decline measured by ALSFRS compared to non-carriers. No pharmacogenetic interactions were demonstrated in the valproic acid trial, however, there was a pharmacognetic interaction between creatine treatment and the A allele of MOBP, whilst a dose response was observed for the C allele of UNC13A improving survival outcomes (Van Eijk et al., 2019). This highlights the importance of taking genetic information into account in clinical trials to enrich trial populations for potential responders. Identifying new genetic variations that may explain changes in gene expression in sALS patients will therefore be extremely useful to help inform both primary and secondary end points in clinical trial, and may improve the likelihood of clinical trial success.
The methodologies currently used to elucidate ALS pathogenesis and inform drug development have not delivered effective therapeutic strategies to date. In our view, continuing to perform further genome wide association studies is unlikely to provide the breakthroughs that are urgently needed. Genome wide studies can sometimes identify biochemical pathways involved in disease and indicate genes associated with fALS and sALS, however, in-depth characterization of these implicated regions may identify SVs that influence ALS susceptibility. Limitations of GWAS and even whole genome sequencing in identifying ALS risk must be recognized, since allele frequencies of variants or SNPs may not differ between patient cohorts and controls in these complex diseases. With increasing likelihood that SVs do indeed contribute to ALS risk, future investigations will need to incorporate SVs into genetic studies. It is possible that a combination of variants occurring frequently throughout healthy populations will collectively contribute to the vulnerability of motor neurons, and that this could be further exacerbated by both physiological and environmental insults. Particular SVs may better account for the variability in phenotypes and progression across ALS patient cohorts, and could be critical targets that can inform drug development. As our current molecular understanding of ALS has proven largely ineffective in easing the burden of ALS, clinical trials are likely to continue to fail if analyses are restricted to conventional strategies and platforms.
In silico investigations by our laboratory group reveal that there are a number of under-characterized genomic regions in ALS genes. Genetic data-bases including National Center for Biotechnology Information1, Ensembl genome browser 972 and University of California Santa Cruz genome browser3 have multiple sequence entries logged for genomic loci under different RS numbers. Sequencing technologies, whilst sometimes precise, have limited accuracy (Roses, 2016), as they only provide the location but not the specifics of each variant in individual patients. These poorly characterized regions of the genome could therefore contribute to the missing heritability of ALS. In most cases, the variation in allele length and allele frequency remains unclear, ultimately leading to the question, “how significant a role do SVs play in complex diseases, such as ALS?” We have focused on the development of accurate assays to genotype SVs. For example, investigation of the SOD1 region has led to the characterization of SV1, a variant located within the 3′ untranslated region of SCAF4, a downstream gene that was previously overlooked. The function of SCAF4 has recently been elucidated, with the protein being necessary for accurate termination of transcription by ensuring correct polyadenylation site selection (Gregersen et al., 2019). This SV appears to influence SOD1 expression, possibly through a toxic gain of function, and could more broadly influence ALS pathogenesis (Roses Allen, 2019). Continued investigations into the function of SV1 are presently underway in our laboratory. In addition, other variants appear to stratify sALS patients on the basis of survival and may in fact act as modifiers of gene expression (unpublished data). If this data is confirmed, it will not only indicate novel mechanisms contributing to ALS, but also allow patient stratification for enrichment of ALS clinical trials. Although SVs may not always be a viable drug target, they may indicate pathways that can be targeted to inform drug development. It is our belief that we need to re-assess these regions of the genome in order to identify some of the missing heritability of ALS and explain the phenotypic variability seen across this disease.
FT, RA, and PA contributed to the content addressed in the perspective. FT wrote the first draft of the manuscript. All authors contributed to the manuscript revision, read, and approved the submitted version.
This work was funded by the Perron Institute for Neurological and Translational Science and the Giumelli Foundation. The funders had no role in the preparation of the manuscript and the decision to publish.
DB and AS are employed by the company Zinfandel Pharmaceuticals. The patent for “Methods for detecting structural variants in neurodegenerative disease,” invented by Allen D. Roses, is now owned by AS.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
We would like to acknowledge the patients that contribute to our ongoing work. We would also like to acknowledge the lifetime of mentoring and wisdom that the late Professor Allen Roses has given to our team and this field of work. It is an honor and privilege to have worked with him for over 27 years and through our research we continue the work we started together.
Agah, E., Saleh, F., Moghaddam, H. S., Saghazadeh, A., Tafakhori, A., and Rezaei, N. (2018). CSF and blood biomarkers in amyotrophic lateral sclerosis: protocol for a systematic review and meta-analysis. Syst. Rev. 7:237. doi: 10.1186/s13643-018-0913-4
Aggarwal, S. P., Zinman, L., Simpson, E., McKinley, J., Jackson, K. E., Pinto, H., et al. (2010). Safety and efficacy of lithium in combination with riluzole for treatment of amyotrophic lateral sclerosis: a randomised, double-blind, placebo-controlled trial. Lancet Neurol. 9, 481–488. doi: 10.1016/s1474-4422(10)70068-5
Al-Chalabi, A., Calvo, A., Chio, A., Colville, S., Ellis, C. M., Hardiman, O., et al. (2014). Analysis of amyotrophic lateral sclerosis as a multistep process: a population-based modelling study. Lancet Neurol. 13, 1108–1113. doi: 10.1016/S1474-4422(14)70219-4
Al-Chalabi, A., Fang, F., Hanby, M. F., Leigh, P. N., Shaw, C. E., Ye, W., et al. (2010). An estimate of amyotrophic lateral sclerosis heritability using twin data. J. Neurol. Neurosurg. Psychiatry 81, 1324–1326. doi: 10.1136/jnnp.2010.207464
Al-Chalabi, A., and Hardiman, O. (2013). The epidemiology of ALS: a conspiracy of genes, environment and time. Nat. Rev. Neurol. 9, 617–628. doi: 10.1038/nrneurol.2013.203
Al-Chalabi, A., Jones, A., Troakes, C., King, A., Al-Sarraj, S., and van den Berg, L. H. (2012). The genetics and neuropathology of amyotrophic lateral sclerosis. Acta Neuropathol. 124, 339–352. doi: 10.1007/s00401-012-1022-4
Al-Chalabi, A., Van Den Berg, L. H., and Veldink, J. (2017). Gene discovery in amyotrophic lateral sclerosis: implications for clinical management. Nat. Rev. Neurol. 13, 96–104. doi: 10.1038/nrneurol.2016.182
Al-Chalabi, A., and Visscher, P. M. (2014). Motor neuron disease: common genetic variants and the heritability of ALS. Nat. Rev. Neurol. 10, 549–550. doi: 10.1038/nrneurol.2014.166
Alkan, C., Coe, B. P., and Eichler, E. E. (2011). Genome structural variation discovery and genotyping. Nat. Rev. Genet. 12, 363–376. doi: 10.1038/nrg2958
Al-Saif, A., Al-Mohanna, F., and Bohlega, S. (2011). A mutation in sigma-1 receptor causes juvenile amyotrophic lateral sclerosis. Ann. Neurol. 70, 913–919. doi: 10.1002/ana.22534
Andersen, P. M., and Al-Chalabi, A. (2011). Clinical genetics of amyotrophic lateral sclerosis: what do we really know? Nat. Rev. Neurol. 7, 603–615. doi: 10.1038/nrneurol.2011.150
Arthur, K. C., Calvo, A., Price, T. R., Geiger, J. T., Chio, A., and Traynor, B. J. (2016). Projected increase in amyotrophic lateral sclerosis from 2015 to 2040. Nat. Commun. 7:12408. doi: 10.1038/ncomms12408
Balendra, R., and Isaacs, A. M. (2018). C9orf72-mediated ALS and FTD: multiple pathways to disease. Nat. Rev. Neurol. 14, 544–558. doi: 10.1038/s41582-018-0047-2
Bannwarth, S., Ait-El-Mkadem, S., Chaussenot, A., Genin, E. C., Lacas-Gervais, S., Fragaki, K., et al. (2014). A mitochondrial origin for frontotemporal dementia and amyotrophic lateral sclerosis through CHCHD10 involvement. Brain 137, 2329–2345. doi: 10.1093/brain/awu138
Beck, J., Poulter, M., Hensman, D., Rohrer, J. D., Mahoney, C. J., Adamson, G., et al. (2013). Large C9orf72 hexanucleotide repeat expansions are seen in multiple neurodegenerative syndromes and are more frequent than expected in the UK population. Am. J. Hum. Genet. 92, 345–353. doi: 10.1016/j.ajhg.2013.01.011
Byrne, S., Elamin, M., Bede, P., Shatunov, A., Walsh, C., Corr, B., et al. (2012). Cognitive and clinical characteristics of patients with amyotrophic lateral sclerosis carrying a C9orf72 repeat expansion: a population-based cohort study. Lancet Neurol. 11, 232–240. doi: 10.1016/S1474-4422(12)70014-5
Cameron, D. L., Di Stefano, L., and Papenfuss, A. T. (2019). Comprehensive evaluation and characterisation of short read general-purpose structural variant calling software. Nat. Commun. 10:3240. doi: 10.1038/s41467-019-11146-4
Carmo-Silva, S., Nobrega, C., de Almeida, L. P., and Cavadas, C. (2017). Unraveling the role of ataxin-2 in metabolism. Trends Endocrinol. Metab. 28, 309–318. doi: 10.1016/j.tem.2016.12.006
Chaisson, M. J., Sanders, A. D., Zhao, X., Malhotra, A., Porubsky, D., Rausch, T., et al. (2019). Multi-platform discovery of haplotype-resolved structural variation in human genomes. Nat. Commun. 10:1784. doi: 10.1038/s41467-018-08148-z
Chen, Y.-Z., Bennett, C. L., Huynh, H. M., Blair, I. P., Puls, I., Irobi, J., et al. (2004). DNA/RNA helicase gene mutations in a form of juvenile amyotrophic lateral sclerosis (ALS4). Am. J. Hum. Genet. 74, 1128–1135. doi: 10.1086/421054
Chiang, C., Scott, A. J., Davis, J. R., Tsang, E. K., Li, X., Kim, Y., et al. (2017). The impact of structural variation on human gene expression. Nat. Genet. 49, 692–699. doi: 10.1038/ng.3834
Chiò, A., Borghero, G., Calvo, A., Capasso, M., Caponnetto, C., Corbo, M., et al. (2010). Lithium carbonate in amyotrophic lateral sclerosis: lack of efficacy in a dose-finding trial. Neurology 75, 619–625. doi: 10.1212/WNL.0b013e3181ed9e7c
Chow, C. Y., Landers, J. E., Bergren, S. K., Sapp, P. C., Grant, A. E., Jones, J. M., et al. (2009). Deleterious variants of FIG4, a phosphoinositide phosphatase, in patients with ALS. Am. J. Hum. Genet. 84, 85–88. doi: 10.1016/j.ajhg.2008.12.010
Cirulli, E. T., Lasseigne, B. N., Petrovski, S., Sapp, P. C., Dion, P. A., Leblond, C. S., et al. (2015). Exome sequencing in amyotrophic lateral sclerosis identifies risk genes and pathways. Science 347, 1436–1441. doi: 10.1126/science.aaa3650
Cleary, J. D., and Ranum, L. P. (2014). Repeat associated non-ATG (RAN) translation: new starts in microsatellite expansion disorders. Curr. Opin. Genet. Dev. 26, 6–15. doi: 10.1016/j.gde.2014.03.002
Cleary, J. D., and Ranum, L. P. (2017). New developments in RAN translation: insights from multiple diseases. Curr. Opin. Genet. Dev. 44, 125–134. doi: 10.1016/j.gde.2017.03.006
Cook, C., and Petrucelli, L. (2019). Genetic convergence brings clarity to the enigmatic red line in ALS. Neuron 101, 1057–1069. doi: 10.1016/j.neuron.2019.02.032
Cooper-Knock, J., Shaw, P. J., and Kirby, J. (2014). The widening spectrum of C9ORF72-related disease; genotype/phenotype correlations and potential modifiers of clinical phenotype. Acta Neuropathol. 127, 333–345. doi: 10.1007/s00401-014-1251-9
Corcia, P., Mayeux-Portas, V., Khoris, J., de Toffol, B., Autret, A., Müh, J. P., et al. (2002). Abnormal SMN1 gene copy number is a susceptibility factor for amyotrophic lateral sclerosis. Ann. Neurol. 51, 243–246. doi: 10.1002/ana.10104
Corder, E. H., Saunders, A. M., Strittmatter, W. J., Schmechel, D. E., Gaskell, P. C., Small, G., et al. (1993). Gene dose of apolipoprotein E type 4 allele and the risk of Alzheimer’s disease in late onset families. Science 261, 921–923. doi: 10.1126/science.8346443
Couthouis, J., Hart, M. P., Erion, R., King, O. D., Diaz, Z., Nakaya, T., et al. (2012). Evaluating the role of the FUS/TLS-related gene EWSR1 in amyotrophic lateral sclerosis. Hum. Mol. Genet. 21, 2899–2911. doi: 10.1093/hmg/dds116
Daoud, H., Belzil, V., Martins, S., Sabbagh, M., Provencher, P., Lacomblez, L., et al. (2011). Association of long ATXN2 CAG repeat sizes with increased risk of amyotrophic lateral sclerosis. Arch. Neurol. 68, 739–742. doi: 10.1001/archneurol.2011.111
DeJesus-Hernandez, M., Mackenzie, I. R., Boeve, B. F., Boxer, A. L., Baker, M., Rutherford, N. J., et al. (2011). Expanded GGGGCC hexanucleotide repeat in noncoding region of C9ORF72 causes chromosome 9p-linked FTD and ALS. Neuron 72, 245–256. doi: 10.1016/j.neuron.2011.09.011
Deng, H.-X., Chen, W., Hong, S.-T., Boycott, K. M., Gorrie, G. H., Siddique, N., et al. (2011). Mutations in UBQLN2 cause dominant X-linked juvenile and adult-onset ALS and ALS/dementia. Nature 477, 211–215. doi: 10.1038/nature10353
D’Erchia, A. M., Gallo, A., Manzari, C., Raho, S., Horner, D. S., Chiara, M., et al. (2017). Massive transcriptome sequencing of human spinal cord tissues provides new insights into motor neuron degeneration in ALS. Sci. Rep. 7:10046. doi: 10.1038/s41598-017-10488-7
Dharmadasa, T., Henderson, R. D., Talman, P. S., Macdonell, R. A., Mathers, S., Schultz, D. W., et al. (2017). Motor neurone disease: progress and challenges. Med. J. Aust. 206, 357–362.
Doble, A. (1996). The pharmacology and mechanism of action of riluzole. Neurology 47(6 Suppl. 4), 233S–241S. doi: 10.1212/wnl.47.6_suppl_4.233s
Droppelmann, C. A., Wang, J., Campos-Melo, D., Keller, B., Volkening, K., Hegele, R. A., et al. (2013). Detection of a novel frameshift mutation and regions with homozygosis within ARHGEF28 gene in familial amyotrophic lateral sclerosis. Amyotroph. Lateral Scler. Frontotemporal Degener. 14, 444–451. doi: 10.3109/21678421.2012.758288
Ebbert, M. T. W., Jensen, T. D., Jansen-West, K., Sens, J. P., Reddy, J. S., Ridge, P. G., et al. (2019). Systematic analysis of dark and camouflaged genes reveals disease-relevant genes hiding in plain sight. Genome Biol. 20:97. doi: 10.1186/s13059-019-1707-2
Elden, A. C., Kim, H.-J., Hart, M. P., Chen-Plotkin, A. S., Johnson, B. S., Fang, X., et al. (2010). Ataxin-2 intermediate-length polyglutamine expansions are associated with increased risk for ALS. Nature 466, 1069–1075. doi: 10.1038/nature09320
Farg, M. A., Sundaramoorthy, V., Sultana, J. M., Yang, S., Atkinson, R. A., Levina, V., et al. (2014). C9ORF72, implicated in amytrophic lateral sclerosis and frontotemporal dementia, regulates endosomal trafficking. Hum. Mol. Genet. 23, 3579–3595. doi: 10.1093/hmg/ddu068
Fecto, F., Yan, J., Vemula, S. P., Liu, E., Yang, Y., Chen, W., et al. (2011). SQSTM1 mutations in familial and sporadic amyotrophic lateral sclerosis. JAMA Neurol. 68, 1440–1446.
Feuk, L., Carson, A. R., and Scherer, S. W. (2006). Structural variation in the human genome. Nat. Rev. Genet. 7, 85–97.
Figlewicz, D. A., Rouleau, G. A., Krizus, A., and Julien, J.-P. (1993). Polymorphism in the multi-phosphorylation domain of the human neurofilament heavy-subunit-encoding gene. Gene 132, 297–300. doi: 10.1016/0378-1119(93)90211-k
Fornai, F., Longone, P., Cafaro, L., Kastsiuchenka, O., Ferrucci, M., Manca, M. L., et al. (2008). Lithium delays progression of amyotrophic lateral sclerosis. Proc. Natl. Acad. Sci. U.S.A. 105, 2052–2057. doi: 10.1073/pnas.0708022105
Freischmidt, A., Wieland, T., Richter, B., Ruf, W., Schaeffer, V., Müller, K., et al. (2015). Haploinsufficiency of TBK1 causes familial ALS and fronto-temporal dementia. Nat. Neurosci. 18, 631–636. doi: 10.1038/nn.4000
Gitcho, M. A., Baloh, R. H., Chakraverty, S., Mayo, K., Norton, J. B., Levitch, D., et al. (2008). TDP-43 A315T mutation in familial motor neuron disease. Ann. Neurol. 63, 535–538.
Gitler, A. D., and Tsuiji, H. (2016). There has been an awakening: emerging mechanisms of C9orf72 mutations in FTD/ALS. Brain Res. 1647, 19–29. doi: 10.1016/j.brainres.2016.04.004
Greenway, M. J., Alexander, M. D., Ennis, S., Traynor, B. J., Corr, B., Frost, E., et al. (2004). A novel candidate region for ALS on chromosome 14q11.2. Neurology 63, 1936–1938. doi: 10.1212/01.wnl.0000144344.39103.f6
Greenway, M. J., Andersen, P. M., Russ, C., Ennis, S., Cashman, S., Donaghy, C., et al. (2006). ANG mutations segregate with familial and ‘sporadic’ amyotrophic lateral sclerosis. Nat. Genet. 38, 411–413. doi: 10.1038/ng1742
Gregersen, L. H., Mitter, R., Ugalde, A. P., Nojima, T., Proudfoot, N. J., Agami, R., et al. (2019). SCAF4 and SCAF8, mRNA anti-terminator proteins. Cell 177, 1797–1813.e18. doi: 10.1016/j.cell.2019.04.038
Hadano, S., Hand, C. K., Osuga, H., Yanagisawa, Y., Otomo, A., Devon, R. S., et al. (2001). A gene encoding a putative GTPase regulator is mutated in familial amyotrophic lateral sclerosis 2. Nat. Genet. 29, 166–173. doi: 10.1038/ng1001-166
Huang, C. R. L., Schneider, A. M., Lu, Y., Niranjan, T., Shen, P., Robinson, M. A., et al. (2010). Mobile interspersed repeats are major structural variants in the human genome. Cell 141, 1171–1182. doi: 10.1016/j.cell.2010.05.026
Hutten, S., and Dormann, D. (2019). RAN translation down. Nat. Neurosci. 22, 1379–1380. doi: 10.1038/s41593-019-0482-4
Jin, P., and Warren, S. T. (2000). Understanding the molecular basis of fragile X syndrome. Hum. Mol. Genet. 9, 901–908. doi: 10.1093/hmg/9.6.901
Johnson, J. O., Mandrioli, J., Benatar, M., Abramzon, Y., Van Deerlin, V. M., Trojanowski, J. Q., et al. (2010). Exome sequencing reveals VCP mutations as a cause of familial ALS. Neuron 68, 857–864. doi: 10.1016/j.neuron.2010.11.036
Johnson, J. O., Pioro, E. P., Boehringer, A., Chia, R., Feit, H., Renton, A. E., et al. (2014). Mutations in the Matrin 3 gene cause familial amyotrophic lateral sclerosis. Nat. Neurosci. 17, 664–666. doi: 10.1038/nn.3688
Kayatekin, C., Matlack, K. E., Hesse, W. R., Guan, Y., Chakrabortee, S., Russ, J., et al. (2014). Prion-like proteins sequester and suppress the toxicity of huntingtin exon 1. Proc. Natl. Acad. Sci. U.S.A. 111, 12085–12090. doi: 10.1073/pnas.1412504111
Kenna, K. P., van Doormaal, P. T. C., Dekker, A. M., Ticozzi, N., Kenna, B. J., Diekstra, F. P., et al. (2016). NEK1 variants confer susceptibility to amyotrophic lateral sclerosis. Nat. Genet. 48, 1037–1042. doi: 10.1038/ng.3626
Khalil, M., Teunissen, C. E., Otto, M., Piehl, F., Sormani, M. P., Gattringer, T., et al. (2018). Neurofilaments as biomarkers in neurological disorders. Nat. Rev. Neurol. 14, 577–589. doi: 10.1038/s41582-018-0058-z
Kim, H. J., Kim, N. C., Wang, Y.-D., Scarborough, E. A., Moore, J., Diaz, Z., et al. (2013). Mutations in prion-like domains in hnRNPA2B1 and hnRNPA1 cause multisystem proteinopathy and ALS. Nature 495, 467–473. doi: 10.1038/nature11922
Kinsley, L., and Siddique, T. (2015). “Amyotrophic lateral sclerosis overview,” in GeneReviews® [Internet], eds R. A. Ardinger, M. P. Pagon, H. H. Adam, S. E. Amemiya, A. Wallace, L. J. H. Bean, et al. (Seattle, WA: University of Washington).
Lambrechts, D., Storkebaum, E., Morimoto, M., Del-Favero, J., Desmet, F., Marklund, S. L., et al. (2003). VEGF is a modifier of amyotrophic lateral sclerosis in mice and humans and protects motoneurons against ischemic death. Nat. Genet. 34, 383–394. doi: 10.1038/ng1211
Landers, J. E., Melki, J., Meininger, V., Glass, J. D., Van Den Berg, L. H., Van Es, M. A., et al. (2009). Reduced expression of the Kinesin-Associated Protein 3 (KIFAP3) gene increases survival in sporadic amyotrophic lateral sclerosis. Proc. Natl. Acad. Sci. 106, 9004–9009. doi: 10.1073/pnas.0812937106
Lattante, S., Millecamps, S., Stevanin, G., Rivaud-Péchoux, S., Moigneu, C., Camuzat, A., et al. (2014). Contribution of ATXN2 intermediary polyQ expansions in a spectrum of neurodegenerative disorders. Neurology 83, 990–995. doi: 10.1212/WNL.0000000000000778
Leung, C. L., He, C. Z., Kaufmann, P., Chin, S. S., Naini, A., Liem, R. K. H., et al. (2004). A pathogenic peripherin gene mutation in a patient with amyotrophic lateral sclerosis. Brain Pathol. 14, 290–296. doi: 10.1111/j.1750-3639.2004.tb00066.x
Ly, C. V., and Miller, T. M. (2018). Emerging antisense oligonucleotide and viral therapies for amyotrophic lateral sclerosis. Curr. Opin. Neurol. 31, 648–654. doi: 10.1097/WCO.0000000000000594
Lyall, D. M., Royle, N. A., Harris, S. E., Bastin, M. E., Maniega, S. M., Murray, C., et al. (2013). Alzheimer’s disease susceptibility genes APOE and TOMM40, and hippocampal volumes in the Lothian birth cohort 1936. PLoS One 8:e80513. doi: 10.1371/journal.pone.0080513
Mahmoud, M., Gobet, N., Cruz-Dávalos, D. I., Mounier, N., Dessimoz, C., and Sedlazeck, F. J. (2019). Structural variant calling: the long and the short of it. Genome Biol. 20:246. doi: 10.1186/s13059-019-1828-7
Maruyama, H., Morino, H., Ito, H., Izumi, Y., Kato, H., Watanabe, Y., et al. (2010). Mutations of optineurin in amyotrophic lateral sclerosis. Nature 465, 223–226. doi: 10.1038/nature08971
Mejzini, R., Flynn, L. L., Pitout, I. L., Fletcher, S., Wilton, S. D., and Akkari, P. A. (2019). ALS genetics, mechanisms, and therapeutics: where are we now? Front. Neurosci. 13:1310. doi: 10.3389/fnins.2019.01310
Miller, R., Moore, D., Forshew, D., Katz, J., Barohn, R., Valan, M., et al. (2011). Phase II screening trial of lithium carbonate in amyotrophic lateral sclerosis: examining a more efficient trial design. Neurology 77, 973–979. doi: 10.1212/WNL.0b013e31822dc7a5
Mis, M. S. C., Brajkovic, S., Tafuri, F., Bresolin, N., Comi, G. P., and Corti, S. (2017). Development of therapeutics for C9ORF72 ALS/FTD-related disorders. Mol. Neurobiol. 54, 4466–4476. doi: 10.1007/s12035-016-9993-0
Mitchell, J., Paul, P., Chen, H.-J., Morris, A., Payling, M., Falchi, M., et al. (2010). Familial amyotrophic lateral sclerosis is associated with a mutation in D-amino acid oxidase. Proc. Natl. Acad. Sci. U.S.A. 107, 7556–7561.
Mitsumoto, H., Brooks, B. R., and Silani, V. (2014). Clinical trials in amyotrophic lateral sclerosis: why so many negative trials and how can trials be improved? Lancet Neurol. 13, 1127–1138. doi: 10.1016/S1474-4422(14)70129-2
Mitsumoto, H., and Saito, T. (2018). A prognostic biomarker in amyotrophic lateral sclerosis. Clin. Neurol. 58, 729–736. doi: 10.5692/clinicalneurol.cn-001220
Morita, M., Al-Chalabi, A., Andersen, P., Hosler, B., Sapp, P., Englund, E., et al. (2006). A locus on chromosome 9p confers susceptibility to ALS and frontotemporal dementia. Neurology 66, 839–844. doi: 10.1212/01.wnl.0000200048.53766.b4
Münch, C., Sedlmeier, R., Meyer, T., Homberg, V., Sperfeld, A., Kurt, A., et al. (2004). Point mutations of the p150 subunit of dynactin (DCTN1) gene in ALS. Neurology 63, 724–726. doi: 10.1212/01.wnl.0000134608.83927.b1
Nguyen, H. P., Van Broeckhoven, C., and van der Zee, J. (2018). ALS genes in the genomic era and their implications for FTD. Trends Genet. 34, 404–423. doi: 10.1016/j.tig.2018.03.001
Nicolas, A., Kenna, K. P., Renton, A. E., Ticozzi, N., Faghri, F., Chia, R., et al. (2018). Genome-wide analyses identify KIF5A as a novel ALS gene. Neuron 97, 1268–1283.e6. doi: 10.1016/j.neuron.2018.02.027
Nishimura, A. L., Mitne-Neto, M., Silva, H. C., Richieri-Costa, A. N., Middleton, S., Cascio, D., et al. (2004). A mutation in the vesicle-trafficking protein VAPB causes late-onset spinal muscular atrophy and amyotrophic lateral sclerosis. Am. J. Hum. Genet. 75, 822–831. doi: 10.1086/425287
Okamoto, K., Hirai, S., Amari, M., Watanabe, M., and Sakurai, A. (1993). Bunina bodies in amyotrophic lateral sclerosis immunostained with rabbit anti-cystatin C serum. Neurosci. Lett. 162, 125–128. doi: 10.1016/0304-3940(93)90576-7
Orlacchio, A., Babalini, C., Borreca, A., Patrono, C., Massa, R., Basaran, S., et al. (2010). SPATACSIN mutations cause autosomal recessive juvenile amyotrophic lateral sclerosis. Brain 133, 591–598. doi: 10.1093/brain/awp325
Parkinson, N., Ince, P., Smith, M., Highley, R., Skibinski, G., Andersen, P., et al. (2006). ALS phenotypes with mutations in CHMP2B (charged multivesicular body protein 2B). Neurology 67, 1074–1077. doi: 10.1212/01.wnl.0000231510.89311.8b
Petrov, D., Mansfield, C., Moussy, A., and Hermine, O. (2017). ALS clinical trials review: 20 years of failure. Are we any closer to registering a new treatment? Front. Aging Neurosci. 9:68. doi: 10.3389/fnagi.2017.00068
Renton, A. E., Chiò, A., and Traynor, B. J. (2014). State of play in amyotrophic lateral sclerosis genetics. Nat. Neurosci. 17, 17–23. doi: 10.1038/nn.3584
Renton, A. E., Majounie, E., Waite, A., Simón-Sánchez, J., Rollinson, S., Gibbs, J. R., et al. (2011). A hexanucleotide repeat expansion in C9ORF72 is the cause of chromosome 9p21-linked ALS-FTD. Neuron 72, 257–268. doi: 10.1016/j.neuron.2011.09.010
Robberecht, W., and Philips, T. (2013). The changing scene of amyotrophic lateral sclerosis. Nat. Rev. Neurosci. 14, 248–264. doi: 10.1038/nrn3430
Rose, A. M., Shah, A. Z., Venturini, G., Krishna, A., Chakravarti, A., Rivolta, C., et al. (2016). Transcriptional regulation of PRPF31 gene expression by MSR1 repeat elements causes incomplete penetrance in retinitis pigmentosa. Sci. Rep. 6:19450. doi: 10.1038/srep19450
Rosen, D. R., Siddique, T., Patterson, D., Figlewicz, D. A., Sapp, P., Hentati, A., et al. (1993). Mutations in Cu/Zn superoxide dismutase gene are associated with familial amyotrophic lateral sclerosis. Nature 362, 59–62.
Roses, A. (2016). Polyallelic structural variants can provide accurate, highly informative genetic markers focused on diagnosis and therapeutic targets: accuracy vs. Precision. Clin. Pharmacol. Ther. 99, 169–171. doi: 10.1002/cpt.288
Roses, A., Lutz, M., Amrine-Madsen, H., Saunders, A., Crenshaw, D., Sundseth, S., et al. (2010). A TOMM40 variable-length polymorphism predicts the age of late-onset Alzheimer’s disease. Pharmacogenomics J. 10, 375–384. doi: 10.1038/tpj.2009.69
Roses, A. D., Akkari, P. A., Chiba-Falek, O., Lutz, M. W., Gottschalk, W. K., Saunders, A. M., et al. (2016). Structural variants can be more informative for disease diagnostics, prognostics and translation than current SNP mapping and exon sequencing. Expert Opin. Drug Metab. Toxicol. 12, 135–147. doi: 10.1517/17425255.2016.1133586
Roses Allen, D. (2019). Methods for detecting structural variants in neurodegenerative disease. US Patent No 201,716,308,315.
Rothstein, J. D. (2017). Edaravone: a new drug approved for ALS. Cell 171:725. doi: 10.1016/j.cell.2017.10.011
Saul, R., Lutz, M. W., Burns, D. K., Roses, A. D., and Chiba-Falek, O. (2016). The SSV evaluation system: a tool to prioritize short structural variants for studies of possible regulatory and causal variants. Hum. Mutat. 37, 877–883. doi: 10.1002/humu.23023
Schymick, J. C., Yang, Y., Andersen, P. M., Vonsattel, J. P., Greenway, M., Momeni, P., et al. (2007). Progranulin mutations and amyotrophic lateral sclerosis or amyotrophic lateral sclerosis-frontotemporal dementia phenotypes. J. Neurol. Neurosurg. Psychiatry 78, 754–756.
Scoles, D. R., Ho, M. H., Dansithong, W., Pflieger, L. T., Petersen, L. W., Thai, K. K., et al. (2015). Repeat associated non-AUG translation (RAN translation) dependent on sequence downstream of the ATXN2 CAG repeat. PLoS One 10:e0128769. doi: 10.1371/journal.pone.0128769
Sebat, J., Lakshmi, B., Troge, J., Alexander, J., Young, J., Lundin, P., et al. (2004). Large-scale copy number polymorphism in the human genome. Science 305, 525–528. doi: 10.1126/science.1098918
Shi, Y., Lin, S., Staats, K. A., Li, Y., Chang, W.-H., Hung, S.-T., et al. (2018). Haploinsufficiency leads to neurodegeneration in C9ORF72 ALS/FTD human induced motor neurons. Nat. Med. 24, 313–325. doi: 10.1038/nm.4490
Simpson, C. L., Lemmens, R., Miskiewicz, K., Broom, W. J., Hansen, V. K., van Vught, P. W., et al. (2008). Variants of the elongator protein 3 (ELP3) gene are associated with motor neuron degeneration. Hum. Mol. Genet. 18, 472–481. doi: 10.1093/hmg/ddn375
Slowik, A., Tomik, B., Wolkow, P., Partyka, D., Turaj, W., Malecki, M., et al. (2006). Paraoxonase gene polymorphisms and sporadic ALS. Neurology 67, 766–770. doi: 10.1212/01.wnl.0000219565.32247.11
Smith, B. N., Ticozzi, N., Fallini, C., Gkazi, A. S., Topp, S., Kenna, K. P., et al. (2014). Exome-wide rare variant analysis identifies TUBA4A mutations associated with familial ALS. Neuron 84, 324–331. doi: 10.1016/j.neuron.2014.09.027
Smith, B. N., Topp, S. D., Fallini, C., Shibata, H., Chen, H.-J., Troakes, C., et al. (2017). Mutations in the vesicular trafficking protein annexin A11 are associated with amyotrophic lateral sclerosis. Sci. Transl. Med. 9:eaad9157. doi: 10.1126/scitranslmed.aad9157
Sproviero, W., Shatunov, A., Stahl, D., Shoai, M., van Rheenen, W., Jones, A. R., et al. (2017). ATXN2 trinucleotide repeat length correlates with risk of ALS. Neurobiol. Aging 51, 178.e1–178.e9. doi: 10.1016/j.neurobiolaging.2016.11.010
Staats, K., Seah, C., Sahimi, A., Wang, Y., Koutsodendris, N., Lin, S., et al. (2019). Small molecule inhibition of PIKFYVE kinase rescues gain-and loss-of-function C9ORF72 ALS/FTD disease processes in vivo. bioRxiv [Preprint] doi: 10.1101/685800
Staats, K. A., Van Helleputte, L., Jones, A. R., Bento-Abreu, A., Van Hoecke, A., Shatunov, A., et al. (2013). Genetic ablation of phospholipase C delta 1 increases survival in SOD1G93A mice. Neurobiol. Dis. 60, 11–17. doi: 10.1016/j.nbd.2013.08.006
Sudmant, P. H., Rausch, T., Gardner, E. J., Handsaker, R. E., Abyzov, A., Huddleston, J., et al. (2015). An integrated map of structural variation in 2,504 human genomes. Nature 526, 75–81. doi: 10.1038/nature15394
Talman, P., Duong, T., Vucic, S., Mathers, S., Venkatesh, S., Henderson, R., et al. (2016). Identification and outcomes of clinical phenotypes in amyotrophic lateral sclerosis/motor neuron disease: Australian national motor neuron disease observational cohort. Br. Med. J. Open 6:e012054. doi: 10.1136/bmjopen-2016-012054
Ticozzi, N., Vance, C., Leclerc, A., Keagle, P., Glass, J., McKenna-Yasek, D., et al. (2011). Mutational analysis reveals the FUS homolog TAF15 as a candidate gene for familial amyotrophic lateral sclerosis. Am. J. Med. Genet. Part B Neuropsychiatr. Genet. 156, 285–290. doi: 10.1002/ajmg.b.31158
UKMND-LiCALS Study Group (2013). Lithium in patients with amyotrophic lateral sclerosis (LiCALS): a phase 3 multicentre, randomised, double-blind, placebo-controlled trial. Lancet Neurol. 12, 339–345. doi: 10.1016/S1474-4422(13)70037-1
Van Damme, P., Veldink, J. H., van Blitterswijk, M., Corveleyn, A., van Vught, P. W., Thijs, V., et al. (2011). Expanded ATXN2 CAG repeat size in ALS identifies genetic overlap between ALS and SCA2. Neurology 76, 2066–2072. doi: 10.1212/WNL.0b013e31821f445b
Van Eijk, R. P., Eijkemans, M. J., Nikolakopoulos, S., Jansen, M. D., Westeneng, H.-J., Van Eijk, K. R., et al. (2019). Pharmacogenetic interactions in amyotrophic lateral sclerosis: a step closer to a cure? Pharmacogenomics J. doi: 10.1038/s41397-019-0111-3 [Epub ahead of print].
Van Eijk, R. P., Jones, A. R., Sproviero, W., Shatunov, A., Shaw, P. J., Leigh, P. N., et al. (2017). Meta-analysis of pharmacogenetic interactions in amyotrophic lateral sclerosis clinical trials. Neurology 89, 1915–1922. doi: 10.1212/WNL.0000000000004606
Van Es, M. A., Van Vught, P. W., Blauw, H. M., Franke, L., Saris, C. G., Andersen, P. M., et al. (2007). ITPR2 as a susceptibility gene in sporadic amyotrophic lateral sclerosis: a genome-wide association study. Lancet Neurol. 6, 869–877.
Van Es, M. A., Van Vught, P. W., Blauw, H. M., Franke, L., Saris, C. G., Van Den Bosch, L., et al. (2008). Genetic variation in DPP6 is associated with susceptibility to amyotrophic lateral sclerosis. Nat. Genet. 40, 29–31.
Van Es, M. A., Veldink, J. H., Saris, C. G., Blauw, H. M., van Vught, P. W., Birve, A., et al. (2009). Genome-wide association study identifies 19p13. 3 (UNC13A) and 9p21. 2 as susceptibility loci for sporadic amyotrophic lateral sclerosis. Nat. Genet. 41, 1083–1087. doi: 10.1038/ng.442
Van Rheenen, W., Shatunov, A., Dekker, A. M., McLaughlin, R. L., Diekstra, F. P., Pulit, S. L., et al. (2016). Genome-wide association analyses identify new risk variants and the genetic architecture of amyotrophic lateral sclerosis. Nat. Genet. 48, 1043–1048. doi: 10.1038/ng.3622
Vance, C., Rogelj, B., Hortobágyi, T., De Vos, K. J., Nishimura, A. L., Sreedharan, J., et al. (2009). Mutations in FUS, an RNA processing protein, cause familial amyotrophic lateral sclerosis type 6. Science 323, 1208–1211. doi: 10.1126/science.1165942
Vejux, A., Namsi, A., Nury, T., Moreau, T., and Lizard, G. (2018). Biomarkers of amyotrophic lateral sclerosis: current status and interest of oxysterols and phytosterols. Front. Mol. Neurosci. 11:12. doi: 10.3389/fnmol.2018.00012
Verstraete, E., Veldink, J. H., Huisman, M. H., Draak, T., Uijtendaal, E. V., van der Kooi, A. J., et al. (2012). Lithium lacks effect on survival in amyotrophic lateral sclerosis: a phase IIb randomised sequential trial. J. Neurol. Neurosurg. Psychiatry 83, 557–564. doi: 10.1136/jnnp-2011-302021
Wang, X.-S., Lee, S., Simmons, Z., Boyer, P., Scott, K., Liu, W., et al. (2004). Increased incidence of the Hfe mutation in amyotrophic lateral sclerosis and related cellular consequences. J. Neurol. Sci. 227, 27–33. doi: 10.1016/j.jns.2004.08.003
Williams, K. L., Topp, S., Yang, S., Smith, B., Fifita, J. A., Warraich, S. T., et al. (2016). CCNF mutations in amyotrophic lateral sclerosis and frontotemporal dementia. Nat. Commun. 7:11253. doi: 10.1038/ncomms11253
Wu, C.-H., Fallini, C., Ticozzi, N., Keagle, P. J., Sapp, P. C., Piotrowska, K., et al. (2012). Mutations in the profilin 1 gene cause familial amyotrophic lateral sclerosis. Nature 488, 499–503. doi: 10.1038/nature11280
Yamada, S. B., Gendron, T. F., Niccoli, T., Genuth, N. R., Grosely, R., Shi, Y., et al. (2019). RPS25 is required for efficient RAN translation of C9orf72 and other neurodegenerative disease-associated nucleotide repeats. Nat. Neurosci. 22, 1383–1388. doi: 10.1038/s41593-019-0455-7
Keywords: amyotrophic lateral sclerosis, structural variant, genetic marker, missing heritability, clinical trial stratification
Citation: Theunissen F, Flynn LL, Anderton RS, Mastaglia F, Pytte J, Jiang L, Hodgetts S, Burns DK, Saunders A, Fletcher S, Wilton SD and Akkari PA (2020) Structural Variants May Be a Source of Missing Heritability in sALS. Front. Neurosci. 14:47. doi: 10.3389/fnins.2020.00047
Received: 21 October 2019; Accepted: 13 January 2020;
Published: 31 January 2020.
Edited by:
Francesca Trojsi, University of Campania Luigi Vanvitelli, ItalyReviewed by:
Kim A. Staats, University of Southern California, United StatesCopyright © 2020 Theunissen, Flynn, Anderton, Mastaglia, Pytte, Jiang, Hodgetts, Burns, Saunders, Fletcher, Wilton and Akkari. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Patrick Anthony Akkari, QW50aG9ueS5ha2thcmlAcGVycm9uLnV3YS5lZHUuYXU=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.