- Molecular Neuropsychiatry Research Branch, NIH/NIDA Intramural Research Program, Baltimore, MD, United States
Opioid use disorder (OUD) is characterized by compulsive drug taking despite adverse life consequences. Here, we sought to identify neurobiological consequences associated with the behavioral effects of contingent footshocks administered after escalation of oxycodone self-administration. To reach these goals, Sprague-Dawley rats were trained to self-administer oxycodone for 4 weeks and were then exposed to contingent electric footshocks. This paradigm helped to dichotomize rats into two distinct behavioral phenotypes: (1) those that reduce lever pressing (shock-sensitive) and (2) others that continue lever pressing (shock-resistant) for oxycodone during contingent punishment. The rats were euthanized at 2-h after the last oxycodone plus footshock session. The dorsal striata and prefrontal cortices were dissected for use in western blot and RT-qPCR analyses. All oxycodone self-administration rats showed significant decreased expression of Mu and Kappa opioid receptor proteins only in the dorsal striatum. However, expression of Delta opioid receptor protein was decreased in both brain regions. RT-qPCR analyses documented significant decreases in the expression of c-fos, fosB, fra2, junB, egr1, and egr2 mRNAs in the dorsal striatum (but not in PFC) of the shock-sensitive rats. In the PFC, junD expression was reduced in both phenotypes. However, egr3 mRNA expression was increased in the PFC of only shock-resistant rats. These results reveal that, similar to psychostimulants and alcohol, footshocks can dichotomize rats that escalated their intake of oxycodone into two distinct behavioral phenotypes. These animals also show significant differences in the mRNA expression of immediate early genes, mainly, in the dorsal striatum. The increases in PFC egr3 expression in the shock-resistant rats suggest that Egr3 might be involved in the persistence of oxycodone-associated memory under aversive conditions. This punishment-driven model may help to identify neurobiological substrates of persistent oxycodone taking and abstinence in the presence of adverse consequences.
Introduction
Opioid use disorders (OUDs) continue to constitute a public health crisis (Cicero et al., 2005; Skolnick, 2017). OUDs are characterized by compulsive consumption of large amounts of opioids despite adverse life consequences (American Psychiatric Association and Dsm-5 Task Force, 2013). Adverse consequences of OUDs are influenced by widespread use of opioids such as oxycodone for the treatment of various pain syndromes (Gaskell et al., 2016; Schmidt-Hansen et al., 2017) and their over-prescription by medical professionals (Van Zee, 2009). These have led to substantial increases in the number of deaths caused by opioid overdoses (Skolnick, 2017). To develop more effective treatment strategies for OUDs (Blanco and Volkow, 2019; Patel and Kosten, 2019), animal models that meet multiple DSM criteria are needed to increase their translational validity (Cadet, 2019).
Efforts to elucidate the molecular neurobiology of substance use disorders (SUDs), including OUDs, have largely depended on drug self-administration (SA) models (Wade et al., 2015; Mavrikaki et al., 2017; Blackwood et al., 2018). However, as suggested by several investigators (Chen et al., 2013; Belin-Rauscent et al., 2016; Cadet, 2019), drug self-administration alone represents only one DSM criterion and fails to replicate the complex behavioral syndromes termed SUDs (American Psychiatric Association and Dsm-5 Task Force, 2013). Some groups have tried to remedy this conundrum by adding contingent punishment during the performance of self-administration of alcohol, cocaine, and methamphetamine by rodents (Chen et al., 2013; Vanderschuren et al., 2017; Marchant et al., 2018; Cadet et al., 2019). In these models, footshocks are used to segregate rats that continue to self-administer drugs [shock-resistant (SR), addicted] from those that significantly reduce or stop [shock-sensitive (SS), non-addicted users] their intake in the presence of punishment (Chen et al., 2013; Vanderschuren et al., 2017; Marchant et al., 2018; Cadet et al., 2019). Those rats that significantly reduced or stopped their drug intake may represent a group of individuals who use illicit drugs without meeting DSM criteria for SUD (Korf et al., 2010; Zaaijer et al., 2014). Similar to observations with psychostimulants, rats, given long access to oxycodone, escalate their intake and exhibit compulsive drug seeking behaviors (Wade et al., 2015; Blackwood et al., 2018; Bossert et al., 2018). However, it remains to be determined if punishment could also help to dichotomize rats that escalated their oxycodone intake into SR and SS rats as reported for other drugs (Chen et al., 2013; Marchant et al., 2018; Cadet et al., 2019).
Behaviors measured during drug self-administration have been shown to depend on interactions of interconnected brain regions that include the dorsal striatum and the prefrontal cortex (PFC) among others (Everitt, 2014; Moorman et al., 2015; Smith and Laiks, 2018). On the one hand, the dorsal striatum has been reported to play prominent roles in the mediation of habitual drug taking behaviors (Belin and Everitt, 2008; Everitt and Robbins, 2016; Hodebourg et al., 2018). On the other hand, the PFC is involved in drug seeking, reinstatement of drug seeking, and other complex cognitive behaviors including decision making in relation to drug taking behaviors (Bossert et al., 2011; Perry et al., 2011; Hu et al., 2019). Human studies have also documented abnormalities in these regions of humans who suffer from SUDs (Bolla et al., 2004; Goldstein and Volkow, 2011; Cadet et al., 2014; Cadet and Bisagno, 2015). Of further relevance to investigations of oxycodone use disorder and other OUDs, in general, is the fact that these brain regions contain high concentration of opioid receptors. For example, using receptor autoradiographic techniques, Mansour et al. (1987) had reported large concentration of mu and delta opioid receptors in the frontal cortex and dorsal striatum of rats. The dorsal striatum also contains high concentration of kappa opioid receptors (Mansour et al., 1987). These regions also contain mRNAs that code for these receptors (Mansour et al., 1994). Human studies using PET and radiolabeled carfentanil have also identified mu opioid receptors in these brain regions (Frost et al., 1985; Nummenmaa et al., 2018; Burns et al., 2019). Of significance to the presence study, post-mortem studies have reported decreased expression of mu opioid receptors in the striatum (Sillivan et al., 2013) and PFC of heroin abusers (Ferrer-Alcon et al., 2004b). Other post-mortem abnormalities in signaling pathways have also been reported in the PFC of opioid users (Ferrer-Alcon et al., 2004a; Keller et al., 2017).
Thus, we reasoned that biochemical and molecular adaptations in these brain regions might play important roles in the behavioral manifestations of punishment-induced separation of rats into resistant and sensitive rats. This idea is supported by our recent demonstration that decreases in the expression of Mu opioid receptor protein levels in the dorsal striatum correlated with escalated drug intake and drug seeking behavior in oxycodone self-administering rats (Blackwood et al., 2018). This reasoning is also consonant with our findings of dose-dependent changes in the mRNA expression of immediate early genes (IEGs) following oxycodone seeking after a month of withdrawal from escalated oxycodone SA (Blackwood et al., 2019). We reasoned further that shock-resistant and shock-sensitive rats may show differential expression of opioid receptors in the dorsal striatum and of IEGs in both the dorsal striatum and PFC. Changes in IEG expression have been reported in several models of psychostimulant and OUDs (Bisagno and Cadet, 2019), supporting the possibility that IEGs could be used as markers of the two shock-induced divergent phenotypes.
As predicted, footshocks did help to separate oxycodone SA rats into SR and SS phenotypes. These two phenotypes showed differential expression of IEGs in the dorsal striatum and PFC, with greater changes occurring in the striatum. These data further implicate the dorsal striatum as a major node in the chemical neuroanatomical pathways of habitual compulsive oxycodone taking.
Materials and Methods
Subjects
Male Sprague-Dawley rats (Charles River, Raleigh, NC, United States), weighing 350–400 g before surgery, were used in all experiments. Rats were maintained on a 12-h reversed light/dark cycle with food and water available ad libitum. This study was carried out in accordance with the principles and guidelines outlined in the National Institutes of Health (NIH) Guide for the Care and Use of Laboratory Animals (eighth Edition1) as approved by the NIDA (National Institute of Drug Abuse) Animal Care and Use Committee at the Intramural Research Program (IRP).
Intravenous Surgery and Self-Administration Training
Surgical implantations of intravenous catheters were done as previously described (Cadet et al., 2017). Briefly, we anesthetized the rats with an intraperitoneal injection of ketamine (50 mg/kg) and xylazine (5 mg/kg) and inserted a polyurethane catheter (SAI Infusion Technologies, Lake Villa, IL, United States) into the jugular vein. One end of the catheters was inserted in the jugular vein while the other end was attached to a modified 22-gauge cannula (Plastics One, Roanoke, VA, United States) that was mounted to the back of each rat. The modified cannulae were connected to a fluid swivel (Instech, Plymouth, PA, United States) via polyethylene-50 tubing that was protected by a metal spring. These cannulae served as infusion ports for the catheters. The ports were closed using dust caps (PlasticOne, Roanoke, VA, United States). Thereafter, the catheters were flushed every 48-h with gentamicin (0.05 mg/kg, Henry Schein, Melville, NY, United States) in sterile saline to maintain patency. Injections of buprenorphine (0.1 mg/kg) were used post-surgery to relieve pain.
Rats were allowed to recover for 5–7 days before oxycodone self-administration (SA) training. Rats were trained in SA chambers located inside sound-attenuated cabinets and controlled by a Med Associates System (Med Associates, St Albans, VT, United States). Each chamber was equipped with two levers located 8.5 cm above the grid floor. Presses on the retractable active lever activated the infusion pump and tone-light cue. Presses on the inactive lever had no reinforced consequences.
Training and Punishment Phases
Rats (n = 28) were housed in Med Associates SA chambers and were randomly assigned to either saline (Sal) (n = 8) or oxycodone (n = 20) conditions. Rats were given long access to oxycodone and were trained for two 3-h sessions during days 1–5, followed by three 3-h sessions during days 6–22 (Figure 1A). Each of the 3-h sessions was separated by 30-min intervals during which rats remained in operant chambers but had no access to the levers to press for oxycodone. Lever presses were reinforced using a fixed ratio-1 with a 20-s timeout accompanied by a 5-s compound tone-light cue. Rats self-administered oxycodone at a dose of 0.1 mg/kg per infusion given over 3.5-s (0.1 ml per infusion). The house light was turned off and the active lever retracted at the end of the 3-h session. After training rats for 22 days, oxycodone rats that escalated their oxycodone intake equal to or greater than 50 daily infusions underwent the punishment phase.

Figure 1. Long access to oxycodone self-administration and contingent punishment dichotomize rats into shock-resistant and shock-sensitive phenotypes. (A) Experimental timeline of oxycodone self-administration training and footshock phases. Rats were trained to self-administer oxycodone using a long access (LgA) paradigms of 6-h for 1–5 days, 9-h for 6–22 days, followed by oxycodone SA and contingent footshocks (0.18–0.36 mA) for 9-h for 6 more days. (B) All rats escalate their intake of oxycodone during long access self-administration training (n = 20). (C) During the 9-h training paradigm rats showed significantly higher levels of oxycodone on the last day compared to the first day (n = 20). (D) Footshocks caused decreased lever pressing significantly more in the shock-sensitive (SS) than in the shock-resistant (SR) rats (n = 5, SS; n = 6, SR). (E) SS rats took substantially less oxycodone than SR rats. Key to statistics: ∗, ∗∗, ∗∗∗p < 0.05, 0.01, 0.001, respectively, in comparison to saline rats or last 2 days before shocks in the SR subgroup; #, ##, ###p < 0.05, 0.01, 0.001, respectively, in comparison to SR rats or last 2 days of shocks in the SR subgroup. !!!p < 0.001, in comparison to first day of 9-h training. Stats were performed by two-way ANOVA followed by Bonferroni post hoc tests or Student’s t-test.
During the punishment phase, rats continued to self-administer oxycodone every day (three 3-h sessions/day separated by 30 min off intervals) as described above. During that phase, ∼50% of the reinforced lever-presses also resulted in the delivery of a 0.5-s footshock through the grid floor. We set the initial footshock at 0.18 mA and increased the shock intensity by 0.06 mA to a final value of 0.36 mA over the course of 6 punishment days.
Collection of Tissues
We euthanized rats 2-h after the last oxycodone plus punishment day. Brains were removed from skulls and the dorsal striata and PFC were dissected and snap frozen on dry ice. Dissections were performed as previously described (Blackwood et al., 2018). In brief, we used stereotaxic coordinates to dissect the dorsal striatum (A/P + 2 to −2 mm bregma, M/L ± 2 to 5 mm, D/V −3 to −6 mm) and the PFC (A/P + 2.7 to + 1.7 mm bregma, M/L 0 to + 4 mm, D/V + 7 to + 9 mm) using the Atlas by Paxinos and Watson (1998). We also followed distinguishable anatomical structures (olfactory bulb, corpus callosum, and lateral ventricle) for further accuracy. Tissues were then processed for western blotting and quantitative RT-PCR analyses.
Quantitative RT-PCR
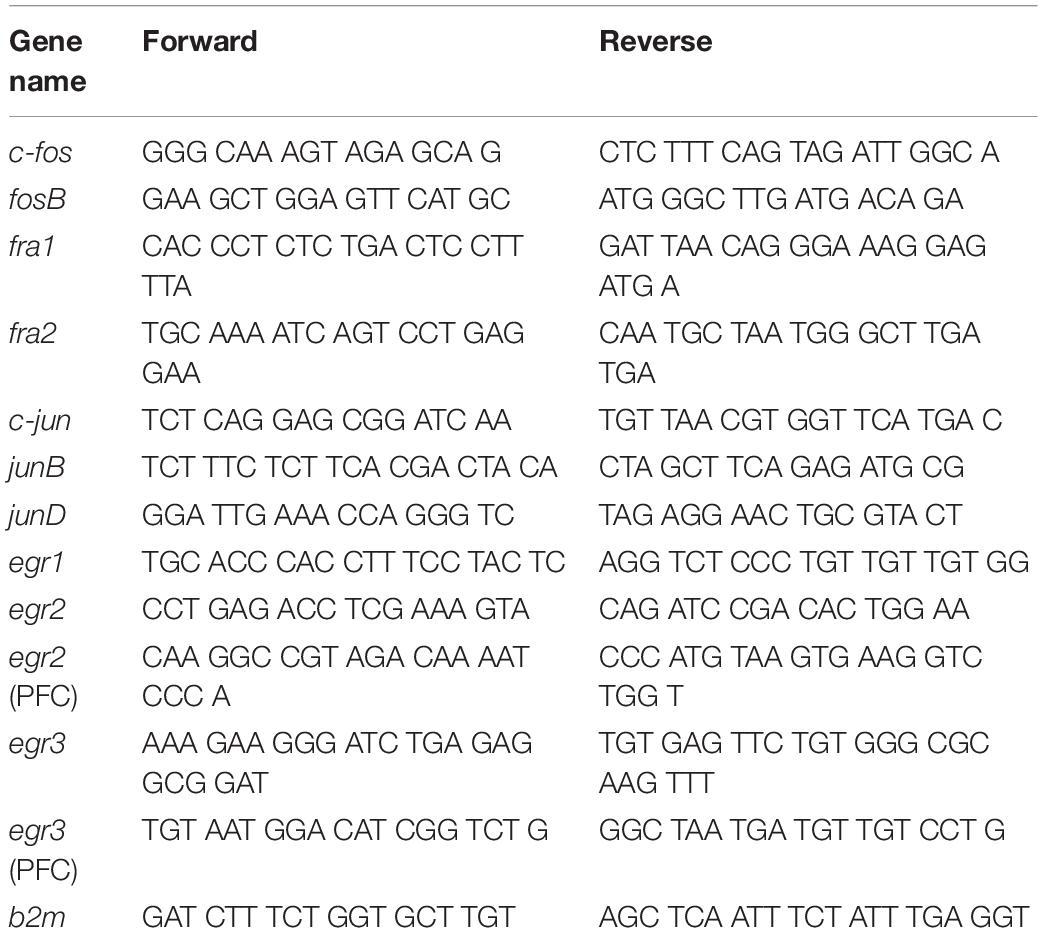
Total RNA was isolated from one hemisphere of the dorsal striatum and PFC using RNeasy Mini Kit (Qiagen, Valencia, CA, United States). Total RNA (0.5 μg) was reverse-transcribed (RT) with oligo dT primers using Advantage RT-for-PCR kit (Clontech, Mountain View, CA, United States). RT-qPCR was performed as previously described (Cadet et al., 2017; Blackwood et al., 2019) with Roche LightCycler 480 II (Roche Diagnostics, Indianapolis, IN, United States) using iQ SYBR Green Supermix (Bio-Rad, Hercules, CA, United States). Custom-designed primers were made and HPLC-purified by the Synthesis and Sequence Facility of the Johns Hopkins University (Baltimore, MD, United States). Primer sequences are listed in Table 1.
Western Blotting
Western blotting was performed essentially as previously described (Blackwood et al., 2018). In brief, soluble protein lysates were prepared in solutions that contained 1x NuPage LDS Sample Buffer (Thermo Fisher Scientific, Waltham, MA, United States), and 1% β-Mercaptoethanol (Sigma, St. Louis, MO, United States). Samples were then boiled and resolved using NuPage 10% Bis-Tris Protein Gels (Thermo Fisher Scientific, Waltham, MA, United States). Proteins were electrophoretically transferred onto Trans-Blot® TurboTM Midi Nitrocellulose membranes using the Trans-Blot® TurboTM system (Bio-Rad, Hercules, CA, United States). Primary rabbit antibodies used were anti-OPRM1 (1:5000, ab17934), anti-OPRK1 (1:10000, ab183825), anti-OPRD1 (1:1000, ab176324), and anti-Cyclophilin B (CYPB) (1:10000, ab16045) purchased from Abcam (Cambridge, MA, United States). Secondary antibodies used were goat anti-rabbit (1:500, Sc-1404) conjugated HRP purchased from Santa Cruz Biotechnology (Dallas, TX, United States). Following secondary antibody incubation, ECL clarity (Bio-Rad, Hercules, CA, United States) was used to detect gel bands on ChemiDoc Touch Imaging System (Bio-Rad, Hercules, CA, United States), and intensities were quantified with Image Lab version 6.0 (Bio-Rad, Hercules, CA, United States) software.
Statistical Analyses
Behavioral data were analyzed using two-way analysis of variance (ANOVA). Dependent variables were the number of oxycodone infusions on training days. Independent variables were between-subject factor reward types (saline, Oxy, SR, SS), within-subject factor SA day (training days 1–22 or shock days 1–6), and their interactions. If the main effects were significant (p ≤ 0.05), Bonferroni post hoc tests were used to compare reward types on each training/shock day. Biochemical data were analyzed using one-way ANOVA followed by the Fisher’s PLSD post hoc test if the main effect was significant. Genes that showed a trend toward significance using ANOVA were also analyzed by Student’s t-test. Linear regression analyses were performed to detect potential relationships between the total amount of oxycodone taken during the last 3 days of footshocks and protein/mRNA expression. The null hypothesis was rejected at p ≤ 0.05. Behavioral data were analyzed with SPSS version 24 (IBM, Armonk, NY, United States), Prism 8.2.0 (GraphPad Software, San Diego, CA, United States) while biochemical data were analyzed using StatView version 4.0 (SAS, Cary, NC, United States).
Results
Footshocks Separate Oxycodone Self-Administering Rats Into Resistant and Sensitive Phenotypes
Figure 1 shows the timeline and results of the behavioral studies. The repeated-measures ANOVA for reward earned included the between-subject factor group (Saline and oxycodone) and the within-subject factor of SA day (training days 1–22), and the group × day interaction. This analysis showed statistically significant effects of group [F(1,572) = 272.8, p < 0.0001], non-significant effects of day [F(21,572) = 1.133, p = 0.3081], and significant group × day interaction [F(21,572) = 1.756, p = 0.0201]. Oxycodone rats increased their drug intake substantially after training day 6 when compared to saline rats [F(1,416) = 230.6, p = 0.0001] (Figure 1B). There was also a significant uptake of oxycodone between day 6 and day 22 during the 9-h training period (Figure 1C).
Similar to our previous reports (Cadet et al., 2016; Krasnova et al., 2017; Torres et al., 2017), the introduction of footshocks allowed for the separation of rats that had escalated their oxycodone intake into (1) shock-resistant (SR) animals that continue to press the lever slightly less than before and (2) shock-sensitive (SS) that markedly decreased their intake of the drug (Figure 1D). Fifty five percent (55%) of the oxycodone SA rats were classified as SR while 45% were denoted SS animals. Respectively, SR and SS exhibited 38 and 78% suppression of drug infusion during the last 2 days of footshocks in comparison to the last 2 days before the introduction of shocks (Figure 1D), indicating different degrees of suppression of individual oxycodone intake by the contingent shocks. For these data, the repeated measures ANOVA for reward earned included the SR and SS rats, and the within-subject factor days of footshocks (1–6 days), and the group × day interaction. We found statistically significant effects of shocks [F(1,54) = 75.0, p < 0.0001] and day [F(5,54) = 2.4, p = 0.0484], and non-significant effect of group × day interaction [F(5,54) = 0.1, p = 0.9857] (Figure 1D). A similar ANOVA model for reward earned prior to the punishment phase found statistically significant effects of group [F(1,196) = 40.25, p < 0.0001], day [F(21,196) = 4.369, p < 0.0001], and non-significant group × day interaction [F(21,196) = 0.9484, p = 0.2076]. Further analyses revealed no significant differences between SS and SR rats during training days 1–22. We also compared the amount of oxycodone taken during the last 2 days of SA training alone to the amount taken during the phase of oxycodone SA plus footshocks with shock intensity at 0.36 mA. Figure 1E shows that the amount of oxycodone (mg/kg) consumed during the last 2 days of punishment was significantly decreased in both SR and SS rats [F(3,36) = 41.30, p < 0.0001], with the SR rats consuming much more oxycodone than the SS rats.
Effects of Oxycodone Self-Administration and Footshocks on Opioid Receptor Protein Expression
Oxycodone exerts its actions by binding to opioid receptors in the brain (Williams et al., 2013). We had previously reported that rats that escalated their intake of oxycodone showed decreased Mu opioid receptor protein levels in the dorsal striatum even after a month of withdrawal (Blackwood et al., 2018). In contrast, oxycodone-exposed rats showed comparable expression of Delta and Kappa opioid receptor proteins to control rats (Blackwood et al., 2018). Here, we tested the possibility that there might be differences in the expression of the 3 opioid receptors in the dorsal striatum and PFC of rats euthanized at 2-h after the last session.
There were significant decreases in striatal Mu (OPRM1) [F(2,16) = 5.352, p = 0.0166], Delta (OPRD1) [F(2,16) = 15.45, p = 0.0002], and Kappa (OPRK1) [F(2,16) = 8.230, p = 0.0035] protein levels in both SR and SS rats compared to the saline SA animals (Figures 2A,C,E). In contrast, there were no substantial changes in the protein expression of OPRM1 (p = 0.0837) and OPRK1 (p = 0.1030) in the PFC (Figures 2B,F). However, significantly decreases in OPRD1 [F(2,16) = 9.219, p = 0.0022] protein levels were observed in both SR and SS subgroups compared to the saline group (Figure 2D) in the PFC. These data suggest region-specific regulation of opioid receptor expression in the brain.
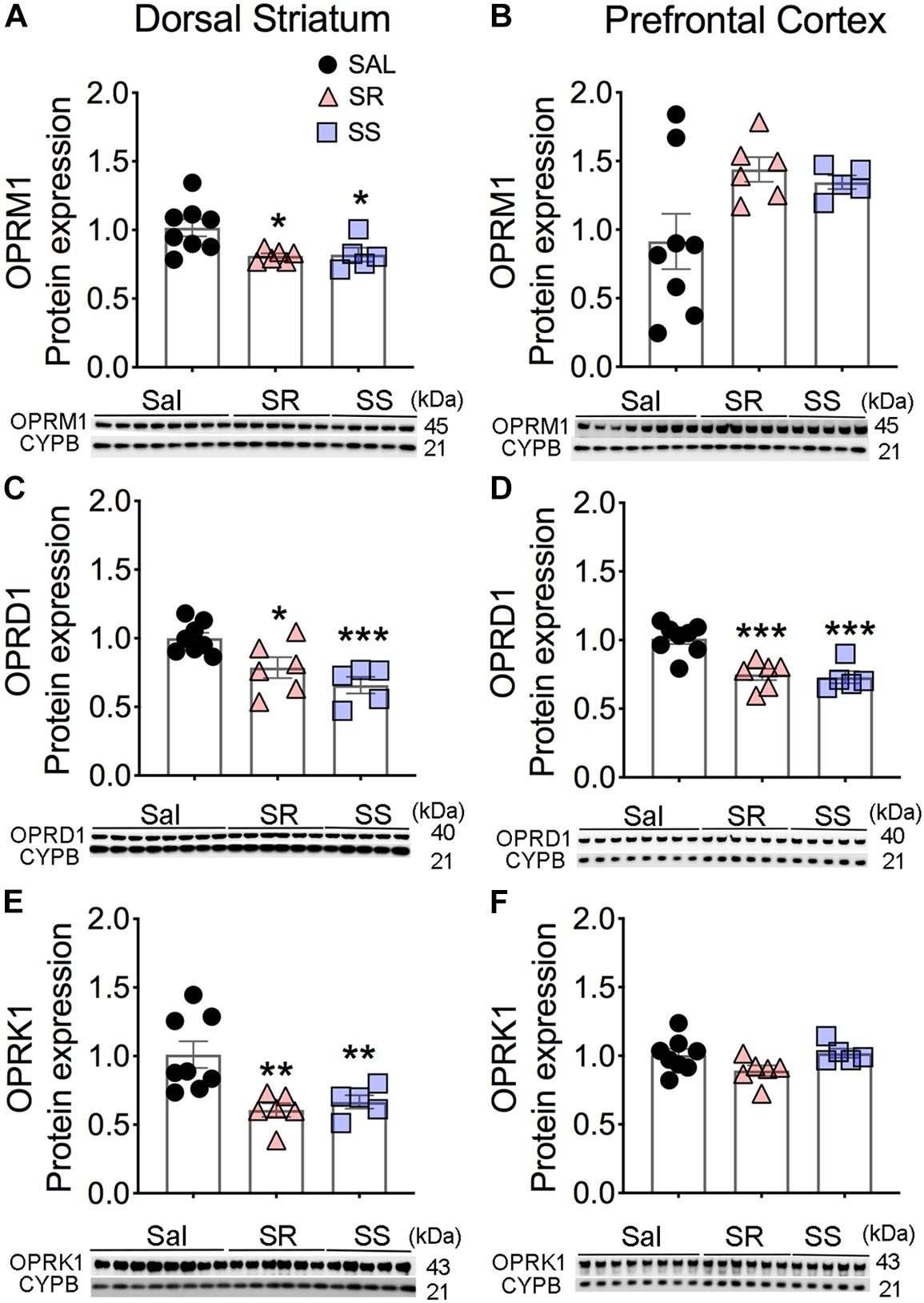
Figure 2. Differential expression of Mu, Delta, and Kappa opioid receptor proteins in the dorsal striatum and prefrontal cortex (PFC) of oxycodone- and shock-exposed rats. (A–F) Quantitative measures and representative images showing Western blot analyses of Mu (OPRM1), Delta (OPRD1), and Kappa (OPRK1) in the SR and SS rats show decreased OPRM1 (A), OPRD1 (C), and OPRK1 (E) in the dorsal striatum. There were also significant decreases in OPRD1 (D), but not of OPRM1 (B) and OPRK1 (F), receptor protein levels in the PFC (n = 8, Sal; n = 6, SR; n = 5, SS). Key to statistics: ∗, ∗∗, ∗∗∗p < 0.05, 0.01, 0.001, respectively, in comparison to saline-exposed rats or SR rats. Stats were performed by one-way ANOVA and Fisher’s PLSD post hoc tests.
Effects of Oxycodone SA and Footshocks on Members of Fos Family of IEGs
Dorsal Striatum
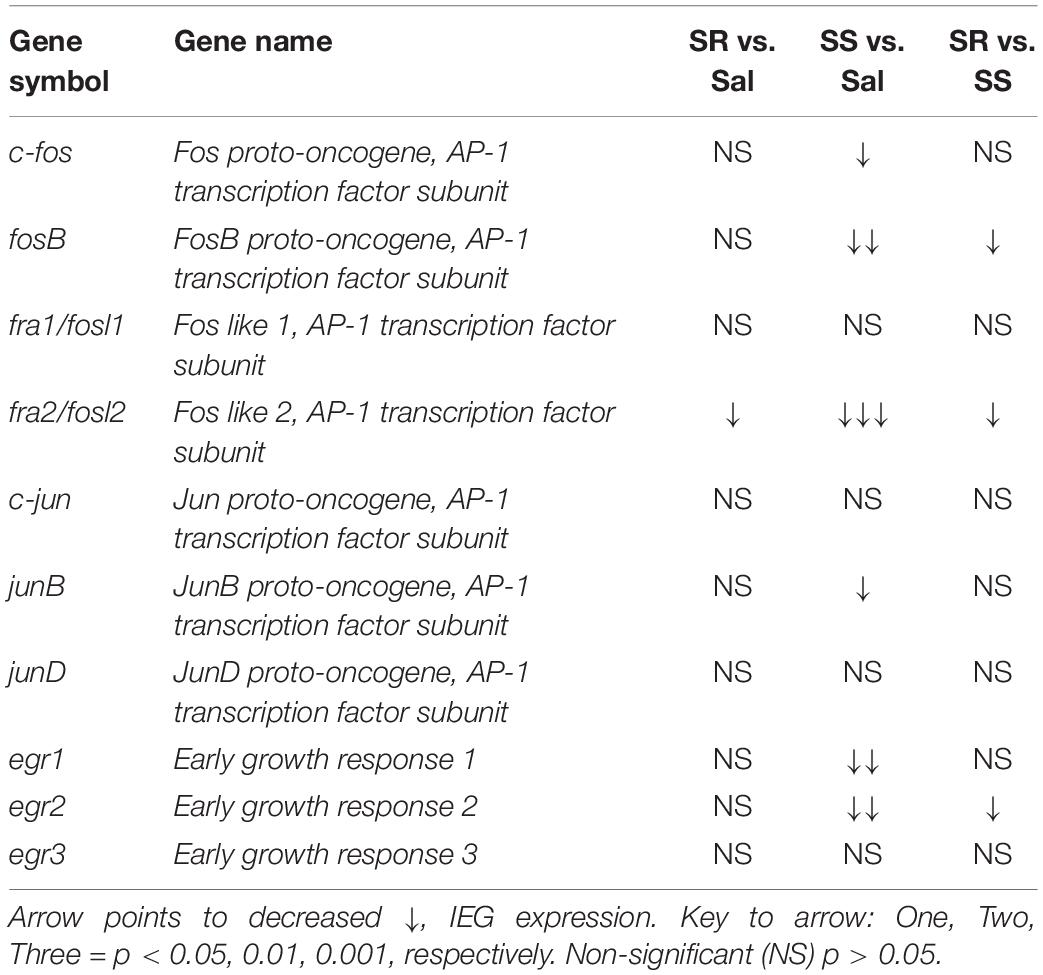
As noted in the introduction, because the expression of several IEGs is known to be impacted by administration of opioid drugs (Bisagno and Cadet, 2019; Blackwood et al., 2019), we reasoned that differential expression of some IEGs of the Fos family might occur in the SR and SS oxycodone SA phenotypes, with the SR showing higher mRNA expression. Figure 3 shows the effects of footshocks and oxycodone SA on c-fos, fosB, fra1, and fra2 mRNA levels in the dorsal striatum of the SR and SS rats. We found that the expression of c-fos mRNA levels showed a trend toward significance [F(2,13) = 3.47, p = 0.0621]. Planned t-test between the oxycodone-exposed rats and control rats revealed significant differences in c-fos mRNA expression between the SS subgroup compared to controls (p = 0.0212) but not compared to the SR subgroup (Figure 3A). Regression analysis revealed that the levels of mRNA expression were significantly related to the amount of total oxycodone taken during the experiment (Figure 3B). We also observed significant decreases in striatal mRNA levels of fosB [F(2,13) = 6.554, p = 0.0108] (Figure 3C) and fra2 [F(2,13) = 14.326, p = 0.0005] (Figure 3G), but not of fra1 (Figure 3E) in SS rats in comparison to control and SR rats. The fra2 mRNA levels also showed significant decreases in the SR rats compared to saline rats. The mRNA expression levels of c-fos, fosB and fra2 also showed significant linear correlation with total oxycodone taken (Figures 3B,D,H) whereas fra1 expression did not show any significant correlation with oxycodone SA (Figure 3F).
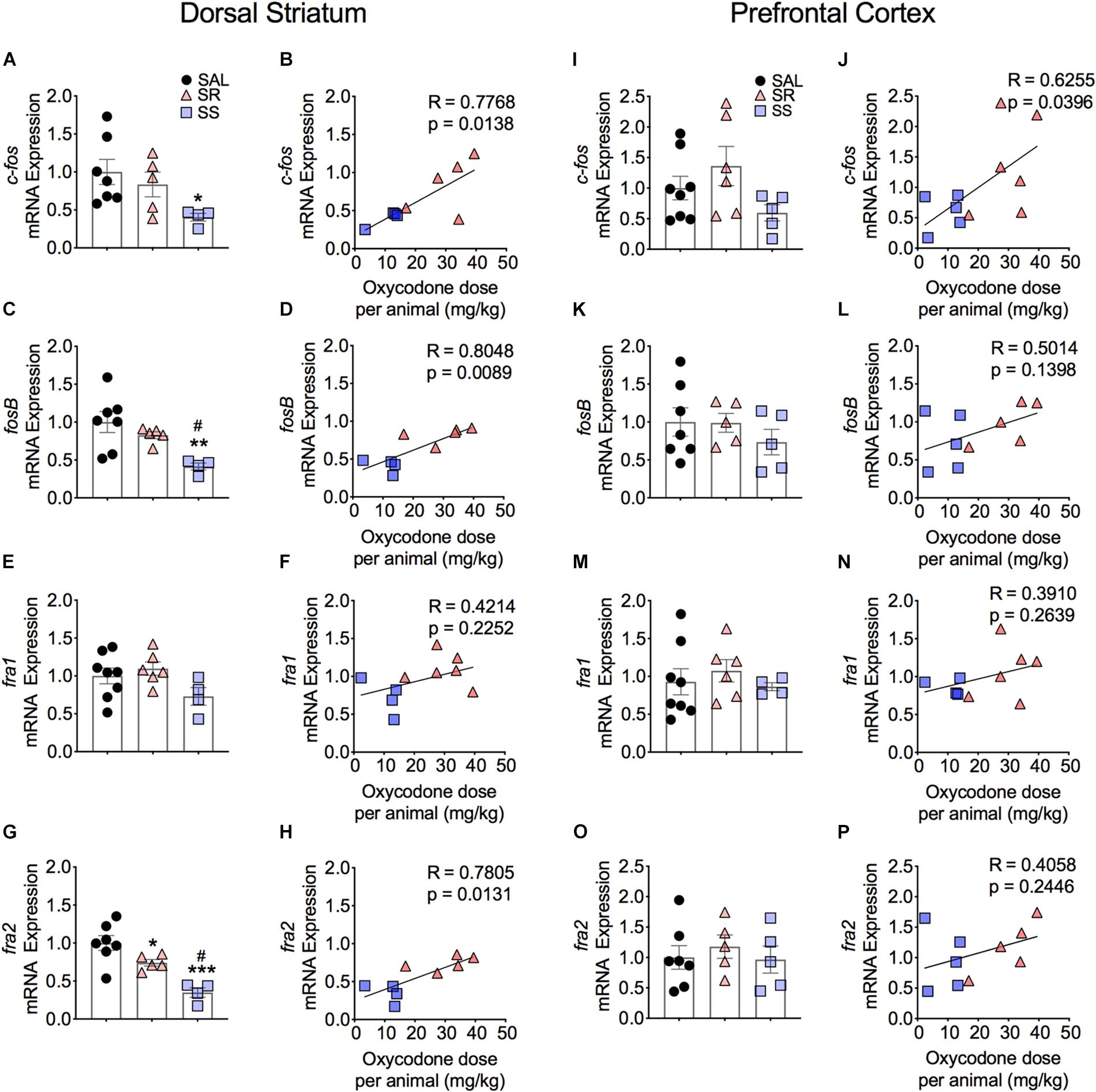
Figure 3. Striatal Fos Family members show decreased mRNA expression. SS rats show decreased striatal c-fos (A), fosB (C), and fra2 (G) mRNA levels. mRNA expression of c-fos, fosB, and fra2 correlated with doses of oxycodone (B,D,H). Striatal fra1 (E,F) show no significant changes in mRNA expression. No significant changes in c-fos (I), fosB (K,L), fra1 (M,N), and fra2 (O,P) mRNA levels were detected in the PFC. The expression of c-fos correlated with doses of oxycodone (J) (n = 7–8, Sal; n = 5–6, SR; n = 4–5, SS). Key to statistics: ∗, ∗∗, ∗∗∗p < 0.05, 0.01, 0.001, respectively, in comparison to saline rats. #p < 0.05, in comparison to SR rats. Stats were performed by one-way ANOVA, Fisher’s PLSD post hoc, or Student’s t-test.
Prefrontal Cortex
Figure 3 also illustrates the results of footshocks on c-fos, fosB, fra1, and fra2 mRNA levels in the PFC. In the PFC, although the mRNA expression of c-fos did trend toward significance (p = 0.0701) between the SR and SS subgroups, we detected no significant changes (Figure 3I). However, the regression analysis revealed significant relationship of c-fos mRNA expression to amount of oxycodone taken (Figure 3J). There was no significant change in fosB, fra1 or fra2 (Figures 3K,M,O). Moreover, regression analysis revealed no significant correlations between these genes expression and the amount of oxycodone taken (Figures 3L,N,P).
Differential Expression of Members of Jun IEG Family in the Dorsal Striatum and PFC of Oxycodone Exposed Rats
Dorsal Striatum
Figure 4 shows the observations for JUN family of IEGs in the dorsal striatum. The mRNA levels of c-jun or junD showed no significant changes between the subgroups (Figures 4A,E) and no correlation to oxycodone doses (Figures 4B,F). In contrast, we observed significantly decreased mRNA levels of junB [F(2,15) = 3.743, p = 0.0480) in SS rats compared to controls (Figure 4C). However, there was no correlation between mRNA expression of junB and the amount of oxycodone taken (Figure 4D).
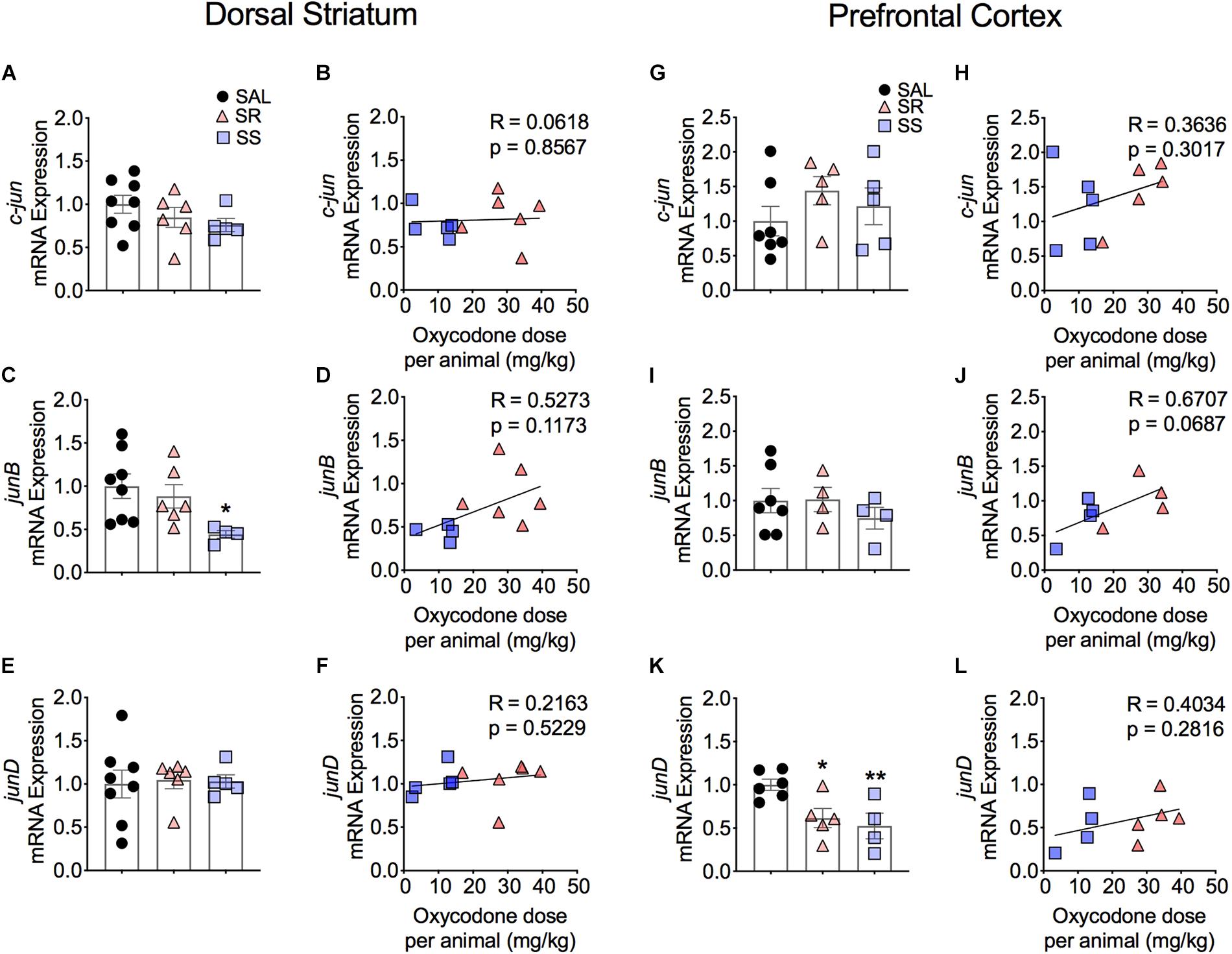
Figure 4. Striatal junB and cortical junD mRNA expression is significantly decreased in shock-sensitive rats. There were no significant changes in mRNA levels of c-jun in the dorsal striatum (A,B) and PFC (G,H). SS rats show decreased junB in the dorsal striatum (C) but not in the PFC (I). No significant correlations between junB and doses of oxycodone (D,J). The expression of junD mRNA was not affected in the dorsal striatum (E) but was significantly decreased in the PFC of both SR and SS rats (K). There was no significant correlation in JunD (F,L) (n = 7–8, Sal; n = 5–6, SR; n = 4–5, SS). Key to statistics: ∗, ∗∗p < 0.05, 0.01, respectively, in comparison to saline rats. Stats were performed by one-way ANOVA and Fisher’s PLSD post hoc tests.
Prefrontal Cortex
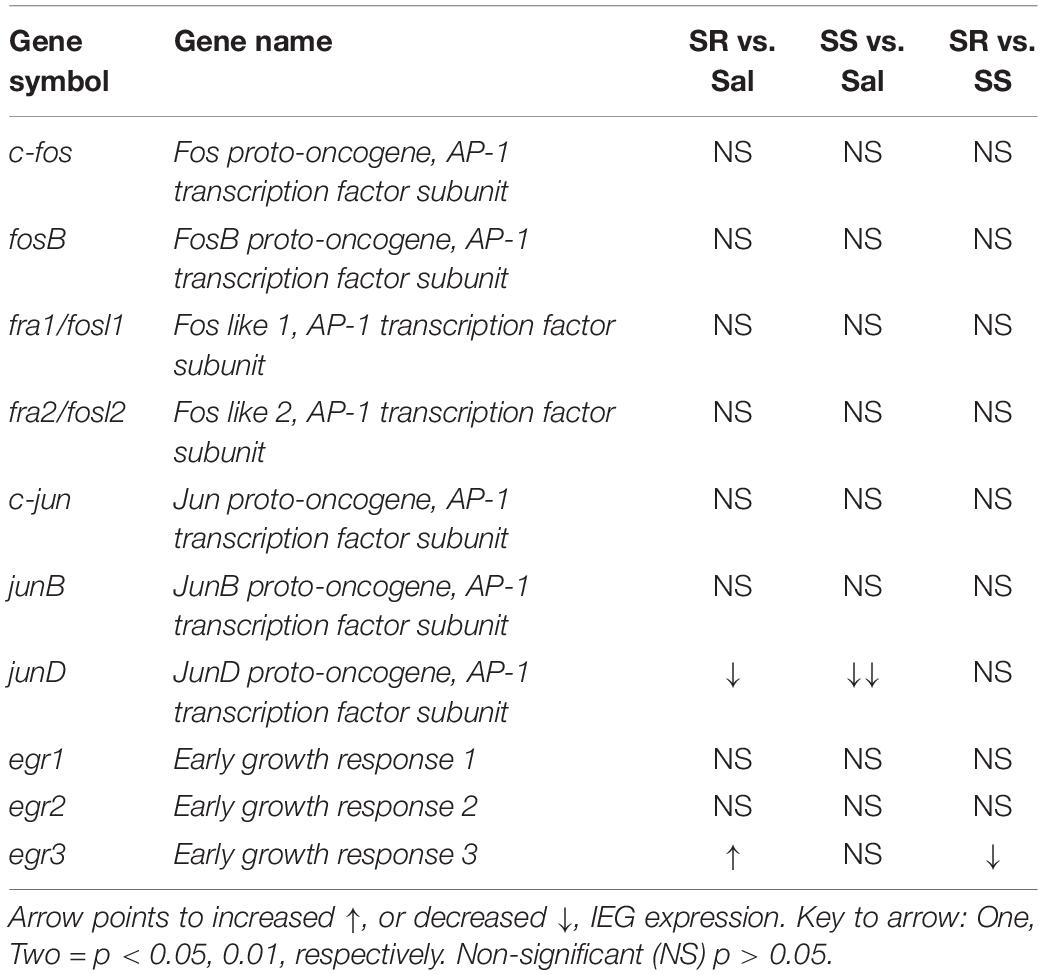
Figure 4 also shows the quantification of JUN family IEGs in the PFC. The mRNA expression of c-jun and junB showed no significant changes (Figures 4G,I) and no correlation to the total amount of infused oxycodone (Figures 4H,J). In contrast, we found significantly decreased mRNA levels of junD in the SR and SS rats [F(2,12) = 6.331, p = 0.0133] (Figure 4K). However, there was no relationship between the mRNA expression of junD and infused oxycodone (Figure 4L).
Effects of Oxycodone SA and Footshocks on the Expression of Members of Egr Family of IEGs
Dorsal Striatum
Figure 5 shows the effects of footshocks from oxycodone SA on egr1, egr2, and egr3 mRNA levels in the dorsal striatum. There were significantly decreases in mRNA levels of striatal egr1 [F(2,14) = 7.588, p = 0.0059] and egr2 [F(2,13) = 8.019, p = 0.0054] in the SS rats compared to saline rats (Figures 5A,C). Striatal egr2 expression in the SS rats was also significantly decreased in comparison to SR rats (Figure 5C). We found no changes in egr3 mRNA expression (Figure 5E). Moreover, there were no significant correlations between mRNA levels of egr1, egr2, and egr3 and the amount of oxycodone taken (Figures 5B,D,F).
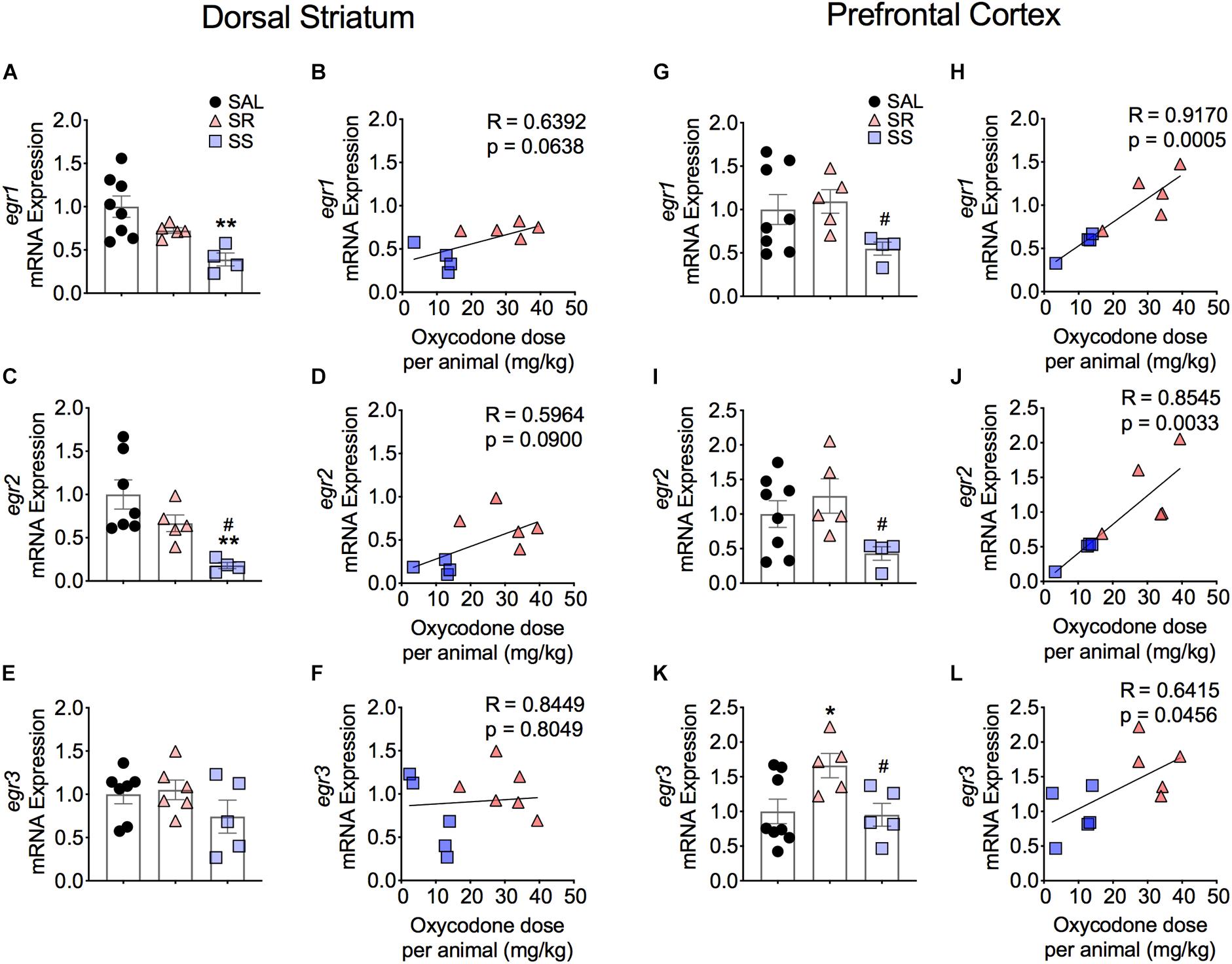
Figure 5. Differential expression of Egr family of IEGs in the dorsal striatum and PFC of shock-resistant and shock-sensitive rats. SS rats show decreased striatal egr1 (A) and egr2 (C) mRNA levels. There were no significant changes in mRNA levels of egr1 (B) and egr2 (D) in the PFC. Striatal egr3 (E) showed no changes in mRNA expression in either SR or SS rats. SR rats show increased egr3 (F) mRNA levels of in the PFC. SS rats showed significant correlation and decreased mRNA levels of egr1 (G,H), egr2 (I,J), and egr3 (K,L) in the PFC (n = 7–8, Sal; n = 5–6, SR; n = 4–5, SS). Key to statistics: ∗, ∗∗p < 0.05, 0.01, respectively, in comparison to saline. #p < 0.05, in comparison to SR rats. Stats were performed by one-way ANOVA and Fisher’s PLSD post hoc tests.
Prefrontal Cortex
Figure 5 also illustrates the results of Egr family of IEGs in the PFC. There were significantly decreases in mRNA levels of egr1 (p = 0.0141) and egr2 (p = 0.0256) in the SS rats compared to SR rats (Figures 5G,I). These changes showed a significant correlation between the mRNA expression and the amount of oxycodone taken (Figures 5H,J). In addition, we detected significant increases in egr3 [F(2,13) = 4.314, p = 0.0331] in the SR rats in comparison to both saline rats and SS rats (Figure 5K). A significant correlation was also found between the mRNA expression of egr3 and total amount of infused oxycodone (Figure 5L).
Discussion
The current opioid crisis (Boscarino et al., 2010; Rudd et al., 2016; Fornili, 2018) has prompted renewed calls to develop better treatment strategies for patients who misuse these drugs. We have therefore begun to investigate the neurobiological consequences of oxycodone SA behaviors in rats. We have reported recently that rats, given long access to oxycodone, will escalate their intake and show incubation of drug-seeking behaviors after several weeks of withdrawal (Blackwood et al., 2018). As discussed elsewhere (Cadet, 2019), because repeated drug use in humans is not enough to reach a SUD DSM-V diagnosis, we have added contingent footshocks during drug SA to encompass one additional DMS criterion, namely, compulsive drug taking in the presence of adverse consequences. This approach is consistent with the observations that, in clinical situations, only small percentages of individuals continue to misuse drugs when faced with legal and financial consequences (Anthony et al., 1994; Miller and Flaherty, 2000; Santiago Rivera et al., 2018). It needs to be also noted that there exists a substantial number of individuals who use opioids without meeting criteria for OUD (Korf et al., 2010; Zaaijer et al., 2014).
The major findings of the present study include the following: (1) the introduction of contingent punishment to rats that had escalated their intake of oxycodone helped to dichotomize them into two shock-induced phenotypes, with one group of resistant animals that continued to press the active lever for the drug and another group of sensitive rats that stopped or reduced their intake substantially; (2) all oxycodone-exposed rats showed significant decreased striatal protein levels of the three opioid receptors; (3) SS rats showed reduced striatal mRNA expression of several IEGs; whereas (4) egr3 mRNA expression was significantly increased in the PFC of SR rats. These data are summarized in Tables 2, 3 for readers to refer to. In what follows, we discuss the potential role of some of these changes in gene and protein expression in mediating shock-induced suppression and/or continuation of compulsive oxycodone SA in these rats.
The present behavioral results are consistent with those of previous papers that had reported that punishment can cause some rats to significantly reduce or stop to self-administer methamphetamine, cocaine, or alcohol (Chen et al., 2013; Marchant et al., 2018; Cadet et al., 2019). The present findings expand the punishment-induced phenomenon to opioids. Our results support the notion that there is not an one-to-one correspondence between escalated drug taking behaviors and a diagnosis of SUD (Cadet, 2019) and supports the idea of using these two phenotypes to attempt to understand the neurobiology of continued drug taking behaviors or abstinence in the presence of adverse consequences.
The present observations of decreased expression of the three opioid receptors in the dorsal striatum suggest that repeated exposure to oxycodone can cause downregulation of these receptors when measured after a short withdrawal interval from oxycodone SA. These findings are consistent, in part, with those of Blackwood et al. (2018) who had reported that the protein levels of only striatal Mu opioid receptors were downregulated in rats that had self-administered oxycodone over a period of 20 days, and that were subsequently withdrawn from the SA experiment for a period of 1 month. When taken together, these observations suggest that there might exist certain regional molecular mechanisms that drive the permanence of the decreased expression of Mu opioid receptors whereas no such mechanisms appear to exist for Delta and Kappa receptors whose expression had returned to normal after 1 month of withdrawal (Blackwood et al., 2018). The relative permanence of decreased levels of striatal Mu receptor protein after long-term exposure to oxycodone might be due, in part, to oxycodone-induced changes in the stability, degradation, or trafficking of these receptors (Williams et al., 2013). These reduced levels might, in part, drive oxycodone SA and/or cue-induced incubation of oxycodone seeking (Blackwood et al., 2018). These mechanisms might not be in play for delta and kappa receptor proteins, of which expression had normalized after a month of oxycodone withdrawal (Blackwood et al., 2018). This idea will need to be tested in future studies using diverse models of OUDs.
It is of interest to note, at this juncture, that clinical studies have reported that several single nucleotide polymorphisms (SNPs) in Mu receptors might influence the effects of oxycodone-induced euphoric responses (Jones et al., 2019) and vulnerability to OUDs (Drakenberg et al., 2006; Nielsen et al., 2008; Levy et al., 2015; Randesi et al., 2016). One SNP of interest to our present discussion is the OPRM A118G, which is associated with increased risk for heroin addiction (Bond et al., 1998; Zhang et al., 2015; see Burns et al., 2019, for a review of other SNPs linked to opioid addiction). This SNP results in reduced maximum binding of the mu opioid receptor ligand, DAMGO, in vitro (Bond et al., 1998; Zhang et al., 2005) and in human tissues (Zhang et al., 2005; Weerts et al., 2013). Mice that possess the human equivalent of this SNP have been reported to exhibit decreased expression of both mRNA and protein levels of Mu opioid receptor protein (Mague et al., 2009). Of relevance to the present study, these mice self-administered more heroin than control mice (Zhang et al., 2015). Thus, the possibility exists that the rats that continue to take more oxycodone during footshocks might express a SNP similar to the human OPRM A118G and/or other pro-addiction SNPs. This supposition will need to be tested in future genetic experiments.
Although the changes in the expression of opioid receptors were similar in both resistant and sensitive rats, we found significant differences in the mRNA expression of several IEGs including fosB, fra2, and egr2 that showed decreased expression in the dorsal striatum, but not the PFC, of sensitive rats compared to control and resistant rats. It is important to note that the SR and SS phenotypes began to separate almost immediately after the first day of the punishment phase (see Figure 1D). Thus, it is possible to suggest that some of the changes observed in IEG expression might be related to compensatory responses that might have occurred in the SS but not in the SR rats. This argument suggests that there is a need for more studies focusing on quantifying the expression of IEGs at various time points during oxycodone SA and the application of contingent footshocks, once there is measurable evidence of a split between SR and SS rats. It is worth noting that, in our studies of methamphetamine SA and footshocks, we have not been able to identify any effects of footshocks alone on IEG expression (Krasnova et al., 2017; Cadet et al., 2019). It is also possible that rats with differential OPRM1 SNPs might respond differential to contingent footshocks in terms of oxycodone SA and resulting changes in IEG expression during the punishment phase of the study, with the end result being abstention from lever pressing in order to avoid footshocks.
Differential alterations in the expression of several mRNAs and proteins have previously been reported in the dorsal striatum of the SR and SS phenotypes in the case of methamphetamine SA and footshocks (Cadet et al., 2016; Torres et al., 2017). Therefore, we had predicted that the SR rats might show acute increased expression of some IEGs because they were taking oxycodone just 2-h before they were euthanized. We had, indeed, previously found increased expression of c-fos in the dorsal striatum of rats that were euthanized after 1 month of withdrawal from escalated oxycodone intake (Blackwood et al., 2019). We also thought that the levels of expression of IEGs might be normal or might be reduced in the SS group in comparison to the SR rats. We found, however, that striatal IEG mRNA levels were mostly decreased in the SS animals without there being any major changes in the SR rats. Of all our observations, the decreases in fosB mRNA expression in the dorsal striatum are of interest because fosB expression has been reported to be altered after exposure to methamphetamine (Krasnova et al., 2013; Cadet et al., 2015), cocaine (Hope et al., 1992; Larson et al., 2010), opioid receptor agonists (Liu et al., 1994), and nicotine (Saint-Preux et al., 2013), with most papers reporting increased fosB expression. Thus, our findings of decreased fosB in the shock-sensitive rats suggest that reduced fosB expression might participate in a molecular cascade that helps to suppress oxycodone-taking behaviors, possibly via a SNP-dependent fashion (see section “Discussion” above). This conclusion is consistent with the report that overexpression of delta fosB, an alternative splicing product of fosB mRNA in which Exon IV is truncated (Alibhai et al., 2007), can enhance the rewarding effects of cocaine when mice were tested in the conditioned place preference procedure (Kelz et al., 1999). Because these authors used cocaine in that study, the effects of this genetic manipulation will need to be tested in future oxycodone SA experiments. It is important to indicate that there are several fosB target genes that may be relevant to this discussion (Nestler, 2015). These include the glutamate AMPA receptor, GluA2/GluR2, the opioid peptide, dynorphin, and CAMKIIalpha that have been implicated in models of SUDs (Nestler, 2015).
Although the above discussion has focused on the potential role of decreased FosB and/or delta FosB in suppressing oxycodone SA, similar arguments could be put forward for fra2 and egr2 that also showed significant decreases in the dorsal striatum of sensitive rats in comparison to the resistant animals. It is also of interest that the changes in fosB, fra2, and egr2 mRNA levels occurred only in the dorsal striatum, further implicating that brain region in the manifestation of compulsive habit-like behaviors (Everitt and Robbins, 2016; Hodebourg et al., 2018) in oxycodone self-administering rats (Blackwood et al., 2018). Although this discussion has focused on IEG expression, several recent papers on oxycodone self-administration have identified other genes that might be relevant to oxycodone use disorder (Zhang et al., 2014, 2017, 2018). These included mRNAs coding for GABA-, glutamate, and dopamine receptors that were downregulated in the dorsal striatum (Zhang et al., 2018). In contrast, NPY5 receptor and the glycine receptor, alpha 4 subunit, were reported to show increases after oxycodone SA in the dorsal striatum of mice (Zhang et al., 2015). In addition, some structural genes, including integrins were also affected in the dorsal striatum of oxycodone self-administering mice (Yuferov et al., 2018). Because these authors had used all oxycodone-exposed animals in their studies, it will be important to test whether the shock-induced SR and SS phenotypes will show differential expression of some of these genes.
It is also noteworthy that egr3 (Patwardhan et al., 1991) was the only gene that showed increased mRNA expression in the PFC of resistant rats in comparison to control and sensitive rats. Egr3 expression is rapidly regulated in hippocampal and cortical neurons by electroconvulsive seizures (Yamagata et al., 1994). Changes in the expression of this IEG may be relevant to the effects of drugs of abuse because egr3 mRNA expression is induced by acute administration of psychostimulants including cocaine and methamphetamine (O’Donovan and Baraban, 1999; Courtin et al., 2006; McCoy et al., 2011). Repeated injections of cocaine also induced Egr3 expression (Jouvert et al., 2002) in D1-containing neurons in the ventral striatum (Chandra et al., 2015). Importantly, Egr3 knockdown in D1-containing neurons reduced cocaine-associated conditioned place preference (CPP) and cocaine-induced locomotor hyperactivity. Taken together, our observations of increased egr3 expression in the PFC of rats that took oxycodone compulsively extends the role of Egr3 to opioid-driven behaviors, possibly, by regulating executive functions (Duverne and Koechlin, 2017; Jayachandran et al., 2019) that may include decisions to continue to take oxycodone compulsively in the presence of adverse consequences.
Conclusion
The present study provides the first evidence that contingent footshocks can dichotomize oxycodone SA rats into two phenotypes of resistant and sensitive animals in a fashion similar to what has been reported for alcohol, cocaine, and methamphetamine. Our findings of significant decreases in striatal fosB, fra2, and egr2 mRNA levels in the sensitive in comparison to the resistant rats suggest that IEGs of diverse classes might participate in molecular networks that drive long-term changes in striatal neuronal structures and functions that might serve to suppress habitual drug-taking behaviors. In contrast, increased egr3 expression in the PFC, a structure that has been implicated in decision making and memory functions (Duverne and Koechlin, 2017; Jayachandran et al., 2019), supports the notion that Egr3 may regulate rewarding effects of psychostimulants (Chandra et al., 2015) and the propensity to continue to take oxycodone in the presence of punishment. These ideas will need to be tested to identify the specific roles that these diverse IEGs might play in the regulation of punishment-induced abstinence or compulsive oxycodone taking.
Data Availability Statement
All datasets generated for this study are included in the article/supplementary material.
Ethics Statement
The animal study was reviewed and approved by the NIDA (National Institute of Drug Abuse) Animal Care and Use Committee at the Intramural Research Program (IRP).
Author Conributions
CB, MM, and BL performed self-administration and punishment test. CB and MM performed western blot and RT-qPCR experiments. CB and JC prepared the manuscript. JC supervised the overall project.
Funding
This work was supported by funds of the Intramural Research Program of the HHS/NIH/NIDA.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The authors are grateful to several reviewers whose suggestions helped us to write a better manuscript.
Footnotes
References
Alibhai, I. N., Green, T. A., Potashkin, J. A., and Nestler, E. J. (2007). Regulation of fosB and DeltafosB mRNA expression: in vivo and in vitro studies. Brain Res. 1143, 22–33. doi: 10.1016/j.brainres.2007.01.069
American Psychiatric Association and Dsm-5 Task Force (2013). Diagnostic and Statistical Manual of Mental Disorders, 5th Edn. Washington, DC: American Psychiatric Association.
Anthony, C. J., Warner, L. A., and Kessler, R. C. (1994). Comparative epidemiology of dependence on tobacco, alcohol, controlled substances, and inhalants: basic findings from the national comorbidity survey. Exp. Clin. Psychopharmacol. 2, 244–268. doi: 10.1037/1064-1297.2.3.244
Belin, D., and Everitt, B. J. (2008). Cocaine seeking habits depend upon dopamine-dependent serial connectivity linking the ventral with the dorsal striatum. Neuron 57, 432–441. doi: 10.1016/j.neuron.2007.12.019
Belin-Rauscent, A., Fouyssac, M., Bonci, A., and Belin, D. (2016). How preclinical models evolved to resemble the diagnostic criteria of drug addiction. Biol. Psychiatry 79, 39–46. doi: 10.1016/j.biopsych.2015.01.004
Bisagno, V., and Cadet, J. L. (2019). Expression of immediate early genes in brain reward circuitries: differential regulation by psychostimulant and opioid drugs. Neurochem. Int. 124, 10–18. doi: 10.1016/j.neuint.2018.12.004
Blackwood, C. A., Hoerle, R., Leary, M., Schroeder, J., Job, M. O., McCoy, M. T., et al. (2018). Molecular adaptations in the rat dorsal striatum and hippocampus following abstinence-induced incubation of drug seeking after escalated oxycodone self-administration. Mol. Neurobiol. 56, 3603–3615. doi: 10.1007/s12035-018-1318-z
Blackwood, C. A., Leary, M., Salisbury, A., McCoy, M. T., and Cadet, J. L. (2019). Escalated oxycodone self-administration causes differential striatal mRNA expression of FGFs and IEGs following abstinence-associated incubation of oxycodone craving. Neuroscience 415, 173–183. doi: 10.1016/j.neuroscience.2019.07.030
Blanco, C., and Volkow, N. D. (2019). Management of opioid use disorder in the USA: present status and future directions. Lancet 393, 1760–1772. doi: 10.1016/S0140-6736(18)33078-2
Bolla, K., Ernst, M., Kiehl, K., Mouratidis, M., Eldreth, D., Contoreggi, C., et al. (2004). Prefrontal cortical dysfunction in abstinent cocaine abusers. J. Neuropsychiatry Clin. Neurosci. 16, 456–464. doi: 10.1176/jnp.16.4.456
Bond, C., LaForge, K. S., Tian, M., Melia, D., Zhang, S., Borg, L., et al. (1998). Single-nucleotide polymorphism in the human mu opioid receptor gene alters beta-endorphin binding and activity: possible implications for opiate addiction. Proc. Natl. Acad. Sci. U.S.A. 95, 9608–9613. doi: 10.1073/pnas.95.16.9608
Boscarino, J. A., Rukstalis, M., Hoffman, S. N., Han, J. J., Erlich, P. M., Gerhard, G. S., et al. (2010). Risk factors for drug dependence among out-patients on opioid therapy in a large US health-care system. Addiction 105, 1776–1782. doi: 10.1111/j.1360-0443.2010.03052.x
Bossert, J. M., Hoots, J. K., Fredriksson, I., Adhikary, S., Zhang, M., Venniro, M., et al. (2018). Role of mu, but not delta or kappa, opioid receptors in context-induced reinstatement of oxycodone seeking. Eur. J. Neurosci. 50, 2075–2085. doi: 10.1111/ejn.13955
Bossert, J. M., Stern, A. L., Theberge, F. R., Cifani, C., Koya, E., Hope, B. T., et al. (2011). Ventral medial prefrontal cortex neuronal ensembles mediate context-induced relapse to heroin. Nat. Neurosci. 14, 420–422. doi: 10.1038/nn.2758
Burns, J. A., Kroll, D. S., Feldman, D. E., Kure Liu, C., Manza, P., Wiers, C. E., et al. (2019). Molecular imaging of opioid and dopamine systems: insights into the pharmacogenetics of opioid use disorders. Front. Psychiatry 10:626. doi: 10.3389/fpsyt.2019.00626
Cadet, J. L. (2019). Animal models of addiction: compulsive drug taking and cognition. Neurosci. Biobehav. Rev. 106, 5–6. doi: 10.1016/j.neubiorev.2019.05.026
Cadet, J. L., and Bisagno, V. (2015). Neuropsychological consequences of chronic drug use: relevance to treatment approaches. Front. Psychiatry 6:189. doi: 10.3389/fpsyt.2015.00189
Cadet, J. L., Bisagno, V., and Milroy, C. M. (2014). Neuropathology of substance use disorders. Acta Neuropathol. 127, 91–107. doi: 10.1007/s00401-013-1221-7
Cadet, J. L., Brannock, C., Jayanthi, S., and Krasnova, I. N. (2015). Transcriptional and epigenetic substrates of methamphetamine addiction and withdrawal: evidence from a long-access self-administration model in the rat. Mol. Neurobiol. 51, 696–717. doi: 10.1007/s12035-014-8776-8
Cadet, J. L., Brannock, C., Krasnova, I. N., Jayanthi, S., Ladenheim, B., McCoy, M. T., et al. (2017). Genome-wide DNA hydroxymethylation identifies potassium channels in the nucleus accumbens as discriminators of methamphetamine addiction and abstinence. Mol. Psychiatry 22, 1196–1204. doi: 10.1038/mp.2016.48
Cadet, J. L., Krasnova, I. N., Walther, D., Brannock, C., Ladenheim, B., McCoy, M. T., et al. (2016). Increased expression of proenkephalin and prodynorphin mRNAs in the nucleus accumbens of compulsive methamphetamine taking rats. Sci. Rep. 6:37002. doi: 10.1038/srep37002
Cadet, J. L., Patel, R., and Jayanthi, S. (2019). Compulsive methamphetamine taking and abstinence in the presence of adverse consequences: epigenetic and transcriptional consequences in the rat brain. Pharmacol. Biochem. Behav. 179, 98–108. doi: 10.1016/j.pbb.2019.02.009
Chandra, R., Francis, T. C., Konkalmatt, P., Amgalan, A., Gancarz, A. M., Dietz, D. M., et al. (2015). Opposing role for Egr3 in nucleus accumbens cell subtypes in cocaine action. J. Neurosci. 35, 7927–7937. doi: 10.1523/JNEUROSCI.0548-15.2015
Chen, B. T., Yau, H. J., Hatch, C., Kusumoto-Yoshida, I., Cho, S. L., Hopf, F. W., et al. (2013). Rescuing cocaine-induced prefrontal cortex hypoactivity prevents compulsive cocaine seeking. Nature 496, 359–362. doi: 10.1038/nature12024
Cicero, T. J., Inciardi, J. A., and Munoz, A. (2005). Trends in abuse of Oxycontin and other opioid analgesics in the United States: 2002-2004. J. Pain 6, 662–672. doi: 10.1016/j.jpain.2005.05.004
Courtin, C., Crete, D., Canestrelli, C., Noble, F., and Marie-Claire, C. (2006). Regulation of genes involved in dopamine transporter modulation by acute cocaine in rat striatum. Neurosci. Lett. 398, 235–240. doi: 10.1016/j.neulet.2006.01.001
Drakenberg, K., Nikoshkov, A., Horvath, M. C., Fagergren, P., Gharibyan, A., Saarelainen, K., et al. (2006). Mu opioid receptor A118G polymorphism in association with striatal opioid neuropeptide gene expression in heroin abusers. Proc. Natl. Acad. Sci. U.S.A. 103, 7883–7888. doi: 10.1073/pnas.0600871103
Duverne, S., and Koechlin, E. (2017). Rewards and cognitive control in the human prefrontal cortex. Cereb. Cortex 27, 5024–5039. doi: 10.1093/cercor/bhx210
Everitt, B. J. (2014). Neural and psychological mechanisms underlying compulsive drug seeking habits and drug memories–indications for novel treatments of addiction. Eur. J. Neurosci. 40, 2163–2182. doi: 10.1111/ejn.12644
Everitt, B. J., and Robbins, T. W. (2016). Drug addiction: updating actions to habits to compulsions ten years on. Annu. Rev. Psychol. 67, 23–50. doi: 10.1146/annurev-psych-122414-033457
Ferrer-Alcon, M., Garcia-Fuster, M. J., La Harpe, R., and Garcia-Sevilla, J. A. (2004a). Long-term regulation of signalling components of adenylyl cyclase and mitogen-activated protein kinase in the pre-frontal cortex of human opiate addicts. J. Neurochem. 90, 220–230. doi: 10.1111/j.1471-4159.2004.02473.x
Ferrer-Alcon, M., La Harpe, R., and Garcia-Sevilla, J. A. (2004b). Decreased immunodensities of micro-opioid receptors, receptor kinases GRK 2/6 and beta-arrestin-2 in postmortem brains of opiate addicts. Brain Res. Mol. Brain Res. 121, 114–122. doi: 10.1016/j.molbrainres.2003.11.009
Fornili, K. (2018). The opioid crisis, suicides, and related conditions: multiple clustered syndemics, not singular epidemics. J. Addict. Nurs. 29, 214–220. doi: 10.1097/JAN.0000000000000240
Frost, J. J., Wagner, H. N. Jr., Dannals, R. F., Ravert, H. T., Links, J. M., Wilson, A. A., et al. (1985). Imaging opiate receptors in the human brain by positron tomography. J. Comput. Assist. Tomogr. 9, 231–236. doi: 10.1097/00004728-198503000-00001
Gaskell, H., Derry, S., Stannard, C., and Moore, R. A. (2016). Oxycodone for neuropathic pain in adults. Cochrane Database Syst. Rev. 7:CD010692. doi: 10.1002/14651858.CD010692.pub3
Goldstein, R. Z., and Volkow, N. D. (2011). Dysfunction of the prefrontal cortex in addiction: neuroimaging findings and clinical implications. Nat. Rev. Neurosci. 12, 652–669. doi: 10.1038/nrn3119
Hodebourg, R., Murray, J. E., Fouyssac, M., Puaud, M., Everitt, B. J., and Belin, D. (2018). Heroin seeking becomes dependent on dorsal striatal dopaminergic mechanisms and can be decreased by N-acetylcysteine. Eur. J. Neurosci. 50, 2036–2044. doi: 10.1111/ejn.13894
Hope, B., Kosofsky, B., Hyman, S. E., and Nestler, E. J. (1992). Regulation of immediate early gene expression and AP-1 binding in the rat nucleus accumbens by chronic cocaine. Proc. Natl. Acad. Sci. U.S.A. 89, 5764–5768. doi: 10.1073/pnas.89.13.5764
Hu, Y., Salmeron, B. J., Krasnova, I. N., Gu, H., Lu, H., Bonci, A., et al. (2019). Compulsive drug use is associated with imbalance of orbitofrontal- and prelimbic-striatal circuits in punishment-resistant individuals. Proc. Natl. Acad. Sci. U.S.A. 116, 9066–9071. doi: 10.1073/pnas.1819978116
Jayachandran, M., Linley, S. B., Schlecht, M., Mahler, S. V., Vertes, R. P., and Allen, T. A. (2019). Prefrontal pathways provide top-down control of memory for sequences of events. Cell Rep. 28, 640–654.e6. doi: 10.1016/j.celrep.2019.06.053
Jones, J. D., Mumtaz, M., Manubay, J. M., Mogali, S., Sherwin, E., Martinez, S., et al. (2019). Assessing the contribution of opioid- and dopamine-related genetic polymorphisms to the abuse liability of oxycodone. Pharmacol. Biochem. Behav. 186:172778. doi: 10.1016/j.pbb.2019.172778
Jouvert, P., Dietrich, J. B., Aunis, D., and Zwiller, J. (2002). Differential rat brain expression of EGR proteins and of the transcriptional corepressor NAB in response to acute or chronic cocaine administration. Neuromolecular Med. 1, 137–151. doi: 10.1385/nmm:1:2:137
Keller, B., La Harpe, R., and Garcia-Sevilla, J. A. (2017). Upregulation of IRAS/nischarin (I1-imidazoline receptor), a regulatory protein of mu-opioid receptor trafficking, in postmortem prefrontal cortex of long-term opiate and mixed opiate/cocaine abusers. Neurochem. Int. 108, 282–286. doi: 10.1016/j.neuint.2017.04.017
Kelz, M. B., Chen, J., Carlezon, W. A. Jr., Whisler, K., Gilden, L., Beckmann, A. M., et al. (1999). Expression of the transcription factor deltaFosB in the brain controls sensitivity to cocaine. Nature 401, 272–276. doi: 10.1038/45790
Korf, D. J., van Ginkel, P., and Benschop, A. (2010). How to find non-dependent opiate users: a comparison of sampling methods in a field study of opium and heroin users. Int. J. Drug Policy 21, 215–221. doi: 10.1016/j.drugpo.2009.08.005
Krasnova, I. N., Chiflikyan, M., Justinova, Z., McCoy, M. T., Ladenheim, B., Jayanthi, S., et al. (2013). CREB phosphorylation regulates striatal transcriptional responses in the self-administration model of methamphetamine addiction in the rat. Neurobiol. Dis. 58, 132–143. doi: 10.1016/j.nbd.2013.05.009
Krasnova, I. N., Gerra, M. C., Walther, D., Jayanthi, S., Ladenheim, B., McCoy, M. T., et al. (2017). Compulsive methamphetamine taking in the presence of punishment is associated with increased oxytocin expression in the nucleus accumbens of rats. Sci. Rep. 7:8331. doi: 10.1038/s41598-017-08898-8
Larson, E. B., Akkentli, F., Edwards, S., Graham, D. L., Simmons, D. L., Alibhai, I. N., et al. (2010). Striatal regulation of DeltaFosB, FosB, and cFos during cocaine self-administration and withdrawal. J. Neurochem. 115, 112–122. doi: 10.1111/j.1471-4159.2010.06907.x
Levy, B., Paulozzi, L., Mack, K. A., and Jones, C. M. (2015). Trends in opioid analgesic-prescribing rates by specialty, U.S., 2007-2012. Am. J. Prev. Med. 49, 409–413. doi: 10.1016/j.amepre.2015.02.020
Liu, J., Nickolenko, J., and Sharp, F. R. (1994). Morphine induces c-fos and junB in striatum and nucleus accumbens via D1 and N-methyl-D-aspartate receptors. Proc. Natl. Acad. Sci. U.S.A. 91, 8537–8541. doi: 10.1073/pnas.91.18.8537
Mague, S. D., Isiegas, C., Huang, P., Liu-Chen, L. Y., Lerman, C., and Blendy, J. A. (2009). Mouse model of OPRM1 (A118G) polymorphism has sex-specific effects on drug-mediated behavior. Proc. Natl. Acad. Sci. U.S.A. 106, 10847–10852. doi: 10.1073/pnas.0901800106
Mansour, A., Fox, C. A., Burke, S., Meng, F., Thompson, R. C., Akil, H., et al. (1994). Mu, delta, and kappa opioid receptor mRNA expression in the rat CNS: an in situ hybridization study. J. Comp. Neurol. 350, 412–438. doi: 10.1002/cne.903500307
Mansour, A., Khachaturian, H., Lewis, M. E., Akil, H., and Watson, S. J. (1987). Autoradiographic differentiation of mu, delta, and kappa opioid receptors in the rat forebrain and midbrain. J. Neurosci. 7, 2445–2464.
Marchant, N. J., Campbell, E. J., and Kaganovsky, K. (2018). Punishment of alcohol-reinforced responding in alcohol preferring P rats reveals a bimodal population: implications for models of compulsive drug seeking. Prog. Neuropsychopharmacol. Biol. Psychiatry 87(Pt A), 68–77. doi: 10.1016/j.pnpbp.2017.07.020
Mavrikaki, M., Pravetoni, M., Page, S., Potter, D., and Chartoff, E. (2017). Oxycodone self-administration in male and female rats. Psychopharmacology 234, 977–987. doi: 10.1007/s00213-017-4536-6
McCoy, M. T., Jayanthi, S., Wulu, J. A., Beauvais, G., Ladenheim, B., Martin, T. A., et al. (2011). Chronic methamphetamine exposure suppresses the striatal expression of members of multiple families of immediate early genes (IEGs) in the rat: normalization by an acute methamphetamine injection. Psychopharmacology 215, 353–365. doi: 10.1007/s00213-010-2146-7
Miller, N. S., and Flaherty, J. A. (2000). Effectiveness of coerced addiction treatment (alternative consequences): a review of the clinical research. J. Subst. Abuse Treat. 18, 9–16. doi: 10.1016/s0740-5472(99)00073-2
Moorman, D. E., James, M. H., McGlinchey, E. M., and Aston-Jones, G. (2015). Differential roles of medial prefrontal subregions in the regulation of drug seeking. Brain Res. 1628(Pt A), 130–146. doi: 10.1016/j.brainres.2014.12.024
Nestler, E. J. (2015). FosB: a transcriptional regulator of stress and antidepressant responses. Eur. J. Pharmacol. 753, 66–72. doi: 10.1016/j.ejphar.2014.10.034
Nielsen, D. A., Ji, F., Yuferov, V., Ho, A., Chen, A., Levran, O., et al. (2008). Genotype patterns that contribute to increased risk for or protection from developing heroin addiction. Mol. Psychiatry 13, 417–428. doi: 10.1038/sj.mp.4002147
Nummenmaa, L., Saanijoki, T., Tuominen, L., Hirvonen, J., Tuulari, J. J., Nuutila, P., et al. (2018). mu-opioid receptor system mediates reward processing in humans. Nat. Commun. 9:1500. doi: 10.1038/s41467-018-03848-y
O’Donovan, K. J., and Baraban, J. M. (1999). Major Egr3 isoforms are generated via alternate translation start sites and differ in their abilities to activate transcription. Mol. Cell Biol. 19, 4711–4718. doi: 10.1128/mcb.19.7.4711
Patel, B., and Kosten, T. R. (2019). Keeping up with clinical advances: opioid use disorder. CNS Spectr. 24, 14–24. doi: 10.1017/S109285291900110X
Patwardhan, S., Gashler, A., Siegel, M. G., Chang, L. C., Joseph, L. J., Shows, T. B., et al. (1991). EGR3, a novel member of the Egr family of genes encoding immediate-early transcription factors. Oncogene 6, 917–928.
Paxinos, G., and Watson, C. (1998). The Rat Brain in Stereotaxic Coordinates, 6th Edn. Burlington, MA: Academic Press.
Perry, J. L., Joseph, J. E., Jiang, Y., Zimmerman, R. S., Kelly, T. H., Darna, M., et al. (2011). Prefrontal cortex and drug abuse vulnerability: translation to prevention and treatment interventions. Brain Res. Rev. 65, 124–149. doi: 10.1016/j.brainresrev.2010.09.001
Randesi, M., van den Brink, W., Levran, O., Blanken, P., Butelman, E. R., Yuferov, V., et al. (2016). Variants of opioid system genes are associated with non-dependent opioid use and heroin dependence. Drug Alcohol Depend. 168, 164–169. doi: 10.1016/j.drugalcdep.2016.08.634
Rudd, R. A., Aleshire, N., Zibbell, J. E., and Gladden, R. M. (2016). Increases in drug and opioid overdose deaths–United States, 2000-2014. MMWR Morb. Mortal. Wkly. Rep. 64, 1378–1382. doi: 10.15585/mmwr.mm6450a3
Saint-Preux, F., Bores, L. R., Tulloch, I., Ladenheim, B., Kim, R., Thanos, P. K., et al. (2013). Chronic co-administration of nicotine and methamphetamine causes differential expression of immediate early genes in the dorsal striatum and nucleus accumbens of rats. Neuroscience 243, 89–96. doi: 10.1016/j.neuroscience.2013.03.052
Santiago Rivera, O. J., Havens, J. R., Parker, M. A., and Anthony, J. C. (2018). Risk of heroin dependence in newly incident heroin users. JAMA Psychiatry 75, 863–864. doi: 10.1001/jamapsychiatry.2018.1214
Schmidt-Hansen, M., Bennett, M. I., Arnold, S., Bromham, N., and Hilgart, J. S. (2017). Oxycodone for cancer-related pain. Cochrane Database Syst. Rev. 8:CD003870. doi: 10.1002/14651858.CD003870.pub6
Sillivan, S. E., Whittard, J. D., Jacobs, M. M., Ren, Y., Mazloom, A. R., Caputi, F. F., et al. (2013). ELK1 transcription factor linked to dysregulated striatal mu opioid receptor signaling network and OPRM1 polymorphism in human heroin abusers. Biol. Psychiatry 74, 511–519. doi: 10.1016/j.biopsych.2013.04.012
Skolnick, P. (2017). The opioid epidemic: crisis and solutions. Annu. Rev. Pharmacol. Toxicol. 58, 143–159. doi: 10.1146/annurev-pharmtox-010617-052534
Smith, R. J., and Laiks, L. S. (2018). Behavioral and neural mechanisms underlying habitual and compulsive drug seeking. Prog. Neuropsychopharmacol. Biol. Psychiatry 87(Pt A), 11–21. doi: 10.1016/j.pnpbp.2017.09.003
Torres, O. V., Jayanthi, S., Ladenheim, B., McCoy, M. T., Krasnova, I. N., and Cadet, J. L. (2017). Compulsive methamphetamine taking under punishment is associated with greater cue-induced drug seeking in rats. Behav. Brain Res. 326, 265–271. doi: 10.1016/j.bbr.2017.03.009
Van Zee, A. (2009). The promotion and marketing of oxycontin: commercial triumph, public health tragedy. Am. J. Public Health 99, 221–227. doi: 10.2105/AJPH.2007.131714
Vanderschuren, L., Minnaard, A. M., Smeets, J., and Lisscher, M. (2017). Punishment models of addictive behavior. Behav. Sci. 13, 77–84. doi: 10.1016/j.cobeha.2016.10.007
Wade, C. L., Vendruscolo, L. F., Schlosburg, J. E., Hernandez, D. O., and Koob, G. F. (2015). Compulsive-like responding for opioid analgesics in rats with extended access. Neuropsychopharmacology 40, 421–428. doi: 10.1038/npp.2014.188
Weerts, E. M., McCaul, M. E., Kuwabara, H., Yang, X., Xu, X., Dannals, R. F., et al. (2013). Influence of OPRM1 Asn40Asp variant (A118G) on [11C]carfentanil binding potential: preliminary findings in human subjects. Int. J. Neuropsychopharmacol. 16, 47–53. doi: 10.1017/S146114571200017X
Williams, J. T., Ingram, S. L., Henderson, G., Chavkin, C., von Zastrow, M., Schulz, S., et al. (2013). Regulation of mu-opioid receptors: desensitization, phosphorylation, internalization, and tolerance. Pharmacol. Rev. 65, 223–254. doi: 10.1124/pr.112.005942
Yamagata, K., Kaufmann, W. E., Lanahan, A., Papapavlou, M., Barnes, C. A., Andreasson, K. I., et al. (1994). Egr3/Pilot, a zinc finger transcription factor, is rapidly regulated by activity in brain neurons and colocalizes with Egr1/zif268. Learn. Mem. 1, 140–152.
Yuferov, V., Zhang, Y., Liang, Y., Zhao, C., Randesi, M., and Kreek, M. J. (2018). Oxycodone self-administration induces alterations in expression of integrin, semaphorin and ephrin genes in the mouse striatum. Front. Psychiatry 9:257. doi: 10.3389/fpsyt.2018.00257
Zaaijer, E. R., Bruijel, J., Blanken, P., Hendriks, V., Koeter, M. W., Kreek, M. J., et al. (2014). Personality as a risk factor for illicit opioid use and a protective factor for illicit opioid dependence. Drug Alcohol Depend. 145, 101–105. doi: 10.1016/j.drugalcdep.2014.09.783
Zhang, Y., Liang, Y., Levran, O., Randesi, M., Yuferov, V., Zhao, C., et al. (2017). Alterations of expression of inflammation/immune-related genes in the dorsal and ventral striatum of adult C57BL/6J mice following chronic oxycodone self-administration: a RNA sequencing study. Psychopharmacology 234, 2259–2275. doi: 10.1007/s00213-017-4657-y
Zhang, Y., Liang, Y., Randesi, M., Yuferov, V., Zhao, C., and Kreek, M. J. (2018). Chronic oxycodone self-administration altered reward-related genes in the ventral and dorsal striatum of C57BL/6J mice: an RNA-seq analysis. Neuroscience 393, 333–349. doi: 10.1016/j.neuroscience.2018.07.032
Zhang, Y., Mayer-Blackwell, B., Schlussman, S. D., Randesi, M., Butelman, E. R., Ho, A., et al. (2014). Extended access oxycodone self-administration and neurotransmitter receptor gene expression in the dorsal striatum of adult C57BL/6 J mice. Psychopharmacology 231, 1277–1287. doi: 10.1007/s00213-013-3306-3
Zhang, Y., Picetti, R., Butelman, E. R., Ho, A., Blendy, J. A., and Kreek, M. J. (2015). Mouse model of the OPRM1 (A118G) polymorphism: differential heroin self-administration behavior compared with wild-type mice. Neuropsychopharmacology 40, 1091–1100. doi: 10.1038/npp.2014.286
Keywords: oxycodone, opioid receptors, protein, mRNA, footshocks, prefrontal cortex, dorsal striatum
Citation: Blackwood CA, McCoy MT, Ladenheim B and Cadet JL (2020) Escalated Oxycodone Self-Administration and Punishment: Differential Expression of Opioid Receptors and Immediate Early Genes in the Rat Dorsal Striatum and Prefrontal Cortex. Front. Neurosci. 13:1392. doi: 10.3389/fnins.2019.01392
Received: 15 August 2019; Accepted: 10 December 2019;
Published: 09 January 2020.
Edited by:
George Panagis, University of Crete, GreeceReviewed by:
Elena Chartoff, McLean Hospital, United StatesMarek Schwendt, University of Florida, United States
Copyright © 2020 Blackwood, McCoy, Ladenheim and Cadet. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jean Lud Cadet, amNhZGV0QGludHJhLm5pZGEubmloLmdvdg==
 Christopher A. Blackwood
Christopher A. Blackwood Michael T. McCoy
Michael T. McCoy Bruce Ladenheim
Bruce Ladenheim Jean Lud Cadet
Jean Lud Cadet