- Department of Biological Sciences, School of Science and Technology, Sunway University, Bandar Sunway, Malaysia
The accumulating knowledge of the host-microbiota interplay gives rise to the microbiota-gut-brain (MGB) axis. The MGB axis depicts the interkingdom communication between the gut microbiota and the brain. This communication process involves the endocrine, immune and neurotransmitters systems. Dysfunction of these systems, along with the presence of gut dysbiosis, have been detected among clinically depressed patients. This implicates the involvement of a maladaptive MGB axis in the pathophysiology of depression. Depression refers to symptoms that characterize major depressive disorder (MDD), a mood disorder with a disease burden that rivals that of heart diseases. The use of probiotics to treat depression has gained attention in recent years, as evidenced by increasing numbers of animal and human studies that have supported the antidepressive efficacy of probiotics. Physiological changes observed in these studies allow for the elucidation of probiotics antidepressive mechanisms, which ultimately aim to restore proper functioning of the MGB axis. However, the understanding of mechanisms does not yet complete the endeavor in applying probiotics to treat MDD. Other challenges remain which include the heterogeneous nature of both the gut microbiota composition and depressive symptoms in the clinical setting. Nevertheless, probiotics offer some advantages over standard pharmaceutical antidepressants, in terms of residual symptoms, side effects and stigma involved. This review outlines antidepressive mechanisms of probiotics based on the currently available literature and discusses therapeutic potentials of probiotics for depression.
Introduction
Approximately 1014 microbes, also known as gut microbiota, reside in the human gastrointestinal tract. The majority of these microbes belong to the Firmicutes, Bacteroidetes, Actinobacteria and Proteobacteria phyla. The gut microbiota flourishes in a symbiotic alliance with the host and, as such, has eminent regulatory effects on the host physiology. The gut microbiota actively engages with the proper development and functioning of both the immune system and brain. This is mediated by the microbiota–gut–brain (MGB) axis that lays the foundation for the intricate communicative pathways between gut microbiota and the nervous, immune and endocrine systems. However, the diversity and richness of gut microbiota are susceptible to change based on the host’s lifestyle. An adverse change induces a gut dysbiosis which disrupts the symbiosis maintained by the MGB axis. Indeed, a gut dysbiosis has been linked to various health conditions, such as obesity, IBS, schizophrenia, Parkinson’s disease and MDD (Sherwin et al., 2016; Thursby and Juge, 2017; van de Guchte et al., 2018).
Major depressive disorder is currently the leading cause of disability worldwide and is expected to outrank heart diseases as the number one disease burden by 2030 (Reddy, 2010; Tucci and Moukaddam, 2017). According to the Diagnostic and Statistical Manual of Mental Disorders-5, MDD is diagnosed when a person experiences most of the following symptoms for at least 2 weeks: depressed mood, anhedonia, excessive guilt, suicidal ideation, changes in appetite and sleep, psychomotor retardation, poor concentration and fatigue. Among these criteria, either depressed mood or anhedonia (or both) must be present for a diagnosis of MDD (American Psychiatric Association, 2013). In this review, the term “depression” would be used to refer to symptoms that characterize MDD.
A causal relationship potentially exists between the gut microbiota and MDD. Germ-free (GF) rodents developed depressive-like behaviors following fecal microbiota transplantation from MDD patients, but not from healthy people (Kelly et al., 2016; Zheng et al., 2016). As compared to healthy individuals, MDD patients have a different gut microbiota profile. The decrease in Faecalibacterium, Bifidobacterium, Lactobacillus (Aizawa et al., 2016), and Dialister (Kelly et al., 2016), and increase in Clostridium, Streptococcus, Klebsiella, Oscillibacter, Allistipes (Naseribafrouei et al., 2014; Jiang et al., 2015; Lin et al., 2017; Rong et al., 2019), Eggerthella, Holdemania, Gelria, Turicibacter, Paraprevotella, and Anaerofilum (Kelly et al., 2016) genera have been found among MDD patients. This shift in the gut microbiota composition may contribute to a shift in the regulation of the host physiology (Luan et al., 2017). It is, thus, worthwhile to tackle MDD from the MGB axis standpoint, with an emphasis on the gut microbiota.
Probiotics are microbes (usually lactic acid bacteria such as Lactobacilli and Bifidobacteria) that benefit the host physiology upon ingestion. Probiotics are marketed in the form of capsules, powder or fermented products. The global market size of probiotics amount to billions and is increasing annually due to consumers’ interest in optimizing their health with functional foods (Di Cerbo and Palmieri, 2015). Probiotics have been utilized to modulate the MGB axis in an attempt to treat diseases, including MDD. Meta-analyses and systematic reviews have already supported the efficacy of probiotics in reducing clinical depression and depressive-like symptoms in MDD patients and healthy individuals, respectively (Huang et al., 2016; Pirbaglou et al., 2016; Wang et al., 2016; McKean et al., 2017; Wallace and Milev, 2017).
To what extent are probiotics viable tools to treat MDD/depression? This review addresses this question by first outlining the workings of MGB axis and process by which this axis becomes maladaptive, leading to the development of depression. Antidepressive mechanisms of probiotics are further elucidated by drawing parallels between the physiological outcomes that accompanied the behavioral changes to the MGB axis from animal and human research. Lastly, in light of the heterogeneous nature of both the gut microbiota composition and depression subtypes in the clinical setting, challenges and potentials in translating probiotics for clinical use are discussed.
The MGB Axis and Depression
Signaling Pathways of the MGB Axis: Neural and Humoral Routes
The first point of contact between the gut microbiota and host nervous system is likely via the enteric nervous system (ENS). The ENS has been described as “the second brain” due to its neuronal complexity on par with the brain and its ability to function as an independent, discrete unit to regulate gut-related activities and the immune system (Furness, 2012; Breit et al., 2018). Without gut microbiota, the excitability of enteric neurons would likely be attenuated, based on data observed in GF mice (McVey Neufeld et al., 2013). Through the ENS, gut microbiota and the brain communicate bidirectionally through neural and humoral (systemic circulation) pathways (Luan et al., 2017). Parasympathetic vagus afferents carry neural information from internal organs, including the gut, to the brain (Breit et al., 2018). The vagus nerve also consists of motor neurons that innervate nearly all enteric neurons (Powley, 2000). This enables the brain to influence the activity of ENS to some extent, particularly the state of intestinal permeability and gut inflammation. Sympathetic spinal nerves also connect enteric neurons to the brain, albeit to a lesser extent than vagal nerves (Lomax et al., 2010; Breit et al., 2018). Additionally, the humoral route allows microbial metabolites to enter the systemic circulation and exert its effects elsewhere, including the brain. Likewise, the brain also sends chemical messengers, such as cytokines and glucocorticoids, via the humoral route to regulate the gut physiology (Luan et al., 2017).
Signaling Mechanisms of the MGB Axis: Immune, Endocrine, and Neurotransmitter Systems
The gastrointestinal tract contains approximately 70% of the immune system (Vighi et al., 2008). Immune cells express TLRs that respond to foreign antigens, such as LPS, as they penetrate the intestinal mucosal barrier. This promptly triggers production of inflammatory cytokines, mainly ILs, tumor necrosis factor (TNF)-α and IFN-γ (Sherwin et al., 2016). These cytokines enter the brain through various pathways. The humoral pathway enables cytokines to enter circumventricular organs or permeable regions of the BBB or bind to carrier proteins that cross the BBB. The neural pathway allows gut cytokines to stimulate specific brain areas such as the brainstem, hypothalamus and limbic structures via vagus and spinal afferents. The cellular pathway allows cytokines to be transported into the brain by the action of monocytes or macrophages. These cytokines could also bind to receptors on astrocytes and microglia, and subsequently trigger cytokine production within the brain (Schiepers et al., 2005; Miller and Raison, 2016).
When proinflammatory signals reach the brain, the hypothalamic-pituitary-adrenal (HPA) axis, a sympathetic-neuroendocrine system, is activated to restore homeostasis. In response to stress, the hypothalamic paraventricular nucleus (PVN) synthesizes and releases corticotropin-releasing factor (CRF) to stimulate the anterior pituitary gland to release adrenocorticotropic hormone (ACTH) into the systemic circulation. ACTH stimulates the adrenal cortex to release glucocorticoids (cortisol in humans and corticosterone in rodents) which inhibit the release of CRF, establishing a negative feedback loop. Glucocorticoids are core effectors of the HPA axis that travel by the humoral route to exert its adaptive effects elsewhere; for instance, to reduce gut inflammation (Tsigos and Chrousos, 2002; Schiepers et al., 2005).
Furthermore, neurotransmitters in the brain serve indispensable roles in maintaining proper brain functions. Neurotransmitters such as GABA, glutamate (Glu), serotonin (5-HT), DA, NE, histamine and acetylcholine (ACh) are known to be synthesized by the gut microbiota (Oleskin et al., 2016). Notably, Lactobacillus, a prominent probiotic genus, produces multiple neurotransmitters in a species-dependent manner in vitro (Table 1). It should be noted that gut-derived neurotransmitters are functionally different from brain-derived neurotransmitters (Mittal et al., 2017). The bioavailability of precursors for these neurotransmitters is also regulated by the gut microbiota. For example, carbohydrate-fermenting microbes secrete butyrate (a SCFA) that stimulates 5-HT synthesis from intestinal enterochromaffin cells (ECs) (Reigstad et al., 2015; Yano et al., 2015; Lund et al., 2018). In contrast, Clostridia metabolites, such as 4-cresol and 4-hydroxyphenylacetate (4-HPA), inhibit dopamine-β-hydroxylase (an enzyme that converts DA to NE in the brain) (Shaw, 2017). These microbial neuroactive molecules likely modulate local ENS signaling, which ultimately influence the MGB axis (Karl et al., 2018).
Dysregulated MGB Axis in Depression: Chronic Stress Response Loop
Acute psychological stress increases the release of ACh from cholinergic nerves (Saunders et al., 1997; Kiliaan et al., 1998) and glucocorticoids from the HPA axis (Alonso et al., 2012; Zheng et al., 2013; Vanuytsel et al., 2014), both of which loosen tight junctions of the intestinal barrier (Figure 1). Other stressors such as poor diet, sleep deprivation, antibiotics, environmental pollutants and excessive exercise also increase the intestinal permeability (Karl et al., 2018). Additionally, exposure to stress stimulates sympathetic spinal nerves to release NE into the gut which expedites quorum sensing systems and iron uptake of bacteria, leading to increased virulence and growth of pathogenic bacteria (e.g., Escherichia coli, Salmonella, Campylobacter, etc.) (Lomax et al., 2010; Freestone, 2013). These factors facilitate penetration of bacteria and their toxins, such as LPS, through the weakened intestinal barrier. Administration of LPS increased proinflammatory cytokines and caused anxiety and depression in healthy males in a dose-dependent manner (Grigoleit et al., 2011). This phenomenon is only transient due to the adaptive response of the immune system and HPA axis. However, chronic stress prevents this homeostatic restoration and causes prolonged inflammation and HPA axis overactivity, both of which aggravate the disrupted intestinal barrier. During this process, chronic inflammation renders the immune system insensitive to inhibitory signals from glucocorticoids (de Punder and Pruimboom, 2015). Excess proinflammatory cytokines, in turn, disrupt the negative feedback inhibition of circulating glucocorticoids of the HPA axis (Schiepers et al., 2005; Miller et al., 2009). Indeed, MDD patients often show increased intestinal barrier permeability (Stevens et al., 2018; Calarge et al., 2019; Ohlsson et al., 2019) and elevated serum antibodies against LPS (Maes et al., 2008).
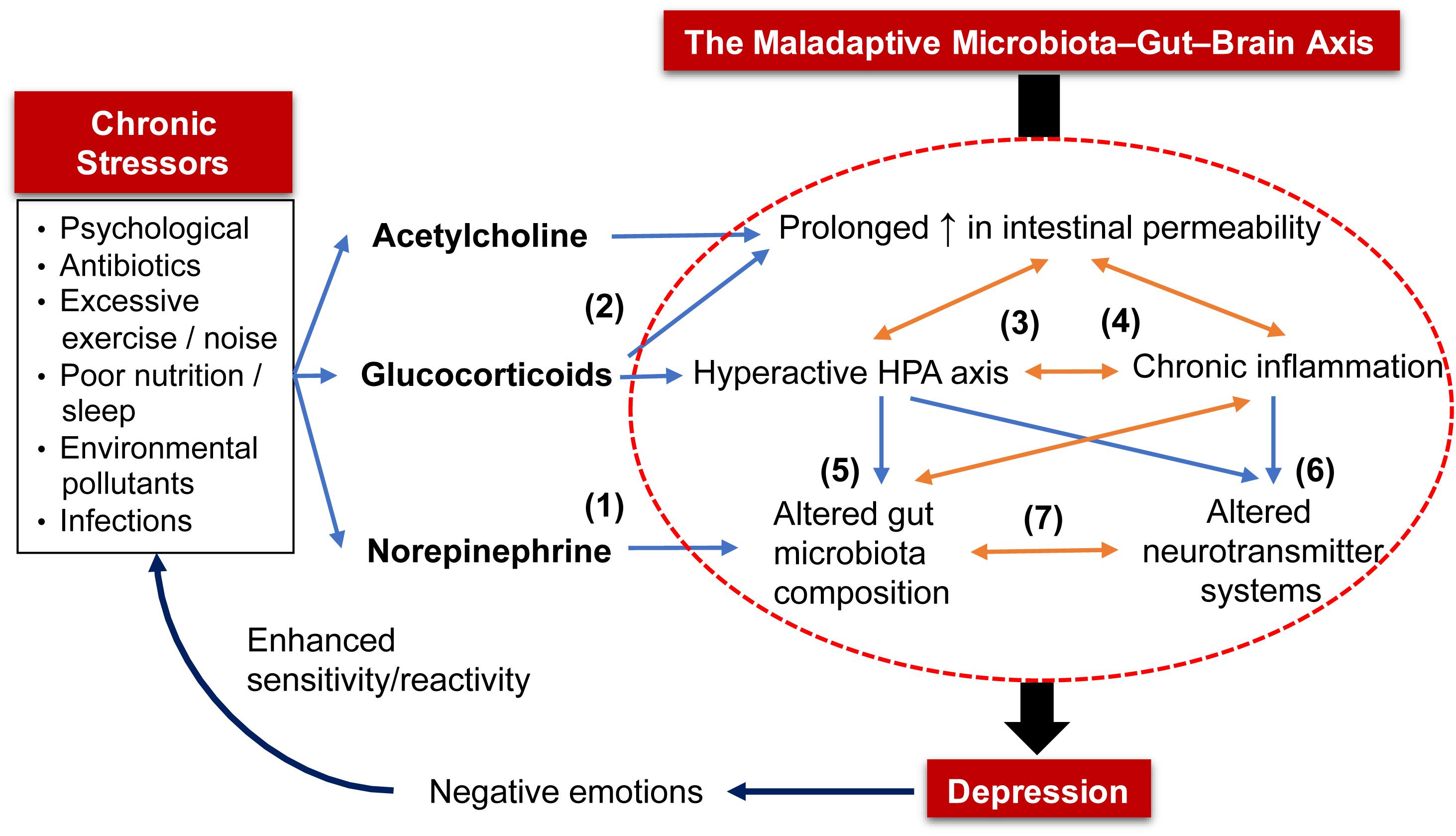
Figure 1. The maladaptive microbiota–gut–brain (MGB) axis in the pathophysiology of depression. Chronic exposure to stressors (e.g., psychological, poor nutrition) triggers prolonged release of (1) norepinephrine that alters gut microbiota composition by shifting to one that is enriched with pathogenic bacteria, and (2) acetylcholine and glucocorticoids that increase intestinal barrier permeability. The increased intestinal permeability allows bacteria and their toxins to enter systemic circulation, triggering stress responses from the HPA axis and immune system that, when excessive; (3) leads to chronic inflammation and HPA axis overactivity; (4) aggravate intestinal permeability; (5) alter composition of gut microbiota; and (6) disrupt neurotransmitter systems. Altered gut microbiota also results in an inflamed gut and (7) a shift in the production of bioactive molecules that regulate host neurotransmitter systems and gut motor functions. As a proof of concept, these five factors (in the circle) that depict the maladaptive MGB axis are often detected in MDD patients. Lastly, the constant negative emotions displayed by depressed patients further trigger a stronger reaction or sensitivity to various stressors.
Excessive glucocorticoids hyperactivate monoamine oxidases (MAOs; enzymes that degrade 5-HT, NE, and DA) (Grunewald et al., 2012). An overactive HPA axis can also induce gut dysbiosis (Murakami et al., 2017) and impairment of brain neurotransmitter systems (Pacak et al., 1993; Smith et al., 1995; Lopez et al., 1998; Hewitt et al., 2009). Higher baseline levels of cortisol, an indicator of an overactive HPA axis, were detected in more than 70% of MDD patients (Vreeburg et al., 2009; Lok et al., 2012). Proinflammatory cytokines and glucocorticoids upregulate indoleamine 2,3-dioxygenase (IDO) and tryptophan-2,3-dioxygenase (TDO) enzymes, respectively (Schimke et al., 1965; Young, 1981). Both enzymes metabolize TRP into KYN and quinolinic acid, which reduce the bioavailability of TRP to cross the BBB, thereby lowering 5-HT synthesis (Reus et al., 2015). This is evidenced by low plasma TRP levels that were also correlated to a heightened proinflammatory state found in MDD patients (Maes et al., 1993, 1994). Furthermore, proinflammatory cytokines can decrease levels of DA, 5-HT and NE in the brain by upregulating their reuptake via presynaptic transporters and downregulating enzymatic cofactors required for their synthesis (Miller and Raison, 2016). Indeed, administration of cytokines consistently induced neurotransmitter imbalances in the brain and behavioral changes that are reminiscent of depression in animals and humans (Miller et al., 2009). Similarly, higher levels of proinflammatory cytokines were observed in depressed individuals as reported using meta-analyses of the data available in the literature (Howren et al., 2009; Dowlati et al., 2010).
A stress-induced inflamed gut adversely alters the relative abundances of preexisting bacteria in the gut (Figure 1). Acute psychological stress stimulated the release of inflammatory mediators that were correlated with the lowered abundance of Coprococcus, Pseudobutyrivibrio, Dorea, and Lactobacillus in mice. This, in turn, allowed the proliferation of Clostridium species in the gut (Bailey et al., 2011). The gut microbiota of chronic-stressed mice also deviated from the baseline, whereby an increase in proinflammatory bacteria, such as Helicobacter and Streptococcus, and a decrease in butyrate-producing bacteria, such as Roseburia and Lachnospiraceae species, were observed (Gao et al., 2018). Altered gut microbiota composition consequently exacerbates gut inflammation and further increases intestinal permeability and production of proinflammatory cytokines (van de Guchte et al., 2018). The precise mechanism underlying vulnerability of certain bacteria to inflammation remains poorly understood. It is hypothesized that inflammation disrupts β-oxidation of intestinal epithelial cells (IECs, both enterocytes and colonocytes) to increase oxygen content in the gut lumen. This promotes formate oxidation that favors the growth of facultative anaerobes, such as E. coli, that are pathogenic and inflammatory at the cost of obligate anaerobes, such as Bacteroides and Firmicutes (Hughes et al., 2017).
A dysregulated gut microbiota translates to a shift in the production of neuroactive metabolites and alters host neurotransmitter circuitry. This corresponds with disrupted levels of neurotransmitters in the brain of GF mice (Diaz Heijtz et al., 2011; Neufeld et al., 2011; Clarke et al., 2013; Pan et al., 2019). Altered neurotransmitter profile (e.g., GABA, Glu, 5-HT, DA, and NE) has been associated with the pathophysiology of depression. Therefore, pharmaceutical antidepressants function to restore synaptic levels of neurotransmitters (Harald and Gordon, 2012). In addition, impaired neurotransmitter systems within the ENS may alter gut motor function. This has direct implications as gut motility is a determining factor in the size and diversity of gut microbiota (Quigley, 2011). Therefore, chronic stress sets up a vicious cycle of increased intestinal permeability, chronic inflammation, hyperactive HPA axis, altered gut microbiota profile and neurotransmitter imbalances – forming a maladaptive MGB axis (Figure 1). Furthermore, MDD patients perceive stress as more threatening and challenging to cope with compared to healthy individuals (Farabaugh et al., 2004; Salomon et al., 2009). These negative emotions can increase their sensitivity to stressors, such as an elevated cortisol response (Mendonca-de-Souza et al., 2007). To restore this malfunctioned axis, probiotics have been demonstrated by meta-analyses and systematic reviews as a potential treatment for MDD/depression (Huang et al., 2016; Pirbaglou et al., 2016; Wang et al., 2016; McKean et al., 2017; Wallace and Milev, 2017). Potential antidepressive mechanisms of probiotics are elucidated in the following section.
Delineating the Antidepressive Mechanisms of Probiotics
Probiotics secrete a wide range of signaling molecules that operate via distinct pathways to exert their effects, be it antidepressive, immunomodulatory or modulation of neurotransmission (Luan et al., 2017). This review classifies probiotic-associated signaling molecules into four types: neurotransmitters, bacterial secreted proteins, butyrate and other bioactive molecules (Figure 2). Some probiotics can secrete signaling molecules of different types. In this regard, the mechanisms of individual probiotics will be presented in the order of pertinence and similarity to each other.
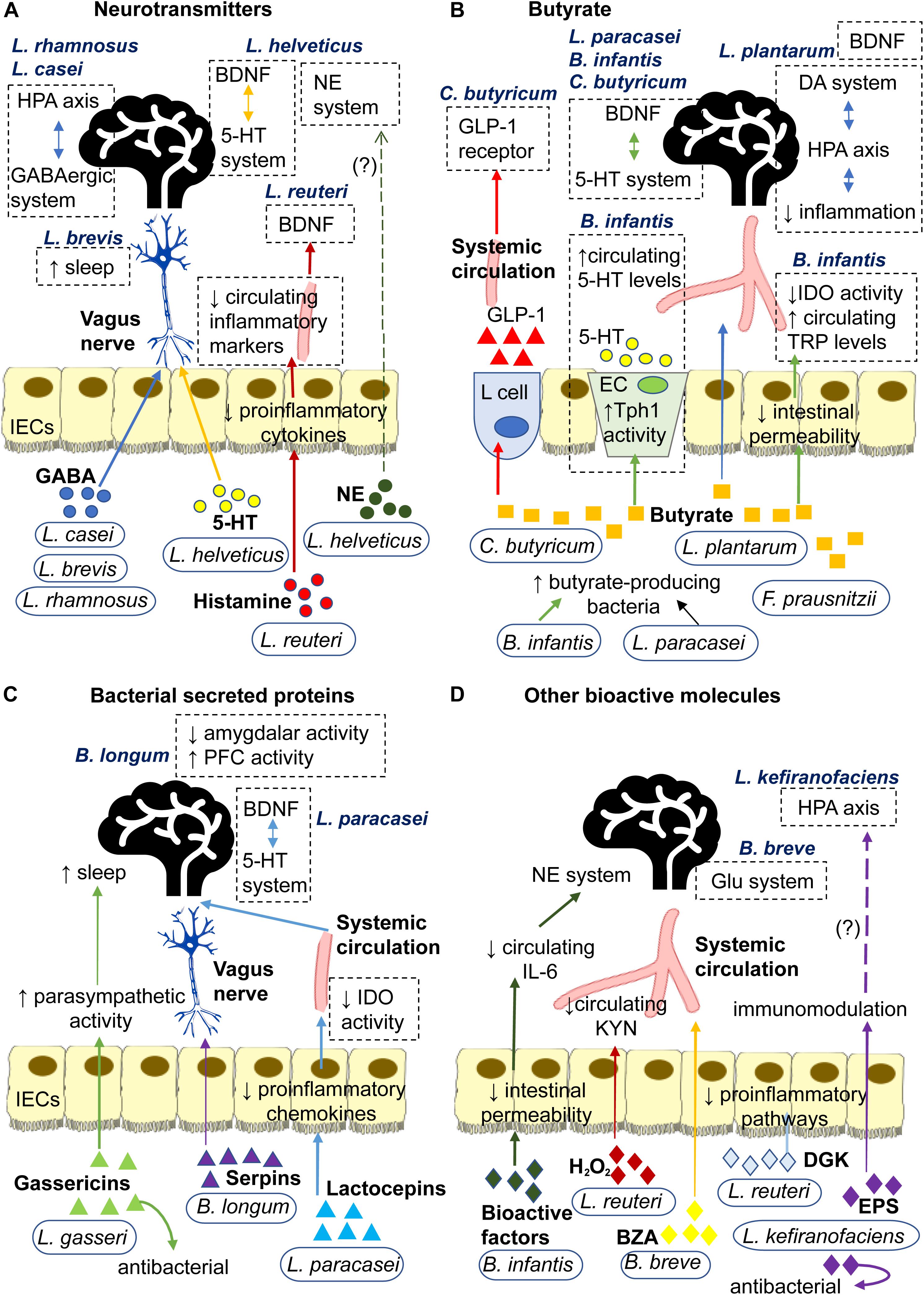
Figure 2. Signaling mechanisms underlying antidepressive effects of probiotics mediated through secretion of (A) Neurotransmitters: L. rhamnosus and L. casei secrete GABA that may signal central GABAergic system and HPA axis via the neural route. L. brevis secretes GABA that enhances sleep. L. helveticus secrete 5-HT that may signal the central 5-HT system via the neural route. L helveticus also secretes NE that may affect the central NE system. L. reuteri secretes histamine that decreases secretion of proinflammatory cytokines by IECs. This may reduce circulating inflammatory markers, such as LPS, IL-6 and corticosterone, and subsequently prevent the inflammation-induced decrease in hippocampal BDNF. (B) Butyrate: L. plantarum produces butyrate that strengthens intestinal barrier and diffuses through the circulation to regulate BDNF expression and reduce inflammation in the brain. The latter consequently regulates the HPA axis and its regulator, the DA system. C. butyricum produces butyrate that influences central 5-HT and BDNF systems and stimulates L cell to secrete GLP-1 into the bloodstream which increases expression of GLP-1 receptors. F. prausnitzii produces butyrate that strengthens the intestinal barrier. B. infantis and L. paracasei promote growth of butyrate-producing bacteria. Through butyrate, B. infantis upregulates Tph1 activity of EC which increases circulating 5-HT and strengthens intestinal barrier to lower IDO activity and increase circulating TRP, both of which affect the central 5-HT system and BDNF expression. Through butyrate, L. paracasei may influence the central 5-HT system and BDNF expression. (C) Bacterial secreted proteins: L. gasseri secretes gassericins that increase parasympathetic activity to facilitate sleep and improves gut microbiota composition. B. longum secretes serpins that alter neural activities in the brain via the neural route. L. paracasei secretes lactocepins that decrease proinflammatory chemokines in IECs. This lowers IDO activity which, in turn, affects the central 5-HT system and BDNF expression. (D) Other bioactive molecules: B. infantis secretes bioactive factors (likely polysaccharides) that decrease circulating IL-6 which affects the central NE system. L. reuteri secretes H2O2 that decreases IDO activity and circulating KYN, and dgk that inhibits the initiation of proinflammatory pathways. B. breve converts albiflorin into BZA which affects the Glu system via the humoral route. L. kefiranofaciens secretes exopolysaccharides that have immunomodulatory and antibacterial properties, which may potentially prevent HPA axis overactivity. 5-HT, 5-hydroxytryptamine or serotonin; BDNF, brain-derived neurotrophic factor; DA, dopamine; BZA, benzoic acids; dgk, diacylglycerol kinase; ECs, enterochromaffin cells; EPS, exopolysaccharide; GABA, gamma-Aminobutyric acid; GLP-1, glucagon-like peptide-1; Glu, glutamate or glutaminergic; H2O2, hydrogen peroxide; HPA, hypothalamic-pituitary-adrenal; IECs, intestinal epithelial cells; IDO, indoleamine 2,3-dioxygenase; IL-6, interleukin-6; KYN, kynurenine; NE, norepinephrine; LPS, lipopolysaccharides; Tph1, tryptophan hydroxylase 1; TRP, tryptophan.
Lactobacillus rhamnosus
Lactobacillus rhamnosus JB-1, the typical experimental strain of L. rhamnosus, was formerly referred to as Lactobacillus reuteri. Orally administered L. rhamnosus reduced depressive-like behaviors in normal, healthy mice (Bravo et al., 2011) and chronic-stressed mice (McVey Neufeld et al., 2018). Postpartum women (Slykerman et al., 2017) and obese individuals (Sanchez et al., 2017) that were supplemented with L. rhamnosus reported lower depressive thoughts compared to the control group. In vagotomized rats, behavioral and physiological benefits of L. rhamnosus were abolished (Bravo et al., 2011). This substantiates the vagus nerve as an essential conduit in the signaling pathway of L. rhamnosus. Introduction of L. rhamnosus into the gut lumen heightened the firing rate of vagus nerve and enteric neurons in mice (Perez-Burgos et al., 2013, 2014). These findings suggest that L. rhamnosus signals to the brain via the neural route, which may influence the central GABAergic system and HPA axis to manifest an antidepressive effect (Figure 2A). However, it is unclear whether neurotransmitters, cytokines or other molecules are involved in the neural signaling of L. rhamnosus.
Microbial GABA, Central GABAergic System, and HPA Axis
Glutamine is a precursor to Glu while Glu is a precursor to GABA. Reduced levels of GABA and Glx (Glu + glutamine) have been consistently reported in cortical regions of MDD patients (Sanacora et al., 1999; Hasler et al., 2007; Bhagwagar et al., 2008; Moriguchi et al., 2018; Godlewska et al., 2019). A dysfunctional glutaminergic system, that is partly responsible by a decreased GABAergic tone, is also implicated in MDD (Murrough et al., 2017). N-acetyl aspartate (NAA) is regarded as a marker for neuronal vitality. In MDD patients, decreased NAA levels in the PFC and hippocampus have been detected (Gonul et al., 2006; Olvera et al., 2010; Lefebvre et al., 2017). These neurochemical (i.e., Glx, NAA, and GABA) levels in the PFC and hippocampus of mice increased when administered with L. rhamnosus (Janik et al., 2016), implicating its antidepressive potential.
Intake of L. rhamnosus altered the central mRNA expression of GABAA and GABAB receptors while reducing depressive- and anxiety-like behaviors in mice. These effects were also dependent on an intact vagus nerve (Bravo et al., 2011). With prebiotics, L. rhamnosus intake decreased hippocampal GABAAα2 mRNA expression in stressed mice (McVey Neufeld et al., 2017). L. rhamnosus produced GABA and Glu efficiently from microbial glutamate decarboxylase and glutaminase, respectively, in vitro (Stromeck et al., 2011; Liao et al., 2013; Lin, 2013). These biosynthetic machineries utilized by microbes to synthesize Glu and GABA are mutual in neurons (Mathews and Diamond, 2003), which support the interkingdom communication of microbial GABA (Lyte, 2011). It was demonstrated in vitro that gut microbial GABA can cross the intestinal barrier via H+/GABA symporter (Thwaites et al., 2000; Nielsen et al., 2012). The microbial GABA may subsequently interact with GABA receptors and transporters that are widely expressed on enteric neurons and vagus afferents (Hyland and Cryan, 2010).
Administration of L. rhamnosus reduced stress-induced plasma corticosterone levels in mice that averted depression (Bravo et al., 2011; McVey Neufeld et al., 2018). This could be due to the innervation of PVN neurons by GABAergic synapses that can be desensitized by acute stress (Hewitt et al., 2009). Inhibited GABA signals allow continuous release of CRF by PVN neurons, which ultimately leads to cortisol overproduction and HPA axis overactivity (Cullinan et al., 2008). Impairment of GABA receptors also inhibits hippocampal neurogenesis, which has been shown to activate the HPA axis and induce depression in mice (Earnheart et al., 2007; Schloesser et al., 2009). Such effects may be possibly prevented by the production of GABA by L. rhamnosus.
Lactobacillus casei Strain Shirota
Individuals with low mood reported feeling happier after consuming milk containing L. casei, but not the placebo (Benton et al., 2007). Intake of mixed-species probiotics that included L. casei also reduced clinical depression and depressive-like symptoms in MDD patients (Akkasheh et al., 2016) and healthy individuals (Steenbergen et al., 2015; Mohammadi et al., 2016), respectively. Similar to L. rhamnosus, evidence suggests that L. casei may also regulate the HPA axis via the neural route (Figure 2A).
Microbial GABA and HPA Axis
Intake of L. casei stimulated vagus afferents and decreased both the activity and quantity of CRF-expressing cells in PVN of rats (Takada et al., 2016). Intragastric injection of L. casei downregulated the activity of sympathetic efferents to adrenal glands and liver, and this effect ceased upon vagotomy (Tanida et al., 2014). In clinical trials, L. casei supplementation lowered salivary cortisol levels, feelings of stress and frequency of abdominal- and flu-related symptoms in stressed individuals (Kato-Kataoka et al., 2016; Takada et al., 2016). These studies imply that L. casei prevents HPA axis overactivity via the vagus nerve, which may consequently lower stress-related feelings and illnesses. L. casei produced GABA in vitro (Oleskin et al., 2014), indicating a possibility that it may share an antidepressive mechanism of L. rhamnosus. Stressed individuals that consumed L. casei showed improvements in mental health and gut microbiota composition, characterized by increased Lactobacillus and Bifidobacterium populations (Rao et al., 2009; Kato-Kataoka et al., 2016). As most of the antidepressive probiotics belong to Lactobacillus and Bifidobacterium genera, the potential antidepressive capacity of L. casei is highly supported.
Lactobacillus brevis
Similar to L. rhamnosus and L. casei, L. brevis produces GABA via glutamate decarboxylase in substantial amounts (Yokoyama et al., 2002; Siragusa et al., 2007; Barrett et al., 2012; Ko et al., 2013; Yunes et al., 2016). This indicates that L. brevis may share a mutual mechanism of action with L. rhamnosus and L. casei (Figure 2A). Although L. brevis has been shown to influence neither the central GABAergic system nor the HPA axis, L. brevis appears to promote sleep.
Microbial GABA and Sleep
Milk fermented with L. brevis had increased GABA content. This L. brevis-fermented milk demonstrated an antidepressive potency on par with fluoxetine, a SSRI, in depressed rats (Ko et al., 2013). Intriguingly, intake of L. brevis-produced GABA improved sleep duration in mice (Han et al., 2017). Another study also showed that dietary L. brevis enhanced sleep quality and voluntary physical activity in mice (Miyazaki et al., 2014). GABA is the main inhibitory neurotransmitter that is widely associated with sleep, and GABA receptors are frequent targets for pharmaceutical drugs, such as benzodiazepine, to treat insomnia (Gottesmann, 2002). GABA-enriched foods and GABA extract have also been shown to improve sleep quality in insomniacs (Byun et al., 2018) and healthy individuals (Yamatsu et al., 2015). Therefore, L. brevis has therapeutic value for insomnia, which reflects one of the diagnostic criteria for MDD (American Psychiatric Association, 2013).
Lactobacillus reuteri
Treatment of L. reuteri ameliorated depressive-like behaviors in chronic-stressed (Marin et al., 2017) and immobilization-stressed mice (Jang et al., 2019). The former study further elucidated the mechanism of L. reuteri which involves regulation of IDO, a rate-limiting enzyme of immune cells that catabolizes TRP to KYN (Reus et al., 2015). It is also well documented that L. reuteri exhibits anti-inflammatory activities (Thomas et al., 2012; Gao et al., 2015; Ganesh et al., 2018). It is, thus, conceivable that L. reuteri may also prevent activation of IDO by proinflammatory cytokines (Reus et al., 2015).
Microbial Hydrogen Peroxide and Kynurenine Pathway
The etiology of depression is partly attributed to a dysregulated KYN/TRP pathway (Reus et al., 2015). An elevated ratio of plasma KYN/TRP often correlates positively with the depression severity in human (Maes et al., 2002; Gabbay et al., 2010; Baranyi et al., 2013; Zhou et al., 2019). It was demonstrated that L. reuteri intake improved behaviors of depressed mice by reversing the stress-induced (1) decrease in fecal H2O2 levels and Lactobacillus populations, and (2) increase in intestinal IDO1 expression and plasma KYN levels (Marin et al., 2017). KYN administration attenuated this antidepressive effect, which indicates that L. reuteri ameliorates depression by reducing plasma KYN levels. This study also showed that L. reuteri generated high amounts of H2O2 in vitro, and the author proposed that H2O2 is the key metabolite in mediating antidepressive effect of L. reuteri (Marin et al., 2017). This is because H2O2 catalyzes peroxidase-mediated reactions that inhibit IDO activity (Freewan et al., 2013). H2O2 is transported by aquaporin-3 transporters that are expressed on IECs (Thiagarajah et al., 2017) and immune cells (Moon et al., 2004). These findings suggest that microbial H2O2 can potentially cross the intestinal barrier to suppress IDO activity in immune cells, which would lower circulating KYN levels (Figure 2D).
Microbial Histamine, Diacylglycerol Kinase, and Brain-Derived Neurotrophic Factor (BDNF) Expression
Lactobacillus reuteri possesses histidine decarboxylase that converts dietary L-histidine to histamine, which inhibits the production of TNF-α in vitro (Thomas et al., 2012; Hemarajata et al., 2013). The microbial histamine suppressed proinflammatory cytokine activities in IECs via the histamine-2 receptor signaling pathway in mice. This effect disappeared when the histidine decarboxylase gene of L. reuteri was inactivated by mutagenesis (Gao et al., 2015). Intriguingly, microbial histamine also activated histamine-1 receptors to initiate downstream proinflammatory pathways in mice (Ganesh et al., 2018). However, the substrate for this pathway, diacylglycerol, is metabolized to phosphatidic acid by diacylglycerol kinase produced by L. reuteri. Thus, L. reuteri secretes both histamine and diacylglycerol kinase that act on histamine receptors to produce an anti-inflammatory effect (Ganesh et al., 2018). Orally administered L. reuteri simultaneously alleviated colitis and behaviors indicative of anxiety and depression in stressed mice. These effects were also accompanied by a decrease in colon inflammation and blood levels of LPS, interleukin-6 (IL-6) and corticosterone. In the same study, this reduction in peripheral inflammation prevented the infiltration of activated microglia into the hippocampus and increased hippocampal BDNF expression (Jang et al., 2019; Figure 2A). BDNF has been extensively studied for its vital role in neuronal function and its causal link to depression. Antidepressants such as SSRI and ketamine also increase hippocampal BDNF expression as part of their mechanism of action (Bjorkholm and Monteggia, 2016). Furthermore, this anti-inflammatory effect of L. reuteri may prevent IDO activation by proinflammatory cytokines (Reus et al., 2015).
Lactobacillus plantarum
Lactobacillus plantarum supplementation decreased depressive-like symptoms in chronic-stressed mice (Liu Y.W. et al., 2016; Dhaliwal et al., 2018) and stressed adults with mild depression (Lew et al., 2018), though the latter study did not reach statistical significance. Following L. plantarum intake, reduction in plasma corticosterone levels and inflammation were seen in mice with reduced depressive-like behaviors (Liu Y.W. et al., 2016). Another study reported that mice fed with L. plantarum displayed an increase in cecum SCFAs levels (acetic and butyric), and a decrease in intestinal permeability and level of MAOs in the brain (Dhaliwal et al., 2018). These physiological changes can be unified into a mutual mechanism that L. plantarum likely mitigates systemic inflammation (Figure 2B).
Butyrate, Intestinal Barrier, and BDNF Expression
Chronic-stressed mice fed with L. plantarum exhibited reduced depressive-like behaviors, coupled with an increase in butyrate and butyrate-producing bacteria, such as Lactobacillus, Bacteroidetes, and Roseburia (Dhaliwal et al., 2018). L. plantarum synthesizes butyrate via fatty acid synthase II–thioesterase, a glutamine-mediated butyrogenic pathway (Botta et al., 2017). Butyrate can enter IECs through cholesterol-rich microdomains and/or monocarboxylate transporter 1 protein (Suzuki et al., 2008; Goncalves et al., 2011; Nedjadi et al., 2014), and promote synthesis and assembly of tight junction proteins of IECs (Bordin et al., 2004; Ohata et al., 2005; Peng et al., 2009; Wang et al., 2012; Yan and Ajuwon, 2017). Butyrate also has anti-inflammatory properties; for instance, butyrate inhibited proinflammatory activities of IECs in vitro (Elce et al., 2017) and interacted with IECs to regulate host T cell responses (Lew et al., 2018; Xu et al., 2018). Butyrate may also diffuse into the systemic circulation to exert anti-inflammatory effects on various organs and tissues, including the brain (McNabney and Henagan, 2017; Matt et al., 2018). Indeed, butyrate has been shown to normalize behavior of depressed rodents through epigenetic regulations of hippocampal BDNF expression (Han et al., 2014; Wei et al., 2014; Sun et al., 2016). These outcomes are consistent with the finding that L. plantarum intake increased hippocampal BDNF expression and cecum butyrate levels in chronic stress-induced depressed mice (Dhaliwal et al., 2018).
HPA Axis and Central DA System
Lactobacillus plantarum supplementation decreased MAOs levels in brain tissues of mice with reduced depression (Dhaliwal et al., 2018). This is in line with another finding that L. plantarum intake in mice increased levels of DA and its metabolites (HVA and 3,4-dihydroxyphenylacetic acid, DOPAC) in the PFC, along with reduced depressive-like behaviors (Liu Y.W. et al., 2016). However, another study showed that L. plantarum increased DA levels in the striatum of mice while alleviating anxiety-like behaviors (Liu W.H. et al., 2016). These studies suggest that L. plantarum likely affects the central DA system in a context-dependent manner. It was also proposed that L. plantarum increases DA levels in the PFC to prevent HPA axis overactivation (Liu Y.W. et al., 2016). DA neurons in the PFC and ventral tegmental area (VTA) form the mesocortical pathway which regulates reward-seeking behaviors (Pariyadath et al., 2016) and the HPA axis (Sullivan and Dufresne, 2006). Glucocorticoids from the HPA axis can also influence the DA system either directly or indirectly, via epigenetic control and MAOs inhibition, respectively (Feenstra et al., 1992; Grunewald et al., 2012; Butts and Phillips, 2013). Taken together, L. plantarum may regulate both the DA system and HPA axis by attenuating glucocorticoid-induced MAOs activity.
Faecalibacterium prausnitzii (Previously Known as Fusobacterium prausnitzii)
Recently, it was discovered that oral gavage of F. prausnitzii exerted antidepressive and anxiolytic effects in chronic-stressed mice (Hao et al., 2019). F. prausnitzii, as the sole species of Faecalibacterium genera (Duncan, 2002), represents around 5% of the total human gut microbiota (Hold et al., 2003). Low populations of F. prausnitzii correlated with the disease severity of those with MDD (Jiang et al., 2015) and bipolar depression (Evans et al., 2017). In a recent large cohort study, fecal levels of F. prausnitzii correlated negatively with depressed mood and positively with quality of life (Valles-Colomer et al., 2019). Therefore, F. prausnitzii seems to have pertinent contributions to mental health.
Butyrate, Microbial Anti-inflammatory Molecules, and Peripheral Inflammation
Faecalibacterium prausnitzii produces butyrate in large quantities from fermenting glucose and fiber (Duncan, 2002; Hold et al., 2003). F. prausnitzii also secretes microbial anti-inflammatory molecules that suppress the proinflammatory nuclear factor (NF)-κB pathway in IECs (Sokol et al., 2008; Quevrain et al., 2016a, b). These immunomodulatory effects are consistent with neurochemical changes observed in F. prausnitzii-treated depressed mice, whereby cecum SCFAs and plasma IL-10 levels increased, while corticosterone and IL-6 levels decreased (Hao et al., 2019). Moreover, intragastric administration of F. prausnitzii decreased colonic cytokine levels and intestinal permeability in mice with colitis (Laval et al., 2015; Martin et al., 2015). Thus, butyrate produced by F. prausnitzii potentially strengthens the intestinal barrier (similar to L. plantarum; Figure 2B). However, whether local immunomodulatory effects of F. prausnitzii extend to the brain remains unknown. Nevertheless, the ability of F. prausnitzii to attenuate gut inflammation is sufficient to reduce depressive- and anxiety-like behaviors in mice (Hao et al., 2019).
Lactobacillus helveticus
Lactobacillus helveticus intake enabled the recovery of chronic- and subchronic-stressed rodents from their state of depression (Liang et al., 2015; Maehata et al., 2019). Probiotic sticks containing L. helveticus, in addition to Bifidobacterium longum, reduced clinical depression and depressive-like symptoms in MDD patients (Kazemi et al., 2019) and healthy individuals (Messaoudi et al., 2011), respectively. Most of the animal and human studies also showed that L. helveticus intake enhanced memory and, sometimes, attention and learning (Ohland et al., 2013; Chung et al., 2014; Luo et al., 2014; Liang et al., 2015; Ohsawa et al., 2018). Cognitive impairments, such as poor memory and concentration, represent one major cluster of MDD symptoms (Sharpley and Bitsika, 2014). Evidence suggests that L. helveticus may modulate the central NE system and HPA axis to improve cognition, and the central 5-HT system and BDNF expression to reduce depression (Liang et al., 2015) (Figure 2A).
Microbial NE, Central NE System, and HPA Axis
Supplementation of L. helveticus improved memory and cognitive performance in chronic-stressed rats, comparable to the SSRI citalopram-treated rats. This memory improvement correlated with increased plasma IL-10 and hippocampal NE levels, and reduced plasma corticosterone and ACTH levels (Liang et al., 2015). A previous study also showed that ingestion of L. helveticus enhanced memory and mitigated gut inflammation in neuroinflammation-induced rats (Luo et al., 2014). However, another study reported that memory improvement in L. helveticus-treated mice did not correlate with the state of gut inflammation (Ohland et al., 2013). Despite this discrepancy, it is well established that the hippocampal NE system and HPA axis both interact to regulate hippocampal glucose metabolism for memory consolidation (Osborne et al., 2015). This mechanism may be affected by microbial NE as L. helveticus produced NE in vitro in amounts that exceed the human bloodstream (Oleskin et al., 2014). It was also shown in vivo that gut bacteria are responsible for converting conjugated NE into its biologically active form (Asano et al., 2012). This neuroactive NE likely influences the MGB axis, but the exact mechanism remains unknown (Lyte, 2011).
Microbial 5-HT and Central 5-HT-BDNF System
Liang et al. (2015) showed that elevated hippocampal 5-HT levels correlated with reduced depression severity in L. helveticus-fed rats. The same study also demonstrated that treatment with SSRI citalopram alleviated depression and increased hippocampal BDNF expression and 5-HT levels (Liang et al., 2015). Hence, the antidepressive mechanism appears similar between L. helveticus and citalopram. Cultures of L. helveticus produced 5-HT at concentrations close to that in the human bloodstream (Oleskin et al., 2014). As shown in vivo, the gut microbiota has an indispensable function in deconjugating glucuronide-conjugated 5-HT to generate their free, biologically active counterparts in considerable amounts (Hata et al., 2017). It is hypothesized that gut luminal 5-HT may sensitize 5-HT 3A receptors of enteric neurons by stimulating the glial cell-derived neurotrophic factor of IECs (Hata et al., 2017). 5-HT3 receptors are also expressed on IECs (Hasler, 2009) and vagal afferents (Hillsley and Grundy, 1998). Therefore, it can be speculated that L. helveticus influences the central 5-HT circuitry via the neural route. This is supported by a recent study showing that L. helveticus intake increased expression of 5-HT 1A receptors in the nucleus accumbens while restoring behaviors of depressed mice (Maehata et al., 2019).
Chronic-stressed mice that ingested L. helveticus displayed an increase in hippocampal BDNF levels (Liang et al., 2015) and neurogenesis in the nucleus accumbens (Maehata et al., 2019). Nucleus accumbens is a brain region implicated in reward behavior. The central BDNF and 5-HT systems are synergistic, whereby 5-HT upregulates hippocampal BDNF–TrkB signaling to increase expression and synthesis of BDNF. The elevated BDNF, in turn, facilitates neurogenesis of 5-HT neurons (Martinowich and Lu, 2008; Bjorkholm and Monteggia, 2016). Therefore, L. helveticus likely increases hippocampal BDNF levels via modulation of 5-HT circuitry, in a similar manner to SSRIs (Liang et al., 2015).
Lactobacillus paracasei
Dietary intervention of heat-killed L. paracasei prevented mood deterioration in times of stress in healthy individuals (Murata et al., 2018). In corticosterone-induced depressed mice, oral gavage of either live or heat-killed L. paracasei exhibited antidepressive efficacy equivalent to or better than fluoxetine. The same study also showed that live and heat-killed L. paracasei operated via different mechanisms. Live L. paracasei increased 5-HT levels whereas heat-killed L. paracasei increased DA levels in the brain (Wei et al., 2019). The signaling mechanism of L. paracasei appears independent of the HPA axis (Wei et al., 2019) or vagus afferents (Tanida and Nagai, 2011). The remaining evidence suggests that L. paracasei potentially functions via an immune-mediated humoral pathway.
Lactocepin, Butyrate, and Central 5-HT-BDNF System
Lactobacillus paracasei secretes lactocepin, a PrtP-encoded serine protease, that selectively degrades proinflammatory chemokines in inflamed ileal tissue of mice (von Schillde et al., 2012). Lactocepin is most likely a heat-labile cell surface protein unique to L. paracasei (Hoermannsperger et al., 2009; von Schillde et al., 2012). Mice fed with live L. paracasei exhibited lower inflammatory markers in serum, such as increased IL-10 and glutathione peroxidase and decreased TNF-α and MCP-1 (Huang et al., 2018). Another study showed that oral gavage of live L. paracasei with its bacterial products prevented adverse effect of stress on intestinal permeability in rats (Eutamene et al., 2007). This can be linked to a suppressed IDO activity, resulting in higher TRP bioavailability for 5-HT synthesis in the brain (Reus et al., 2015). Following this, it was shown that live L. paracasei delivered via gavage increased 5-HT and 5-HIAA (the main metabolite of 5-HT) levels in the hippocampus and striatum of mice (Huang et al., 2018; Wei et al., 2019). As 5-HT facilitates BDNF synthesis (Martinowich and Lu, 2008), the upregulated central 5-HT expression presumably explains the accompanying increase in hippocampal BDNF expression of mice alleviated of depression from L. paracasei intake (Wei et al., 2019). Therefore, L. paracasei may upregulate the central 5-HT-BDNF system (similar to L. helveticus; Figure 2C).
Treatment of live L. paracasei also increased fecal Bifidobacterium populations while normalizing behaviors of depressed mice (Wei et al., 2019). The gut microbiota profile, inflammatory markers and levels of acetate and butyrate were improved in IBS patients supplemented with live L. paracasei (Bertani et al., 2017; Cremon et al., 2018). Reduction in systemic inflammation, coupled with an improvement in hippocampal function, was also observed in obese rats fed with live L. paracasei (Chunchai et al., 2018). Thus, live L. paracasei may facilitate the colonization of butyrate-producing bacteria to reduce systemic inflammation (similar to L. plantarum) and increase 5-HT secretion from ECs (similar to Bifidobacterium infantis; Figure 2B).
Bifidobacterium infantis
In naïve rats, intake of B. infantis was shown to alter depression-related biomarkers (Desbonnet et al., 2008). The same group later showed that chronic-stressed mice no longer displayed depressive-like behaviors after B. infantis intake (Desbonnet et al., 2010). In flood victims with IBS, B. infantis consumption did not affect their IBS symptoms but improved their mental health instead (Murata et al., 2018). B. infantis did not influence corticosterone levels in mice (Desbonnet et al., 2008, 2010), implying that the effect of B. infantis is likely to be independent of the HPA axis. Evidence suggests that B. infantis has immunomodulatory effects that regulate the central NE system (Desbonnet et al., 2010). A recent study also provided support for the antidepressive mechanism of B. infantis that involves the hippocampal 5-HT system (Tian et al., 2019).
Bioactive Factors, IL-6, and Central NE System
Bifidobacterium infantis treatment manifested two physiological changes in vivo. First, B. infantis decreased plasma IL-6 levels in mice (Desbonnet et al., 2008, 2010) and patients with inflammatory conditions (Groeger et al., 2013). In depressed mice, the IL-6 release also correlated positively with the severity of depression (Desbonnet et al., 2010). Second, B. infantis increased NE levels in the murine brainstem (Desbonnet et al., 2010) containing the majority of NE neurons (Schwarz and Luo, 2015). Therefore, B. infantis likely regulates plasma IL-6 and central NE system to exert an antidepressive effect.
Bifidobacterium infantis secretes bioactive factors (probably polysaccharides) that enhance transepithelial resistance of IECs (Ewaschuk et al., 2008). Other studies involving rodents also showed that B. infantis treatment enhanced the intestinal barrier by strengthening the formation of tight junction proteins and anti-inflammatory activities of immune cells (Lomasney et al., 2014; Zuo et al., 2014; Javed et al., 2016). Indeed, bacterial DNA translocation from the gut lumen into the circulation was reduced in B. infantis-fed rodents (Osman et al., 2006; Gómez-Hurtado et al., 2012). Bacterial DNA is a potent inducer of TLRs which facilitate the release of proinflammatory cytokines, including IL-6 (Gutierrez et al., 2016). Administration of IL-6 induced depression in mice, and this outcome was prevented by pharmaceutical blockage of NE neurons in the brainstem (Kurosawa et al., 2016). Hence, B. infantis potentially modulates the NE system via an immune-mediated humoral route to reduce depression (Figure 2D). This mechanism appears to be independent of the vagus nerve as oral gavage of B. infantis also decreased proinflammatory cytokine (including IL-6) levels in vagotomized mice with an inflamed colon (van der Kleij et al., 2008).
Butyrate, TRP, and Central 5-HT-BDNF System
Treatment of B. infantis upregulated mRNA expression of Tph1 in RIN14B cells, a cell line that mimics ECs (Tian et al., 2019). Tph1 converts TRP to 5-hydroxytryptophan (5-HTP) and aromatic amino acid decarboxylase subsequently converts 5-HTP to 5-HT. B. infantis-fed mice displayed reduced depressive-like behaviors, along with an increase in TRP biosynthesis and hippocampal 5-HT and 5-HTP levels. In the same study, B. infantis increased cecum butyrate levels and the abundance of butyrate-producing Bifidobacterium. The elevated butyrate levels also correlated with increased hippocampal 5-HTP and PFC BDNF levels (Tian et al., 2019). This could be due to the ability of butyrate and other SCFAs to increase Tph1 activity of ECs, thereby promoting 5-HTP and 5-HT secretions (Reigstad et al., 2015; Yano et al., 2015; Lund et al., 2018). This is consequential as ECs contribute about 95% of the bodily 5-HT (El-Merahbi et al., 2015), and that mice with a gut microbiota had 2.8-fold higher plasma 5-HT levels than GF mice (Wikoff et al., 2009). The evidence for the ability of 5-HT to cross the BBB is conflicting (Brust et al., 2000; Wakayama et al., 2002; Nakatani et al., 2008; El-Merahbi et al., 2015). In contrast, 5-HTP readily crosses the BBB and can be converted into 5-HT. Therapeutic 5-HTP has also been shown to treat clinical depression with a potency equivalent to or better than SSRIs (Birdsall, 1998; Jangid et al., 2013; Jacobsen et al., 2016).
Furthermore, B. infantis intake increased plasma TRP levels in healthy rats (Desbonnet et al., 2008), but another study with chronic-stressed rats reported otherwise (Desbonnet et al., 2010). The author then suggested that B. infantis regulates TRP metabolism differently, depending on the rat strain (Desbonnet et al., 2010). Therapeutic TRP can improve symptoms of mood, sleep and cognitive disorders as TRP readily passes through BBB to regulate numerous brain functions, such as 5-HT synthesis (Richard et al., 2009). The elevated plasma TRP levels from B. infantis intake is most likely a result of reduced proinflammatory cytokines (Desbonnet et al., 2008, 2010), which reduces IDO activity and prevents over-catabolism of TRP (Reus et al., 2015). Thus, B. infantis may upregulate the hippocampal 5-HT system via modulation of peripheral 5-HTP, 5-HT and/or TRP levels. As 5-HT promotes BDNF synthesis (Martinowich and Lu, 2008), this presumably explains the concomitant increase in BDNF levels in PFC of rats ameliorated of depression with B. infantis treatment (Tian et al., 2019). Taken together, L. helveticus, L. paracasei and B. infantis upregulate the central 5-HT-BDNF system as their mutual antidepressive mechanism, although via different pathways (Figure 2B).
Clostridium butyricum
Treatment of C. butyricum improved depressive-like behaviors in chronic-stressed mice. These treated mice also showed upregulated central 5-HT, BDNF and GLP-1 receptors in the brain (Sun et al., 2018). Remarkably, the combination of C. butyricum with antidepressants reduced depression in about 70% of treatment-resistant MDD patients, of which 30% achieved remission (Miyaoka et al., 2018). These studies support the antidepressive efficacy of non-pathogenic C. butyricum. It should be noted that certain strains of C. butyricum are pathogenic which may cause botulism and necrotizing enterocolitis (Cassir et al., 2016).
Butyrate, Central 5-HT-BDNF System, and GLP-1
Clostridium butyricum, as a resident of healthy gut microbiota, produces butyrate from carbohydrate fermentation (Araki et al., 2002; He et al., 2005; Liu J. et al., 2015). Treatment of C. butyricum increased central 5-HT levels and BDNF expression in mice with reduced depression (Sun et al., 2018). Another study also reported that C. butyricum intake upregulated neurogenesis-related pathways, such as BDNF, via butyrate production in mice (Liu J. et al., 2015). Additionally, intragastric inoculation of C. butyricum increased intestinal secretion of GLP-1 and the central expression of GLP-1 receptors in mice alleviated from depression (Sun et al., 2018). This effect may also be mediated by butyrate as SCFAs can bind to receptors expressed on intestinal L cells to stimulate GLP-1 secretion into the bloodstream (Tolhurst et al., 2012). GLP-1 is known for appetite and glucose control, but the activation of central GLP-1 receptors has been shown to regulate the central 5-HT system and reduce anxiety- and depressive-like behaviors in rats (Anderberg et al., 2016). Therefore, antidepressive mechanism of C. butyricum potentially involves a butyrate-mediated upregulation of central BDNF-5-HT system (similar to L. paracasei and B. infantis) and GLP-1 receptor expression (Figure 2B).
Lactobacillus kefiranofaciens
Lactobacillus kefiranofaciens is isolated from kefir, a type of fermented milk. Oral gavage of L. kefiranofaciens improved behaviors of chronic-stressed, depressed mice. These treated mice also showed several physiological alterations. Levels of circulating TRP, splenic IL-10 and beneficial gut bacteria (e.g., Lachnospiraceae, Bifidobacteriaceae, and Akkermansia) increased, and KYN/TRP ratio, splenic IL-6 and IFN-γ levels and Proteobacteria abundance decreased (Sun et al., 2019). What factors mediate such broad effects of L. kefiranofaciens on the TRP/KYN pathway, immune system, HPA axis and gut microbiota remain unclear, but exopolysaccharide is potentially a candidate (Figure 2D).
Exopolysaccharide, Peripheral Inflammation, and Gut Microbiota
The only known metabolite of L. kefiranofaciens is an exopolysaccharide called kefiran (Maeda et al., 2004; Xing et al., 2017). The intake of kefiran modulated the gut mucosal immune system of mice (Vinderola et al., 2006), which could potentially account for changes in splenic cytokines seen in depressed mice (Sun et al., 2019). Kefiran was also shown to protect human enterocyte cell lines from adhesion and damage inflicted by toxins of pathogenic bacteria (Santos et al., 2003; Medrano et al., 2008). A further study discovered that L. kefiranofaciens produces a novel exopolysaccharide (not kefiran) that is bactericidal toward enteropathogens Listeria monocytogenes and Salmonella enteritidis (Jeong et al., 2017a). It may be possible that the antibacterial effects of this exopolysaccharide extend to other species in the gut microbiota. This supports the finding that L. kefiranofaciens supplementation ameliorated depressive-like behaviors in chronic-stressed mice by regulating gut microbiota content, which included the decreased abundance of Proteobacteria, a phylum that includes pathogens such as Salmonella (Sun et al., 2019). Other mice studies also supported the role of L. kefiranofaciens in modulating gut microbiota composition (Jeong et al., 2017b; Xing et al., 2018). Collectively, these changes in gut microbiota profile prevent gut dysbiosis that could lead to chronic inflammation, HPA axis overactivity and depression (Jeong et al., 2017b).
Bifidobacterium breve
Bifidobacterium breve treatment improved symptoms of depression in innately anxious mice (Savignac et al., 2014), chronic-stressed mice (Tian et al., 2019) and schizophrenic patients with depression (Okubo et al., 2019). B. breve supplementation also improved mood and cognition in elderly people with mild cognitive impairment (Kobayashi et al., 2019). However, none of the accompanying physiological changes among these studies overlapped, making it difficult to identify an exact mechanism of B. breve. In spite of this, one study demonstrated that antidepressive mechanism of B. breve involves the generation of benzoic acid (Zhao et al., 2018; Figure 2D).
Benzoic Acid and Central Glu System
Among the 18 bacterial strains isolated from gut microbiota, B. breve was the most efficient converter of albiflorin to benzoic acid via microbial carboxylesterase, at the rate of 75% as compared to L. casei, Lactobacillus acidophilus and B. longum at about 5%. The same study further showed that orally administered benzoic acid alleviated depression in mice (Zhao et al., 2018). Benzoic acid readily crosses the intestinal barrier and BBB to inhibit D-amino acid oxidase that catabolizes D-serine, a co-agonist of N-methyl-D-aspartate receptor (NMDAR, a type of Glu receptor) (Zhao et al., 2018). Both D-serine and NMDARs are therapeutic targets in neuropsychiatric disorders, such as depression, schizophrenia and cognitive impairment (Durrant and Heresco-Levy, 2014). Indeed, a dysfunctional Glu system is linked to the pathophysiology of depression (Pytka et al., 2016). In line with this, B. breve intake increased Glu synapses in chronic-stressed mice while treating its depressive-like behaviors (Tian et al., 2019).
Bifidobacterium longum
Bifidobacterium longum treatment decreased depressive-like symptoms in innately anxious mice (Savignac et al., 2014) and IBS patients with mild to moderate depression and/or anxiety (Pinto-Sanchez et al., 2017). B. longum supplementation also presented anxiolytic efficacy in numerous human and animal studies (Bercik et al., 2010, 2011; Allen et al., 2016; Orikasa et al., 2016). However, B. longum did not affect the gut inflammatory state in animals and humans, indicating a lack of immunomodulatory function (Bercik et al., 2010, 2011; Pinto-Sanchez et al., 2017). Other physiological changes, such as BDNF expression and plasma KYN/TRP ratio, seen in B. longum-treated mice and humans were inconsistent (Bercik et al., 2010, 2011; Orikasa et al., 2016; Pinto-Sanchez et al., 2017). Collectively, these data suggest that brain neural activity and HPA axis are possible targets of B. longum signaling mechanisms (Figure 2C).
Serpin, Central Neural Activity, and HPA Axis
Both in vitro and in vivo studies showed that B. longum weakened the excitability of murine myenteric neurons (Bercik et al., 2011; Khoshdel et al., 2013). Mice with inflamed intestines that were fed with B. longum demonstrated reduced anxiety-like behaviors, and this effect ceased upon vagotomy (Bercik et al., 2011). Intriguingly, B. longum intake also alleviated anxiety in colon-inflamed mice that were vagotomized before treatment (Bercik et al., 2010). The author postulated that vagus afferents are an essential conduit when B. longum signals enterocytes, but not colonocytes (Bercik et al., 2011). The genome of B. longum encodes serpin, a serine protease inhibitor (Ivanov et al., 2006; Mkaouar et al., 2016). Serpin can inhibit the activation of enteric neurons by suppressing the secretion of elastase-like proteases from IECs (Ivanov et al., 2006; Buhner et al., 2018). These studies support the premise that B. longum interacts with the host via the neural pathway (similar to L. rhamnosus). Following this, the neural activity and HPA axis of the brain may be altered. Individuals consuming B. longum had increased neural activity in the PFC and decreased neural activity in the amygdala and fronto-limbic regions (Allen et al., 2016; Pinto-Sanchez et al., 2017). Anomalies in the anatomy and activity of the amygdala and PFC are also commonly observed among depressed patients (Liu W. et al., 2017). Furthermore, B. longum intake exerted simultaneous glucocorticoids-lowering and anxiolytic effects in humans and mice (Allen et al., 2016; Orikasa et al., 2016), suggesting that B. longum potentially modulates the HPA axis.
Lactobacillus gasseri
Supplementation of L. gasseri improved mood (Sashihara et al., 2013) and depressive-like symptoms (Sawada et al., 2017) in stressed individuals. However, no studies have evaluated the effect of L. gasseri on clinically depressed individuals. Interestingly, L. gasseri is the only dietary probiotic which showed consistent sleep-enhancing effects in humans (Nishida et al., 2017a, b; Sawada et al., 2017). Irregular sleeping patterns are frequently associated with MDD (American Psychiatric Association, 2013; Wallace and Milev, 2017), supporting the use of L. gasseri as a potential treatment for MDD-related sleep disturbances.
Gassericins, Gut Microbiota, and Parasympathetic Activity in Sleep
Stressed individuals that were given probiotic-based milk containing either heat-killed or live L. paracasei showed alterations in the gut microbiota profile. Heat-killed L. gasseri decreased Bacteroides vulgatus and increased Dorea longicatena populations (Nishida et al., 2017a), whereas live L. gasseri decreased growth of inflammatory Enterobacteriaceae and Veillonella (Sawada et al., 2017). Both studies also showed that L. gasseri enhanced sleep quality of participants. Another study reported that heat-killed L. gasseri (in milk) increased the population of Clostridium cluster IV group and SCFAs levels in individuals with altered bowel movements (Sawada et al., 2016). Using a similar methodology, decreased Clostridium cluster IV and increased Bifidobacterium populations were found in another group of participants (Sugawara et al., 2016). Taken together, these results suggest that heat-killed L. gasseri does not have a specific microbial target, but rather modifies the preexisting gut microbiota that is unique to each individual. Nevertheless, these changes in the gut microbiota composition favor an anti-inflammatory state (Sawada et al., 2016; Sugawara et al., 2016; Nishida et al., 2017a). L. gasseri likely alters the gut microbiota profile through its unique, heat-resistant gassericins A and T with potent antibacterial properties against enteric pathogens (Pandey et al., 2013).
Heat-killed L. gasseri decreased expression of leukocytic stress-responsive microRNAs and salivary cortisol levels in stressed individuals (Nishida et al., 2017b). L. gasseri intake also prevented downregulation of EIF2-related genes in IBS patients (Nobutani et al., 2017). These studies suggest that L. gasseri confers protection against detrimental effects of stress. Moreover, heat-killed L. gasseri intake promoted parasympathetic nerve activity while improving sleep quality of stressed individuals (Nishida et al., 2017b). In healthy individuals, administration of either live or heat-killed L. gasseri increased their parasympathetic activity (Otomi et al., 2015; Sugawara et al., 2016). Therefore, L. gasseri may modify the gut microbiota profile in such a way that lowers gut inflammation and stress response, which may consequently promote parasympathetic activity to facilitate sleep (Figure 2C).
Challenges and Perspectives for Probiotics as Treatment for Depression
The existence of different gut microbiota compositions, depression subtypes and probiotic formulations complicate treatment outcomes and necessitate an individualized approach when using probiotics to treat depression. Despite these challenges, probiotics confer some benefits over antidepressant drugs, and there are more promising candidate probiotics that can potentially treat depression.
Heterogeneity of Gut Microbiota Composition
Several factors are known to influence the gut microbiota composition, such as diet, medications, genetics, age, geographical location and smoking (Thursby and Juge, 2017). Recently, approximately 1000 gut-derived putative bacterial species that do not belong to any existing genus were discovered in humans (Almeida et al., 2019). Such tremendous diversity complicates the understanding of how introduced probiotics affect the overall gut microbiota. One study showed that tolerability of individuals’ gut microbiota toward the colonization of probiotics ranges from permissive to resistant (Zmora et al., 2018). This appears to depend on the baseline abundance of probiotic species in the host gut microbiota. For instance, those who were permissive toward the colonization of Lactobacillus had prior low levels of Lactobacillus populations before treatment (Zmora et al., 2018). Similarly, B. longum colonized the gut for a longer period in 30% of users who initially had low levels of B. longum (Maldonado-Gomez et al., 2016). Another study showed that the antidepressive effect of multi-species probiotics (MSP) only manifests when the administered MSP successfully colonized the gut of rats (Abildgaard et al., 2019). This is consistent with the observation that lower levels of two main probiotic genera, Lactobacillus and Bifidobacterium, are commonly found in individuals with MDD (Aizawa et al., 2016).
Despite most studies supported the effectiveness of probiotic supplements in reducing depression, not all randomized controlled trials reported the same outcome (Table 2). For instance, L. rhamnosus did not affect scores of anxiety, depressions, sleep, cognition, inflammatory and stress responses among healthy adults (Kelly et al., 2017). L. rhamnosus also did not affect perceptions of wellbeing, anxiety and stress among healthy older adults (Ostlund-Lagerstrom et al., 2016). In healthy individuals, L. helveticus exhibited no antidepressive effect (Chung et al., 2014; Ohsawa et al., 2018). These results imply that probiotics are less efficacious among the healthy population, which agree with a meta-analysis that reported an insignificant effect of probiotics on mood, particularly in healthy individuals (Ng et al., 2018). Therefore, probiotics could be generally more effective in colonizing gut microbiota of depressed individuals that are different from healthy people (Jiang et al., 2015; Zheng et al., 2016). In some cases, probiotic colonization may be optional for their effects to manifest. For instance, heat-killed L. paracasei benefited the human and animal host, in terms of neurochemical and behavioral changes (Corpuz et al., 2018; Murata et al., 2018; Wei et al., 2019). Some probiotics, such as L. reuteri, L. paracasei, L. plantarum, L. gasseri, L. kefiranofaciens, B. breve, and B. infantis, promoted the colonization of other beneficial microbes that contributed to the reduction of depressive-like symptoms in animals (Marin et al., 2017; Dhaliwal et al., 2018; Jang et al., 2019; Sun et al., 2019; Tian et al., 2019; Wei et al., 2019).
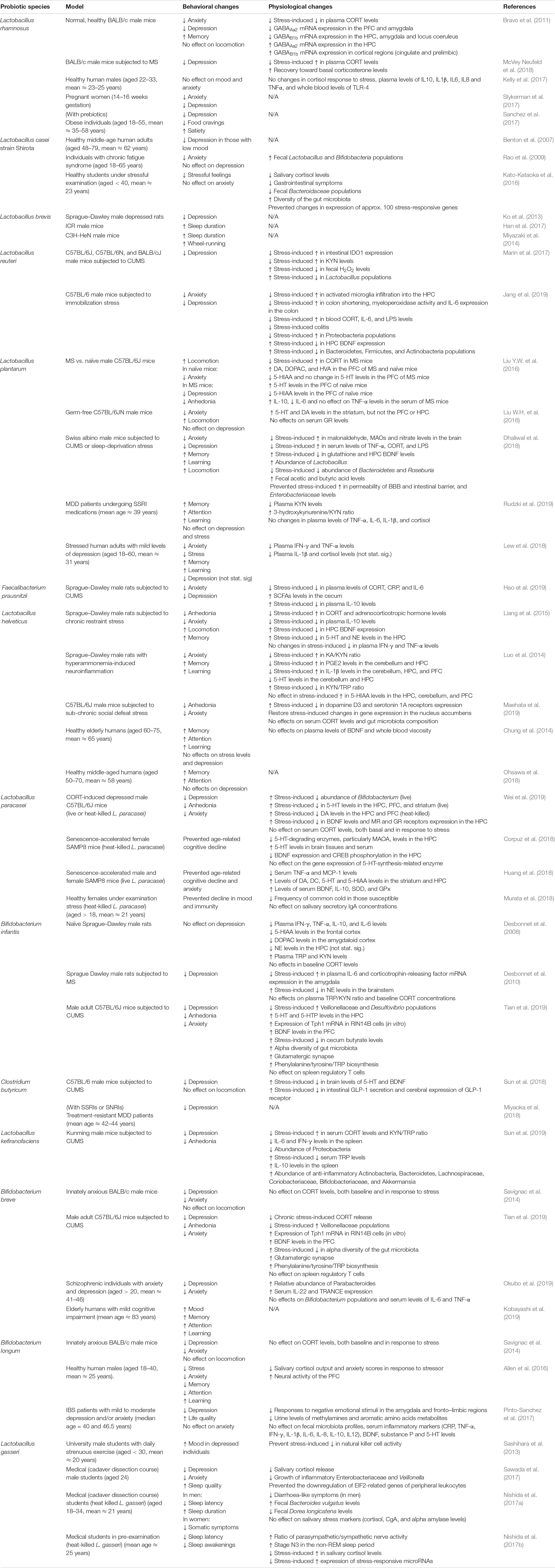
Table 2. Selected preclinical and clinical studies on the behavioral and physiological effects of single-species probiotics.
Heterogeneity of Depression
Major depressive disorder is characterized by depressed mood and/or anhedonia, in addition to excessive guilt, suicidal ideation, changes in appetite and sleep, psychomotor retardation, poor concentration and fatigue (American Psychiatric Association, 2013). From these diagnostic criteria, approximately a thousand combinations of symptoms (Ostergaard et al., 2011) and 19 depression subtypes (Harald and Gordon, 2012; Sharpley and Bitsika, 2014) can be derived. These subtypes of depression are often grouped as a single term, namely depression, which should not be the case when evaluating therapeutic potential of probiotics.
Some associations can be drawn by matching behavioral benefits of probiotics to the characteristics of depression subtypes (Table 2). For instance, the sucrose preference test in rodents reflects the anhedonia subtype (Dedic et al., 2011). Probiotics that have been shown to improve the outcome of this test include L. helveticus (Liang et al., 2015), L. plantarum (Liu Y.W. et al., 2016), L. paracasei (Wei et al., 2019), L. kefiranofaciens (Sun et al., 2019), B. infantis (Tian et al., 2019), and B. breve (Tian et al., 2019). Among these probiotics, L. plantarum (Liu Y.W. et al., 2016) and L. paracasei (Wei et al., 2019) also modulated the central DA system, whereas B. infantis and B. breve upregulated tyrosine (precursor to DA) biosynthesis (Tian et al., 2019). An impaired DA system represents the hallmark pathophysiology of anhedonia (Dunlop and Nemeroff, 2007). This provides a proof of concept that these probiotics may be effective in treating anhedonia.
Somatic depression subtype is characterized by psychomotor agitation/retardation (i.e., locomotion), changes in weight/appetite, insomnia/hypersomnia and fatigue without physical exertion (Sharpley and Bitsika, 2014). Probiotics that improved locomotor activity of rodents include L. plantarum (Liu W.H. et al., 2016; Dhaliwal et al., 2018), L. helveticus (Liang et al., 2015) and L. brevis (Miyazaki et al., 2014). Intake of L. brevis increased sleep duration in healthy mice (Miyazaki et al., 2014; Han et al., 2017), and L. gasseri enhanced sleep quality in medical students with mild depression (Nishida et al., 2017a, b). L. rhamnosus supplementation modulated appetite-associated genes and attenuated appetite in zebrafish (Falcinelli et al., 2016, 2017). In combination with prebiotics, L. rhamnosus exerted antidepressive effect and appetite control in obese individuals (Sanchez et al., 2017). Hence, symptoms of somatic depression are rather distinct and may be improved differently with different probiotics.
Cognitive depression subtype is distinguished by poor concentration and memory function as well as indecisiveness (Sharpley and Bitsika, 2014). Behavioral assessments for memory function in mice include the Morris water maze, Barnes maze and other behavioral tests (Dedic et al., 2011). Administration of probiotics including L. helveticus (Ohland et al., 2013; Luo et al., 2014; Liang et al., 2015), L. plantarum (Dhaliwal et al., 2018), and L. paracasei (Corpuz et al., 2018; Huang et al., 2018) enabled animals to perform these memory test more effectively. Attention, memory and learning behaviors in humans are assessed by cognitive tests, such as the Stroop, verbal-learning and digit-symbol tests. Improvements in these tests have been shown with the intake of (1) L. helveticus (Chung et al., 2014; Ohsawa et al., 2018) and B. longum (Allen et al., 2016) in healthy adults; (2) L. plantarum in MDD patients (Rudzki et al., 2019) and stressed adults with mild depression (Lew et al., 2018); and (3) B. breve in elderly with mild cognitive impairment (Kobayashi et al., 2019). Thus, some probiotics appear to improve cognition regardless of depression.
Anxious depression subtype refers to major depression that comorbid with high levels of anxiety (Harald and Gordon, 2012). In mice, anxiety can be measured by behavioral tests, such as the elevated plus maze and open field tests (Dedic et al., 2011). In humans, anxiety is generally assessed with questionnaires. Probiotics that exhibit anxiolytic effect include L. rhamnosus (Bravo et al., 2011; Bharwani et al., 2017; McVey Neufeld et al., 2017; Slykerman et al., 2017), L. helveticus (Ohland et al., 2013; Luo et al., 2014; Liang et al., 2015), L. plantarum (Liu W.H. et al., 2016; Liu Y.W. et al., 2016; Dhaliwal et al., 2018; Lew et al., 2018), B. longum (Bercik et al., 2010, 2011; Savignac et al., 2014; Allen et al., 2016) and B. breve (Savignac et al., 2014; Okubo et al., 2019; Tian et al., 2019). Moreover, MSPs intake often decreased depression and anxiety simultaneously in randomized controlled trials (Mohammadi et al., 2016; Kouchaki et al., 2017; Jamilian et al., 2018; Raygan et al., 2018; Ostadmohammadi et al., 2019; Salami et al., 2019).
Conventional SSRIs that target the 5-HT system often fail to treat anhedonic patients and, in some cases, worsen their symptoms (Dunlop and Nemeroff, 2007). Antidepressant drugs (e.g., SSRI and SNRI) are also ineffective against other depression subtypes, namely the somatic (Tylee and Gandhi, 2005), cognitive (Shilyansky et al., 2016) and anxious depression (Ionescu et al., 2014). Therefore, certain probiotics may serve as an adjuvant or alternative treatment for MDD and its subtypes. A pilot study showed that MSP, together with a magnesium supplement, decreased depression in SSRI treatment-resistant patients (Bambling et al., 2017). A clinical trial also reported that the combination of B. longum and L. helveticus decreased depression in MDD patients with prior use of standard antidepressants (Kazemi et al., 2019).
Single-Species and Multi-Species Probiotic
In studies that investigated behavioral effects of probiotics, about 60% of animal studies and 50% of human studies used single-species probiotics (SSPs) (Joseph and Law, 2019). Studies with SSPs promote a better understanding of the function and contribution of individual probiotic, which is difficult to measure in MSPs. However, MSPs may have higher potency in humans. In MDD patients, SSP (L. plantarum) did not reduce depression but improved cognition (Lew et al., 2018), whereas MSPs had repeatedly shown antidepressive efficacy (Akkasheh et al., 2016; Bambling et al., 2017; Ghorbani et al., 2018; Kazemi et al., 2019). MSPs often gave better therapeutic efficacy compared to that of SSPs in gut-related disorders and pathogen infections, which could be explained by an overall higher dosage (Chapman et al., 2011, 2012). Indeed, MSPs with a higher dosage improved symptoms of depression and anxiety in healthy individuals compared to that of a lower dosage (Tran et al., 2019). MSPs are also hypothesized to exhibit synergistic effects that would have an expanded effect on the host physiology (Chapman et al., 2012). In contrast, SSPs are speculated to promote better colonization as it does not have to compete for nutrient or adhesion sites in the host (Chapman et al., 2011). This highlights the need for more studies to understand how probiotics in MSPs interact with each other and with existing gut microbiota, and which probiotic(s) is suitable in formulation of MSPs for antidepressive efficacy.
Advantages of Probiotics as Antidepressive Treatment
Probiotics are generally safe for consumption, except for immune-compromised and critically sick individuals wherein probiotics may cause sepsis, pneumonia, endocarditis and allergies (Didari et al., 2014). Still, it has been viewed by some that more human trials are required to establish the dosage efficacy and long-term safety profile of probiotics (Kothari et al., 2018). For antidepressant drugs such as SSRIs, side effects occur in 40-60% of users which include sexual dysfunction, suicidality, emotional numbness and addiction (Read and Williams, 2018). A meta-analysis data showed that users of antidepressant drugs were associated with a 33% increased risk of mortality (Maslej et al., 2017). On the other hand, probiotics possess fewer side effects than antidepressant drugs. For instance, rats fed with L. brevis-fermented milk exhibited comparable antidepressive efficacy to fluoxetine-treated rats, but without side effects of fluoxetine (decreased appetite and weight loss) (Ko et al., 2013).
Antidepressant usage is also associated with stigma, such as being perceived as emotionally weak and dependent on drugs, which contributes to the disease severity and poor adherence to treatment (Castaldelli-Maia et al., 2011). In a survey study, 77% of depressed patients prefer to hide their use of antidepressant medication from others (Martinez et al., 2018). However, the prevalence of perceived stigma against antidepressants differs based on the population studied (Castaldelli-Maia et al., 2011). To this end, probiotics may help as an alternative treatment for depression, given that probiotics have not been associated with any perceived social stigma (Wallace and Milev, 2017).
Candidate Probiotics With Potential Antidepressive Effect
Bifidobacterium pseudocatenulatum is known for its regulation of obesity-related changes in metabolism and the immune system (Cano et al., 2013; Moya-Perez et al., 2014, 2015; Sanchis-Chorda et al., 2018). B. pseudocatenulatum intake reversed diet-induced obesity, depression, high corticosterone and low hippocampal 5-HT levels in mice (Agusti et al., 2018). However, a high-fat diet model is meant to study the pathophysiology of obesity and type 2 diabetes (Winzell and Ahren, 2004; Wang and Liao, 2012). It is, thus, unclear if B. pseudocatenulatum would decrease depression in mice without obesity. Another study showed that anxiety-like behaviors diminished in chronic-stressed mice fed with B. pseudocatenulatum, but depressive-like behaviors were unevaluated (Moya-Perez et al., 2017). Therefore, further studies are required to determine whether B. pseudocatenulatum has an independent antidepressive effect.
Bacillus coagulans supplementation relieved symptoms of both IBS and depression in patients diagnosed with IBS and MDD. This clinical recovery is accompanied by a decrease in serum myeloperoxidase, an inflammatory marker (Majeed et al., 2018). However, patients might have experienced less depression as a result of reduced IBS symptoms. Interestingly, B. coagulans intake increased levels of circulating IL-10, fecal F. prausnitzii and SCFAs in older adults (Nyangale et al., 2014, 2015). As F. prausnitzii and butyrate are associated with antidepressive properties (Hao et al., 2019), B. coagulans may also indirectly reduce depression and improve gut health.
Bifidobacterium bifidum and L. acidophilus were often included in the formulation of MSPs to treat depressive symptoms in patients with MDD (Akkasheh et al., 2016; Bambling et al., 2017; Ghorbani et al., 2018) and other health conditions, such as polycystic ovarian syndrome, multiple sclerosis and IBS (Kouchaki et al., 2017; Ostadmohammadi et al., 2019; Zhang et al., 2019). Surprisingly, B. bifidum and L. acidophilus have not been tested independently for its antidepressive effect. B. bifidum intake improved mood and reduced symptoms of abdominal pain, diarrhea and constipation in patients with gastrointestinal disorders (Urita et al., 2015). However, the mood elevation could be due to recovery of gastrointestinal symptoms rather than effect of probiotics solely. Both in vitro and in vivo models showed that L. acidophilus protects the intestinal barrier integrity by preventing pathogen adherence and release of proinflammatory cytokines (Chen et al., 2009; Justino et al., 2015; Alamdary et al., 2018; Lepine et al., 2018; Najarian et al., 2019). Taken together, B. bifidum and L. acidophilus potentially exhibit antidepressive effect and their direct influence on depression warrants further investigation.
Bacteroides fragilis has been proposed as a potential probiotic, although its pathogenicity needs to be taken into consideration. B. fragilis secretes polysaccharide A and expresses sphingolipids that benefit the host gut health and immune system (Troy and Kasper, 2010; Tan et al., 2019). Bacteroides genus is likely to be the largest GABA producer amongst human gut microbiota, with B. fragilis produces GABA at low pH. The same study also found that neural patterns of a typical MDD patient correlated with low fecal levels of Bacteroides (Strandwitz et al., 2019). Hence, antidepressive potential of B. pseudocatenulatum, B. coagulans, B. bifidum, L. acidophilus, and B. fragilis warrants further investigation. It is also worth noting that Bifidobacterium adolescentis’s antidepressive capability may be a new probiotic candidate (Jang et al., 2019). Evidently, an increasing number of probiotics are being presented as a potential treatment for depression. This provides a wide repository of available probiotics, with different species combinations, that can be assessed for clinical efficacy against depression.
Conclusion
The MGB axis enables the bidirectional communication between the gut microbiota and the brain. When this axis becomes maladaptive, the host physiology is adversely affected which may lead to the development of depression. Probiotics have shown clinical efficacy in the treatment of depression by modulating the MGB axis. Yet, the complexity of gut microbiota and heterogeneity of depression presents a challenge to explain the underlying mechanisms that contribute to this clinical efficacy. Nonetheless, cumulative evidence suggests the therapeutic potential of probiotics for certain depression subtypes, with fewer side effects and less stigma compared to standard antidepressants.
Limitations of this review include: (1) inferences of probiotic mechanisms were derived from preclinical and in vitro data; (2) interactions of probiotics with other members of gut microbiota were unexplored, therefore the mechanisms of MSPs was unable to be explored; (3) strain-specific effects of bacterial species were neglected; (4) potential applications for probiotics for depression subtypes are hypothesized, however, clinical evidence is limited; (5) effect sizes of probiotics as antidepressants was not evaluated. Notwithstanding these caveats, this review adds further understanding to the potential antidepressive effects and therapeutic potentials of probiotics. Venema (2017) stated that it is imperative to grasp the underlying molecular mechanisms of the MGB axis, and which microbial populations are pertinent for this intervention, to advance the marketability of probiotics.
Author Contributions
SY wrote and edited the manuscript. TT, JC, and WL conceptualized and edited the manuscript. All authors contributed to the intellectual input and critical revision of the manuscript.
Funding
This work was supported by the Sunway University Research Internal Grants (INT-2018-SST-DBS-05).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The authors would like to thank Tong Xen Leong and Bahaa Abdella for providing their initial input to the work.
Abbreviations
5-HIAA, 5-hydroxyindoleacetic acid; 5-HT, 5-hydroxytryptamine (serotonin); 5-HTP, 5-hydroxytryptamine; BBB, blood-brain barrier; BDNF, brain-derived neurotropic factor; CgA, salivary chromogranin A; CORT, corticosterone; CREB, cAMP response element binding protein; CRP, C-reactive protein; CUMS, chronic unpredictable mild stress; DA, dopamine; DC, dihydroxyphenylacetic acid; EIF2, eukaryotic initiation factor 2; GABA, gamma-aminobutyric acid; GLP-1, glucagon-like peptide-1; GPx, glutathione peroxidase; GR, glucocorticoid; H2O2, hydrogen peroxide; HPC, hippocampus; HVA, homovanillic acid; IBS, irritable bowel syndrome; IDO, indolamine 2,3-dioxyhydrogenase; IFN, interferon; IgA, immunoglobin A; IL, interleukin; KA, kynurenic acid; KYN, kynurenine; LPS, lipopolysaccharides; MAOA, monoamine oxygenase A; MCP-1, monocyte chemotactic protein-1; MDD, major depressive disorder; MR, mineralocorticoid; MS, maternal separation model; NE, norepinephrine; PFC, prefrontal cortex; PGE2, prostaglandin E2; REM, rapid eye movement; SCFA, short-chain fatty acids; SNRI, serotonin-noradrenaline reuptake inhibitor; SOD, superoxide dismutase; SSRI, selective serotonin reuptake inhibitor; TLR, toll-like receptor; TNF-α, tumor necrosis factor-α; Tph1, tryptophan hydroxylase 1; TRANCE, TNF-related activation-induced cytokine; TRP, tryptophan.
References
Abildgaard, A., Kern, T., Pedersen, O., Hansen, T., Wegener, G., and Lund, S. (2019). The antidepressant-like effect of probiotics and their faecal abundance may be modulated by the cohabiting gut microbiota in rats. Eur. Neuropsychopharmacol. 29, 98–110. doi: 10.1016/j.euroneuro.2018.10.011
Agusti, A., Moya-Perez, A., Campillo, I., Montserrat-de la Paz, S., Cerrudo, V., Perez-Villalba, A., et al. (2018). Bifidobacterium pseudocatenulatum CECT 7765 ameliorates neuroendocrine alterations associated with an exaggerated stress response and anhedonia in obese mice. Mol. Neurobiol. 55, 5337–5352. doi: 10.1007/s12035-017-0768-z
Aizawa, E., Tsuji, H., Asahara, T., Takahashi, T., Teraishi, T., Yoshida, S., et al. (2016). Possible association of Bifidobacterium and Lactobacillus in the gut microbiota of patients with major depressive disorder. J. Affect. Disord. 202, 254–257. doi: 10.1016/j.jad.2016.05.038
Akkasheh, G., Kashani-Poor, Z., Tajabadi-Ebrahimi, M., Jafari, P., Akbari, H., Taghizadeh, M., et al. (2016). Clinical and metabolic response to probiotic administration in patients with major depressive disorder: a randomized, double-blind, placebo-controlled trial. Nutrition 32, 315–320. doi: 10.1016/j.nut.2015.09.003
Alamdary, S. Z., Bakhshi, B., and Soudi, S. (2018). The anti-apoptotic and anti-inflammatory effect of Lactobacillus acidophilus on Shigella sonnei and Vibrio cholerae interaction with intestinal epithelial cells: a comparison between invasive and non-invasive bacteria. PLoS One 13:e0196941. doi: 10.1371/journal.pone.0196941
Allen, A. P., Hutch, W., Borre, Y. E., Kennedy, P. J., Temko, A., Boylan, G., et al. (2016). Bifidobacterium longum 1714 as a translational psychobiotic: modulation of stress, electrophysiology and neurocognition in healthy volunteers. Transl. Psychiatry 6:e939. doi: 10.1038/tp.2016.191
Almeida, A., Mitchell, A. L., Boland, M., Forster, S. C., Gloor, G. B., Tarkowska, A., et al. (2019). A new genomic blueprint of the human gut microbiota. Nature 568, 499–504. doi: 10.1038/s41586-019-0965-1
Alonso, C., Guilarte, M., Vicario, M., Ramos, L., Rezzi, S., Martinez, C., et al. (2012). Acute experimental stress evokes a differential gender-determined increase in human intestinal macromolecular permeability. Neurogastroenterol. Motil. 24, 740–746, e348–e349. doi: 10.1111/j.1365-2982.2012.01928.x
American Psychiatric Association, (2013). Diagnostic and Statistical Manual of Mental Disorders, the Edition: DSM-5. Washington, DC: American Psychiatric Publishing.
Anderberg, R. H., Richard, J. E., Hansson, C., Nissbrandt, H., Bergquist, F., and Skibicka, K. P. (2016). GLP-1 is both anxiogenic and antidepressant; divergent effects of acute and chronic GLP-1 on emotionality. Psychoneuroendocrinology 65, 54–66. doi: 10.1016/j.psyneuen.2015.11.021
Araki, Y., Andoh, A., Fujiyama, Y., Takizawa, J., Takizawa, W., and Bamba, T. (2002). Oral administration of a product derived from Clostridium butyricum in rats. Int. J. Mol. Med. 9, 53–57.
Asano, Y., Hiramoto, T., Nishino, R., Aiba, Y., Kimura, T., Yoshihara, K., et al. (2012). Critical role of gut microbiota in the production of biologically active, free catecholamines in the gut lumen of mice. Am. J. Physiol. Gastrointest. Liver Physiol. 303, G1288–G1295. doi: 10.1152/ajpgi.00341.2012
Bailey, M. T., Dowd, S. E., Galley, J. D., Hufnagle, A. R., Allen, R. G., and Lyte, M. (2011). Exposure to a social stressor alters the structure of the intestinal microbiota: implications for stressor-induced immunomodulation. Brain Behav. Immun. 25, 397–407. doi: 10.1016/j.bbi.2010.10.023
Bambling, M., Edwards, S. C., Hall, S., and Vitetta, L. (2017). A combination of probiotics and magnesium orotate attenuate depression in a small SSRI resistant cohort: an intestinal anti-inflammatory response is suggested. Inflammopharmacology 25, 271–274. doi: 10.1007/s10787-017-0311-x
Baranyi, A., Meinitzer, A., Stepan, A., Putz-Bankuti, C., Breitenecker, R. J., Stauber, R., et al. (2013). A biopsychosocial model of interferon-alpha-induced depression in patients with chronic hepatitis C infection. Psychother. Psychosom. 82, 332–340. doi: 10.1159/000348587
Barrett, E., Ross, R. P., O’Toole, P. W., Fitzgerald, G. F., and Stanton, C. (2012). gamma-Aminobutyric acid production by culturable bacteria from the human intestine. J. Appl. Microbiol. 113, 411–417. doi: 10.1111/j.1365-2672.2012.05344.x
Benton, D., Williams, C., and Brown, A. (2007). Impact of consuming a milk drink containing a probiotic on mood and cognition. Eur. J. Clin. Nutr. 61, 355–361. doi: 10.1038/sj.ejcn.1602546
Bercik, P., Park, A. J., Sinclair, D., Khoshdel, A., Lu, J., Huang, X., et al. (2011). The anxiolytic effect of Bifidobacterium longum NCC3001 involves vagal pathways for gut-brain communication. Neurogastroenterol. Motil. 23, 1132–1139. doi: 10.1111/j.1365-2982.2011.01796.x
Bercik, P., Verdu, E. F., Foster, J. A., Macri, J., Potter, M., Huang, X., et al. (2010). Chronic gastrointestinal inflammation induces anxiety-like behavior and alters central nervous system biochemistry in mice. Gastroenterology 139, 2102.e1–2112.e1. doi: 10.1053/j.gastro.2010.06.063
Bertani, L., Gambaccini, D., Pancetti, A., Guglielmetti, S., Mastorci, F., Gemignani, A., et al. (2017). Lactobacillus Casei DG® in patients with irritable bowel syndrome: not only a change of the gut microbiota. A Pilot Study. Gastroenterology 152(Suppl. 1):S819. doi: 10.1016/s0016-5085(17)32830-5
Bhagwagar, Z., Wylezinska, M., Jezzard, P., Evans, J., Boorman, E., Matthews, P., et al. (2008). Low GABA concentrations in occipital cortex and anterior cingulate cortex in medication-free, recovered depressed patients. Int. J. Neuropsychopharmacol. 11, 255–260. doi: 10.1017/S1461145707007924
Bharwani, A., Mian, M. F., Surette, M. G., Bienenstock, J., and Forsythe, P. (2017). Oral treatment with Lactobacillus rhamnosus attenuates behavioural deficits and immune changes in chronic social stress. BMC Med. 15:7. doi: 10.1186/s12916-016-0771-7
Birdsall, T. C. (1998). 5-Hydroxytryptophan: a clinically-effective serotonin precursor. Altern. Med. Rev. 3, 271–280.
Bjorkholm, C., and Monteggia, L. M. (2016). BDNF - a key transducer of antidepressant effects. Neuropharmacology 102, 72–79. doi: 10.1016/j.neuropharm.2015.10.034
Bordin, M., D’Atri, F., Guillemot, L., and Citi, S. (2004). Histone deacetylase inhibitors up-regulate the expression of tight junction proteins. Mol. Cancer Res. 2, 692–701.
Botta, C., Acquadro, A., Greppi, A., Barchi, L., Bertolino, M., Cocolin, L., et al. (2017). Genomic assessment in Lactobacillus plantarum links the butyrogenic pathway with glutamine metabolism. Sci. Rep. 7:15975. doi: 10.1038/s41598-017-16186-8
Bravo, J. A., Forsythe, P., Chew, M. V., Escaravage, E., Savignac, H. M., Dinan, T. G., et al. (2011). Ingestion of Lactobacillus strain regulates emotional behavior and central GABA receptor expression in a mouse via the vagus nerve. Proc. Natl. Acad. Sci. U.S.A. 108, 16050–16055. doi: 10.1073/pnas.1102999108
Breit, S., Kupferberg, A., Rogler, G., and Hasler, G. (2018). Vagus nerve as modulator of the brain-gut axis in psychiatric and inflammatory disorders. Front. Psychiatry 9:44. doi: 10.3389/fpsyt.2018.00044
Brust, P., Friedrich, A., Krizbai, I. A., Bergmann, R., Roux, F., Ganapathy, V., et al. (2000). Functional expression of the serotonin transporter in immortalized rat brain microvessel endothelial cells. J. Neurochem. 74, 1241–1248. doi: 10.1046/j.1471-4159.2000.741241.x
Buhner, S., Hahne, H., Hartwig, K., Li, Q., Vignali, S., Ostertag, D., et al. (2018). Protease signaling through protease activated receptor 1 mediate nerve activation by mucosal supernatants from irritable bowel syndrome but not from ulcerative colitis patients. PLoS One 13:e0193943. doi: 10.1371/journal.pone.0193943
Butts, K. A., and Phillips, A. G. (2013). Glucocorticoid receptors in the prefrontal cortex regulate dopamine efflux to stress via descending glutamatergic feedback to the ventral tegmental area. Int. J. Neuropsychopharmacol. 16, 1799–1807. doi: 10.1017/S1461145713000187
Byun, J. I., Shin, Y. Y., Chung, S. E., and Shin, W. C. (2018). Safety and efficacy of gamma-aminobutyric acid from fermented rice germ in patients with insomnia symptoms: a randomized, double-blind trial. J. Clin. Neurol. 14, 291–295. doi: 10.3988/jcn.2018.14.3.291
Calarge, C. A., Devaraj, S., and Shulman, R. J. (2019). Gut permeability and depressive symptom severity in unmedicated adolescents. J. Affect. Disord. 246, 586–594. doi: 10.1016/j.jad.2018.12.077
Cano, P. G., Santacruz, A., Trejo, F. M., and Sanz, Y. (2013). Bifidobacterium CECT 7765 improves metabolic and immunological alterations associated with obesity in high-fat diet-fed mice. Obesity 21, 2310–2321. doi: 10.1002/oby.20330
Carhart-Harris, R. L., and Nutt, D. J. (2017). Serotonin and brain function: a tale of two receptors. J. Psychopharmacol. 31, 1091–1120. doi: 10.1177/0269881117725915
Cassir, N., Benamar, S., and La Scola, B. (2016). Clostridium butyricum: from beneficial to a new emerging pathogen. Clin. Microbiol. Infect. 22, 37–45. doi: 10.1016/j.cmi.2015.10.014
Castaldelli-Maia, J. M., Scomparini, L. B., Andrade, A. G., Bhugra, D., de Toledo Ferraz Alves, T. C., and D’Elia, G. (2011). Perceptions of and attitudes toward antidepressants: stigma attached to their use–a review. J. Nerv. Ment. Dis. 199, 866–871. doi: 10.1097/NMD.0b013e3182388950
Chapman, C. M., Gibson, G. R., and Rowland, I. (2011). Health benefits of probiotics: are mixtures more effective than single strains? Eur. J. Nutr. 50, 1–17. doi: 10.1007/s00394-010-0166-z
Chapman, C. M., Gibson, G. R., and Rowland, I. (2012). In vitro evaluation of single- and multi-strain probiotics: inter-species inhibition between probiotic strains, and inhibition of pathogens. Anaerobe 18, 405–413. doi: 10.1016/j.anaerobe.2012.05.004
Chen, C. C., Chiu, C. H., Lin, T. Y., Shi, H. N., and Walker, W. A. (2009). Effect of probiotics Lactobacillus acidophilus on Citrobacter rodentium colitis: the role of dendritic cells. Pediatr. Res. 65, 169–175. doi: 10.1203/PDR.0b013e31818d5a06
Chunchai, T., Thunapong, W., Yasom, S., Wanchai, K., Eaimworawuthikul, S., Metzler, G., et al. (2018). Decreased microglial activation through gut-brain axis by prebiotics, probiotics, or synbiotics effectively restored cognitive function in obese-insulin resistant rats. J. Neuroinflamm. 15:11. doi: 10.1186/s12974-018-1055-2
Chung, Y.-C., Jin, H.-M., Cui, Y., Kim, D. S., Jung, J. M., Park, J.-I., et al. (2014). Fermented milk of Lactobacillus helveticus IDCC3801 improves cognitive functioning during cognitive fatigue tests in healthy older adults. J. Funct. Foods 10, 465–474. doi: 10.1016/j.jff.2014.07.007
Clarke, G., Grenham, S., Scully, P., Fitzgerald, P., Moloney, R. D., Shanahan, F., et al. (2013). The microbiome-gut-brain axis during early life regulates the hippocampal serotonergic system in a sex-dependent manner. Mol. Psychiatry 18, 666–673. doi: 10.1038/mp.2012.77
Corpuz, H. M., Ichikawa, S., Arimura, M., Mihara, T., Kumagai, T., Mitani, T., et al. (2018). Long-term diet supplementation with Lactobacillus paracasei K71 prevents age-related cognitive decline in senescence-accelerated mouse prone 8. Nutrients 10:E762. doi: 10.3390/nu10060762
Cremon, C., Guglielmetti, S., Gargari, G., Taverniti, V., Castellazzi, A. M., Valsecchi, C., et al. (2018). Effect of Lactobacillus paracasei CNCM I-1572 on symptoms, gut microbiota, short chain fatty acids, and immune activation in patients with irritable bowel syndrome: a pilot randomized clinical trial. United Eur. Gastroenterol. J. 6, 604–613. doi: 10.1177/2050640617736478
Cullinan, W. E., Ziegler, D. R., and Herman, J. P. (2008). Functional role of local GABAergic influences on the HPA axis. Brain Struct. Funct. 213, 63–72. doi: 10.1007/s00429-008-0192-2
de Punder, K., and Pruimboom, L. (2015). Stress induces endotoxemia and low-grade inflammation by increasing barrier permeability. Front. Immunol. 6:223. doi: 10.3389/fimmu.2015.00223
Dedic, N., Walser, S. M., and Deussing, J. M. (2011). “Mouse models of depression,” in Psychiatric Disorders - Trends and Developments, ed. T. Uehara (London: InTech).
Desbonnet, L., Garrett, L., Clarke, G., Bienenstock, J., and Dinan, T. G. (2008). The probiotic Bifidobacteria infantis: an assessment of potential antidepressant properties in the rat. J. Psychiatr. Res. 43, 164–174. doi: 10.1016/j.jpsychires.2008.03.009
Desbonnet, L., Garrett, L., Clarke, G., Kiely, B., Cryan, J. F., and Dinan, T. G. (2010). Effects of the probiotic Bifidobacterium infantis in the maternal separation model of depression. Neuroscience 170, 1179–1188. doi: 10.1016/j.neuroscience.2010.08.005
Dhaliwal, J., Singh, D. P., Singh, S., Pinnaka, A. K., Boparai, R. K., Bishnoi, M., et al. (2018). Lactobacillus plantarum MTCC 9510 supplementation protects from chronic unpredictable and sleep deprivation-induced behaviour, biochemical and selected gut microbial aberrations in mice. J. Appl. Microbiol. 125, 257–269. doi: 10.1111/jam.13765
Di Cerbo, A., and Palmieri, B. (2015). Review: the market of probiotics. Pak. J. Pharm. Sci. 28, 2199–2206.
Diaz Heijtz, R., Wang, S., Anuar, F., Qian, Y., Bjorkholm, B., Samuelsson, A., et al. (2011). Normal gut microbiota modulates brain development and behavior. Proc. Natl. Acad. Sci. U.S.A. 108, 3047–3052. doi: 10.1073/pnas.1010529108
Didari, T., Solki, S., Mozaffari, S., Nikfar, S., and Abdollahi, M. (2014). A systematic review of the safety of probiotics. Expert Opin. Drug Saf. 13, 227–239. doi: 10.1517/14740338.2014.872627
Dowlati, Y., Herrmann, N., Swardfager, W., Liu, H., Sham, L., Reim, E. K., et al. (2010). A meta-analysis of cytokines in major depression. Biol. Psychiatry 67, 446–457. doi: 10.1016/j.biopsych.2009.09.033
Duncan, S. H. (2002). Growth requirements and fermentation products of Fusobacterium prausnitzii, and a proposal to reclassify it as Faecalibacterium prausnitzii gen. nov., comb. nov. Int. J. Syst. Evol. Microbiol. 52, 2141–2146. doi: 10.1099/ijs.0.02241-0
Dunlop, B. W., and Nemeroff, C. B. (2007). The role of dopamine in the pathophysiology of depression. Arch. Gen. Psychiatry 64, 327–337. doi: 10.1001/archpsyc.64.3.327
Durrant, A. R., and Heresco-Levy, U. (2014). D-Serine in neuropsychiatric disorders: new advances. Adv. Psychiatry 2014, 1–16. doi: 10.1155/2014/859735
Earnheart, J. C., Schweizer, C., Crestani, F., Iwasato, T., Itohara, S., Mohler, H., et al. (2007). GABAergic control of adult hippocampal neurogenesis in relation to behavior indicative of trait anxiety and depression states. J. Neurosci. 27, 3845–3854. doi: 10.1523/JNEUROSCI.3609-06.2007
Elce, A., Amato, F., Zarrilli, F., Calignano, A., Troncone, R., Castaldo, G., et al. (2017). Butyrate modulating effects on pro-inflammatory pathways in human intestinal epithelial cells. Benef. Microbes 8, 841–847. doi: 10.3920/BM2016.0197
El-Merahbi, R., Loffler, M., Mayer, A., and Sumara, G. (2015). The roles of peripheral serotonin in metabolic homeostasis. FEBS Lett. 589, 1728–1734. doi: 10.1016/j.febslet.2015.05.054
Eutamene, H., Lamine, F., Chabo, C., Theodorou, V., Rochat, F., Bergonzelli, G. E., et al. (2007). Synergy between Lactobacillus paracasei and its bacterial products to counteract stress-induced gut permeability and sensitivity increase in rats. J. Nutr. 137, 1901–1907. doi: 10.1093/jn/137.8.1901
Evans, S. J., Bassis, C. M., Hein, R., Assari, S., Flowers, S. A., Kelly, M. B., et al. (2017). The gut microbiome composition associates with bipolar disorder and illness severity. J. Psychiatr. Res. 87, 23–29. doi: 10.1016/j.jpsychires.2016.12.007
Ewaschuk, J. B., Diaz, H., Meddings, L., Diederichs, B., Dmytrash, A., Backer, J., et al. (2008). Secreted bioactive factors from Bifidobacterium infantis enhance epithelial cell barrier function. Am. J. Physiol. Gastrointest. Liver Physiol. 295, G1025–G1034. doi: 10.1152/ajpgi.90227.2008
Falcinelli, S., Rodiles, A., Hatef, A., Picchietti, S., Cossignani, L., Merrifield, D. L., et al. (2017). Dietary lipid content reorganizes gut microbiota and probiotic L. rhamnosus attenuates obesity and enhances catabolic hormonal milieu in zebrafish. Sci. Rep. 7:5512. doi: 10.1038/s41598-017-05147-w
Falcinelli, S., Rodiles, A., Unniappan, S., Picchietti, S., Gioacchini, G., Merrifield, D. L., et al. (2016). Probiotic treatment reduces appetite and glucose level in the zebrafish model. Sci. Rep. 6:18061. doi: 10.1038/srep18061
Farabaugh, A. H., Mischoulon, D., Fava, M., Green, C., Guyker, W., and Alpert, J. (2004). The potential relationship between levels of perceived stress and subtypes of major depressive disorder (MDD). Acta Psychiatr. Scand. 110, 465–470. doi: 10.1111/j.1600-0447.2004.00377.x
Feenstra, M. G., Kalsbeek, A., and van Galen, H. (1992). Neonatal lesions of the ventral tegmental area affect monoaminergic responses to stress in the medial prefrontal cortex and other dopamine projection areas in adulthood. Brain Res. 596, 169–182. doi: 10.1016/0006-8993(92)91545-p
Freestone, P. (2013). Communication between bacteria and their hosts. Scientifica 2013, 361073. doi: 10.1155/2013/361073
Freewan, M., Rees, M. D., Plaza, T. S., Glaros, E., Lim, Y. J., Wang, X. S., et al. (2013). Human indoleamine 2,3-dioxygenase is a catalyst of physiological heme peroxidase reactions: implications for the inhibition of dioxygenase activity by hydrogen peroxide. J. Biol. Chem. 288, 1548–1567. doi: 10.1074/jbc.M112.410993
Furness, J. B. (2012). The enteric nervous system and neurogastroenterology. Nat. Rev. Gastroenterol. Hepatol. 9, 286–294. doi: 10.1038/nrgastro.2012.32
Gabbay, V., Klein, R. G., Katz, Y., Mendoza, S., Guttman, L. E., Alonso, C. M., et al. (2010). The possible role of the kynurenine pathway in adolescent depression with melancholic features. J. Child Psychol. Psychiatry 51, 935–943. doi: 10.1111/j.1469-7610.2010.02245.x
Ganesh, B. P., Hall, A., Ayyaswamy, S., Nelson, J. W., Fultz, R., Major, A., et al. (2018). Diacylglycerol kinase synthesized by commensal Lactobacillus reuteri diminishes protein kinase C phosphorylation and histamine-mediated signaling in the mammalian intestinal epithelium. Mucosal Immunol. 11, 380–393. doi: 10.1038/mi.2017.58
Gao, C., Major, A., Rendon, D., Lugo, M., Jackson, V., Shi, Z., et al. (2015). Histamine H2 receptor-mediated suppression of intestinal inflammation by probiotic Lactobacillus reuteri. mBio 6:e01358-15. doi: 10.1128/mBio.01358-15
Gao, X., Cao, Q., Cheng, Y., Zhao, D., Wang, Z., Yang, H., et al. (2018). Chronic stress promotes colitis by disturbing the gut microbiota and triggering immune system response. Proc. Natl. Acad. Sci. U.S.A. 115, E2960–E2969. doi: 10.1073/pnas.1720696115
Ghorbani, Z., Nazari, S., Etesam, F., Nourimajd, S., Ahmadpanah, M., and Razeghi Jahromi, S. (2018). The effect of synbiotic as an adjuvant therapy to fluoxetine in moderate depression: a randomized multicenter trial. Arch. Neurosci. 5:e60507. doi: 10.5812/archneurosci.60507
Girvin, G. T., and Stevenson, J. W. (1954). Cell free choline acetylase from Lactobacillus plantarum. Can. J. Biochem. Physiol. 32, 131–146. doi: 10.1139/y54-015
Godlewska, B. R., Emir, U. E., Masaki, C., Bargiotas, T., and Cowen, P. J. (2019). Changes in brain Glx in depressed bipolar patients treated with lamotrigine: a proton MRS study. J. Affect. Disord. 246, 418–421. doi: 10.1016/j.jad.2018.12.092
Gómez-Hurtado, I., Zapater, P., Peiró, G., González-Navajas, J. M., Pérez-Mateo, M., Such, J., et al. (2012). Oral administration of B. infantis favors a reduction in mesenteric lymph node bacterial DNA translocation episodes in mice with carbon tetrachloride-induced cirrhosis. J. Hepatol. 56(Suppl. 2), S229. doi: 10.1016/s0168-8278(12)60589-3
Goncalves, P., Araujo, J. R., and Martel, F. (2011). Characterization of butyrate uptake by nontransformed intestinal epithelial cell lines. J. Membr. Biol. 240, 35–46. doi: 10.1007/s00232-011-9340-3
Gonul, A. S., Kitis, O., Ozan, E., Akdeniz, F., Eker, C., Eker, O. D., et al. (2006). The effect of antidepressant treatment on N-acetyl aspartate levels of medial frontal cortex in drug-free depressed patients. Prog. Neuropsychopharmacol. Biol. Psychiatry 30, 120–125. doi: 10.1016/j.pnpbp.2005.08.017
Gottesmann, C. (2002). GABA mechanisms and sleep. Neuroscience 111, 231–239. doi: 10.1016/s0306-4522(02)00034-9
Grigoleit, J. S., Kullmann, J. S., Wolf, O. T., Hammes, F., Wegner, A., Jablonowski, S., et al. (2011). Dose-dependent effects of endotoxin on neurobehavioral functions in humans. PLoS One 6:e28330. doi: 10.1371/journal.pone.0028330
Groeger, D., O’Mahony, L., Murphy, E. F., Bourke, J. F., Dinan, T. G., Kiely, B., et al. (2013). Bifidobacterium infantis 35624 modulates host inflammatory processes beyond the gut. Gut Microbes 4, 325–339. doi: 10.4161/gmic.25487
Grunewald, M., Johnson, S., Lu, D., Wang, Z., Lomberk, G., Albert, P. R., et al. (2012). Mechanistic role for a novel glucocorticoid-KLF11 (TIEG2) protein pathway in stress-induced monoamine oxidase A expression. J. Biol. Chem. 287, 24195–24206. doi: 10.1074/jbc.M112.373936
Gutierrez, A., Zapater, P., Juanola, O., Sempere, L., Garcia, M., Laveda, R., et al. (2016). Gut bacterial DNA translocation is an independent risk factor of flare at short term in patients With Crohn’s disease. Am. J. Gastroenterol. 111, 529–540. doi: 10.1038/ajg.2016.8
Han, A., Sung, Y. B., Chung, S. Y., and Kwon, M. S. (2014). Possible additional antidepressant-like mechanism of sodium butyrate: targeting the hippocampus. Neuropharmacology 81, 292–302. doi: 10.1016/j.neuropharm.2014.02.017
Han, S. H., Hong, K. B., and Suh, H. J. (2017). Biotransformation of monosodium glutamate to gamma-aminobutyric acid by isolated strain Lactobacillus brevis L-32 for potentiation of pentobarbital-induced sleep in mice. Food Biotechnol. 31, 80–93. doi: 10.1080/08905436.2017.1301821
Hao, Z., Wang, W., Guo, R., and Liu, H. (2019). Faecalibacterium prausnitzii (ATCC 27766) has preventive and therapeutic effects on chronic unpredictable mild stress-induced depression-like and anxiety-like behavior in rats. Psychoneuroendocrinology 104, 132–142. doi: 10.1016/j.psyneuen.2019.02.025
Harald, B., and Gordon, P. (2012). Meta-review of depressive subtyping models. J. Affect. Disord. 139, 126–140. doi: 10.1016/j.jad.2011.07.015
Hasler, G., van der Veen, J. W., Tumonis, T., Meyers, N., Shen, J., and Drevets, W. C. (2007). Reduced prefrontal glutamate/glutamine and gamma-aminobutyric acid levels in major depression determined using proton magnetic resonance spectroscopy. Arch. Gen. Psychiatry 64, 193–200. doi: 10.1001/archpsyc.64.2.193
Hasler, W. L. (2009). Serotonin and the GI tract. Curr. Gastroenterol. Rep. 11, 383–391. doi: 10.1007/s11894-009-0058-7
Hata, T., Asano, Y., Yoshihara, K., Kimura-Todani, T., Miyata, N., Zhang, X. T., et al. (2017). Regulation of gut luminal serotonin by commensal microbiota in mice. PLoS One 12:e0180745. doi: 10.1371/journal.pone.0180745
He, G. Q., Kong, Q., Chen, Q. H., and Ruan, H. (2005). Batch and fed-batch production of butyric acid by clostridium butyricum ZJUCB. J. Zhejiang Univ. Sci. B 6, 1076–1080. doi: 10.1631/jzus.2005.B1076
Hemarajata, P., Gao, C., Pflughoeft, K. J., Thomas, C. M., Saulnier, D. M., Spinler, J. K., et al. (2013). Lactobacillus reuteri-specific immunoregulatory gene rsiR modulates histamine production and immunomodulation by Lactobacillus reuteri. J. Bacteriol. 195, 5567–5576. doi: 10.1128/JB.00261-13
Hewitt, S. A., Wamsteeker, J. I., Kurz, E. U., and Bains, J. S. (2009). Altered chloride homeostasis removes synaptic inhibitory constraint of the stress axis. Nat. Neurosci. 12, 438–443. doi: 10.1038/nn.2274
Hillsley, K., and Grundy, D. (1998). Serotonin and cholecystokinin activate different populations of rat mesenteric vagal afferents. Neurosci. Lett. 255, 63–66. doi: 10.1016/s0304-3940(98)00690-9
Hoermannsperger, G., Clavel, T., Hoffmann, M., Reiff, C., Kelly, D., Loh, G., et al. (2009). Post-translational inhibition of IP-10 secretion in IEC by probiotic bacteria: impact on chronic inflammation. PLoS One 4:e4365. doi: 10.1371/journal.pone.0004365
Hold, G. L., Schwiertz, A., Aminov, R. I., Blaut, M., and Flint, H. J. (2003). Oligonucleotide probes that detect quantitatively significant groups of butyrate-producing bacteria in human feces. Appl. Environ. Microbiol. 69, 4320–4324. doi: 10.1128/aem.69.7.4320-4324.2003
Howren, M. B., Lamkin, D. M., and Suls, J. (2009). Associations of depression with C-reactive protein, IL-1, and IL-6: a meta-analysis. Psychosom. Med. 71, 171–186. doi: 10.1097/PSY.0b013e3181907c1b
Huang, R., Wang, K., and Hu, J. (2016). Effect of probiotics on depression: a systematic review and meta-analysis of randomized controlled trials. Nutrients 8:E483. doi: 10.3390/nu8080483
Huang, S. Y., Chen, L. H., Wang, M. F., Hsu, C. C., Chan, C. H., Li, J. X., et al. (2018). Lactobacillus paracasei PS23 delays progression of age-related cognitive decline in senescence accelerated mouse prone 8 (SAMP8) mice. Nutrients 10:894. doi: 10.3390/nu10070894
Hughes, E. R., Winter, M. G., Duerkop, B. A., Spiga, L., Furtado de Carvalho, T., Zhu, W., et al. (2017). Microbial respiration and formate oxidation as metabolic signatures of inflammation-associated dysbiosis. Cell Host Microbe 21, 208–219. doi: 10.1016/j.chom.2017.01.005
Hyland, N. P., and Cryan, J. F. (2010). A gut feeling about GABA: focus on GABA(B) receptors. Front. Pharmacol. 1:124. doi: 10.3389/fphar.2010.00124
Ionescu, D. F., Niciu, M. J., Richards, E. M., and Zarate, C. A. Jr. (2014). Pharmacologic treatment of dimensional anxious depression: a review. Prim Care Companion CNS Disord. 16:CC.13r01621. doi: 10.4088/PCC.13r01621
Ivanov, D., Emonet, C., Foata, F., Affolter, M., Delley, M., Fisseha, M., et al. (2006). A serpin from the gut bacterium Bifidobacterium longum inhibits eukaryotic elastase-like serine proteases. J. Biol. Chem. 281, 17246–17252. doi: 10.1074/jbc.M601678200
Jacobsen, J. P., Rudder, M. L., Roberts, W., Royer, E. L., Robinson, T. J., Oh, A., et al. (2016). SSRI augmentation by 5-Hydroxytryptophan slow release: mouse pharmacodynamic proof of concept. Neuropsychopharmacology 41, 2324–2334. doi: 10.1038/npp.2016.35
Jamilian, M., Mansury, S., Bahmani, F., Heidar, Z., Amirani, E., and Asemi, Z. (2018). The effects of probiotic and selenium co-supplementation on parameters of mental health, hormonal profiles, and biomarkers of inflammation and oxidative stress in women with polycystic ovary syndrome. J. Ovarian Res. 11:80. doi: 10.1186/s13048-018-0457-1
Jang, H. M., Lee, K. E., and Kim, D. H. (2019). The preventive and curative effects of Lactobacillus reuteri NK33 and Bifidobacterium adolescentis NK98 on immobilization stress-induced anxiety/depression and colitis in mice. Nutrients 11:E819. doi: 10.3390/nu11040819
Jangid, P., Malik, P., Singh, P., Sharma, M., and Gulia, A. K. (2013). Comparative study of efficacy of l-5-hydroxytryptophan and fluoxetine in patients presenting with first depressive episode. Asian J. Psychiatr. 6, 29–34. doi: 10.1016/j.ajp.2012.05.011
Janik, R., Thomason, L. A. M., Stanisz, A. M., Forsythe, P., Bienenstock, J., and Stanisz, G. J. (2016). Magnetic resonance spectroscopy reveals oral Lactobacillus promotion of increases in brain GABA, N-acetyl aspartate and glutamate. Neuroimage 125, 988–995. doi: 10.1016/j.neuroimage.2015.11.018
Javed, N. H., Alsahly, M. B., and Khubchandani, J. (2016). Oral feeding of probiotic bifidobacterium infantis: colonic morphological changes in rat model of TNBS-Induced Colitis. Scientifica 2016, 9572596. doi: 10.1155/2016/9572596
Jeong, D., Kim, D.-H., Kang, I.-B., Kim, H., Song, K.-Y., Kim, H.-S., et al. (2017a). Characterization and antibacterial activity of a novel exopolysaccharide produced by Lactobacillus kefiranofaciens DN1 isolated from kefir. Food Control 78, 436–442. doi: 10.1016/j.foodcont.2017.02.033
Jeong, D., Kim, D. H., Kang, I. B., Kim, H., Song, K. Y., Kim, H. S., et al. (2017b). Modulation of gut microbiota and increase in fecal water content in mice induced by administration of Lactobacillus kefiranofaciens DN1. Food Funct. 8, 680–686. doi: 10.1039/c6fo01559j
Jiang, H., Ling, Z., Zhang, Y., Mao, H., Ma, Z., Yin, Y., et al. (2015). Altered fecal microbiota composition in patients with major depressive disorder. Brain Behav. Immun. 48, 186–194. doi: 10.1016/j.bbi.2015.03.016
Joseph, J. M., and Law, C. (2019). Cross-species examination of single- and multi-strain probiotic treatment effects on neuropsychiatric outcomes. Neurosci. Biobehav. Rev. 99, 160–197. doi: 10.1016/j.neubiorev.2018.11.010
Julio-Pieper, M., O’Connor, R. M., Dinan, T. G., and Cryan, J. F. (2013). Regulation of the brain-gut axis by group III metabotropic glutamate receptors. Eur. J. Pharmacol. 698, 19–30. doi: 10.1016/j.ejphar.2012.10.027
Justino, P. F., Melo, L. F., Nogueira, A. F., Morais, C. M., Mendes, W. O., Franco, A. X., et al. (2015). Regulatory role of Lactobacillus acidophilus on inflammation and gastric dysmotility in intestinal mucositis induced by 5-fluorouracil in mice. Cancer Chemother. Pharmacol. 75, 559–567. doi: 10.1007/s00280-014-2663-x
Kano, M., Fukudo, S., Tashiro, A., Utsumi, A., Tamura, D., Itoh, M., et al. (2004). Decreased histamine H1 receptor binding in the brain of depressed patients. Eur. J. Neurosci. 20, 803–810. doi: 10.1111/j.1460-9568.2004.03540.x
Karl, J. P., Hatch, A. M., Arcidiacono, S. M., Pearce, S. C., Pantoja-Feliciano, I. G., Doherty, L. A., et al. (2018). Effects of psychological, environmental and physical stressors on the gut microbiota. Front. Microbiol. 9:2013. doi: 10.3389/fmicb.2018.02013
Kato-Kataoka, A., Nishida, K., Takada, M., Kawai, M., Kikuchi-Hayakawa, H., Suda, K., et al. (2016). Fermented milk containing lactobacillus casei strain shirota preserves the diversity of the gut microbiota and relieves abdominal dysfunction in healthy medical students exposed to academic stress. Appl. Environ. Microbiol. 82, 3649–3658. doi: 10.1128/AEM.04134-15
Kazemi, A., Noorbala, A. A., Azam, K., and Djafarian, K. (2019). Effect of prebiotic and probiotic supplementation on circulating pro-inflammatory cytokines and urinary cortisol levels in patients with major depressive disorder: a double-blind, placebo-controlled randomized clinical trial. J. Funct. Foods 52, 596–602. doi: 10.1016/j.jff.2018.11.041
Kelly, J. R., Allen, A. P., Temko, A., Hutch, W., Kennedy, P. J., Farid, N., et al. (2017). Lost in translation? The potential psychobiotic Lactobacillus rhamnosus (JB-1) fails to modulate stress or cognitive performance in healthy male subjects. Brain Behav. Immun. 61, 50–59. doi: 10.1016/j.bbi.2016.11.018
Kelly, J. R., Borre, Y., O’ Brien, C., Patterson, E., El Aidy, S., Deane, J., et al. (2016). Transferring the blues: depression-associated gut microbiota induces neurobehavioural changes in the rat. J. Psychiatr. Res. 82, 109–118. doi: 10.1016/j.jpsychires.2016.07.019
Khoshdel, A., Verdu, E. F., Kunze, W., McLean, P., Bergonzelli, G., and Huizinga, J. D. (2013). Bifidobacterium longum NCC3001 inhibits AH neuron excitability. Neurogastroenterol. Motil. 25, e478–e484. doi: 10.1111/nmo.12147
Kiliaan, A. J., Saunders, P. R., Bijlsma, P. B., Berin, M. C., Taminiau, J. A., Groot, J. A., et al. (1998). Stress stimulates transepithelial macromolecular uptake in rat jejunum. Am. J. Physiol. 275(5 Pt 1), G1037–G1044. doi: 10.1152/ajpgi.1998.275.5.G1037
Ko, C. Y., Lin, H.-T. V., and Tsai, G. J. (2013). Gamma-aminobutyric acid production in black soybean milk by Lactobacillus brevis FPA 3709 and the antidepressant effect of the fermented product on a forced swimming rat model. Process Biochem. 48, 559–568. doi: 10.1016/j.procbio.2013.02.021
Kobayashi, Y., Kinoshita, T., Matsumoto, A., Yoshino, K., Saito, I., and Xiao, J. Z. (2019). Bifidobacterium Breve A1 supplementation improved cognitive decline in older adults with mild cognitive impairment: an open-label, single-arm study. J. Prev. Alzheimers Dis. 6, 70–75. doi: 10.14283/jpad.2018.32
Komatsuzaki, N., Shima, J., Kawamoto, S., Momose, H., and Kimura, T. (2005). Production of γ-aminobutyric acid (GABA) by Lactobacillus paracasei isolated from traditional fermented foods. Food Microbiol. 22, 497–504. doi: 10.1016/j.fm.2005.01.002
Kothari, D., Patel, S., and Kim, S. K. (2018). Probiotic supplements might not be universally-effective and safe: a review. Biomed. Pharmacother. 111, 537–547. doi: 10.1016/j.biopha.2018.12.104
Kouchaki, E., Tamtaji, O. R., Salami, M., Bahmani, F., Daneshvar Kakhaki, R., Akbari, E., et al. (2017). Clinical and metabolic response to probiotic supplementation in patients with multiple sclerosis: a randomized, double-blind, placebo-controlled trial. Clin. Nutr. 36, 1245–1249. doi: 10.1016/j.clnu.2016.08.015
Kurosawa, N., Shimizu, K., and Seki, K. (2016). The development of depression-like behavior is consolidated by IL-6-induced activation of locus coeruleus neurons and IL-1beta-induced elevated leptin levels in mice. Psychopharmacology 233, 1725–1737. doi: 10.1007/s00213-015-4084-x
Laval, L., Martin, R., Natividad, J. N., Chain, F., Miquel, S., Desclee de Maredsous, C., et al. (2015). Lactobacillus rhamnosus CNCM I-3690 and the commensal bacterium Faecalibacterium prausnitzii A2-165 exhibit similar protective effects to induced barrier hyper-permeability in mice. Gut Microbes 6, 1–9. doi: 10.4161/19490976.2014.990784
Lefebvre, D., Langevin, L. M., Jaworska, N., Harris, A. D., Lebel, R. M., Jasaui, Y., et al. (2017). A pilot study of hippocampal N-acetyl-aspartate in youth with treatment resistant major depression. J. Affect. Disord. 207, 110–113. doi: 10.1016/j.jad.2016.05.077
Leonard, B. E. (2001). Stress, norepinephrine and depression. J. Psychiatry Neurosci. 26(Suppl.), S11–S16.
Lepine, A. F. P., de Wit, N., Oosterink, E., Wichers, H., Mes, J., and de Vos, P. (2018). Lactobacillus acidophilus attenuates Salmonella-induced stress of epithelial cells by modulating tight-junction genes and cytokine responses. Front. Microbiol. 9:1439. doi: 10.3389/fmicb.2018.01439
Lew, L. C., Hor, Y. Y., Yusoff, N. A. A., Choi, S. B., Yusoff, M. S. B., Roslan, N. S., et al. (2018). Probiotic Lactobacillus plantarum P8 alleviated stress and anxiety while enhancing memory and cognition in stressed adults: a randomised, double-blind, placebo-controlled study. Clin. Nutr. 38, 2053–2064. doi: 10.1016/j.clnu.2018.09.010
Liang, S., Wang, T., Hu, X., Luo, J., Li, W., Wu, X., et al. (2015). Administration of Lactobacillus helveticus NS8 improves behavioral, cognitive, and biochemical aberrations caused by chronic restraint stress. Neuroscience 310, 561–577. doi: 10.1016/j.neuroscience.2015.09.033
Liao, W.-C., Wang, C.-Y., Shyu, Y.-T., Yu, R.-C., and Ho, K.-C. (2013). Influence of preprocessing methods and fermentation of adzuki beans on γ-aminobutyric acid (GABA) accumulation by lactic acid bacteria. J. Funct. Foods 5, 1108–1115. doi: 10.1016/j.jff.2013.03.006
Lin, P., Ding, B., Feng, C., Yin, S., Zhang, T., Qi, X., et al. (2017). Prevotella and Klebsiella proportions in fecal microbial communities are potential characteristic parameters for patients with major depressive disorder. J. Affect. Disord. 207, 300–304. doi: 10.1016/j.jad.2016.09.051
Lin, Q. (2013). Submerged fermentation of Lactobacillus rhamnosus YS9 for gamma-aminobutyric acid (GABA) production. Braz. J. Microbiol. 44, 183–187. doi: 10.1590/S1517-83822013000100028
Liu, J., Sun, J., Wang, F., Yu, X., Ling, Z., Li, H., et al. (2015). Neuroprotective effects of Clostridium butyricum against vascular dementia in mice via metabolic butyrate. Biomed. Res. Int. 2015:412946. doi: 10.1155/2015/412946
Liu, W., Ge, T., Leng, Y., Pan, Z., Fan, J., Yang, W., et al. (2017). The role of neural plasticity in depression: from hippocampus to prefrontal cortex. Neural Plast. 2017:6871089. doi: 10.1155/2017/6871089
Liu, W. H., Chuang, H. L., Huang, Y. T., Wu, C. C., Chou, G. T., Wang, S., et al. (2016). Alteration of behavior and monoamine levels attributable to Lactobacillus plantarum PS128 in germ-free mice. Behav. Brain Res. 298(Pt B), 202–209. doi: 10.1016/j.bbr.2015.10.046
Liu, Y. W., Liu, W. H., Wu, C. C., Juan, Y. C., Wu, Y. C., Tsai, H. P., et al. (2016). Psychotropic effects of Lactobacillus plantarum PS128 in early life-stressed and naive adult mice. Brain Res. 1631, 1–12. doi: 10.1016/j.brainres.2015.11.018
Lok, A., Mocking, R. J., Ruhe, H. G., Visser, I., Koeter, M. W., Assies, J., et al. (2012). Longitudinal hypothalamic-pituitary-adrenal axis trait and state effects in recurrent depression. Psychoneuroendocrinology 37, 892–902. doi: 10.1016/j.psyneuen.2011.10.005
Lomasney, K. W., Cryan, J. F., and Hyland, N. P. (2014). Converging effects of a Bifidobacterium and Lactobacillus probiotic strain on mouse intestinal physiology. Am. J. Physiol. Gastrointest. Liver Physiol. 307, G241–G247. doi: 10.1152/ajpgi.00401.2013
Lomax, A. E., Sharkey, K. A., and Furness, J. B. (2010). The participation of the sympathetic innervation of the gastrointestinal tract in disease states. Neurogastroenterol. Motil. 22, 7–18. doi: 10.1111/j.1365-2982.2009.01381.x
Lopez, J. F., Chalmers, D. T., Little, K. Y., and Watson, S. J. (1998). A.E. Bennett Research Award. Regulation of serotonin1A, glucocorticoid, and mineralocorticoid receptor in rat and human hippocampus: implications for the neurobiology of depression. Biol. Psychiatry 43, 547–573. doi: 10.1016/s0006-3223(97)00484-8
Luan, H., Wang, X., and Cai, Z. (2017). Mass spectrometry-based metabolomics: targeting the crosstalk between gut microbiota and brain in neurodegenerative disorders. Mass Spectrom. Rev. 38, 22–33. doi: 10.1002/mas.21553
Lund, M. L., Egerod, K. L., Engelstoft, M. S., Dmytriyeva, O., Theodorsson, E., Patel, B. A., et al. (2018). Enterochromaffin 5-HT cells - A major target for GLP-1 and gut microbial metabolites. Mol. Metab. 11, 70–83. doi: 10.1016/j.molmet.2018.03.004
Luo, J., Wang, T., Liang, S., Hu, X., Li, W., and Jin, F. (2014). Ingestion of Lactobacillus strain reduces anxiety and improves cognitive function in the hyperammonemia rat. Sci. China Life Sci. 57, 327–335. doi: 10.1007/s11427-014-4615-4
Luscher, B., Shen, Q., and Sahir, N. (2011). The GABAergic deficit hypothesis of major depressive disorder. Mol. Psychiatry 16, 383–406. doi: 10.1038/mp.2010.12
Lyte, M. (2011). Probiotics function mechanistically as delivery vehicles for neuroactive compounds: microbial endocrinology in the design and use of probiotics. Bioessays 33, 574–581. doi: 10.1002/bies.201100024
Maeda, H., Zhu, X., Suzuki, S., Suzuki, K., and Kitamura, S. (2004). Structural characterization and biological activities of an exopolysaccharide kefiran produced by Lactobacillus kefiranofaciens WT-2B(T). J. Agric. Food Chem. 52, 5533–5538. doi: 10.1021/jf049617g
Maehata, H., Kobayashi, Y., Mitsuyama, E., Kawase, T., Kuhara, T., Xiao, J. Z., et al. (2019). Heat-killed Lactobacillus helveticus strain MCC1848 confers resilience to anxiety or depression-like symptoms caused by subchronic social defeat stress in mice. Biosci. Biotechnol. Biochem. 83, 1239–1247. doi: 10.1080/09168451.2019.1591263
Maes, M., Kubera, M., and Leunis, J. C. (2008). The gut-brain barrier in major depression: intestinal mucosal dysfunction with an increased translocation of LPS from gram negative enterobacteria (leaky gut) plays a role in the inflammatory pathophysiology of depression. Neuro Endocrinol. Lett. 29, 117–124.
Maes, M., Meltzer, H. Y., Scharpé, S., Bosmans, E., Suy, E., De Meester, I., et al. (1993). Relationships between lower plasma L-tryptophan levels and immune-inflammatory variables in depression. Psychiatry Res. 49, 151–165. doi: 10.1016/0165-1781(93)90102-M
Maes, M., Scharpe, S., Meltzer, H. Y., Okayli, G., Bosmans, E., D’Hondt, P., et al. (1994). Increased neopterin and interferon-gamma secretion and lower availability of L-tryptophan in major depression: further evidence for an immune response. Psychiatry Res. 54, 143–160. doi: 10.1016/0165-1781(94)90003-5
Maes, M., Verkerk, R., Bonaccorso, S., Ombelet, W., Bosmans, E., and Scharpe, S. (2002). Depressive and anxiety symptoms in the early puerperium are related to increased degradation of tryptophan into kynurenine, a phenomenon which is related to immune activation. Life Sci. 71, 1837–1848. doi: 10.1016/s0024-3205(02)01853-2
Majeed, M., Nagabhushanam, K., Arumugam, S., Majeed, S., and Ali, F. (2018). Bacillus coagulans MTCC 5856 for the management of major depression with irritable bowel syndrome: a randomised, double-blind, placebo controlled, multi-centre, pilot clinical study. Food Nutr. Res. 62. doi: 10.29219/fnr.v62.1218
Maldonado-Gomez, M. X., Martinez, I., Bottacini, F., O’Callaghan, A., Ventura, M., van Sinderen, D., et al. (2016). Stable engraftment of Bifidobacterium longum AH1206 in the human gut depends on individualized features of the resident microbiome. Cell Host Microbe 20, 515–526. doi: 10.1016/j.chom.2016.09.001
Marin, I. A., Goertz, J. E., Ren, T., Rich, S. S., Onengut-Gumuscu, S., Farber, E., et al. (2017). Microbiota alteration is associated with the development of stress-induced despair behavior. Sci. Rep. 7:43859. doi: 10.1038/srep43859
Martin, R., Miquel, S., Chain, F., Natividad, J. M., Jury, J., Lu, J., et al. (2015). Faecalibacterium prausnitzii prevents physiological damages in a chronic low-grade inflammation murine model. BMC Microbiol. 15:67. doi: 10.1186/s12866-015-0400-1
Martinez, L. R., Xu, S., and Hebl, M. (2018). Utilizing education and perspective taking to remediate the stigma of taking antidepressants. Commun. Ment. Health J. 54, 450–459. doi: 10.1007/s10597-017-0174-z
Martinowich, K., and Lu, B. (2008). Interaction between BDNF and serotonin: role in mood disorders. Neuropsychopharmacology 33, 73–83. doi: 10.1038/sj.npp.1301571
Maslej, M. M., Bolker, B. M., Russell, M. J., Eaton, K., Durisko, Z., Hollon, S. D., et al. (2017). The mortality and myocardial effects of antidepressants are moderated by preexisting cardiovascular disease: a meta-analysis. Psychother. Psychosom. 86, 268–282. doi: 10.1159/000477940
Mathews, G. C., and Diamond, J. S. (2003). Neuronal glutamate uptake contributes to GABA synthesis and inhibitory synaptic strength. J. Neurosci. 23, 2040–2048. doi: 10.1523/jneurosci.23-06-02040.2003
Matt, S. M., Allen, J. M., Lawson, M. A., Mailing, L. J., Woods, J. A., and Johnson, R. W. (2018). Butyrate and dietary soluble fiber improve neuroinflammation associated with aging in mice. Front. Immunol. 9:1832. doi: 10.3389/fimmu.2018.01832
McKean, J., Naug, H., Nikbakht, E., Amiet, B., and Colson, N. (2017). Probiotics and subclinical psychological symptoms in healthy participants: a systematic review and meta-analysis. J. Altern. Complement. Med. 23, 249–258. doi: 10.1089/acm.2016.0023
McNabney, S. M., and Henagan, T. M. (2017). Short chain fatty acids in the colon and peripheral tissues: a focus on butyrate, colon cancer, obesity and insulin resistance. Nutrients 9:E1348. doi: 10.3390/nu9121348
McVey Neufeld, K. A., Kay, S., and Bienenstock, J. (2018). Mouse Strain affects behavioral and neuroendocrine stress responses following administration of probiotic Lactobacillus rhamnosus JB-1 or traditional antidepressant fluoxetine. Front. Neurosci. 12:294. doi: 10.3389/fnins.2018.00294
McVey Neufeld, K. A., Mao, Y. K., Bienenstock, J., Foster, J. A., and Kunze, W. A. (2013). The microbiome is essential for normal gut intrinsic primary afferent neuron excitability in the mouse. Neurogastroenterol. Motil. 25:183-e88. doi: 10.1111/nmo.12049
McVey Neufeld, K. A., O’Mahony, S. M., Hoban, A. E., Waworuntu, R. V., Berg, B. M., Dinan, T. G., et al. (2017). Neurobehavioural effects of Lactobacillus rhamnosus GG alone and in combination with prebiotics polydextrose and galactooligosaccharide in male rats exposed to early-life stress. Nutr. Neurosci. 22, 425–434. doi: 10.1080/1028415X.2017.1397875
Medrano, M., Perez, P. F., and Abraham, A. G. (2008). Kefiran antagonizes cytopathic effects of Bacillus cereus extracellular factors. Int. J. Food Microbiol. 122, 1–7. doi: 10.1016/j.ijfoodmicro.2007.11.046
Mendonca-de-Souza, A. C., Souza, G. G., Vieira, A., Fischer, N. L., Souza, W. F., Rumjanek, V. M., et al. (2007). Negative affect as a predisposing factor for cortisol release after an acute stress–the impact of unpleasant priming. Stress 10, 362–367. doi: 10.1080/10253890701379999
Messaoudi, M., Lalonde, R., Violle, N., Javelot, H., Desor, D., Nejdi, A., et al. (2011). Assessment of psychotropic-like properties of a probiotic formulation (Lactobacillus helveticus R0052 and Bifidobacterium longum R0175) in rats and human subjects. Br. J. Nutr. 105, 755–764. doi: 10.1017/S0007114510004319
Miller, A. H., Maletic, V., and Raison, C. L. (2009). Inflammation and its discontents: the role of cytokines in the pathophysiology of major depression. Biol. Psychiatry 65, 732–741. doi: 10.1016/j.biopsych.2008.11.029
Miller, A. H., and Raison, C. L. (2016). The role of inflammation in depression: from evolutionary imperative to modern treatment target. Nat. Rev. Immunol. 16, 22–34. doi: 10.1038/nri.2015.5
Mittal, R., Debs, L. H., Patel, A. P., Nguyen, D., Patel, K., O’Connor, G., et al. (2017). Neurotransmitters: the critical modulators regulating gut-brain axis. J. Cell Physiol. 232, 2359–2372. doi: 10.1002/jcp.25518
Miyaoka, T., Kanayama, M., Wake, R., Hashioka, S., Hayashida, M., Nagahama, M., et al. (2018). Clostridium butyricum MIYAIRI 588 as adjunctive therapy for treatment-resistant major depressive disorder: a prospective open-label trial. Clin. Neuropharmacol. 41, 151–155. doi: 10.1097/WNF.0000000000000299
Miyazaki, K., Itoh, N., Yamamoto, S., Higo-Yamamoto, S., Nakakita, Y., Kaneda, H., et al. (2014). Dietary heat-killed Lactobacillus brevis SBC8803 promotes voluntary wheel-running and affects sleep rhythms in mice. Life Sci. 111, 47–52. doi: 10.1016/j.lfs.2014.07.009
Mkaouar, H., Akermi, N., Mariaule, V., Boudebbouze, S., Gaci, N., Szukala, F., et al. (2016). Siropins, novel serine protease inhibitors from gut microbiota acting on human proteases involved in inflammatory bowel diseases. Microb. Cell Fact. 15:201. doi: 10.1186/s12934-016-0596-2
Mohammadi, A. A., Jazayeri, S., Khosravi-Darani, K., Solati, Z., Mohammadpour, N., Asemi, Z., et al. (2016). The effects of probiotics on mental health and hypothalamic-pituitary-adrenal axis: a randomized, double-blind, placebo-controlled trial in petrochemical workers. Nutr. Neurosci. 19, 387–395. doi: 10.1179/1476830515Y.0000000023
Montgomery, S., and Briley, M. (2011). Noradrenergic symptom cluster in depression. Neuropsychiatr. Dis. Treat. 7(Suppl. 1), 1–2. doi: 10.2147/NDT.S19611
Moon, C., Rousseau, R., Soria, J. C., Hoque, M. O., Lee, J., Jang, S. J., et al. (2004). Aquaporin expression in human lymphocytes and dendritic cells. Am. J. Hematol. 75, 128–133. doi: 10.1002/ajh.10476
Moriguchi, S., Takamiya, A., Noda, Y., Horita, N., Wada, M., Tsugawa, S., et al. (2018). Glutamatergic neurometabolite levels in major depressive disorder: a systematic review and meta-analysis of proton magnetic resonance spectroscopy studies. Mol. Psychiatry 24, 952–964. doi: 10.1038/s41380-018-0252-9
Moya-Perez, A., Neef, A., and Sanz, Y. (2015). Bifidobacterium pseudocatenulatum CECT 7765 reduces obesity-associated inflammation by restoring the lymphocyte-macrophage balance and gut microbiota structure in high-fat diet-fed mice. PLoS One 10:e0126976. doi: 10.1371/journal.pone.0126976
Moya-Perez, A., Perez-Villalba, A., Benitez-Paez, A., Campillo, I., and Sanz, Y. (2017). Bifidobacterium CECT 7765 modulates early stress-induced immune, neuroendocrine and behavioral alterations in mice. Brain Behav. Immun. 65, 43–56. doi: 10.1016/j.bbi.2017.05.011
Moya-Perez, A., Romo-Vaquero, M., Tomas-Barberan, F., Sanz, Y., and Garcia-Conesa, M. T. (2014). Hepatic molecular responses to Bifidobacterium pseudocatenulatum CECT 7765 in a mouse model of diet-induced obesity. Nutr. Metab. Cardiovasc. Dis. 24, 57–64. doi: 10.1016/j.numecd.2013.04.011
Murakami, T., Kamada, K., Mizushima, K., Higashimura, Y., Katada, K., Uchiyama, K., et al. (2017). Changes in intestinal motility and gut microbiota composition in a rat stress model. Digestion 95, 55–60. doi: 10.1159/000452364
Murata, M., Kondo, J., Iwabuchi, N., Takahashi, S., Yamauchi, K., Abe, F., et al. (2018). Effects of paraprobiotic Lactobacillus paracasei MCC1849 supplementation on symptoms of the common cold and mood states in healthy adults. Benef. Microbes 9, 855–864. doi: 10.3920/BM2017.0197
Murrough, J. W., Abdallah, C. G., and Mathew, S. J. (2017). Targeting glutamate signalling in depression: progress and prospects. Nat. Rev. Drug Discov. 16, 472–486. doi: 10.1038/nrd.2017.16
Najarian, A., Sharif, S., and Griffiths, M. W. (2019). Evaluation of protective effect of Lactobacillus acidophilus La-5 on toxicity and colonization of Clostridium difficile in human epithelial cells in vitro. Anaerobe 55, 142–151. doi: 10.1016/j.anaerobe.2018.12.004
Nakatani, Y., Sato-Suzuki, I., Tsujino, N., Nakasato, A., Seki, Y., Fumoto, M., et al. (2008). Augmented brain 5-HT crosses the blood-brain barrier through the 5-HT transporter in rat. Eur. J. Neurosci. 27, 2466–2472. doi: 10.1111/j.1460-9568.2008.06201.x
Naseribafrouei, A., Hestad, K., Avershina, E., Sekelja, M., Linlokken, A., Wilson, R., et al. (2014). Correlation between the human fecal microbiota and depression. Neurogastroenterol. Motil. 26, 1155–1162. doi: 10.1111/nmo.12378
Nedjadi, T., Moran, A. W., Al-Rammahi, M. A., and Shirazi-Beechey, S. P. (2014). Characterization of butyrate transport across the luminal membranes of equine large intestine. Exp. Physiol. 99, 1335–1347. doi: 10.1113/expphysiol.2014.077982
Neufeld, K. M., Kang, N., Bienenstock, J., and Foster, J. A. (2011). Reduced anxiety-like behavior and central neurochemical change in germ-free mice. Neurogastroenterol. Motil. 23, 255–264, e119. doi: 10.1111/j.1365-2982.2010.01620.x
Ng, Q. X., Peters, C., Ho, C. Y. X., Lim, D. Y., and Yeo, W. S. (2018). A meta-analysis of the use of probiotics to alleviate depressive symptoms. J. Affect. Disord. 228, 13–19. doi: 10.1016/j.jad.2017.11.063
Nielsen, C. U., Carstensen, M., and Brodin, B. (2012). Carrier-mediated gamma-aminobutyric acid transport across the basolateral membrane of human intestinal Caco-2 cell monolayers. Eur. J. Pharm. Biopharm. 81, 458–462. doi: 10.1016/j.ejpb.2012.03.007
Nishida, K., Sawada, D., Kawai, T., Kuwano, Y., Fujiwara, S., and Rokutan, K. (2017a). Para-psychobiotic Lactobacillus gasseri CP2305 ameliorates stress-related symptoms and sleep quality. J. Appl. Microbiol. 123, 1561–1570. doi: 10.1111/jam.13594
Nishida, K., Sawada, D., Kuwano, Y., Tanaka, H., Sugawara, T., Aoki, Y., et al. (2017b). Daily administration of paraprobiotic Lactobacillus gasseri CP2305 ameliorates chronic stress-associated symptoms in Japanese medical students. J. Funct. Foods 36, 112–121. doi: 10.1016/j.jff.2017.06.031
Nobutani, K., Sawada, D., Fujiwara, S., Kuwano, Y., Nishida, K., Nakayama, J., et al. (2017). The effects of administration of the Lactobacillus gasseri strain CP2305 on quality of life, clinical symptoms and changes in gene expression in patients with irritable bowel syndrome. J. Appl. Microbiol. 122, 212–224. doi: 10.1111/jam.13329
Nyangale, E. P., Farmer, S., Cash, H. A., Keller, D., Chernoff, D., and Gibson, G. R. (2015). Bacillus coagulans GBI-30, 6086 modulates Faecalibacterium prausnitzii in older men and women. J. Nutr. 145, 1446–1452. doi: 10.3945/jn.114.199802
Nyangale, E. P., Farmer, S., Keller, D., Chernoff, D., and Gibson, G. R. (2014). Effect of prebiotics on the fecal microbiota of elderly volunteers after dietary supplementation of Bacillus coagulans GBI-30, 6086. Anaerobe 30, 75–81. doi: 10.1016/j.anaerobe.2014.09.002
Ohata, A., Usami, M., and Miyoshi, M. (2005). Short-chain fatty acids alter tight junction permeability in intestinal monolayer cells via lipoxygenase activation. Nutrition 21, 838–847. doi: 10.1016/j.nut.2004.12.004
Ohland, C. L., Kish, L., Bell, H., Thiesen, A., Hotte, N., Pankiv, E., et al. (2013). Effects of Lactobacillus helveticus on murine behavior are dependent on diet and genotype and correlate with alterations in the gut microbiome. Psychoneuroendocrinology 38, 1738–1747. doi: 10.1016/j.psyneuen.2013.02.008
Ohlsson, L., Gustafsson, A., Lavant, E., Suneson, K., Brundin, L., Westrin, A., et al. (2019). Leaky gut biomarkers in depression and suicidal behavior. Acta Psychiatr. Scand. 139, 185–193. doi: 10.1111/acps.12978
Ohsawa, K., Nakamura, F., Uchida, N., Mizuno, S., and Yokogoshi, H. (2018). Lactobacillus helveticus-fermented milk containing lactononadecapeptide (NIPPLTQTPVVVPPFLQPE) improves cognitive function in healthy middle-aged adults: a randomised, double-blind, placebo-controlled trial. Int. J. Food Sci. Nutr. 69, 369–376. doi: 10.1080/09637486.2017.1365824
Okubo, R., Koga, M., Katsumata, N., Odamaki, T., Matsuyama, S., Oka, M., et al. (2019). Effect of bifidobacterium breve A-1 on anxiety and depressive symptoms in schizophrenia: a proof-of-concept study. J. Affect. Disord. 245, 377–385. doi: 10.1016/j.jad.2018.11.011
Oleskin, A., Zhilenkova, O., Shenderov, B., Amerhanova, A., Kudrin, V., and Klodt, P. (2014). Lactic-Acid bacteria supplement fermented dairy products with human behavior-modifying neuroactive compounds. J. Pharm. Nutr. Sci. 4, 199–206. doi: 10.6000/1927-5951.2014.04.03.5
Oleskin, A. V., El’-Registan, G. I., and Shenderov, B. A. (2016). Role of neuromediators in the functioning of the human microbiota: “Business talks” among microorganisms and the microbiota-host dialogue. Microbiology 85, 1–22. doi: 10.1134/s0026261716010082
Olvera, R. L., Caetano, S. C., Stanley, J. A., Chen, H. H., Nicoletti, M., Hatch, J. P., et al. (2010). Reduced medial prefrontal N-acetyl-aspartate levels in pediatric major depressive disorder: a multi-voxel in vivo(1)H spectroscopy study. Psychiatry Res. 184, 71–76. doi: 10.1016/j.pscychresns.2010.07.008
Orikasa, S., Nabeshima, K., Iwabuchi, N., and Xiao, J. Z. (2016). Effect of repeated oral administration of Bifidobacterium longum BB536 on apomorphine-induced rearing behavior in mice. Biosci. Microb. Food Health 35, 141–145. doi: 10.12938/bmfh.2016-004
Osborne, D. M., Pearson-Leary, J., and McNay, E. C. (2015). The neuroenergetics of stress hormones in the hippocampus and implications for memory. Front. Neurosci. 9:164. doi: 10.3389/fnins.2015.00164
Osman, N., Adawi, D., Molin, G., Ahrne, S., Berggren, A., and Jeppsson, B. (2006). Bifidobacterium infantis strains with and without a combination of oligofructose and inulin (OFI) attenuate inflammation in DSS-induced colitis in rats. BMC Gastroenterol. 6:31. doi: 10.1186/1471-230X-6-31
Ostadmohammadi, V., Jamilian, M., Bahmani, F., and Asemi, Z. (2019). Vitamin D and probiotic co-supplementation affects mental health, hormonal, inflammatory and oxidative stress parameters in women with polycystic ovary syndrome. J. Ovarian Res. 12:5. doi: 10.1186/s13048-019-0480-x
Ostergaard, S. D., Jensen, S. O., and Bech, P. (2011). The heterogeneity of the depressive syndrome: when numbers get serious. Acta Psychiatr. Scand. 124, 495–496. doi: 10.1111/j.1600-0447.2011.01744.x
Ostlund-Lagerstrom, L., Kihlgren, A., Repsilber, D., Bjorksten, B., Brummer, R. J., and Schoultz, I. (2016). Probiotic administration among free-living older adults: a double blinded, randomized, placebo-controlled clinical trial. Nutr. J. 15:80. doi: 10.1186/s12937-016-0198-1
Otomi, K., Ymaguchi, T., Watanabe, S., Kobayashi, A., Kobayashi, H., and Hashiguchi, N. (2015). Effects of yogurt containing Lactobacillus gasseri OLL2716 on autonomic nerve activities and physiological functions. Health 07, 397–405. doi: 10.4236/health.2015.73045
Özogul, F. (2011). Effects of specific lactic acid bacteria species on biogenic amine production by foodborne pathogen. Int. J. Food Sci. Technol. 46, 478–484. doi: 10.1111/j.1365-2621.2010.02511.x
Özoğul, F., Kuley, E., Özoğul, Y., and Özoul, I. (2012). The function of lactic acid bacteria on biogenic amines production by food-borne pathogens in arginine decarboxylase broth. Food Sci. Technol. Res. 18, 795–804. doi: 10.3136/fstr.18.795
Pacak, K., Kvetnansky, R., Palkovits, M., Fukuhara, K., Yadid, G., Kopin, I. J., et al. (1993). Adrenalectomy augments in vivo release of norepinephrine in the paraventricular nucleus during immobilization stress. Endocrinology 133, 1404–1410. doi: 10.1210/endo.133.3.8396018
Pan, J. X., Deng, F. L., Zeng, B. H., Zheng, P., Liang, W. W., Yin, B. M., et al. (2019). Absence of gut microbiota during early life affects anxiolytic behaviors and monoamine neurotransmitters system in the hippocampal of mice. J. Neurol. Sci. 400, 160–168. doi: 10.1016/j.jns.2019.03.027
Pandey, N., Malik, R. K., Kaushik, J. K., and Singroha, G. (2013). Gassericin A: a circular bacteriocin produced by lactic acid bacteria Lactobacillus gasseri. World J. Microbiol. Biotechnol. 29, 1977–1987. doi: 10.1007/s11274-013-1368-3
Pariyadath, V., Gowin, J. L., and Stein, E. A. (2016). Resting state functional connectivity analysis for addiction medicine: from individual loci to complex networks. Prog. Brain Res. 224, 155–173. doi: 10.1016/bs.pbr.2015.07.015
Peng, L., Li, Z. R., Green, R. S., Holzman, I. R., and Lin, J. (2009). Butyrate enhances the intestinal barrier by facilitating tight junction assembly via activation of AMP-activated protein kinase in Caco-2 cell monolayers. J. Nutr. 139, 1619–1625. doi: 10.3945/jn.109.104638
Perez-Burgos, A., Mao, Y. K., Bienenstock, J., and Kunze, W. A. (2014). The gut-brain axis rewired: adding a functional vagal nicotinic “sensory synapse”. FASEB J. 28, 3064–3074. doi: 10.1096/fj.13-245282
Perez-Burgos, A., Wang, B., Mao, Y. K., Mistry, B., McVey Neufeld, K. A., Bienenstock, J., et al. (2013). Psychoactive bacteria Lactobacillus rhamnosus (JB-1) elicits rapid frequency facilitation in vagal afferents. Am. J. Physiol. Gastrointest. Liver Physiol. 304, G211–G220. doi: 10.1152/ajpgi.00128.2012
Pinto-Sanchez, M. I., Hall, G. B., Ghajar, K., Nardelli, A., Bolino, C., Lau, J. T., et al. (2017). Probiotic Bifidobacterium longum NCC3001 reduces depression scores and alters brain activity: a pilot study in patients with irritable bowel syndrome. Gastroenterology 153, 448.e8–459.e8. doi: 10.1053/j.gastro.2017.05.003
Pirbaglou, M., Katz, J., de Souza, R. J., Stearns, J. C., Motamed, M., and Ritvo, P. (2016). Probiotic supplementation can positively affect anxiety and depressive symptoms: a systematic review of randomized controlled trials. Nutr. Res. 36, 889–898. doi: 10.1016/j.nutres.2016.06.009
Pytka, K., Dziubina, A., Mlyniec, K., Dziedziczak, A., Zmudzka, E., Furgala, A., et al. (2016). The role of glutamatergic, GABA-ergic, and cholinergic receptors in depression and antidepressant-like effect. Pharmacol. Rep. 68, 443–450. doi: 10.1016/j.pharep.2015.10.006
Quevrain, E., Maubert, M. A., Michon, C., Chain, F., Marquant, R., Tailhades, J., et al. (2016a). Identification of an anti-inflammatory protein from Faecalibacterium prausnitzii, a commensal bacterium deficient in Crohn’s disease. Gut 65, 415–425. doi: 10.1136/gutjnl-2014-307649
Quevrain, E., Maubert, M. A., Sokol, H., Devreese, B., and Seksik, P. (2016b). The presence of the anti-inflammatory protein MAM, from Faecalibacterium prausnitzii, in the intestinal ecosystem. Gut 65:882. doi: 10.1136/gutjnl-2015-311094
Quigley, E. M. (2011). Microflora modulation of motility. J. Neurogastroenterol. Motil. 17, 140–147. doi: 10.5056/jnm.2011.17.2.140
Rao, A. V., Bested, A. C., Beaulne, T. M., Katzman, M. A., Iorio, C., Berardi, J. M., et al. (2009). A randomized, double-blind, placebo-controlled pilot study of a probiotic in emotional symptoms of chronic fatigue syndrome. Gut Pathog. 1:6. doi: 10.1186/1757-4749-1-6
Raygan, F., Ostadmohammadi, V., Bahmani, F., and Asemi, Z. (2018). The effects of vitamin D and probiotic co-supplementation on mental health parameters and metabolic status in type 2 diabetic patients with coronary heart disease: a randomized, double-blind, placebo-controlled trial. Prog. Neuropsychopharmacol. Biol. Psychiatry 84(Pt A), 50–55. doi: 10.1016/j.pnpbp.2018.02.007
Read, J., and Williams, J. (2018). Adverse effects of antidepressants reported by a large international cohort: emotional blunting, suicidality, and withdrawal effects. Curr. Drug Saf. 13, 176–186. doi: 10.2174/1574886313666180605095130
Reddy, M. S. (2010). Depression: the disorder and the burden. Indian J. Psychol. Med. 32, 1–2. doi: 10.4103/0253-7176.70510
Reigstad, C. S., Salmonson, C. E., Rainey, J. F. III, Szurszewski, J. H., Linden, D. R., Sonnenburg, J. L., et al. (2015). Gut microbes promote colonic serotonin production through an effect of short-chain fatty acids on enterochromaffin cells. FASEB J. 29, 1395–1403. doi: 10.1096/fj.14-259598
Reus, G. Z., Jansen, K., Titus, S., Carvalho, A. F., Gabbay, V., and Quevedo, J. (2015). Kynurenine pathway dysfunction in the pathophysiology and treatment of depression: evidences from animal and human studies. J. Psychiatr. Res. 68, 316–328. doi: 10.1016/j.jpsychires.2015.05.007
Richard, D. M., Dawes, M. A., Mathias, C. W., Acheson, A., Hill-Kapturczak, N., and Dougherty, D. M. (2009). L-tryptophan: basic metabolic functions, behavioral research and therapeutic indications. Int. J. Tryptophan. Res. 2, 45–60.
Rong, H., Xie, X. H., Zhao, J., Lai, W. T., Wang, M. B., Xu, D., et al. (2019). Similarly in depression, nuances of gut microbiota: evidences from a shotgun metagenomics sequencing study on major depressive disorder versus bipolar disorder with current major depressive episode patients. J. Psychiatr. Res. 113, 90–99. doi: 10.1016/j.jpsychires.2019.03.017
Rowatt, E. (1948). The relation of pantothenic acid to acetylcholine formation by a strain of Lactobacillus plantarum. Microbiology 2, 25–30. doi: 10.1099/00221287-2-1-25
Rudzki, L., Ostrowska, L., Pawlak, D., Malus, A., Pawlak, K., Waszkiewicz, N., et al. (2019). Probiotic Lactobacillus Plantarum 299v decreases kynurenine concentration and improves cognitive functions in patients with major depression: a double-blind, randomized, placebo controlled study. Psychoneuroendocrinology 100, 213–222. doi: 10.1016/j.psyneuen.2018.10.010
Salami, M., Kouchaki, E., Asemi, Z., and Tamtaji, O. R. (2019). How probiotic bacteria influence the motor and mental behaviors as well as immunological and oxidative biomarkers in multiple sclerosis? A double blind clinical trial. J. Funct. Foods 52, 8–13. doi: 10.1016/j.jff.2018.10.023
Salomon, K., Clift, A., Karlsdottir, M., and Rottenberg, J. (2009). Major depressive disorder is associated with attenuated cardiovascular reactivity and impaired recovery among those free of cardiovascular disease. Health Psychol. 28, 157–165. doi: 10.1037/a0013001
Sanacora, G., Mason, G. F., Rothman, D. L., Behar, K. L., Hyder, F., Petroff, O. A., et al. (1999). Reduced cortical gamma-aminobutyric acid levels in depressed patients determined by proton magnetic resonance spectroscopy. Arch. Gen. Psychiatry 56, 1043–1047.
Sanchez, M., Darimont, C., Panahi, S., Drapeau, V., Marette, A., Taylor, V. H., et al. (2017). Effects of a diet-based weight-reducing program with probiotic supplementation on satiety efficiency, eating behaviour traits, and psychosocial behaviours in obese Individuals. Nutrients 9:E284. doi: 10.3390/nu9030284
Sanchis-Chorda, J., Del Pulgar, E. M. G., Carrasco-Luna, J., Benitez-Paez, A., Sanz, Y., and Codoner-Franch, P. (2018). Bifidobacterium pseudocatenulatum CECT 7765 supplementation improves inflammatory status in insulin-resistant obese children. Eur. J. Nutr. 58, 2789–2800. doi: 10.1007/s00394-018-1828-5
Santos, A., San Mauro, M., Sanchez, A., Torres, J. M., and Marquina, D. (2003). The antimicrobial properties of different strains of Lactobacillus spp. isolated from kefir. Syst. Appl. Microbiol. 26, 434–437. doi: 10.1078/072320203322497464
Sashihara, T., Nagata, M., Mori, T., Ikegami, S., Gotoh, M., Okubo, K., et al. (2013). Effects of Lactobacillus gasseri OLL2809 and alpha-lactalbumin on university-student athletes: a randomized, double-blind, placebo-controlled clinical trial. Appl. Physiol. Nutr. Metab. 38, 1228–1235. doi: 10.1139/apnm-2012-0490
Saunders, P. R., Hanssen, N. P., and Perdue, M. H. (1997). Cholinergic nerves mediate stress-induced intestinal transport abnormalities in Wistar-Kyoto rats. Am. J. Physiol. 273(2 Pt 1), G486–G490. doi: 10.1152/ajpgi.1997.273.2.G486
Savignac, H. M., Kiely, B., Dinan, T. G., and Cryan, J. F. (2014). Bifidobacteria exert strain-specific effects on stress-related behavior and physiology in BALB/c mice. Neurogastroenterol. Motil. 26, 1615–1627. doi: 10.1111/nmo.12427
Sawada, D., Kawai, T., Nishida, K., Kuwano, Y., Fujiwara, S., and Rokutan, K. (2017). Daily intake of Lactobacillus gasseri CP2305 improves mental, physical, and sleep quality among Japanese medical students enrolled in a cadaver dissection course. J. Funct. Foods 31, 188–197. doi: 10.1016/j.jff.2017.01.042
Sawada, D., Sugawara, T., Ishida, Y., Aihara, K., Aoki, Y., Takehara, I., et al. (2016). Effect of continuous ingestion of a beverage prepared with Lactobacillus gasseri CP2305 inactivated by heat treatment on the regulation of intestinal function. Food Res. Int. 79, 33–39. doi: 10.1016/j.foodres.2015.11.032
Schiepers, O. J., Wichers, M. C., and Maes, M. (2005). Cytokines and major depression. Prog. Neuropsychopharmacol. Biol. Psychiatry 29, 201–217. doi: 10.1016/j.pnpbp.2004.11.003
Schimke, R. T., Sweeney, E. W., and Berlin, C. M. (1965). The roles of synthesis and degradation in the control of rat liver tryptophan pyrrolase. J. Biol. Chem. 240, 322–331.
Schloesser, R. J., Manji, H. K., and Martinowich, K. (2009). Suppression of adult neurogenesis leads to an increased hypothalamo-pituitary-adrenal axis response. Neuroreport 20, 553–557. doi: 10.1097/WNR.0b013e3283293e59
Schwarz, L. A., and Luo, L. (2015). Organization of the locus coeruleus-norepinephrine system. Curr. Biol. 25, R1051–R1056. doi: 10.1016/j.cub.2015.09.039
Sharpley, C. F., and Bitsika, V. (2014). Validity, reliability and prevalence of four ‘clinical content’ subtypes of depression. Behav. Brain Res. 259, 9–15. doi: 10.1016/j.bbr.2013.10.032
Shaw, W. (2017). Elevated urinary glyphosate and clostridia metabolites with altered dopamine metabolism in triplets with autistic spectrum disorder or suspected seizure disorder: a case study. Integr. Med. 16, 50–57.
Sherwin, E., Sandhu, K. V., Dinan, T. G., and Cryan, J. F. (2016). May the force be with you: the light and dark sides of the microbiota-gut-brain axis in neuropsychiatry. CNS Drugs 30, 1019–1041. doi: 10.1007/s40263-016-0370-3
Shilyansky, C., Williams, L. M., Gyurak, A., Harris, A., Usherwood, T., and Etkin, A. (2016). Effect of antidepressant treatment on cognitive impairments associated with depression: a randomised longitudinal study. Lancet Psychiatry 3, 425–435. doi: 10.1016/s2215-0366(16)00012-2
Siragusa, S., De Angelis, M., Di Cagno, R., Rizzello, C. G., Coda, R., and Gobbetti, M. (2007). Synthesis of gamma-aminobutyric acid by lactic acid bacteria isolated from a variety of Italian cheeses. Appl. Environ. Microbiol. 73, 7283–7290. doi: 10.1128/AEM.01064-07
Slykerman, R. F., Hood, F., Wickens, K., Thompson, J. M. D., Barthow, C., Murphy, R., et al. (2017). Effect of Lactobacillus rhamnosus HN001 in pregnancy on postpartum symptoms of depression and anxiety: a randomised double-blind placebo-controlled trial. EBioMedicine 24, 159–165. doi: 10.1016/j.ebiom.2017.09.013
Smith, M. A., Makino, S., Kvetnansky, R., and Post, R. M. (1995). Stress and glucocorticoids affect the expression of brain-derived neurotrophic factor and neurotrophin-3 mRNAs in the hippocampus. J. Neurosci. 15(3 Pt 1), 1768–1777. doi: 10.1523/jneurosci.15-03-01768.1995
Sokol, H., Pigneur, B., Watterlot, L., Lakhdari, O., Bermudez-Humaran, L. G., Gratadoux, J. J., et al. (2008). Faecalibacterium prausnitzii is an anti-inflammatory commensal bacterium identified by gut microbiota analysis of Crohn disease patients. Proc. Natl. Acad. Sci. U.S.A. 105, 16731–16736. doi: 10.1073/pnas.0804812105
Steenbergen, L., Sellaro, R., van Hemert, S., Bosch, J. A., and Colzato, L. S. (2015). A randomized controlled trial to test the effect of multispecies probiotics on cognitive reactivity to sad mood. Brain Behav. Immun. 48, 258–264. doi: 10.1016/j.bbi.2015.04.003
Stevens, B. R., Goel, R., Seungbum, K., Richards, E. M., Holbert, R. C., Pepine, C. J., et al. (2018). Increased human intestinal barrier permeability plasma biomarkers zonulin and FABP2 correlated with plasma LPS and altered gut microbiome in anxiety or depression. Gut 67, 1555–1557. doi: 10.1136/gutjnl-2017-314759
Strandwitz, P., Kim, K. H., Terekhova, D., Liu, J. K., Sharma, A., Levering, J., et al. (2019). GABA-modulating bacteria of the human gut microbiota. Nat. Microbiol. 4, 396–403. doi: 10.1038/s41564-018-0307-3
Stromeck, A., Hu, Y., Chen, L., and Ganzle, M. G. (2011). Proteolysis and bioconversion of cereal proteins to glutamate and gamma-Aminobutyrate (GABA) in Rye malt sourdoughs. J. Agric. Food Chem. 59, 1392–1399. doi: 10.1021/jf103546t
Sugawara, T., Sawada, D., Ishida, Y., Aihara, K., Aoki, Y., Takehara, I., et al. (2016). Regulatory effect of paraprobiotic Lactobacillus gasseri CP2305 on gut environment and function. Microb. Ecol. Health Dis. 27:30259. doi: 10.3402/mehd.v27.30259
Sullivan, R. M., and Dufresne, M. M. (2006). Mesocortical dopamine and HPA axis regulation: role of laterality and early environment. Brain Res. 1076, 49–59. doi: 10.1016/j.brainres.2005.12.100
Sun, J., Wang, F., Hong, G., Pang, M., Xu, H., Li, H., et al. (2016). Antidepressant-like effects of sodium butyrate and its possible mechanisms of action in mice exposed to chronic unpredictable mild stress. Neurosci. Lett. 618, 159–166. doi: 10.1016/j.neulet.2016.03.003
Sun, J., Wang, F., Hu, X., Yang, C., Xu, H., Yao, Y., et al. (2018). Clostridium butyricum attenuates chronic unpredictable mild stress-induced depressive-like behavior in mice via the gut-brain axis. J. Agric. Food Chem. 66, 8415–8421. doi: 10.1021/acs.jafc.8b02462
Sun, Y., Geng, W., Pan, Y., Wang, J., Xiao, P., and Wang, Y. (2019). Supplementation with Lactobacillus kefiranofaciens ZW3 from Tibetan Kefir improves depression-like behavior in stressed mice by modulating the gut microbiota. Food Funct. 10, 925–937. doi: 10.1039/c8fo02096e
Suzuki, T., Yoshida, S., and Hara, H. (2008). Physiological concentrations of short-chain fatty acids immediately suppress colonic epithelial permeability. Br. J. Nutr. 100, 297–305. doi: 10.1017/S0007114508888733
Takada, M., Nishida, K., Kataoka-Kato, A., Gondo, Y., Ishikawa, H., Suda, K., et al. (2016). Probiotic Lactobacillus casei strain Shirota relieves stress-associated symptoms by modulating the gut-brain interaction in human and animal models. Neurogastroenterol. Motil. 28, 1027–1036. doi: 10.1111/nmo.12804
Tan, H., Zhai, Q., and Chen, W. (2019). Investigations of Bacteroides spp. towards next-generation probiotics. Food Res. Int. 116, 637–644. doi: 10.1016/j.foodres.2018.08.088
Tanida, M., Imanishi, K., Akashi, H., Kurata, Y., Chonan, O., Naito, E., et al. (2014). Injection of Lactobacillus casei strain Shirota affects autonomic nerve activities in a tissue-specific manner, and regulates glucose and lipid metabolism in rats. J. Diabetes Investig. 5, 153–161. doi: 10.1111/jdi.12141
Tanida, M., and Nagai, K. (2011). Electrophysiological analysis of the mechanism of autonomic action by lactobacilli. Biosci. Microflora 30, 99–109. doi: 10.12938/bifidus.30.99
Thiagarajah, J. R., Chang, J., Goettel, J. A., Verkman, A. S., and Lencer, W. I. (2017). Aquaporin-3 mediates hydrogen peroxide-dependent responses to environmental stress in colonic epithelia. Proc. Natl. Acad. Sci. U.S.A. 114, 568–573. doi: 10.1073/pnas.1612921114
Thomas, C. M., Hong, T., van Pijkeren, J. P., Hemarajata, P., Trinh, D. V., Hu, W., et al. (2012). Histamine derived from probiotic Lactobacillus reuteri suppresses TNF via modulation of PKA and ERK signaling. PLoS One 7:e31951. doi: 10.1371/journal.pone.0031951
Thursby, E., and Juge, N. (2017). Introduction to the human gut microbiota. Biochem. J. 474, 1823–1836. doi: 10.1042/BCJ20160510
Thwaites, D. T., Basterfield, L., McCleave, P. M., Carter, S. M., and Simmons, N. L. (2000). Gamma-Aminobutyric acid (GABA) transport across human intestinal epithelial (Caco-2) cell monolayers. Br. J. Pharmacol. 129, 457–464. doi: 10.1038/sj.bjp.0703069
Tian, P., Wang, G., Zhao, J., Zhang, H., and Chen, W. (2019). Bifidobacterium with the role of 5-hydroxytryptophan synthesis regulation alleviates the symptom of depression and related microbiota dysbiosis. J. Nutr. Biochem. 66, 43–51. doi: 10.1016/j.jnutbio.2019.01.007
Tolhurst, G., Heffron, H., Lam, Y. S., Parker, H. E., Habib, A. M., Diakogiannaki, E., et al. (2012). Short-chain fatty acids stimulate glucagon-like peptide-1 secretion via the G-protein-coupled receptor FFAR2. Diabetes 61, 364–371. doi: 10.2337/db11-1019
Torrealba, F., Riveros, M. E., Contreras, M., and Valdes, J. L. (2012). Histamine and motivation. Front. Syst. Neurosci. 6:51. doi: 10.3389/fnsys.2012.00051
Tran, N., Zhebrak, M., Yacoub, C., Pelletier, J., and Hawley, D. (2019). The gut-brain relationship: investigating the effect of multispecies probiotics on anxiety in a randomized placebo-controlled trial of healthy young adults. J. Affect. Disord. 252, 271–277. doi: 10.1016/j.jad.2019.04.043
Troy, E. B., and Kasper, D. L. (2010). Beneficial effects of Bacteroides fragilis polysaccharides on the immune system. Front. Biosci. 15:25–34. doi: 10.2741/3603
Tsigos, C., and Chrousos, G. P. (2002). Hypothalamic-pituitary-adrenal axis, neuroendocrine factors and stress. J. Psychosom. Res. 53, 865–871. doi: 10.1016/s0022-3999(02)00429-4
Tucci, V., and Moukaddam, N. (2017). We are the hollow men: the worldwide epidemic of mental illness, psychiatric and behavioral emergencies, and its impact on patients and providers. J. Emerg. Trauma Shock 10, 4–6. doi: 10.4103/0974-2700.199517
Tylee, A., and Gandhi, P. (2005). The importance of somatic symptoms in depression in primary care. Prim Care Companion J. Clin. Psychiatry 7, 167–176. doi: 10.4088/pcc.v07n0405
Urita, Y., Goto, M., Watanabe, T., Matsuzaki, M., Gomi, A., Kano, M., et al. (2015). Continuous consumption of fermented milk containing Bifidobacterium bifidum YIT 10347 improves gastrointestinal and psychological symptoms in patients with functional gastrointestinal disorders. Biosci. Microb. Food Health 34, 37–44. doi: 10.12938/bmfh.2014-017
Valles-Colomer, M., Falony, G., Darzi, Y., Tigchelaar, E. F., Wang, J., Tito, R. Y., et al. (2019). The neuroactive potential of the human gut microbiota in quality of life and depression. Nat. Microbiol. 4, 623–632. doi: 10.1038/s41564-018-0337-x
van de Guchte, M., Blottiere, H. M., and Dore, J. (2018). Humans as holobionts: implications for prevention and therapy. Microbiome 6:81. doi: 10.1186/s40168-018-0466-8
van der Kleij, H., O’Mahony, C., Shanahan, F., O’Mahony, L., and Bienenstock, J. (2008). Protective effects of Lactobacillus rhamnosus [corrected] and Bifidobacterium infantis in murine models for colitis do not involve the vagus nerve. Am. J. Physiol. Regul. Integr. Comp. Physiol. 295, R1131–R1137. doi: 10.1152/ajpregu.90434.2008
Vanuytsel, T., van Wanrooy, S., Vanheel, H., Vanormelingen, C., Verschueren, S., Houben, E., et al. (2014). Psychological stress and corticotropin-releasing hormone increase intestinal permeability in humans by a mast cell-dependent mechanism. Gut 63, 1293–1299. doi: 10.1136/gutjnl-2013-305690
Venema, K. (2017). Foreword - Probiotics and prebiotics - important dietary components for health. Benef. Microbes 8, 1–2. doi: 10.3920/BM2017.x001
Vighi, G., Marcucci, F., Sensi, L., Di Cara, G., and Frati, F. (2008). Allergy and the gastrointestinal system. Clin. Exp. Immunol. 153(Suppl. 1), 3–6. doi: 10.1111/j.1365-2249.2008.03713.x
Vinderola, G., Perdigon, G., Duarte, J., Farnworth, E., and Matar, C. (2006). Effects of the oral administration of the exopolysaccharide produced by Lactobacillus kefiranofaciens on the gut mucosal immunity. Cytokine 36, 254–260. doi: 10.1016/j.cyto.2007.01.003
von Schillde, M. A., Hormannsperger, G., Weiher, M., Alpert, C. A., Hahne, H., Bauerl, C., et al. (2012). Lactocepin secreted by Lactobacillus exerts anti-inflammatory effects by selectively degrading proinflammatory chemokines. Cell Host Microbe 11, 387–396. doi: 10.1016/j.chom.2012.02.006
Vreeburg, S. A., Hoogendijk, W. J., van Pelt, J., Derijk, R. H., Verhagen, J. C., van Dyck, R., et al. (2009). Major depressive disorder and hypothalamic-pituitary-adrenal axis activity: results from a large cohort study. Arch. Gen. Psychiatry 66, 617–626. doi: 10.1001/archgenpsychiatry.2009.50
Wakayama, K., Ohtsuki, S., Takanaga, H., Hosoya, K., and Terasaki, T. (2002). Localization of norepinephrine and serotonin transporter in mouse brain capillary endothelial cells. Neurosci. Res. 44, 173–180. doi: 10.1016/s0168-0102(02)00120-7
Wallace, C. J. K., and Milev, R. (2017). The effects of probiotics on depressive symptoms in humans: a systematic review. Ann. Gen. Psychiatry 16:14. doi: 10.1186/s12991-017-0138-2
Wang, C. Y., and Liao, J. K. (2012). A mouse model of diet-induced obesity and insulin resistance. Methods Mol. Biol. 821, 421–433. doi: 10.1007/978-1-61779-430-8_27
Wang, H., Lee, I. S., Braun, C., and Enck, P. (2016). Effect of probiotics on central nervous system functions in animals and humans: a systematic review. J. Neurogastroenterol. Motil. 22, 589–605. doi: 10.5056/jnm16018
Wang, H. B., Wang, P. Y., Wang, X., Wan, Y. L., and Liu, Y. C. (2012). Butyrate enhances intestinal epithelial barrier function via up-regulation of tight junction protein Claudin-1 transcription. Dig. Dis. Sci. 57, 3126–3135. doi: 10.1007/s10620-012-2259-4
Wei, C. L., Wang, S., Yen, J. T., Cheng, Y. F., Liao, C. L., Hsu, C. C., et al. (2019). Antidepressant-like activities of live and heat-killed Lactobacillus paracasei PS23 in chronic corticosterone-treated mice and possible mechanisms. Brain Res. 1711, 202–213. doi: 10.1016/j.brainres.2019.01.025
Wei, Y., Melas, P. A., Wegener, G., Mathe, A. A., and Lavebratt, C. (2014). Antidepressant-like effect of sodium butyrate is associated with an increase in TET1 and in 5-hydroxymethylation levels in the Bdnf gene. Int. J. Neuropsychopharmacol. 18:yu032. doi: 10.1093/ijnp/pyu032
Weingand-Ziadé, A., Gerber-Décombaz, C., and Affolter, M. (2003). Functional characterization of a salt- and thermotolerant glutaminase from Lactobacillus rhamnosus. Enzyme Microb. Technol. 32, 862–867. doi: 10.1016/s0141-0229(03)00059-0
Wikoff, W. R., Anfora, A. T., Liu, J., Schultz, P. G., Lesley, S. A., Peters, E. C., et al. (2009). Metabolomics analysis reveals large effects of gut microflora on mammalian blood metabolites. Proc. Natl. Acad. Sci. U.S.A. 106, 3698–3703. doi: 10.1073/pnas.0812874106
Winzell, M. S., and Ahren, B. (2004). The high-fat diet-fed mouse: a model for studying mechanisms and treatment of impaired glucose tolerance and type 2 diabetes. Diabetes 53(Suppl. 3), S215–S219.
Xing, Z., Geng, W., Li, C., Sun, Y., and Wang, Y. (2017). Comparative genomics of Lactobacillus kefiranofaciens ZW3 and related members of Lactobacillus. spp reveal adaptations to dairy and gut environments. Sci. Rep. 7:12827. doi: 10.1038/s41598-017-12916-0
Xing, Z., Tang, W., Yang, Y., Geng, W., Rehman, R. U., and Wang, Y. (2018). Colonization and gut flora modulation of Lactobacillus kefiranofaciens ZW3 in the intestinal tract of mice. Probiot. Antimicrob. Proteins 10, 374–382. doi: 10.1007/s12602-017-9288-4
Xu, L., Ma, C., Huang, X., Yang, W., Chen, L., Bilotta, A. J., et al. (2018). Microbiota metabolites short-chain fatty acid butyrate conditions intestinal epithelial cells to promote development of Treg cells and T cell IL-10 production. J. Immunol. 200(1 Suppl.), 53.16.
Yamatsu, A., Yamashita, Y., Maru, I., Yang, J., Tatsuzaki, J., and Kim, M. (2015). The improvement of sleep by oral intake of GABA and Apocynum venetum leaf extract. J. Nutr. Sci. Vitaminol. 61, 182–187. doi: 10.3177/jnsv.61.182
Yan, H., and Ajuwon, K. M. (2017). Butyrate modifies intestinal barrier function in IPEC-J2 cells through a selective upregulation of tight junction proteins and activation of the Akt signaling pathway. PLoS One 12:e0179586. doi: 10.1371/journal.pone.0179586
Yano, J. M., Yu, K., Donaldson, G. P., Shastri, G. G., Ann, P., Ma, L., et al. (2015). Indigenous bacteria from the gut microbiota regulate host serotonin biosynthesis. Cell 161, 264–276. doi: 10.1016/j.cell.2015.02.047
Yokoyama, S., Hiramatsu, J., and Hayakawa, K. (2002). Production of gamma-aminobutyric acid from alcohol distillery lees by Lactobacillus brevis IFO-12005. J. Biosci. Bioeng. 93, 95–97. doi: 10.1263/jbb.93.95
Young, S. N. (1981). Mechanism of decline in rat brain 5-hydroxytryptamine after induction of liver tryptophan pyrrolase by hydrocortisone: roles of tryptophan catabolism and kynurenine synthesis. Br. J. Pharmacol. 74, 695–700. doi: 10.1111/j.1476-5381.1981.tb10480.x
Yunes, R. A., Poluektova, E. U., Dyachkova, M. S., Klimina, K. M., Kovtun, A. S., Averina, O. V., et al. (2016). GABA production and structure of gadB/gadC genes in Lactobacillus and Bifidobacterium strains from human microbiota. Anaerobe 42, 197–204. doi: 10.1016/j.anaerobe.2016.10.011
Zalán, Z., Hudáček, J., Štětina, J., Chumchalová, J., and Halász, A. (2009). Production of organic acids by Lactobacillus strains in three different media. Eur. Food Res. Technol. 230, 395–404. doi: 10.1007/s00217-009-1179-9
Zareian, M., Ebrahimpour, A., Bakar, F. A., Mohamed, A. K., Forghani, B., Ab-Kadir, M. S., et al. (2012). A glutamic acid-producing lactic acid bacteria isolated from Malaysian fermented foods. Int. J. Mol. Sci. 13, 5482–5497. doi: 10.3390/ijms13055482
Zhang, L., Liu, Y. X., Wang, Z., Wang, X. Q., Zhang, J. J., Jiang, R. H., et al. (2019). Clinical characteristic and fecal microbiota responses to probiotic or antidepressant in patients with diarrhea-predominant irritable bowel syndrome with depression comorbidity: a pilot study. Chin. Med. J. 132, 346–351. doi: 10.1097/CM9.0000000000000071
Zhao, Z. X., Fu, J., Ma, S. R., Peng, R., Yu, J. B., Cong, L., et al. (2018). Gut-brain axis metabolic pathway regulates antidepressant efficacy of albiflorin. Theranostics 8, 5945–5959. doi: 10.7150/thno.28068
Zheng, G., Wu, S. P., Hu, Y., Smith, D. E., Wiley, J. W., and Hong, S. (2013). Corticosterone mediates stress-related increased intestinal permeability in a region-specific manner. Neurogastroenterol. Motil. 25, e127–e139. doi: 10.1111/nmo.12066
Zheng, P., Zeng, B., Zhou, C., Liu, M., Fang, Z., Xu, X., et al. (2016). Gut microbiome remodeling induces depressive-like behaviors through a pathway mediated by the host’s metabolism. Mol. Psychiatry 21, 786–796. doi: 10.1038/mp.2016.44
Zhou, Y., Zheng, W., Liu, W., Wang, C., Zhan, Y., Li, H., et al. (2019). Cross-sectional relationship between kynurenine pathway metabolites and cognitive function in major depressive disorder. Psychoneuroendocrinology 101, 72–79. doi: 10.1016/j.psyneuen.2018.11.001
Zmora, N., Zilberman-Schapira, G., Suez, J., Mor, U., Dori-Bachash, M., Bashiardes, S., et al. (2018). Personalized gut mucosal colonization resistance to empiric probiotics Is associated with unique host and microbiome features. Cell 174, 1388.e21–1405.e21. doi: 10.1016/j.cell.2018.08.041
Keywords: microbiota-gut-brain axis, gut microbiota, major depressive disorder, probiotics, inflammation
Citation: Yong SJ, Tong T, Chew J and Lim WL (2020) Antidepressive Mechanisms of Probiotics and Their Therapeutic Potential. Front. Neurosci. 13:1361. doi: 10.3389/fnins.2019.01361
Received: 28 April 2019; Accepted: 02 December 2019;
Published: 14 January 2020.
Edited by:
Elisa L. Hill-Yardin, RMIT University, AustraliaReviewed by:
Ben Nephew, Worcester Polytechnic Institute, United StatesJolanta B. Zawilska, Medical University of Lodz, Poland
Copyright © 2020 Yong, Tong, Chew and Lim. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Wei Ling Lim, d2VpbGluZ2xAc3Vud2F5LmVkdS5teQ==
 Shin Jie Yong
Shin Jie Yong Tommy Tong
Tommy Tong Jactty Chew
Jactty Chew Wei Ling Lim
Wei Ling Lim