
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CORRECTION article
Front. Neurosci., 29 January 2020
Sec. Neurogenomics
Volume 13 - 2019 | https://doi.org/10.3389/fnins.2019.01344
This article is part of the Research TopicMulti-“omics” in Neurodevelopmental DisordersView all 5 articles
This article is a correction to:
2-Pyrrolidinone and Succinimide as Clinical Screening Biomarkers for GABA-Transaminase Deficiency: Anti-seizure Medications Impact Accurate Diagnosis
 Adam D. Kennedy1†
Adam D. Kennedy1† Kirk L. Pappan1
Kirk L. Pappan1 Taraka Donti2†
Taraka Donti2† Mauricio R. Delgado3
Mauricio R. Delgado3 Marwan Shinawi4
Marwan Shinawi4 Toni S. Pearson5
Toni S. Pearson5 Seema R. Lalani2
Seema R. Lalani2 William J. Craigen2
William J. Craigen2 V. Reid Sutton2
V. Reid Sutton2 Anne M. Evans1
Anne M. Evans1 Qin Sun2
Qin Sun2 Lisa T. Emrick2,6
Lisa T. Emrick2,6 Sarah H. Elsea2*
Sarah H. Elsea2*A Corrigendum on
2-Pyrrolidinone and Succinimide as Clinical Screening Biomarkers for GABA-Transaminase Deficiency: Anti-seizure Medications Impact Accurate Diagnosis
by Kennedy, A. D., Pappan, K. L., Donti, T., Delgado, M. R., Shinawi, M., Pearson, T. S., et al. (2019). Front. Neurosci. 13:394. doi: 10.3389/fnins.2019.00394
In the original article, there was a mistake in Figure 1 and Figure 3A as published. “Succinamic acid” was omitted from the pathway in Figure 1 and Figure 3A. The corrected figures and figure legends appear below.
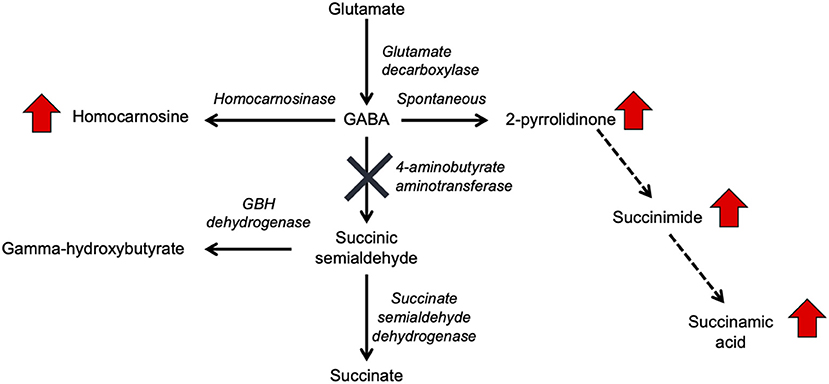
Figure 1. GABA metabolism pathways are altered due to GABA-transaminase deficiency and treatment affecting GABA metabolism. The entire pathway from glutamate conversion to GABA through succinate formation is represented along with the respective enzymes for each step. Due to tissue-specific expression of the enzymes, not all molecules are detected in each biological matrix (e.g., homocarnosine is present below the limit of detection in plasma).
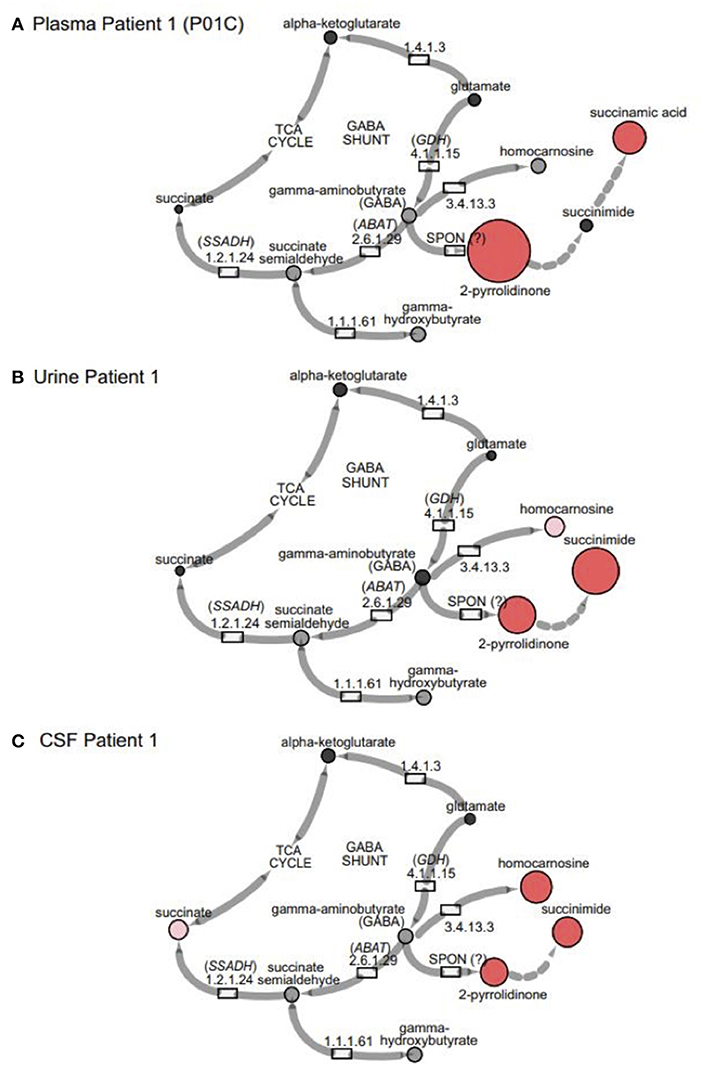
Figure 3. GABA metabolites are altered in GABA-transaminase deficiency and in use of treatments affecting GABA metabolism. Representative pathway images are shown for GABA-transaminase deficiency Patient 1 in (A) EDTA Plasma, (B) Urine, and (C) CSF. Each image shows the relative accumulation of biochemicals (red circles) or trending increases (pink circles, 1.5 ≤ Z < 2). The size of each of the circles is representative of the Z-score for that biochemical. Black circles represent molecules with Z-scores between −1.5 and 1.5 (−1.5 < Z < 1.5) or detected rare molecules for which a Z-score could not be calculated. Gray circles represent biochemicals in the library but not detected in the samples using Cytoscape to delineate biochemical pathways (http://cytoscape.org) (Shannon et al., 2003). All enzymes in the pathway are denoted by their EC designations. GDH, glutamate dehydrogenase; SSADH, succinic semialdehyde dehydrogenase; ABAT, aminobutyrate aminotransferase; SPON, spontaneous.
Additionally, there was a mistake in Figure 4B as published. “Succinimide” was mistakenly used as the primary biomarker in plasma for the original data analysis, but “succinamic acid” is the proper biomarker. The data have been reanalyzed with succinamic acid to reflect this error. The corrected figure and figure legend appears below.
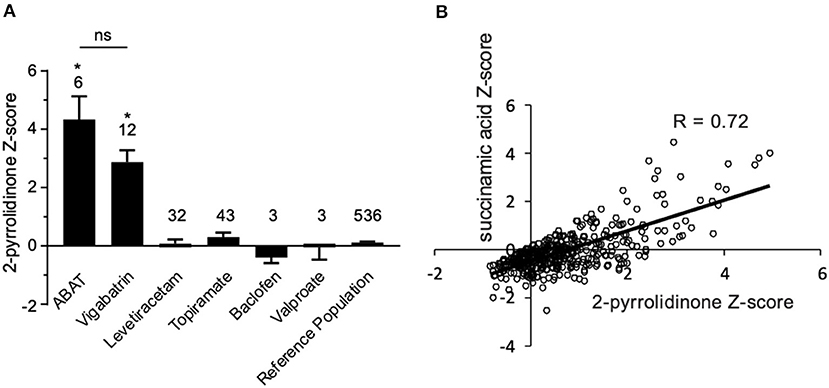
Figure 4. Box plot profile for 2-pyrrolidinone levels detected in plasma for all clinical samples. (A) Z-scores for all clinical EDTA plasma samples are plotted using a box plot format. ABAT cases show elevated Z-scores for 2-pyrrolidinone versus those patients without GABA-transaminase deficiency and treated with vigabatrin, topiramate, and/or valproate. If patients were receiving vigabatrin in addition to other treatments, they were grouped with vigabatrin. Bars represent the mean +/– the standard error of the mean (SEM) of the Z-scores for 2-pyrrolidinone for each group. The numbers above the bars represent the number of unique patient samples for each group. *p < 0.05. NS indicates a comparison which was not statically significant, p > 0.05. (B) Correlation of 2-pyrolidinone levels to succinamic acid levels in clinical plasma samples. Samples which had both 2-pyrrolidinone and succinamic acid Z-scored (n = 409) are plotted showing a significant positive correlation with these two molecules.
Table 1 and Table 2 have also been updated to reflect these changes:
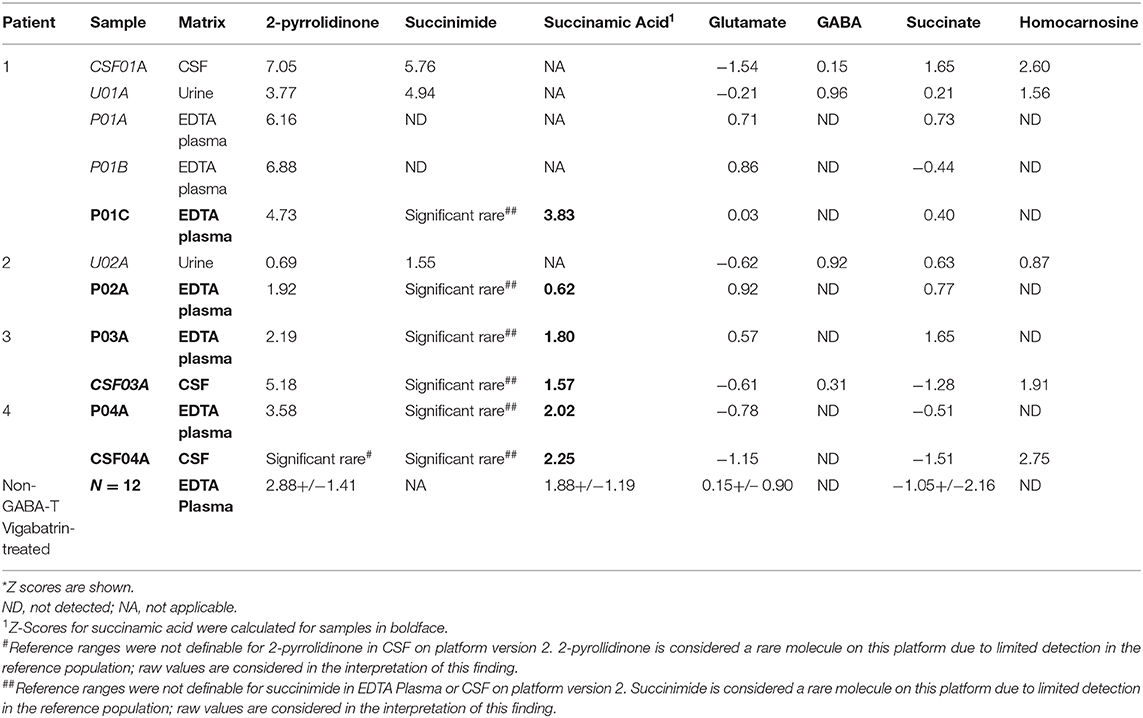
Table 2. Metabolomics identifies altered levels of molecules connected to GABA metabolism in GABA-transaminase deficiency patients*.
In line with the changes made above, a correction has also been made to the Abstract:
“Broad-scale untargeted biochemical phenotyping is a technology that supplements widely accepted assays, such as organic acid, amino acid, and acylcarnitine analyses typically utilized for the diagnosis of inborn errors of metabolism. In this study, we investigate the analyte changes associated with 4-aminobutyrate aminotransferase (ABAT, GABA transaminase) deficiency and treatments that affect GABA metabolism. GABA-transaminase deficiency is a rare neurodevelopmental and neurometabolic disorder caused by mutations in ABAT and resulting in accumulation of GABA in the cerebrospinal fluid (CSF). For that reason, measurement of ABA in CSF is currently the primary approach to diagnosis. GABA-transaminase deficiency results in severe developmental delay with intellectual disability, seizures, and movement disorder, and is often associated with death in childhood. Using an untargeted metabolomics platform, we analyzed EDTA plasma, urine, and CSF specimens from four individuals with GABA-transaminase deficiency to identify biomarkers by comparing the biochemical profile of individual patient samples to a pediatric-centric population cohort. Metabolomic analyses of over 1,000 clinical plasma samples revealed a rich source of biochemical information. Three out of four patients showed significantly elevated levels of the molecule 2-pyrrolidinone (Z-score ≥ 2) in plasma, and whole exome sequencing revealed variants of uncertain significance in ABAT. Additionally, these same patients also had elevated levels of succinimide or its ring-opened form, succinamic acid, in plasma, urine, and CSF and/or homocarnosine in urine and CSF. In the analysis of clinical EDTA plasma samples, the levels of succinamic acid and 2-pyrrolidinone showed a high level of correlation (R = 0.72), indicating impairment in GABA metabolism and further supporting the association with GABA-transaminase deficiency and the pathogenicity of the ABAT variants. Further analysis of metabolomic data across our patient population revealed the association of elevated levels of 2-pyrrolidinone with administration of vigabatrin, a commonly used anti-seizure medication and a known inhibitor of GABA-transaminase. These data indicate that anti-seizure medications may alter the biochemical and metabolomic data, potentially impacting the interpretation and diagnosis for the patient. Further, these data demonstrate the power of combining broad scale genotyping and phenotyping technologies to diagnose inherited neurometabolic disorders and support the use of metabolic phenotyping of plasma to screen for GABA-transaminase deficiency.”
to the Introduction, paragraph two:
“GABA has a relatively short half-life in plasma, is rapidly absorbed, and has both endocrine and hormonal effects (Abel and McCandless, 1992; Li et al., 2015; Maguire et al., 2015). GABA serves as the primary inhibitory neurotransmitter in the human nervous system, and GABA metabolism to succinic semialdehyde helps to regulate its levels and neurotransmitter activity. The accumulation of GABA, either through enzymatic inactivity of ABAT or medical intervention, can result in elevated levels of 2-pyrrolidinone, due to cyclization of GABA (Callery et al., 1978). Conversely, 2-pyrrolidinone can be converted to GABA when it is administered intravenously (Callery et al., 1979) or orally (Fasolato et al., 1988). 2-pyrrolidinone can be converted to succinimide through a two-step reaction (Bandle et al., 1984), and hydrolytic ring opening of cyclic imides such as succinimide can occur through enzymatic (Maguire and Dudley, 1978) and non-enzymatic (Kurono et al., 2008; Lerner et al., 2013) routes.”
and to the Results, paragraph three:
“By the time additional samples from subsequent ABAT patients were acquired, the configuration of the metabolomics platform had matured. The description of the platform configuration is outlined in the Materials and Methods section, and Table 1 delineates which samples were run on the respective configurations of the platform. Four plasma samples, one from each of the four patients, were subsequently analyzed. 2-pyrrolidinone Z-scores ranged from 1.92 to 4.73 across these four EDTA plasma samples (Table 2). For the EDTA plasma sample from Patient 1, succinamic acid, or ring-opened succinimide, showed a relative elevation (Z = 3.83), and Z-scores for succinamic acid in EDTA plasma and CSF samples from the other GABA-transaminase deficiency patients ranged from 0.62 to 2.25 (Table 2). Succinimide was detected in all EDTA plasma and CSF samples from GABA-transaminase deficiency patients analyzed on platform 2 but was not detected in enough of the healthy reference control population samples to permit the calculation of Z-score reference ranges. Of all clinical EDTA plasma samples run on platform version 2, Z-scores were obtained for both succinamic acid and 2-pyrrolidinone in 409 samples. Correlation of the values within these samples for succinamic acid and 2-pyrrolidinone showed a high degree of correlation (R = 0.72, Figure 4B). The considerable correlation between 2-pyrrolidinone and succinamic acid is suggestive of a product-substrate relationship, even though the biological mechanism leading to the conversion of one to the other is only partially understood.”
Lastly, an author name was incorrectly spelled as “William E. Craigen”. The correct spelling is “William J. Craigen”.
The authors apologize for these errors and state that they do not change the scientific conclusions of the article in any way. The original article has been updated.
Kurono, M., Itogawa, A., Noguchi, H., and Sanjoba, M. (2008). Stability and hydrolysis kinetics of spirosuccinimide type inhibitors of aldose reductase in aqueous solution and retardation of their hydrolysis by the target enzyme. J. Pharm. Sci. 97, 1468–1483. doi: 10.1002/jps.21049
Lerner, E., Hilzenrat, G., Amir, D., Tauber, E., Garini, Y., and Haas, E. (2013). Preparation of homogeneous samples of double-labelled protein suitable for single-molecule FRET measurements. Anal. Bioanal. Chem. 405, 5983–5991. doi: 10.1007/s00216-013-7002-2
Keywords: 2-pyrrolidinone, vigabatrin, GABA, neurometabolic, inborn error of metabolism, neurotransmitter, 4-aminobutyrate aminotransferase deficiency, GABA-transaminase deficiency
Citation: Kennedy AD, Pappan KL, Donti T, Delgado MR, Shinawi M, Pearson TS, Lalani SR, Craigen WJ, Sutton VR, Evans AM, Sun Q, Emrick LT and Elsea SH (2020) Corrigendum: 2-Pyrrolidinone and Succinimide as Clinical Screening Biomarkers for GABA-Transaminase Deficiency: Anti-seizure Medications Impact Accurate Diagnosis. Front. Neurosci. 13:1344. doi: 10.3389/fnins.2019.01344
Received: 09 November 2019; Accepted: 28 November 2019;
Published: 29 January 2020.
Edited and reviewed by: Cristian Bonvicini, Centro San Giovanni di Dio Fatebenefratelli (IRCCS), Italy
Copyright © 2020 Kennedy, Pappan, Donti, Delgado, Shinawi, Pearson, Lalani, Craigen, Sutton, Evans, Sun, Emrick and Elsea. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Sarah H. Elsea, U2FyYWguRWxzZWFAYmNtLmVkdQ==; ZWxzZWFAYmNtLmVkdQ==
†Present address: Adam D. Kennedy, Baebies, Inc., Morrisville, NC, United States
Taraka Donti, PerkinElmer Genetics, Pittsburgh, PA, United States
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.