- Key Laboratory of Ethnomedicine for Ministry of Education, Center on Translational Neuroscience, College of Life and Environmental Sciences, Minzu University of China, Beijing, China
Background: Guillain Barré Syndrome (GBS) is an autoimmune disorder caused by the immune-mediated damage of the peripheral nervous system. Increasing evidence suggests that inflammatory cytokines are important mediators for the onset and progression of GBS. A number of clinical studies have demonstrated elevated levels of T helper-1 (Th1-), Th2-, and Th17-related cytokines in patients with GBS; however, the results were inconsistent across studies.
Methods: We performed a systematic review and a meta-analysis of studies comparing the levels of inflammatory cytokines in the cerebrospinal fluid and peripheral blood between patients with GBS and healthy individuals, using Comprehensive Meta-Analysis Version 2 software. A database search identified 30 studies comprising 1,302 patients with GBS and 1,073 healthy controls.
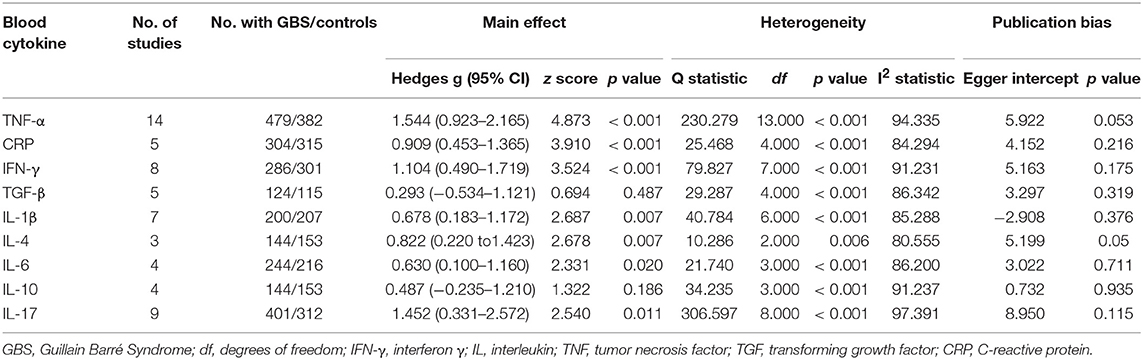
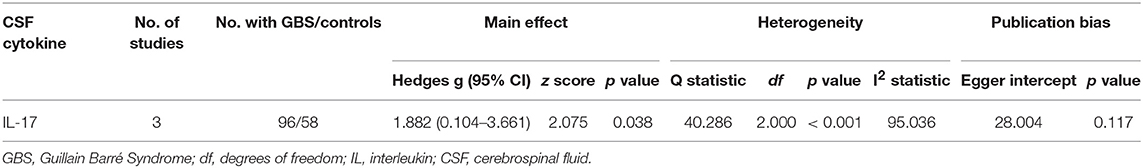
Results: The random-effects meta-analysis demonstrated that peripheral blood tumor necrosis factor-α (Hedges g, 1.544; 95% confidence interval (CI), 0.923–2.165; p < 0.001), interleukin-1β (IL-1β; Hedges g, 0.678; 95% CI, 0.183–1.172; p = 0.007), IL-6 (Hedges g, 0.630; 95% CI, 0.100–1.160; p = 0.02), IL-4 (Hedges g, 0.822; 95% CI, 0.220–1.423; p = 0.007), IL-17 (Hedges g, 1.452; 95% CI, 0.331–2.573; p = 0.011), interferon-γ (Hedges g, 1.104; 95% CI, 0.490–1.719; p < 0.001), and C-reactive protein (Hedges g, 0.909; 95% CI, 0.453–1.365; p < 0.001) levels were significantly increased in patients with GBS when compared with healthy controls. Contrastingly, the blood IL-10 and transforming growth factor-β levels were not significantly associated with GBS. Furthermore, the meta-analysis found that cerebrospinal fluid IL-17 levels were significantly associated with GBS (Hedges g, 1.882; 95% CI, 0.104–3.661; p = 0.038).
Conclusion: Altogether, our results clarified the circulating inflammatory cytokine profile in patients with GBS, and revealed that Th1-, Th2-, and Th17-related cytokines were highly elevated in the GBS patients, suggesting the potential use of these cytokines as biomarkers for GBS.
Introduction
Guillain Barré Syndrome (GBS) is an immune-mediated disorder of the peripheral nervous system characterized by muscle weakness (Eldar and Chapman, 2014). GBS has a number of subtypes, among which the most common subtype is the acute inflammatory demyelinating polyradiculoneuropathy, followed by acute motor axonal neuropathy (van den Berg et al., 2014). The disease prevalence has been reported to be one or two cases per 100,000 individuals every year, and most of the patients had an infection before the onset of the disease (van den Berg et al., 2014). Although it is generally believed that the post-infectious immune dysfunction-mediated demyelination of the peripheral nervous system is the cause of GBS, the precise etiology of the disease is still inadequately understood.
It has been proposed that a series of immune responses including macrophage and complement activation, and T-cell mediated cytotoxicity causes the demyelination of the peripheral nervous system and axonal damage leading to the onset of GBS (Hartung and Toyka, 1990; Wanschitz et al., 2003; Debnath et al., 2018b). It is well-known that the integrated actions of various cytokines play crucial roles in the differentiation and activation of immune cells such as B lymphocytes, T lymphocytes, and macrophages. Thus, cytokines are thought to be important mediators in the upstream and downstream processes of many inflammatory diseases. Indeed, various studies suggest that cytokines produced by various T-cell lineages including Th1, Th2, and Th17 play important roles in the onset and progression of GBS (Zhang et al., 2013; Li et al., 2014). Several studies demonstrated that the levels of inflammatory cytokines–tumor necrosis factor-α (TNF-α), interleukin-6 (IL-6), interferon-γ (IFN-γ), and IL-17–were elevated in the patients with GBS compared to healthy controls (Hohnoki et al., 1998; Ossege et al., 2000; Nyati et al., 2011; Han et al., 2014). However, other studies did not show significant differences between patients with GBS and controls for these cytokines (Exley et al., 1994; Press et al., 2001; Beppu et al., 2015), and moreover, one study showed that blood IL-17 levels were significantly down-regulated in the patients with GBS (Doncel-Perez et al., 2016). Therefore, a systematic review and meta-analysis is necessary to address the inconsistencies in available data.
In this study, we performed a systematic review of literature on cytokines in the cerebrospinal fluid (CSF) and blood of patients with GBS and compared the data with that of healthy controls, and pooled the individual cytokine data from included studies with a meta-analytic technique. Moreover, we used sub-group and meta-regression analyses to address the between-study heterogeneity found in this meta-analysis.
Materials and Methods
This systematic review and meta-analysis was performed according to the PRISMA (Preferred Reporting Items for Systematic reviews and Meta-analysis) guidelines (Moher et al., 2009).
Search Strategy and Study Selection
A systematic review of peer-reviewed English articles was performed by two independent investigators, and the PubMed and Web of Science databases were searched until November 2018. The search terms used in this systematic review were as follows: (inflammation or cytokine or chemokine or tumor necrosis factor or interleukin or interferon or C-reactive protein) and (GBS). We included studies with reported data on CSF and blood cytokine levels in patients with GBS and healthy, control individuals. The exclusion criteria were as follows: (1) Ex vivo data with reported cytokine concentrations; (2) data without a control group; (3) single case study, and (4) Cytokines were measured in less than three studies.
Data Extraction
The data were extracted by one investigator and checked by another. We extracted data on sample size, mean cytokine concentration, standard deviation (SD), and p-value to calculate the effective size (ES). We also extracted age, gender, sample source, disease duration, disease severity, diagnosis, and medication status for potential moderator analysis (Supplementary Table 1).
Data Analysis
The statistical analyses for the cytokine differences between cases and controls in the meta-analysis were performed using Comprehensive Meta-Analysis Version 2 software (Biostat Inc., Englewood, NJ, USA) (Du et al., 2018). The sample size, mean cytokine concentration, and SD were used to calculate the ES. If cytokine concentration data were not available, the ESs were generated by sample size and p-value. We calculated the ES as standardized mean differences (SMD) of cytokine concentrations between patients with GBS and healthy individuals, and converted the values to Hedges g, which adjusted the ES based on the sample size (Wei et al., 2018). Each blood or CSF cytokine analyzed in this study was subjected to meta-analysis for providing an ES estimate. The random-effects meta-analysis was chosen in this study because both between-study and within-study heterogeneities were postulated to influence the true ES (Qin et al., 2016). Moreover, we used a sensitivity analysis, by removing one study at a time, to determine whether the statistical significance was influenced by any single study.
The Cochrane's Q-test and I2 index were used to determine the between-study heterogeneity as described previously (Chen et al., 2018). It should be noted that for the Cochrane's Q-test, p < 0.1 was considered statistically significant; I2 index of 075, 0.50, and 0.25 denoted high, moderate, and small levels of heterogeneity, respectively. Furthermore, we used unrestricted maximum-likelihood random-effects meta-regressions of ES to assess whether age, gender (proportion of male), and publication year influenced the outcomes of the meta-analysis. Additionally, we evaluated publication bias using Egger's test which assesses the asymmetry of the funnel plot.
We considered p < 0.05 to be statistically significant except where noted.
Results
The initial search retrieved 761 records from PubMed and 427 records from Web of Science. After screening the titles and abstracts of the 1,188 records, 60 relevant studies were selected for full text scrutiny. Thirty of the 60 studies were excluded after reading the full text because they lacked the necessary data (n = 7); lack of a control group (n = 9); reported ex vivo cytokine data (n = 4); single case reports (n = 2), and blood or CSF cytokines were analyzed in less than three studies (n = 8). Thus, a total of 30 studies comprising 1,302 patients with GBS and 1,073 healthy individuals were included in the meta-analysis (Sharief et al., 1993; Exley et al., 1994; Sindern et al., 1996; Creange et al., 1998; Hohnoki et al., 1998; Ossege et al., 2000; Press et al., 2001; Radhakrishnan et al., 2003, 2004; Deng et al., 2008; Nyati et al., 2010, 2011; Sainaghi et al., 2010; Li et al., 2012, 2013, 2014, 2018a,b; Liang et al., 2012; Wang et al., 2012; Han et al., 2014; Beppu et al., 2015; Du et al., 2015; Chang et al., 2016; Doncel-Perez et al., 2016; Zhang et al., 2016; Kharwar et al., 2017; Debnath et al., 2018a,b; Ethemoglu and Calik, 2018) (Flowchart see Figure 1).
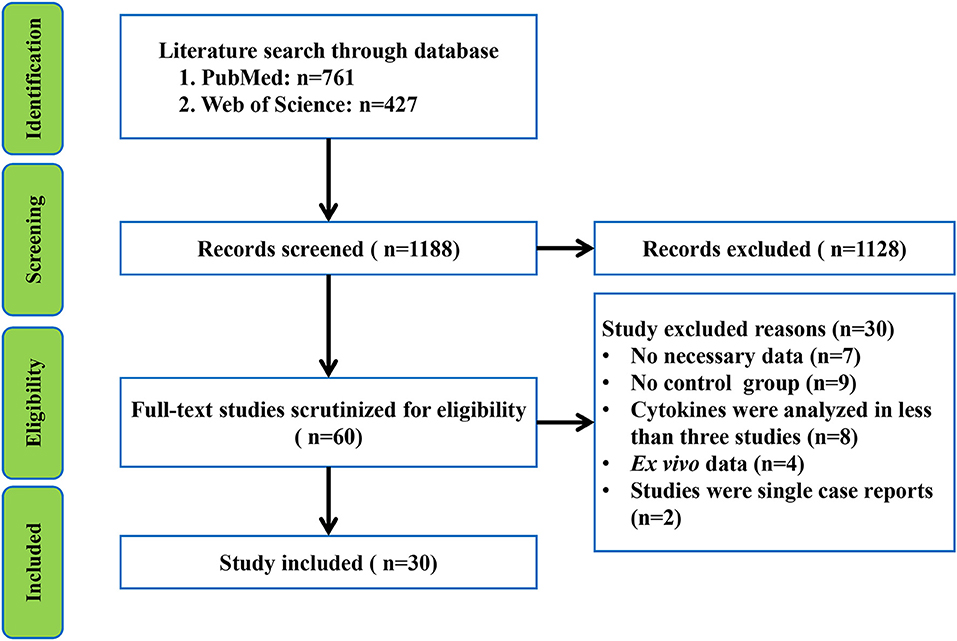
Figure 1. Preferred Reporting Items for Systematic reviews and Meta-analysis flowchart of the literature search.
Main Associations of GBS With Blood Cytokines
Random-effects meta-analysis showed that patients with GBS had significantly elevated TNF-α (Hedges g, 1.544; 95% confidence interval (CI), 0.923–2.165; p < 0.001), IL-1β (IL-1β; Hedges g, 0.678; 95% CI, 0.183–1.172; p = 0.007), IL-6 (Hedges g, 0.630; 95% CI, 0.100–1.160; p = 0.02), IL-4 (Hedges g, 0.822; 95% CI, 0.220–1.423; p = 0.007), IL-17 (Hedges g, 1.452; 95% CI, 0.331–2.572; p = 0.011), IFN-γ (Hedges g, 1.104; 95% CI, 0.490–1.719; p < 0.001), and C-reactive protein (CRP; Hedges g, 0.909; 95% CI, 0.453–1.365; p < 0.001) levels when compared with healthy controls (Figures 2, 3; Table 1). However, blood IL-10 and transforming growth factor- β (TGF-β) levels did not show significant differences between the cases and controls (Table 1).

Figure 2. Studies of blood tumor necrosis factor-α (TNF-α), interferon-γ (IFN-γ), and interleukin-17 (IL-17) in Guillain Barré Syndrome. Forest plot displaying random-effects meta-analysis results of the association between IFN-γ (A), TNF-α (B), IL-17 (C) and Guillain Barré Syndrome. The sizes of the squares are proportional to study weights.
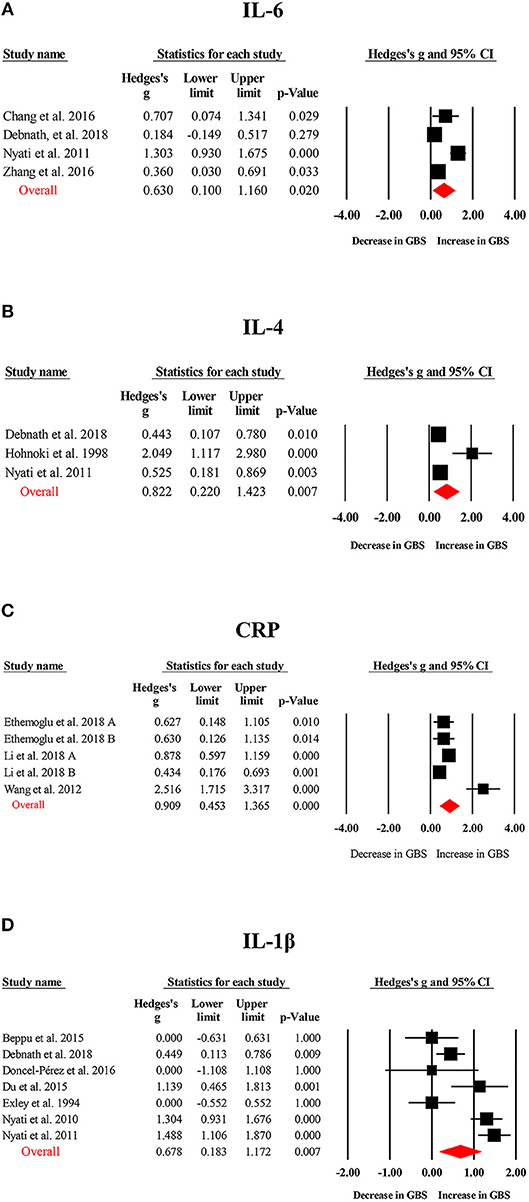
Figure 3. Studies of blood IL-1β, IL-4, IL-6, and CRP in Guillain Barré Syndrome. Forest plot displaying random-effects meta-analysis results of the association between IL-6 (A), IL-4 (B), CRP (C), IL-1β (D) and Guillain Barré Syndrome. The sizes of the squares are proportional to study weights.
Main Associations of GBS With CSF Cytokines
Only CSF IL-17 were analyzed in the three studies included in this meta-analysis, and the results from the meta-analysis indicated that CSF IL-17 levels were significantly increased in patients with GBS compared to the healthy controls (Figure 4; Table 2).
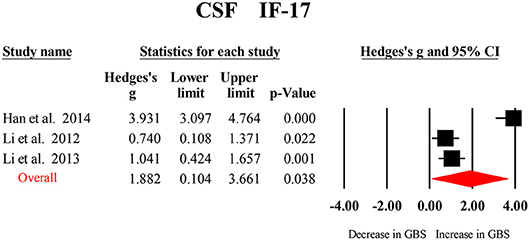
Figure 4. Studies of cerebrospinal fluid IL-17 in Guillain Barré Syndrome. Forest plot displaying random-effects meta-analysis result of the association between IL17 and Guillain Barré Syndrome. The sizes of the squares are proportional to study weights.
Investigation of Heterogeneity
Our analyses suggested that all the blood and CSF cytokines had high levels of between-study heterogeneity (Tables 1, 2). Subsequently, we tried to perform sub-group and meta-regression analyses to address whether the relevant categorical and continuous variables would affect the between-study heterogeneity. As shown in the Supplementary Table 1, the data on disease duration, disease severity, and medication status were limited, and therefore, meta-regressions were performed on age, gender, and publication year. Additionally, since most cytokines were not analyzed in less than 10 studies, blood TNF-α was chosen for sub-group and meta-regression analyses.
Meta-regression analyses suggested that age, gender, and publication year did not significantly affect the outcomes of the meta-analysis (p > 0.05 in all the analyses).
Sub-group analyses based on sampling source performed for blood TNF-α did not change the impact of heterogeneity (Q = 165.152, df = 8, p < 0.001, I2 = 95.156), and the significance of the association between elevated serum TNF-α levels and GBS was retained (Hedges g, 1.869; 95% CI, 0.948–2.790; p < 0.001). Contrastingly, there was no significant association between plasma TNF-α levels and GBS (Hedges g, 0.660; 95% CI, −0.068–1.388; p = 0.076), whereas, a mild reduction was noted in the impact of heterogeneity (Q = 12.465, df = 2, p = 0.002, I2 = 83.955).
A sensitivity analysis was additionally performed, and the results indicated that the significant association between blood TNF-α and GBS was not influenced by any single study. Furthermore, the Egger test suggested no significant publication bias risk for most cytokines included in the meta-analysis (Tables 1, 2).
Discussion
Our results demonstrate that blood inflammatory cytokines—TNF-α, IFN-γ, IL-1β, IL-4, IL-6, IL-17, and CRP levels—are significantly increased in the patients with GBS. We found elevated levels of IL-17 in the CSF of patients with GBS. Contrastingly, no significant association was found between blood levels of IL-10 and TGF-β and GBS. For cytokines significantly associated with GBS, the values of ES associated with the results of CSF IL-17, blood TNF-α, IFN-γ, IL-4, IL-17, and CRP were large, and medium to large for blood IL-1β and IL-6. Sensitivity analysis suggested that a significant association between blood TNF-α and GBS was not influenced by any individual study, and no significant publication bias risks were observed for most cytokines included in the meta-analysis. Therefore, the results demonstrate the robustness of the outcomes of the meta-analysis. Although the literature provided inconsistent clinical data for cytokine aberrations in the patients with GBS, this study used the meta-analytic technique to pool the cytokine data and clarify the profile of inflammatory cytokines in GBS patients. Altogether, our results show that Th1-, Th2-, and Th17-related cytokines were highly elevated in patients with GBS, and therefore, provides strong clinical evidence for better understanding the etiology of GBS.
Results from this meta-analysis suggest that blood IL-10 and TGF-β has no significant association with GBS. However, it is unknown whether the non-significant associations were due to the limited number of studies with small sample sizes for these cytokines. For example, a previous meta-analysis by Swardfager et al. published in 2010 could only show the significant increase in the CSF TGF-β levels in patients with Alzheimer's disease (Swardfager et al., 2010), whereas, a recent meta-analysis by Chen et al. included more studies and suggested that CSF TGF-β, MCP-1, and YLK-40 levels were elevated in the patients with Alzheimer's disease (Chen et al., 2018). In addition to the increased circulating levels of inflammatory cytokines in the patients with Alzheimer's diseases, previous meta-analyses also assessed the cytokine levels in other neurodegenerative diseases of the central nervous system, including Parkinson's disease (Qin et al., 2016) and amyotrophic lateral sclerosis (Hu et al., 2017). However, the values of ES associated with the results of cytokines were mostly small to medium, presenting difficulties in the use of cytokines for informed diagnosis, prognosis, and treatment response for Alzheimer's disease, Parkinson's disease, and amyotrophic lateral sclerosis. Contrastingly, our meta-analysis indicated that the ES values associated with the results of cytokines were mostly large. The large ES values for the associations between blood cytokines and GBS are justified, since GBS is an autoimmune disease which damages the peripheral nervous system, suggesting that cytokines are closely associated with the pathophysiology of GBS. In fact, it has been demonstrated that the infiltrating T cell-produced TNF-α has a direct myelinotoxic effect on myelinated fibers which leads to demyelination in nerve cells, and TNF-α can also affect the synthesis of myelin protein and glycolipids (Lisak et al., 1997; Nyati and Prasad, 2014). Furthermore, in an animal model of GBS-experimental autoimmune neuritis (EAN), it has been shown that the severity of clinical signs of EAN was associated with IL-17 accumulation in the sciatic nerve, and administration of IL-17 exacerbated the clinical signs of the acute phase in chronic EAN (Pelidou et al., 2000). The potential role of IL-17 in the pathogenesis of GBS was further supported by the results suggesting that down-regulation of IL-17 in peripheral blood and /or sciatic nerves contributed to the treatment effects of FTY720, atorvastatin and AUY954 on EAN (Wu et al., 2016). Altogether, these results suggest that cytokine aberrations are pathogenic in GBS.
High levels of between-study heterogeneity were found for all the cytokines analyzed in this meta-analysis. We tried to adjust for potential confounders which may explain the between-study heterogeneity with sub-groups and the results of the meta-regression analyses. However, meta-regression analyses based on age, gender, and publication year did not address the between-study heterogeneity. Although the sub-group analyses indicated a sampling source to partially explain the between-study heterogeneity, while the lower heterogeneity in plasma cytokine-based studies may be due to the low power of the test for heterogeneity in meta-analyses with a smaller number of studies. Moreover, we found that serum TNF-α levels we significantly elevated in the patients with GBS, whereas no significant association between plasma TNF-α levels and GBS was observed, suggesting that the difference of sample sources may influence results of cytokine study. However, it is likely that the small number of studies on plasma TNF-α levels in GBS made it difficult to observe statistical significance. Other clinical variables such as disease duration, disease severity, and medication status may contribute to the between-study heterogeneity in the meta-analysis. In fact, data from Deng et al. show increased Th1-related cytokine, IL-12p70, levels in the serum of patients with GBS, and intravenous immunoglobulin therapy down-regulated the IL-12p70 levels into healthy controls (Deng et al., 2008). Similarly, Radhakrishnan et al. demonstrated that intravenous immunoglobulin therapy in patients with GBS reduced the blood TNF-α levels (Radhakrishnan et al., 2004). However, most studies included in this meta-analysis did not reveal the medication status. This limitation prevented us from performing a meta-analysis to investigate the usefulness of cytokines as biomarkers of treatment responses in GBS.
Another limitation of this meta-analysis is that most of the included studies did not provide information on the stage of GBS. Indeed, Nyati et al. showed that in comparison to healthy controls, blood TNF-α and IL-1β levels were significantly increased in patients in the progressive phase of GBS, whereas, TNF-α and IL-1β levels did not show significant differences between GBS patients and healthy individuals at the recovery phase (Nyati et al., 2010). Therefore, it is likely that the between-study heterogeneity found in this meta-analysis was contributed by the different stages of patients across studies. Nevertheless, these results highlight the need for continuous investigations into the cytokine aberrations in patients with GBS and controlling the relevant clinical and methodological variables to better understand the mechanisms underlying the disease. The last limitation of this study is that only English–language articles were included. However, owing to the limited number of non-English records in the literature on the associations between cytokines and GBS, the outcomes of our meta-analysis are unlikely to be significantly influenced by the non-English articles.
In conclusion, this is the first systematic review and meta-analysis to assess the dysfunctions of blood and CSF inflammatory cytokines in patients with GBS. Our analysis indicates that the peripheral blood levels of inflammatory cytokines are highly elevated in patients with GBS. Therefore, the cytokines have potential to be used as biomarkers to inform diagnosis, prognosis, or treatment responses in GBS, and future studies are necessary to validate this hypothesis.
Author Contributions
YC and QL conceived and designed the study. TS and XC performed the literature search and data analysis. YC drafted the manuscript with critical revisions from TS, XC, SS, and QL.
Funding
This study was supported by the National Science Foundation of China (81703492, 81774006), Beijing Natural Science Foundation (7182092), and the Minzu University Research Fund (2018CXTD03) and the MUC 111 project.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnins.2019.00717/full#supplementary-material
References
Beppu, M., Sawai, S., Misawa, S., Sogawa, K., Mori, M., Ishige, T., et al. (2015). Serum cytokine and chemokine profiles in patients with chronic inflammatory demyelinating polyneuropathy. J. Neuroimmunol. 279, 7–10. doi: 10.1016/j.jneuroim.2014.12.017
Chang, K. H., Lyu, R. K., Ro, Y. S., Chen, Y. C., Ro, L. S., Chang, H. S., et al. (2016). Increased serum concentrations of transforming growth factor-beta1 (TGF-beta1) in patients with Guillain-Barre syndrome. Clin. Chim. Acta 461, 8–13. doi: 10.1016/j.cca.2016.07.013
Chen, X., Hu, Y., Cao, Z., Liu, Q., and Cheng, Y. (2018). Cerebrospinal fluid inflammatory cytokine aberrations in Alzheimer's disease, Parkinson's disease and amyotrophic lateral sclerosis: a systematic review and meta-analysis. Front. Immunol. 9:2122. doi: 10.3389/fimmu.2018.02122
Creange, A., Belec, L., Clair, B., Degos, J. D., Raphael, J. C., and Gherardi, R. K. (1998). Circulating transforming growth factor beta 1 (TGF-beta1) in Guillain-Barre syndrome: decreased concentrations in the early course and increase with motor function. J. Neurol. Neurosurg. Psychiatr. 64, 162–165. doi: 10.1136/jnnp.64.2.162
Debnath, M., Nagappa, M., Subbanna, M., Sundaravadivel, P., Talukdar, P. M., Shivakumar, V., et al. (2018a). Th17 pathway signatures in a large Indian cohort of Guillain Barre syndrome. J. Neuroimmunol. 323, 125–130. doi: 10.1016/j.jneuroim.2018.08.001
Debnath, M., Nagappa, M., Talukdar, P. M., Subbanna, M., Sundaravadivel, P., Shivakumar, V., et al. (2018b). Comprehensive cytokine profiling provides evidence for a multi-lineage Th response in Guillain Barre Syndrome. Cytokine 110, 58–62. doi: 10.1016/j.cyto.2018.04.026
Deng, H., Yang, X., Jin, T., Wu, J., Hu, L. S., Chang, M., et al. (2008). The role of IL-12 and TNF-alpha in AIDP and AMAN. Eur. J. Neurol. 15, 1100–1105. doi: 10.1111/j.1468-1331.2008.02261.x
Doncel-Perez, E., Mateos-Hernandez, L., and Pareja, E. (2016). Expression of early growth response gene-2 and regulated cytokines correlates with recovery from Guillain-Barre syndrome. 196, 1102–1107. doi: 10.4049/jimmunol.1502100
Du, Y., Wu, H. T., Qin, X. Y., Cao, C., Liu, Y., Cao, Z. Z., et al. (2018). Postmortem brain, cerebrospinal fluid, and blood neurotrophic factor levels in Alzheimer's disease: a systematic review and meta-analysis. J. Mol. Neurosci. 65, 289–300. doi: 10.1007/s12031-018-1100-8
Du, Y., Zhang, G., Zhang, Z., Wang, Q., Ma, R., Zhang, L., et al. (2015). Toll-like receptor 2 and−4 are involved in the pathogenesis of the Guillain-Barre syndrome. Mol. Med. Rep. 12, 3207–3213. doi: 10.3892/mmr.2015.3730
Eldar, A. H., and Chapman, J. (2014). Guillain Barre syndrome and other immune mediated neuropathies: diagnosis and classification. Autoimmun. Rev. 13, 525–530. doi: 10.1016/j.autrev.2014.01.033
Ethemoglu, O., and Calik, M. (2018). Effect of serum inflammatory markers on the prognosis of adult and pediatric patients with Guillain-Barre syndrome. Neuropsychiatr. Dis. Treat. 14, 1255–1260. doi: 10.2147/NDT.S162896
Exley, A. R., Smith, N., and Winer, J. B. (1994). Tumour necrosis factor-alpha and other cytokines in Guillain-Barre syndrome. J. Neurol. Neurosurg. Psychiatr. 57, 1118–1120. doi: 10.1136/jnnp.57.9.1118
Han, R. K., Cheng, Y. F., Zhou, S. S., Guo, H., He, R. D., Chi, L. J., et al. (2014). Increased circulating Th17 cell populations and elevated CSF osteopontin and IL-17 concentrations in patients with Guillain-Barre syndrome. J. Clin. Immunol. 34, 94–103. doi: 10.1007/s10875-013-9965-3
Hartung, H. P., and Toyka, K. V. (1990). T-cell and macrophage activation in experimental autoimmune neuritis and Guillain-Barre syndrome. Ann. Neurol. 27(Suppl.), S57–63. doi: 10.1002/ana.410270716
Hohnoki, K., Inoue, A., and Koh, C. S. (1998). Elevated serum levels of IFN-gamma, IL-4 and TNF-alpha/unelevated serum levels of IL-10 in patients with demyelinating diseases during the acute stage. J. Neuroimmunol. 87, 27–32. doi: 10.1016/S0165-5728(98)00053-8
Hu, Y., Cao, C., Qin, X. Y., Yu, Y., Yuan, J., Zhao, Y., et al. (2017). Increased peripheral blood inflammatory cytokine levels in amyotrophic lateral sclerosis: a meta-analysis study. Sci. Rep. 7:9094. doi: 10.1038/s41598-017-09097-1
Kharwar, N. K., Prasad, K. N., Singh, K., Paliwal, V. K., and Modi, D. R. (2017). Polymorphisms of IL-17 and ICAM-1 and their expression in Guillain-Barre syndrome. Int. J. Neurosci. 127, 680–687. doi: 10.1080/00207454.2016.1231186
Li, C., Zhao, P., Sun, X., Che, Y., and Jiang, Y. (2013). Elevated levels of cerebrospinal fluid and plasma interleukin-37 in patients with Guillain-Barre syndrome. Mediators Inflamm. 2013:639712. doi: 10.1155/2013/639712
Li, S., Jin, T., Zhang, H. L., and Yu, H. (2014). Circulating Th17, Th22, and Th1 cells are elevated in the Guillain-Barre syndrome and downregulated by IVIg treatments. 2014:740947. doi: 10.1155/2014/740947
Li, S., Yu, M., Li, H., Zhang, H., and Jiang, Y. (2012). IL-17 and IL-22 in cerebrospinal fluid and plasma are elevated in Guillain-Barre syndrome. Mediators Inflamm. 2012:260473. doi: 10.1155/2012/260473
Li, X., Li, W., Luo, Y., Qin, L., Su, Q., and Mo, W. (2018a). Can we assess severity of Guillain-Barre syndrome using absolute monocyte count? Int. J. Lab. Hematol. 40, 488–492. doi: 10.1111/ijlh.12845
Li, X., Li, W., Shi, X., Mo, L., Luo, Y., Qin, L., et al. (2018b). Is serum bilirubin associated with the severity of Guillain-Barre syndrome? Int. J. Neurosci. 128, 595–599. doi: 10.1080/00207454.2017.1404465
Liang, S. L., Wang, W. Z., Huang, S., Wang, X. K., Zhang, S., and Wu, Y. (2012). Th17 helper cell and T-cell immunoglobulin and mucin domain 3 involvement in Guillain-Barre syndrome. Immunopharmacol. Immunotoxicol. 34, 1039–1046. doi: 10.3109/08923973.2012.697469
Lisak, R. P., Skundric, D., Bealmear, B., and Ragheb, S. (1997). The role of cytokines in Schwann cell damage, protection, and repair. J. Infect. Dis. 176(Suppl. 2), S173–S179. doi: 10.1086/513788
Moher, D., Liberati, A., Tetzlaff, J., Altman, D. G., and Group, P. (2009). Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 6:e1000097. doi: 10.1371/journal.pmed.1000097
Nyati, K. K., and Prasad, K. N. (2014). Role of cytokines and Toll-like receptors in the immunopathogenesis of Guillain-Barre syndrome. Mediators Inflamm. 2014:758639. doi: 10.1155/2014/758639
Nyati, K. K., Prasad, K. N., Rizwan, A., Verma, A., and Paliwal, V. K. (2011). TH1 and TH2 response to Campylobacter jejuni antigen in Guillain-Barre syndrome. Arch. Neurol. 68, 445–452. doi: 10.1001/archneurol.2011.51
Nyati, K. K., Prasad, K. N., Verma, A., and Paliwal, V. K. (2010). Correlation of matrix metalloproteinases-2 and−9 with proinflammatory cytokines in Guillain-Barre syndrome. J. Neurosci. Res. 88, 3540–3546. doi: 10.1002/jnr.22514
Ossege, L. M., Sindern, E., Voss, B., and Malin, J. P. (2000). Expression of TNFalpha and TGFbeta1 in Guillain-Barre syndrome: correlation of a low TNFalpha-/TGFbeta1-mRNA ratio with good recovery and signs for immunoregulation within the cerebrospinal fluid compartment. Eur. J. Neurol. 7, 17–25. doi: 10.1046/j.1468-1331.2000.00005.x
Pelidou, S. H., Zou, L. P., Deretzi, G., Oniding, C., Mix, E., and Zhu, J. (2000). Enhancement of acute phase and inhibition of chronic phase of experimental autoimmune neuritis in Lewis rats by intranasal administration of recombinant mouse interleukin 17: potential immunoregulatory role. Exp. Neurol. 163, 165–172. doi: 10.1006/exnr.2000.7357
Press, R., Deretzi, G., Zou, L. P., Zhu, J., Fredman, P., Lycke, J., et al. (2001). IL-10 and IFN-gamma in Guillain-Barre syndrome. Network members of the Swedish Epidemiological Study Group. J. Neuroimmunol. 112, 129–138. doi: 10.1016/S0165-5728(00)00388-X
Qin, X. Y., Zhang, S. P., Cao, C., Loh, Y. P., and Cheng, Y. (2016). Aberrations in peripheral inflammatory cytokine levels in Parkinson disease: a systematic review and meta-analysis. JAMA Neurol. 73, 1316–1324. doi: 10.1001/jamaneurol.2016.2742
Radhakrishnan, V. V., Sumi, M. G., Reuben, S., Mathai, A., and Nair, M. D. (2003). Circulating tumour necrosis factor alpha and soluble TNF receptors in patients with Guillain-Barre syndrome. Indian J. Med. Res. 117, 216–220.
Radhakrishnan, V. V., Sumi, M. G., Reuben, S., Mathai, A., and Nair, M. D. (2004). Serum tumour necrosis factor-alpha and soluble tumour necrosis factor receptors levels in patients with Guillain-Barre syndrome. Acta Neurol. Scand. 109, 71–74. doi: 10.1034/j.1600-0404.2003.00179.x
Sainaghi, P. P., Collimedaglia, L., Alciato, F., Leone, M. A., Naldi, P., Molinari, R., et al. (2010). The expression pattern of inflammatory mediators in cerebrospinal fluid differentiates Guillain-Barre syndrome from chronic inflammatory demyelinating polyneuropathy. Cytokine 51, 138–143. doi: 10.1016/j.cyto.2010.05.005
Sharief, M. K., McLean, B., and Thompson, E. J. (1993). Elevated serum levels of tumor necrosis factor-alpha in Guillain-Barre syndrome. Ann. Neurol. 33, 591–596. doi: 10.1002/ana.410330606
Sindern, E., Schweppe, K., Ossege, L. M., and Malin, J. P. (1996). Potential role of transforming growth factor-beta 1 in terminating the immune response in patients with Guillain-Barre syndrome. J. Neurol. 243, 264–268. doi: 10.1007/BF00868524
Swardfager, W., Lanctot, K., Rothenburg, L., Wong, A., Cappell, J., and Herrmann, N. (2010). A meta-analysis of cytokines in Alzheimer's disease. Biol. Psychiatry 68, 930–941. doi: 10.1016/j.biopsych.2010.06.012
van den Berg, B., Walgaard, C., Drenthen, J., Fokke, C., Jacobs, B. C., and van Doorn, P. A. (2014). Guillain-Barre syndrome: pathogenesis, diagnosis, treatment and prognosis. Nat. Rev. Neurol. 10, 469–82. doi: 10.1038/nrneurol.2014.121
Wang, Y. Z., Liang, Q. H., Ramkalawan, H., Wang, Y. L., Yang, Y. F., Zhou, W. B., et al. (2012). Expression of Toll-like receptors 2, 4 and 9 in patients with Guillain-Barre syndrome. Neuroimmunomodulation. 19, 60–68. doi: 10.1159/000328200
Wanschitz, J., Maier, H., Lassmann, H., Budka, H., and Berger, T. (2003). Distinct time pattern of complement activation and cytotoxic T cell response in Guillain-Barre syndrome. Brain. 126, 2034–2042. doi: 10.1093/brain/awg207
Wei, Z., Li, X., Li, X., Liu, Q., and Cheng, Y. (2018). Oxidative stress in Parkinson's disease: a systematic review and meta-analysis. Front. Mol. Neurosci. 11:236. doi: 10.3389/fnmol.2018.00236
Wu, X., Wang, J., Liu, K., Zhu, J., and Zhang, H. L. (2016). Are Th17 cells and their cytokines a therapeutic target in Guillain-Barre syndrome? Expert Opin. Ther. Targets 20, 209–222. doi: 10.1517/14728222.2016.1086751
Zhang, D. Q., Wang, R., Li, T., Zhou, J. P., Chang, G. Q., Zhao, N., et al. (2016). Reduced soluble RAGE is associated with disease severity of axonal Guillain-Barre syndrome. Sci. Rep. 6:21890. doi: 10.1038/srep21890
Keywords: inflammation, cytokine, Guillain Barré Syndrome, peripheral blood, cerebrospinal fluid, meta-analysis
Citation: Sun T, Chen X, Shi S, Liu Q and Cheng Y (2019) Peripheral Blood and Cerebrospinal Fluid Cytokine Levels in Guillain Barré Syndrome: A Systematic Review and Meta-Analysis. Front. Neurosci. 13:717. doi: 10.3389/fnins.2019.00717
Received: 21 February 2019; Accepted: 25 June 2019;
Published: 16 July 2019.
Edited by:
Vincenzo La Bella, University of Palermo, ItalyReviewed by:
Ghulam Md. Ashraf, King Abdulaziz University, Saudi ArabiaRuoyu Zhang, Zhejiang University, China
Kenichi Kaida, National Defense Medical College, Japan
Carmelo Rodolico, University of Messina, Italy
Copyright © 2019 Sun, Chen, Shi, Liu and Cheng. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Sha Shi, WXVubmFuc3NAMTI2LmNvbQ==; Qingshan Liu, bmxxc2hAMTYzLmNvbQ==; Yong Cheng, eW9uZ2NoZW5nQG11Yy5lZHUuY24=
 Ting Sun
Ting Sun Xi Chen
Xi Chen Qingshan Liu
Qingshan Liu Yong Cheng
Yong Cheng