
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Neurosci. , 10 July 2019
Sec. Brain Imaging Methods
Volume 13 - 2019 | https://doi.org/10.3389/fnins.2019.00618
This article is part of the Research Topic Dynamic Functioning of Resting State Networks in Physiological and Pathological Conditions View all 39 articles
Functional magnetic resonance imaging (fMRI) at resting state (RS) has been widely used to characterize the main brain networks. Functional connectivity (FC) has been mostly assessed assuming that FC is static across the whole fMRI examination. However, FC is highly variable at a very fast time-scale, as demonstrated by neurophysiological techniques. Time-varying functional connectivity (TVC) is a novel approach that allows capturing reoccurring patterns of interaction among functional brain networks. Aim of this review is to provide a description of the methods currently used to assess TVC on RS fMRI data, and to summarize the main results of studies applying TVC in healthy controls and patients with multiple sclerosis (MS). An overview of the main results obtained in neurodegenerative and psychiatric conditions is also provided. The most popular TVC approach is based on the so-called “sliding windows,” in which the RS fMRI acquisition is divided in small temporal segments (windows). A window of fixed length is shifted over RS fMRI time courses, and data within each window are used to calculate FC and its variability over time. Sliding windows can be combined with clustering techniques to identify recurring FC states or used to assess global TVC properties of large-scale functional networks or specific brain regions. TVC studies have used heterogeneous methodologies so far. Despite this, similar results have been obtained across investigations. In healthy subjects, the default-mode network (DMN) exhibited the highest degree of connectivity dynamism. In MS patients, abnormal global TVC properties and TVC strengths were found mainly in sensorimotor, DMN and salience networks, and were associated with more severe structural MRI damage and with more severe physical and cognitive disability. Conversely, abnormal TVC measures of the temporal network were correlated with better cognitive performances and less severe fatigue. In patients with neurodegenerative and psychiatric conditions, TVC abnormalities of the DMN, attention and executive networks were associated to more severe clinical manifestations. TVC helps to provide novel insights into fundamental properties of functional networks, and improves the understanding of brain reorganization mechanisms. Future technical advances might help to clarify TVC association with disease prognosis and response to treatment.
The human brain at resting state (RS) exhibits highly structured spontaneous fluctuations in functional magnetic resonance imaging (fMRI) data, which reflect the underlying network architecture (Biswal et al., 2010). RS functional connectivity (FC) captures the temporal associations between such fluctuations, and has been successfully used to characterize the main networks of the brain and map abnormalities of functional network architecture occurring in different neurological conditions. In healthy controls, RS FC strength was found to be associated to age, with RS fluctuations being strongest in adulthood and lowest in children and elderly (Mak et al., 2017). A dependency of connectivity from sex (Biswal et al., 2010; Mak et al., 2017), as well as from cognitive, emotional, and behavioral variables was also detected (Kelly et al., 2012).
Multiple sclerosis (MS) is an inflammatory and neurodegenerative disease of the central nervous system leading to a progressive increase over time of clinical disability and cognitive impairment (Filippi et al., 2017, 2018). Reorganization of brain functional networks in MS has been shown from the first RS fMRI studies (Lowe et al., 2002, 2008; Rocca et al., 2010; Roosendaal et al., 2010), which is thought to limit the clinical consequences of widespread tissue damage (Filippi et al., 2013a; Sbardella et al., 2015). Cortical reorganization has been demonstrated to be variable across the different stages of the disease, and a progressive exhaustion or inefficiency of the adaptive properties of the cerebral cortex is likely to be among the factors responsible for the worsening of clinical disability (Rocca et al., 2010, 2018; Roosendaal et al., 2010; Loitfelder et al., 2011). In neurodegenerative conditions, RS FC studies showed a progressive and gradual spreading of connectivity changes from a target brain network, reflecting specific behavioral and cognitive dysfunctions (Zhou et al., 2017). In psychiatric diseases, disruption of fronto-parietal network connectivity seems to be the common fingerprint across distinct forms of pathology (Baker et al., 2019).
However, current understanding of the role of functional abnormalities in neurological and psychiatric disorders is still incomplete, mostly due to inconsistencies in the findings from several studies. Specifically, in MS some investigations found trends toward lower RS FC vs. healthy controls in the default-mode (Rocca et al., 2010, 2012, 2018; Bonavita et al., 2011), sensorimotor (Rocca et al., 2018) and subcortical (Liu et al., 2011; Rocca et al., 2018) networks, while in other studies the opposite trends were observed (Roosendaal et al., 2010; Tona et al., 2014; Schoonheim et al., 2015). Similarly, even if RS FC abnormalities were principally located in the core regions hit by pathology, a certain variability of brain areas involved by RS FC changes was detected in neurodegenerative and psychiatric conditions (Busatto, 2013; Weiner et al., 2017).
The wide spectrum of clinical characteristics of MS patients has been considered as one of the main causes for the discrepancies described in RS fMRI literature (Filippi et al., 2013a; Sbardella et al., 2015). However, technical factors might also bias connectivity estimation, including scanner-related signal instabilities, an inappropriate control of confounding covariates, and the application of analysis methods based on inaccurate assumptions.
For instance, one of the main assumptions of classical RS FC assessment methods is that connectivity is static across the entire fMRI examination, e.g., it can be assessed by calculating the mean correlation between whole-length RS fMRI time series (Biswal et al., 2010). However, as widely evident by neurophysiological techniques, brain FC is highly variable at a very fast time-scale. The functioning human brain during any state of wakefulness repeatedly changes between different combinations of cognitive, sensorimotor, attentional, emotional, auditory, and visual-related tasks. Notably, the majority of brain regions experience continuous functional changes even during sleep (Tagliazucchi and van Someren, 2017). Thus, studying time-varying RS FC patterns is likely to shed light not only on physiological processes occurring in healthy subjects, but also to understand clinical manifestations of different neurological and psychiatric conditions. In fact, clinical symptoms associated to these diseases are likely to depend not only from damage to specific brain regions, but also from delayed (or abnormal) communication between brain areas. The study of the temporal reconfigurations of FC occurring within RS fMRI sessions has been defined as time-varying functional connectivity (TVC) (Hutchison et al., 2013; Calhoun et al., 2014; Preti et al., 2017).
The main goal of this review is to summarize the main results obtained using TVC in healthy and diseased populations. A particular focus is given to studies of patients with MS; however, the main findings of investigations performed in neurodegenerative and psychiatric conditions are also reported. The review is structured as follows: in section Methods Used to Assess Time-Varying Functional Connectivity, we present the main approaches developed to investigate TVC using fMRI data, with a main emphasis on the methods applied to study MS patients. Then, we summarize the results obtained applying these methods in healthy controls (section Application of TVC to Healthy Subjects) and in patients with MS (section Application of TVC Techniques to MS). An overview of the results derived from other neurological and psychiatric conditions is also given (section Application of Time-Varying FC Techniques to Psychiatric and Other Neurological Diseases). In the final part (section Current Limitations and Future Directions), current TVC methodological limitations are discussed and possible future developments are presented.
Several analysis strategies have been applied so far to quantify temporal variations of blood oxygenation level dependent (BOLD) signal fluctuations (Hutchison et al., 2013; Preti et al., 2017). Some strategies aim at capturing variations in inter-regional associations between pairs of brain areas (Sakoglu et al., 2010; Allen et al., 2014), while others try to detect changing patterns of temporal synchrony at a multivariate level, e.g., considering all brain regions at once (Tagliazucchi et al., 2012; Liu and Duyn, 2013). One of the most popular methods for TVC analysis, which is based on the use of the so-called “sliding windows” (Sakoglu et al., 2010; Allen et al., 2014), belongs to the first category, since it relies on the calculation of a series of pairwise correlation coefficients over small shifting segments of fMRI time series.
Despite the great variability of available pipelines, TVC analysis usually requires the performance of the following steps: (1) selection of a set of regions of interest (ROIs) in the brain; (2) assessment of time-varying correlations among the selected ROIs; and (3) extraction of features quantifying connectivity changes over time, as described in details in the next paragraphs.
It is important to properly identify the ROIs (which may be areas of the brain, or even entire functional networks) that will be included in TVC analysis. Several factors can influence the choice of ROIs: spatial resolution, the use of a priori hypotheses or data-driven strategies, and the rationale of the experiment, which may focus on selected functional circuits or on the whole brain.
The large majority of studies assessing TVC in MS patients mainly relied on the use of a priori atlases, such as the Automatic Anatomical Labeling (AAL) (Tzourio-Mazoyer et al., 2002) or the Desikan (Desikan et al., 2006) cortical atlas (Leonardi et al., 2013; Lin et al., 2018; van Geest et al., 2018a,b). Some studies built ad-hoc ROIs centered in critical nodes of large-scale brain networks (Bosma et al., 2018). However, a widely used approach in previous literature consists in a ROI data-driven selection through independent component analysis (ICA; Rocca et al., 2010, 2019; Sakoglu et al., 2010; Filippi et al., 2013b; Allen et al., 2014; Damaraju et al., 2014; Yang et al., 2014; Zalesky et al., 2014; Bisecco et al., 2018; Castellazzi et al., 2018; d'Ambrosio et al., 2019) (Figure 1). The broad application of ICA in previous TVC studies can be explained by the flexibility of this approach, which allows to extract ROIs at different spatial resolution according to ICA dimensionality, to perfectly fit the data (avoiding non-linear registrations with a priori atlases, which may be challenging in diseased populations) and to reduce the impact of physiological and motion-related noise.
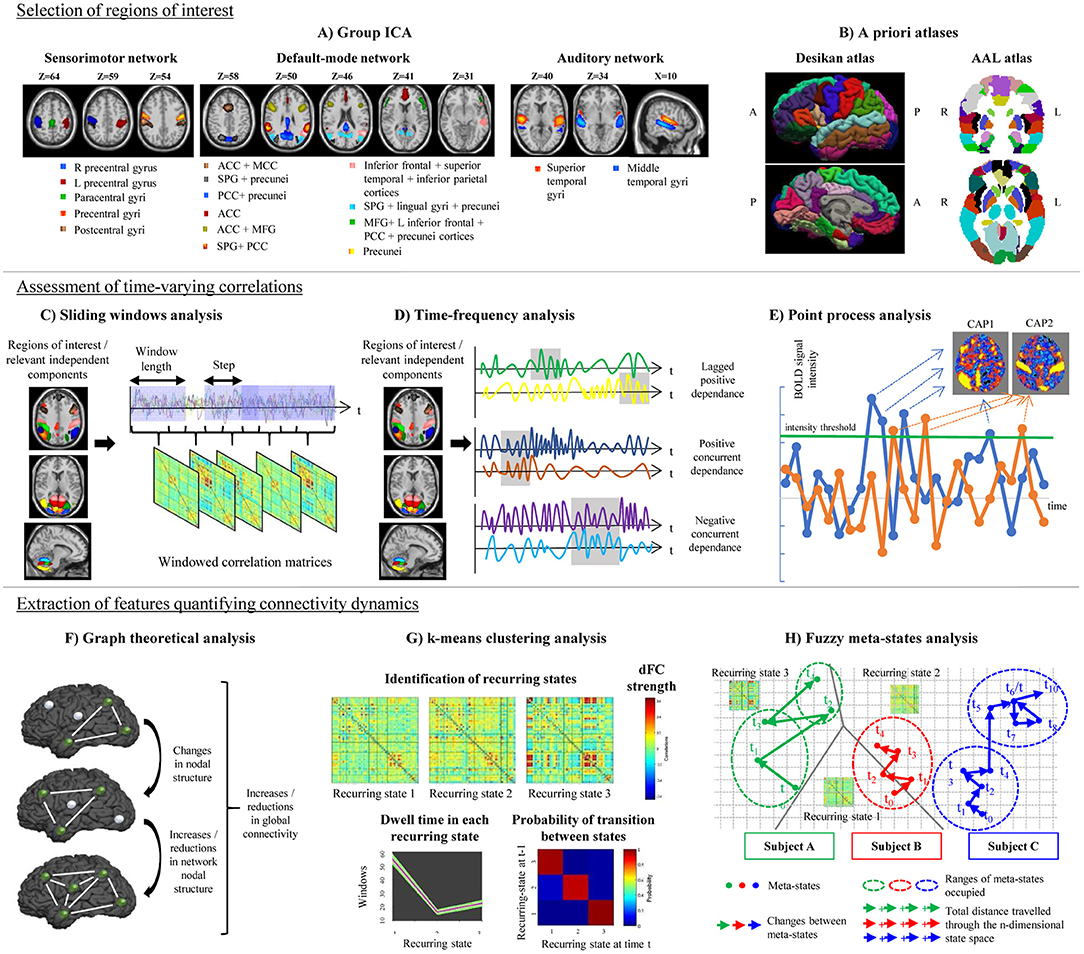
Figure 1. Schematic representation of the post-processing steps used in the assessment of time-varying functional connectivity (TVC). Top row: selection of regions of interest for TVC analysis, which can be done using data-driven approaches (e.g., independent component analysis, A) or using a priori atlases (B). Middle row: assessment of time-varying correlations between fMRI time series. The most popular approach consists in using a sliding-window analysis (C); alternative approaches, such as time-frequency analysis (D) or point-process analysis (E) have been also proposed. Bottom row: extraction of features quantifying connectivity changes over time, which can be done using several techniques, such as graph theory (F), k-means clustering to estimate recurring TVC states (G), or fuzzy meta-state analysis (H). ICA, independent component analysis; AAL, automatic anatomical labeling; ACC, anterior cingulate cortex; CAP, co-activation pattern; MCC, middle cingulate cortex; PCC, posterior cingulate cortex; SPG, superior parietal gyrus; MFG, middle frontal gyrus; R, right; L, left.
Since ROIs identified by “static” a priori atlases may not reflect significant connectivity variations occurring within brain regions at short time scales (Ryyppo et al., 2018), recent studies have suggested that incorporating information of time-varying connectivity between neighboring voxels to parcellate the brain may improve accuracy of TVC analyses (Preti and Van De Ville, 2017; Ryyppo et al., 2018).
The most popular strategy used to examine time-varying correlations between RS fMRI time series relies on the use of sliding windows (Sakoglu et al., 2010; Allen et al., 2014). In this approach, a time window of fixed length is selected, and correlations between pairs of fMRI time series are calculated using data within that window. Then, the window is shifted in time by a certain number of time points, and correlations are re-assessed on the new data. This procedure results in a series of pair-wise correlation matrices that describe the time-resolved behavior of connectivity over the entire duration of the fMRI experiment (Allen et al., 2014; Figure 1).
The choice of an appropriate length for sliding windows is crucial: too short time segments may introduce spurious fluctuations associated with intrinsic fMRI signal instability, while with increased window size TVC estimation may become too similar to the classic static FC (Leonardi and Van De Ville, 2015; Preti et al., 2017). Different validation analyses recommended to set window length around 30–60 s (or the equivalent time expressed as repetition times, TRs), demonstrating consistent reproducibility of the obtained results (Allen et al., 2014; Damaraju et al., 2014; Rashid et al., 2014, 2016; Zalesky et al., 2014; Leonardi and Van De Ville, 2015; Qin et al., 2015; Zalesky and Breakspear, 2015; Choe et al., 2017; Zhang C. et al., 2018).
Once sliding windows correlation matrices have been produced, different strategies can be applied to extract features describing connectivity reorganization through time inside the data (Leonardi et al., 2013; Allen et al., 2014; Miller et al., 2016), as described in details in section Extraction of Features Quantifying Time-Varying Connectivity.
A variety of approaches alternative to sliding windows have been developed to quantify TVC in fMRI data (Preti et al., 2017). For instance, time-frequency decomposition has been used to represent correlations between two fMRI time series in the joint time and frequency domain (Chang and Glover, 2010; Yaesoubi et al., 2015a; Figure 1). Point-process analysis allowed to detect recurring patterns of co-activation between brain regions from a small fraction of the total scans of a RS fMRI experiment (Tagliazucchi et al., 2012; Liu and Duyn, 2013). Phase coherence connectivity has been proposed to calculate RS FC at each recorded fMRI time point (Deco and Kringelbach, 2016).
In MS studies, two alternative methods to sliding windows have been applied. One study (Bosma et al., 2018) used dynamic conditional correlations (DCC) to quantify TVC. DCC were originally proposed to study fluctuations of financial time series (Engle, 2002) and subsequently adapted to neuroimaging data to quantify time-varying variances and correlations between multivariate RS fMRI time series (Lindquist et al., 2014). DCC overcome some limitations intrinsic to sliding-window techniques, since they do not depend from any arbitrary window length and do not give the same weight to all time points within the window, ignoring older observations. Moreover, DCC are not easily confused by changes of correlation occurring in fMRI time series merely due to random noise (Lindquist et al., 2014).
Another study (Zhou et al., 2016) quantified connectivity reorganization over time using brain entropy (BEN). Entropy is a statistical and physical index that measures irregularity of a time-varying system (Sandler, 2006). In RS fMRI data, voxel-wise assessments of entropy were performed by calculating sample entropy, defined as the negative logarithm of the probability that if two time series of length m have a correlation < r, then two time series of length m+1 also have a correlation < r. A higher entropy indicates increased randomness of a system, meaning that the time-varying system activity is less predictable and less organized (Wang et al., 2014).
Sliding-window (or alternative) techniques produce a large amount of correlation data, calculated on several time segments. Some features have then to be extracted from this big data mass, to summarize to what extent functional relationships reorganize through time. The simplest summary TVC statistic is standard deviation (or variance) of sliding-window correlation time series (Sakoglu et al., 2010; Choe et al., 2017) or of DCC time series (Lindquist et al., 2014; Bosma et al., 2018). The mean TVC (Huang et al., 2019) or the sum of absolute differences in pair-wise connectivity between consecutive windows have also been used as a summary TVC measure (van Geest et al., 2018a,b). Another interesting metric assessing temporal variability of BOLD fluctuations is the so-called amplitude of low-frequency fluctuations functional connectivity (ALFF-FC) (Shen et al., 2016), which sums up the spectral content of low-frequency RS fluctuations through consecutive sliding windows.
Flexibility metrics quantifying time-varying global and regional network properties were also calculated using a graph theory framework (Lin et al., 2018), as described in section Graph Theoretical Analysis. More complex strategies rely on the identification of connectivity patterns that reoccur over time during the course of the experiment. Reoccurring RS FC patterns, often called “states,” can be determined using clustering techniques (Allen et al., 2014), principal component analysis (Leonardi et al., 2013) or tensor decomposition (Mokhtari et al., 2018a), as detailed in section Definition of Reoccurring Connectivity States. Finally, approaches overcoming a rigid data decomposition into “fixed” connectivity states have been recently proposed (Miller et al., 2016), as described in detail in section Fuzzy Meta-State Analysis.
Graph theory analysis can be applied to series of matrices derived from sliding-window analysis (Figure 1). Besides the classical network metrics (Rubinov and Sporns, 2010), which can be quantified as a function of time (Fukushima and Sporns, 2018), more specific metrics can be used to assess time-varying network structure. For instance, network power measures the summed values of TVC pairs in all windows, and density estimates how dense, on average, connections are over time. Specific time-resolved network features include network variation, which describes how different are connectivity values between two adjacent windows, flexibility of homologous, non-homologous and intra-hemispheric connections, which quantify connectivity differences between two consecutive windows for the specified type of connections (Lin et al., 2018), or the Fiedler value, which summarizes how well-connected a network is (Cai J. et al., 2018). Recently, novel approaches have been proposed to improve modeling of brain network TVC using graph theory (Khambhati et al., 2018). Such modeling strategies aim to assess time-varying patterns of connectivity (e.g., dynamic community detection or non-negative matrix factorization), time-varying patterns of activity, or a combination of both. A detailed review of these methods is reported in Khambhati et al. (2018).
One of the most diffuse approaches used to identify reoccurring FC states from sliding-window matrices is based on hard-clustering algorithms (Preti et al., 2017), such as the k-means algorithm (Allen et al., 2014). In this approach, data are partitioned into different connectivity states by maximizing a cluster validity index, which describes the between-cluster/within-cluster distance ratio. In this way, identified recurring connectivity states have a minimal degree of overlap (Allen et al., 2014). The amount of time spent in each recurring state (dwell time) and the number of transitions between states can be calculated and compared between groups (Figure 1). Between-group comparisons can be also performed on pair-wise TVC strengths within each detected state (Allen et al., 2014).
Other ways to identify FC states from sliding-window data rely on principal component analysis (PCA) (Leonardi et al., 2013) or tensor decomposition (Mokhtari et al., 2018a). PCA is able to decompose sliding-window matrices into patterns of correlated connectivities (called “eigenconnectivities”) between brain regions. Each eigenconnectivity pattern is characterized by a “contribution” (which can be thought as the equivalent of dwell times for k-means clustering analysis), which varies over time across subjects. Between-group comparisons of such contributions may allow to characterize TVC abnormalities in patients' populations (Leonardi et al., 2013). Similarly, tensor decomposition (Mokhtari et al., 2018a) is able to decompose sliding-window connectivity matrices in a set of components, each with an associated weight, which explain the majority of data content.
In hard-clustering analysis, windowed correlation matrices are forced to fit into determined TVC recurring states. However, the existence of just one state at each time point may be a too rigid assumption. A more flexible approach is to consider the possibility that multiple states might be represented to varying degrees at the same time point. The contribution of each state for a specific time is characterized by a vector that is called a “meta-state” (Miller et al., 2016). Four different measures of neural reorganization over time can be associated to such meta-states and can be calculated for each study subject: (1) the total number of distinct meta-states that a subject assumes during the experiment; (2) the number of changes between distinct meta-states; (3) the range of meta-states occupied in the n-dimensional meta-state space during the entire RS fMRI experiment; and (4) the total distance traveled in the n-dimensional state space (Figure 1).
The results of the main studies assessing TVC in healthy controls are summarized in Table 1.
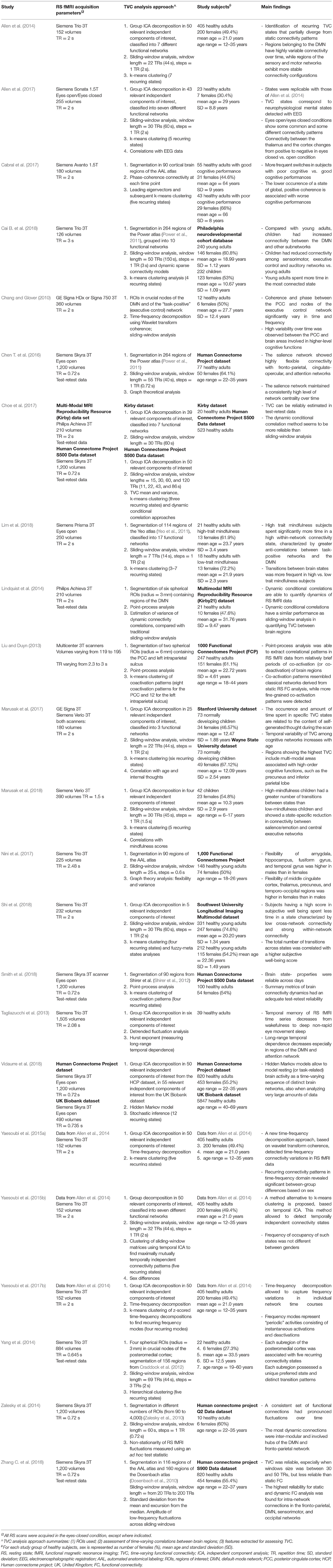
Table 1. Summary of studies assessing time-varying resting state functional connectivity in healthy subjects and simulated data.
In healthy subjects, it was always possible to identify a certain number of recurring connectivity configurations (from 3 to 12, depending on the method applied and on RS fMRI sequence settings). The DMN was one of the functional networks showing the highest degree of connectivity change over time, both when analyzing within-DMN TVC (Chang and Glover, 2010; Liu and Duyn, 2013; Lindquist et al., 2014; Zalesky et al., 2014) and when considering connections between the DMN and other crucial cognitive networks (Chang and Glover, 2010; Liu and Duyn, 2013; Allen et al., 2014; Marusak et al., 2017; Vidaurre et al., 2018). High dynamism was also observed in multimodal brain regions, involved in high-order emotional and cognitive processing (Yang et al., 2014; Zalesky et al., 2014; Chen S. et al., 2016; Marusak et al., 2017; Vidaurre et al., 2018). Such quick temporal reconfigurations may be required to facilitate transient psychological states between different brain functions (starting, maintenance or conclusion of the different attentional, cognitive, and executive tasks). Conversely, networks involved in sensory and motor processing showed more “static” connectivity profiles (Allen et al., 2014; Zalesky et al., 2014).
TVC was also useful to characterize age- and sex-related features. For instance, it was shown that children have higher TVC between the DMN and other subnetworks than young adults, but that young adults have stronger TVC than children among sensorimotor, executive control, and auditory networks (Cai B. et al., 2018). Moreover, variability of TVC among cognitive networks increased with age (Marusak et al., 2017). Overall, these results suggest that maturation is associated with a higher flexibility of functional connections. More discrepancies were found when analyzing sex-related characteristics of connectivity dynamics (Yaesoubi et al., 2015a,b; Nini et al., 2017). While some studies found no differences in TVC configurations between males and females (Yaesoubi et al., 2015b), other studies found that connectivity configurations were different between genders (Yaesoubi et al., 2015a,b; Nini et al., 2017): males showed a higher connectivity flexibility than females in the amygdala, hippocampus, fusiform, and temporal gyrus, whereas the opposite trend was found in the middle cingulate cortex, thalamus, precuneus, and some temporal-occipital regions (Nini et al., 2017).
TVC constitutes a complex and novel methodology. Studies from healthy controls also served to test how reliable and reproducible TVC results were across scanning sessions. This was the goal of some recent investigations (Choe et al., 2017; Smith et al., 2018; Zhang C. et al., 2018), which found that TVC metrics were reliable across days (Smith et al., 2018) and had an overall good reproducibility (Choe et al., 2017; Smith et al., 2018; Zhang C. et al., 2018), even if lower than that of the corresponding static FC metrics (Zhang C. et al., 2018). The highest reliability was found for intra-network connections in the DMN, fronto-parietal, sensorimotor, and occipital networks (Zhang C. et al., 2018).
To better investigate the intrinsic nature of TVC states and their electrophysiological correlates, simultaneously acquired electroencephalography (EEG) and RS fMRI data were analyzed and concurrent temporal variations were assessed (Allen et al., 2017). Results indicated that connectivity states detected by TVC analysis correspond to neuro-electric brain activity with distinct spectral signatures. Moreover, eyes open/eyes closed conditions show some common and some different connectivity patterns, with a greater integration within sensory systems, as well as reduced modularity and increased global efficiency, in the eyes-closed compared to the eyes-open condition (Allen et al., 2017). These results integrate and complete previous EGG/RS fMRI studies, which showed a variable TVC configuration between wakefulness and different stages of sleep (Tagliazucchi et al., 2013), with temporal memory and long-range temporal dependencies decreasing from wakefulness to deep non-rapid eye movement sleep.
To date, correlations between TVC measures and cognitive performances in healthy controls have been evaluated by one study (Cabral et al., 2017), which found that worse cognitive performance in healthy elderly was associated with a lower permanence in a TVC state characterized by strong, positive connectivity. These results suggest that a more static pattern of TVC may characterize poor vs. good performers.
Another study (Shi et al., 2018) analyzed the correlation between TVC and scores obtained at questionnaires of subjective well-being, and found that subjects with higher well-being scores spent less time in low cross-network and strong within-network connectivity states. The total number of transitions between states was also higher in subjects with high well-being scores, suggesting a more efficient transfer of information between networks in this group (Shi et al., 2018). Finally, two studies assessing the relationship between TVC and mindfulness in healthy adults (Lim et al., 2018) and children (Marusak et al., 2018) had similar conclusions, showing that high-mindfulness subjects spent more time in highly-connected states and switched more frequently between states than low-mindfulness subjects, suggesting a more efficient and flexible connectivity in the first group.
The main studies assessing TVC abnormalities in MS patients are summarized in Table 2. As it is evident from this table, TVC methodologies applied in MS investigations were quite heterogeneous. Despite this, results of different studies share some common points.
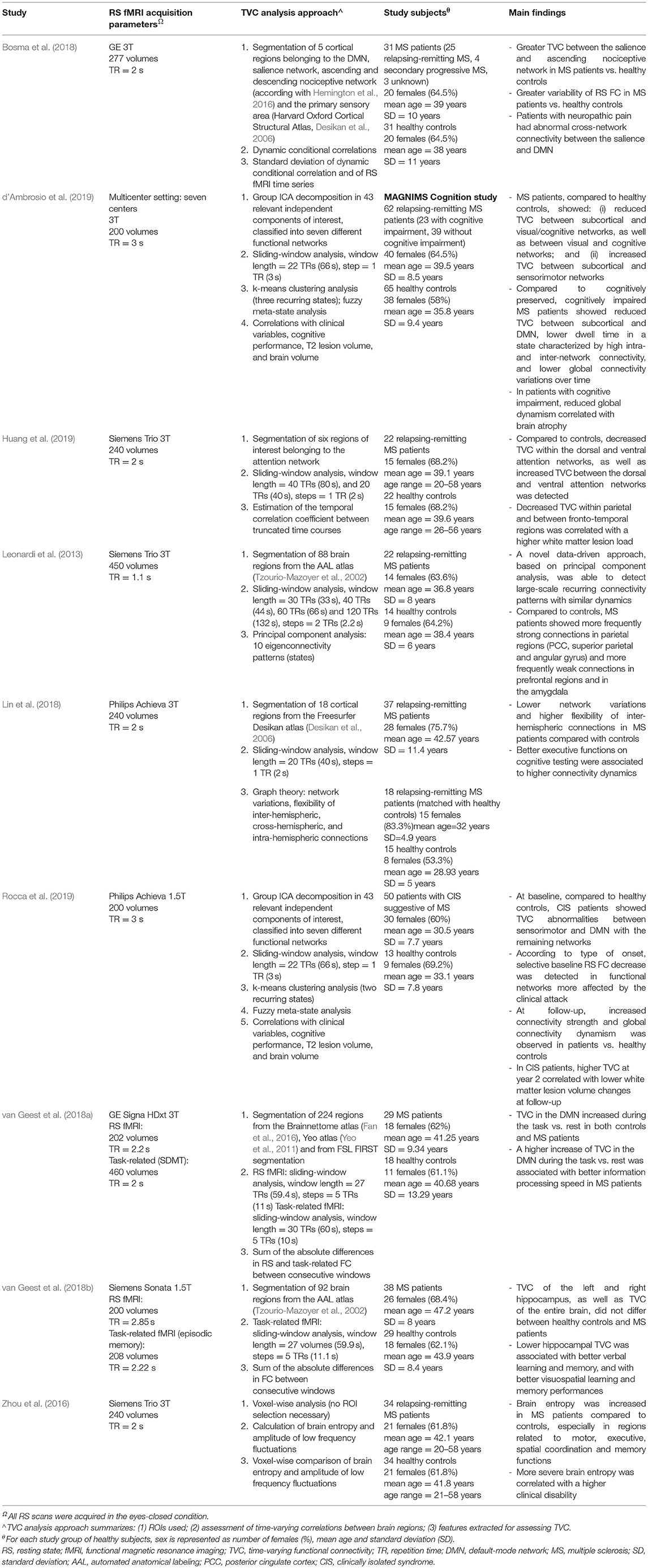
Table 2. Summary of studies assessing time-varying resting state functional connectivity modifications in multiple sclerosis (MS).
First of all, networks showing the greatest amount of TVC abnormalities in MS patients in comparison to healthy subjects were the DMN, salience, executive and sensorimotor networks (Leonardi et al., 2013; Zhou et al., 2016; Bosma et al., 2018; Lin et al., 2018; d'Ambrosio et al., 2019; Rocca et al., 2019).
The regional pattern of TVC abnormalities was quite complex, and regions involved by TVC changes were variable across studies, probably depending from the used TVC approach and patients' clinical characteristics. The analysis of eigenconnectivity patterns helped to identify the presence of stronger TVC in parietal regions and weaker TVC in frontal/subcortical regions in relapsing-remitting MS patients with mild to moderate disability compared to healthy controls (Leonardi et al., 2013). These patients also showed more frequently strong connections in temporal and parietal (angular gyrus) regions as well as weaker connections in motor and amygdalar regions vs. control subjects (Leonardi et al., 2013). Another study assessing TVC abnormalities in relapsing-remitting MS patients with mild disability found an increased BEN (corresponding to an increased connectivity disorganization) of regions involved in motor, executive and spatial coordination, as well as reduced BEN in memory brain areas (including temporal and hippocampal cortices) and relay areas as the cerebellum or the brainstem compared to healthy subjects (Zhou et al., 2016).
A recent study using DCC to quantify TVC (Bosma et al., 2018) confirmed the results obtained by Leonardi et al. (2013), and found an increased BOLD signal variability in posterior regions of the DMN in MS patients vs. controls. The same study also found an increased TVC between the salience network and the ascending nociceptive pathway. Conversely, divergent results were obtained by Lin et al. (2018), who showed an overall reduction of network variation in MS patients compared to healthy controls, suggesting a globally more “static” FC configuration, but at the same time found an increased flexibility of interhemispheric connections, which was interpreted as a compensatory mechanism for the decreased global connectivity. A complex pattern of increased and decreased TVC was also shown by d'Ambrosio et al. (2019), who found, in a multicenter study, a selective TVC increase between subcortical and visual/cognitive networks, and a TVC decrease between subcortical and sensorimotor networks in relapsing-remitting MS patients compared to healthy controls (d'Ambrosio et al., 2019).
Specific investigations of crucial systems involved in cognitive functions were performed by Van Geest et al., who studied TVC of the hippocampal network (Lin et al., 2018; van Geest et al., 2018a,b) and of the DMN (Lin et al., 2018; van Geest et al., 2018a,b) and by Huang et al., who investigated the attention network (Huang et al., 2019). Overall, hippocampal and DMN TVC were not different between MS patients and control subjects; however, including TVC measures in multivariate statistical models contributed to explain the performance of MS patients at visuospatial memory (Lin et al., 2018; van Geest et al., 2018a,b) and information processing speed (Lin et al., 2018; van Geest et al., 2018a,b) tasks. Huang et al. detected a complex pattern of TVC abnormalities, which was characterized by a TVC decrease within the dorsal and ventral attention networks, as well as TVC increase between the same networks (Huang et al., 2019).
Changes in TVC at the earliest stages of the disease have been rarely assessed, but interesting results have been observed. Patients with clinically isolated syndrome (CIS) suggestive of MS exhibited, early after the first demyelinating attack, reduced TVC in the functional networks more affected by the clinical onset, compared to healthy controls (Rocca et al., 2019). These patients also showed, in the first 2 years after the clinical event, a progressive increase over time of TVC strength, mainly between the DMN and sensorimotor/visual/cognitive networks, combined with a progressive increase over time of global fuzzy meta-state dynamism (Rocca et al., 2019).
Overall, these results suggest that, at the beginning of the disease, TVC dysfunctions have a specific correspondence with clinical symptoms. Then, a progressive increase of TVC oscillations occurs, probably trying to compensate disease-related damage. This initial phase seems to be followed by a loss of coordination and flexibility among brain regions in MS patients (Leonardi et al., 2013; Zhou et al., 2016; Lin et al., 2018; d'Ambrosio et al., 2019), which may be compensated by local increased fluctuations between specific areas (Lin et al., 2018;van Geest et al., 2018a,b).
Recent studies tried to investigate TVC changes in MS populations affected by specific clinical manifestations. In details, cognitive impairment in patients with MS was associated to reduced TVC between subcortical and DMN areas, as well as to reduced global dynamism, compared to cognitively preserved patients (d'Ambrosio et al., 2019). Patients with MS suffering from neuropathic pain expressed selectively reduced TVC strength in the salience-descending nociceptive circuit (Bosma et al., 2018), whereas in patients without such neuropathic pain, TVC strength was increased in the same network (Bosma et al., 2018).
The large majority of the above-mentioned studies assessed TVC changes in relapsing-remitting MS patients, while detailed investigations of TVC abnormalities occurring in progressive MS phenotypes or over the course of the disease, are still missing.
Different correlation analyses have been performed in MS patients, in order to understand the possible association between TVC abnormalities and motor and cognitive performances, as well as with specific clinical symptoms such as fatigue.
A higher expanded disability status scale (EDSS) score, reflecting more severe clinical disability, was found to be correlated with increased BEN in the bilateral supplementary motor area and in the right precentral operculum (Zhou et al., 2016), as well as with a more rigid (less fluid) global connectivity in MS patients (Lin et al., 2018). Conversely, other studies failed to show significant associations between TVC abnormalities and disability, probably because of the relatively low sample size and/or a narrow EDSS range (Leonardi et al., 2013).
Several correlations have been detected between TVC abnormalities and MS patients' cognitive performances. In particular, better scores in tests involving executive control functions and processing speed ability were correlated with a higher global network dynamism (Lin et al., 2018). Similar findings were shown by van Geest et al. (2018a), who found that a higher dynamism in the DMN during an information processing speed task vs. a resting state condition was associated with better information processing speed performances. These results are in agreement with the reduced network dynamics observed in cognitively impaired vs. preserved MS patients (d'Ambrosio et al., 2019). Conversely, a lower hippocampal TVC contributed to explain, at least partially, better verbal learning, visuospatial learning, and memory performances (van Geest et al., 2018b).
Lower fatigue was associated with reduced TVC in the parahippocampal gyrus, right posterior cerebellum, and brainstem (Zhou et al., 2016). Pain interference has been associated with increased TVC in the posterior cingulate cortex, an associative region involved in the salience and nociceptive networks and DMN (Bosma et al., 2018).
A few studies investigated the relationship between TVC and white matter lesions or MS-related structural damage. Decreased TVC between parietal and fronto-temporal regions of the attention network was associated with an higher lesion load in relapsing-remitting MS patients (Huang et al., 2019). A significant association has been demonstrated between reduced global dynamism in cognitively impaired MS patients and brain atrophy (d'Ambrosio et al., 2019), as well as between increased TVC and diffuse microstructural damage in relapsing-remitting MS patients, quantified as a higher mean diffusivity on diffusion-tensor MRI (Zhou et al., 2016). At the earliest stages of MS, a progressive increase of TVC over 2 years of follow-up was associated with a lower white matter lesion volume change over the same period of time (Rocca et al., 2019).
In summary, in MS patients, abnormal global TVC properties of the sensorimotor, DMN and salience networks were associated with more severe tissue damage at structural MRI, more severe clinical disability, worse cognitive performance and pain interference, evidencing a maladaptive neuronal response to direct disease-related damage. Conversely, abnormal TVC properties of the temporal network and relay areas as the cerebellum and brainstem were correlated with better cognitive performances and less severe fatigue, suggesting a compensatory role of TVC changes.
The main studies discussed in this section are summarized in Table 3.

Table 3. Summary of studies assessing time-varying resting state functional connectivity modifications in different psychiatric and neurological pathologies (excluding multiple sclerosis).
Several studies tried to characterize TVC abnormalities present in different psychiatric and neurological diseases, sometimes looking for an early diagnostic biomarker (Du et al., 2018; Mennigen et al., 2018). Modification of TVC strength, dwell time or number of transitions between states varied according to the disease status in patients affected by bipolar disorder (Rashid et al., 2014, 2016), schizophrenia (Yu et al., 2015; Cetin et al., 2016; Rashid et al., 2016; Gazula et al., 2018; Yue et al., 2018; Zhang W. et al., 2018), depression (Liao et al., 2018; Qiu et al., 2018; Zhi et al., 2018), autism (He et al., 2018; Rashid et al., 2018a), stroke (Chen et al., 2018), mild traumatic brain injury (Vergara et al., 2018), epilepsy (Ridley et al., 2017; Klugah-Brown et al., 2018), Alzheimer's disease (Quevenco et al., 2017; Jie et al., 2018), and Parkinson's disease (Engels et al., 2018).
In psychiatric diseases, widespread TVC abnormalities have been found. Patients with bipolar disorder and major depression expressed TVC abnormalities mainly in executive (Rashid et al., 2014; Du et al., 2017), amygdala/salience (Qiu et al., 2018; Zhi et al., 2018), and salience/executive regions (Mokhtari et al., 2018a,b). Schizophrenia patients showed a complex pattern of decreased and increased TVC mainly in the DMN (Sakoglu et al., 2010; Abrol et al., 2017), and in frontal, parietal, auditory (Damaraju et al., 2014; Rashid et al., 2014; Du et al., 2017, 2018; Sun et al., 2018), visual (Fu et al., 2018; Rashid et al., 2018b; Sun et al., 2018), and thalamic areas (Damaraju et al., 2014; Rashid et al., 2014, 2018b; Du et al., 2018). Schizophrenia patients spent less time and made fewer transitions between states characterized by weak correlations between the thalami and sense-related brain regions (Damaraju et al., 2014). They also showed more lagged correlations between the DMN and sensory networks (Yaesoubi et al., 2017a) and a higher occupancy rate of globally disconnected states (Yu et al., 2015; Cetin et al., 2016; Rashid et al., 2016; Gazula et al., 2018; Yue et al., 2018; Zhang W. et al., 2018). In children with autism spectrum disorders, TVC was mainly decreased in DMN and insular areas (Falahpour et al., 2016; Guo et al., 2018; He et al., 2018; Rashid et al., 2018a).
In neurological disorders, TVC abnormalities have been mainly observed in areas directly affected by the disease. For example, subcortical stroke and mild traumatic brain injury patients showed TVC abnormalities in sensorimotor networks (Chen et al., 2018; Vergara et al., 2018). Patients with myoclonic/frontal lobe epilepsy showed reduced TVC mainly in frontal and parietal brain regions, whereas patients with temporal lobe epilepsy experienced TVC decrease mainly in temporal regions (Ridley et al., 2017; Klugah-Brown et al., 2018; Wang et al., 2018). Generalized epilepsy was related to TVC strength changes mainly in the DMN and cognitive networks (Liu et al., 2017; Li et al., 2018). Patients suffering from Alzheimer's disease had reduced regional (nodal) TVC (Alderson et al., 2018) and alterations in inter-network TVC of the anterior and posterior regions of the DMN (Jones et al., 2012; Quevenco et al., 2017), the frontal cortex and temporal areas (Jie et al., 2018). Patients with Parkinson's disease showed TVC changes mainly in sensorimotor, executive, cognitive (Liu et al., 2018), visual, and DMN areas (Diez-Cirarda et al., 2018), combined with reduced global and nodal TVC (Cai J. et al., 2018; Diez-Cirarda et al., 2018).
In schizophrenia patients, reduced global time-resolved graph metrics have been related to structural disease-related damage (Yu et al., 2015), while abnormalities in TVC of auditory brain regions have been correlated with the presence of auditory hallucinations (Sun et al., 2018). Hallucinations were also correlated with a more rigid, reduced global dynamism (Miller et al., 2016; Mennigen et al., 2018). Autistic behavior and diagnosis were associated with longer dwell times in a globally disconnected state (Rashid et al., 2018a).
In patients with temporal lobe epilepsy, recurring states characterized by high inter-network TVC expressed reduced dwell time and correlated with an early seizure onset (Klugah-Brown et al., 2018). Interestingly, reduced TVC in the ictal irritative zone was associated to an intracranial EEG connectivity increase in the same epileptic region in alpha, beta and gamma bands (Ridley et al., 2017). In patients with Alzheimer's disease, TVC abnormalities between the anterior and the posterior DMN areas correlated with poorer episodic memory performance (Quevenco et al., 2017), while reductions in global TVC were associated with microstructural tissue damage (Alderson et al., 2018). In patients with Parkinson's disease, TVC abnormalities in the DMN have been associated with memory performance (Engels et al., 2018), while TVC alterations in the putamen were associated with clinical disability (Liu et al., 2018).
At this moment, TVC approaches are applicable only at a group level. However, some preliminary investigations have successfully used TVC abnormalities to classify schizophrenia patients from bipolar patients and/or healthy controls (Cetin et al., 2016; Rashid et al., 2016), suggesting a future application of TVC at an individual level.
The field of TVC is relatively new: all main technical developments have been achieved in the last 9 years. Nonetheless, in such short period of time TVC has provided greater insights into fundamental properties of functional networks, and has improved knowledge of the pathophysiological brain reorganization occurring in MS and other neurological and psychiatric diseases.
However, TVC methodology presents some inherent limitations that are likely to be overcome in the next future. Further investigations are also needed to better understand the physiological meaning of TVC fluctuations and their electrophysiological correlates.
One of the main pitfalls of current TVC analysis approaches consists in the fact that the mere presence of signal fluctuations in an fMRI time series is often taken as an evidence of TVC (Hindriks et al., 2016). This might be not necessarily true: FC values fluctuating over time might be observed just because of noise, or statistical uncertainty. Several measures have been employed to test the effective presence of FC variability in fMRI time series, including variance (Sakoglu et al., 2010), standard deviation (Chang and Glover, 2010), kurtosis (Laumann et al., 2017), or more complex, non-linear measures (Zalesky et al., 2014). Usually, these metrics are compared between real fMRI data and simulated data, constructed ad-hoc to have a static FC. If the test is significant, the null hypothesis of stationarity can be rejected, and TVC can be considered to be effectively present in the data.
Results of studies assessing evidence of TVC in RS fMRI data were quite disappointing, showing that the power of TVC detection in typical 10-min RS fMRI acquisitions was relatively low (Leonardi and Van De Ville, 2015; Hindriks et al., 2016; Zhang C. et al., 2018). Solutions to improve the likelihood of detecting TVC might be the choice of appropriate lengths for sliding windows (Leonardi and Van De Ville, 2015) or the concatenation of more RS fMRI sessions (Hindriks et al., 2016). On the other hand, it is possible that measures used to test the hypothesis of dynamism so far might be not fully appropriate (Miller et al., 2018). Indeed, novel wavelet-based metrics (Miller et al., 2018) seem to be more sensitive to capture non-stationarities present in real RS fMRI data.
Results of TVC also depend upon the temporal resolution used to acquire fMRI data. TVC studies usually investigate modifications in RS FC occurring within seconds, by using fMRI volumes acquired with TRs ranging from 1 s to 3 s (Chang and Glover, 2010; Allen et al., 2017; Cabral et al., 2017; Nini et al., 2017; Yaesoubi et al., 2017b; Marusak et al., 2018). Investigations performed on RS fMRI data acquired with a higher sampling rate, e.g., thanks to the use of simultaneous multi-slice imaging techniques, may be more powerful in detecting changing connectivity reconfigurations over time (Choe et al., 2017). Also, the use of ultra-fast fMRI acquisition techniques, such as inverse imaging (Lin et al., 2006), generalized inverse imaging (Boyacioglu and Barth, 2013), or multi-slab echo-volumar imaging (Posse et al., 2013), might constitute an important improvement for TVC. Ultra-fast fMRI allows to acquire a single functional volume covering the whole brain in <300 ms, resembling the results of magnetoencephalography studies (Asslander et al., 2013). Therefore, fMRI scans acquired with ultra-fast techniques do not include physiological aliasing and allow the detection of more accurate BOLD signal responses to neural activity. Seminal studies already showed that ultra-fast fMRI significantly enhanced the sensitivity of mapping RS FC dynamics (Posse et al., 2013).
Regardless of the analysis method, the signal-to-noise ratio of the BOLD signal in RS fMRI is low, especially in small temporal segments (Handwerker et al., 2012). Non-neural processes contaminating RS fMRI time series can affect TVC estimates (Hutchison et al., 2013; Murphy et al., 2013; Preti and Van De Ville, 2017). These confounds often include the effects of motion, cardiac and respiratory activity, and fluctuations in arterial CO2 concentration (Hutchison et al., 2013; Murphy et al., 2013; Nikolaou et al., 2016; Glomb et al., 2018). Global signal regression (GSR) may be useful to better denoise RS fMRI time series (Murphy and Fox, 2017); however, it was shown to slightly reduce reliability of the estimated TVC connectivity states (Smith et al., 2018). Moreover, the impact of GSR was spatially heterogeneous across brain regions and was dependent from the amount of global signal magnitude across windows (Xu et al., 2018). As such, caution is suggested in applying GSR to sliding-window correlation analyses, and a control of subjects' mental fluctuations during RS fMRI scanning is recommended (Xu et al., 2018). By applying accurate pre-processing steps on the fMRI data, the rate of artifacts present in the TVC analyses will be minimized (Murphy et al., 2013), thus increasing the quality of the observed findings.
Improvements can still be done not only to pre-processing of RS fMRI time series, but also to TVC post-processing, e.g., by implementing new, accurate methods to estimate changing connectivity over time. Recent papers proposed new approaches to analyse TVC, which aim at capturing change points of connectivity in functional correlation matrices (Cribben et al., 2012; Jeong et al., 2016; Kundu et al., 2018). Other studies introduced tensor-based multilayer community detection algorithms, which are able to describe how organization of functional networks evolves over time (Al-Sharoa et al., 2019). All these methods might be useful to complement TVC information obtained by using more standard, state-of-art methods, such as sliding-window analysis. Finally, improvements can still be done in statistical thresholding strategies. TVC assessment relies on the use of a massive amount of pairwise correlations, stored in series of connectivity matrices, and the best way to perform a proper adjustment for multiple comparisons is still an open issue. Traditional methods of correction for multiple comparisons (Friston et al., 1994) may be too conservative and may suppress all significant results; therefore, different approaches of adjustment for multiple comparisons might be more suitable. For instance, network-based statistic (NBS, Zalesky et al., 2010) was proposed as an alternative method of multiple comparison correction in studies using graph theory, which suffers of similar drawbacks as TVC. NBS has been rarely applied in TVC studies (Diez-Cirarda et al., 2018), probably because the process to construct “components” is not straightforward when connectivity matrices change over time. Future studies investigating new strategies of adjustment for multiple comparisons may propose new solutions for this issue.
Although some studies have tried to provide a functional interpretation of TVC output, several questions remain to be answered by future work. Preliminary data found some degree of correspondence between EEG rhythms and TVC frequency content (Allen et al., 2017) and hypothesized that some of the TVC states observed in healthy subjects, especially at the end of RS fMRI sessions, might be related to drowsiness or light sleep (Allen et al., 2014, 2017). A preliminary study assessing contemporary TVC and EEG registrations confirmed the presence of connectivity changes over different phases of sleep, with long-range temporal dependencies becoming weaker during deep sleep (Tagliazucchi et al., 2013). Still, it is not clear why larger TVC oscillations have been registered in functional networks at the beginning of RS fMRI sessions (Allen et al., 2014, 2017). Theories hypothesizing the brain functional “anticipation” (e.g., brain predisposition to switch quickly between different psychological states; Zalesky et al., 2014) might partially explain why more “specialized” functional networks (sensorimotor, auditory, visual) express more constant TVC behavior, while more complex, multi-modal networks express more dynamism. On the other hand, constant TVC oscillations in sensorimotor, auditory and visual networks might only reflect their lower activity during RS (Syed et al., 2017).
From this perspective, additional multi-modal studies integrating information from imaging and electrophysiological modalities are necessary for a better comprehension of the neural origin, mechanisms and function of temporal FC variations, as well as of the physiological meaning of TVC states.
The analysis of time-varying FC has contributed to provide significant information on intrinsic brain functional organization, both in healthy and diseased conditions, which complements data produced by static FC approaches. TVC seems to be an intrinsic property of the brain with a neural origin, although some open questions still remain about the correct interpretation of TVC output.
In MS patients, TVC helped to better understand the pathophysiological functional reorganization occurring in the brain, with a peculiar involvement of the DMN, salience, sensorimotor, and fronto-temporal networks. TVC abnormalities were partially correlated with more severe tissue damage and more severe clinical disability, while more extensive correlations were found with abnormal cognitive performances. In patients with neurodegenerative and psychiatric conditions, TVC abnormalities of the DMN, attention and executive networks were also associated to stronger clinical manifestations. Overall, these results suggest a maladaptive neuronal response to disease-related damage.
There are still several unmet needs in neurological and psychiatric conditions that TVC analysis may help to address. First, TVC may be useful to identify multi-modal regions, crucial for functional network plasticity, which may constitute possible targets for motor and cognitive neurorehabilitation protocols, as well as for symptomatic or disease-modifying treatments. Second, trajectories of TVC changes over time during the disease course need to be better defined, both in MS and in psychiatric/other neurodegenerative disorders. This may be the topic of future longitudinal studies, or of cross-sectional studies enrolling patients at different disease phases. Finally, it is still unclear whether TVC abnormalities may have a prognostic value on future disease course. The collection of clinical data at medium- or long-term follow-up may allow to define whether some TVC abnormalities are associated with a more favorable/worse disease prognosis.
MH wrote the first draft of the manuscript. PV, MF, and MR drafted/revised all sections of the manuscript. MR contributed to the study concept and acted as study supervisor. All authors contributed intellectually to manuscript revision, read, and approved the submitted version.
PV received speakers' honoraria from ExceMed. MF is Editor-in-Chief of the Journal of Neurology; received compensation for consulting services and/or speaking activities from Biogen Idec, Merck-Serono, Novartis, Teva Pharmaceutical Industries; and receives research support from Biogen Idec, Merck-Serono, Novartis, Teva Pharmaceutical Industries, Roche, Italian Ministry of Health, Fondazione Italiana Sclerosi Multipla, and ARiSLA (Fondazione Italiana di Ricerca per la SLA). MR received speakers honoraria from Biogen Idec, Novartis, Genzyme, Sanofi-Aventis, Teva, Merck Serono, and Roche and receives research support from the Italian Ministry of Health and Fondazione Italiana Sclerosi Multipla.
The remaining author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Abrol, A., Rashid, B., Rachakonda, S., Damaraju, E., and Calhoun, V. D. (2017). Schizophrenia shows disrupted links between brain volume and dynamic functional connectivity. Front. Neurosci. 11:624. doi: 10.3389/fnins.2017.00624
Alderson, T. H., Bokde, A. L. W., Kelso, J. A. S., Maguire, L., and Coyle, D. (2018). Metastable neural dynamics in Alzheimer's disease are disrupted by lesions to the structural connectome. Neuroimage 183, 438–455. doi: 10.1016/j.neuroimage.2018.08.033
Allen, E. A., Damaraju, E., Eichele, T., Wu, L., and Calhoun, V. D. (2017). EEG signatures of dynamic functional network connectivity states. Brain Topogr. 31, 101–116. doi: 10.1007/s10548-017-0546-2
Allen, E. A., Damaraju, E., Plis, S. M., Erhardt, E. B., Eichele, T., and Calhoun, V. D. (2014). Tracking whole-brain connectivity dynamics in the resting state. Cereb. Cortex 24, 663–676. doi: 10.1093/cercor/bhs352
Al-Sharoa, E., Al-Khassaweneh, M., and Aviyente, S. (2019). Tensor based temporal and multi-layer community detection for studying brain dynamics during resting state fMRI. IEEE Trans. Biomed. Eng. 66, 659–709. doi: 10.1109/TBME.2018.2854676
Asslander, J., Zahneisen, B., Hugger, T., Reisert, M., Lee, H. L., LeVan, P., et al. (2013). Single shot whole brain imaging using spherical stack of spirals trajectories. Neuroimage 73, 59–70. doi: 10.1016/j.neuroimage.2013.01.065
Baker, J. T., Dillon, D. G., Patrick, L. M., Roffman, J. L., Brady, R. O. Jr., Pizzagalli, D. A., et al. (2019). Functional connectomics of affective and psychotic pathology. Proc. Natl. Acad. Sci. U.S.A. 116, 9050–9059. doi: 10.1073/pnas.1820780116
Bisecco, A., Nardo, F. D., Docimo, R., Caiazzo, G., d'Ambrosio, A., Bonavita, S., et al. (2018). Fatigue in multiple sclerosis: the contribution of resting-state functional connectivity reorganization. Mult. Scler. 24, 1696–1705. doi: 10.1177/1352458517730932
Biswal, B. B., Mennes, M., Zuo, X. N., Gohel, S., Kelly, C., Smith, S. M., et al. (2010). Toward discovery science of human brain function. Proc. Natl. Acad. Sci. U.S.A. 107, 4734–4739. doi: 10.1073/pnas.0911855107
Bonavita, S., Gallo, A., Sacco, R., Corte, M. D., Bisecco, A., Docimo, R., et al. (2011). Distributed changes in default-mode resting-state connectivity in multiple sclerosis. Mult. Scler. 17, 411–422. doi: 10.1177/1352458510394609
Bosma, R. L., Kim, J. A., Cheng, J. C., Rogachov, A., Hemington, K. S., Osborne, N. R., et al. (2018). Dynamic pain connectome functional connectivity and oscillations reflect multiple sclerosis pain. Pain 159, 2267–2276. doi: 10.1097/j.pain.0000000000001332
Boyacioglu, R., and Barth, M. (2013). Generalized INverse imaging (GIN): ultrafast fMRI with physiological noise correction. Magn. Reson. Med. 70, 962–971. doi: 10.1002/mrm.24528
Busatto, G. F. (2013). Structural and functional neuroimaging studies in major depressive disorder with psychotic features: a critical review. Schizophr. Bull. 39, 776–786. doi: 10.1093/schbul/sbt054
Cabral, J., Vidaurre, D., Marques, P., Magalhaes, R., Silva Moreira, P., Miguel Soares, J., et al. (2017). Cognitive performance in healthy older adults relates to spontaneous switching between states of functional connectivity during rest. Sci. Rep. 7:5135. doi: 10.1038/s41598-017-05425-7
Cai, B., Zille, P., Stephen, J. M., Wilson, T. W., Calhoun, V. D., and Wang, Y. P. (2018). Estimation of dynamic sparse connectivity patterns from resting state fMRI. IEEE Trans. Med. Imaging 37, 1224–1234. doi: 10.1109/TMI.2017.2786553
Cai, J., Liu, A., Mi, T., Garg, S., Trappe, W., McKeown, M. J., et al. (2018). Dynamic graph theoretical analysis of functional connectivity in parkinson's disease: the importance of fiedler value. IEEE J. Biomed. Health Inform. doi: 10.1109/JBHI.2018.2875456. [Epub ahead of print].
Calhoun, V. D., Miller, R., Pearlson, G., and Adali, T. (2014). The chronnectome: time-varying connectivity networks as the next frontier in fMRI data discovery. Neuron 84, 262–274. doi: 10.1016/j.neuron.2014.10.015
Castellazzi, G., Debernard, L., Melzer, T. R., Dalrymple-Alford, J. C., D'Angelo, E., Miller, D. H., et al. (2018). Functional connectivity alterations reveal complex mechanisms based on clinical and radiological status in mild relapsing remitting multiple sclerosis. Front. Neurol. 9:690. doi: 10.3389/fneur.2018.00690
Cetin, M. S., Houck, J. M., Rashid, B., Agacoglu, O., Stephen, J. M., Sui, J., et al. (2016). Multimodal classification of schizophrenia patients with MEG and fMRI data using static and dynamic connectivity measures. Front. Neurosci. 10:466. doi: 10.3389/fnins.2016.00466
Chang, C., and Glover, G. H. (2010). Time-frequency dynamics of resting-state brain connectivity measured with fMRI. Neuroimage 50, 81–98. doi: 10.1016/j.neuroimage.2009.12.011
Chen, J., Sun, D., Shi, Y., Jin, W., Wang, Y., Xi, Q., et al. (2018). Alterations of static functional connectivity and dynamic functional connectivity in motor execution regions after stroke. Neurosci. Lett. 686, 112–121. doi: 10.1016/j.neulet.2018.09.008
Chen, S., Langley, J., Chen, X., and Hu, X. (2016). Spatiotemporal modeling of brain dynamics using resting-state functional magnetic resonance imaging with gaussian hidden markov model. Brain Connect. 6, 326–334. doi: 10.1089/brain.2015.0398
Chen, T., Cai, W., Ryali, S., Supekar, K., and Menon, V. (2016). Distinct global brain dynamics and spatiotemporal organization of the salience network. PLoS Biol. 14:e1002469. doi: 10.1371/journal.pbio.1002469
Choe, A. S., Nebel, M. B., Barber, A. D., Cohen, J. R., Xu, Y., Pekar, J. J., et al. (2017). Comparing test-retest reliability of dynamic functional connectivity methods. Neuroimage 158, 155–175. doi: 10.1016/j.neuroimage.2017.07.005
Craddock, R. C., James, G. A., Holtzheimer, P. E. 3rd, Hu, X. P., and Mayberg, H. S. (2012). A whole brain fMRI atlas generated via spatially constrained spectral clustering. Hum. Brain Mapp. 33, 1914–1928. doi: 10.1002/hbm.21333
Cribben, I., Haraldsdottir, R., Atlas, L. Y., Wager, T. D., and Lindquist, M. A. (2012). Dynamic connectivity regression: determining state-related changes in brain connectivity. Neuroimage 61, 907–920. doi: 10.1016/j.neuroimage.2012.03.070
Damaraju, E., Allen, E. A., Belger, A., Ford, J. M., McEwen, S., Mathalon, D. H., et al. (2014). Dynamic functional connectivity analysis reveals transient states of dysconnectivity in schizophrenia. Neuroimage Clin. 5, 298–308. doi: 10.1016/j.nicl.2014.07.003
d'Ambrosio, A., Valsasina, P., Gallo, A., De Stefano, N., Pareto, D., Barkhof, F., et al. (2019). Reduced dynamics of functional connectivity and cognitive impairment in multiple sclerosis. Mult. Scler. doi: 10.1177/1352458519837707. [Epub ahead of print].
Deco, G., and Kringelbach, M. L. (2016). Metastability and coherence: extending the communication through coherence hypothesis using a whole-brain computational perspective. Trends Neurosci. 39, 125–135. doi: 10.1016/j.tins.2016.01.001
Desikan, R. S., Segonne, F., Fischl, B., Quinn, B. T., Dickerson, B. C., Blacker, D., et al. (2006). An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage 31, 968–980. doi: 10.1016/j.neuroimage.2006.01.021
Destrieux, C., Fischl, B., Dale, A., and Halgren, E. (2010). Automatic parcellation of human cortical gyri and sulci using standard anatomical nomenclature. Neuroimage 53, 1–15. doi: 10.1016/j.neuroimage.2010.06.010
Diez-Cirarda, M., Strafella, A. P., Kim, J., Pena, J., Ojeda, N., Cabrera-Zubizarreta, A., et al. (2018). Dynamic functional connectivity in Parkinson's disease patients with mild cognitive impairment and normal cognition. Neuroimage Clin. 17, 847–855. doi: 10.1016/j.nicl.2017.12.013
Dosenbach, N. U., Nardos, B., Cohen, A. L., Fair, D. A., Power, J. D., Church, J. A., et al. (2010). Prediction of individual brain maturity using fMRI. Science 329, 1358–1361. doi: 10.1126/science.1194144
Du, Y., Fryer, S. L., Fu, Z., Lin, D., Sui, J., Chen, J., et al. (2018). Dynamic functional connectivity impairments in early schizophrenia and clinical high-risk for psychosis. Neuroimage 180(Pt B), 632–645. doi: 10.1016/j.neuroimage.2017.10.022
Du, Y., Pearlson, G. D., Lin, D., Sui, J., Chen, J., Salman, M., et al. (2017). Identifying dynamic functional connectivity biomarkers using GIG-ICA: application to schizophrenia, schizoaffective disorder, and psychotic bipolar disorder. Hum. Brain Mapp. 38, 2683–2708. doi: 10.1002/hbm.23553
Engels, G., Vlaar, A., McCoy, B., Scherder, E., and Douw, L. (2018). Dynamic functional connectivity and symptoms of parkinson's disease: a resting-state fMRI study. Front. Aging Neurosci. 10:388. doi: 10.3389/fnagi.2018.00388
Engle, R. (2002). Dynamic conditional correlation: a simple class of multivariate generalized autoregressive conditional heteroskedasticity models. J. Bus. Econ. Stat. 20, 339–350. doi: 10.1198/073500102288618487
Evans, A. C., Collins, D. L., and Milner, B. (1992). An MRI-based stereotactic atlas from 250 young normal subjects. Soc. Neurosci. 18:408.
Falahpour, M., Thompson, W. K., Abbott, A. E., Jahedi, A., Mulvey, M. E., Datko, M., et al. (2016). Underconnected, but not broken? Dynamic functional connectivity MRI shows underconnectivity in autism is linked to increased intra-individual variability across time. Brain Connect. 6, 403–414. doi: 10.1089/brain.2015.0389
Fan, L., Chu, C., Li, H., Chen, L., Xie, S., Zhang, Y., et al. (2016). The human brainnetome atlas: a new brain atlas based on connectional architecture. Cereb. Cortex 26, 3508–3526. doi: 10.1093/cercor/bhw157
Filippi, M., Agosta, F., Spinelli, E. G., and Rocca, M. A. (2013a). Imaging resting state brain function in multiple sclerosis. J. Neurol. 260, 1709–1713. doi: 10.1007/s00415-012-6695-z
Filippi, M., Bar-Or, A., Piehl, F., Preziosa, P., Solari, A., Vukusic, S., et al. (2018). Multiple sclerosis. Nat. Rev. Dis. Primers 4:43. doi: 10.1038/s41572-018-0050-3
Filippi, M., Preziosa, P., and Rocca, M. A. (2017). Brain mapping in multiple sclerosis: Lessons learned about the human brain. Neuroimage 190, 32–45. doi: 10.1016/j.neuroimage.2017.09.021
Filippi, M., Valsasina, P., Misci, P., Falini, A., Comi, G., and Rocca, M. A. (2013b). The organization of intrinsic brain activity differs between genders: a resting-state fMRI study in a large cohort of young healthy subjects. Hum. Brain Mapp. 34, 1330–1343. doi: 10.1002/hbm.21514
Friston, K. J., Worsley, K. J., Frackowiak, R. S., Mazziotta, J. C., and Evans, A. C. (1994). Assessing the significance of focal activations using their spatial extent. Hum. Brain Mapp. 1, 210–220. doi: 10.1002/hbm.460010306
Fu, Z., Tu, Y., Di, X., Du, Y., Pearlson, G. D., Turner, J. A., et al. (2018). Characterizing dynamic amplitude of low-frequency fluctuation and its relationship with dynamic functional connectivity: an application to schizophrenia. Neuroimage 180(Pt B), 619–631. doi: 10.1016/j.neuroimage.2017.09.035
Fukushima, M., and Sporns, O. (2018). Comparison of fluctuations in global network topology of modeled and empirical brain functional connectivity. PLoS Comput. Biol. 14:e1006497. doi: 10.1371/journal.pcbi.1006497
Gazula, H., Baker, B. T., Damaraju, E., Plis, S. M., Panta, S. R., Silva, R. F., et al. (2018). Decentralized analysis of brain imaging data: voxel-based morphometry and dynamic functional network connectivity. Front. Neuroinform. 12:55. doi: 10.3389/fninf.2018.00055
Glomb, K., Ponce-Alvarez, A., Gilson, M., Ritter, P., and Deco, G. (2018). Stereotypical modulations in dynamic functional connectivity explained by changes in BOLD variance. Neuroimage 171, 40–54. doi: 10.1016/j.neuroimage.2017.12.074
Guo, X., Duan, X., Suckling, J., Chen, H., Liao, W., Cui, Q., et al. (2018). Partially impaired functional connectivity states between right anterior insula and default mode network in autism spectrum disorder. Hum. Brain Mapp. 40, 1264–1275. doi: 10.1002/hbm.24447
Handwerker, D. A., Roopchansingh, V., Gonzalez-Castillo, J., and Bandettini, P. A. (2012). Periodic changes in fMRI connectivity. Neuroimage 63, 1712–1719. doi: 10.1016/j.neuroimage.2012.06.078
He, C., Chen, Y., Jian, T., Chen, H., Guo, X., Wang, J., et al. (2018). Dynamic functional connectivity analysis reveals decreased variability of the default-mode network in developing autistic brain. Autism Res. 11, 1479–1493. doi: 10.1002/aur.2020
Hemington, K. S., Wu, Q., Kucyi, A., Inman, R. D., and Davis, K. D. (2016). Abnormal cross-network functional connectivity in chronic pain and its association with clinical symptoms. Brain Struct. Funct. 221, 4203–4219. doi: 10.1007/s00429-015-1161-1
Hindriks, R., Adhikari, M. H., Murayama, Y., Ganzetti, M., Mantini, D., Logothetis, N. K., et al. (2016). Can sliding-window correlations reveal dynamic functional connectivity in resting-state fMRI? Neuroimage 127, 242–256. doi: 10.1016/j.neuroimage.2015.11.055
Huang, M., Zhou, F., Wu, L., Wang, B., Guo, L., Zhao, Y., et al. (2019). White matter lesion loads associated with dynamic functional connectivity within attention network in patients with relapsing-remitting multiple sclerosis. J. Clin. Neurosci. 65, 59–65. doi: 10.1016/j.jocn.2019.03.034
Hutchison, R. M., Womelsdorf, T., Allen, E. A., Bandettini, P. A., Calhoun, V. D., Corbetta, M., et al. (2013). Dynamic functional connectivity: promise, issues, and interpretations. Neuroimage 80, 360–378. doi: 10.1016/j.neuroimage.2013.05.079
Jeong, S. O., Pae, C., and Park, H. J. (2016). Connectivity-based change point detection for large-size functional networks. Neuroimage 143, 353–363. doi: 10.1016/j.neuroimage.2016.09.019
Jie, B., Liu, M., and Shen, D. (2018). Integration of temporal and spatial properties of dynamic connectivity networks for automatic diagnosis of brain disease. Med. Image Anal. 47, 81–94. doi: 10.1016/j.media.2018.03.013
Jones, D. T., Vemuri, P., Murphy, M. C., Gunter, J. L., Senjem, M. L., Machulda, M. M., et al. (2012). Non-stationarity in the “resting brain's” modular architecture. PLoS ONE 7:e39731. doi: 10.1371/journal.pone.0039731
Kelly, C., Biswal, B. B., Craddock, R. C., Castellanos, F. X., and Milham, M. P. (2012). Characterizing variation in the functional connectome: promise and pitfalls. Trends Cogn. Sci. 16, 181–188. doi: 10.1016/j.tics.2012.02.001
Khambhati, A. N., Sizemore, A. E., Betzel, R. F., and Bassett, D. S. (2018). Modeling and interpreting mesoscale network dynamics. Neuroimage 180(Pt B), 337–349. doi: 10.1016/j.neuroimage.2017.06.029
Klugah-Brown, B., Luo, C., He, H., Jiang, S., Armah, G. K., Wu, Y., et al. (2018). Altered dynamic functional network connectivity in frontal lobe epilepsy. Brain Topogr. 32, 394–404. doi: 10.1007/s10548-018-0678-z
Kundu, S., Ming, J., Pierce, J., McDowell, J., and Guo, Y. (2018). Estimating dynamic brain functional networks using multi-subject fMRI data. Neuroimage 183, 635–649. doi: 10.1016/j.neuroimage.2018.07.045
Laumann, T. O., Snyder, A. Z., Mitra, A., Gordon, E. M., Gratton, C., Adeyemo, B., et al. (2017). On the stability of BOLD fMRI correlations. Cereb. Cortex 27, 4719–4732. doi: 10.1093/cercor/bhw265
Leonardi, N., Richiardi, J., Gschwind, M., Simioni, S., Annoni, J. M., Schluep, M., et al. (2013). Principal components of functional connectivity: a new approach to study dynamic brain connectivity during rest. Neuroimage 83, 937–950. doi: 10.1016/j.neuroimage.2013.07.019
Leonardi, N., and Van De Ville, D. (2015). On spurious and real fluctuations of dynamic functional connectivity during rest. Neuroimage 104, 430–436. doi: 10.1016/j.neuroimage.2014.09.007
Li, R., Wang, L., Chen, H., Guo, X., Liao, W., Tang, Y. L., et al. (2018). Abnormal dynamics of functional connectivity density in children with benign epilepsy with centrotemporal spikes. Brain Imaging Behav. doi: 10.1007/s11682-018-9914-0. [Epub ahead of print].
Liao, W., Li, J., Duan, X., Cui, Q., Chen, H., and Chen, H. (2018). Static and dynamic connectomics differentiate between depressed patients with and without suicidal ideation. Hum. Brain Mapp. 39, 4105–4118. doi: 10.1002/hbm.24235
Lim, J., Teng, J., Patanaik, A., Tandi, J., and Massar, S. A. A. (2018). Dynamic functional connectivity markers of objective trait mindfulness. Neuroimage 176, 193–202. doi: 10.1016/j.neuroimage.2018.04.056
Lin, F. H., Wald, L. L., Ahlfors, S. P., Hamalainen, M. S., Kwong, K. K., and Belliveau, J. W. (2006). Dynamic magnetic resonance inverse imaging of human brain function. Magn. Reson. Med. 56, 787–802. doi: 10.1002/mrm.20997
Lin, S. J., Vavasour, I., Kosaka, B., Li, D. K. B., Traboulsee, A., MacKay, A., et al. (2018). Education, and the balance between dynamic and stationary functional connectivity jointly support executive functions in relapsing-remitting multiple sclerosis. Hum. Brain Map. 39, 5039–5049. doi: 10.1002/hbm.24343
Lindquist, M. A., Xu, Y., Nebel, M. B., and Caffo, B. S. (2014). Evaluating dynamic bivariate correlations in resting-state fMRI: a comparison study and a new approach. Neuroimage 101, 531–546. doi: 10.1016/j.neuroimage.2014.06.052
Liu, A., Lin, S. J., Mi, T., Chen, X., Chan, P., Wang, Z. J., et al. (2018). Decreased subregional specificity of the putamen in Parkinson's Disease revealed by dynamic connectivity-derived parcellation. Neuroimage Clin. 20, 1163–1175. doi: 10.1016/j.nicl.2018.10.022
Liu, F., Wang, Y., Li, M., Wang, W., Li, R., Zhang, Z., et al. (2017). Dynamic functional network connectivity in idiopathic generalized epilepsy with generalized tonic-clonic seizure. Hum. Brain Mapp. 38, 957–973. doi: 10.1002/hbm.23430
Liu, X., and Duyn, J. H. (2013). Time-varying functional network information extracted from brief instances of spontaneous brain activity. Proc. Natl. Acad. Sci. U.S.A. 110, 4392–4397. doi: 10.1073/pnas.1216856110
Liu, Y., Liang, P., Duan, Y., Jia, X., Yu, C., Zhang, M., et al. (2011). Brain plasticity in relapsing-remitting multiple sclerosis: evidence from resting-state fMRI. J. Neurol. Sci. 304, 127–131. doi: 10.1016/j.jns.2011.01.023
Loitfelder, M., Fazekas, F., Petrovic, K., Fuchs, S., Ropele, S., Wallner-Blazek, M., et al. (2011). Reorganization in cognitive networks with progression of multiple sclerosis: insights from fMRI. Neurology 76, 526–533. doi: 10.1212/WNL.0b013e31820b75cf
Lowe, M. J., Beall, E. B., Sakaie, K. E., Koenig, K. A., Stone, L., Marrie, R. A., et al. (2008). Resting state sensorimotor functional connectivity in multiple sclerosis inversely correlates with transcallosal motor pathway transverse diffusivity. Hum. Brain Mapp. 29, 818–827. doi: 10.1002/hbm.20576
Lowe, M. J., Phillips, M. D., Lurito, J. T., Mattson, D., Dzemidzic, M., and Mathews, V. P. (2002). Multiple sclerosis: low-frequency temporal blood oxygen level-dependent fluctuations indicate reduced functional connectivity initial results. Radiology 224, 184–192. doi: 10.1148/radiol.2241011005
Mak, L. E., Minuzzi, L., MacQueen, G., Hall, G., Kennedy, S. H., and Milev, R. (2017). The default mode network in healthy individuals: a systematic review and meta-analysis. Brain Connect. 7, 25–33. doi: 10.1089/brain.2016.0438
Marusak, H. A., Calhoun, V. D., Brown, S., Crespo, L. M., Sala-Hamrick, K., Gotlib, I. H., et al. (2017). Dynamic functional connectivity of neurocognitive networks in children. Hum. Brain Mapp. 38, 97–108. doi: 10.1002/hbm.23346
Marusak, H. A., Elrahal, F., Peters, C. A., Kundu, P., Lombardo, M. V., Calhoun, V. D., et al. (2018). Mindfulness and dynamic functional neural connectivity in children and adolescents. Behav. Brain Res. 336, 211–218. doi: 10.1016/j.bbr.2017.09.010
Mennigen, E., Miller, R. L., Rashid, B., Fryer, S. L., Loewy, R. L., Stuart, B. K., et al. (2018). Reduced higher-dimensional resting state fMRI dynamism in clinical high-risk individuals for schizophrenia identified by meta-state analysis. Schizophr. Res. 201, 217–223. doi: 10.1016/j.schres.2018.06.007
Miller, R. L., Abrol, A., Adali, T., Levin-Schwarz, Y., and Calhoun, V. D. (2018). Resting-state fMRI dynamics and null models: perspectives, sampling variability, and simulations. Front. Neurosci. 12:551. doi: 10.3389/fnins.2018.00551
Miller, R. L., Yaesoubi, M., Turner, J. A., Mathalon, D., Preda, A., Pearlson, G., et al. (2016). Higher Dimensional meta-state analysis reveals reduced resting fMRI connectivity dynamism in schizophrenia patients. PLoS ONE 11:e0149849. doi: 10.1371/journal.pone.0149849
Mokhtari, F., Laurienti, P. J., Rejeski, W. J., and Ballard, G. (2018a). Dynamic fMRI connectivity tensor decomposition: a new approach to analyze and interpret dynamic brain connectivity. Brain Connect. 9, 95–112. doi: 10.1089/brain.2018.0605
Mokhtari, F., Rejeski, W. J., Zhu, Y., Wu, G., Simpson, S. L., Burdette, J. H., et al. (2018b). Dynamic fMRI networks predict success in a behavioral weight loss program among older adults. Neuroimage 173, 421–433. doi: 10.1016/j.neuroimage.2018.02.025
Murphy, K., Birn, R. M., and Bandettini, P. A. (2013). Resting-state fMRI confounds and cleanup. Neuroimage 80, 349–359. doi: 10.1016/j.neuroimage.2013.04.001
Murphy, K., and Fox, M. D. (2017). Towards a consensus regarding global signal regression for resting state functional connectivity MRI. Neuroimage 154, 169–173. doi: 10.1016/j.neuroimage.2016.11.052
Nikolaou, F., Orphanidou, C., Papakyriakou, P., Murphy, K., Wise, R. G., and Mitsis, G. D. (2016). Spontaneous physiological variability modulates dynamic functional connectivity in resting-state functional magnetic resonance imaging. Philos. Trans. Ser. A Math. Phys. Eng. Sci. 374:20150183. doi: 10.1098/rsta.2015.0183
Nini, M., Hongna, Z., Zhiying, L., Li, Y., and Xia, W. (2017). “Gender differences in dynamic functional connectivity based on resting-state fMRI,” in Conference proceedings: Annual International Conference of the IEEE Engineering in Medicine and Biology Society (Stougthon, WI: The Printing House, Inc.) 2017, 2940–2943.
Posse, S., Ackley, E., Mutihac, R., Zhang, T., Hummatov, R., Akhtari, M., et al. (2013). High-speed real-time resting-state FMRI using multi-slab echo-volumar imaging. Front. Hum. Neurosci. 7:479. doi: 10.3389/fnhum.2013.00479
Power, J. D., Cohen, A. L., Nelson, S. M., Wig, G. S., Barnes, K. A., Church, J. A., et al. (2011). Functional network organization of the human brain. Neuron 72, 665–678. doi: 10.1016/j.neuron.2011.09.006
Preti, M. G., Bolton, T. A., and Van De Ville, D. (2017). The dynamic functional connectome: State-of-the-art and perspectives. Neuroimage 160, 41–54. doi: 10.1016/j.neuroimage.2016.12.061
Preti, M. G., and Van De Ville, D. (2017). Dynamics of functional connectivity at high spatial resolution reveal long-range interactions and fine-scale organization. Sci. Rep. 7:12773. doi: 10.1038/s41598-017-12993-1
Qin, J., Chen, S. G., Hu, D., Zeng, L. L., Fan, Y. M., Chen, X. P., et al. (2015). Predicting individual brain maturity using dynamic functional connectivity. Front. Hum. Neurosci. 9:418. doi: 10.3389/fnhum.2015.00418
Qiu, L., Xia, M., Cheng, B., Yuan, L., Kuang, W., Bi, F., et al. (2018). Abnormal dynamic functional connectivity of amygdalar subregions in untreated patients with first-episode major depressive disorder. J. Psychiatry Neurosci. 43, 262–272. doi: 10.1503/jpn.170112
Quevenco, F. C., Preti, M. G., van Bergen, J. M., Hua, J., Wyss, M., Li, X., et al. (2017). Memory performance-related dynamic brain connectivity indicates pathological burden and genetic risk for Alzheimer's disease. Alzheimers Res. Ther. 9:24. doi: 10.1186/s13195-017-0249-7
Rashid, B., Arbabshirani, M. R., Damaraju, E., Cetin, M. S., Miller, R., Pearlson, G. D., et al. (2016). Classification of schizophrenia and bipolar patients using static and dynamic resting-state fMRI brain connectivity. Neuroimage 134, 645–657. doi: 10.1016/j.neuroimage.2016.04.051
Rashid, B., Blanken, L. M. E., Muetzel, R. L., Miller, R., Damaraju, E., Arbabshirani, M. R., et al. (2018a). Connectivity dynamics in typical development and its relationship to autistic traits and autism spectrum disorder. Hum. Brain Mapp. 39, 3127–3142. doi: 10.1002/hbm.24064
Rashid, B., Chen, J., Rashid, I., Damaraju, E., Liu, J., Miller, R., et al. (2018b). A framework for linking resting-state chronnectome/genome features in schizophrenia: a pilot study. Neuroimage 184, 843–854. doi: 10.1016/j.neuroimage.2018.10.004
Rashid, B., Damaraju, E., Pearlson, G. D., and Calhoun, V. D. (2014). Dynamic connectivity states estimated from resting fMRI Identify differences among Schizophrenia, bipolar disorder, and healthy control subjects. Front. Hum. Neurosci. 8:897. doi: 10.3389/fnhum.2014.00897
Ridley, B., Wirsich, J., Bettus, G., Rodionov, R., Murta, T., Chaudhary, U., et al. (2017). Simultaneous intracranial EEG-fMRI shows inter-modality correlation in time-resolved connectivity within normal areas but not within epileptic regions. Brain Topogr. 30, 639–655. doi: 10.1007/s10548-017-0551-5
Rocca, M., Hidalgo de la Cruz, M., Valsasina, P., Mesaros, S., Martinovic, V., Ivanovic, J., et al. (2019). Two-year dynamic functional network connectivity in clinically isolated syndrome. Mult. Scler. doi: 10.1177/1352458519837704. [Epub ahead of print].
Rocca, M. A., Valsasina, P., Absinta, M., Riccitelli, G., Rodegher, M. E., Misci, P., et al. (2010). Default-mode network dysfunction and cognitive impairment in progressive MS. Neurology 74, 1252–1259. doi: 10.1212/WNL.0b013e3181d9ed91
Rocca, M. A., Valsasina, P., Leavitt, V. M., Rodegher, M., Radaelli, M., Riccitelli, G. C., et al. (2018). Functional network connectivity abnormalities in multiple sclerosis: correlations with disability and cognitive impairment. Mult. Scler. 24, 459–471. doi: 10.1177/1352458517699875
Rocca, M. A., Valsasina, P., Martinelli, V., Misci, P., Falini, A., Comi, G., et al. (2012). Large-scale neuronal network dysfunction in relapsing-remitting multiple sclerosis. Neurology 79, 1449–1457. doi: 10.1212/WNL.0b013e31826d5f10
Roosendaal, S. D., Schoonheim, M. M., Hulst, H. E., Sanz-Arigita, E. J., Smith, S. M., Geurts, J. J., et al. (2010). Resting state networks change in clinically isolated syndrome. Brain 133(Pt 6), 1612–1621. doi: 10.1093/brain/awq058
Rubinov, M., and Sporns, O. (2010). Complex network measures of brain connectivity: uses and interpretations. Neuroimage 52, 1059–1069. doi: 10.1016/j.neuroimage.2009.10.003
Ryyppo, E., Glerean, E., Brattico, E., Saramaki, J., and Korhonen, O. (2018). Regions of Interest as nodes of dynamic functional brain networks. Netw. Neurosci. 2, 513–535. doi: 10.1162/netn_a_00047
Sakoglu, U., Pearlson, G. D., Kiehl, K. A., Wang, Y. M., Michael, A. M., and Calhoun, V. D. (2010). A method for evaluating dynamic functional network connectivity and task-modulation: application to schizophrenia. Magma 23, 351–366. doi: 10.1007/s10334-010-0197-8
Sandler, S. I. (2006). Chemical, Biochemical and Engineering Thermodynamics, 4th Edn. Hoboken, NJ: John Wiley & Sons, Inc.
Sbardella, E., Petsas, N., Tona, F., and Pantano, P. (2015). Resting-State fMRI in MS: general concepts and brief overview of its application. Biomed Res. Int. 2015:212693. doi: 10.1155/2015/212693
Schoonheim, M. M., Hulst, H. E., Brandt, R. B., Strik, M., Wink, A. M., Uitdehaag, B. M., et al. (2015). Thalamus structure and function determine severity of cognitive impairment in multiple sclerosis. Neurology 84, 776–783. doi: 10.1212/WNL.0000000000001285
Shen, H., Li, Z., Qin, J., Liu, Q., Wang, L., Zeng, L. L., et al. (2016). Changes in functional connectivity dynamics associated with vigilance network in taxi drivers. Neuroimage 124(Pt A), 367–378. doi: 10.1016/j.neuroimage.2015.09.010
Shi, L., Sun, J., Wu, X., Wei, D., Chen, Q., Yang, W., et al. (2018). Brain networks of happiness: dynamic functional connectivity among the default, cognitive and salience networks relates to subjective well-being. Soc. Cogn. Affect. Neurosci. 13, 851–862. doi: 10.1093/scan/nsy059
Shirer, W. R., Ryali, S., Rykhlevskaia, E., Menon, V., and Greicius, M. D. (2012). Decoding subject-driven cognitive states with whole-brain connectivity patterns. Cereb. Cortex 22, 158–165. doi: 10.1093/cercor/bhr099
Smith, D. M., Zhao, Y., Keilholz, S. D., and Schumacher, E. H. (2018). Investigating the intersession reliability of dynamic brain-state properties. Brain Connect. 8, 255–267. doi: 10.1089/brain.2017.0571
Sun, Y., Collinson, S. L., Suckling, J., and Sim, K. (2018). Dynamic reorganization of functional connectivity reveals abnormal temporal efficiency in schizophrenia. Schizophr. Bull. 45, 659–669. doi: 10.1093/schbul/sby077
Syed, M. F., Lindquist, M. A., Pillai, J. J., Agarwal, S., Gujar, S. K., Choe, A. S., et al. (2017). Dynamic functional connectivity states between the dorsal and ventral sensorimotor networks revealed by dynamic conditional correlation analysis of resting-state functional magnetic resonance imaging. Brain Connect. 7, 635–642. doi: 10.1089/brain.2017.0533
Tagliazucchi, E., Balenzuela, P., Fraiman, D., and Chialvo, D. R. (2012). Criticality in large-scale brain FMRI dynamics unveiled by a novel point process analysis. Front. Physiol. 3:15. doi: 10.3389/fphys.2012.00015
Tagliazucchi, E., and van Someren, E. J. W. (2017). The large-scale functional connectivity correlates of consciousness and arousal during the healthy and pathological human sleep cycle. Neuroimage 160, 55–72. doi: 10.1016/j.neuroimage.2017.06.026
Tagliazucchi, E., von Wegner, F., Morzelewski, A., Brodbeck, V., Jahnke, K., and Laufs, H. (2013). Breakdown of long-range temporal dependence in default mode and attention networks during deep sleep. Proc. Natl. Acad. Sci. U.S.A. 110, 15419–15424. doi: 10.1073/pnas.1312848110
Tona, F., Petsas, N., Sbardella, E., Prosperini, L., Carmellini, M., Pozzilli, C., et al. (2014). Multiple sclerosis: altered thalamic resting-state functional connectivity and its effect on cognitive function. Radiology 271, 814–821. doi: 10.1148/radiol.14131688
Tzourio-Mazoyer, N., Landeau, B., Papathanassiou, D., Crivello, F., Etard, O., Delcroix, N., et al. (2002). Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage 15, 273–289. doi: 10.1006/nimg.2001.0978
van Geest, Q., Douw, L., van 't Klooster, S., Leurs, C. E., Genova, H. M., Wylie, G. R., et al. (2018a). Information processing speed in multiple sclerosis: Relevance of default mode network dynamics. Neuroimage Clin. 19, 507–515. doi: 10.1016/j.nicl.2018.05.015
van Geest, Q., Hulst, H. E., Meijer, K. A., Hoyng, L., Geurts, J. J. G., and Douw, L. (2018b). The importance of hippocampal dynamic connectivity in explaining memory function in multiple sclerosis. Brain Behav. 8:e00954. doi: 10.1002/brb3.954
Vergara, V. M., Mayer, A. R., Damaraju, E., Kiehl, K. A., and Calhoun, V. (2017). Detection of mild traumatic brain injury by machine learning classification using resting state functional network connectivity and fractional anisotropy. J. Neurotrauma 34, 1045–1053. doi: 10.1089/neu.2016.4526
Vergara, V. M., Mayer, A. R., Kiehl, K. A., and Calhoun, V. D. (2018). Dynamic functional network connectivity discriminates mild traumatic brain injury through machine learning. Neuroimage Clin. 19, 30–37. doi: 10.1016/j.nicl.2018.03.017
Vidaurre, D., Abeysuriya, R., Becker, R., Quinn, A. J., Alfaro-Almagro, F., Smith, S. M., et al. (2018). Discovering dynamic brain networks from big data in rest and task. Neuroimage 180(Pt B), 646–656. doi: 10.1016/j.neuroimage.2017.06.077
Wang, Y., Berglund, I. S., Uppman, M., and Li, T. Q. (2018). Juvenile myoclonic epilepsy has hyper dynamic functional connectivity in the dorsolateral frontal cortex. Neuroimage Clin. 21:101604. doi: 10.1016/j.nicl.2018.11.014
Wang, Z., Li, Y., Childress, A. R., and Detre, J. A. (2014). Brain entropy mapping using fMRI. PLoS ONE 9:e89948. doi: 10.1371/journal.pone.0089948
Weiner, M. W., Veitch, D. P., Aisen, P. S., Beckett, L. A., Cairns, N. J., Green, R. C., et al. (2017). Recent publications from the Alzheimer's disease neuroimaging initiative: reviewing progress toward improved AD clinical trials. Alzheimers Dement. 13, e1–e85. doi: 10.1016/j.jalz.2016.11.007
Xu, H., Su, J., Qin, J., Li, M., Zeng, L. L., Hu, D., et al. (2018). Impact of global signal regression on characterizing dynamic functional connectivity and brain states. Neuroimage 173, 127–145. doi: 10.1016/j.neuroimage.2018.02.036
Yaesoubi, M., Allen, E. A., Miller, R. L., and Calhoun, V. D. (2015a). Dynamic coherence analysis of resting fMRI data to jointly capture state-based phase, frequency, and time-domain information. Neuroimage 120, 133–142. doi: 10.1016/j.neuroimage.2015.07.002
Yaesoubi, M., Miller, R. L., Bustillo, J., Lim, K. O., Vaidya, J., and Calhoun, V. D. (2017a). A joint time-frequency analysis of resting-state functional connectivity reveals novel patterns of connectivity shared between or unique to schizophrenia patients and healthy controls. Neuroimage Clin. 15, 761–768. doi: 10.1016/j.nicl.2017.06.023
Yaesoubi, M., Miller, R. L., and Calhoun, V. D. (2015b). Mutually temporally independent connectivity patterns: a new framework to study the dynamics of brain connectivity at rest with application to explain group difference based on gender. Neuroimage 107, 85–94. doi: 10.1016/j.neuroimage.2014.11.054
Yaesoubi, M., Miller, R. L., and Calhoun, V. D. (2017b). Time-varying spectral power of resting-state fMRI networks reveal cross-frequency dependence in dynamic connectivity. PLoS ONE 12:e0171647. doi: 10.1371/journal.pone.0171647
Yang, Z., Craddock, R. C., Margulies, D. S., Yan, C. G., and Milham, M. P. (2014). Common intrinsic connectivity states among posteromedial cortex subdivisions: Insights from analysis of temporal dynamics. Neuroimage 93(Pt 1), 124–137. doi: 10.1016/j.neuroimage.2014.02.014
Yeo, B. T., Krienen, F. M., Sepulcre, J., Sabuncu, M. R., Lashkari, D., Hollinshead, M., et al. (2011). The organization of the human cerebral cortex estimated by intrinsic functional connectivity. J. Neurophysiol. 106, 1125–1165. doi: 10.1152/jn.00338.2011
Yu, Q., Erhardt, E. B., Sui, J., Du, Y., He, H., Hjelm, D., et al. (2015). Assessing dynamic brain graphs of time-varying connectivity in fMRI data: application to healthy controls and patients with schizophrenia. Neuroimage 107, 345–355. doi: 10.1016/j.neuroimage.2014.12.020
Yue, J. L., Li, P., Shi, L., Lin, X., Sun, H. Q., and Lu, L. (2018). Enhanced temporal variability of amygdala-frontal functional connectivity in patients with schizophrenia. Neuroimage Clin. 18, 527–532. doi: 10.1016/j.nicl.2018.02.025
Zalesky, A., and Breakspear, M. (2015). Towards a statistical test for functional connectivity dynamics. Neuroimage 114, 466–470. doi: 10.1016/j.neuroimage.2015.03.047
Zalesky, A., Fornito, A., and Bullmore, E. T. (2010). Network-based statistic: identifying differences in brain networks. Neuroimage 53, 1197–1207. doi: 10.1016/j.neuroimage.2010.06.041
Zalesky, A., Fornito, A., Cocchi, L., Gollo, L. L., and Breakspear, M. (2014). Time-resolved resting-state brain networks. Proc. Natl. Acad. Sci. U.S.A. 111, 10341–10346. doi: 10.1073/pnas.1400181111
Zhang, C., Baum, S. A., Adduru, V. R., Biswal, B. B., and Michael, A. M. (2018). Test-retest reliability of dynamic functional connectivity in resting state fMRI. Neuroimage 183, 907–918. doi: 10.1016/j.neuroimage.2018.08.021
Zhang, W., Li, S., Wang, X., Gong, Y., Yao, L., Xiao, Y., et al. (2018). Abnormal dynamic functional connectivity between speech and auditory areas in schizophrenia patients with auditory hallucinations. Neuroimage Clin. 19, 918–924. doi: 10.1016/j.nicl.2018.06.018
Zhi, D., Calhoun, V. D., Lv, L., Ma, X., Ke, Q., Fu, Z., et al. (2018). Aberrant dynamic functional network connectivity and graph properties in major depressive disorder. Front. Psychiatry 9:339. doi: 10.3389/fpsyt.2018.00339
Zhou, F., Zhuang, Y., Gong, H., Zhan, J., Grossman, M., and Wang, Z. (2016). Resting state brain entropy alterations in relapsing remitting multiple sclerosis. PLoS ONE 11:e0146080. doi: 10.1371/journal.pone.0146080
Zhou, J., Liu, S., Ng, K. K., and Wang, J. (2017). Applications of resting-state functional connectivity to neurodegenerative disease. Neuroimaging Clin. N. Am. 27, 663–683. doi: 10.1016/j.nic.2017.06.007
Keywords: multiple sclerosis, neurodegenerative conditions, time-varying, functional connectivity, resting state, fMRI
Citation: Valsasina P, Hidalgo de la Cruz M, Filippi M and Rocca MA (2019) Characterizing Rapid Fluctuations of Resting State Functional Connectivity in Demyelinating, Neurodegenerative, and Psychiatric Conditions: From Static to Time-Varying Analysis. Front. Neurosci. 13:618. doi: 10.3389/fnins.2019.00618
Received: 25 February 2019; Accepted: 29 May 2019;
Published: 10 July 2019.
Edited by:
Roberto Esposito, A.O. Ospedali Riuniti Marche Nord, ItalyReviewed by:
Hui Shen, National University of Defense Technology, ChinaCopyright © 2019 Valsasina, Hidalgo de la Cruz, Filippi and Rocca. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Maria A. Rocca, cm9jY2EubWFyYUBoc3IuaXQ=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.