
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Neurosci., 21 December 2018
Sec. Neuropharmacology
Volume 12 - 2018 | https://doi.org/10.3389/fnins.2018.00974
This article is part of the Research TopicSerotonin, Receptors and Transporters: Exploring New and Known Signaling Pathways to Improve the Efficacy of Antidepressant TreatmentView all 10 articles
 Juliane Zemdegs1,2,3
Juliane Zemdegs1,2,3 Quentin Rainer2
Quentin Rainer2 Cindy P. Grossmann3
Cindy P. Grossmann3 Delphine Rousseau-Ralliard4,5
Delphine Rousseau-Ralliard4,5 Alain Grynberg5
Alain Grynberg5 Eliane Ribeiro1
Eliane Ribeiro1 Bruno P. Guiard2,3*
Bruno P. Guiard2,3*Despite significant advances in the understanding of the therapeutic activity of antidepressant drugs, treatment-resistant depression is a public health issue prompting research to identify new therapeutic strategies. Evidence strongly suggests that nutrition might exert a significant impact on the onset, the duration and the severity of major depression. Accordingly, preclinical and clinical investigations demonstrated the beneficial effects of omega-3 fatty acids in anxiety and mood disorders. Although the neurobiological substrates of its action remain poorly documented, basic research has shown that omega-3 increases brain-derived neurotrophic factor (BDNF) levels in brain regions associated with depression, as antidepressant drugs do. In contrast, low BDNF levels and hippocampal atrophy were observed in animal models of depression. In this context, the present study compared the effects of long-lasting fish oil-enriched diet, an important source of omega-3 fatty acids, between heterozygous BDNF+/- mice and their wild-type littermates. Our results demonstrated lower activation of Erk in BDNF+/- mice whereas this deficit was rescued by fish oil-enriched diet. In parallel, BDNF+/- mice displayed elevated hippocampal extracellular 5-HT levels in relation with a local decreased serotonin transporter protein level. Fish oil-enriched diet restored normal serotonergic tone by increasing the protein levels of serotonin transporter. At the cellular level, fish oil-enriched diet increased the pool of immature neurons in the dentate gyrus of BDNF+/- mice and the latter observations coincide with its ability to promote anxiolytic- and antidepressant-like response in these mutants. Collectively, our results demonstrate that the beneficial effects of long-term exposure to fish oil-enriched diet in behavioral paradigms known to recapitulate diverse abnormalities related to the depressive state specifically in mice with a partial loss of BDNF. These findings contrast with the mechanism of action of currently available antidepressant drugs for which the full manifestation of their therapeutic activity depends on the enhancement of serotoninergic and BDNF signaling. Further studies are warranted to determine whether fish oil supplementation could be used as an add-on strategy to conventional pharmacological interventions in treatment-resistant patients and relevant animal models.
Major depressive disorder (MDD) is an important public health concern worldwide. The lifetime prevalence of MDD is nowadays 15–20% of the population, and is expected to become the second most prevalent cause of illness-induced disability by 2020 (Lecrubier, 2001). These epidemiological data prompt research to identify the cellular and molecular mechanisms underpinning these mental disorders and to develop innovative treatments with better therapeutic effects than currently available medications. Indeed, despite their therapeutic activity, antidepressant drugs, including selective serotonin reuptake inhibitors (SSRIs), alleviate depression symptoms in only a limited percentage of patients, and remain insufficiently effective in treatment responders (Hamon and Blier, 2013).
Omega-3 polyunsaturated fatty acids (PUFAs) deficiency has been associated with several pathologies such as mood disorders, cardiovascular diseases, and stroke (Hibbeln et al., 2006). Mammals are unable to synthesize omega-3 and its supply depends on dietary intake. Fish oils represent the main source of omega-3 PUFAs [(i.e., eicosapentaenoic (EPA) and docosahexaenoic (DHA)] (Calder, 1998). Interestingly, it has been reported that depressed patients display low plasma and brain levels of omega-3 PUFAs (McNamara et al., 2007; Lin et al., 2010). Such deficits were also found in other populations with mental disorders: e.g., lower DHA and total omega-3 PUFAs in postpartum depression (De Vriese et al., 2003) and lower DHA in bipolar disorders (Chiu et al., 2003). Conversely, multiple sources of evidence suggested that consumption of omega-3 PUFAs produces antidepressant activity in patients with MDD (Peet et al., 1998; Marangell et al., 2003; Silvers et al., 2005; Freeman et al., 2006; Lin and Su, 2007; Owen et al., 2008; Su et al., 2008) or bipolar disorders (Montgomery and Richardson, 2008). A recent meta-analysis also revealed a beneficial overall effect of omega-3 PUFAs in patients under antidepressant drugs treatment (Mocking et al., 2016), suggesting that supplementation with these fatty acids could be used as an “add-on” strategy to reduce treatment resistance, and potentiate treatment response (Peet and Horrobin, 2002; Jazayeri et al., 2008; Gertsik et al., 2012). Consistent with these clinical studies, research in rodents showed that omega-3 PUFAs elicits a robust anxiolytic-like activity in the elevated plus maze (EPM) (Pérez et al., 2013) and an antidepressant-like activity in the forced swim and tail suspension tests (Blondeau et al., 2009; Venna et al., 2009; Moranis et al., 2012; Park et al., 2012; Vines et al., 2012). Moreover, omega-3 PUFAs were shown to improve anxiety-like and depressive-like phenotypes in various animal models of depression (Pérez et al., 2013; Pudell et al., 2014; Tang et al., 2015; Wu et al., 2016) and their combination with SSRIs appeared to be more effective than antidepressant drugs alone for reducing depression-like behaviors (Lakhwani et al., 2007; Laino et al., 2010; Able et al., 2014).
Antidepressant drugs activity is associated with the stimulation of brain serotonergic neurotransmission (Gardier et al., 1996) accompanied with an enhancement of adult hippocampal neurogenesis. On the contrary, disruption of hippocampal neurogenecis prevents the behavioral effects of various classes of antidepressant in mice (Schmidt and Duman, 2007). A number of factors have been proposed to participate in adult hippocampal neurogenesis and SSRI response including Brain-Derived Neurotrophic Factor (BDNF) (Nibuya et al., 1995). A single bilateral infusion of BDNF into the dentate gyrus of hippocampus produced antidepressant-like effects in naive mice (Deltheil et al., 2009) or in animal models of depression such as the learned helplessness (Shirayama et al., 2002). Interestingly, in heterozygous BDNF+/- mice or in inducible BDNF KO lines of mice, deletion of BDNF in adults does not impact on depression-like behavior evaluated in the forced swim test (FST) (MacQueen et al., 2001; Saarelainen et al., 2003; Monteggia et al., 2007). However, these mutants display signs of antidepressant drugs resistance, notably at the behavioral and neurochemical levels (Saarelainen et al., 2003; Monteggia et al., 2004; Daws et al., 2007; Monteggia et al., 2007; Guiard et al., 2008; Ibarguen-Vargas et al., 2009). In an attempt to clarify the relationship between BDNF and the serotonergic system, alterations in behaviors regulated by serotonin such as hyperphagia and weight gain were demonstrated in BDNF+/- mice (Lyons et al., 1999). BDNF+/- mice also exhibit accelerated age-related loss of serotonergic innervation to the hippocampus (Lyons et al., 1999; Luellen et al., 2007) and increased expression of 5-HT transporter (Guiard et al., 2008). The latter effects likely contribute to dampen serotonergic neurotransmission (Siuciak et al., 1996; Mamounas et al., 2000) and strongly suggest that normal BDNF signaling is essential for antidepressant efficacy in mice.
Interestingly, the time course of omega-3 PUFAs-induced antidepressant-like effects in rodents is compatible with molecular and morphological changes taking place in the hippocampus. In particular, it has been reported that prolonged omega-3 PUFAs exposure stimulated BDNF expression and adult hippocampal neurogenesis in mice (Wu et al., 2004; Rao et al., 2007; Blondeau et al., 2009; Venna et al., 2009). In this context, the present study was designed to determine to what extent fish oil-enriched diet containing omega-3 PUFAs influence serotonergic tone and markers of hippocampal plasticity in BDNF+/- mice and their wild-type littermates. Using behavioral paradigms assessing anxiolytic/antidepressant-like activities, we also examined whether fish oil-enriched diet represents an alternative therapeutic strategy to currently available antidepressant drugs in BDNF+/- mice.
Experiments were performed in accordance with the European Union (86/609/EEC) and the French National Committee of Ethics (87/848) policies regarding the care and use of laboratory animals. BDNF+/- mice and their wild-type littermates initially bred on a mixed S129/Sv x C57BL/6 genetic background (Korte et al., 1995) were backcrossed to 129Sv strain, mated and raised at the animal facility of the Université Paris-Sud (Châtenay-Malabry, France) or at the Universidade Federal de São Paulo (Sao Paulo, Brazil). One-month-old male mice were genotyped by polymerase chain reaction and were randomly assigned to receive either a control diet or a fish oil-enriched diet for 12 weeks. Diets were prepared according to the recommendations of the American Institute of Nutrition (AIN-93) for rodents (Reeves, 1997) and were isocaloric and normolipidic, i.e., diets had identical energy and lipid content. The source of fat was soybean oil in the control diet and fish oil (Sigma-Aldrich, St. Louis, MO, United States). Both diets met the minimum suggested requirement for rodents of 2 g/kg diet of alpha-linolenic acid (ALA). The fatty acids composition of diets is depicted in Supplementary Table S1. Animals were housed in groups of five mice per cage under standard conditions (12:12 h light-dark cycle, 22 ± 1°C ambient temperature, 60% relative humidity), with ad libitum access to food and water. Experimental timeline is depicted in Figure 1. Procedures were conducted in conformity with the institutional guidelines in compliance with national and policy (Council directive #87–848, October 19, 1987, Ministère de l’Agriculture et de la Forêt, Service Vétérinaire de la Santé et de la Protection Animale, permission #92.196).
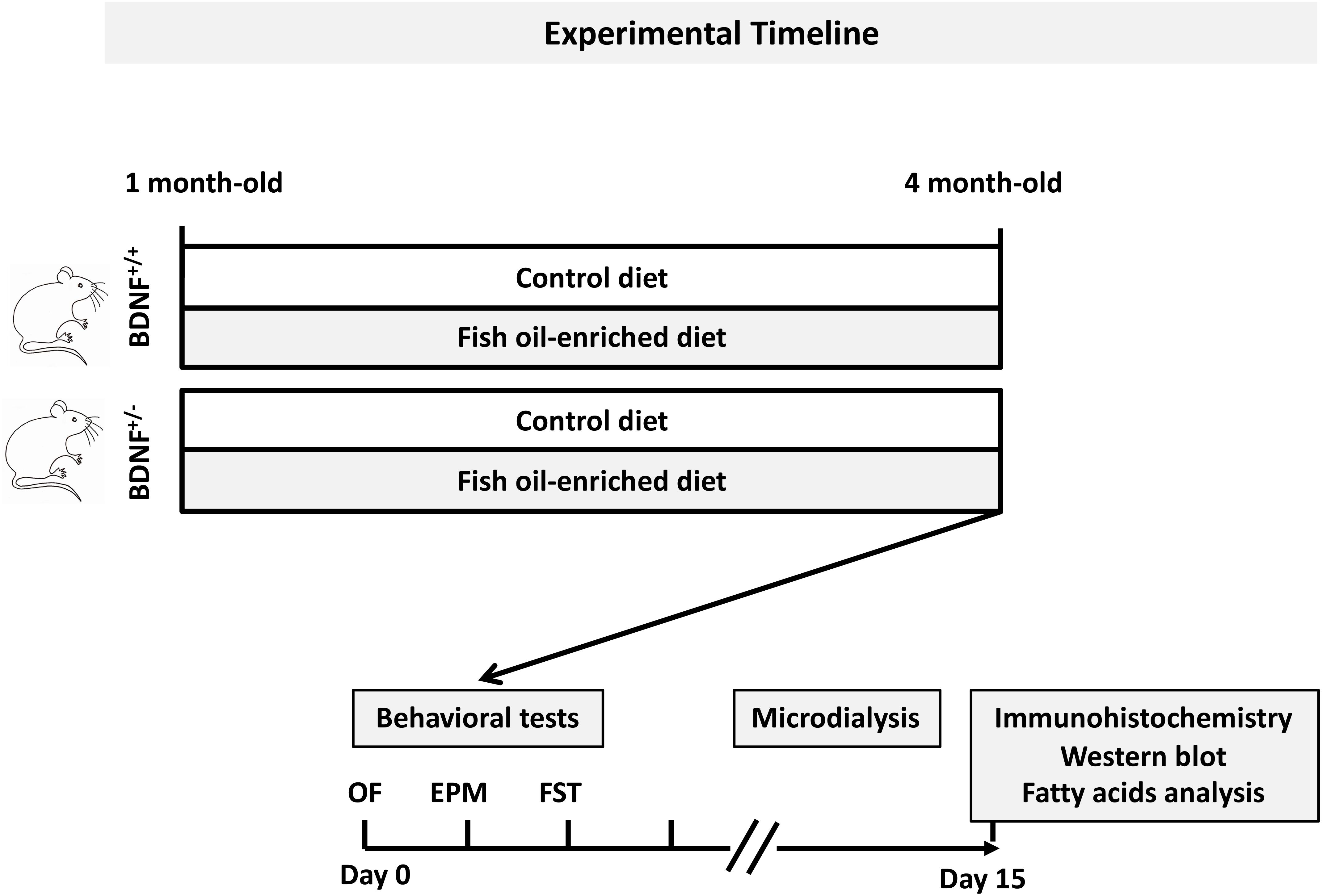
Figure 1. General procedure and experimental groups. Mice from each genotype were fed a control or fish oil-enriched diet for 12 weeks. In vivo procedures (i.e., behavioral and microdialysis experiments) were conducted while the animals were maintained under their respective diets. Given that it has been demonstrated that the interval between behavioral tests could be as little as 1 day, with a weak effect on overall performance (Paylor et al., 2006), in the present study, a 2-day recovery period between each test was provided. Moreover, to avoid the interference of behavioral testing on microdialysis experiments and notably the fact that the FST represents a strong stressor for the animals, the collection of dialysate samples was conducted 10 days after the behavioral assessment. The next day, animals were euthanized for immunohistochemistry, Western-blot and biochemical analyses.
Mice were killed by cervical dislocation. The brains were withdrawn and rinsed in saline (NaCl 0.9%). The hippocampi were finely dissected, weighed and stored at -80°C in CHCl3-MeOH (v/v, 2/1) to further determine its fatty acids profile. The lipids were extracted with chloroform/methanol (2/1), according to an adaptation of the method previously described (Rousseau et al., 2003). The phospholipids (PL) were separated from non-phosphorous lipids on silica acid cartridges. After the separation, the phospholipid fractions, mostly representative of the membranes, were transmethylated with boron trifluoride methanol 7% (Sigma-Aldrich, Saint Quentin Fallavier, France). The methyl esters of phospholipid fatty acids were analyzed by gas chromatography coupled to FID (Auto Sampling 8410 Gas Chromatograph 3900; Varian, Les Ulis, France) on an Econo-Cap EC-WAX capillary column (30-m, 0.32-mm internal diameter, 0.25-μm Film, ref 19654, ALLTECH Associates Inc., Templemars, France), using heptadecanoic acid (margaric acid, C17:0) as internal standard. Fatty acid composition was expressed as the percentage of total fatty acid weight. Desaturase activity was estimated according to Warensjo et al. (2009).
Under anesthesia (chloral hydrate, 400 mg/kg, i.p.), mice were stereotaxically implanted with concentric microdialysis probes (active membrane length: 2.0 mm, molecular weight cut-off: 4.5 kD) in the ventral hippocampus (coordinates in mm from bregma: AP: -3.4, L: ± 3.4, V: 4.0). The next day, mice were connected to a swivel system and the probes were connected to a microinjection pump, allowing a continuous perfusion of artificial cerebrospinal fluid (composition: NaCl 147 mM, KCl 3.5 mM, CaCl2 1.26 mM, MgCl2 1.2 mM, NaH2PO4 1.0 mM, NaHCO3 25.0 mM; pH 7.4 ± 0.2) at a flow rate of 1.5 μl/ min. A 2 h-perfusion was performed to allow stabilization of 5-HT concentrations and microdialysis samples were then collected every 15 min. Microdialysates were kept at -80°C until analysis of 5-HT content by high performance liquid chromatography (HPLC) coupled to an amperometric detector (VT03; Antec Leyden, Netherlands). The amounts of 5-HT in microdialysates (19 μl) were calculated by measurement of peak heights relative to external standards. The limit of sensitivity for 5-HT was ∼ 0.5 fmol/sample (signal-to-noise ratio = 2).
The hippocampi were homogenized in lysis buffer (1% Triton X-100, 100 mM Tris-HCl pH 7.4, 100 mM sodium pyrophosphate, 100 mM sodium fluoride, 10 mM EDTA, 10 mM sodium orthovanadate, 2.0 mM phenylmethylsulfonyl fluoride and 0.1 mg aprotinin/ml). Protein concentrations were determined using a commercial kit (BioAgency, Brazil). Equal amounts of proteins (50 μg) were loaded and separated on 10% SDS polyacrylamide gels and transferred onto nitrocellulose membranes (Amersham Biosciences, GE Healthcare, United States). Membranes were saturated with a blocking solution containing 1% BSA in TPBS (10 mM Tris, 150 mM NaCl and 0.02% Tween 20). Protein blots were incubated in 1% BSA in TPBS, overnight with the following primary antibodies: anti-phospho-Akt, anti-phospho-p44/p42 Erk, anti-SERT, and anti-alpha-tubulin. Primary antibodies were purchased from Cell Signaling (anti-phospho-Akt, anti phospho-p44/p42 Erk) or Santa Cruz Biotechnology (St. Louis, MO, United States) (serotoninergic and alpha-tubulin). After washing, membranes were incubated with the appropriate HRP-conjugated secondary antibodies (Sigma-Aldrich, St. Louis, MO, United States). Staining was revealed using the ECL-Plus Western blotting detection system (Thermo Scientific, Rockford IL, United States). Chemiluminescence was quantified by Scion Image software. After each revelation, membranes were incubated in stripping solution (62.6 mM Tris-HCl, 2% SDS, 100 mM b-mercaptoethanol, pH 6.8) for 30 min at 45°C and reblotted. Results are presented as the ratio of the protein, or phosphoprotein levels, to alpha-tubulin and are expressed as a percentage of the controls (wild-type under the control diet).
A new cohort of mice was used for adult neurogenesis experiments. Just before the beginning of the dietary treatment, mice received i.p. injections of 5-bromo-2-deoxyuridine BrdU (150 mg/kg; 2 times/day) dissolved in saline (0.9% NaCl) for 4 days. After 12 weeks of dietary treatment, mice were deeply anesthetized with ketamine and transcardially perfused with 0.9% sodium chloride followed by 4% paraformaldehyde (PFA) in 0.1 M phosphate saline buffer (PBS). Brains were removed and postfixed overnight in 4% PFA at 4°C. Brains equilibrated in 30% sucrose 0.1 M phosphate buffer were embedded in Tissue-Tek OCT (Sakura, United States) and frozen. Coronal 40 μm-thick sections were obtained with a cryostat (Leica, Bensheim, Germany) and stored in cryoprotectant at -20°C until use.
One in six series of coronal sections (spaced 240 μm) throughout the rostrocaudal extent of the hippocampus was used for BrdU staining to evaluate new cell survival. Free-floating brain sections were rinsed in PB containing 0.9% NaCl and 0.25% Triton X-100 (PBST) before inactivation of endogenous peroxidases with 3% H2O2 in 10% methanol in PBS. Sections were incubated in 2N HCl in PBST for 50 min to denature DNA and then neutralized in 0.1M borate buffer (pH 8.5). Sections were then blocked in PBST containing 5% normal goat serum for 60 min, followed by overnight incubation in primary antibody monoclonal rat anti-BrdU (1:400; OBT-0030, Harlan Seralab, Loughborough, United Kingdom) in PBST with 0.1% sodium azide containing 5% normal goat serum. After incubation in goat anti-rat-biotinylated antibody (1:100, BA9400 Vector) for 1 h at room temperature, sections were incubated in the avidin-biotin complex (1:400 in PBS-T; Vector Laboratories ABC Elite Kit) and staining was visualized with DAB-Ni.
One in twelve series of coronal sections (spaced 480 μm) of the rostrocaudal extent of the hippocampus was used for doublecortin (DCX) staining to evaluate maturation of newborn neurons. Sections were incubated in 0.1M phosphate buffered saline with 0.5% Triton X-100 and 10% normal donkey serum (NDS), followed by goat anti-doublecortin primary antibody (1:500; Santa Cruz Biotechnology, SC8066, Santa Cruz, CA, United States) in TBS/Tx/NDS for 24 h at 4°C. Sections were then incubated in biotinylated donkey anti-goat secondary antibody (1:500; Jackson ImmunoResearch, West Grove, PA, United States) in TBS/NDS for 1 h at room temperature, followed by a 1 h amplification step using an avidin-biotin complex (Vector Laboratories ABC Elite Kit) and diaminobenzidine (DAB; Vectastain DAB Kit) as previously described (Quesseveur et al., 2013).
Slides were coded before analysis; the experimenter was blind to genotype and diet until all samples were counted. Quantification of BrdU-immunoreactive (BrdU+) and DCX-immunoreactive (DCX+) cells was conducted using Olympus BX51 microscope (Olympus Deutschland GmbH, Hamburg, Germany). The corresponding surface area of the granule cell layer (GCL) sampled for counting was measured using the Mercator stereology system (Explora Nova, La Rochelle, France). Density of positive cells was then calculated by dividing the number of positive cells by the GCL area sampled. Results were expressed as the number of positive cells/mm2.
Behavioral tests were performed between 9:00 and 11:00 am in a low light condition. Studies in animals are reported in accordance with the ARRIVE guidelines (McGrath et al., 2010). Thus, each mouse was subjected the open-field (OF), the EPM and the FST. This sequence was applied to minimize the impact of stress across tests and a 2-day recovery period between each test was provided (Figure 1). It is noteworthy that reducing the inter-test interval reduces the possible effect of dietary administration on tests.
Open Field was performed in Plexiglas setups (MED Associates, France) during a 30-min session. Entries count and total time in the center were measured by an automated system (MED Associated, France). Total ambulatory distance was also measured to ensure the absence of any locomotor effect of genotypes and/or diets.
Elevated Plus Maze was performed in a Plexiglas apparatus (MED Associates, France) during a 5-min session. Mice were placed in the center of the EPM facing an open arm and entries as well as time spent in the open and closed arms were measured by an automated system (ANY-maze, Stoelting Co., Wood Dale, IL, United States).
Forced Swim Test was performed in plastic buckets (20 cm diameter, 23 cm deep) filled up to two thirds with water at 23–25°C. FST was videotaped for a 6-min session period and the last 4 min were scored for active (climbing and swimming) and passive (immobility) behaviors by an experimenter blind to both genotypes and diets.
Statistical analysis was performed using GraphPad Prism software (Version 5, San Diego, CA, United States). Comparisons between groups were made using an analysis of variance (ANOVA), followed by Tukey’s post hoc analysis when warranted. Significance was set at p < 0.05.
In the present study, two diets equivalent in total fat, protein, carbohydrate and caloric content were formulated. The control diet contained the fatty acid ALA, the precursor of omega-3 PUFAs, and the fish oil-enriched diet presented higher levels of EPA and DHA, two omega-3 PUFAs components (Supplementary Table S1). The fish oil-enriched diet increased levels of omega-3 PUFAs [F(3,10) = 83.1; p < 0.001] and decreased the ratio omega-6 to omega-3 [F(3,10) = 568.2; p < 0.001] in the hippocampus of both wild-type and BDNF+/- mice. Hence, the partial genetic inactivation of BDNF did not prevent the incorporation of omega-3 fatty acids into hippocampal phospholipid membranes (Table 1).
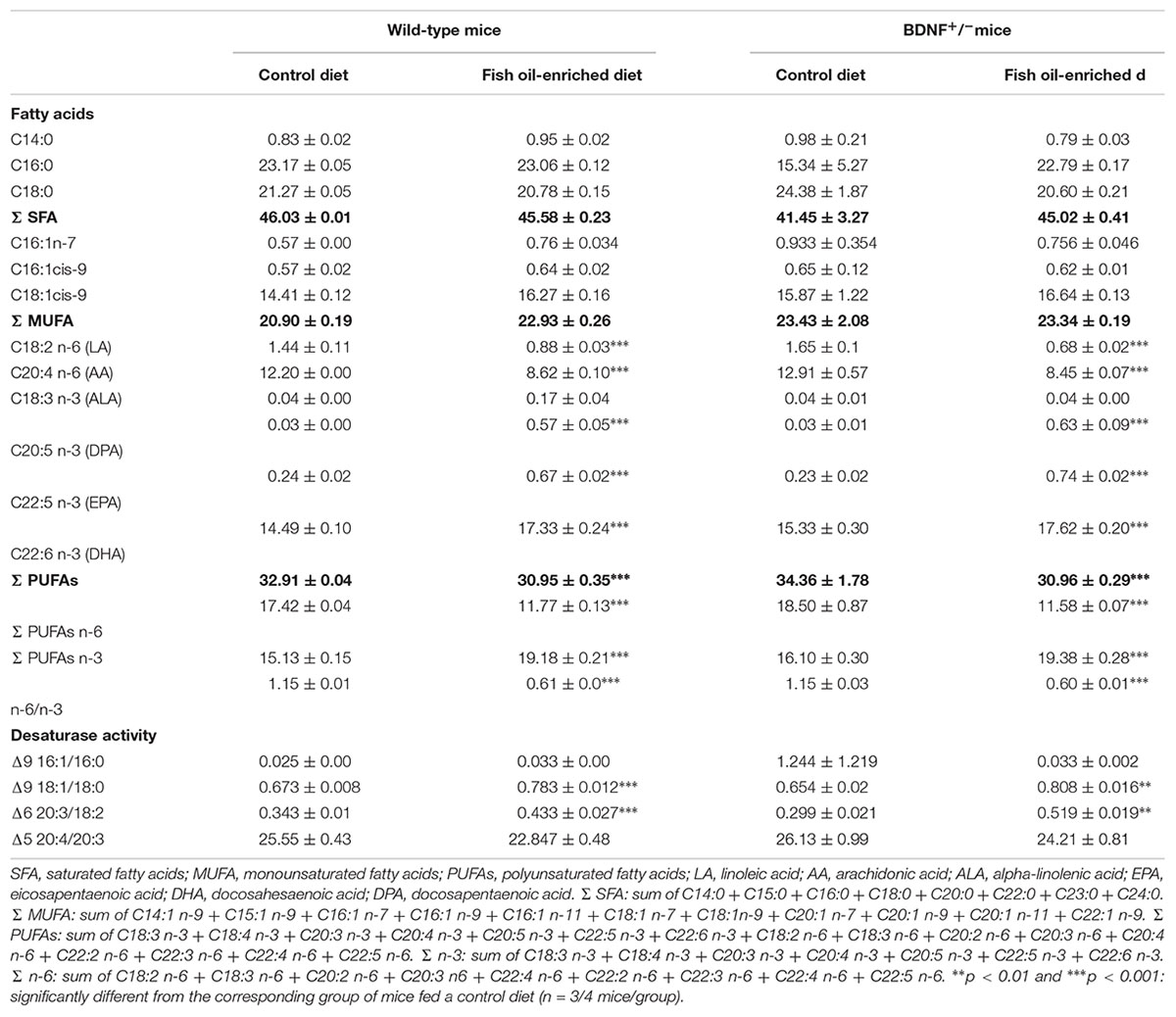
Table 1. Fatty acids profile (% of total fatty acids) of hippocamrpus membrane phospholipids of wild-type and BDNF+/- mice treated with control or fish oil-enriched diet.
Because initial studies demonstrated that forebrain BDNF mRNA and protein levels in BDNF+/- mice were ≈50% of the wild-type (Ernfors et al., 1994; Korte et al., 1995; Kolbeck et al., 1999), the activation of ErK and Akt in the hippocampus was used in the present study as an indirect marker of changes in BDNF signaling (Schmidt and Duman, 2010; Quesseveur et al., 2013; Lepack et al., 2016). Under control diet, a significant reduction in p-Erk protein levels was unveiled in BDNF+/- mice compared to wild-type littermates (p = 0.04, Figure 2A). Fish oil restored p-Erk protein to normal levels (p = 0.04) in BDNF+/- mice, whereas it had no impact on p-Erk in wild-type animals (p = 0.7; Figure 2A). We also monitored the expression of p-Akt and found that partial BDNF depletion had no impact this parameter. Moreover, no significant effects were detected in either wild-type nor BDNF+/- mice fed a fish oil-enriched diet [F(3,16) = 0.9; p = 0.4; Figure 2B]. Altogether, these data support the fact that the partial BDNF depletion leads to impairment of Erk signaling pathway, a deficit which can be rescued by fish oil-enriched diet.
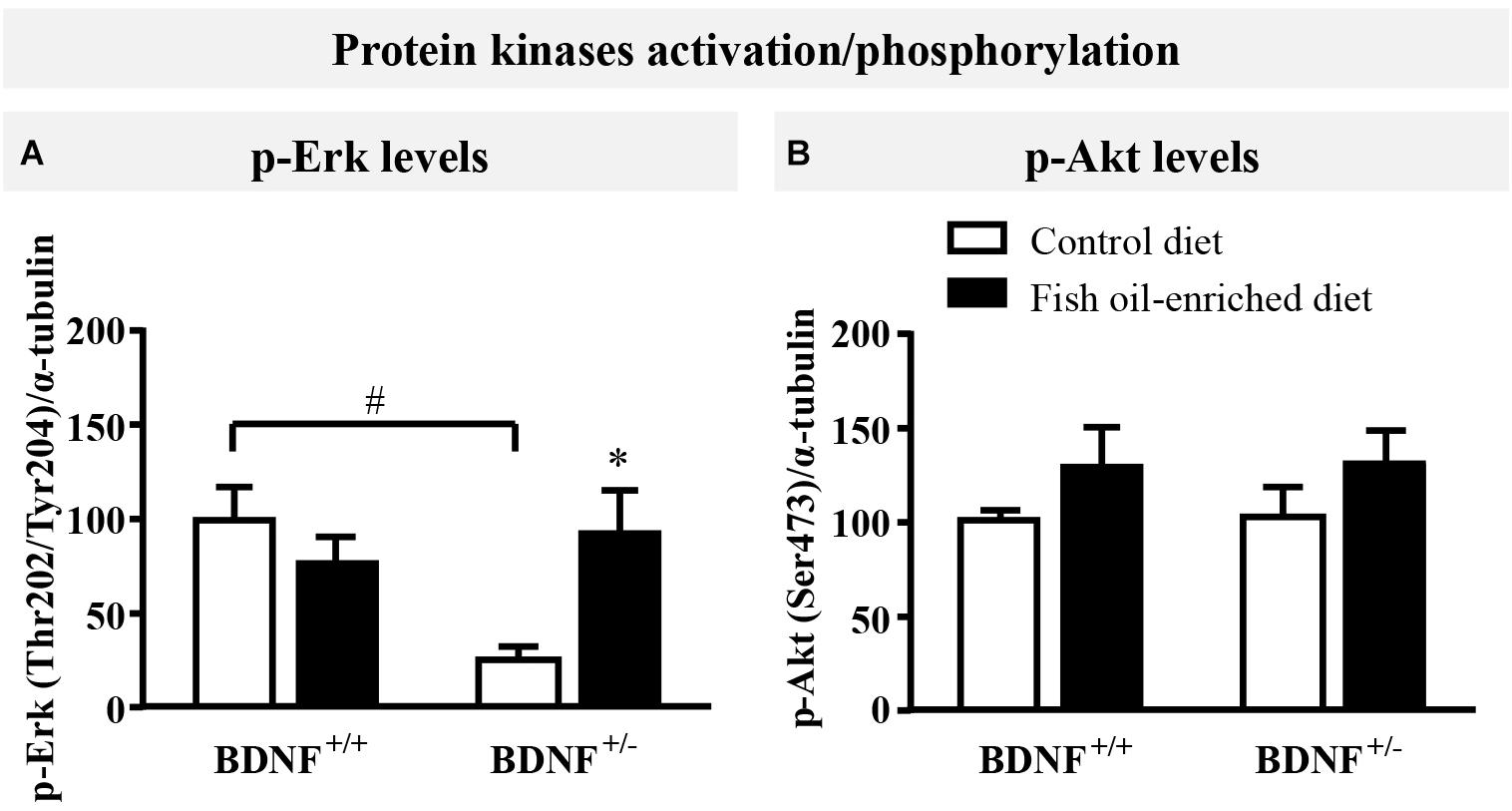
Figure 2. Fish oil-enriched diet increases p-Erk (Thr202/Tyr204) in the hippocampus of BDNF+/- mice. (A) Densitometric quantification of immunoblot analysis from p-Erk (Thr202/Tyr204) (ANOVA: [F(3,16) = 4.2; p = 0.02]) and (B) p-Akt (Ser473) (ANOVA: [F(3,16) = 0.9; p = 0.4]) in the hippocampus of wild-type and BDNF+/- mice fed a control (white bars) or fish oil-enriched diet (black bars) for 12 weeks. Data are expressed as means ± SEM of the ratio p-Erk/α-tubulin or p-Akt/α-tubulin (% of wild-type mice fed a control diet). ∗p < 0.05: diet effect, #p < 0.05: genotype effect (n = 5 mice/group).
We then sought to determine whether fish oil-enriched diet influenced hippocampal serotonergic tone by first assessing the expression of the 5-HT transporter SERT in the hippocampus of wild-type and BDNF+/- mice. Under control diet, a significant decrease in SERT protein levels was observed in BDNF+/- compared to wild-type mice (p = 0.015). Although fish oil had no effect on SERT protein levels in wild-type mice (p = 0.7), this diet rescued SERT protein to control levels in BDNF+/- mice (p = 0.02; Figure 3A). In light of these findings, we tested the possibility that serotonergic tone might be differentially modified in wild-type and BDNF+/- mice. Accordingly, under control diet a significant increase in extracellular 5-HT levels ([5-HT]ext) was detected in the ventral hippocampus of BDNF+/- mice compared to their wild-type littermates (p < 0.001). Fish oil-enriched diet normalized this parameter in BDNF+/- mice (p < 0.001; Figure 3B). These results indicate that the partial BDNF depletion is responsible for an enhancement of serotonergic tone in the hippocampus whereas fish oil-enriched diet restored normal [5-HT]ext levels in BDNF+/- mice by heightening SERT protein expression.
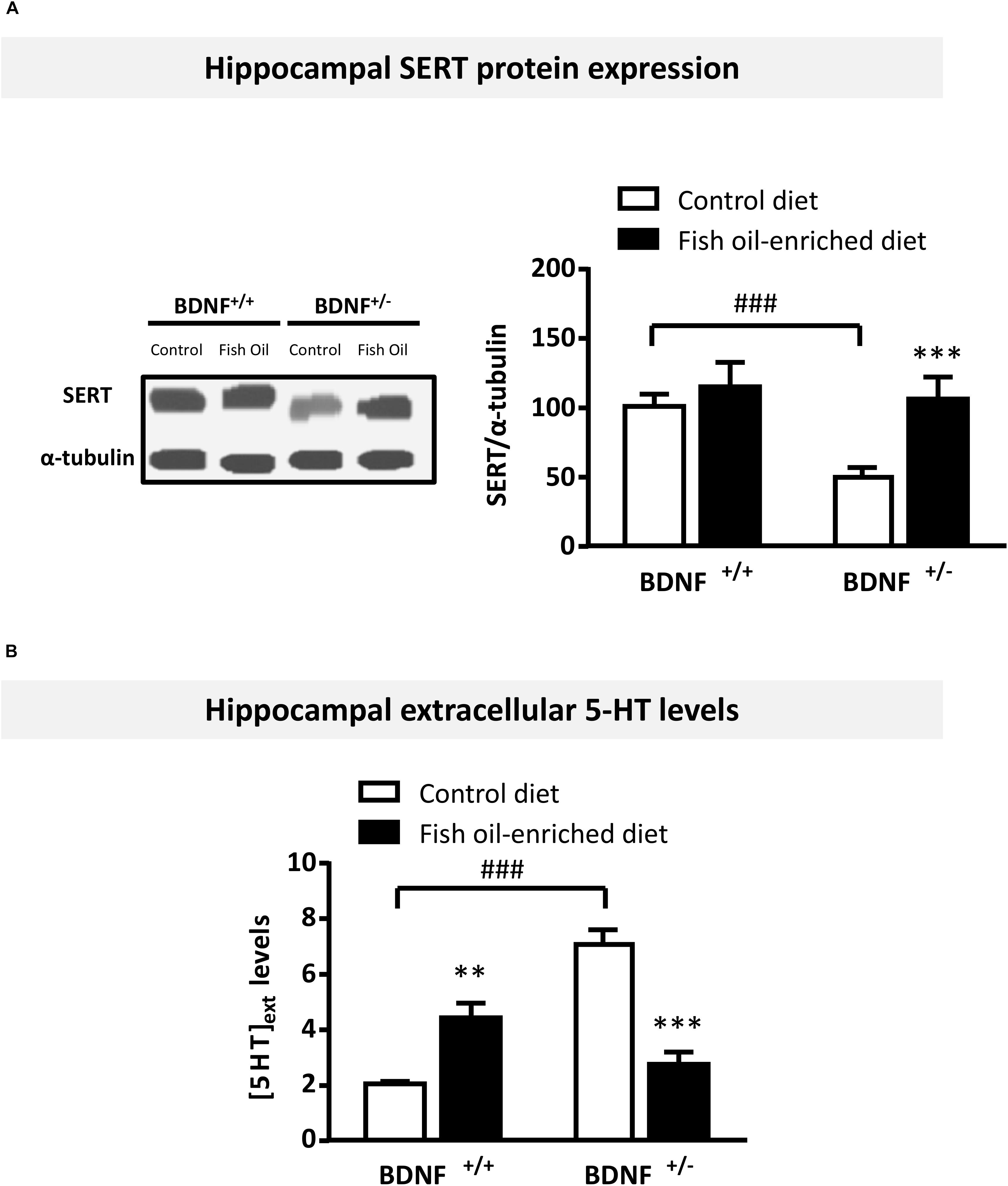
Figure 3. Fish oil-enriched diet decreases serotonergic tone in the hippocampus of BDNF+/- mice. (A) Protein expression of the Serotonin Transporter (SERT). (Left panel) Representative blots from SERT. (Right panel) Densitometric quantification of immunoblot analysis from SERT (ANOVA: [F(3,29) = 5.2; p = 0.002]) in the hippocampus of wild-type and BDNF+/- mice fed a control (white bars) or fish oil-enriched diet (black bars) for 12 weeks. Data are means ± SEM of the ratio SERT/α-tubulin (% of wild-type mice fed a control diet). ∗p < 0.05: diet effect; #p < 0.05: genotype effect significantly different from wild-type mice fed a control diet (n = 8–9 mice/group). (B) Basal extracellular 5-HT levels ([5-HT]ext) in the hippocampus. Data are means ± SEM of basal [5-HT]ext (fmol/19 μL) measured for a 60 min-period in the hippocampus of mice from both genotypes fed a control (white) or fish oil-enriched diet (black) for 12 weeks (ANOVA: [F(3,25) = 67.23]; p < 0.001). ∗∗p < 0.01 and ∗∗∗p < 0.001: diet effect; ###p < 0.001: significantly different from wild-type mice fed a control diet (n = 6–9 mice/group).
Because previous reports pointed out a role for 5-HT and BDNF signaling in the control of hippocampal plasticity, particularly regarding its ability to influence new cell survival and neuronal differentiation (Nibuya et al., 1995; Quesseveur et al., 2013), we examined the effects of fish oil-enriched diet on these parameters in wild-type and BDNF+/- mice. To this end, we quantified BrdU- and DCX-labeled (BrdU+ and DCX+) cells in the dentate gyrus of mice from both genotypes. Densities of 12-week-old BrdU+ cells were not significantly different among experimental groups (Figures 4A,B), indicating that partial BDNF depletion has no long-term impact on hippocampal new cell survival. As regards the density of DCX+ cells, under control diet, neuronal differentiation was not different between wild-type and BDNF+/- mice. Interestingly, although fish-oil diet failed to alter this parameter, a significant increase in the density of DCX+ cells was observed in BDNF+/- mice (p = 0.05; Figures 4C,D). These results suggest that fish-oil enriched diet increases the pool of immature neurons in the dentate gyrus of BDNF+/- mice.
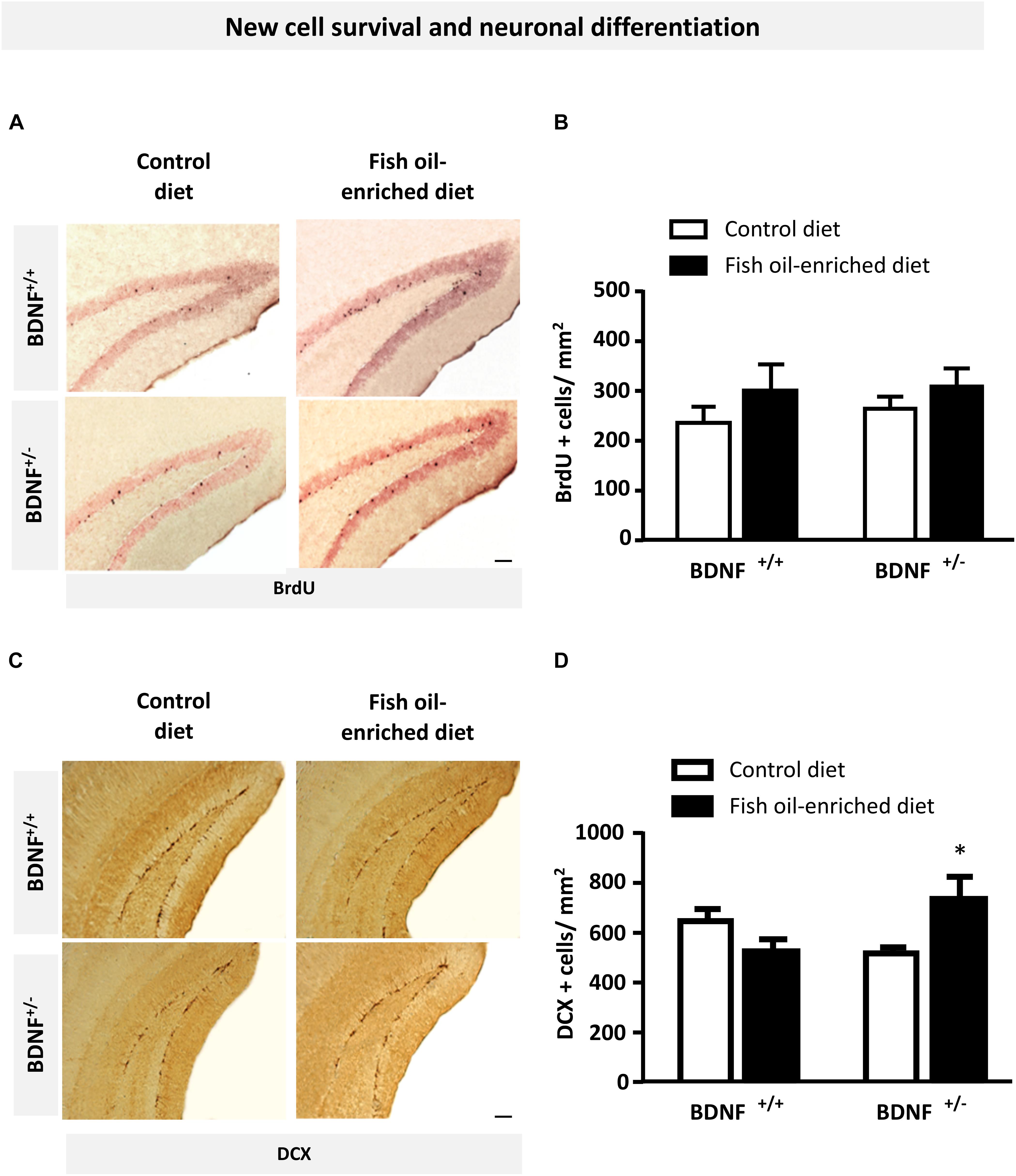
Figure 4. Fish oil-enriched diet does not affect cell survival but increases the density of immature neurons in the hippocampus of BDNF+/- mice. (A) Representative images of the dentate gyrus after 5-bromo-2-deoxyuridine (BrdU) immunostaining in each experimental group. Scale bar: 50 μm. (B) Density of BrdU-labeled (BrdU+) cells 4 weeks after BrdU injection indicative of new cell survival. Data are means ± SEM of BrdU+ cell counts per mm2 (ANOVA: [F(3,19) = 0.8; p = 0.4]; n = 5–6 mice/group) in the dentate gyrus of wild-type and BDNF+/- mice fed a control (white) or fish oil-enriched diet (black) for 12 weeks. (C) Representative images of the dentate gyrus showing doublecortin-labeled (DCX+) cells in each experimental group. Scale bar: 50 μm. (D) Density of DCX+ cells in the dentate gyrus, indicative of the presence of immature neurons. Data are means ± SEM of DCX+ cell counts per mm2 (ANOVA: [F(3,26) = 3.6; p = 0.025]) in the dentate gyrus of wild-type and BDNF+/- mice fed a control (white) or fish oil-enriched diet (black) for 12 weeks. ∗p < 0.05: diet effect (n = 6–9 mice/group).
Finally, we tested the effects of fish oil-enriched diet on mice from both genotypes submitted to behavioral paradigms designed to evaluate different symptoms of depressive state such as anxiety in the OF and EPM and despair in the FST.
In the OF test, numbers of entries in the center were not statistically different between wild-type and BDNF+/- mice fed a control diet (p = 0.06). In mice fed the fish oil-enriched diet, the number of entries (p < 0.01) was significantly increased in BDNF+/- mice (p < 0.01), but not in wild-type littermates (p = 0.7; Figure 5A). Interestingly, similar results were obtained on the time spent in the center (Supplementary Figure S1A). To eliminate a putative bias, we verified that the locomotor activity was not different between groups (wild-type control diet: 3195 ± 461 cm during 30 min; BDNF+/- control diet: 2694 ± 209 cm; wild-type fish oil-enriched diet: 2794 ± 305 cm and BDNF+/- fish oil-enriched diet: 3201 ± 154 cm [F(3,20) = 0.7; p = 0.5491]).
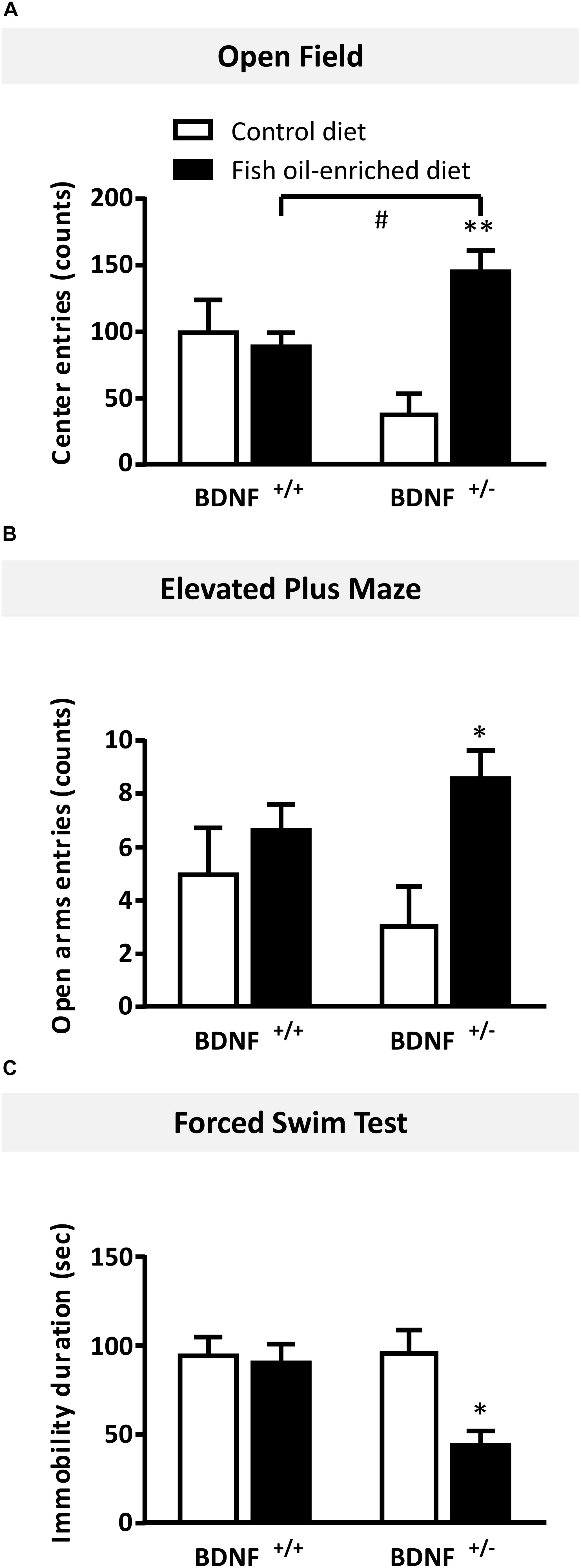
Figure 5. Fish oil-enriched diet induces anxiolytic- and antidepressant-like activities in BDNF+/- mice. (A) Anxiety evaluated in the open field. Data are means ± SEM of the number of entries in the center of the arena (ANOVA: [F(3,20) = 7.1; p = 0.002]). (B) Anxiety evaluated in the elevated plus maze. Data are means ± SEM of the number of entries in the open arms (ANOVA: [F(3,20 = 2.8; p = 0.11]). (C) Antidepressant-like activity evaluated in the forced swim test (FST). Data represent means ± SEM of the immobility time of wild-type and BDNF+/- mice fed a control (white) or fish oil-enriched diet (black) for 12 weeks (ANOVA: [F(3,20) = 7.1; p = 0.002]). ∗p < 0.05, ∗∗p < 0.01: diet effect; #p < 0.05: genotype effect (n = 6 mice/group).
Likewise, in the elevated plus maze, under control diet, no differences were detected between BDNF+/- and their wild-type littermates in the number of entries in the open arms (p = 0.4). However, in mice fed the fish oil-enriched diet, this parameter was significantly increased in BDNF+/- mice (p < 0.01) but not in wild-type littermates (p = 0.4; Figure 5B). Again, these results are consistent with those obtained on the time spent in the open arms (Supplementary Figure S1B).
Finally, in the FST, under control diet, wild-type and BDNF+/- mice displayed the same duration of immobility (p = 0.9). Fish oil significantly decreased the time of immobility in BDNF+/- mice (p < 0.01), while it had no effect in wild-type mice fed a control diet (p = 0.8; Figure 5C).
Overall, these set of behavioral data indicate that fish oil-enriched diet elicited anxiolytic- and antidepressant-like activities specifically in BDNF+/- mice.
The present study evaluated the effects of prolonged exposure to fish oil-enriched diet in wild-type and BDNF+/- mice at the molecular, cellular and behavioral levels. BDNF+/- mice offer a good model to study non-conventional therapeutic strategies for anxiety and depression since these mice are less prone to respond to currently available antidepressant drugs including SSRIs (Daws et al., 2007; Guiard et al., 2008; Ibarguen-Vargas et al., 2009). One of the most remarkable results obtained herein is that under control diet, the partial BDNF depletion produced significant changes in the hippocampus, including a decreased activation of the MAP kinase Erk along with an elevated serotonergic tone. However, these effects were not sufficient to impact cell survival and neuronal differentiation in the hippocampus and to impact anxio-depressive-like behaviors. In a marked contrast, long-term exposure to fish oil-enriched diet in BDNF+/- but not in BDNF+/+ mice increased the activation of Erk and decreased serotonergic tone. These molecular changes were accompanied by an enhancement of neuronal differentiation along with reproducible anxiolytic responses and robust antidepressant-like effects. Together these results led us to envision that fish oil could exert its beneficial effects on mood specifically in patients displaying decreased BDNF signaling in the hippocampus.
The effects of fish oil-enriched diet, an omega-3 PUFAs source, on anxiety have been poorly documented. The limited data available in rodents show that supplementation of diet with omega-3 PUFAs favors anxiolytic-like activities (Pérez et al., 2013; Pudell et al., 2014), whereas their deprivation produces opposite effects (Lafourcade et al., 2011; Larrieu et al., 2012). The latter findings are not consistent with the present results since we failed to unveil beneficial effects of fish oil in wild-type BDNF+/+ mice submitted to the OF and EPM. One possible explanation for this relies on the fact that the control diet used in the present study was not deficient in omega-3. Indeed, the therapeutic benefits of fish oil supplementation have been found only in omega-3 deficient individuals, while those without baseline deficits were less prone to benefit from supplementation (Horrobin and Bennett, 1999). Alternatively, one would expect that fish oil-enriched diet specifically dampens anxiety under pathological conditions. In agreement with this hypothesis, evidence demonstrated that the anxiolytic-like effects of omega-3 PUFAs are detectable after acute or chronic stress (Harauma and Moriguchi, 2011; Mathieu et al., 2011; Ferreira et al., 2013). Of particularly interest in the context of the present study unveiling anxiolytic-like effects of fish oil in BDNF+/- mice, it has been reported that such diet displays similar behavioral properties in bulbectomized rats, an animal model of depression also characterized by reduced hippocampal BDNF levels (Pudell et al., 2014). In keeping with the latter findings, omega-3 PUFAs supplementation was shown to improve social interaction in a strain of mice displaying a reduction of BDNF levels in various brain regions (Pietropaolo et al., 2014). Collectively, these results suggest that the down-regulation of BDNF may be a prerequisite for the manifestation of fish oil’s anxiolytic-like activity. These findings are important since previous studies reported that BDNF+/- mice are not responsive to chronic imipramine treatment (Ibarguen-Vargas et al., 2009) or to acute paroxetine administration (Guiard et al., 2008). Hence, fish oil-enriched diet might be used either alone or as an add-on strategy to antidepressant drugs in treatment-resistant patients (Laino et al., 2010, 2014; Hou and Lai, 2016; Mayor, 2016).
To unravel the putative links between BDNF deficiency and behavioral effects of fish oil-enriched diet, we examined the functional activity of two proteins kinases in the hippocampus (i.e., Erk and Akt). Doing so, we observed that BDNF+/- mice displayed a significant reduction in the level of Erk phosphorylation/activation whereas fish oil diet increased this deficit. Such reversal effect might have contributed, at least in part, to the anxiolytic properties of fish oil diet in these mutants. In support of this hypothesis, we recently demonstrated that an increase in hippocampal p-Erk correlates with a decrease in the latency to feed in the novelty suppressed feeding test (Quesseveur et al., 2015). Conversely, evidence showed that rats microinjected with a specific inhibitor of Erk in the hippocampus for seven consecutive days display anxiety-like behaviors in the open field and the elevated plus maze (Qi et al., 2009). Having shown that fish oil diet increases p-Erk and exert anxiolytic effects specifically in BDNF+/- mice, we then explored to what extent the serotonergic system could be involved an additional and possible component in the behavioral characteristic of fish oil-enriched diet. Here, we report an increase in basal [5-HT]ext in the ventral hippocampus of BDNF+/- mice, which is normalized in response to fish oil-enriched diet. Interestingly, evidence indicated that an abnormally elevated 5-HT tone favors anxiety through the activation of specific post-synaptic 5-HT receptors including the 5HT1A, 5-HT2A/C or 5-HT3 subtypes (Hamon, 1994). Although the impact of fish oil-enriched diet on hippocampal [5-HT]ext levels remains elusive, a recent study pointed out that omega-3 PUFAs supplementation in diet increases tissue 5-HT contents in hippocampus and cortex associated to reduced 5-HIAA levels in 3 months-old rats (Vines et al., 2012). The latter findings are relevant in light of our microdialysis data since an accumulation of tissue (i.e., intracellular) 5-HT in fish oil fed animals could reflect lower [5-HT]ext, notably if the release process is dampened (Kodas et al., 2004). It is noteworthy that the putative inhibitory influence of fish oil-enriched diet on [5-HT]ext levels in the hippocampus of BDNF+/- mice may also result from mechanisms involving the tryptophan hydroxylase 2 (TpH2), the rate-limiting enzyme of 5-HT synthesis, and/or the monoamine oxydase (MAO), an enzyme important in the catabolism of 5-HT. However, the observations that omega-3 PUFAs enhance the expression of tryptophan hydroxylase-2 (TPH-2) (McNamara et al., 2009), while attenuating that of MAO-A/B in the brain (Delion et al., 1997; Chalon et al., 1998; Naveen et al., 2013) are not compatible with our neurochemical data. Because the serotonin transporter SERT is an alternative target through which 5-HT tone may be regulated, we studied this molecular element in the hippocampus. As previously described, we found that BDNF+/- mice exhibited lower levels of SERT protein expression in the hippocampus (Guiard et al., 2008) thereby resulting in an elevated 5-HT tone. Fish oil-enriched diet restored normal hippocampal SERT expression, a process that contributed to normalize 5-HT tone in the hippocampus in BDNF+/- mice and probably in other brain regions. Hence, we demonstrated that fish oil-enriched diet corrected SERT down-regulation directed at minimizing the basal hyperserotonergic phenotype reported in BDNF+/- mice. It is noteworthy that increased anxiety-related behaviors were observed in adult SERT-/- mice (Holmes et al., 2003; Kalueff et al., 2010; Sakakibara et al., 2014) which display spontaneous higher [5-HT]ext. A corollary of this observation is that a functional SERT is necessary to promote long-term fish oil-induced anxiolysis as reported herein. In humans, a short promoter variant in the SERT gene is linked to lower SERT expression, leading to a reduced 5-HT reuptake (Bengel et al., 1998; Murphy and Lesch, 2008). This short variant has also been associated with anxiety-related personality traits (Lesch et al., 1996; Hariri and Holmes, 2006; Canli and Lesch, 2007) and it would be relevant to determine whether long-term exposure to fish oil is effective in this specific population of patients. Again, fish oil-enriched diet had no effect on serotonergic activity in wild-type animals. This is likely due to the fact that these mice display normal levels of anxiety and 5-HT transmission at baseline.
As regards the antidepressant-like effects of fish oil in BDNF+/- mice, it is difficult to envision that decreased immobility observed in the FST relates to the decreased serotonergic tone since an activation of 5-HT neurotransmission is required to hinder behavioral despair in this paradigm (Page et al., 1999). Alternative mechanisms are likely responsible for the antidepressant response induced by long-term exposure to fish oil-enriched diet. It is noteworthy that changes in hippocampal TrkB/BDNF transmission strongly influence MAP kinases signaling pathways, which in turn regulates depressive-related symptoms (Schmidt and Duman, 2010; Lepack et al., 2016). For example, interventions producing antidepressant-like effects such as electroconvulsive shocks or chronic administration of antidepressant drugs are generally associated with an up-regulation of BDNF and downstream signaling pathways in various brain regions including the hippocampus (Nibuya et al., 1995; Balu et al., 2008; Quesseveur et al., 2013). Based on this evidence, we can infer that the ability of fish oil to increase p-Erk levels in BDNF+/- mice leading to a complete recovery of initial hippocampal levels played an important role in the induction of antidepressant-like effects. This is consistent with the observation that addition of DHA to rat primary culture of cortical astrocytes induced BDNF protein expression, an effect blocked by a MAPK inhibitor (Rao et al., 2007). Moreover, it is noteworthy that blunted Erk activation has been observed both in depressed patients and in relevant animal models of depression (Dwivedi et al., 2001; Feng et al., 2003; Gourley et al., 2008; Yuan et al., 2010) whereas inhibition of kinases such as MEK or Erk produced despair-like behaviors and prevented the antidepressant-like effects of SSRIs in rodents (Shirayama et al., 2002; Duman et al., 2007). The observation that fish oil-enriched diet had no effect on Erk activation in wild-type animals is consistent with the lack of effects of this diet on behavioral parameters. The putative enhancement of BDNF signaling, as suggested by Erk phosphorylation/activation in response to fish-oil enriched diet in BDNF+/- mice, also draw our attention because MAP kinases play an important role in the regulation of adult hippocampal neurogenesis including the stimulation of proliferation/differentiation of neural progenitor cells (Lee et al., 2002; Sairanen et al., 2005; Li et al., 2008; Taliaz et al., 2010), the maturation of newborn neurons and their survival (Lee et al., 2002; Sairanen et al., 2005; Rossi et al., 2006; Wang et al., 2015). However, comparing densities of surviving BrdU+ cells, no differences were detected between wild-type and BDNF+/- mice fed a control or a fish oil-enriched diet. However, from these results we cannot provide definitive conclusion on cell survival as long as the number of new generated cells is not assessed in all experimental groups. Given that some studies have described a neurogenic effect of omega-3 PUFAs through its ability to stimulate neuronal maturation (Grundy et al., 2014; McCall et al., 2015), we also quantified immature neurons, using DCX immunolabeling. Interestingly, we observed increased numbers of DCX+ cells in the dentate gyrus of BDNF+/- mice fed a fish oil-enriched diet, suggesting that adult neurogenesis is impacted. Whether increased numbers of DCX+ cells reflect a preferential engagement of newborn cells toward a neuronal fate or a delay in terminal neuronal differentiation remains to be explored.
In an attempt to identify the putative beneficial effects of fish oil, we have to take into consideration the possibility that it might act by modulating inflammatory processes. Indeed, systemic administration of (LPS), widely used to create neuroinflammation, is known to precipitate depression-related behaviors in rodents (Dantzer et al., 2008) whereas evidence indicates that the antidepressant-like effects of fish-oil result, at least in part, from its ability to attenuate this state (Moranis et al., 2012; Delpech et al., 2015; Fourrier et al., 2017). A recent study reported that the TrkB agonist 7,8-dihydroxyflavone (7,8-DHF) reversed LPS-induced depression-like phenotype and morphological changes (i.e., spine density) in the mouse hippocampus (Zhang et al., 2014). These findings suggest that the enhancement of BDNF signaling could be a prerequisite to decrease neuroinflammation (Xu et al., 2017) and to promote the beneficial effects of fish oil in BDNF+/- mice. Nevertheless, different reports indicated that BDNF+/- mice are protected from inflammation not only in the whole brain (Javeri et al., 2010) but also in peripheral tissues such as the heart and the gut (Yang et al., 2010; Halade et al., 2013). Although these findings argue against the fact that BDNF+/- mice could display hallmarks of neuroinflammation, further investigations are warranted to determine to what extent inflammatory processes such as increases in the expression of pro-inflammatory cytokines, activation of ubiquitous indoleamine 2,3-dioxygenase (IDO) or recruitment of microglial cells are altered in these mutants and whether fish-oil enriched diet positively reverberates on these specific markers in the hippocampus.
Our data demonstrate that BDNF+/- mice were more sensitive to the effects of fish oil-enriched diet than wild-type mice. As depicted in Figure 6, the present study strongly suggests that fish oil positively reverberates on emotionality through its ability to decrease hippocampal extracellular 5-HT levels and to increase the activation of Erk that might contribute by itself to stimulate neuronal plasticity. It should be borne in mind that fish-oil is a mix of omega-3 fatty acids including, for example, eicosapentaenoic acid (EPA), docosahexaenoic acid (DHA) or α-linolenic acid (ALA). Given that these components display distinct effects on behavioral paradigms assessing antidepressant-like activities (Jin and Park, 2015; Choi and Park, 2017), it would be interesting to precise which of them interfere specifically on neurobehavior. This will help optimize an “add-on” strategy based on the combination of fish oil and SSRI in animal models resistant to conventional monoaminergic antidepressant drugs. In particular, it will be interesting to determine the effects of fish oil-enriched diet in mice exposed to chronic stress (e.g., restraint stress, unpredictable chronic mild stress or even social defeat).
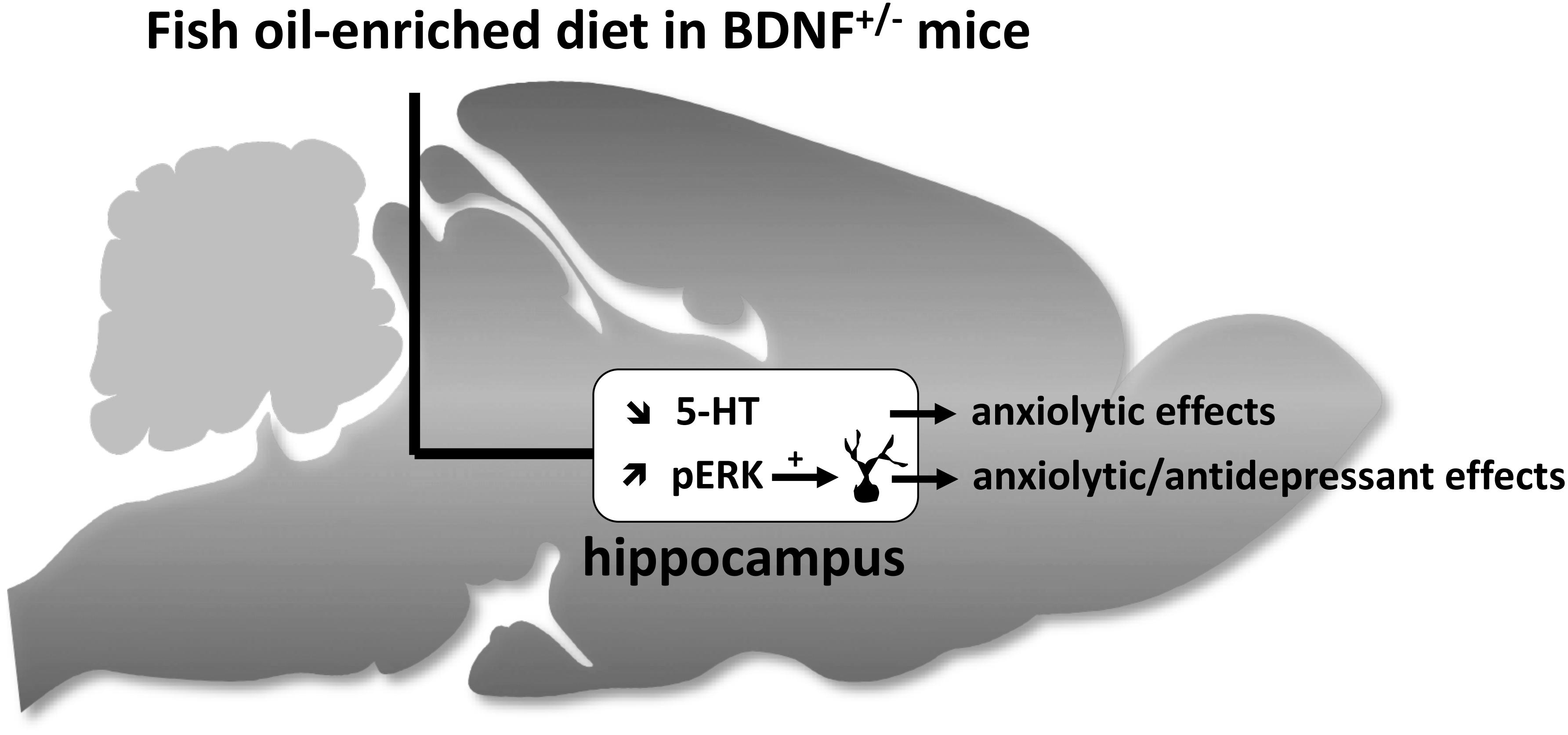
Figure 6. Schematic representation of hippocampal neurochemical, molecular and cellular targets of fish oil-enriched diet and its behavioral effects in BDNF+/- mice. In BDNF+/- mice, a decrease in the extracellular 5-HT concentrations and an increase in the level of Erk phosphorylation are observed in response to long-term exposure to a fish oil-enriched diet. These effects might have contributed to positively reverberate on anxiety and despair. However, because the enhancement of BDNF synthesis/release and related-signaling (Erk activation) in the hippocampus relies on the activation of local 5-HT tone, the results presented herein cannot draw a cause and effect relationship between BDNF signaling and 5-HT neurotransmission. We propose that the beneficial behavioral effects of fish-oil in BDNF+/- mice involved two distinct mechanisms leading on one hand, to decrease extracellular 5-HT concentrations (favorable for anxiolysis) and on the other hand, to stimulate BDNF signaling and neuronal maturation (favorable for anxiolysis and antidepressant response).
The in vivo part of this study was conducted in 2013 and the procedures were conducted in conformity with the institutional guidelines in compliance with national and policy (Council directive #87–848, October 19, 1987, Ministère de l’Agriculture et de la Forêt, Service Vétérinaire de la Santé et de la Protection Animale, permission #92.196). The in vitro part of this study, not subjected to an ethical committee, was conducted between 2015 and 2017.
JZ conducted all the experiments in BDNF wild-type and mutant mice fed a fish oil diet and wrote the manuscript. QR and CG provided their assistance for the behavioral and Western-blot analyses. DR-R and AG prepared the fish oil diet and analyses. ER and BG were the principal investigators of this study and contributed to the analyses of the results.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The authors are thankful to M. Pereira (Sao Paulo) and P. Robert (Châtenay-Malabry) for animal care. JZ was supported by “Coordenação de Aperfeiçoamento de Pessoal de Nível Superior” (CAPES, Brazil) and “Conselho Nacional de Desenvolvimento Científico e Tecnológico” (CNPq, Brazil). The authors greatly acknowledge “the Mouse Behavioural Phenotyping Core” (Center of Integrative Biology, Toulouse, France) and more particularly Sebastien Lopez for his expertise and assistance in setting up behavioral apparatus and procedures. The authors also thank Prs A. M. Gardier and D. David for their helpful comments in the preparation of this manuscript.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnins.2018.00974/full#supplementary-material
FIGURE S1 | Fish oil-enriched diet induces anxiolytic-like activities in BDNF+/- mice. (A) Anxiety evaluated in the open field. Data are means ± SEM of the time spent in the center of the arena (ANOVA: [F(3,20) = 7.1; p = 0.002]). (B) Anxiety evaluated in the elevated plus maze. Data are means ± SEM of the time spent in the open arms (ANOVA: [F(3,20) = 2.8; p = 0.11]). ∗p < 0.05: significantly different from the corresponding group fed a control diet (n = 6 mice/group).
TABLE S1 | Fatty acids content in control and fish oil-enriched diet.
Able, J. A., Liu, Y., Jandacek, R., Rider, T., Tso, P., and McNamara, R. K. (2014). Omega-3 fatty acid deficient male rats exhibit abnormal behavioral activation in the forced swim test following chronic fluoxetine treatment: association with altered 5-HT1A and alpha2A adrenergic receptor expression. J. Psychiatr. Res. 50, 42–50. doi: 10.1016/j.jpsychires.2013.11.008
Balu, D. T., Hoshaw, B. A., Malberg, J. E., Rosenzweig-Lipson, S., Schechter, L. E., and Lucki, I. (2008). Differential regulation of central BDNF protein levels by antidepressant and non-antidepressant drug treatments. Brain Res. 1211, 37–43. doi: 10.1016/j.brainres.2008.03.023
Bengel, D., Murphy, D. L., Andrews, A. M., Wichems, C. H., Feltner, D., Heils, A., et al. (1998). Altered brain serotonin homeostasis and locomotor insensitivity to 3, 4-methylenedioxymethamphetamine (“Ecstasy”) in serotonin transporter-deficient mice. Mol. Pharmacol. 53, 649–655. doi: 10.1124/mol.53.4.649
Blondeau, N., Nguemeni, C., Debruyne, D. N., Piens, M., Wu, X., Pan, H., et al. (2009). Subchronic alpha-linolenic acid treatment enhances brain plasticity and exerts an antidepressant effect: a versatile potential therapy for stroke. Neuropsychopharmacology 34, 2548–2559. doi: 10.1038/npp.2009.84
Calder, P. C. (1998). Immunoregulatory and anti-inflammatory effects of n-3 polyunsaturated fatty acids. Braz. J. Med. Biol. Res. 31, 467–490. doi: 10.1590/S0100-879X1998000400002
Canli, T., and Lesch, K. P. (2007). Long story short: the serotonin transporter in emotion regulation and social cognition. Nat. Neurosci. 10, 1103–1109. doi: 10.1038/nn1964
Chalon, S., Delion-Vancassel, S., Belzung, C., Guilloteau, D., Leguisquet, A. M., Besnard, J. C., et al. (1998). Dietary fish oil affects monoaminergic neurotransmission and behavior in rats. J. Nutr. 128, 2512–2519. doi: 10.1093/jn/128.12.2512
Chiu, C. C., Huang, S. Y., Su, K. P., Lu, M. L., Huang, M. C., Chen, C. C., et al. (2003). Polyunsaturated fatty acid deficit in patients with bipolar mania. Eur. Neuropsychopharmacol. 13, 99–103. doi: 10.1016/S0924-977X(02)00130-X
Choi, J. E., and Park, Y. (2017). EPA and DHA, but not ALA, have antidepressant effects with 17β-estradiol injection via regulation of a neurobiological system in ovariectomized rats. J. Nutr. Biochem. 49, 101–109. doi: 10.1016/j.jnutbio.2017.07.012
Dantzer, R., O’Connor, J. C., Freund, G. G., Johnson, R. W., and Kelley, K. W. (2008). From inflammation to sickness and depression: when the immune system subjugates the brain. Nat. Rev. Neurosci. 9, 46–56. doi: 10.1038/nrn2297
Daws, L. C., Munn, J. L., Valdez, M. F., Frosto-Burke, T., and Hensler, J. G. (2007). Serotonin transporter function, but not the expression, is dependent on brain-derived neurotrophic factor (BDNF): in vivo studies in BDNF-deficient mice. J. Neurochem. 101, 641–651. doi: 10.1111/j.1471-4159.2006.04392.x
De Vriese, S. R., Christophe, A. B., and Maes, M. (2003). Lowered serum n-3 polyunsaturated fatty acid (PUFA) levels predict the occurrence of postpartum depression: further evidence that lowered n-PUFAs are related to major depression. Life Sci. 73, 3181–3187. doi: 10.1016/j.lfs.2003.02.001
Delion, S., Chalon, S., Guilloteau, D., Lejeune, B., Besnard, J. C., and Durand, G. (1997). Age-related changes in phospholipid fatty acid composition and monoaminergic neurotransmission in the hippocampus of rats fed a balanced or an n-3 polyunsaturated fatty acid-deficient diet. J. Lipid Res. 38, 680–689.
Delpech, J. C., Thomazeau, A., Madore, C., Bosch-Bouju, C., Larrieu, T., Lacabanne, C., et al. (2015). Dietary n-3 PUFAs deficiency increases vulnerability to inflammation-induced spatial memory impairments. Neuropsychopharmacology 40, 2274–2287. doi: 10.1038/npp.2015.127
Deltheil, T., Tanaka, K., Repérant, C., Hen, R., David, D. J., and Gardier, A. M. (2009). Synergistic neurochemical and behavioural effects of acute intra-hippocampal injection of brain-derived neurotrophic factor and antidepressants in adult mice. Int. J. Neuropsychopharmacol. 12, 905–915. doi: 10.1017/S1461145709000017
Duman, C. H., Schlesinger, L., Kodama, M., Russell, D. S., and Duman, R. S. (2007). A role for MAP kinase signaling in behavioral models of depression and antidepressant treatment. Biol. Psychiatry 61, 661–670. doi: 10.1016/j.biopsych.2006.05.047
Dwivedi, Y., Rizavi, H. S., Roberts, R. C., Conley, R. C., Tamminga, C. A., and Pandey, G. N. (2001). Reduced activation and expression of ERK1/2 MAP kinase in the post-mortem brain of depressed suicide subjects. J. Neurochem. 77, 916–928. doi: 10.1046/j.1471-4159.2001.00300.x
Ernfors, P., Lee, K. F., and Jaenisch, R. (1994). Mice lacking brain-derived neurotrophic factor develop with sensory deficits. Nature 368, 147–150. doi: 10.1038/368147a0
Feng, P., Guan, Z., Yang, X., and Fang, J. (2003). Impairments of ERK signal transduction in the brain in a rat model of depression induced by neonatal exposure of clomipramine. Brain Res. 991, 195–205. doi: 10.1016/j.brainres.2003.08.018
Ferreira, C. F., Bernardi, J. R., Krolow, R., Arcego, D. M., Fries, G. R., de Aguiar, B. W., et al. (2013). Vulnerability to dietary n-3 polyunsaturated fatty acid deficiency after exposure to early stress in rats. Pharmacol. Biochem. Behav. 107, 11–19. doi: 10.1016/j.pbb.2013.03.006
Fourrier, C., Remus-Borel, J., Greenhalgh, A. D., Guichardant, M., Bernoud-Hubac, N., Lagarde, M., et al. (2017). Docosahexaenoic acid-containing choline phospholipid modulates LPS-induced neuroinflammation in vivo and in microglia in vitro. J. Neuroinflamm. 14:170. doi: 10.1186/s12974-017-0939-x
Freeman, M. P., Hibbeln, J. R., Wisner, K. L., Brumbach, B. H., Watchman, M., and Gelenberg, A. J. (2006). Randomized dose-ranging pilot trial of omega-3 fatty acids for postpartum depression. Acta Psychiatr. Scand. 113, 31–35. doi: 10.1111/j.1600-0447.2005.00660.x
Gardier, A. M., Malagié, I., Trillat, A. C., Jacquot, C., and Artigas, F. (1996). Role of 5-HT1A autoreceptors in the mechanism of action of serotoninergic antidepressant drugs: recent findings from in vivo microdialysis studies. Fundam. Clin. Pharmacol. 10, 16–27. doi: 10.1111/j.1472-8206.1996.tb00145.x
Gertsik, L., Poland, R. E., Bresee, C., and Rapaport, M. H. (2012). Omega-3 fatty acid augmentation of citalopram treatment for patients with major depressive disorder. J. Clin. Psychopharmacol. 32, 61–64. doi: 10.1097/JCP.0b013e31823f3b5f
Gourley, S. L., Wu, F. J., Kiraly, D. D., Ploski, J. E., Kedves, A. T., Duman, R. S., et al. (2008). Regionally specific regulation of ERK MAP kinase in a model of antidepressant-sensitive chronic depression. Biol. Psychiatry 63, 353–359. doi: 10.1016/j.biopsych.2007.07.016
Grundy, T., Toben, C., Jaehne, E. J., Corrigan, F., and Baune, B. T. (2014). Long-term omega-3 supplementation modulates behavior, hippocampal fatty acid concentration, neuronal progenitor proliferation and central TNF-α expression in 7 month old unchallenged mice. Front. Cell. Neurosci. 8:399. doi: 10.3389/fncel.2014.00399
Guiard, B. P., David, D. J., Deltheil, T., Chenu, F., Le Maître, E., Renoir, T., et al. (2008). Brain-derived neurotrophic factor-deficient mice exhibit a hippocampal hyperserotonergic phenotype. Int. J. Neuropsychopharmacol. 11, 79–92. doi: 10.1017/S1461145707007857
Halade, G. V., Ma, Y., Ramirez, T. A., Zhang, J., Dai, Q., Hensler, J. G., et al. (2013). Reduced BDNF attenuates inflammation and angiogenesis to improve survival and cardiac function following myocardial infarction in mice. Am. J. Physiol. Heart Circ. Physiol. 305, H1830-18-42. doi: 10.1152/ajpheart.00224.2013
Hamon, M. (1994). Neuropharmacology of anxiety: perspectives and prospects. Trends Pharmacol. Sci. 15, 36–39. doi: 10.1016/0165-6147(94)90104-X
Hamon, M., and Blier, P. (2013). Monoamine neurocircuitry in depression and strategies for new treatments. Prog. Neuropsychopharmacol. Biol. Psychiatry 45, 54–63. doi: 10.1016/j.pnpbp.2013.04.009
Harauma, A., and Moriguchi, T. (2011). Dietary n-3 fatty acid deficiency in mice enhances anxiety induced by chronic mild stress. Lipids 46, 409–416. doi: 10.1007/s11745-010-3523-z
Hariri, A. R., and Holmes, A. (2006). Genetics of emotional regulation: the role of the serotonin transporter in neural function. Trends Cogn. Sci. 10, 182–191. doi: 10.1016/j.tics.2006.02.011
Hibbeln, J. R., Ferguson, T. A., and Blasbalg, T. L. (2006). Omega-3 fatty acid deficiencies in neurodevelopment, aggression and autonomic dysregulation: opportunities for intervention. Int. Rev. Psychiatry 18, 107–118. doi: 10.1080/09540260600582967
Holmes, A., Yang, R. J., Lesch, K. P., Crawley, J. N., and Murphy, D. L. (2003). Mice lacking the serotonin transporter exhibit 5-HT(1A) receptor-mediated abnormalities in tests for anxiety-like behavior. Neuropsychopharmacology 28, 2077–2088. doi: 10.1038/sj.npp.1300266
Horrobin, D. F., and Bennett, C. N. (1999). Depression and bipolar disorder: relationships to impaired fatty acid and phospholipid metabolism and to diabetes, cardiovascular disease, immunological abnormalities, cancer, ageing and osteoporosis, Possible candidate genes, Prostaglandins Leukot. Essent. Fatty Acids 60, 217–234. doi: 10.1054/plef.1999.0037
Hou, Y. C., and Lai, C. H. (2016). Enhancing therapeutic effects after augmentation of omega-3 fatty acid with long-term antidepressant therapy in a chronic case of panic disorder. Psychiatry Clin. Neurosci. 70, 72–83. doi: 10.1111/pcn.12373
Ibarguen-Vargas, Y., Surget, A., Vourc’h, P., Leman, S., Andres, C. R., Gardier, A. M., et al. (2009). Deficit in BDNF does not increase vulnerability to stress but dampens antidepressant-like effects in the unpredictable chronic mild stress. Behav. Brain Res. 202, 245–251. doi: 10.1016/j.bbr.2009.03.040
Javeri, S., Rodi, M., Tary-Lehmann, M., Lehmann, P. V., Addicks, K., and Kuerten, S. (2010). Involvement of brain-derived neurotrophic factor (BDNF) in MP4-induced autoimmune encephalomyelitis. Clin. Immunol. 137, 181–189. doi: 10.1016/j.clim.2010.08.001
Jazayeri, S., Tehrani-Doost, M., Keshavarz, S. A., Hosseini, M., Djazayery, A., Amini, H., et al. (2008). Comparison of therapeutic effects of omega-3 fatty acid eicosapentaenoic acid and fluoxetine, separately and in combination, in major depressive disorder. Aust. N. Z. J. Psychiatry 42, 192–198. doi: 10.1080/00048670701827275
Jin, Y., and Park, Y. (2015). N-3 polyunsaturated fatty acids and 17β-estradiol injection induce antidepressant-like effects through regulation of serotonergic neurotransmission in ovariectomized rats. J. Nutr. Biochem. 26, 970–977. doi: 10.1016/j.jnutbio.2015.04.005
Kalueff, A. V., Olivier, J. D., Nonkes, L. J., and Homberg, J. R. (2010). Conserved role for the serotonin transporter gene in rat and mouse neurobehavioral endophenotypes. Neurosci. Biobehav. Rev. 34, 373–386. doi: 10.1016/j.neubiorev.2009.08.003
Kodas, E., Galineau, L., Bodard, S., Vancassel, S., Guilloteau, D., Besnard, J. C., et al. (2004). Serotoninergic neurotransmission is affected by n-3 polyunsaturated fatty acids in the rat. J. Neurochem. 89, 695–702. doi: 10.1111/j.1471-4159.2004.02401.x
Kolbeck, R., Bartke, I., Eberle, W., and Barde, Y. A. (1999). Brain-derived neurotrophic factor levels in the nervous system of wild-type and neurotrophin gene mutant mice. J. Neurochem. 75, 1930–1938. doi: 10.1046/j.1471-4159.1999.0721930.x
Korte, M., Carroll, P., Wolf, E., Brem, G., Thoenen, H., and Bonhoeffer, T. (1995). Hippocampal long-term potentiation is impaired in mice lacking brain-derived neurotrophic factor. Proc. Natl. Acad. Sci. U.S.A. 92, 8856–8860. doi: 10.1073/pnas.92.19.8856
Lafourcade, M., Larrieu, T., Mato, S., Duffaud, A., Sepers, M., Matias, I., et al. (2011). Nutritional omega-3 deficiency abolishes endocannabinoid-mediated neuronal functions. Nat. Neurosci. 14, 345–350. doi: 10.1038/nn.2736
Laino, C. H., Fonseca, C., Sterin-Speziale, N., Slobodianik, N., and Reinés, A. (2010). Potentiation of omega-3 fatty acid antidepressant-like effects with low non-antidepressant doses of fluoxetine and mirtazapine. Eur. J. Pharmacol. 648, 117–126. doi: 10.1016/j.ejphar.2010.08.047
Laino, C. H., Garcia, P., Podestá, M. F., Höcht, C., Slobodianik, N., and Reinés, A. (2014). Fluoxetine potentiation of omega-3 fatty acid antidepressant effect: evaluating pharmacokinetic and brain fatty acid-related aspects in rodents. J. Pharm. Sci. 103, 3316–3325. doi: 10.1002/jps.24123
Lakhwani, L., Tongia, S. K., Pal, V. S., Agrawal, R. P., Nyati, P., and Phadnis, P. (2007). Omega-3 fatty acids have antidepressant activity in forced swimming test in Wistar rats. Acta Pol. Pharm. 64, 271–276.
Larrieu, T., Madore, C., Joffre, C., and Layé, S. (2012). Nutritional n-3 polyunsaturated fatty acids deficiency alters cannabinoid receptor signaling pathway in the brain and associated anxiety-like behavior in mice. J. Physiol. Biochem. 68, 671–681. doi: 10.1007/s13105-012-0179-6
Lecrubier, Y. (2001). Prescribing patterns for depression and anxiety worldwide. J. Clin. Psychiatry 62 (Suppl. 13), 31–36; discussion 37–38.
Lee, J., Duan, W., and Mattson, M. P. (2002). Evidence that brain-derived neurotrophic factor is required for basal neurogenesis and mediates, in part, the enhancement of neurogenesis by dietary restriction in the hippocampus of adult mice. J. Neurochem. 82, 1367–1375. doi: 10.1046/j.1471-4159.2002.01085.x
Lepack, A. E., Bang, E., Lee, B., Dwyer, J. M., and Duman, R. S. (2016). Fast-acting antidepressants rapidely stimulate ERK signaling and BDNF release in primary neuronal cultures. Neuropharmacology 111, 242–252. doi: 10.1016/j.neuropharm.2016.09.011
Lesch, K. P., Bengel, D., Heils, A., Sabol, S. Z., Greenberg, B. D., Petri, S., et al. (1996). Association of anxiety-related traits with a polymorphism in the serotonin transporter gene regulatory region. Science 274, 1527–1531. doi: 10.1126/science.274.5292.1527
Li, Y., Luikart, B. W., Birnbaum, S., Chen, J., Kwon, C. H., Kernie, S. G., et al. (2008). TrkB regulates hippocampal neurogenesis and governs sensitivity to antidepressive treatment. Neuron 59, 399–412; Erratum in: Neuron 2008 60, 730. doi: 10.1016/j.neuron.2008.06.023
Lin, P. Y., Huang, S. Y., and Su, K. P. (2010). A meta-analytic review of polyunsaturated fatty acid compositions in patients with depression. Biol. Psychiatry 15, 140–147. doi: 10.1016/j.biopsych.2010.03.018
Lin, P. Y., and Su, K. P. (2007). A meta-analytic review of double-blind, placebo-controlled trials of antidepressant efficacy of omega-3 fatty acids. J. Clin. Psychiatry 68, 1056–1061. doi: 10.4088/JCP.v68n0712
Luellen, B. A., Bianco, L. E., Schneider, L. M., and Andrews, A. M. (2007). Reduced brain-derived neurotrophic factor is associated with a loss of serotonergic innervation in the hippocampus of aging mice. Gene Brain Behav. 6, 482–490. doi: 10.1111/j.1601-183X.2006.00279.x
Lyons, W. E., Mamounas, L. A., Ricaurte, G. A., Coppola, V., Reid, S. W., Bora, S. H., et al. (1999). Brain-derived neurotrophic factor-deficient mice develop aggressiveness and hyperphagia in conjunction with brain serotonergic abnormalities. Proc. Natl. Acad. Sci. U.S.A. 96, 15239–15244. doi: 10.1073/pnas.96.26.15239
MacQueen, G. M., Ramakrishnan, K., Croll, S. D., Siuciak, J. A., Yu, G., Young, L. T., et al. (2001). Performance of heterozygous brain-derived neurotrophic factor knockout mice on behavioral analogues of anxiety, nociception, and depression. Behav. Neurosci. 115, 1145–1153. doi: 10.1037/0735-7044.115.5.1145
Mamounas, L. A., Altar, C. A., Blue, M. E., Kaplan, D. R., Tessarollo, L., and Lyons, W. E. (2000). BDNF promotes the regenerative sprouting, but not survival, of injured serotonergic axons in the adult rat brain. J. Neurosci. 20, 771–782. doi: 10.1523/JNEUROSCI.20-02-00771.2000
Marangell, L. B., Martinez, J. M., Zboyan, H. A., Kertz, B., Kim, H. F., and Puryear, L. J. (2003). A double-blind, placebo-controlled study of the omega-3 fatty acid docosahexaenoic acid in the treatment of major depression. Am. J. Psychiatry 160, 996–998. doi: 10.1176/appi.ajp.160.5.996
Mathieu, G., Oualian, C., Denis, I., Lavialle, M., Gisquet-Verrier, P., and Vancassel, S. (2011). Dietary n-3 polyunsaturated fatty acid deprivation together with early maternal separation increases anxiety and vulnerability to stress in adult rats. Prostaglandins Leukot Essent Fatty Acids 85, 129–136. doi: 10.1016/j.plefa.2011.07.001
Mayor, S. (2016). Supplements such as fish oils improve antidepressant effectiveness, review finds. BMJ 27:i2418. doi: 10.1136/bmj.i2418
McCall, N., Mahadevia, D., Corriveau, J. A., and Glenn, M. J. (2015). Adult emotionality and neural plasticity as a function of adolescent nutrient supplementation in male rats. Pharmacol. Biochem. Behav. 132, 125–135. doi: 10.1016/j.pbb.2015.03.004
McGrath, J., Drummond, G., Kilkenny, C., and Wainwright, C. (2010). Guidelines for reporting experiments involving animals: the ARRIVE guidelines. Br. J. Pharmacol. 160, 1573–1576. doi: 10.1111/j.1476-5381.2010.00873.x
McNamara, R. K., Able, J., Liu, Y., Jandacek, R., Rider, T., Tso, P., et al. (2009). Omega-3 fatty acid deficiency during perinatal development increases serotonin turnover in the prefrontal cortex and decreases midbrain tryptophan hydroxylase-2 expression in adult female rats: dissociation from estrogenic effects. J. Psychiatr. Res. 43, 656–663. doi: 10.1016/j.jpsychires.2008.09.011
McNamara, R. K., Hahn, C. G., Jandacek, R., Rider, T., Tso, P., Stanford, K. E., et al. (2007). Selective deficits in the omega-3 fatty acid docosahexaenoic acid in the postmortem orbitofrontal cortex of patients with major depressive disorder. Biol. Psychiatry 62, 17–24. doi: 10.1016/j.biopsych.2006.08.026
Mocking, R. J., Harmsen, I., Assies, J., Koeter, M. W., Ruhé, H. G., and Schene, A. H. (2016). Meta-analysis and meta-regression of omega-3 polyunsaturated fatty acid supplementation for major depressive disorder. Transl. Psychiatry 6:e756. doi: 10.1038/tp.2016.29
Monteggia, L. M., Barrot, M., Powell, C. M., Berton, O., Galapis, V., Gemelli, T., et al. (2004). Essential role of brain-derived neurotrophic factor in adult hippocampal function. Proc. Natl. Acad. Sci. U.S.A. 101, 10827–10832. doi: 10.1073/pnas.0402141101
Monteggia, L. M., Luikart, B., Barrot, M., Theobold, D., Malkovska, I., Nef, S., et al. (2007). Brain-derived neurotrophic factor conditional knockouts show gender differences in depression-related behaviors. Biol. Psychiatry 61, 187–197. doi: 10.1016/j.biopsych.2006.03.021
Montgomery, P., and Richardson, A. J. (2008). Omega-3 fatty acids for bipolar disorder. Cochrane Database Syst Rev. 2:CD005169. doi: 10.1002/14651858.CD005169.pub2
Moranis, A., Delpech, J. C., De Smedt-Peyrusse, V., Aubert, A., Guesnet, P., Lavialle, M., et al. (2012). Long term adequate n-3 polyunsaturated fatty acid diet protects from depressive-like behavior but not from working memory disruption and brain cytokine expression in aged mice. Brain Behav. Immun. 26, 721–731. doi: 10.1016/j.bbi.2011.11.001
Murphy, D. L., and Lesch, K. P. (2008). Targeting the murine serotonin transporter: insights into human neurobiology. Nat. Rev. Neurosci. 9, 85–96. doi: 10.1038/nrn2284
Naveen, S., Siddalingaswamy, M., Singsit, D., and Khanum, F. (2013). Anti-depressive effect of polyphenols and omega-3 fatty acid from pomegranate peel and flax seed in mice exposed to chronic mild stress. Psychiatry Clin. Neurosci. 67, 501–508. doi: 10.1111/pcn.12100
Nibuya, M., Morinobu, S., and Duman, R. S. (1995). Regulation of BDNF and trkB mRNA in rat brain by chronic electroconvulsive seizure and antidepressant drug treatments. J. Neurosci. 15, 7539–7547. doi: 10.1523/JNEUROSCI.15-11-07539.1995
Owen, C., Rees, A. M., and Parker, G. (2008). The role of fatty acids in the development and treatment of mood disorders. Curr. Opin. Psychiatry 21, 19–24. doi: 10.1097/YCO.0b013e3282f29841
Page, M. E., Detke, M. J., Dalvi, A., Kirby, L. G., and Lucki, I. (1999). Serotonergic mediation of the effects of fluoxetine, but not desipramine, in the rat forced swimming test. Psychopharmacology (Berl.) 147, 162–167. doi: 10.1007/s002130051156
Park, Y., Moon, H. J., and Kim, S. H. (2012). N-3 polyunsaturated fatty acid consumption produces neurobiological effects associated with prevention of depression in rats after the forced swimming test. J. Nutr. Biochem. 23, 924–928. doi: 10.1016/j.jnutbio.2011.04.018
Paylor, R., Spencer, C. M., Yuva-Paylor, L. A., and Pieke-Dahl, S. (2006). The use of behavioral test batteries II: effect of test interval. Physiol. Behav. 87, 95–102. doi: 10.1016/j.physbeh.2005.09.002
Peet, M., and Horrobin, D. F. (2002). A dose-ranging study of the effects of ethyl-eicosapentaenoate in patients with ongoing depression despite apparently adequate treatment with standard drugs. Arch. Gen. Psychiatry 59, 913–919. doi: 10.1001/archpsyc.59.10.913
Peet, M., Murphy, B., Shay, J., and Horrobin, D. (1998). Depletion of omega-3 fatty acid levels in red blood cell membranes of depressive patients. Biol. Psychiatry 43, 315–319. doi: 10.1016/S0006-3223(97)00206-0
Pérez, M. Á., Terreros, G., and Dagnino-Subiabre, A. (2013). Long-term ω-3 fatty acid supplementation induces anti-stress effects and improves learning in rats. Behav. Brain Funct. 9:25. doi: 10.1186/1744-9081-9-25
Pietropaolo, S., Goubran, M. G., Joffre, C., Aubert, A., Lemaire-Mayo, V., Crusio, W. E., et al. (2014). Dietary supplementation of omega-3 fatty acids rescues fragile X phenotypes in Fmr1-Ko mice. Psychoneuroendocrinology 49, 119–129. doi: 10.1016/j.psyneuen.2014.07.002
Pudell, C., Vicente, B. A., Delattre, A. M., Carabelli, B., Mori, M. A., Suchecki, D., et al. (2014). Fish oil improves anxiety-like, depressive-like and cognitive behaviors in olfactory bulbectomised rats. Eur. J. Neurosci. 39, 266–274. doi: 10.1111/ejn.12406
Qi, X., Lin, W., Wang, D., Pan, Y., Wang, W., and Sun, M. (2009). A role for the extracellular signal-regulated kinase signal pathway in depressive-like behavior. Behav. Brain Res. 199, 203–209. doi: 10.1016/j.bbr.2008.11.051
Quesseveur, G., David, D. J., Gaillard, M. C., Pla, P., Wu, M. V., Nguyen, H. T., et al. (2013). BDNF overexpression in mouse hippocampal astrocytes promotes local neurogenesis and elicits anxiolytic-like activities. Transl. Psychiatry 3:e253. doi: 10.1038/tp.2013.30
Quesseveur, G., Portal, B., Basile, J. A., Ezan, P., Mathou, A., Halley, H., et al. (2015). Attenuated levels of hippocampal connexin 43 and its phosphorylation correlate with antidepressant- and anxiolytic-like activities in mice. Front. Cell Neurosci. 9:490. doi: 10.3389/fncel.2015.00490
Rao, J. S., Ertley, R. N., Lee, H. J., DeMar, J. C. Jr., Arnold, J. T., Rapoport, S. I., et al. (2007). n-3 polyunsaturated fatty acid deprivation in rats decreases frontal cortex BDNF via a p38 MAPK-dependent mechanism. Mol. Psychiatry 12, 36–46. doi: 10.1038/sj.mp.4001888
Reeves, P. G. (1997). Components of the AIN-93 diets as improvements in the AIN-76A diet. J. Nutr. 27(5 Suppl.), 838S–841S. doi: 10.1093/jn/127.5.838S
Rossi, C., Angelucci, A., Costantin, L., Braschi, C., Mazzantini, M., Babbini, F., et al. (2006). Brain-derived neurotrophic factor (BDNF) is required for the enhancement of hippocampal neurogenesis following environmental enrichment. Eur. J. Neurosci. 24, 1850–1856. doi: 10.1111/j.1460-9568.2006.05059.x
Rousseau, D., Helies-Toussaint, C., Moreau, D., Raederstorff, D., and Grynberg, A. (2003). Dietary n-3 PUFAs affect the blood pressure rise and cardiac impairments in a hyperinsulinemia rat model in vivo. Am. J. Physiol. Heart Circ. Physiol. 285, H1294–H1302. doi: 10.1152/ajpheart.00651.2002
Saarelainen, T., Hendolin, P., Lucas, G., Koponen, E., Sairanen, M., MacDonald, E., et al. (2003). Activation of TrkB neurotrophin receptor is induced by antidepressant drugs and is required for antidepressant-induced behavioral effects. J. Neurosci. 23, 349–357. doi: 10.1523/JNEUROSCI.23-01-00349.2003
Sairanen, M., Lucas, G., Ernfors, P., Castren, M., and Castren, E. (2005). Brain-derived neurotrophic factor and antidepressant drugs have different but coordinated effects on neuronal turnover, proliferation, and survival in the adult dentate gyrus. J. Neurosci. 25, 1089–1094. doi: 10.1523/JNEUROSCI.3741-04.2005
Sakakibara, Y., Kasahara, Y., Hall, F. S., Lesch, K. P., Murphy, D. L., Uhl, G. R., et al. (2014). Developmental alterations in anxiety and cognitive behavior in serotonin transporter mutant mice. Psychopharmacology (Berl.) 231, 4119–4133. doi: 10.1007/s00213-014-3554-x
Schmidt, H. D., and Duman, R. S. (2007). The role of neurotrophic factors in adult hippocampal neurogenesis, antidepressant treatements and animal models of depressive-like behavior. Behav. Pharmacol. 18, 391–418. doi: 10.1097/FBP.0b013e3282ee2aa8
Schmidt, H. D., and Duman, R. S. (2010). Peripheral BDNF produces antidepressant-like effects in cellular and behavioral models. Neuropsychopharmacology 35, 2378–2391. doi: 10.1038/npp.2010.114
Shirayama, Y., Chen, A. C., Nakagawa, S., Russell, D. S., and Duman, R. S. (2002). Brain-derived neurotrophic factor produces antidepressant effects in behavioral models of depression. J. Neurosci. 22, 3251–3261. doi: 10.1523/JNEUROSCI.22-08-03251.2002
Silvers, K. M., Woolley, C. C., Hamilton, F. C., Watts, P. M., and Watson, R. A. (2005). Randomised double-blind placebo-controlled trial of fish oil in the treatment of depression. Prostaglandins Leukot Essent Fatty Acids 72, 211–218. doi: 10.1016/j.plefa.2004.11.004
Siuciak, J. A., Boylan, C., Fritsche, M., Altar, C. A., and Lindsay, R. M. (1996). BDNF increases monoaminergic activity in rat brain following intracerebroventricular or intraparenchymal administration. Brain Res. 710, 11–20. doi: 10.1016/0006-8993(95)01289-3
Su, K. P., Huang, S. Y., Chiu, T. H., Huang, K. C., Huang, C. L., Chang, H. C., et al. (2008). Omega-3 fatty acids for major depressive disorder during pregnancy: results from a randomized, double-blind, placebo-controlled trial. J. Clin. Psychiatry 69, 644–651. doi: 10.4088/JCP.v69n0418
Taliaz, D., Stall, N., Dar, D. E., and Zangen, A. (2010). Knockdown of brain-derived neurotrophic factor in specific brain sites precipitates behaviors associated with depression and reduces neurogenesis. Mol. Psychiatry 15, 80–92. doi: 10.1038/mp.2009.67
Tang, M., Jiang, P., Li, H., Cai, H., Liu, Y., Gong, H., et al. (2015). Antidepressant-like effect of n-3 PUFAs in CUMS rats: role of tPA/PAI-1 system. Physiol. Behav. 139, 210–215. doi: 10.1016/j.physbeh.2014.11.054
Venna, V. R., Deplanque, D., Allet, C., Belarbi, K., Hamdane, M., and Bordet, R. (2009). PUFA induce antidepressant-like effects in parallel to structural and molecular changes in the hippocampus. Psychoneuroendocrinology 34, 199–211. doi: 10.1016/j.psyneuen.2008.08.025
Vines, A., Delattre, A. M., Lima, M. M., Rodrigues, L. S., Suchecki, D., Machado, R. B., et al. (2012). The role of 5-HT1A receptors in fish oil-mediated increased BDNF expression in the rat hippocampus and cortex: a possible antidepressant mechanism. Neuropharmacology 62, 184–191. doi: 10.1016/j.neuropharm.2011.06.017
Wang, L., Chang, X., She, L., Xu, D., Huang, W., and Poo, M. M. (2015). Autocrine action of BDNF on dendrite development of adult-born hippocampal neurons. J. Neurosci. 3, 8384–8393. doi: 10.1523/JNEUROSCI.4682-14.2015
Warensjo, E., Rosell, M., Hellenius, M. L., Vessby, B., De Faire, U., and Riserus, U. (2009). Associations between estimated fatty acid desaturase activities in serum lipids and adipose tissue in humans: links to obesity and insulin resistance. Lipids Health Dis. 8:37. doi: 10.1186/1476-511X-8-37
Wu, A., Ying, Z., and Gomez-Pinilla, F. (2004). Dietary omega-3 fatty acids normalize BDNF levels, reduce oxidative damage, and counteract learning disability after traumatic brain injury in rats. J. Neurotrauma 21, 1457–1467. doi: 10.1089/neu.2004.21.1457
Wu, Y. Q., Dang, R. L., Tang, M. M., Cai, H. L., Li, H. D., Liao, D. H., et al. (2016). Long chain omega-3 polyunsaturated fatty acid supplementation alleviates doxorubicin-induced depressive-like behaviors and neurotoxicity in rats: involvement of oxidative stress and neuroinflammation. Nutrients 8:243. doi: 10.3390/nu8040243
Xu, D., Lian, D., Wu, J., Liu, Y., Zhu, M., Sun, J., et al. (2017). Brain-derived neurotrophic factor reduces inflammation and hippocampal apoptosis in experimental Streptococcus pneumoniae meningitis. J. Neuroinflamm. 14:156. doi: 10.1186/s12974-017-0930-6
Yang, J., Yu, Y., Yu, H., Zuo, X., Liu, C., Gao, L., et al. (2010). The role of brain-derived neurotrophic factor in experimental inflammation of mouse gut. Eur. J. Pain. 14, 574–579. doi: 10.1016/j.ejpain.2009.10.007
Yuan, P., Zhou, R., Wang, Y., Li, X., Li, J., Chen, G., et al. (2010). Altered levels of extracellular signal-regulated kinase signaling proteins in post-mortem frontal cortex of individuals with mood disorders and schizophrenia. J. Affect. Disord. 124, 164–169. doi: 10.1016/j.jad.2009.10.017
Keywords: brain-derived neurotrophic factor (BDNF), neurobehavior, antidepressant, anxiolytic fish oil (n-3) fatty acids, serotonin
Citation: Zemdegs J, Rainer Q, Grossmann CP, Rousseau-Ralliard D, Grynberg A, Ribeiro E and Guiard BP (2018) Anxiolytic- and Antidepressant-Like Effects of Fish Oil-Enriched Diet in Brain-Derived Neurotrophic Factor Deficient Mice. Front. Neurosci. 12:974. doi: 10.3389/fnins.2018.00974
Received: 28 September 2018; Accepted: 05 December 2018;
Published: 21 December 2018.
Edited by:
Nasser Haddjeri, Institut National de la Santé et de la Recherche Médicale (INSERM), FranceReviewed by:
Amandine Gautier-Stein, INRA – Centre Auvergne-Rhône-Alpes, FranceCopyright © 2018 Zemdegs, Rainer, Grossmann, Rousseau-Ralliard, Grynberg, Ribeiro and Guiard. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Bruno P. Guiard, YnJ1bm8uZ3VpYXJkQHVuaXYtdGxzZTMuZnI=; YnJ1bm8uZ3VpYXJkQHUtcHN1ZC5mcg==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.