
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Neurosci., 21 August 2018
Sec. Auditory Cognitive Neuroscience
Volume 12 - 2018 | https://doi.org/10.3389/fnins.2018.00581
The role of the spatial separation between the stimulating electrodes (electrode separation) in sequential stream segregation was explored in cochlear implant (CI) listeners using a deviant detection task. Twelve CI listeners were instructed to attend to a series of target sounds in the presence of interleaved distractor sounds. A deviant was randomly introduced in the target stream either at the beginning, middle or end of each trial. The listeners were asked to detect sequences that contained a deviant and to report its location within the trial. The perceptual segregation of the streams should, therefore, improve deviant detection performance. The electrode range for the distractor sounds was varied, resulting in different amounts of overlap between the target and the distractor streams. For the largest electrode separation condition, event-related potentials (ERPs) were recorded under active and passive listening conditions. The listeners were asked to perform the behavioral task for the active listening condition and encouraged to watch a muted movie for the passive listening condition. Deviant detection performance improved with increasing electrode separation between the streams, suggesting that larger electrode differences facilitate the segregation of the streams. Deviant detection performance was best for deviants happening late in the sequence, indicating that a segregated percept builds up over time. The analysis of the ERP waveforms revealed that auditory selective attention modulates the ERP responses in CI listeners. Specifically, the responses to the target stream were, overall, larger in the active relative to the passive listening condition. Conversely, the ERP responses to the distractor stream were not affected by selective attention. However, no significant correlation was observed between the behavioral performance and the amount of attentional modulation. Overall, the findings from the present study suggest that CI listeners can use electrode separation to perceptually group sequential sounds. Moreover, selective attention can be deployed on the resulting auditory objects, as reflected by the attentional modulation of the ERPs at the group level.
Many daily listening scenarios involve multiple sound sources. Thus, to selectively listen to a single person’s voice among many, or to a melody in a complex musical arrangement, the listener needs to parse the sounds in the complex auditory scene and group them into meaningful auditory objects or streams (e.g., McDermott, 2009). This process is known as auditory scene analysis (Bregman, 1990). Hearing impairment may affect the process of object formation and thus, hearing-impaired (HI) listeners generally perform worse than normal-hearing (NH) listeners in complex listening scenarios (e.g., Mackersie et al., 2001; Oxenham, 2008). This is the case even when hearing aids or cochlear implants (CIs) are used to make the signals audible (e.g., Nelson et al., 2003). Most current CIs convey spectral information through place cues, whereby different frequency bands of the acoustic signal are used to stimulate different electrodes at a given pulse rate. It is unclear to what extent CI listeners can use place cues in the process of object formation. Thus, a better understanding of the role of place cues in the process of object formation would be beneficial to overcome the challenges that CI listeners experience in complex listening scenarios.
Two main processes have been described in auditory scene analysis: auditory stream integration (the perceptual grouping of multiple sound events into a single stream) and auditory stream segregation (the perceptual grouping of multiple sound events into several streams). In NH listeners, these processes have been studied in experiments that employ sequences of repeating, sequentially-presented sounds. These sounds differ in various acoustic properties, such as frequency content (e.g., Bregman and Campbell, 1971; Van Noorden, 1975) or temporal envelope (e.g., Iverson, 1995; Cusack and Roberts, 2000). Large differences between the sounds facilitate segregation, whereas small differences promote integration. Furthermore, it has been suggested that sequential stream segregation may be directly related to the degree of the perceptual difference between the sounds (Moore and Gockel, 2002, 2012).
In addition to stimulus-driven effects, stream segregation can be influenced by cognitive factors, such as attention. Van Noorden (1975) investigated the perceptual organization of sounds using sequences of alternating low- and high-frequency pure tones. The segregation threshold varied with the intention of the listener: a smaller frequency difference was needed when the listeners were encouraged to segregate the sounds (i.e., voluntary stream segregation) than when the listeners were encouraged to integrate them (i.e., obligatory stream segregation). From these results, Van Noorden defined the fission boundary as the frequency separation below which the sounds could no longer be segregated and the temporal coherence boundary as the frequency separation above which the sounds could no longer be integrated. Thus, the temporal coherence boundary and the fission boundary represent the thresholds of obligatory and voluntary stream segregation, respectively. The probability of perceiving a segregated percept has been reported to increase over time. This is often referred to as the build-up effect of stream segregation (e.g., Van Noorden, 1975; Bregman, 1978; Anstis and Saida, 1985). The build-up effect has been reported to occur both under integration-promoting listening instructions (e.g., Roberts et al., 2008; Thompson et al., 2011) and under segregation-promoting listening instructions (Micheyl et al., 2005; Nie and Nelson, 2015).
Several studies have investigated obligatory stream segregation in CI listeners (e.g., Cooper and Roberts, 2009; Duran et al., 2012; Tejani et al., 2017). These studies varied either the spatial separation between the stimulating electrodes (i.e., place cues) or the pulse rate of stimulation at a fixed electrode (i.e., temporal cues). The manipulation of these parameters is known to elicit perceptual differences in CI listeners (e.g., Eddington et al., 1978; Landsberger et al., 2016). Moreover, it has been suggested that stream segregation may be related to the degree of the perceptual difference between the streams, regardless of whether such perceptual difference was elicited by varying the place or the rate of stimulation (Paredes-Gallardo et al., 2018b). The results from Tejani et al. (2017) were consistent with those from studies in NH listeners, i.e., the probability of experiencing a two-stream percept increased by increasing the electrode separation. Similarly, the results from Duran et al. (2012) suggest that CI listeners experience obligatory stream segregation when only temporal cues are provided. However, Cooper and Roberts (2009) did not observe a build-up effect, suggesting that not all elements of obligatory stream segregation may be experienced by CI listeners.
Cooper and Roberts (2009) also assessed voluntary stream segregation abilities of CI listeners using a melody discrimination task. The listeners were asked to identify a pattern of sequentially activated electrodes (melody) in the presence of interleaved, random distractor sounds. Their results showed that CI listeners were not able to segregate the melody from the distractor sounds, regardless of the electrode separation between the streams. Conversely, other studies suggest that CI listeners can use either electrode separation, pulse rate differences or amplitude modulation (AM) rate differences to voluntarily segregate sequences composed of two repeating and alternating sounds (Hong and Turner, 2009; Paredes-Gallardo et al., 2018a,b). Moreover, a build-up effect has been reported to occur during voluntary stream segregation when using either place or temporal cues (Paredes-Gallardo et al., 2018a,b).
There are several differences between the study of Cooper and Roberts (2009) and those of Hong and Turner (2009) and Paredes-Gallardo et al. (2018a,b). The sequences of sounds were shorter in the study by Cooper and Roberts (2009) than those used in the studies of Hong and Turner (2009) and Paredes-Gallardo et al. (2018a,b). Thus, it is possible that the poor performance reported in the study of Cooper and Roberts (2009) reflects the long time that CI listeners need to build up a segregated percept, even when the attention of the listener is directed toward segregation (Paredes-Gallardo et al., 2018a). Another difference is that Cooper and Roberts used streams composed of different sounds, resulting in a more complex task than those employed in the studies of Hong and Turner (2009) and Paredes-Gallardo et al. (2018a,b) Finally, the stimuli used by Hong and Turner (2009) and Paredes-Gallardo et al. (2018a,b) might have facilitated segregation due to the inclusion of rhythmic cues (for a review, see Bendixen, 2014). Thus, it is unclear whether the poor performance reported by Cooper and Roberts reflects that CI listeners need longer time to build up a two-stream percept or that CI listeners are not able to segregate streams composed of different sounds in the absence of rhythmic cues.
It has been suggested that selective attention operates as a form of sensory gain control, modulating the neural representations of signals in the auditory cortex. Specifically, selective attention has been shown to enhance the event-related potentials (ERPs) evoked by attended sounds and to suppress those evoked by ignored sounds (e.g., Hillyard et al., 1973, 1998; Picton and Hillyard, 1974). Thus, several studies have used recordings of ERPs to investigate the process of object selection and selective attention, tightly related to stream segregation (e.g., Alain and Arnott, 2000; Choi et al., 2013). Moreover, the amount of attentional modulation of the ERPs has been shown to correlate with the listener’s ability to perform an auditory selective-attention task (Choi et al., 2014; Dai and Shinn-Cunningham, 2016; Dai et al., 2018), suggesting a strong link to perception. However, to the authors’ knowledge, no previous study has investigated whether selective attention modulates the ERPs in CI listeners and whether such attentional modulation would correlate with performance in an auditory selective-attention task. If this would be the case, the attentional modulation of the ERPs could be used as an objective tool to assess stream segregation and selective attention in CI listeners.
The present study investigated, in CI listeners, (1) whether electrode separation is a cue for the segregation of streams composed of different sounds, (2) whether a two-stream percept builds-up over time, (3) whether selective auditory attention modulates the amplitude of the ERPs, and (4) whether such attentional modulation of the ERP reflects individual stream segregation abilities and therefore, whether the attentional modulation of the ERPs can be used as an objective tool to assess voluntary stream segregation. Behavioral detection performance was measured in a paradigm where the listeners were required to attend to a series of sounds in the presence of interleaved distractor sounds. A deviant was randomly introduced in the target stream either at the beginning, middle or end of each trial. The listeners were asked to detect sequences that contained a deviant and to report its location within the trial. As in the task described by Cooper and Roberts (2009), the perceptual segregation of the streams should improve performance in the deviant detection task. It was hypothesized that if CI listeners can use electrode separation as a cue to segregate the streams, performance in the deviant detection task would improve with increasing electrode separation between the target and the distractor streams. If CI listeners need time to build up a segregated percept, detection performance should be highest for deviants presented late in the trial. Furthermore, ERPs to the same stimuli were recorded while the listeners performed the behavioral task (active listening) and while they watched a muted movie (passive listening). It was hypothesized that if CI listeners can segregate the streams, then the ERPs evoked by the target stream should be enhanced in the active listening condition compared to the passive listening condition. Conversely, ERPs evoked by the distractor stream should be suppressed in the active listening condition with respect to the passive listening condition.
The experiments took place in a sound-attenuating and electrically shielded booth at the Bionics Institute of Australia and at the Technical University of Denmark. The experiments were conducted in three sessions, each lasting 2 h including short breaks. The first session comprised categorical loudness scaling and loudness matching of the different stimuli, a pitch ranking task, a test run of the detection task in the absence of the distractor stream and a 15–20 min training on the segregation task. The behavioral experiment and the recording of the ERPs took place in the second and third sessions, respectively.
Twelve CI listeners participated in this study. The listeners were aged between 20 and 82 years (mean: 61.3 years, SD: 22.2 years; see Table 1) and had no residual hearing in the implanted ear. For the bimodal listeners, the contralateral ear was unaided and blocked with an earplug during the experiments. All listeners performed the behavioral task. Listener CI-10 did not participate in the ERP recording session. All listeners provided written informed consent prior to the study and all experiments were approved by the Human Research Ethics Committee of the Royal Victorian Eye & Ear Hospital (reference 14.1180H) and the Science-Ethics Committee for the Capital Region of Denmark (reference H-16036391). All listeners were users of the Cochlear Ltd. (Sydney, Australia) implant. The electrode array of the Cochlear Ltd. implant consists of 22 electrodes where electrode 1 is the most basal electrode and electrode 22 is the most apical one.
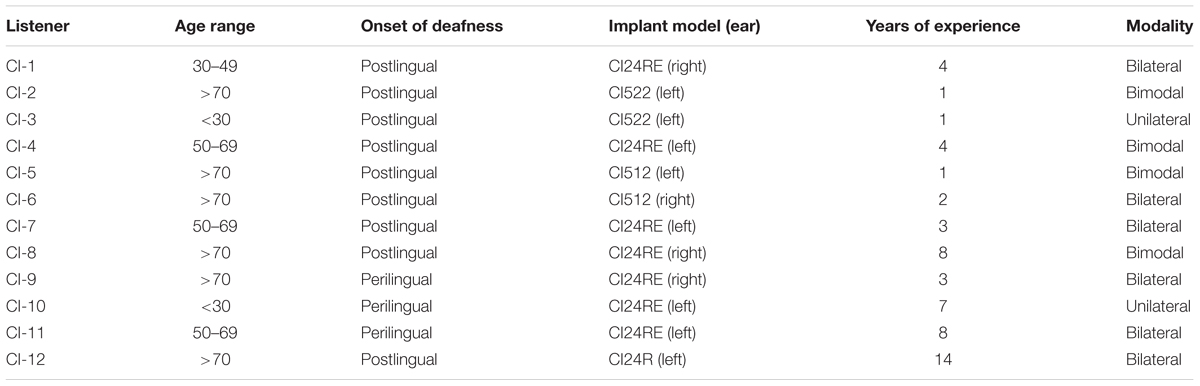
TABLE 1. Information about the participants regarding age, gender, onset of deafness, implanted ear, number of years of experience and modality of rehabilitation.
The listeners were asked to perform a detection task, illustrated in Figure 1. The target stream consisted on a pattern of sounds, presented on electrodes 9, 11, and 13 (i.e., a triplet). On each trial, three triplets were presented consecutively in the presence of interleaved, random distractor sounds which the listeners were asked to ignore. Both the target and the distractor streams extended over a range of five electrodes. The electrode range of the distractor stream was varied across conditions, as illustrated in Figure 2. In the no overlap condition, the distractor stream was presented through electrodes 16–20, resulting in a separation of three or more electrodes between the streams. In the apical overlap condition, the distractor stream was presented through electrodes 13–17, resulting in an overlap with the most apical electrode of the target stream. In the basal overlap condition, the distractor stream was presented through electrodes 5–9, such that there was an overlap with the most basal electrode of the target stream. Finally, in the full overlap condition, the electrode range of both target and distractor streams was identical (i.e., electrodes 9–13).
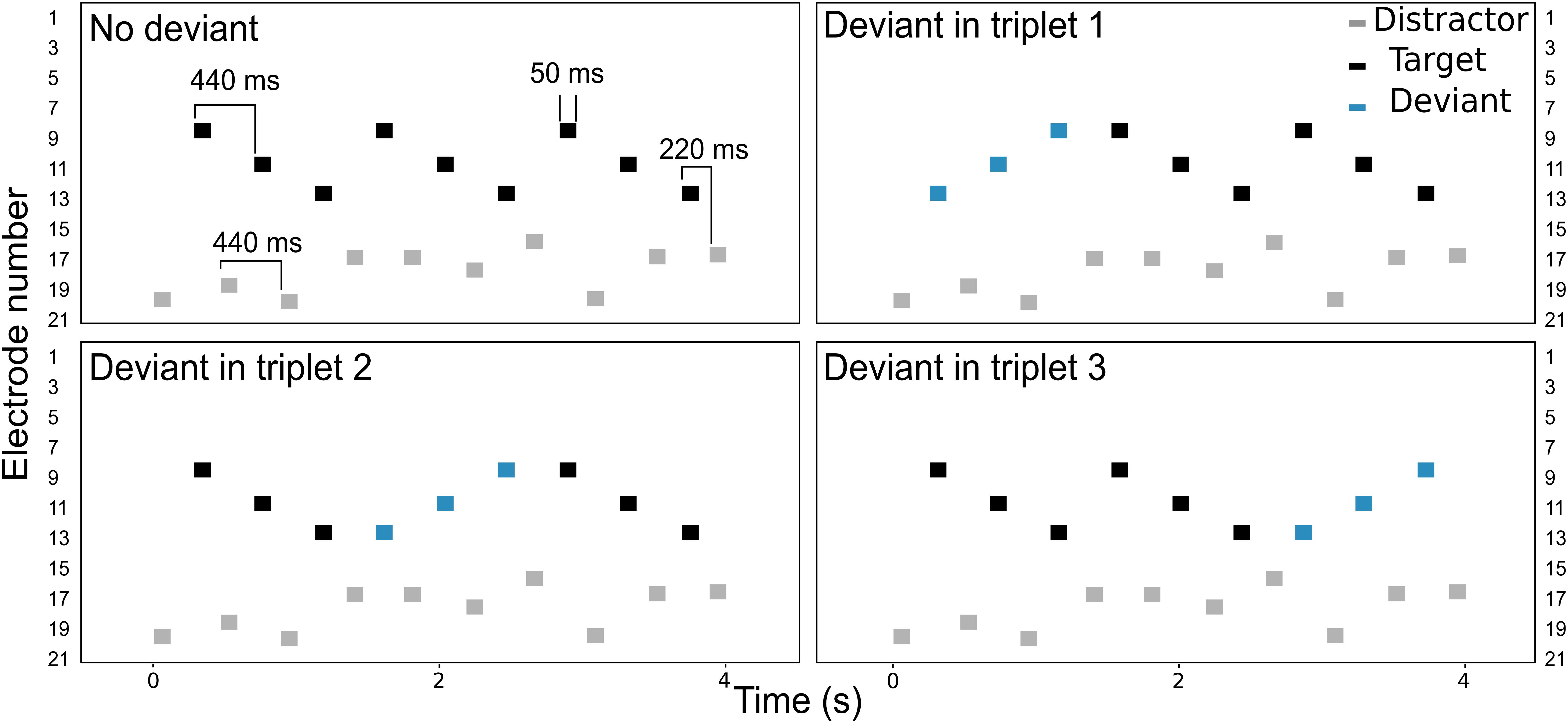
FIGURE 1. Schematic representation of the electrodogram for each of the four deviant conditions. Black and gray markers represent the target and the distractor sounds, respectively. The deviant triplet is shown with blue markers.
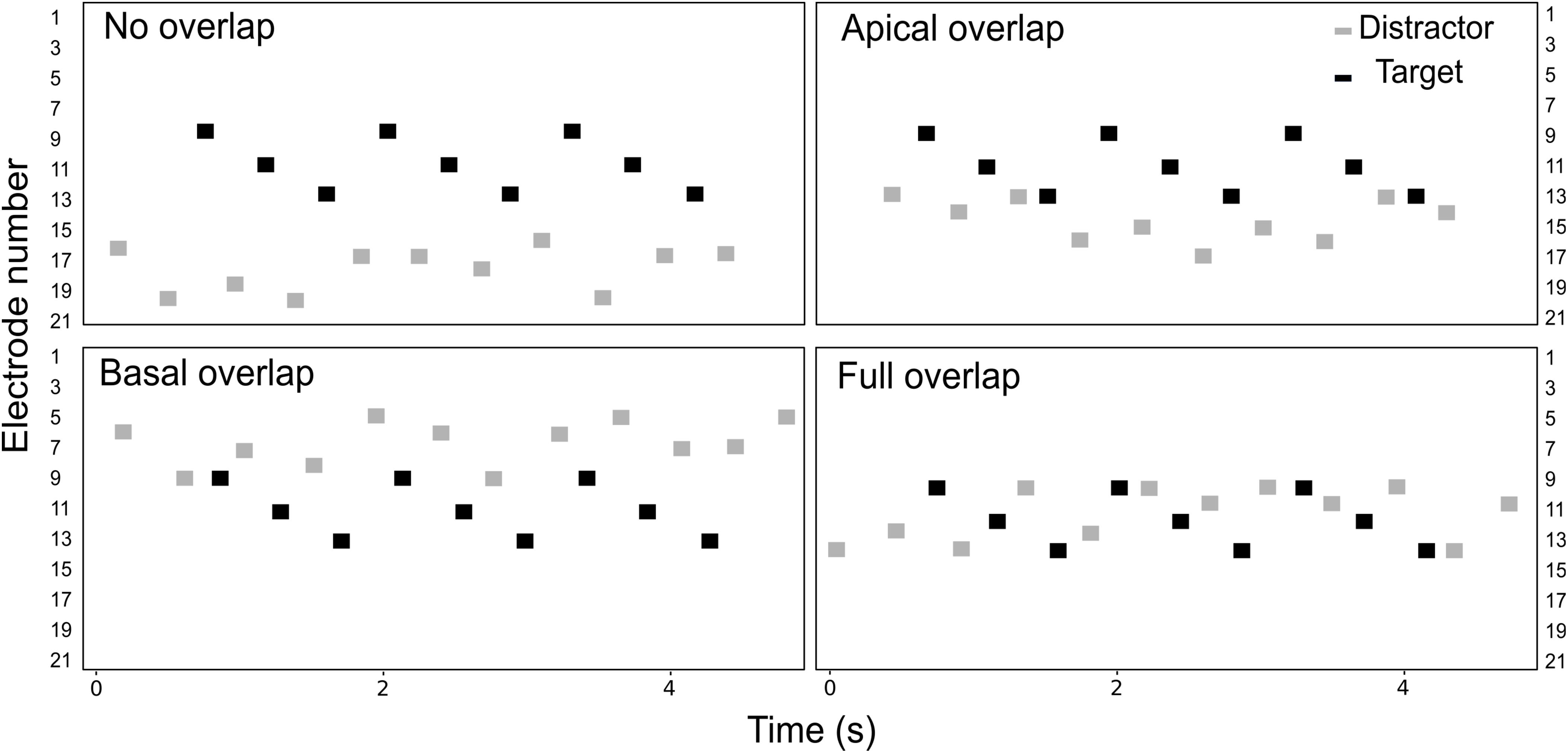
FIGURE 2. Schematic representation of the electrodogram for each of the four electrode separation conditions. Black and gray markers represent the target and the distractor sounds, respectively. On each trial, the distractor stream started before the target stream and ended after the target stream, with a random number of one to four sounds.
Each triplet began with a target sound and ended with a distractor sound. A deviant triplet was randomly introduced in 75% of the trials by reversing the electrode sequence in one of the three triplets (i.e., electrodes 13, 11, and 9). In the remaining 25% of the trials, no deviant was presented, i.e., the three triplets were identical (no deviant condition, see Figure 1). The four deviant conditions (i.e., deviant triplet 1, 2, 3 or no deviant) were presented in random order. The listeners were asked to detect sequences that contained a deviant and to report its location within the trial in a one-interval, four-alternative forced-choice paradigm.
Behavioral responses during both the training and the data collection were recorded using a custom-made user interface in Python. Four response buttons were used to record the listener’s response 200 ms after each trial. The duration of the inter-trial interval was randomized between 1.5 and 2.5 s. Feedback was provided after each trial.
Each sound consisted of a 50 ms burst of biphasic pulses presented at a given electrode. Each biphasic pulse had a phase width of 25 μs and an inter-phase gap of 8 μs. The pulse rate was fixed at 900 pps. The inter-stimulus interval (ISI) was 440 ms between two consecutive target or distractor sounds, and 220 ms between two consecutive target-distractor sounds (see Figure 1). The stimuli were presented in monopolar mode through the Nucleus Implant Communicator research interface (NIC v3, Cochlear Ltd., Sydney) and a research speech processor (L34) provided by Cochlear Ltd.
Previous studies have suggested that loudness could be an effective cue for the segregation of sounds for CI listeners (e.g., Cooper and Roberts, 2009; Marozeau et al., 2013). To ensure that the listeners did not rely on loudness cues to segregate the sounds, the stimuli of the present study were loudness-balanced. A total of 16 electrodes (from electrode 5 to electrode 20) were used in the present study. Categorical loudness scaling was used to find the most comfortable level (MCL) for six electrodes (i.e., electrodes 5, 8, 11, 14, 17, and 20) using an 11-step attribute scale, as described in Paredes-Gallardo et al. (2018a). The MCL for the remaining electrodes (i.e., electrodes 6, 7, 9, 10, 12, 13, 15, 16, 18, and 19) was obtained by linear interpolation. All electrodes were then loudness matched to a reference electrode (electrode 11) by the listeners, using a simple user interface. The interface allowed the increase and the decrease of the test-sound intensity in steps of 0.15, 0.3, or 0.45 dB.
Most CI listeners report a monotonic relation between the place of stimulation and the corresponding pitch percept (e.g., Eddington et al., 1978; Tong et al., 1980; Shannon, 1983; Townshend et al., 1987). However, several previous studies reported instances where the pitch percept did not follow a monotonic function (e.g., Nelson et al., 1995; Collins et al., 1997). In the present study, local pitch reversals could hinder the performance in the detection task. Thus, a pitch ranking experiment was conducted with eight odd-numbered electrodes (between electrodes 5 and 20) using the midpoint comparison procedure (Long et al., 2005; Macherey and Carlyon, 2010). All twelve listeners exhibited monotonic pitch ranks. Furthermore, to ensure that all listeners were able to perform the detection task, a test run with 20 presentations of each of the four different conditions (see Figure 1) was performed in the absence of the distractor stream. A minimum average performance of 90% correct was achieved by all listeners.
In the behavioral experiment, the four electrode separation conditions between the target and the distractor streams were tested (i.e., no overlap, apical overlap, basal overlap, and full overlap conditions – see Figure 2). The target stream was always presented on electrodes 9, 11, and 13, whereas the electrode range of the distractor stream was varied.
In each trial, a random number of one to four distractor sounds was played before and after the target stream (i.e., inducer sounds). Thus, the listeners did not have a priori knowledge about the starting point of the target stream. This was done to encourage the listeners to attend to the full duration of the trial instead of listening for a specific time point. The duration of each trial ranged between 4 and 6.65 s.
Prior to the behavioral experiment, the listeners underwent 15–20 min of training in the stream segregation task. The training began with the detection task in the absence of the distractor stream. Once the listeners were familiarized with the sequences, the distractor stream was introduced at a soft, but audible, level (no overlap condition). The level of the distractor stream was increased in steps of 0.45 dB every third sequence until both streams where played at the listener’s MCL. The training procedure was repeated with the three remaining distractor sets, i.e., apical, basal and full overlap.
In the behavioral experiment, a total of 20 trials were presented for each electrode separation and each of the four deviant conditions. The resulting 320 trials were divided into eight blocks. A block consisted of 10 trials of each of the four deviant conditions for a given electrode separation condition. The order of the blocks was randomized.
The sensitivity measure (d′) was calculated using equation (1) for each of the three deviant triplet locations (i), where z represents the z-transformation, NHi and NFAi the number of hits and false alarms, respectively, and H and FA the maximum number of hits and false alarms (20 and 60, respectively). The log-linear rule was used to avoid undefined extremes when the hit or the false alarm rates take the values of zero or one (Hautus, 1995; Verde et al., 2006).
Statistical inference was performed by fitting a mixed-effects linear model to the d’ scores. The experimental variables and their interactions were treated as fixed effects whereas listener-related effects were treated as random effects with random intercepts and slopes. The model was implemented in R (R Core Team, 2015) using the lme4 library (Bates et al., 2014) and the model selection was carried out with the lmerTest library (Kuznetsova et al., 2017) following the backward selection approach based on step-wise deletion of model terms with high p-values (Kuznetsova et al., 2015). The p-values for the fixed effects were calculated from F-tests based on Satterthwaite’s approximation of denominator degrees of freedom and the p-values for the random effects were calculated based on likelihood ratio tests (Kuznetsova et al., 2015). The post hoc analysis was performed through contrasts of least-square means using the lsmeans library (Lenth, 2016). The p-values were corrected for multiple comparisons using the Tukey method.
The no overlap condition was chosen for the recording of the ERPs. Thus, the target stream was presented at electrodes 9, 11, and 13 and the distractor stream comprised electrodes 16 to 20. It has been suggested that the first sound of a sequence may draw attention exogenously (e.g., Choi et al., 2014; Dai et al., 2018). In order to ensure that the listeners deployed top-down attention to the target stream, on each trial, a single distractor sound was played before the first triplet. Thus, in the present study, the target stream was always the lagging stream.
A 50 ms burst of pulses on electrode 11, followed by a 750 ms silence was played before each trial. This burst was not relevant for the behavioral task, and the listeners were not given specific listening instructions (whether to attend or ignore it). The burst was included to normalize the N1 amplitude for the remaining sounds across listeners (e.g., Choi et al., 2014). It was hypothesized that the N1 response elicited by this burst would reflect individual differences in the N1 amplitude but would not be affected by attention. However, the N1 responses to this burst were affected by attention, and this effect was variable across listeners. Thus, the responses to this pre-trial burst were not used for the normalization of the individual N1 amplitudes.
Two attention conditions were tested in the ERP recording session: an active listening condition and a passive listening condition. During the active listening condition, the scalp electroencephalogram (EEG) was recorded while the listeners performed the behavioral detection task. In the passive listening condition, the EEG signal was recorded in response to the same sounds while the listeners watched a muted movie with captions. Overall, a total of 55 trials were recorded for each attention (active/passive) and deviant condition. For the active listening condition, the 220 trials were divided into two blocks of 110 trials each (∼15 min). For the passive listening condition, the 220 trials were recorded in a single block (∼30 min).
The EEG data were recorded using the Biosemi ActiveTwoTM system at a sampling rate of 8192 Hz. The hardware anti-aliasing filter bandwidth follows a 5th order sinc response, with the -3 dB point located at 1600 Hz. 68 electrodes were used for the recording: 64 electrodes mounted on an elastic headcap according to the international 10–20 electrode configuration, two electrodes at the left and right mastoids, one electrode near the outer canthus of the eye and one electrode below the eye contralateral to the CI. The electrodes directly over the coil were not used in the recording and all electrode wires were directed away from the coil to minimize radio frequency (RF) artifact. The offsets of the recording electrodes were kept below 20 mV in all recordings.
The data were processed using the Fieldtrip toolbox (Oostenveld et al., 2011) and customized Matlab scripts. The continuous EEG data were re-referenced to the average mastoids, highpass-filtered at 1 Hz (FIR with zero-phase lag, 1 Hz transition bandwidth) and lowpass-filtered at 100 Hz (FIR with zero-phase lag, 1 Hz transition bandwidth). The data were downsampled to 256 Hz and epoched from -1.3 to 4.7 s relative to the onset of the first distractor sound. The total duration of the epoch was used for baseline subtraction. Epochs containing unique, non-stereotyped artifacts were manually rejected. Infomax independent component analysis (ICA) was then applied to the remaining epochs. Equivalent current dipole modeling was computed for all independent components (ICs) on each condition. ICs representing eye blinks and saccadic eye movement were manually identified based on their scalp topography, waveform and power-spectrum. Components representing the RF artifact from the implant were automatically identified with a custom implementation of the procedure described in Viola et al. (2012) (see Supplementary Material for more details). Artefactual components were removed from all datasets and data were back-projected to the sensor space. After artifact correction, a second baseline subtraction was performed. The time interval between -1.05 and -0.85 s was used for this second baseline subtraction. Epochs were lowpass-filtered at 20 Hz (FIR, zero-phase lag, 1 Hz transition bandwidth and 1 s zero-padding both before and after the epoch).
Only the correctly answered trials were processed. Since there were not enough correct trials to analyze the data for each of the four deviant conditions, the epochs were grouped in early and late deviant conditions. The early deviant group contained the epochs where either the first or the second triplets were a deviant. The late deviant group contained the epochs where the deviant was in the third triplet or absent (i.e., the epochs where the listeners had to sustain selective attention throughout the full duration of the sequence). A minimum of 64 correct trials was available for each listener, deviant condition (early vs. late) and attention condition (active vs. passive). Thus, the first 64 correctly answered trials for each listener, deviant condition and attention condition were analyzed. The deviant count was balanced within each deviant condition for all listeners except for CI-6 (i.e., 32 trials per deviant triplet). For CI-6, only 25 correct trials of the first deviant triplet were available, which were pooled with 39 trials of the second deviant triplet on the early deviant condition.
For each condition, listener and electrode, the amplitude of the N1 ERP component was calculated as the local minimum in the time window from 70 to 170 ms after each sound onset (i.e., both for the target and the distractor sounds). For each listener, the across-electrode N1 amplitude was calculated by averaging the amplitudes from nine front-central electrodes (Fz, AFz, FCz, F1, F2, FC1, FC2, AF3, and AF4). This average measure will be referred to as N1 amplitude. The attentional modulation of the ERPs was quantified for each listener as the difference in N1 amplitude between the active and passive listening conditions for each sound. Thus, a negative value indicates a larger N1 response in the active than in the passive listening condition. These values were averaged across all target sounds to obtain a single estimate per listener. The single value was used to compute the Kendall rank correlations between the behavioral performance and the attentional modulation of the ERPs.
A mixed-effects linear model was used for the statistical analysis. N1 amplitude differences between the active and the passive listening conditions were modeled following the approach described in the section “Data Analysis.”
The results from the behavioral experiment are shown in Figure 3. The d′ scores are shown for each combination of electrode separation (A) and deviant triplet location (B). Different electrode separation conditions are shown with different colors. Statistically significant differences between conditions are illustrated with letters. Conditions sharing one or more letters are not significantly different. Detailed statistics from the post hoc analysis are provided in the Supplementary Material.
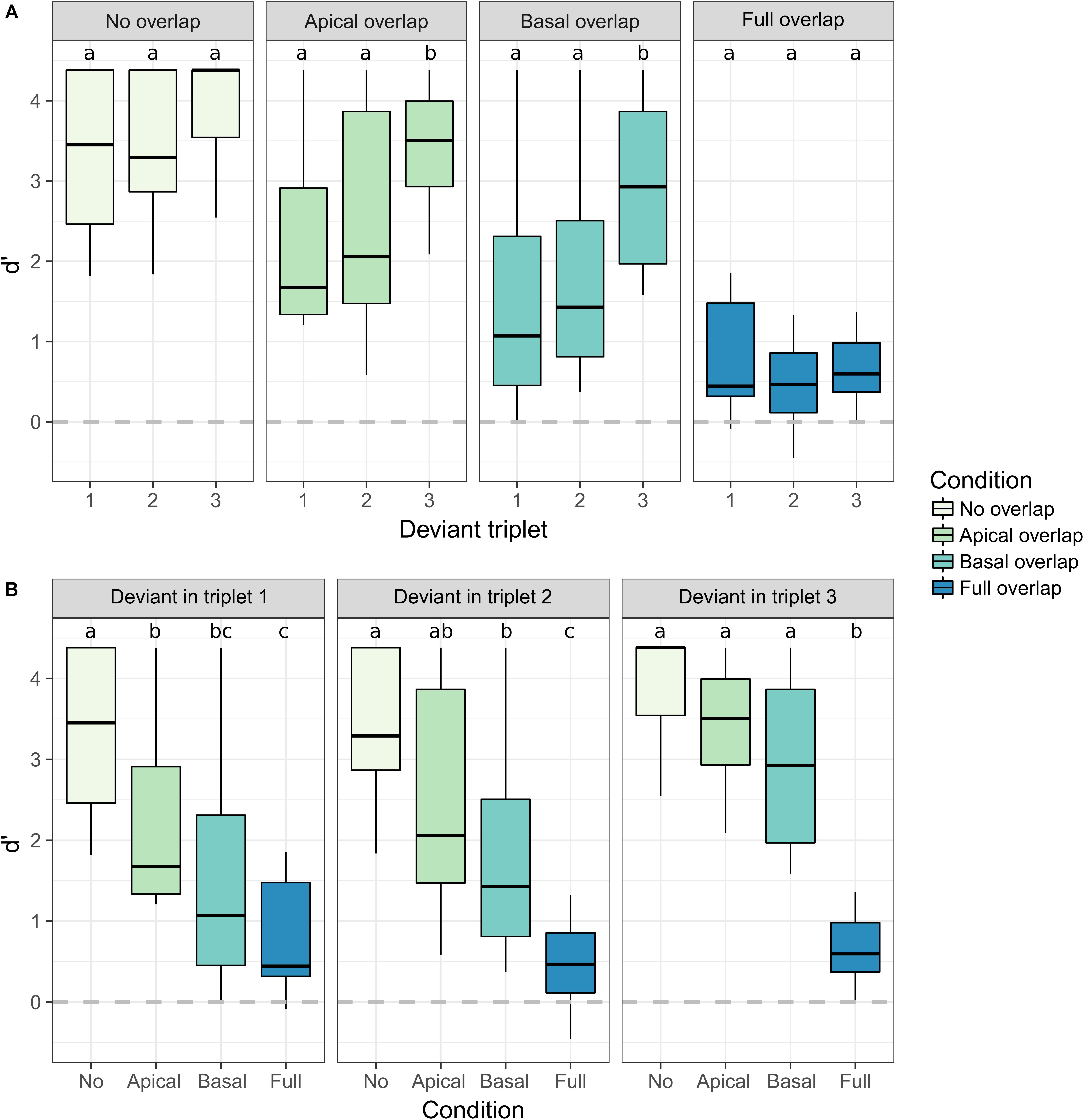
FIGURE 3. Boxplot of the sensitivity scores (d′) to the deviant triplet for each electrode separation and deviant triplet location. The color of the boxes represents the electrode separation condition. (A) Effect of the deviant triplet for each electrode separation condition. (B) Effect of the electrode separation for each deviant triplet location. Results from the statistical contrasts are indicated with lowercase letters. Conditions sharing one or more letters are not significantly different (significance level α = 0.05).
Overall, the d′ scores increased the later the deviant triplet occurred [F(2,11.75) = 16.423, p < 0.001] and decreased with increasing electrode overlap between the streams [F(3,11.39) = 73.484, p < 0.001]. Moreover, a significant interaction was found between the deviant triplet location and the electrode overlap between the streams [F(6,88.00) = 5.811, p < 0.001], indicating that the effect of the deviant triplet location was not the same for all electrode separation conditions.
The location of deviant triplet did not affect the d′ scores for the no overlap condition (Figure 3A). However, the d′ scores were at ceiling for this condition, preventing any effect of the deviant triplet location to be observed. Similarly, no effect of the deviant triplet location was observed for the full overlap condition, where the d′ scores were close to zero. The largest effect of the deviant triplet location was observed for the apical and basal overlap conditions. In these conditions, significantly larger d′ scores were achieved when the deviant triplet happened at the end of the sequence.
When the deviant occurred in the first triplet, the d′ scores for the no overlap condition were significantly larger than the ones achieved for any of the other conditions (Figure 3B). The difference between the no overlap condition and the apical and basal overlap conditions was reduced when the deviant occurred in the second triplet. No significant difference was observed between these three conditions when the deviant occurred in the third triplet. The d′ scores were generally lower for the basal overlap than for the apical overlap condition. However, no significant difference was observed between these two conditions for any of the deviant triplet locations.
The grand average waveform across all listeners is shown in Figure 4 (averaged across all deviant conditions) and in Figure 5 (in separate panels for the early and late deviant conditions). Red and blue solid lines represent the active and the passive listening conditions, respectively. Blue and gray shaded areas indicate the N1 response time window for the target and the distractor sounds, respectively. Sharp oscillations before the N1 time window are likely to represent the residual CI artifact, and should not be mistaken for a P1 response. The scalp distribution of the response to one target and one distractor sound is also shown for each listening condition and their difference. The scalp distributions were obtained by averaging the response over the N1 time window for each of the 64 electrodes. Blue and red colors represent negative and positive values, respectively.
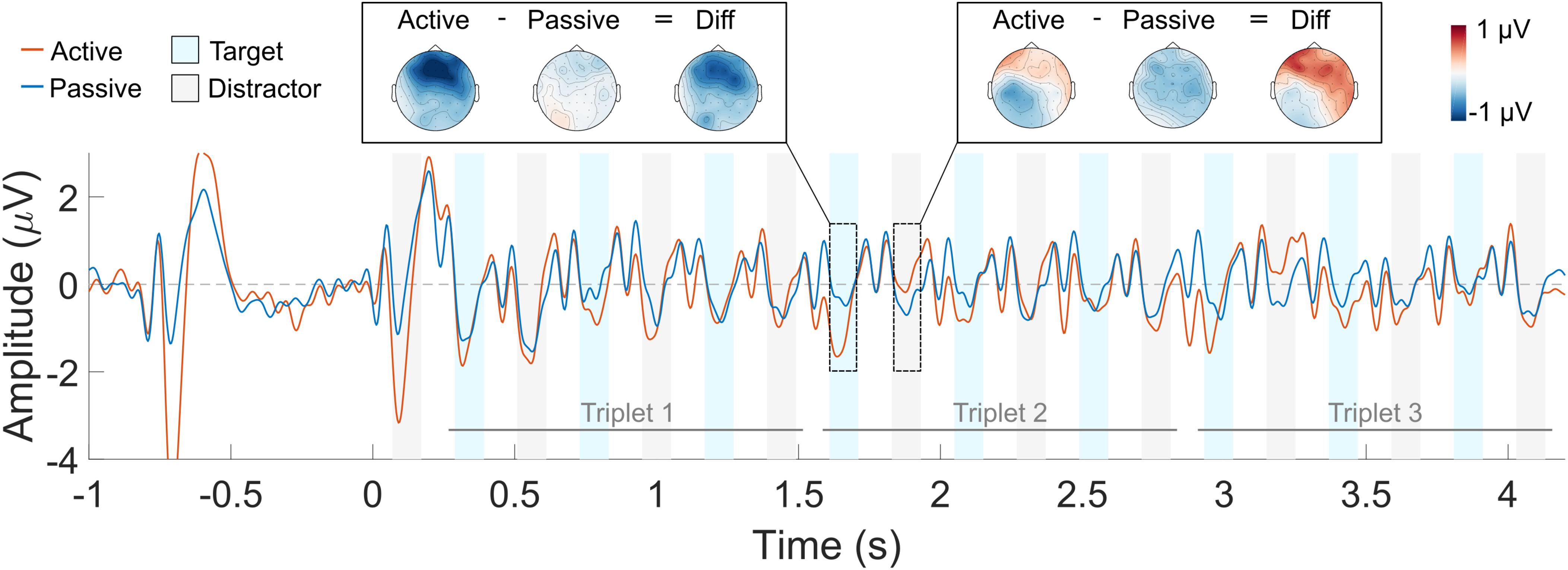
FIGURE 4. Averaged ERP waveform across the four deviant triplet conditions. The active listening condition is shown in red and the passive listening condition in blue. The blue and gray shaded areas indicate the N1 ERP component time window for the target and the distractor sounds, respectively. Each trace represents the average across nine front-central electrodes (Fz, AFz, FCz, F1, F2, FC1, FC2, AF3, AF4). The scalp topography of the response to a target and a distractor sound is shown for each of the listening conditions and their difference.
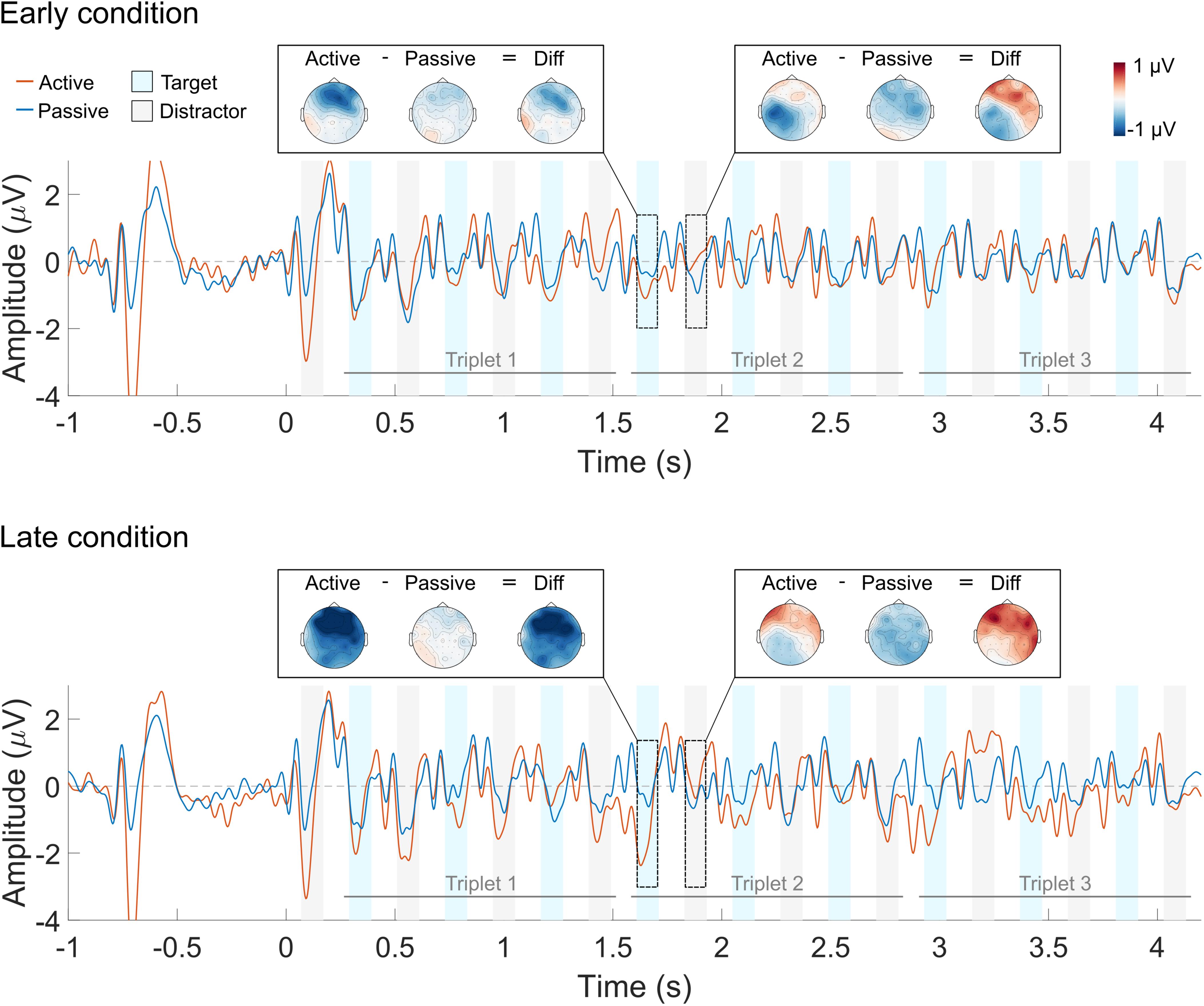
FIGURE 5. Averaged ERP waveform for the early (top) and late (bottom) deviant conditions. The active listening condition is shown in red and the passive listening condition in blue. The blue and gray shaded areas indicate the N1 ERP component time window for the target and the distractor sounds, respectively. Each trace represents the average across nine front-central electrodes (Fz, AFz, FCz, F1, F2, FC1, FC2, AF3, AF4). The scalp topography of the response to a target and a distractor sound is shown for each of the listening conditions and their difference.
N1 responses to the first triplet are, qualitatively, similar for the active and the passive listening condition (Figure 4). This was the case for both the target and the distractor sounds. Conversely, the N1 attentional modulation seem, qualitatively, different for the target and the distractor sounds in the second and third triplets: selective attention enhanced the N1 responses to the target sounds (i.e., the N1 amplitude is more negative in the active vs. the passive listening condition) and suppressed the N1 responses to the distractor sounds (i.e., the N1 amplitude is more negative in the passive vs. the active listening condition). However, this was only observed for the first two target sounds and the first distractor sound of the second and third triplets. Similar patterns can be seen in Figure 5, both for the early and for the late deviant conditions. Nevertheless, the effect of attention is largest for the late deviant condition. This is apparent when comparing the topography of the N1 responses to a target and a distractor sound in Figures 4, 5.
The individual N1 attentional modulation for each deviant condition (early vs. late deviant), sound type (target vs. distractor), triplet number and sound number (first, second, or third sound of a triplet) was modeled using a mixed-effects statistical model. The first sound of the sequence was not part of any of the triplets and therefore, was excluded from the analysis. The model revealed a significant main effect of the sound type [F(1,356) = 20.051, p < 0.001]. Moreover, a significant interaction was found between the triplet number, the deviant condition and the sound type [F(2,356) = 4.557, p = 0.011] and between the sound number, the deviant condition and the sound type [F(2,356) = 3.111, p = 0.046]. No significant interaction was found between the triplet number, the sound number and the deviant condition [F(4,344) = 0.464, p = 0.763] or between the triplet number, the sound number, the sound type and the deviant condition [F(4,340) = 1.049, p = 0.382].
A post hoc analysis revealed that the N1 responses to the target sounds were, on average, enhanced by 0.623 μV in the active vs. the passive listening condition [t(14.9) = 4.336, p = 0.001]. Conversely, the difference in the N1 responses elicited by the distractor sounds was not statistically significant [estimate = 0.075 μV, t(14.9) = 0.524, p = 1]. This was also the case when including the responses to the first sound of the sequence (i.e., a distractor sound) in the analysis.
The significant interactions from the statistical model are illustrated in Figure 6, where the N1 attentional modulation is shown for each sound type and deviant condition. In Figure 6A, the N1 attentional modulation is averaged across the three sounds of each triplet, illustrating the interaction between the triplet number, the deviant condition and the sound type. For the early deviant condition, no significant modulation of the N1 responses was observed for either the target or the distractor sounds in any of the three triplets. For the late deviant condition, a significant N1 enhancement was observed for the first triplet of the distractor stream [t(26.97) = 2.719, p = 0.034] and for the second [t(26.97) = 3.315, p = 0.008] and third [t(26.97) = 2.672, p = 0.038] triplets of the target stream. In Figure 6B, the N1 attentional modulation is averaged across the three triplets for each sound, illustrating the interaction between the sound number, the deviant condition and the sound type. As in Figure 6A, no significant modulation of the N1 responses to any of the sounds was observed for the early deviant condition. However, a significant N1 enhancement was observed for the first [t(26.97) = 3.763, p = 0.003] and second [t(26.97) = 3.451, p = 0.006] target sounds for the late deviant condition.
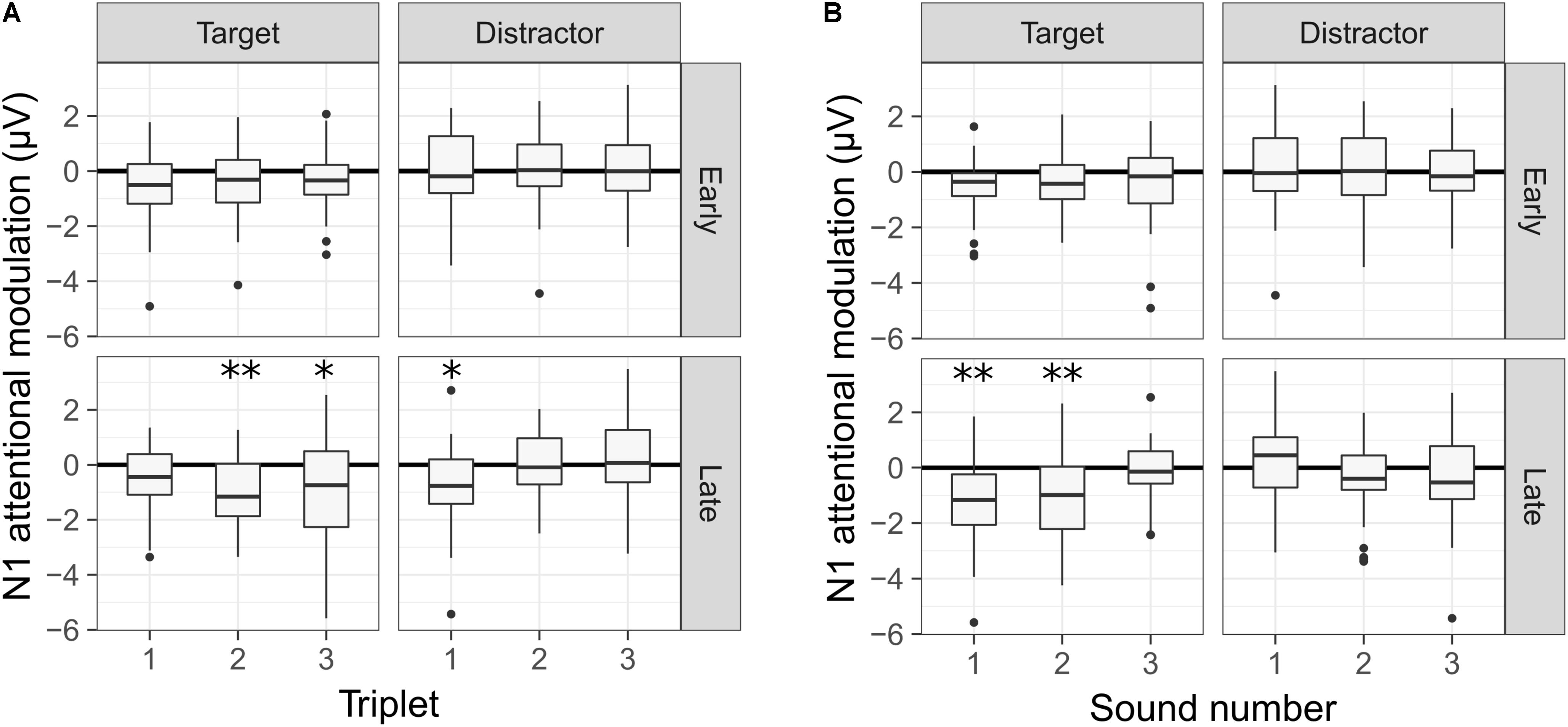
FIGURE 6. N1 attentional modulation of the target and distractor sounds for each deviant condition. A negative value represents an enhanced N1 response in the active condition. (A) Averaged N1 attentional modulation across the three sounds of each triplet. (B) Averaged N1 attentional modulation across the three triplets for each sound. A statistically significant difference from zero is indicated by one asterisk if 0.05 > p > 0.01, two asterisks if 0.01 > p > 0.001 and three asterisks if p < 0.001. The p-values were corrected for multiple comparisons using the Bonferroni correction.
The relation between the individual d′ scores and the N1 attentional modulation of the target sounds is shown in Figure 7. No significant correlation was found between the d′ scores achieved in the active listening condition and the N1 attentional modulation (Figure 7A) [τ = -0.037, p = 0.876]. Kendall rank correlation scores were also computed for the N1 attentional modulation and the d′ scores from the behavioral session. No significant correlation was found for the no overlap condition (Figure 7B) [τ = 0.112, p = 0.637], for the apical overlap condition (Figure 7C) [τ = 0.127, p = 0.648] or for the basal overlap condition (Figure 7D) [τ = 0.273, p = 0.283].
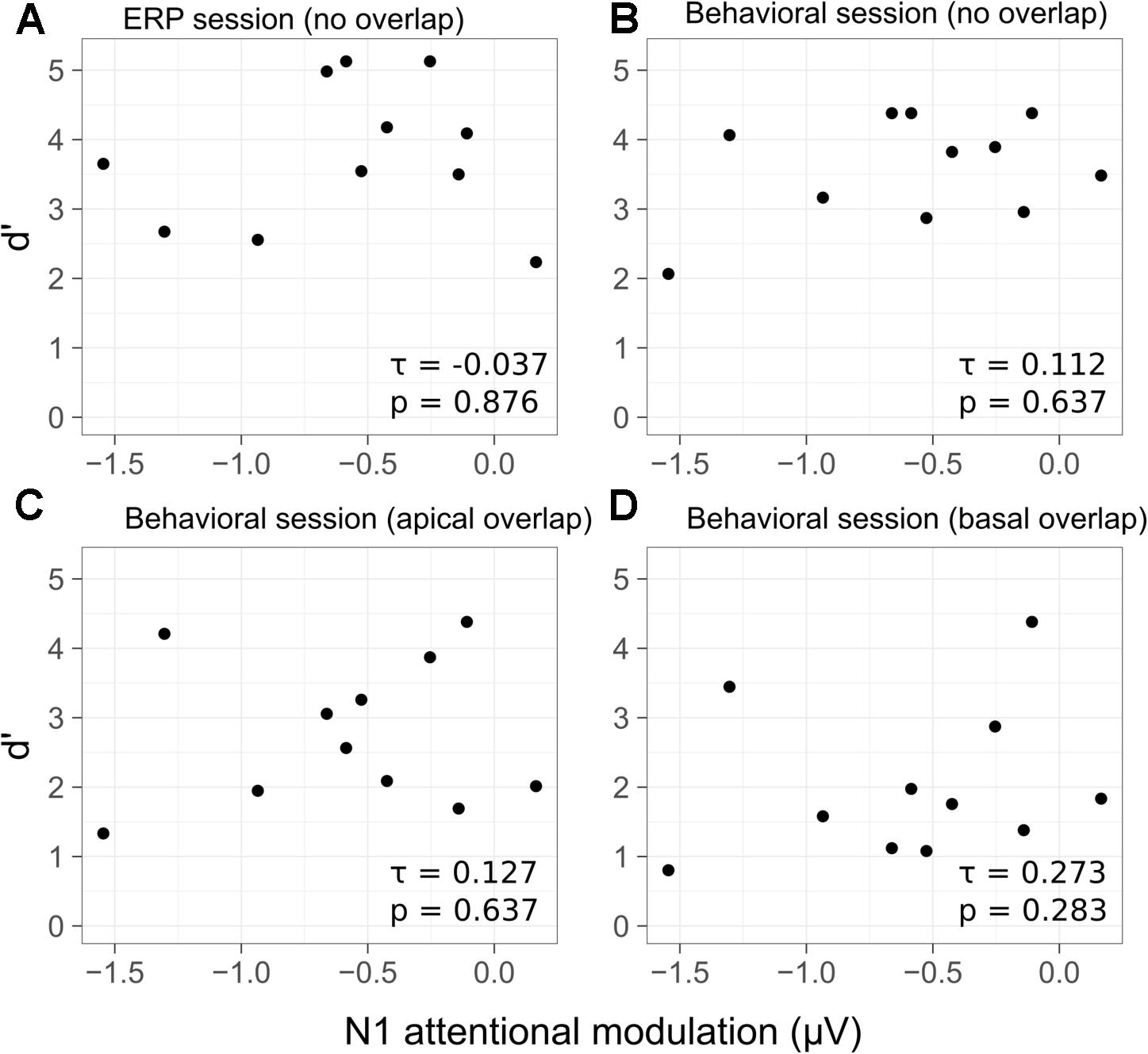
FIGURE 7. Scatterplots of the d′ scores as a function of the average N1 attentional modulation for each listener and condition. Behavioral d′ scores from the ERP session are shown in (A) (no overlap condition). The d′ scores from the behavioral session are shown in (B) for the no overlap condition, in (C) for the apical overlap condition and in (D) for the basal overlap condition. Kendall rank correlation coefficients and p-values are shown in the bottom-right corner of each panel.
Performance in the detection task was assumed to improve when the target and the distractor streams were perceptually segregated. Overall, the d′ scores obtained by the listeners increased with increasing electrode separation between the streams. The d′ scores obtained by the listeners were near chance-level for the full overlap condition, indicating that the listeners could not segregate the streams in the absence of place cues. Conversely, performance was at ceiling for the no overlap condition, suggesting that the listeners were able to use place cues to segregate the streams. These findings are consistent with previous work suggesting that electrode separation facilitates stream segregation for CI listeners (Chatterjee et al., 2006; Hong and Turner, 2006; Böckmann-Barthel et al., 2014; Tejani et al., 2017; Paredes-Gallardo et al., 2018a).
Previous studies generally assessed stream segregation using sequences composed of two repeating and alternating sounds. In such paradigms, the listeners need to segregate the target sound from the distractor sound. In contrast, in the present study, each stream was composed of multiple and different sounds, increasing the complexity of the task: the listeners had to integrate different sounds to form a representation of the target and the distractor streams and to maintain these representations segregated over time. Despite the differences between the paradigms, the results of the present study were consistent with those from Paredes-Gallardo et al. (2018a), which suggested that most CI listeners can segregate streams separated by three electrodes. In the present study, all listeners could segregate the streams in the no overlap condition, where the minimum electrode separation between the streams was three electrodes.
The d′ scores obtained by the listeners increased for deviants happening late in the sequence. This suggests that a two stream percept built up over time (for a review, see Moore and Gockel, 2002, 2012). These results are consistent with earlier reports suggesting that CI listeners may experience a build-up when attention is directed toward segregation, i.e., voluntary stream segregation (Paredes-Gallardo et al., 2018a,b). Moreover, the effect of the deviant triplet location was found to be dependent on the electrode separation between the streams. The largest effect of the deviant triplet location (i.e., build-up) was observed for the apical overlap and the basal overlap conditions, where the performance was not at ceiling or at chance level. This is consistent with previous reports from studies with NH listeners (e.g., Bregman, 1978; Anstis and Saida, 1985) and CI listeners (Böckmann-Barthel et al., 2014), that reported a build-up for intermediate frequency differences between the sounds. However, Böckmann-Barthel et al. (2014) did not provide specific listening instructions to the participants, who directly reported their perception. It has been suggested that the results from such paradigms may reflect pitch or electrode discrimination instead of stream segregation (Chatterjee et al., 2006; Cooper and Roberts, 2007). This uncertainty was avoided in the present study by using a detection task which was facilitated by the segregation of the sounds (e.g., Dowling, 1973; Cusack and Roberts, 2000; Cooper and Roberts, 2009).
In the behavioral session, a random number of distractor sounds was played, in each trial, before and after the target stream (i.e., inducer sounds). It has been suggested that inducer sounds can trigger the build-up and therefore, may facilitate stream segregation (e.g., Rogers and Bregman, 1993; Roberts et al., 2008). To evaluate whether the presence of a random number of inducer sounds facilitated stream segregation, the d′ scores from the behavioral full overlap condition were compared with those from the ERP recording session, where no inducer sounds were presented. The results from a mixed-effects linear model showed no significant effect of the inducer sounds [F(1,11) = 2.701, p = 0.129], indicating that these did not affect the d′ scores.
Despite the similarity of the paradigms, the findings from the present study appear to be inconsistent with those reported by Cooper and Roberts (2009). In their study, the electrode separation between the streams did not affect the performance in the melody discrimination task. Moreover, most of their listeners performed near-chance level unless the distractor sounds were attenuated by at least 50% of the listener’s dynamic range. In contrast, in the present study, all listeners were able to use electrode separation to segregate the streams when the target and the distractor stream were presented at the same loudness. Cooper and Roberts employed sequences with a fixed duration of 2.2 s whereas in the present study, the sequences had a duration which ranged between 4 and 6.65 s. Therefore, one might argue that in the study from Cooper and Roberts, the listeners did not have enough time to build up a segregated percept, as suggested by Paredes-Gallardo et al. (2018a). If this was the case, in the present study, near-chance performance would be expected for the first deviant triplet, which happened between 1.3 and 2.6 s. Instead, the results from the behavioral experiment indicate that the effect of the electrode separation on the d′ scores was largest for the first deviant triplet: the d′ scores ranged from near-chance in the full overlap condition to near-ceiling in the no overlap condition.
In the study of Cooper and Roberts (2009) pitch direction judgments were required to identify the target melody. In contrast, in the present study, the listeners were not required to identify the melodic contour of the target stream, and could, instead, perform the task by detecting a change (i.e., deviant) in the target stream. Kong et al. (2004), suggested that as many as 32 independent frequency bands are needed to recognize familiar melodies. The spectral resolution of CIs is limited both by the number of electrodes (typically 22 or less) and the interaction of the electrical current across electrodes. Thus, current CIs may not provide enough spectral resolution to support melody recognition (e.g., Kong et al., 2004; Mehta and Oxenham, 2017). Therefore, the poor performance observed by Cooper and Roberts (2009) could reflect inherent limitations of CI listeners to recognize familiar melodies and may not be related to poor stream segregation.
The N1 responses to the target and the distractor sounds were recorded while the listeners performed the behavioral task (i.e., active listening) and when the listeners watched a muted movie (i.e., passive listening). Even though the same physical stimuli were presented in both conditions, at the group level and when averaged across all deviant conditions, the N1 amplitudes were different in the active and in the passive listening conditions. Thus, selective auditory attention modulated the ERPs. Consistent with previous work in NH and HI listeners, the N1 responses to the target sounds were enhanced when the listeners performed the behavioral task vs. when they passively listened to the sounds (e.g., Choi et al., 2014; Dai and Shinn-Cunningham, 2016; Dai et al., 2018). However, attention did not significantly modulate the responses to the distractor sounds. This is consistent with previous studies suggesting that hearing impairment may affect the ability to suppress irrelevant sounds (Petersen et al., 2017; Dai et al., 2018).
The effect of selective attention on the N1 responses was largest in the late deviant condition, where the listeners had to sustain selective attention throughout the full duration of the sequence. In this condition, the N1 responses to the target sounds were enhanced during the second and third triplets, but not during the first one. Conversely, the N1 responses to the distractor sounds were enhanced for the first triplet, but not for the second and third ones (Figure 6A). Thus, whereas the attentional modulation of the N1 responses to the target sounds became more robust over time, the attentional modulation of the N1 responses to the distractor sounds diminished over time. These results suggest that CI listeners become more effective at selectively listening to the target stream over time, in agreement with previous reports with NH listeners (e.g., Shinn-Cunningham and Best, 2008; Choi et al., 2014; Dai et al., 2018). Moreover, only the N1 responses to the first two sounds of the target stream were enhanced in the active listening condition (Figure 6B). Given the design of the stimuli, only the first two sounds of each triplet were necessary to detect the deviant. Thus, these results suggest that the listeners may have generally relied on the first two target sounds of each triplet to perform the task.
It has been suggested that the use of a cue to direct the listeners’ attention toward a specific attribute of the sound (e.g., location or pitch) leads to anticipatory modulation of the cortical responses (Hill and Miller, 2010; Lee et al., 2013). As a result, the responses evoked by a sound will be larger when its attributes match those of the cue than when there is a mismatch. However, other studies have suggested that the inherent salience of sudden onsets may override this anticipatory modulation, leading to similar ERP responses in the active and the passive listening conditions (e.g., Dai et al., 2018). In the present study, the target stream was temporally predictable (i.e., it was always the lagging stream) and the listeners were familiar with both the target and the distractor stream. Thus, one would expect either a suppression effect on the N1 response to the first sound of the sequences (i.e., a distractor sound) or a lack of any attentional modulation. Instead, a larger N1 response to the first sound was observed for the active than for the passive listening condition (Figures 4, 5). These results suggest that CI listeners might not be able to ignore the leading stream and therefore deploy top-down attention to the first distractor sound. As a consequence, they need to switch their attention from the distractor to the target stream during the trial. Consequently, the N1 attentional modulation of the distractor stream is significantly different from zero during the first triplet whereas the N1 attentional modulation of the target stream is significantly different than zero during the second and third triplets.
It has been suggested that selective attention can operate at early stages of sensory analysis, even before the features of the stimulus are bound together, or conjoined (e.g., Woods et al., 1994, 1998). In addition, it has been suggested that when attention is focused on a particular stimulus feature, all coherent perceptual features may be bound together forming a stream (e.g., Shamma et al., 2011, 2013). Other studies have suggested that selective attention may operate at the level of auditory objects, even if attention is initially focused on a particular feature (e.g., Alain and Arnott, 2000; Shinn-Cunningham, 2008; Bressler et al., 2014). The presence of a salient perceptual feature in the target stream may, therefore, facilitate the process of object formation. A clear object representation might, in turn, make the process of selective attention more effective. Correspondingly, several studies have found a significant correlation between the attentional modulation of cortical responses and the behavioral performance in a selective auditory attention task (e.g., Choi et al., 2014; Dai and Shinn-Cunningham, 2016; Dai et al., 2018). In the present study, no significant correlation was found between the individual performance in the deviant detection task and the amount of N1 attentional modulation. This was the case for all electrode separation conditions (Figure 7). The d′ scores in the no overlap condition (Figures 7A,B) were, overall, at ceiling. This limited the individual variability of the d′ scores, presumably contributing to the non-significant correlation between the behavioral performance and the N1 attentional modulation. In order to avoid ceiling effects in the behavioral performance while ensuring that enough correct trials are available to estimate the N1 response amplitude, Choi et al. (2014) correlated the behavioral performance in a challenging task with the N1 attentional modulation recorded under a less challenging condition. Choi et al. (2014) found a significant correlation between the behavioral performance and the amount of N1 attentional modulation. In contrast, in the present study, no significant correlation was found between the behavioral performance in the apical and basal overlap conditions, where no ceiling effects were present, and the amount of N1 attentional modulation (Figures 7C,D).
The lack of a significant correlation between the d′ scores and the N1 attentional modulation does not necessarily imply the independence of these two measures. Instead, it might reflect the limitations imposed by the conditions and paradigm chosen for the ERP recordings. The d′ scores from the ERP recordings (no overlap condition) were at ceiling for most listeners, suggesting that the task was not demanding. Thus, it is possible that some listeners could have achieved high d′ scores in the task without selectively attending to the target stream (e.g., they could have switched their attention between the streams). If this was the case, the individual differences in the N1 attentional modulation would not reflect differences in the ability to perceptually group the sounds. Moreover, the ERP recordings took place in the last of the three sessions. As a result, all listeners were familiar with the target stream at the time of the ERP recordings. During the passive listening condition, the listeners watched a muted movie and after the recording session, the listeners were asked informal questions about the movies. However, the individual level of engagement in the movie was not quantified. Thus, some listeners might have been engaged in the movie whereas other might have attended to the sound sequences. As a result, the N1 attentional modulation estimates from the present study might not be precise at an individual level.
The present study combined a behavioral deviant detection task with ERP recordings to investigate the role of electrode separation in voluntary stream segregation for CI listeners. The results suggested that CI listeners can voluntarily segregate streams composed of multiple and different sounds when only electrode separation cues are provided. Moreover, a two-stream percept was found to build up over time. The results from the ERP recordings showed that auditory selective attention modulates the cortical responses in CI listeners. Specifically, selective attention enhanced the responses to the target sounds whereas responses to the distractor sounds remained unchanged. However, no correlation was found between the behavioral performance in the detection task and the attentional modulation of the ERPs.
Overall, the results from the present study suggest that CI listeners can perceptually group sequentially presented sounds into streams on the basis of electrode separation. Moreover, the effects of selective attention could be measured in CI listeners using EEG recordings (at the group level). This suggests that CI listeners, like NH listeners, are able to selectively attend to a target stream as long as its distinctive feature is sufficiently salient. The results from the present study also suggest that CI listeners might experience limitations in their ability to ignore a competing stream and to initially select the stream of interest, which might contribute to their poor performance in complex listening scenarios.
The data generated for the present study is publicly available at https://doi.org/10.5281/zenodo.1211574. The code used for the CI artifact correction is publicly available at https://doi.org/10.5281/zenodo.1303275.
AP-G, HI-B, SM, TD, and JM designed the research. AP-G performed the research. AP-G and HI-B analyzed the data. AP-G, HI-B, SM, TD, and JM wrote the paper.
This work was supported by the Oticon Centre of Excellence for Hearing and Speech Sciences (CHESS), the Carlsberg Foundation, the National Health and Medical Research Council (Australia) Early-Career Research Fellowship Grant No. 1069999, and The Marian and E.H. Flack Trust. The Bionics Institute acknowledges the support from the Victorian Government through its Operational Infrastructure Support Program.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
We would like to thank all volunteers who participated in this study. We thank the two reviewers and the editor for the helpful and constructive comments on an earlier version of the article. We thank Colette McKay for her help, advice, and constructive criticism during the design of the experiments; Darren Mao for his help with the EEG setup; and our colleagues from the Hearing Systems Group and the Translational Hearing Group for valuable comments and stimulating discussions. We also thank Cochlear Ltd. for providing us with some of the research equipment for this study.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnins.2018.00581/full#supplementary-material
Alain, C., and Arnott, S. R. (2000). Selectively attending to auditory objects. Front. Biosci. 5:D202–D212. doi: 10.2741/Alain
Anstis, S. M., and Saida, S. (1985). Adaptation to auditory streaming of frequency-modulated tones. J. Exp. Psychol. Hum. Percept. Perform. 11, 257–271. doi: 10.1037/0096-1523.11.3.257
Bates, D., Mächler, M., Bolker, B., and Walker, S. (2014). Fitting linear mixed-effects models using lme4. J. Statis. Softw. 67:51. doi: 10.18637/jss.v067.i01
Bendixen, A. (2014). Predictability effects in auditory scene analysis: a review. Front. Neurosci. 8:60. doi: 10.3389/fnins.2014.00060
Böckmann-Barthel, M., Deike, S., Brechmann, A., Ziese, M., and Verhey, J. L. (2014). Time course of auditory streaming: do CI users differ from normal-hearing listeners? Front. Psychol. 5:775. doi: 10.3389/fpsyg.2014.00775
Bregman, A. S. (1978). Auditory streaming is cumulative. J. Exp. Psychol. Hum. Percept. Perform. 4, 380–387. doi: 10.1037/0096-1523.4.3.380
Bregman, A. S. (1990). Auditory Scene Analysis: The Perceptual Organization of Sound. Cambridge, MA: The MIT Press.
Bregman, A. S., and Campbell, J. (1971). Primary auditory stream segregation and perception of order in rapid sequences of tones. J. Exp. Psychol. 89, 244–249. doi: 10.1037/h0031163
Bressler, S., Masud, S., Bharadwaj, H., and Shinn-Cunningham, B. (2014). Bottom-up influences of voice continuity in focusing selective auditory attention. Psychol. Res. 78, 349–360. doi: 10.1007/s00426-014-0555-7
Chatterjee, M., Sarampalis, A., and Oba, S. I. (2006). Auditory stream segregation with cochlear implants: a preliminary report. Hear. Res. 222, 100–107. doi: 10.1016/j.heares.2006.09.001
Choi, I., Rajaram, S., Varghese, L. A., and Shinn-Cunningham, B. G. (2013). Quantifying attentional modulation of auditory-evoked cortical responses from single-trial electroencephalography. Front. Hum. Neurosci. 7:115. doi: 10.3389/fnhum.2013.00115
Choi, I., Wang, L., Bharadwaj, H., and Shinn-Cunningham, B. (2014). Individual differences in attentional modulation of cortical responses correlate with selective attention performance. Hear. Res. 314, 10–19. doi: 10.1016/j.heares.2014.04.008
Collins, L. M., Zwolan, T. A., and Wakefield, G. H. (1997). Comparison of electrode discrimination, pitch ranking, and pitch scaling data in postlingually deafened adult cochlear implant subjects. J. Acoust. Soc. Am. 101, 440–455. doi: 10.1121/1.417989
Cooper, H. R., and Roberts, B. (2007). Auditory stream segregation of tone sequences in cochlear implant listeners. Hear. Res. 225, 11–24. doi: 10.1016/j.heares.2006.11.010
Cooper, H. R., and Roberts, B. (2009). Auditory stream segregation in cochlear implant listeners: measures based on temporal discrimination and interleaved melody recognition. J. Acoust. Soc. Am. 126, 1975–1987. doi: 10.1121/1.3203210
Cusack, R., and Roberts, B. (2000). Effects of differences in timbre on sequential grouping. Percept. Psychophys. 62, 1112–1120. doi: 10.3758/BF03212092
Dai, L., Best, V., and Shinn-Cunningham, B. G. (2018). Sensorineural hearing loss degrades behavioral and physiological measures of human spatial selective auditory attention. Proc. Natl. Acad. Sci. U.S.A. 115, E3286–E3295. doi: 10.1073/pnas.1721226115
Dai, L., and Shinn-Cunningham, B. G. (2016). Contributions of sensory coding and attentional control to individual differences in performance in spatial auditory selective attention tasks. Front. Hum. Neurosci. 10:530. doi: 10.3389/fnhum.2016.00530
Dowling, W. J. (1973). The perception of interleaved melodies. Cogn. Psychol. 5, 322–337. doi: 10.1016/0010-0285(73)90040-6
Duran, S. I., Collins, L. M., and Throckmorton, C. S. (2012). Stream segregation on a single electrode as a function of pulse rate in cochlear implant listeners. J. Acoust. Soc. Am. 132, 3849–3855. doi: 10.1121/1.4764875
Eddington, D. K., Dobelle, W. H., Brackmann, D. E., Mladejovsky, M. G., and Parkin, J. (1978). Place and periodicity pitch by stimulation of multiple scala tympani electrodes in deaf volunteers. Trans. Am. Soc. Artif. Int. Organs 24, 1–5. Available at: http://www.ncbi.nlm.nih.gov/pubmed/715981
Hautus, M. J. (1995). Corrections for extreme proportions and their biasing effects on estimated values of d′. Behav. Res. Methods Instrum. Comput. 27, 46–51. doi: 10.3758/BF03203619
Hill, K. T., and Miller, L. M. (2010). Auditory attentional control and selection during cocktail party listening. Cereb. Cortex 20, 583–590. doi: 10.1093/cercor/bhp124
Hillyard, S., Hink, R., Schewent, V., and Picton, T. (1973). Electrical signs of selective attention in the human brain. Science 182, 177–180. doi: 10.1126/science.182.4108.177
Hillyard, S. A., Vogel, E. K., and Luck, S. J. (1998). Sensory gain control (amplification) as a mechanism of selective attention: electrophysiological and neuroimaging evidence. Philos. Trans. R. Soc. Lon. Ser. B Biol. Sci. 353, 1257–1270. doi: 10.1098/rstb.1998.0281
Hong, R. S., and Turner, C. W. (2006). Pure-tone auditory stream segregation and speech perception in noise in cochlear implant recipients. J. Acoust. Soc. Am. 120, 360–374. doi: 10.1121/1.2204450
Hong, R. S., and Turner, C. W. (2009). Sequential stream segregation using temporal periodicity cues in cochlear implant recipients. J. Acoust. Soc. Am. 126, 291–299. doi: 10.1121/1.3140592
Iverson, P. (1995). Auditory stream segregation by musical timbre: effects of static and dynamic acoustic attributes. J. Exp. Psychol. Hum. Percept. Perform. 21, 751–763. doi: 10.1121/1.403521
Kong, Y.-Y., Cruz, R., Jones, J. A., and Zeng, F.-G. (2004). Music perception with temporal cues in acoustic and electric hearing. Ear. Hear. 25, 173–185.
Kuznetsova, A., Brockhoff, P. B., and Christensen, R. H. B. (2017). lmerTest package: tests in linear mixed effects models. J. Statist. Softw. 82, 1–26. doi: 10.18637/jss.v082.i13
Kuznetsova, A., Christensen, R. H. B., Bavay, C., and Brockhoff, P. B. (2015). Automated mixed ANOVA modeling of sensory and consumer data. Food Qual. Preferen. 40, 31–38. doi: 10.1016/j.foodqual.2014.08.004
Landsberger, D. M., Vermeire, K., Claes, A., Van Rompaey, V., and Van de Heyning, P. (2016). Qualities of single electrode stimulation as a function of rate and place of stimulation with a cochlear implant. Ear Hear. 37, e149–e159. doi: 10.1097/AUD.0000000000000250
Lee, A. K. C., Rajaram, S., Xia, J., Bharadwaj, H., Larson, E., Hämäläinen, M. S., et al. (2013). Auditory selective attention reveals preparatory activity in different cortical regions for selection based on source location and source pitch. Front. Neurosci. 6:1–9. doi: 10.3389/fnins.2012.00190
Lenth, R. V. (2016). Least-squares means: The {R} package {lsmeans}. J. Statis. Softw. 69, 1–33. doi: 10.18637/jss.v069.i01
Long, C. J., Nimmo-Smith, I., Baguley, D. M., O’Driscoll, M., Ramsden, R., Otto, S. R., et al. (2005). Optimizing the clinical fit of auditory brain stem implants. Ear Hear. 26, 251–262. doi: 10.1097/00003446-200506000-00002
Macherey, O., and Carlyon, R. P. (2010). Temporal pitch percepts elicited by dual-channel stimulation of a cochlear implant. J. Acoust. Soc. Am. 127, 339–349. doi: 10.1121/1.3269042
Mackersie, C. L., Prida, T. L., and Stiles, D. (2001). The role of sequential stream segregation and frequency selectivity in the perception of simultaneous sentences by listeners with sensorineural hearing loss. J. Speech Lang. Hear. Res. 44, 19–28. doi: 10.1044/1092-4388(2001/002)
Marozeau, J., Innes-Brown, H., and Blamey, P. J. (2013). The acoustic and perceptual cues affecting melody segregation for listeners with a cochlear implant. Front. Psychol. 4:790. doi: 10.3389/fpsyg.2013.00790
McDermott, J. H. (2009). The cocktail party problem. Curr. Biol. CB 19, R1024–R1027. doi: 10.1016/j.cub.2009.09.005
Mehta, A. H., and Oxenham, A. J. (2017). Vocoder simulations explain complex pitch perception limitations experienced by cochlear implant users. JARO – J. Assoc. Res. Otolaryngol. 18, 789–802. doi: 10.1007/s10162-017-0632-x
Micheyl, C., Carlyon, R. P., Cusack, R., and Moore, B. C. J. (2005). “Performance measures of auditory organization,” in Auditory Signal Processing, eds D. Pressnitzer, A. de Cheveigné, S. McAdams, and L. Collet (New York, NY: Springer).
Moore, B. C. J., and Gockel, H. E. (2002). Factors influencing sequential stream segregation. Acta Acust. Unit. Acust. 88, 320–333.
Moore, B. C. J., and Gockel, H. E. (2012). Properties of auditory stream formation. Philos. Trans. R. Soc. B Biol. Sci. 367, 919–931. doi: 10.1098/rstb.2011.0355
Nelson, D. A., Van Tasell, D. J., Schroder, A. C., Soli, S., and Levine, S. (1995). Electrode ranking of place pitch and speech recognition in electrical hearing. J. Acoust. Soc. Am. 98, 1987–1999.
Nelson, P. B., Jin, S.-H., Carney, A. E., and Nelson, D. A. (2003). Understanding speech in modulated interference: cochlear implant users and normal-hearing listeners. J. Acoust. Soc. Am. 113, 961–968. doi: 10.1121/1.1531983
Nie, Y., and Nelson, P. (2015). Auditory stream segregation using amplitude modulated bandpass noise. J. Acoust. Soc. Am. 127:1809. doi: 10.1121/1.3384104
Oostenveld, R., Fries, P., Maris, E., and Schoffelen, J. M. (2011). FieldTrip: open source software for advanced analysis of MEG, EEG, and invasive electrophysiological data. Comput. Intell. Neurosci. 2011:156869. doi: 10.1155/2011/156869
Oxenham, A. J. (2008). Pitch perception and auditory stream segregation: implications for hearing loss and cochlear implants. Trends Amplif. 12, 316–331. doi: 10.1177/1084713808325881
Paredes-Gallardo, A., Madsen, S. M. K., Dau, T., and Marozeau, J. (2018a). The role of place cues in voluntary stream segregation for cochlear implant users. Trends Hear. 22, 1–13. doi: 10.1177/2331216517750262
Paredes-Gallardo, A., Madsen, S. M. K., Dau, T., and Marozeau, J. (2018b). The role of temporal cues on voluntary stream segregation in cochlear implant users. Trends Hear. 22, 1–13. doi: 10.1177/2331216518773226
Petersen, E. B., Wöstmann, M., Obleser, J., and Lunner, T. (2017). Neural tracking of attended versus ignored speech is differentially affected by hearing loss. J. Neurophysiol. 117, 18–27. doi: 10.1152/jn.00527.2016
Picton, T. W., and Hillyard, S. A. (1974). Human auditory evoked potentials. II: effects of attention. Electroencephal. Clin. Neurophysiol. 36, 191–199. doi: 10.1016/0013-4694(74)90156-4
R Core Team (2015). R: A Language and Environment for Statistical Computing. Vienna: R Foundation for Statistical Computing. https://www.r-project.org/
Roberts, B., Glasberg, B. R., and Moore, B. C. J. (2008). Effects of the build-up and resetting of auditory stream segregation on temporal discrimination. J. Exp. Psychol. Hum. Percept. Perform. 34, 992–1006. doi: 10.1037/0096-1523.34.4.992
Rogers, W. L., and Bregman, A. S. (1993). An experimental evaluation of three theories of auditory stream segregation. Percept. Psychophys. 53, 179–189. doi: 10.3758/BF03211728
Shamma, S., Elhilali, M., Ma, L., Micheyl, C., Oxenham, A. J., Pressnitzer, D., et al. (2013). Temporal coherence and the streaming of complex sounds. Basic Aspects Hear. Physiol. Percept. 787, 535–543. doi: 10.1007/978-1-4614-1590-9_59
Shamma, S. A., Elhilali, M., and Micheyl, C. (2011). Temporal coherence and attention in auditory scene analysis. Trends Neurosci. 34, 114–123. doi: 10.1016/j.tins.2010.11.002
Shannon, R. (1983). Multichannel electrical stimulation of the auditory nerve in man. I. Basic psychophysics. Hear. Res. 11, 157–189. doi: 10.1016/0378-5955(83)90077-1
Shinn-Cunningham, B. G. (2008). Object-based auditory and visual attention. Trends Cognit. Sci. 12, 182–186. doi: 10.1016/j.tics.2008.02.003
Shinn-Cunningham, B. G., and Best, V. (2008). Selective attention in normal and impaired hearing. Trends Amplifi. 12, 283–299. doi: 10.1177/1084713808325306
Tejani, V. D., Schvartz-Leyzac, K. C., and Chatterjee, M. (2017). Sequential stream segregation in normally-hearing and cochlear-implant listeners. J. Acoust. Soc. Am. 141, 50–64. doi: 10.1121/1.4973516
Thompson, S. K., Carlyon, R. P., and Cusack, R. (2011). An objective measurement of the build-up of auditory streaming and of its modulation by attention. J. Exp. Psychol. Hum. Percept. Perform. 37, 1253–1262. doi: 10.1037/a0021925
Tong, Y. C., Clark, G. M., Blamey, P. J., Busby, P. A., and Dowell, R. C. (1980). Psychophysical studies on two multiple channel cochlear implant patients. J. Acoust. Soc. Am. 71, 153–160. doi: 10.1121/1.387342
Townshend, B., Cotter, N., Compernolle, D., Van, and White, R. L. (1987). Pitch perception by cochlear implant subjects. J. Acoust. Soc. Am. 82, 106–115. doi: 10.1121/1.395554
Van Noorden, L. P. A. S. (1975). Temporal Coherence in the Perception of Tone Sequences. Eindhoven: Technische Hogeschool. doi: 10.6100/IR152538
Verde, M. F., Macmillan, N. A., and Rotello, C. M. (2006). Measures of sensitivity based on a single hit rate and false alarm rate: The accuracy, precision, and robustness of, d’ Az, and A’. Percept. Psychophys. 68, 643–654. doi: 10.3758/BF03208765
Viola, F. C., De Vos, M., Hine, J., Sandmann, P., Bleeck, S., Eyles, J., et al. (2012). Semi-automatic attenuation of cochlear implant artifacts for the evaluation of late auditory evoked potentials. Hear. Res. 284, 6–15. doi: 10.1016/j.heares.2011.12.010
Woods, D. L., Alain, C., and Ogawa, K. H. (1998). Conjoining auditory and visual features during high-rate serial presentation: processing and conjoining two features can be faster than processing one. Percept. Psychophys. 60, 239–249. doi: 10.3758/BF03206033
Keywords: auditory scene analysis, segregation, cochlear implant, auditory attention, event-related potentials, build-up
Citation: Paredes-Gallardo A, Innes-Brown H, Madsen SMK, Dau T and Marozeau J (2018) Auditory Stream Segregation and Selective Attention for Cochlear Implant Listeners: Evidence From Behavioral Measures and Event-Related Potentials. Front. Neurosci. 12:581. doi: 10.3389/fnins.2018.00581
Received: 01 May 2018; Accepted: 02 August 2018;
Published: 21 August 2018.
Edited by:
Claude Alain, Rotman Research Institute (RRI), CanadaReviewed by:
Andrew Dimitrijevic, Sunnybrook Health Sciences Centre, CanadaCopyright © 2018 Paredes-Gallardo, Innes-Brown, Madsen, Dau and Marozeau. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Andreu Paredes-Gallardo, YXBhZ2FAZWxla3Ryby5kdHUuZGs=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.