- 1Department of Medical Psychology, Chaohu Clinical Medical College, Anhui Medical University, Hefei, China
- 2Department of Substance-Related Disorders, Anhui Mental Health Center, Hefei, China
- 3Key Laboratory of Brain Function and Disease, School of Life Sciences, University of Science and Technology of China, Chinese Academy of Sciences, Hefei, China
Alcohol use disorders (AUDs) represent a severe, world-wide problem, and are usually comorbid with psychiatric disorders, comorbidity increases the risks associated with AUDs, and results in more serious consequences for patients. However, currently the underlying mechanisms of comorbid psychiatric disorders in AUDs are not clear. Studies investigating comorbidity could help us understand the neural mechanisms of AUDs. In this review, we explore three comorbidities in AUDs, including schizophrenia, major depressive disorder (MDD), and personality disorders (PDs). They are all co-morbidities of AUDs with rate of 33.7, 28, and 50–70%, respectively. The rate is significantly higher than other diseases. Therefore we review and analyze relevant literature to explore whether these three diseases are the risk factors of AUDs, focusing on studies assessing cognitive function and those using neural imaging. We found that memory deficits, impairment of cognitive control, negative emotion, and impulsivity may increase an individual's vulnerability to AUDs. This comorbidity may indicate the neural basis of AUDs and reveal characteristics associated with different types of comorbidity, leading to further development of new treatment approaches for AUDs.
Introduction
Alcohol use disorders (AUDs) are chronic and relapsing illnesses that contribute to around 4% of the global burden of disease (Margolese et al., 2004; Rehm et al., 2009). The prevalence of 12-month and lifetime AUD (DSM-5) has been reported to be 13.9% and 29.1%, respectively (Grant et al., 2016). AUDs increase the risk of many adverse consequences, including injury, disease, death (Barbosa et al., 2010; Dawson and Grant, 2011), and substantial financial cost (Joshua, 2017).
The neural mechanisms of AUDs remain uncertain. According to previous studies, there are multiple possible causes of AUDs, including genetic, psychological, an environment factors (Kendler et al., 2011). However, there are complex factors in identification of genetic risk variants, involved in alcohol-related phenotypes. Until recently, gene finding efforts have mainly focused on candidate genes. Certain factors play a role in alcohol-related phenotypes in addition to genetic factors. For example, disadvantageous life circumstances, including early life stress and stressful life events (e.g., death of a loved one, divorce) (Holgate and Bartlett, 2015). Similar to stress, poor life satisfaction has been associated with alcohol use and heavy drinking (Peltzer and Pengpid, 2016). Individuals with psychiatric disorders often exhibit comorbidity with AUDs (Sher, 2006). The close relationship between psychiatric disorders, such as schizophrenia, mood disorders, and personality disorders, and AUDs suggests that psychiatric disorders may increase the risk of exposure to alcohol, exacerbating other early life risk factors for AUDs (Fink et al., 2016). Studies on the comorbidity of AUDs in psychiatric disorders might be useful to help us understand the neural mechanisms of AUDs. Therefore, it is important to enhance our understanding of these comorbidities.
Alternatively, it is plausible that the AUDs would induce some psychiatric disorders. The early study found that AUDs were related to impulsive behavior, negative affect and weaken self-regulation (Drevets et al., 1997) which in turn caused other mental disorders. Additionally, the self-medication hypothesis suggests that AUDs promote individuals to continue using alcohol to reduce negative emotional and mental symptoms, which predicted greater risk factors for comorbiding with other psychiatric disorders (Abrams et al., 2001).
However, this review focuses on revealing the risk factors of AUD, therefore we present current studies of AUDs and their associated comorbidities, including MDD, schizophrenia, and PDs, with a particular focus on the effect of these disorders on the AUD.
Schizophrenia and AUDs
Over one-third of patients with schizophrenia meet the criteria for an AUD diagnosis, which is more than three times the prevalence in the population at large (Regier et al., 1990; Green and Brown, 2006). The Epidemiologic Catchment Area (ECA) study found that the co-morbidity rate of the two diseases was as high as 33.7% (Regier et al., 1990). These results suggest that schizophrenia may act as a vulnerability factor to AUDs.
Studies show that significant predictors of comorbid AUDs in patients with schizophrenia are being male, severity of negative symptoms, severity of depression (Meszaros et al., 2011), low educational attainment, previous violent offending, and a family history of substance use disorders (Apantaku-Olajide et al., 2014).
However, there is an important risk factor for relapse in abstinent alcoholics psychological stress, as well as the neural mechanisms, by which stress induces relapse are fairly well established. Chronic alcohol using, especially in stress, results in neuroadaptations. Thus alcoholic patients show dysfunction of stress [e.g., sympathetic adrenomedullary axis (SAM) and hypothalamic pituitary adrenocortical axis (HPA)] pathways, characterized by (for example) dysregulation of the cortisol response (Kreek and Koob, 1998), and/or deficits in emotional regulation (Sinha, 2001). These neuroadaptations may lead to patients showing increases in craving for alcohol in response to stress, and thus being particularly at risk of relapse.
The co-morbidity of AUDs in patients with schizophrenia is associated with greater severity of psychopathology (Margolese et al., 2004) and neurocognitive dysfunction (Manning et al., 2009). Several studies suggest that individuals with schizophrenia and comorbid AUDs have exacerbated impairments in memory, including working memory (Bowie et al., 2005; Potvin et al., 2008), episodic memory (Smith et al., 2011), and verbal learning (Manning et al., 2009).
Volume loss within localized regions of the hippocampus (Sinha, 2001) and subcortical structures, such as the thalamus (Byne et al., 2009), striatum and globus pallidus (Brandt and Bonelli, 2008), can be seen as schizophrenia characteristic.Smith and colleagues (Smith et al., 2011) reported that schizophrenia patients with AUD had a similar a more widespread pattern of hippocampal deformity with inward deformation near the head and tail in the right hemisphere and inward deformations in the left head that extended more medially. Within the hippocampus and subcorticalstructures, experts have suggested earlier that addiction and schizophrenia may have overlapping neurobiological substrates, which may place schizophrenia patients at increased risk for developing a substance use disorder (Green et al., 2002).
In addition developmental dysfunction of the hippocampus also leads to a reward circuit dysfunction gets further validation in a rodent model of preadolescent hippocampal lesioning-the neonatal ventral hippocampal lesion rat (NVHL rat), in which normal hippocampal signaling (particularly to the frontal cortex and the nucleus accumbens) is altered during development (Tseng et al., 2009). NVHL rats not only exhibit a schizophrenia-like phenotype, but they also have a dysregulated reward circuit. Importantly, they also demonstrate increased consumption of alcohol (Jeanblanc et al., 2015). Data from research on this animal model suggest that brain reward circuit dysfunctions increase the vulnerability for substance use in patients with schizophrenia, which combined with potentially accentuated reinforcing properties of the substances, may make patients with schizophrenia especially vulnerable to initiating substance use.
Moreover, individuals at an elevated risk of schizophrenia have been suggested to be particularly vulnerable to the effects of alcohol on the frontal lobe and hippocampus (Welch et al., 2011), which are implicated in memory processes. These neural imaging studies suggest that brain regions associated with memory may also support the neural mechanisms of AUDs. Consistently, Luo and colleagues recently reported that an associative memory retrieval-extinction procedure decreases reinstatement of cocaine and heroin seeking in rats, and heroin craving in humans (Luo et al., 2015). The authors conclude that retrieval-extinction manipulations, started 1 day after cocaine self-administration, decreased reinstatement (cocaine priming), spontaneous recovery, and renewal (context-induced reinstatement) of cocaine seeking.
Additionally, cognitive dysfunction, in particular executive dysfunction (Manning et al., 2009), is another major symptom in schizophrenic patients with comorbid AUDs. This suggests that executive dysfunction is a risk factor of AUDs. In a study of non-alcoholic addiction, individuals with cannabis-related impairment in executive function have been found to have trouble learning and applying the skills required for successful recovery, putting them at an increased risk of relapsing to cannabis use (Crean et al., 2011). Similarly, this may hinder an individual's ability to benefit from behavioral therapies, increasing the risk of relapse to alcohol use (Aharonovich et al., 2008). An array of higher cognitive functions is vital for overriding and inhibiting responses that otherwise would be automatic or require little thought, such as continued substance abuse.
In summary, a number of studies (Smith et al., 2011; Johnson et al., 2013) have shown that schizophrenia results in severe abnormalities in the hippocampus and related brain regions that affect memory and executive function. Disorganized memory and the inability to inhibit craving may be risk factors for inducing alcohol-seeking and subsequent alcohol-dependence.
Depressive Disorders and AUDs
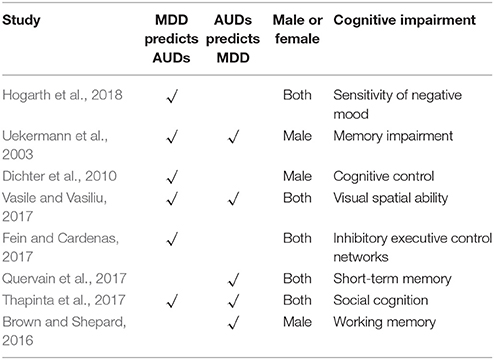
MDD as well as alcohol use disorders (alcohol abuse and alcohol dependence) are widespread in the general population and constitute a huge public health burden worldwide (World Health Organization, 2011). In addition, comorbidity of alcohol use disorders and MDD is high (Boschloo et al., 2011). Studies in the general population show that people with depressive disorders have a 2- to 3-fold increased risk of AUDs (Hasin et al., 2007). With respect to 12-month comorbidity among respondents with a diagnosis of alcohol dependence, 29% of respondents had at least one affective disorder and the most common was major depression (28%) (Burns and Teesson, 2002). It is suggested that MDD is likely to be a pathogenic factor in triggering AUDs. Likewise, chronic drinking may promote depression indirectly as well. For example by contributing to stressful life circumstances (e.g., partner-relationship disruptions) that in turn are known to promote depression (Conner et al., 2009) (See Table 1).
The self-medication hypothesis (Kalén et al., 1990) suggests that people with depression misuse alcohol to reduce their distressing symptoms, This theory has been corroborated by another research (Bolton et al., 2006). Studies examining clinical characteristics show that people with comorbid alcohol dependence have more depressive symptoms (Rae et al., 2002). One possible explanation is that distressing circumstances, such as having no partner or feelings of loneliness, in combination with a neurotic personality may increase the likelihood of an individual to reduce distress with alcohol.
Structural and functional neuroimaging (Solomon and Corbit, 1974; Walker et al., 2011) has provided evidence in support of some degree of neuropathological convergence in AUDs and mood disorders. In chronic alcoholics, prefrontal regions show abnormally reduced glucose metabolism or blood flow in medial, dorsolateral, and orbitofrontal regions (Beaunieux et al., 2013). Similarly, in mood disorders, dysregulation of glucose metabolism and blood flow particularly affects the prefrontal cortex (PFC), temporal cortex, and amygdale (Videbech, 2000).
A therapeutic study (Dichter et al., 2010) found that cognitive control of MDD decreased significantly. Cognitive biases, in the context of addiction, can be broadly defined as automatically triggered cognitive processes, such as paying attention to and approaching drug cues. One such bias, attentional bias, is the degree to which attention is drawn to motivationally relevant stimuli as opposed to neutral stimuli. This can be quantified through reaction time tasks and measured in a laboratory setting. Attentional bias for drug cues has been studied extensively (Dependence, 2008) and is (modestly) related to craving (Fadardi and Cox, 2009). Furthermore, attentional bias has been related to relapse and escalation of drug problems. As such attentional bias for drug cues is thought to be an important behavioral marker for (problematic) drug use (Stacy and Wiers, 2010).
Attentional bias to social threats, as an initiator of cognitive distortion, is central to social fear (Dudeney et al., 2015). The role of attentional bias to threat has been implicated in the development, maintenance, and remediation of anxiety pathology (Dudeney et al., 2015). The heightened salience of these cues can “grab” attention leading to drug-seeking, a cascade effect that may not even require conscious awareness of the drug cues (Rose et al., 2008).
Imaging studies show when patients were urged to pay attention to their own heartbeats, they exhibited notable responses in the lateral orbitofrontal cortex (OFC), which found that lateral OFC hyperactivity may be responsible for anxiety-laden cognitions (Hahn et al., 2011). Previous a study has shown that the gray matter volume of the PFC reduced in familial bipolar disorder and familial MDD (Drevets et al., 1997). Another study suggests that patients with MDD have reduced OFC gray matter volumes (Lacerda et al., 2004). Further, the defect of the OFC in MDD may result in attention deviation.
These results suggest that the defect of PFC (especially in the lateral OFC) leading to the deficient cognitive control in MDD possibly increases the risk of developing co-morbid AUDs.
Neurobiological studies also support the self-medication hypothesis. In the context of the opponent-process theory (Solomon and Corbit, 1974), activation of the associated receptor is proposed to underpin the “negative” component, such as dysphoria and MDD (Yan and Roth, 2004), which drives drug-seeking behavior and consumption. Consistent with this theory, in preclinical models of AUD, antagonism of the κ-opioid receptor function, with nalmefene for example (Quelch et al., 2017), reduces alcohol consumption (Walker et al., 2011).
Furthermore, a previous study (Uekermann et al., 2003) found that co-morbid AUDs in patients with MDD have memory impairment on the behavior. The lateral habenula (LHb) plays a very important role in memory, and interestingly enough, both AUDs (Mathis and Lecourtier, 2017) and MDD (Park et al., 2017) show dysfunction of the LHb. These studies suggest lesions of memory and related brain area LHb might be a reason of this comorbidity. The LHb is a core brain region related to memory process and adaptive behavior selection (Lee and Huang, 1988; Goutagny et al., 2013). First, a large number of animal studies have shown the LHb is in a key position to process memory as it is connected with the main networks mediating such a function. The LHb is indirectly connected with the dorsal hippocampus (dHPC) (Goutagny et al., 2013) and projects to the mediodorsal nucleus of the thalamus (Araki et al., 1988). Moreover, it is involved in modulating hormones associated with memory, such as dopamine (Brown and Shepard, 2016), serotonin (Kalén et al., 1990), as well as raphe serotonergic neurons (Wang and Aghajanian, 1977). Third, recently, different studies have suggested that the LHb may take part in memory processes and perhaps play an important role in the selection of the most adapted “memory-based behavior” (Mathis and Lecourtier 2017).
We know that the patients with depression and AUD have more stressful events that are more prone to negative emotions. Consequently, given the well-known detrimental effects of altered stress coping on learning and memory (Quervain et al., 2017), the marked memory impairments observed could partly be the consequence of such a failure to cope with stress. The LHb has been involved in not only participating in the online processing of contextual information but also the processing of the emotional valance of incoming information in order to adapt to particularly stressful situations. Then, they produce risky behaviors such as drinking.
In short, negative emotion, deficient cognitive control and dysfunction of the LHb may increase the risk of developing alcohol-seeking behavior, potentially as a result of overlapping neural mechanisms.
Personality Disorders and AUDs
PDs are highly comorbid with AUDs in both general and clinical populations (Trull et al., 2000), especially in cluster-B alcoholics (Mccarter et al., 2016). The strongest and most consistent links reported in the literature have been found between externalizing-related personality pathology (antisocial personality disorder [ASPD] and borderline personality disorder [BPD]) and AUDs (Jahng et al., 2011). Approximately 50–70% of psychiatric inpatients with BPD also meet diagnostic criteria for substance use disorders, most commonly alcohol use disorder (Zanarini et al., 2004), The study found the patients who met the DSM criteria for ASPD were 21 times more likely to develop alcohol abuse and dependence at some point during their lives than were the people who did not have ASPD (Regier et al., 1990).
Studies on cognitive function suggest that alcohol-seeking in PDs may be associated with impulsivity (Trull et al., 2000). Self-report measures of impulsivity were higher in cluster-B alcoholics than in alcoholics without PD (Dom et al., 2006). Impulsivity in the early years is related to greater risk of future alcohol dependence, and adults with high impulsivity scores are more likely to be diagnosed with alcoholism (Prince van Leeuwen et al., 2011). Impulsivity increases the vulnerability to AUDs through negative emotion (Shin et al., 2015), worse cognitive performance (Haaland et al., 2009), and impaired executive function (Stevens et al., 2003). The study also found that AUDs was strongly and negatively correlated with BPD, by contributing to unemployment, poor school performance, promiscuity (Gregory et al., 2008) and neuroticism–negative affectivity (Trull et al., 2004).
Impulsivity predicts development of AUDs, which is higher in family-history positive (FHPs) with more alcohol-dependent relatives (Dick et al., 2010). Neuroimaging Go/No-Go studies have identified functional abnormalities consistent with increases in aspects of impulsivity (e.g., poor response inhibition) in FHPs. But there is no consensus on the brain regions of alcohol—related impulses, and these studies have different conclusions. The study finds that bilateral junction of the anterior insula and inferior frontal gyrus (insula/IFG) were associated with impulsivity and addiction and Go/No-Go task activation (Stevens et al., 2007). The insula/IFG is integral to response inhibition (Stevens et al., 2007). The insula has been proposed to be relevant to addiction, given its role in interoception and integration of information from regions important for cognitive-control and affective processes (Naqvi and Bechara, 2010).
In a previous study that utilized SPECT, alcoholics with a comorbid diagnosis of ASPD exhibited reductions in regional cerebral blood flow within the frontal lobes (Vural, 1996). In another study (Soloff et al., 2003), PET technology was used to demonstrate reduced regional glucose metabolism in the prefrontal cortex (orbitofrontal, anterior medial, superior frontal). These studies show that the prefrontal lobe is the key part of comorbid the cluster-B PDs in patients with AUDs, which are indeed associated with addiction, especially inferior frontal gyrus (IFG).
Devito (Devito et al., 2013) thinks that activity in the left insula/IFG cluster correlated with aspects of impulsivity, which, in turn, were associated with alcohol use measures. His study also confirms this view when successfully inhibiting responses on “No-Go” trials, FHPs activated the left insula/IFG region more robustly than FHNs (Devito et al., 2013).
In summary, impulsivity is the obvious characteristic of Cluster B personality disorder, further impulsivity is associated with abnormality of the prefrontal lobe (especially in IFG), having an injury in IFG of Cluster B personality disorder might be risk factors for alcohol-seeking behavior.
Conclusions
The close relationship between psychiatric disorders (e.g., Schizophrenia, mood disorders, PDs) and AUDs suggests that psychiatric disorders are predisposing factors for AUDs. In this review, we investigated three comorbid disorders with AUDs, focusing on cognitive function in these disorders and neural imaging studies. We found that memory deficits, cognitive control, negative emotion, impulsivity, and affective instability may increase an individual's vulnerability to AUDs. This review may indicate the neural basis and clinical subdivisions of AUDs, as well as suggest new approaches for the preclinical treatment of AUDs.
Author Contributions
PY and XZ work for substantial contributions to the conception or design; YW work for drafting the work or revising it critically for important intellectual content; RT and CH work for final approval of the version to be published; CH contribution for refining the ideas; SL contribution for looking up the literature for this article.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The authors appreciate YW and XZ for their careful reading of the manuscript. This work was supported by the National Natural Science Foundation of China (31471071, 31230032,31171083, 31500917) and the National Key Basic Research Program (2016YFA0400900) and the Fundamental Research Funds for the Central Universities of China, and MOE-Microsoft Key Laboratory of USTC, the National Key Basic Research Program (2016YFA0400900); the National Natural Science Foundation of China (31771221, 31471071); the Fundamental Research Funds for the Central Universities of China, the Anhui Province university natural Grants (KJ2018A0659) and the Anhui Province Natural Grants(KJ2016SD30).
References
Abrams, K., Kushner, M., Medina, K. L., and Voight, A. (2001). The pharmacologic and expectancy effects of alcohol on social anxiety in individuals with social phobia. Drug Alcohol Depend. 64, 219–231. doi: 10.1016/S0376-8716(01)00125-9
Aharonovich, E., Brooks, A. C., Nunes, E. V., and Hasin, D. S. (2008). Cognitive deficits in marijuana users: effects on motivational enhancement therapy plus cognitive behavioral therapy treatment outcome. Drug Alcohol Depend. 95:279. doi: 10.1016/j.drugalcdep.2008.01.009
Apantaku-Olajide, T., James, P. D., and Smyth, B. P. (2014). Association of educational attainment and adolescent substance use disorder in a clinical sample. J. Child Adolesc. Subst. Abuse 23, 169–176. doi: 10.1080/1067828X.2013.786921
Araki, M., Mcgeer, P. L., and Kimura, H. (1988). The efferent projections of the rat lateral habenular nucleus revealed by the PHA-L anterograde tracing method. Brain Res. 441, 319–330. doi: 10.1016/0006-8993(88)91410-2
Barbosa, C., Taylor, B., Godfrey, C., Rehm, J., Parrott, S., Drummond, C., et al. (2010). Modelling lifetime QALYs and health care costs from different drinking patterns over time: a Markov model. Int. J. Methods Psychiatr. Res. 19, 97–109. doi: 10.1002/mpr.306
Beaunieux, H., Ritz, L., Segobin, S., Berre, A. P. L., Lannuzel, C., Boudehent, C., et al. (2013). Alcohol-related neuropsychological deficits: an explanation of relapse? Rev. Neuropsychol. 5, 159–165.
Bolton, J., Cox, B., Clara, I., and Sareen, J. (2006). Use of alcohol and drugs to self-medicate anxiety disorders in a nationally representative sample. J. Nerv. Mental Dis. 194, 818–825. doi: 10.1097/01.nmd.0000244481.63148.98
Boschloo, L., Vogelzangs, N., Smit, J. H., Van der Brink, W., Veltman, D. J., Beekman, A. T., et al. (2011). Comorbidity and risk indicators for alcohol use disorders among persons with anxiety and/or depressive disorders: findings from the Netherlands Study of Depression and Anxiety (NESDA). J. Affect. Disord. 131, 233–242. doi: 10.1016/j.jad.2010.12.014
Bowie, C. R., Serper, M. R., Riggio, S., and Harvey, P. D. (2005). Neurocognition, symptomatology, and functional skills in older alcohol-abusing schizophrenia patients. Schizophr. Bull. 31, 175–182. doi: 10.1093/jschbul/sbi001
Brandt, G. N., and Bonelli, R. M. (2008). Structural neuroimaging of the basal ganglia in schizophrenic patients: a review. Wiener Medizinisc. Wochenschr. 158, 84–90. doi: 10.1007/s10354-007-0478-7
Brown, P. L., and Shepard, P. D. (2016). Functional evidence for a direct excitatory projection from the lateral habenula to the ventral tegmental area in the rat. J. Neurophysiol. 116, 1161–1174. doi: 10.1152/jn.00305.2016
Burns, L., and Teesson, M. (2002). Alcohol use disorders comorbid with anxiety, depression and drug use disorders: findings from the Australian national survey of mental health and well being. Drug Alcohol Depend. 68, 299–307. doi: 10.1016/S0376-8716(02)00220-X
Byne, W., Hazlett, E. A., Buchsbaum, M. S., and Kemether, E. (2009). The thalamus and schizophrenia: current status of research. Acta Neuropathol. 117, 347–368. doi: 10.1007/s00401-008-0404-0
Conner, K. R., Pinquart, M., and Gamble, S. A. (2009). Meta-analysis of depression and substance use among individuals with alcohol use disorders. J. Subst. Abuse Treat. 37, 127–137. doi: 10.1016/j.jsat.2008.11.007
Crean, R. D., Crane, N. A., and Mason, B. J. (2011). An evidence based review of acute and long-term effects of cannabis use on executive cognitive functions. J. Addict. Med. 5, 1–8. doi: 10.1097/ADM.0b013e31820c23fa
Dawson, D. A., and Grant, B. F. (2011). The “gray area” of consumption between moderate and risk drinking. J. Stud. Alcohol Drugs 72, 453–458. doi: 10.15288/jsad.2011.72.453
Dependence, D. (2008). Attentional bias in addictive behaviors: a review of its development, causes, and consequences. Drug Alcohol Depend. 97, 1–20. doi: 10.1016/j.drugalcdep.2008.03.030
Devito, E. E., Meda, S. A., Jiantonio, R., Potenza, M. N., Krystal, J. H., and Pearlson, G. D. (2013). Neural correlates of impulsivity in healthy males and females with family histories of alcoholism. Neuropsychopharmacology 38, 1854–1863. doi: 10.1038/npp.2013.92
Dichter, G. S., Felder, J. N., and Smoski, M. J. (2010). The effects of brief behavioral activation therapy for depression on cognitive control in affective contexts: an fMRI investigation. J. Affect. Disord. 126, 236–244. doi: 10.1016/j.jad.2010.03.022
Dick, D. M., Smith, G., Olausson, P., Mitchell, S. H., Leeman, R. F., O'Malley, S. S., et al. (2010). Understanding the construct of impulsivity and its relationship to alcohol use disorders. Addict. Biol. 15, 217–226. doi: 10.1111/j.1369-1600.2009.00190.x
Dom, G., Wilde, B. D., Hulstijn, W., Brink, W., and Sabbe, B. (2006). Decision-making deficits in alcohol-dependent patients with and without comorbid personality disorder. Alcohol Clini. Exp. Res. 30, 1670–1677. doi: 10.1111/j.1530-0277.2006.00202.x
Drevets, W. C., Price, J. L., Simpson, J. R., Todd, R. D., Reich, T., Vannier, M., et al. (1997). Subgenual prefrontal cortex abnormalities in mood disorders. Nature 386, 824–827. doi: 10.1038/386824a0
Dudeney, J., Sharpe, L., and Hunt, C. (2015). Attentional bias towards threatening stimuli in children with anxiety: a meta-analysis. Clin. Psychol. Rev. 40, 66–75. doi: 10.1016/j.cpr.2015.05.007
Fadardi, J. S., and Cox, W. M. (2009). Reversing the sequence: reducing alcohol consumption by overcoming alcohol attentional bias. Drug Alcohol Depend. 101, 137–145. doi: 10.1016/j.drugalcdep.2008.11.015
Fein, G., and Cardenas, V. A. (2017). P3b amplitude is not reduced in abstinent alcoholics with a current MDD. Alcohol 63, 33–42. doi: 10.1016/j.alcohol.2017.03.004
Fink, D. S., Gallaway, M. S., Tamburrino, M. B., Liberzon, I., Chan, P., Cohen, G. H., et al. (2016). Onset of alcohol use disorders and comorbid psychiatric disorders in a military cohort: are there critical periods for prevention of alcohol use disorders? Prev. Sci. 17, 1–10. doi: 10.1007/s11121-015-0624-1
Goutagny, R., Loureiro, M., Jackson, J., Chaumont, J., Williams, S., Isope, P., et al. (2013). Interactions between the Lateral Habenula and the hippocampus: implication for spatial memory processes. Neuropsychopharmacology 38, 2418–2426. doi: 10.1038/npp.2013.142
Grant, B. F., Goldstein, R. B., Saha, T. D., Chou, S. P., Jung, J., Zhang, H., et al. (2016). Epidemiology of DSM-5 alcohol use disorder: results from the national epidemiologic survey on alcohol and related conditions III. J. Clini. Psychiatry 84:3102013. doi: 10.1001/jamapsychiatry.2015.0584
Green, A. I., and Brown, E. S. (2006). Comorbid schizophrenia and substance abuse. J. Clin. Psychiatry 67:e08. doi: 10.4088/JCP.0906e08
Green, A. I., Salomon, M. S., Brenner, M. J., and Rawlins, K. (2002). Treatment of schizophrenia and comorbid substance use disorder. Curr. Drug Targets Cns Neurol. Disord. 1, 129–139. doi: 10.2174/1568007024606230
Gregory, R. J., Chlebowski, S., Kang, D., Remen, A. L., Soderberg, M. G., Stepkovitch, J., et al. (2008). A controlled trial of psychodynamic psychotherapy for co-occurring borderline personality disorder and alcohol use disorder. Psychotherapy 45:28. doi: 10.1037/0033-3204.45.1.28
Haaland, V. Ø., Esperaas, L., and Landrø, N. I. (2009). Selective deficit in executive functioning among patients with borderline personality disorder. Psychol. Med. 39, 1733–1743. doi: 10.1017/S0033291709005285
Hahn, A., Stein, P., Windischberger, C., Weissenbacher, A., Spindelegger, C., and Moser, E. (2011). Reuced resting-state functional connectivity between amygdala and orbitofrontal cortex in social anxiety disorder. Neuroimage 56, 881–889. doi: 10.1016/j.neuroimage.2011.02.064
Hasin, D. S., Stinson, F. S., Ogburn, E., and Grant, B. F. (2007). Prevalence, correlates, disability, and comorbidity of DSM-IV alcohol abuse and dependence in the United States: results from the National Epidemiologic Survey on Alcohol and Related Conditions. Arch. Gen. Psychiatry 64:830–842. doi: 10.1001/archpsyc.64.7.830
Hogarth, L., Hardy, L., Mathew, A. R., and Hitsman, B. (2018). Negative mood-induced alcohol-seeking is greater in young adults who report depression symptoms, drinking to cope, and subjective reactivity. Exp. Clin. Psychopharmacol. 26, 138–146. doi: 10.1037/pha0000177
Holgate, J. Y., and Bartlett, S. E. (2015). Early life stress, nicotinic acetylcholine receptors and alcohol use disorders. Brain Sci. 5, 258–274. doi: 10.3390/brainsci5030258
Jahng, S., Trull, T. J., Wood, P. K., Tragesser, S. L., Tomko, R., Grant, J. D., et al. (2011). Distinguishing general and specific personality disorder features and implications for substance dependence comorbidity. J. Abnorm. Psychol. 120, 656–669. doi: 10.1037/a0023539
Jeanblanc, J., Balguerie, K., Coune, F., Legastelois, R., Jeanblanc, V., and Naassila, M. (2015). Light alcohol intake during adolescence induces alcohol addiction in a neurodevelopmental model of schizophrenia. Addict. Biol. 20, 490–499. doi: 10.1111/adb.12146
Johnson, S. L., Wang, L., Alpert, K. I., Greenstein, D., Clasen, L., Lalonde, F., et al. (2013). Hippocampal shape abnormalities of patients with childhood-onset schizophrenia and their unaffected siblings. J. Am. Acad. Child Adolesc. Psychiatry 52, 527.e2–536.e2. doi: 10.1016/j.jaac.2013.02.003
Joshua, J. (2017). The Market and the Social and Private Costs of Alcohol Abuse. Cham: Springer International Publishing.
Kalén, P., Nilsson, O. G., Cenci, M. A., Rosengren, E., Lindvall, O., and Björklund, A. (1990). Intracerebral microdialysis as a tool to monitor transmitter release from grafted cholinergic and monoaminergic neurons. J. Neurosci. Methods 34, 107–115. doi: 10.1016/0165-0270(90)90048-K
Kendler, K. S., Gardner, C., and Dick, D. M. (2011). Predicting alcohol consumption in adolescence from alcohol-specific and general externalizing genetic risk factors, key environmental exposures and their interaction. Psychol. Med. 41, 1507–1516. doi: 10.1017/S003329171000190X
Kreek, M. J., and Koob, G. F. (1998). Drug dependence: stress and dysregulation of brain reward pathways. Drug Alcohol Depend. 51, 23–47. doi: 10.1016/S0376-8716(98)00064-7
Lacerda, A. L. T., Keshavan, M. S., Hardan, A. Y., Yorbik, O., Brambilla, P., Sassi, R. B., et al. (2004). Anatomic evaluation of the orbitofrontal cortex in major depressive disorder. Biol. Psychiatry 55, 353–358. doi: 10.1016/j.biopsych.2003.08.021
Lee, E. H., and Huang, S. L. (1988). Role of lateral habenula in the regulation of exploratory behavior and its relationship to stress in rats. Behav. Brain Res. 30, 265–271. doi: 10.1016/0166-4328(88)90169-6
Luo, Y. X., Xue, Y. X., Liu, J. F., Shi, H. S., Jian, M., Han, Y., et al. (2015). A novel UCS memory retrieval-extinction procedure to inhibit relapse to drug seeking. Nat. Commun. 6:7675. doi: 10.1038/ncomms8675
Manning, V., Betteridge, S., Wanigaratne, S., Best, D., Strang, J., and Gossop, M. (2009). Cognitive impairment in dual diagnosis inpatients with schizophrenia and alcohol use disorder. Schizophr. Res. 114, 98–104. doi: 10.1016/j.schres.2009.05.020
Margolese, H. C., Malchy, L., Negrete, J. C., Tempier, R., and Gill, K. (2004). Drug and alcohol use among patients with schizophrenia and related psychoses: levels and consequences. Schizophr. Res. 67, 157–166. doi: 10.1016/S0920-9964(02)00523-6
Mathis, V., and Lecourtier, L. (2017). Role of the lateral habenula in memory through online processing of information. Pharmacol. Biochem. Behav. 162, 69–78. doi: 10.1016/j.pbb.2017.07.004
Mccarter, K. L., Halpin, S. A., Baker, A. L., Kaylambkin, F. J., Lewin, T. J., Thornton, L. K., et al. (2016). Associations between personality disorder characteristics and treatment outcomes in people with co-occurring alcohol misuse and depression. BMC Psychiatry 16:210. doi: 10.1186/s12888-016-0937-z
Meszaros, Z. S., Dimmock, J. A., Ploutzsnyder, R., Abdulmalak, Y., Leontieva, L., Canfield, K., et al. (2011). Predictors of smoking severity in patients with schizophrenia and alcohol use disorders. Am. J. Addict. 20, 462–467. doi: 10.1111/j.1521-0391.2011.00150.x
Naqvi, N. H., and Bechara, A. (2010). The insula and drug addiction: an interoceptive view of pleasure, urges, and decision-making. Brain Struct. Funct. 214, 435–450. doi: 10.1007/s00429-010-0268-7
Park, H., Rhee, J., Lee, S., and Chung, C. (2017). Selectively impaired endocannabinoid-dependent long-term depression in the lateral habenula in an animal model of depression. Cell Rep. 20, 289–296. doi: 10.1016/j.celrep.2017.06.049
Peltzer, K., and Pengpid, S. (2016). Heavy drinking and social and health factors in university students from 24 low, middle income and emerging economy countries. Commun. Ment. Health J. 52, 239–244. doi: 10.1007/s10597-015-9925-x
Potvin, S., Pampoulova, T., Lipp, O., Ait Bentaleb, L., Lalonde, P., Stip, E., et al. (2008). Working memory and depressive symptoms in patients with schizophrenia and substance use disorders. Cogn. Neuropsychiatry 13, 357–366. doi: 10.1080/13546800802264330
Prince van Leeuwen, A., Creemers, H. E., Verhulst, F. C., Ormel, J., and Huizink, A. C. (2011). Are adolescents gambling with cannabis use? A longitudinal study of impulsivity measures and adolescent substance use: the TRAILS study. J. Stud. Alcohol Drugs 72, 70–78. doi: 10.15288/jsad.2011.72.70
Quelch, D. R., Mick, I., Mcgonigle, J., Ramos, A. C., Flechais, R. S. A., Bolstridge, M., et al. (2017). Nalmefene reduces reward anticipation in alcohol dependence: an experimental functional magnetic resonance imaging study. Biol. Psychiatry 81, 941–948. doi: 10.1016/j.biopsych.2016.12.029
Quervain, D. D., Schwabe, L., and Roozendaal, B. (2017). Stress, glucocorticoids and memory: implications for treating fear-related disorders. Nat. Rev. Neurosci. 18, 7–19. doi: 10.1038/nrn.2016.155
Rae, A. M., Joyce, P. R., Luty, S. E., and Mulder, R. T. (2002). The effect of a history of alcohol dependence in adult major depression. J. Affect. Disord. 70, 281–290. doi: 10.1016/S0165-0327(01)00365-2
Regier, D. A., Farmer, M. E., Rae, D. S., Locke, B. Z., Keith, S. J., Judd, L. L., et al. (1990). Comorbidity of mental disorders with alcohol and other drug abuse. Results from the Epidemiologic Catchment Area (ECA) Study. J. Am. Med. Assoc. 264:2511–2518. doi: 10.1001/jama.1990.03450190043026
Rehm, J., Mathers, C., Popova, S., Thavorncharoensap, M., Teerawattananon, Y., and Patra, J. (2009). Global burden of disease and injury and economic cost attributable to alcohol use and alcohol-use disorders. Lancet 373, 2223–2233. doi: 10.1016/S0140-6736(09)60746-7
Rose, C. A., Ehrman, R. N., Wang, Z., Yin, L., Nathan, S., Jonathan, H., et al. (2008). Prelude to passion: limbic activation by “unseen” drug and sexual cues. PLoS ONE 3:e1506. doi: 10.1371/journal.pone.0001506
Sher, L. (2006). Alcoholism and suicidal behavior: a clinical overview. Acta Psychiatr. Scand. 113, 13–22. doi: 10.1111/j.1600-0447.2005.00643.x
Shin, S. H., Lee, S., Jeon, S. M., and Wills, T. A. (2015). Childhood emotional abuse, negative emotion-driven impulsivity, and alcohol use in young adulthood. Child Abuse Negl. 50, 94–103. doi: 10.1016/j.chiabu.2015.02.010
Sinha, R. (2001). How does stress increase risk of drug abuse and relapse? Psychopharmacology 158, 343–359. doi: 10.1007/s002130100917
Smith, M. J., Wang, L., Cronenwett, W., Goldman, M. B., Mamah, D., Barch, D. M., et al. (2011). Alcohol use disorders contribute to hippocampal and subcortical shape differences in schizophrenia. Schizophr. Res. 131, 174–183. doi: 10.1016/j.schres.2011.05.014
Soloff, P. H., Meltzer, C. C., Becker, C., Greer, P. J., Kelly, T. M., and Constantine, D. (2003). Impulsivity and prefrontal hypometabolism in borderline personality disorder. Psychiatry Res. Neuroimaging 123, 153–163. doi: 10.1016/S0925-4927(03)00064-7
Solomon, R. L., and Corbit, J. D. (1974). An opponent-process theory of motivation: I. Temporal dynamics of affect. Psychol. Rev. 81, 119–145.
Stacy, A. W., and Wiers, R. W. (2010). Implicit cognition and addiction: a tool for explaining paradoxical behavior. Annu. Rev. Clin. Psychol. 6, 551–575. doi: 10.1146/annurev.clinpsy.121208.131444
Stevens, M. C., Kaplan, R. F., and Hesselbrock, V. M. (2003). Executive-cognitive functioning in the development of antisocial personality disorder. Addict. Behav. 28, 285–300. doi: 10.1016/S0306-4603(01)00232-5
Stevens, M. C., Kiehl, K. A., Pearlson, G. D., and Calhoun, V. D. (2007). Functional neural networks underlying response inhibition in adolescents and adults. Behav. Brain Res. 181, 12–22. doi: 10.1016/j.bbr.2007.03.023
Thapinta, D., Skulphan, S., Kitsumban, V., and Longchoopol, C. (2017). Cognitive behavior therapy self-help booklet to decrease depression and alcohol use among people with alcohol dependence in Thailand. Issues Ment. Health Nurs. 38, 964–970. doi: 10.1080/01612840.2017.1332700
Trull, T. J., Sher, K. J., Minksbrown, C., Durbin, J., and Burr, R. (2000). Borderline personality disorder and substance use disorders: a review and integration. Clin. Psychol. Rev. 20, 235–253. doi: 10.1016/S0272-7358(99)00028-8
Trull, T. J., Waudby, C. J., and Sher, K. J. (2004). Alcohol, tobacco, and drug use disorders and personality disorder symptoms. Exp. Clin. Psychopharmacol. 12, 65–75. doi: 10.1037/1064-1297.12.1.65
Tseng, K. Y., Chambers, R. A., and Lipska, B. K. (2009). The neonatal ventral hippocampal lesion as a heuristic neurodevelopmental model of schizophrenia. Behav. Brain Res. 204, 295–305. doi: 10.1016/j.bbr.2008.11.039
Uekermann, J., Daum, I., Schlebusch, P., Wiebel, B., and Trenckmann, U. (2003). Depression and cognitive functioning in alcoholism. Addiction 98, 1521–1529. doi: 10.1046/j.1360-0443.2003.00526.x
Vasile, D., and Vasiliu, O. (2017). Cognitive-behavioral therapy in young adults with major depression and alcohol dependence. Eur. Psychiatry 41:S397. doi: 10.1016/j.eurpsy.2017.02.459
Videbech, P. (2000). PET measurements of brain glucose metabolism and blood flow in major depressive disorder: a critical review. Acta Psychiatr. Scand. 101, 11–20. doi: 10.1034/j.1600-0447.2000.101001011.x
Vural, G. U. (1996). Single photon emission computerised tomography in chronic alcoholism. Br. J. Psychiatry 169, 348–354.
Walker, B. M., Zorrilla, E. P., and Koob, G. F. (2011). Systemic κ-opioid receptor antagonism by nor-binaltorphimine reduces dependence-induced excessive alcohol self-administration in rats. Addict. Biol. 16, 116–119. doi: 10.1111/j.1369-1600.2010.00226.x
Wang, R. Y., and Aghajanian, G. K. (1977). Physiological evidence for habenula as Major link between forebrain and midbrain raphe. Science 197, 89–91. doi: 10.1126/science.194312
Welch, K. A., Mcintosh, A. M., Job, D. E., Whalley, H. C., Moorhead, T. W., Hall, J., et al. (2011). The impact of substance use on brain structure in people at high risk of developing schizophrenia. Schizophr. Bull. 37, 1066–1076. doi: 10.1093/schbul/sbq013
World Health Organization (2011). Day Against Alcohol Abuse “Alcohol and Your Bounds - Find Them!”. World Health Organization.
Yan, F., and Roth, B. L. (2004). Salvinorin A: a novel and highly selective κ-opioid receptor agonist. Life Sci. 75, 2615–2619. doi: 10.1016/j.lfs.2004.07.008
Keywords: alcohol use disorders (AUDs), comorbidity, major depressive disorder (MDD), schizophrenia, personality disorders (PDs)
Citation: Yang P, Tao R, He C, Liu S, Wang Y and Zhang X (2018) The Risk Factors of the Alcohol Use Disorders—Through Review of Its Comorbidities. Front. Neurosci. 12:303. doi: 10.3389/fnins.2018.00303
Received: 08 October 2017; Accepted: 18 April 2018;
Published: 11 May 2018.
Edited by:
Min-Fang Kuo, Leibniz Research Centre for Working Environment and Human Factors (LG), GermanyReviewed by:
Po See Chen, National Cheng Kung University, TaiwanMirjana Ratko Jovanovic, Department of Psychiatry, Faculty of Medical Sciences, University of Kragujevac, Serbia
Catarine Conti, Federal University of Espírito Santo, Brazil
Copyright © 2018 Yang, Tao, He, Liu, Wang and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ying Wang, d3kxOTg3QHVzdGMuZWR1LmNu
Xiaochu Zhang, enhjdXN0Y0B1c3RjLmVkdS5jbg==
†Co-first author.
 Ping Yang1†
Ping Yang1† Rui Tao
Rui Tao Xiaochu Zhang
Xiaochu Zhang