
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
HYPOTHESIS AND THEORY article
Front. Neurosci. , 21 March 2018
Sec. Social and Evolutionary Neuroscience
Volume 12 - 2018 | https://doi.org/10.3389/fnins.2018.00150
This article is part of the Research Topic Language at the Interface Between the Immune System and the Brain View all 4 articles
 Guglielmo Lucchese1,2*
Guglielmo Lucchese1,2* Benjamin Stahl2,3,4,5
Benjamin Stahl2,3,4,5The present study seeks to determine potential associations between viral infections and neuropsychiatric diseases. To address this issue, we investigated the peptide commonalities between viruses that have been related to psychiatric and neurological disorders—such as rubella, human immunodeficiency virus, and herpesviruses—and human distal-less homeobox (DLX) proteins expressed in developing brain—namely, DLX1, DLX2, DLX5, and DLX6. Peptide matching analyses revealed a high degree of pentapeptide sharing. From an immunological perspective, this overlap is relevant because pentapeptides are endowed with immunogenicity and antigenicity—that is, they are immune determinants. Moreover, infection-induced immune cross-reactions might have functional, spatial, and temporal implications related to the functions and expression patterns of DLX1 and DLX5 in the fetal and adult human brain. In sum, our data support the hypothesis that viral infections may be linked to neuropsychiatric diseases through autoimmune cross-reactions caused by molecular mimicry between viral proteins and brain-specific DLX self-antigens.
Infections, neuropsychiatric diseases, and language disorders are often concomitant pathological events that can have early etiological roots in fetal life and then become apparent in any stage across the life span of the individual, from the postnatal period to the late age (Yolken and Torrey, 1995; Coplan et al., 1998; Arias et al., 2012; Brown, 2012, 2015; Khandaker et al., 2013). However, it is unclear how these pathological events are mechanistically interlinked and temporally related, most likely because the wide spectrum of infectious agents and the varying extent of the numerous neuropsychiatric symptoms make it difficult to dissect the molecular correlations between infections and brain damage (Ludlow et al., 2016).
During the past few decades, scientific-clinical research examined the assumption that infections may relate to neuropsychiatric disturbances through autoimmune mechanisms (Knuesel et al., 2014; Severance et al., 2014; Blomström et al., 2015; Estes and McAllister, 2016; de Haan et al., 2017). More recently, it was suggested that anti-pathogen immune responses cross-reacting with the human NMDA receptor 2A subunit—alterations of which are involved in language dysfunctions (Carvill et al., 2013; Turner et al., 2015)—might represent a pathologic background for infections and many neurodegenerative disorders, ranging from schizophrenia to frontotemporal dementia (Lucchese, 2016).
The current study is extended to four members of the DLX transcription factor (TF) family—namely DLX1, DLX2, DLX5, and DLX6—that have been thoroughly investigated in numerous studies on neurodevelopment. Indeed, the four TFs are expressed during early fetal neurodevelopment (Merlo et al., 2000; Panganiban and Rubenstein, 2002) and are associated with the specification of γ-aminobutyric acid (GABA)ergic interneurons in the vertebrate forebrain subventricular zone (SVZ) as well as with granule neurons in the subgranular zone (SGZ) (Simeone et al., 1994; Anderson et al., 1997a,b). The issue appears to be important especially when considering that cognitive and emotional tasks occur in the neurogenic areas (Aimone et al., 2011; Ming and Song, 2011; Miller and Hen, 2015), and that altered adult neurogenesis and hyppocampal lesions have been repeatedly related to neuropsychiatric conditions (Parent and Murphy, 2008; Gonçalves et al., 2016; Inta et al., 2016; Kang et al., 2016; Yun et al., 2016; Kohman and Rhodes, 2017) and language disturbances (Sass et al., 1992; MacKay et al., 1998; Covington and Duff, 2016; Piai et al., 2016).
In this context, we hypothesized that an infection-induced maternal immune response may cross-react with DLX proteins, thus possibly causing a first subclinical immune-mediated damage of the developing nervous system. Later, successive encounters in adulthood with pathogens able to induce cross-reactions with DLX proteins might further damage regions of the adult brain (the subventricular zone and dentate gyrus), where DLX proteins may be expressed (Lim and Alvarez-Buylla, 2016) thus triggering the onset of neuropsychiatric clinical manifestations.
Focusing on infections as a trigger of DLX alterations and seeking for a possible mechanism, we proceeded along three steps. Firstly, we investigated whether or not infectious pathogens have the molecular basis to react with human DLX proteins—that is, we searched for shared peptides that might lead to cross-reactions. Secondly, we analyzed the immunological potential of the viral vs. DLX peptide overlap. Thirdly, we collected data on the expression level of DLX proteins in the fetal and adult human brain.
The primary amino acid (aa) sequences of human DXL1 (Uniprot: P56177, 256 aa), DLX2 (Uniprot: Q07687, 328 aa), DLX5 (Uniprot: P56178, 289 aa), and DLX6 (Uniprot: P56179; 175 aa) were dissected into pentapeptides offset by one aa residue: MTMTT, TMTTM, MTTMP, and so forth. Then, each of the resulting pentapeptides was analyzed for matches to a sample library of 25 viral proteomes using the Protein Information Resource (PIR) match program (https://research.bioinformatics.udel.edu/peptidematch/batchpeptidematch.jsp) (Chen et al., 2013), as previously described (Kanduc et al., 2008; Lucchese et al., 2014; Lucchese, 2016, 2017). Neuronal Regeneration-Related Protein (NREP, Uniprot: Q16612, 68 aa) was used as a control neural protein. Glutamate decarboxylases 1 (Uniprot: Q99259; GAD-67; 594 aa) and 2 (Uniprot: Q05329; GAD-65; 585 aa) were additionally investigated as DLX-related proteins.
The virus library consisted of 25 proteomes derived from the viruses listed as follows (with Abbreviation, NCBI Tax ID, number of proteins, and aa length in parentheses): Borna disease virus (BDV; 928296; 6 proteins; 3014 aa); Epstein-Barr virus (EBV; 82830; 56 proteins; 30727 aa); Hendra virus (HeV; 928303; 9 proteins; 6956 aa); Hepatitis B virus genotype C subtype ayr (HBV-C; 928302; 5 proteins; 1792 aa); Hepatitis C virus genotype 1a (HCVH; 11108; 11 proteins; 3011 aa); Human cytomegalovirus (HCMV; 295027; 168 proteins; 63460 aa); Human herpesvirus 1 (HHV1; 10299; 73 proteins; 38368 aa); Human herpesvirus 2 (HHV2; 10315; 72 proteins; 38122 aa); Human herpesvirus 6A (HHV6A; 10370; 101 proteins; 43629 aa); Human herpesvirus 6B (HHV6B; 36351; 98 proteins; 43692 aa); Human immunodeficiency virus type 1 group M subtype A (HIV-1; 11697; 9 proteins; 3585 aa); Human parvovirus B19 (HPV-B19; 648237; 5 proteins; 1701 aa); Influenza A virus, H5N5 (IVA, H5N5; 93838; 12 proteins; 4809 aa); Influenza A virus, H1N1 (IVA, H1N1; 211044; 12 proteins; 4788 aa); Influenza A virus, H7N7 (IVA, H7N7; 384493; 12 proteins; 4802 aa); Influenza B virus (FLUBV; 518987; 11 proteins; 4718 aa); Influenza C virus (FLUCV; 11553; 8 proteins; 4259 aa); Measles virus (MeV; 645098; 8 proteins; 5202 aa); Rotavirus A (RV-A; 450149; 12 proteins; 5897 aa); Rotavirus C (RV-C; 31567; 11 proteins; 5608 aa); Rotavirus X (RV ADRV-N; 335103; 11 proteins; 5679 aa); Rubella virus (RUBV; 11045; 5 proteins; 3179 aa); Vaccinia virus (VACV; 10254; 217 proteins; 56795 aa); Varicella-zoster virus (HHV-3; 10338; 69 proteins; 35782 aa); and Zika virus (ZIKV; 64320; 13 proteins; 3419 aa).
The peptide sharing was investigated for immunologic potential using the Immune Epitope Database (IEDB; www.iedb.org) resource (Vita et al., 2015). Epitopes that have been experimentally validated as immunopositive in the human host were considered.
Laser microdissection microarray data on DLXs and NREP transcript expression in fetal and adult human brain were obtained from the online database of the Allen Institute for Brain Science (http://www.brainspan.org/; http://human.brain-map.org/) (Lein et al., 2007; Miller et al., 2014). Data on DLX and NREP protein expression in adult human brain were retrieved from https://www.proteinatlas.org/humanproteome (Uhlén et al., 2015; Thul et al., 2017).
A sample library formed by 25 virus proteomes was analyzed for pentapeptide sharing with DLX proteins. Pentapeptides were used as probes, for five aa residues represent a minimal immune-biological determinant in humoral and cellular immune recognition (Kanduc, 2012, 2013 and further refs. therein). NREP, a protein involved in neuronal regeneration (Fujitani et al., 2004), was used as a neural control protein.
The analyzed viral set consisted of pathogens that have been reported in studies on language disorders and other cognitive dysfunctions, bipolar disorders, and schizophrenia (Yolken and Torrey, 1995; Coplan et al., 1998; Brown et al., 2004, 2006; Torrey et al., 2006; Baillargeon et al., 2008; Buka et al., 2008; Mortensen et al., 2010; Arias et al., 2012; Brown, 2012; Hornig et al., 2012; Khandaker et al., 2013; Blomström et al., 2015; Canuti et al., 2015; Lucchese, 2016; Ludlow et al., 2016; Soltani et al., 2016).
The quantitative and qualitative pentapeptide overlap between the four human TF DLX proteins and NREP vs. the set of 25 virus proteomes is shown in Table 1.
At a first glance, the following points become apparent when considering Table 1:
• the neural proteins, including the neuronal regeneration-related protein NREP, have pentapeptides in common with all viral proteomes, excluding Borna disease virus and Influenza B virus;
• DLX2 is the main target of the peptide sharing by being 49% the level of DLX2 pentapeptide similarity to the 25 proteomes, i.e., 159 out of 324 DLX2 pentapeptides are shared with the viral proteomic ensemble;
• the peptide sharing mostly occurs with herpesviruses in general, and with HCMV in particular. Instead, the peptide sharing with HeV, HBV-C, RV-C, and RV ADRV-N is restricted to a few pentapeptides thus indicating a scarce contribution of such infectious agents in crossreactivity-triggered DLX alterations and consequent neurological manifestations;
• the viral pentapeptide distribution is not stochastic. For example, Vaccinia virus pentapeptides represent 27% of NREP peptide sharing (i.e., 3 out of 13 pentapeptides) and 4.4% of the DLX2 peptide sharing (i.e., 7 out of 159 pentapeptides);
• the extent of the peptide sharing is independent of the virus protein length. For example, Rubella virus (3,179 aa) and the eleven times longer HHV3 (35,782 aa) share exactly the same number of pentapeptides—namely, three—with DLX1 protein;
• quantitatively, the extent of the peptide sharing is unexpectedly high compared to the mathematical expected value of the pentapeptide sharing between the five neural proteins and the 25 viral proteomes. The expected value can be calculated as follows: given two protein entities (for example, DLX1, and the set of 25 viral proteomes) and assuming that all aa occur with the same frequency, the expected probability for the two entities to share a pentapeptide is expressed by the formula
where: m is the number of pentapeptides present in the DLX1 protein (i.e., 251); n is the number of pentapeptides present in the set formed by the 25 viral proteomes (i.e., 418,854), and N is the number of all possible pentapeptides. Since each residue can be any of 20 aa, then N is 205 (i.e., 3,200,000). Developing the equation, the expected pentapeptide sharing between DLX1 and the 25 viral proteomes is equal to 1,02668314453125e-5 or, practically, zero.
The pentapeptide matching between viral and neural proteins illustrated in Table 1 has an experimentally documented immunologic potential. As a matter of fact, exploration of the Immune Epitope DataBase (IEDB; www.immuneepitope.org; Vita et al., 2015) shows that almost all shared pentapeptides are also part of hundreds of epitopes that have been experimentally validated as immunopositive in humans. Using the epitope aa length as a filter, Table 2 is restricted to n-mer sequences with n < 12.
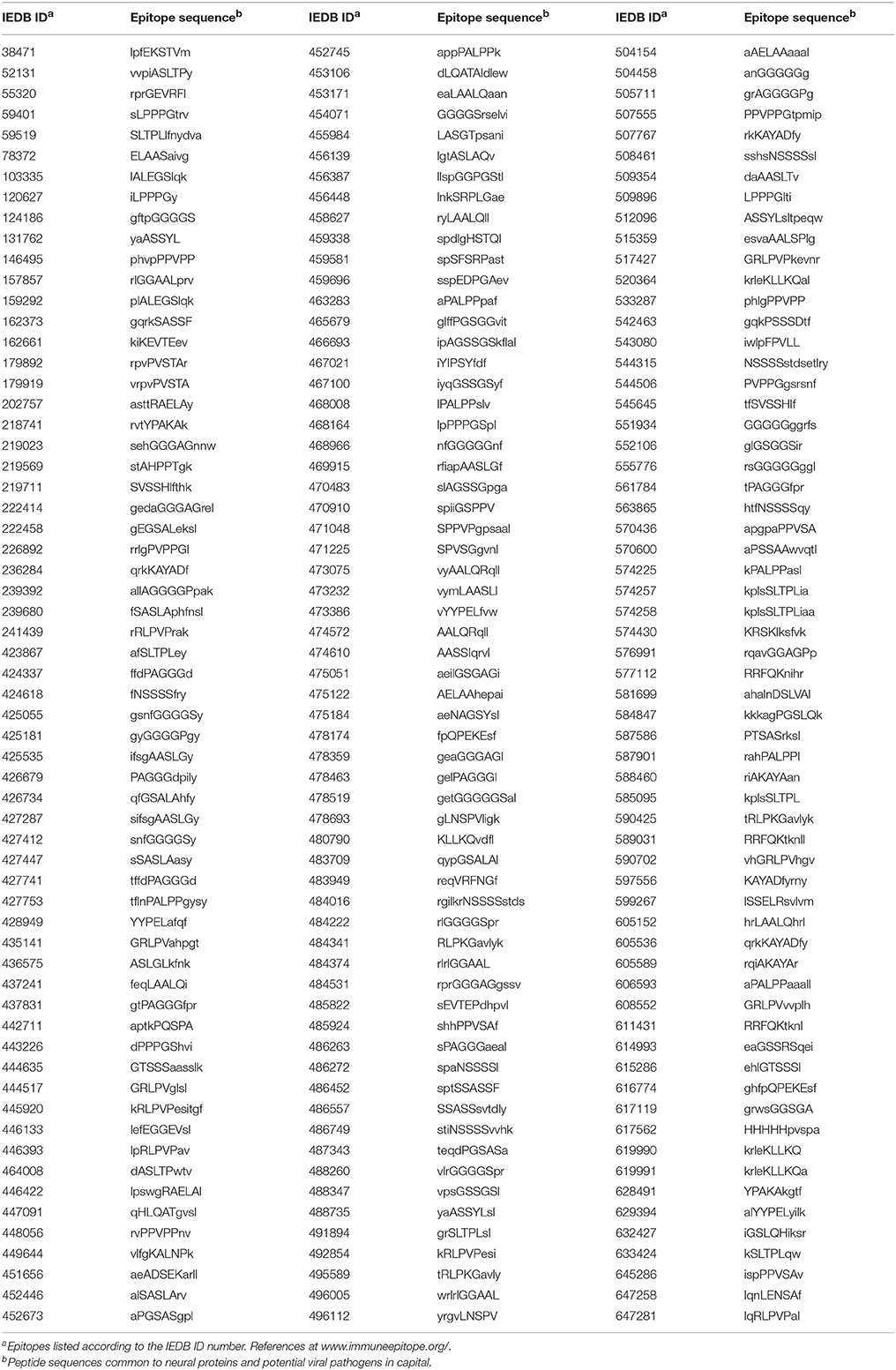
Table 2. Epitopes experimentally validated as immunopositive in the human host and containing pentapeptides common to the analyzed neural and viral proteins.
A comparative analysis of DLX expression in fetal and adult neurogenic areas of the human brain was conducted using the online database and resources of the Allen Institute for Brain Science (Lein et al., 2007; Miller et al., 2014). Figure 1 reports laser microdissection microarray analyses showing that the transcript expression of the four TFs ranges from medium to high in the fetal transient structures of forebrain (ventricular zone and ganglionic eminence) (Figure 1A), and reaches the lowest but still detectable value in the adult neurogenic dentate gyrus (Figure 1B, subareas CA1, CA2, CA3, and CA4). Notably, only DLX5 and DLX6 appear to be expressed in basal ganglia of adult brain, that is, substantia innominata, caudate nucleus, nucleus accumbens, and putamen (Figure 1B). The control neuronal regeneration-related protein NREP is widely expressed through almost all fetal and adult brain areas, except the fetal ventricular zone and ganglionic eminence (Figure 1A) as well as the dentate gyrus area in the adult brain (Figure 1B).
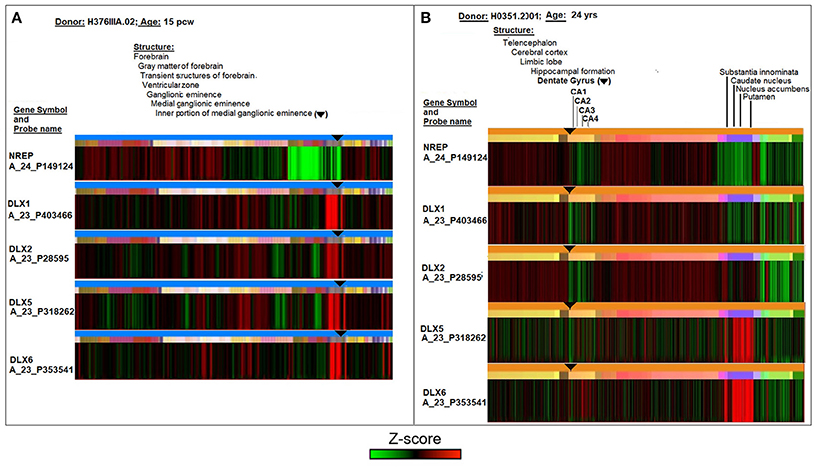
Figure 1. Comparative analyses of DLX1, DLX2, DLX5, and DLX6 transcript expression in fetal (15 post-conception weeks, pcw; A) and adult (24 years; B) human brain. The symbol ▾ localizes neurogenic areas of developing and adult brain. The figure assembles images and data from the Allen Institute. Further details on donors, DNA probes, complete transcriptome profiles, and methodology can be found at http://www.brainspan.org/ and http://human.brain-map.org/ (Lein et al., 2007; Miller et al., 2014).
On the whole, Figure 1 theoretically supports the possibility that the cross-reactivity scenario outlined in Tables 1, 2 may occur in neurogenic areas in the fetal life of an individual and then possibly recur in adulthood. However, from an immunological point of view, a condition that is necessary for the cross-reactivity hypothesis to be biologically plausible depends on sufficient DLX antigen expression in the brain. In other words, data of Figure 1 need to be substantiated in a protein context.
Actually, few data are available on DLX protein expression in humans. Rakic and Zecevic (2003) studied DLX expression in the late human embryonic period (Carnegie stages 19–20) and showed that DLX2 protein was widely distributed through the ganglionic eminence and dorsal telencephalon. Moreover, immunocytochemistry based on a pan-DLX antibody that recognizes DLX 1, 2, 5, and 6 revealed that, in the developing brain, 11 gestational week, DLX protein expression is present in all cortical layers, including layer I and the subpial granular layer (SGL). Almost all small GABAergic cells of the SGL were labeled with the pan-DLX antibody. Successively, using the same pan-DLX antibody, Jakovcevski et al. (2011) showed labeling of the neocortical VZ/SVZ and of the cortical plate in human fetal forebrains during the first half of gestation.
Such experimental results obtained in human fetal developing brain are flanked by data collected from the Human Protein Atlas (https://www.proteinatlas.org/on DLX1 and DLX5 protein expression in the adult human brain. The data are shown in Figure 2. It can be seen that human DLX1 and DLX5 have a protein expression from low to medium level, that is, sufficient to sustain immune cross-reactions. No protein expression data were available for DLX2 and DLX6 proteins. The control NREP had the highest levels of protein expression (from medium to high in the cerebral cortex and the cerebellum).
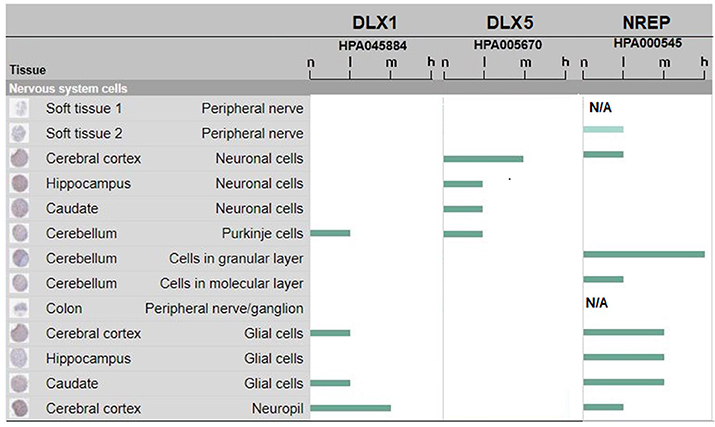
Figure 2. DLX1, DLX5, and NREP protein expression in adult human brain. Estimate of the protein expression are: not detected (n), low (l), medium (m), or high (h). Data for DLX2 and DLX6 proteins were not available or pending. The figure assembles images and data from www.proteinatlas.org (Uhlén et al., 2015; Thul et al., 2017).
In essence, we found a vast and unexpected peptide sharing between DLX proteins and numerous infectious agents that constellate human life, from prenatal time to adulthood. The peptide platform defined in Table 1 has also a high immunologic potential, as documented in Table 2, so that, on the whole, data from Tables 1, 2 show the existence of a wide immunologic peptide platform common to viral and human DLX proteins. Moreover, data on protein expression from literature (Rakic and Zecevic, 2003; Jakovcevski et al., 2011) and Figure 2 (www.proteinatlas.org; Uhlén et al., 2015; Thul et al., 2017), although few in numbers and fragmentary, support the possibility that mild, subclinical anti-DLX autoimmune damage in the fetal brain structures evoked by maternal viral infections (and consequent maternal immune activation) may be followed in the adult brain by additional damage after a second encounter with the same pathogen or novel infection with a different agent sharing the same epitopic sequences.
In this context, different immunological pathogenic mechanisms might be theoretically account for the neuronal damage according to the type of immune response, i.e., humoral vs. cell-mediated, and the timing of infection-induce maternal immune activation in relationship to the expression patterns of DLX proteins in the fetus (see Figure 1). The main hypothesis that we considered here relates to maternal infection and consequent immune activation that may also be antecedent to pregnancy and are followed by antibody–mediated neuronal damage in the fetus due to cross-reactions with DLX proteins. In such a scenario, the passage in the fetus of maternal memory B cells (Vernochet et al., 2005, 2007) against epitopes shared between the pathogen(s) and DLX proteins might induce an immune response targeting the developing nervous systems, where DLX proteins are expressed early (see Figure 1). Cellular damage from (auto)antibodies targeting intracellular antigens, like the DLX family of TFs may be, not only plays a pathogenic role in a variety of autoimmune diseases (Racanelli et al., 2011) but even represents a promising therapeutic strategy for cancer treatment (Weisbart et al., 2012; Wang et al., 2015; Chan et al., 2016). The notion that autoantibodies can penetrate living cells is not new. Alarcon-Segovia et al. (1978) showed that antibodies can penetrate living cells. In more recent years, more evidence has accumulated showing autoantibody penetration into different types cell, including neurons, and proposing mechanisms that may explain a pathogenic role of internalized immunoglobulins in autoimmune diseases (Koren et al., 1995; Koscec et al., 1997; Portales-Pérez et al., 1998; Ruíz-Argüelles et al., 1998, 2003, 2007; Ronda et al., 2002; Proulx et al., 2009). Moreover, a nuclear-penetrating lupus anti-DNA autoantibody, 3E10, has been found to inhibit DNA repair and selectively kill certain cancer cells that are highly vulnerable to DNA damage (Weisbart et al., 2012), and, of special importance, nuclear-penetrating anti-dsDNA autoantibodies could possibly function as a pathogenic autoimmune factor for lupus nephritis (Im et al., 2015). Bearing even more relevance to the present discussion, antibodies targeting intracellular antigens, like for instance the glutamic acid decarboxylase, appear to be also involved specifically in neuropsychiatric disorders, like CNS lupus (Karimifar et al., 2013), limbic encephalitis (Matà et al., 2008), schizophrenia (Najjar et al., 2012), and autism (Rout et al., 2012). Indeed, the glutamic acid decarboxylase isoforms (Gad1 and Gad2), which regulate GABA synthesis from the excitatory neurotransmitter glutamate and whose expression is activated by DLX1 and/or DLX2 (Stühmer et al., 2002a,b; Le et al., 2017), share numerous pentapeptides with the 25 viral proteomes (see Supplementary Table 1). Hence, a scenario emerges where immune responses following infections might cause a cascade of multiple crossreactions at multiple levels (i.e., DLX, GAD) of the intracellular mechanisms regulating the function of GABAergic neurons and altering the excitation and inhibition ratio, which is necessary for normal neural circuit function and whose imbalance contributes to neurodevelopmental diseases (Kang, 2017; Maffei et al., 2017; Ye and Kaszuba, 2017; Catavero et al., 2018; Garret et al., 2018).
On the other hand, a cell-mediated mechanism could also theoretically be implied in the cross-reactive immune-mediated subclinical damage of the fetal nervous systems, since memory T-cell trafficking between mother and fetus is also a well-known phenomenon (Jeanty et al., 2014). Nevertheless, the hypothesis of a cell-mediated response would need to take into account the late MHC expression in the fetal CNS (Elmer and McAllister, 2012; Zhang et al., 2013, 2015; McAllister, 2014) that might not sit well with the very early pattern of expression of the DLX-proteins in the fetus seen in Figure 1. However a later cell-mediated damage, and even the possible occurrence of both humoral and cell-mediated responses at different stages of the fetal neural development, can still be hypothesized.
Based on data from Figure 2 and, consequently, confining our discussion to DLX1 and DLX5 proteins, we observe that infection-induced immune cross-reactions might have functional, spatial, and temporal implications:
• Functionally, infection-induced immune cross-reactions would affect two TFs that, according to numerous studies, are implicated in crucial functions and fundamental processes during neurodevelopment and adult neurogenesis, and are potentially relevant to language competence and other higher cognitive functions (see Box 1);
• Spatially, infection-induced immune cross-reactions would damage brain structures where adult neurogenesis occurs and that are involved in the neural circuitry of language and memory, and in cognitive and emotional functions (Ming and Song, 2011). Altered human neurogenesis is linked to neuropsychiatric conditions and to impaired cognition (Aimone et al., 2014). Also, alterations of the SVZ and hippocampus have been specifically related to some of the pathogenic and symptomatic aspects of schizophrenia (Reif et al., 2006, 2007; Duan et al., 2007; Toro and Deakin, 2007; Christian et al., 2010; Aimone et al., 2014; Allen et al., 2016; Kang et al., 2016; Yun et al., 2016; Iannitelli et al., 2017; Terrillion et al., 2017).
• Temporally, infection-induced immune cross-reactions suggest a two-hit model that, depending on the DLX protein expression profiles, comprehends targets allocated in two time-windows in the life of an individual with a subclinical damage in fetal life and clinical onset in adulthood.
Box 1. DLX1 and DLX5 functions and relevance to neuropsychiatric disturbances.
DLX1:
• regulates the development of the ventral forebrain, craniofacial patterning, and the early diencephalic subdivision (Eisenstat et al., 1999; Merlo et al., 2000; Letinic and Rakic, 2001; Letinic et al., 2002).
• regulates neurite maturation and migration (Cobos et al., 2007) and interneuron differentiation (Wonders and Anderson, 2005).
• regulates the fate switch between cortical and striatal interneurons: cells that ordinarily would become cortical interneurons transform toward a subtype of GABAergic striatal interneurons, thus reducing glutamatergic input to the hippocampus (McKinsey et al., 2013).
• its loss leads to subtype-specific loss of inhibitory interneurons with a reduction in inhibitory currents and generalized seizures in mice (Cobos et al., 2005; Jones et al., 2011).
• contributes to promote cortical interneuron migration from the basal forebrain by direct repression of the semaphorin receptor neuropilin-2 (Le et al., 2017).
• when altered, might be related to Mowat-Wilson syndrome (McKinsey et al., 2013).
• is downregulated or altered in autism spectrum disorders (ASD; Liu et al., 2009; Voineagu et al., 2011; Benítez-Burraco et al., 2016).
• has low thalamic expression in psychosis (Kromkamp et al., 2003).
• has been proposed as a language-associated protein (Boeckx and Benítez-Burraco, 2014; Benítez-Burraco et al., 2016; Murphy and Benítez-Burraco, 2016, 2017) and relates to language deficits in schizophrenia (Murphy and Benítez-Burraco, 2016).
DLX5:
• promotes neuronal differentiation in SVZ (Shu et al., 2010).
• its loss leads to defective neuronogenesis (Perera et al., 2004).
• contributes to convert fibroblasts into induced GABAergic interneurons (Colasante et al., 2015).
• regulates development of peripheral and central components of the olfactory system (Long et al., 2003).
• is a candidate genes for autism (Nakashima et al., 2010).
• is involved in Rett syndrome (Itaba-Matsumoto et al., 2007; Itoh et al., 2007).
• participates to the regulation of the expression of the glutamic acid decarboxylases (Stühmer et al., 2002a).
• its loss preferentially reduces the number of mature parvalbumin- interneurons (Wang et al., 2010).
In synthesis, the present study confirms previous reports (Kanduc et al., 2008; Lucchese et al., 2014; Lucchese, 2017) and supports the hypothesis that an autoimmune connection exists at the molecular level among infections, autoimmune reactions and neuropsychiatric disorders. Such a connection implies a vast viral vs. neural proteins peptide overlap and operates through cross-reactivity mechanisms. To test this hypothesis in vivo, the possibility of obtaining animal models of neuropsychiatric disorders by immunizing pregnant animals with DLX proteins could be examined. Moreover, analyses of sera from human patients with neuropsychiatric diseases, for example schizophrenia, are warranted to measure immunoreactivity against the peptides shared between viruses and DLX proteins. Possibly, the results of these joint basic and clinical in vivo approaches might also help design new therapeutic approaches in neuropsychiatry.
All authors listed have made a substantial, direct and intellectual contribution to the work, and approved it for publication.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
We acknowledge support by the German Research Foundation and the OpenAccess Publication Funds of the Freie Universität Berlin. We thank the two reviewers for the constructive criticism that contributed to increase the clarity and quality of this article.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnins.2018.00150/full#supplementary-material
Aimone, J. B., Deng, W., and Gage, F. H. (2011). Resolving new memories: a critical look at the dentate gyrus, adult neurogenesis, and pattern separation. Neuron 70, 589–596. doi: 10.1016/j.neuron.2011.05.010
Aimone, J. B., Li, Y., Lee, S. W., Clemenson, G. D., Deng, W., and Gage, F. H. (2014). Regulation and function of adult neurogenesis: from genes to cognition. Physiol. Rev. 94, 991–1026. doi: 10.1152/physrev.00004.2014
Alarcon-Segovia, D., Ruiz-Arguelles, A., and Fishbein, E. (1978). Antibody to nuclear ribonucleoprotein penetrates live human mononuclear cells through Fc receptors. Nature 271, 67–69. doi: 10.1038/271067a0
Allen, K. M., Fung, S. J., and Weickert, C. S. (2016). Cell proliferation is reduced in the hippocampus in schizophrenia. Aust. N. Z. J. Psychiatry 50, 473–480. doi: 10.1177/0004867415589793
Anderson, S. A., Eisenstat, D. D., Shi, L., and Rubenstein, J. L. (1997a). Interneuron migration from basal forebrain to neocortex: dependence on Dlx genes. Science 278, 474–476. doi: 10.1126/science.278.5337.474
Anderson, S. A., Qiu, M., Bulfone, A., Eisenstat, D. D., Meneses, J., Pedersen, R., et al. (1997b). Mutations of the homeobox genes Dlx-1 and Dlx-2 disrupt the striatal subventricular zone and differentiation of late born striatal neurons. Neuron 19, 27–37. doi: 10.1016/S0896-6273(00)80345-1
Arias, I., Sorlozano, A., Villegas, E., de Dios Luna, J., McKenney, K., Cervilla, J., et al. (2012). Infectious agents associated with schizophrenia: a meta-analysis. Schizophr. Res. 136, 128–136. doi: 10.1016/j.schres.2011.10.026
Baillargeon, J. G., Paar, D. P., Wu, H., Giordano, T. P., Murray, O., Raimer, B. G., et al. (2008). Psychiatric disorders, HIV infection and HIV/hepatitis co-infection in the correctional setting. AIDS Care 20, 124–129. doi: 10.1080/09540120701426532
Benítez-Burraco, A., Lattanzi, W., and Murphy, E. (2016). Language impairments in ASD resulting from a failed domestication of the human brain. Front. Neurosci. 10:373. doi: 10.3389/fnins.2016.00373
Blomström, Ä., Gardner, R. M., Dalman, C., Yolken, R. H., and Karlsson, H. (2015). Influence of maternal infections on neonatal acute phase proteins and their interaction in the development of non-affective psychosis. Transl. Psychiatry 5:e502. doi: 10.1038/tp.2014.142
Boeckx, C., and Benítez-Burraco, A. (2014). Globularity and language-readiness: generating new predictions by expanding the set of genes of interest. Front. Psychol. 5:1324. doi: 10.3389/fpsyg.2014.01324
Brown, A. S. (2012). Epidemiologic studies of exposure to prenatal infection and risk of schizophrenia and autism. Dev. Neurobiol. 72, 1272–1276. doi: 10.1002/dneu.22024
Brown, A. S. (2015). The Kraepelinian dichotomy from the perspective of prenatal infectious and immunologic insults. Schizophr. Bull. 41, 786–791. doi: 10.1093/schbul/sbv063
Brown, A. S., Begg, M. D., Gravenstein, S., Schaefer, C. A., Wyatt, R. J., Bresnahan, M., et al. (2004). Serologic evidence of prenatal influenza in the etiology of schizophrenia. Arch. Gen. Psychiatry 61, 774–780. doi: 10.1001/archpsyc.61.8.774
Brown, A. S., Schaefer, C. A., Quesenberry, C. P. Jr., Shen, L., and Susser, E. S. (2006). No evidence of relation between maternal exposure to herpes simplex virus type 2 and risk of schizophrenia? Am. J. Psychiatry 163, 2178–2180. doi: 10.1176/ajp.2006.163.12.2178
Buka, S. L., Cannon, T. D., Torrey, E. F., and Yolken, R. H. (2008). Maternal exposure to herpes simplex virus and risk of psychosis among adult offspring. Biol. Psychiatry 63, 809–815. doi: 10.1016/j.biopsych.2007.09.022
Canuti, M., Buka, S., Jazaeri Farsani, S. M., Oude Munnink, B. B., Jebbink, M. F., van Beveren, N. J., et al. (2015). Reduced maternal levels of common viruses during pregnancy predict offspring psychosis: potential role of enhanced maternal immune activity? Schizophr. Res. 166, 248–254. doi: 10.1016/j.schres.2015.04.037
Carvill, G. L., Regan, B. M., Yendle, S. C., O'Roak, B. J., Lozovaya, N., Bruneau, N., et al. (2013). GRIN2A mutations cause epilepsy-aphasia spectrum disorders. Nat. Genet. 45, 1073–1076. doi: 10.1038/ng.2727
Catavero, C., Bao, H., and Song, J. (2018). Neural mechanisms underlying GABAergic regulation of adult hippocampal neurogenesis. Cell Tissue Res. 371, 33–46. doi: 10.1007/s00441-017-2668-y
Chan, G., Jordaan, G., Nishimura, R. N., and Weisbart, R. H. (2016). Combining intracellular antibodies to restore function of mutated p53 in cancer. Int. J. Cancer 138, 182–186. doi: 10.1002/ijc.29685
Chen, C., Li, Z., Huang, H., Suzek, B. E., and Wu, C. H. (2013). A fast peptide match service for uniprot knowledgebase. Bioinformatics 29, 2808–2809. doi: 10.1093/bioinformatics/btt484
Christian, K., Song, H., and Ming, G. L. (2010). Adult neurogenesis as a cellular model to study schizophrenia. Cell Cycle 9, 636–637. doi: 10.4161/cc.9.4.10932
Cobos, I., Borello, U., and Rubenstein, J. L. (2007). Dlx transcription factors promote migration through repression of axon and dendrite growth. Neuron 54, 873–888. doi: 10.1016/j.neuron.2007.05.024
Cobos, I., Calcagnotto, M. E., Vilaythong, A. J., Thwin, M. T., Noebels, J. L., Baraban, S. C., et al. (2005). Mice lacking Dlx1 show subtype-specific loss of interneurons, reduced inhibition and epilepsy. Nat. Neurosci. 8, 1059–1068. doi: 10.1038/nn1499
Colasante, G., Lignani, G., Rubio, A., Medrihan, L., Yekhlef, L., Sessa, A., et al. (2015). Rapid conversion of fibroblasts into functional forebrain GABAergic interneurons by direct genetic reprogramming. Cell Stem Cell 17, 719–734. doi: 10.1016/j.stem.2015.09.002
Coplan, J., Contello, K. A., Cunningham, C. K., Weiner, L. B., Dye, T. D., Roberge, L., et al. (1998). Early language development in children exposed to or infected with human immunodeficiency virus. Pediatrics 102:e8. doi: 10.1542/peds.102.1.e8
Covington, N. V., and Duff, M. C. (2016). Expanding the language network: direct contributions from the hippocampus. Trends Cogn. Sci. 20, 869–870. doi: 10.1016/j.tics.2016.10.006
de Haan, P., Klein, H. C., and t Hart, B. A. (2017). Autoimmune aspects of neurodegenerative and psychiatric diseases: a template for innovative therapy. Front. Psychiatry 8:46. doi: 10.3389/fpsyt.2017.00046
Duan, X., Chang, J. H., Ge, S., Faulkner, R. L., Kim, J. Y., Kitabatake, Y., et al. (2007). Disrupted-in-schizophrenia 1 regulates integration of newly generated neurons in the adult brain. Cell 130, 1146–1158. doi: 10.1016/j.cell.2007.07.010
Eisenstat, D. D., Liu, J. K., Mione, M., Zhong, W., Yu, G., Anderson, S. A., et al. (1999). DLX-1, DLX-2, and DLX-5 expression define distinct stages of basal forebrain differentiation. J. Comp. Neurol. 414, 217–237. doi: 10.1002/(SICI)1096-9861(19991115)414:2<217::AID-CNE6>3.0.CO;2-I
Elmer, B. M., and McAllister, A. K. (2012). Major histocompatibility complex class I proteins in brain development and plasticity. Trends Neurosci. 35, 660–670. doi: 10.1016/j.tins.2012.08.001
Estes, M. L., and McAllister, A. K. (2016). Maternal immune activation: implications for neuropsychiatric disorders. Science 353, 772–777. doi: 10.1126/science.aag3194
Fujitani, M., Yamagishi, S., Che, Y. H., Hata, K., Kubo, T., Ino, H., et al. (2004). P311 accelerates nerve regeneration of the axotomized facial nerve. J. Neurochem. 91, 737–744. doi: 10.1111/j.1471-4159.2004.02738.x. [Epub ahead of print].
Garret, M., Du, Z., Chazalon, M., Cho, Y. H., and Baufreton, J. (2018). Alteration of GABAergic neurotransmission in Huntington's disease. CNS Neurosci. Ther. doi: 10.1111/cns.12826
Gonçalves, J. T., Schafer, S. T., and Gage, F. H. (2016). Adult neurogenesis in the hippocampus: from stem cells to behavior. Cell 167, 897–914. doi: 10.1016/j.cell.2016.10.021
Hornig, M., Briese, T., Licinio, J., Khabbaz, R. F., Altshuler, L. L., Potkin, S. G., et al. (2012). Absence of evidence for bornavirus infection in schizophrenia, bipolar disorder and major depressive disorder. Mol. Psychiatry 17, 486–493. doi: 10.1038/mp.2011.179
Iannitelli, A., Quartini, A., Tirassa, P., and Bersani, G. (2017). Schizophrenia and neurogenesis: a stem cell approach. Neurosci. Biobehav. Rev. 80, 414–442. doi: 10.1016/j.neubiorev.2017.06.010
Im, S. R., Im, S. W., Chung, H. Y., Pravinsagar, P., and Jang, Y. J. (2015). Cell- and nuclear-penetrating anti-dsDNA autoantibodies have multiple arginines in CDR3 of VH and increase cellular level of pERK and Bcl-2 in mesangial cells. Mol. Immunol. 67, 377–387. doi: 10.1016/j.molimm.2015.06.025
Inta, D., Lang, U. E., Borgwardt, S., Meyer-Lindenberg, A., and Gass, P. (2016). Adult neurogenesis in the human striatum: possible implications for psychiatric disorders. Mol. Psychiatry 21, 446–447. doi: 10.1038/mp.2016.8
Itaba-Matsumoto, N., Maegawa, S., Yamagata, H., Kondo, I., Oshimura, M., and Nanba, E. (2007). Imprinting status of paternally imprinted DLX5 gene in Japanese patients with Rett syndrome. Brain Dev. 29, 491–495. doi: 10.1016/j.braindev.2007.01.006
Itoh, M., Ide, S., Takashima, S., Kudo, S., Nomura, Y., Segawa, M., et al. (2007). Methyl CpG-binding protein 2 (a mutation of which causes Rett syndrome) directly regulates insulin-like growth factor binding protein 3 in mouse and human brains. J. Neuropathol. Exp. Neurol. 66, 117–123. doi: 10.1097/nen.0b013e3180302078
Jakovcevski, I., Mayer, N., and Zecevic, N. (2011). Multiple origins of human neocortical interneurons are supported by distinct expression of transcription factors. Cereb. Cortex 21, 1771–1782. doi: 10.1093/cercor/bhq245
Jeanty, C., Derderian, S. C., and Mackenzie, T. C. (2014). Maternal-fetal cellular trafficking: clinical implications and consequences. Curr. Opin. Pediatr. 26, 377–382. doi: 10.1097/MOP.0000000000000087
Jones, D. L., Howard, M. A., Stanco, A., Rubenstein, J. L., and Baraban, S. C. (2011). Deletion of Dlx1 results in reduced glutamatergic input to hippocampal interneurons. J. Neurophysiol. 105, 1984–1991. doi: 10.1152/jn.00056.2011
Kanduc, D. (2012). Homology, similarity, and identity in peptide epitope immunodefinition. J. Pept. Sci. 18, 487–494. doi: 10.1002/psc.2419
Kanduc, D. (2013). Pentapeptides as minimal functional units in cell biology and immunology. Curr. Protein Pept. Sci. 14, 111–120. doi: 10.2174/1389203711314020003
Kanduc, D., Stufano, A., Lucchese, G., and Kusalik, A. (2008). Massive peptide sharing between viral and human proteomes. Peptides 29, 1755–1766. doi: 10.1016/j.peptides.2008.05.022
Kang, E., Wen, Z., Song, H., Christian, K. M., and Ming, G. L. (2016). Adult neurogenesis and psychiatric disorders. Cold Spring Harb. Perspect. Biol. 8:a019026. doi: 10.1101/cshperspect.a019026
Kang, J. Q. (2017). Defects at the crossroads of GABAergic signaling in generalized genetic epilepsies. Epilepsy Res. 137, 9–18. doi: 10.1016/j.eplepsyres.2017.08.013
Karimifar, M., Sharifi, I., and Shafiey, K. (2013). Anti-ribosomal P antibodies related to depression in early clinical course of systemic lupus erythematosus. J. Res. Med. Sci. 18, 860–864.
Khandaker, G. M., Zimbron, J., Lewis, G., and Jones, P. B. (2013). Prenatal maternal infection, neurodevelopment and adult schizophrenia: a systematic review of population-based studies. Psychol. Med. 43, 239–257. doi: 10.1017/S0033291712000736
Knuesel, I., Chicha, L., Britschgi, M., Schobel, S. A., Bodmer, M., Hellings, J. A., et al. (2014). Maternal immune activation and abnormal brain development across CNS disorders. Nat. Rev. Neurol. 10, 643–660. doi: 10.1038/nrneurol.2014.187
Kohman, R. A., and Rhodes, J. S. (2017). The contribution of adult hippocampal neurogenesis to the progression of psychiatric disorders. Modern Trends Pharmacopsychol. 31, 124–151. doi: 10.1159/000470812
Koren, E., Koscec, M., Wolfson-Reichlin, M., Ebling, F. M., Tsao, B., Hahn, B. H., et al. (1995). Murine and human antibodies to native DNA that cross-react with the A and D SnRNP polypeptides cause direct injury of cultured kidney cells. J. Immunol. 154, 4857–4864.
Koscec, M., Koren, E., Wolfson-Reichlin, M., Fugate, R. D., Trieu, E., Targoff, I. N., et al. (1997). Autoantibodies to ribosomal P proteins penetrate into live hepatocytes and cause cellular dysfunction in culture. J. Immunol. 159, 2033–2041.
Kromkamp, M., Uylings, H. B., Smidt, M. P., Hellemons, A. J., Burbach, J. P., and Kahn, R. S. (2003). Decreased thalamic expression of the homeobox gene DLX1 in psychosis. Arch. Gen. Psychiatry 60, 869–874. doi: 10.1001/archpsyc.60.9.869
Le, T. N., Zhou, Q. P., Cobos, I., Zhang, S., Zagozewski, J., Japoni, S., et al. (2017). GABAergic interneuron differentiation in the basal forebrain is mediated through direct regulation of glutamic acid decarboxylase isoforms by Dlx homeobox transcription factors. J. Neurosci. 37, 8816–8829. doi: 10.1523/JNEUROSCI.2125-16.2017
Lein, E. S., Hawrylycz, M. J., Ao, N., Ayres, M., Bensinger, A., Bernard, A., et al. (2007). Genome-wide atlas of gene expression in the adult mouse brain. Nature 445, 168–176. doi: 10.1038/nature05453
Letinic, K., and Rakic, P. (2001). Telencephalic origin of human thalamic GABAergic neurons. Nat. Neurosci. 4, 931–936. doi: 10.1038/nn0901-931
Letinic, K., Zoncu, R., and Rakic, P. (2002). Origin of GABAergic neurons in the human neocortex. Nature 417, 645–649. doi: 10.1038/nature00779
Lim, D. A., and Alvarez-Buylla, A. (2016). The adult ventricular-subventricular zone (V-SVZ) and olfactory bulb (OB) neurogenesis. Cold Spring Harb. Perspect. Biol. 8:a018820. doi: 10.1101/cshperspect.a018820
Liu, X., Novosedlik, N., Wang, A., Hudson, M. L., Cohen, I. L., Chudley, A. E., et al. (2009). The DLX1and DLX2 genes and susceptibility to autism spectrum disorders. Eur. J. Hum. Genet. 17, 228–235. doi: 10.1038/ejhg.2008.148
Long, J. E., Garel, S., Depew, M. J., Tobet, S., and Rubenstein, J. L. (2003). DLX5 regulates development of peripheral and central components of the olfactory system. J. Neurosci. 23, 568–578.
Lucchese, G. (2016). Understanding neuropsychiatric diseases, analyzing the peptide sharing between infectious agents and the language-associated NMDA 2A protein. Front. Psychiatry 7:60. doi: 10.3389/fpsyt.2016.00060
Lucchese, G. (2017). From toxoplasmosis to schizophrenia via NMDA dysfunction: peptide overlap between Toxoplasma gondii and N-Methyl-d-aspartate receptors as a potential mechanistic link. Front. Psychiatry 8:37. doi: 10.3389/fpsyt.2017.00037
Lucchese, G., Capone, G., and Kanduc, D. (2014). Peptide sharing between influenza A H1N1 hemagglutinin and human axon guidance proteins. Schizophr. Bull. 40, 362–375. doi: 10.1093/schbul/sbs197
Ludlow, M., Kortekaas, J., Herden, C., Hoffmann, B., Tappe, D., Trebst, C., et al. (2016). Neurotropic virus infections as the cause of immediate and delayed neuropathology. Acta Neuropathol. 131, 159–184. doi: 10.1007/s00401-015-1511-3
MacKay, D. G., Stewart, R., and Burke, D. M. (1998). H.M. revisited: relations between language comprehension, memory, and the hippocampal system. J. Cogn. Neurosci. 10, 377–394. doi: 10.1162/089892998562807
Maffei, A., Charrier, C., Caiati, M. D., Barberis, A., Mahadevan, V., Woodin, M. A., et al. (2017). Emerging mechanisms underlying dynamics of GABAergic synapses. J. Neurosci. 37, 10792–10799. doi: 10.1523/JNEUROSCI.1824-17.2017
Matà, S., Muscas, G. C., Naldi, I., Rosati, E., Paladini, S., Cruciatti, B., et al. (2008). Non-paraneoplastic limbic encephalitis associated with anti-glutamic acid decarboxylase antibodies. J. Neuroimmunol. 199, 155–159. doi: 10.1016/j.jneuroim.2008.05.015
McAllister, A. K. (2014). Major histocompatibility complex I in brain development and schizophrenia. Biol. Psychiatry 75, 262–268. doi: 10.1016/j.biopsych.2013.10.003
McKinsey, G. L., Lindtner, S., Trzcinski, B., Visel, A., Pennacchio, L. A., Huylebroeck, D., et al. (2013). Dlx1&2-dependent expression of Zfhx1b (Sip1, Zeb2) regulates the fate switch between cortical and striatal interneurons. Neuron 77, 83–98. doi: 10.1016/j.neuron.2012.11.035
Merlo, G. R., Zerega, B., Paleari, L., Trombino, S., Mantero, S., and Levi, G. (2000). Multiple functions of Dlx genes. Int. J. Dev. Biol. 44, 619–626.
Miller, B. R., and Hen, R. (2015). The current state of the neurogenic theory of depression and anxiety. Curr. Opin. Neurobiol. 30, 51–58. doi: 10.1016/j.conb.2014.08.012
Miller, J. A., Ding, S. L., Sunkin, S. M., Smith, K. A., Ng, L., Szafer, A., et al. (2014). Transcriptional landscape of the prenatal human brain. Nature 508, 199–206. doi: 10.1038/nature13185
Ming, G. L., and Song, H. (2011). Adult neurogenesis in the mammalian brain: significant answers and significant questions. Neuron 70, 687–702. doi: 10.1016/j.neuron.2011.05.001
Mortensen, P. B., Pedersen, C. B., Hougaard, D. M., Nørgaard-Petersen, B., Mors, O., Børglum, A. D., et al. (2010). A Danish national birth cohort study of maternal HSV-2 antibodies as a risk factor for schizophrenia in their offspring. Schizophr. Res. 122, 257–263. doi: 10.1016/j.schres.2010.06.010
Murphy, E., and Benítez-Burraco, A. (2016). Bridging the gap between genes and language deficits in schizophrenia: an oscillopathic approach. Front. Hum. Neurosci. 10:422. doi: 10.3389/fnhum.2016.00422
Murphy, E., and Benítez-Burraco, A. (2017). Language deficits in schizophrenia and autism as related oscillatory connectomopathies: an evolutionary account. Neurosci. Biobehav. Rev. 83, 742–764. doi: 10.1101/044198
Najjar, S., Pearlman, D., Zagzag, D., Golfinos, J., and Devinsky, O. (2012). Glutamic acid decarboxylase autoantibody syndrome presenting as schizophrenia. Neurologist 18, 88–91. doi: 10.1097/NRL.0b013e318247b87d
Nakashima, N., Yamagata, T., Mori, M., Kuwajima, M., Suwa, K., and Momoi, M. Y. (2010). Expression analysis and mutation detection of DLX5 and DLX6 in autism. Brain Dev. 32, 98–104. doi: 10.1016/j.braindev.2008.12.021
Panganiban, G., and Rubenstein, J. L. (2002). Developmental functions of the Distal-less/Dlx homeobox genes. Development 129, 4371–4386.
Parent, J. M., and Murphy, G. G. (2008). Mechanisms and functional significance of aberrant seizure-induced hippocampal neurogenesis. Epilepsia 49(Suppl. 5), 19–25. doi: 10.1111/j.1528-1167.2008.01634.x
Perera, M., Merlo, G. R., Verardo, S., Paleari, L., Corte, G., and Levi, G. (2004). Defective neuronogenesis in the absence of Dlx5. Mol. Cell. Neurosci. 25, 153–161. doi: 10.1016/j.mcn.2003.10.004
Piai, V., Anderson, K. L., Lin, J. J., Dewar, C., Parvizi, J., Dronkers, N. F., et al. (2016). Direct brain recordings reveal hippocampal rhythm underpinnings of language processing. Proc. Natl. Acad. Sci. U.S.A. 113, 11366–11371. doi: 10.1073/pnas.1603312113
Portales-Pérez, D., Alarcón-Segovia, D., Llorente, L., Ruíz-Argüelles, A., Abud-Mendoza, C., Baranda, L., et al. (1998). Penetrating anti-DNA monoclonal antibodies induce activation of human peripheral blood mononuclear cells. J. Autoimmun. 11, 563–571. doi: 10.1006/jaut.1998.0218
Proulx, D. P., Aubin, E., Lemieux, R., and Bazin, R. (2009). Spontaneous internalization of IVIg in activated B cells. Immunol. Lett. 124, 18–26. doi: 10.1016/j.imlet.2009.03.012
Racanelli, V., Prete, M., Musaraj, G., Dammacco, F., and Perosa, F. (2011). Autoantibodies to intracellular antigens: generation and pathogenetic role. Autoimmun. Rev. 10, 503–508. doi: 10.1016/j.autrev.2011.03.001
Rakic, S., and Zecevic, N. (2003). Emerging complexity of layer I in human cerebral cortex. Cereb. Cortex 13, 1072–1083. doi: 10.1093/cercor/13.10.1072
Reif, A., Fritzen, S., Finger, M., Strobel, A., Lauer, M., Schmitt, A., et al. (2006). Neural stem cell proliferation is decreased in schizophrenia, but not in depression. Mol. Psychiatry 11, 514–522. doi: 10.1038/sj.mp.4001791
Reif, A., Schmitt, A., Fritzen, S., and Lesch, K. P. (2007). Neurogenesis and schizophrenia: dividing neurons in a divided mind? Eur. Arch. Psychiatry Clin. Neurosci. 257, 290–299. doi: 10.1007/s00406-007-0733-3
Ronda, N., Gatti, R., Giacosa, R., Raschi, E., Testoni, C., Meroni, P. L., et al. (2002). Antifibroblast antibodies from systemic sclerosis patients are internalized by fibroblasts via a caveolin-linked pathway. Arthritis Rheum. 46, 1595–1601. doi: 10.1002/art.10362
Rout, U. K., Mungan, N. K., and Dhossche, D. M. (2012). Presence of GAD65 autoantibodies in the serum of children with autism or ADHD. Eur. Child Adolesc. Psychiatry 21, 141–147. doi: 10.1007/s00787-012-0245-1
Ruíz-Argüelles, A., Brito, G. J., Reyes-Izquierdo, P., Pérez-Romano, B., and Sánchez-Sosa, S. (2007). Apoptosis of melanocytes in vitiligo results from antibody penetration. J. Autoimmun. 29, 281–286. doi: 10.1016/j.jaut.2007.07.012
Ruíz-Argüelles, A., Pérez-romano, B., Llorente, L., Alarcón-Segovia, D., and Castellanos, J. M. (1998). Penetration of anti-DNA antibodies into immature live cells. J. Autoimmun. 11, 547–556. doi: 10.1006/jaut.1998.0216
Ruíz-Argüelles, A., Rivadeneyra-Espinoza, L., and Alarcón-Segovia, D. (2003). Antibody penetration into living cells: pathogenic, preventive and immuno-therapeutic implications. Curr. Pharm. Des. 9, 1881–1887. doi: 10.2174/1381612033454379
Sass, K. J., Sass, A., Westerveld, M., Lencz, T., Novelly, R. A., Kim, J. H., et al. (1992). Specificity in the correlation of verbal memory and hippocampal neuron loss: dissociation of memory, language, and verbal intellectual ability. J. Clin. Exp. Neuropsychol. 14, 662–672. doi: 10.1080/01688639208402854
Severance, E. G., Gressitt, K. L., Buka, S. L., Cannon, T. D., and Yolken, R. H. (2014). Maternal complement C1q and increased odds for psychosis in adult offspring. Schizophr. Res. 159, 14–19. doi: 10.1016/j.schres.2014.07.053
Shu, H. F., Gao, F. Y., Zhang, C. Q., Liu, S. Y., Zhang, Z. Y., Song, Y. C., et al. (2010). Rat Dlx5 is expressed in the subventricular zone and promotes neuronal differentiation. Braz. J. Med. Biol. Res. 43, 176–185. doi: 10.1590/S0100-879X2009007500034
Simeone, A., Acampora, D., Pannese, M., D'Esposito, M., Stornaiuolo, A., Gulisano, M., et al. (1994). Cloning and characterization of two members of the vertebrate Dlx gene family. Proc. Natl. Acad. Sci. U.S.A. 91, 2250–2254. doi: 10.1073/pnas.91.6.2250
Soltani, H., Mohammadzadeh, S., Makvandi, M., Pakseresht, S., and Samarbaf-Zadeh, A. (2016). Detection of Borna Disease Virus (BDV) in patients with first episode of schizophrenia. Iran. J. Psychiatry 11, 257–261.
Stühmer, T., Anderson, S. A., Ekker, M., and Rubenstein, J. L. (2002a). Ectopic expression of the Dlx genes induces glutamic acid decarboxylase and Dlx expression. Development 129, 245–252.
Stühmer, T., Puelles, L., Ekker, M., and Rubenstein, J. L. (2002b). Expression from a Dlx gene enhancer marks adult mouse cortical GABAergic neurons. Cereb. Cortex 12, 75–85. doi: 10.1093/cercor/12.1.75
Terrillion, C. E., Abazyan, B., Yang, Z., Crawford, J., Shevelkin, A. V., Jouroukhin, Y., et al. (2017). DISC1 in astrocytes influences adult neurogenesis and hippocampus-dependent behaviors in mice. Neuropsychopharmacology 42, 2242–2251. doi: 10.1038/npp.2017.129
Thul, P. J., Åkesson, L., Wiking, M., Mahdessian, D., Geladaki, A., Ait Blal, H., et al. (2017). A subcellular map of the human proteome. Science 356:eaal3321. doi: 10.1126/science.aal3321
Toro, C. T., and Deakin, J. F. (2007). Adult neurogenesis and schizophrenia: a window on abnormal early brain development? Schizophr. Res. 90, 1–14. doi: 10.1016/j.schres.2006.09.030
Torrey, E. F., Leweke, M. F., Schwarz, M. J., Mueller, N., Bachmann, S., Schroeder, J., et al. (2006). Cytomegalovirus and schizophrenia. CNS Drugs 20, 879–885. doi: 10.2165/00023210-200620110-00001
Turner, S. J., Mayes, A. K., Verhoeven, A., Mandelstam, S. A., Morgan, A. T., and Scheffer, I. E. (2015). GRIN2A: an aptly named gene for speech dysfunction. Neurology 84, 586–593. doi: 10.1212/WNL.0000000000001228
Uhlén, M., Fagerberg, L., Hallstrom, B. M., Lindskog, C., Oksvold, P., Mardinoglu, A., et al. (2015). Proteomics. Tissue-based map of the human proteome. Science 347:1260419. doi: 10.1126/science.1260419
Vernochet, C., Caucheteux, S. M., Gendron, M. C., Wantyghem, J., and Kanellopoulos-Langevin, C. (2005). Affinity-dependent alterations of mouse B cell development by noninherited maternal antigen. Biol. Reprod. 72, 460–469. doi: 10.1095/biolreprod.104.035048
Vernochet, C., Caucheteux, S. M., and Kanellopoulos-Langevin, C. (2007). Bi-directional cell trafficking between mother and fetus in mouse placenta. Placenta 28, 639–649. doi: 10.1016/j.placenta.2006.10.006
Vita, R., Overton, J. A., Greenbaum, J. A., Ponomarenko, J., Clark, J. D., Cantrell, J. R., et al. (2015). The immune epitope database (IEDB) 3.0. Nucleic Acids Res. 43, D405–D412. doi: 10.1093/nar/gku938
Voineagu, I., Wang, X., Johnston, P., Lowe, J. K., Tian, Y., Horvath, S., et al. (2011). Transcriptomic analysis of autistic brain reveals convergent molecular pathology. Nature 474, 380–384. doi: 10.1038/nature10110
Wang, Y., Dye, C. A., Sohal, V., Long, J. E., Estrada, R. C., Roztocil, T., et al. (2010). Dlx5 and Dlx6 regulate the development of parvalbumin-expressing cortical interneurons. J. Neurosci. 30, 5334–5345. doi: 10.1523/JNEUROSCI.5963-09.2010
Wang, Y., Wang, X., Ferrone, C. R., Schwab, J. H., and Ferrone, S. (2015). Intracellular antigens as targets for antibody based immunotherapy of malignant diseases. Mol. Oncol. 9, 1982–1993. doi: 10.1016/j.molonc.2015.10.019
Weisbart, R. H., Gera, J. F., Chan, G., Hansen, J. E., Li, E., Cloninger, C., et al. (2012). A cell-penetrating bispecific antibody for therapeutic regulation of intracellular targets. Mol. Cancer Ther. 11, 2169–2173. doi: 10.1158/1535-7163.MCT-12-0476-T
Wonders, C., and Anderson, S. (2005). Beyond migration: Dlx1 regulates interneuron differentiation. Nat. Neurosci. 8, 979–981. doi: 10.1038/nn0805-979
Ye, H., and Kaszuba, S. (2017). Inhibitory or excitatory? Optogenetic interrogation of the functional roles of GABAergic interneurons in epileptogenesis. J. Biomed. Sci. 24:93. doi: 10.1186/s12929-017-0399-8
Yolken, R. H., and Torrey, E. F. (1995). Viruses, schizophrenia, and bipolar disorder. Clin. Microbiol. Rev. 8, 131–145.
Yun, S., Reynolds, R. P., Masiulis, I., and Eisch, A. J. (2016). Re-evaluating the link between neuropsychiatric disorders and dysregulated adult neurogenesis. Nat. Med. 22, 1239–1247. doi: 10.1038/nm.4218
Zhang, A., Yu, H., He, Y., Shen, Y., Pan, N., Liu, J., et al. (2013). The spatio-temporal expression of MHC class I molecules during human hippocampal formation development. Brain Res. 1529, 26–38. doi: 10.1016/j.brainres.2013.07.001
Keywords: viral infections, neuropsychiatric diseases, language disorders, fetal and adult neurogenesis, DLX proteins, peptide sharing, cross-reactivity, autoimmunity
Citation: Lucchese G and Stahl B (2018) Peptide Sharing Between Viruses and DLX Proteins: A Potential Cross-Reactivity Pathway to Neuropsychiatric Disorders. Front. Neurosci. 12:150. doi: 10.3389/fnins.2018.00150
Received: 24 June 2017; Accepted: 26 February 2018;
Published: 21 March 2018.
Edited by:
Antonio Benítez-Burraco, Universidad de Sevilla, SpainReviewed by:
Peter De Haan, Amarna Therapeutics BV, NetherlandsCopyright © 2018 Lucchese and Stahl. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Guglielmo Lucchese, Z3VnbGllbG1vLmx1Y2NoZXNlQHVuaS1ncmVpZnN3YWxkLmRl
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.