- 1Department of Neurology, Xinhua Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai, China
- 2School of Biotechnology and Food Engineering, Hefei University of Technology, Hefei, China
- 3Shanghai Laboratory Animal Center, Chinese Academy of Sciences, Shanghai, China
Increasing evidences show that the etiology of Parkinson's disease (PD) is multifactorial. Studying the combined effect of several factors is becoming a hot topic in PD research. On one hand, iron is one of the essential trace metals for human body; on the other hand, iron may be involved in the etiopathogenesis of PD. In our present study, the rats with increased neonatal iron (120 μg/g bodyweight) supplementation were treated with rotenone (0.5 mg/kg) when they were aged to 14 weeks. We observed that iron and rotenone co-treatment induced significant behavior deficits (time-dependent) and striatal dopamine depletion in the male and female rats, while they did not do so when they were used alone. No significant change in striatal 5-hydroxytryptamine content was observed in the male and female rats with iron and rotenone co-treatment. Also, iron and rotenone co-treatment significantly decreased substantia nigra TH expression in the male rats. Furthermore, co-treatment with iron and rotenone significantly induced malondialdehyde increase and glutathione decrease in the substantia nigra of male and female rats. There was no significant change in cerebellar malondialdehyde and glutathione content of the rats co-treated with iron and rotenone. Interestingly, biochanin A significantly attenuated striatal dopamine depletion and improved behavior deficits (dose-dependently) in the male and female rats with iron and rotenone co-treatment. Biochanin A treatment also significantly alleviated substantia nigra TH expression reduction in the male rats co-treated with iron and rotenone. Finally, biochanin A significantly decreased malondialdehyde content and increased glutathione content in the substantia nigra of male and female rats with iron and rotenone co-treatment. Our results indicate that iron and rotenone co-treatment may result in aggravated neurochemical and behavior deficits through inducing redox imbalance and increased neonatal iron supplementation may participate in the etiopathogenesis of PD. Moreover, biochanin A may exert dopaminergic neuroprotection by maintaining redox balance.
Introduction
Parkinson's disease (PD) is a common neurodegenerative disorder clinically characterized by four major hallmarks including resting tremor, rigidity, bradykinesia and postural instability (Lang and Lozano, 1998; Dauer and Przedborski, 2003; Fiesel et al., 2015). Accumulating studies have showed that the etiology of Parkinson's disease (PD) is multifactorial (Kidd, 2000; Connolly and Lang, 2014; Mitsuyama et al., 2015; Richter et al., 2017). Environmental factors are shown to play an important role in the etiopathogenesis of PD (Olanow and Tatton, 1999; Tanner et al., 1999; Ascherio and Schwarzschild, 2016). In recent years, studying the combined effect of several risk factors is being become a hot topic in PD research.
Iron is one of the essential trace metals for human body especially for neural development. Iron deficiency negatively impacts on myelinogenesis, synthesis of neurotransmitters and construction of neural connections (Beard, 2003; Stankiewicz et al., 2007). Insufficient iron supplement also results in a number of neurological and psychiatric conditions such as pediatric restless leg syndrome and attention deficit hyperactivity disorder (Millichap, 2008; Benton, 2010). In addition, anemia due to iron deficiency in infants contributes to cognitive and social impairments (Carter et al., 2010; Wang L. et al., 2016). Because of the essential role of iron in neurological as well as overall development, it has been recommended that breast milk which contains iron nutrition is preferred benefit for infants and may be replaced with iron-fortified formula to those who cannot receive breastfeed. However, studies have shown that increased neonatal iron supplementation could result in increased iron content in the substantia nigra and subsequent nigrostriatal dopaminergic neurodegeneration in aging rats (Kaur et al., 2007; Chen H. et al., 2015). Rotenone, a mitochondrial complex I inhibitor, has been extensively used as pesticides in a rural environment. Rotenone is being widely employed into PD models because of its highly selective toxicity on dopaminergic neurons and effective reproduction of pathological and clinical features of PD (Betarbet et al., 2000; Sherer et al., 2003b; Cannon et al., 2009; Sanders and Greenamyre, 2013; Jagmag et al., 2016). However, little is known about whether increased neonatal iron supplementation enhances susceptibility of dopaminergic neurons to subsequent exposure of rotenone.
In our present study, the rats with increased neonatal iron (120 μg/g bodyweight) supplementation were treated with rotenone (0.5 mg/kg) for 35 days when they were aged to 14 weeks. We investigated the combined effect of iron and rotenone treatment and mechanism of action on behavioral and neurochemical indexes in male and female rats. Biochanin A, an O-methylated isoflavone, is classified as phytoestrogen due to its similar chemical structure with mammalian estrogen. Moreover, biochanin A has been suggested to be protective in several in vitro and in vivo models (Chen et al., 2007; Su et al., 2013; Wang J. et al., 2016). Therefore, in this study, we also investigated biochanin A's effect and mechanism of action in male and female rats co-treated with iron and rotenone.
Materials and Methods
Animals and Treatment
All animals were from Sino-British SIPPR/BK Lab Animal LTD (Shanghai, People's Republic of China). Sprague-Dawley rat pups were fed either saline vehicle or carbonyl iron daily by oral gavage from days 10 to 17 post-partum. Based on the previous studies (Kaur et al., 2007; Stankiewicz et al., 2007; Chen H. et al., 2015), the rat pups were fed with increased iron (120 μg/g bodyweight). The rats were aged to 14 weeks. Then, rotenone, emulsified in sunflower oil at 0.5 mg/mL, was given intraperitoneally, at 1 mL/kg once a day for 35 days, to the rats (Wang et al., 2015). Biochanin A was dissolved in dimethyl sulfoxide (DMSO) and administered intraperitoneally (0.1 ml/100 g/day) to different groups of rats at two different concentrations of 3 and 30 mg/kg. Together with rotenone injection, the rats were continuously treated with biochanin A for 35 days. To investigate the combined effect of iron and rotenone treatment and mechanism of action on behavioral and neurochemical indexes in male and female rats, rats (male and female) were randomly divided into four groups: Veh group (rats co-treated with saline and sunflower oil), Ir group (rats co-treated with iron and sunflower oil), Rot group (rats co-treated with saline and rotenone), and Ir+Rot group (rats co-treated with iron and rotenone). To investigate biochanin A's effect and mechanism of action in male and female rats co-treated with iron and rotenone, rats (male and female) were randomly divided into five groups: Veh group (rats co-treated with saline, sunflower oil and DMSO), Ir+Rot group (rats co-treated with iron, rotenone and DMSO), Ir+Rot+BA3 group [rats co-treated with iron, rotenone and biochanin A (3 mg/kg)], Ir+Rot+BA30 group [rats co-treated with iron, rotenone and biochanin A (30 mg/kg)], and BA30 group [rats co-treated with saline, sunflower oil and biochanin A (30 mg/kg)]. For research involving biohazards, biological select agents, toxins (including rotenone), restricted materials or reagents, the standard biosecurity or institutional safety procedures were carried out in our experiments. The use of toxic or biohazards substances was approved by the Health, Safety and environment (HSE) Committee of Xinhua Hospital Affiliated to Shanghai Jiao Tong University School of Medicine. This study was reviewed and approved by the Ethical Committee of Xinhua Hospital Affiliated to Shanghai Jiao Tong University School of Medicine. All experiments were carried out in accordance with the approved guidelines and regulations of the National Institutes of Health for the care and use of laboratory animals. All attempts were made to minimize the number of animals used and their suffering.
Behavior Tests
Rotarod and open-field tests were performed to evaluate rat behavior on the 15th and 45th day after the last injection of rotenone. The rotarod apparatus required a roller, a power source to turn the roller and four separators that divided the roller into equal-sized compartments. Rats were placed onto the rotating rod and trained at accelerated speeds of 5, 10, and 15 rotations per minute (rpm). After completing the training, each rat was given three trials at each rotarod speed and the latency time to fall was recorded. To assess the locomotor activity, the rats were tested in an open field chamber (100 × 100 × 50 cm3) with the floor divided into 25 equal squares of 20 × 20 cm2, which is made of wood covered with impermeable formica. Each rat was initially placed in the center of the open field to acclimatize for 10 min and then behavioral parameters including crossing number (entering of another square with all four paws) and rearing number (rearing with and without wall contact namely standing only on hind legs) were measured during a period of 30 min.
Neurochemical Analysis
High-performance liquid chromatography (HPLC) with electrochemical detection (ECD) (HPLC-ECD) was used to the biochemical analysis of neurotransmitters in the rat striata as previously described (McNaught et al., 2004). Briefly, rat striata were quickly removed on ice and weighed. Striata were homogenized (10% wt/vol) in ice-cold homogenization buffer containing perchloric acid (0.1 mol/L) using sonication and 3,4-dihydroxybenzylamine is applied to be an internal control. After lysis, samples were centrifuged at 4°C for 10 min and the collected supernatants were then assayed for dopamine and 5-hydroxytryptamine content through HPLC-ECD equipped with a column of 5 μm spherical C18 particles. The mobile phase consisted of 4.5% acetonitrile, 0.1 M phosphate buffer (pH 2.6) containing 2.5%methanol and 0.2 mM octane sulfonic acid. The content of each neurotransmitter was expressed as ng/g equivalent striatal tissue.
Western Blotting
The substantia nigra tissues of rats were separated and homogenized in cold lysis buffer. Protein lysates were adjusted to equal protein concentrations using bicinchoninic acid (BCA) protein assay kit (Beyotime Institute of Biotechnology, Shanghai, China). After being separated by 10% sodium dodecyl sulfate-polyacrylamide gels (SDS-PAGE), protein samples were transferred onto polyvinylidene fluoride membranes (Millipore, Bedford, MA, USA). Then, the membranes were blocked with blocking solution for 1 h and incubated with primary rabbit anti-tyrosine hydroxylase (TH) antibody (Abcam, Cambridge, UK) or rabbit anti-β-Actin antibody (Abcam) at 4°C overnight. Subsequently, the membranes were washed and incubated with horseradish peroxidase (HRP)-conjugated secondary antibodies for another 1 h at room temperature. Finally, detection of protein bands was performed by using an enhanced chemiluminescence (ECL) assay kit (EMD Millipore, Billerica, MA, USA). The density of bands was quantified by using ImageJ software (National Institutes of Health, USA) and results presented the ratio of density of the target protein to β-actin as densitometric relative units.
Determination of Malondialdehyde and Glutathione Levels
The levels of malondialdehyde and glutathione were measured by commercially available kits (Cayman Chemical Co., Ann Arbor, MI, USA) according to the manufacturer's instructions. Substantia nigra tissues were quickly removed on ice and homogenized (10% wt/vol) with radioimmunoprecipitation assay (RIPA) homogenizing buffer containing a protease inhibitor for protein extraction. Then samples were centrifuged at 1,600 g for 10 min at 4°C and supernatants were collected. For malondialdehyde assay, thiobarbituric acid (TBA) reacts with malondialdehyde to generate a thiobarbituric acid reactive substance which can be quantified. Samples or standards (100 μl) were added to trichloroacetic acid and thiobarbituric acid reactive substances reagent and then the mixture solutions were boiled for 1 h. After incubating on the ice for 10 min to stop reaction, samples were centrifuged and the absorbance values of the supernatants were read at 540 nm. For glutathione assay, the determination was based on the enzymatic recycling method. Since glutathione reductase is used in the Cayman GSH assay, both GSH and GSSG are measured and the assay reflects total glutathione. Briefly, 100 μl of supernatant from substantia nigra sample was deproteinated with 100 μl metaphosphoric acid reagent, added by triethanolamine reagent (50 μl/ml, 4 M) and pipetted 50 μl of the solution. Then, this was followed by the addition of 150 μl of freshly prepared Assay Cocktail consisting of 11.25 ml of MES Buffer, 2.1 ml of reconstituted Enzyme Mixture, 0.45 ml of reconstituted Cofactor Mixture, 0.45 ml of reconstituted DTNB [5,5′-dithio-bis-(2-nitrobenzoic acid)] and 2.3 ml of water and incubated for 25 min. The absorbance value was read at 405 nm.
Statistical Analysis
Data were expressed as the mean ± standard error of the mean (SEM). Results were analyzed by two-tailed Student's t-test for comparison between two groups and an analysis of variance (ANOVA) followed by Bonferroni post hoc test for comparison between more than two groups. Normality of sample distribution and homogeneity of variances were tested before each ANOVA. A value of p < 0.05 was considered to be statistically significant.
Results
Effect of Iron and Rotenone Co-treatment on Behavioral and Neurochemical Indexes of Male and Female Rats
To evaluate the effect of iron and rotenone co-treatment on motor behavior in male and female rats, the rotarod and open field tests were performed on rats on the 15th and 45th day after the last injection of rotenone. As shown in Figures 1, 2, no significant behavior change was observed in the male and female rats treated with iron (or rotenone) alone compared with the vehicle-treated rats on both the 15th and the 45th day after rotenone injection. However, iron and rotenone co-treatment significantly decreased the latency time [male and female: p < 0.01 (5 and 15 rpm); p < 0.05(10 rpm)] in rotarod test and the number of crossing and rearing [male: p < 0.01 (crossing and rearing); female: p < 0.05 (crossing), p < 0.01 (rearing)] in open field test in the male and female rats on the 45th day but did not significantly decrease them on the 15th day compared with the rats treated with vehicle, iron or rotenone (Figures 1, 2). Next, we investigated the effect of iron and rotenone co-treatment on striatal neurotransmitters of male and female rats. In accordance with behavior tests, no significant striatal dopamine depletion was observed in the male and female rats treated with iron (or rotenone) alone in comparison with the vehicle-treated rats (Figure 3A). However, iron and rotenone co-treatment significantly decreased striatal dopamine content in the male and female rats compared with the rats treated with vehicle, iron or rotenone (Figure 3A) (p < 0.01). Even though male and female rats were co-treated with iron and rotenone, no significant change in striatal 5-hydroxytryptamine level was observed in the rats compared with those treated with vehicle, iron or rotenone (Figure 3B). In addition, iron and rotenone co-treatment significantly decreased substantia nigra TH expression in the male rats compared with the rats treated with vehicle, iron, or rotenone (p < 0.01, Figures 4A,C). No significant change was observed in the substantia nigra TH expression of male rats treated with iron (or rotenone) alone in comparison with the vehicle-treated rats (Figures 4A,C).
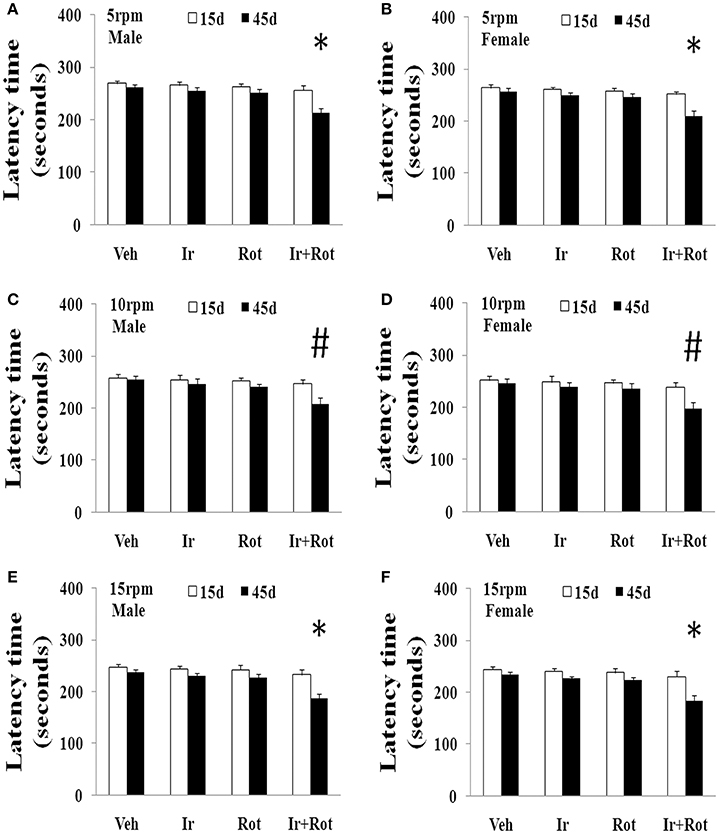
Figure 1. Effect of iron and rotenone co-treatment on motor behavior of male (A,C,E) and female (B,D,F) rats in rotarod test (A,B: 5 rpm; C,D: 10 rpm; E,F: 15 rpm). Results are expressed as mean ± SEM. N = 9. Latency time was analyzed using multi-factor ANOVA followed by Bonferroni post hoc test. #p < 0.05, compared with the rats treated with vehicle, iron or rotenone; *p < 0.01, compared with the rats treated with vehicle, iron or rotenone. Veh, vehicle; Ir, iron; Rot, rotenone.
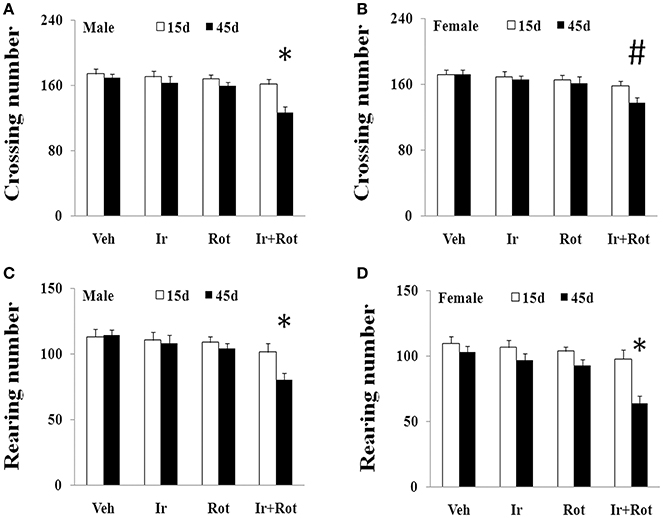
Figure 2. Effect of iron and rotenone co-treatment on motor behavior of male (A,C) and female (B,D) rats in open field test (A,B: crossing number; C,D: rearing number). Results are expressed as mean ± SEM. N = 9. Crossing and rearing number were analyzed using multi-factor ANOVA followed by Bonferroni post hoc test. #p < 0.05, compared with the rats treated with vehicle, iron or rotenone; *p < 0.01, compared with the rats treated with vehicle, iron or rotenone. Veh, vehicle; Ir, iron; Rot, rotenone.
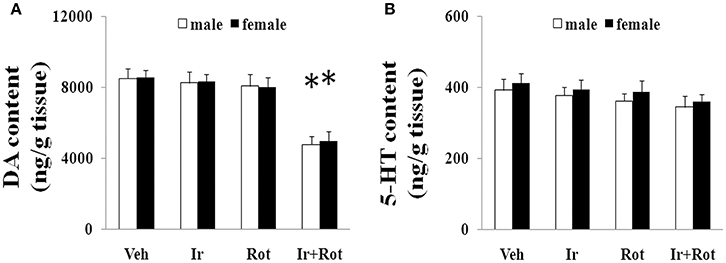
Figure 3. Effect of iron and rotenone co-treatment on striatal dopamine (A) and 5-hydroxytryptamine (B) conent in male and female rats. Results are expressed as mean ± SEM. N = 9. DA and 5-HT content were analyzed using multi-factor ANOVA followed by Bonferroni post hoc test. *p < 0.01, compared with the rats treated with vehicle, iron or rotenone. Veh, vehicle; Ir, iron; Rot, rotenone. DA, dopamine; 5-HT, 5-hydroxytryptamine.
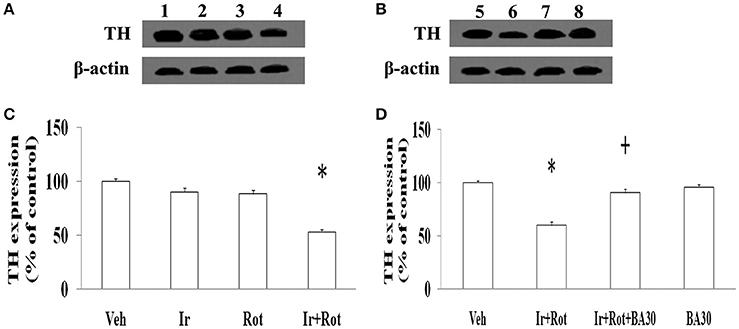
Figure 4. Effect of iron, rotenone and biochanin A co-treatment on substantia nigra TH expression in male rats. Results are expressed as mean ± SEM. N = 9. TH expression was analyzed using multi-factor ANOVA followed by Bonferroni post hoc test. *p < 0.01(C), compared with the rats treated with vehicle, iron or rotenone. *p < 0.01(D), compared with the rats treated with vehicle. +p < 0.01, compared with the rats co-treated with iron and rotenone. 1, Veh1(saline)+Veh2(sunflower oil); 2, Ir+Veh2; 3, Veh1+Rot; 4, Ir+Rot; 5, Veh1+Veh2+Veh3(DMSO); 6, Ir+Rot+Veh3; 7, Ir+Rot+BA30; 8, Veh1+Veh2+BA30; Veh, vehicle; Ir, iron; Rot, rotenone; BA30, biochanin A (30 mg/kg).
Effect of Iron and Rotenone Co-treatment on Substantia Nigra Malondialdehyde and Glutathione Content in Male and Female Rats
Oxidative stress has been shown to play an important role in iron (or rotenone)-induced neurodegeneration (Betarbet et al., 2000; Stankiewicz et al., 2007), so we measured the content of malondialdehyde and glutathione in the substantia nigra of male and female rat to investigate the potential mechanism underlying behavioral and neurochemical deficits induced by iron and rotenone co-treatment. As shown in Figure 5, no significant change in the content of malondialdehyde and glutathione was observed in the substantia nigra of male and female rats treated with iron (or rotenone) alone in comparison with the vehicle-treated rats. However, iron and rotenone co-treatment significantly increased malondialdehyde content (p < 0.01) and decreased glutathione content (p < 0.01) in the substantia nigra of male and female rats compared with the rats treated with vehicle, iron or rotenone (Figure 5). In addition, even though male and female rats were co-treated with iron and rotenone, no significant change in malondialdehyde and glutathione content was observed in the cerebellum of rats compared with the rats treated with vehicle, iron or rotenone (Figure 5).
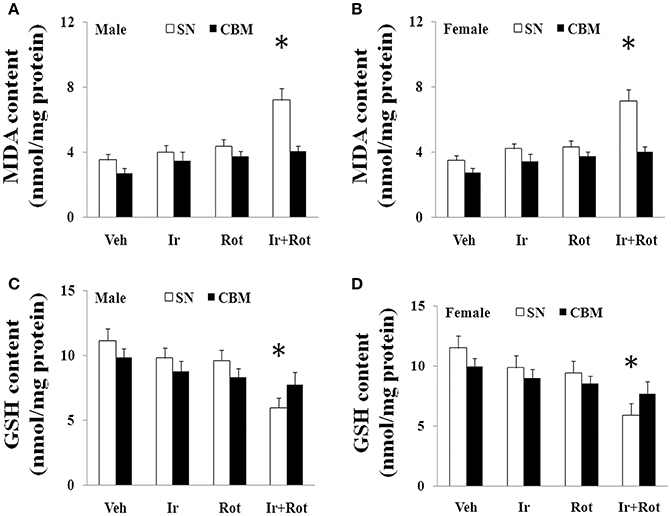
Figure 5. Effect of iron and rotenone co-treatment on malondialdehyde (A,B) and glutathione (C,D) conent in the substantia nigra and cerebellum of male (A,C) and female (B,D) rats. Results are expressed as mean ± SEM. N = 9. MDA and GSH content were analyzed using multi-factor ANOVA followed by Bonferroni post hoc test. *p < 0.01, compared with the rats treated with vehicle, iron or rotenone. Veh, vehicle; Ir, iron; Rot, rotenone. MDA, malondialdehyde; GSH, glutathione; SN, substantia nigra; CBM, cerebellum.
Effect of Biochanin A on Behavioral and Neurochemical Indexes in Male and Female Rats with Iron and Rotenone Co-treatment
Biochanin A has been suggested to be protective in several in vitro and in vivo models (Chen et al., 2007; Su et al., 2013; Wang J. et al., 2016). Therefore, we further investigated the effect of biochanin A in the rats co-treated with iron and rotenone. As shown in Figures 6, 7, biochanin A (30 mg/kg) administration significantly alleviated the reduction of latency time (male: 5 rpm: p < 0.01, 10, and 15 rpm: p < 0.05; female: p < 0.05) and crossing and rearing number (p < 0.05) in the male and female rats co-treated with iron and rotenone, although no significant behavior change but a trend toward improvement was observed in the rats co-treated with iron and rotenone after biochanin A (3 mg/kg) administration compared with the vehicle-treated rats. In accordance with behavior tests, biochanin A treatment significantly alleviated striatal dopamine depletion [p < 0.05 (3 mg/kg), p < 0.01 (30 mg/kg)] in the male and female rats co-treated with iron and rotenone in comparison with the Ir+Rot group (Figure 8). In addition, there was significant difference (p < 0.01) in striatal dopamine content between the Ir+Rot+BA3 and Ir+Rot+BA30 group, indicating that biochanin A's effect on striatal dopamine content was dose-dependent in the male and female rats co-treated with iron and rotenone. Furthermore, biochanin A (30 mg/kg) treatment significantly alleviated substantia nigra TH expression reduction (p < 0.01) in the male rats co-treated with iron and rotenone compared with the Ir+Rot group (Figures 4B,D).
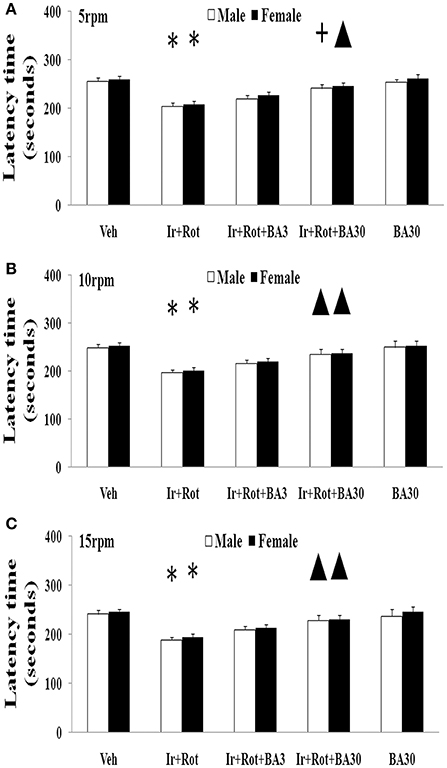
Figure 6. Effect of biochanin A on motor behavior (rotarod test) in male and female rats with iron and rotenone co-treatment (A: 5 rpm; B: 10 rpm; C: 15 rpm). Results are expressed as mean ± SEM. N = 9. Latency time was analyzed using multi-factor ANOVA followed by Bonferroni post hoc test. *p < 0.01 compared with the vehicle-treated rats. ▲p < 0.05, compared with the rats co-treated with iron and rotenone. +p < 0.01, compared with the rats co-treated with iron and rotenone. Veh, vehicle; Ir, iron; Rot, rotenone; BA3, biochanin A (3 mg/kg); BA30, biochanin A (30 mg/kg).
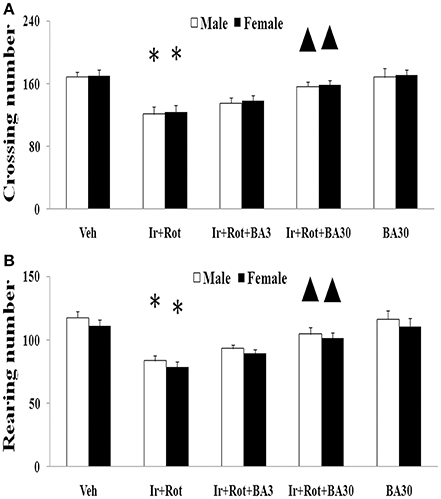
Figure 7. Effect of biochanin A on motor behavior (open field test) in male and female rats with iron and rotenone co-treatment (A: crossing number; B: rearing number). Results are expressed as mean ± SEM. N = 9. Crossing and rearing number were analyzed using multi-factor ANOVA followed by Bonferroni post hoc test. *p < 0.01 compared with the vehicle-treated rats. ▲p < 0.05 compared with the rats co-treated with iron and rotenone. Veh, vehicle; Ir, iron; Rot, rotenone; BA3, biochanin A (3 mg/kg); BA30, biochanin A (30 mg/kg).
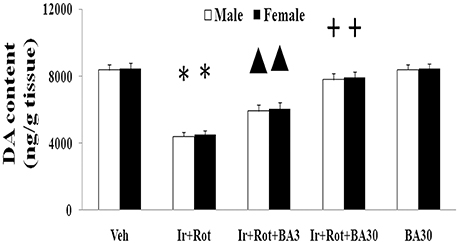
Figure 8. Effect of biochanin A on striatal dopamine conent in male and female rats with iron and rotenone co-treatment. Results are expressed as mean ± SEM. N = 9. DA content was analyzed using multi-factor ANOVA followed by Bonferroni post hoc test. *p < 0.01, compared with the vehicle-treated rats. ▲p < 0.05, compared with the rats co-treated with iron and rotenone. +p < 0.01, compared with the rats co-treated with iron and rotenone. DA, dopamine; Veh, vehicle; Ir, iron; Rot, rotenone; BA3, biochanin A (3 mg/kg); BA30, biochanin A (30 mg/kg).
Effect of Biochanin A on Substantia Nigra Malondialdehyde and Glutathione Conent in Male and Female Rats with Iron and Rotenone Co-treatment
Finally, we investigated the potential mechanism underlying biochanin A's neuroprotection in the male and female rats with iron and rotenone co-treatment. As shown in Figure 9, treatment with biochanin A significantly decreased malondialdehyde [p < 0.05 (3 mg/kg), p < 0.01 (30 mg/kg)] content and increased glutathione (p < 0.01) content in the substantia nigra of male and female rats co-treated with iron and rotenone compared with the Ir+Rot group.
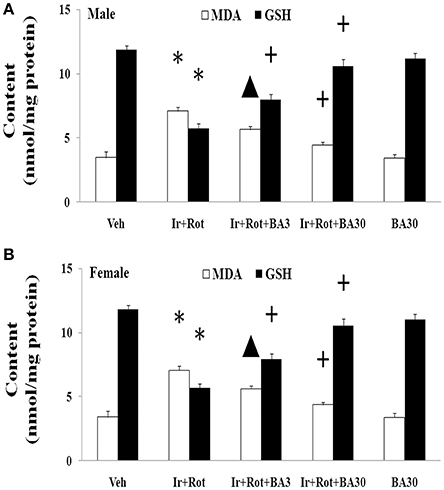
Figure 9. Effect of biochanin A on substantia nigra malondialdehyde and glutathione conent in male (A) and female (B) rats with iron and rotenone co-treatment. Results are expressed as mean ± SEM. N = 9. MDA and GSH content were analyzed using multi-factor ANOVA followed by Bonferroni post hoc test. *p < 0.01, compared with the vehicle-treated rats. ▲p < 0.05, compared with the rats co-treated with iron and rotenone. +p < 0.01, compared with the rats co-treated with iron and rotenone. MDA, malondialdehyde; GSH, glutathione; Veh, vehicle; Ir, iron; Rot, rotenone; BA3, biochanin A (3 mg/kg); BA30, biochanin A (30 mg/kg).
Discussion
Up to now, it remains unclear about the etiology and pathogenesis of PD. The “multiple hit” hypothesis that multiple risk factors may act together to induce PD neurodegeneration is a widely-accepted potential mechanism for PD (Sulzer, 2007; Ghanbari et al., 2016; Kim, 2017). Researchers estimated ambient exposures to the pesticides in California's heavily agricultural central valley, showing patients exposed to paraquat, ziram, and maneb together experienced increased risk for PD, suggesting that pesticides, which are key environmental factors, may act together to induce dopaminergic neurodegeneration (Wang A. et al., 2011). Nowadays, studying the combined effect of several risk factors is being become a hot topic in PD research.
In our present study, the rats with increased neonatal iron (120 μg/g bodyweight) supplementation were treated with a relatively low dose of rotenone (0.5 mg/kg) for 35 days when they were aged to 14 weeks. We observed that although no significant behavioral and neurochemical change was observed in the male and female rats treated with iron (or rotenone) alone compared with the vehicle-treated rats, iron and rotenone co-treatment significantly induced behavioral and neurochemical deficits in the male and female rats on the 45th day after the last rotenone injection. Our results suggest that increased neonatal iron supplement may enhance susceptibility of dopaminergic neurons to subsequent exposure of environmental toxins and increase risk for the development of PD, even though environmental toxins may be at a relatively low dose which is not harmful for ordinary individuals in our daily life. In addition, no significant behavioral change was observed in the male and female rats co-treated with iron and rotenone on the 15th day after the last rotenone injection until on the 45th day, suggesting that the effect of iron and rotenone co-treatment on behavior indexes was in a time-dependent manner in the male and female rats. Clinical and epidemiologic studies show that there are distinctions in incidence and disease progression of PD between men and women (Baldereschi et al., 2000; Taylor et al., 2007; Alves et al., 2009; Pavon et al., 2010). The data that men are more likely to develop PD and more severe phenotype than women highlight the importance of dissecting gender disparities in PD research. Therefore, based on previous studies (Dluzen et al., 1996; Chen H. et al., 2015; Wu et al., 2016), male and female rats were respectively, compared in our study. In the present study, no significant difference was observed in the sensitivity to iron and rotenone co-treatment between male and female rats. The difference of our results from others' may be attributable to different experimental samples and conditions. Some gender differences in epidemiologic and clinical features of PD may be attributable to many factors including gender-related biological and environmental differences. Although iron is important for human body especially for neural development, disruption of iron metabolism may be involved in the etiopathogenesis of PD, which is indicated by the evidence that iron levels are selectively increased in the substantia nigra of PD patients and pharmacological or genetic chelation of iron could exert neuroprotective effect in animal models of PD (Sofic et al., 1991; Griffiths et al., 1999; Kaur et al., 2003; Gaeta and Hider, 2005; Stayte and Vissel, 2014; Ward et al., 2014). In addition, direct injection of ferric iron into the substantia nigra was observed to lead to behavioral changes associated with dopamine depletion in the striatum (Lin and Ho, 2000; Junxia et al., 2003). Peripheral iron overload resulted in loss of dopaminergic neurons in rats (Jiang et al., 2007). Moreover, the correlation between increased dietary iron supplementation and PD has also been reported in aging rats (Kaur et al., 2007; Chen H. et al., 2015). At present, infant iron-fortified formula is increasingly popular as an alternative to breastfeeding for iron supplementation (Hare et al., 2015). Therefore, the possible neurotoxicity of increased dietary iron supplementation should be seriously taken into consideration. Further studies are needed to determine optimal dose of iron for infancy dietary intake.
Oxidative stress is believed to play a crucial role in the pathogenesis and development of PD (Jensen and Oliveira, 2014; Niranjan, 2014; Javed et al., 2016; Jiang et al., 2016; Niedzielska et al., 2016; Xie and Chen, 2016; Tio et al., 2017; Zhao et al., 2017). Oxidative stress is the result of an imbalance between pro-oxidant and antioxidant causing the generation of toxic reactive oxygen species (Barnham et al., 2004; Metherell et al., 2016; Hefti et al., 2017). In recent years, multiple studies have suggested that iron, as well as rotenone, potentially results in the formation of reactive oxygen species (ROS) such as superoxide free radical and may consequently contribute to inducing selective and progressive dopaminergic neurotoxicity (Gao et al., 2002; Sherer et al., 2003a; Kaur et al., 2007; Sanders and Greenamyre, 2013; Zhang et al., 2014). In the present study, co-treatment with iron and rotenone significantly induced malondialdehyde (a product of oxidative damage to lipids) increase and glutathione (an antioxidant) decrease in the substantia nigra of male and female rats, indicating that iron and rotenone co-treatment may act together to induce behavioral and neurochemical deficits through inducing redox imbalance. Moreover, there was no significant change in cerebellar malondialdehyde and glutathione content of the rats co-treated with iron and rotenone, suggesting regional selectivity of redox imbalance induced by co-treatment with iron and rotenone. Of the various populations of neurons in the brain, substantia nigra dopaminergic neurons are particularly vulnerable to oxidative insult. Enhanced susceptibility of dopaminergic neurons to oxidative damage so far has been mainly attributed to their decreased intrinsic antioxidant capacity (Jenner and Olanow, 1998; Gao et al., 2002; Wang X. J. et al., 2011). In the present study, iron and rotenone co-treatment significantly decreased substantia nigra TH expression while no significant change was observed in the substantia nigra TH expression of male rats treated with iron (or rotenone) alone. Therefore, we infer that elevated neonatal iron supplement may produce moderate oxidative stress, which does not induce significant dopaminergic neurotoxicity. It is triggered by a second hit such as rotenone in later life, and then exaggerated oxidative stress may seriously impair dopaminergic neurons. Collectively, redox imbalance may be a mechanism for dopaminergic neurotoxicity induced by iron and rotenone co-treatment. Further studies will be needed to investigate precise mechanism underlying dopaminergic neurotoxicity induced by iron and rotenone co-treatment.
Biochanin A, an O-methylated isoflavone, is extracted from soy, chickpea or red clover. Being a natural compound, biochanin A is traditionally used as a carminative, antispasmodic, expectorant, and emmenagogue in some countries (Wang et al., 2014). Moreover, biochanin A may improve lipid profile as a peroxisome proliferator-activated receptor gamma (PPARγ) agonist and provide beneficial effects on metabolic syndrome such as hyperglycaemia (Wang et al., 2014; Park et al., 2016). It has been reported that biochanin A may be useful in the prevention and treatment of breast cancer, prostate cancer and hepatocellular carcinoma (Sun et al., 1998; Khan et al., 2011; Chen J. et al., 2015; Youssef et al., 2016). In addition, biochanin A was shown to exert neuroprotective effect in neurodegenerative diseases via inhibiting microglia activation or attenuating L-glutamate cytotoxicity (Chen et al., 2007; Tan et al., 2013; Wang J. et al., 2016). Here, we showed that biochanin A could improve behavioral and neurochemical deficits in male and female rats with iron and rotenone co-treatment in a dose-dependent manner. Malondialdehyde content decrease and glutathione content increase were also observed in the substantia nigra of male and female rats co-treated with iron and rotenone after the administration of biochanin A, indicating that maintaining redox balance may be a potential mechanism for neuroprotection by biochanin A.
In conclusion, our results show that co-treatment with iron and rotenone may result in aggravated neurochemical and behavioral deficits in male and female rats through inducing redox imbalance. Increased neonatal iron supplementation may enhance susceptibility of dopaminergic neurons to subsequent exposure of rotenone. Biochanin A may exert dopaminergic neuroprotection by maintaining redox balance.
Author Contributions
XW conceived the research and designed the experiments. XW, LY, HC, ZY, MW, and YL performed the experiments and collected the results. XW, LY, and HC statistically analyzed the data. XW, LY, and HC interpreted the results. The manuscript was written and revised by XW and LY. All the authors critically reviewed the manuscript and approved the final submitted manuscript.
Funding
This work was supported by the Projects of the National Natural Science Foundation of China (No. 81671273, 81171204, 30772280, 81400925, 81471148, 81771211, and 81703852), the Project of Shanghai Municipal Education Commission of China (No. 14YZ046), the Project of Shanghai Municipal Health and Family Planning Commission of China (No. 20134049), the Project of Shanghai Jiao Tong University of China (No. YG2013MS22), the Project of National Eastern Tech-transfer Center (201713972877), and the Projects of Shanghai Committee of Science and Technology (17401901000), National key R & D Program of China (2017YFC1310300), SHSMU-ION Research Center for Brain Disorders(2015NKX007).
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
Alves, G., Müller, B., Herlofson, K., HogenEsch, I., Telstad, W., Aarsland, D., et al. (2009). Incidence of Parkinson's disease in Norway: the Norwegian ParkWest study. J. Neurol. Neurosurg. Psychiatr. 80, 851–857. doi: 10.1136/jnnp.2008.168211
Ascherio, A., and Schwarzschild, M. A. (2016). The epidemiology of Parkinson's disease: risk factors and prevention. Lancet Neurol. 15, 1257–1272. doi: 10.1016/S1474-4422(16)30230-7
Baldereschi, M., Di Carlo, A., Rocca, W. A., Vanni, P., Maggi, S., Perissinotto, E., et al. (2000). Parkinson's disease and parkinsonism in a longitudinal study: two-fold higher incidence in men. ILSA working group. Italian longitudinal study on aging. Neurology 55, 1358–1363. doi: 10.1212/WNL.55.9.1358
Barnham, K. J., Masters, C. L., and Bush, A. I. (2004). Neurodegenerative diseases and oxidative stress. Nat. Rev. Drug Discov. 3, 205–214. doi: 10.1038/nrd1330
Beard, J. (2003). Iron deficiency alters brain development and functioning. J. Nutr. 133, 1468S−1472S.
Benton, D. (2010). The influence of dietary status on the cognitive performance of children. Mol. Nutr. Food Res. 54, 457–470. doi: 10.1002/mnfr.200900158
Betarbet, R., Sherer, T. B., MacKenzie, G., Garcia-Osuna, M., Panov, A. V., and Greenamyre, J. T. (2000). Chronic systemic pesticide exposure reproduces features of Parkinson's disease. Nat. Neurosci. 3, 1301–1306. doi: 10.1038/81834
Cannon, J. R., Tapias, V., Na, H. M., Honick, A. S., Drolet, R. E., and Greenamyre, J. T. (2009). A highly reproducible rotenone model of Parkinson's disease. Neurobiol. Dis. 34, 279–290. doi: 10.1016/j.nbd.2009.01.016
Carter, R. C., Jacobson, J. L., Burden, M. J., Armony-Sivan, R., Dodge, N. C., Angelilli, M. L., et al. (2010). Iron deficiency anemia and cognitive function in infancy. Pediatrics 126, e427–e434. doi: 10.1542/peds.2009-2097
Chen, H. Q., Jin, Z. Y., and Li, G. H. (2007). Biochanin A protects dopaminergic neurons against lipopolysaccharide-induced damage through inhibition of microglia activation and proinflammatory factors generation. Neurosci. Lett. 417, 112–117. doi: 10.1016/j.neulet.2006.11.045
Chen, H., Wang, X., Wang, M., Yang, L., Yan, Z., Zhang, Y., et al. (2015). Behavioral and neurochemical deficits in aging rats with increased neonatal iron intake: silibinin's neuroprotection by maintaining redox balance. Front. Aging Neurosci. 7:206. doi: 10.3389/fnagi.2015.00206
Chen, J., Ge, B., Wang, Y., Ye, Y., Zeng, S., and Huang, Z. (2015). Biochanin A promotes proliferation that involves a feedback loop of microRNA-375 and estrogen receptor alpha in breast cancer cells. Cell. Physiol. Biochem. 35, 639–646. doi: 10.1159/000369725
Connolly, B. S., and Lang, A. E. (2014). Pharmacological treatment of Parkinson disease: a review. JAMA 311, 1670–1683. doi: 10.1001/jama.2014.3654
Dauer, W., and Przedborski, S. (2003). Parkinson's disease: mechanisms and models. Neuron 39, 889–909. doi: 10.1016/S0896-6273(03)00568-3
Dluzen, D. E., McDermott, J. L., and Liu, B. (1996). Estrogen as a neuroprotectant against MPTP-induced neurotoxicity in C57/B1 mice. Neurotoxicol. Teratol. 18, 603–606. doi: 10.1016/0892-0362(96)00086-4
Fiesel, F. C., Caulfield, T. R., Moussaud-Lamodière, E. L., Ogaki, K., Dourado, D. F., Flores, S. C., et al. (2015). Structural and functional impact of parkinson disease-associated mutations in the E3 ubiquitin ligase parkin. Hum. Mutat. 36, 774–786. doi: 10.1002/humu.22808
Gaeta, A., and Hider, R. C. (2005). The crucial role of metal ions in neurodegeneration: the basis for a promising therapeutic strategy. Br. J. Pharmacol. 146, 1041–1059. doi: 10.1038/sj.bjp.0706416
Gao, H. M., Hong, J. S., Zhang, W., and Liu, B. (2002). Distinct role for microglia in rotenone-induced degeneration of dopaminergic neurons. J. Neurosci. 22, 782–790.
Ghanbari, M., Darweesh, S. K., de Looper, H. W., van Luijn, M. M., Hofman, A., Ikram, M. A., et al. (2016). Genetic variants in microRNAs and their binding sites are associated with the risk of parkinson disease. Hum. Mutat. 37, 292–300. doi: 10.1002/humu.22943
Griffiths, P. D., Dobson, B. R., Jones, G. R., and Clarke, D. T. (1999). Iron in the basal ganglia in Parkinson's disease. an in vitro study using extended X-ray absorption fine structure and cryo-electron microscopy. Brain 122(Pt 4), 667–673. doi: 10.1093/brain/122.4.667
Hare, D. J., Arora, M., Jenkins, N. L., Finkelstein, D. I., Doble, P. A., and Bush, A. I. (2015). Is early-life iron exposure critical in neurodegeneration? Nat. Rev. Neurol. 11, 536–544. doi: 10.1038/nrneurol.2015.100
Hefti, E., Bard, J., and Blanco, J. G. (2017). Analysis of heteroplasmic variants in the cardiac mitochondrial genome of individuals with down syndrome. Hum. Mutat. 38, 48–54. doi: 10.1002/humu.23071
Jagmag, S. A., Tripathi, N., Shukla, S. D., Maiti, S., and Khurana, S. (2016). Evaluation of models of Parkinson's disease. Front. Neurosci. 19:503. doi: 10.3389/fnins.2015.00503
Javed, H., Azimullah, S., Haque, M. E., and Ojha, S. K. (2016). Cannabinoid type 2 (CB2) receptors activation protects against oxidative stress and neuroinflammation associated dopaminergic neurodegeneration in rotenone model of Parkinson's Disease. Front. Neurosci. 10:321. doi: 10.3389/fnins.2016.00321
Jenner, P., and Olanow, C. W. (1998). Understanding cell death in Parkinson's disease. Ann. Neurol. 44, S72–S84. doi: 10.1002/ana.410440712
Jensen, N., and Oliveira, J. R. (2014). Basal ganglia vulnerability to oxidative stress. Front. Neurosci. 21:80. doi: 10.3389/fnins.2014.00080
Jiang, H., Song, N., Wang, J., Ren, L. Y., and Xie, J. X. (2007). Peripheral iron dextran induced degeneration of dopaminergic neurons in rat substantia nigra. Neurochem. Int. 51, 32–36. doi: 10.1016/j.neuint.2007.03.006
Jiang, T., Sun, Q., and Chen, S. (2016). Oxidative stress: a major pathogenesis and potential therapeutic target of antioxidative agents in Parkinson's disease and Alzheimer's disease. Prog. Neurobiol. 147, 1–19. doi: 10.1016/j.pneurobio.2016.07.005
Junxia, X., Hong, J., Wenfang, C., and Ming, Q. (2003). Dopamine release rather than content in the caudate putamen is associated with behavioral changes in the iron rat model of Parkinson's disease. Exp. Neurol. 182, 483–489. doi: 10.1016/S0014-4886(03)00123-7
Kaur, D., Peng, J., Chinta, S. J., Rajagopalan, S., Di Monte, D. A., Cherny, R. A., et al. (2007). Increased murine neonatal iron intake results in Parkinson-like neurodegeneration with age. Neurobiol. Aging 28, 907–913. doi: 10.1016/j.neurobiolaging.2006.04.003
Kaur, D., Yantiri, F., Rajagopalan, S., Kumar, J., Mo, J. Q., Boonplueang, R., et al. (2003). Genetic or pharmacological iron chelation prevents MPTP-induced neurotoxicity in vivo: a novel therapy for Parkinson's disease. Neuron 37, 899–909. doi: 10.1016/S0896-6273(03)00126-0
Khan, S. I., Zhao, J., Khan, I. A., Walker, L. A., and Dasmahapatra, A. K. (2011). Potential utility of natural products as regulators of breast cancer-associated aromatase promoters. Reprod. Biol. Endocrinol. 9:91. doi: 10.1186/1477-7827-9-91
Kidd, P. M. (2000). Parkinson's disease as multifactorial oxidative neurodegeneration: implications for integrative management. Altern. Med. Rev. 5, 502–529.
Kim, J. M. (2017). Pathophysiologic effects of CHCHD2 variants associated with late-onset Parkinson disease. Hum. Mutat. 38, 903. doi: 10.1002/humu.23264
Lang, A. E., and Lozano, A. M. (1998). Parkinson's disease. First of two parts. N. Engl. J. Med. 339, 1044–1053. doi: 10.1056/NEJM199810083391506
Lin, A. M., and Ho, L. T. (2000). Melatonin suppresses iron-induced neurodegeneration in rat brain. Free Radic. Biol. Med. 28, 904–911. doi: 10.1016/S0891-5849(00)00169-6
McNaught, K. S., Perl, D. P., Brownell, A. L., and Olanow, C. W. (2004). Systemic exposure to proteasome inhibitors causes a progressive model of Parkinson's disease. Ann. Neurol. 56, 149–162. doi: 10.1002/ana.20186
Metherell, L. A., Guerra-Assunção, J. A., Sternberg, M. J., and David, A. (2016). Three-dimensional model of human nicotinamide nucleotide transhydrogenase (NNT) and sequence-structure analysis of its disease-causing variations. Hum. Mutat. 37, 1074–1084. doi: 10.1002/humu.23046
Millichap, J. G. (2008). Etiologic classification of attention-deficit/hyperactivity disorder. Pediatrics 121, e358–e365. doi: 10.1542/peds.2007-1332
Mitsuyama, S., Ohtsubo, M., Minoshima, S., and Shimizu, N. (2015). The KM-parkin-DB: a Sub-set mutationview database specialized for PARK2 (PARKIN) variants. Hum. Mutat. 36, E2430–E2440. doi: 10.1002/humu.22803
Niedzielska, E., Smaga, I., Gawlik, M., Moniczewski, A., Stankowicz, P., Pera, J., et al. (2016). Oxidative stress in neurodegenerative diseases. Mol. Neurobiol. 53, 4094–4125. doi: 10.1007/s12035-015-9337-5
Niranjan, R. (2014). The role of inflammatory and oxidative stress mechanisms in the pathogenesis of Parkinson's disease: focus on astrocytes. Mol. Neurobiol. 49, 28–38. doi: 10.1007/s12035-013-8483-x
Olanow, C. W., and Tatton, W. G. (1999). Etiology and pathogenesis of Parkinson's disease. Annu. Rev. Neurosci. 22, 123–144. doi: 10.1146/annurev.neuro.22.1.123
Park, H. S., Hur, H. J., Kim, S. H., Park, S. J., Hong, M. J., Sung, M. J., et al. (2016). Biochanin A improves hepatic steatosis and insulin resistance by regulating the hepatic lipid and glucose metabolic pathways in diet-induced obese mice. Mol. Nutr. Food Res. 60, 1944–1955. doi: 10.1002/mnfr.201500689
Pavon, J. M., Whitson, H. E., and Okun, M. S. (2010). Parkinson's disease in women: a call for improved clinical studies and for comparative effectiveness research. Maturitas 65, 352–358. doi: 10.1016/j.maturitas.2010.01.001
Richter, F., Gabby, L., McDowell, K. A., Mulligan, C. K., De La Rosa, K., Sioshansi, P. C., et al. (2017). Effects of decreased dopamine transporter levels on nigrostriatal neurons and paraquat/maneb toxicity in mice. Neurobiol. Aging 51, 54–66. doi: 10.1016/j.neurobiolaging.2016.11.015
Sanders, L. H., and Greenamyre, J. T. (2013). Oxidative damage to macromolecules in human Parkinson disease and the rotenone model. Free. Radic. Biol. Med. 62, 111–120. doi: 10.1016/j.freeradbiomed.2013.01.003
Sherer, T. B., Betarbet, R., Kim, J. H., and Greenamyre, J. T. (2003a). Selective microglial activation in the rat rotenone model of Parkinson's disease. Neurosci. Lett. 341, 87–90. doi: 10.1016/S0304-3940(03)00172-1
Sherer, T. B., Kim, J. H., Betarbet, R., and Greenamyre, J. T. (2003b). Subcutaneous rotenone exposure causes highly selective dopaminergic degeneration and alpha-synuclein aggregation. Exp. Neurol. 179, 9–16. doi: 10.1006/exnr.2002.8072
Sofic, E., Paulus, W., Jellinger, K., Riederer, P., and Youdim, M. B. (1991). Selective increase of iron in substantia nigra zona compacta of parkinsonian brains. J. Neurochem. 56, 978–982. doi: 10.1111/j.1471-4159.1991.tb02017.x
Stankiewicz, J., Panter, S. S., Neema, M., Arora, A., Batt, C. E., and Bakshi, R. (2007). Iron in chronic brain disorders: imaging and neurotherapeutic implications. Neurotherapeutics 4, 371–386. doi: 10.1016/j.nurt.2007.05.006
Stayte, S., and Vissel, B. (2014). Advances in non-dopaminergic treatments for Parkinson's disease. Front. Neurosci. 8:113. doi: 10.3389/fnins.2014.00113
Su, S. J., Yeh, Y. T., Su, S. H., Chang, K. L., Shyu, H. W., Chen, K. M., et al. (2013). Biochanin a promotes osteogenic but inhibits adipogenic differentiation: evidence with primary adipose-derived stem cells. Evid. Based. Complement. Alternat. Med. 2013:846039. doi: 10.1155/2013/846039
Sulzer, D. (2007). Multiple hit hypotheses for dopamine neuron loss in Parkinson's disease. Trends Neurosci. 30, 244–250. doi: 10.1016/j.tins.2007.03.009
Sun, X. Y., Plouzek, C. A., Henry, J. P., Wang, T. T., and Phang, J. M. (1998). Increased UDP-glucuronosyltransferase activity and decreased prostate specific antigen production by biochanin A in prostate cancer cells. Cancer Res. 58, 2379–2384.
Tan, J. W., Tham, C. L., Israf, D. A., Lee, S. H., and Kim, M. K. (2013). Neuroprotective effects of biochanin A against glutamate-induced cytotoxicity in PC12 cells via apoptosis inhibition. Neurochem. Res. 38, 512–518. doi: 10.1007/s11064-012-0943-6
Tanner, C. M., Ottman, R., Goldman, S. M., Ellenberg, J., Chan, P., Mayeux, R., et al. (1999). Parkinson disease in twins: an etiologic study. JAMA 281, 341–346. doi: 10.1001/jama.281.4.341
Taylor, K. S., Cook, J. A., and Counsell, C. E. (2007). Heterogeneity in male to female risk for Parkinson's disease. J. Neurol. Neurosurg. Psychiatry 78, 905–906. doi: 10.1136/jnnp.2006.104695
Tio, M., Wen, R., Lim, Y. L., Zukifli, Z. H. B., Xie, S., Ho, P., et al. (2017). Varied pathological and therapeutic response effects associated with CHCHD2 mutant and risk variants. Hum. Mutat. 38, 978–987. doi: 10.1002/humu.23234
Wang, A., Costello, S., Cockburn, M., Zhang, X., Bronstein, J., and Ritz, B. (2011). Parkinson's disease risk from ambient exposure to pesticides. Eur. J. Epidemiol. 26, 547–555. doi: 10.1007/s10654-011-9574-5
Wang, J., Wu, W. Y., Huang, H., Li, W. Z., Chen, H. Q., and Yin, Y. Y. (2016). Biochanin A protects against lipopolysaccharide-induced damage of dopaminergic neurons both in vivo and in vitro via inhibition of microglial activation. Neurotox. Res. 30, 486–498. doi: 10.1007/s12640-016-9648-y
Wang, L., Almeida, L. E. F., de Souza Batista, C. M., Khaibullina, A., Xu, N., Albani, S., et al. (2016). Cognitive and behavior deficits in sickle cell mice are associated with profound neuropathologic changes in hippocampus and cerebellum. Neurobiol. Dis. 85, 60–72. doi: 10.1016/j.nbd.2015.10.004
Wang, L., Waltenberger, B., Pferschy-Wenzig, E. M., Blunder, M., Liu, X., Malainer, C., et al. (2014). Natural product agonists of peroxisome proliferator-activated receptor gamma (PPARgamma): a review. Biochem. Pharmacol. 92, 73–89. doi: 10.1016/j.bcp.2014.07.018
Wang, X., Guan, Q., Wang, M., Yang, L., Bai, J., Yan, Z., et al. (2015). Aging-related rotenone-induced neurochemical and behavioral deficits: role of SIRT2 and redox imbalance, and neuroprotection by AK-7. Drug Des. Devel. Ther. 9, 2553–2563. doi: 10.2147/DDDT.S81539
Wang, X. J., Zhang, S., Yan, Z. Q., Zhao, Y. X., Zhou, H. Y., Wang, Y., et al. (2011). Impaired CD200-CD200R-mediated microglia silencing enhances midbrain dopaminergic neurodegeneration: roles of aging, superoxide, NADPH oxidase, and p38 MAPK. Free Radic. Biol. Med. 50, 1094–1106. doi: 10.1016/j.freeradbiomed.2011.01.032
Ward, R. J., Zucca, F. A., Duyn, J. H., Crichton, R. R., and Zecca, L. (2014). The role of iron in brain ageing and neurodegenerative disorders. Lancet Neurol. 13, 1045–1060. doi: 10.1016/S1474-4422(14)70117-6
Wu, Q., Yang, X., Zhang, Y., Zhang, L., and Feng, L. (2016). Chronic mild stress accelerates the progression of Parkinson's disease in A53T α-synuclein transgenic mice. Exp. Neurol. 285(Pt A), 61–71. doi: 10.1016/j.expneurol.2016.09.004
Xie, Y., and Chen, Y. (2016). microRNAs: emerging targets regulating oxidative stress in the models of Parkinson's disease. Front. Neurosci. 28:298. doi: 10.3389/fnins.2016.00298
Youssef, M. M., Tolba, M. F., Badawy, N. N., Liu, A. W., El-Ahwany, E., Khalifa, A. E., et al. (2016). Novel combination of sorafenib and biochanin-A synergistically enhances the anti-proliferative and pro-apoptotic effects on hepatocellular carcinoma cells. Sci. Rep. 6:30717. doi: 10.1038/srep30717
Zhang, W., Yan, Z. F., Gao, J. H., Sun, L., Huang, X. Y., Liu, Z., et al. (2014). Role and mechanism of microglial activation in iron-induced selective and progressive dopaminergic neurodegeneration. Mol. Neurobiol. 49, 1153–1165. doi: 10.1007/s12035-013-8586-4
Keywords: parkinson's disease, iron, roteone, redox balance, biochanin A
Citation: Yu L, Wang X, Chen H, Yan Z, Wang M and Li Y (2017) Neurochemical and Behavior Deficits in Rats with Iron and Rotenone Co-treatment: Role of Redox Imbalance and Neuroprotection by Biochanin A. Front. Neurosci. 11:657. doi: 10.3389/fnins.2017.00657
Received: 16 June 2017; Accepted: 10 November 2017;
Published: 23 November 2017.
Edited by:
Sadayuki Hashioka, Shimane University, JapanReviewed by:
Luke Esposito, Allen Institute for Brain Science, United StatesRebecca Jane Rylett, University of Western Ontario, Canada
Xuebing Cao, Tongji Medical College, Huazhong University of Science and Technology, China
Copyright © 2017 Yu, Wang, Chen, Yan, Wang and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xijin Wang, d2FuZ3hpamluQHhpbmh1YW1lZC5jb20uY24=
 Lijia Yu
Lijia Yu Xijin Wang
Xijin Wang Hanqing Chen
Hanqing Chen Zhiqiang Yan3
Zhiqiang Yan3