
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
MINI REVIEW article
Front. Neurosci., 05 September 2017
Sec. Neuroenergetics and Brain Health
Volume 11 - 2017 | https://doi.org/10.3389/fnins.2017.00498
Celiac disease (CD) can be considered a complex multi-organ disorder with highly variable extra-intestinal, including neurological, involvement. Cerebellar ataxia, peripheral neuropathy, seizures, headache, cognitive impairment, and neuropsychiatric diseases are complications frequently reported. These manifestations may be present at the onset of the typical disease or become clinically evident during its course. However, CD subjects with subclinical neurological involvement have also been described, as well as patients with clear central and/or peripheral nervous system and intestinal histopathological disease features in the absence of typical CD manifestations. Based on these considerations, a sensitive and specific diagnostic method that is able to detect early disease process, progression, and complications is desirable. In this context, neurophysiological techniques play a crucial role in the non-invasive assessment of central nervous system (CNS) excitability and conductivity. Moreover, some of these tools are known for their valuable role in early diagnosis and follow-up of several neurological diseases or systemic disorders, such as CD with nervous system involvement, even at the subclinical level. This review provides an up-to-date summary of the neurophysiological basis of CD using electroencephalography (EEG), multimodal evoked potentials, and transcranial magnetic stimulation (TMS). The evidence examined here seems to converge on an overall profile of “hyperexcitable celiac brain,” which partially recovers after institution of a gluten-free diet (GFD). The main translational correlate is that in case of subclinical neurological involvement or overt unexplained symptoms, neurophysiology could contribute to the diagnosis, assessment, and monitoring of a potentially underlying CD.
Celiac disease (CD) is an autoimmune disorder triggered by the ingestion of gluten that, in genetically predisposed individuals, leads to damage of the small intestine and consequent malabsorption. Most patients (95% of them) are carriers of the DQ2 or DQ8 haplotype of the histocompatibility complex class II human leukocyte antigen (Lebwohl et al., 2017). Tissue transglutaminase (tTG) is the main auto-antigen (Alaedini and Green, 2008), whereas gut histopathology shows variable degrees of small bowel mucosal villi atrophy. CD affects 0.3–1.5% of the general population (approximately 1 of 120–300 people in Europe and America) (Morello et al., 2003; Bingley et al., 2004). To date, the only established therapy is a lifetime dietary gluten restriction, which is usually followed by relief of several clinical manifestations, normalization of histological and serological markers, as well as decreased risk of associated malignant and non-malignant complications (Holmes, 1996, 2002).
Clinically, although diarrhea and other gastro-intestinal symptoms can commonly be observed at disease onset or in its early phases in both pediatric patients and young adults, they are not as frequent as in the past (Campagna et al., 2017). Many adults (>50%) exhibit significant extra-intestinal involvement even without typical CD manifestations (Cooke and Smith, 1966; Hadjivassiliou et al., 2002a, 2010, 2014; Bushara, 2005; Uygur-Bayramicli and Ozel, 2011; Castillo et al., 2015). Therefore, CD is considered a complex systemic disorder with multifactorial pathogenesis that should be investigated from genetic, biological, and environmental perspectives.
Currently, little is known about the neurophysiology of central nervous system (CNS) damage in CD. This review aims to summarize the electrophysiological evidence on CNS functioning and pathology, including the clinical and instrumental response to a gluten-free diet (GFD).
Nowadays, it is widely accepted that the typical disease represents a small proportion of the so-called “CD iceberg,” because 5–6 fold more patients present with atypical or silent forms (Green et al., 2005). Neurological manifestations may either precede or follow the disease, or be present at its onset (Hadjivassiliou et al., 2002a, 2010, 2014; Briani et al., 2008). Therefore, a sensitive and specific diagnostic method able to recognize early disease process, progression, and complication would be desirable.
To date, both the causative factors and pathophysiological mechanisms of neurological involvement in CD remain a matter of debate. According to the literature, the nervous system may be one of the elective sites of gluten-mediated pathogenesis, including cross-reacting antibodies, immune-complex deposition, direct neurotoxicity, other immune-mediated factors, and deficiency of vitamin and other nutrients secondary to chronic malabsorption (Zelnik et al., 2004; Bushara, 2005; Abenavoli, 2010; Parisi et al., 2015). Recently, studies using single photon emission computed tomography showed regional changes in cerebral perfusion, with regression after institution of a GFD (De Santis et al., 1997; Usai et al., 2004). The authors argued that the cerebral hypoperfusion might be related to intestinal hyperemia from immune-mediated or endothelial damage due to immune-complex deposition likely involving antibodies against gliadin (De Santis et al., 1997). Alternatively, cortical brain hypoperfusion could reflect focal vasculitis secondary to perivascular inflammation (Usai et al., 2004).
“Gluten ataxia” is one of the first recognized symptoms (Cooke and Smith, 1966) and the most frequent neurological disturbance in CD (Hadjivassiliou et al., 2015). Dysarthria, cortical-spinal signs, eye and gaze movement disorders, and cerebellar ataxia are representative presentations. Recent studies showed deposits of antibodies against tTG on cerebellar blood vessels, adding support to a blood-brain barrier (BBB) dysfunction in CD (Hadjivassiliou et al., 2006, 2015). Interestingly, gluten ataxia is not usually related to intestinal manifestations or vitamin deficiency, and improvement with a GFD is possible (Hadjivassiliou et al., 2006).
Peripheral neuropathy is the second most common neurological manifestation of CD (up to half of patients) after cerebellar ataxia, and can appear even before diagnosis (Chin et al., 2003; Chin and Latov, 2005). Studies on the effect of a GFD on peripheral neuropathy are conflicting, with some authors reporting clinical improvement whereas others concluding a lack of relevant response (Luostarinen et al., 2003; Siqueira Neto et al., 2004). A previous study on 32 consecutive adult patients complaining of peripheral neuropathy, autonomic dysfunction, or both, and showing anti-neuronal antibodies found no response despite the adoption of a GFD (Tursi et al., 2006).
A bidirectional link between epilepsy and CD has been established in several studies, although not all (Vieira et al., 2013), with rates of prevalence from 3.5 to 7.2% (Cooke and Smith, 1966; Zelnik et al., 2004; Bushara, 2005; Uygur-Bayramicli and Ozel, 2011; Hadjivassiliou et al., 2014; Parisi et al., 2015). A large population-based cohort study observed an increased risk of CD in subjects of all ages, including children, even when epilepsy was independently restricted to patients receiving the diagnosis of epilepsy and those with prescriptions of antiepileptic drugs (Ludvigsson et al., 2012). The hypotheses accounting for epilepsy in CD included a gluten-mediated toxicity, an immune-induced cortical damage, the presence of cerebral calcifications, and vitamins/trace elements malabsorption. GFD usually controls seizures refractory to antiepileptic drugs (Hadjivassiliou et al., 2002a; Canales et al., 2006). CD-related progressive ataxia is associated with stimulus-sensitive myoclonus, opsoclonus-myoclonus, and sometimes with seizures (Borg, 2006; Deconinck et al., 2006).
It has been reported that a GFD results in complaints of less severe headache symptoms by celiac patients (Hadjivassiliou et al., 2001). Accordingly, structural and functional neuroimaging studies were in favor of an association between migraine and CD, with relief after gluten restriction (Hadjivassiliou et al., 2001). However, when headaches in CD patients were compared with those in the general population, a conclusive association was not proven (Nikpour, 2012).
Adult CD patients often complain of mild cognitive symptoms called “brain fog,” which improves when gluten-restriction is started, but re-appears with dietary contamination (Lichtwark et al., 2014; Yelland, 2017). Concentration and attention difficulties, episodic memory deficits, word-retrieval problems, reduced mental acuity, and episodes of confusion or disorientation are the commonly reported features (Lurie et al., 2008). In some severely affected patients, dementia can develop as acalculia, confusion, amnesia, and personality disorders (Collin et al., 1991; Hu et al., 2006; Lurie et al., 2008; Casella et al., 2012). Despite long-term administration of a GFD, patients older than 65 years exhibited worse cognitive performance than age- and sex-matched controls (Casella et al., 2012).
Several psychiatric symptoms, including depression, bipolar disorder, apathy (Carta et al., 2003, 2015; Cicarelli et al., 2003), excessive anxiety (Bushara, 2005; Campagna et al., 2017), irritability (Hernanz and Polanco, 1991), schizophrenia (De Santis et al., 1997; Bushara, 2005), eating disorders (Addolorato et al., 2001), attention-deficit/hyperactivity disorder (Karwautz et al., 2008), autism (Niederhofer and Pittschieler, 2006), and sleep complaints (Barcia et al., 2008) have been associated with CD.
Reactive anxiety that usually ameliorates with a GFD is the predominant form of anxiety disorder in these patients. Depressive disturbances, which affect a relevant number of subjects, may significantly impair quality of life, and are a good predictor of lack of dietary compliance (Zingone et al., 2010). Therefore, screening patients for depression is of pivotal importance both at diagnosis and follow-up in order to advice psychological support and/or pharmacological therapy. Possible causative factors of mood disorders might be tryptophan deficiency secondary to chronic malabsorption (Hallert et al., 1982; Hernanz and Polanco, 1991) or co-morbidity with thyroid disease (Carta et al., 2002). Decreases in levels of serotonin, dopamine, and noradrenaline metabolites in cerebrospinal fluid as well as tryptophan and other monoamine precursors in serum were observed in untreated patients (Hallert et al., 1982; Hernanz and Polanco, 1991).
Clinical improvement was reported only after long-term administration of a GFD (>5 years) (van Hees et al., 2013), highlighting the importance of prolonged alimentary restriction on extra-intestinal CD symptoms as well.
The spectrum of electroencephalography (EEG) features associated with CD is rather wide, although focal activity in terms of unilateral or bilateral spike or slow waves, mainly localized in the occipital regions, have been reported in most of the wakefulness EEG studies (Magaudda et al., 1993; Labate et al., 2001; Pratesi et al., 2003; Ranua et al., 2005; Lionetti et al., 2010; Licchetta et al., 2011; Aksoy et al., 2016). However, as recommended (Parisi et al., 2014), EEG patterns should not be considered disease-specific.
A recent prospective study among 307 CD children compared with 197 age- and sex-matched controls observed that patients were more prone to epileptiform activities on EEG (spike/sharp-wave discharges, especially in the occipital lobes but also in the central-temporal sites and in diffuse distribution). However, early and strict adherence to a GFD effectively decreased these findings (Işıkay et al., 2015a). In addition, a positive correlation between tTG level and epileptiform changes during sleep and awake EEG was found (Işıkay et al., 2015a). The concept that the occipital region is frequently involved in CD seems to be supported by the evidence of occipital calcium deposition, occipital lobe semiology, and EEG findings. The preferential involvement of this lobe may lie on several factors, such as its vulnerability to some metabolic circumstances (e.g., hypoglycemia and hypoxia) and its thinner morphological structure than other cortical regions (Işıkay et al., 2015b). However, the opposite scenario (the occurrence of CD in patients with “posterior” epileptic semiology) is not always true because in a group of 90 pediatric epileptic patients with occipital EEG abnormalities, tTG antibody was positive in only two (Canales et al., 2006).
CD-associated epilepsy has also been reported in association with other neurological signs, such as ataxia, tremor, and progressive myoclonus (Javed et al., 2012; Sarrigiannis et al., 2014). In these cases, epilepsy was usually refractory, and EEG demonstrated spike and waves in the right anterior and mid-temporal lobes, as well as bilateral slow and sharp waves. Some of these spike waves were present in association with localized jerks of the upper or lower limb, although without periodic complexes (Javed et al., 2012; Sarrigiannis et al., 2014). Finally, the fixation-off sensitivity phenomenon could be observed (Casciato et al., 2015).
In regard to the impact of dietary restriction, a recent study in 19 children with biopsy-proven CD revealed abnormal EEG findings in 48% of them that were no longer evident in most of the patients after 6 months of GFD (Parisi et al., 2015). However, some asymptomatic children and adolescents still manifested hyperexcitability to EEG despite the diet (Parisi et al., 2015).
In summary, CD screening should be performed in patients with cryptogenic and/or refractory epilepsy, or in the presence of unexplained EEG findings. Gluten restriction is usually effective in ameliorating the clinical-instrumental correlates.
Few studies, most of which used somatosensory evoked potentials (SEPs), have explored CD with evoked potentials. Di Lazzaro and co-workers reported a patient whose lower limb SEPs presented enlargement of lumbar waves and bilateral lack of cortical responses, suggesting an impaired somatosensory conduction along the spinal dorsal columns; GFD induced complete clinical-instrumental recovery (Di Lazzaro et al., 2010). Another case presented one child with prolonged central conduction time among 27 treated children (Cakir et al., 2007). In patients with cerebellar ataxia associated with subclinical CD responding to a GFD, normal SEPs were reported (Pellecchia et al., 1999). However, in a large cohort of adult ataxic patients, more than half had loss or delayed P40 cortical response, suggesting dorsal column degeneration (Bürk et al., 2001). In regard to SEPs in cortical mycolonus, a previous study in two CD-proven subjects with myoclonic ataxic syndrome showed giant and time-locked cortical responses that preceded the myoclonus (Tijssen et al., 2000). The authors speculated that, in spite of the neurophysiological evidence of cerebral cortical involvement, the hyperexcitability was mainly located in the cerebellum, and that the effects on sensory-motor cortex represented a remote influence from cerebellar dysfunction (Tijssen et al., 2000). Conversely, the cortical electrophysiological origin of the myoclonus was argued by other researchers who found their patients responded poorly to a GFD and worsened progressively (Lu et al., 1986; Tison et al., 1989; Bhatia et al., 1995).
Although CD may impact the auditory system (Bürk et al., 2001), brainstem auditory evoked potentials (BAEPs) and vestibular evoked myogenic potentials were reported to be normal (Pawlak-Osińska et al., 2007). More recently, it was found that only 1 of 25 patients had abnormalities in BAEPs in terms of moderate sensorineural hearing loss (Aksoy et al., 2016).
Complications affecting visual pathways may develop in CD and be evidenced by visual evoked potentials (VEPs) (Bürk et al., 2001; Freeman, 2008; Hadjivassiliou et al., 2010). In particular, patients can show abnormalities on VEPs without evident lesions at neuroimaging (Aksoy et al., 2016). A previous case described a slight increase of P100 wave latency bilaterally at pattern-reversal VEPs that reverted back to normal after a GFD (Pellecchia et al., 1999). Given their role in detecting even preclinical pathology in subjects with normal ophthalmological and brain imaging exams, VEPs may provide useful insights in neurologically asymptomatic CD patients (Aksoy et al., 2016).
In 1999, Pellecchia and co-workers first reported motor evoked responses to transcranial magnetic stimulation (TMS) in a CD patient who exhibited reduced amplitude in the rectus femoris muscle that improved with diet; however, motor responses remained undetectable in the tibialis anterior muscle (Pellecchia et al., 1999). A year later, report of delayed motor response in the left tibialis anterior muscle and abnormal cortical inhibition was published in one of three CD patients with cortical myoclonus (Tijssen et al., 2000).
Specific TMS studies before and after GFD were published more recently. TMS is an electrophysiological tool able to non-invasively explore the excitation state of motor cortical areas and conductivity of the pyramidal tract in vivo. Moreover, it is capable of unveiling subclinical central motor involvement in different neurological and psychiatric diseases or systemic disorders affecting the CNS (Bella et al., 2011a,b, 2016; Pennisi et al., 2011a,b, 2015, 2017; Concerto et al., 2013; Lanza et al., 2013, 2015a,b, 2017a,b; Cantone et al., 2014, 2017), also providing prognostic (Bella et al., 2013; Pennisi et al., 2016) and therapeutic implications (Spampinato et al., 2013; Concerto et al., 2015; Bordet et al., 2017). Lastly, the so-called “pharmaco-TMS” can selectively probe the functioning of different central neurotransmission pathways, such as glutamate, gamma-aminobutyric-acid (GABA), monoamine, and acetylcholine, by testing their pharmacological agonists or antagonists (Paulus et al., 2008; Ziemann et al., 2015).
The first TMS study investigated 20 de novo CD patients without apparent neurological involvement and 20 age-matched controls (Pennisi et al., 2014). TMS revealed cortical motor disinhibition and hyperfacilitation, which is a profile compatible with dysfunctional GABAergic and glutamatergic transmissions, in patients. The authors hypothesized that an imbalance of excitatory and inhibitory circuits within the motor cortex might be the neurochemical correlate of the cross-interaction between antibodies against gliadin and specific neuronal antigens. An alternative explanation was the deposition of tTG-immunoglobulin leading to an abnormal ion levels across neuronal membrane. Likewise, antibodies synthesized within the CNS and directed against glutamic acid decarboxylase might disrupt the functioning of GABAergic interneurons (Pennisi et al., 2014).
The same cohort of patients underwent re-evaluation after a relatively short time of a GFD (median of 16 months) (Bella et al., 2015). Their gastrointestinal symptoms were ameliorated but, unexpectedly, the cortical excitability to TMS further increased. This finding was hypothesized to represent a plastic re-organization of the cerebral cortex triggered by gluten exposure and independent of GFD. On the other hand, diet duration or compliance might not have been enough to induce an adequate remission (Bella et al., 2015). A recent cross-sectional TMS study after a much longer GFD (mean period of 8.35 years) showed that a more prolonged period of gluten restriction was required to revert the cortical changes in adult CD patients. Nevertheless, regardless of diet, some specific excitatory features to TMS remained, probably suggesting an intracortical synaptic rearrangement, mostly involving glutamate-mediated interneurons (Pennisi et al., 2017).
The main translational value of this review is that clinical neurophysiology can contribute to the diagnosis, assessment, and monitoring of CD even in patients with subclinical CNS involvement or unexplained neurological symptoms (Table 1, Figure 1). In particular, the majority of electrophysiological changes are often subclinical (“celiac iceberg”), and these need to be strictly monitored because of the possibility of progression to clinically visible neurological syndrome in both young and adult patients (“symptomatic celiac disease”). Accordingly, it has been shown that there is an increased risk of neurological complications in atypical or silent CD forms, especially in older patients or those older at diagnosis (Aksoy et al., 2016). It is worth noting that, despite their valuable role, anti-ganglioside antibodies and neuronal antigens are not always specifically linked to the neurological manifestations and their progression in the course of CD (Kaplan et al., 1988; Gobbi et al., 1992; Hadjivassiliou et al., 1998, 2002b; Aksoy et al., 2016).
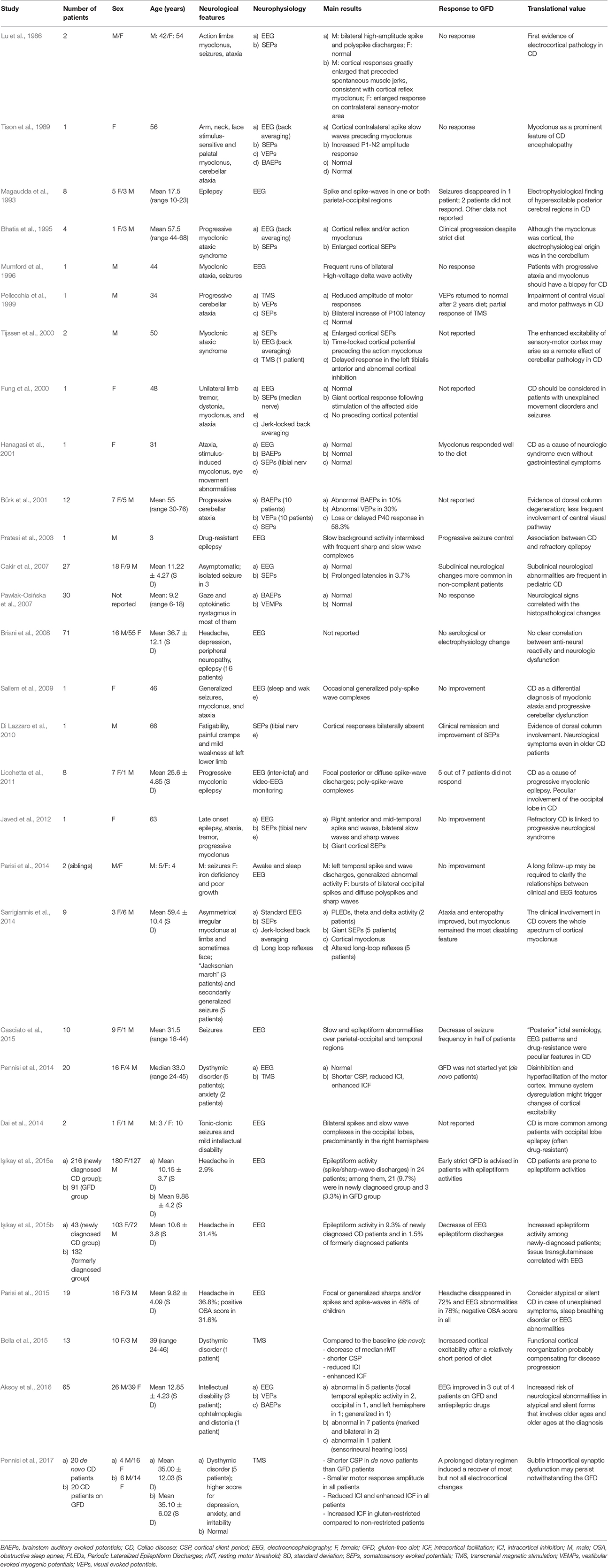
Table 1. Studies using electrophysiological techniques probing the central nervous system involvement in patients with celiac disease.
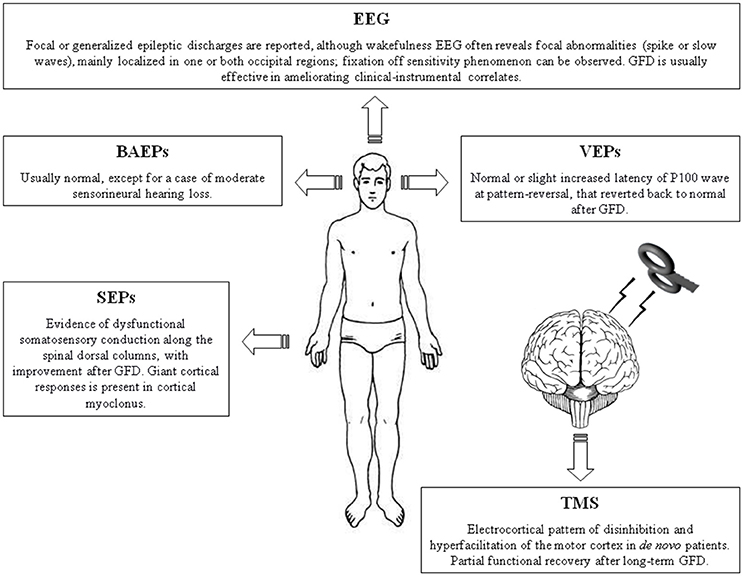
Figure 1. Summary figure illustrating the main neurophysiological findings in patients with celiac disease. BAEPs, brainstem auditory evoked potentials; EEG, electroencephalography. GFD, gluten-free diet; SEPs, somatosensory evoked potentials; TMS, Transcranial magnetic stimulation; VEPs, visual evoked potentials.
From a pure neurophysiological perspective, findings from EEG, SEPs, and TMS seem to converge on an overall profile of “hyperexcitable celiac brain,” albeit this may not be confined to the cerebral cortex. Indeed, an increase in cerebral cortical excitability may arise from enhanced inputs from the cerebellum (Tijssen et al., 2000). In this context, it is important to remember that malabsorption syndrome (with the consequent deficiency of vitamins and other nutrients) probably does not account for these cortical manifestations, given that it takes place in the most severely affected patients whose intestinal mucosa are seriously damaged and do not recover after institution of a GFD (Pennisi et al., 2014).
Regarding humoral autoimmunity to neuronal antigens, deposits of anti-tTG2 and anti-tTG6 antibodies have been found not only in the small intestine but also in different CNS sites (cerebellum, pons, medulla, brain blood vessels) (Hadjivassiliou et al., 2008). Furthermore, a possible BBB lesion, secondary to diffuse infiltration of T-lymphocytes and inflammatory cells within the perivascular cuffing might expose cerebral tissues to antibodies (Hadjivassiliou et al., 2010). The result may be a vicious circle that eventually leads to a prevailing synaptic hyperexcitation and a weaker inhibition at the cortical level (Pennisi et al., 2014). The increased excitability may also be the correlate of a glutamate-induced cortical rearrangement or a dysfunctional control of GABAergic inhibitory interneurons. In particular, because glutamate is of pivotal importance in synaptic plasticity, it can be speculated that immune system dysregulation triggered by gluten ingestion, might result in a long-standing activation of post-synaptic glutamate receptors accounting for the enhanced hyperexcitability (Bella et al., 2015).
The neurophysiological-based approach to CD should take into account potential pitfalls and critical aspects related to both the techniques themselves and methodological biases in the studies reviewed here. First, as mentioned, electrophysiological changes are not disease-specific. Second, an association finding does not mean causative relationship. For instance, an association between CD and amyotrophic lateral sclerosis was previously reported in different investigations (Turner et al., 2007, 2013; Brown et al., 2010; Bersano et al., 2015; Gadoth et al., 2015) but not confirmed in a large population-based cohort study (Ludvigsson et al., 2014). Finally, it is mandatory to make a differential diagnosis between hyperexcitability-related seizures and incidental EEG findings in neurologically asymptomatic CD subjects. In the latter case, EEG changes represent a confounding factor and a long follow-up is required (Parisi et al., 2014).
The response of neurological symptoms to a GFD is still controversial. Current knowledge encompasses an initial phase when patients are “gluten-sensitive” and a subsequent stage characterized by “gluten-insensitivity” (Tursi et al., 2006). An older age at diagnosis or a prolonged period of gluten ingestion may account for persistent neurological symptoms after a relatively short period of GFD (Bella et al., 2015). Moreover, gluten restriction is not usually effective in patients with refractory CD and in those with an associated autoimmune disease or some neurological complications (Hadjivassiliou et al., 2010; Castillo et al., 2015; Campagna et al., 2017). It is reasonable to conclude that some neurological aspects improve after diet restriction whereas others persist, supporting the concept that the more prolonged the GFD, the more likely clinical and neurophysiological remission may occur. However, given that neurological impairment may develop despite an adequate adherence to a GFD (Luostarinen et al., 2003; Chin and Latov, 2005; Tursi et al., 2006; Bürk et al., 2009), other causative factors have to contribute (McKeon et al., 2014): (a) accidental minimal gluten contamination despite a good dietary compliance (Green and Jabri, 2003); (b) direct gliadin-mediated inflammatory attack; (c) other components that are independent of GFD (Tijssen et al., 2000).
Based on further understanding of the pathogenesis and treatment of CD, neurophysiology-targeted non-dietary therapies are in development (Schuppan et al., 2009). Similarly, modern rehabilitation approach involves organizational measures that promote not only clinical recovery but also a better quality of life (Sabel'nikova et al., 2013) through the support of different medical and non-medical specialists (Usanova et al., 2012).
In conclusion, neurophysiology, together with clinical, serological, and imaging data, can help in disentangling the multifaceted physiopathological and neurobiological mechanisms coupling gut and brain in CD. The eventual identification of neurophysiological markers might be useful in the diagnosis and monitoring of CD, aiming to improve the healthcare of both single subjects and the global community.
All authors provided substantial contributions to the conception, drafting, critical revision for important intellectual content, final approval, and agreement to be accountable for all aspects of the work. In particular, MP and AB conceived and designed the study, MC and GL reviewed the literature and drafted the manuscript, and GP and RB critically reviewed and finalized the paper.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
We would like to thank Charlesworth Author Services (http://www.charlesworthauthorservices.com) for English language editing.
Abenavoli, L. (2010). Nervous system in the gluten syndrome: a close relationship. Med. Hypotheses 74, 204–205. doi: 10.1016/j.mehy.2009.08.012
Addolorato, G., Capristo, E., Ghittoni, G., Valeri, C., Mascianà, R., Ancona, C., et al. (2001). Anxiety but not depression decreases in coeliac patients after one-year gluten-free diet: a longitudinal study. Scand. J. Gastroenterol. 36, 502–506. doi: 10.1080/00365520119754
Aksoy, E., Tıraş-Teber, S., Kansu, A., Deda, G., and Kartal, A. (2016). Neurological findings spectrum in Celiac disease. Turk. J. Pediatr. 58, 233–240. doi: 10.24953/turkjped.2016.03.001
Alaedini, A., and Green, P. H. (2008). Autoantibodies in celiac disease. Autoimmunity 41, 19–26. doi: 10.1080/08916930701619219
Barcia, G., Posar, A., Santucci, M., and Parmeggiani, A. (2008). Autism and coeliac disease. J. Autism Dev. Disord. 38, 407–408. doi: 10.1007/s10803-007-0480-3
Bella, R., Cantone, M., Lanza, G., Ferri, R., Vinciguerra, L., Puglisi, V., et al. (2016). Cholinergic circuitry functioning in patients with vascular cognitive impairment–no dementia. Brain Stimul. 9, 225–233. doi: 10.1016/j.brs.2015.09.013
Bella, R., Ferri, R., Cantone, M., Pennisi, M., Lanza, G., Malaguarnera, G., et al. (2011a). Motor cortex excitability in vascular depression. Int. J. Psychophysiol. 82, 248–253. doi: 10.1016/j.ijpsycho.2011.09.006
Bella, R., Ferri, R., Lanza, G., Cantone, M., Pennisi, M., Puglisi, V., et al. (2013). TMS follow-up study in patients with vascular cognitive impairment-no dementia. Neurosci. Lett. 534, 155–159. doi: 10.1016/j.neulet.2012.12.017
Bella, R., Ferri, R., Pennisi, M., Cantone, M., Lanza, G., Malaguarnera, G., et al. (2011b). Enhanced motor cortex facilitation in patients with vascular cognitive impairment-no dementia. Neurosci. Lett. 503, 171–175. doi: 10.1016/j.neulet.2011.08.022
Bella, R., Lanza, G., Cantone, M., Giuffrida, S., Puglisi, V., Vinciguerra, L., et al. (2015). Effect of a gluten-free diet on cortical excitability in adults with Celiac disease. PLoS ONE 10:e0129218. doi: 10.1371/journal.pone.0129218
Bersano, E., Stecco, A., D'Alfonso, S., Corrado, L., Sarnelli, M. F., Solara, V., et al. (2015). Coeliac disease mimicking amyotrophic lateral sclerosis. Amyotroph. Lateral Scler. Frontotemporal Degener. 16, 277–279. doi: 10.3109/21678421.2014.980614
Bhatia, K. P., Brown, P., Gregory, R., Lennox, G. G., Manji, H., Thompson, P. D., et al. (1995). Progressive myoclonic ataxia associated with coeliac disease. The myoclonus is of cortical origin, but the pathology is in the cerebellum. Brain 118, 1087–1093. doi: 10.1093/brain/118.5.1087
Bingley, P. J., Williams, A. J., Norcross, A. J., Unsworth, D. J., Lock, R. J., Ness, A. R., et al. (2004). Undiagnosed coeliac disease at age seven: population based prospective birth cohort study. BMJ 328, 322–323. doi: 10.1136/bmj.328.7435.322
Bordet, R., Ihl, R., Korczyn, A. D., Lanza, G., Jansa, J., Hoerr, R., et al. (2017). Towards the concept of disease-modifier in post-stroke or vascular cognitive impairment: a consensus report. BMC Med. 15:107. doi: 10.1186/s12916-017-0869-6
Borg, M. (2006). Symptomatic myoclonus. Neurophysiol. Clin. 36, 309–318. doi: 10.1016/j.neucli.2006.12.006
Briani, C., Zara, G., Alaedini, A., Grassivaro, F., Ruggero, S., Toffanin, E., et al. (2008). Neurological complications of celiac disease and autoimmune mechanisms: a prospective study. J. Neuroimmunol. 195, 171–175. doi: 10.1016/j.jneuroim.2008.01.008
Brown, K. J., Jewells, V., Herfarth, H., and Castillo, M. (2010). White matter lesions suggestive of amyotrophic lateral sclerosis attributed to celiac disease. Am. J. Neuroradiol. 31, 880–881. doi: 10.3174/ajnr.A1826
Bürk, K., Bösch, S., Müller, C. A., Melms, A., Zühlke, C., Stern, M., et al. (2001). Sporadic cerebellar ataxia associated with gluten sensitivity. Brain 124, 1013–1019. doi: 10.1093/brain/124.5.1013
Bürk, K., Farecki, M. L., Lamprecht, G., Roth, G., Decker, P., Weller, M., et al. (2009). Neurological symptoms in patients with biopsy proven celiac disease. Mov. Disord. 24, 2358–2362. doi: 10.1002/mds.22821
Bushara, K. O. (2005). Neurologic presentation of celiac disease. Gastroenterology 128, S92–S97. doi: 10.1053/j.gastro.2005.02.018
Cakir, D., Tosun, A., Polat, M., Celebisoy, N., Gokben, S., Aydogdu, S., et al. (2007). Subclinical neurological abnormalities in children with celiac disease receiving a gluten-free diet. J. Pediatr. Gastroenterol. Nutr. 45, 366–369. doi: 10.1097/MPG.0b013e31806907e8
Campagna, G., Pesce, M., Tatangelo, R., Rizzuto, A., La Fratta, I., and Grilli, A. (2017). The progression of coeliac disease: its neurological and psychiatric implications. Nutr. Res. Rev. 30, 25–35. doi: 10.1017/S0954422416000214
Canales, P., Mery, V. P., Larrondo, F. J., Bravo, F. L., and Godoy, J. (2006). Epilepsy and celiac disease: favorable outcome with a gluten-free diet in a patient refractory to antiepileptic drugs. Neurologist 12, 318–321. doi: 10.1097/01.nrl.0000250950.35887.6c
Cantone, M., Bramanti, A., Lanza, G., Pennisi, M., Bramanti, P., Pennisi, G., et al. (2017). Cortical plasticity in depression. ASN Neuro 9:1759091417711512. doi: 10.1177/1759091417711512
Cantone, M., Di Pino, G., Capone, F., Piombo, M., Chiarello, D., Cheeran, B., et al. (2014). The contribution of transcranial magnetic stimulation in the diagnosis and in the management of dementia. Clin. Neurophysiol. 125, 1509–1532. doi: 10.1016/j.clinph.2014.04.010
Carta, M. G., Conti, A., Lecca, F., Sancassiani, F., Cossu, G., Carruxi, R., et al. (2015). The burden of depressive and bipolar disorders in Celiac disease. Clin. Pract. Epidemiol. Ment. Health 11, 180–185. doi: 10.2174/1745017901511010180
Carta, M. G., Hardoy, M. C., Boi, M. F., Mariotti, S., Carpiniello, B., and Usai, P. (2002). Association between panic disorder, major depressive disorder and celiac disease: a possible role of thyroid autoimmunity. J. Psychosom. Res. 53, 789–793. doi: 10.1016/S0022-3999(02)00328-8
Carta, M. G., Hardoy, M. C., Usai, P., Carpiniello, B., and Angst, J. (2003). Recurrent brief depression in celiac disease. J. Psychosom. Res. 55, 573–574. doi: 10.1016/S0022-3999(03)00547-6
Casciato, S., Morano, A., Albini, M., Fanella, M., Lapenta, L., Fattouch, J., et al. (2015). Cryptogenic focal epilepsy and “hidden” celiac disease in adulthood: a causal or accidental link? Int. J. Neurosci. 125, 913–917. doi: 10.3109/00207454.2014.983227
Casella, S., Zanini, B., Lanzarotto, F., Ricci, C., Marengoni, A., Romanelli, G., et al. (2012). Cognitive performance is impaired in coeliac patients on gluten free diet: a case-control study in patients older than 65 years of age. Dig. Liver Dis. 44, 729–735. doi: 10.1016/j.dld.2012.03.008
Castillo, N. E., Theethira, T. G., and Leffler, D. A. (2015). The present and the future in the diagnosis and management of celiac disease. Gastroenterol Rep (Oxf) 3, 3–11. doi: 10.1093/gastro/gou065
Chin, R. L., and Latov, N. (2005). Peripheral neuropathy and Celiac disease. Curr. Treat. Options Neurol. 7, 43–48. doi: 10.1007/s11940-005-0005-3
Chin, R. L., Sander, H. W., Brannagan, T. H., Green, P. H., Hays, A. P., Alaedini, A., et al. (2003). Celiac neuropathy. Neurology 60, 1581–1585. doi: 10.1212/01.WNL.0000063307.84039.C7
Cicarelli, G., Della Rocca, G., Amboni, M., Ciacci, C., Mazzacca, G., Filla, A., et al. (2003). Clinical and neurological abnormalities in adult celiac disease. Neurol. Sci. 24, 311–317. doi: 10.1007/s10072-003-0181-4
Collin, P., Pirttilä, T., Nurmikko, T., Somer, H., Erilä, T., and Keyriläinen, O. (1991). Celiac disease, brain atrophy, and dementia. Neurology 41, 372–375. doi: 10.1212/WNL.41.3.372
Concerto, C., Lanza, G., Cantone, M., Ferri, R., Pennisi, G., Bella, R., et al. (2015). Repetitive transcranial magnetic stimulation in patients with drug-resistant major depression: a six-month clinical follow-up study. Int. J. Psychiatry Clin. Pract. 19, 252–258. doi: 10.3109/13651501.2015.1084329
Concerto, C., Lanza, G., Cantone, M., Pennisi, M., Giordano, D., Spampinato, C., et al. (2013). Different patterns of cortical excitability in major depression and vascular depression: a transcranial magnetic stimulation study. BMC Psychiatry 13:300. doi: 10.1186/1471-244X-13-300
Cooke, W. T., and Smith, W. T. (1966). Neurological disorders associated with adult coeliac disease. Brain 89, 683–722. doi: 10.1093/brain/89.4.683
Dai, A. I., Akcali, A., Varan, C., and Demiryürek, A. T. (2014). Prevalence of resistant occipital lobe epilepsy associated with celiac disease in children. Childs. Nerv. Syst. 30, 1091–1098. doi: 10.1007/s00381-014-2387-6
Deconinck, N., Scaillon, M., Segers, V., Groswasser, J. J., and Dan, B. (2006). Opsoclonus-myoclonus associated with celiac disease. Pediatr. Neurol. 34, 312–314. doi: 10.1016/j.pediatrneurol.2005.08.034
De Santis, A., Addolorato, G., Romito, A., Caputo, S., Giordano, A., Gambassi, G., et al. (1997). Schizophrenic symptoms and SPECT abnormalities in a coeliac patient: regression after a gluten-free diet. J. Intern. Med. 242, 421–423. doi: 10.1046/j.1365-2796.1997.00200.x
Di Lazzaro, V., Pilato, F., Batocchi, A. P., Restuccia, D., Cammarota, G., and Profice, P. (2010). Tired legs–a gut diagnosis. Lancet 376, 1798. doi: 10.1016/S0140-6736(10)61163-4
Fung, V. S., Duggins, A., Morris, J. G., and Lorentz, I. T. (2000). Progressive myoclonic ataxia associated with celiac disease presenting as unilateral cortical tremor and dystonia. Mov. Disord. 15, 732–734. doi: 10.1002/1531-8257(200007)15:4<732::AID-MDS1021>3.0.CO;2-J
Freeman, H. J. (2008). Neurological disorders in adult celiac disease. Can. J. Gastroenterol. 22, 909–911. doi: 10.1155/2008/824631
Gadoth, A., Nefussy, B., Bleiberg, M., Klein, T., Artman, I., and Drory, V. E. (2015). Transglutaminase 6 antibodies in the serum of patients with amyotrophic lateral sclerosis. JAMA Neurol. 72, 676–681. doi: 10.1001/jamaneurol.2015.48
Gobbi, G., Bouquet, F., Greco, L., Lambertini, A., Tassinari, C. A., Ventura, A., et al. (1992). Coeliac disease, epilepsy, and cerebral calcifications. the Italian working group on coeliac disease and epilepsy. Lancet 340, 439–443. doi: 10.1016/0140-6736(92)91766-2
Green, P. H., Alaedini, A., Sander, H. W., Brannagan, T. H. III., Latov, N., and Chin, R. L. (2005). Mechanisms underlying celiac disease and its neurologic manifestations. Cell. Mol. Life Sci. 62, 791–799. doi: 10.1007/s00018-004-4109-9
Green, P. H., and Jabri, B. (2003). Coeliac disease. Lancet 362, 383–391. doi: 10.1016/S0140-6736(03)14027-5
Hadjivassiliou, M., Aeschlimann, P., Strigun, A., Sanders, D. S., Woodroofe, N., and Aeschlimann, D. (2008). Autoantibodies in gluten ataxia recognize a novel neuronal transglutaminase. Ann. Neurol. 64, 332–343. doi: 10.1002/ana.21450
Hadjivassiliou, M., Grünewald, R. A., and Davies-Jones, G. A. (2002a). Gluten sensitivity as a neurological illness. J. Neurol. Neurosurg. Psychiatr. 72, 560–563. doi: 10.1136/jnnp.72.5.560
Hadjivassiliou, M., Boscolo, S., Davies-Jones, G. A., Grünewald, R. A., Not, T., Sanders, D. S., et al. (2002b). The humoral response in the pathogenesis of gluten ataxia. Neurology 58, 1221–1226. doi: 10.1212/WNL.58.8.1221
Hadjivassiliou, M., Duker, A. P., and Sanders, D. S. (2014). Gluten-related neurologic dysfunction. Handb. Clin. Neurol. 120, 607–619. doi: 10.1016/B978-0-7020-4087-0.00041-3
Hadjivassiliou, M., Grünewald, R. A., Chattopadhyay, A. K., Davies-Jones, G. A., Gibson, A., Jarratt, J. A., et al. (1998). Clinical, radiological, neurophysiological, and neuropathological characteristics of gluten ataxia. Lancet 352, 1582–1585. doi: 10.1016/S0140-6736(98)05342-2
Hadjivassiliou, M., Grünewald, R. A., Lawden, M., Davies-Jones, G. A., Powell, T., and Smith, C. M. (2001). Headache and CNS white matter abnormalities associated with gluten sensitivity. Neurology 56, 385–388. doi: 10.1212/WNL.56.3.385
Hadjivassiliou, M., Mäki, M., Sanders, D. S., Williamson, C. A., Grünewald, R. A., Woodroofe, N. M., et al. (2006). Autoantibody targeting of brain and intestinal transglutaminase in gluten ataxia. Neurology 66, 373–377. doi: 10.1212/01.wnl.0000196480.55601.3a
Hadjivassiliou, M., Sanders, D. D., and Aeschlimann, D. P. (2015). Gluten-related disorders: gluten ataxia. Dig. Dis. 33, 264–268. doi: 10.1159/000369509
Hadjivassiliou, M., Sanders, D. S., Grünewald, R. A., Woodroofe, N., Boscolo, S., and Aeschlimann, D. (2010). Gluten sensitivity: from gut to brain. Lancet Neurol. 9, 318–330. doi: 10.1016/S1474-4422(09)70290-X
Hallert, C., Aström, J., and Sedvall, G. (1982). Psychic disturbances in adult coeliac disease. III. Reduced central monoamine metabolism and signs of depression. Scand J. Gastroenterol. 17, 25–28. doi: 10.3109/00365528209181039
Hanagasi, H. A., Gürol, E., Sahin, H. A., and Emre, M. (2001). Atypical neurological involvement associated with celiac disease. Eur. J. Neurol. 8, 67–69. doi: 10.1046/j.1468-1331.2001.00155.x
Hernanz, A., and Polanco, I. (1991). Plasma precursor amino acids of central nervous system monoamines in children with coeliac disease. Gut 32, 1478–1481. doi: 10.1136/gut.32.12.1478
Holmes, G. K. (1996). Non-malignant complications of coeliac disease. Acta Paediatr. Suppl. 412, 68–75. doi: 10.1111/j.1651-2227.1996.tb14257.x
Holmes, G. K. (2002). Coeliac disease and malignancy. Dig. Liver Dis. 34, 229–237. doi: 10.1016/S1590-8658(02)80198-0
Hu, W. T., Murray, J. A., Greenaway, M. C., Parisi, J. E., and Josephs, K. A. (2006). Cognitive impairment and celiac disease. Arch. Neurol. 63, 1440–1446. doi: 10.1001/archneur.63.10.1440
Işıkay, S., Kocamaz, H., Sezer, S., Özkars, M. Y., Işıkay, N., Filik, B., et al. (2015a). The frequency of epileptiform discharges in Celiac disease. Pediatr. Neurol. 53, 78–82. doi: 10.1016/j.pediatrneurol.2015.02.006
Işıkay, S., Hizli, Ş., Çoşkun, S., and Yilmaz, K. (2015b). Increased tissue transglutaminase levels are associated with increased epileptiform activity in electroencephalography among patients with celiac disease. Arq. Gastroenterol. 52, 272–277. doi: 10.1590/S0004-28032015000400005
Javed, S., Safdar, A., Forster, A., Selvan, A., Chadwick, D., Nicholson, A., et al. (2012). Refractory coeliac disease associated with late onset epilepsy, ataxia, tremor and progressive myoclonus with giant cortical evoked potentials–a case report and review of literature. Seizure 21, 482–485. doi: 10.1016/j.seizure.2012.04.003
Kaplan, J. G., Pack, D., Horoupian, D., DeSouza, T., Brin, M., and Schaumburg, H. (1988). Distal axonopathy associated with chronic gluten enteropathy: a treatable disorder. Neurology 38, 642–645. doi: 10.1212/WNL.38.4.642
Karwautz, A., Wagner, G., Berger, G., Sinnreich, U., Grylli, V., and Huber, W. D. (2008). Eating pathology in adolescents with celiac disease. Psychosomatics 49, 399–406. doi: 10.1176/appi.psy.49.5.399
Labate, A., Gambardella, A., Messina, D., Tammaro, S., Le Piane, E., Pirritano, D., et al. (2001). Silent celiac disease in patients with childhood localization-related epilepsies. Epilepsia 42, 1153–1155. doi: 10.1046/j.1528-1157.2001.45700.x
Lanza, G., Bachmann, C. G., Ghorayeb, I., Wang, Y., Ferri, R., and Paulus, W. (2017a). Central and peripheral nervous system excitability in restless legs syndrome. Sleep Med. 31, 49–60. doi: 10.1016/j.sleep.2016.05.010
Lanza, G., Bella, R., Giuffrida, S., Cantone, M., Pennisi, G., Spampinato, C., et al. (2013). Preserved transcallosal inhibition to transcranial magnetic stimulation in nondemented elderly patients with leukoaraiosis. Biomed Res. Int. 2013:351680. doi: 10.1155/2013/351680
Lanza, G., Bramanti, P., Cantone, M., Pennisi, M., Pennisi, G., and Bella, R. (2017b). Vascular cognitive impairment through the looking glass of transcranial magnetic stimulation. Behav. Neurol. 2017:1421326. doi: 10.1155/2017/1421326
Lanza, G., Cantone, M., Lanuzza, B., Pennisi, M., Bella, R., Pennisi, G., et al. (2015a). Distinctive patterns of cortical excitability to transcranial magnetic stimulation in obstructive sleep apnea syndrome, restless legs syndrome, insomnia, and sleep deprivation. Sleep Med. Rev. 19, 39–50. doi: 10.1016/j.smrv.2014.04.001
Lanza, G., Lanuzza, B., Aricò, D., Cantone, M., Cosentino, F. I., Pennisi, M., et al. (2015b). Direct comparison of cortical excitability to transcranial magnetic stimulation in obstructive sleep apnea syndrome and restless legs syndrome. Sleep Med. 16, 138–142. doi: 10.1016/j.sleep.2014.08.016
Lebwohl, B., Sanders, D. S., and Green, P. H. R. (2017). Coeliac disease. Lancet doi: 10.1016/S0140-6736(17)31796-8. [Epub ahead of print].
Licchetta, L., Bisulli, F., Di Vito, L., La Morgia, C., Naldi, I., Volta, U., et al. (2011). Epilepsy in coeliac disease: not just a matter of calcifications. Neurol. Sci. 32, 1069–1074. doi: 10.1007/s10072-011-0629-x
Lichtwark, I. T., Newnham, E. D., Shepherd, S. J., Hosking, P., Gibson, P. R., et al. (2014). Cognitive impairment in coeliac disease improves on a gluten-free diet and correlates with histological and serological indices of disease severity. Aliment. Pharmacol. Ther. 40, 160–170. doi: 10.1111/apt.12809
Lionetti, E., Francavilla, R., Pavone, P., Pavone, L., Francavilla, T., Pulvirenti, A., et al. (2010). The neurology of coeliac disease in childhood: what is the evidence? A systematic review and meta-analysis. Dev. Med. Child Neurol. 52, 700–707. doi: 10.1111/j.1469-8749.2010.03647.x
Lu, C. S., Thompson, P. D., Quinn, N. P., Parkes, J. D., and Marsden, C. D. (1986). Ramsay Hunt syndrome and coeliac disease: a new association? Mov. Disord. 1, 209–219. doi: 10.1002/mds.870010306
Ludvigsson, J. F., Mariosa, D., Lebwohl, B., and Fang, F. (2014). No association between biopsy-verified celiac disease and subsequent amyotrophic lateral sclerosis–a population-based cohort study. Eur. J. Neurol. 21, 976–982. doi: 10.1111/ene.12419
Ludvigsson, J. F., Zingone, F., Tomson, T., Ekbom, A., and Ciacci, C. (2012). Increased risk of epilepsy in biopsy-verified celiac disease: a population-based cohort study. Neurology 78, 1401–1407. doi: 10.1212/WNL.0b013e3182544728
Luostarinen, L., Himanen, S. L., Luostarinen, M., Collin, P., and Pirttilä, T. (2003). Neuromuscular and sensory disturbances in patients with well treated coeliac disease. J. Neurol. Neurosurg. Psychiatr. 74, 490–494. doi: 10.1136/jnnp.74.4.490
Lurie, Y., Landau, D. A., Pfeffer, J., and Oren, R. (2008). Celiac disease diagnosed in the elderly. J. Clin. Gastroenterol. 42, 59–61. doi: 10.1097/01.mcg.0000247995.12087.7b
Magaudda, A., Dalla Bernardina, B., De Marco, P., Sfaello, Z., Longo, M., Colamaria, V., et al. (1993). Bilateral occipital calcification, epilepsy and coeliac disease: clinical and neuroimaging features of a new syndrome. J. Neurol. Neurosurg. Psychiatr. 56, 885–889. doi: 10.1136/jnnp.56.8.885
McKeon, A., Lennon, V. A., Pittock, S. J., Kryzer, T. J., and Murray, J. (2014). The neurologic significance of celiac disease biomarkers. Neurology 83, 1789–1796. doi: 10.1212/WNL.0000000000000970
Morello, F., Ronzani, G., and Cappellari, F. (2003). Migraine, cortical blindness, multiple cerebral infarctions and hypocoagulopathy in celiac disease. Neurol. Sci. 24, 85–89.
Mumford, C. J., Fletcher, N. A., Ironside, J. W., and Warlow, C. P. (1996). Progressive ataxia, focal seizures, and malabsorption syndrome in a 41 year old woman. J. Neurol. Neurosurg. Psychiatr. 60, 225–230. doi: 10.1136/jnnp.60.2.225
Niederhofer, H., and Pittschieler, K. (2006). A preliminary investigation of ADHD symptoms in persons with celiac disease. J. Atten. Disord. 10, 200–204. doi: 10.1177/1087054706292109
Nikpour, S. (2012). Neurological manifestations, diagnosis, and treatment of celiac disease: a comprehensive review. Iran J. Neurol. 11, 59–64.
Parisi, P., Pietropaoli, N., Ferretti, A., Nenna, R., Mastrogiorgio, G., Del Pozzo, M., et al. (2015). Role of the gluten-free diet on neurological-EEG findings and sleep disordered breathing in children with celiac disease. Seizure 25, 181–183. doi: 10.1016/j.seizure.2014.09.016
Parisi, P., Principessa, L., Ferretti, A., D'Onofrio, D., Del Giudice, E., Pacchiarotti, C., et al. (2014). “EEG abnormalities” may represent a confounding factor in celiac disease: a 4-year follow-up family report. Epilepsy Behav. Case Rep. 2, 40–42. doi: 10.1016/j.ebcr.2014.01.008
Paulus, W., Classen, J., Cohen, L. G., Large, C. H., Di Lazzaro, V., Nitsche, M., et al. (2008). State of the art: pharmacologic effects on cortical excitability measures tested by transcranial magnetic stimulation. Brain Stimul. 1, 151–163. doi: 10.1016/j.brs.2008.06.002
Pawlak-Osińska, K., Kaźmierczak, H., Kuczyńska, R., and Szaflarska-Popławska, A. (2007). Looking for the auditory and vestibular pathology in celiac disease. Otolaryngol. Pol. 61, 178–183. doi: 10.1016/S0030-6657(07)70409-2
Pellecchia, M. T., Scala, R., Perretti, A., De Michele, G., Santoro, L., Filla, A., et al. (1999). Cerebellar ataxia associated with subclinical celiac disease responding to gluten-free diet. Neurology 53, 1606–1608. doi: 10.1212/WNL.53.7.1606-a
Pennisi, G., Bella, R., and Lanza, G. (2015). Motor cortex plasticity in subcortical ischemic vascular dementia: what can TMS say? Clin. Neurophysiol. 126, 851–852. doi: 10.1016/j.clinph.2014.09.001
Pennisi, G., Ferri, R., Cantone, M., Lanza, G., Pennisi, M., Vinciguerra, L., et al. (2011a). A review of transcranial magnetic stimulation in vascular dementia. Dement. Geriatr. Cogn. Disord. 31, 71–80. doi: 10.1159/000322798
Pennisi, G., Ferri, R., Lanza, G., Cantone, M., Pennisi, M., Puglisi, V., et al. (2011b). Transcranial magnetic stimulation in Alzheimer's disease: a neurophysiological marker of cortical hyperexcitability. J. Neural. Transm. (Vienna) 118, 587–598. doi: 10.1007/s00702-010-0554-9
Pennisi, G., Lanza, G., Giuffrida, S., Vinciguerra, L., Puglisi, V., Cantone, M., et al. (2014). Excitability of the motor cortex in de novo patients with celiac disease. PLoS ONE 9:e102790. doi: 10.1371/journal.pone.0102790
Pennisi, M., Lanza, G., Cantone, M., Ricceri, R., Ferri, R., D'Agate, C. C., et al. (2017). Cortical involvement in celiac disease before and after long-term gluten-free diet: a transcranial magnetic stimulation study. PLoS ONE 12:e0177560. doi: 10.1371/journal.pone.0177560
Pennisi, M., Lanza, G., Cantone, M., Ricceri, R., Spampinato, C., Pennisi, G., et al. (2016). Correlation between motor cortex excitability changes and cognitive impairment in vascular depression: pathophysiological insights from a longitudinal TMS study. Neural Plast. 2016:8154969. doi: 10.1155/2016/8154969
Pratesi, R., Modelli, I. C., Martins, R. C., Almeida, P. L., and Gandolfi, L. (2003). Celiac disease and epilepsy: favorable outcome in a child with difficult to control seizures. Acta Neurol. Scand. 108, 290–293. doi: 10.1034/j.1600-0404.2003.00082.x
Ranua, J., Luoma, K., Auvinen, A., Mäki, M., Haapala, A. M., Peltola, J., et al. (2005). Celiac disease-related antibodies in an epilepsy cohort and matched reference population. Epilepsy Behav. 6, 388–392. doi: 10.1016/j.yebeh.2005.01.007
Sabel'nikova, E. A., Krums, L. M., Parfenov, A. I., Vorob'eva, N. N., and Gudkova, R. B. (2013). Specific features of rehabilitation in patients with gluten-sensitivity celiac disease. Ter. Arkh. 85, 42–47.
Sallem, F. S., Castro, L. M., Jorge, C., Marchiori, P., and Barbosa, E. (2009). Gluten sensitivity presenting as myoclonic epilepsy with cerebellar syndrome. Mov. Disord. 24, 2162–2163. doi: 10.1002/mds.22576
Sarrigiannis, P. G., Hoggard, N., Aeschlimann, D., Sanders, D. S., Grünewald, R. A., Unwin, Z. C., et al. (2014). Myoclonus ataxia and refractory coeliac disease. Cerebellum Ataxia 1:11. doi: 10.1186/2053-8871-1-11
Schuppan, D., Junker, Y., and Barisani, D. (2009). Celiac disease: from pathogenesis to novel therapies. Gastroenterology 137, 1912–1933. doi: 10.1053/j.gastro.2009.09.008
Siqueira Neto, J. I., Costa, A. C., Magalhães, F. G., and Silva, G. S. (2004). Neurological manifestations of celiac disease. Arq. Neuropsiquiatr. 62, 969–972. doi: 10.1590/S0004-282X2004000600007
Spampinato, C., Aguglia, E., Concerto, C., Pennisi, M., Lanza, G., Bella, R., et al. (2013). Transcranial magnetic stimulation in the assessment of motor cortex excitability and treatment of drug-resistant major depression. IEEE Trans. Neural Syst. Rehabil. Eng. 21, 391–403. doi: 10.1109/TNSRE.2013.2256432
Tijssen, M. A., Thom, M., Ellison, D. W., Wilkins, P., Barnes, D., Thompson, P. D., et al. (2000). Cortical myoclonus and cerebellar pathology. Neurology 54, 1350–1356. doi: 10.1212/WNL.54.6.1350
Tison, F., Arne, P., and Henry, P. (1989). Myoclonus and adult coeliac disease. J. Neurol. 236, 307–308. doi: 10.1007/BF00314464
Turner, M. R., Chohan, G., Quaghebeur, G., Greenhall, R. C., Hadjivassiliou, M., and Talbot, K. (2007). A case of celiac disease mimicking amyotrophic lateral sclerosis. Nat. Clin. Pract. Neurol. 3, 581–584. doi: 10.1038/ncpneuro0631
Turner, M. R., Goldacre, R., Ramagopalan, S., Talbot, K., and Goldacre, M. J. (2013). Autoimmune disease preceding amyotrophic lateral sclerosis: an epidemiologic study. Neurology 81, 1222–1225. doi: 10.1212/WNL.0b013e3182a6cc13
Tursi, A., Giorgetti, G. M., Iani, C., Arciprete, F., Brandimarte, G., Capria, A., et al. (2006). Peripheral neurological disturbances, autonomic dysfunction, and antineuronal antibodies in adult celiac disease before and after a gluten-free diet. Dig. Dis. Sci. 51, 1869–1874. doi: 10.1007/s10620-005-9054-4
Usai, P., Serra, A., Marini, B., Mariotti, S., Satta, L., Boi, M. F., et al. (2004). Frontal cortical perfusion abnormalities related to gluten intake and associated autoimmune disease in adult coeliac disease: 99mTc-ECD brain SPECT study. Dig. Liver Dis. 36, 513–518. doi: 10.1016/j.dld.2004.03.010
Usanova, E. P., Shapkina, O. A., Matkivskiǐ, R. A., Fedulova, É. N., and Uspenskaia, I. D. (2012). The organization of comprehensive rehabilitation of the children presenting with inflammatory intestinal diseases and celiacia under the conditions of a health resort. Vopr. Kurortol. Fizioter. Lech. Fiz. Kult. 4, 37–40.
Uygur-Bayramicli, O., and Ozel, A. M. (2011). Celiac disease is associated with neurological syndromes. Dig. Dis. Sci. 56, 1587–1588. doi: 10.1007/s10620-011-1663-5
van Hees, N. J., Van der Does, W., and Giltay, E. J. (2013). Coeliac disease, diet adherence and depressive symptoms. J. Psychosom. Res. 74, 155–160. doi: 10.1016/j.jpsychores.2012.11.007
Vieira, C., Jatobá, I., Matos, M., Diniz-Santos, D., and Silva, L. R. (2013). Prevalence of celiac disease in children with epilepsy. Arq. Gastroenterol. 50, 290–296. doi: 10.1590/S0004-28032013000400010
Yelland, G. W. (2017). Gluten-induced cognitive impairment (“brain fog”) in coeliac disease. J. Gastroenterol. Hepatol. 32, 90–93. doi: 10.1111/jgh.13706
Zelnik, N., Pacht, A., Obeid, R., and Lerner, A. (2004). Range of neurologic disorders in patients with celiac disease. Pediatrics 113, 1672–1676. doi: 10.1542/peds.113.6.1672
Ziemann, U., Reis, J., Schwenkreis, P., Rosanova, M., Strafella, A., Badawy, R., et al. (2015). TMS and drugs revisited 2014. Clin. Neurophysiol. 126, 1847–1868. doi: 10.1016/j.clinph.2014.08.028
Keywords: celiac disease, cortical excitability, electroencephalography, evoked potentials, transcranial magnetic stimulation, neuroplasticity
Citation: Pennisi M, Bramanti A, Cantone M, Pennisi G, Bella R and Lanza G (2017) Neurophysiology of the “Celiac Brain”: Disentangling Gut-Brain Connections. Front. Neurosci. 11:498. doi: 10.3389/fnins.2017.00498
Received: 28 June 2017; Accepted: 23 August 2017;
Published: 05 September 2017.
Edited by:
Benjamin Boutrel, University of Lausanne, SwitzerlandReviewed by:
Clara Rossetti-Marcon, Centre Hospitalier Universitaire Vaudois (CHUV), SwitzerlandCopyright © 2017 Pennisi, Bramanti, Cantone, Pennisi, Bella and Lanza. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Giuseppe Lanza, Z2xhbnphQG9hc2kuZW4uaXQ=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.