- 1Bioimaging Core, Faculty of Health Sciences, University of Macau, Macau, China
- 2Beijing Hospital of Traditional Chinese Medicine, Capital Medical University, Beijing Institute of Traditional Chinese Medicine, Beijing, China
- 3Department of Radiology, Beijing Anding Hospital, Capital Medical University, Beijing, China
- 4Faculty of Education, University of Macau, Macau, China
Insomnia is one of the most common health complaints, with a high prevalence of 30~50% in the general population. In particular, neuroimaging research has revealed that widespread dysfunctions in brain regions involved in hyperarousal are strongly correlated with insomnia. However, whether the topology of the intrinsic connectivity is aberrant in insomnia remains largely unknown. In this study, resting-state functional magnetic resonance imaging (rsfMRI) in conjunction with graph theoretical analysis, was used to construct functional connectivity matrices and to extract the attribute features of the small-world networks in insomnia. We examined the alterations in global and local small-world network properties of the distributed brain regions that are predominantly implicated in the frontostriatal network between 30 healthy subjects with insomnia symptoms (IS) and 62 healthy subjects without insomnia symptoms (NIS). Correlations between the small-world properties and clinical measurements were also generated to identify the differences between the two groups. Both the IS group and the NIS group exhibited a small-worldness topology. Meanwhile, the global topological properties didn't show significant difference between the two groups. By contrast, participants in the IS group showed decreased regional degree and efficiency in the left inferior frontal gyrus (IFG) compared with subjects in the NIS group. More specifically, significantly decreased nodal efficiency in the IFG was found to be negatively associated with insomnia scores, whereas the abnormal changes in nodal betweenness centrality of the right putamen were positively correlated with insomnia scores. Our findings suggested that the aberrant topology of the salience network and frontostriatal connectivity is linked to insomnia, which can serve as an important biomarker for insomnia.
Introduction
Insomnia is the most prevalent sleep disorder and is characterized by difficulties in falling asleep at bedtime, frequent awakenings in the middle of the night, and waking up too early in the morning (Morin and Benca, 2012; Cheung et al., 2013; Riedner et al., 2015). It has a high prevalence, in which 30~50% of the general population suffers from insomnia symptoms, with an increased incidence rate with older age, and up to 10% of the population meets the clinical criteria for insomnia disorder (Ohayon, 2002; Moore, 2012; Morin and Benca, 2012; Kronholm et al., 2015). More importantly, insomnia has been recognized to be a substantially increased potential trigger for the development of other mental illnesses, such as depression. Previous work has demonstrated that insomnia individuals are approximately 10 times more likely to develop depression as compared with good sleepers, and approximately 90% of patients with depression also suffer from insomnia (Taylor et al., 2005; Kaneita et al., 2006; Ohayon and Hong, 2006; Benca and Peterson, 2008; Wulff et al., 2010; Mayer et al., 2011). In particular, insomnia is strongly associated with feeling fatigued in the performance of academic functioning, a reduced working memory performance, feeling anxiety in complex tasks, the overuse of drugs and alcohol, the presence of suicidal ideation, and a poor life quality in adults, children and adolescents (Short et al., 2013; Kronholm et al., 2015). Accordingly, insomnia is also associated with a marked increase in health-care consumption, poor occupational performance, and high costs for society (Kucharczyk et al., 2012; Moore, 2012; Lian et al., 2015). Despite the huge socio-economic impact caused by insomnia, the neurobiological mechanisms that underlie insomnia remain unclear, thereby hampering the development of effective treatments.
Recently, numerous structural and functional neuroimaging studies have examined widespread dysfunctions in the fronto-striato-limbic-thalamic regions in insomnia (Drummond et al., 2004; Spiegelhalder et al., 2013, 2015; Riemann et al., 2015). For example, using high-resolution 3D T1-weighted magnetic resonance imaging (MRI), Altena et al. discovered that the gray matter volume (GMV) in the left orbitofrontal cortex (OFC) was decreased in 24 insomnia patients, and the GMV of the OFC was strongly positively associated with insomnia scores (Altena et al., 2010). Additionally, Stoffers et al. used the whole-brain voxel-based morphometry (VBM) to access the gray matter density of 65 healthy subjects with insomnia symptoms, in which a negative relationship between the gray matter density in the left OFC and earlier morning awakening was identified (Stoffers et al., 2012). Joo et al. also found a reduced GMV in multiple brain regions including the bilateral frontal lobes based on measurements from 27 patients with insomnia (Joo et al., 2013). In particular, a further VBM study revealed GMV reductions in the bilateral OFC and the adjacent bilateral inferior frontal gyrus (IFG) pars orbitalis were correlated with increased sleep fragmentation, which was objectively quantified via actigraphy in 141 older community-dwelling adults (Lim et al., 2015). Further, the GMV reduction of frontal areas was also identified, which was related to decreased integrity of white matter tracts in the anterior internal capsule, as demonstrated by a recent diffusion tensor imaging study in 24 insomnia patients (Spiegelhalder et al., 2014).
In addition to the changes in the GMV, using task-based functional MRI (fMRI), Altena et al. found a hypoactivation in the inferior prefrontal cortex during daytime task performance in 21 patients with insomnia (Altena et al., 2008). Interestingly, findings from Drummond et al.'s analysis suggested that decreased activations in the frontal and thalamic brain regions were present during the performance of working memory tasks for 25 individuals with insomnia, which demonstrated dysfunctions in the fronto-striatal networks for insomnia (Drummond et al., 2013). In addition, based on a seed-based functional connectivity analysis method, Huang et al. identified a decreased connectivity between the frontal regions and the limbic areas in 10 medication-naive insomnia patients, which illustrated impairments in the fronto-limbic systems (Huang et al., 2012). Further, Nie et al. found that compared with good sleepers, patients with insomnia exhibited a significantly decreased functional connectivity between the medial prefrontal cortex [a core region of the default-mode network (DMN)] and the right medial temporal lobe (Nie et al., 2015). Li et al. used the resting-state fMRI (rsfMRI) to investigate 55 insomnia patients, which unveiled decreased amplitude of low-frequency fluctuation (ALFF) in the left OFC/IFG and the right middle frontal gyrus in insomnia (Li et al., 2016). An additional ALFF study performed by Zhou et al. revealed that significantly decreased ALFF regions were mainly detected in the prefrontal cortex and DMN sub-regions, which indicated that abnormal sensory input and intrinsic information processing could be a hyperarousal state in insomnia (Zhou et al., 2016). More recently, Stoffers et al. used an executive task to evaluate fronto-striatal functioning in 24 disturbed sleep subjects because the fronto-striatal networks play an important role in sleep, arousal regulation and executive function, and their findings revealed that a reduced recruitment of the left caudate nucleus was involved in the pathophysiology of insomnia (Stoffers et al., 2014).
Taken together, the important neuroimaging findings described above have indicated that dysfunctions in the frontal-based circuits and striatal-based systems played an essential role in identification of the neurobiological mechanism of insomnia. To date, most previous work on the analysis of disrupted networks in insomnia has predominately centered on the seed-based method. For this method, the seed was first defined according to the previous knowledge regarding the anatomical information or task-based activation, and the intrinsic functional connectivity was generated based on the correlation between this seed and all other brain areas. However, the seed-based connectivity measurement is unable to investigate the disconnectivity without a prediction of a priori regions of interest (ROIs). This is particularly challenging in the identification of accurate seeds/ROIs within the large, heterogeneous, and insomnia-related regions, such as the frontal cortex and the striatum. In contrast, graph theoretical analysis, as an alternative data-driven approach, provides the basis for improving understanding of the topological properties of brain networks independent of a priori seeds. It delineated the brain as a large-scale network that consists of nodes (brain regions) and edges (functional connections between pairs of nodes) (Bullmore and Sporns, 2009). The high-level topological properties may be sensitive to influences that cannot be detected by seed-based methods. Importantly, graph theoretical analysis can be adopted to investigate the functional abnormalities in insomnia at both the global and nodal levels. For example, at the global level, Watts and Strogatz demonstrated that the small-world network may be characterized by a higher clustering coefficient (the ratio of the number of existing interconnections of a node with its node neighbors to the maximum of all possible connections) and a similar shorter path length (the average number of minimum links that are required to travel between two nodes) compared with a random network (Watts and Strogatz, 1998). By contrast, at the nodal level, the node degree measured the connectedness of an isolated node with all other nodes, which may identify highly connected nodes that may play key roles in information integration in a network.
More importantly, evidences from neuroimaging studies have demonstrated that the human brain has a small-world network topology that may be disrupted in psychiatric and neurological disorders, such as depression (Meng et al., 2014; Long et al., 2015), schizophrenia (Liu F et al., 2016), Alzheimer's disease (Wang et al., 2013), epilepsy (Zhang Z et al., 2011), obsessive-compulsive disorder (Zhang T et al., 2011), autism (Itahashi et al., 2014), attention-deficit/hyperactivity disorder (Wang L et al., 2009), multiple sclerosis (He et al., 2009), post-traumatic stress disorder (Long et al., 2013), and spinal cord injury (De Vico Fallani et al., 2007). In this study, we determined to investigate whether the functional brain networks in insomnia also have small-world properties. We examined the topological properties of the whole brain networks between healthy subjects with insomnia symptoms (IS) and healthy subjects without insomnia symptoms (NIS). In particular, we explored the functional brain networks and the small-world network properties of insomnia generated by using rsfMRI data and graph theoretical analysis. Specifically, we hypothesized that (1) both the IS group and the NIS group should exhibit small-world properties; (2) the IS group can exhibit a disrupted functional topological organization and show altered local network properties of distributed regions predominantly within the frontal and striatal regions compared with the NIS group; and (3) an altered topology of the frontal and striatal regions should be involved in the salience network and frontostriatal circuits for the IS group.
To test these hypotheses, rsfMRI was used to explore the intrinsic brain activity in 30 healthy subjects with insomnia symptoms and 62 healthy subjects without insomnia symptoms using spontaneous low-frequency (0.01~0.1 Hz) fluctuations in the blood oxygen level-dependent signal during the resting state (Biswal et al., 1995, 1997). In addition, graph theoretical analysis was also used to explore the topological organization of the functional connectivity networks. The global and nodal network properties were examined and compared between the IS and NIS groups, and the clinical relevance of the aberrant brain network topologies was also evaluated. Certainly, this pilot work will pave a new avenue for an improved understanding of the neural mechanism that underlies insomnia.
Materials and Methods
Participants
One hundred and two right-handed, healthy subjects (53 females, 49 males; mean age ± SD: 36.83 ± 12.36 years; years of education: 15.00 ± 3.51 years) were recruited to participate in the study. The subjects were screened by two psychiatrists (LRT and CLT) by using the Non-Patient Structured Clinical Interview for the Diagnostic and Statistical Manual of Mental Disorders (DSM-IV) (SCID). Participants with a current or lifetime history of psychiatric or neurological illnesses, such as depression, anxiety disorders, schizophrenia, epilepsy, chronic pain or traumatic brain damage, were excluded from this study. All participants had no histories of alcohol, substance abuse or dependence. Written informed consents were collected for all participants prior to the experimental tests. All clinical tests and protocol for the present work were approved by the Medical Ethics Committee of Beijing Anding Hospital with the Capital Medical University, the Imaging Center for Brain Research with the Beijing Normal University, and the Biomedical Ethics Board with the Faculty of Health Sciences at the University of Macau (Macao SAR, China) in accordance with the approved guidelines.
Clinical Measurements
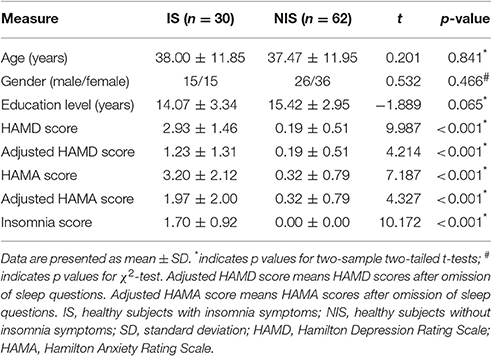
The 17-item Hamilton Depression Rating Scale (HAMD-17) and the Hamilton Anxiety Rating Scale (HAMA) were used to measure the severity of depression and anxiety (Hamilton, 1967). Insomnia symptoms were assessed according to the total score of the three-item sleep subscale on the HAMD-17. Three items which were scored 0, 1, or 2, were applied to evaluate the difficulty in initiating sleep (DIS, early insomnia), the difficulty in maintaining sleep (DMS, middle insomnia), and the early morning awakening (EMA, late insomnia), respectively. In particular, DIS is defined as a difficulty in falling asleep, DMS is denoted as a difficulty in maintaining sleep that leads to frequent awakenings or a difficulty in returning to sleep after awakenings, and EMA indicates early morning awakening with an inability to return to sleep. The participants who had a total score equal to or greater than 1 were considered to possess insomnia symptoms. It is noted here that the HAMD-17 sleep items were adopted to evaluate the three stages of insomnia instead of the Pittsburgh Insomnia Rating Scale that involved not only insomnia but also the subjective sleep quality, the sleep latency and duration, the use of sleep medication, and daytime dysfunction account (Trivedi et al., 2013). However, previous work showed that the sleep items measured by the HAMD were better correlated with sleep diaries (Manber et al., 2005). More notably, we generated the adjusted HAMD scores and adjusted HAMA scores without using the insomnia-related items to reduce the potential influence of insomnia via the HAMD and HAMA on our diagnostic results.
Data Acquisition
Data acquisition was performed on a 3.0-Tesla MRI scanner (Siemens Medical Solutions, Erlangen, Germany) in the National Key Laboratory for Cognitive Neuroscience and Learning, Beijing Normal University. In particular, no medicine were taken for all participates before the MRI recordings. The rsfMRI data were collected using an echo-planar imaging (EPI) sequence with the following scanning parameters: TR = 2 s, TE = 30 ms, flip angle = 90°, FOV = 220 × 220 mm, in-plane matrix size = 64 × 64, number of slices = 33, slice thickness = 3.5 mm, and inter-slice gap = 0.6 mm. All participants were instructed to completely relax without thinking of special things, simply rest quietly with their eyes closed, remain still, and remain awake during the data recording. For each subject, the overall resting-state data acquisition lasted 8 min, resulting in 240 volumes. No subject dropped out of the experiment during the scanning.
Data Preprocessing
All single-subject resting-state data preprocessing steps were performed using the Data Processing Assistant for Rest-State fMRI (DPARSF) toolbox (Chao-Gan and Yu-Feng, 2010). The first 10 volumes were discarded from each participant in favor of steady-state longitudinal magnetization. The remaining resting-state fMRI data were first slice-timing corrected and then realigned to the first of the remaining volumes for head-motion correction. Following these corrections, the functional images were spatially normalized into the standard Montreal Neurological Institute (MNI) space with a resliced resolution at 3 × 3 × 3 mm3. No spatial smoothing was applied to avert the introduction of an artificial local spatial correlation according to previous studies (Salvador et al., 2005; Achard et al., 2006; Achard and Bullmore, 2007; Wang J et al., 2009; Liao et al., 2010b). To remove the several spurious sources of variance, the six head motion parameters and the averaged signals from the white matter signals and cerebrospinal fluid signals were regressed out using the CompCor method (Behzadi et al., 2007). No global signal was regressed out to avoid yielding spurious negative correlations (Murphy et al., 2009). In addition, the resulting time series was detrended to remove the linear trend and then temporally bandpass filtered (0.01~0.1 Hz) to reduce the effects of low-frequency drifts and high-frequency physiological noises. Furthermore, a “scrubbing” method together with a frame-wise displacements (FD) threshold of 0.5 mm were implicated to remove the contaminated time points and reduce the impact of motion artifacts induced by systematic biases on the time series (Power et al., 2012). Specifically, at each time point t, the estimation of the motion FD was computed from three head translations (dtx, dty, dtz) and three head rotations (dtα, dtβ, dtγ) using the formula: FDt = |Δfdtx|+|Δfdty|+|Δfdtz|+|Δfdtα|+|Δfdtβ|+|Δfdtγ|, in which the Δfdtx = fd(t−1)x−fdtx, and the similarity calculation procedure was performed for the other rigid body parameters (dty, dtz, dtα, dtβ, dtγ). In particular, the angle rotational displacements were generated and transformed from degrees to millimeters by applying a radius of r = 50 mm, which is approximately the mean distance between the center of the MNI space and the cortex. Recordings at time points when FDt > 0.3 mm were considered to be potentially contaminated with motion artifacts and should be eliminated from the time series for further analysis (Power et al., 2012). The root mean squared variance of the voxels was also calculated to measure the degree to which the intensity of a brain image has changed compared with previous time points (Power et al., 2012). Finally, no data exceeded ± 2 mm or ± 2° For their translational or rotational parameters, respectively. However, the data from 10 subjects were discarded from further analysis because their data were cut off by more than 50% of their original volumes after scrubbing procedures.
Network Construction
To generate a brain network, it is essential to define the nodes and edges of the networks. To determine the nodes and edges of brain functional connectivity networks, the following procedure was applied as methods previously described (Gong et al., 2009; Zhang J et al., 2011).
Network Node Definition
To define the nodes of brain networks, the automated anatomical labeling (AAL) atlas (Tzourio-Mazoyer et al., 2002) was utilized to parcellate the whole brain into 90 anatomical ROIs, with 45 ROIs per hemisphere, and each ROI represented a node of the network. Detailed information on the ROIs is given in Table S1 as the supplementary material.
Network Edge Definition
To define the edges of the brain networks, a representative time series of each ROI from each subject was generated by averaging the time series of all voxels within the ROI. A 90 by 90 temporal correlation matrix was then constructed for each subject by calculating the Pearson's correlation coefficients between the time series from all pairs of ROIs. Further, a Fisher's r-to-z transformation was applied to the correlation matrices, and the individual correlation matrices were thresholded into binarized matrices with a sparsity value T (defined as the ratio of the total number of edges in a network to the maximum possible number of edges to ensure that the networks from two comparison groups have the same number of edges or wiring costs). When the Pearson's correlation coefficient was greater than T, the corresponding edge was considered as existence in brain networks.
Network Analysis
Threshold Selection
Instead of selecting a single threshold T, we applied a range of sparsity values from 0.11 to 0.30 with an interval of 0.01 according to the criteria suggested in previous studies (Zhang J et al., 2011). Specifically, (1) the minimum sparsity was selected to ensure that the mean degree across all nodes of each thresholded network was greater than 2 × log(N), where N = 90 is the number of nodes, and (2) the maximum sparsity was selected to ensure that the small-worldness scalar of each thresholded network was greater than 1.1 for all participants.
Small-World Network Measurements
Graph theoretical analysis was carried out using the graph theoretical network analysis (GRETNA) toolbox (Version 1.2, https://www.nitrc.org/projects/gretna/) to measure the network topological properties and examine the functional connectivity organization (Rubinov and Sporns, 2010; Wang et al., 2015). The small-world network properties, including the clustering coefficient of the network, Cp, and the path length, Lp, were also generated by using the method initially proposed by Watts and Strogatz (Watts and Strogatz, 1998).
To quantify the small-world characteristics, 100 random networks were constructed by using a Markov-chain algorithm at each sparsity threshold for each individual network with the same degree distribution as the functional connectivity networks (Liao et al., 2010a). Typically, we scaled Cp and Lp of the examined functional connectivity networks to the averaged Crandom and Lrandom from all 100 random networks (i.e., the normalized clustering coefficient, lambda , and the normalized characteristic path length, gamma ). These two parameters can also be integrated into a quantitative measurement, sigma , which is denoted as small-worldness. A real network is recognized to own small-world network properties if it satisfies the following conditions: (1) γ > 1 and λ ≈ 1 (Watts and Strogatz, 1998); (2) σ > 1 (namely, the small-world network has a higher clustering coefficient and a similar path length compared with the random network) (Humphries et al., 2006; Liu et al., 2008). The descriptions of the measurements were provided in Table S2.
Regional Nodal Measurements
The regional nodal properties may be characterized by a number of key measurements, including the nodal degree Degi, the nodal efficiency Enodal, and the nodal betweenness BCi (Table S2). The nodal degree is defined as the number of nodes in a subgraph Gi, which is the graph that includes the nodes that are direct neighbors of node i. The nodal degree evaluates the extent to which the node is connected to the remaining nodes in a network. The nodal efficiency Enodal is the inverse of the harmonic mean of the length between node i and all other nodes in the network to address the disconnected graphs, non-sparse graphs, or both factors, which measures the level of information propagation of a node with all other nodes in the network. The nodal betweenness BCi is denoted as the fraction of all the shortest paths in the network that pass through node i, which estimates the influence of a node over the information flow with the remaining nodes in a network.
Efficiency of Small-World Networks
There are two indicators to define the network efficiency: global efficiency Eglo and local efficiency Eloc (Achard and Bullmore, 2007) (Table S2). We first calculated the six global network parameters, Cp, Lp, λ, γ, Eglo, and Eloc, and three regional nodal properties, Degi, Enodal, and BCi. Then, we generated a summarized scalar for the topological characterization of brain functional networks by computing the area under the curve (AUC) based on each network parameter (Zhang J et al., 2011). Detailed descriptions on the definition and calculation of the network properties were provided in the supplementary materials.
Statistical Analysis
Differences in the Network Properties
In this study, the remaining 92 subjects were divided into two groups: the IS group (n = 30), who had insomnia scores greater than or equivalent to 1, and the NIS group (n = 62), who had insomnia scores equivalent to 0. Subjects from the two groups were matched in age, gender, and educational level, and their demographic information was provided in Table 1. To compare the global network topological properties (Cp, Lp, Eglo, Eloc, λ, γ, and σ) between the IS and NIS groups, a series of two-sample t-tests (p < 0.05, uncorrected) were performed for each property across the preselected sparsity thresholds, 0.11 ≤ T ≤ 0.30, with an interval of 0.01. To show the distinctions in the regional nodal properties (Degi, Enodal and BCi) between the two groups, nonparametric permutation tests (5,000 iterations, p < 0.05, uncorrected, controlling for age, gender, educational level, adjusted HAMA score, and adjusted HAMD score) were also performed on the AUC generated from each nodal property (Bullmore et al., 1999). Briefly, we first computed the actual between-group differences in the AUC of each network metric. Then, we put this difference into a null permutation distribution of the differences to recalculate by chance by randomly assigning the values of each subject to two randomized groups with the same sizes as the IS and NIS groups. This procedure was repeated for 5,000 permutations. Before the permutation tests, a multiple regression analysis was conducted with age, gender, educational level, adjusted HAMA score, and adjusted HAMD score as the dependent variables and the AUC of each network metric as the independent variable as well. A p < 0.05 for multiple comparisons was considered significant. Furthermore, Pearson's correlation analysis was utilized to inspect the relationship in the AUC of the nodal network properties between the insula and other brain regions that showed significant difference between the IS and NIS groups.
Correlations between the Network Properties and Insomnia Scores
Pearson's correlation analysis was performed to assess the relationship between the network properties (both global and nodal) and insomnia scores in the IS and NIS groups with age, gender, educational level, adjusted HAMA score, and adjusted HAMD score as covariates (independent variables: AUC of each network property; dependent variables: insomnia scores of all subjects). A value of 1/number of nodes = 1/90 = 0.011 was considered as a significant threshold for false-positive correction for all analyses.
Results
Demographic and Clinical Comparisons between the IS and NIS Groups
Table 1 presented the information on the demographics and clinical variables for the two groups. Both the original HAMD scores and the original HAMA scores in the IS group were higher than that in the NIS group (p < 0.001; Table 1). Additionally, the adjusted HAMD scores and the adjusted HAMA scores of the IS group were also higher as compared to that from the NIS group (p < 0.001; Table 1).
Differences in the Global Network Properties between the Two Groups
It was discovered from Figure 1 that the functional brain networks of both the IS and NIS groups exhibited small-world properties, in which the small-worldness index of sigma was greater than 1.1 for all threshold values ranged from 0.11 to 0.30. In addition, as displayed in Figure 1, all other global network properties didn't show significantly difference between the two groups across the entire scopes of sparsity for the correlation coefficients.
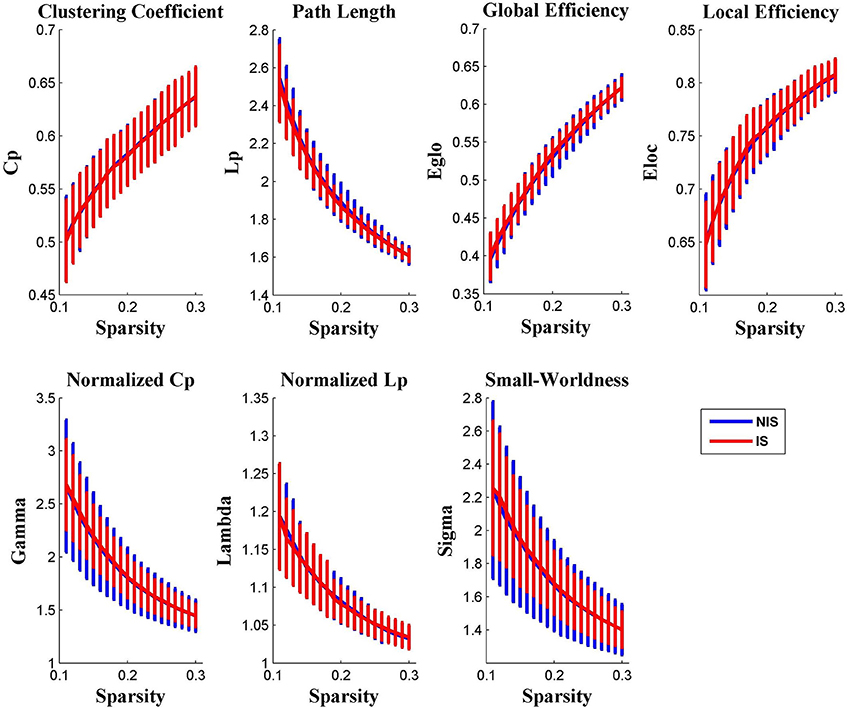
Figure 1. Group comparison of global network topological properties (Cp, Lp, Eglo, Eloc, λ, γ, and σ) between the IS and NIS group (two-sample t-tests, p < 0.05, uncorrected). The small-worldness suggests a small-world topology for functional brain networks of both the IS and NIS group. The error bar represents the standard deviation (SD). Cp, clustering coefficient; Lp, path length; Eglo, global efficiency; Eloc, local efficiency. IS, healthy participants with insomnia symptoms, NIS, healthy participants without insomnia symptoms.
Alterations in Regional Nodal Network Properties of Salience Networks
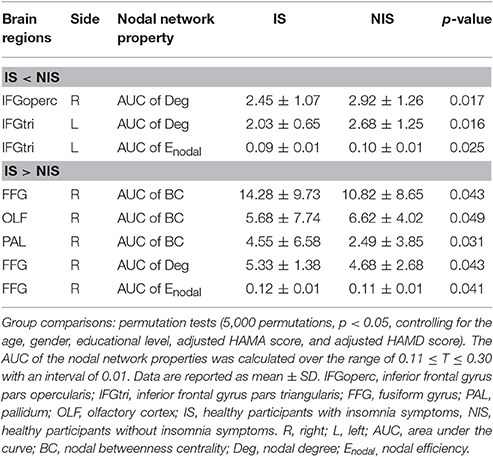
Alterations in the nodal betweenness centrality, nodal degree and nodal efficiency were identified in several brain regions of the subjects in the IS group (p < 0.05, uncorrected) (Figure 2 and Table 2). In particular, compared with the NIS group, the IS group exhibited increased nodal betweenness centrality in the right fusiform (FFG), right pallidum (PAL), and right olfactory cortex (Figure 2A and Table 2). Meanwhile, decreased nodal degree in the left IFG including the right inferior frontal gyrus pars opercularis (IFGoperc) and the left inferior frontal gyrus pars triangularis (IFGtri), and increased nodal degree in the right FFG were identified in the IS group compared with that from the NIS group (Figure 2B and Table 2). In addition, the IS group also showed decreased nodal efficiency in the left IFGtri and increased nodal efficiency in the right FFG compared with the NIS group (Figure 2C and Table 2). Intriguingly, regarding the regional nodal degree, we discovered that for the IS group, the right IFGoperc was positively correlated with the right insula of the brain (r = 0.274, p = 0.008). Further, it was also observed from Table 2 that insomnia scores were associated with the aberrant nodal network topology of frontostriatal connectivity.
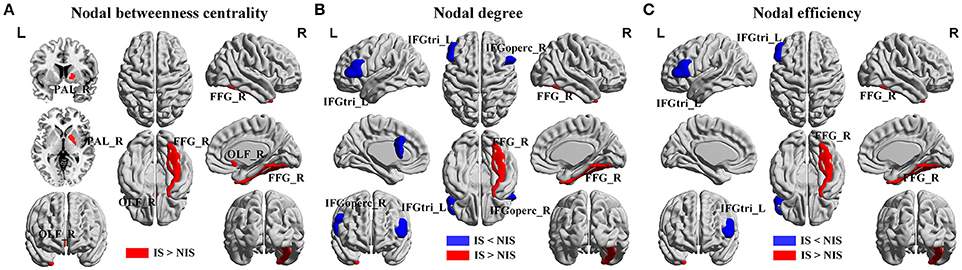
Figure 2. Brain regions showing altered local network properties in IS group as compared with NIS group. The aberrant (A) nodal betweenness centrality, (B) nodal degree, and (C) nodal efficiency were observed in IS group. Group comparisons were based on permutation tests (5,000 permutations, p < 0.05, controlling for the age, gender, educational level, adjusted HAMA score, and adjusted HAMD score). Colored brain areas indicate the significantly altered local network properties in IS group. The blue and red regions represent significantly decreased and increased nodal network properties in IS group as compared with NIS group, respectively. The more detailed information were presented in Table 2. IS, healthy participants with insomnia symptoms; NIS, healthy participants without insomnia symptoms; L, left; R, right; FFG, fusiform gyrus; PAL, pallidum; OLF, olfactory cortex; IFGoperc, inferior frontal gyrus pars opercularis; IFGtri, inferior frontal gyrus pars triangularis. Figures were visualized using the BrainNet Viewer software (https://www.nitrc.org/projects/bnv/).
No significant correlation was identified between the global network metrics (Cp, Lp, Eglo, Eloc, λ, γ, and σ) and the insomnia scores across all subjects. However, the AUC of the nodal efficiency in left IFGtri was negatively correlated with the insomnia scores (p < 0.05, uncorrected) (Figure 3A and Table 3). What's more, the AUC of the nodal betweenness centrality of the right putamen (p < 0.011) (Figure 3B and Table 3), the right fusiform gyrus (FFG) (p < 0.05) (Table 3), and the right middle frontal gyrus (MFG) (p < 0.05) (Table 3) were found to be positively associated with the insomnia scores. In particular, the altered nodal network topology in the IFGtri and putamen was identified to be related with the abnormal frontostriatal connectivity in insomnia.
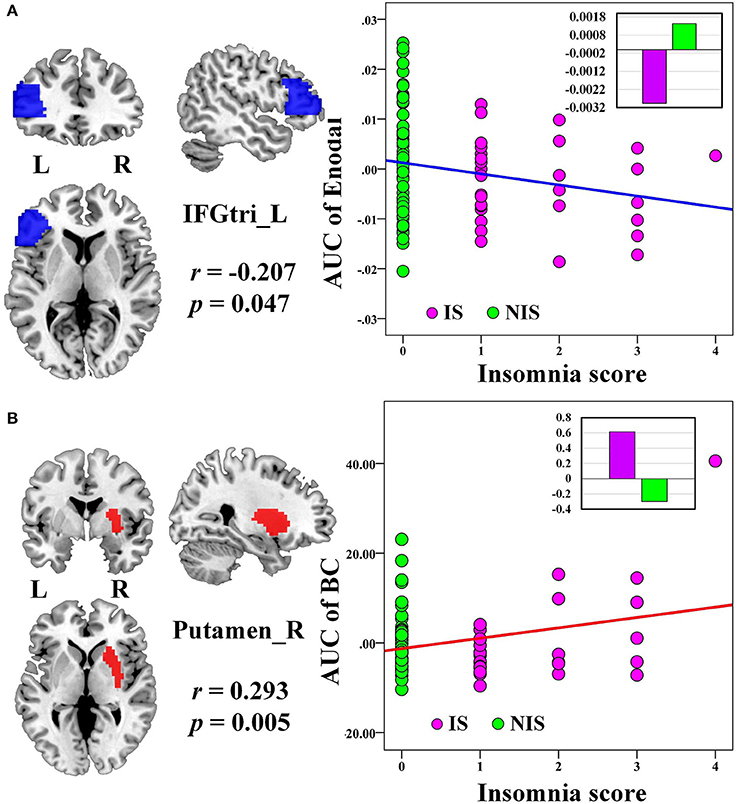
Figure 3. The Pearson correlation between the AUC of the nodal network properties with insomnia scores in all the subjects (p < 0.05, uncorrected, controlling for the age, gender, educational level, adjusted HAMA score, and adjusted HAMD score). The AUC of each nodal topology was calculated over the range of 0.11 ≤ T ≤ 0.30 with an interval of 0.01. The red color reveals the positive correlation while the blue color represents the negative correlation. The violet circles stand for the IS group while the green circles indicate the NIS group. The bar plots stand for the mean value of each AUC of nodal network properties in corresponding brain regions for IS and NIS groups. The more detailed information were presented in Table 3. L, left; R, right; AUC, area under the curve; BC, nodal betweenness centrality; Enodal, nodal efficiency; IFGtri, inferior frontal gyrus pars triangularis; IS, healthy participants with insomnia symptoms; NIS, healthy participants without insomnia symptoms.
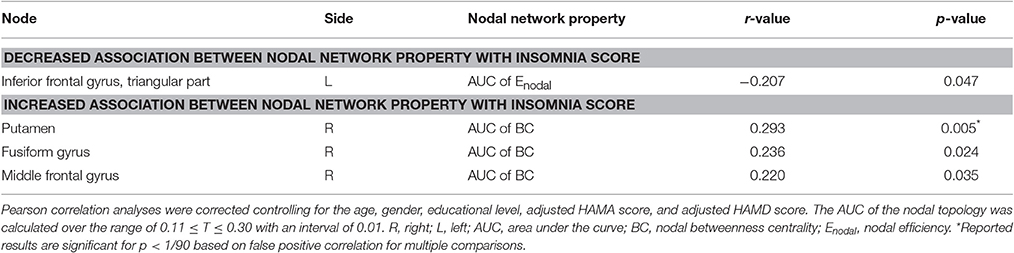
Table 3. Significant correlations between nodal network properties and insomnia scores across all subjects.
Discussion
In the current study, we examined how the topological organization of the intrinsic brain networks is associated with insomnia in 30 healthy individuals with insomnia and 62 healthy individuals without insomnia. The comparison of the measurement of small-worldness indicated that the differences in the small-world properties of the brain activation networks between the IS and NIS groups were not significant. However, compared with the NIS group, the IS group exhibited decreased nodal degree in the IFG including the right IFGoperc and the left IFGtri, and decreased nodal efficiency in the left IFGtri. Specifically, it is noted that the nodal efficiency of the left IFGtri was significantly negatively correlated with insomnia scores, whereas the nodal betweenness centrality in the right putamen was positively correlated with insomnia scores. In particular, the insomnia score was found to be selectively associated with the aberrant nodal network properties in the left IFGtri and right putamen. By contrast, the regional nodal degree in right IFGoperc was positively associated with the right insula, a key component of the salience network. The identified neural markers demonstrated that the insomnia without any underlying psychiatric comorbidity can be characterized by the altered network topology of the IFG of the salience network. These novel findings also provided evidence that disrupted topological organizations of the frontostriatal connectivity and salience network were associated with insomnia, suggesting that these networks contributed to the neural circuitry that underlies insomnia. In addition, the identified neural markers were independent of the age, gender or educational level, which are controlled for in the group comparison. Further, previous work showed that insomnia has close link with depression and/or anxiety disorders (Ford and Kamerow, 1989; Gillin, 1998; Riemann and Voderholzer, 2003; Staner, 2010). However, in the preset study, the neuroimaging results were also controlled for the adjusted HAMA scores and adjusted HAMD scores to eliminate the influence of depression and/or anxiety disorders, indicating that insomnia rather than anxiety disorders or depression is associated with an altered topology of the frontostriatal connectivity. Consequently, our novel findings suggested that altered nodal network properties in areas of the salience network and frontostriatal connectivity are only linked with insomnia.
Consistent with our hypothesis, we discovered that the IS group showed a disrupted functional topological organization compared with the NIS group. A decreased nodal degree and nodal efficiency of the distributed brain regions were identified predominantly within the left IFGtri in insomnia, whereas decreased nodal degree was also revealed in the right IFGoperc for the NIS group. In addition, the nodal efficiency of the left IFGtri was negatively associated with the insomnia score. As such, our results exhibited decreased nodal degree and nodal efficiency and revealed a negative association between the nodal network topology in the IFG and insomnia, indicating that more severe insomnia was associated with a more disrupted IFG and a weaker IFG hubness for information integration.
The prefrontal cortex has demonstrated its important roles in alertness, attention, and higher-order cognitive processes for insomnia (Thomas et al., 2000; Spiegelhalder et al., 2015). Recently, Li et al. demonstrated reduced ALFF values in the left IFG and right MFG in primary insomnia (PI) patients. In addition, they also revealed a negative correlation between the duration of PI and ALFF values in the left IFG (Li et al., 2016). Using task-based fMRI, Martin et al. reported that poor quality sleepers showed smaller activations in the right IFG, right MFG, and bilateral insula compared with good quality sleepers during an immediate and impulsive monetary decision task (Martin et al., 2015). By using the superior parietal lobe (SPL) as a seed, Li et al. illustrated that insomnia patients exhibited increased connectivity between the SPL and the right IFGtri, right insula lobe, right ventral anterior cingulate cortex (ACC), left ventral posterior cingulate cortex, and right splenium of the corpus callosum, which represent brain regions critical for spatial and verbal working memory (Li et al., 2014). Moreover, Altena et al. discovered that insomnia patients showed a hypoactivation in the IFG during performance in a category and letter fluency task, which recovered after sleep therapy. These findings suggested that insomnia disrupted cognitive functions (Altena et al., 2008). In Spira's study, the subjects with a sleep duration of less than 7 h exhibited increased rates of subsequent gray matter atrophy in the IFG and MFG compared with those with more than 7 h of sleep (Spira et al., 2015). Liu et al. found that a sleep deprivation group showed increased resting-state functional connectivity between the left cerebellum posterior lobe and the right IFG compared with the good sleepers group (Liu et al., 2015). Further, Shao et al. demonstrated that following total sleep deprivation, subjects with insomnia exhibited a significant decrease in the functional connectivity between the amygdala and several executive control regions, including the right IFG, right dorsal ACC, and left dorsolateral prefrontal cortex, indicating disturbances in the IFG-amygdala circuits in sleep (Shao et al., 2014). Using PET recordings, Wu et al. found a significant reduction in glucose metabolism in the IFG, MFG, medial frontal gyrus, and superior frontal gyrus after sleep deprivation (Wu et al., 2006). Consequently, the identified neural markers for the present work are consistent with those previous findings. Decreased nodal network topology of the IFG in this study indicated its weakened role in coordinating whole-brain networks, which sheds light on the understanding of the pathophysiology of insomnia.
In addition, a positive correlation was identified between the nodal degree of the IFGoperc and the insula. Notably, the anterior insula is adjacent to the IFG, which may be jointly referred to as the insula/IFG cortex. The anterior insula associated with IFG is the key hub of the salience network, which is interconnected with diverse cortical regions including the amygdala, medial temporal lobe, and basal ganglia (Kelly et al., 2012; Tahmasian et al., 2016). As a result, it is not surprising that the insula plays key roles in saliency detection, decision-making, motor/sensory processes, emotion and attention regulation, and cognition (Cauda et al., 2012; Uddin, 2015). Importantly, it should be pointed out that the insula is parcellated into two regions: (1) the anterior region, which is connected to the frontal cortex, ACC, parietal, and limbic regions, is mainly involved in salience detection and other emotional aspects; and (2) the posterior region, which is linked to the premotor, sensorimotor, temporal and posterior cingulate regions, is responsible for processing perception, emotion, sensorimotor integration, and interoception (Cauda et al., 2011, 2012). In particular, previous work has revealed that insomnia subjects exhibit insula abnormalities. For example, our previous findings showed decreased fractional ALFF in the left anterior insula and bilateral posterior insula, which can offer an explanation for the sleep state misperception and hyperarousal in insomnia (Liu C. H et al., 2016). Stoffers et al. demonstrated that diminished gray matter density in the left inferior orbitofrontal cortex borders the insula in patients with earlier morning awakening (Stoffers et al., 2012). Huang et al. found that decreased functional connectivity was identified mainly between the amygdala and the insula, striatum and thalamus in patients with insomnia (Huang et al., 2012). In a recent study, Chen et al. discovered that female insomniacs had increased activation of the anterior insula with salience networks, and they further revealed the relationship between blood-oxygen-level dependent (BOLD) in insula and the EEG gamma frequency power (Chen et al., 2014). Li et al. also reported a stronger connectivity between the bilateral superior parietal lobe and the right insular lobe in insomnia (Li et al., 2014). More recently, Wang et al. found an increased regional homogeneity (ReHo) value in the left insula in insomnia patients (Wang et al., 2016). These inconsistent results may be due to the diversity of the sample size of the insomnia subjects, gender differentiation, and methodological difference used. Interestingly, although the present study did not identify an altered topology in the insula in healthy subjects with insomnia, our results on decreased nodal network topology of the IFG were consistent with previous findings that revealed the relationship between altered insula activity and insomnia. More importantly, because the IFG is connected to the anterior insula and the aberrant IFG topology is also identified in insomnia for the present work, we speculated that the IFG may comprise an important biomarker for the hyperarousal pathophysiology of insomnia. Taken together, it is suggested that insomnia may disturb the role of the IFG in the maintenance of alertness and cognitive process functions.
More interestingly, a positive relationship between the nodal betweenness centrality of the right putamen and the insomnia score was also identified, indicating that a stronger putamen centrality control of information flow within the network was associated with more severe insomnia. The putamen is a key component of the striatum and is a critical component of the fronto-striatum circuits as well. Importantly, the insula and thalamus are strongly preferentially associated with the intrinsic coactivation pattern of the right putamen (Postuma and Dagher, 2006; Di Martino et al., 2008). The insula of the salience network plays an essential role in saliency detection, decision-making, motor/sensory processes, emotion and attention regulation, and cognition insomnia. Meanwhile, the thalamus has been confirmed to play crucial roles in various functions, including arousal functions, cognitive functions, affective and reward processing and motor organizations (Saalmann, 2014). In particular, the thalamus is connected to the ventral striatum, amygdala and medial prefrontal cortex, which is implicated in diverse functions, including reward-based decision-making, motivation drive, planning, and monitoring decisions for the development and expression of reward-based behaviors (Akert and Hartmann-von Monakow, 1980; Haber and Calzavara, 2009; Krebs et al., 2012). Moreover, the thalamus is a complex association of central nuclei that are closely correlated and interposed with the arousal and frontal systems, thus linking to the levels of vigilance and wakefulness of sleep (Bridoux et al., 2015). Overall, the insula of the salience network and the thalamus of the hyperarousal system are essential for insomnia, which indicates the hub of the insula and thalamus in sleep-related activity (Spiegelhalder et al., 2015). Using task-based fMRI, Bell-McGinty et al. also discovered increased activation in the right putamen in subjects after total sleep deprivation (TSD), which revealed that changes in the arousal levels may affect performance following TSD (Bell-McGinty et al., 2004). As a result, although the insula did not appear in our mapping results, the altered putamen revealed in this study is of considerable importance in insomnia. According to the identified IFG and putamen, we concluded that the topology of the fronto-striatum connectivity develops distinctively in insomnia. Specifically, our findings demonstrated that the insomnia may either result from, or contribute to the disturbed fronto-striatum network connectivity.
Limitations
To appropriately access the present findings, several limitations should be discussed. First, the Pittsburgh Sleep Quality Index or Duke structured interview for insomnia or sleep disorders were not adopted in this study, instead, we used the HAMD-17 with its three-item sleep subscale, which was better correlated with sleep diaries (Manber et al., 2005). Second, the functional brain networks in this study were only constructed at a regional level by parcellating the whole brain into 90 regions based on the AAL atlas. Previous studies have revealed that different parcellation strategies can exhibit distinct topological properties. Future studies should plan to systematically examine the most appropriate parcellation strategy to map the brain network topology in insomnia.
Conclusions
To the best of our knowledge, this is the first study to examine the aberrant topological organization of intrinsic brain networks in insomnia. We discovered that both healthy subjects with and without insomnia symptoms exhibited the small-worldness topology. No significant differences were identified between the IS and NIS groups according to the measures of global topological properties. However, altered regional network properties of the salience and frontostriatal networks were strongly linked with insomnia. Consequently, our findings presented novel evidence that disrupted topological organizations of the frontostriatal connectivity and salience networks may be a cause or consequence of insomnia.
Author Contributions
FL, CL, SL, LT, CT, and ZY conceived and designed the experiments. SL, LT, and CT acquired the data, which FL and ZY analyzed the data. FL, CL, JZ, and ZY wrote the article, which all authors reviewed and approved for submission.
Funding
This work was supported by MYRG2014-00093-FHS, MYRG2015-00036-FHS and MYRG2016-00110-FHS grants from the University of Macau and FDCT 026/2014/A1 and FDCT 025/2015/A1 grants from Macao government in Macau. This work was also supported by the National Natural Science Foundation of China (grant 81471389) and the High-Level Health Technical Personnel in Beijing (grant 2014-3-095).
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The authors gratefully appreciate the volunteers for participating in this study, the staffs from the Beijing Normal University Imaging Center for Brain Research and Prof. Yu-Feng Zang for their contributions in recruitment and MRI data acquisition.
Supplementary Material
The Supplementary Material for this article can be found online at: http://journal.frontiersin.org/article/10.3389/fnins.2017.00214/full#supplementary-material
References
Achard, S., and Bullmore, E. (2007). Efficiency and cost of economical brain functional networks. PLoS Comput. Biol. 3:e17. doi: 10.1371/journal.pcbi.0030017
Achard, S., Salvador, R., Whitcher, B., Suckling, J., and Bullmore, E. (2006). A resilient, low-frequency, small-world human brain functional network with highly connected association cortical hubs. J. Neurosci. 26, 63–72. doi: 10.1523/JNEUROSCI.3874-05.2006
Akert, K., and Hartmann-von Monakow, K. (1980). Relationships of precentral premotor and prefrontal cortex to the mediodorsal and intralaminar nuclei of the monkey thalamus. Acta Neurobiol. Exp. (Wars). 40, 7–25.
Altena, E., Van Der Werf, Y. D., Sanz-Arigita, E. J., Voorn, T. A., Rombouts, S., Kuijer, J., et al. (2008). Prefrontal hypoactivation and recovery in insomnia. Sleep 31, 1271–1276.
Altena, E., Vrenken, H., Van Der Werf, Y. D., van den Heuvel, O. A., and Van Someren, E. J. (2010). Reduced orbitofrontal and parietal gray matter in chronic insomnia: a voxel-based morphometric study. Biol. Psychiatry 67, 182–185. doi: 10.1016/j.biopsych.2009.08.003
Behzadi, Y., Restom, K., Liau, J., and Liu, T. T. (2007). A component based noise correction method (CompCor) for BOLD and perfusion based fMRI. Neuroimage 37, 90–101. doi: 10.1016/j.neuroimage.2007.04.042
Bell-McGinty, S., Habeck, C., Hilton, H. J., Rakitin, B., Scarmeas, N., Zarahn, E., et al. (2004). Identification and differential vulnerability of a neural network in sleep deprivation. Cereb. Cortex 14, 496–502. doi: 10.1093/cercor/bhh011
Benca, R. M., and Peterson, M. J. (2008). Insomnia and depression. Sleep Med. 9, S3–S9. doi: 10.1016/S1389-9457(08)70010-8
Biswal, B. B., Kylen, J. V., and Hyde, J. S. (1997). Simultaneous assessment of flow and BOLD signals in resting-state functional connectivity maps. NMR Biomed. 10, 165–170. doi: 10.1002/(SICI)1099-1492(199706/08)10:4/5<165::AID-NBM454>3.0.CO;2-7
Biswal, B. B., Yetkin, F. Z., Haughton, V. M., and Hyde, J. S. (1995). Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magn. Reson. Med. 34, 537–541. doi: 10.1002/mrm.1910340409
Bridoux, A., Drouot, X., Sangare, A., Al-Ani, T., Brignol, A., Charles-Nelson, A., et al. (2015). Bilateral thalamic stimulation induces insomnia in patients treated for intractable tremor. Sleep 38, 473–478. doi: 10.5665/sleep.4512
Bullmore, E., and Sporns, O. (2009). Complex brain networks: graph theoretical analysis of structural and functional systems. Nat. Rev. Neurosci. 10, 186–198. doi: 10.1038/nrn2575
Bullmore, E. T., Suckling, J., Overmeyer, S., Rabe-Hesketh, S., Taylor, E., and Brammer, M. J. (1999). Global, voxel, and cluster tests, by theory and permutation, for a difference between two groups of structural MR images of the brain. IEEE Trans. Med. Imaging 18, 32–42. doi: 10.1109/42.750253
Cauda, F., Costa, T., Torta, D. M., Sacco, K., D'Agata, F., Duca, S., et al. (2012). Meta-analytic clustering of the insular cortex: characterizing the meta-analytic connectivity of the insula when involved in active tasks. Neuroimage 62, 343–355. doi: 10.1016/j.neuroimage.2012.04.012
Cauda, F., D'Agata, F., Sacco, K., Duca, S., Geminiani, G., and Vercelli, A. (2011). Functional connectivity of the insula in the resting brain. Neuroimage 55, 8–23. doi: 10.1016/j.neuroimage.2010.11.049
Chao-Gan, Y., and Yu-Feng, Z. (2010). DPARSF: a MATLAB toolbox for “pipeline” data analysis of resting-state fMRI. Front. Syst. Neurosci. 4:13. doi: 10.3389/fnsys.2010.00013
Chen, M. C., Chang, C., Glover, G. H., and Gotlib, I. H. (2014). Increased insula coactivation with salience networks in insomnia. Biol. Psychol. 97, 1–8. doi: 10.1016/j.biopsycho.2013.12.016
Cheung, J. M., Bartlett, D. J., Armour, C. L., and Saini, B. (2013). The insomnia patient perspective, a narrative review. Behav. Sleep Med. 11, 369–389. doi: 10.1080/15402002.2012.694382
Di Martino, A., Scheres, A., Margulies, D. S., Kelly, A. M., Uddin, L. Q., Shehzad, Z., et al. (2008). Functional connectivity of human striatum: a resting state fMRI study. Cereb. Cortex 18, 2735–2747. doi: 10.1093/cercor/bhn041
Drummond, S. P., Smith, M. T., Orff, H. J., Chengazi, V., and Perlis, M. L. (2004). Functional imaging of the sleeping brain: review of findings and implications for the study of insomnia. Sleep Med. Rev. 8, 227–242. doi: 10.1016/j.smrv.2003.10.005
Drummond, S., Walker, M., Almklov, E., Campos, M., Anderson, D. E., and Straus, L. D. (2013). Neural correlates of working memory performance in primary insomnia. Sleep 36, 1307–1316. doi: 10.5665/sleep.2952
De Vico Fallani, F., Astolfi, L., Cincotti, F., Mattia, D., Marciani, M. G., Salinari, S., et al. (2007). Cortical functional connectivity networks in normal and spinal cord injured patients: evaluation by graph analysis. Hum. Brain Mapp. 28, 1334–1346. doi: 10.1002/hbm.20353
Ford, D. E., and Kamerow, D. B. (1989). Epidemiologic study of sleep disturbances and psychiatric disorders: an opportunity for prevention? J. Am. Med. Assoc. 262, 1479–1484. doi: 10.1001/jama.1989.03430110069030
Gillin, J. C. (1998). Are sleep disturbances risk factors for anxiety, depressive and addictive disorders? Acta Psychiatr. Scand. 98, 39–43. doi: 10.1111/j.1600-0447.1998.tb05965.x
Gong, G., He, Y., Concha, L., Lebel, C., Gross, D. W., Evans, A. C., et al. (2009). Mapping anatomical connectivity patterns of human cerebral cortex using in vivo diffusion tensor imaging tractography. Cereb. Cortex 19, 524–536. doi: 10.1093/cercor/bhn102
Haber, S. N., and Calzavara, R. (2009). The cortico-basal ganglia integrative network: the role of the thalamus. Brain Res. Bull. 78, 69–74. doi: 10.1016/j.brainresbull.2008.09.013
Hamilton, M. (1967). Development of a rating scale for primary depressive illness. Br. J. Soc. Clin. Psychol. 6, 278–296. doi: 10.1111/j.2044-8260.1967.tb00530.x
He, Y., Dagher, A., Chen, Z., Charil, A., Zijdenbos, A., Worsley, K., et al. (2009). Impaired small-world efficiency in structural cortical networks in multiple sclerosis associated with white matter lesion load. Brain 132, 3366–3379. doi: 10.1093/brain/awp089
Huang, Z., Liang, P., Jia, X., Zhan, S., Li, N., Ding, Y., et al. (2012). Abnormal amygdala connectivity in patients with primary insomnia: evidence from resting state fMRI. Eur. J. Radiol. 81, 1288–1295. doi: 10.1016/j.ejrad.2011.03.029
Humphries, M. D., Gurney, K., and Prescott, T. J. (2006). The brainstem reticular formation is a small-world, not scale-free, network. Proc. R. Soc. Biol. Sci. 273, 503–511. doi: 10.1098/rspb.2005.3354
Itahashi, T., Yamada, T., Watanabe, H., Nakamura, M., Jimbo, D., Shioda, S., et al. (2014). Altered network topologies and hub organization in adults with autism: a resting-state fMRI study. PLoS ONE 9:e94115. doi: 10.1371/journal.pone.0094115
Joo, E. Y., Noh, H. J., Kim, J.-S., Koo, D. L., Kim, D., Hwang, K. J., et al. (2013). Brain gray matter deficits in patients with chronic primary insomnia. Sleep 36, 999. doi: 10.5665/sleep.2796
Kaneita, Y., Ohida, T., Uchiyama, M., Takemura, S., Kawahara, K., Yokoyama, E., et al. (2006). The relationship between depression and sleep disturbances: a Japanese nationwide general population survey. J. Clin. Psychiatry 67, 196–203. doi: 10.4088/JCP.v67n0204
Kelly, C., Toro, R., Di Martino, A., Cox, C. L., Bellec, P., Castellanos, F. X., et al. (2012). A convergent functional architecture of the insula emerges across imaging modalities. Neuroimage 61, 1129–1142. doi: 10.1016/j.neuroimage.2012.03.021
Krebs, R. M., Boehler, C. N., Roberts, K. C., Song, A. W., and Woldorff, M. G. (2012). The involvement of the dopaminergic midbrain and cortico-striatal-thalamic circuits in the integration of reward prospect and attentional task demands. Cereb. Cortex 22, 607–615. doi: 10.1093/cercor/bhr134
Kronholm, E., Puusniekka, R., Jokela, J., Villberg, J., Urrila, A. S., Paunio, T., et al. (2015). Trends in self-reported sleep problems, tiredness and related school performance among Finnish adolescents from 1984 to 2011. J. Sleep Res. 24, 3–10. doi: 10.1111/jsr.12258
Kucharczyk, E. R., Morgan, K., and Hall, A. P. (2012). The occupational impact of sleep quality and insomnia symptoms. Sleep Med. Rev. 16, 547–559. doi: 10.1016/j.smrv.2012.01.005
Li, C., Ma, X., Dong, M., Yin, Y., Hua, K., Li, M., et al. (2016). Abnormal spontaneous regional brain activity in primary insomnia: a resting-state functional magnetic resonance imaging study. Neuropsychiatr. Dis. Treat. 12, 1371–1378. doi: 10.2147/NDT.S109633
Li, Y., Wang, E., Zhang, H., Dou, S., Liu, L., Tong, L., et al. (2014). Functional connectivity changes between parietal and prefrontal cortices in primary insomnia patients: evidence from resting-state fMRI. Eur. J. Med. Res. 19, 1. doi: 10.2147/NDT.S109633
Lian, Y., Xiao, J., Liu, Y., Ning, L., Guan, S., Ge, H., et al. (2015). Associations between insomnia, sleep duration and poor work ability. J. Psychosom. Res. 78, 45–51. doi: 10.1016/j.jpsychores.2014.09.009
Liao, W., Chen, H., Feng, Y., Mantini, D., Gentili, C., Pan, Z., et al. (2010a). Selective aberrant functional connectivity of resting state networks in social anxiety disorder. Neuroimage 52, 1549–1558. doi: 10.1016/j.neuroimage.2010.05.010
Liao, W., Zhang, Z., Pan, Z., Mantini, D., Ding, J., Duan, X., et al. (2010b). Altered functional connectivity and small-world in mesial temporal lobe epilepsy. PLoS ONE 5:e8525. doi: 10.1371/journal.pone.0008525
Lim, A., Fleischman, D. A., Dawe, R. J., Yu, L., Arfanakis, K., Buchman, A. S., et al. (2015). Regional neocortical gray matter structure and sleep fragmentation in older adults. Sleep 39, 227–235. doi: 10.1016/j.sleep.2015.02.077
Liu, C.-H., Liu, C.-Z., Zhang, J., Yuan, Z., Tang, L.-R., Tie, C.-L., et al. (2016). Reduced spontaneous neuronal activity in the insular cortex and thalamus in healthy adults with insomnia symptoms. Brain Res. 1648(Pt A), 317–324. doi: 10.1016/j.brainres.2016.07.024
Liu, F., Zhuo, C., and Yu, C. (2016). Altered cerebral blood flow covariance network in schizophrenia. Front. Neurosci. 10:308. doi: 10.3389/fnins.2016.00308
Liu, X., Yan, Z., Wang, T., Yang, X., Feng, F., Fan, L., et al. (2015). Connectivity pattern differences bilaterally in the cerebellum posterior lobe in healthy subjects after normal sleep and sleep deprivation: a resting-state functional MRI study. Neuropsychiatr. Dis. Treat. 11, 1279–1289. doi: 10.2147/NDT.S84204
Liu, Y., Liang, M., Zhou, Y., He, Y., Hao, Y., Song, M., et al. (2008). Disrupted small-world networks in schizophrenia. Brain 131(Pt 4), 945–961. doi: 10.1093/brain/awn018
Long, Z., Duan, X., Wang, Y., Liu, F., Zeng, L., Zhao, J. P., et al. (2015). Disrupted structural connectivity network in treatment-naive depression. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 56, 18–26. doi: 10.1016/j.pnpbp.2014.07.007
Long, Z., Duan, X., Xie, B., Du, H., Li, R., Xu, Q., et al. (2013). Altered brain structural connectivity in post-traumatic stress disorder: a diffusion tensor imaging tractography study. J. Affect. Disord. 150, 798–806. doi: 10.1016/j.jad.2013.03.004
Manber, R., Blasey, C., Arnow, B., Markowitz, J. C., Thase, M. E., Rush, A. J., et al. (2005). Assessing insomnia severity in depression: comparison of depression rating scales and sleep diaries. J. Psychiatr. Res. 39, 481–488. doi: 10.1016/j.jpsychires.2004.12.003
Martin, L. E., Pollack, L., McCune, A., Schulte, E., Savage, C. R., and Lundgren, J. D. (2015). Comparison of obese adults with poor versus good sleep quality during a functional neuroimaging delay discounting task: a pilot study. Psychiatry Res. 234, 90–95. doi: 10.1016/j.pscychresns.2015.08.011
Mayer, G., Jennum, P., Riemann, D., and Dauvilliers, Y. (2011). Insomnia in central neurologic diseases - occurrence and management. Sleep Med. Rev. 15, 369–378. doi: 10.1016/j.smrv.2011.01.005
Meng, C., Brandl, F., Tahmasian, M., Shao, J., Manoliu, A., Scherr, M., et al. (2014). Aberrant topology of striatum's connectivity is associated with the number of episodes in depression. Brain 137, 598–609. doi: 10.1093/brain/awt290
Moore, M. (2012). Looking for a good night's sleep. Lancet 380, 322–323. doi: 10.1016/S0140-8046736(12)61220-3
Morin, C. M., and Benca, R. (2012). Chronic insomnia. Lancet 379, 1129–1141. doi: 10.1016/S0140-6736(11)60750-2
Murphy, K., Birn, R. M., Handwerker, D. A., Jones, T. B., and Bandettini, P. A. (2009). The impact of global signal regression on resting state correlations: are anti-correlated networks introduced? Neuroimage 44, 893–905. doi: 10.1016/j.neuroimage.2008.09.036
Nie, X., Shao, Y., Liu, S. Y., Li, H. J., Wan, A. L., Nie, S., et al. (2015). Functional connectivity of paired default mode network subregions in primary insomnia. Neuropsychiatr. Dis. Treat. 11, 3085. doi: 10.2147/NDT.S95224
Ohayon, M. M. (2002). Epidemiology of insomnia: what we know and what we still need to learn. Sleep Med. Rev. 6, 97–111. doi: 10.1053/smrv.2002.0186
Ohayon, M. M., and Hong, S.-C. (2006). Prevalence of major depressive disorder in the general population of South Korea. J. Psychiatr. Res. 40, 30–36. doi: 10.1016/j.jpsychires.2005.02.003
Postuma, R. B., and Dagher, A. (2006). Basal ganglia functional connectivity based on a meta-analysis of 126 positron emission tomography and functional magnetic resonance imaging publications. Cereb. Cortex 16, 1508–1521. doi: 10.1093/cercor/bhj088
Power, J. D., Barnes, K. A., Snyder, A. Z., Schlaggar, B. L., and Petersen, S. E. (2012). Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. Neuroimage 59, 2142–2154. doi: 10.1016/j.neuroimage.2011.10.018
Riedner, B. A., Goldstein, M. R., Plante, D. T., Rumble, M. E., Ferrarelli, F., Tononi, G., et al. (2015). Regional patterns of elevated alpha and high-frequency electroencephalographic activity during nonrapid eye movement sleep in chronic insomnia: a pilot study. Sleep 39, 801–812. doi: 10.5665/sleep.5632
Riemann, D., Nissen, C., Palagini, L., Otte, A., Perlis, M. L., and Spiegelhalder, K. (2015). The neurobiology, investigation, and treatment of chronic insomnia. Lancet Neurol. 14, 547–558. doi: 10.1016/S1474-4422(15)00021-6
Riemann, D., and Voderholzer, U. (2003). Primary insomnia: a risk factor to develop depression? J. Affect. Disord. 76, 255–259. doi: 10.1016/S0165-0327(02)00072-1
Rubinov, M., and Sporns, O. (2010). Complex network measures of brain connectivity: uses and interpretations. Neuroimage 52, 1059–1069. doi: 10.1016/j.neuroimage.2009.10.003
Saalmann, Y. B. (2014). Intralaminar and medial thalamic influence on cortical synchrony, information transmission and cognition. Front. Syst. Neurosci. 8:83. doi: 10.3389/fnsys.2014.00083
Salvador, R., Suckling, J., Coleman, M. R., Pickard, J. D., Menon, D., and Bullmore, E. (2005). Neurophysiological architecture of functional magnetic resonance images of human brain. Cereb. Cortex 15, 1332–1342. doi: 10.1093/cercor/bhi016
Shao, Y., Lei, Y., Wang, L., Zhai, T., Jin, X., Ni, W., et al. (2014). Altered resting-state amygdala functional connectivity after 36 hours of total sleep deprivation. PLoS ONE 9:e112222. doi: 10.1371/journal.pone.0112222
Short, M. A., Gradisar, M., Lack, L. C., and Wright, H. R. (2013). The impact of sleep on adolescent depressed mood, alertness and academic performance. J. Adolesc. 36, 1025–1033. doi: 10.1016/j.adolescence.2013.08.007
Spiegelhalder, K., Regen, W., Baglioni, C., Nissen, C., Riemann, D., and Kyle, S. D. (2015). Neuroimaging insights into insomnia. Curr. Neurol. Neurosci. Rep. 15, 1–7. doi: 10.1007/s11910-015-0527-3
Spiegelhalder, K., Regen, W., Baglioni, C., Riemann, D., and Winkelman, J. W. (2013). Neuroimaging studies in insomnia. Curr. Psychiatry Rep. 15, 1–6. doi: 10.1007/s11920-013-0405-0
Spiegelhalder, K., Regen, W., Prem, M., Baglioni, C., Nissen, C., Feige, B., et al. (2014). Reduced anterior internal capsule white matter integrity in primary insomnia. Hum. Brain Mapp. 35, 3431–3438. doi: 10.1002/hbm.22412
Spira, A. P., Gonzalez, C. E., Venkatraman, V. K., Wu, M. N., Pacheco, J., Simonsick, E. M., et al. (2015). Sleep duration and subsequent cortical thinning in cognitively normal older adults. Sleep 39, 1121–1128. doi: 10.5665/sleep.5768
Staner, L. (2010). Comorbidity of insomnia and depression. Sleep Med. Rev. 14, 35–46. doi: 10.1016/j.smrv.2009.09.003
Stoffers, D., Altena, E., van der Werf, Y. D., Sanz-Arigita, E. J., Voorn, T. A., Astill, R. G., et al. (2014). The caudate: a key node in the neuronal network imbalance of insomnia? Brain 137, 610–620. doi: 10.3389/fneur.2012.00105
Stoffers, D., Moens, S., Benjamins, J., van Tol, M.-J., Penninx, B. W. H. J., Veltman, D. J., et al. (2012). Orbitofrontal gray matter relates to early morning awakening: a neural correlate of insomnia complaints? Front. Neurol. 3:105. doi: 10.3389/fneur.2012.00105
Tahmasian, M., Rosenzweig, I., Eickhoff, S. B., Sepehry, A. A., Laird, A. R., Fox, P. T., et al. (2016). Structural and functional neural adaptations in obstructive sleep apnea: an activation likelihood estimation meta-analysis. Neurosci. Biobehav. Rev. 65, 142–156. doi: 10.1016/j.neubiorev.2016.03.026
Taylor, D. J., Lichstein, K. L., Durrence, H. H., Reidel, B. W., and Bush, A. J. (2005). Epidemiology of insomnia, depression, and anxiety. Sleep 28, 1457.
Thomas, M., Sing, H., Belenky, G., Holcomb, H., Mayberg, H., Dannals, R., et al. (2000). Neural basis of alertness and cognitive performance impairments during sleepiness. I. Effects of 24 h of sleep deprivation on waking human regional brain activity. J. Sleep Res. 9, 335–352. doi: 10.1046/j.1365-2869.2000.00225.x
Trivedi, M. H., Bandelow, B., Demyttenaere, K., Papakosts, G. I., Szamosi, J., Earley, W., et al. (2013). Evaluation of the effects of extended release quetiapine fumarate monotherapy on sleep disturbance in patients with major depressive disorder: a pooled analysis of four randomized acute studies. Int. J. Neuropsychopharmacol. 16, 1733–1744. doi: 10.1017/S146114571300028X
Tzourio-Mazoyer, N., Landeau, B., Papathanassiou, D., Crivello, F., Etard, O., Delcroix, N., et al. (2002). Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage 15, 273–289. doi: 10.1006/nimg.2001.0978
Uddin, L. Q. (2015). Salience processing and insular cortical function and dysfunction. Nat. Rev. Neurosci. 16, 55–61. doi: 10.1038/nrn3857
Wang, J., Wang, L., Zang, Y., Yang, H., Tang, H., Gong, Q., et al. (2009). Parcellation-dependent small-world brain functional networks: a resting-state fMRI study. Hum. Brain Mapp. 30, 1511–1523. doi: 10.1002/hbm.20623
Wang, J., Wang, X., Xia, M., Liao, X., Evans, A., and He, Y. (2015). GRETNA: a graph theoretical network analysis toolbox for imaging connectomics. Front. Hum. Neurosci. 9:386. doi: 10.3389/fnhum.2015.00386
Wang, J., Zuo, X., Dai, Z., Xia, M., Zhao, Z., Zhao, X., et al. (2013). Disrupted functional brain connectome in individuals at risk for Alzheimer's disease. Biol. Psychiatry 73, 472–481. doi: 10.1016/j.biopsych.2012.03.026
Wang, L., Zhu, C., He, Y., Zang, Y., Cao, Q., Zhang, H., et al. (2009). Altered small-world brain functional networks in children with attention-deficit/hyperactivity disorder. Hum. Brain Mapp. 30, 638–649. doi: 10.1002/hbm.20530
Wang, T., Li, S., Jiang, G., Lin, C., Li, M., Ma, X., et al. (2016). Regional homogeneity changes in patients with primary insomnia. Eur. Radiol. 26, 1292–1300. doi: 10.1007/s00330-015-3960-4
Watts, D. J., and Strogatz, S. H. (1998). Collective dynamics of “small-world” networks. Nature 393, 440–442. doi: 10.1038/30918
Wu, J. C., Gillin, J. C., Buchsbaum, M. S., Chen, P., Keator, D. B., Khosla Wu, N., et al. (2006). Frontal lobe metabolic decreases with sleep deprivation not totally reversed by recovery sleep. Neuropsychopharmacology 31, 2783–2792. doi: 10.1038/sj.npp.1301166
Wulff, K., Gatti, S., Wettstein, J. G., and Foster, R. G. (2010). Sleep and circadian rhythm disruption in psychiatric and neurodegenerative disease. Nat. Rev. Neurosci. 11, 589–599. doi: 10.1038/nrn2868
Zhang, J., Wang, J., Wu, Q., Kuang, W., Huang, X., He, Y., et al. (2011). Disrupted brain connectivity networks in drug-naive, first-episode major depressive disorder. Biol. Psychiatry 70, 334–342. doi: 10.1016/j.biopsych.2011.05.018
Zhang, T., Yang, Y., Li, B., Yue, Q., and Zang, Y. (2011). Abnormal small-world architecture of top-down control networks in obsessive-compulsive disorder. J. Psychiatry Neurosci. 36, 23. doi: 10.1503/jpn.100006
Zhang, Z., Liao, W., Chen, H., Mantini, D., Ding, J.-R., Xu, Q., et al. (2011). Altered functional–structural coupling of large-scale brain networks in idiopathic generalized epilepsy. Brain 134, 2912–2928. doi: 10.1093/brain/awr223
Keywords: insomnia, resting-state fMRI, graph theory, salience network, frontostriatal connectivity
Citation: Lu F-M, Liu C-H, Lu S-L, Tang L-R, Tie C-L, Zhang J and Yuan Z (2017) Disrupted Topology of Frontostriatal Circuits Is Linked to the Severity of Insomnia. Front. Neurosci. 11:214. doi: 10.3389/fnins.2017.00214
Received: 13 January 2017; Accepted: 30 March 2017;
Published: 19 April 2017.
Edited by:
J. Michael Williams, Drexel University, USAReviewed by:
Jeffrey Bedwell, University of Central Florida, USAIsabel Maria Martin Monzon, University of Seville, Spain
Copyright © 2017 Lu, Liu, Lu, Tang, Tie, Zhang and Yuan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhen Yuan, emhlbnl1YW5AdW1hYy5tbw==
 Feng-Mei Lu
Feng-Mei Lu Chun-Hong Liu
Chun-Hong Liu Shun-Li Lu3
Shun-Li Lu3 Juan Zhang
Juan Zhang Zhen Yuan
Zhen Yuan