
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Neurosci. , 31 August 2016
Sec. Child and Adolescent Psychiatry
Volume 10 - 2016 | https://doi.org/10.3389/fnins.2016.00388
This article is part of the Research Topic Advanced neuroimaging methods for studying autism disorder View all 14 articles
Autism is a neurodevelopmental disorder that mainly affects social interaction and communication. Evidence from behavioral and functional MRI studies supports the hypothesis that dysfunctional mechanisms involving social brain structures play a major role in autistic symptomatology. However, the investigation of anatomical abnormalities in the brain of people with autism has led to inconsistent results. We investigated whether specific brain regions, known to display functional abnormalities in autism, may exhibit mutual and peculiar patterns of covariance in their gray-matter concentrations. We analyzed structural MRI images of 32 young men affected by autistic disorder (AD) and 50 healthy controls. Controls were matched for sex, age, handedness. IQ scores were also monitored to avoid confounding. A multivariate Source-Based Morphometry (SBM) was applied for the first time on AD and controls to detect maximally independent networks of gray matter. Group comparison revealed a gray-matter source that showed differences in AD compared to controls. This network includes broad temporal regions involved in social cognition and high-level visual processing, but also motor and executive areas of the frontal lobe. Notably, we found that gray matter differences, as reflected by SBM, significantly correlated with social and behavioral deficits displayed by AD individuals and encoded via the Autism Diagnostic Observation Schedule scores. These findings provide support for current hypotheses about the neural basis of atypical social and mental states information processing in autism.
Autism Spectrum Disorder (ASD) is a category of pervasive developmental disorders (PDD) that affect 1 in 150 children (Rapin and Tuchman, 2008). Autistic symptomatology is characterized by severe impairments that mainly affect social interaction, communication, while sparing basic cognitive skills (Misra, 2014), and not implying emotional disturbance (Rapin and Tuchman, 2008). The term ASD was introduced by Allen (1988) and included: autistic disorder (AD), Asperger syndrome (AS), and PDD not otherwise specified (Levy et al., 2009; Pina-Camacho et al., 2012). Recently, the DSM V revised the conceptualization of those disorders and currently diagnostic classifications only include Autism Spectrum Disorders (ASD) (Lord and Bishop, 2015). Nevertheless, the diagnostic criteria adopted in the current study still refer to DSM IV, and we focused primarily on autism disorder (AD). Consequently, we use the words “autism” and “autistic” to specifically refer to AD.
The mind-blindness theory proposed that characteristic problem in social interaction arises because AD have difficulties in mentalizing and understanding psychological dynamics in other people and in oneself (Baron-Cohen et al., 1985; Frith et al., 1991). Support for this model comes from behavioral studies showing that autistics poorly perform on tasks that require theory of mind (ToM) abilities (Baron-Cohen et al., 1985; Misra, 2014). The poor performance of people with autism on ToM task is thought to be due to a conceptual deficit or to processing peculiarities. In normally developing children ToM tasks elicit intuitive social insights into people. In contrast, autistic children treat these tasks as logical-reasoning problems relying on language and non-social cognitive functions (Tager-Flusberg et al., 2009). In line with this hypothesis, functional MRI investigations of AD have highlighted reduced responses in cortical areas related to social interaction (Frith, 2003; Di Martino et al., 2009; Lombardo et al., 2011; Gliga et al., 2014), and the engagement of areas associated with general problem solving abilities (Frith, 2003). Moreover, functional connectivity in a group with autism shows abnormal pattern in areas mediating ToM and in the mirror neuron system (MNS) as well (Fishman et al., 2014; Cheng et al., 2015; Kana et al., 2015).
On the other hand, studies of volumetric changes in the autistic brain resulted in inconsistent brain structures across studies: prefrontal cortex (Courchesne et al., 2011b), cerebellum (Sparks et al., 2002), temporal lobe (Palmen et al., 2006), both the frontal (Herbert et al., 2004; Jiao et al., 2010) and parietal lobes (Courchesne et al., 1993) and amygdala (Schumann et al., 2004). Some inconsistencies in the structural MRI literature may be attributed to differences in methodology, age, heterogeneity of the disorder (Geschwind, 2009), or diffuse structural abnormalities. Diffuse structural abnormalities in autism could reflect impairment of many, if not most, brain networks (Müller, 2007).
Recently, predictive models of autism based on pattern recognition in structural MR images have been successfully developed. Ecker et al. (2010) adopted a support vector machine (SVM) method to discriminate ASD individuals from controls. Brain areas in the temporal lobe, precuneus, hippocampal, and fusiform gyri were crucial for discrimination. Neural abnormalities discovered with such methodologies, however, rarely correlate with clinical criteria, or correlate only with generic total scores, and not with specific subscales (Eliez and Reiss, 2000; Lord et al., 2000; Hardan et al., 2006a,b; Ecker et al., 2010; Griebling et al., 2010). In other words, these correlations may refer more to a general severity of pathology than to specific deficits.
We suggest that neuroanatomical markers of autism disorder may be better understood by studying large-scale anatomical networks (Minshew and Williams, 2007; Schaer et al., 2013). SBM is a data-driven multivariate alternative to the standard Voxel-Based Morphometry (VBM), and it may be particularly suitable to the investigation of anatomical changes in autism. SBM takes into account information across different voxels and identifies unpredicted, naturally occurring patterns of covariance across brain regions (Xu et al., 2009). Notably, such anatomical covariance has been shown to reflect functional connectivity (Evans, 2013). For these reasons, we expect SBM to individuate large anatomical networks of gray-matter which show aberrant patterns of covariance in AD, as compared to control. We also expect those networks to include brain areas that previous studies showed to be anatomically or functionally abnormal.
Given the heterogeneity of AD and the need for large-scale samples of MR and clinical measures, the present study utilizes the ABIDE (Autism Brain Imaging Data Exchange) database.
Structural MRI of 82 participants (32 AD and 50 controls) were extracted from Autism Brain Imaging Data Exchange (ABIDE). Details of acquisition, informed consent, site-specific protocols, specific diagnostic criteria for each data set can be found at http://fcon_1000.projects.nitrc.org/indi/abide/index.html. The following committees approved the protocols of each site: the Human Subjects Protection Committee of the California Institute of Technology (CAL), the Institutional Review Board at the University of Pittsburgh (PBG) and the University of Utah School of Medicine (USM).
From around 3000 subjects available, we carefully selected participants by gender (males), age (range: 18–39 years old), as well as parameters of the MR scanners (image type: T1; magnetic field strength: 3T). This first selection resulted in a dataset composed by structural MRI of 283 subject acquired with 12 different MRI scanners. Next, the 283 subjects were screened on the basis of the DSV-IV for either the absence of any neuropsychiatric disorders (control group), or the diagnosis of Autism (AD) (patients group). Further inclusion criteria were: (a) the indication of the IQ score computed on the basis of Wechsler abbreviated scale of intelligence (WASI); (b) scores on at least three specific subscales of the Autism diagnostic observation schedule (ADOS) for AD participants. Finally, individuals with any other pathology or comorbidity were rejected. An in house made Dori script iteratively sampled the database until controls and AD group were balanced for age and there was no association between groups and a particular scanner.
This procedure yielded a dataset comprising 82 participants (32 AD and 50 controls) tested on one of the two MR scanners, with the same MR sequence (Table 1 in Supplementary Material). The scanners belonged to the same vendor (i.e., Siemens MAGNETOM TrioTim, Siemens MAGNETOM Allegra), and participants were equally distributed between scanners (See Table 1 in Supplementary Material). The two groups did not differed for age [t(31) = 0.2953, p = 0.7728]. However, there was a difference in IQ scores [t(31) = 2.9586, p = 0.0041]. See Table 1 in Supplementary Material. This is in line with previous observations for which the prevalence of mental retardation in autism is ~60% groups, and AD naturally differ from normal controls for IQ (Amaral et al., 2008).
Source-based morphometry (SBM) is a multivariate technique that takes advantage of independent component analysis (ICA) (Lee, 1998; Xu et al., 2009). ICA is a statistical technique that is widely used in many fields of biomedical research for signal analysis. In the field of neuroscience ICA has found important application in EEG/MEG/fMRI data analyses to isolate noisy artifacts.
Pulling images from different MR scanners is known to lead to confounding, even though MR sequences are the same (Han et al., 2006). This is especially true for a massive univariate analysis, such as VBM. However, SBM can decompose the MR signal in several maximally independent sources, or independent components (ICs). In this study, few sources may reflect signal differences among MR scanners, while the majority of ICs individuate networks of gray-matter that share patterns of covariance among subjects (Xu et al., 2009; Kaspárek et al., 2010; Kubera et al., 2014). Artifactual components are usually easy to detect because are asymmetric, do not follow the anatomical organization of the brain, and do not exhibit any coherent patterns. In addition, the distribution of subjects throughout scanners is similar in both controls and AD, and this should guarantee that group differences are not due to artifact components.
The preprocessing of images is identical to the procedure adopted for classical VBM analyses. Brain extraction and robust center estimation was automatically carried out using FSL Brain extraction tool (BET) (Smith, 2002). For normalization and segmentation we used the SPM toolbox VBM8. Images were spatially normalized to the 152 average T1 MNI (Montreal Neurological Institute) template, and segmented into gray-matter (GM), white-matter (WM), and cerebrospinal fluid (CSF). The normalized gray-matter images were smoothed with 8-mm full width at half-maximum (FWHM) Gaussian kernel to establish spatial correspondence between the different brains.
Source-based morphometry analysis was carried out using the GIFT toolbox (http://icatb.sourceforge.net) (Xu et al., 2009). The minimum description length (MDL) principle was used to estimate a number of independent components. MDL found eight reliable ICs. We performed ICA using a neural network algorithm (Infomax) that attempts to minimize the mutual information of the network outputs to identify naturally grouping and maximally independent sources (Bell and Sejnowski, 1995). ICA was repeated 20 times in ICASSO (http://research.ics.aalto.fi/ica/icasso/) and the resulting components were clustered to ensure the consistency and reliability of the results. Reliability is quantified using a quality index Iq, ranging from 0 to 1 and reflecting the difference between intra-cluster and extra-cluster similarity (Himberg et al., 2004). All the 8 components extracted from the GM images were found to be associated with an Iq > 0.97 indicating a highly stable ICA decomposition.
SBM involves converting each gray-matter volume into a vector. As a result, we obtained a matrix where the 82 rows represent the 82 subjects (the first 50 rows represent controls, while the other 32 AD), and each column indicates a voxel. This matrix was decomposed into two matrices by ICA. The first matrix is named “mixing matrix” and it is composed by a subject per row and an IC per column. Therefore, the mixing matrix indicates how much a subject expresses a given component. For this reason, values in the mixing matrix are called “loading coefficients.” The second matrix is named source matrix and it specifies the relation between the ICs and the voxels. For gray-matter volume component visualization the source matrix was reshaped back to a three-dimensional image, scaled to unit standard deviations (Z maps) and thresholded at Z > 2.5.
We used the mixing matrix to verify whether components are differently expressed between controls and AD. A two sample t-test without assuming equal variances (F-test revealed unequal variances) was used to test whether all the ICs are similarity expressed by either of the groups. Similarly, we used the loading coefficients in the mixing matrix to test a linear relation among ADOS scores and the level of components' expression. All the results were thresholded at p < 0.05 corrected for Family Wise Error (FWE).
We extracted eight independent components (Figure 1). However, only the 7th component was significantly different [t(31) = 2.9482, p(FWE) = 0.0042] between AD and controls. We call this component “autism-specific structural network” (ASN). Anatomical labels of the regions composing ASN were obtained using the WFU PickAtlas (Tzourio-Mazoyer et al., 2002). Among regions that differ between AD and controls we found: inferior, middle, superior temporal gyri, fusiform gyrus, parahippocampal gyrus, paracentral lobule, precuneus, cerebellar tonsil, and portions of the inferior, middle, and superior frontal gyri (Figure 2). All the gray-matter regions of ASN are presented in Table 2 in Supplementary Material, and in Figure 2.
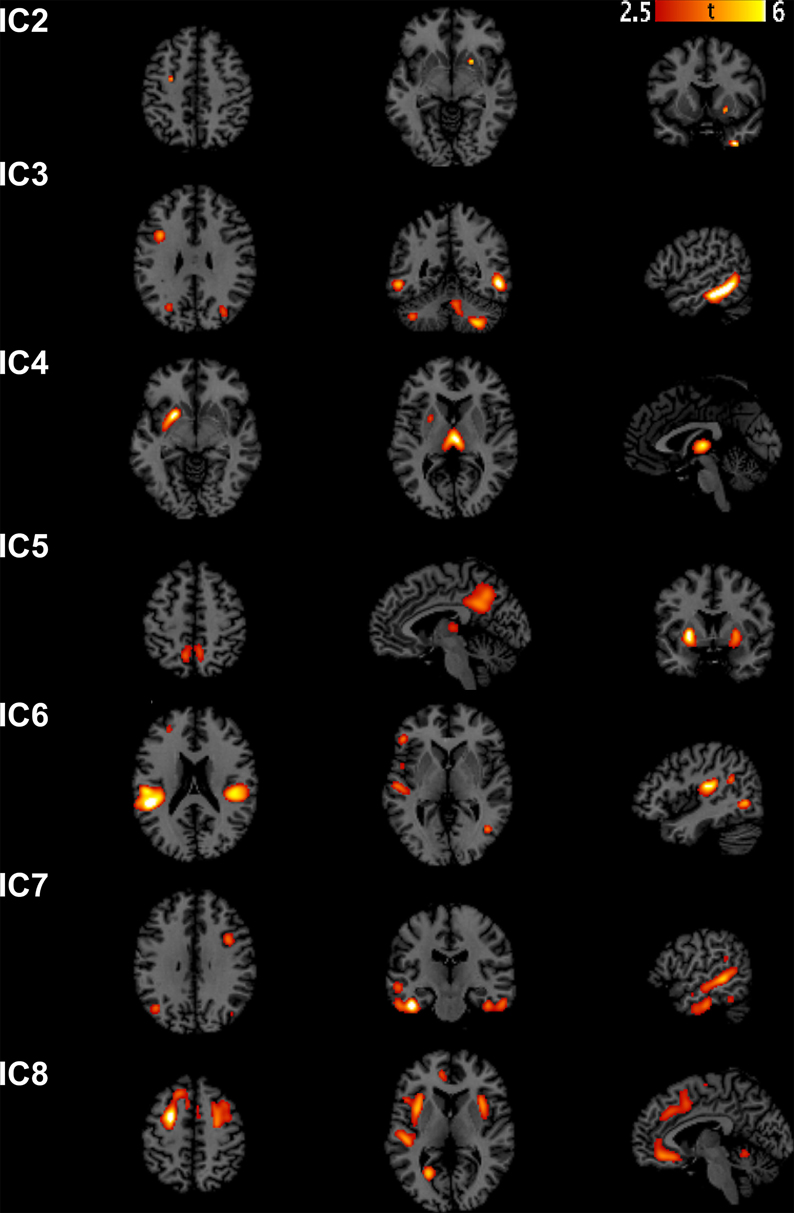
Figure 1. Sources discovered by SBM. According to the estimation of the number of components, eight independent components were extracted. Note that IC1 is not graphically represented because no voxel survived after thresholding for Z > 2.5.
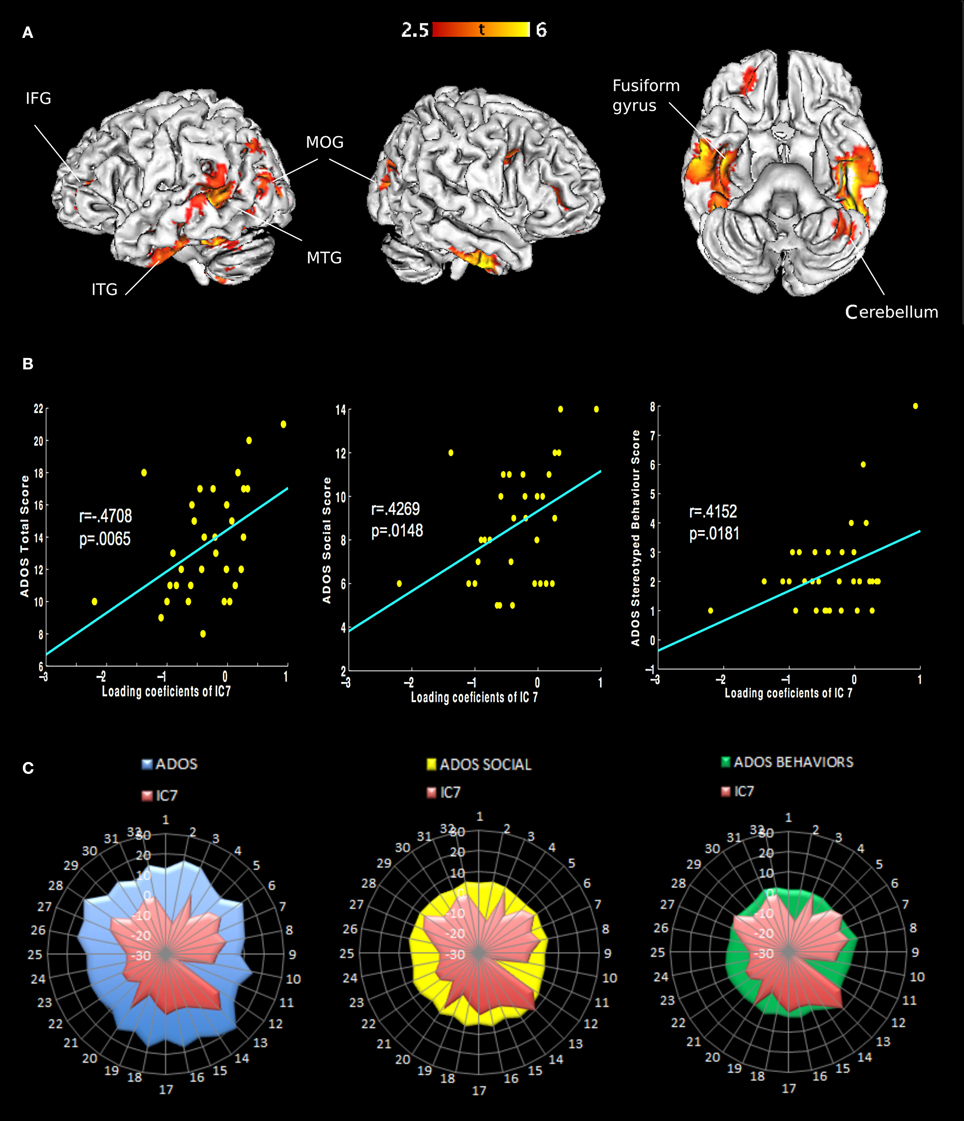
Figure 2. Representation of ASN in the brain and behavior in AD. (A) Only the seventh component shows different loading coefficient between ASD and controls. The regions involved are: inferior frontal gyrus, middle frontal gyrus, superior frontal gyrus, inferior temporal gyrus, middle temporal gyrus and superior temporal gyrus, fusiform gyrus, parahippocampal gyrus, paracentral lobule, precuneus and cerebellar tonsil (Table 2 in Supplementary Material). (B) Correlations of behavioral measures with loading coeficients values in IC 7: Classic Total ADOS Score (communication subscore + social interaction subscore); Social Total subscore: Stereotyped behaviors and restricted interest. (C) Covariance plots showing each subjects' ADOS scores and individual ASN loading coefficients.
To ensure that differences in IQ (see Table 1 in Supplementary Material) did not account for brain differences we correlated ASN loading coefficients (i.e., 7th column of the mixing matrix) against IQ values. The correlation was not significant (p = 0.6336), thus excluding the relevance of IQ on this component.
Notably, ASN significantly correlated with the total scores of ADOS, and also with two ADOS subscales measuring highly relevant impairments in AD, i.e., difficulties in social interactions and stereotyped behavior. Both variables show significant correlation with the loading coefficient of ASN, after Bonferroni correction for multiple comparisons. Classic Total ADOS Score: r = 0.4708, p(FWE) = 0.0065. Social Total subscore: r = 0.4269, p(FWE) = 0.0148. Stereotyped behaviors and restricted interest: r = 0.4152, p(FWE) = 0.0181. See Figure 2.
In this study we presented for the first time a whole brain morphometric method (SBM), based on independent component analysis, which shows alterations in gray-matter between AD individuals and controls. This innovative multivariate procedure was applied to detect brain networks that exhibit abnormal pattern of gray-matter covariance in AD. We showed that morphometric changes in autism, as detected by SBM, are significantly associated with observable social and behavioral deficits (ADOS scores).
SBM addresses a different question in comparison with massive univariate approaches to morphological changes, as Voxel-Base-Morphometry (VBM) or ROI differences between groups. Univariate approaches detect voxel- or ROI-based changes in gray-matter concentrations, while SBM individuates different levels of expression in maximally independent gray-matter networks. Differences in network expression in turn implicate differences in gray-matter concentrations, distributed along patterns of voxel-covariation. SBM is particularly suitable to study autism as spectrum disorder, since anatomical changes are likely to be distributed along networks of brain regions. In this scenario, such anatomical changes may not be locally detectable by univariate approaches.
In the present study we found an autism-specific structural network (ASN) which covers brain regions found to exhibit functional and structural abnormalities in previous studies compared with controls (Castelli et al., 2002; Saxe and Wexler, 2005; Amaral et al., 2008; Courchesne et al., 2011b; Ecker et al., 2012; Nickl-Jockschat et al., 2012; Pappaianni et al., 2016).
The core of ASN is localized in temporal regions involved in processing and integrating social stimuli, such as faces and intention-related movements.
ASN is centered in vast portions of the temporal lobes, suggesting that the temporal lobe may have particular relevance in the autistic disorder. ASN includes parts of the inferior, medial and superior temporal sulci, the fusiform gyrus, and the parahippocampal gyrus. Temporal lobe regions are implicated in social perception, auditory processing, language, and theory of mind. These abilities were shown to be the most damaged in autism (Gendry Meresse et al., 2005). In the present study inferior temporal and fusiform gyri are the brain regions which disclosed the greatest differences between AD and controls. These are the areas involved in high-level visual processing and object recognition, in particular face recognition (Rossion et al., 2003). Furthermore, the posterior superior temporal sulcus (STS) is a core region for perception of social acts (Zilbovicius et al., 2006). STS appears to respond also to biological motion and to how another person's motion is related to his/her intentions (Vander Wyk et al., 2009). Pelphrey et al. (2007) individuated a functional deficit in ASD regarding neural mechanisms for processing emotional facial expressions and biological motion. This system of regions referring to the deficit includes the amygdala, posterior STS and fusiform gyrus. This evidence provides further support for the idea that autism disorder relates to an impairment in processing social-relevant information (Baron-Cohen et al., 1985).
ASN includes bilateral clusters in both the superior frontal gyrus (BA6) and in the precentral gyrus (BA4). Those areas correspond to premotor, supplementary, and primary motor cortices. Action processing impairments and repetitive movements are commonly observed among subjects affected by autism. Even though motor impairments are not considered one of the main symptoms of ASD, recently increasing attention has been directed to motor aspects aiming to improve a diagnostic process (Torres et al., 2013).
Our results also showed abnormalities in the inferior part of the frontal gyrus, a brain region implied in executive processes and language (Gotts et al., 2012; Libero et al., 2014). Since AD are largely characterized by deficits in imitation, language, ToM and empathy, a theory known as “broken mirror” hypothesis of ASD, has suggested that a dysfunctional MNS is an important factor in AD pathogenesis (Oberman et al., 2005). Sustaining this hypothesis, some recent studies have found abnormal pattern of functional connectivity in networks believed to underlie social abilities, as MNS and ToM systems (Fishman et al., 2014). However, the role of the mirror system in understanding AD symptomatology is still controversial (Grecucci et al., 2013; Hamilton, 2013; Enticott et al., 2014).
Cerebellar pathology is usually reported in autism (Courchesne et al., 1994, 2011a; Cauda et al., 2011; Rogers et al., 2013), both at structural and functional level (Fatemi et al., 2012). We also found cerebellum to be a part of the autism-specific structural network. Evidence suggests that the cerebellum supports cognitive functions, including language and executive functions (Becker and Stoodley, 2013), which are typically damaged in autism.
Previous meta-analyses of VBM in ASD have proposed that the amygdala and the insula are two brain regions frequently associated with abnormalities in autism (Cauda et al., 2011). In our study, we did not find structural changes in those regions. Our interpretation is that the amygdala and the insula are likely to play a role in autism, though these areas belong to a broader limbic system that, considered as a whole, may be sufficiently preserved in AD. Therefore, the neurostructural configuration of the limbic system was not markedly different between AD and controls.
Finally, we found that the autism-specific structural network (ASN loading coefficients) significantly correlates with ADOS social and ADOS stereotypic behavior scores. Such evidence further supports the relation between the structural differences in ASN and the behavioral deficits displayed by AD individuals. These findings provide support for current hypotheses about the neural basis of atypical social and mental states information processing in autism.
Autism is a behaviorally diagnosed pathology, although evidence of brain abnormalities in AD, such as atypical neural “connectivity” (Ecker et al., 2012), are increasing. MRI investigation is a precious tool for shedding light on both the neurological causes and the neurodevelopment of AD. This knowledge is needed to get fast and objective diagnosis, but also for developing appropriate treatments. The present study, along with increasing evidence (Ecker et al., 2010; Jiao et al., 2010), suggests that structural MRI may become a diagnostic instrument useful to improve the traditional behavior-based diagnosis.
AG: Experimental design, data collection and writing. DR: Experimental design, data collection, analyses and writing. RS: Experimental design, data collection, analyses, and writing. LS: writing of the paper. RJ: writing of the paper.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
We would like to thank the Comune of Rovereto and the Neuropsychoanalysis Foundation for grants that supported the present study. Special thanks go to Aidas Aglinskas for precious advice.
The Supplementary Material for this article can be found online at: http://journal.frontiersin.org/article/10.3389/fnins.2016.00388
Allen, D. A. (1988). Austistic spectrum disorders: clinical presentation in preschool children. J. Child Neurol. 3(Suppl.), S48–S56. doi: 10.1177/088307388800300110
Amaral, D. G., Schumann, C. M., and Nordahl, C. W. (2008). Neuroanatomy of autism. Trends Neurosci. 31, 137–145. doi: 10.1016/j.tins.2007.12.005
Baron-Cohen, S., Leslie, A. M., and Frith, U. (1985). Does the autistic child have a “theory of mind”? Cognition 21, 37–46.
Becker, E. B. E., and Stoodley, C. J. (2013). Autism spectrum disorder and the cerebellum. Int. Rev. Neurobiol. 113, 1–34. doi: 10.1016/B978-0-12-418700-9.00001-0
Bell, A. J., and Sejnowski, T. J. (1995). An information-maximization approach to blind separation and blind deconvolution. Neural Comput. 7, 1129–1159.
Castelli, F., Frith, C., Happé, F., and Frith, U. (2002). Autism, Asperger syndrome and brain mechanisms for the attribution of mental states to animated shapes. Brain 125(Pt 8), 1839–1849. doi: 10.1093/brain/awf189
Cauda, F., Geda, E., Sacco, K., D'Agata, F., Duca, S., Geminiani, G., et al. (2011). Grey matter abnormality in autism spectrum disorder: an activation likelihood estimation meta-analysis study. J. Neurol. Neurosurg. Psychiatr. 82, 1304–1313. doi: 10.1136/jnnp.2010.239111
Cheng, W., Rolls, E. T., Gu, H., Zhang, J., and Feng, J. (2015). Autism: reduced connectivity between cortical areas involved in face expression, theory of mind, and the sense of self. Brain 138(Pt 5), 1382–1393. doi: 10.1093/brain/awv051
Courchesne, E., Campbell, K., and Solso, S. (2011a). Brain growth across the life span in autism: age-specific changes in anatomical pathology. Brain Res. 1380, 138–145. doi: 10.1016/j.brainres.2010.09.101
Courchesne, E., Mouton, P. R., Calhoun, M. E., Semendeferi, K., Ahrens-Barbeau, C., Hallet, M. J., et al. (2011b). Neuron number and size in prefrontal cortex of children with autism. JAMA 306, 2001–2010. doi: 10.1001/jama.2011.1638
Courchesne, E., Press, G. A., and Yeung-Courchesne, R. (1993). Parietal lobe abnormalities detected with MR in patients with infantile autism. Am. J. Roentgenol. 160, 387–393. doi: 10.2214/ajr.160.2.8424359
Courchesne, E., Townsend, J., Akshoomoff, N. A., Saitoh, O., Yeung-Courchesne, R., Lincoln, A. J., et al. (1994). Impairment in shifting attention in autistic and cerebellar patients. Behav. Neurosci. 108, 848–865. doi: 10.1037/0735-7044.108.5.848
Di Martino, A., Ross, K., Uddin, L. Q., Sklar, A. B., Castellanos, F. X., and Milham, M. P. (2009). Functional brain correlates of social and nonsocial processes in autism spectrum disorders: an activation likelihood estimation meta-analysis. Biol. Psychiatry 65, 63–74. doi: 10.1016/j.biopsych.2008.09.022
Ecker, C., Rocha-Rego, V., Johnston, P., Mourao-Miranda, J., Marquand, A., Daly, E. M., et al. (2010). Investigating the predictive value of whole-brain structural MR scans in autism: a pattern classification approach. Neuroimage 49, 44–56. doi: 10.1016/j.neuroimage.2009.08.024
Ecker, C., Suckling, J., Deoni, S. C., Lombardo, M. V., Bullmore, E. T., Baron-Cohen, S., et al. (2012). Brain anatomy and its relationship to behavior in adults with autism spectrum disorder: a multicenter magnetic resonance imaging study. Arch. Gen. Psychiatry 69, 195–209. doi: 10.1001/archgenpsychiatry.2011.1251
Eliez, S., and Reiss, A. L. (2000). MRI neuroimaging of childhood psychiatric disorders: a selective review. J. Child Psychol. Psychiatry 41, 679–694. doi: 10.1111/1469-7610.00656
Enticott, P. G., Kennedy, H. A., Johnston, P. J., Rinehart, N. J., Tonge, B. J., Taffe, J. R., et al. (2014). Emotion recognition of static and dynamic faces in autism spectrum disorder. Cogn. Emot. 28, 1110–1118. doi: 10.1080/02699931.2013.867832
Evans, A. C. (2013). Networks of anatomical covariance. Neuroimage 80, 489–504. doi: 10.1016/j.neuroimage.2013.05.054
Fatemi, S. H., Aldinger, K. A., Ashwood, P., Bauman, M. L., Blaha, C. D., Blatt, G. J., et al. (2012). Consensus paper: pathological role of the cerebellum in autism. Cerebellum 11, 777–807. doi: 10.1007/s12311-012-0355-9
Fishman, I., Keown, C. L., Lincoln, A. J., Pineda, J. A., and Müller, R.-A. (2014). Atypical cross talk between mentalizing and mirror neuron networks in autism spectrum disorder. JAMA Psychiatry 71, 751–760. doi: 10.1001/jamapsychiatry.2014.83
Frith, C. (2003). What do imaging studies tell us about the neural basis of autism? Novartis Found. Symp. 251, 149–66; discussion 166–76, 281–97.
Frith, U., Morton, J., and Leslie, A. M. (1991). The cognitive basis of a biological disorder: autism. Trends Neurosci. 14, 433–438.
Gendry Meresse, I., Zilbovicius, M., Boddaert, N., Robel, L., Philippe, A., Sfaello, I., et al. (2005). Autism severity and temporal lobe functional abnormalities. Ann. Neurol. 58, 466–469. doi: 10.1002/ana.20597
Geschwind, D. H. (2009). Advances in autism. Annu. Rev. Med. 60, 367–380. doi: 10.1146/annurev.med.60.053107.121225
Gliga, T., Jones, E. J. H., Bedford, R., Charman, T., and Johnson, M. H. (2014). From early markers to neuro-developmental mechanisms of autism. Dev. Rev. 34, 189–207. doi: 10.1016/j.dr.2014.05.003
Gotts, S. J., Simmons, W. K., Milbury, L. A., Wallace, G. L., Cox, R. W., and Martin, A. (2012). Fractionation of social brain circuits in autism spectrum disorders. Brain 135(Pt 9), 2711–2725. doi: 10.1093/brain/aws160
Grecucci, A., Brambilla, P., Siugzdaite, R., Londero, D., Fabbro, F., and Rumiati, R. I. (2013). Emotional resonance deficits in autistic children. J. Autism Dev. Disord. 43, 616–628. doi: 10.1007/s10803-012-1603-z
Griebling, J., Minshew, N. J., Bodner, K., Libove, R., Bansal, R., Konasale, P., et al. (2010). Dorsolateral prefrontal cortex magnetic resonance imaging measurements and cognitive performance in autism. J. Child Neurol. 25, 856–863. doi: 10.1177/0883073809351313
Hamilton, A. F. (2013). Reflecting on the mirror neuron system in autism: a systematic review of current theories. Dev. Cogn. Neurosci. 3, 91–105. doi: 10.1016/j.dcn.2012.09.008
Han, X., Jovicich, J., Salat, D., van der Kouwe, A., Quinn, B., Czanner, S., et al. (2006). Reliability of MRI-derived measurements of human cerebral cortical thickness: the effects of field strength, scanner upgrade and manufacturer. Neuroimage 32, 180–194. doi: 10.1016/j.neuroimage.2006.02.051
Hardan, A. Y., Girgis, R. R., Adams, J., Gilbert, A. R., Keshavan, M. S., and Minshew, N. J. (2006a). Abnormal brain size effect on the thalamus in autism. Psychiatry Res. 147, 145–151. doi: 10.1016/j.pscychresns.2005.12.009
Hardan, A. Y., Girgis, R. R., Lacerda, A. L. T., Yorbik, O., Kilpatrick, M., Keshavan, M. S., et al. (2006b). Magnetic resonance imaging study of the orbitofrontal cortex in autism. J. Child Neurol. 21, 866–871.
Herbert, M. R., Ziegler, D. A., Makris, N., Filipek, P. A., Kemper, T. L., Normandin, J. J., et al. (2004). Localization of white matter volume increase in autism and developmental language disorder. Ann. Neurol. 55, 530–540. doi: 10.1002/ana.20032
Himberg, J., Hyvärinen, A., and Esposito, F. (2004). Validating the independent components of neuroimaging time series via clustering and visualization. Neuroimage 22, 1214–1222. doi: 10.1016/j.neuroimage.2004.03.027
Jiao, Y., Chen, R., Ke, X., Chu, K., Lu, Z., and Herskovits, E. H. (2010). Predictive models of autism spectrum disorder based on brain regional cortical thickness. Neuroimage 50, 589–599. doi: 10.1016/j.neuroimage.2009.12.047
Kana, R. K., Maximo, J. O., Williams, D. L., Keller, T. A., Schipul, S. E., Cherkassky, V. L., et al. (2015). Aberrant functioning of the theory-of-mind network in children and adolescents with autism. Mol. Autism 6, 59. doi: 10.1186/s13229-015-0052-x
Kaspárek, T., Marecek, R., Schwarz, D., Prikryl, R., Vanícek, J., Mikl, M., et al. (2010). Source-based morphometry of gray matter volume in men with first-episode schizophrenia. Hum. Brain Mapp. 31, 300–310. doi: 10.1002/hbm.20865
Kubera, K. M., Sambataro, F., Vasic, N., Wolf, N. D., Frasch, K., Hirjak, D., et al. (2014). Source-based morphometry of gray matter volume in patients with schizophrenia who have persistent auditory verbal hallucinations. Prog. Neuropsychopharmacol. Biol. Psychiatry 50, 102–109. doi: 10.1016/j.pnpbp.2013.11.015
Lee, T.-W. (1998). “Independent component analysis,” in Independent Component Analysis (Boston, MA: Springer US), 27–66. doi: 10.1007/978-1-4757-2851-4_2
Levy, S. E., Mandell, D. S., and Schultz, R. T. (2009). Autism. Lancet 374, 1627–1638. doi: 10.1016/S0140-6736(09)61376-3
Libero, L. E., Maximo, J. O., Deshpande, H. D., Klinger, L. G., Klinger, M. R., and Kana, R. K. (2014). The role of mirroring and mentalizing networks in mediating action intentions in autism. Mol. Autism 5:50. doi: 10.1186/2040-2392-5-50
Lombardo, M. V., Chakrabarti, B., Bullmore, E. T., and Baron-Cohen, S. (2011). Specialization of right temporo-parietal junction for mentalizing and its relation to social impairments in autism. Neuroimage 56, 1832–1838. doi: 10.1016/j.neuroimage.2011.02.067
Lord, C., and Bishop, S. L. (2015). Recent advances in autism research as reflected in DSM-5 criteria for autism spectrum disorder. Annu. Rev. Clin. Psychol. 11, 53–70. doi: 10.1146/annurev-clinpsy-032814-112745
Lord, C., Cook, E. H., Leventhal, B. L., and Amaral, D. G. (2000). Autism spectrum disorders. Neuron 28, 355–363.
Minshew, N. J., and Williams, D. L. (2007). The new neurobiology of autism: cortex, connectivity, and neuronal organization. Arch. Neurol. 64, 945–950. doi: 10.1001/archneur.64.7.945
Misra, V. (2014). The social brain network and autism. Ann. Neurosci. 21, 69–73. doi: 10.5214/ans.0972.7531.210208
Müller, R.-A. (2007). The study of autism as a distributed disorder. Ment. Retard. Dev. Disabil. Res. Rev. 13, 85–95. doi: 10.1002/mrdd.20141
Nickl-Jockschat, T., Habel, U., Michel, T. M., Manning, J., Laird, A. R., Fox, P. T., et al. (2012). Brain structure anomalies in autism spectrum disorder–a meta-analysis of VBM studies using anatomic likelihood estimation. Hum. Brain Mapp. 33, 1470–1489. doi: 10.1002/hbm.21299
Oberman, L. M., Hubbard, E. M., McCleery, J. P., Altschuler, E. L., Ramachandran, V. S., and Pineda, J. A. (2005). EEG evidence for mirror neuron dysfunction in autism spectrum disorders. Brain Res. 24, 190–198. doi: 10.1016/j.cogbrainres.2005.01.014
Palmen, S. J. M. C., Durston, S., Nederveen, H., and Van Engeland, H. (2006). No evidence for preferential involvement of medial temporal lobe structures in high-functioning autism. Psychol. Med. 36, 827–834. doi: 10.1017/S0033291706007215
Pappaianni, E., Siugzdaite, R., and Grecucci, A. (2016). An abnormal cerebellar network in children with autistic spectrum disorder: a morphometric study. Autism Open Access 6:178. doi: 10.4172/2165-7890.1000178
Pelphrey, K. A., Morris, J. P., McCarthy, G., and Labar, K. S. (2007). Perception of dynamic changes in facial affect and identity in autism. Soc. Cogn. Affect Neurosci. 2, 140–149. doi: 10.1093/scan/nsm010
Pina-Camacho, L., Villero, S., Fraguas, D., Boada, L., Janssen, J., Navas-Sánchez, F. J., et al. (2012). Autism spectrum disorder: does neuroimaging support the DSM-5 proposal for a symptom dyad? A systematic review of functional magnetic resonance imaging and diffusion tensor imaging studies. J. Autism Dev. Disord. 42, 1326–1341. doi: 10.1007/s10803-011-1360-4
Rapin, I., and Tuchman, R. F. (2008). Autism: definition, neurobiology, screening, diagnosis. Pediatr. Clin. North Am. 55, 1129–1146, viii. doi: 10.1016/j.pcl.2008.07.005
Rogers, T. D., McKimm, E., Dickson, P. E., Goldowitz, D., Blaha, C. D., and Mittleman, G. (2013). Is autism a disease of the cerebellum? An integration of clinical and pre-clinical research. Front. Syst. Neurosci. 7:15. doi: 10.3389/fnsys.2013.00015
Rossion, B., Caldara, R., Seghier, M., Schuller, A.-M., Lazeyras, F., and Mayer, E. (2003). A network of occipito-temporal face-sensitive areas besides the right middle fusiform gyrus is necessary for normal face processing. Brain 126(Pt 11), 2381–2395. doi: 10.1093/brain/awg241
Saxe, R., and Wexler, A. (2005). Making sense of another mind: the role of the right temporo-parietal junction. Neuropsychologia 43, 1391–1399. doi: 10.1016/j.neuropsychologia.2005.02.013
Schaer, M., Ottet, M.-C., Scariati, E., Dukes, D., Franchini, M., Eliez, S., et al. (2013). Decreased frontal gyrification correlates with altered connectivity in children with autism. Front. Hum. Neurosci. 7:750. doi: 10.3389/fnhum.2013.00750
Schumann, C. M., Hamstra, J., Goodlin-Jones, B. L., Lotspeich, L. J., Kwon, H., Buonocore, M. H., et al. (2004). The amygdala is enlarged in children but not adolescents with autism; the hippocampus is enlarged at all ages. J. Neurosci. 24, 6392–6401. doi: 10.1523/JNEUROSCI.1297-04.2004
Smith, S. M. (2002). Fast robust automated brain extraction. Hum. Brain Mapp. 17, 143–155. doi: 10.1002/hbm.10062
Sparks, B. F., Friedman, S. D., Shaw, D. W., Aylward, E. H., Echelard, D., Artru, A. A., et al. (2002). Brain structural abnormalities in young children with autism spectrum disorder. Neurology 59, 184–192. doi: 10.1212/WNL.59.2.184
Tager-Flusberg, H., Rogers, S., Cooper, J., Landa, R., Lord, C., Paul, R., et al. (2009). Defining spoken language benchmarks and selecting measures of expressive language development for young children with autism spectrum disorders. J. Speech Lang. Hear. Res. 52, 643–652. doi: 10.1044/1092-4388(2009/08-0136)
Torres, E. B., Isenhower, R. W., Yanovich, P., Rehrig, G., Stigler, K., Nurnberger, J., et al. (2013). Strategies to develop putative biomarkers to characterize the female phenotype with autism spectrum disorders. J. Neurophysiol. 110, 1646–1662. doi: 10.1152/jn.00059.2013
Tzourio-Mazoyer, N., Landeau, B., Papathanassiou, D., Crivello, F., Etard, O., Delcroix, N., et al. (2002). Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. NeuroImage 15, 273–289. doi: 10.1006/nimg.2001.0978
Vander Wyk, B. C., Hudac, C. M., Carter, E. J., Sobel, D. M., and Pelphrey, K. A. (2009). Action understanding in the superior temporal sulcus region. Psychol. Sci. 20, 771–777. doi: 10.1111/j.1467-9280.2009.02359.x
Xu, L., Groth, K. M., Pearlson, G., Schretlen, D. J., and Calhoun, V. D. (2009). Source-based morphometry: the use of independent component analysis to identify gray matter differences with application to schizophrenia. Hum. Brain Mapp. 30, 711–724. doi: 10.1002/hbm.20540
Keywords: autism, morphometric analysis, social deficits, neuroscience, developmental disabilities
Citation: Grecucci A, Rubicondo D, Siugzdaite R, Surian L and Job R (2016) Uncovering the Social Deficits in the Autistic Brain. A Source-Based Morphometric Study. Front. Neurosci. 10:388. doi: 10.3389/fnins.2016.00388
Received: 11 May 2016; Accepted: 09 August 2016;
Published: 31 August 2016.
Edited by:
Ahmet O. Caglayan, Yale University, USACopyright © 2016 Grecucci, Rubicondo, Siugzdaite, Surian and Job. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Alessandro Grecucci, YWxlc3NhbmRyby5ncmVjdWNjaUB1bml0bi5pdA==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.