
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
MINI REVIEW article
Front. Neurosci., 12 January 2016
Sec. Neuroendocrine Science
Volume 9 - 2015 | https://doi.org/10.3389/fnins.2015.00499
This article is part of the Research TopicChemicals in the environment and brain development: importance of neuroendocrinological approachesView all 16 articles
Astrocytes comprise a large population of cells in the brain and are important partners to neighboring neurons, vascular cells, and other glial cells. Astrocytes not only form a scaffold for other cells, but also extend foot processes around the capillaries to maintain the blood–brain barrier. Thus, environmental chemicals that exist in the blood stream could have potentially harmful effects on the physiological function of astrocytes. Although astrocytes are not electrically excitable, they have been shown to function as active participants in the development of neural circuits and synaptic activity. Astrocytes respond to neurotransmitters and contribute to synaptic information processing by releasing chemical transmitters called “gliotransmitters.” State-of-the-art optical imaging techniques enable us to clarify how neurotransmitters elicit the release of various gliotransmitters, including glutamate, D-serine, and ATP. Moreover, recent studies have demonstrated that the disruption of gliotransmission results in neuronal dysfunction and abnormal behaviors in animal models. In this review, we focus on the latest technical approaches to clarify the molecular mechanisms of gliotransmitter exocytosis, and discuss the possibility that exposure to environmental chemicals could alter gliotransmission and cause neurodevelopmental disorders.
Astrocytes are the most abundant glial cells in the central nervous system (CNS) of mammals (Ventura and Harris, 1999). Based on electron microscopic analyses, astrocytes are located near to neurons and blood vessels (Figure 1A). Regarding vasculature, capillary endothelial cells are surrounded by pericytes and basal lamina, and astrocytes tightly wrap these microvascular structures (Abbott et al., 2006). Together with pericytes, astrocytes are an essential component of the blood–brain barrier (BBB), which selects and transports molecules from the bloodstream, and allows for the transfer of nutrients to neurons (Figure 1B). Regarding their relationship with neurons, astrocytic foot processes make close contact with pre- and post-synaptic areas, forming structures called “tripartite synapses” (Araque et al., 1999; Halassa et al., 2007). Indeed, in the hippocampus, 57% of synapses are associated with astrocytes (Ventura and Harris, 1999), suggesting that astrocytes might contribute to neural information processing in the CNS.
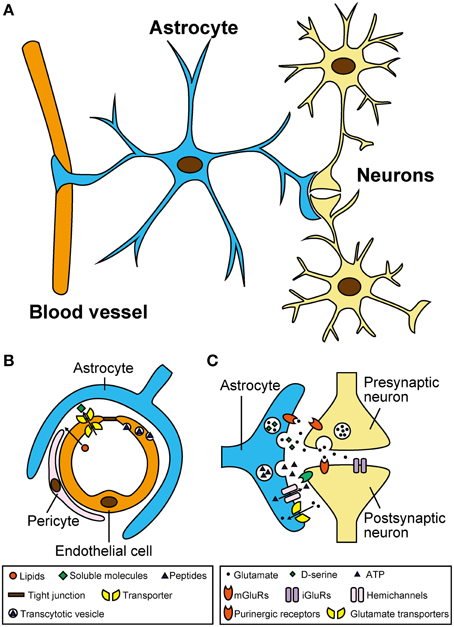
Figure 1. Astrocytes have close morphological and functional associations with microvasculature and neurons. (A) Location of astrocytes around blood vessels and neurons in the central nervous system. Note that single astrocytes make contact with a large number of blood vessels and neurons through their numerous processes. (B) Schematic diagram showing the blood–brain barrier and its functions in selecting and transporting various molecules from the blood stream. Although, vascular endothelial cells form robust tight junctions that prevent infiltration of most soluble molecules, hydrophobic lipids can penetrate across the plasma membrane. In addition, certain soluble molecules such as glucose are actively transported across the endothelial cells via their specific transporters, and some peptides are taken up by selective vesicular transcytosis. (C) Schematic diagram showing the tripartite synapse and complex signaling interactions mediated by neurotransmitters and gliotransmitters. Neurotransmitters released from presynaptic terminals such as glutamate act not only on postsynapses but also on astrocytes. Activated astrocytes release gliotransmitters including glutamate, D-serine, and ATP, via vesicular exocytosis (and also possibly via hemichannels for ATP). Released gliotransmitters bind to presynaptic and postsynaptic receptors to regulate synaptic transmission. Astrocytes also take part in clearance of extracellular glutamate via glutamate transporters.
Despite their morphological characteristics described above, astrocytes have long been considered as mere metabolic supporters that nurture adjacent neurons (Halassa et al., 2007; Wang and Bordey, 2008; Calì et al., 2009). However, recent electrophysiology and optical imaging analyses have provided strong evidence that astrocytes respond to neurotransmitters and release chemical transmitters called “gliotransmitters” (Li et al., 2013). Gliotransmitters, including glutamate, D-serine, and ATP, bind to their respective receptors on neurons to modulate their firing frequency and/or synaptic transmission (Figure 1C; Halassa et al., 2007; Koizumi, 2010). In fact, the dysfunction of gliotransmitter release-related proteins (e.g., vesicular transporters and vesicle-associated membrane proteins) in astrocytes can cause serious brain disorders and abnormal behaviors (Rossi et al., 2011; Verkhratsky et al., 2014). At the same time, traumatic injury, stroke, or infection-induced astrogliosis (also known as reactive astrocytes). These reactive astrocytes produce and release neurotoxic levels of glutamate (Rossi et al., 2011; Verkhratsky et al., 2014). Astrocytes also contribute to proper development of the BBB by aligning endothelial cells and pericytes, transporting molecules selected from the bloodstream to neurons (Abbott et al., 2006), and providing a protective barrier against toxic substances (Pentreath and Slamon, 2000; Calabrese, 2008). Thus, chronic exposure to environmental chemicals, or inflammatory molecules from vasculature, may potentially affect the function of astrocytes and gliotransmitter release (Kim et al., 2014; Orellana et al., 2014; Avendano et al., 2015).
In this review, we present the latest methods that enable scientists to decipher the molecular mechanisms of gliotransmitter secretion. In particular, we focus on the vesicular exocytosis of gliotransmitters from astrocytes using optical microscopic imaging. We further discuss how genetic alterations, acute injuries, and chronically toxic conditions (including exposure to stress in utero) could impair gliotransmission and consequently lead to neuronal and behavioral disorders.
There have been two major methodological breakthroughs that have allowed for profound understanding of astrocytic activities including gliotransmission: calcium imaging and advanced optical microscopy (Li et al., 2013). The initial discovery made by using chemical calcium indicators was that astrocytes exhibit increased intracellular calcium concentration ([Ca2+]i), which spreads to adjacent astrocytes. This phenomenon is called Ca2+ waves (Cornell-Bell et al., 1990; Charles et al., 1991; Rusakov et al., 2014). Genetically encoded calcium indicators have enabled more detailed analysis of astrocyte functions (Shigetomi et al., 2013).
Two-photon microscopy enabled scientists to observe fluorescence with superior penetration depth. Thus, studies on astrocytes have been expanded to experiments using brain slices and in vivo models (Nimmerjahn et al., 2004; Nishida and Okabe, 2007). Moreover, thanks to total internal reflection fluorescence microscopy, which can visualize fluorescent molecule behaviors beneath the plasma membrane, the interaction between [Ca2+]i elevation and subsequent vesicular trafficking became precisely clarified (Bezzi et al., 2004; Shigetomi et al., 2012; Oya et al., 2013).
Because of these experimental advancements, accumulating evidence suggests the paradigm that: (1) inositol 1,4,5-trisphosphate-mediated Ca2+ release from endoplasmic reticulum causes [Ca2+]i increases in astrocytes in response to the activity of adjacent astrocytes and neurons; (2) elicited [Ca2+]i elevation induces release of gliotransmitters (Halassa et al., 2007; Oya et al., 2013; Khakh and McCarthy, 2015). Although the exact mechanisms of gliotransmission are unclear, recent studies have partially revealed the release mechanisms of glutamate, D-serine, and ATP in astrocytes (Figure 2; Gucek et al., 2012; Li et al., 2013).
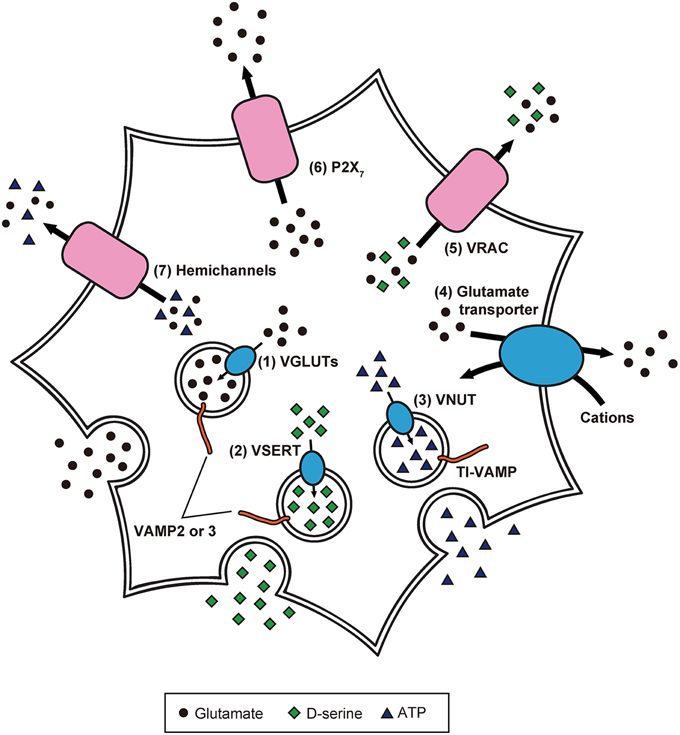
Figure 2. Precise intracellular machinery involved in the release of glutamate, D-serine, and ATP from astrocytes. Glutamate and D-serine are taken up into synaptic-like vesicles through (1) VGLUT and (2) vesicular D-serine transporters (VSERT), respectively. These synaptic-like vesicles fuse to the plasma membrane, mediated by SNARE proteins including VAMP2 or VAMP3, in response to [Ca2+]i increase. In contrast, ATP is released through secretory lysosomes. Storage of ATP into secretory lysosomes is achieved by (3) VNUT. Through the interaction of SNARE proteins including TI-VAMP, ATP-containing secretory lysosomes are Ca2+-dependently exocytosed. Moreover, the existence of other release mechanisms has been discovered: (4) reverse operation of plasma membrane glutamate transporters, (5) cell swelling-induced anion transporter (VRAC) opening, (6) release via P2X7 receptors, and (7) gap junction channels (hemichannels) on the cell surface of astrocytes.
Although, glutamate is well-known as a neurotransmitter, it also acts as a gliotransmitter. Application of bradykinin to cultured astrocytes induces glutamate release and influences adjacent neurons through N-methyl-D-aspartate (NMDA) receptors (Parpura et al., 1994). In contrast, application of clostridium, tetanus, and botulinum neurotoxins, which differentially cleave the exocytosis-regulating soluble N-ethylmaleimide-sensitive factor attachment protein receptor (SNARE) proteins, reduces Ca2+-dependent glutamate release. These findings suggest that the SNARE proteins, including vesicle-associated membrane protein-2 (VAMP2), syntaxin-1, and synaptosome-associated protein-23, mediate Ca2+-dependent glutamate release (Montana et al., 2006; Parpura and Zorec, 2010).
The uptake of cytoplasmic glutamate into exocytotic vesicles is mediated by vesicular glutamate transporters (VGLUTs), which are driven by a proton gradient produced by vacuolar-type H+ ATPases (V-ATPases; Takamori et al., 2000; Gucek et al., 2012). Inhibition of V-ATPases blocks Ca2+-dependent glutamate release (Parpura and Zorec, 2010). Furthermore, VGLUT1 and 2 are colocalized with synaptic-like vesicles (Bezzi et al., 2004), suggesting that glutamate is packaged into synaptic-like vesicles and released from astrocytes in a Ca2+-dependent manner.
Meanwhile, other release mechanisms have been identified: (1) reverse operation of plasma membrane glutamate transporters (Longuemare and Swanson, 1995); (2) cell swelling-induced anion transporter opening (Kimelberg et al., 1990); (3) release via P2X7 receptors (Duan et al., 2003); (4) gap junction channels (i.e., hemichannels) on the cell surface of astrocytes (Ye et al., 2003). However, it is not clear how often and to what extent astrocytes employ these different mechanisms. Further studies will be needed to clarify whether there are specific release mechanisms that operate under particular conditions.
The discovery of D-serine as a gliotransmitter was remarkable because it was long thought that mammalian tissues only produced L-isomers of amino acids (Oliet and Mothet, 2006; Henneberger et al., 2012). D-serine is thought to be produced from L-serine by serine racemase (de Miranda et al., 2002). In cultured astrocytes, application of glutamate enhanced Ca2+-dependent secretion of D-serine via the activation of α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA)/kainate receptors (AMPA/KARs) and metabotropic glutamate receptors (Mothet et al., 2005). Correspondingly, agonists for AMPA/KARs and metabotropic glutamate receptors were found to increase [Ca2+]i as well as subsequent secretion of D-serine, which is reduced by inhibition of these receptors. Furthermore, tetanus neurotoxins and V-ATPase inhibitors suppress agonist-evoked secretion of D-serine, and VAMP2/3 and VGLUT2-containing vesicles that are colocalized with D-serine. These results suggest that D-serine is stored in the synaptic-like vesicles and released from the vesicles in a Ca2+-dependent manner (Martineau et al., 2013).
Although ATP is the primary energy currency of the cells, ATP can also act as a signaling molecule through purinergic receptors. A recent study showed that culture medium from cultured astrocytes exhibiting Ca2+ waves contained more ATP than control culture medium. Interestingly, the addition of collected culture medium to astrocytes induced Ca2+ waves that were inhibited by purinergic receptor antagonists (Guthrie et al., 1999). Thus, the ATP released from astrocytes induces Ca2+ waves, which astrocytes use to communicate with each other. However, the ATP release mechanisms still remain controversial; several lines of investigation have suggested various putative models for ATP release from astrocytes (Koizumi, 2010).
Connexin 43 (Cx43) assembles into a hemichannel which constitutes gap junctions in astrocytes, and exchanges signaling molecules, including Ca2+ and inositol 1,4,5-trisphosphate, between adjacent astrocytes (Orellana and Stehberg, 2014). Bioluminescence imaging of ATP combined with single channel recording showed that Cx43 hemichannels in rat glioma C6 cells and CA1 hippocampal astrocytes are permeable to ATP (Kang et al., 2008). Consistent with this finding, glutamate evoked [Ca2+]i increase and ATP release in astrocytes of hippocampal slices, which were inhibited by application of a hemichannel blocker and in Cx43/Cx30 knockout mice (Torres et al., 2012), suggesting that ATP is released extracellularly through Cx43 hemichannels.
However, some studies have shown the involvement of secretory lysosomes in ATP release from astrocytes. In fact, primary cultured astrocytes express a secretory lysosome marker called vesicle-associated membrane protein-7 (also called TI-VAMP), and TI-VAMP-positive secretory lysosomes contain ATP which is Ca2+-dependently released (Verderio et al., 2012). In an experiment using primary cultured astrocytes and C6 cells, vesicular nucleotide transporter (VNUT)-positive lysosomes were labeled with fluorescent ATP, and application of VNUT inhibitor reduced the number of fluorescent ATP-containing vesicles. Observation by total internal reflection fluorescence microscopy revealed exocytotic events of secretory lysosomes in the cells following the application of a calcium ionophore, ATP, and glutamate. Thus, ATP is stored in lysosomes and released from lysosomes in a Ca2+-dependent manner (Oya et al., 2013).
Release of gliotransmitters regulates synaptic transmission between neurons and the extracellular environment in the brain. It is known that glutamate and D-serine excite synaptic transmission. However, whether ATP potentiates or inhibits synaptic transmission is still under debate because adenosine, a metabolite synthesized from ATP, usually inhibits synaptic activity via adenosine A1 receptors (Koizumi, 2010; Nam et al., 2012; Delekate et al., 2014). It is therefore reasonable to speculate that imbalance in the release of these gliotransmitters could result in altered neuronal activity. Various pathological conditions, including CNS diseases, traumatic brain injuries, developmental disorders, and prenatal exposure to deleterious molecules have been reported to be closely associated with impairment of gliotransmission.
Many CNS diseases are attributed to hyperactivity of neurons or unregulated neuronal cell death. Although such conditions have long been the focus of “neurocentric” studies, recent progress in the study of astrocytic gliotransmission has provided accumulating evidence for the contribution of astrocytes (Rossi et al., 2011; Verkhratsky et al., 2014).
Epilepsy is one of the most common CNS diseases, and is characterized by sudden and frequent seizures resulting from excessive firings by neurons (Wetherington et al., 2008). In slices from epilepsy model mice, astrocytic glutamate release was found to cause abnormal and prolonged depolarization in neurons (Tian et al., 2005). Furthermore, tumor necrosis factor-α (TNFα) and prostaglandins (PGs), released from astrocytes under traumatic events, can reactivate their calcium signaling, and can cause increased glutamate release (Bezzi et al., 1998, 2001; Domercq et al., 2006).
Reactive astrocytes are also involved in the pathogenesis of other neuronal disorders. In a mouse model of Huntington's disease, cultured astrocytes exhibited hyperactivated Ca2+-dependent glutamate release. This activity was owing to increased expression of pyruvate carboxylase (Lee et al., 2013), or reduced expression of glutamate transporter-1 and Kir4.1 K+ channels, which are key regulators for the clearance of extracellular glutamate and maintenance of membrane potentials, respectively (Behrens et al., 2002; Tong et al., 2014). In addition to overpotentiating neuronal activity, excessive accumulation of extracellular glutamate causes cytotoxicity. For instance, mice with genetic deletion in glutamate transporter-1 exhibit reduced glutamate clearance, and consequently display abnormal cell death in motor neurons, reminiscent of amyotrophic lateral sclerosis (Staats and Van Den Bosch, 2009).
In Alzheimer's disease (AD) mouse models, reactive astrocytes are detected near β-amyloid plaques (Nagele et al., 2004). Although chronic rise in [Ca2+]i is a well-known phenomenon in reactive astrocytes in AD, its underlying mechanisms remain unclear. A recent study demonstrated that purinergic signaling through Cx43 hemichannels and P2Y1 receptors mediated the hyperactivity of astrocytes in AD (Delekate et al., 2014). Consistent with this finding, upregulation of Cx43 hemichannels was observed in an AD mouse model (Mei et al., 2010), and AD patients displayed higher levels of ATP in brain regions surrounding β-amyloid plaques (Mecheri et al., 1997; Mandal et al., 2012).
Gliotransmitter release from astrocytes is also required for correct development of neuronal circuits. In particular, glial-neuronal communication through NMDA receptors is an essential process for proper dendritic morphogenesis and establishment of synaptic connections (Rabacchi et al., 1992; Sin et al., 2002; Espinosa et al., 2009). Although NMDA receptors are activated by both glutamate and D-serine, recent discoveries suggest that D-serine plays an important role in dendritic development and long-term potentiation (Henneberger et al., 2010; Devito et al., 2011; Balu and Coyle, 2012; Diniz et al., 2012). Mice with a deletion of serine racemase showed reduced levels of brain D-serine and brain-derived neurotrophic factor, and loss of glutamatergic neurotransmission, and consequently had less complex dendrites (Morita et al., 2007; Balu and Coyle, 2012). Because NMDA receptor malfunction has been considered to be responsible for schizophrenia, deficiency in D-serine secretion from astrocytes can be a potent schizophrenia risk factor (Van Horn et al., 2013). Indeed, association studies of schizophrenia patients revealed several mutations in genes for serine racemase, as well as D-amino acid oxidase and its interacting protein G72 (Boks et al., 2007; Morita et al., 2007; Müller et al., 2011; Caldinelli et al., 2013).
Acute brain insults, caused by ischemia or infection, affect neuronal circuitry through direct inflammatory responses in neurons and through signals from glial cells (Vesce et al., 2007; Calì et al., 2009). Astrocytes under acute inflammatory conditions undergo reactive astrogliosis similarly to those in CNS diseases, albeit with differences in gene expression and cell structure (Khakh and Sofroniew, 2015). Upon injury or ischemia, damaged neurons, endothelial cells and glial cells are known to release considerable amounts of ATP (Cook and McCleskey, 2002; Wang et al., 2004; Davalos et al., 2005; Nedergaard et al., 2010). Increased levels of extracellular ATP activate purinergic receptors on astrocytes, particularly P2Y1 (Domercq et al., 2006), thereby inducing [Ca2+]i elevation and release of glutamate, as well as ATP (Domercq et al., 2006; Nedergaard et al., 2010). Furthermore, inflammatory molecules including TNFα, interleukin-1β, and PGs, are profoundly engaged in these responses. Not only the activated microglia converge to the site of injury and secrete cytokines; astrocytes themselves synthesize TNFα and PGs (Domercq et al., 2006; Santello et al., 2011). TNFα and PGs either interact with certain processes in the stimulus–secretion coupling machinery within astrocytes (Domercq et al., 2006; Santello et al., 2011), or bind to TNFα and PGs receptors on astrocytes after secretion (Bezzi et al., 2001; Vesce et al., 2007).
Increasing evidence shows significant correlations between environmentally deleterious chemicals and the risk of neurodevelopmental disorders (Feng et al., 1990; Leonardsson and Ny, 1997). Previous studies have focused on the effects of toxic substances on neurons, but recently it was suggested that astrocytes are also involved in the pathogenesis of those conditions.
Owing to their close connections with microvascular units via BBB, astrocytes tend to be chronically exposed to noxious molecules in circulation. Probably because of their interactions with environmental toxins, astrocytes possess more resilient and adaptive machinery against toxic molecules compared with neurons (Pentreath and Slamon, 2000; Calabrese, 2008). These protective systems include the glutathione system, superoxide dismutase, and hemeoxygenase (Dwyer et al., 1995; Huang and Philbert, 1995; Blaauwgeers et al., 1996; Pentreath and Slamon, 2000). Nevertheless, excessive passage of harmful substances across the BBB seriously affects astrocyte homeostasis and functionality.
The toxicological effects of heavy metals (e.g., mercury, zinc, manganese, and aluminum) on neurons and glial cells have been studied for decades (Calabrese, 2008; De Keyser et al., 2008). However, it is unclear how these metals affect gliotransmitter release. Some studies have shown that lead and manganese induce cytotoxic cell death by impairing glutamate uptake in astrocytes (Normandin and Hazell, 2002; Struzynska et al., 2005). However, pathological effects on gliotransmission by lifestyle-associated factors, such as smoking, drinking, and insufficient sleep, are becoming the focus of growing interest. Because nicotinic acetylcholine receptors are expressed on astrocytes, they exhibit nicotine sensitivity and [Ca2+]i increase (Oikawa et al., 2005; Delbro et al., 2009). Ethanol causes reactive oxygen species production, and [Ca2+]i increase and glutamate secretion from astrocytes (Salazar et al., 2008). Astrocytes exposed to ethanol also exhibit alterations in Golgi complex morphology, secretory vesicle biogenesis, and expression levels of Rab GTPases and motor proteins (Tomas et al., 2005), which may be an additional factor for the dysfunction of brain development caused by ethanol.
Because adenosine plays a critical role in the control of sleep-wakefulness (Thakkar et al., 2003), and chronic alcoholism is frequently accompanied by sleep disorders (Brower, 2001), changes in sleep pattern may also induce alteration in gliotransmitter release. Interestingly, hypothalamic astrocytes from rats following sleep deprivation exhibited different proteome profiles, and the expression of VAMP2, which is an essential protein for vesicular exocytosis (Kim et al., 2014), was significantly increased. These findings suggest a strong association between alcohol intake, sleep disorders, and astrocytic gliotransmission.
Additionally, certain ambient ultrafine particles, which are defined as particulate substances with a diameter less than 100 nm, are emerging as another toxic substance that may deleteriously affect brain function (Block and Calderón-Garcidueñas, 2009; Loane et al., 2013). In a recent study, ultrafine carbon black, a surrogate for ultrafine particles, was shown to induce the release of glutamate and ATP from astrocytes by activating Cx43 and pannexin-1 hemichannels (Wei et al., 2014).
Recent epidemiological and experimental studies have demonstrated that children born from mothers who are exposed to infections or are addicted to alcohol or drugs have a higher risk of neuronal disorders and abnormal behaviors (Jacobsen et al., 2006; Stringari et al., 2008; Boksa, 2010; Brolese et al., 2015). However, the effects of these agents on astrocytes still remain largely unknown. Some studies have shown that prenatal exposure to lipopolysaccharides or nicotine together with postnatal high-fat/cholesterol diet result in enhancement of Cx43 hemichannel activity, and consequently increases the release of glutamate and ATP (Orellana et al., 2014; Avendano et al., 2015).
Over several decades, researchers have attempted to understand the properties and pathologies of the CNS by focusing solely on neurons; however, recent improvements in molecular and cellular imaging techniques are increasingly indicating that this neurocentric approach needs to be revised. In addition to neurons, glial cells including astrocytes are important elements for brain functions. Astrocytes are located in close morphological and functional relationships with blood vessels and neurons, and various genetic or environmental factors are implicated in gliotransmission impairment. Considering these characteristics of astrocytes, further studies will provide new insight on the significance of gliotransmitter release for fetal neurodevelopment. Thus, new therapies can be developed to overcome environmental chemical-induced neurodevelopmental disorders.
KH, TK, and TT wrote the paper.
This work was funded in part by a research grant from The Grant of National Center for Child Health and Development, Tokyo, Japan (grant numbers 27-9), and by a Grant-in-Aid for Science Research from the Ministry of Education, Culture, Sports, Science, and Technology of Japan.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The authors thank Dr. Fumihiko Maekawa, Dr. Kazuaki Nakamura, and Dr Manami Oya for their helpful discussion and comments.
Abbott, N. J., Rönnback, L., and Hänsson, E. (2006). Astrocyte-endothelial interactions at the blood-brain barrier. Nat. Rev. Neurosci. 7, 41–53. doi: 10.1038/nrn.1824
Araque, A., Parpura, V., Sanzgiri, R. P., and Haydon, P. G. (1999). Tripartite synapses: glia, the unacknowledged partner. Trends Neurosci. 22, 208–215. doi: 10.1016/S0166-2236(98)01349-6
Avendaño, B. C., Montero, T. D., Chávez, C. E., Von Bernhardi, R., and Orellana, J. A. (2015). Prenatal exposure to inflammatory conditions increases Cx43 and Panx1 unopposed channel opening and activation of astrocytes in the offspring effect on neuronal survival. Glia. 63, 2058–2072. doi: 10.1002/glia.22877
Balu, D. T., and Coyle, J. T. (2012). Neuronal D-serine regulates dendritic architecture in the somatosensory cortex. Neurosci. Lett. 517, 77–81. doi: 10.1016/j.neulet.2012.04.020
Behrens, P. F., Franz, P., Woodman, B., Lindenberg, K. S., and Landwehrmeyer, G. B. (2002). Impaired glutamate transport and glutamate-glutamine cycling: downstream effects of the Huntington mutation. Brain 125, 1908–1922. doi: 10.1093/brain/awf180
Bezzi, P., Carmignoto, G., Pasti, L., Vesce, S., Rossi, D., Rizzini, B. L., et al. (1998). Prostaglandins stimulate calcium-dependent glutamate release in astrocytes. Nature 391, 281–285. doi: 10.1038/34651
Bezzi, P., Domercq, M., Brambilla, L., Galli, R., Schols, D., De Clercq, E., et al. (2001). CXCR4-activated astrocyte glutamate release via TNFalpha: amplification by microglia triggers neurotoxicity. Nat. Neurosci. 4, 702–710. doi: 10.1038/89490
Bezzi, P., Gundersen, V., Galbete, J. L., Seifert, G., Steinhäuser, C., Pilati, E., et al. (2004). Astrocytes contain a vesicular compartment that is competent for regulated exocytosis of glutamate. Nat. Neurosci. 7, 613–620. doi: 10.1038/nn1246
Blaauwgeers, H. G., Vianney De Jong, J. M., Verspaget, H. W., Van Den Berg, F. M., and Troost, D. (1996). Enhanced superoxide dismutase-2 immunoreactivity of astrocytes and occasional neurons in amyotrophic lateral sclerosis. J. Neurol. Sci. 140, 21–29. doi: 10.1016/0022-510X(96)00110-4
Block, M. L., and Calderon-Garciduenas, L. (2009). Air pollution: mechanisms of neuroinflammation and CNS disease. Trends Neurosci. 32, 506–516. doi: 10.1016/j.tins.2009.05.009
Boks, M. P., Rietkerk, T., Van De Beek, M. H., Sommer, I. E., De Koning, T. J., and Kahn, R. S. (2007). Reviewing the role of the genes G72 and DAAO in glutamate neurotransmission in schizophrenia. Eur. Neuropsychopharmacol. 17, 567–572. doi: 10.1016/j.euroneuro.2006.12.003
Boksa, P. (2010). Effects of prenatal infection on brain development and behavior: a review of findings from animal models. Brain Behav. Immun. 24, 881–897. doi: 10.1016/j.bbi.2010.03.005
Brolese, G., Lunardi, P., De Souza, D. F., Lopes, F. M., Leite, M. C., and Goncalves, C. A. (2015). Pre- and postnatal exposure to moderate levels of ethanol can have long-lasting effects on hippocampal glutamate uptake in adolescent offspring. PLoS ONE 10:e0127845. doi: 10.1371/journal.pone.0127845
Calabrese, E. J. (2008). Astrocytes: adaptive responses to low doses of neurotoxins. Crit. Rev. Toxicol. 38, 463–471. doi: 10.1080/10408440802004023
Caldinelli, L., Sacchi, S., Molla, G., Nardini, M., and Pollegioni, L. (2013). Characterization of human DAAO variants potentially related to an increased risk of schizophrenia. Biochim. Biophys. Acta 1832, 400–410. doi: 10.1016/j.bbadis.2012.11.019
Calì, C., Marchaland, J., Spagnuolo, P., Gremion, J., and Bezzi, P. (2009). Regulated exocytosis from astrocytes physiological and pathological related aspects. Int. Rev. Neurobiol. 85, 261–293. doi: 10.1016/S0074-7742(09)85020-4
Charles, A. C., Merrill, J. E., Dirksen, E. R., and Sanderson, M. J. (1991). Intercellular signaling in glial cells: calcium waves and oscillations in response to mechanical stimulation and glutamate. Neuron 6, 983–992. doi: 10.1016/0896-6273(91)90238-U
Cook, S. P., and McCleskey, E. W. (2002). Cell damage excites nociceptors through release of cytosolic ATP. Pain 95, 41–47. doi: 10.1016/S0304-3959(01)00372-4
Cornell-Bell, A. H., Finkbeiner, S. M., Cooper, M. S., and Smith, S. J. (1990). Glutamate induces calcium waves in cultured astrocytes: long-range glial signaling. Science 247, 470–473. doi: 10.1126/science.1967852
Davalos, D., Grutzendler, J., Yang, G., Kim, J. V., Zuo, Y., Jung, S., et al. (2005). ATP mediates rapid microglial response to local brain injury in vivo. Nat. Neurosci. 8, 752–758. doi: 10.1038/nn1472
De Keyser, J., Mostert, J. P., and Koch, M. W. (2008). Dysfunctional astrocytes as key players in the pathogenesis of central nervous system disorders. J. Neurol. Sci. 267, 3–16. doi: 10.1016/j.jns.2007.08.044
Delbro, D., Westerlund, A., Björklund, U., and Hansson, E. (2009). In inflammatory reactive astrocytes co-cultured with brain endothelial cells nicotine-evoked Ca(2+) transients are attenuated due to interleukin-1beta release and rearrangement of actin filaments. Neuroscience 159, 770–779. doi: 10.1016/j.neuroscience.2009.01.005
Delekate, A., Füchtemeier, M., Schumacher, T., Ulbrich, C., Foddis, M., and Petzold, G. C. (2014). Metabotropic P2Y1 receptor signalling mediates astrocytic hyperactivity in vivo in an Alzheimer's disease mouse model. Nat. Commun. 5, 5422. doi: 10.1038/ncomms6422
de Miranda, J., Panizzutti, R., Foltyn, V. N., and Wolosker, H. (2002). Cofactors of serine racemase that physiologically stimulate the synthesis of the N-methyl-D-aspartate (NMDA) receptor coagonist D-serine. Proc. Natl. Acad. Sci. U.S.A. 99, 14542–14547. doi: 10.1073/pnas.222421299
Devito, L. M., Balu, D. T., Kanter, B. R., Lykken, C., Basu, A. C., Coyle, J. T., et al. (2011). Serine racemase deletion disrupts memory for order and alters cortical dendritic morphology. Genes Brain Behav. 10, 210–222. doi: 10.1111/j.1601-183X.2010.00656.x
Diniz, L. P., Almeida, J. C., Tortelli, V., Vargas Lopes, C., Setti-Perdigão, P., Stipursky, J., et al. (2012). Astrocyte-induced synaptogenesis is mediated by transforming growth factor beta signaling through modulation of D-serine levels in cerebral cortex neurons. J. Biol. Chem. 287, 41432–41445. doi: 10.1074/jbc.M112.380824
Domercq, M., Brambilla, L., Pilati, E., Marchaland, J., Volterra, A., and Bezzi, P. (2006). P2Y1 receptor-evoked glutamate exocytosis from astrocytes: control by tumor necrosis factor-alpha and prostaglandins. J. Biol. Chem. 281, 30684–30696. doi: 10.1074/jbc.M606429200
Duan, S., Anderson, C. M., Keung, E. C., Chen, Y., and Swanson, R. A. (2003). P2X7 receptor-mediated release of excitatory amino acids from astrocytes. J. Neurosci. 23, 1320–1328.
Dwyer, B. E., Nishimura, R. N., and Lu, S. Y. (1995). Differential expression of heme oxygenase-1 in cultured cortical neurons and astrocytes determined by the aid of a new heme oxygenase antibody. Response to oxidative stress. Brain Res. Mol. Brain Res. 30, 37–47. doi: 10.1016/0169-328X(94)00273-H
Espinosa, J. S., Wheeler, D. G., Tsien, R. W., and Luo, L. (2009). Uncoupling dendrite growth and patterning: single-cell knockout analysis of NMDA receptor 2B. Neuron 62, 205–217. doi: 10.1016/j.neuron.2009.03.006
Feng, P., Ohlsson, M., and Ny, T. (1990). The structure of the TATA-less rat tissue-type plasminogen activator gene. Species-specific sequence divergences in the promoter predict differences in regulation of gene expression. J. Biol. Chem. 265, 2022–2027.
Gucek, A., Vardjan, N., and Zorec, R. (2012). Exocytosis in astrocytes: transmitter release and membrane signal regulation. Neurochem. Res. 37, 2351–2363. doi: 10.1007/s11064-012-0773-6
Guthrie, P. B., Knappenberger, J., Segal, M., Bennett, M. V., Charles, A. C., and Kater, S. B. (1999). ATP released from astrocytes mediates glial calcium waves. J. Neurosci. 19, 520–528.
Halassa, M. M., Fellin, T., and Haydon, P. G. (2007). The tripartite synapse: roles for gliotransmission in health and disease. Trends Mol. Med. 13, 54–63. doi: 10.1016/j.molmed.2006.12.005
Henneberger, C., Bard, L., and Rusakov, D. A. (2012). D-Serine: a key to synaptic plasticity? Int. J. Biochem. Cell Biol. 44, 587–590. doi: 10.1016/j.biocel.2012.01.005
Henneberger, C., Papouin, T., Oliet, S. H., and Rusakov, D. A. (2010). Long-term potentiation depends on release of D-serine from astrocytes. Nature 463, 232–236. doi: 10.1038/nature.08673
Huang, J., and Philbert, M. A. (1995). Distribution of glutathione and glutathione-related enzyme systems in mitochondria and cytosol of cultured cerebellar astrocytes and granule cells. Brain Res. 680, 16–22. doi: 10.1016/0006-8993(95)00209-9
Jacobsen, L. K., Slotkin, T. A., Westerveld, M., Mencl, W. E., and Pugh, K. R. (2006). Visuospatial memory deficits emerging during nicotine withdrawal in adolescents with prenatal exposure to active maternal smoking. Neuropsychopharmacology 31, 1550–1561. doi: 10.1038/sj.npp.1300981
Kang, J., Kang, N., Lovatt, D., Torres, A., Zhao, Z., Lin, J., et al. (2008). Connexin 43 hemichannels are permeable to ATP. J. Neurosci. 28, 4702–4711. doi: 10.1523/JNEUROSCI.5048-07.2008
Khakh, B. S., and McCarthy, K. D. (2015). Astrocyte Calcium Signaling: from observations to functions and the challenges therein. Cold Spring Harb. Perspect. Biol. 7:a020404. doi: 10.1101/cshperspect.a020404
Khakh, B. S., and Sofroniew, M. V. (2015). Diversity of astrocyte functions and phenotypes in neural circuits. Nat. Neurosci. 18, 942–952. doi: 10.1038/nn.4043
Kim, J. H., Cho, Y. E., Baek, M. C., Jung, J. Y., Lee, M. G., Jang, I. S., et al. (2014). Chronic sleep deprivation-induced proteome changes in astrocytes of the rat hypothalamus. J. Proteome Res. 13, 4047–4061. doi: 10.1021/pr.500431j
Kimelberg, H. K., Goderie, S. K., Higman, S., Pang, S., and Waniewski, R. A. (1990). Swelling-induced release of glutamate, aspartate, and taurine from astrocyte cultures. J. Neurosci. 10, 1583–1591.
Koizumi, S. (2010). Synchronization of Ca2+ oscillations: involvement of ATP release in astrocytes. FEBS J. 277, 286–292. doi: 10.1111/j.1742-4658.2009.07438.x
Lee, W., Reyes, R. C., Gottipati, M. K., Lewis, K., Lesort, M., Parpura, V., et al. (2013). Enhanced Ca(2+)-dependent glutamate release from astrocytes of the BACHD Huntington's disease mouse model. Neurobiol. Dis. 58, 192–199. doi: 10.1016/j.nbd.2013.06.002
Leonardsson, G., and Ny, T. (1997). Characterisation of the rat tissue-type plasminogen activator gene promoter – identification of a TAAT-containing promoter element. Eur. J. Biochem. 248, 676–683. doi: 10.1111/j.1432-1033.1997.t01-1-00676.x
Li, D., Agulhon, C., Schmidt, E., Oheim, M., and Ropert, N. (2013). New tools for investigating astrocyte-to-neuron communication. Front. Cell. Neurosci. 7:193. doi: 10.3389/fncel.2013.00193
Loane, C., Pilinis, C., Lekkas, T. D., and Politis, M. (2013). Ambient particulate matter and its potential neurological consequences. Rev. Neurosci. 24, 323–335. doi: 10.1515/revneuro-2013-0001
Longuemare, M. C., and Swanson, R. A. (1995). Excitatory amino acid release from astrocytes during energy failure by reversal of sodium-dependent uptake. J. Neurosci. Res. 40, 379–386. doi: 10.1002/jnr.490400312
Mandal, P. K., Akolkar, H., and Tripathi, M. (2012). Mapping of hippocampal pH and neurochemicals from in vivo multi-voxel 31P study in healthy normal young male/female, mild cognitive impairment, and Alzheimer's disease. J. Alzheimers. Dis. 31(Suppl. 3), S75–S86. doi: 10.3233/JAD-2012-120166
Martineau, M., Shi, T., Puyal, J., Knolhoff, A. M., Dulong, J., Gasnier, B., et al. (2013). Storage and uptake of D-serine into astrocytic synaptic-like vesicles specify gliotransmission. J. Neurosci. 33, U3413–U3605. doi: 10.1523/jneurosci.3497-12.2013
Mecheri, G., Marie-Cardine, M., Sappey-Marinier, D., Bonmartin, H., Albrand, G., Ferry, G., et al. (1997). In vivo hippocampal (31)P NMR metabolites in Alzheimer's disease and ageing. Eur. Psychiatry 12, 140–148. doi: 10.1016/S0924-9338(97)80203-9
Mei, X., Ezan, P., Giaume, C., and Koulakoff, A. (2010). Astroglial connexin immunoreactivity is specifically altered at beta-amyloid plaques in beta-amyloid precursor protein/presenilin1 mice. Neuroscience 171, 92–105. doi: 10.1016/j.neuroscience.2010.08.001
Montana, V., Malarkey, E. B., Verderio, C., Matteoli, M., and Parpura, V. (2006). Vesicular transmitter release from astrocytes. Glia 54, 700–715. doi: 10.1002/glia.20367
Morita, Y., Ujike, H., Tanaka, Y., Otani, K., Kishimoto, M., Morio, A., et al. (2007). A genetic variant of the serine racemase gene is associated with schizophrenia. Biol. Psychiatry 61, 1200–1203. doi: 10.1016/j.biopsych.2006.07.025
Mothet, J. P., Pollegioni, L., Ouanounou, G., Martineau, M., Fossier, P., and Baux, G. (2005). Glutamate receptor activation triggers a calcium-dependent and SNARE protein-dependent release of the gliotransmitter D-serine. Proc. Natl. Acad. Sci. U.S.A. 102, 5606–5611. doi: 10.1073/pnas.0408483102
Müller, D. J., Zai, C. C., Shinkai, T., Strauss, J., and Kennedy, J. L. (2011). Association between the DAOA/G72 gene and bipolar disorder and meta-analyses in bipolar disorder and schizophrenia. Bipolar Disord. 13, 198–207. doi: 10.1111/j.1399-5618.2011.00905.x
Nagele, R. G., Wegiel, J., Venkataraman, V., Imaki, H., and Wang, K. C. (2004). Contribution of glial cells to the development of amyloid plaques in Alzheimer's disease. Neurobiol. Aging 25, 663–674. doi: 10.1016/j.neurobiolaging.2004.01.007
Nam, H. W., Mciver, S. R., Hinton, D. J., Thakkar, M. M., Sari, Y., Parkinson, F. E., et al. (2012). Adenosine and glutamate signaling in neuron-glial interactions: implications in alcoholism and sleep disorders. Alcohol. Clin. Exp. Res. 36, 1117–1125. doi: 10.1111/j.1530-0277.2011.01722.x
Nedergaard, M., Rodríguez, J. J., and Verkhratsky, A. (2010). Glial calcium and diseases of the nervous system. Cell Calcium 47, 140–149. doi: 10.1016/j.ceca.2009.11.010
Nimmerjahn, A., Kirchhoff, F., Kerr, J. N. D., and Helmchen, F. (2004). Sulforhodamine 101 as a specific marker of astroglia in the neocortex in vivo. Nat. Methods 1, 31–37. doi: 10.1038/nmeth.706
Nishida, H., and Okabe, S. (2007). Direct astrocytic contacts regulate local maturation of dendritic spines. J. Neurosci. 27, 331–340. doi: 10.1523/JNEUROSCI.4466-06.2007
Normandin, L., and Hazell, A. S. (2002). Manganese neurotoxicity: an update of pathophysiologic mechanisms. Metab. Brain Dis. 17, 375–387. doi: 10.1023/A:1021970120965
Oikawa, H., Nakamichi, N., Kambe, Y., Ogura, M., and Yoneda, Y. (2005). An increase in intracellular free calcium ions by nicotinic acetylcholine receptors in a single cultured rat cortical astrocyte. J. Neurosci. Res. 79, 535–544. doi: 10.1002/jnr.20398
Oliet, S. H., and Mothet, J. P. (2006). Molecular determinants of D-serine-mediated gliotransmission: from release to function. Glia 54, 726–737. doi: 10.1002/glia.20356
Orellana, J. A., Busso, D., Ramírez, G., Campos, M., Rigotti, A., Eugenín, J., et al. (2014). Prenatal nicotine exposure enhances Cx43 and Panx1 unopposed channel activity in brain cells of adult offspring mice fed a high-fat/cholesterol diet. Front. Cell. Neurosci. 8:403. doi: 10.3389/fncel.2014.00403
Orellana, J. A., and Stehberg, J. (2014). Hemichannels: new roles in astroglial function. Front. Physiol. 5:193. doi: 10.3389/fphys.2014.00193
Oya, M., Kitaguchi, T., Pais, R., Reimann, F., Gribble, F., and Tsuboi, T. (2013). The G protein-coupled receptor family C group 6 subtype A (GPRC6A) receptor is involved in amino acid-induced glucagon-like peptide-1 secretion from GLUTag cells. J. Biol. Chem. 288, 4513–4521. doi: 10.1074/jbc.M112.402677
Parpura, V., Basarsky, T. A., Liu, F., Jeftinija, K., Jeftinija, S., and Haydon, P. G. (1994). Glutamate-mediated astrocyte-neuron signalling. Nature 369, 744–747. doi: 10.1038/369744a0
Parpura, V., and Zorec, R. (2010). Gliotransmission: exocytotic release from astrocytes. Brain Res. Rev. 63, 83–92. doi: 10.1016/j.brainresrev.2009.11.008
Pentreath, V. W., and Slamon, N. D. (2000). Astrocyte phenotype and prevention against oxidative damage in neurotoxicity. Hum. Exp. Toxicol. 19, 641–649. doi: 10.1191/096032700676221595
Rabacchi, S., Bailly, Y., Delhaye-Bouchaud, N., and Mariani, J. (1992). Involvement of the N-methyl D-aspartate (NMDA) receptor in synapse elimination during cerebellar development. Science 256, 1823–1825. doi: 10.1126/science.1352066
Rossi, D., Martorana, F., and Brambilla, L. (2011). Implications of gliotransmission for the pharmacotherapy of CNS disorders. CNS Drugs 25, 641–658. doi: 10.2165/11593090-000000000-00000
Rusakov, D. A., Bard, L., Stewart, M. G., and Henneberger, C. (2014). Diversity of astroglial functions alludes to subcellular specialisation. Trends Neurosci. 37, 228–242. doi: 10.1016/j.tins.2014.02.008
Salazar, M., Pariente, J. A., Salido, G. M., and González, A. (2008). Ethanol induces glutamate secretion by Ca2+ mobilization and ROS generation in rat hippocampal astrocytes. Neurochem. Int. 52, 1061–1067. doi: 10.1016/j.neuint.2007.11.001
Santello, M., Bezzi, P., and Volterra, A. (2011). TNFalpha controls glutamatergic gliotransmission in the hippocampal dentate gyrus. Neuron 69, 988–1001. doi: 10.1016/j.neuron.2011.02.003
Shigetomi, E., Bushong, E. A., Haustein, M. D., Tong, X. P., Jackson-Weaver, O., Kracun, S., et al. (2013). Imaging calcium microdomains within entire astrocyte territories and endfeet with GCaMPs expressed using adeno-associated viruses. J. Gen. Physiol. 141, 633–647. doi: 10.1085/jgp.201210949
Shigetomi, E., Tong, X., Kwan, K. Y., Corey, D. P., and Khakh, B. S. (2012). TRPA1 channels regulate astrocyte resting calcium and inhibitory synapse efficacy through GAT-3. Nat. Neurosci. 15, 70–80. doi: 10.1038/nn.3000
Sin, W. C., Haas, K., Ruthazer, E. S., and Cline, H. T. (2002). Dendrite growth increased by visual activity requires NMDA receptor and Rho GTPases. Nature 419, 475–480. doi: 10.1038/nature00987
Staats, K. A., and Van Den Bosch, L. (2009). Astrocytes in amyotrophic lateral sclerosis: direct effects on motor neuron survival. J. Biol. Phys. 35, 337–346. doi: 10.1007/s10867-009-9141-4
Stringari, J., Nunes, A. K., Franco, J. L., Bohrer, D., Garcia, S. C., Dafre, A. L., et al. (2008). Prenatal methylmercury exposure hampers glutathione antioxidant system ontogenesis and causes long-lasting oxidative stress in the mouse brain. Toxicol. Appl. Pharmacol. 227, 147–154. doi: 10.1016/j.taap.2007.10.010
Struzynska, L., Chalimoniuk, M., and Sulkowski, G. (2005). The role of astroglia in Pb-exposed adult rat brain with respect to glutamate toxicity. Toxicology 212, 185–194. doi: 10.1016/j.tox.2005.04.013
Takamori, S., Rhee, J. S., Rosenmund, C., and Jahn, R. (2000). Identification of a vesicular glutamate transporter that defines a glutamatergic phenotype in neurons. Nature 407, 189–194. doi: 10.1038/35025070
Thakkar, M. M., Winston, S., and McCarley, R. W. (2003). A1 receptor and adenosinergic homeostatic regulation of sleep-wakefulness: effects of antisense to the A1 receptor in the cholinergic basal forebrain. J. Neurosci. 23, 4278–4287.
Tian, G. F., Azmi, H., Takano, T., Xu, Q., Peng, W., Lin, J., et al. (2005). An astrocytic basis of epilepsy. Nat. Med. 11, 973–981. doi: 10.1038/nm.1277
Tomás, M., Marín, P., Megías, L., Egea, G., and Renau-Piqueras, J. (2005). Ethanol perturbs the secretory pathway in astrocytes. Neurobiol. Dis. 20, 773–784. doi: 10.1016/j.nbd.2005.05.012
Tong, X., Ao, Y., Faas, G. C., Nwaobi, S. E., Xu, J., Haustein, M. D., et al. (2014). Astrocyte Kir4.1 ion channel deficits contribute to neuronal dysfunction in Huntington's disease model mice. Nat. Neurosci. 17, 694–703. doi: 10.1038/nn.3691
Torres, A., Wang, F. S., Xu, Q. W., Fujita, T., Dobrowolski, R., Willecke, K., et al. (2012). Extracellular Ca2+ acts as a mediator of communication from neurons to glia. Sci. Signal. 5:ra8. doi: 10.1126/scisignal.2002160
Van Horn, M. R., Sild, M., and Ruthazer, E. S. (2013). D-serine as a gliotransmitter and its roles in brain development and disease. Front. Cell. Neurosci. 7:39. doi: 10.3389/fncel.2013.00039
Ventura, R., and Harris, K. M. (1999). Three-dimensional relationships between hippocampal synapses and astrocytes. J. Neurosci. 19, 6897–6906.
Verderio, C., Cagnoli, C., Bergami, M., Francolini, M., Schenk, U., Colombo, A., et al. (2012). TI-VAMP/VAMP7 is the SNARE of secretory lysosomes contributing to ATP secretion from astrocytes. Biol. Cell 104, 213–228. doi: 10.1111/boc.201100070
Verkhratsky, A., Parpura, V., Pekna, M., Pekny, M., and Sofroniew, M. (2014). Glia in the pathogenesis of neurodegenerative diseases. Biochem. Soc. Trans. 42, 1291–1301. doi: 10.1042/BST20140107
Vesce, S., Rossi, D., Brambilla, L., and Volterra, A. (2007). Glutamate release from astrocytes in physiological conditions and in neurodegenerative disorders characterized by neuroinflammation. Int. Rev. Neurobiol. 82, 57–71. doi: 10.1016/S0074-7742(07)82003-4
Wang, D. D., and Bordey, A. (2008). The astrocyte odyssey. Prog. Neurobiol. 86, 342–367. doi: 10.1016/j.pneurobio.2008.09.015
Wang, X., Arcuino, G., Takano, T., Lin, J., Peng, W. G., Wan, P., et al. (2004). P2X7 receptor inhibition improves recovery after spinal cord injury. Nat. Med. 10, 821–827. doi: 10.1038/nm.1082
Wei, H., Deng, F., Chen, Y., Qin, Y., Hao, Y., and Guo, X. (2014). Ultrafine carbon black induces glutamate and ATP release by activating connexin and pannexin hemichannels in cultured astrocytes. Toxicology 323, 32–41. doi: 10.1016/j.tox.2014.06.005
Wetherington, J., Serrano, G., and Dingledine, R. (2008). Astrocytes in the epileptic brain. Neuron 58, 168–178. doi: 10.1016/j.neuron.2008.04.002
Keywords: astrocytes, exocytosis, glial cell, gliotransmitter, neurodevelopmental disorders, optical imaging, synaptic activity
Citation: Harada K, Kamiya T and Tsuboi T (2016) Gliotransmitter Release from Astrocytes: Functional, Developmental, and Pathological Implications in the Brain. Front. Neurosci. 9:499. doi: 10.3389/fnins.2015.00499
Received: 04 November 2015; Accepted: 15 December 2015;
Published: 12 January 2016.
Edited by:
Fumihiko Maekawa, National Institute for Environmental Studies, JapanReviewed by:
Robert Pawlak, University of Exeter, UKCopyright © 2016 Harada, Kamiya and Tsuboi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Takashi Tsuboi, dGFrYXRzdWJvaUBiaW8uYy51LXRva3lvLmFjLmpw
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.