
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Neurosci. , 28 November 2014
Sec. Neuroendocrine Science
Volume 8 - 2014 | https://doi.org/10.3389/fnins.2014.00387
This article is part of the Research Topic Reproductive Neuroendocrinology and Social Behavior View all 33 articles
Gonadotropin-releasing hormone (GnRH) neurons form the final common pathway for the central regulation of reproduction. Gamma-amino butyric acid (GABA) has long been implicated as one of the major players in the regulation of GnRH neurons. Although GABA is typically an inhibitory neurotransmitter in the mature adult central nervous system, most mature GnRH neurons show the unusual characteristic of being excited by GABA. While many reports have provided much insight into the contribution of GABA to the activity of GnRH neurons, the precise physiological role of the excitatory action of GABA on GnRH neurons remains elusive. This brief review presents the current knowledge of the role of GABA signaling in GnRH neuronal activity. We also discuss the modulation of GABA signaling by neurotransmitters and neuromodulators and the functional consequence of GABAergic inputs to GnRH neurons in both the physiology and pathology of reproduction.
Gonadotropin-releasing hormone (GnRH) neurons constitute the final output neurons in the neuroendocrine control of reproduction (Freeman, 2006). Pulsatile GnRH release stimulates the secretion of the gonadotropins, luteinizing hormone (LH) and follicle-stimulating hormone (FSH) from the pituitary. LH and FSH stimulate the development of mature eggs and sperm and also the synthesis of the gonadal hormones; estrogen and progesterone from the ovaries and androgens from the testes. The gonadal steroids feedback to the hypothalamus and pituitary to decrease GnRH and gonadotropin secretion throughout the estrous cycle, except during the afternoon of proestrus, when elevated levels of estradiol, released by maturing ovarian follicles, initiate the preovulatory GnRH/LH surge that causes ovulation.
The hypothalamus contains a relatively small number of GnRH neurons and these are diffusely scattered throughout the hypothalamus. Hence the mechanisms enabling GnRH neurons to generate the discrete episodes of GnRH secretion remain unknown. GnRH release is closely related to the activity of GnRH neurons, which are regulated by neurotransmitters, steroid hormones, and growth factors (Freeman, 2006). GnRH neurons express both GABAA(Sim et al., 2000; Temple and Wray, 2005) and GABAB receptors (Zhang et al., 2009) and receive GABAergic inputs that express estrogen receptors (Leranth et al., 1985); therefore, GABA has long been implicated as a major player in the regulation of GnRH neuron activity and secretion. In this brief review, we focus on the action of GABA on GnRH neurons.
The majority of in vivo whole animal studies have reported inhibitory actions of GABA on GnRH/LH secretion, although some reports have suggested excitatory effects of GABA (Donoso et al., 1992; Bilger et al., 2001). GABA infusion into the preoptic area or intraperitoneal injection of the GABAA receptor agonist, muscimol, blocked the LH surge (Adler and Crowley, 1986; Herbison and Dyer, 1991), while GABAA receptor antagonist, bicuculline, advanced the timing of the LH surge (Kimura and Jinnai, 1994). GABA release in the preoptic area was decreased prior to and during the time of the LH surge (Jarry et al., 1995). GABA is synthesized primarily from glutamate by the enzyme glutamate decarboxylase, two isoforms of which exist, GAD65 and GAD67 (Soghomonian and Martin, 1998). GAD67 mRNA levels in the preoptic area were decreased prior to the LH surge (Herbison et al., 1992). The number of terminals containing vesicular GABA transporter (VGAT, a marker of GABAergic neurons) was decreased in GnRH neurons at the time of the LH surge (Ottem et al., 2004). Injection of GABA or muscimol inhibited pulsatile LH release (Herbison et al., 1991; Jarry et al., 1991; Hiruma et al., 1994). The suppression of pulsatile LH release induced by infection stress was inhibited by bicuculline (Lin et al., 2012). From these in vivo studies, it is thought that GABA acts to inhibit the LH surge and pulsatile LH release via GABAAreceptors. The origins of GABAergic inputs to GnRH neurons are not well established, but the anteroventral periventricular area (AVPV) (Ottem et al., 2004) and the suprachiasmatic nucleus (SCN) are candidate regions (Christian and Moenter, 2007). This is because GABAergic neurons both in the AVPV and SCN express ERα, while GABAergic neurons in the AVPV exhibit changes in GAD67 gene expression that parallel GABA release on the day of LH surge release (Curran-Rauhut and Petersen, 2002). However, from these experiments, it is difficult to clarify the direct actions of GABA on GnRH neurons. Because GnRH neurons lack any specific identifying morphology, and owing to their diffuse location (Herbison, 2006), it is difficult to directly study the cellular and molecular mechanisms in single, functional GnRH neurons. The direct action of GABA on GnRH neurons has been studied using an immortalized GnRH neuronal cell line (GT1). GT1 cells were generated by genetically targeted tumorigenesis in transgenic mice (Mellon et al., 1990). GT1 cells are thought to preserve many characteristics of native GnRH neurons. They generate spontaneous action potentials, exhibit transient oscillations of the intracellular Ca2+ concentration ([Ca2+]i) (Hales et al., 1994; Charles and Hales, 1995), and secrete GnRH in a pulsatile manner (Wetsel et al., 1992; Martínez de la Escalera et al., 1994). GT1 cells synthesize GABA (Ahnert-Hilger et al., 1998) and express functional GABAA receptors (Favit et al., 1993). The activation of GABAA receptors in GT1 cells depolarizes the membrane potential, which activates voltage-gated Ca2+ channels, thereby facilitating Ca2+ influx and increasing [Ca2+]i and GnRH release (Favit et al., 1993; Hales et al., 1994; Martínez de la Escalera et al., 1994; Spergel et al., 1995). Although GT1 cells are still useful, especially in biochemical and molecular biology experiments, which often require many cells with uniform characteristics, the immortalized nature of these cells may interfere with normal differentiated functions and the study of the neural circuitry that regulates GnRH neurons, such as afferent inputs, cannot be accomplished in GT1 cells. To overcome these barriers, transgenic mice and rats expressing enhanced green fluorescent protein (EGFP) under the control of the GnRH promoter were generated (Spergel et al., 1999; Suter et al., 2000; Watanabe et al., 2009a). Using these mice and rats, the direct action of GABA on EGFP-tagged living GnRH neurons has been studied (Herbison and Moenter, 2011). Activation of GABAA receptors excited mouse GnRH neurons in acutely prepared slices through the hypothalamus (DeFazio et al., 2002) and evoked increases in [Ca2+]i in GnRH-Pericam transgenic mice (Constantin et al., 2010). The reversal potential of GABAA receptor current (EGABA) was more positive than the resting potential in mouse GnRH neurons (EGABA = −36.5 ± 1.2 mV, Vrest = −50.7 ± 1.7 mV) (DeFazio et al., 2002). Therefore, GABA caused depolarization in GnRH neurons. The GABAA receptor antagonist, bicuculline, or picrotoxin decreased the firing rate of GnRH neurons in the presence of ionotropic glutamate receptor antagonists, AP5 and CNQX, which excluded glutamatergic transmission (Moenter and DeFazio, 2005). Activation of somatic/proximal dendritic GABAA receptors in GnRH neurons caused robust action potential discharges by the activation of L-type calcium channels (Hemond et al., 2012). Furthermore, activation of GABAA receptors increased [Ca2+]i in isolated GnRH neurons from prepubertal and adult rats (Watanabe et al., 2009a) (Figure 1). Bicuculline inhibited the [Ca2+]i increase induced by GABA. GABA-induced [Ca2+]i increase was inhibited in Ca2+-free solution. EGABA of rat adult GnRH neurons was more positive than resting potential (EGABA = −26 ± 1.4 mV, Vrest = −60 to −50 mV) (Yin et al., 2008). Therefore, GABA also depolarized rat GnRH neurons. However, contradictory results on the actions of GABA have been demonstrated using transgenic mice in which GnRH neurons express beta-galactosidase (GnRH-lacZ mice). The beta-galactosidase can convert substrates to a fluorescent state enabling the visualization of GnRH neurons. The effect of GABA on GnRH neurons switched from depolarization to hyperpolarization at puberty in females (Han et al., 2002). A GABAA receptor antagonist increased the firing rate of GnRH neurons (Han et al., 2004); however, the recording was performed in the absence of CNQX and AP5. The GABAA receptor antagonist acts on all cells in the brain slice; therefore, it removes GABAergic inhibitory signaling and causes disinhibition in most neurons. Therefore, to remove the effect of disinhibition of glutamatergic neurons in the network that regulates GnRH neurons, glutamatergic signaling needs to be blocked. The presence of a tonic GABAA receptor current in GnRH neurons was also reported as inhibitory. GABA and THIP, a GABAA δ receptor agonist, hyperpolarized the membrane potential in adult GnRH neurons (Bhattarai et al., 2011). GABA has also been reported to act to GnRH neurons at the level of GnRH nerve terminals in the median eminence. The conditional activation of GABA release near GnRH nerve terminals disrupted the estrous cycle and reduced fertility in rats (Bilger et al., 2001). Recent reports show that GnRH neurons have unique morphology; long-distance projections to the median eminence function simultaneously as axons and dendrites (Herde et al., 2013). These GnRH projections have functional GABAA receptors and the activation of GABAA receptors depolarized the membrane potential and initiated action potentials at the median eminence. GABA is also excitatory to GnRH neurons in a variety of species, such as goldfish and sea lamprey (Trudeau et al., 2000; Reed et al., 2002; Root et al., 2004; Popesku et al., 2008). In an adult teleost fish, the dwarf gourami, GABAA receptor activation induced excitation in the terminal nerve-GnRH neurons (Nakane and Oka, 2010). From these results, GABA might regulate the excitability of GnRH neurons at GnRH cell bodies as well as at the median eminence.
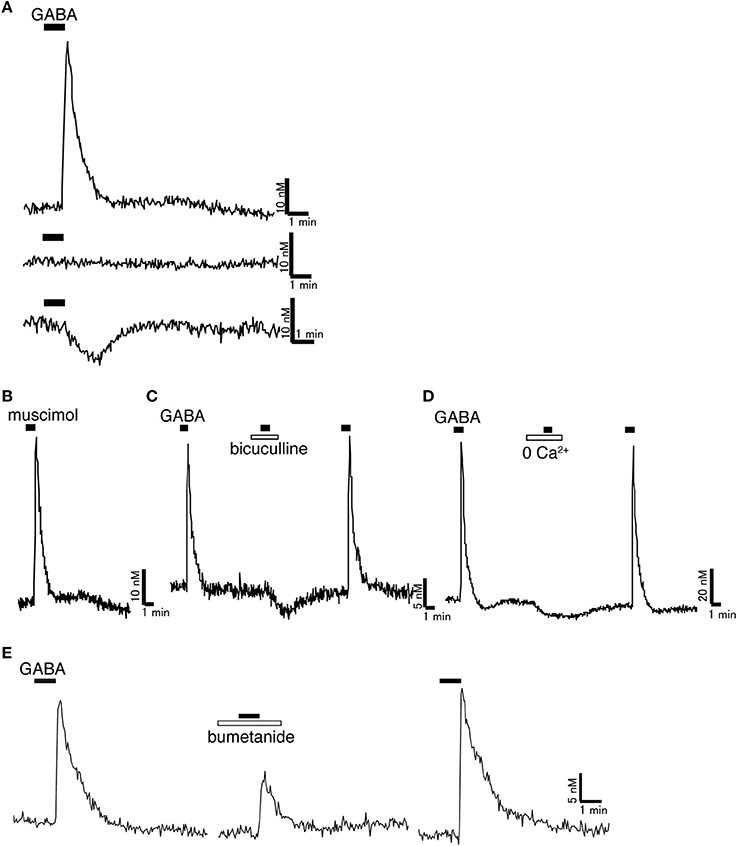
Figure 1. Excitatory action of GABA on rat GnRH neurons. (A) Representative [Ca2+]i response to 100 μM GABA. Most GnRH neurons showed [Ca2+]i increase in response to GABA. Some GnRH neurons did not respond to GABA. Some GnRH neurons showed [Ca2+]i decrease in response to GABA. (B) Muscimol (100 μM), a GABAA receptor agonist, increased [Ca2+]i in GnRH neurons. (C) Bicuculline (100 μM), a GABAA receptor antagonist, inhibited the [Ca2+]i increase induced by GABA. (D) GABA-induced [Ca2+]i increase was inhibited in Ca2+-free solution. (E) Bumetanide (100 μM), a blocker of NKCC1, reduced the GABA-induced [Ca2+]i increase. Muscimol and GABA were applied as indicated with horizontal bars. Bicuculline, Ca2+-free solution, and bumetanide were applied as indicated with open bars (Originally published in Watanabe et al., 2009a).
Recently, the first electrical recording of GnRH neurons in vivo in the anesthetized mouse was reported. Whereas muscimol evoked excitatory, inhibitory, or mixed effects on GnRH neuron firing, picrotoxin resulted in a consistent suppression of firing (Constantin et al., 2013). This study also reported that the effects of GABA on GnRH neurons were critically dependent upon the orientation within the slice (Constantin et al., 2012b). GABA was excitatory to GnRH neurons in coronal slices but inhibitory in the anterior hypothalamic area in horizontal slices. This is because of the direct activation of GABAA or GABAB receptors. GABAB receptors also modulate the excitability of GnRH neurons. GABAB R1 and R2 subunits are expressed in GnRH neurons (Zhang et al., 2009), and the GABAB receptor agonist baclofen hyperpolarized GnRH neurons through activation of an inwardly rectifying K+ current (Lagrange et al.1995; Zhang et al., 2009). Therefore, the net GABA effects are likely to be determined by the balance of GABAA vs. GABAB receptor-mediated effects along the GnRH neuron soma and dendrite (Constantin et al., 2013).
Few studies have investigated the effect of GABA on gene expression in GnRH neurons. Intracerebroventricular injection of muscimol induced a pronounced decrease of GnRH mRNA levels in the preoptic area. Injection of baclofen had no effect on GnRH mRNA levels (Bergen et al., 1991; Leonhardt et al., 1999). But opposite results have also been reported (Kang et al., 1995; Cho and Kim, 1997). Further work is needed to clarify this point.
From these results, although the action of GABA on GnRH neurons is still controversial, most GnRH neurons appear to be excited by GABA. However, GnRH neurons may exhibit heterogeneity in their GABA response depending on their location in the hypothalamus. Clarification of this point requires further study.
Because Cl− is the most permeant ion through the GABAA receptor ion channel, the intracellular Cl− concentration ([Cl−]i) determines the polarity of the GABA response (Ben-Ari, 2002). A hyperpolarizing and generally inhibitory action of GABA occurs when [Cl−]i is low, whereas a depolarizing and generally excitatory action of GABA is seen when [Cl−]i is high. In most neurons, the GABA response switches from a depolarization to a hyperpolarization during early postnatal development. Among the many molecules involved in [Cl−]i homeostasis, the exclusively neuronal subtype of the K+-Cl− cotransporter (KCC2), which couples the K+ electrochemical gradient to Cl− extrusion, is the principal molecule which maintains low [Cl−]i in mature neurons (Blaesse et al., 2009). In contrast, the neuronal subtype of the Na+-K+-2Cl− cotransporter (NKCC1), which mediates inward transport of Cl−, maintains high [Cl−]i (Figure 2). Because GABA excites in most GnRH neurons, one would predict the expression of KCC2 to be low and that of NKCC1 to be high in GnRH neurons. Actually, bumetanide, a blocker of NKCC1, reduced the GABA-induced [Ca2+]i increase in rat GnRH neurons (Figure 1). GT1 cells do not express detectable levels of KCC2 mRNA or protein but do express NKCC1 mRNA and protein (DeFazio et al., 2002). Adult rat GnRH neurons do not express KCC2 protein and express low levels of NKCC1 protein. KCC2 mRNA was expressed in 4.9% of GnRH neurons, whereas NKCC1 mRNA was expressed in 13.5% of GnRH neurons (DeFazio et al., 2002). A similar expression of KCC2 and NKCC1 mRNAs was shown in adult mouse GnRH neurons. The vast majority of KCC2 and NKCC1 expressing GnRH neurons are located in the anterior region of the preoptic area where the greatest concentration of neuroendocrine GnRH neurons is normally observed. Heterogeneous expression of KCC2 in mouse GnRH neurons has also been reported with 34% of GnRH neurons expressing KCC2 mRNA (Leupen et al., 2003). This proportion was similar in females and males. However, females exhibited a marked rostrocaudal gradient of colocalization that was not seen in males. Protein levels and the function of KCC2 and NKCC1 are rapidly modulated by intracellular and extracellular substrates (Blaesse et al., 2009). The activity, cell surface stability, and membrane trafficking of KCC2 are modulated by the phosphorylation of serine, threonine, and tyrosine residues in the C terminal region (Watanabe et al., 2009b; Kahle et al., 2013). KCC2 expression levels are reduced in response to various pathophysiological conditions (Kahle et al., 2008), including axotomy (Nabekura et al., 2002; Toyoda et al., 2003), global ischemia (Reid et al., 2000) chronic pain (Eto et al., 2012), interictal activity (Rivera et al., 2004), and neuronal stress (Wake et al., 2007) with resulting increases in [Cl−]i and a shift of GABA-mediated responses from hyperpolarizing to depolarizing. Therefore, it is reasonable to speculate that the functional expression of NKCC1 and/or KCC2 is changed according to estrous cycle stage and is different between males and females. These changes may modulate the response to GABA in GnRH neurons. Further studies are needed to clarify this point. In immature or injured neurons when GABA is also excitatory, this excitation can result in action potentials, [Ca2+]i oscillations, and synchronized patterns of activity (Ben-Ari, 2002; Toyoda et al., 2003). GnRH neurons also show spontaneous activity and [Ca2+]i oscillations (Constantin et al., 2012a) and the frequency of calcium oscillation in GnRH neurons was reduced by a GABAA receptor antagonist. Therefore, the excitatory action of GABA in GnRH neurons may contribute to the synchronous activity that generates discrete episodes of GnRH secretion.
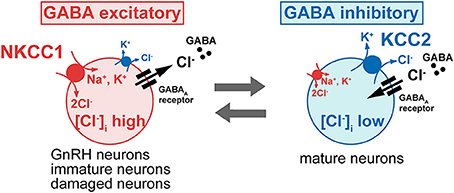
Figure 2. The intracellular Cl− concentration determines the polarity of GABA response. The GABA action is excitatory in immature neurons because [Cl−]i is high, owing to high levels of the Na+-K+-2Cl− cotransporter (NKCC1), which mediates inward transport of Cl−, and to low levels of the K+-Cl− cotransporter (KCC2), which excludes Cl− from the cell. In most neurons, the GABA response switches from excitation to inhibition during early postnatal development, due to the developmental decrease of the NKCC1 and increase of the KCC2. However, even in the mature neurons, neuronal damage down regulates the KCC2 and elevated Cl− concentration shifts GABA response from hyperpolarization to depolarization, occasionally excitation. Most GnRH neurons show the unusual characteristic of being excited by GABA in the adult brain.
Several neurotransmitters have been reported to regulate the activity of GABA neurons. Kisspeptin is a potent stimulator of GnRH release via G protein-coupled receptor 54 (GPR54) (Gottsch et al., 2004; Dungan et al., 2007; Mayer and Boehm, 2011). GnRH neurons express GPR54 (Messager et al., 2005) and kisspeptin acts directly on GnRH neurons (Han et al., 2005; Pielecka-Fortuna and Moenter, 2010). Kisspeptin also acts indirectly to modulate GnRH neurons via a change in GABAergic transmission. Kisspeptin increased the frequency and amplitude of GABAergic postsynaptic currents in GnRH neurons in an estradiol-dependent manner at the time of estradiol negative feedback (Pielecka-Fortuna and Moenter, 2010). Metabotropic glutamate receptors (mGluRs) also regulate GABA transmission to GnRH neurons. The endogenous activation of presynaptic mGluRs decreased the frequency of GABAA-mediated spontaneous postsynaptic currents in GnRH neurons and decreased GnRH neuron firing rate (Chu and Moenter, 2005). These effects occur through group II/III mGluRs and are mimicked by GnRH neural activity, suggesting a role for mGluRs in feedback regulation. The adipose-derived hormone, leptin, regulates GABAergic signaling. Acute fasting decreased the frequency of spontaneous GABAergic postsynaptic currents in GnRH neurons and GnRH neuronal activity (Sullivan et al., 2003; Sullivan and Moenter, 2004a). Because GnRH neurons do not express leptin receptors, the leptin effect was indirect (Quennell et al., 2009). GABAergic signaling seems to communicate information about metabolic status to the GnRH neurons indirectly. Retrograde endocannabinoid signaling reduces GABAergic synaptic transmission to GnRH neurons via the activation of presynaptic CB1 receptors, resulting in inhibition of GnRH neuron firing activity (Farkas et al., 2010). The depolarization of GnRH neurons induced short-term inhibition of GABAergic afferents via endocannabinoids and glia derived prostaglandins, and this interaction was steroid and likely sex dependent (Glanowska and Moenter, 2011). GnRH itself also regulated the activity of GABA neurons. GABAergic neurons express the type-1 GnRH receptor. Low levels of GnRH reduced the frequency of GABAergic postsynaptic currents in GnRH neurons, suggesting that low-dose GnRH suppressed GnRH firing in part by decreasing GABAergic transmission to GnRH neurons (Chen and Moenter, 2009). The pineal hormone, melatonin, is involved in the regulation of reproductive function, including the timing of the LH surge. Melatonin modulates GABAA receptor currents in GnRH neurons isolated from GnRH-EGFP transgenic rats, positively in males and negatively in females (Sato et al., 2008).
GABAergic transmission is also regulated by a nonclassical action of the ovarian steroid, estradiol. Estrogen receptor α (ERα) agonists reduced the frequency of GABA transmission to GnRH neurons (Chu et al., 2009). A nonclassical action of estradiol via ERα on GnRH neurons that caused phosphorylation of ERK1/2 and consequently CREB was blocked by a GABAA receptor antagonist (Kwakowsky et al., 2014). In contrast, ERβ agonists increased GABA transmission and postsynaptic response. Estrogen interacted with the classical ERα at the level of the GABAergic nerve terminal to regulate action potential-independent GABA release (Romanò et al., 2008). Steroid metabolites known as neurosteroids also modulate the function of the GABAA receptor. Specifically, the progesterone derivative allopregnanolone is an allosteric agonist, whereas the androgen, dehydroepiandrosterone sulfate (DHEAS), is an allosteric antagonist. Allopregnanolone increased GABAergic miniature postsynaptic current frequency, amplitude and decay time. DHEAS reduced mPSC frequency and amplitude but did not alter decay time (Sullivan and Moenter, 2003). Also, in rat GnRH neurons, GABAA currents were augmented by allopregnanolone and 3α,21-dihydroxy-5α-pregnan-20-one (Yin et al., 2008).
Therefore, several neurotransmitters and hormones modulate GABAergic transmission to GnRH neurons, and this modulation may mediate various physiological stimuli that regulate GnRH neuronal activity.
The precise physiological role of direct GABAA receptor activation in GnRH neurons in vivo remains to be investigated. Although the near complete abolition of GABAA receptor signaling by knockout of the GABAA receptor γ2 subunit in GnRH neurons was found to have no major effect on fertility in vivo (Lee et al., 2010), there are many reports that propose a role for GABA in multiple aspects of GnRH neuronal physiology. These range from embryonic migration to a role in puberty and both estrogen negative and positive feedback.
GABA plays a key developmental role in the regulation of GnRH neuron migration from the olfactory placodes into the forebrain during fetal development. Like GT1 cells, a subset of embryonic GnRH neurons can produce GABA during migration (Tobet et al., 1996; Ahnert-Hilger et al., 1998). GABA is also present in cells and fibers along the GnRH migratory route throughout the nasal compartment (Tobet et al., 1996; Wray et al., 1996). GAD65 is expressed exclusively in undifferentiated neuronal progenitors confined to the proliferative zones of the sensory vomeronasal and olfactory epithelia (Vastagh et al., 2014). In contrast, GAD67 is expressed in a subregion of the nonsensory epithelium/vomeronasal organ epithelium containing the putative GnRH progenitors and GnRH neurons migrating from this region. Muscimol inhibited GnRH neuron migration and decreased extension of GnRH fibers. Bicuculline led to a disorganized distribution of GnRH neurons in the forebrain (Bless et al., 2000). Transgenic mice that selectively over-express GAD67 in GnRH neurons had more GnRH neurons in aberrant locations in the cerebral cortex and fewer neurons reaching the forebrain (Heger et al., 2003). Consequently, hypothalamic GnRH content was low during the second postnatal week, while in adult mice disrupted the estrous cycle and litter sizes were reduced. In contrast, in GABA deficient mice (GAD 67 knockout mice), GnRH neurons reached the nasal/forebrain junction earlier and entered the forebrain earlier (Lee et al., 2008). From these results, GABA production within GnRH neurons alters the migratory fate of these neurons and the timely termination of GABA production within the GnRH neuronal network is required for normal reproductive function. The role of GABAergic inputs on GnRH neuronal migration was also evaluated using olfactory explants. Mouse embryonic GnRH neurons in olfactory pit explant cultures express GABAA receptors and activation of GABAA receptors resulted in membrane depolarization (Kusano et al., 1995) and increased [Ca2+]i (Moore and Wray, 2000). Muscimol inhibited GnRH migration and bicuculline or picrotoxin increased GnRH migration (Fueshko et al., 1998). Stromal derived growth factor (SDF-1) and GABA synergistically regulate the rate of GnRH migration (Casoni et al., 2012). SDF-1 accelerated migration by hyperpolarization via changes in potassium, while GABA slowed migration by depolarization via changes in chloride. These studies demonstrate that GABAergic activity in nasal regions has effects on migration of GnRH neurons and that GABA participates in appropriate timing of GnRH neuronal migration into the developing brain.
GABA has been reported to have a role in mediating puberty. In most neurons of the central nervous system, the GABA response switches from a depolarization to a hyperpolarization during early postnatal development (Ben-Ari, 2002). One group reported that the switch of GABAA receptor signaling in GnRH neurons was delayed until the time of puberty (Han et al., 2002). The expression patterns of GABAA receptor subunit mRNAs in GnRH neurons change during the developmental period. In juvenile and prepubertal female mice, α1-5, β 1-3, and γ2,3 subunits are broadly expressed in a heterogeneous manner. Adult female mouse GnRH neurons of the rostral preoptic area express predominantly α1, α5, β 1, and γ2 subunits and those of the medial septum express α1, α3, α5, β 1, β 3, and γ2 subunits (Sim et al., 2000). These changes appear to involve the activation of the GnRH neurons at puberty. In female rhesus monkeys, a reduction of GABA inhibition is thought to be critical for the mechanism initiating puberty onset, because chronic infusion of bicuculline into the stalk-median eminence significantly increased GnRH release and accelerated the timing of the menarche and first ovulation (Terasawa et al., 2011). Bicuculline dramatically stimulated kisspeptin release in the medial basal hypothalamus of prepubertal monkeys but had little effect on kisspeptin release in midpubertal monkeys (Kurian et al., 2012). This implies that a reduction in tonic GABA inhibition of GnRH release is, at least in part, mediated through kisspeptin neurons.
GABA plays a critical role in mediating both estradiol negative and positive feedback and appears to control the timing of the switch in estradiol feedback action. The frequency of GABA transmission to GnRH neurons is directly correlated with estradiol negative and positive feedback. Frequency of GABAergic postsynaptic currents was low during negative feedback but frequency and amplitude of GABAergic postsynaptic currents was increased at surge onset (Christian and Moenter, 2007). This indicates that estradiol induces diurnal shifts in GABA transmission at appropriate times to generate changes in GnRH neuronal firing activity and hormone release characteristic of both negative and positive feedback. Adult mice lacking functional GABAB receptors (GABAB1KO) displayed disruption of cyclicity and fertility (Catalano et al., 2005). GABAB1KO mice showed increased Gnrh1 and Gad1 expression but decreased Kiss1 expression in the medial basal hypothalamus of neonatal mice (Di Giorgio et al., 2013). Thus, GABA signaling via GABAB receptors is also important for regulating the estrous cycle.
Metabolic signals have influences on fertility. GABA neuron-specific leptin receptor knock-out female and male mice show significantly delayed puberty onset (Zuure et al., 2013). Female mice lacking functional leptin receptors in GABAergic neurons have hypogonadotropic hypogonadism (Martin et al., 2014). Adult leptin receptor knockout mice showed decreased fecundity. There results suggest that leptin signaling in GABAergic neurons plays a critical role in the timing of puberty onset and is involved in fertility regulation. Therefore, GABAergic afferents integrate metabolic signals for delivery to GnRH neurons.
In human, GABAergic axons exhibiting VGAT immunoreactivity innervate the soma and dendrites of GnRH neurons (Hrabovszky et al., 2012). A change in GABAergic transmission is associated with the hypothalamic abnormalities of fertility disorders. In polycystic ovary syndrome model mice, which were exposed to androgen in utero, the size and frequency of GABAergic postsynaptic currents were increased (Sullivan and Moenter, 2004b). From these data, increased GnRH pulse frequency observed in polycystic ovary syndrome may be attributable to androgen-induced increases in GABAergic drive to GnRH neurons.
Although the importance of GABAergic inputs has been demonstrated in in vitro studies, further work is needed to determine the precise functional roles of direct GABAergic inputs to GnRH neurons in vivo. Because most GnRH neurons show the unusual characteristic of being excited by GABA, the excitatory action of GABA might make a major contribution to the regulation of GnRH neuron activity and secretion. As aberrant central GABAergic signaling is seen in polycystic ovary syndrome model mice, change in neuronal GABA activity appears to alter reproductive status both physiologically and pathologically. Therefore, determination of the precise role of GABAergic transmission in the regulation of GnRH neurons is important for understanding the regulation of normal reproduction as well as the hypothalamic abnormalities of fertility disorders.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Adler, B. A., and Crowley, W. R. (1986). Evidence for gamma-aminobutyric acid modulation of ovarian hormonal effects on luteinizing hormone secretion and hypothalamic catecholamine activity in the female rat. Endocrinology 118, 91–97. doi: 10.1210/endo-118-1-91
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Ahnert-Hilger, G., John, M., Kistner, U., Wiedenmann, B., and Jarry, H. (1998). Immortalized gonadotropin-releasing hormone neurons secrete gamma-aminobutyric acid-evidence for an autocrine regulation. Eur. J. Neurosci. 10, 1145–1152. doi: 10.1046/j.1460-9568.1998.00129.x
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Ben-Ari, Y. (2002). Excitatory actions of GABA during development: the nature of the nurture. Nat. Rev. Neurosci. 3, 728–739. doi: 10.1038/nrn920
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Bergen, H., Hejtmancik, J., and Pfaff, D. (1991). Effects of gamma-aminobutyric acid receptor agonists and antagonist on LHRH-synthesizing neurons as detected by immunocytochemistry and in situ hybridization. Exp. Brain Res. 87, 46–56. doi: 10.1007/BF00228505
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Bhattarai, J. P., Park, S. A., Park, J. B., Lee, S. Y., Herbison, A. E., Ryu, P. D., et al. (2011). Tonic extrasynaptic GABAA receptor currents control gonadotropin-releasing hormone neuron excitability in the mouse. Endocrinology 152, 1551–1561. doi: 10.1210/en.2010-1191
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Bilger, M., Heger, S., Brann, D. W., Paredes, A., and Ojeda, S. R. (2001). A conditional tetracycline-regulated increase in Gamma amino butyric acid production near luteinizing hormone-releasing hormone nerve terminals disrupts estrous cyclicity in the rat. Endocrinology 142, 2102–2114. doi: 10.1210/endo.142.5.8166
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Blaesse, P., Airaksinen, M. S., Rivera, C., and Kaila, K. (2009). Cation-chloride cotransporters and neuronal function. Neuron 61, 820–838. doi: 10.1016/j.neuron.2009.03.003
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Bless, E. P., Westaway, W. A., Schwarting, G. A., and Tobet, S. A. (2000). Effects of gamma-aminobutyric acidA receptor manipulation on migrating gonadotropin-releasing hormone neurons through the entire migratory route in vivo and in vitro. Endocrinology 141, 1254–1262. doi: 10.1210/endo.141.3.7348
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Casoni, F., Hutchins, B. I., Donohue, D., Fornaro, M., Condie, B. G., and Wray, S. (2012). SDF and GABA interact to regulate axophilic migration of GnRH neurons. J. Cell Sci. 125, 5015–5025. doi: 10.1242/jcs.101675
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Catalano, P., Bonaventura, M., Silveyra, P., Bettler, B., Libertun, C., and Lux-Lantos, V. A. (2005). GABAB1 knockout mice reveal alterations in prolactin levels, gonadotropic axis, and reproductive function. Neuroendocrinology 82, 294–305. doi: 10.1159/000093128
Charles, C., and Hales, T. G. (1995). Mechanisms of spontaneous calcium oscillations and action potentials in immortalized hypothalamic (GTl-7). J. Neurophysiol. 73, 56–64.
Chen, P., and Moenter, S. M. (2009). GABAergic transmission to gonadotropin-releasing hormone (GnRH) neurons is regulated by GnRH in a concentration-dependent manner engaging multiple signaling pathways. J. Neurosci. 29, 9809–9818. doi: 10.1523/JNEUROSCI.2509-09.2009
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Cho, B. N., and Kim, K. (1997). Differential effect of baclofen on hypothalamic GnRH and pituitary LHβ gene expression in steroid-treated rats. Mol. Cells 7, 605–609.
Christian, C. A., and Moenter, S. M. (2007). Estradiol induces diurnal shifts in GABA transmission to gonadotropin-releasing hormone neurons to provide a neural signal for ovulation. J. Neurosci. 27, 1913–1921. doi: 10.1523/JNEUROSCI.4738-06.2007
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Chu, Z., Andrade, J., Shupnik, M. A., and Moenter, S. M. (2009). Differential regulation of gonadotropin-releasing hormone neuron activity and membrane properties by acutely applied estradiol: dependence on dose and estrogen receptor subtype. J. Neurosci. 29, 5616–5627. doi: 10.1523/JNEUROSCI.0352-09.2009
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Chu, Z., and Moenter, S. M. (2005). Endogenous activation of metabotropic glutamate receptors modulates GABAergic transmission to gonadotropin-releasing hormone neurons and alters their firing rate: a possible local feedback circuit. J. Neurosci. 25, 5740–5749. doi: 10.1523/JNEUROSCI.0913-05.2005
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Constantin, S., Iremonger, K. J., and Herbison, A. E. (2013). In vivo recordings of GnRH neuron firing reveal heterogeneity and dependence upon GABAA receptor signaling. J. Neurosci. 33, 9394–9401. doi: 10.1523/JNEUROSCI.0533-13.2013
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Constantin, S., Jasoni, C., Romanò, N., Lee, K., and Herbison, A. E. (2012a). Understanding calcium homeostasis in postnatal gonadotropin-releasing hormone neurons using cell-specific Pericam transgenics. Cell Calcium 51, 267–276. doi: 10.1016/j.ceca.2011.11.005
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Constantin, S., Jasoni, C. L., Wadas, B., and Herbison, A. E. (2010). Gamma-aminobutyric acid and glutamate differentially regulate intracellular calcium concentrations in mouse gonadotropin-releasing hormone neurons. Endocrinology 151, 262–270. doi: 10.1210/en.2009-0817
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Constantin, S., Piet, R., Iremonger, K., Hwa Yeo, S., Clarkson, J., Porteous, R., et al. (2012b). GnRH neuron firing and response to GABA in vitro depend on acute brain slice thickness and orientation. Endocrinology 153, 3758–3769. doi: 10.1210/en.2012-1126
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Curran-Rauhut, M. A., and Petersen, S. L. (2002). Regulation of glutamic acid decarboxylase 65 and 67 gene expression by ovarian steroids: identification of two functionally distinct populations of GABA neurones in the preoptic area. J. Neuroendocrinol. 14, 310–317. doi: 10.1046/j.1365-2826.2002.00780.x
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
DeFazio, R. A., Heger, S., Ojeda, S. R., and Moenter, S. M. (2002). Activation of A-type gamma-aminobutyric acid receptors excites gonadotropin-releasing hormone neurons. Mol. Endocrinol. 16, 2872–2891. doi: 10.1210/me.2002-0163
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Di Giorgio, N., Catalano, P., López, P., González, B., Semaan, S., López, G., et al. (2013). Lack of functional GABAB receptors alters Kiss1, Gnrh1 and Gad1 mRNA expression in the medial basal hypothalamus at postnatal day 4. Neuroendocrinology 98, 212–223. doi: 10.1159/000355631
Donoso, A. O., Lopez, F. J., and Negro-Vilar, A. (1992). Cross-talk between excitatory and inhibitory amino acids in the regulation of luteinizing hormone-releasing hormone secretion. Endocrinology 31, 1559–1561.
Dungan, H. M., Gottsch, M. L., Zeng, H., Gragerov, A., Bergmann, J. E., Vassilatis, D. K., et al. (2007). The role of kisspeptin-GPR54 signaling in the tonic regulation and surge release of gonadotropin-releasing hormone/luteinizing hormone. J. Neurosci. 27, 12088–12095. doi: 10.1523/JNEUROSCI.2748-07.2007
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Eto, K., Ishibashi, H., Yoshimura, T., Watanabe, M., Miyamoto, A., Ikenaka, K., et al. (2012). Enhanced GABAergic activity in the mouse primary somatosensory cortex is insufficient to alleviate chronic pain behavior with reduced expression of neuronal potassium-chloride cotransporter. J. Neurosci. 32, 16552–16559. doi: 10.1523/JNEUROSCI.2104-12.2012
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Farkas, I., Kalló, I., Deli, L., Vida, B., Hrabovszky, E., Fekete, C., et al. (2010). Retrograde endocannabinoid signaling reduces GABAergic synaptic transmission to gonadotropin-releasing hormone neurons. Endocrinology 151, 5818–5829. doi: 10.1210/en.2010-0638
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Favit, A., Wetsel, W. C., and Negro-Vilar, A. (1993). Differential expression of gamma-aminobutyric acid receptors in immortalized luteinizing hormone-releasing hormone neurons. Endocrinology 133, 1983–1989.
Freeman, M. E. (2006). “Neuroendocrine control of the ovarian cycle of the rat,” in The Physiology of Reproduction, 3rd Edn., eds E. Knobil and J. D. Neill (New York, NY: Raven Press), 2328–2388.
Fueshko, S. M., Key, S., and Wray, S. (1998). GABA inhibits migration of luteinizing hormone-releasing hormone neurons in embryonic olfactory explants. J. Neurosci. 18, 2560–2569.
Glanowska, K. M., and Moenter, S. M. (2011). Endocannabinoids and prostaglandins both contribute to GnRH neuron-GABAergic afferent local feedback circuits. J. Neurophysiol. 106, 3073–3081. doi: 10.1152/jn.00046.2011
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Gottsch, M. L., Cunningham, M. J., Smith, J. T., Popa, S. M., Acohido, B. V., Crowley, W. F., et al. (2004). A role for kisspeptins in the regulation of gonadotropin secretion in the mouse. Endocrinology 145, 4073–4077. doi: 10.1210/en.2004-0431
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Hales, T. G., Sanderson, M. J., and Charles, A. C. (1994). GABA has excitatory actions on GnRH-secreting immortalized hypothalamic (GT1-7) neurons. Neuroendocrinology 59, 297–308. doi: 10.1159/000126671
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Han, S. K., Abraham, I. M., and Herbison, A. E. (2002). Effect of GABA on GnRH neurons switches from depolarization to hyperpolarization at puberty in the female mouse. Endocrinology 143, 1459–1466. doi: 10.1210/endo.143.4.8724
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Han, S. K., Gottsch, M. L., Lee, K. J., Popa, S. M., Smith, J. T., Jakawich, S. K., et al. (2005). Activation of gonadotropin-releasing hormone neurons by kisspeptin as a neuroendocrine switch for the onset of puberty. J. Neurosci. 25, 11349–11356. doi: 10.1523/JNEUROSCI.3328-05.2005
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Han, S. K., Todman, M. G., and Herbison, A. E. (2004). Endogenous GABA release inhibits the firing of adult gonadotropin-releasing hormone neurons. Endocrinology 145, 495–499. doi: 10.1210/en.2003-1333
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Heger, S., Seney, M., Bless, E., Schwarting, G. A., Bilger, M., Mungenast, A., et al. (2003). Overexpression of glutamic acid decarboxylase-67 (GAD-67) in gonadotropin-releasing hormone neurons disrupts migratory fate and female reproductive function in mice. Endocrinology 144, 2566–2579. doi: 10.1210/en.2002-221107
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Hemond, P. J., O'Boyle, M. P., Roberts, C. B., Delgado-Reyes, A., Hemond, Z., and Suter, K. J. (2012). Simulated GABA synaptic input and L-type calcium channels form functional microdomains in hypothalamic gonadotropin-releasing hormone neurons. J. Neurosci. 32, 8756–8766. doi: 10.1523/JNEUROSCI.4188-11.2012
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Herbison, A. E. (2006). “Physiology of gonadotropin-releasing hormone neuronal network,” in The Physiology of Reproduction, 3rd Edn., eds E. Knobil and J. D. Neill (New York, NY: Raven Press), 1415–1482.
Herbison, A. E., Augood, S. J., and McGowan, E. M. (1992). Expression of glutamic acid decarboxylase messenger RNA in rat medial preoptic area neurones during the oestrous cycle and after ovariectomy. Brain Res. Mol. Brain Res. 14, 310–316. doi: 10.1016/0169-328X(92)90098-V
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Herbison, A. E., Chapman, C., and Dyer, R. G. (1991). Role of medial preoptic GABA neurones in regulating luteinising hormone secretion in the ovariectomised rat. Exp. Brain Res. 87, 345–352. doi: 10.1007/BF00231851
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Herbison, A. E., and Dyer, R. G. (1991). Effect on luteinizing hormone secretion of GABA receptor modulation in the medial preoptic area at the time of proestrous luteinizing hormone surge. Neuroendocrinology 53, 317–320. doi: 10.1159/000125735
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Herbison, A. E., and Moenter, S. M. (2011). Depolarising and hyperpolarising actions of GABAA receptor activation on gonadotrophin-releasing hormone neurones: towards an emerging consensus. J. Neuroendocrinol. 23, 557–569. doi: 10.1111/j.1365-2826.2011.02145.x
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Herde, M. K., Iremonger, K. J., Constantin, S., and Herbison, A. E. (2013). GnRH neurons elaborate a long-range projection with shared axonal and dendritic functions. J. Neurosci. 33, 12689–12697. doi: 10.1523/JNEUROSCI.0579-13.2013
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Hiruma, H., Sano, A., and Kimura, F. (1994). Injection of bicuculline elicits firing of luteinizing hormone releasing hormone pulse generator in muscimol-treated ovariectomized rats. Brain Res. 641, 191–197. doi: 10.1016/0006-8993(94)90145-7
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Hrabovszky, E., Molnár, C. S., Nagy, R., Vida, B., Borsay, B. Á., Rácz, K., et al. (2012). Glutamatergic and GABAergic innervation of human gonadotropin-releasing hormone-I neurons. Endocrinology 153, 2766–2776. doi: 10.1210/en.2011-2106
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Jarry, H., Leonhardt, S., Schwarze, T., and Wuttke, W. (1995). Preoptic rather than mediobasal hypothalamic amino acid neurotransmitter release regulates GnRH secretion during the estrogen-induced LH surge in the ovariectomized rat. Neuroendocrinology 62, 479–486. doi: 10.1159/000127037
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Jarry, H., Leonhardt, S., and Wuttke, W. (1991). Gamma-aminobutyric acid neurons in the preoptic/anterior hypothalamic area synchronize the phasic activity of the gonadotropin-releasing hormone pulse generator in ovariectomized rats. Neuroendocrinology 53, 261–267. doi: 10.1159/000125727
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Kahle, K. T., Deeb, T. Z., Puskarjov, M., Silayeva, L., Liang, B., Kaila, K., et al. (2013). Modulation of neuronal activity by phosphorylation of the K-Cl cotransporter KCC2. Trends Neurosci. 36, 726–737. doi: 10.1016/j.tins.2013.08.006
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Kahle, K. T., Staley, K. J., Nahed, B. V., Gamba, G., Hebert, S. C., Lifton, R. P., et al. (2008). Roles of the cation-chloride cotransporters in neurological disease. Nat. Clin. Pract. Neurol. 4, 490–503. doi: 10.1038/ncpneuro0883
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Kang, S., Seong, J., Cho, S., Cho, H., and Kim, K. (1995). Acute increase of GABAergic neurotransmission exerts a stimulatory effect on GnRH gene expression in the preoptic/anterior hypothalamic area of ovariectomized, estrogen- and progesterone-treated adult female rats. Neuroendocrinology 61, 486–492. doi: 10.1159/000126871
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Kimura, F., and Jinnai, K. (1994). Bicuculline infusions advance the timing of luteinizing hormone surge in proestrous rats: comparisons with naloxone effects. Horm. Behav. 28, 424–430. doi: 10.1006/hbeh.1994.1039
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Kurian, J. R., Keen, K. L., Guerriero, K. A., and Terasawa, E. (2012). Tonic control of kisspeptin release in prepubertal monkeys: implications to the mechanism of puberty onset. Endocrinology 153, 3331–3336. doi: 10.1210/en.2012-1221
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Kusano, K., Fueshko, S., Gainer, H., and Wray, S. (1995). Electrical and synaptic properties of embryonic luteinizing hormone-releasing hormone neurons in explant cultures. Proc. Natl. Acad. Sci. U.S.A. 92, 3918–3922. doi: 10.1073/pnas.92.9.3918
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Kwakowsky, A., Cheong, R. Y., Herbison, A. E., and Ábrahám, I. M. (2014). Non-classical effects of estradiol on cAMP responsive element binding protein phosphorylation in gonadotropin-releasing hormone neurons: mechanisms and role. Front. Neuroendocrinol. 35, 31–41. doi: 10.1016/j.yfrne.2013.08.002
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Lagrange, A. H., Rønnekleiv, O. K., and Kelly, M. J. (1995). Estradiol-17 beta and mu-opioid peptides rapidly hyperpolarize GnRH neurons: a cellular mechanism of negative feedback? Endocrinology 136, 2341–2344. doi: 10.1210/endo.136.5.7720682
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Lee, J. M., Tiong, J., Maddox, D. M., Condie, B. G., and Wray, S. (2008). Temporal migration of gonadotrophin-releasing hormone-1 neurones is modified in GAD67 knockout mice. J. Neuroendocrinol. 20, 93–103. doi: 10.1111/j.1365-2826.2007.01623.x
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Lee, K., Porteous, R., Campbell, R. E., Lüscher, B., and Herbison, A. E. (2010). Knockdown of GABAA receptor signaling in GnRH neurons has minimal effects upon fertility. Endocrinology 151, 4428–4436. doi: 10.1210/en.2010-0314
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Leonhardt, S., Shahab, M., Luft, H., Wuttke, W., and Jarry, H. (1999). Reduction of luteinzing hormone secretion induced by long-term feed restriction in male rats is associated with increased expression of GABA-synthesizing enzymes without alterations of GnRH gene expression. J. Neuroendocrinol. 11, 613–619. doi: 10.1046/j.1365-2826.1999.00377.x
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Leranth, C., MacLusky, N. J., Sakamoto, H., Shanabrough, M., and Naftolin, F. (1985). Glutamic acid decarboxylase-containing axons synapse on LHRH neurons in the rat medial preoptic area. Neuroendocrinology 40, 536–539. doi: 10.1159/000124127
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Leupen, S. M., Tobet, S. A., Crowley, W. F., and Kaila, K. (2003). Heterogeneous expression of the potassium-chloride cotransporter KCC2 in gonadotropin-releasing hormone neurons of the adult mouse. Endocrinology 144, 3031–3036. doi: 10.1210/en.2002-220995
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Lin, Y. S., Li, X. F., Shao, B., Hu, M. H., Goundry, L. R., Jeyaram, A., et al. (2012). The role of GABAergic signalling in stress-induced suppression of gonadotrophin-releasing hormone pulse generator frequency in female rats. J. Neuroendocrinol. 24, 477–488. doi: 10.1111/j.1365-2826.2011.02270.x
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Martin, C., Navarro, V. M., Simavli, S., Vong, L., Carroll, R. S., Lowell, B. B., et al. (2014). Leptin-responsive GABAergic neurons regulate fertility through pathways that result in reduced kisspeptinergic tone. J. Neurosci. 34, 6047–6056. doi: 10.1523/JNEUROSCI.3003-13.2014
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Martínez de la Escalera, G., Choi, A. L., and Weiner, R. I. (1994). Biphasic gabaergic regulation of GnRH secretion in GT1 cell lines. Neuroendocrinology 59, 420–425. doi: 10.1159/000126687
Mayer, C., and Boehm, U. (2011). Female reproductive maturation in the absence of kisspeptin/GPR54 signaling. Nat. Neurosci. 14, 704–710. doi: 10.1038/nn.2818
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Mellon, P. L., Windle, J. J., Goldsmith, P. C., Padula, C. A., Roberts, J. L., and Weiner, R. I. (1990). Immortalization of hypothalamic GnRH neurons by genetically targeted tumorigenesis. Neuron 5, 1–10. doi: 10.1016/0896-6273(90)90028-E
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Messager, S., Chatzidaki, E. E., Ma, D., Hendrick, A. G., Zahn, D., Dixon, J., et al. (2005). Kisspeptin directly stimulates gonadotropin-releasing hormone release via G protein-coupled receptor 54. Proc. Natl. Acad. Sci. U.S.A. 102, 1761–1766. doi: 10.1073/pnas.0409330102
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Moenter, S. M., and DeFazio, R. A. (2005). Endogenous gamma-aminobutyric acid can excite gonadotropin-releasing hormone neurons. Endocrinology 146, 5374–5379. doi: 10.1210/en.2005-0788
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Moore, J. P., and Wray, S. (2000). Biosynthesis and secretion in embryonic LHRH neurons. Endocrinology 141, 4486–4495. doi: 10.1210/endo.141.12.7814
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Nabekura, J., Ueno, T., Okabe, A., Furuta, A., Iwaki, T., Shimizu-Okabe, C., et al. (2002). Reduction of KCC2 expression and GABAA receptor-mediated excitation after in vivo axonal injury. J. Neurosci. 22, 4412–4417.
Nakane, R., and Oka, Y. (2010). Excitatory action of GABA in the terminal nerve gonadotropin-releasing hormone neurons. J. Neurophysiol. 103, 1375–1384. doi: 10.1152/jn.00910.2009
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Ottem, E. N., Godwin, J. G., Krishnan, S., and Petersen, S. L. (2004). Dual-phenotype GABA/glutamate neurons in adult preoptic area: sexual dimorphism and function. J. Neurosci. 24, 8097–8105. doi: 10.1523/JNEUROSCI.2267-04.2004
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Pielecka-Fortuna, J., and Moenter, S. M. (2010). Kisspeptin increases gamma-aminobutyric acidergic and glutamatergic transmission directly to gonadotropin-releasing hormone neurons in an estradiol-dependent manner. Endocrinology 151, 291–300. doi: 10.1210/en.2009-0692
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Popesku, J. T., Martyniuk, C. J., Mennigen, J., Xiong, H., Zhang, D., Xia, X., et al. (2008). The goldfish (Carassius auratus) as a model for neuroendocrine signaling. Mol. Cell. Endocrinol. 293, 43–56. doi: 10.1016/j.mce.2008.06.017
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Quennell, J. H., Mulligan, A. C., Tups, A., Liu, X., Phipps, S. J., Kemp, C. J., et al. (2009). Leptin indirectly regulates gonadotropin-releasing hormone neuronal function. Endocrinology 150, 2805–2812. doi: 10.1210/en.2008-1693
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Reed, K., MacIntyre, J., Tobet, S., Rudeau, V., MacEachern, L., Rubin, B., et al. (2002). The spatial relationship of gamma-aminobutyric acid (GABA) neurons and gonadotropin-releasing hormone (GnRH) neurons in larval and adult sea lamprey, Petromyzon marinus. Brain Behav. Evol. 60, 1–12. doi: 10.1159/000064117
Reid, K. H., Guo, S. Z., and Iyer, V. G. (2000). Agents which block potassium-chloride cotransport prevent sound-triggered seizures in post-ischemic audiogenic seizure-prone rats. Brain Res. 864, 134–137. doi: 10.1016/S0006-8993(00)02121-1
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Rivera, C., Voipio, J., Thomas-Crusells, J., Li, H., Emri, Z., Sipilä, S., et al. (2004). Mechanism of activity-dependent downregulation of the neuron-specific K-Cl cotransporter KCC2. J. Neurosci. 24, 4683–4691. doi: 10.1523/JNEUROSCI.5265-03.2004
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Romanò, N., Lee, K., Abrahám, I. M., Jasoni, C. L., and Herbison, A. E. (2008). Nonclassical estrogen modulation of presynaptic GABA terminals modulates calcium dynamics in gonadotropin-releasing hormone neurons. Endocrinology 149, 5335–5344. doi: 10.1210/en.2008-0424
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Root, A. R., Sanford, J. D., Kavanaugh, S. I., and Sower, S. A. (2004). In vitro and in vivo effects of GABA, muscimol, and bicuculline on lamprey GnRH concentration in the brain of the sea lamprey (Petromyzon marinus). Comp. Biochem. Physiol. A Mol. Integr. Physiol. 138, 493–501. doi: 10.1016/j.cbpb.2004.06.011
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Sato, S., Yin, C., Teramoto, A., Sakuma, Y., and Kato, M. (2008). Sexually dimorphic modulation of GABAA receptor currents by melatonin in rat gonadotropin-releasing hormone neurons. J. Physiol. Sci. 58, 317–322. doi: 10.2170/physiolsci.RP006208
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Sim, J. A., Skynner, M. J., Pape, J. R., and Herbison, A. E. (2000). Late postnatal reorganization of GABAA receptor signalling in native GnRH neurons. Eur. J. Neurosci. 12, 3497–3504. doi: 10.1046/j.1460-9568.2000.00261.x
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Soghomonian, J., and Martin, D. L. (1998). Two isoforms of glutamate decarboxylase: why? Trends Pharmacol. Sci. 19, 500–505. doi: 10.1016/S0165-6147(98)01270-X
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Spergel, D. J., Krsmanovic, L. Z., Stojilkovic, S. S., and Catt, K. J. (1995). L-type Ca2+ channels mediate joint modulation by gamma-amino-butyric acid and glutamate of [Ca2+]i and neuropeptide secretion in immortalized gonadodropin-releasing hormone neurons. Neuroendocrinology 61, 499–508. doi: 10.1159/000126873
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Spergel, D. J., Krüth, U., Hanley, D. F., Sprengel, R., and Seeburg, P. H. (1999). GABA- and glutamate-activated channels in green fluorescent protein-tagged gonadotropin-releasing hormone neurons in transgenic mice. J. Neurosci. 19, 2037–2050.
Sullivan, S. D., DeFazio, R. A., and Moenter, S. M. (2003). Metabolic regulation of fertility through presynaptic and postsynaptic signaling to gonadotropin-releasing hormone neurons. J. Neurosci. 23, 8578–8585.
Sullivan, S. D., and Moenter, S. M. (2003). Neurosteroids alter gamma-aminobutyric acid postsynaptic currents in gonadotropin-releasing hormone neurons: a possible mechanism for direct steroidal control. Endocrinology 144, 4366–4375. doi: 10.1210/en.2003-0634
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Sullivan, S. D., and Moenter, S. M. (2004a). Gamma-aminobutyric acid neurons integrate and rapidly transmit permissive and inhibitory metabolic cues to gonadotropin-releasing hormone neurons. Endocrinology 145, 1194–1202. doi: 10.1210/en.2003-1374
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Sullivan, S. D., and Moenter, S. M. (2004b). Prenatal androgens alter GABAergic drive to gonadotropin-releasing hormone neurons: implications for a common fertility disorder. Proc. Natl. Acad. Sci. U.S.A. 101, 7129–7134. doi: 10.1073/pnas.0308058101
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Suter, K. J., Song, W. J., Sampson, T. L., Wuarin, J. P., Saunders, J. T., Dudek, F. E., et al. (2000). Genetic targeting of green fluorescent protein to gonadotropin-releasing hormone neurons: characterization of whole-cell electrophysiological properties and morphology. Endocrinology 141, 412–419. doi: 10.1210/endo.141.1.7279
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Temple, J. L., and Wray, S. (2005). Developmental changes in GABA receptor subunit composition within the gonadotrophin-releasing hormone-1 neuronal system. J. Neuroendocrinol. 17, 591–599. doi: 10.1111/j.1365-2826.2005.01348.x
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Terasawa, E., Kurian, J. R., Guerriero, K. A., Kenealy, B. P., Erika, D., and Keen, K. L. (2011). Recent discoveries on the control of GnRH neurons in nonhuman primates. J. Neuroendocrinol. 22, 630–638. doi: 10.1111/j.1365-2826.2010.02027.x
Tobet, S. A., Chickering, T. W., King, J. C., Stopa, E. G., Kim, K., Kuo-Leblank, V., et al. (1996). Expression of γ-aminobutyric acid and gonadotropin- releasing hormone during neuronal migration through the olfactory system. Endocrinology 137, 5415–5420.
Toyoda, H., Ohno, K., Yamada, J., Ikeda, M., Okabe, A., Sato, K., et al. (2003). Induction of NMDA and GABAA receptor-mediated Ca2+ oscillations with KCC2 mRNA downregulation in injured facial motoneurons. J. Neurophysiol. 89, 1353–1362. doi: 10.1152/jn.00721.2002
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Trudeau, V., Spanswick, D., Fraserm, E., Larivière, K., Crump, D., Chiu, S., et al. (2000). The role of amino acid neurotransmitters in the regulation of pituitary gonadotropin release in fish. Biochem. Cell Biol. 78, 241–259. doi: 10.1139/o99-075
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Vastagh, C., Schwirtlich, M., Kwakowsky, A., Erdélyi, F., Margolis, F. L., Yanagawa, Y., et al. (2014). The spatio-temporal segregation of GAD forms defines distinct GABA signaling functions in the developing mouse olfactory system and provides novel insights into the origin and migration of GnRH neurons. Dev. Neurobiol. doi: 10.1002/dneu.22222. [Epub ahead of print].
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Wake, H., Watanabe, M., Moorhouse, A. J., Kanematsu, T., Horibe, S., Matsukawa, N., et al. (2007). Early changes in KCC2 phosphorylation in response to neuronal stress result in functional downregulation. J. Neurosci. 27, 1642–1650. doi: 10.1523/JNEUROSCI.3104-06.2007
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Watanabe, M., Sakuma, Y., and Kato, M. (2009a). GABAA receptors mediate excitation in adult rat GnRH neurons. Biol. Reprod. 81, 327–332. doi: 10.1095/biolreprod.108.074583
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Watanabe, M., Wake, H., Moorhouse, A. J., and Nabekura, J. (2009b). Clustering of neuronal K+-Cl− cotransporters in lipid rafts by tyrosine phosphorylation. J. Biol. Chem. 284, 27980–27988. doi: 10.1074/jbc.M109.043620
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Wetsel, W. C., Valen, M. M., Merchenthalert, I., Lipositstt, Z., Lpez, F. J., Weiner, R. I., et al. (1992). Intrinsic pulsatile secretory activity of immortalized luteinizing hormone-releasing hormone-secreting neurons. Proc. Natl. Acad. Sci. U.S.A. 89, 4149–4153. doi: 10.1073/pnas.89.9.4149
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Wray, S., Fueshko, S. M., Kusano, K., and Gainer, H. (1996). GABAergic neurons in the embryonic olfactory pit/vomeronasal organ: maintenance of functional GABAergic synapses in olfactory explants. Dev. Biol. 180, 631–645. doi: 10.1006/dbio.1996.0334
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Yin, C., Ishii, H., Tanaka, N., Sakuma, Y., and Kato, M. (2008). Activation of A-type gamma-amino butyric acid receptors excites gonadotrophin-releasing hormone neurones isolated from adult rats. J. Neuroendocrinol. 20, 566–575. doi: 10.1111/j.1365-2826.2008.01697.x
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Zhang, C., Bosch, M., Ronnekleiv, O. K., and Kelly, M. J. (2009). GABAB receptor mediated inhibition of GnRH neurons is suppressed by Kisspeptin-GPR54 signaling. Endocrinology 150, 2388–2394. doi: 10.1210/en.2008-1313
Zuure, W. A., Roberts, A. L., Quennell, J. H., and Anderson, G. M. (2013). Leptin signaling in GABA neurons, but not glutamate neurons, is required for reproductive function. J. Neurosci. 33, 17874–17883. doi: 10.1523/JNEUROSCI.2278-13.2013
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Keywords: GnRH neuron, GABA, KCC2, NKCC1, LH surge
Citation: Watanabe M, Fukuda A and Nabekura J (2014) The role of GABA in the regulation of GnRH neurons. Front. Neurosci. 8:387. doi: 10.3389/fnins.2014.00387
Received: 30 August 2014; Accepted: 12 November 2014;
Published online: 28 November 2014.
Edited by:
Ishwar Parhar, Monash University, MalaysiaReviewed by:
Oline K. Ronnekleiv, Oregen Health & Science University, USACopyright © 2014 Watanabe, Fukuda and Nabekura. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Miho Watanabe, Department of Neurophysiology, Hamamatsu University School of Medicine, 1-20-1 Handayama, Higashi-ku, Hamamatsu, Shizuoka 431-3192, Japan e-mail:bWlob3dAaGFtYS1tZWQuYWMuanA=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.