- 1 Division of Systems Medical Science, Institute for Comprehensive Medical Science, Fujita Health University, Toyoake, Japan
- 2 Japan Science and Technology Agency, Core Research for Evolutional Science and Technology, Kawaguchi, Japan
- 3 Japan Science and Technology Agency, Institute for Bioinformatics Research and Development, Kawaguchi, Japan
- 4 Center for Genetic Analysis of Behavior, National Institute for Physiological Sciences, Okazaki, Japan
The dentate gyrus produces new granule neurons throughout adulthood in mammals from rodents to humans. During granule cell maturation, defined markers are expressed in a highly regulated sequential process, which is necessary for directed neuronal differentiation. In the present study, we show that α-amino-3-hydroxy-5-methy-4-isoxazole propionate (AMPA) receptor subunits GluR1 and GluR2 are expressed in differentiated granule cells, but not in stem cells, in neonatal, and adult dentate gyrus. Using markers for neural progenitors, immature and mature granule cells, we found that GluR1 and GluR2 were expressed mainly in mature cells and in some immature cells. A time-course analysis of 5-bromo-2′-deoxyuridine staining revealed that granule cells express GluR1 around 3 weeks after being generated. In mice heterozygous for the alpha-isoform of calcium/calmodulin-dependent protein kinase II, a putative animal model of schizophrenia and bipolar disorder in which dentate gyrus granule cells fail to mature normally, GluR1 and GluR2 immunoreactivities were substantially downregulated in the dentate gyrus granule cells. In the granule cells of mutant mice, the expression of both presynaptic and postsynaptic markers was decreased, suggesting that GluR1 and GluR2 are also associated with synaptic maturation. Moreover, GluR1 and GluR2 were also expressed in mature granule cells of the neonatal dentate gyrus. Taken together, these findings indicate that GluR1 and GluR2 expression closely correlates with the neuronal maturation state, and that GluR1 and GluR2 are useful markers for mature granule cells in the dentate gyrus.
Introduction
New neurons are generated in the subgranular zone of the hippocampal dentate gyrus and in the anterior subventricular zone adjacent to the lateral ventricles throughout life (Abrous et al., 2005; Duan et al., 2008; Zhao et al., 2008). In the adult hippocampus, neural stem cells exist near the border between the hilus and the dentate gyrus granule cell layer. Postmitotic granule cells in the subgranular zone migrate radially into the granule cell layer and are integrated into the deepest part of the granule cell layer, where they differentiate into granule cells, extending dendrites and axons and receiving synaptic inputs. During development, new granule cells express several marker proteins depending on the level of cell differentiation (Duan et al., 2008; Zhao et al., 2008).
In the adult dentate granule cell, tryptophan 2,3-dioxygenase is expressed with increasing cell age (Ohira et al., 2010), and expression is substantially downregulated in the granule cells of mice heterozygous for the alpha-isoform of calcium/calmodulin-dependent protein kinase II (αCaMKII; Yamasaki et al., 2008; Ohira et al., 2010). These mutant mice have an endophenotype called “immature dentate gyrus” (Yamasaki et al., 2008), in which almost all hippocampal granule cells remain in an immature state. Immunohistochemical analysis revealed that these mutants have increased expression of polysialic acid NCAM, a marker for late-stage progenitor cells and immature neurons, and calretinin, a marker for immature neurons, and decreased expression of calbindin, a marker for mature neurons. The maturation failure of granule cells in αCaMKII± mice and the higher GluR expression in the hippocampus compared with several regions of the central nervous system (Rogers et al., 1991; Hampson et al., 1992; Medvedev et al., 2008) suggest that GluR are expressed only after a certain stage of granule cell differentiation in the normal mouse. Electrophysiological analysis revealed that dentate granule cells of these mutants have high input resistance, high excitability, small spike amplitude, and a decreased number of spikes during sustained depolarization, findings that suggest that the dentate gyrus of αCaMKII± mice is also electrophysiologically immature. These mutant mice also exhibit some behavioral abnormalities, including a severe working memory deficit and an exaggerated infradian rhythm, similar to symptoms observed in patients with schizophrenia, bipolar mood disorder, and other psychiatric disorders (Yamasaki et al., 2008). These observations suggest the association between granule cell maturation failure and these behavioral phenotypes.
The AMPA-type glutamate receptor, which is involved in fast excitatory transmission in the mammalian central nervous system, comprises four subunits, GluR1-4. Neither GluR1- nor GLuR2/3-immunolabeling cells are observed in the rat cerebral cortex and hippocampus at the embryonic stage, but they appear during the postnatal stage (Arai et al., 1997). Thus, developmental changes of the receptor may well correlate with synaptogenesis and consolidation of synaptic connections.
Impaired signal transduction through GluR is significantly related with neuronal disorders. Hippocampal GluR expression are altered under pathologic conditions; GluR1 and GluR2 expression is decreased in ischemia (Dos-Anjos et al., 2009; Montori et al., 2010), and either increased or decreased in epilepsy (de Lanerolle et al., 1998; Ying et al., 1998; Tang et al., 2005; Solomonia et al., 2010). Also, GluR1 seems to be involved in psychiatric disorders, such as bipolar disorder (Du et al., 2004, 2008) and schizophrenia (Schmitt et al., 2005; Wiedholz et al., 2008; Sanderson et al., 2009; Erickson et al., 2010). Administration of the antimanic agents reduce synaptic GluR1 in hippocampal neurons, and reduction in GluR1 phosphorylation at its cAMP-dependent protein kinase A site by the mimics of antimanic agents induce manic-like behaviors, indicating GluR1 trafficking play an important role in the pathophysiology and treatment of manic-like behaviors (Du et al., 2004, 2008). GluR1 knockout mice exhibit schizophrenia-like behaviors (Schmitt et al., 2005; Wiedholz et al., 2008; Sanderson et al., 2009; Erickson et al., 2010). AMPA receptors are critically involved in spatial working memory (Schmitt et al., 2005; Sanderson et al., 2009; Erickson et al., 2010). GluR1 deletion profoundly impairs hippocampus-dependent spatial working memory, one of the core disordered functions in patients with schizophrenia (Silver et al., 2003) and in model animals (Yamasaki et al., 2008; Matsuo et al., 2009), and genetically expressed GluR1 in GluR1-deficient mice restores the spatial working memory (Schmitt et al., 2005). These findings reveal a critical association of hippocampal GluR1-dependent synaptic plasticity and memory processing.
In the present study, to clarify the GluR1 and GluR2 expression patterns in the hippocampal dentate gyrus of neonatal and adult mice during granule cell maturation, we conducted single- and double-immunofluorescence analyses of GluR1 and GluR2 with markers for proliferative cells, neural progenitor cells, immature and mature granule cells, and showed that GluR1 and GluR2 were expressed in mature granule cells. Furthermore, we confirmed the usefulness of GluR1 and GluR2 as maturation markers of dentate granule cells by investigating GluR expression and its correlation with synaptic integration in the hippocampus of αCaMKII± mice that have an “immature dentate gyrus.”
Results
GLuR1 Expression in Mature Granule Cells
GluR1 was abundantly expressed in the hippocampus of the adult mouse brain, including the dentate gyrus and Ammon’s horn regions (Figures 1A–E), consistent with previous studies using different antibodies (Rogers et al., 1991; Hampson et al., 1992). In the dentate gyrus, GluR1 immunoreactivity was observed in the plasma membrane-like structures of granule cells in the granule cell layer and their dendrites extending to the molecular layer, and in some interneurons and neurites in the hilus (Figure 1A). Some interneurons strongly labeled with GluR1 antibody in the hilus also contained GAD67 (Figure 1B). The subgranular zone where neural stem cells/progenitor cells exist (brackets in Figure 1A) lacked any GluR1 immunoreactivity, suggesting that GluR1 expression is gradually increased during granule cell development.
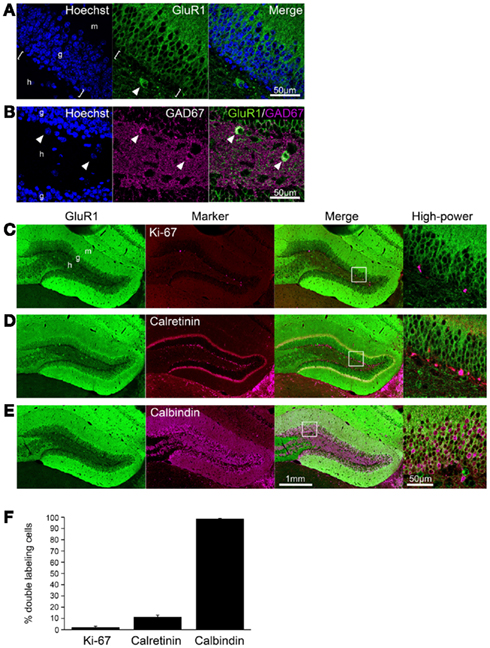
Figure 1. GluR1 expression in mature granule cells. (A,B) GluR1 immunoreactivity was abundant in the granule cells and hilar interneurons (arrowheads) in the adult dentate gyrus (A). Note that there was no GluR1 immunoreactive signal in the subgranular zone [brackets in (A)]. Some interneurons labeled with GluR1 antibody in the hilus were GAD67-positive [arrowheads in (B)]. To visualize the nucleus, cells were counterstained with Hoechst. (C–E) Co-labeling of GluR1 with Ki-67, neural progenitor cells (C), calretinin, immature granule cells (D) or calbindin, mature granule cells (E). Higher magnifications of the boxed-in area in the merged images are shown on the right-hand side. (F) The co-labeled cells were quantified. Values are given as the mean ± SEM of the analysis based on the results of three mice. g, Granule cell layer; h, hilus; m, molecular layer.
To evaluate the GluR1 expression pattern during the development of granule cells in the adult hippocampus, immunofluorescence analysis was performed by laser scanning confocal microscopy. All progenitors are proliferative and therefore contain the cell proliferation marker Ki-67, which is a nuclear protein expressed in all phases of the cell cycle except the resting phase (Scholzen and Gerdes, 2000). Ki-67-positive cells were restricted to the subgranular zone, and there were few Ki-67/GluR1 double-positive cells (2.1 ± 0.93%, n = 3 mice; Figures 1C,F). After exiting the proliferative phase, immature granule cells can be distinguished from progenitors by the expression of calretinin and their location in the deepest granule cell layer (Brandt et al., 2003; Duan et al., 2008; Zhao et al., 2008). On the other hand, granule cells express calbindin as they mature and migrate into the granule cell layer (Kempermann et al., 1997; Duan et al., 2008; Zhao et al., 2008). Therefore, immature and mature granule cells can be distinguished by the expression of the cell markers calretinin and calbindin, respectively. Calretinin-positive cells were observed in the subgranular zone and in the deep part of the granule cell layer and rarely expressed GluR1 (11.3 ± 1.70%, n = 3 mice; Figures 1D,F). Almost all calbindin-positive cells were located in the granule cell layer and expressed GluR1 (98.7 ± 0.16%, n = 3 mice; Figures 1E,F). These results suggest that GluR1 is expressed in some immature granule cells that might be at the late-stage of development and in mature granule cells.
Using a double-labeling method, we determined whether GluR1 was expressed in neurons or glial cells. NeuN, a soluble nuclear protein, is observed in most neuronal cell types, but not in non-neuronal cells (Wolf et al., 1996). Almost all GluR1-positive cells in the granule cell layer were stained by anti-NeuN antibody (Figure 2A). No GluR1-positive cells expressed glial fibrillary acidic protein (GFAP), a mature astrocyte marker, and F4/80, the plasma membrane glycoprotein expressed exclusively on macrophages and microglia (Austyn and Gordon, 1981; Figures 2B,C). These findings indicate that GluR1 is expressed specifically in neurons in the adult mouse hippocampus.
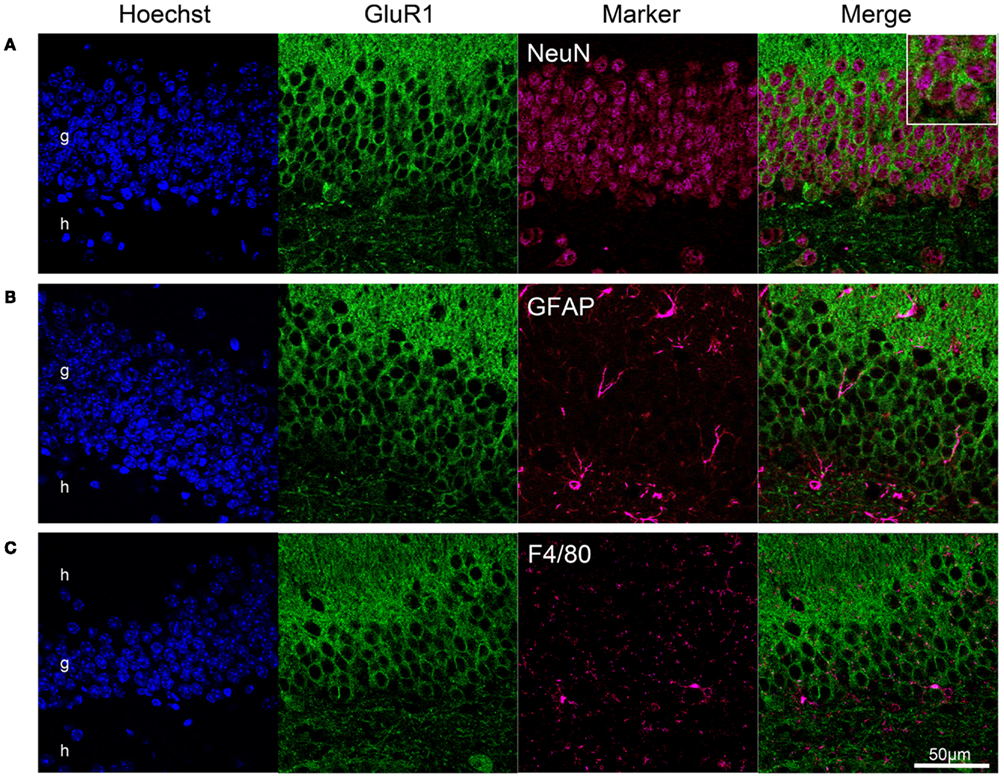
Figure 2. GluR1 expression in neurons but not in glial cells. Neuronal expression of GluR1 in the dentate gyrus. Immunofluorescence analysis revealed GluR1 expression in neurons labeled with the NeuN antibody (A), but not in GFAP-positive astrocytes (B), and F4/80-positive microglia (C). Almost all NeuN-positive neurons were immunoreactive for GluR1. g, Granule cell layer; h, hilus.
Time-Course Analysis with 5-Bromo-2′-Deoxyuridine (BrdU)
To investigate when GluR1 was expressed during the development of granule cells in adult animals, granule cells were labeled with BrdU. Almost all BrdU-positive cells were observed in the subgranular zone at 1 week after BrdU administration, whereas they were integrated into the granule cell layer at 4 weeks (Figure 3A). The rate of cells that were GluR1- and BrdU-double positive was 9.64 ± 1.00% at 1 week, 32.7 ± 3.02% at 2 weeks, 74.2 ± 6.73% at 3 weeks, and 83.5 ± 1.61% at 4 weeks after BrdU injection (Figure 3B, n = 3–4 mice for each time point; 1 versus 2 weeks, p = 0.016; 2 versus 3 weeks, p = 3.20 × 10−4; 2 versus 3 weeks, p = 0.18, Tukey’s post hoc test). GluR1 expression dramatically increased around 3 weeks of age. These results seem to be consistent with the calretinin and calbindin staining results (Figures 1D,E) and previous reports that calretinin expression begins 2 weeks after granule generation and ceases by 4 weeks, followed by the expression of calbindin at 4 weeks (Kempermann et al., 1997; Brandt et al., 2003; Duan et al., 2008; Zhao et al., 2008). These findings indicate that GluR1 expression appears around 3 weeks after the generation of granule cells, although a few granule cells express GluR1 at approximately 2 weeks.
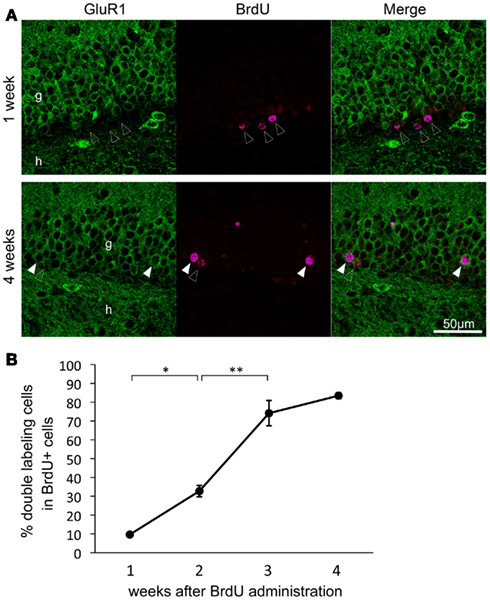
Figure 3. Time course of the double-labeling of GluR1 with bromodeoxyuridine (BrdU) in the dentate gyrus granule cells. (A) Representative images of BrdU and GluR1 double-labeling at 1 week (upper panel) and 4 weeks (lower panel) after BrdU injection. Closed and open arrowheads indicate BrdU-positive cells with or without co-expression of GluR1, respectively. g, Granule cell layer; h, hilus. (B) Changes in co-labeling rates of BrdU with GluR1 in the dentate gyrus over a period of 4 weeks after BrdU injection. Values are given as the means ± SEM of the analysis based on the results of three mice. Asterisks indicate statistically significant differences: **p < 0.01 (one-way ANOVA and Tukey’s post hoc test).
GLuR2 Expression in Mature Granule Cells
We next performed double-immunolabeling of GluR2 with Ki-67, calretinin, or calbindin to investigate whether GluR2 subunit expression is regulated during granule cell maturation. Few proliferative cells positive for Ki-67 were labeled with GluR2 (3.6 ± 0.79%, n = 3 mice; Figures 4B,E). Calretinin-positive immature granule cells rarely expressed GluR2 (19.8 ± 2.31%, n = 3 mice; Figures 4C,E), and almost all of the calbindin-positive mature granule cells expressed GluR2 (98.9 ± 0.11%, n = 3 mice; Figures 4D,E).
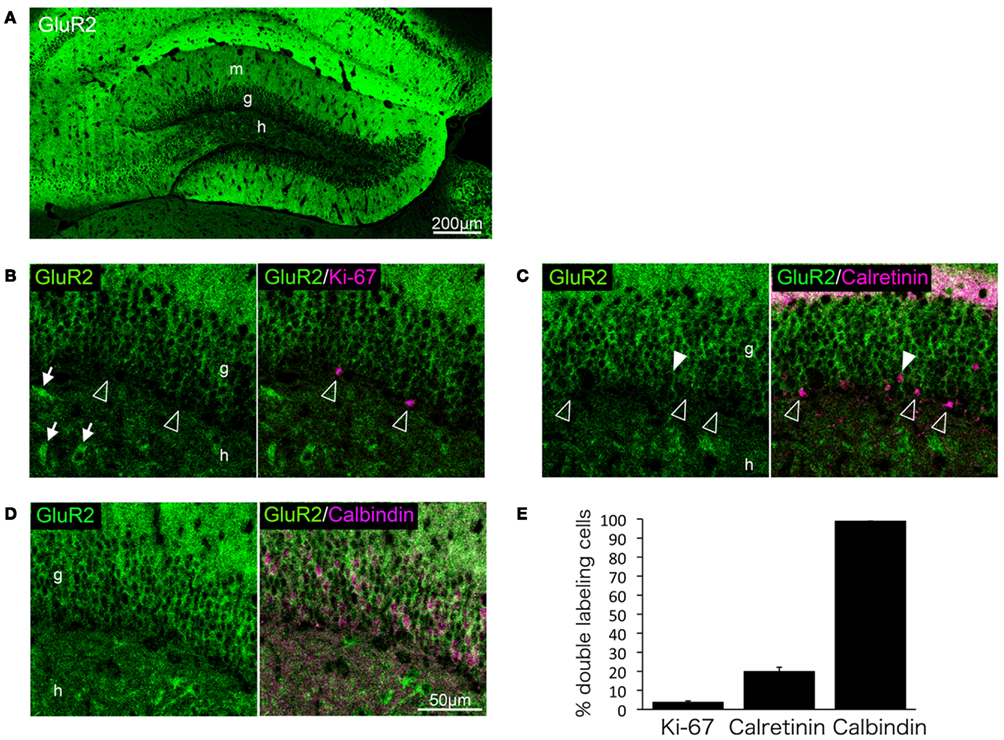
Figure 4. GluR2 expression in mature granule cells. GluR2 is expressed throughout the adult hippocampus (A). Some hilar interneurons also express GluR2 [arrows in (B)]. Ki-67 (B), calretinin (C), and calbindin (D) were co-labeled with GluR2. Open arrowheads indicate GluR2-negative cells. Filled arrowheads indicate GluR2-positive cells. (E) The co-labeled cells were quantified. Values are given as the mean ± SEM of the analysis based on the results of three mice. g, Granule cell layer; h, hilus; m, molecular layer.
Reduced GLuR1 and GLuR2 Expression in the Dentate Granule Cells of αCaMKII± Mice
The observations described above indicate that GluR1 is expressed mainly in mature granule cells. We next examined GluR1 expression in αCaMKII± mice, in which most of dentate gyrus granule cells do not express calbindin, and electrophysiologic and morphologic features are strikingly similar to those of normal immature dentate gyrus granule cells (Yamasaki et al., 2008). GluR1 expression was significantly reduced in the dentate gyrus of αCaMKII± mice compared to those of wild-type littermate mice (Figures 5A–C). GluR1 expression in a subfield of the hippocampus of αCaMKII± mice was quantified based on immunohistochemical staining (Figures 5A,B). GluR1 immunoreactivity in the granule cell layer and molecular layer of αCaMKII± mice was drastically reduced to 30% that of wild-type mice (Figure 5B, n = 4 mice for each genotype; ML: p = 2.64 × 10−7; GCL: p = 1.61 × 10−6; hilus: p = 7.52 × 10−3, Student’s t-test). Interestingly, in the CA1 region of the mutant mice, GluR1 immunoreactivity was significantly increased in the pyramidal cell layer (Py: p = 6.40 × 10−3), but not in the radiatum layer or the oriens layer (Rad: p = 0.081; Or: p = 0.257; Figure 5B). Very low GluR1 expression levels were detected in the mutant granule cells (Figure 5C). GluR1-positive interneurons located in the dentate gyrus were clearly visualized due to reduced GluR1 expression in the mutant granule cells (Figure 5C). In the mutants, the expression of a neuronal-specific protein, NeuN, was also decreased. NeuN is usually used as a marker of mature neurons, thus the decline in NeuN expression also indicates disturbed maturation of the mutant granule cells (Figure 5D). In addition, GluR1 expression in the cerebellum of adult αCaMKII± mice was examined. The expression of AMPA receptor subunits in the cerebellum is developmentally regulated. GluR1 are primarily expressed in the molecular layer of adult cerebellum as a result of developmental changes in the expression that shifted from Purkinje cells to Bergmann glia cells (Ripellino et al., 1998; Douyard et al., 2007). In the cerebellum of adult αCaMKII± mice, GluR1 immunoreactivity was observed in the radial processes of Bergmann glial cells in the molecular layer, and immunoreactivity was hardly affected in the mutant mice (Figure 5E).
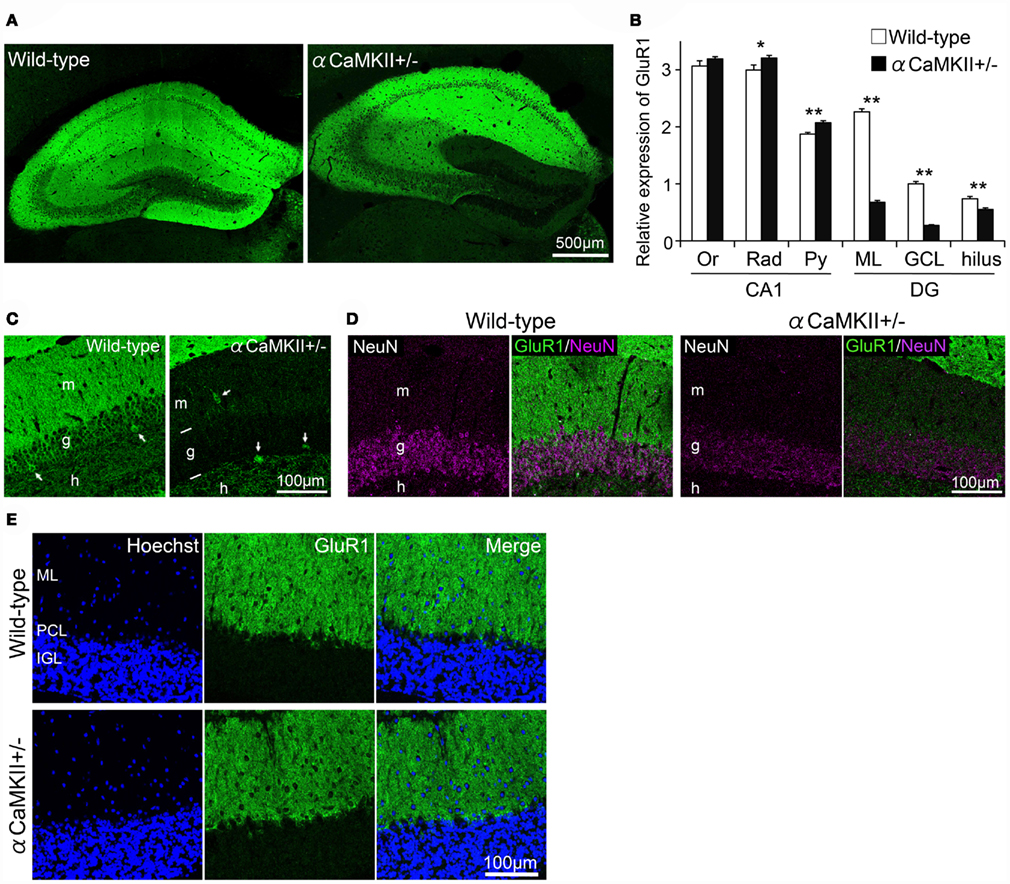
Figure 5. Reduced expression of GluR1 in the dentate gyrus of αCaMKII± mice. (A,B) Immunohistochemical analysis revealed alterations in GluR1 expression in the hippocampus of the mutant mice. GluR1 immunoreactivity was quantified based on histochemical staining in a hippocampal subfield of αCaMKII± mice (B). Values represent fluorescence intensity normalized to that in the granule cell layer of wild-type mice and are given as the mean ± SEM of the analysis based on the results of four mice. Asterisks indicate statistically significant differences: *p < 0.05; **p < 0.01 (Student’s t-test). (C) GluR1 was expressed in some hilar interneurons of the mutants. As a result of reduced GluR1 expression in the granule cells in the mutants, GluR1-positive interneurons located in the dentate gyrus were clearly visualized (arrows). (D) Expression level of neuron-specific protein NeuN was diminished in the granule cells of the mutants. (E) Parasagittal sections of adult αCaMKII± mice cerebellum were stained with GluR1 antibody. Immunoreactivity for GluR1 in Bergmann glial cells was hardly affected in the mutant mice. DG, dentate gyrus; GCL, granule cell layer; IGL, internal granule cell layer; ML, molecular layer; Or, oriens layer; PCL, Purkinje cell layer; Py, pyramidal cell layer; Rad, radiatum layer.
GluR2 expression in hippocampal subfields of the αCaMKII± mice was quantified based on immunohistochemical staining (Figures 6A,B). GluR2 immunoreactivity in the granule cell and molecular layers in αCaMKII± mice was significantly reduced to 50% that of wild-type mice (Figure 4D, n = 4 mice for each genotype; ML: p = 1.03 × 10−3; GCL: p = 1.50 × 10−3; hilus: p = 0.22, Student’s t-test). In the CA1 region of the mutant mice, GluR2 immunoreactivity was not significantly changed in the pyramidal cell layer (Py: p = 0.47), in the radiatum layer (Rad: p = 0.66), or in the oriens layer (Or: p = 0.47; Figure 6B). Some hilar interneurons also expressed GluR2 (arrowheads in Figure 6C).
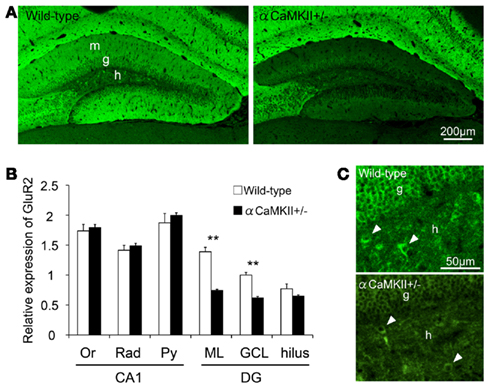
Figure 6. Reduced GluR2 expression in the dentate gyrus of αCaMKII± mice. (A,B) Immunohistochemical analysis revealed reduced GluR2 expression in the dentate gyrus of the mutant mice. GluR2 immunoreactivity was quantified based on histochemical staining in the hippocampal subfields of αCaMKII± mice (B) Values represent fluorescence intensity normalized to that in the granule cell layer of wild-type mice and are given as the mean ± SEM of the analysis based on the results of four mice. Asterisks indicate statistically significant differences: **p < 0.01 (Student’s t-test). (C) GluR2 is expressed in some hilar interneurons of the mutants. Arrowheads indicate examples of GluR2-labeled hilar interneurons. DG, dentate gyrus; GCL, granule cell layer; IGL, internal granule cell layer; ML, molecular layer; Or, oriens layer; PCL, Purkinje cell layer; Py, pyramidal cell layer; Rad, radiatum layer.
Concomitant Decrease in GluR Expression and Synaptic Markers in the Granule Cells of αCaMKII± Mice
In the αCaMKII± mouse dentate gyrus, in which the expression of GluR1 and GluR2 is downregulated, synaptic marker expression was examined to gain insight into the relation between GluR expression and synaptic maturation. Immunoblot analysis revealed decreased GluR1 expression in the mutant dentate gyrus (Figures 7A,B, n = 3 mice for each genotype; p = 0.029) with a concomitant decrease in the expression of the postsynaptic marker PSD95 (Figures 7A,C, n = 3 mice for each genotype; p = 0.026, Student’s t-test). The decrease was observed in both the molecular and granule cell layers of the mutants (Figure 7D). The presynaptic protein synaptophysin was also decreased in the presynaptic terminals of mossy fibers within the stratum lucidum of CA3 (Figures 7E,F, n = 3 mice for each genotype; p = 0.0023). These findings suggest that synaptic maturation of the mutant granule cells is incomplete and that GluR expression correlates with synaptic maturation.
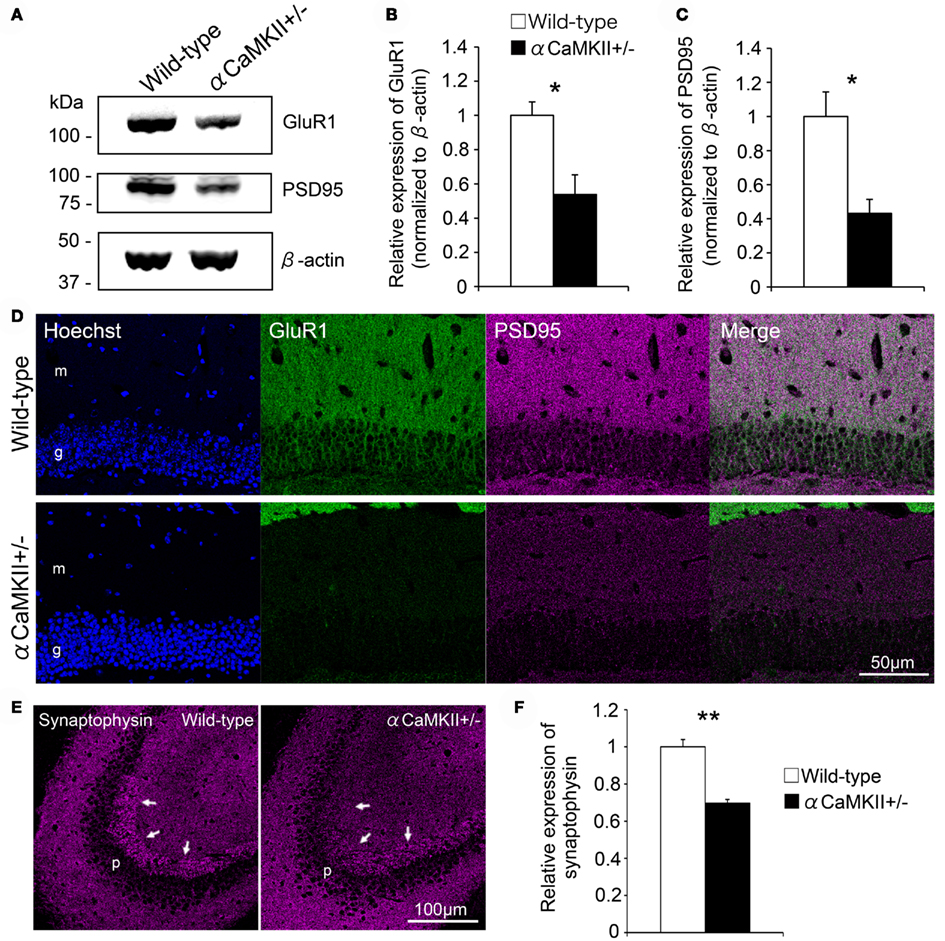
Figure 7. Reduced expression of pre- and post-synaptic markers in the granule cells of αCaMKII± mice. (A–C) Immunoblotting analysis revealed that GluR1 expression was greatly reduced in the dentate gyrus of αCaMKII± mice compared to that of their wild-type littermates. Dissected dentate gyri of the mice were processed for immunoblotting analysis, and representative images are shown (A). GluR1 (B) and PSD95 (C) expression levels were quantified. Values represent band intensity as a ratio of αCaMKII± mice to wild-type mice and are given as the mean ± SEM of the analysis based on the results of three mice. To correct for differences in loading, individual values were normalized to β-actin measured in the same blot. Asterisks indicate statistically significant differences: *p < 0.05 (Student’s t-test). (D) Immunostaining revealed that the expression of a postsynaptic marker, PSD95, was downregulated in both the granule cell and molecular layers of the mutants. (E,F) In the mutants, expression of a presynaptic marker, synaptophysin, was also decreased in mossy fiber terminals within the stratum lucidum of CA3 [arrows in (E)]. Synaptophysin immunoreactivity in CA3 was quantified (F). Values represent fluorescent intensity as a ratio of αCaMKII± mice to wild-type mice and are given as the mean ± SEM of the analysis based on the results of three mice. Asterisks indicate statistically significant differences: **p < 0.01 (Student’s t-test). g, Granule cell layer; h, hilus; m, molecular layer; p, pyramidal cell layer.
GluR1 and GluR2 Expression Patterns in the Neonatal Dentate Gyrus
We investigated GluR1 expression in the dentate gyrus of 5-day-old mice. GluR1 was expressed throughout the hippocampus in the neonatal mice, and the highest GluR1 immunoreactivity was detected in lacunosum moleculare (Figure 8A). In the CA regions, GluR1 was expressed abundantly in the pyramidal cell layer, the oriens layer, and the radiatum layer. In the dentate gyrus, GluR1 was expressed only in the small population of granule cells that are located in the outer part of the granule cell layer, which reduced the GluR1 immunoreactivity in the dentate molecular layer. In the neonatal hippocampus, Ki-67 immunoreactivity was observed in sparsely distributed cells, unlike in adult hippocampus in which it is concentrated in the subgranular zone (Schwab et al., 2000; Kronenberg et al., 2003). These Ki-67-positive cells rarely expressed GluR1 (Figure 8C). The granule cell layer in the neonatal dentate gyrus contained a large number of calretinin-positive immature granule cells that occupied the inner two-thirds of the layer (Figure 8D). These calretinin-positive cells did not express GluR1. On the other hand, calbindin-positive mature granule cells that existed in the outer part of granule cell layer expressed GluR1 (Figure 8E).
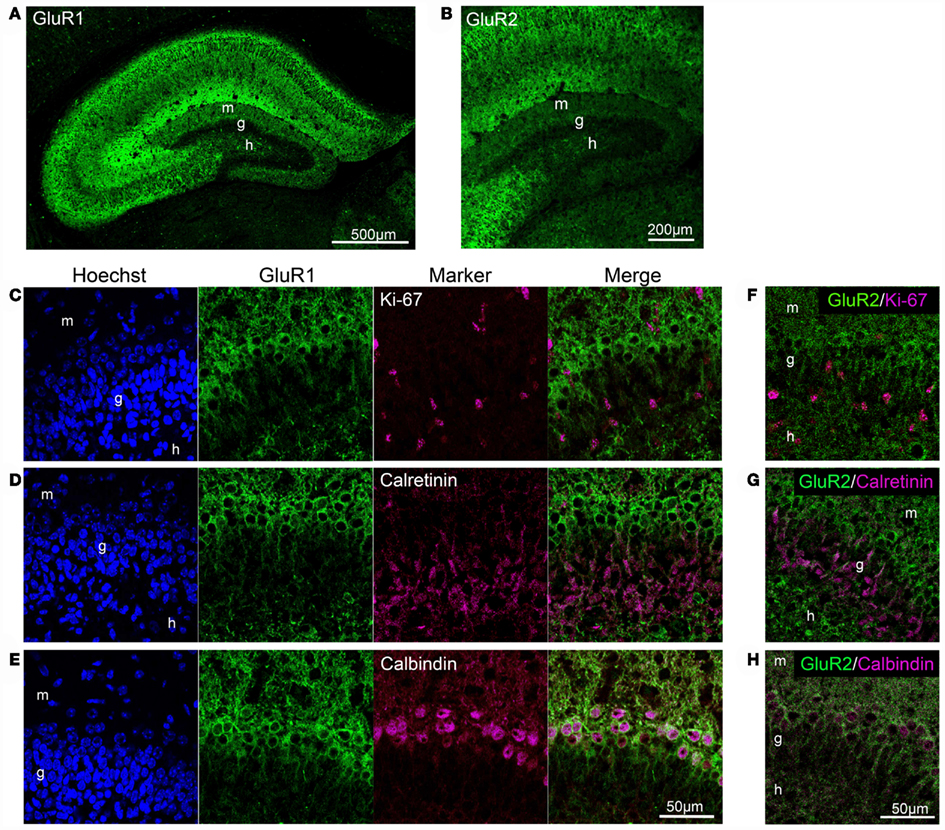
Figure 8. GluR1 and GluR2 expression in the neonatal dentate gyrus. (A,B) GluR1 (A) and GluR2 (B) immunostaining in the neonatal hippocampus. (C–E) Co-labeling of GluR1 with Ki-67, neural progenitor cells (C), calretinin, immature granule cells (D), or calbindin, mature granule cells (E) in the dentate gyrus. (F–H) Co-labeling of GluR2 with Ki-67 (F), calretinin (G), and calbindin (H). g, Granule cell layer; h, hilus; m, molecular layer.
It is likely that the GluR2 expression pattern in mature granule cells in the neonatal dentate gyrus, but not immature cells, is similar to that of GluR1 (Figures 8B,F–H). Neither Ki-67-positive (Figure 8F) nor calretinin-positive cells (Figure 8G) expressed GluR2, whereas calbindin-positive cells expressed GluR2 (Figure 8H). These findings indicate that GluR2 is expressed in mature granule cells in the dentate gyrus of neonatal and adult mice.
Discussion
The findings of the present study indicate that GluR1 and GluR2 are expressed in the mature granule cells of the mouse dentate gyrus. Immunofluorescence staining for GluR1 combined with BrdU labeling revealed that expression of GluR1 began at the late-phase of granule cell development, around 3 weeks after their generation. Most of the GluR1- or GluR2-positive cells co-expressed the mature granule cell marker calbindin. Furthermore, GluR1 and GluR2 immunoreactivities were drastically decreased in the dentate gyrus of αCaMKII± mice, in which almost all the granule cells remain in the immature state (Yamasaki et al., 2008). Together these results indicate that GluR1 and GluR2 are expressed mainly in mature granule cells during their development and suggest that GluR1 and GluR2 are useful markers for mature granule cells.
Usefulness of GLuR1 and GLuR2 as Maturation Markers of Dentate Granule Cells
Calbindin is generally used as a mature granule cell marker, and is widely and strongly expressed in the hippocampal formation, including the dentate gyrus, CA regions, and interneurons (Kempermann et al., 1997; Duan et al., 2008; Zhao et al., 2008). In the present study, GluR1 and GluR2 expression was observed in more than 98% of calbindin-positive mature granule cells. In addition, GluR1 and GluR2 were rarely expressed in Ki-67-positive progenitor cells and calretinin-positive immature granule cells. These findings indicate that GluR1 and GluR2 can be used as markers for mature granule cells. To confirm the usefulness of GluR1 and GluR2 as dentate granule cell maturation markers, we investigated their expression patterns in αCaMKII± mice. We previously reported that almost all of the neurons in the dentate gyrus of αCaMKII± mice fail to mature at molecular, morphological, and electrophysiological levels (Yamasaki et al., 2008). At the molecular level, both DNA array and immunohistochemical analyses revealed that the expression of calbindin and tryptophan 2,3-dioxygenase was drastically reduced in the dentate gyrus of these mutant mice (Yamasaki et al., 2008; Hagihara et al., 2009; Ohira et al., 2010). Electrophysiological studies indicated that dentate granule cell maturity in the mutant mice is characterized by an increased input resistance, high excitability, and a reduced spike latency. In particular, increased input resistance was inversely correlated with granule cell maturation, and is a proposed index of maturity (Ye et al., 2005). In the present study, GluR1 and GluR2 expression was strikingly attenuated in the granule cells of these mutant mice, and their expression patterns in the hippocampus of adult αCaMKII± mice were similar to those in naive neonatal mice, in which GluR1 and GluR2 expression was low in the dentate gyrus (Figures 4C and 5A). These findings suggest that GluR1 and GluR2 expression is closely correlated to the neuronal maturation state and that GluR1 and GluR2 are useful markers for mature granule cells.
We further investigated whether GluR1 was expressed in non-neuronal cells. Increasing evidence suggests that glutamate receptors are expressed not only in neurons, but also in glial cells. Although GluR2/3 is expressed in astrocytes in the juvenile mouse hippocampus (Seifert et al., 1997) and in the mouse spinal cord (Brand-Schieber et al., 2004), weak GluR1 labeling in hypertrophic astrocytes appears after transient ischemia (Gottlieb and Matute, 1997). In the present study, no GFAP-positive astrocytes showed obvious GluR1 expression in the intact adult hippocampus. The lack of GluR1-positive microglial cells observed in this study is consistent with a previous report that microglial cells express various types of glutamate receptors, but not GluR1 (Noda et al., 2000). Taken together, these findings suggest that the selective expression of GluR1 in the dentate granule cells reflects the maturity of the cells.
GluR expression might be correlated with synaptic integration. Granule cells begin to profoundly express GluR1 at around 3 weeks after cell division. Many lines of evidence indicate that synaptic integration of adult-born neurons occurs at the same period of time. Electrophysiological and morphological studies reveal that new neurons receive their first glutamatergic input on dendritic spines at the end of the second week after birth (Espósito et al., 2005; Ge et al., 2006; Zhao et al., 2006). The new neurons project their first glutamatergic output onto area CA3 during the same period of time (Faulkner et al., 2008; Toni et al., 2008). Between 3 and 6 weeks of age, adult-born neurons show increased synaptic plasticity (Schmidt-Hieber et al., 2004; Ge et al., 2007). Therefore, GluR1 expression might also correlate with synaptic integration during granule cell maturation.
Possible Functional Role of GLuR Expressed in Mature Dentate Granule Cells
The subunit composition of AMPA receptor channels determines their permeability properties. GluR1 and GluR3 form Ca2+-permeable channels, whereas GluR2, expressed alone or in combination with GluR1 or GluR3, forms channels that are Ca2+-impermeable (Hollmann et al., 1991; Hume et al., 1991; Burnashev et al., 1992). Electrophysiological studies using ligand application or perforant path stimulation of hippocampal slices demonstrate an association between AMPA receptor properties and dentate granule cell maturation. In addition, peak AMPA current amplitudes in granule cells change with cell age (Liu et al., 1996; Ye et al., 2000, 2005). Granule cells at the earliest stage of their generation have exclusively N-methyl-D-aspartate (NMDA) currents evoked by medial perforant path stimulation and progressively acquire AMPA currents with maturation. Peak NMDA and AMPA currents both increase as the cells mature, whereas the ratio of NMDA to AMPA currents decreases (Liu et al., 1996; Ye et al., 2000, 2005). Thus, dentate granule cells show a maturational shift from NMDA-dominated to AMPA-dominated glutamatergic transmission. Taken together, these findings indicate that AMPA receptors mediating Ca2+ permeability and peak currents are tightly correlated with granule cell maturation. Based on these facts, the attenuated expression of GluR1 in the dentate granule cells of αCaMKII± mice (Figures 4A–D) raises the possibility that the impaired Ca2+-permeability in dentate granule cells underlies the deficit in hippocampus-dependent spatial working memory.
GLuR in Neuropathologic Conditions
Accumulating evidence indicates that dysfunctional glutamatergic neurotransmission is associated with the pathophysiology of various neuropathologic conditions, such as ischemia, epilepsy, and psychiatric disorders.
Transient global brain ischemia results in a remarkable decrease in GluR1, concomitant with a remarkable decrease in GluR2 in the hippocampus and each hippocampal subregion (Dos-Anjos et al., 2009; Montori et al., 2010). Electrophysiological studies of the postischemic brain indicate that GluR1/GluR2 ratios affect AMPA excitatory postsynaptic currents and suggest that the control of long-term changes in Ca2+-permeability might be a potential therapeutic target (Noh et al., 2005).
Alterations in GluR expression in the hippocampus have been reported in the human epileptic brain (de Lanerolle et al., 1998; Ying et al., 1998) and in animal epilepsy models (Tang et al., 2005; Solomonia et al., 2010). In many cases, increased GluR1 expression is observed in the hippocampus of patients. Extensive evidence suggests that the hippocampus is a key structure involved in the initiation and propagation of temporal lobe seizures (Sperling et al., 1992). In particular, the dentate gyrus is believed to act as a tightly regulated filter limiting inputs into the hippocampus, because dentate granule cells are strongly inhibited by multiple inputs from various interneurons (Heinemann et al., 1992; Lothman et al., 1992). Breakdown of the inhibition is hypothesized to induce the initiation and/or propagation of seizures (Heinemann et al., 1992; Lothman et al., 1992). In this process, alterations in GluR1 expression may contribute to enhance the glutamatergic response of these neurons, which is suggested to contribute to the process of epileptogenesis.
Knockout mice lacking the GluR1 subunit exhibit behavioral abnormalities, such as impaired working memory, locomotor hyperactivity in the open field, and deficits in prepulse inhibition, suggesting an association between GluR1 and certain symptoms of schizophrenia (Schmitt et al., 2005; Wiedholz et al., 2008; Sanderson et al., 2009; Erickson et al., 2010). We previously reported that αCaMKII± mice exhibit behavioral abnormalities such as severe working memory deficits and an exaggerated infradian rhythm, similar to symptoms observed in patients with schizophrenia, bipolar mood disorder, and other psychiatric disorders (Yamasaki et al., 2008; Matsuo et al., 2009). GluR1 and GluR2 expression was drastically downregulated in the dentate granule cell in mutant mice. Thus, impaired GluR function in these cells may be associated with psychiatric disorder-associated behaviors. Despite a working memory deficit in GluR1−/− mice and αCaMKII± mice, acquisition of reference memory tasks appears to be normal (Yamasaki et al., 2008; Sanderson et al., 2009). These similarities in behavioral phenotypes suggest that GluR1 expression in the hippocampus, especially in the dentate gyrus, is critical for the acquisition of short-term spatial memory.
GluR1 is also related to stress-related psychiatric disorders such as major depression. Schmidt et al. (2010) reported that genetic variations in the GluR1 subunit are linked to stress vulnerability and that vulnerability could be predicted by both short-term spatial memory, an AMPA receptor-dependent behavior, and the AMPA receptor subunit ratio in the hippocampus. These findings suggest that the expression and function of GluR1 expressed in hippocampus and/or dentate gyrus are linked with psychiatric disorders. Thus, GluR1 in the dentate gyrus may serve as a promising molecular biomarker for these disorders.
Materials and Methods
Animals
All animal treatments, procedures, and care were approved by the Institutional Animal Care and Use Committee of Fujita Health University (#I0723), based on the Law for the Humane Treatment and Management of Animals (2005) and Standards Relating to the Care and Management of Laboratory Animals and Relief of Pain (2006). Every effort was made to minimize the number of animals used. Neonatal (5-day-old) and adult (8- to 14-week-old) male C57BL/6J mice (Charles River Laboratories International Japan Inc., Shiga, Japan) were used in this study. We used αCaMKII± mice obtained from Jackson Laboratories (Bar Harbor, ME, USA), which were also used in our previous study (Yamasaki et al., 2008). Heterozygous mice were crossed with C57BL/6 mice for at least 16 generations. We used heterozygous αCaMKII-knockout mice, because it is difficult to obtain homozygotes due to mating problems between heterozygous male and heterozygous female mice. The animals were maintained under a normal light–dark cycle (12 h light/12 h dark) and had free access to water and food.
Western Blot Analysis
Adult (12- to 14-week-old) αCaMKII± mice and their wild-type littermates were deeply anesthetized and their brains were removed and immersed in ice-cold phosphate buffered saline (PBS). The dentate gyrus was dissected out (Hagihara et al., 2009) and stored at −80°C until use. The isolated tissue was homogenized in lysis buffer (Sigma-Aldrich, St. Louis, MO, USA) containing a proteinase inhibitor cocktail (Roche Applied Science, Indianapolis, IN, USA). Protein homogenates were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and transferred to polyvinylidene difluoride membranes. Membranes were preincubated in 5% skim milk in PBS containing 0.05% Tween-20 (PBST) for 1 h at room temperature, and incubated overnight at 4°C in primary antibodies, rabbit anti-GluR1 antibody (AB1504; Millipore, Temecula, CA, USA), mouse monoclonal anti-PSD95 antibody (MA1-046; Affinity BioReagents, Golden, CO, USA), and mouse anti-β-actin antibody (A5316; Sigma-Aldrich), diluted in PBST. They were then incubated with a secondary antibodies conjugated with Alexa488 or Alexa647 (Molecular Probes, Eugene, OR, USA) diluted with PBST at room temperature for 1 h. Immunoreactivity was visualized by scanning the membranes with a Typhoon 9400 fluorescence scanner (GE Healthcare, Buckinghamshire, UK).
BrdU Labeling
Eight-week-old C57BL/6J mice were injected intraperitoneally with BrdU (Sigma-Aldrich; 50 mg/kg body weight) every 24 h over 5 days to label newborn neurons. At 1, 2, 3, and 4 weeks after the last BrdU injection, animals were deeply anesthetized and transcardially perfused with 4% paraformaldehyde in PBS.
Immunohistochemistry
Adult (10- to 14-week-old) animals were deeply anesthetized and transcardially perfused with 4% paraformaldehyde in PBS. The brains were dissected, immersed overnight in the same fixative, and transferred to 30% sucrose in PBS for at least 3 days for cryoprotection. Neonatal (5-day-old) mice were deeply anesthetized and the brains were dissected followed by immersion in the fixative and cryoprotection. Brains were mounted in Tissue-Tek (Miles, Elkhart, IN, USA), frozen, and cut into 8-μm thick coronal sections using a microtome (CM1850; Leica Microsystems, Wetzlar, Germany).
In this study, we used the following antibodies as primary antibodies: mouse monoclonal antibody for calbindin (300; Swant, Bellinzona, Switzerland), calretinin (6B3; Swant), GAD67 (MAB5406; Millipore), GFAP (G3893; Sigma-Aldrich), NeuN (MAB377; Millipore), PSD95 (MA1-046; Affinity BioReagents), and synaptophysin (S5768; Sigma-Aldrich); rabbit polyclonal antibody for GluR1 (AB1504; Millipore) and GluR2 (AB1768; Millipore); rat monoclonal antibody for BrdU (OBT0030; AbD Serotec, Oxford, UK) and F4/80 (T2008; BMA Biomedicals, Augst, Switzerland), Ki-67 (M7249; DAKO, Carpinteria, CA, USA). The rabbit polyclonal anti-GluR1 antibody used in this study was raised against a 13-amino acid peptide (SHSSGMPLGATGL), corresponding to amino acids 894-906 of rat GluR1 protein. This anti-GluR1 antibody recognized a single band corresponding to the molecular weight ∼110 kDa in Western blot analysis of mouse dentate gyrus (Figure 7A and manufacturer’s technical information). Moreover, this antibody has been characterized by light and electron microscopic techniques (Das et al., 2008; Nedelescu et al., 2010), and staining with this antibody is completely absent in GluR1 knockout mice (Zamanillo et al., 1999). The anti-GluR2 antibody used in this study was raised against a 16-amino acid peptide (VAKNPQNINPSSSQNS), corresponding to amino acids 827–842 of rat GluR2 protein. Based on both Western blot and immunocytochemical analysis (Petralia et al., 1997; Kienzler et al., 2009), this anti-GluR2 antibody did not recognize any other GluR subunits.
For immunostaining of GluR2, sections were incubated at 80°C for 30 min in 10 mM citrate buffer, pH 9.0, to retrieve and enhance their antigenicities and staining intensities. When double-staining with BrdU was performed, the sections were further incubated at 4°C for 10 min in 0.1 N HCl and then at 37°C for 30 min in 2 N HCl. After washing in PBS, the sections were preincubated for 30 min at room temperature in 5% skim milk in PBST, and then incubated overnight at 4°C in PBS containing the primary antibodies. Immunoreactivity to the antigen was visualized using Alexa594- or Alexa488-conjugated secondary antibodies (Molecular Probes). Nuclear staining was performed with Hoechst 33258 (Polyscience, Warrington, PA, USA). We used a confocal microscope (LSM 510 META; Zeiss, Göttingen, Germany) to obtain images of the stained sections.
Quantification
Analysis of immunostaining images was performed using ZEN software (Zeiss). A minimum of 200 BrdU-, calbindin-, calretinin-, or Ki-67-positive cells was examined for co-labeling with GluR in each animal and at each time point. In the GluR co-labeling assay, we identified cells containing an apparent nucleus-surrounding structure as GluR-positive cells after subtracting the background fluorescence. Background fluorescence was defined as the signal intensity of sections incubated without primary antibodies. Data are presented as the mean percentage of BrdU-positive cells co-labeled with GluR (mean ± SEM). The quantification of double-positive cells was analyzed with a one-way ANOVA. If a significant main effect was detected by ANOVA, a Tukey’s post hoc test was used to determine the source of the detected significance in the ANOVA. The mean fluorescence intensity of the GluR immunostaining in the region of interest was measured using ZEN software (Zeiss). At least six sections from each animal were processed for quantification. GluR1 expression level assessed by Western blotting was quantified by measuring the fluorescence intensity taken from each band using ImageQuant TL software (GE Healthcare). Statistical analysis was performed by Student’s t-test.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
Abrous, D. N., Koehl, M., and Le Moal, M. (2005). Adult neurogenesis: from precursors to network and physiology. Physiol. Rev. 85, 523–569.
Arai, Y., Mizuguchi, M., and Takashima, S. (1997). Developmental changes of glutamate receptors in the rat cerebral cortex and hippocampus. Anat. Embryol. 195, 65–70.
Austyn, J. M., and Gordon, S. (1981). F4/80, a monoclonal antibody directed specifically against the mouse macrophage. Eur. J. Immunol. 11, 805–815.
Brand-Schieber, E., Lowery, S. L., and Werner, P. (2004). Select ionotropic glutamate AMPA/kainate receptors are expressed at the astrocyte-vessel interface. Brain Res. 1007, 178–182.
Brandt, M. D., Jessberger, S., Steiner, B., Kronenberg, G., Reuter, K., Bick-Sander, A., von der Behrens, W., and Kempermann, G. (2003). Transient calretinin expression defines early postmitotic step of neuronal differentiation in adult hippocampal neurogenesis of mice. Mol. Cell. Neurosci. 24, 603–613.
Burnashev, N., Monyer, H., Seeburg, P. H., and Sakmann, B. (1992). Divalent ion permeability of AMPA receptor channels is dominated by the edited form of a single subunit. Neuron 8, 189–198.
Das, P., Scott, M. L., Zerda, R., Gunning, W. T., Alvarez, F. J., and Tietz, E. I. (2008). Increased AMPA receptor GluR1 subunit incorporation in rat hippocampal CA1 synapses during benzodiazepine withdrawal. J. Comp. Neurol. 511, 832–846.
de Lanerolle, N. C., Eid, T., von Campe, G., Kovacs, I., Spencer, D. D., and Brines, M. (1998). Glutamate receptor subunits GluR1 and GluR2/3 distribution shows reorganization in the human epileptogenic hippocampus. Eur. J. Neurosci. 10, 1687–1703.
Dos-Anjos, S., Martínez-Villayandre, B., Montori, S., Regueiro-Purriños, M. M., Gonzalo-Orden, J. M., and Fernández-López, A. (2009). Global ischemia-induced modifications in the expression of AMPA receptors and inflammation in rat brain. Brain Res. 1287, 20–27.
Douyard, J., Shen, L., Huganir, R. L., and Maria, E. R. (2007). Differential neuronal and glial expression of GluR1 AMPA receptor subunit and the scaffolding proteins SAP97 and 4.1N during rat cerebellar development. J. Comp. Neurol. 502, 141–156.
Du, J., Creson, T. K., Wu, L., Ren, M., Gray, N. A., Falke, C., Wei, Y., Wang, Y., Blumenthal, R., Machado-Vieira, R., Yuan, P., Chen, G., Zhuo, M., and Manji, H. K. (2008). The role of hippocampal GluR1 and GluR2 receptors in manic-like behavior. J. Neurosci. 28, 68–79.
Du, J., Gray, N. A., Falke, C. A., Chen, W., Yuan, P., Szabo, S. T., Einat, H., and Manji, H. K. (2004). Modulation of synaptic plasticity by antimanic agents: the role of AMPA glutamate receptor subunit 1 synaptic expression. J. Neurosci. 24, 6578–6589.
Duan, X., Kang, E., Liu, C. Y., Ming, G., and Song, H. (2008). Development of neural stem cell in the adult brain. Curr. Opin. Neurobiol. 18, 108–115.
Erickson, M. A., Maramara, L. A., and Lisman, J. (2010). A single brief burst induces GluR1-dependent associative short-term potentiation: a potential mechanism for short-term memory. J. Cogn. Neurosci. 22, 2530–2540.
Espósito, M. S., Piatti, V. C., Laplagne, D. A., Morgenstern, N. A., Ferrari, C. C., Pitossi, F. J., and Schinder, A. F. (2005). Neuronal differentiation in the adult hippocampus recapitulates embryonic development. J. Neurosci. 25, 10074–10086.
Faulkner, R. L., Jang, M. H., Liu, X. B., Duan, X., Sailor, K. A., Kim, J. Y., Ge, S., Jones, E. G., Ming, G. L., Song, H., and Cheng, H. J. (2008). Development of hippocampal mossy fiber synaptic outputs by new neurons in the adult brain. Proc. Natl. Acad. Sci. U.S.A. 105, 14157–14162.
Ge, S., Goh, E. L. K., Sailor, K. A., Kitabatake, Y., Ming, G., and Song, H. (2006). GABA regulates synaptic integration of newly generated neurons in the adult brain. Nature 439, 589–593.
Ge, S., Yang, C. H., Hsu, K. S., Ming, G. L., and Song, H. (2007). A critical period for enhanced synaptic plasticity in newly generated neurons of the adult brain. Neuron 54, 559–566.
Gottlieb, M., and Matute, C. (1997). Expression of ionotropic glutamate receptor subunits in glial cells of the hippocampal CA1 area following transient forebrain ischemia. J. Cereb. Blood Flow Metab. 17, 290–300.
Hagihara, H., Toyama, K., Yamasaki, N., and Miyakawa, T. (2009). Dissection of hippocampal dentate gyrus from adult mouse. J. Vis. Exp. 33, pii: 1543.
Hampson, D. R., Huang, X. P., Oberdorfer, M. D., Goh, J. W., Auyeung, A., and Wenthold, R. J. (1992). Localization of AMPA receptors in the hippocampus and cerebellum of the rat using an anti-receptor monoclonal antibody. Neuroscience 50, 11–22.
Heinemann, U., Beck, H., Dreier, J. P., Ficker, E., Stabel, J., and Zhang, C. L. (1992). The dentate gyrus as a regulated gate for the propagation of epileptiform activity. Epilepsy Res. Suppl. 7, 273–280.
Hollmann, M., Hartley, M., and Heinemann, S. (1991). Ca2+ permeability of KA-AMPA – gated glutamate receptor channels depends on subunit composition. Science 252, 851–853.
Hume, R. I., Dingledine, R., and Heinemann, S. F. (1991). Identification of a site in glutamate receptor subunits that controls calcium permeability. Science 253, 1028–1031.
Kempermann, G., Kuhn, H. G., and Gage, F. H. (1997). More hippocampal neurons in adult mice living in an enriched environment. Nature 386, 493–495.
Kienzler, F., Norwood, B. A., and Sloviter, R. S. (2009). Hippocampal injury, atrophy, synaptic reorganization, and epileptogenesis after perforant pathway stimulation-induced status epilepticus in the mouse. J. Comp. Neurol. 515, 181–196.
Kronenberg, G., Reuter, K., Steiner, B., Brandt, M. D., Jessberger, S., Yamaguchi, M., and Kempermann, G. (2003). Subpopulations of proliferating cells of the adult hippocampus respond differently to physiologic neurogenic stimuli. J. Comp. Neurol. 467, 455–463.
Liu, Y. B., Lio, P. A., Pasternak, J. F., and Trommer, B. L. (1996). Developmental changes in membrane properties and postsynaptic currents of granule cells in rat dentate gyrus. J. Neurophysiol. 76, 1074–1088.
Lothman, E. W., Stringer, J. L., and Bertram, E. H. (1992). The dentate gyrus as a control point for seizures in the hippocampus and beyond. Epilepsy Res. Suppl. 7, 301–313.
Matsuo, N., Yamasaki, N., Ohira, K., Takao, K., Toyama, K., Eguchi, M., Yamaguchi, S., and Miyakawa, T. (2009). Neural activity changes underlying the working memory deficit in alpha-CaMKII heterozygous knockout mice. Front. Behav. Neurosci. 3:20. doi: 10.3389/neuro.08.020.2009
Medvedev, N. I., Rodríguez-Arellano, J. J., Popov, V. I., Davies, H. A., Tigaret, C. M., Schoepfer, R., and Stewart, M. G. (2008). The glutamate receptor 2 subunit controls post-synaptic density complexity and spine shape in the dentate gyrus. Eur. J. Neurosci. 27, 315–325.
Montori, S., Dos Anjos, A., Ríos-Granja, M. A., Pérez-García, C. C., Fernández-López, A., and Martínez-Villayandre, B. (2010). AMPA receptor downregulation induced by ischaemia/reperfusion is attenuated by age and blocked by meloxicam. Neuropathol. Appl. Neurobiol. 36, 436–447.
Nedelescu, H., Kelso, C. M., Lázaro-Muñoz, G., Purpura, M., Cain, C. K., Ledoux, J. E., and Aoki, C. (2010). Endogenous GluR1-containing AMPA receptors translocate to asymmetric synapses in the lateral amygdala during the early phase of fear memory formation: an electron microscopic immunocytochemical study. J. Comp. Neurol. 518, 4723–4739.
Noda, M., Nakanishi, H., Nabekura, J., and Akaike, N. (2000). AMPA-kainate subtypes of glutamate receptor in rat cerebral microglia. J. Neurosci. 20, 251–258.
Noh, K., Yokota, H., Mashiko, T., Castillo, P. E., Zukin, R. S., and Bennett, M. V. L. (2005). Blockade of calcium-permeable AMPA receptors protects hippocampal neurons against global ischemia-induced death. Proc. Natl. Acad. Sci. U.S.A. 102, 12230–12235.
Ohira, K., Hagihara, H., Toyama, K., Takao, K., Kanai, M., Funakoshi, H., Nakamura, T., and Miyakawa, T. (2010). Expression of tryptophan 2,3-dioxygenase in mature granule cells of the adult mouse dentate gyrus. Mol. Brain 3, 26.
Petralia, R. S., Wang, Y. X., Mayat, E., and Wenthold, R. J. (1997). Glutamate receptor subunit 2-selective antibody shows a differential distribution of calcium-impermeable AMPA receptors among populations of neurons. J. Comp. Neurol. 385, 456–476.
Ripellino, J. A., Neve, R. L., and Howe, J. R. (1998). Expression and heteromeric interactions of non-N-methyl-D-aspartate glutamate receptor subunits in the developing and adult cerebellum. Neuroscience 82, 485–497.
Rogers, S. W., Hughes, T. E., Hollmann, M., Gasic, G. P., Deneris, E. S., and Heinemann, S. (1991). The characterization and localization of the glutamate receptor subunit GluR1 in the rat brain. J. Neurosci. 11, 2713–2724.
Sanderson, D. J., Good, M. A., Skelton, K., Sprengel, R., Seeburg, P. H., Rawlins, J. N. P., and Bannerman, D. M. (2009). Enhanced long-term and impaired short-term spatial memory in GluA1 AMPA receptor subunit knockout mice: evidence for a dual-process memory model. Learn. Mem. 16, 379–386.
Schmidt, M. V., Trümbach, D., Weber, P., Wagner, K., Scharf, S. H., Liebl, C., Datson, N., Namendorf, C., Gerlach, T., Kühne, C., Uhr, M., Deussing, J. M., Wurst, W., Binder, E. B., Holsboer, F., and Müller, M. B. (2010). Individual stress vulnerability is predicted by short-term memory and AMPA receptor subunit ratio in the hippocampus. J. Neurosci. 30, 16949–16958.
Schmidt-Hieber, C., Jonas, P., and Bischofberger, J. (2004). Enhanced synaptic plasticity in newly generated granule cells of the adult hippocampus. Nature 429, 184–187.
Schmitt, W. B., Sprengel, R., Mack, V., Draft, R. W., Seeburg, P. H., Deacon, R. M. J., Rawlins, J. N. P., and Bannerman, D. M. (2005). Restoration of spatial working memory by genetic rescue of GluR-A-deficient mice. Nat. Neurosci. 270–272.
Scholzen, T., and Gerdes, J. (2000). The Ki-67 protein: from the known and the unknown. J. Cell. Physiol. 182, 311–322.
Schwab, M. H., Bartholomae, A., Heimrich, B., Feldmeyer, D., Druffel-Augustin, S., Goebbels, S., Naya, F. J., Zhao, S., Frotscher, M., Tsai, M., and Nave, K. (2000). Neuronal basic helix-loop-helix proteins (NEX and BETA2/Neuro D) regulate terminal granule cell differentiation in the hippocampus. J. Neurosci. 20, 3714–3724.
Seifert, G., Rehn, L., Weber, M., and Steinhäuser, C. (1997). AMPA receptor subunits expressed by single astrocytes in the juvenile mouse hippocampus. Brain Res. Mol. Brain Res. 47, 286–294.
Silver, H., Feldman, P., Bilker, W., and Gur, R. C. (2003). Working memory deficit as a core neuropsychological dysfunction in schizophrenia. Am. J. Psychiatry 160, 1809–1816.
Solomonia, R., Mikautadze, E., Nozadze, M., Kuchiashvili, N., Lepsveridze, E., and Kiguradze, T. (2010). Myo-inositol treatment prevents biochemical changes triggered by kainate-induced status epilepticus. Neurosci. Lett. 468, 277–281.
Sperling, M. R., O’Connor, M. J., Saykin, A. J., Phillips, C. A., Morrell, M. J., Bridgman, P. A., French, J. A., and Gonatas, N. (1992). A noninvasive protocol for anterior temporal lobectomy. Neurology 42, 416–422.
Tang, F. R., Chia, S. C., Zhang, S., Chen, P. M., Gao, H., Liu, C. P., Khanna, S., and Lee, W. L. (2005). Glutamate receptor 1-immunopositive neurons in the gliotic CA1 area of the mouse hippocampus after pilocarpine-induced status epilepticus. Eur. J. Neurosci. 21, 2361–2374.
Toni, N., Laplagne, D. A., Zhao, C., Lombardi, G., Ribak, C. E., Gage, F. H., and Schinder, A. F. (2008). Neurons born in the adult dentate gyrus form functional synapses with target cells. Nat. Neurosci. 11, 901–907.
Wiedholz, L. M., Owens, W. A., Horton, R. E., Feyder, M., Karlsson, R.-M., Hefner, K., Sprengel, R., Celikel, T., Daws, L. C., and Holmes, A. (2008). Mice lacking the AMPA GluR1 receptor exhibit striatal hyperdopaminergia and “schizophrenia-related” behaviors. Mol. Psychiatry 13, 631–640.
Wolf, H. K., Buslei, R., Schmidt-Kastner, R., Schmidt-Kastner, P. K., Pietsch, T., Wiestler, O. D., and Blümcke, I. (1996). NeuN: a useful neuronal marker for diagnostic histopathology. J. Histochem. Cytochem. 44, 1167–1171.
Yamasaki, N., Maekawa, M., Kobayashi, K., Kajii, Y., Maeda, J., Soma, M., Takao, K., Tanda, K., Ohira, K., Toyama, K., Kanazaki, K., Fukunaga, K., Sudo, Y., Ichinose, H., Ikeda, M., Iwata, N., Ozaki, N., Suzuki, H., Higuchi, M., Suhara, T., Yuasa, S., and Miyakawa, T. (2008). Alpha-CaMKII deficiency causes immature dentate gyrus, a novel candidate endophenotype of psychiatric disorders. Mol. Brain 1, 6.
Ye, G., Yi, S., Gamkrelidze, G., Pasternak, J. F., and Trommer, B. L. (2005). AMPA and NMDA receptor-mediated currents in developing dentate gyrus granule cells. Brain Res. Dev. Brain Res. 155, 26–32.
Ye, G. L., Song Liu, X., Pasternak, J. F., and Trommer, B. L. (2000). Maturation of glutamatergic neurotransmission in dentate gyrus granule cells. Brain Res. Dev. Brain Res. 124, 33–42.
Ying, Z., Babb, T. L., Comair, Y. G., Bushey, M., and Touhalisky, K. (1998). Increased densities of AMPA GluR1 subunit proteins and presynaptic mossy fiber sprouting in the fascia dentata of human hippocampal epilepsy. Brain Res. 798, 239–246.
Zamanillo, D., Sprengel, R., Hvalby, O., Jensen, V., Burnashev, N., Rozov, A., Kaiser, K. M., Köster, H. J., Borchardt, T., Worley, P., Lübke, J., Frotscher, M., Kelly, P. H., Sommer, B., Andersen, P., Seeburg, P. H., and Sakmann, B. (1999). Importance of AMPA receptors for hippocampal synaptic plasticity but not for spatial learning. Science 284, 1805–1811.
Zhao, C., Deng, W., and Gage, F. H. (2008). Mechanisms and functional implications of adult neurogenesis. Cell 132, 645–660.
Keywords: GluR1, GluR2, dentate gyrus, granule cell, maturation, αCaMKII
Citation: Hagihara H, Ohira K, Toyama K and Miyakawa T (2011) Expression of the AMPA receptor subunits GluR1 and GluR2 is associated with granule cell maturation in the dentate gyrus. Front. Neurosci. 5:100. doi: 10.3389/fnins.2011.00100
Received: 17 May 2011;
Accepted: 15 August 2011;
Published online: 08 September 2011.
Edited by:
Joseph LoTurco, University of Connecticut, USAReviewed by:
Linda Overstreet-Wadiche, University of Alabama at Birmingham, USAJoseph LoTurco, University of Connecticut, USA
Copyright: © 2011 Hagihara, Ohira, Toyama and Miyakawa. This is an open-access article subject to a non-exclusive license between the authors and Frontiers Media SA, which permits use, distribution and reproduction in other forums, provided the original authors and source are credited and other Frontiers conditions are complied with.
*Correspondence: Tsuyoshi Miyakawa, Division of Systems Medical Science, Institute for Comprehensive Medical Science, Fujita Health University, 1-98 Dengakugakubo Kutsukake-cho, Toyoake, Aichi 470-1192, Japan. e-mail:bWl5YWthd2FAZnVqaXRhLWh1LmFjLmpw



