
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Neurosci. , 08 December 2010
Sec. Neurodegeneration
Volume 4 - 2010 | https://doi.org/10.3389/fnins.2010.00205
Drosophila models of Parkinson’s disease are characterized by two principal phenotypes: the specific loss of dopaminergic (DA) neurons in the aging brain and defects in motor behavior. However, an age-related analysis of these baseline parameters in wildtype Drosophila is lacking. Here we analyzed the DA system and motor behavior in aging Drosophila. DA neurons in the adult brain can be grouped into bilateral symmetric clusters, each comprising a stereotypical number of cells. Analysis of TH > mCD8::GFP and cell type-specific MARCM clones revealed that DA neurons show cluster-specific, stereotypical projection patterns with terminal arborization in target regions that represent distinct functional areas of the adult brain. Target areas include the mushroom bodies, involved in memory formation and motivation, and the central complex, involved in the control of motor behavior, indicating that similar to the mammalian brain, DA neurons in the fly brain are involved in the regulation of specific behaviors. Behavioral analysis revealed that Drosophila show an age-related decline in startle-induced locomotion and negative geotaxis. Motion tracking however, revealed that walking activity, and exploration behavior, but not centrophobism increase at late stages of life. Analysis of TH > Dcr2, mCD8::GFP revealed a specific effect of Dcr2 expression on walking activity but not on exploratory or centrophobic behavior, indicating that the siRNA pathway may modulate distinct DA behaviors in Drosophila. Moreover, DA neurons were maintained between early- and late life, as quantified by TH > mCD8::GFP and anti-TH labeling, indicating that adult onset, age-related degeneration of DA neurons does not occur in the aging brain of Drosophila. Taken together, our data establish baseline parameters in Drosophila for the study of Parkinson’s disease as well as other disorders affecting DA neurons and movement control.
Parkinson’s disease (PD) is the most common neurodegenerative movement disorder characterized by the progressive loss of dopaminergic (DA) neurons in the substantia nigra of the ventral midbrain, although neuropathology is not limited to this region (Braak and Del Tredici, 2008). DA cell loss is usually associated with the presence of intraneuronal inclusions known as Lewy bodies, which are composed principally of alpha-synuclein (Obeso et al., 2010). Loss of DA innervation of the putamen gives rise to the characteristic locomotor abnormalities associated with the disease, including slowness or absence of movement (bradykinesia and akinesia respectively), muscular rigidity, unstable posture, and resting tremor (Lees et al., 2009). Disease prevalence increases with age, with approximately 1% of the population being affected at 65 years, increasing to 4–5% at 85 years (Farrer, 2006). Males appear to be 1.5 times more likely to develop PD than females, although it is not currently known why this is the case (Lees et al., 2009). PD is progressive and incurable, and current treatment is symptomatic only. The majority of cases of PD appear to be sporadic, likely to be caused by a combination of genetic and environmental risk factors, the most evident being increasing age.
In addition to the majority of sporadic cases, there are also rare familial disease forms caused by gene mutations which show similar clinical and neuropathological features. Although these inherited forms of PD only account for 5–10% of all PD cases, studies of the function of the affected genes have provided insights into the mechanisms underlying PD pathogenesis. Several genetic loci have been identified that are affected or at least associated with familial forms of PD. These include alpha-synuclein (asyn), parkin, ubiquitin carboxy-terminal hydrolase L1 (UCHL1), phosphatase and tensin homolog (PTEN)-induced kinase 1, (PINK1), DJ-1, leucine-rich-repeat kinase 2 (LRRK2), High temperature requirement protein A2 (HTRA2), glucocerebrosidase (GBA), polymerase gamma and tau (for review see Farrer, 2006; Thomas and Beal, 2007; Hardy et al., 2009). Functional analyses of these genes identified three major types of cellular dysfunction implicated in familial and sporadic PD: abnormal protein aggregation, mitochondrial dysfunction and oxidative stress (Hardy et al., 2009; Schapira, 2009). These studies also revealed that DA neurons are specifically vulnerable to degeneration in PD, although the reason why this is so is currently unknown (Obeso et al., 2010).
The limitations of human genetic studies make it difficult to analyze genes and pathways in any further detail, because of complex patterns of inheritance, lack of sufficient family pedigree data and population-based genetic heterogeneity. Therefore, model systems are used to study specific functional aspects of the genes/proteins identified in neurodegenerative diseases and to gain in vivo insights into the underlying pathogenic mechanisms. Drosophila provides an excellent model system to study PD owing to its elegant and sophisticated genetics. Homologs for PD genes exist in Drosophila, with the exception of asyn (Hirth, 2010). Synthesis of the neurotransmitter dopamine is conserved between Drosophila and human and distinct clusters of DA neurons are detectable in the developing and adult fly brain (Monastirioti, 1999). Comparable to the human condition, the Drosophila DA system is also involved in locomotor control (Yellman et al., 1997; Lima and Miesenböck, 2005), although the details of the underlying neural circuit(s) are unknown. A variety of approaches have been used to model aspects of PD in Drosophila, including pharmacological insults, generation of mutant flies, and overexpression or knockdown by RNAi of familial PD gene homologs (Hirth, 2010). Significantly, these flies exhibit typical Parkinson’s disease-like phenotypes analogous to symptoms in PD patients, including locomotor defects and specific loss of DA neurons. However, for some of these models in Drosophila, contradicting results have been reported (for review see Hirth, 2010), and a systematic, age-related analysis of baseline parameters for DA neuron numbers and motor behavior is lacking.
Here we characterize the DA system and locomotor behavior in an age-related manner in Drosophila, in order to provide reliable baseline parameters for Drosophila as a model system in the study of PD. We show that bilaterally symmetric clusters of DA neurons comprise stereotypical number of cells, with cluster-specific projections onto target regions that represent distinct functional areas of the adult brain. Our data reveal that DA neurons do not degenerate with age, although Drosophila display an age-related decline in startle-induced locomotion and negative geotaxis, whereas walking speed is unaffected, and activity rather increases at late stages of life. Moreover, we provide initial evidence for a role of Dicer-2 (Dcr2), hence the siRNA pathway, in the regulation of distinct DA-dependent motor behaviors. Our findings establish baseline parameters for cluster-specific DA brain neuron numbers and locomotor behavior in aging Drosophila, which can serve for future studies into the pathogenic mechanisms underlying PD, as well as other disorders affecting DA neurons and movement control.
Flies were obtained from the Bloomington stock center unless otherwise stated. Flies stocks were maintained at 25°C on standard cornmeal media seeded with dried active yeast in a humid incubator (LMS) with a 12 h light/dark cycle except in aging studies where flies were maintained on 15% sugar/yeast (S/Y) food. Control lines used were wild type Oregon R and w1118. w; UAS-mCD8::GFP; TH1-Gal4 (TH > mCD8:: GFP) was generated using w; P{UAS-mCD8::GFP.L}LL5; + and w; +; TH1-Gal4 (Friggi-Grelin et al., 2003, a gift from S. Birman). TH > mCD8:: GFP flies were always used in the heterozygous condition, which was achieved by crossing with w1118 controls to generate w; UAS-mCD8::GFP/+; TH1-Gal4/+. For mosaic analysis with a repressible cell marker (MARCM), w, FRT19A, tubP-Gal80, hs-FLP; UAS-mCD8::GFP; TH-Gal4 was generated from P{neoFRT}19A, P{tubP-Gal80}LL1, P{hs-FLP}1, w[*]; P{UAS-mCD8::GFP.L}LL5; + (a gift from A. Gould and B. Bello) and w; +; TH1-Gal4. The MARCM receiver line was w, sn, FRT19A; +; +.
Crosses to generate heterozygous TH-MARCM flies were set up under standard conditions at 25°C. Flies were placed in egg collection chambers one or two days prior to timed egg collections. After pre-lays, plates were replaced at 4 h intervals (10 am–2 pm, 2 pm–6 pm) in order to restrict the developmental window in which clones were generated. Following incubation of plates at 25°C for 24 h, L1 larvae were placed into vials containing standard cornmeal media, and development was allowed to continue at 25°C. Heat-shocks for 1 h in a 37°C water bath were carried out at 22–30 and 26–30 h to target early first instar larvae (L1), 40–44 h to target late L1, 50–54 h to target early second instar larvae (L2) and 74–78 h to target early third instar larvae (L3). 2–5 day old adult female brains were dissected, fixed, and labeled.
Adult flies were anesthetized with CO2, then placed in ethanol for up to 1 min to remove the waxy coating from the cuticle. Flies were then transferred to cold phosphate buffer (PB) preceding dissection. Brains were dissected in cold PB by gentle forceps manipulation under a dissecting microscope, and then transferred to a 0.5 ml microcentrifuge tube containing PB on ice for a maximum of 30 min. The tissue was then fixed by replacing PB with ∼600 μl phosphate lysine buffer with 2% para-formaldehyde (PLP) and agitating on a rotator for 1 h at room temperature. Brains were then washed 3 times for 5 min in PB with 0.5% v/v Triton-X100 (PBT), and then blocked for 15 min in PBT containing 10% v/v normal goat serum (NGS) in order to prevent non-specific antibody binding. Brains were then incubated with the appropriate concentration of primary antibody diluted in PBT-NGS overnight at 4°C. A further 3 times 5 min washes in PBT preceded incubation with the secondary antibody, also diluted in PBT-NGS, for 2–3 h under agitation at room temperature (in the dark to prevent photo-bleaching of fluorescent label). Brains were then washed two times for 15 min in PBT, followed by 2 times 15 min in PB. The buffer was then replaced with 2–3 drops Vectashield mounting media (Vector Laboratories), either standard or with DAPI if visualization of cell nuclei was required, and incubated overnight at 4°C before mounting. Brains were mounted on a glass slide covered by a 22 × 22 mm, 0.13–0.17 mm thickness cover slip (Menzel) sealed with clear nail varnish. The cover slip was supported at the corners by a small amount of vacuum grease, to prevent the brains from being squashed. Primary antibodies used were mouse anti-TH 1:50 (Immunostar) and mouse anti-bruchpilot 1:50 (NC82, DSHB). Goat anti-mouse conjugated to 568 (red) fluorescent dye was obtained from Invitrogen Molecular Probes. Secondary antibodies were pre-absorbed against Drosophila embryos at a dilution of 1:15, and used at a final dilution of 1:150.
Confocal microscopy and image processing was performed using a Leica TCS SP5 confocal microscope and Leica Application Suite Advanced Fluorescence (LAS AF) version 2.0.2 software. Images were acquired at a resolution of 512 × 512 or 1024 × 1024, with a slice thickness of 1 μm and a line-average of 4. Different channels were scanned sequentially in order to avoid bleed-through. The major processes of DA neurons labeled with mCD8::GFP were traced through confocal Z-stacks using the Simple Neurite Tracer plugin for ImageJ1. Z-projections were created using ImageJ and processed using Adobe Photoshop.
Whole-mount adult brains from heterozygous female TH > mCD8::GFP flies were labeled with anti-TH-568 and scanned sequentially in order to avoid bleed-through. GFP and anti-TH labeled cells were counted manually through each Z-stack using the point selection tool in Image J. Numbers of cells were recorded per hemisphere for PPL1, PPL2, PPM1/2, PPM3, and PAL clusters. The mean number of cells per cluster and per hemisphere was calculated for up to 24 hemispheres. Comparison of means was carried out using independent samples t-tests.
Sugar yeast (SY) media used in aging experiments was 15% w/v sucrose, 15% w/v dried yeast (Allinson), 1.5% w/v agar, 0.3% v/v propionic acid, and 0.3% nipagen (dissolved in ethanol). Flies were kept under 12 h light/dark conditions at 25°C in a humidified incubator (LMS). Flies of the same starting age were obtained by collecting larvae from egg collections on fruit-agar plates at 25°C. Approximately 100 flies were placed into egg collection chambers one or two days prior to egg collections, with plates being replaced at 4 h intervals (10 am–2 pm, 2 pm–6 pm) and overnight. Following incubation of plates at 25°C for up to a maximum of 48 h, L1 or early L2 larvae were placed into a vial containing ∼10 ml of 15% SY media at a density of 50 larvae per vial.
Newly eclosed flies were allowed to mature and mate for 48 h before the flies were briefly anesthetized with CO2 so that females could be separated and selected for aging experiments. 100-200 adult female flies of the same starting age were then transferred into fresh vials at a density of 10 flies per vial, each vial containing 15% SY media to begin the aging experiments. Subsequently, flies were transferred onto fresh food twice weekly and their survival recorded. This process was followed until all flies were dead. Individual flies were censored from the results if they escaped during tipping or if they died of a non-age-related cause, for example getting stuck in the food. Analysis of survival data was performed using the Kaplan–Meier method, which takes account of censored data. The Kaplan–Meier estimate is the non-parametric maximum likelihood of survival at a given time-point S(t):
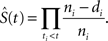
Where ni is the number of survivors less the number of censored cases and di is the number of deaths at time-point ti. A plot of the Kaplan–Meier estimate is a series of horizontal steps of declining magnitude which, when a large enough sample is taken, approaches the true survival function for that population.
Startle-induced locomotion and negative geotaxis tests were carried out under constant temperature at 25°C during the day. Adult female flies of the same starting age were maintained on 15% SY media at a density of 10 flies per vial and transferred onto fresh food twice weekly, until reaching the appropriate age. Fresh (not previously tested) flies were used at each time-point. 60 flies were aged per time-point, in order to ensure that 45 flies were available at each time-point for the assay. Thus, 3× 15 appropriately aged flies per condition/genotype were anesthetized with CO2 and placed in adapted 25 ml pipettes to be used as vertical climbing columns: The top was removed and bunged with cotton wool; the tip was sealed with Parafilm and closed with a 0.5 ml microcentrifuge tube with the lid removed. Flies were allowed to recover for 30 min in a 25°C incubator prior to starting the assay. Flies were tapped to the bottom of the pipette (always with the same number and intensity of taps) and allowed to climb for 45 s. At 45 s the number of flies at the top (above 25 ml line) and the number of flies at the bottom (below 2 ml line) of the pipette were recorded. This was repeated a further 2 consecutive times for each sample and pipette. The performance index (PI) was calculated for each group of 15 flies (average of 3 trials) using the formula PI = 0.5 × (ntotal + ntop − nbottom)/ntotal described previously (Coulom and Birman, 2004), where ntotal is the total number of flies, ntop is the total number of flies at the top, and nbottom is the total number of flies at the bottom. If all flies climb to the top of the tube, the score is 1, and if no flies climb the score is 0. The mean PI for the three groups of flies for each genotype was subsequently calculated. Comparisons were made using either independent samples t-tests or, in the case of three or more means, one-way analysis of variance (ANOVA). Following ANOVA, pairwise comparisons were carried out using the post hoc Tukey honestly significant difference (HSD) test.
Fly tracking arenas were either modified 6-well plates (3.5 cm diameter wells) or 9 cm diameter Petri dish lids (Corning) filled with Sylgard transparent silicon elastomer (Dow Corning), leaving a 3-mm gap so that flies could walk freely and breathe normally but could not fly. Arenas were placed above an array of white LEDs within a temperature-controlled incubator (Stuart Scientific). A CCD camera positioned above the arenas was connected to a PC via a VCR and television monitor. Experimental flies were cooled on ice for 3–4 min to subdue them before either one female per well (for 3.5 cm arenas), or 12 females per plate (for 9 cm arenas) were placed into the incubator at 25°C and flies were left to acclimatize for 60 min, before being moved under the camera for recording. 12 flies were recorded simultaneously at 5 frames per second for 10 or 30 min for TH > mCD8::GFP or TH > Dcr2, mCD8::GFP, respectively. Recorded movement trajectories from 9000 frames were analyzed using Ctrax software (Branson et al., 2009) which is freely available from the Dickinson lab at Caltech2. Distances within the image sequence were calibrated based on a known measurement (here, diameter of an arena = 3.5 cm, or = 9 cm). Tracked data were exported in a Matlab compatible format and analyzed using GNU Octave3 to calculate whole-genotype means. Activity was calculated from distance traveled per frame; based on image sequences and distance data, a threshold was established (0.25 mm/s) below which flies were moving but not walking (e.g., grooming) and above which they were walking. In frames where distance was below threshold (<0.25 mm/s), a score of 0 was given, and above the threshold (>0.25 mm/s), the score was 1.
Mean walking activity scores for each animal were calculated and these were combined with all other recorded flies/genotype to obtain a mean value for all flies of that genotype. Exploration for each genotype was calculated by dividing the arena by 100 × 100 lines – equivalent to approximately 7854 boxes (π × 502) – and counting the number of boxes each fly entered over a 10-min period. Percentage exploration was deduced: 100× count/total boxes. Mean exploration scores for each fly were combined with other flies of the same genotype to obtain a mean value for all flies of that genotype. Centrophobism was represented as the amount of time each fly was in a region 75% from the center of the arena. Smaller values indicate that the fly is more centrophobic. In the case of 9 cm arena this is an area 35.8 cm2 in a total area of 63.6 cm2. XY co-ordinates were zeroed to the center of the arena and converted into polar co-ordinates in order to use the radial coordinate as a measure of distance from the center. The percentage of time in the central region was calculated as time less than 75% from the center divided by total time (30 min). Mean centrophobism scores for each fly were combined with other flies of the same genotype to obtain a mean value for all flies of that genotype.
Statistical analysis was carried out using PASW Statistics 18 for Windows. Comparison of mean numbers of DA neurons was carried out using independent samples t-tests. Analysis of survival data was performed using the Kaplan–Meier method.
We first characterized the number, position, and projection patterns of DA neurons in the adult brain of Drosophila. To visualize DA neurons and their projections, we used an antibody specific to the rate-limiting enzyme tyrosine hydroxylase (TH) and a TH-specific Gal4 line (Friggi-Grelin et al., 2003) in combination with a membrane-tethered GFP-reporter (UAS-mCD8::GFP). The expression pattern of TH > mCD8::GFP was determined in the adult brain of 2-day-old heterozygous female TH > mCD8::GFP flies (Figures 1A–D). Consistent with previous findings (Friggi-Grelin et al., 2003), whole-mount TH > mCD8::GFP brains co-labeled with anti-TH showed significant, but not complete overlap of GFP and endogenous TH expression in the adult central brain (Figure 1E). In addition, anti-TH also labeled cells in the visual system were DA is known to play a significant role in phototransduction (Nässel and Elekes, 1992; Chyb et al., 1999). We focussed on six major clusters in the central brain that can be annotated according to their anatomical position (Figures 1B,D): paired posterior lateral 1 and 2 (PPL1 and PPL2); paired posterior medial 1 and 2 (PPM1/2) which are often grouped together because of their close proximity; paired posterior medial 3 (PPM3); paired anterior lateral (PAL), and paired anterior medial (PAM). The PAM cluster, however, is not well targeted by TH-Gal4 (not shown), and therefore not included in our analysis. DA neuron numbers within defined clusters were manually counted through confocal Z-stacks, which revealed that each DA neuron cluster is comprised of distinct number of cells. As compared to anti-TH labeling, significantly higher mean numbers of GFP-labeled cells were observed in all clusters (Figure 1F; Table 1), indicating ectopic activity of TH-Gal4.
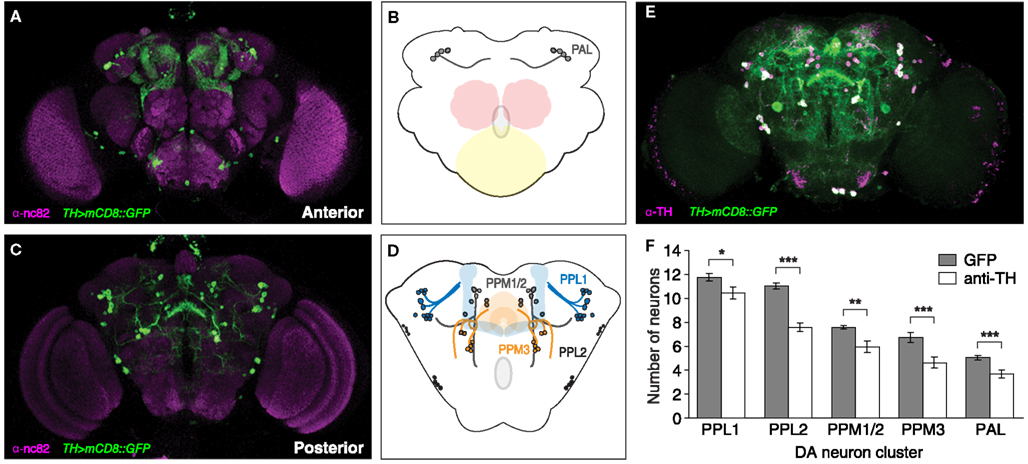
Figure 1. Dopaminergic neurons in the adult Drosophila brain define distinct clusters which comprise defined numbers of cells. (A–D) Confocal Z-stack of TH > mCD8::GFP brain; anti-nc82 immunoreactivity together with GFP labeling reveals dopaminergic neurons in the anterior (A,B), and posterior (C,D) brain. DA neurons can be grouped into the PAL cluster (B), which is located superior to the subesophageal ganglion (yellow) and the antennal glomeruli (red), as well as (D) the PPM1/2, PPL1, PPL2, and PPM3 clusters; their axonal projections target major neuropil structures of the adult brain, including the mushroom body (blue) and central complex (orange). (E) TH > mCD8::GFP brain co-labeled with anti-TH reveals overlap, although incomplete, between GFP- and anti-TH labeled DA neurons, with (F) significant differences in numbers per cluster and hemisphere (n = 12; *p < 0.05; **p < 0.01; ***p < 0.001).
We next traced the major processes of DA neurons belonging to defined clusters using Simple Neurite Tracer plugin for ImageJ. Axonal projections were traced through confocal Z-stacks of whole-mount adult brains from a heterozygous female TH > mCD8::GFP fly dissected at day 2. PAL neurons were found to project across the midline, PPM1/2 neurons were found to project ventrally, PPL1 neurons were observed to project medially and dorsally toward the mushroom body and, PPM3 neurons were observed to project toward the central complex (data not shown). These data suggest that DA neurons in the adult brain of Drosophila can be grouped into bilateral symmetric clusters, each comprising a stereotypical number of cells. Each cluster is arranged in a spatial organization that represents distinct DA pathways.
However, the complex and dense arborization patterns of TH > mCD8::GFP-labeled DA neurons rendered it impossible to observe the specific targets of clusters or individual neurons using the method described above. In order to gain further insights into the targets of these neurons, we carried out MARCM (Wu and Luo, 2006) to visualize the individual projection patterns of randomly labeled DA neurons in the adult Drosophila brain.
The GFP-labeled MARCM clones of DA neurons were generated at late L1, early L2, and early L3, and analyzed in 2 day old adult brains (Figure 2). To identify innervation target areas, we used the anti-bruchpilot (nc82) antibody that labels brain neuropil structures (Ito and Awasaki, 2008; Hofbauer et al., 2009). The observed innervation target areas of the different DA neuron types labeled by TH-specific MARCM clones are summarized in Figure 2K. DA MARCM clones observed in the PPM1/2 cluster showed two large, divergent fields of arborization in the ventral lateral protocerebrum (Figures 2A–C), and a subset of PAL neurons were found to innervate the optic tubercle and medial protocerebrum, in addition to the superior medial and inferior medial protocerebrum (Figures 2D,E). PPL1 neurons were found to innervate distinct regions of the mushroom bodies, which are known to be involved in learning and memory (Zars, 2000). Single cell projections were observed to target the α-lobe, the peduncle and the calyx of the mushroom body, as well as innervating the superior lateral and superior medial protocerebrum (Figures 2F–H). The ipsilateral arborizations of these PPL1 subtypes were mirrored on in the contra-lateral hemisphere. In both cases a clear connective fiber could be seen crossing the midline (Figure 2H, arrow).
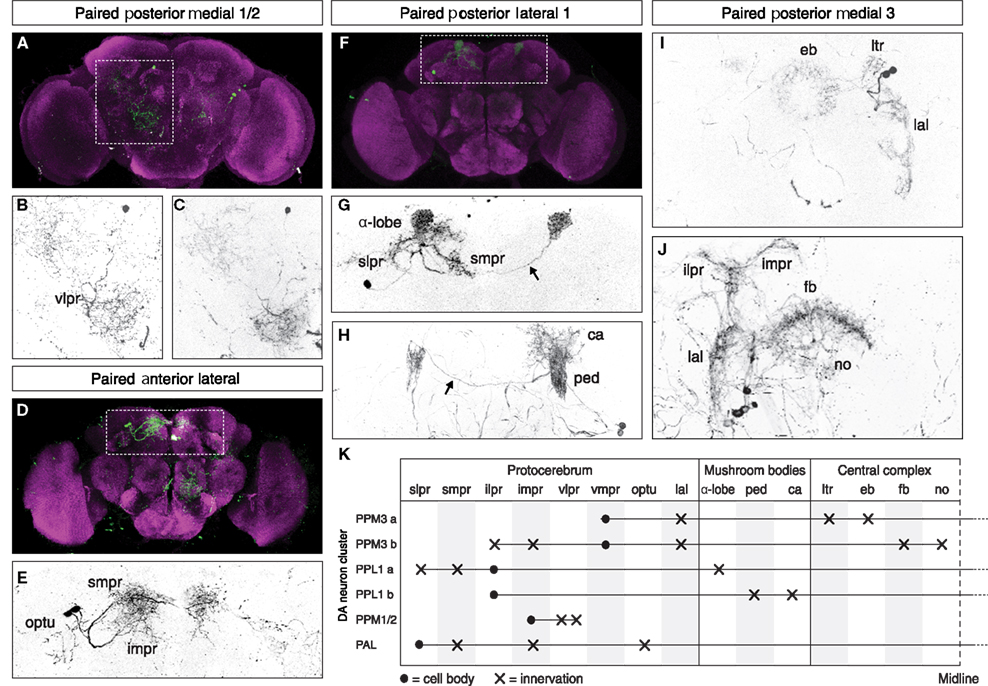
Figure 2. Dopaminergic neurons show cluster-specific projections in target regions that represent distinct functional areas of the adult Drosophila brain. MARCM analysis reveals that (A–C) the PPM1/2 cluster projections cover a large area in the ventral lateral protocerebrum (vlpr); (D,E) PAL cluster neurons innervate the optic tubercle (optu) and the superior medial (smpr) and inferior medial protocerebrum (impr); (F–H) DA neurons of the PPL1 cluster project onto the alpha-lobe (α-lobe), the peduncle (ped), and calyx (ca) of the mushroom body, as well as to the superior lateral (slpr) and superior medial protocerebrum (smpr), with projections also to the contra-lateral side (arrows). (I,J) DA neurons of the PPM3 cluster project to the ellipsoid body (eb) via the lateral triangle (ltr) and the lateral accessory lobe (lal), as well as to the fan-shaped body (fb), the noduli (no) and the ipsilateral (ilpr), and inferior medial protocerebrum (impr). The eb, fb, and no neuropiles are part of the central complex which is involved in the higher control of locomotor behavior. Anatomical regions were identified using the anti-bruchpilot (nc82) antibody which labels brain neuropil structures [(A,D,F) magenta]. (K) Summary of the location of cluster-specific DA cell bodies and the terminal arborization of their axonal projections in the adult Drosophila brain.
Paired posterior medial 3 neurons were found to innervate substructures of the central complex, which is described as a higher center for the control of locomotion (Strauss, 2002). Clonally related PPM3 DA neurons were observed to project to the ellipsoid body via the lateral triangle, as well as the lateral accessory lobe (Figure 2I). The arborizations in the ipsilateral hemisphere were mirrored in the contra-lateral hemisphere in both the lateral triangle and lateral accessory lobe. An apparently distinct clonally related group of DA cells in the PPM3 cluster were observed to project to the fan-shaped body and noduli of the central complex (Figure 2J). These cells also sent projections to the inferior lateral and inferior medial protocerebrum, in the vicinity of the mushroom body. In contrast to the cells projecting to the ellipsoid body, these cells showed no arborization in the contra-lateral hemisphere. Taken together, our MARCM data suggest that DA neurons in the adult brain of Drosophila show cluster-specific, stereotypical projection patterns with terminal arborization in target regions that represent distinct functional areas of the adult Drosophila brain.
Parkinson’s disease is an adult-onset, age-related neurodegenerative disorder mainly characterized by locomotor dysfunction due to progressive loss of DA neurons in the brain. These parameters, age, locomotion and numbers of DA neurons in the brain, are therefore essential parameters in the study of PD. Hence, we characterized DA cell numbers and locomotor behavior of aging Drosophila. In a first set of lifespan experiments, we compared female wildtype Oregon R flies fed on standard cornmeal food, with female w1118 flies that were fed on 15% SY food (Figure 3A). We chose to use 15% SY food since it has been described as a reliable protocol for lifespan experiments (Bass et al., 2007; Grandison et al., 2009). Oregon R flies showed a mean lifespan of 97 days, (max = 128 days; n = 93), whereas w1118 flies showed a mean lifespan of 83 days (max = 104 days; n = 100), suggesting that both genetic background and nutrition likely caused a reduction in mean lifespan by 14% in w1118 flies. In a second set of experiments, we changed the environment to an incubator with higher humidity and compared female w1118 flies with female transgenic TH > mCD8::GFP flies, both raised on 15% SY food (Figure 3B). This time w1118 flies showed a mean lifespan of 78 days (max = 92 days; n = 93), and heterozygous female TH > mCD8::GFP flies showed a mean lifespan of 66 days (max = 83 days; n = 100). These results suggest that genetic background and environmental factors such as nutrition and humidity significantly impact on Drosophila lifespan. In the following, all aging experiments were carried out in the same incubator using 15% SY media.
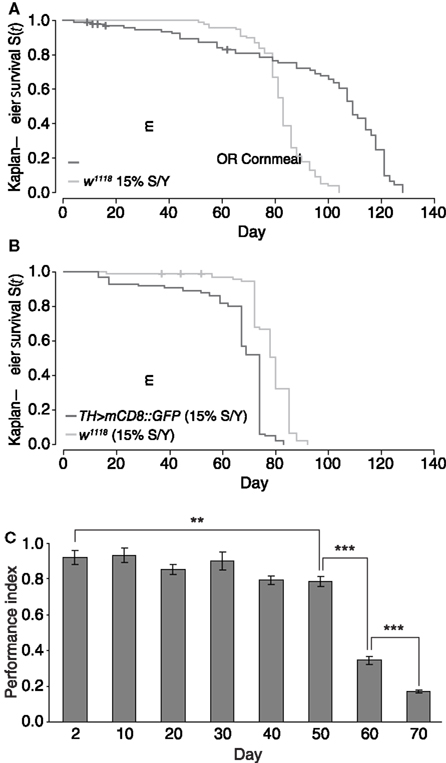
Figure 3. Drosophila lifespan is dependent on genetic background and environment; startle-induced locomotion and negative geotaxis decline with age. (A) Kaplan–Meier survival curve of female wildtype Oregon R flies maintained on standard cornmeal media (OR cornmeal), and female w1118 flies maintained on 15% sugar yeast (SY) media. Maximum survival of OR was 128 days (mean = 97), whereas w1118 flies maintained on 15% SY revealed a maximum survival of 104 days (mean = 83). (B) Kaplan–Meier survival curve of heterozygous female TH > mCD8::GFP and female w1118 flies that were maintained on 15% SY media and in an incubator with higher humidity than in (A). Maximum survival was 83 days (mean = 66) for TH > mCD8::GFP, and 92 days (mean = 78) for w1118 flies. (C) Startle-induced locomotion and negative geotaxis of heterozygous TH > mCD8::GFP maintained on 15% SY media; mean value of performance for consecutive days. A gradual decline can be observed from day 2 to day 50 (**p = 0.002), with a more pronounced performance decline toward the end of life between days 50, 60, and 70 (***p < 0.001). Error bars are standard deviations.
Next we determined locomotor behavior in aging flies and analyzed inborn startle-induced locomotion and negative geotaxis as an assay for climbing performance. Startle-induced locomotion and negative geotaxis was recorded in aging heterozygous female TH > mCD8::GFP flies at day 2, then from day 10 onwards every 10 days throughout life. A gradual decline in performance of 14% from 0.92 to 0.79 was observed from day 2 to day 50, with a more pronounced reduction in performance toward the end of life at days 60 and 70 (Figure 3C). A one-way ANOVA showed that the observed decline was significant (p < 0.001). Post hoc Tukey HSD comparisons showed that whilst the drop in performance from day 2 to day 50 was significant (p = 0.002), there was no strongly significant decline between consecutive time-points until day 60. Between day 50 and 60, performance declined by 57% from 0.79 to 0.34 (p < 0.001), and then again at day 70 by 50% to 0.17 (p < 0.001), after which there were not enough flies remaining alive to carry out the number of replicates required to generate meaningful statistics. These data suggest that startle-induced locomotion and negative geotaxis continuously decline in aging Drosophila.
In PD, loss of the nigrostriatal pathway severely affects movement, and causes a behavioral deficit that is reflected by impaired walking activity. Moreover, PD patients frequently suffer from neuropsychiatric symptoms including anxiety and apathy, which predate motor symptoms by several years (Aarsland et al., 2009). In Drosophila, walking activity and exploration can be measured as a behavioral read-out of DA dysfunction using an open-field paradigm. In the open-field paradigm, flies are kept in an arena, where they are left undisturbed, and can walk freely (see Materials and Methods). Walking is recorded as movement per time which is described by velocity. From this, mean speed and distance walked can be calculated for the recorded period. Anxiety or fear and apathy can be inversely correlated to ambulation and exploratory movement, which can be further characterized by the time spent exposed in the center of an open field. Drosophila display exploratory behavior and centrophobism (also called thigmotaxis) where they actively explore an open arena but tend to avoid the center (Götz and Biesinger, 1985; Besson and Martin, 2005; Liu et al., 2007).
We measured walking activity, speed and distance, as well as exploration and centrophobism in mid-age and old flies suitable for targeted, Gal4/UAS-mediated genetic manipulation. We chose two different genotypes, TH > mCD8::GFP and TH > Dcr2, mCD8::GFP that allow the simultaneous visualization and targeted genetic manipulation of DA neurons in vivo. In Drosophila, Dcr2 is mainly required for short interfering RNA (siRNA) directed mRNA cleavage by facilitating RNA-induced silencing (Kim et al., 2006; Carthew and Sontheimer, 2009), thereby downregulating targeted gene products; its ectopic expression in Drosophila is commonly used to enhance the efficacy of Gal4/UAS-mediated RNA interference (Dietzl et al., 2007), for example for the targeted knockdown of fly homologs of human PD-related genes (Hirth, 2010). Moreover, in mammals, the single Dicer homolog has been implicated in DA neuron survival and function (Cuellar et al., 2008; Huang et al., 2010). We therefore wanted to assess the impact of TH-specific Dcr2 expression on motor behavior in Drosophila.
Thus, video-assisted motion tracking was carried out using heterozygous female TH > mCD8::GFP and TH > Dcr2, mCD8::GFP flies at day 26 and 31, and day 62 and 61, respectively. Analysis of video-assisted movement tracking (Figure 4) revealed that both TH > mCD8::GFP and TH > Dcr2, mCD8::GFP flies showed an increase in walking activity when compared between day 26/31 and day 62/61, respectively (Figures 4A,B). For both genotypes, we did not detect significant changes in mean speed (Figure 4C) but increased activity resulted in greater total distance traveled (Figure 4D) at later stages in life. In addition, old flies also showed a significant increase in exploration behavior (Figure 4E), whereas we did not detect any significant differences in the time spent in the center of the arena as a measure for centrophobism (Figure 4F) for both time-points and genotypes. The age-related increase in walking activity, exploration and distance traveled was unexpected, but significant for both genotypes, even though total numbers differed.
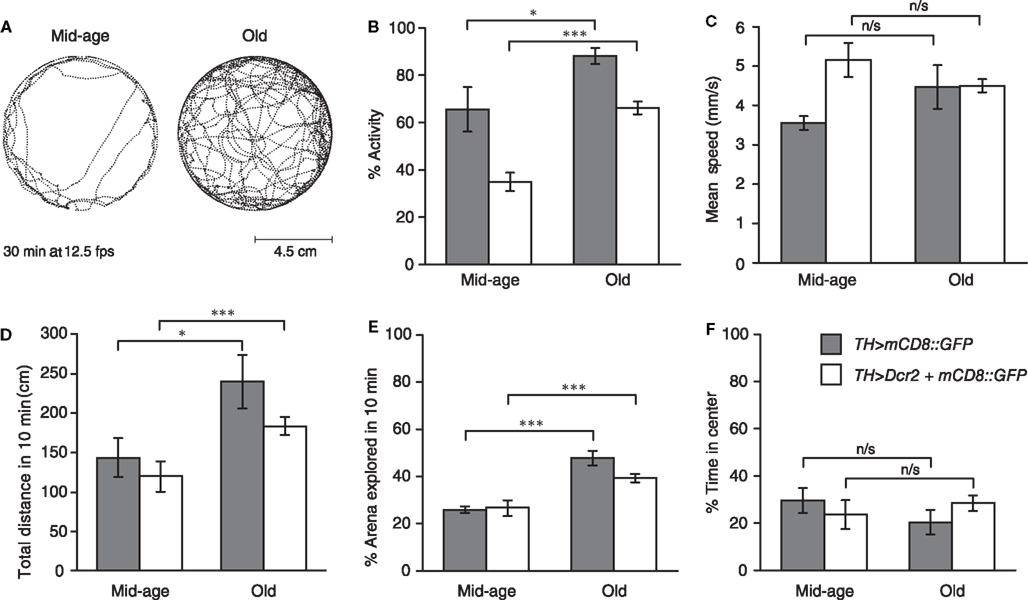
Figure 4. Drosophila walking activity and exploration behavior but not centrophobism increase in late life and TH-specific Dcr2 expression affects distinct motor behavior. (A) Movement trajectories of mid-age (26 days) and old (62 days) heterozygous female TH > Dcr2, mCD8::GFP flies recorded for 30 min at 5 frames per second (fps) in an arena (9 cm diameter). (B) Heterozygous female TH > Dcr2, mCD8::GFP and TH > mCD8::GFP flies show in late life an increase in walking activity, but (C) not mean speed (calculated from speeds when fly is active), resulting (D) in a greater distance traveled. (E) These flies also show increased exploration behavior, but (F) not centrophobism in late life. In addition to these age-related differences, expression of Dcr2 in DA neurons in mid-age and old flies affected walking activity (B). Mid-age flies expressing Dcr2 moved faster, though this difference was no longer apparent in old flies (C). Expression of Dcr2 in DA neurons did not affect exploratory behavior (E), nor the time flies spend in the center of the arena (F). P values, *p < 0.05; ***p < 0.005; n/s = not significant. Errors bars represent standard errors of the mean, SEM.
In addition to these age-related differences, we also observed behavioral differences between the two genotypes studied. TH-specific expression of Dcr2 had a significant effect on walking activity. In mid-age and old flies, TH > Dcr2, mCD8::GFP flies were less active than TH > mCD8::GFP flies (Figure 4B). Furthermore, we observed a significant difference in mean speed in mid-age flies, though this difference was no longer apparent in old flies (Figure 4C). These alterations in motor behavior caused by Dcr2 expression appeared to be specific, since we did not observe any differences in total distance traveled (Figure 4D), nor in exploratory behavior (Figure 4E) nor centrophobism (Figure 4F). Taken together, these data suggest that in contrast to startle-induced locomotion and negative geotaxis, Drosophila walking activity and exploratory behavior do not decline with age but rather increase at late stages of life. Moreover, TH-specific Dcr2 expression specifically affects walking activity, indicating that the siRNA pathway may modulate distinct DA motor behaviors.
Finally, we determined DA neuron numbers in the aging brain of Drosophila. For this we used heterozygous female TH > mCD8::GFP flies maintained in standard conditions on 15% SY media which were analyzed at day 2 and day 60 (Figure 5). Based on our previous lifespan data, these two time-points were selected as appropriate early and late life time-points for neuron quantification, and also because at day 60, flies showed a marked decrease in startle-induced locomotion and negative geotaxis. For each time-point, we examined n = 24 hemispheres to count DA neuron numbers per cluster. Thus, anti-TH labeled and GFP-positive cells belonging to defined DA clusters per brain hemisphere were manually counted based on confocal Z-stacks of whole-mount adult brains. For TH > mCD8::GFP flies (Figure 5A), we detected on average per hemisphere 12 cells per PPL1 cluster (mean day 2 = 11.78 and mean day 60 = 12.93; SD d2 = 1.53 and SD d60 = 1.40), ten cells per PPL2 cluster (mean d2 = 11.04 and mean d60 = 9.41; SD d2 = 1.19 and SD d60 = 1.63), seven cells per PPM1/2 cluster (mean d2 = 7.58 and mean d60 = 7.16; SD d2 = 0.77 and SD d60 = 1.27), six cells per PPM3 cluster (mean d2 = 6.75 and mean d60 = 5.40; SD d2 = 1.96 and SD d60 = 1.94), and five cells per PAL cluster (mean d2 = 11.78 and mean d60 = 12.93; SD d2 = 1.53 and SD d60 = 1.40).
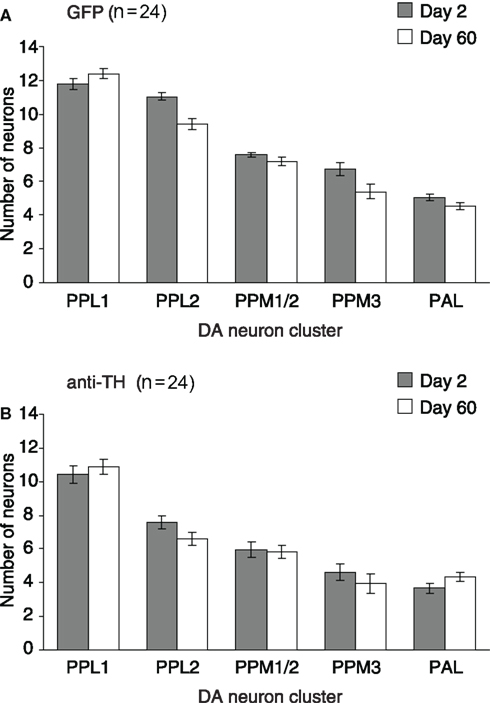
Figure 5. Dopaminergic neurons do not degenerate in the aging brain of Drosophila. Mean number of DA neurons per hemisphere for the protocerebral cell clusters PPL1, PPL2, PPM1/2, PPM3, and PAL. For each, day 2 and day 60, n = 24 hemispheres were examined to count the DA neuron numbers/cluster of heterozygous female TH > mCD8::GFP brains. Neuron numbers are given for day 2 and day 60, quantification was based on either (A) GFP, or (B) anti-TH labeling. Error bars are standard error of the mean, SEM.
Counting anti-TH labeled cells in TH > mCD8::GFP flies (Figure 5B), we detected on average per hemisphere ten cells per PPL1 cluster (mean d2 = 10.43 and mean d60 = 10.86; SD d2 = 2.38 and SD d60 = 2.18), seven cells per PPL2 cluster (mean d2 = 7.58 and mean d60 = 6.58; SD d2 = 1.76 and SD d60 = 1.90), six cells per PPM1/2 cluster (mean d2 = 5.95 and mean d60 = 5.83; SD d2 = 2.33 and SD d60 = 1.88), four cells per PPM3 cluster (mean d2 = 4.62 and mean d60 = 3.95; SD d2 = 2.31 and SD d60 = 2.59), and four cells per PAL cluster (mean d2 = 3.66 and mean d60 = 4.33; SD d2 = 1.52 and SD d60 = 1.23). Taken together, these results show that comparable numbers of TH > mCD8::GFP and anti-TH labeled DA neurons can be found in the brains of young and old age Drosophila. These data suggest that adult onset, age-related degeneration of DA neurons does not occur in the aging brain of Drosophila.
Drosophila models of PD are characterized by two principal phenotypes related to parkinsonism: the specific loss of subsets of DA neurons in the aging brain and locomotion defects (Hirth, 2010). Here we provide a thorough analysis of DA neuron numbers and locomotor behavior in aging wildtype and transgenic flies suitable for targeted, Gal4/UAS-mediated genetic manipulation. Our data establish baseline parameters in Drosophila melanogaster for the study of PD, as well as other diseases affecting DA neurons and movement control.
Previous studies in Drosophila described the DA system in the adult brain using dopamine and anti-TH immunoreactivity (Budnik and White, 1988; Nässel and Elekes, 1992). These studies led to the characterization of individual clusters which were named according to their anatomical position in the brain (Monastirioti, 1999). These experimental tools were subsequently complemented by the identification of regulatory sequences of the rate-limiting enzyme for DA synthesis, TH, which made it possible to generate a TH-specific GAL4 line (Friggi-Grelin et al., 2003). Although TH-Gal4 does not completely overlap with anti-TH labeling (Figure 1E), TH > mCD8::GFP targets the majority of DA neurons, allowing the visualization and genetic manipulation of DA neurons throughout development and adulthood. More recently, lineage analysis with a repressible cell marker provided an initial annotation of individual DA neurons and their projection patterns in the adult brain of Drosophila (Mao and Davis, 2009). Our data confirm these findings and extend them to the aging fly. Our analysis focussed on five prominent DA clusters in the adult protocerebrum, and reveals that cluster-specific DA neurons project to distinct functional areas (summarized in Figure 2K).
Comparable to the data of Mao and Davis (2009), we found that DA neurons of the PPL1 cluster specifically project onto the mushroom body, a higher center for experience-dependent activity like associative memory formation (Heisenberg, 2003). Most recent data also implicate PPL1 DA neurons in aversive reinforcement and appetitive motivation (Riemensperger et al., 2005; Claridge-Chang et al., 2009; Krashes et al., 2009; Aso et al., 2010), suggesting that combinations of DA neuronal activity might represent a motivational state similar to that proposed in mammals (Waddell, 2010). Our MARCM analysis also revealed that PPM3 neurons specifically project onto the fan-shaped body and ellipsoid body, neuropil structures that form part of the central complex. The central complex has been identified as a higher center for the control of locomotor behavior (Strauss, 2002), and DA input onto the fan-shaped body and ellipsoid body modulates goal-directed movement (Lebestky et al., 2009; Kong et al., 2010). Together these data indicate that DA neurons in the fly brain are involved in the regulation of specific behaviors, which in turn can be localized to defined DA clusters, such as a role in motor behavior to PPM3 neurons in the Drosophila central brain.
A similar situation has been observed in the mammalian brain which comprises nine clusters of DA neurons that are localized to the mes- and diencephalon, and the olfactory bulb (Björklund and Dunnett, 2007). The most prominent DA pathways are the nigrostriatal neurons projecting from the substantia nigra to the caudate putamen, which is primarily affected in PD, as well as the mesolimbic, mesocortical, and the hypothalamus-specific tuberoinfundibular pathway (Vallone et al., 2000). DA activity in mammals modulates locomotion, motivational states, and cognitive function, and cluster-specific dysfunction of DA neurons has been correlated with specific behavioral deficits in mammals (Costa, 2007; Palmiter, 2008). In Drosophila, specific loss of PPL1 neurons was reported in several models of PD including mutants for PINK1 (Park et al., 2006) and parkin (Whitworth et al., 2005), although the functional impact on mushroom body and hence, memory function and motivation of these flies was not determined. Also, PPM3-specific neurodegeneration was reported in Drosophila PD models affecting motor control. These models include treatment with either rotenone (Coulom and Birman, 2004), which affects mitochondrial complex I activity, or paraquat (Chaudhuri et al., 2007) which leads to oxidative stress in a concentration-dependant manner. Neurodegeneration of PPM3 was also observed in Drosophila LRRK2 gain-of-function (Liu et al., 2008; Ng et al., 2009), and Gal4/UAS-mediated RNA interference of Drosophila PINK1 (Wang et al., 2006).
For some Drosophila models of PD, however, there exist conflicting data as to whether loss of the fly homolog of LRRK2 or PINK1 or overexpression of human asyn causes parkinsonian-like DA neurodegeneration or not (see Feany and Bender, 2000; Pesah et al., 2005; Yang et al., 2006; Liu et al., 2008; Wang et al., 2008; Venderova et al., 2009). These discrepancies might be attributable, at least to some extent, to differences in the underlying methods used to analyze DA neuron numbers, including differences in tissue preparation (paraffin sections or whole-mount analysis), differences in how DA neurons are visualized (TH > mCD8::GFP or anti-TH labeled cells), and differences in Gal4 driver- and/or transgene activity, as well as the inherent artificial situation of Gal4-mediated protein overload which is added to the endogenous protein level. The apparent differences emphasize the necessity for reliable baseline parameters when using Drosophila in the study of PD, but also for other disease models.
Increasing age remains the most evident risk factor for the development of PD. Thus, the relatively short lifespan of Drosophila allows the study of disease-relevant processes throughout life. To determine lifespan data, we choose w1118 as compared to TH > mCD8::GFP, because the majority of transposon insertion lines, as well as deficiency lines used in Drosophila, are generated in this genetic background, including models of PD and other diseases. Our results show that both w1118 and TH > mCD8::GFP have a mean lifespan between 70 and 80 days when maintained on 15% SY media. These data are in agreement with recent results revealing significant differences in lifespan for three wildtype Drosophila strains. Sanz et al. (2010) reported mean lifespan of 79 days for Dahomey females, 69 days for Canton S females, and 86 days for Oregon R females (Sanz et al., 2010). It is obvious from these differences that genetic background and environmental factors such as nutrition and stress significantly impact on Drosophila lifespan (for review, see Partridge et al., 2005; Vermeulen and Loeschcke, 2007), with males being more susceptible to dietary restriction and environmental stressors (Sanz et al., 2010).
We did not detect any age-related DA cell loss under normal laboratory conditions, which contrasts with a previous study indicating increased degeneration of DA neurons with aging (Neckameyer et al., 2000). Based on high-performance liquid chromatography, this study provided evidence that DA levels decreased between day 5 and day 20, whereas western blotting revealed unchanged TH protein levels. Moreover, Neckameyer et al. (2000) used anti-TH labeling and observed TH immunoreactivity in the antennal glomeruli that diminished between day 1 and day 20, which was considered suggestive of degenerative changes in DA neuron numbers. In contrast to these observations, we (data not shown) and others (Nässel and Elekes, 1992; Monastirioti, 1999) never detected any anti-TH immunoreactivity in the antennal glomeruli. This discrepancy might be due to the fact that we used a high affinity monoclonal mouse antibody that shows proven cross-reactivity with TH, whereas Neckameyer et al. used a rabbit polyclonal anti-TH antibody that may consequently have weaker affinity for TH leading to more variable staining. Yet, our TH > mCD8::GFP tracing never visualized any GFP labeling in the antennal region (Figure 1A), indicating that TH is not expressed in this brain area. In addition, our quantification of TH > mCD8::GFP but also anti-TH labeling comparing day 2 and day 60 (Figure 5) did not reveal any significant differences in cell numbers in the PPL1, PPL2, PPM1/2, PPM3, and PAL clusters. We therefore conclude that adult onset, age-related degeneration of DA neurons does not occur in the PPL1, PPL2, PPM1/2, PPM3, and PAL clusters of the aging Drosophila brain.
Interestingly, however, the lack of DA neurodegeneration was in contrast to the age-related decline of startle-induced locomotion and negative geotaxis (Figure 3C). In Drosophila, negative geotaxis and locomotor activity have been shown to decline with age (Le Bourg, 1987; Martin et al., 1999; Fernández et al., 1999; Gargano et al., 2005), probably due to functional senescence intrinsically related to age-related deterioration of body systems. However, it is currently not known what causes the age-related decline in negative geotaxis (Grotewiel et al., 2005). Although DA neurons have been shown to mediate locomotor behavior in Drosophila (Yellman et al., 1997; Lima and Miesenböck, 2005; Hirsh et al., 2010), it might be possible that startle-induced locomotion and negative geotaxis are not influenced by the physiological activity of DA cells such as the PPM3 neurons innervating the central complex. However, the mere presence of TH > mCD8::GFP and anti-TH immunolabeled DA neurons in old flies does not necessarily mean that these neurons are fully functional. Age-related activity decline of DA neurons has been reported in normal aging human (Volkow et al., 1998a,b; Kaasinen et al., 2000; Reeves et al., 2002), as well as in mouse models of PD that show locomotor dysfunction without nigral cell loss (Colebrooke et al., 2006). It is conceivable that physiological senescence of DA neurons also occurs in the Drosophila brain, which might be due to age-related decrease in synaptic efficacy (Martinez et al., 2007) and/or waning dopamine 2-like receptor activity (Draper et al., 2007), as well as mitochondrial dysfunction (Morrow and Tanguay, 2008).
Surprisingly, and in stark contrast to the age-related decline in startle-induced geotaxis, we detected a marked increase in walking activity near the end of life in both experimental conditions, TH > mCD8::GFP and TH > Dcr2, mCD8::GFP. It is conceivable that near-death increase in walking activity might be attributable to genetic background. However, similar observations have been made for inbred Drosophila strains (Fernández et al., 1999), as well as for old mice (Wax and Goodrick, 1978) and old rats (Martin et al., 1986). In both cases, motor activity markedly increased during photophase/daylight starting up to 1 week before death, indicating de-regulated circadian motor control. Our movement tracking was carried out during daylight at day 61 and 62, respectively, which was less than 1 week before death of the animals. The observed changes in walking included increased activity and exploration, and hence total distance traveled, but did not affect mean speed of activity nor centrophobism, demonstrating that these aged, near-death flies were de facto more active. Our findings therefore suggest that walking activity and startle-induced locomotion are regulated by distinct neural circuits which also differ in their susceptibility to age-related functional decline.
In addition to these age-related changes, we also observed alterations in motor behavior due to Dcr2 expression. Walking activity, both in mid-age and old TH > Dcr2, mCD8::GFP flies was changed when compared to TH > mCD8::GFP flies. The presence of Dcr2 in DA neurons also affected walking speed in mid-age flies, an effect that was no longer detectable in old flies. We did not detect any alterations in total distance traveled, and both exploratory behavior and centrophobism were unaffected in TH > Dcr2, mCD8::GFP flies. Previous studies in mice showed that the only Dicer homolog is required for DA neuron differentiation (Huang et al., 2010). loss of Dicer targeted to midbrain DA neurons caused ataxia as well as front and hind limb clasping (Cuellar et al., 2008), suggesting that Dicer is necessary for midbrain DA neuron function and maintenance. Our data in Drosophila provide initial evidence that TH-specific expression of Dcr2 and thus, gain-of-function of the siRNA pathway targeted to DA neurons, has a significant and specific effect on motor behavior in Drosophila. It is conceivable, however, that positional effects of UAS-Dcr2 activity potentially affected DA neuron activity. Yet, TH-driven Dcr2 expression led to very specific changes in locomotion, suggesting that specific subsets of DA-dependent behaviors might be modulated by siRNAs. Further experiments are required to determine the exact role of siRNAs in DA function and behaviors. It will be interesting to see whether also in Drosophila Dicer function is required for DA neuron survival, and how small RNAs differentially modulate DA-dependent behaviors.
In summary, we have analyzed DA neuron numbers in the aging brain of Drosophila together with age-related locomotor behavior in wildtype and transgenic flies suitable for targeted, Gal4/UAS-mediated genetic manipulation. Our findings establish baseline parameters for two principal phenotypes related to parkinsonism, as well as other neurological disorders affecting DA neurons and movement control. It will be interesting to see how these parameters are affected in disorders that cause locomotor dysfunction as well as age-related and cell type-specific neurodegeneration.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
We thank Serge Birman, B. Bello, A. Gould, and the Bloomington Stock Centre for flies; Z. Ludlow and J. Hughes for suggestions on motion tracking analysis; and the Dickinson lab at Caltech for making Ctrax freely available. This work was supported by grants from the UK Medical Research Council (G070149), Parkinson’s UK (G-0714), the Royal Society (Hirth/2007/R2), the Wellcome Trust, and King’s College NHS Medical Research Trust (JRC19/2007) to Frank Hirth.
Aarsland, D., Marsh, L., and Schrag, A. (2009). Neuropsychiatric symptoms in Parkinson’s disease. Mov. Disord. 24, 2175–2186.
Aso, Y., Siwanowicz, I., Bräcker, L., Ito, K., Kitamoto, T., and Tanimoto, H. (2010). Specific dopaminergic neurons for the formation of labile aversive memory. Curr. Biol. 20, 1445–1451.
Bass, T. M., Grandison, R. C., Wong, R., Martinez, P., Partridge, L., and Piper, M. D. (2007). Optimization of dietary restriction protocols in Drosophila. J. Gerontol. A Biol. Sci. Med. Sci. 62, 1071–1081.
Besson, M., and Martin, J. R. (2005). Centrophobism/thigmotaxis, a new role for the mushroom bodies in Drosophila. J. Neurobiol. 62, 386–396.
Björklund, A., and Dunnett, S. B. (2007). Dopamine neuron systems in the brain: an update. Trends Neurosci. 30, 194–202.
Braak, H., and Del Tredici, K. (2008). Invited article: nervous system pathology in sporadic Parkinson disease. Neurology 70, 1916–1925.
Branson, K., Robie, A. A., Bender, J., Perona, P., and Dickinson, M. H. (2009). High-throughput ethomics in large groups of Drosophila. Nat. Methods 6, 451–457.
Budnik, V., and White, K. (1988). Catecholamine-containing neurons in Drosophila melanogaster: distribution and development. J. Comp. Neurol. 268, 400–413.
Carthew, R. W., and Sontheimer, E. J. (2009). Origins and mechanisms of miRNAs and siRNAs. Cell 136, 642–655.
Chaudhuri, A., Bowling, K., Funderburk, C., Lawal, H., Inamdar, A., Wang, Z., and O’Donnell, J. M. (2007). Interaction of genetic and environmental factors in a Drosophila parkinsonism model. J. Neurosci. 27, 2457–2467.
Chyb, S., Hevers, W., Forte, M., Wolfgang, W. J., Selinger, Z., and Hardie, R. C. (1999). Modulation of the light response by cAMP in Drosophila photoreceptors. J. Neurosci. 19, 8799–8807.
Claridge-Chang, A., Roorda, R. D., Vrontou, E., Sjulson, L., Li, H., Hirsh, J., and Miesenböck, G. (2009). Writing memories with light-addressable reinforcement circuitry. Cell 139, 405–415.
Colebrooke, R. E., Humby, T., Lynch, P. J., McGowan, D. P., Xia, J., and Emson, P. C. (2006). Age-related decline in striatal dopamine content and motor performance occurs in the absence of nigral cell loss in a genetic mouse model of Parkinson’s disease. Eur. J. Neurosci. 24, 2622–2630.
Costa, R. M. (2007). Plastic corticostriatal circuits for action learning: what’s dopamine got to do with it? Ann. N. Y. Acad. Sci. 1104, 172–191.
Coulom, H., and Birman, S. (2004). Chronic exposure to rotenone models sporadic Parkinson’s disease in Drosophila melanogaster. J. Neurosci. 24, 10993–10998.
Cuellar, T. L., Davis, T. H., Nelson, P. T., Loeb, G. B., Harfe, B. D., Ullian, E., and McManus, M. T. (2008). Dicer loss in striatal neurons produces behavioral and neuroanatomical phenotypes in the absence of neurodegeneration. Proc. Natl. Acad. Sci. U.S.A. 105, 5614–5619.
Dietzl, G., Chen, D., Schnorrer, F., Su, K. C., Barinova, Y., Fellner, M., Gasser, B., Kinsey, K., Oppel, S., Scheiblauer, S., Couto, A., Marra, V., Keleman, K., and Dickson, B. J. (2007). A genome-wide transgenic RNAi library for conditional gene inactivation in Drosophila. Nature 448, 151–156.
Draper, I., Kurshan, P. T., McBride, E., Jackson, F. R., and Kopin, A. S. (2007). Locomotor activity is regulated by D2-like receptors in Drosophila: an anatomic and functional analysis. Dev. Neurobiol. 67, 378–393.
Farrer, M. J. (2006). Genetics of Parkinson disease: paradigm shifts and future prospects. Nat. Rev. Genet. 7, 306–318.
Feany, M. B., and Bender, W. W. (2000). A Drosophila model of Parkinson’s disease. Nature 404, 394–398.
Fernández, J. R., Grant, M. D., Tulli, N. M., Karkowski, L. M., and McClearn, G. E. (1999). Differences in locomotor activity across the lifespan of Drosophila melanogaster. Exp. Gerontol. 34, 621–631.
Friggi-Grelin, F., Coulom, H., Meller, M., Gomez, D., Hirsh, J., and Birman, S. (2003). Targeted gene expression in Drosophila dopaminergic cells using regulatory sequences from tyrosine hydroxylase. J. Neurobiol. 54, 618–627.
Gargano, J. W., Martin, I., Bhandari, P., and Grotewiel, M. S. (2005). Rapid iterative negative geotaxis (RING): a new method for assessing age-related locomotor decline in Drosophila. Exp. Gerontol. 40, 386–395.
Götz, K. G., and Biesinger, R. (1985). Centrophobism in Drosophila melanogaster II. A physiological approach to search and search control. J. Comp. Physiol. A 156, 329–337.
Grandison, R. C., Wong, R., Bass, T. M., Partridge, L., and Piper, M. D. (2009). Effect of a standardised dietary restriction protocol on multiple laboratory strains of Drosophila melanogaster. PLoS ONE 4, e4067. doi: 10.1371/journal.pone.0004067.
Grotewiel, M. S., Martin, I., Bhandari, P., and Cook-Wiens, E. (2005). Functional senescence in Drosophila melanogaster. Ageing Res. Rev. 4, 372–397.
Hardy, J., Lewis, P., Revesz, T., Lees, A., and Paisan-Ruiz, C. (2009). The genetics of Parkinson’s syndromes: a critical review. Curr. Opin. Genet. Dev. 19, 254–265.
Hirsh, J., Riemensperger, T., Coulom, H., Iché, M., Coupar, J., and Birman, S. (2010). Roles of dopamine in circadian rhythmicity and extreme light sensitivity of circadian entrainment. Curr. Biol. 20, 209–214.
Hirth, F. (2010). Drosophila melanogaster in the study of human neurodegeneration. CNS Neurol Disord Drug Targets 9, 504–523.
Hofbauer, A., Ebel, T., Waltenspiel, B., Oswald, P., Chen, Y. C., Halder, P., Biskup, S., Lewandrowski, U., Winkler, C., Sickmann, A., Buchner, S., and Buchner, E. (2009). The Wuerzburg hybridoma library against Drosophila brain. J. Neurogenet. 23, 78–91.
Huang, T., Liu, Y., Huang, M., Zhao, X., and Cheng, L. (2010). Wnt1-cre-mediated conditional loss of Dicer results in malformation of the midbrain and cerebellum and failure of neural crest and dopaminergic differentiation in mice. J. Mol. Cell Biol. 2, 152–163.
Ito, K., and Awasaki, T. (2008). Clonal unit architecture of the adult fly brain. Adv. Exp. Med. Biol. 628, 137–158.
Kaasinen, V., Vilkman, H., Hietala, J., Någren, K., Helenius, H., Olsson, H., Farde, L., and Rinne, J. (2000). Age-related dopamine D2/D3 receptor loss in extrastriatal regions of the human brain. Neurobiol. Aging 21, 683–688.
Kim, K., Lee, Y. S., Harris, D., Nakahara, K., and Carthew, R. W. (2006). The RNAi pathway initiated by Dicer-2 in Drosophila. Cold Spring Harb. Symp. Quant. Biol. 71, 39–44.
Kong, E. C., Woo, K., Li, H., Lebestky, T., Mayer, N., Sniffen, M. R., Heberlein, U., Bainton, R. J., Hirsh, J., and Wolf, F. W. (2010). A pair of dopamine neurons target the D1-like dopamine receptor DopR in the central complex to promote ethanol-stimulated locomotion in Drosophila. PLoS ONE 5, e9954. doi: 10.1371/journal.pone.0009954.
Krashes, M. J., DasGupta, S., Vreede, A., White, B., Armstrong, J. D., and Waddell, S. (2009). A neural circuit mechanism integrating motivational state with memory expression in Drosophila. Cell 139, 416–427.
Lebestky, T., Chang, J. S., Dankert, H., Zelnik, L., Kim, Y. C., Han, K. A., Wolf, F. W., Perona, P., and Anderson, D. J. (2009). Two different forms of arousal in Drosophila are oppositely regulated by the dopamine D1 receptor ortholog DopR via distinct neural circuits. Neuron 64, 522–536.
Le Bourg, E. (1987). The rate of living theory. Spontaneous locomotor activity, aging and longevity in Drosophila melanogaster. Exp. Gerontol. 22, 359–369.
Lima, S. Q., and Miesenböck, G. (2005). Remote control of behavior through genetically targeted photostimulation of neurons. Cell 121, 141–152.
Liu, L., Davis, R. L., and Roman, G. (2007). Exploratory activity in Drosophila requires the kurtz nonvisual arrestin. Genetics 175, 1197–1212.
Liu, Z., Wang, X., Yu, Y., Li, X., Wang, T., Jiang, H., Ren, Q., Jiao, Y., Sawa, A., Moran, T., Ross, C. A., Montell, C., and Smith, W. W. (2008). A Drosophila model for LRRK2-linked parkinsonism. Proc. Natl. Acad. Sci. U.S.A. 105, 2693–2698.
Mao, Z., and Davis, R. L. (2009). Eight different types of dopaminergic neurons innervate the Drosophila mushroom body neuropil: anatomical and physiological heterogeneity. Front. Neural Circuits 3, 5.
Martin, J. R., Ernst, R., and Heisenberg, M. (1999). Temporal pattern of locomotor activity in Drosophila melanogaster. J. Comp. Physiol. A. 184, 73–84.
Martin, J. R., Fuchs, A., Bender, R., and Harting, J. (1986). Altered light/dark activity difference with aging in two rat strains. J. Gerontol. 41, 2–7.
Martinez, V. G., Javadi, C. S., Ngo, E., Ngo, L., Lagow, R. D., and Zhang, B. (2007). Age-related changes in climbing behavior and neural circuit physiology in Drosophila. Dev. Neurobiol. 67, 778–791.
Monastirioti, M. (1999). Biogenic amine systems in the fruit fly Drosophila melanogaster. Microsc. Res. Tech. 45, 106–121.
Morrow, G., and Tanguay, R. M. (2008). Mitochondria and ageing in Drosophila. Biotechnol. J. 3, 728–739.
Nässel, D. R., and Elekes, K. (1992). Aminergic neurons in the brain of blowflies and Drosophila: dopamine- and tyrosine hydroxylase-immunoreactive neurons and their relationship with putative histaminergic neurons. Cell Tissue Res. 267, 147–167.
Neckameyer, W. S., Woodrome, S., Holt, B., and Mayer, A. (2000). Dopamine and senescence in Drosophila melanogaster. Neurobiol. Aging 21, 145–152.
Ng, C., Mok, S. Z. S., Koh, C., Ouyang, X., Fivaz, M. L., Tan, E., Dawson, V. L., Dawson, T. M., Yu, F., and Lim, K. (2009). Parkin protects against LRRK2 G2019S mutant-induced dopaminergic neurodegeneration in Drosophila. J. Neurosci. 29, 11257–11262.
Obeso, J. A., Rodriguez-Oroz, M. C., Goetz, C. G., Marin, C., Kordower, J. H., Rodriguez, M., Hirsch, E. C., Farrer, M., Schapira, A. H., and Halliday, G. (2010). Missing pieces in the Parkinson’s disease puzzle. Nat. Med. 16, 653–661.
Palmiter, R. (2008). Dopamine signaling in the dorsal striatum is essential for motivated behaviors: lessons from dopamine-deficient mice. Ann. N. Y. Acad. Sci. 1129, 35–46.
Park, J., Lee, S. B., Lee, S., Kim, Y., Song, S., Kim, S., Bae, E., Kim, J., Shong, M., Kim, J. M., and Chung, J. (2006). Mitochondrial dysfunction in Drosophila PINK1 mutants is complemented by parkin. Nature 441, 1157–1161.
Partridge, L., Pletcher, S. D., and Mair, W. (2005). Dietary restriction, mortality trajectories, risk and damage. Mech. Ageing Dev. 126, 35–41.
Pesah, Y., Burgess, H., Middlebrooks, B., Ronningen, K., Prosser, J., Tirunagaru, V., Zysk, J., and Mardon, G. (2005). Whole-mount analysis reveals normal numbers of dopaminergic neurons following misexpression of alpha-Synuclein in Drosophila. Genesis 41, 154–159.
Reeves, S., Bench, C., and Howard, R. (2002). Ageing and the nigrostriatal dopaminergic system. Int. J. Geriatr. Psychiatry 17, 359–370.
Riemensperger, T., Völler, T., Stock, P., Buchner, E., and Fiala, A. (2005). Punishment prediction by dopaminergic neurons in Drosophila. Curr. Biol. 15, 1953–1960.
Sanz, A., Fernández-Ayala, D. J., Stefanatos, R. K., and Jacobs, H. T. (2010). Mitochondrial ROS production correlates with, but does not directly regulate lifespan in Drosophila. Aging (Albany NY) 2, 200–223.
Strauss, R. (2002). The central complex and the genetic dissection of locomotor behavior. Curr. Opin. Neurobiol. 12, 633–638.
Vallone, D., Picetti, R., and Borrelli, E. (2000). Structure and function of dopamine receptors. Neurosci. Biobehav. Rev. 24, 125–132.
Venderova, K., Kabbach, G., Abdel-Messih, E., Zhang, Y., Parks, R. J., Imai, Y., Gehrke, S., Ngsee, J., Lavoie, M. J., Slack, R. S., Rao, Y., Zhang, Z., Lu, B., Haque, M. E., and Park, D. S. (2009). Leucine-rich repeat kinase 2 interacts with Parkin, DJ-1 and PINK-1 in a Drosophila melanogaster model of Parkinson’s disease. Hum. Mol. Genet. 18, 4390–4404.
Vermeulen, C. J., and Loeschcke, V. (2007). Longevity and the stress response in Drosophila. Exp. Gerontol. 42, 153–159.
Volkow, N. D., Gur, R. C., Wang, G. J., Fowler, J. S., Moberg, P. J., Ding, Y. S., Hitzemann, R., Smith, G., and Logan, J. (1998a). Association between decline in brain dopamine activity with age and cognitive and motor impairment in healthy individuals. Am. J. Psychiatry 155, 344–349.
Volkow, N. D., Wang, G. J., Fowler, J. S., Ding, Y. S., Gur, R. C., Gatley, J., Logan, J., Moberg, P. J., Hitzemann, R., Smith, G., and Pappas, N. (1998b). Parallel loss of presynaptic and postsynaptic dopamine markers in normal aging. Ann. Neurol. 44, 143–147.
Waddell, S. (2010). Dopamine reveals neural circuit mechanisms of fly memory. Trends Neurosci. 9. [Epub ahead of print].
Wang, D., Qian, L., Xiong, H., Liu, J., Neckameyer, W. S., Oldham, S., Xia, K., Wang, J., Bodmer, R., and Zhang, Z. (2006). Antioxidants protect PINK1-dependent dopaminergic neurons in Drosophila. Proc. Natl. Acad. Sci. U.S.A. 103, 13520–13525.
Wang, D., Tang, B., Zhao, G., Pan, Q., Xia, K., Bodmer, R., and Zhang, Z. (2008). Dispensable role of Drosophila ortholog of LRRK2 kinase activity in survival of dopaminergic neurons. Mol. Neurodegener. 3, 3.
Wax, T. M., and Goodrick, C. L. (1978). Nearness to death and wheelrunning behavior in mice. Exp. Gerontol. 13, 233–236.
Whitworth, A. J., Theodore, D. A., Greene, J. C., Benes, H., Wes, P. D., and Pallanck, L. J. (2005). Increased glutathione S-transferase activity rescues dopaminergic neuron loss in a Drosophila model of Parkinson’s disease. Proc. Natl. Acad. Sci. U.S.A. 102, 8024–8029.
Wu, J. S., and Luo, L. (2006). A protocol for mosaic analysis with a repressible cell marker (MARCM) in Drosophila. Nat. Protoc. 1, 2583–2589.
Yang, Y., Gehrke, S., Imai, Y., Huang, Z., Ouyang, Y., Wang, J., Yang, L., Beal, M. F., Vogel, H., and Lu, B. (2006). Mitochondrial pathology and muscle and dopaminergic degeneration caused by inactivation of Drosophila PINK1 is rescued by Parkin. Proc. Natl. Acad. Sci. U.S.A. 103, 10793–10798.
Yellman, C., Tao, H., He, B., and Hirsh, J. (1997). Conserved and sexually dimorphic behavioral responses to biogenic amines in decapitated Drosophila. Proc. Natl. Acad. Sci. U.S.A. 94, 4131–4136.
Keywords: Parkinson’s disease, Drosophila, brain, dopamine, aging, locomotion, behavior, motor control
Citation: White KE, Humphrey DM and Hirth F (2010) The dopaminergic system in the aging brain of Drosophila. Front. Neurosci. 4:205. doi: 10.3389/fnins.2010.00205
Received: 15 September 2010;
Accepted: 30 October 2010;
Published online: 08 December 2010.
Edited by:
Haung Yu, Columbia University, USAReviewed by:
Ayodeji A. Asuni, New York University, USACopyright: © 2010 White, Humphrey and Hirth. This is an open-access article subject to an exclusive license agreement between the authors and the Frontiers Research Foundation, which permits unrestricted use, distribution, and reproduction in any medium, provided the original authors and source are credited.
*Correspondence: Frank Hirth, Department of Neuroscience, Institute of Psychiatry, Medical Research Council Centre for Neurodegeneration Research, King’s College London, PO Box 37, 16 De Crespigny Park, London SE5 8AF, UK. e-mail:ZnJhbmsuaGlydGhAa2NsLmFjLnVr
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.