
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
FOCUSED REVIEW article
Front. Neurosci., 08 December 2010
Volume 4 - 2010 | https://doi.org/10.3389/fnins.2010.00187
This article mentions parts of:
Living in a dangerous world: the shaping of behavioral profile by early environment and 5-HTT genotype
 Rebecca S. Heiming1,2*
Rebecca S. Heiming1,2* Norbert Sachser1,2*
Norbert Sachser1,2*
This review focuses on how behavioral profile is shaped by early adversity in individuals with varying serotonin transporter (5-HTT) genotype. In a recent study on 5-HTT knockout mice Heiming et al. (2009) simulated a ‘dangerous environment‘ by confronting pregnant and lactating females with odor cues of unfamiliar males, indicating the risk of infant killing. Growing up in a dangerous environment induced increased anxiety-related behavior and decreased exploratory locomotion in the offspring, the effects being most pronounced in mice lacking 5-HTT expression. We argue that these alterations in behavioral profile represent adaptive maternal effects that help the individuals to cope with adversity. In principle, such effects of adversity on behavioral profile should not automatically be regarded as pathological. Rather and in accordance with modern evolutionary theory they may represent adaptations, although individuals with 5-HTT genotype induced susceptibility to adversity may be at risk of developing pathologies.
Individuals may vary considerably in their behavioral profiles. To understand such variation is of major importance because it is frequently related to differences in quality of life (e.g., Broom, 2001), susceptibility to disease (e.g., Henry and Stephens, 1977; Von Holst, 1998; Korte et al., 2005), and finally reproductive success (e.g., Alcock, 2005). Thus, the analysis of variation in behavioral profile is a focus not only in bio-psychological and biomedical research, but also in fields studying the adaptive significance and evolution of behavior.
Individual differences in behavioral profile are shaped by both, genetic and environmental factors (e.g., Gross and Hen, 2004). Concerning the role of the genotype a growing body of evidence identifies for example the genetically encoded variation of the serotonin transporter (5-HTT) as a key element in the regulation of social behaviors (e.g., Canli and Lesch, 2007; Lewejohann et al., 2010), cognitive abilities (e.g., Borg et al., 2009; Olivier et al., 2009), emotional traits (e.g., Lesch et al., 1996; Holmes et al., 2003a; Schinka et al., 2004), and stress responses (e.g., Tjurmina et al., 2002; Gotlib et al., 2008). The 5-HTT regulates the availability of the neurotransmitter serotonin in the brain and its gene appears in two length variants in humans (5-HTT polymorphism). Carriers of at least one short 5-HTT gene variant display higher levels of neuroticism and harm-avoidance (Lesch et al., 1996) as well as a higher trait anxiety (Schinka et al., 2004) than homozygous carriers of the long variant. An ortholog of the human 5-HTT polymorphism is present in rhesus monkeys (Lesch et al., 1997), and consistent with the findings in humans, macaques carrying the short allele are more anxious than homozygous carriers of the long allele (Barr et al., 2003; Spinelli et al., 2007).
Concerning the role of the environment the organism seems to be most susceptible to external influences during early development, that is, the prenatal and early postnatal phase, when brain circuits are highly plastic (Champagne and Curley, 2005; Kaiser and Sachser, 2005). Accordingly, stressors that impinge on the maternal organism during pregnancy evoke high levels of anxiety in the offspring later in life (Vallée et al., 1997; Maccari et al., 2003), as does an adverse postnatal environment (Meaney, 2001; Pryce and Feldon, 2003). Studies in monkeys and humans clearly show that severe early environmental adversity can have profoundly negative consequences for later development and health, eventually resulting in pathology, i.e., forms of illness or disease (for a current view see special topic: Young and Murphy, 2009). For example, when infant rhesus monkeys are reared in complete social isolation for periods of 3–12 months, they display learning impairments, heightened fear-related behaviors, and deficits in virtually every aspect of social behavior. Total social isolation for at least the first 6 months of life enormously damages or destroys subsequent social and sexual behavioral capabilities of these monkeys, leading to detachment from the environment, hostility directed outwardly toward others and inwardly toward the animal’s own body, and inability to form adequate social or heterosexual attachments to others (Harlow et al., 1965; Harlow and Suomi, 1971). Humans who experienced stressful events in early life, e.g., childhood maltreatment or sexual abuse, suffer an increased risk of becoming depressed or suicidal in later life (Brown et al., 1999; Heim and Nemeroff, 2001). Confrontation with severe early caregiver deprivation, e.g., in institutionalized children, leads to persisting disinhibited attachment disturbances in later life, often with co-occurring problems of inattention, developmental delay, as well as emotional, cognitive, and language problems (O’Connor, 2005). In such extreme cases discussion about adaptive function seems inappropriate, since the effects are clearly diagnosable as pathological (Sachser et al., 2010).
Nonetheless, recent experimental animal studies on the effects of moderate levels of adversity on behavioral development suggest an alternative perspective: that variation in behavioral phenotype brought about by stressors can represent an adaptation to the prevailing and/or future environmental situation. From this point of view, deviations from the behavioral and physiological standard should not necessarily be regarded as pathological, but could also be seen as representing adaptations to the offspring’s likely environment (Kaiser and Sachser, 2005, 2009; Sachser et al., 2010).
Here we focus on how behavioral profile is shaped by early adversity in individuals with varying 5-HTT genotype. We argue that, although individuals with genetically induced susceptibility to adversity may be at risk of developing pathologies, the environmental shaping of behavioral profile by adversity is in principle adaptive.
In a recent study (Heiming et al., 2009) we sought to simulate a ‘dangerous environment’ in early life in order to study the effects of early adversity on the behavioral profile of wildtype and 5-HTT knockout mice.
Consistent with the findings in humans, 5-HTT knockout mice, which exhibit either reduced (+/−) or completely absent (−/−) 5-HTT expression (Bengel et al., 1998), demonstrate a range of behavioral and endocrine abnormalities that resemble symptoms of mood and anxiety disorders in humans (Holmes et al., 2003a,b; Lesch, 2005; Zhao et al., 2006; Kalueff et al., 2010; Lewejohann et al., 2010). Given the advantageous ability to control genetic background and environmental circumstances in rodent studies, the 5-HTT knockout mouse provides a valuable model system to study how genetic factors interact with environmental adversity in the development of behavioral profile (Holmes et al., 2003b).
During pregnancy and lactation heterozygous (+/−) 5-HTT knockout dams were repeatedly exposed to olfactory cues of unfamiliar adult males by introducing male-soiled bedding to one corner of their home cage (unfamiliar male bedding, UMB). Control females were instead treated with neutral bedding (NB; Figure 1). Unfamiliar male odor cues indicate the presence of potentially infanticidal males and the resulting risk of infant killing (vom Saal and Howard, 1982; Elwood and Kennedy, 1991; Perrigo et al., 1993; Weber and Olsson, 2008). These olfactory cues provide information about the males’ physiological state and infanticidal potential (Mandillo and D’Amato, 1997) and may even lead to pregnancy disruption in the dams shortly after fertilization (deCatanzaro et al., 1996, 2006), a phenomenon known as “Bruce effect” (Bruce, 1959, 1960). By applying these species-specific adverse stimuli we sought to create a paradigm with high relevance, particularly from an ecological point of view. UMB females spent more time in the quarter of the home cage where the bedding was introduced than NB females, and unlike NB controls, UMB dams did not habituate from the first to the fifth treatment. Thus, UMB females responded strongly and persistently to the male odor cues, suggesting that indeed a threatening environment was created; although confirmation from stress hormone analysis is not yet at hand.
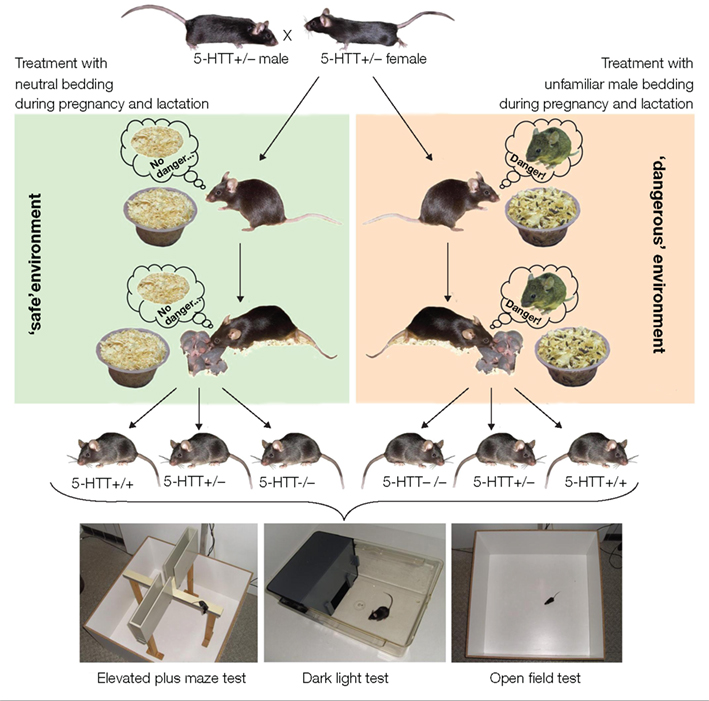
Figure 1. Simulation of a ’dangerous environment’ and subsequent behavioral testing of the offspring. Heterozygous 5-HTT knockout females were mated with 5-HTT +/− males. During pregnancy and lactation dams were repeatedly exposed to olfactory cues of unfamiliar adult males by introducing small amounts of male-soiled bedding to their home cage (‘dangerous environment’). The odor cues of strange males signal the threat of infant killing and thus create a ‘dangerous environment‘ for the mother and her pups. Offspring varying in genotype (5-HTT +/+, +/−, and −/−) were tested for their anxiety-related and exploratory behavior in the Elevated Plus Maze test, Dark Light test, and Open Field test in adulthood.
The offspring were tested in a range of behavioral tests, namely the Elevated Plus Maze test, the Open Field test, and the Dark Light test, in order to assess their anxiety-related and exploratory behavior (see Figure 1). These tests are based on the spontaneous exploratory activity of the mice and on their innate aversion to brightly lit, open, and exposed areas. High levels of exploration in these aversive areas, as opposed to the dark and enclosed areas, are thus interpreted as showing low levels of anxiety-related behavior (e.g., Lister, 1990; Holmes, 2001; Crawley, 2007). Behavioral testing of NB and UMB offspring (5-HTT +/+, +/−, and −/−) revealed a significant effect of the treatment, i.e., compared to NB offspring, UMB offspring showed increased levels of anxiety-related behavior and reduced exploratory locomotion in the Open Field and Dark Light test. Thus, growing up in a threatening environment led to an altered behavioral profile in later life of the mice. There was also a main effect of genotype with 5-HTT −/− mice showing higher levels of anxiety-related behavior and lower levels of exploration than 5-HTT +/− and 5-HTT +/+ mice. Finally, a measure of anxiety-related behavior, the latency to enter the light compartment of the Dark light box, was longest in 5-HTT −/− UMB mice. Thus, behavioral differences between offspring varying in genotype were intensified when their mothers were living in a dangerous world. In summary, applying this newly developed paradigm had a profound effect on the offspring’s behavioral profile, probably owing to the strong ecological relevance of the treatment.
The effects of adversity during early phases of life are predominantly studied by researchers with a psychological or biomedical background, mainly working with animal models of disease. Notably, they often regard the characteristic traits of individuals exposed to environmental stressors during pregnancy and lactation (e.g., heightened levels of anxiety, masculinized behavior in daughters, less pronounced expression of male-typical traits in sons, increased stress responsiveness) as deviations from the norm or even as pathological; i.e., forms of illness or disease. Alternatively, and in accordance with current evolutionary theory, scientists with an evolutionary background regard these traits as adaptive maternal effects (e.g., Mousseau and Fox, 1998; Birkhead et al., 2000; Qvarnström and Price, 2001; Dufty et al., 2002; Kaiser and Sachser, 2005, 2009; Sachser et al., 2010), meaning that mothers maximize their own Darwinian fitness by efficiently adjusting their offspring to current or future environmental conditions.
Heiming et al. (2009) argue that the anxiogenic effects of a threatening environment during early life might represent such adaptive maternal effects. The confrontation with bedding material from several male mice of different strains, alternating every treatment, simulates an environment populated by a high number of potentially infanticidal males, and thus a threatening environment for a female mouse and her young. When living in a dangerous habitat, it is highly adaptive for a mouse to behave careful and unobtrusively. Thus, it would be advantageous for mothers in such an environment to program their offspring to exhibit higher anxiety-related and lower exploratory behavior. Therefore, the behavioral alterations measured in UMB offspring could indeed represent adaptive effects. However, experiments are needed to decide unequivocally whether offspring from adverse conditions during early phases of life cope better with adversity and attain higher reproductive success under conditions of adversity than offspring that have lived in a safe, non-challenging world, and vice versa (Sachser et al., 2010). Adult emotional disorders in humans might not be promoted by early life adversity per se, but by a mismatch of the programmed and the later actual environment in combination with a more vulnerable or resilient genetic predisposition (Schmidt, 2010). In order to test this mismatch hypothesis of disease in mice UMB and NB offspring could be for example confronted with a dangerous and challenging environment in later life by keeping them in semi-naturalistic outdoor pens (e.g., Lewejohann et al., 2004) or in a large semi-naturalistic indoor enclosure (e.g., Lewejohann et al., 2009) populated by several adult males. Vice versa it could be tested whether UMB and NB mice differ concerning their reproductive success in a safe and non-challenging environment. Thus, the congruence of information transmitted by early adversity with the actual environmental conditions the offspring experiences later in life could be systematically varied.
Indications for adaptive shaping of offspring behavioral profile by early adversity are also assumed in other species. In guinea pigs, for instance, daughters and sons whose mothers have lived in a stable social world during pregnancy and lactation show sex-specific differences regarding behavior, endocrine systems, and brain development compared to those whose mothers have lived in an unstable social environment during this phase of life (for a summary see Kaiser and Sachser, 2005). While daughters of mothers living in an unstable social environment show higher amounts of male-typical courtship and play behavior later in life (behavioral masculinization), sons of mothers living in an unstable social environment display behavioral patterns that are usually shown only by very young guinea pigs (behavioral infantilization). Kaiser and Sachser (2009) argue that these are no signs of pathology, but that masculinized daughters and infantilized sons might be better adjusted to high-density populations that are characterized by social instability, while the opposite behavioral profiles may be beneficial when individual numbers are low and the social situation is stable. For example, being more robust and/or competitive, masculinized daughters may be able to defend important resources more efficiently, such as food and shelter, which are scarce in high-density situations. In contrast, under low density conditions sufficient resources are available and it is more beneficial to invest time and energy into reproductive effort rather than to build and maintain a male-typical phenotype to defend resources (for details see Kaiser and Sachser, 2005, 2009).
Another example that adversity in early life is not necessarily linked to a detrimental outcome in later life is a study in rats comparing offspring of high and low maternal care dams, showing either high or low levels of pup licking and grooming (Champagne et al., 2008). Although offspring of low maternal care dams (adverse postnatal environment) generally show lower cognitive performance under basal (non-stress) conditions when compared to offspring of high maternal care dams (Bredy et al., 2003), a different outcome is observed when these rats are tested under stressful conditions, i.e., a situation reminiscent of their early postnatal environment. Within a stressful context (e.g., fear conditioning task), adult offspring which received low maternal care during early phases of life perform better in a cognitive task than adult offspring of high maternal care dams (Champagne et al., 2008; Oitzl et al., 2010).
It is noteworthy that similar arguments for the adaptive value of early adversity in humans have been proposed. For example, Bateson et al. (2004) hypothesize that a period of starvation during pregnancy informs the developing fetus that food is probably going to be scarce in the future. Infants of such mothers often exhibit low body weight and correspondingly modified metabolism. These traits are not necessarily pathological, in as much as they help the infant to cope with environments of low food availability.
There are further studies in humans, rhesus monkeys and mice which investigated how early adversity shapes behavioral profile in individuals with different 5-HTT genotypes (Table 1). Carola et al. (2008) subjected 5-HTT +/− and wildtype mice to poor maternal care, i.e., reduced levels of licking and grooming, and found increased anxiety-related behavior in offspring of all genotypes. This effect has also been shown in other studies in mice (Calatayud et al., 2004; Carola et al., 2006) and in rats (Caldji et al., 1998), where low levels of licking and grooming and arched-back nursing led to increased behavioral fearfulness in the offspring. Several authors argue that the potentiated behavioral reactions (e.g., increased fearfulness; Calatayud et al., 2004; Carola et al., 2006) and higher endocrine responsivity to stress (e.g., exaggerated hypothalamic-pituitary-adrenal [HPA] axis reaction after 20 min restraint stress; Plotsky and Meaney, 1993; Liu et al., 1997; Anisman et al., 1998; Meaney, 2001) in rodents due to poor maternal care might be advantageous under conditions of increased environmental demand (e.g., high predator density or scarce resources), since these responses promote detection of potential threat, avoidance learning, and metabolic responses that are essential under the increased demands of adversity (e.g., Meaney, 2001). In accordance with the findings of Heiming et al. (2009), Carola et al. (2008) found that mice with altered 5-HTT expression were particularly susceptible to the effects of early adversity, since heterozygous 5-HTT knockout mice showed a more pronounced increase of anxiety-related behavior after the experience of low maternal care than wildtype mice.
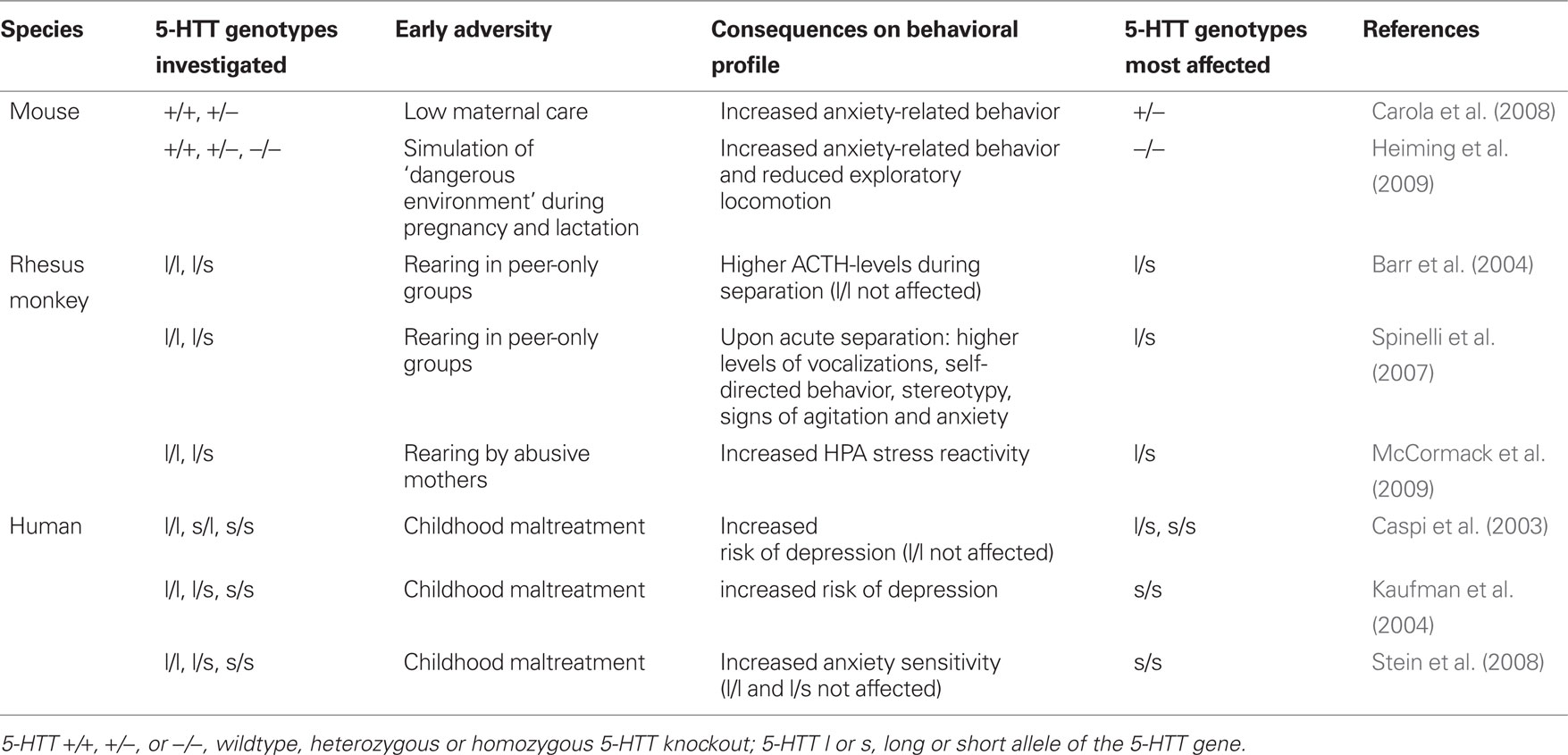
Table 1. Selected studies focusing on the shaping of behavioral profile by early adversity and 5-HTT genotype.
Like in rodents, the behavioral profile of Rhesus monkeys can be shaped by early adversity, which is often created by depriving juveniles of maternal care: Newborn animals are separated from their mother, hand-reared for the first month and then reared together with same age peers (peer-only reared), a procedure which results in decreased exploration and more extreme behavioral and adrenocortical responses to social separation at 6 months of age (Suomi, 1997, 2006). Consistent with the findings in rodents, rhesus monkeys carrying one copy of the low functioning ortholog of the 5-HTT gene (l/s) seem to be more susceptible to the behavioral consequences caused by peer-only rearing, and thus early adversity, than animals carrying two long 5-HTT gene alleles (l/l; Spinelli et al., 2007). For example, peer-only reared l/s monkeys had higher ACTH levels during acute separation from peers (Barr et al., 2004) and showed for example higher levels of vocalizations, self-directed behavior, stereotypy, as well as signs of agitation and anxiety than peer-only reared l/l animals (Spinelli et al., 2007). Similarly, l/s monkeys reared by abusive mothers were found to exhibit increased HPA stress reactivity (McCormack et al., 2009). Whether or not these changes represent adaptations – as discussed in rodents – or rather manifestations of pathology still remains to be studied.
Humans, who experienced early adversity, e.g., severe caregiving deprivation, show disturbances in emotional and behavioral development (O’Connor, 2005). In line with the findings in rodents and monkeys, human carriers of the short 5-HTT gene variant seem to be particularly prone to the effects of adverse early life experience. In a groundbreaking study Caspi et al. (2003) found that, whereas carriers of two long variants were not affected, carriers of the short 5-HTT gene variant experiencing childhood maltreatment suffered an increased risk of depression. In a study by Kaufman et al. (2004) maltreated children were shown to have elevated depression scores, as compared to control children. Notably, children carrying two short 5-HTT alleles were most vulnerable to the consequences of early neglect and abuse, having depression scores that were almost twice as high as in maltreated children with the other genotypes. Consistently, emotional (or physical) childhood maltreatment led to higher anxiety sensitivity in s/s individuals, than in other genotypes, a characteristic predisposing to the development of anxiety disorders and depression (Stein et al., 2008). It should be noted, however, that these results from human studies could not always be replicated (see Risch et al., 2009). In cases where profound early adversity causes severe, detrimental effects in humans, these changes are pathological. However, in cases of moderate effects, e.g., heightened trait anxiety, it remains to be studied whether these changes could represent adaptive effects, as discussed in rodents.
Taken together, these studies show that early adversity shapes behavioral profile, often resulting in increased anxiety-related traits. Such alterations are not necessarily pathologies; rather they may represent adaptations that help the individual to cope with adversity, e.g., by promoting detection of potential threat or avoidance learning. However, an increased susceptibility for adverse environmental influences, for instance caused by reduced levels of 5-HTT expression, may lead to the risk of developing pathologies in some individuals when confronted with early adversity.
How can early adversity effects change from adaptation into pathology? The principle mechanisms mediating adaptation through maternal effects seem to be clear (Figure 2). During the prenatal phase adverse environmental stimuli act on the pregnant female, causing hormonal changes in the maternal organism, which in turn influence the embryonic/fetal endocrine state. Hereby, fetal brain development is affected with major consequence for behavioral profile later in life. For example, the mother can respond with activation of the HPA axis and a consequent release of glucocorticoids. Exposure to high levels of glucocorticoids can permanently affect offspring glucocorticoid receptor expression (Welberg and Seckl, 2001; Bale, 2005), e.g., in the amygdala, which in turn is associated with an anxiogenic behavioral profile in rodents (Welberg et al., 2000).
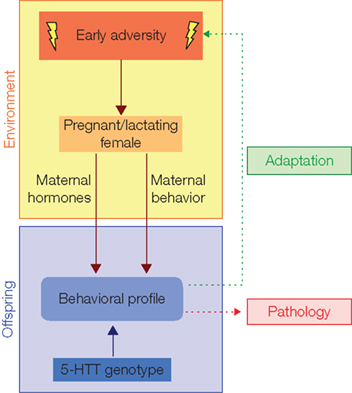
Figure 2. The shaping of behavioral profile by early adversity. Through influencing maternal hormones and maternal behavior early adversity shapes offspring behavioral profile. This process is modulated for instance by the 5-HTT genotype and in principle adjusts the offspring to environmental conditions of adversity (adaptation). An impaired 5-HTT expression, however, increases the risk of emotional disorders (pathology). Notably, a direct influence of early adversity on the offspring, as well as the possible role of father, siblings, or the whole social group is not considered in this schema. Changed after Sachser et al. (2010).
While maternal hormones appear to mediate influences of environmental adversity on offspring behavioral profile during pregnancy, during lactation the presence and behavior of social companions, in particular the mother, are of major importance. Indeed, the variation in the frequency and intensity of maternal care is a major source of inter-individual differences in behavioral profile and stress responsiveness in adulthood (Meaney, 2001; Kaufman et al., 2004; Carola et al., 2008; McCormack et al., 2009). It is well known that adverse conditions can directly influence maternal physiology and behavior (Christian and Davis, 1964; Chapman et al., 2000), and in particular maternal care (Festa-Bianchet et al., 2000; Neumann et al., 2005; Heitor and Vicente, 2008). Thus, it is likely that the effects of adversity during the early postnatal phase on offspring development are mediated by the presence and behavior of social companions, i.e., parents, siblings, or the whole social group (Sachser et al., 2010).
But why are carriers of low functioning 5-HTT genes at increased risk to develop pathologies under comparably adverse conditions? From our point of view there are three major characteristics facilitating the pathogenesis of emotional disorders in these individuals:
• Firstly, low functioning 5-HTT genes enhance the individual’s sensitivity to environmental adversity (Kendler et al., 2005; Mandelli et al., 2007). For example, in humans the short 5-HTT gene allele is related to increased stress reactivity (Gotlib et al., 2008), greater amygdala neuronal activity in response to fearful stimuli (Hariri et al., 2002) and possibly inadequate regulation and integration of amygdala-mediated arousal (Pezawas et al., 2005). Similarly, 5-HTT knockout mice display exaggerated pituitary and adreno-medullary responses to stressors (Li et al., 1999; Murphy et al., 2001; Tjurmina et al., 2002).
• Secondly, low functioning 5-HTT gene variants seem to be related to an impaired long-term extinction of negative experience. For instance, in experiments with Pavlovian fear conditioning 5-HTT −/− mice exhibit a significant deficit in fear extinction recall, that is, the long-term extinction of conditioned fear proceeds in a significantly slower way than in wildtypes (Wellman et al., 2007). Consistently, Jansen et al. (2010) showed that, whereas 5-HTT −/−, +/− and wildtype mice did not differ in anxiety-related behavior after winning a social contest, social defeat led to significantly higher anxiety-related behavior in 5-HTT −/− animals, suggesting that they did not extinguish the negative experience as good as the wildtypes.
• Thirdly, there are indications that low functioning 5-HTT variants are associated with a lower ability to actively cope with an adverse situation. For example, when groups of five male mice (all either 5-HTT −/−, +/−, or wildtype) were housed in spacious terraria connected to an emigration cage, 5-HTT −/− males had to be taken out because of injury more frequently than the other genotypes, although they had the same possibility to emigrate as 5-HTT +/− and +/+ males (Lewejohann et al., 2010). The authors argue that 5-HTT-/− mice manifest a lack of behavioral flexibility when confronted with unfavorable conditions and thus endure adverse situations rather passively. Consistently, 5-HTT −/− mice have been observed to show markedly increased immobility in the forced swim test (Holmes et al., 2002; Lira et al., 2003) and longer latencies to escape foot shock (Lira et al., 2003). Due to their lower ability to actively cope with adverse situations, for example by bringing them to an end through escaping, individuals carrying low functioning 5-HTT variants could be confronted with adverse situations longer than others, which ultimately facilitates the pathogenesis of emotional disorders.
In summary, when discussing early adversity-induced alterations in behavioral profile, the outcomes should not automatically be regarded as pathological, but should also be viewed from an evolutionary perspective. For example, the increased anxiety-related behavior of mice that grew up in a ‘dangerous’ environment provide a good example for the shaping of behavioral profile by early adversity in a possibly adaptive way. Whether these alterations really represent adaptations that help the individual to cope more efficiently with adversity still remains to be investigated. The risk of developing pathologies after experiencing early adversity is increased in individuals with reduced 5-HTT expression. Our hypothesis is that this vulnerability is caused by the combination of increased sensitivity to adversity, an impaired long-term extinction of negative experience and a lower ability to actively cope with an adverse situation.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
We thank Sylvia Kaiser and Lars Lewejohann for critical comments on the manuscript. This focused review was supported by a grant from the German Science Foundation (DFG) to Norbert Sachser (SFB/TRR58, Project A1).
An individual’s behavioral profile is the whole array of his/her characteristics related to behavioral traits, including social behaviors, cognitive abilities, emotions, as well as stress responses.
The 5-HTT is one key regulator of serotonergic neurotransmission, transporting serotonin (5-HT) from the synaptic cleft back into the presynaptic neuron and thereby terminating the serotonergic signal transmission. 5-HT system homeostasis is critical to the genesis, differentiation, and maturation of neuronal cells during early brain development.
The human 5-HTT gene contains a repeat length polymorphism in the upstream regulatory region: the short variant (s) is associated with a lower transcriptional efficiency as compared to the long gene variant (l), resulting in a reduced amount of 5-HTT proteins. Individuals with s/s or s/l genotype were found to have greater anxiety-related personality characteristics (Lesch et al., 1996).
In these mice the 5-HTT gene has been inactivated by an insertion of a non-sense sequence. Individuals carrying two alleles of the modified gene (5-HTT −/−) have no 5-HTT expression at all, whereas animals carrying one intact and one inactivated 5-HTT allele (5-HTT +/−) show a 5-HTT expression that is reduced by approximately 50% as compared to the wildtype (5-HTT +/+) (Bengel et al., 1998).
Maternal effects are a key concept in modern evolutionary theory, meaning that the environment experienced by the mother is translated into phenotypic variation in the offspring (e.g., by maternal hormones/maternal care). Thereby, mothers try to maximize their own Darwinian fitness by adjusting their offspring efficiently to the current environmental conditions (Mousseau and Fox, 1998).
Alcock, J. (2005). Animal Behavior: An Evolutionary Approach, 8th Edn. Sunderland: Sinauer Associates.
Anisman, H., Zaharia, M. D., Meaney, M. J., and Merali, Z. (1998). Do early-life events permanently alter behavioral and hormonal responses to stressors? Int. J. Dev. Neurosci. 16, 149–164.
Bale, T. L. (2005). Is mom too sensitive? Impact of maternal stress during gestation. Front. Neuroendocrinol. 26, 41–49.
Barr, C. S., Newman, T. K., Becker, M. L., Parker, C. C., Champoux, M., Lesch, K. P., Goldman, D., Suomi, S. J., and Higley, J. D. (2003). The utility of the non-human primate; model for studying gene by environment interactions in behavioral research. Genes Brain Behav. 2, 336–340.
Barr, C. S., Newman, T. K., Shannon, C., Parker, C., Dvoskin, R. L., Becker, M. L., Schwandt, M., Champoux, M., Lesch, K. P., Goldman, D., Suomi, S. J., and Higley, J. D. (2004). Rearing condition and rh5-HTTLPR interact to influence limbic–hypothalamic–pituitary–adrenal axis response to stress in infant macaques. Biol. Psychiatry 55, 733–738.
Bateson, P., Barker, D., Clutton-Brock, T., Deb, D., D’Udine, B., Foley, R. A., Gluckman, P., Godfrey, K., Kirkwood, T., Lahr, M. M., McNamara, J., Metcalfe, N. B., Monaghan, P., Spencer, H. G., and Sultan, S. E. (2004). Developmental plasticity and human health. Nature 430, 419–421.
Bengel, D., Murphy, D. L., Andrews, A. M., Wichems, C. H., Feltner, D., Heils, A., Mössner, R., Westphal, H., and Lesch, K.-P. (1998). Altered brain serotonin homeostasis and locomotor insensitivity to 3, 4-methylenedioxymethamphetamine (“ecstasy”) in serotonin transporter-deficient mice. Mol. Pharmacol. 53, 649–655.
Birkhead, T., Schwabl, H., and Burke, T. (2000). Testosterone and maternal effects – integrating mechanisms and function. Trends Ecol. Evol. 15, 86–87.
Borg, J., Henningsson, S., Saijo, T., Inoue, M., Bah, J., Westberg, L., Lundberg, J., Jovanovic, H., Andree, B., Nordstrom, A. L., Halldin, C., Eriksson, E., and Farde, L. (2009). Serotonin transporter genotype is associated with cognitive performance but not regional 5-HT1A receptor binding in humans. Int. J. Neuropsychopharmacol. 12, 783–792.
Bredy, T. W., Humpartzoomian, R. A., Cain, D. P., and Meaney, M. J. (2003). Partial reversal of the effect of maternal care on cognitive function through environmental enrichment. Neuroscience 118, 571–576.
Broom, D. M. (2001). Coping with Challenge: Welfare in Animals Including Humans. Dahlem Workshop Report 87. Berlin: Dahlem University Press.
Brown, J., Cohen, P., Johnson, J. G., and Smailes, E. M. (1999). Childhood abuse and neglect: specificity of effects on adolescent and young adult depression and suicidality. J. Am. Acad. Child Adolesc. Psychiatry 38, 1490–1496.
Bruce, H. M. (1960). A block to pregnancy in the mouse caused by proximity of strange males. J. Reprod. Fertil. 1, 96.
Calatayud, F., Coubard, S., and Belzung, C. (2004). Emotional reactivity in mice may not be inherited but influenced by parent. Physiol. Behav. 80, 465–474.
Caldji, C., Tannenbaum, B., Sharma, S., Francis, D., Plotsky, P. M., and Meaney, M. J. (1998). Maternal care during infancy regulates the development of neural systems mediating the expression of fearfulness in the rat. Proc. Natl. Acad. Sci. U.S.A. 95, 5335–5340.
Canli, T., and Lesch, K.-P. (2007). Long story short: the serotonin transporter in emotion regulation and social cognition. Nat. Neurosci. 10, 1103–1109.
Carola, V., Frazzetto, G., and Gross, C. (2006). Identifying interactions between genes and early environment in the mouse. Genes Brain Behav. 5, 189–199.
Carola, V., Frazzetto, G., Pascucci, T., Audero, E., Puglisi-Allegra, S., Cabib, S., Lesch, K.-P., and Gross, C. (2008). Identifying molecular substrates in a mouse model of the serotonin transporter × environment risk factor for anxiety and depression. Biol. Psychiatry 63, 840–846.
Caspi, A., Sugden, K., Moffitt, T. E., Taylor, A., Craig, I. W., Harrington, H., McClay, J., Mill, J., Martin, J., Braithwaite, A., and Poulton, R. (2003). Influence of life stress on depression: moderation by a polymorphism in the 5-HTT gene. Science 301, 386–389.
Champagne, D. L., Bagot, R. C., van Hasselt, F., Ramakers, G., Meaney, M. J., de Kloet, E. R., Joëls, M., and Krugers, H. (2008). Maternal care and hippocampal plasticity: evidence for experience-dependent structural plasticity, altered synaptic functioning, and differential responsiveness to glucocorticoids and stress. J. Neurosci. 28, 6037–6045.
Champagne, F. A., and Curley, J. P. (2005). How social experiences influence the brain. Curr. Opin. Neurobiol. 15, 704–709.
Chapman, J. C., Christian, J. J., Pawlikowski, M. A., Yasukawa, N., and Michael, S. D. (2000). Female house mice develop a unique ovarian lesion in colonies that are at maximum population density. Proc. Soc. Exp. Biol. Med. 225, 80–90.
Christian, J. J., and Davis, D. E. (1964). Endocrines, behavior, and population. Science 146, 1550–1560.
Crawley, J. N. (2007). What’s Wrong With My Mouse? Behavioral Phenotyping of Transgenic and Knockout Mice, 2nd Edn. Hoboken, NJ: John Wiley & Sons, Inc.
deCatanzaro, D., Beaton, E. A., Khan, A., and Vella, E. (2006). Urinary oestradiol and testosterone levels from novel male mice approach values sufficient to disrupt early pregnancy in nearby inseminated females. Reproduction 132, 309–317.
deCatanzaro, D., Zacharias, R., and Muir, C. (1996). Disruption of early pregnancy by direct and indirect exposure to novel males in mice: comparison of influences of preputialectomized and intact males. J. Reprod. Fertil. 106, 269–274.
Dufty, A. R., Clobert, J., and Møller, A. P. (2002). Hormones, developmental plasticity and adaptation. Trends Ecol. Evol. 17, 190.
Elwood, R. W., and Kennedy, H. F. (1991). Selectivity in paternal and infanticidal responses by male mice: effects of relatedness, location, and previous sexual partners. Behav. Neural Biol. 56, 129–147.
Festa-Bianchet, M., Jorgenson, J. T., and Réale, D. (2000). Early development, adult mass, and reproductive success in bighorn sheep. Behav. Ecol. 11, 633.
Gotlib, I. H., Joormann, J., Minor, K. L., and Hallmayer, J. (2008). HPA axis reactivity: a mechanism underlying the associations among 5-HTTLPR, stress, and depression. Biol. Psychiatry 63, 847–851.
Gross, C., and Hen, R. (2004). The developmental origins of anxiety. Nat. Rev. Neurosci. 5, 545–552.
Hariri, A. R., Mattay, V. S., Tessitore, A., Kolachana, B., Fera, F., Goldman, D., Egan, M. F., and Weinberger, D. R. (2002). Serotonin transporter genetic variation and the response of the human amygdala. Science 297, 400–403.
Harlow, H. F., Dodsworth, R. O., and Harlow, M. K. (1965). Total social isolation in monkeys. Proc. Natl. Acad. Sci. U.S.A. 54, 90–97.
Harlow, H. F., and Suomi, S. J. (1971). Social recovery by isolation-reared monkeys. Proc. Natl. Acad. Sci. U.S.A. 68, 1534–1538.
Heim, C., and Nemeroff, C. B. (2001). The role of childhood trauma in the neurobiology of mood and anxiety disorders: preclinical and clinical studies. Biol. Psychiatry 49, 1023–1039.
Heiming, R. S., Jansen, F., Lewejohann, L., Kaiser, S., Schmitt, A., Lesch, K. P., and Sachser, N. (2009). Living in a dangerous world: the shaping of behavioral profile by early environment and 5-HTT genotype. Front. Behav. Neurosci. 3:26. doi: 10.3389/neuro.08.026.2009.
Heitor, F., and Vicente, L. (2008). Maternal care and foal social relationships in a herd of sorraia horses: influence of maternal rank and experience. Appl. Anim. Behav. Sci. 113, 189–205.
Henry, J. P., and Stephens, P. M. (1977). Stress, Health, and the Social Environment. A Sociobiological Approach to Medicine. New York: Springer-Verlag.
Holmes, A. (2001). Targeted gene mutation approaches to the study of anxiety-like behavior in mice. Neurosci. Biobehav. Rev. 25, 261–273.
Holmes, A., Li, Q., Murphy, D. L., Gold, E., and Crawley, J. N. (2003a). Abnormal anxiety-related behavior in serotonin transporter null mutant mice: the influence of genetic background. Genes Brain Behav. 2, 365–380.
Holmes, A., Murphy, D. L., and Crawley, J. N. (2003b). Abnormal behavioral phenotypes of serotonin transporter knockout mice: parallels with human anxiety and depression. Biol. Psychiatry 54, 953–959.
Holmes, A., Yang, R. J., Murphy, D. L., and Crawley, J. N. (2002). Evaluation of antidepressant-related behavioral responses in mice lacking the serotonin transporter. Neuropsychopharmacology 27, 914–923.
Jansen, F., Heiming, R. S., Lewejohann, L., Touma, C., Palme, R., Schmitt, A., Lesch, K. P., and Sachser, N. (2010). Modulation of behavioural profile and stress response by 5-HTT genotype and social experience in adulthood. Behav. Brain Res. 207, 21–29.
Kaiser, S., and Sachser, N. (2005). The effects of prenatal social stress on behaviour: mechanisms and function. Neurosci. Biobehav. Rev. 29, 283–294.
Kaiser, S., and Sachser, N. (2009). Effects of prenatal social stress on offspring development: pathology or adaptation? Curr. Dir. Psychol. Sci. 18, 118.
Kalueff, A. V., Olivier, J. D. A., Nonkes, L. J. P., and Homberg, J. R. (2010). Conserved role for the serotonin transporter gene in rat and mouse neurobehavioral endophenotypes. Neurosci. Biobehav. Rev. 34, 373–386.
Kaufman, J., Yang, B.-Z., Douglas-Palumberi, H., Houshyar, S., Lipschitz, D., Krystal, J. H., and Gelernter, J. (2004). Social supports and serotonin transporter gene moderate depression in maltreated children. Proc. Natl. Acad. Sci. U.S.A. 101, 17316–17321.
Kendler, K. S., Kuhn, J. W., Vittum, J., Prescott, C. A., and Riley, B. (2005). The interaction of stressful life events and a serotonin transporter polymorphism in the prediction of episodes of major depression: a replication. Arch. Gen. Psychiatry 62, 529–535.
Korte, S. M., Koolhaas, J. M., Wingfield, J. C., and McEwen, B. S. (2005). The Darwinian concept of stress: benefits of allostasis and costs of allostatic load and the trade-offs in health and disease. Neurosci. Biobehav. Rev. 29, 3–38.
Lesch, K.-P. (2005). Genetic alterations of the murine serotonergic gene pathway: the neurodevelopmental basis of anxiety. Handb. Exp. Pharmacol. 169, 71–112.
Lesch, K.-P., Bengel, D., Heils, A., Sabol, S. Z., Greenberg, B. D., Petri, S., Benjamin, J., Müller, C. R., Hamer, D. H., and Murphy, D. L. (1996). Association of anxiety-related traits with a polymorphism in the serotonin transporter gene regulatory region. Science 274, 1527–1531.
Lesch, K. P., Meyer, J., Glatz, K., Flügge, G., Hinney, A., Hebebrand, J., Klauck, S. M., Poustka, A., Poustka, F., Bengel, D., Mössner, R., Riederer, P., and Heils, A. (1997). The 5-HT transporter gene-linked polymorphic region (5-HTTLPR) in evolutionary perspective: alternative biallelic variation in rhesus monkeys. Rapid communication. J. Neural Transm. 104, 1259–1266.
Lewejohann, L., Hoppmann, A. M., Kegel, P., Kritzler, M., Krüger, A., and Sachser, N. (2009). Behavioral phenotyping of a murine model of Alzheimer’s disease in a seminaturalistic environment using RFID tracking. Behav. Res. Methods 41, 850–856.
Lewejohann, L., Kloke, V., Heiming, R. S., Jansen, F., Kaiser, S., Schmitt, A., Lesch, K. P., and Sachser, N. (2010). Social status and day-to-day behaviour of male serotonin transporter knockout mice. Behav. Brain Res. 211, 220–228.
Lewejohann, L., Skryabin, B. V., Sachser, N., Prehn, C., Heiduschka, P., Thanos, S., Jordan, U., Dell’Omo, G., Vyssotski, A. L., Pleskacheva, M. G., Lipp, H.-P., Tiedge, H., Brosius, J., and Prior, H. (2004). Role of a neuronal small non-messenger RNA: behavioural alterations in BC1 RNA-deleted mice. Behav. Brain Res. 273–289.
Li, Q., Wichems, C., Heils, A., Van De Kar, L. D., Lesch, K.-P., and Murphy, D. L. (1999). Reduction of 5-hydroxytryptamine (5-HT)(1A)-mediated temperature and neuroendocrine responses and 5-HT(1A) binding sites in 5-HT transporter knockout mice. J. Pharmacol. Exp. Ther. 291, 999–1007.
Lira, A., Zhou, M., Castanon, N., Ansorge, M. S., Gordon, J. A., Francis, J. H., Bradley-Moore, M., Lira, J., Underwood, M. D., Arango, V., Kung, H. F., Hofer, M. A., Hen, R., and Gingrich, J. A. (2003). Altered depression-related behaviors and functional changes in the dorsal raphe nucleus of serotonin transporter-deficient mice. Biol. Psychiatry 54, 960–971.
Lister, R. G. (1990). Ethologically-based animal models of anxiety disorders. Pharmacol. Ther. 46, 321–340.
Liu, D., Diorio, J., Tannenbaum, B., Caldji, C., Francis, D., Freedman, A., Sharma, S., Pearson, D., Plotsky, P. M., and Meaney, M. J. (1997). Maternal care, hippocampal glucocorticoid receptors, and hypothalamic–pituitary–adrenal responses to stress. Science 277, 1659–1662.
Maccari, S., Darnaudery, M., Morley-Fletcher, S., Zuena, A. R., Cinque, C., and Van Reeth, O. (2003). Prenatal stress and long-term consequences: implications of glucocorticoid hormones. Neurosci. Biobehav. Rev. 27, 119–127.
Mandelli, L., Serretti, A., Marino, E., Pirovano, A., Calati, R., and Colombo, C. (2007). Interaction between serotonin transporter gene, catechol-O-methyltransferase gene and stressful life events in mood disorders. Int. J. Neuropsychopharmacol. 10, 437–447.
Mandillo, S., and D’Amato, F. R. (1997). Effect of strange male odour on parental care in lactating female mice. Anim. Behav. 54, 901–910.
McCormack, K., Newman, T. K., Higley, J. D., Maestripieri, D., and Sanchez, M. M. (2009). Serotonin transporter gene variation, infant abuse, and responsiveness to stress in rhesus macaque mothers and infants. Horm. Behav. 55, 538–547.
Meaney, M. J. (2001). Maternal care, gene expression, and the transmission of individual differences in stress reactivity across generations. Annu. Rev. Neurosci. 24, 1161–1192.
Mousseau, T. A., and Fox, C. W. (1998). The adaptive significance of maternal effects. Trends Ecol. Evol. 13, 403.
Murphy, D. L., Li, Q., Engel, S., Wichems, C., Andrews, A., Lesch, K. -P., and Uhl, G. (2001). Genetic perspectives on the serotonin transporter. Brain Res. Bull. 56, 487–494.
Neumann, I. D., Krömer, S. A., and Bosch, O. J. (2005). Effects of psycho-social stress during pregnancy on neuroendocrine and behavioural parameters in lactation depend on the genetically determined stress vulnerability. Psychoneuroendocrinology 30, 791–806.
O’Connor, T. G. (2005). “Attachment disturbances associated with early severe deprivation,” in Attachment and Bonding. A New Synthesis, eds C. S. Carter, L. Ahnert, K. E. Grossmann, S. B. Hrdy, M. B. Lamb, S. W. Porges, and N. Sachser, (Cambridge, MA: The MIT Press), 257–267.
Oitzl, M. S., Champagne, D. L., van der Veen, R., and de Kloet, E. R. (2010). Brain development under stress: hypotheses of glucocorticoid actions revisited. Neurosci. Biobehav. Rev. 34, 853–866.
Olivier, J. D. A., Jans, L. A. W., Blokland, A., Broers, N. J., Homberg, J. R., Ellenbroek, B. A., and Cools, A. R. (2009). Serotonin transporter deficiency in rats contributes to impaired object memory. Genes Brain Behav. 8, 829–834.
Perrigo, G., Belvin, L., Quindry, P., Kadir, T., Becker, J., van Look, C., Niewoehner, J., and vom Saal, F. S. (1993). Genetic mediation of infanticide and parental behavior in male and female domestic and wild stock house mice. Behav. Genet. 23, 525–531.
Pezawas, L., Meyer-Lindenberg, A., Drabant, E. M., Verchinski, B. A., Munoz, K. E., Kolachana, B. S., Egan, M. F., Mattay, V. S., Hariri, A. R., and Weinberger, D. R. (2005). 5-HTTLPR polymorphism impacts human cingulate–amygdala interactions: a genetic susceptibility mechanism for depression. Nat. Neurosci. 8, 828–834.
Plotsky, P. M., and Meaney, M. J. (1993). Early, postnatal experience alters hypothalamic corticotropin-releasing factor (CRF) mRNA, median eminence CRF content and stress-induced release in adult rats. Brain Res. Mol. Brain Res. 18, 195–200.
Pryce, C. R., and Feldon, J. (2003). Long-term neurobehavioural impact of the postnatal environment in rats: manipulations, effects and mediating mechanisms. Neurosci. Biobehav. Rev. 27, 57–71.
Qvarnström, A., and Price, T. D. (2001). Maternal effects, paternal effects and sexual selection. Trends Ecol. Evol. 16, 95–100.
Risch, N., Herrell, R., Lehner, T., Liang, K.-Y., Eaves, L., Hoh, J., Griem, A., Kovacs, M., Ott, J., and Merikangas, K. R. (2009). Interaction between the serotonin transporter gene (5-HTTLPR), stressful life events, and risk of depression: a meta-analysis. JAMA 301, 2462–2471.
Sachser, N., Hennessy, M. B., and Kaiser, S. (2010). Adaptive modulation of behavioural profiles by social stress during early phases of life and adolescence. Neurosci. Biobehav. Rev. (in press).
Schinka, J. A., Busch, R. M., and Robichaux-Keene, N. (2004). A meta-analysis of the association between the serotonin transporter gene polymorphism (5-HTTLPR) and trait anxiety. Mol. Psychiatry 9, 197–202.
Schmidt, M. V. (2010). Animal models for depression and the mismatch hypothesis of disease. Psychoneuroendocrinology (in press).
Spinelli, S., Schwandt, M. L., Lindell, S. G., Newman, T. K., Heilig, M., Suomi, S. J., Higley, J. D., Goldman, D., and Barr, C. S. (2007). Association between the recombinant human serotonin transporter linked promoter region polymorphism and behavior in rhesus macaques during a separation paradigm. Dev. Psychopathol. 19, 977–987.
Stein, M. B., Schork, N. J., and Gelernter, J. (2008). Gene-by-environment (serotonin transporter and childhood maltreatment) interaction for anxiety sensitivity, an intermediate phenotype for anxiety disorders. Neuropsychopharmacology 33, 312–319.
Suomi, S. J. (1997). Early determinants of behaviour: evidence from primate studies. Br. Med. Bull. 53, 170–184.
Suomi, S. J. (2006). Risk, resilience, and gene × environment interactions in rhesus monkeys. Ann. N. Y. Acad. Sci. 1094, 52–62.
Tjurmina, O. A., Armando, I., Saavedra, J. M., Goldstein, D. S., and Murphy, D. L. (2002). Exaggerated adrenomedullary response to immobilization in mice with targeted disruption of the serotonin transporter gene. Endocrinology 143, 4520–4526.
Vallée, M., Mayo, W., Dellu, F., Le Moal, M., Simon, H., and Maccari, S. (1997). Prenatal stress induces high anxiety and postnatal handling induces low anxiety in adult offspring: correlation with stress-induced corticosterone secretion. J. Neurosci. 17, 2626–2636.
vom Saal, F. S., and Howard, L. S. (1982). The regulation of infanticide and parental behavior: implications for reproductive success in male mice. Science 215, 1270–1272.
Von Holst, D. (1998). “The concept of stress and its relevance for animal behavior,” in Advances of the Study of Behavior, eds D. S. Lehman, R. Hinde, and E. Shaw (London: Academic Press), 1–131.
Weber, E. M., and Olsson, I. A. S. (2008). Maternal behaviour in Mus musculus sp.: an ethological review. Appl. Anim. Behav. Sci. 114, 1.
Welberg, L. A. M., and Seckl, J. R. (2001). Prenatal stress, glucocorticoids and the programming of the brain. J. Neuroendocrinol. 13, 113–128.
Welberg, L. A. M., Seckl, J. R., and Holmes, M. C. (2000). Inhibition of 11beta-hydroxysteroid dehydrogenase, the foeto-placental barrier to maternal glucocorticoids, permanently programs amygdala GR mRNA expression and anxiety-like behaviour in the offspring. Eur. J. Neurosci. 12, 1047–1054.
Wellman, C. L., Izquierdo, A., Garrett, J. E., Martin, K. P., Carroll, J., Millstein, R., Lesch, K.-P., Murphy, D. L., and Holmes, A. (2007). Impaired stress-coping and fear extinction and abnormal corticolimbic morphology in serotonin transporter knock-out mice. J. Neurosci. 27, 684–691.
Young, L. J., and Murphy, A. Z. (2009). Long term consequences of early life experience. Front. Behav. Neurosci.
Keywords: adaptation, behavioral profile, gene × environment interaction, early adversity, serotonin transporter
Citation: Heiming RS and Sachser N (2010) Consequences of serotonin transporter genotype and early adversity on behavioral profile – pathology or adaptation? Front. Neurosci. 4:187. doi: 10.3389/fnins.2010.00187
Received: 23 August 2010;
Accepted: 17 October 2010;
Published online: 08 December 2010.
Edited by:
Larry J. Young, Emory University School of Medicine, USAReviewed by:
Chadi Touma, Max Planck Institute of Psychiatry, GermanyCopyright: © 2010 Heiming and Sachser. This is an open-access article subject to an exclusive license agreement between the authors and the Frontiers Research Foundation, which permits unrestricted use, distribution, and reproduction in any medium, provided the original authors and source are credited.
*Correspondence: Norbert Sachser, Department of Behavioral Biology University of Muenster, Badestraße, 13 D-48149, Muenster, Germany.c2FjaHNlckB1bmktbXVlbnN0ZXIuZGU=;cmViZWNjYWhlaW1pbmdAd2ViLmRl
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.