
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
PERSPECTIVE article
Front. Neurorobot., 16 December 2021
Volume 15 - 2021 | https://doi.org/10.3389/fnbot.2021.750519
This article is part of the Research TopicRehabilitation Robotics: Challenges in Design, Control, and Real ApplicationsView all 16 articles
 Anne D. Koelewijn1*
Anne D. Koelewijn1* Musa Audu2,3
Musa Audu2,3 Antonio J. del-Ama4
Antonio J. del-Ama4 Annalisa Colucci5
Annalisa Colucci5 Josep M. Font-Llagunes6,7
Josep M. Font-Llagunes6,7 Antonio Gogeascoechea8
Antonio Gogeascoechea8 Sandra K. Hnat2,3
Sandra K. Hnat2,3 Nathan Makowski2,9
Nathan Makowski2,9 Juan C. Moreno10
Juan C. Moreno10 Mark Nandor2,11
Mark Nandor2,11 Roger Quinn2,11
Roger Quinn2,11 Marc Reichenbach12,13
Marc Reichenbach12,13 Ryan-David Reyes2,3
Ryan-David Reyes2,3 Massimo Sartori8
Massimo Sartori8 Surjo Soekadar5
Surjo Soekadar5 Ronald J. Triolo2,3
Ronald J. Triolo2,3 Mareike Vermehren5
Mareike Vermehren5 Christian Wenger14
Christian Wenger14 Utku S. Yavuz15
Utku S. Yavuz15 Dietmar Fey13
Dietmar Fey13 Philipp Beckerle16
Philipp Beckerle16Personalization of gait neuroprosthetics is paramount to ensure their efficacy for users, who experience severe limitations in mobility without an assistive device. Our goal is to develop assistive devices that collaborate with and are tailored to their users, while allowing them to use as much of their existing capabilities as possible. Currently, personalization of devices is challenging, and technological advances are required to achieve this goal. Therefore, this paper presents an overview of challenges and research directions regarding an interface with the peripheral nervous system, an interface with the central nervous system, and the requirements of interface computing architectures. The interface should be modular and adaptable, such that it can provide assistance where it is needed. Novel data processing technology should be developed to allow for real-time processing while accounting for signal variations in the human. Personalized biomechanical models and simulation techniques should be developed to predict assisted walking motions and interactions between the user and the device. Furthermore, the advantages of interfacing with both the brain and the spinal cord or the periphery should be further explored. Technological advances of interface computing architecture should focus on learning on the chip to achieve further personalization. Furthermore, energy consumption should be low to allow for longer use of the neuroprosthesis. In-memory processing combined with resistive random access memory is a promising technology for both. This paper discusses the aforementioned aspects to highlight new directions for future research in gait neuroprosthetics.
Gait neuroprostheses aim to restore function in persons with paralysis caused by various injuries, diseases, or dysfunctions in the central or peripheral nervous system, e.g., a stroke, cerebral palsy (CP) or a spinal cord injury (SCI). Interfaces with the brain, spinal cord or the periphery are appropriate (Figure 1), whereas the type and location of the interface is highly dependent on the user's abilities and remaining possibilities for voluntary control. Development of gait neuroprostheses should focus on user-friendliness, and aim to maximize speed and safety, while minimizing fall risk. Furthermore, systems should be portable and easy-to-use to achieve their adoption in real-life environments.
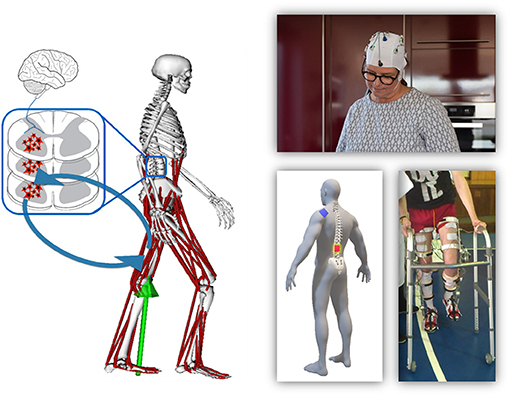
Figure 1. Overview of the different organs involved in gait and their communication structure (left). The right side shows examples of prostheses that interface with these organs, specifically the brain (top), spinal cord (bottom left), or periphery (bottom right).
Despite some individual success using interfaces with the brain (e.g., Ajiboye et al. 2017), spinal cord (e.g., Wagner et al. 2018), and periphery (e.g., Nandor et al. 2021), widespread application of neuroprostheses is still limited. One of the main challenges are differences between and within individuals. Between individuals, there is significant heterogeneity in the target population, which requires personalization of stimulation, stimulus timing and intensity, and the possible extent of additional motorized assistance. With “personalization,” we mean that every patient is provided with an individualized neuroprosthesis. This personalization can be done on the hardware and software level. It can be achieved offline through modeling and simulation, or online through adaptation of the stimulation scheme to the patient's current abilities. Personalizing the intervention is currently a time intensive process of trial and error by a human expert. Automation of this process with expert systems that can learn the optimal combinations of muscular and motor activation for a given individual would increase accessibility of gait neuroprostheses (Seel et al., 2016). Over time, due to fatiguing or improved function after rehabilitation, individuals might benefit from different stimulation patterns, which requires stimulation to be adaptive (Del-Ama et al., 2014).
The development of adaptive interfaces and personalized devices requires an interdisciplinary approach involving clinicians, neuroscientists, engineers, and computer scientists. The effectiveness of gait neuroprostheses can be quantitatively assessed by measuring the gait speed, where desired gait speeds suitable for community use are between 0.8 and 1.2 m/s (Robinett and Vondran, 1988; Lapointe et al., 2001), or by measuring the metabolic energy expenditure while using the device (Asselin et al., 2015; Evans et al., 2015; Miller et al., 2016). Other acceptable metrics could be gait outcomes such as kinematics or symmetry (Hayes et al., 2020). Furthermore, limits for comfortable and tolerable stimulation should be defined for each user. Figure 2 provides an overview of the different aims and future directions that are described in this paper. Movement analyses and simulations are required to understand pathological gait patterns and to tailor assistance, while the design should also be modular and user friendly. Artificial intelligence (AI) would allow control algorithms to be adapted to each individual user, and potentially over the course of rehabilitation. Furthermore, AI has the potential to process data, e.g., electroencephalography and electromyography, accurately in real-time, which is challenging due to the noisiness of these measurements. Adoption of AI in gait neuroprostheses requires development of new software and hardware for efficient on-chip learning, which should also focus on low energy consumption to allow for daily use of the device.
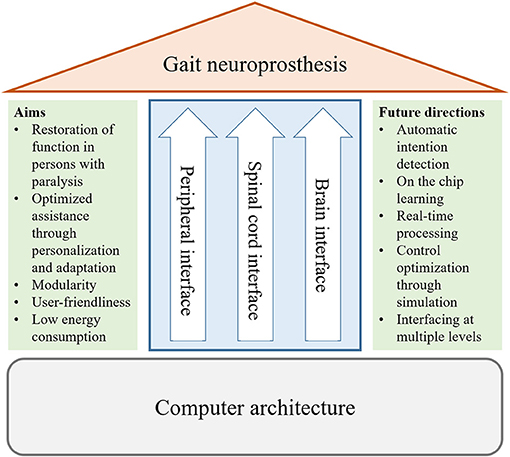
Figure 2. Overview of the topics of this perspective on gait neuroprostheses. We discuss requirements and future directions for interfaces with the periphery and the central nervous system (brain and spinal cord), as well as for the computer architecture.
In this perspective paper, the organizers, speakers, and authors of the mini symposium “Adaptation Strategies for Personalized Gait Neuroprosthetics” which was held during the 10th International IEEE/EMBS Conference on Neural Engineering (NER) in May 2021, summarize and extend their contributions and discussions to point out directions for future research. The workshop comprised talks and discussion from experts working on assistive and rehabilitative neuroprostheses and exoskeletons. This perspective covers three topics: interfacing with the periphery, interfacing with the central nervous system, and requirements of interface computing architectures. We discuss and provide a perspective on current research and future directions.
Interfacing with the peripheral nervous system is a promising method to address specific functional deficits (Wilson et al., 2019). Through user-specific stimulation via surface, percutaneous, or implanted electrodes, muscles can perform a majority of the work for particular tasks. By implementing hybrid actuation approaches combining neural stimulation of affected muscles with robotic assistance, lower extremity function can be restored for patients with gait impairments, who can still drive the main features of the walking cycle by their healthy or preserved muscles. By bringing together neuroprosthetic and wearable robotic approaches, those hybrid concepts combine physiologic benefits by activating the other paralyzed and paretic lower limb muscles through neural stimulation, reducing the demand on external robotic assistance, and augmenting volitional and programmed movements to enhance safety, stability, and endurance.
Neuromechanical simulations of such neuromuscular, biomechanical, and mechanical interactions can be used to optimize the performance of users, hardware design (e.g., reduce size, weight or energy consumption), and controller design (e.g., seamless motor/muscle actuation and appropriate sensory feedback) (Alonso et al., 2012; Crago et al., 2014; García-Vallejo et al., 2016; Uchida et al., 2016; Sreenivasa et al., 2017; Michaud et al., 2019; Sauder et al., 2019). Dynamic simulations of the system composed of the human and the neuroprosthesis can predict the combined human-neuroprosthesis response, allow for device and control customization to maximize walking ability, and improve our understanding of the interaction between human and device for new movement conditions. We advocate to extend computational neuromusculoskeletal models to encompass paralysis-related muscular constraints (Alonso et al., 2012; García-Vallejo et al., 2016) and auxiliary assistive devices (e.g., crutches/walker, orthoses, exoskeletons, etc.) (Febrer-Nafría et al., 2020, 2021) to achieve subject-specific model-based optimization of the device and its control. Then, such simulations might reveal insights into biomechanical effects such as altered recruitment, reduced force production due to atrophy, fatigue effects, or abnormal synergies (Shin et al., 2018). However, creating a personalized neuromusculoskeletal model of a patient is still challenging, since the exact underlying neurological problems of a patient are difficult to extract, and simulations cannot include the variability in muscle responses over time. Experimental validation of simulated clinical outcomes is therefore required (De Groote and Falisse, 2021; Fregly, 2021). Furthermore, even though simulations can provide insight into the required stimulation pattern, the stimulation needs to be adjusted in practice due to the variability in muscle response.
To adapt to the individual user, modular and adjustable hardware and control solutions can be implemented on a joint-need basis, and adapted to different anthropometrics, available muscles and their capabilities, as well as the neurological conditions (Del-Ama et al., 2014; Makowski et al., 2021; Nandor et al., 2021). Control in particular requires an adaptive approach that continuously updates muscle stimulation and electric motor actuation, and potentially prioritizes muscle activity through commanding motors in a trajectory-free ballistic paradigm while monitoring and managing muscle fatigue. To achieve seamless integration of auxiliary assistive devices, control strategies might rely on sensors onboard the human and the machine to measure user-device interaction (Lancini et al., 2016; Ugurlu et al., 2020) and should favor low impedances so the user's muscles can backdrive the device (Foglyano et al., 2016; Beckerle et al., 2017). Wearable kinematic sensing based on inertial measurement units is expected to provide equivalent performance to marker-based approaches. To this end, recent works propose to integrate inertial measurement units into musculoskeletal modeling workflows (Dorschky et al., 2019, 2020; Al Borno et al., 2021; Guidolin et al., 2021). Beyond this, sensory data from the limbs and muscles could be used to improve control and increase muscle perfomance by improved fatigue management compared to current methods based on muscle activity estimation (Alibeji et al., 2017; Mohamad et al., 2017).
Neural interfacing technologies connected to the CNS, the spinal cord (Wagner et al., 2018) and the brain (Ajiboye et al., 2017) have shown promising results, achieving restoration of control of extremities. While myographic control can provide more reliable and versatile assistance, there is increasing evidence that only stimulation at the CNS level can trigger motor recovery (Soekadar et al., 2015; Cervera et al., 2018). Moreover, when there is no voluntary control of the periphery, an interface to the central nervous system might be the only remaining approach to restore movement or communication (Birbaumer et al., 2014). Most neural interfaces focus on either the brain or the spinal cord, but recently, pioneering studies also investigate combinations, either of the brain and the spinal cord, or the brain and the periphery (Dixon et al., 2016; Shulga et al., 2021).
For the spinal cord, a key goal is to interface with specific motor circuitries responsible for locomotion. Commonly, invasive interfaces are used (Hochberg et al., 2006, 2012; Wagner et al., 2018), which require complex setups to infer neural information of movement intention. Noninvasive approaches, which combine high-density electromyography, blind source separation and neuromechanical modeling, could offer an inexpensive alternative. Although high-density electromyography records spatiotemporal myoelectric activity at the periphery level, it underlies interfering information from spinal neural cells (i.e., alpha motor neurons). Blind source separation (Holobar et al., 2009; Negro et al., 2016) enables separating the interfering activity from neural sources, thereby retrieving the activity of actual motor neuron pools. In turn, the decoded neural firing events can be employed to drive comprehensive neuromusculoskeletal models (Sartori et al., 2016, 2017). Such an interface allows for the activity of actual motor neuron pools to be decoded by separating the interference activity from the neural source. This technique is currently used for characterizing motor neurons during voluntary contractions (Farina and Holobar, 2016) and reflex movements (Yavuz et al., 2015) in healthy and impaired (Holobar et al., 2012) individuals. Moreover, other applications include studying the neuromechanical response to external devices (Farina et al., 2014; Gogeascoechea et al., 2020). This technique can therefore extend current open-loop rehabilitation techniques into closed-loop neuro-modulative approaches. However, its use is mostly limited to isometric contractions or slow dynamic contractions, mainly due to computational challenges related to the assumption that motor units are stationary and real-time implementation of the method. Both model-free AI (e.g., machine and deep learning techniques) (Chen et al., 2020; Clarke et al., 2020) and model-based techniques (e.g., data-driven mechanistic modeling) (Sartori and Sawicki, 2021) are explored to enable real-time implementation, which would allow mechanical and neural adaptations to exoskeleton training and neurostimulation to be predicted.
Besides providing intuitive and seamless assistive control, an important goal at the level of the brain is to promote neuroplastic changes and foster functional connectivity between central motoneurons and inactive and/or silent peripheral motoneurons (Donati et al., 2016). By decoding movement intention and gait characteristics in real time, invasive and non-invasive brain-computer interfaces can directly infer the user's intention to move, optimizing rehabilitation outcomes (Soekadar et al., 2015; Mrachacz-Kersting et al., 2016). Non-invasive brain-computer interfaces can assess large-scale brain oscillatory activity directly, through electroencephalography or magnetoencephalography, or indirectly, by measuring the brain's energy expenditure (Liew et al., 2016; Soekadar et al., 2021). Invasive brain-computer interfaces typically exploit the user's ability to train the electrical activity in their brain, which is recorded by electrocorticography or multielectrode arrays (Hochberg et al., 2012; Ajiboye et al., 2017). The future of individualized brain-computer interfaces interventions relies on advanced algorithms for automated detection of brain states and self-adapting neurofeedback, as well as on hybrid neural interfaces, integrating different biosignals, e.g., electroencephalography, electromyography, or electrooculography, to allow for a more robust and safe control in real-life applications (Witkowski et al., 2014; Soekadar et al., 2016). To enable a broad adoption of brain-computer interfaces in real-life environments for clinics and home use, portable and easy-to-use systems need to be designed, requiring comfortable electroencephalography-headsets that minimize preparation time and allow self-applicability. Furthermore, new machine learning approaches are needed to optimize calibration time without inflating the number of sensors.
Instead of focusing on interfacing solely with either the brain, central nervous system, or periphery, the next generation of gait neuroprostheses for movement rehabilitation may aim to develop an interface on multiple levels. The advantage is that the paired activation of pre- and post-synaptic motoneurons at the level of the spinal cord is crucial for facilitating the re-wiring of functional connections after spinal cord injury (Dixon et al., 2016; Shulga et al., 2021). Motor cortex activity can also be used to control spinal cord stimulation (Capogrosso et al., 2016) instead of external control using a mobile app (Wagner et al., 2018). Adapting the parameters of peripheral stimulation to the ongoing neural activity has also proven to play a key role in fostering neuroplastic reorganization (Mrachacz-Kersting et al., 2016; Bonizzato et al., 2018). Therefore, we envision the implementation of brain-computer interfaces that allow for personalized adaptive modulation of brain activity and alpha motor circuitries.
The main requirements for interface computing architectures of gait neuroprosthetics are that the system is personalizable and adaptable, it should consume as little energy as possible, such that the system can be used optimally for at least a full day, and data processing should happen in real-time. Embedded AI for neuroprosthetics is a promising approach to achieve these requirements, as it has the potential to be real-time, while the computing technology and electronics are attached to the body or even implanted inside the body. The importance of a corresponding computer architecture in neuroprosthesis has been emphasized (Vassanelli and Mahmud, 2016; Ielmini and Wong, 2018), specifically to develop a suitable neuronal architecture to interface with the brain. However, no implementations exist so far, and therefore the further development and prototyping of concepts is of utmost importance (Mikhaylov et al., 2020). To achieve personalization and adaptability, a main challenge is to perform online learning on the embedded AI. In contrast, today's embedded AI only allow inference, while networks are trained offline. This training is computationally intensive and consumes much energy. To achieve low energy consumption and real-time processing for training as well as inference on the chip, the embedded AI should be implemented as low-energy circuits.
An important step to achieve low energy consumption in embedded AI is to use in-memory processing, i.e., operations on data are performed analogously or digitally directly in the memory. Low-energy consumption is inherent for analog processing, since no digital conversion is required, while specialized hardware architectures without a central processing unit can greatly decrease energy consumption since long data paths are avoided. Using non-volatile storage ensures that no information is lost when power is switched off, what can be done when no data processing is required, e.g., when a person wearing a neuroprosthetics stops and does not walk. Accordingly, reloading weights after activation is not required. Many international groups currently study applications of non-volatile memory for neural networks and enhanced computing technologies, e.g., utilizing such memory as synaptic elements for artificial neural networks (Hu et al., 2018; Zhang et al., 2020).
Resistive random access memory (ReRAM) has compared to other non-volatile memory technologies, like e.g., Phase Change Memories, the advantage (Ielmini and Wong, 2018) to further decrease energy consumption, due to a unique combination of multi-level programming and a high density of integration (Milo et al., 2019). Furthermore, ReRAMs are potentially compatible with the most commonly used chip technology, complementary metal–oxide–semiconductor process manufacturing. Multi-level programming means that multiple bits can be stored on one ReRAM device. Therefore, weights and matrix multiplications of a neural network can be stored inside one ReRAM cell, and a neural network can be implemented by saving all network weights directly on the chip (Perez et al., 2020). As a result, external memory access is avoided, which can lower the energy consumption drastically (Knödtel et al., 2020). The high-density integration on the chip allows for the ReRAM cells to be closely attached to digital computation units, which decreases energy consumption by avoiding long data paths on the chip. The use of ReRAM technology yielded a 95% reduction in energy consumption for the classification of bio-signals for atrial fibrillation compared to a traditional approach (Pechmann et al., 2021). Therefore, ReRAM technology is a promising candidate for realization of personalized gait neuroprostheses relying on artificial neural networks in digital (e.g., as ReRAM storage for weights) and analog in-memory processing (e.g., as an in-memory processing element itself).
However, several challenges exist for ReRAM technology to be implemented on a neuroprosthesis. Currently, storage of up to three bits on each ReRAM cell is possible (Milo et al., 2021). However, energy consumption for reading out information increases when more bits are stored on a cell. This trade-off between energy consumption for read out and energy saving from in-memory processing should be further investigated to minimize overall energy consumption. Furthermore, storage elements using ReRAM technology have a larger device-to-device variability than traditional storage elements, which use volatile technology. In particular, this device-to-device variability is a problem for multi-bit storing (Fritscher et al., 2021b), which is desired for embedded AI. Also the variability of switching parameters and energy overheads of analog-to-digital and digital-to-analog conversion is still a challenge for the reliable use of ReRAMs as well as their comparatively low endurance, i.e., the number of allowable switching cycles of the ReRAM cells. This is particularly challgengin for neuroprotheses, which have higher time series data analysis requirements than e.g., a ReRAM based analysator for detecting atrial fibrillation. To achieve this, new research should address special training methods that account for tolerable fluctuations by using learning techniques such as noisy training or dropout layers (Fritscher et al., 2021a).
Neuroprostheses can potentially restore function through external activation of the central or peripheral nervous system. We have presented our perspective on current challenges of and future directions in the development of neuroprostheses in stimulation of the peripheral and central nervous systems, and outlined technical approaches to appropriate computer architectures, as also summarized in Figure 2. A paramount challenge is to restore mobility by effectively combining neuroprosthetic and wearable robotic approaches and to align neuroprostheses to the individual user's needs and capabilities.
To this end, predictive simulations of the human-machine system in dynamic tasks appear to be a promising approach to customize design and control. Still, predictive simulations can only provide rough representations of a paralysis and its highly individual constraints, which cannot yet be covered by recent neuromuscular modeling approaches, which, in turn, hampers user-specific control and stimulation. We suggest that future work should focus on improving neuromechanical simulations of user-device interaction based on experimental data, e.g., obtaining muscle parameters on the bench and in real applications, and aim at prioritizing muscle activity over robotic assistance. Combining a deeper understanding of neuromechanical dynamics, particularly muscle-group excitation through the central nervous system, with multimodal sensor networks distributed across the human and the device could foster model-based monitoring and management of muscle fatigue.
For the central nervous system, real-time data processing, ease of use of systems, and combining interfacing at multiple levels are important future directions. Real-time data processing is required to extract useful information from noisy measurements of brain or spinal cord activity in a useful way. AI is a promising approach to enable this real-time data processing, while also allowing for personalization, and limiting the number of sensors required, improving ease of use. Portable and easy-to-use systems should be designed to allow for the adoption of neuroprostheses in real-life. Furthermore, the combination of stimulation at the brain and the spinal cord or the periphery should be further explored, since these combinations were shown to have benefits over stimulation at only one of these levels, and thereby improve rehabilitation outcomes.
Regarding technological advances of the interface to the computing architecture, a main current challenge is to achieve low-energy electronics, real-time data processing, and learning on the chip. Embedded AI has the potential to process data in real-time, and allows for learning and inference on the chip. To achieve low-energy consumption,the embedded AI should use in-memory processing combined with ReRAM technology. To allow the use of ReRAM technology in gait neuroprostheses, we need further research in combining ReRAM technology as multi-bit storage cells with complementary metal–oxide–semiconductor process manufacturing, the commonly-used chip technology. Furthermore, the functionality of the ReRAM cells should be expanded to improve reliability and mitigate the effect its device-to-device variability.
In conclusion, a close cooperation between computer architects, electrical engineers, material scientist, medical experts, and biomechanical experts is required to design appropriate neuroprostheses that are tailored to the user's need, adaptable, easy-to-use, and consume little energy.
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
AK coordinated the writing process, the development of the paper structure, and integration of individual contributions. PB and DF supported this for the whole paper. MA, AJd-A, JMF-L, SH, NM, JCM, MN, RQ, R-DR, RJT, and PB mainly contributed to section 2. AC, AG, MS, SS, MV, USY, and AK mainly contributed to section 3. MR, CW, and DF mainly contributed to section 4. All authors contributed to the article and approved the submitted version.
AK is funded by a faculty endowment by adidas AG. MA, SKH, NM, MN, RJQ, R-DR, RJT are supported by NSF CPS grant 1739800, VA Merit Reviews A2275-R and 3056, and the NIH (5T32EB004314-15, R01 NS040547-13). MS and AG are funded by the European Research Council (ERC) under the European Union's Horizon 2020 research and innovation programme (Grant agreement No. 803035). AJd-A, JMF-L, and JCM are supported by coordinated grants RTI2018-097290-B-C31/C32/C33 (TAILOR project) funded by MCIN/AEI/10.13039/501100011033 and by “ERDF A way of making Europe”. MR is funded by the Lo3-ML project by the Federal Ministry for Education, Science and Technology (BMBF) (Funding No. 16ES1142K). AC, SS, and MV were supported by the European Research Council (ERC) under the project NGBMI (759370), the Einstein Stiftung Berlin, the ERA-NET NEURON project HYBRIDMIND (BMBF, 01GP2121A and -B) and the BMBF project NEO (13GW0483C).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The authors thank the organizers of IEEE/EMBS NER conference in 2021 for the opportunity to hold the mini symposium Adaptation Strategies for Personalized Gait Neuroprosthetics as part of the conference.
Ajiboye, A. B., Willett, F. R., Young, D. R., Memberg, W. D., Murphy, B. A., Miller, J. P., et al. (2017). Restoration of reaching and grasping movements through brain-controlled muscle stimulation in a person with tetraplegia: a proof-of-concept demonstration. Lancet 389, 1821–1830. doi: 10.1016/S0140-6736(17)30601-3
Al Borno, M., O'Day, J., Ibarra, V., Dunne, J., Seth, A., Habib, A., et al. (2021). Opensense: An open-source toolbox for inertial-measurement-unit-based measurement of lower extremity kinematics over long durations. bioRxiv. doi: 10.1101/2021.07.01.450788
Alibeji, N., Kirsch, N., and Sharma, N. (2017). An adaptive low-dimensional control to compensate for actuator redundancy and fes-induced muscle fatigue in a hybrid neuroprosthesis. Control Eng. Pract. 59, 204–219. doi: 10.1016/j.conengprac.2016.07.015
Alonso, J., Romero, F., Pàmies-Vilà, R., Lugrís, U., and Font-Llagunes, J. M. (2012). A simple approach to estimate muscle forces and orthosis actuation in powered assisted walking of spinal cord-injured subjects. Multibody Syst. Dyn. 28, 109–124. doi: 10.1007/s11044-011-9284-5
Asselin, P., Knezevic, S., Kornfeld, S., Cirnigliaro, C., Agranova-Breyter, I., and Bauman, W. A. (2015). Heart rate and oxygen demand of powered exoskeleton-assisted walking in persons with paraplegia. J. Rehabil. Res. Dev. 52, 147. doi: 10.1682/JRRD.2014.02.0060
Beckerle, P., Salvietti, G., Unal, R., Prattichizzo, D., Rossi, S., Castellini, C., et al. (2017). A human-robot interaction perspective on assistive and rehabilitation robotics. Front. Neurorobot. 11:24. doi: 10.3389/fnbot.2017.00024
Birbaumer, N., Gallegos-Ayala, G., Wildgruber, M., Silvoni, S., and Soekadar, S. R. (2014). Direct brain control and communication in paralysis. Brain Topogr. 27, 4–11. doi: 10.1007/s10548-013-0282-1
Bonizzato, M., Pidpruzhnykova, G., DiGiovanna, J., Shkorbatova, P., Pavlova, N., Micera, S., et al. (2018). Brain-controlled modulation of spinal circuits improves recovery from spinal cord injury. Nat. Commun. 9, 1–14. doi: 10.1038/s41467-018-05282-6
Capogrosso, M., Milekovic, T., Borton, D., Wagner, F., Moraud, E. M., Mignardot, J.-B., et al. (2016). A brain-spine interface alleviating gait deficits after spinal cord injury in primates. Nature 539, 284–288. doi: 10.1038/nature20118
Cervera, M. A., Soekadar, S. R., Ushiba, J., Millán, J. d. R., Liu, M., et al. (2018). Brain-computer interfaces for post-stroke motor rehabilitation: a meta-analysis. Ann. Clin. Transl. Neurol. 5, 651–663. doi: 10.1002/acn3.544
Chen, C., Yu, Y., Sheng, X., and Zhu, X. (2020). Analysis of motor unit activities during multiple motor tasks by real-time emg decomposition: perspective for myoelectric control. Annu. Int. Conf. IEEE Eng. Med. Biol. Soc. 2020, 4791–4794. doi: 10.1109/EMBC44109.2020.9176362
Clarke, A. K., Atashzar, S. F., Del Vecchio, A., Barsakcioglu, D., Muceli, S., Bentley, P., et al. (2020). Deep learning for robust decomposition of high-density surface emg signals. IEEE Trans. Biomed. Eng. 68, 526–534. doi: 10.1109/TBME.2020.3006508
Crago, P. E., Makowski, N. S., and Cole, N. M. (2014). Contributions to muscle force and emg by combined neural excitation and electrical stimulation. J Neural Eng. 11, 056022. doi: 10.1088/1741-2560/11/5/056022
De Groote, F., and Falisse, A. (2021). Perspective on musculoskeletal modelling and predictive simulations of human movement to assess the neuromechanics of gait. Proc. R. Soc. B 288, 20202432. doi: 10.1098/rspb.2020.2432
Del-Ama, A. J., Gil-Agudo, Á., Pons, J. L., and Moreno, J. C. (2014). Hybrid gait training with an overground robot for people with incomplete spinal cord injury: a pilot study. Front. Hum. Neurosci. 8:298. doi: 10.3389/fnhum.2014.00298
Dixon, L., Ibrahim, M. M., Santora, D., and Knikou, M. (2016). Paired associative transspinal and transcortical stimulation produces plasticity in human cortical and spinal neuronal circuits. J. Neurophysiol. 116, 904–916. doi: 10.1152/jn.00259.2016
Donati, A. R., Shokur, S., Morya, E., Campos, D. S., Moioli, R. C., Gitti, C. M., et al. (2016). Long-term training with a brain-machine interface-based gait protocol induces partial neurological recovery in paraplegic patients. Sci. Rep. 6, 1–16. doi: 10.1038/srep30383
Dorschky, E., Nitschke, M., Martindale, C. F., van den Bogert, A. J., Koelewijn, A. D., and Eskofier, B. M. (2020). Cnn-based estimation of sagittal plane walking and running biomechanics from measured and simulated inertial sensor data. Front. Bioeng. Biotechnol. 8:604. doi: 10.3389/fbioe.2020.00604
Dorschky, E., Nitschke, M., Seifer, A.-K., van den Bogert, A. J., and Eskofier, B. M. (2019). Estimation of gait kinematics and kinetics from inertial sensor data using optimal control of musculoskeletal models. J. Biomech. 95:109278. doi: 10.1016/j.jbiomech.2019.07.022
Evans, N., Hartigan, C., Kandilakis, C., Pharo, E., and Clesson, I. (2015). Acute cardiorespiratory and metabolic responses during exoskeleton-assisted walking overground among persons with chronic spinal cord injury. Top. Spinal. Cord. Inj. Rehabil. 21, 122–132. doi: 10.1310/sci2102-122
Farina, D., and Holobar, A. (2016). Characterization of human motor units from surface emg decomposition. Proc. IEEE 104, 353–373. doi: 10.1109/JPROC.2015.2498665
Farina, D., Jiang, N., Rehbaum, H., Holobar, A., Graimann, B., Dietl, H., et al. (2014). The extraction of neural information from the surface emg for the control of upper-limb prostheses: emerging avenues and challenges. IEEE Trans. Neural Syst. Rehabil. Eng. 22, 797–809. doi: 10.1109/TNSRE.2014.2305111
Febrer-Nafría, M., Pallarès-López, R., Fregly, B. J., and Font-Llagunes, J. M. (2020). Comparison of different optimal control formulations for generating dynamically consistent crutch walking simulations using a torque-driven model. Mech. Mach. Theory 154:104031. doi: 10.1016/j.mechmachtheory.2020.104031
Febrer-Nafría, M., Pallarès-López, R., Fregly, B. J., and Font-Llagunes, J. M. (2021). Prediction of three-dimensional crutch walking patterns using a torque-driven model. Multibody Syst. Dyn. 51, 1–19. doi: 10.1007/s11044-020-09751-z
Foglyano, K. M., Schnellenberger, J. R., Kobetic, R., Lombardo, L., Pinault, G., Selkirk, S., et al. (2016). Accelerometer-based step initiation control for gait-assist neuroprostheses. J. Rehabil. Res. Dev. 53, 919–932. doi: 10.1682/JRRD.2015.09.0188
Fregly, B. J. (2021). A conceptual blueprint for making neuromusculoskeletal models clinically useful. Appl. Sci. 11:2037. doi: 10.3390/app11052037
Fritscher, M., Knödtel, J., Mallah, M., Pechmann, S., Perez-Bosch Quesada, E., Rizzi, T., et al. (2021a). “Mitigating the effects of RRAM process variation on the accuracy of artifical neural networks,” in Proc. 21st International Conference on Embedded Computer Systems: Architectures, Modeling and Simulation (SAMOS 2021) (Pythagorion).
Fritscher, M., Knödtel, J., Reiser, D., Mallah, M., Pechmann, S., Fey, D., et al. (2021b). “Simulating large neural networks embedding mlc rram as weight storage considering device variations,” in 2021 IEEE 12th Latin America Symposium on Circuits and System (LASCAS), (Arequipa: IEEE), 1–4.
García-Vallejo, D., Font-Llagunes, J. M., and Schiehlen, W. (2016). Dynamical analysis and design of active orthoses for spinal cord injured subjects by aesthetic and energetic optimization. Nonlinear. Dyn. 84, 559–581. doi: 10.1007/s11071-015-2507-1
Gogeascoechea, A., Kuck, A., Van Asseldonk, E., Negro, F., Buitenweg, J. R., Yavuz, U. S., et al. (2020). Interfacing with alpha motor neurons in spinal cord injury patients receiving trans-spinal electrical stimulation. Front. Neurol. 11:493. doi: 10.3389/fneur.2020.00493
Guidolin, M., Petrea, R. A. B., Roberto, O., Reggiani, M., Menegatti, E., and Tagliapietra, L. (2021). “On the accuracy of imus for human motion tracking: a comparative evaluation,” in 2021 IEEE International Conference on Mechatronics (ICM) (Kashiwa: IEEE), 1–6.
Hayes, S. C., White, M., White, H. S. F., and Vanicek, N. (2020). A biomechanical comparison of powered robotic exoskeleton gait with normal and slow walking: an investigation with able-bodied individuals. Clin. Biomech. 80:105133. doi: 10.1016/j.clinbiomech.2020.105133
Hochberg, L. R., Bacher, D., Jarosiewicz, B., Masse, N. Y., Simeral, J. D., Vogel, J., et al. (2012). Reach and grasp by people with tetraplegia using a neurally controlled robotic arm. Nature 485, 372–375. doi: 10.1038/nature11076
Hochberg, L. R., Serruya, M. D., Friehs, G. M., Mukand, J. A., Saleh, M., Caplan, A. H., et al. (2006). Neuronal ensemble control of prosthetic devices by a human with tetraplegia. Nature 442, 164–171. doi: 10.1038/nature04970
Holobar, A., Farina, D., Gazzoni, M., Merletti, R., and Zazula, D. (2009). Estimating motor unit discharge patterns from high-density surface electromyogram. Clin. Neurophysiol. 120, 551–562. doi: 10.1016/j.clinph.2008.10.160
Holobar, A., Glaser, V., Gallego, J., Dideriksen, J. L., and Farina, D. (2012). Non-invasive characterization of motor unit behaviour in pathological tremor. J. Neural Eng. 9, 056011. doi: 10.1088/1741-2560/9/5/056011
Hu, M., Graves, C. E., Li, C., Li, Y., Ge, N., Montgomery, E., et al. (2018). Memristor-based analog computation and neural network classification with a dot product engine. Adv. Mater. 30, 1705914. doi: 10.1002/adma.201705914
Ielmini, D., and Wong, H.-S. P. (2018). In-memory computing with resistive switching devices. Nat. Electron. 1, 333–343. doi: 10.1038/s41928-018-0092-2
Knödtel, J., Fritscher, M., Reiser, D., Fey, D., Breiling, M., and Reichenbach, M. (2020). “A model-to-circuit compiler for evaluation of dnn accelerators based on systolic arrays and multibit emerging memories,” in 2020 9th International Conference on Modern Circuits and Systems Technologies (MOCAST) (Bremen: IEEE), 1–6.
Lancini, M., Serpelloni, M., Pasinetti, S., and Guanziroli, E. (2016). Healthcare sensor system exploiting instrumented crutches for force measurement during assisted gait of exoskeleton users. IEEE Sens. J. 16, 8228–8237. doi: 10.1109/JSEN.2016.2579738
Lapointe, R., Lajoie, Y., Serresse, O., and Barbeau, H. (2001). Functional community ambulation requirements in incomplete spinal cord injured subjects. Spinal Cord. 39, 327–335. doi: 10.1038/sj.sc.3101167
Liew, S.-L., Rana, M., Cornelsen, S., Fortunato de Barros Filho, M., Birbaumer, N., Sitaram, R., et al. (2016). Improving motor corticothalamic communication after stroke using real-time fmri connectivity-based neurofeedback. Neurorehabil. Neural Repair. 30, 671–675. doi: 10.1177/1545968315619699
Makowski, N. S., Campean, A., Lambrecht, J. M., Buckett, J. R., Coburn, J. D., Hart, R. L., et al. (2021). Design and testing of stimulation and myoelectric recording modules in an implanted distributed neuroprosthetic system. IEEE Trans. Biomed. Circ. Syst. 15, 281–293. doi: 10.1109/TBCAS.2021.3066838
Michaud, F., Mouzo, F., Lugrís, U., and Cuadrado, J. (2019). Energy expenditure estimation during crutch-orthosis-assisted gait of a spinal-cord-injured subject. Front. Neurorobot. 13:55. doi: 10.3389/fnbot.2019.00055
Mikhaylov, A., Pimashkin, A., Pigareva, Y., Gerasimova, S., Gryaznov, E., Shchanikov, S., et al. (2020). Neurohybrid memristive cmos-integrated systems for biosensors and neuroprosthetics. Front. Neurosci. 14:358. doi: 10.3389/fnins.2020.00358
Miller, L. E., Zimmermann, A. K., and Herbert, W. G. (2016). Clinical effectiveness and safety of powered exoskeleton-assisted walking in patients with spinal cord injury: systematic review with meta-analysis. Med. Devices (Auckl) 9:455. doi: 10.2147/MDER.S103102
Milo, V., Glukhov, A., Pérez, E., Zambelli, C., Lepri, N., Mahadevaiah, M. K., et al. (2021). Accurate program/verify schemes of resistive switching memory (rram) for in-memory neural network circuits. IEEE Trans. Electron. Devices. 68, 3832–3837. doi: 10.1109/TED.2021.3089995
Milo, V., Zambelli, C., Olivo, P., Pérez, E. K., Mahadevaiah, M., et al. (2019). Multilevel hfo2-based rram devices for low-power neuromorphic networks. APL Mater. 7, 081120. doi: 10.1063/1.5108650
Mohamad, N. Z., Hamzaid, N. A., Davis, G. M., Abdul Wahab, A. K., and Hasnan, N. (2017). Mechanomyography and torque during fes-evoked muscle contractions to fatigue in individuals with spinal cord injury. Sensors 17:1627. doi: 10.3390/s17071627
Mrachacz-Kersting, N., Jiang, N., Stevenson, A. J. T., Niazi, I. K., Kostic, V., Pavlovic, A., et al. (2016). Efficient neuroplasticity induction in chronic stroke patients by an associative brain-computer interface. J. Neurophysiol. 115, 1410–1421. doi: 10.1152/jn.00918.2015
Nandor, M., Kobetic, R., Audu, M., Triolo, R., and Quinn, R. (2021). A muscle-first, electromechanical hybrid gait restoration system in people with spinal cord injury. Front. Rob. AI 8:645588. doi: 10.3389/frobt.2021.645588
Negro, F., Muceli, S., Castronovo, A. M., Holobar, A., and Farina, D. (2016). Multi-channel intramuscular and surface emg decomposition by convolutive blind source separation. J Neural Eng. 13, 026027. doi: 10.1088/1741-2560/13/2/026027
Pechmann, S., Mai, T., Potschka, J., Reiser, D., Reichel, P., Breiling, M., et al. (2021). A low-power RRAM memory block for embedded, multi-level weight and bias storage in artificial neural networks. Micromachines 12:1277. doi: 10.3390/mi12111277
Perez, E., González Ossorio, Ó., Duenas, S., Castan, H., Garcia, H., and Wenger, C. (2020). Programming pulse width assessment for reliable and low-energy endurance performance in al: Hfo2-based rram arrays. Electronics 9, 864. doi: 10.3390/electronics9050864
Robinett, C. S., and Vondran, M. A. (1988). Functional ambulation velocity and distance requirements in rural and urban communities: a clinical report. Phys. Ther. 68, 1371–1373. doi: 10.1093/ptj/68.9.1371
Sartori, M., Lloyd, D. G., and Farina, D. (2016). Neural data-driven musculoskeletal modeling for personalized neurorehabilitation technologies. IEEE Trans. Biomed. Eng. 63, 879–893. doi: 10.1109/TBME.2016.2538296
Sartori, M., and Sawicki, G. S. (2021). Closing the loop between wearable technology and human biology: A new paradigm for steering neuromuscular form and function. Progr. Biomed. Eng. 3, 023001. doi: 10.1088/2516-1091/abe3e0
Sartori, M., Yavuz, U. Ş., and Farina, D. (2017). In vivo neuromechanics: decoding causal motor neuron behavior with resulting musculoskeletal function. Sci. Rep. 7, 1–14. doi: 10.1038/s41598-017-13766-6
Sauder, N. R., Meyer, A. J., Allen, J. L., Ting, L. H., Kesar, T. M., and Fregly, B. J. (2019). Computational design of fastfes treatment to improve propulsive force symmetry during post-stroke gait: a feasibility study. Front. Neurorobot. 13:80. doi: 10.3389/fnbot.2019.00080
Seel, T., Werner, C., Raisch, J., and Schauer, T. (2016). Iterative learning control of a drop foot neuroprosthesis—generating physiological foot motion in paretic gait by automatic feedback control. Control Eng. Pract. 48:87–97. doi: 10.1016/j.conengprac.2015.11.007
Shin, H., Suresh, N. L., Rymer, W. Z., and Hu, X. (2018). Relative contribution of different altered motor unit control to muscle weakness in stroke: a simulation study. J. Neural Eng. 15, 016014. doi: 10.1088/1741-2552/aa925d
Shulga, A., Lioumis, P., Kirveskari, E., Savolainen, S., and Mäkelä, J. P. (2021). A novel paired associative stimulation protocol with a high-frequency peripheral component: A review on results in spinal cord injury rehabilitation. Eur. J. Neurosci. 53, 3242–3257. doi: 10.1111/ejn.15191
Soekadar, S., Witkowski, M., Gómez, C., Opisso, E., Medina, J., Cortese, M., et al. (2016). Hybrid eeg/eog-based brain/neural hand exoskeleton restores fully independent daily living activities after quadriplegia. Sci. Robot 1, 32–96. doi: 10.1126/scirobotics.aag3296
Soekadar, S. R., Birbaumer, N., Slutzky, M. W., and Cohen, L. G. (2015). Brain-machine interfaces in neurorehabilitation of stroke. Neurobiol. Dis. 83, 172–179. doi: 10.1016/j.nbd.2014.11.025
Soekadar, S. R., Kohl, S. H., Mihara, M., and von Lühmann, A. (2021). Optical brain imaging and its application to neurofeedback. Neuroimage Clin. 30:102577. doi: 10.1016/j.nicl.2021.102577
Sreenivasa, M., Millard, M., Felis, M., Mombaur, K., and Wolf, S. I. (2017). Optimal control based stiffness identification of an ankle-foot orthosis using a predictive walking model. Front. Comput. Neurosci. 11:23. doi: 10.3389/fncom.2017.00023
Uchida, T. K., Seth, A., Pouya, S., Dembia, C. L., Hicks, J. L., and Delp, S. L. (2016). Simulating ideal assistive devices to reduce the metabolic cost of running. PLoS ONE 11:e0163417. doi: 10.1371/journal.pone.0163417
Ugurlu, B., Oshima, H., Sariyildiz, E., Narikiyo, T., and Babic, J. (2020). Active compliance control reduces upper body effort in exoskeleton-supported walking. IEEE Trans. Hum. Mach. Syst. 50, 144–153. doi: 10.1109/THMS.2019.2961969
Vassanelli, S., and Mahmud, M. (2016). Trends and challenges in neuroengineering: toward “intelligent” neuroprostheses through brain-“brain inspired systems” communication. Front. Neurosci. 10:438. doi: 10.3389/fnins.2016.00438
Wagner, F. B., Mignardot, J.-B., Le Goff-Mignardot, C. G., Demesmaeker, R., Komi, S., Capogrosso, M., et al. (2018). Targeted neurotechnology restores walking in humans with spinal cord injury. Nature 563, 65–71. doi: 10.1038/s41586-018-0649-2
Wilson, R. D., Bryden, A. M., Kilgore, K. L., Makowski, N., Bourbeau, D., Kowalski, K. E., et al. (2019). Neuromodulation for functional electrical stimulation. Phys. Med. Rehabil. Clin. 30, 301–318. doi: 10.1016/j.pmr.2018.12.011
Witkowski, M., Cortese, M., Cempini, M., Mellinger, J., Vitiello, N., and Soekadar, S. R. (2014). Enhancing brain-machine interface (bmi) control of a hand exoskeleton using electrooculography (eog). J. Neuroeng. Rehabil. 11, 1–6. doi: 10.1186/1743-0003-11-165
Yavuz, U. Ş., Negro, F., Sebik, O., Holobar, A., Frömmel, C., et al. (2015). Estimating reflex responses in large populations of motor units by decomposition of the high-density surface electromyogram. J. Physiol. 593, 4305–4318. doi: 10.1113/JP270635
Keywords: neuroprosthesis, resistive random access memory, neural interface, personalized devices, perspective, embedded artificial intelligence
Citation: Koelewijn AD, Audu M, del-Ama AJ, Colucci A, Font-Llagunes JM, Gogeascoechea A, Hnat SK, Makowski N, Moreno JC, Nandor M, Quinn R, Reichenbach M, Reyes R-D, Sartori M, Soekadar S, Triolo RJ, Vermehren M, Wenger C, Yavuz US, Fey D and Beckerle P (2021) Adaptation Strategies for Personalized Gait Neuroprosthetics. Front. Neurorobot. 15:750519. doi: 10.3389/fnbot.2021.750519
Received: 30 July 2021; Accepted: 18 November 2021;
Published: 16 December 2021.
Edited by:
Zlatko Matjacic, University Rehabilitation Institute (Slovenia), SloveniaReviewed by:
Carlos A. Cifuentes, Escuela Colombiana de Ingenieria Julio Garavito, ColombiaCopyright © 2021 Koelewijn, Audu, del-Ama, Colucci, Font-Llagunes, Gogeascoechea, Hnat, Makowski, Moreno, Nandor, Quinn, Reichenbach, Reyes, Sartori, Soekadar, Triolo, Vermehren, Wenger, Yavuz, Fey and Beckerle. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Anne D. Koelewijn, YW5uZS5rb2VsZXdpam5AZmF1LmRl
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.