
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Neurol. , 11 March 2025
Sec. Dementia and Neurodegenerative Diseases
Volume 16 - 2025 | https://doi.org/10.3389/fneur.2025.1563056
Background: Ginkgo biloba is widely used in some regions as an adjunct therapy for Alzheimer’s disease (AD). Its potential mechanisms include antioxidative and anti-amyloid properties, yet clinical evidence remains mixed.
Objective: We investigated whether combining Ginkgo with donepezil confers additional benefits in amyloid PET-positive AD patients. We also explored changes in the plasma biomarker MDS-Oaβ (Multimer Detection System–Oligomeric Aβ), which reflects the propensity of Aβ monomers to form oligomers.
Methods: This retrospective study included newly diagnosed, drug-naïve AD patients who were amyloid PET-positive and had at least 12 months of follow-up. Participants received either donepezil alone (Donepezil-only) or donepezil plus Ginkgo (Donepezil-Ginkgo). Clinical measures included the Korean version of the Mini-Mental State Examination (K-MMSE) and the Sum of Boxes of the Clinical Dementia Rating (CDR-SB). Plasma MDS-Oaβ was assessed at baseline and at 12 months.
Results: A total of 101 patients were analyzed (60 Donepezil-only, 41 Donepezil-Ginkgo). Baseline demographics and clinical characteristics were similar. After 12 months, the Donepezil-only group showed minimal change in K-MMSE and a slight decrease in MDS-Oaβ. The Donepezil-Ginkgo group demonstrated a significant improvement in K-MMSE (+2.4) and a larger reduction in MDS-Oaβ (−0.15). No significant between-group difference was observed for CDR-SB. Adverse events were mostly mild and did not lead to discontinuation.
Conclusion: The addition of Ginkgo to donepezil may yield superior cognitive outcomes and a greater decrease in plasma MDS-Oaβ compared with donepezil alone in amyloid PET-positive AD patients. Further large-scale, prospective trials are warranted to validate these findings and elucidate Ginkgo’s mechanistic role in AD.
With the global increase in the aging population, the prevalence of Alzheimer’s disease (AD) is also rising. Despite the approval of memantine by the U.S. FDA in October 2003 and numerous research efforts over the past two decades, an effective and cost-efficient medications for AD remains elusive. Consequently, the re-evaluation of existing drugs already used in certain regions for Alzheimer’s disease or related dementia is emerging as another key axis in the development of new treatments.
Ginkgo biloba (Ginkgo; Ginexin-F®) is used in South Korea as an adjunct therapy to help prevent or treat dementia. In this study, we selected Ginexin-F®, a standardized Ginkgo biloba extract that has been approved by the Ministry of Food and Drug Safety (Approval No. MFDS-199702183, Available from: https://nedrug.mfds.go.kr/CEAAA21) for its stringent quality control. Ginexin-F® is standardized to contain 24% flavonoid glycosides and 6% terpene lactones, with ginkgolic acids maintained below 5 ppm, ensuring its compositional equivalence to EGb 761, which has been widely validated in numerous clinical studies. As Ginexin and EGb 761 share similar standardized compositions, findings from studies on EGb 761 are considered relevant. Ginkgo has shown various pharmacological effects, such as inhibiting oxidative stress, improving cerebral blood flow, reducing Aβ toxicity, and modulating neurotransmission and neuroplasticity (1). Despite these potential benefits, questions remain about whether Ginkgo truly confers clinical advantages for AD and, if so, by what mechanisms. While several preclinical studies suggest that Ginkgo biloba may act through antioxidative, anti-inflammatory, and anti-amyloid aggregation pathways (2, 3), the exact molecular basis remains unclear. Therefore, in our clinical study, we employed the plasma MDS-Oaβ biomarker as an indirect measure of amyloid oligomerization, which may partly reflect these underlying mechanisms.
Numerous studies have examined the role of Ginkgo biloba in preventing dementia and treating MCI and AD, yet the findings remain inconsistent. For example, a placebo-controlled pilot study demonstrated significant cognitive benefits when patient compliance was considered (4), whereas the GEM trial using EGb 761 in cognitively healthy elderly found no significant benefit—likely due to low dementia incidence and poor adherence (5). Similarly, the GUIDAGE trial in individuals with subjective memory impairment yielded inconclusive results (6, 7). Early meta-analyses indicated modest benefits (8), but a 2007 Cochrane review cast doubt on significant cognitive improvements (9). In contrast, more recent randomized trials and meta-analyses suggest that EGb 761 can improve cognition, neuropsychiatric symptoms, and activities of daily living in mild-to-moderate dementia (10), with a 12-month placebo-controlled study in China showing a significant reduction in dementia incidence among patients with amnestic MCI (11) and other studies reporting symptomatic improvements in MCI populations (12, 13). Nevertheless, overall results remain mixed, and a recent review concluded that definitive evidence of efficacy is still lacking (14). Possible explanations for these mixed outcomes include: misclassification of patients with non-AD dementia, leading to false positives in clinical trials and the difficulty in detecting small or slowly emerging drug effects within relatively short study periods. Conducting large, well-designed trials focusing on clinical outcomes alone is challenging, particularly given AD’s slow progression.
To address these issues, we designed a study comparing patients receiving donepezil monotherapy versus patients receiving donepezil plus Ginkgo, with two distinctive features: (1) inclusion of only amyloid PET-positive patients to increase diagnostic specificity for AD, and (2) use of the biomarker MDS-Oaβ (Multimer Detection System-Oligomeric Aβ) alongside clinical measures. MDS-Oaβ reflects the oligomerization propensity of Aβ in plasma, potentially capturing treatment effects at the pathological level. Hence, we aimed to determine whether adding Ginkgo to donepezil offers clinical or biomarker-based benefits in AD.
The Dementia Clinic at Soonchunhyang University College of Medicine has maintained the Soonchunhyang Dementia Registry for all dementia patients since March 2020. This registry includes diagnostic evaluations at the time of patient visits: a complete medical history, physical and neurological examinations, comprehensive neuropsychological testing, and routine laboratory tests including ApoE. Magnetic resonance imaging (MRI) is performed within 3 months, and 18F-FC119S PET/computed tomography (CT) is performed when possible in the same period.
From this registry, we identified patients who (1) visited between February 2020 and May 2024, (2) were newly diagnosed with probable AD per the NINCDS-ADRDA criteria (15), (3) tested positive on 18F-FC119S PET (amyloid PET positive), and (4) had at least 12 months of follow-up. All included patients were drug-naive for AD treatments such as cholinesterase inhibitors. After diagnosis, they received either donepezil alone (Donepezil-only group) or a combination of donepezil and Ginkgo (Donepezil-Ginkgo group).
We utilized Ginexin, a standardized Ginkgo biloba extract containing 24% flavonoid glycosides and 6% terpene lactones. For convenience, we refer to it as “Ginkgo.” Ginkgo was administered at a fixed daily dose of 240 mg, and donepezil started at 5 mg/day. The neurologist could raise the donepezil dose to 10 mg/day between 3 and 6 months if tolerated. In this naturalistic study, the clinician determined the dose adjustment method based on their judgment of potential tolerability and efficacy according to the patient’s characteristics. The Korean version of the Mini-Mental State Examination (K-MMSE), the Sum of the Boxes of the Clinical Dementia Rating (CDR-SB) and plasma MDS-Oaβ levels were the primary outcome measures. K-MMSE, CDR-SB, and plasma MDS-Oaβ levels, assessed at baseline and again after approximately 12 months. Because this was a retrospective, naturalistic study, slight variations in follow-up intervals were possible.
This study was based on retrospective registry data. Ethical approval was obtained from the Institutional Review Board of Soonchunhyang University Cheonan Hospital (IRB No. 2023–02–038-002), and informed consent was waived due to anonymized data use. The study was conducted in accordance with the Declaration of Helsinki.
Adverse events were elicited at each visit by inquiring about the patient’s and caregiver’s observations, and by direct clinical observation. All adverse events reported or observed were documented.
Oligomerization of Aβ in plasma was assessed using the MDS-Oaβ method (16, 17). This test, like other assessments, was conducted at the time of initial enrollment according to the registry protocol and was followed up after 12 months. Prior to the assay, plasma samples were initially thawed at a temperature of 37°C for a duration of 15 min. After this, synthetic amyloid-beta (Aβ made by PeopleBio Inc.) was added to the samples, followed by an incubation period of 48 h at 37°C. The incubated plasma sample mixture and serially diluted standard samples were added to their respective wells, and the plates were incubated at room temperature for 1 h. Subsequently, 100 μL/well of an enhanced chemiluminescence substrate solution (Rockland Immunochemicals Inc., Limerick, PA, United States) was added, and the Relative Luminescence Unit (RLU) values were determined using a Victor 3 spectrophotometer to quantify oligomerized Aβ.
Between-group differences (Donepezil-only vs. Donepezil-Ginkgo) were examined using chi-square tests for categorical variables and t-tests for continuous variables. Baseline demographic and clinical variables were compared, and changes in primary outcomes over 12 months were evaluated using independent t-tests. Within-group changes were analyzed using paired t-tests. Statistical analyses were performed using SPSS version 24.0 (SPSS Inc., Chicago, IL, United States).
Among the 101 patients included in the analysis, 60 were treated with donepezil monotherapy, and 41 were treated with a donepezil plus Ginkgo (Figure 1). Although the Donepezil-Ginkgo group tended to have lower baseline K-MMSE scores (21.2 vs. 22.7), the difference was not statistically significant (p = 0.196). No other demographic or clinical differences were observed between the two groups. Baseline MDS-Oaβ values were 0.88 in the Donepezil-only group and 0.87 in the Donepezil-Ginkgo group, both exceeding the cutoff value of 0.78 for AD diagnosis (Table 1) (18).
After 12 months, 56 patients (93.3%) in the Donepezil-only group and 37 (90.2%) in the Donepezil-Ginkgo group were on a 10 mg/day dose of donepezil; the remainder stayed at 5 mg/day. At 12 months, the Donepezil-only group had K-MMSE = 22.5, CDR-SB = 3.6, MDS-Oaβ = 0.86, indicating changes of −0.1, +0.1, and −0.01 from baseline, respectively. Among these, only the MDS-Oaβ change was statistically significant (p = 0.023). In contrast, the Donepezil-Ginkgo group showed K-MMSE = 23.6, CDR-SB = 2.6, and MDS-Oaβ = 0.72, reflecting changes of +2.4, −0.8, and −0.15 from baseline. The improvements in K-MMSE and the reduction in MDS-Oaβ were significant, whereas the change in CDR-SB was not. Moreover, when comparing the two groups at 12 months, K-MMSE (p = 0.021) and MDS-Oaβ (p < 0.001) showed significant between-group differences (Figure 2 and Table 2).
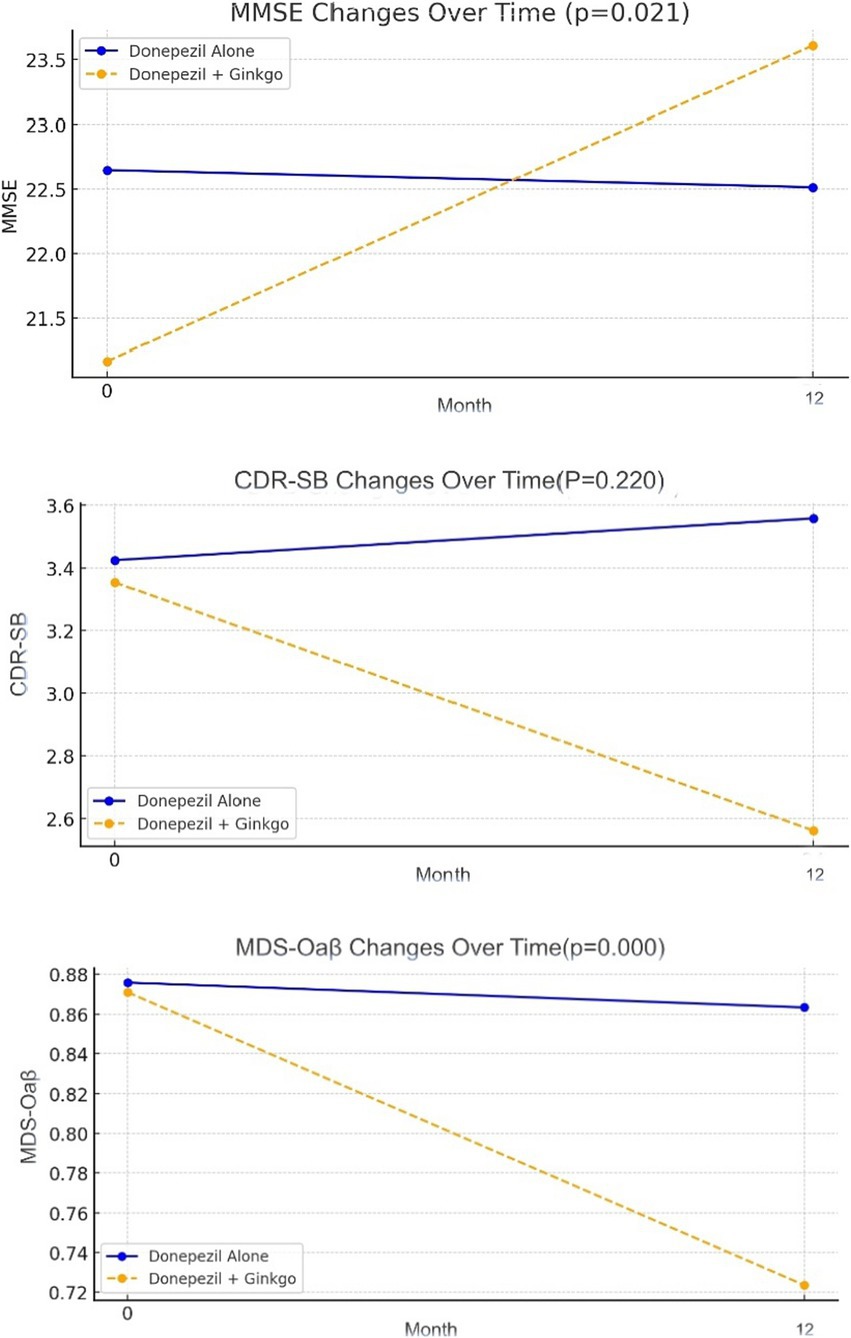
Figure 2. Change of K-MMSE, CDR-SB and MDS-Oaβ in Alzheimer disease patients with donepezil alone and donepezil plus Ginkgo treatment.
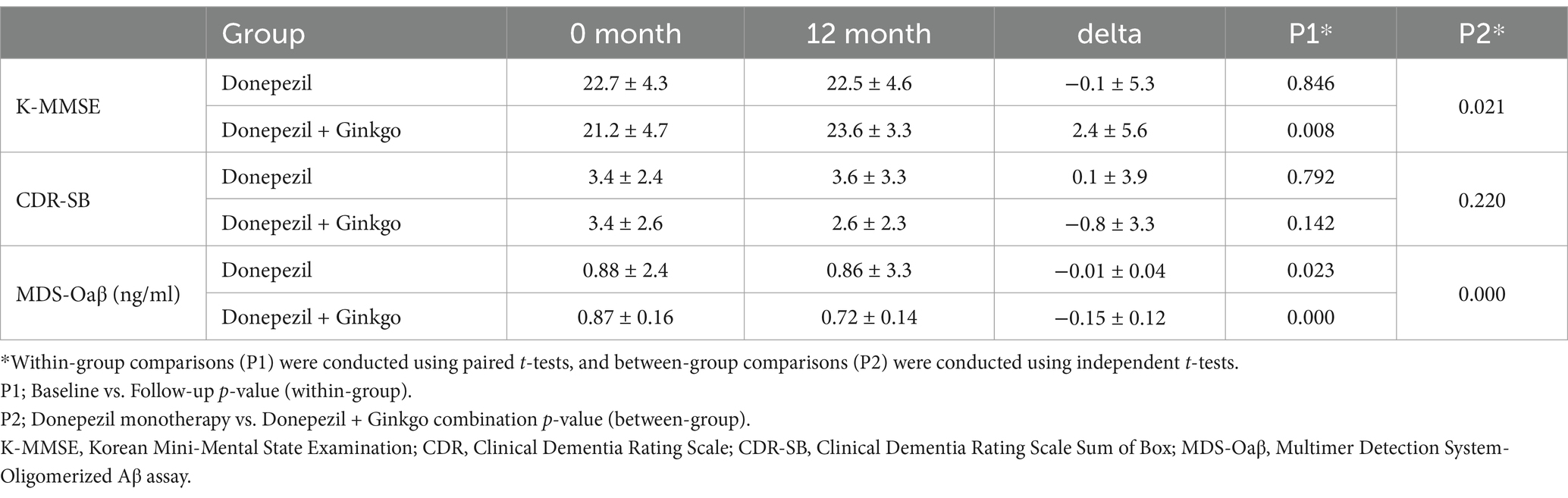
Table 2. Comparison of clinical outcome and MDS-Oaβ between Donepezil alone and Donepezil plus Ginkgo groups over 12 months.
In the Donepezil-only group, 10 patients experienced adverse events, while 9 patients in the Donepezil-Ginkgo group reported adverse events, most of which were gastrointestinal symptoms. In the Donepezil-alone group, dizziness was observed in 3 patients, whereas it was not reported in the Donepezil-Ginkgo group. However, none of these events led to treatment discontinuation (Table 3).
Given the persistent challenges in developing novel treatments for AD, it has become increasingly important to revisit established agents with potential anti-AD properties. Ginkgo is noteworthy for its reported antioxidative, anti-inflammatory, and anti-amyloid effects. However, earlier clinical trials yielded mixed outcomes, possibly due to heterogeneous study populations (e.g., participants without confirmed AD pathology) and the slow, insidious progression of AD that can mask subtle therapeutic effects in short-term studies. To address these issues, our study enrolled only amyloid PET-positive AD patients, thereby confirming pathological involvement, and employed both conventional clinical measures (K-MMSE, CDR-SB) and a biomarker known as MDS-Oaβ. MDS-Oaβ quantifies the propensity of plasma amyloid monomers to form oligomers (17) and correlates with cerebrospinal fluid measures and amyloid PET (19), as well as with overall disease progression (20). Unlike biomarkers such as Aβ42, Aβ40, or tau, which detect specific existing substances, this biomarker evaluates the tendency of how effectively monomeric amyloid in plasma forms into oligomeric amyloid. It provides a comprehensive assessment of the various conditions and stages involved in amyloid oligomerization. This makes it particularly suitable for reflecting the effects of drugs like Ginkgo, which exhibit multifaceted pharmacological actions across different stages of disease progression.
Before analyzing the 12-month outcomes, we first compared baseline characteristics between the Donepezil-only and Donepezil-Ginkgo groups. Although the Donepezil-Ginkgo group tended to have a lower baseline K-MMSE score than the Donepezil-only group (21.2 vs. 22.7), this difference was not statistically significant (p = 0.196), and the gap was not large enough to warrant additional statistical adjustments (Table 1). After 12 months of medication therapy, the Donepezil-only group showed minimal change in K-MMSE (−0.1), aligning with the known trajectories of cholinesterase inhibitors in mild-to-moderate AD (21). Meanwhile, a small but statistically significant reduction in MDS-Oaβ was observed in this group, potentially reflecting a natural course or partial effect of donepezil.
In contrast, the addition of Ginkgo yielded a larger MDS-Oaβ reduction (−0.15) and a meaningful K-MMSE increase (+2.4). This suggests additive or synergistic benefits when combining Ginkgo with donepezil, resonating with previous studies where EGb 761 plus standard AD medications slowed progression more effectively than monotherapy (22, 23). Özge et al. (22) found that EGb 761 administered alongside standard AD medications slowed disease progression more effectively than monotherapy, though their cohort included both mild cognitive impairment (MCI) and AD patients. García-Alberca et al. (23) similarly reported cognitive and neuropsychiatric benefits for amnestic MCI patients treated with EGb 761 plus cholinesterase inhibitors.
Mechanistically, the reduction in MDS-Oaβ may be attributable to Ginkgo’s broad protective actions, including its anti-inflammatory effects (24), inhibition of Aβ1-42 aggregation (25), and attenuation of oxidative stress (26). These effects may be achieved through mechanisms such as scavenging free radicals, enhancing cerebral microperfusion, and inhibiting Aβ fibrillogenesis (27, 28). When combined with the cholinesterase inhibition offered by donepezil, these effects may reduce amyloid oligomer formation and bolster cognitive function. Although CDR-SB showed no significant between-group difference, this likely reflects the scale’s lower sensitivity over a 12-month period in mild-stage AD (mean baseline CDR of 0.7). Additionally, no severe adverse events occurred, suggesting that Ginkgo is generally safe to combine with donepezil.
Our study does have limitations, including its retrospective design and relatively small sample size, which preclude definitive causal conclusions and highlight the need for larger, prospective trials. While a Ginkgo-alone group could have provided additional insights, we did not include this group due to ethical concerns regarding withholding standard-of-care treatment for Alzheimer’s disease. However, our study contributes to the growing body of indirect evidence supporting Ginkgo’s potential role in AD treatment. Based on these findings, we plan to design future prospective studies that explore Ginkgo’s effects within ethically feasible frameworks, such as in earlier disease stages or as an adjunct to other treatments. Furthermore, using K-MMSE alone may not fully capture complex cognitive or neuropsychiatric changes, and other confounders (e.g., comorbidities, adherence, lifestyle) could influence outcomes.
In this retrospective study, our data suggest that combining Ginkgo with donepezil in amyloid PET-positive AD patients may confer tangible cognitive benefits and a more robust decrease in MDS-Oaβ, possibly via reduced amyloid oligomerization tendency. Large-scale, prospective clinical trials with multiple biomarkers, such as tau imaging, inflammation markers, or advanced neuropsychological assessments, are warranted to further validate and clarify Ginkgo’s mechanistic role in AD.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving humans were approved by the Institutional Review Board of Soonchunhyang University Cheonan Hospital (Approval number: 2023-02-038-002). The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and institutional requirements. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
YY: Data curation, Formal analysis, Investigation, Project administration, Resources, Writing – original draft. M-SK: Formal analysis, Methodology, Validation, Writing – review & editing. YK: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing.
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This research was supported by a grant of the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare, Republic of Korea (grant number: HI22C0667).
We would like to acknowledge the Soonchunhyang University Dementia Registry for providing the data used in this study. We also thank the staff of the Dementia Clinic at Soonchunhyang University Cheonan Hospital for their assistance in maintaining and managing the registry.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The authors declare that no Gen AI was used in the creation of this manuscript.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Tabassum, N-E, Das, R, Lami, MS, Islam, MA, Karim, MR, Rahman, MA, et al. Ginkgo biloba: a treasure of functional phytochemicals with multimedicinal applications. Evid Based Complement Altern Med. (2022) 2022:1–30. doi: 10.1155/2022/8288818
2. Pietri, S, Maurelli, E, Drieu, K, and Culcasi, M. Cardioprotective and anti-oxidant effects of the terpenoid constituents of Ginkgo biloba extract (EGb 761). J Mol Cell Cardiol. (1997) 29:733–42. doi: 10.1006/jmcc.1996.0316
3. Luo, Y, Smith, JV, Paramasivam, V, Burdick, A, Curry, KJ, Buford, JP, et al. Inhibition of amyloid-beta aggregation and caspase-3 activation by the Ginkgo biloba extract EGb 761. Proc Natl Acad Sci USA. (2002) 99:12197–202. doi: 10.1073/pnas.182425199
4. Dodge, HH, Zitzelberger, T, Oken, BS, Howieson, D, and Kaye, J. A randomized placebo-controlled trial of Ginkgo biloba for the prevention of cognitive decline. Neurology. (2008) 70:1809–17. doi: 10.1212/01.wnl.0000303814.13509.db
5. DeKosky, ST, Williamson, JD, Fitzpatrick, AL, Kronmal, RA, Ives, DG, Saxton, JA, et al. Ginkgo biloba for prevention of dementia: a randomized controlled trial. JAMA. (2008) 300:2253–62. doi: 10.1001/jama.2008.683
6. Scherrer, B, Andrieu, S, Ousset, PJ, Coley, N, Froelich, L, Jacova, C, et al. Analysing time to event data in dementia prevention trials: the example of the Guid age study of EGb761. J Nutr Health Aging. (2016) 19:1009–11. doi: 10.1007/s12603-015-0661-2
7. Vellas, B, Coley, N, Ousset, PJ, Berr, C, Dartigues, JF, Dubois, B, et al. Long-term use of standardised Ginkgo biloba extract for the prevention of Alzheimer’s disease (Guid age): a randomised placebo-controlled trial. Lancet Neurol. (2012) 11:851–9. doi: 10.1016/S1474-4422(12)70206-5
8. Oken, BS, Storzbach, DM, and Kaye, JA. The efficacy of Ginkgo biloba on cognitive function in Alzheimer disease. Arch Neurol. (1998) 55:1409–15. doi: 10.1001/archneur.55.11.1409
9. Birks, J, and Grimley, EJ. Ginkgo biloba for cognitive impairment and dementia. Cochrane Database Syst Rev. (2007) 2:CD003120. doi: 10.1002/14651858.CD003120.pub2
10. Kandiah, N, Ong, PA, Yuda, T, Das, R, Dominguez, J, Han, SH, et al. Treatment of dementia and mild cognitive impairment with or without cerebrovascular disease: expert consensus on the use of Ginkgo biloba extract, EGb 761®. CNS Neurosci Ther. (2019) 25:288–98. doi: 10.1111/cns.13095
11. Tian, J, Shi, J, Wei, M, Jin, Y, Chen, L, Wang, X, et al. Chinese herbal medicine Qinggongshoutao for the treatment of amnestic mild cognitive impairment: a 52-week randomized controlled trial. Alzheimers Dement. (2019) 5:441–9. doi: 10.1016/j.trci.2019.03.001
12. Zhao, MX, Dong, ZH, Yu, ZH, Xiao, SY, and Li, YM. Effects of Ginkgo biloba extract in improving episodic memory of patients with mild cognitive impairment: a randomized controlled trial. Zhong Xi Yi Jie He Xue Bao. (2012) 10:628–34. doi: 10.3736/jcim20120605
13. Gavrilova, SI, Preuss, UW, Wong, JW, Hoerr, R, Schlaefke, S, Bachinskaya, N, et al. Efficacy and safety of Ginkgo biloba extract EGb 761 in mild cognitive impairment with neuropsychiatric symptoms: a randomized, placebo-controlled, double-blind, multi-center trial. Int J Geriatr Psychiatry. (2014) 29:1087–95. doi: 10.1002/gps.4103
14. Pagotto, GLO, Santos, LMO, Osman, N, Oliveira, TM, Frota, KG, Costa, DC, et al. Ginkgo biloba: a leaf of hope in the fight against Alzheimer’s dementia: clinical trial systematic review. Antioxidants. (2024) 13:651. doi: 10.3390/antiox13060651
15. McKhann, GM, Knopman, DS, Chertkow, H, Hyman, BT, Jack, CR, Kawas, CH, et al. The diagnosis of dementia due to AD: recommendations from the National Institute on Aging-Alzheimer’s association workgroups on diagnostic guidelines for AD. Alzheimers Dement. (2011) 7:263–9. doi: 10.1016/j.jalz.2011.03.005
16. Tomic, JL, Pensalfini, A, Head, E, and Glabe, CG. Soluble fibrillar oligomer levels are elevated in AD brain and correlate with cognitive dysfunction. Neurobiol Dis. (2009) 35:352–8. doi: 10.1016/j.nbd.2009.05.024
17. An, SSA, Lee, BS, Yu, JS, Kang, S, Suh, J, Han, SH, et al. Dynamic changes of oligomeric amyloid β levels in plasma induced by spiked synthetic Aβ₄₂. Alzheimers Res Ther. (2017) 9:86. doi: 10.1186/s13195-017-0310-6
18. Nah, EH, Cho, S, Park, H, Noh, D, Hwang, I, and Cho, HI. Reference interval and the role of plasma oligomeric beta amyloid in screening of risk groups for cognitive dysfunction at health checkups. J Clin Lab Anal. (2021) 35:e23933. doi: 10.1002/jcla.23933
19. Wang, MJ, Yi, S, Han, JY, Kang, SH, Cho, IH, Park, SY, et al. Oligomeric forms of amyloid-beta protein in plasma as a potential blood-based biomarker for AD. Alzheimers Res Ther. (2017) 9:98. doi: 10.1186/s13195-017-0324-0
20. Youn, YC, Kang, S, Suh, J, Koh, SH, Lee, JH, Park, KH, et al. Blood amyloid-β oligomerization associated with neurodegeneration of AD. Alzheimers Res Ther. (2019) 11:40. doi: 10.1186/s13195-019-0499-7
21. Wattmo, C, Minthon, L, and Wallin, AK. Mild versus moderate stages of Alzheimer’s disease: three-year outcomes in a routine clinical setting of cholinesterase inhibitor therapy. Alzheimers Res Ther. (2016) 8:7. doi: 10.1186/s13195-016-0174-1
22. Özge, A, Ghouri, R, Öksüz, N, and Taşdelen, B. Early intervention and adding effective doses of EGb761 like Ginkgo extract slow down dementia progression: insights to the neurovascular unit. Front Neurol. (2023) 14:1240655. doi: 10.3389/fneur.2023.1240655
23. García-Alberca, JM, Gris, E, and Mendoza, S. Combined treatment with Ginkgo biloba extract EGb 761 plus acetylcholinesterase inhibitors improved cognitive function and neuropsychiatric symptoms in patients with mild cognitive impairment. Alzheimers Dement. (2022) 8:e12338. doi: 10.1002/trc2.12338
24. Heneka, MT, Carson, MJ, JEl, K, Landreth, GE, Brosseron, F, Feinstein, DL, et al. Neuroinflammation in Alzheimer’s disease. Lancet Neurol. (2015) 14:388–405. doi: 10.1016/S1474-4422(15)70016-5
25. Mao, P, and Reddy, PH. Aging and amyloid beta-induced oxidative DNA damage and mitochondrial dysfunction in Alzheimer’s disease: implications for early intervention and therapeutics. Biochim Biophys Acta. (2011) 1812:1359–70. doi: 10.1016/j.bbadis.2011.08.005
26. Kapogiannis, D, and Mattson, MP. Disrupted energy metabolism and neuronal circuit dysfunction in cognitive impairment and Alzheimer’s disease. Lancet Neurol. (2011) 10:187–98. doi: 10.1016/S1474-4422(10)70277-5
27. Hoyer, S, Lannert, H, Nöldner, M, and Chatterjee, SS. Damaged neuronal energy metabolism and behavior are improved by Ginkgo biloba extract (EGb 761). J Neural Transm. (1999) 106:1171–88. doi: 10.1007/s007020050232
Keywords: Alzheimer’s disease, amyloid PET, donepezil, Ginkgo biloba, MDS-Oaβ
Citation: Yang Y, Koo M-S and Kwak YT (2025) Efficacy of Ginkgo biloba as an adjunct to donepezil in amyloid PET-positive Alzheimer’s patients. Front. Neurol. 16:1563056. doi: 10.3389/fneur.2025.1563056
Received: 19 January 2025; Accepted: 26 February 2025;
Published: 11 March 2025.
Edited by:
Pei-Ning Wang, National Yang-Ming University, TaiwanReviewed by:
Seung-yeol Nah, Konkuk University, Republic of KoreaCopyright © 2025 Yang, Koo and Kwak. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yong Tae Kwak, a3dha2RyQGdtYWlsLmNvbQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.