
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Neurol. , 25 March 2025
Sec. Neuromuscular Disorders and Peripheral Neuropathies
Volume 16 - 2025 | https://doi.org/10.3389/fneur.2025.1557515
Introduction: Obesity is a worldwide health concern frequently addressed by weight reduction strategies, including bariatric surgery and restricted diets. While effective, these approaches can result in complications, including Guillain-Barré Syndrome (GBS), a rare but serious autoimmune disorder. This study aims to analyze clinical and neurophysiological features of diet-induced GBS and compare them to cases linked with bariatric surgery.
Methods: We retrospectively reviewed medical records of five patients admitted to our institution between August 2012 and August 2022, who developed GBS during active dieting resulting in significant weight loss. Clinical presentations, laboratory results, neurophysiological findings, and nutritional status during treatment were analyzed. Additionally, we performed a literature review comparing these cases with nineteen previously reported instances of bariatric surgery-associated GBS.
Results: All five patients exhibited acute, symmetrical limb weakness primarily affecting the lower extremities, accompanied by diminished tendon reflexes. Neurophysiological assessments revealed axonal damage in all cases, and albuminocytologic dissociation was present in two patients. Three patients received intravenous immunoglobulin (IVIG) therapy, while the remaining two underwent nutritional therapy alone. All patients achieved full recovery within 6 months. Notably, the rate of weight loss observed significantly exceeded recommended safe guidelines.
Discussion: Rapid and substantial weight loss may play a role in triggering GBS, possibly due to nutritional deficiencies or immune dysregulation. Clinicians should recognize the potential neurological risks associated with aggressive weight-loss strategies. Early diagnosis and appropriate intervention are crucial for favorable outcomes and preventing complications.
Obesity is a complex and multifactorial condition that has become a global health epidemic, with far-reaching consequences for both individual health and public health systems (1, 2). It is associated with numerous comorbidities, including cardiovascular diseases, type 2 diabetes, and various metabolic disorders (3–6). The growing prevalence of obesity has led to an increasing burden on healthcare systems worldwide (7). As a result, effective interventions for managing obesity are crucial to reducing associated risks and improving the quality of life for affected individuals (8).
Bariatric surgery has emerged as an effective treatment for severe obesity, leading to substantial weight loss and improvement in comorbid conditions (9–11). However, despite its efficacy, bariatric surgery is not without risks. The incidence of peripheral neuropathies, including Guillain-Barré Syndrome (GBS), following weight loss surgery is approximately 0.06% (12–16). In contrast, diet-induced weight loss is a non-invasive alternative that is widely employed to manage obesity. While it is considered safer than surgical interventions, the potential for diet-induced complications, including GBS, remains underexplored.
GBS is an autoimmune disorder characterized by progressive muscle weakness and paralysis, typically following a triggering event such as an infection, trauma, or surgery (17, 18). Although rare, GBS has been reported in patients undergoing significant weight loss through bariatric surgery (15, 19–22). However, instances of GBS associated with dieting remain exceedingly rare, warranting further investigation.
In this study, we report five cases of GBS that developed during active dieting, providing a detailed analysis of these cases and exploring the potential link between weight loss through dieting and the onset of GBS. Additionally, we compare these cases with nineteen previously reported bariatric surgery-associated GBS cases to elucidate potential differences in clinical features, pathophysiological mechanisms, and outcomes.
This retrospective study included patients diagnosed with GBS who were actively dieting at the time of symptom onset. We reviewed the medical records of five such patients between August 2012 and August 2022. These patients met the diagnostic criteria for GBS, as outlined by Shahrizaila et al. (23). We collected data on their clinical presentation, laboratory findings, and treatment outcomes. Additionally, a comprehensive literature search was performed using PubMed and Google Scholar databases up to January 2025 to identify similar cases reported in the field. The search included terms such as “weight loss,” “Guillain-Barré Syndrome,” “bariatric surgery” and specific types of bariatric procedures. This study was a retrospective analysis of existing patient records without any particular intervention. Nutritional status, including micronutrient levels, was monitored as part of the clinical management of the patients to identify and address any deficiencies. We ensured full protection of patient privacy and adhered to the Helsinki Declaration. Ethical approval was not required for this retrospective study, as it was based on anonymized medical records.
Table 1 summarizes the clinical and paraclinical features, treatments, and outcomes of the five patients in this study. All patients were engaged in dietary weight loss at the time of disease onset. The mean age of the patients was 30.2 ± 12.9 years. The average duration from the onset of neurological symptoms to medical consultation was 14.2 ± 5.2 days, with all cases presenting acute onset (within 4 weeks). Weight loss ranged from 5 to 20 kg, and none of the patients had a history of alcohol consumption, diabetes, peptic ulcer disease, or gastrointestinal surgery.
All patients experienced acute four-limb weakness and altered sensation in either the lower limbs or both upper and lower limbs. Four patients had low albumin levels, indicative of malnutrition, while levels of vitamin B1, vitamin B12, folate, calcium, and vitamin D were normal in all patients. Electrophysiological findings were consistent with GBS, demonstrating pronounced axonal involvement. Cerebrospinal fluid (CSF) analysis revealed albuminocytologic dissociation in two patients. Serum and cerebrospinal fluid tests for metabolic, infectious, and autoimmune causes of acquired peripheral neuropathy were negative. Serum C. jejuni antibodies and stool cultures were also negative. Furthermore, all stool cultures for common diarrhea-associated pathogens were negative. The mean time to reach the disease nadir (signifying rapid clinical deterioration) was 7.0 ± 5.2 days. One patient (Case 3) required support for walking at the peak of disease severity, while the other four presented milder symptoms. Cases 2, 3, and 4 received Intravenous Immunoglobulin (IVIG) (2 g/kg over 3–5 days), whereas Cases 1 and 5 received nutritional therapy without IVIG. At the six-month follow-up, all patients achieved full recovery, with no residual neurological deficits reported.
A systematic literature review identified nineteen cases of GBS following bariatric surgery, summarized in Table 2. These cases primarily involved surgical procedures such as Sleeve Gastrectomy (SG), Laparoscopic Gastric Restriction Surgery (LGRS), Laparoscopic Gastroplasty (LGP), Roux-en-Y Gastric Bypass (RYGB), and Post-Distal Gastrectomy with Gastroduodenal Anastomosis (Post-DG-GDA). The weight loss associated with these cases ranged from 25 kg to 90 kg, with the onset of GBS symptoms occurring between 1 to 12 months post-surgery. Clinical presentations included paraplegia, quadriplegia, paresthesia, areflexia, and in some cases, facial nerve deficits and respiratory failure. Treatments varied among patients, including IVIG, plasma exchange (PE), gabapentin, and nutritional supplementation. Prognoses were generally favorable, with most patients achieving full or nearly full recovery within several months, although some had lingering deficits such as the need for a cane or persistent weakness.
As shown in Table 3, several distinctions emerge when comparing the clinical characteristics and treatment outcomes of dietary weight loss-induced GBS cases with those of bariatric surgery-related GBS. The diet-induced cases had a higher incidence of preceding infections, a higher rate of hypoalbuminemia, slower weight loss rates, and a lower incidence of nutrient deficiencies. Additionally, diet-induced cases generally involved less severe neurological deficits at nadir, with recovery around 6 months appearing more favorable. In contrast, bariatric surgery-associated cases often presented with more severe symptoms, such as quadriplegia and respiratory failure, and required more intensive treatments like PE and prolonged hospitalizations. Despite these differences, both groups shared similar neurophysiological findings, with most cases being of the axonal-type GBS. CSF protein levels were mostly normal, and most patients received IVIG treatment, with overall good prognoses.
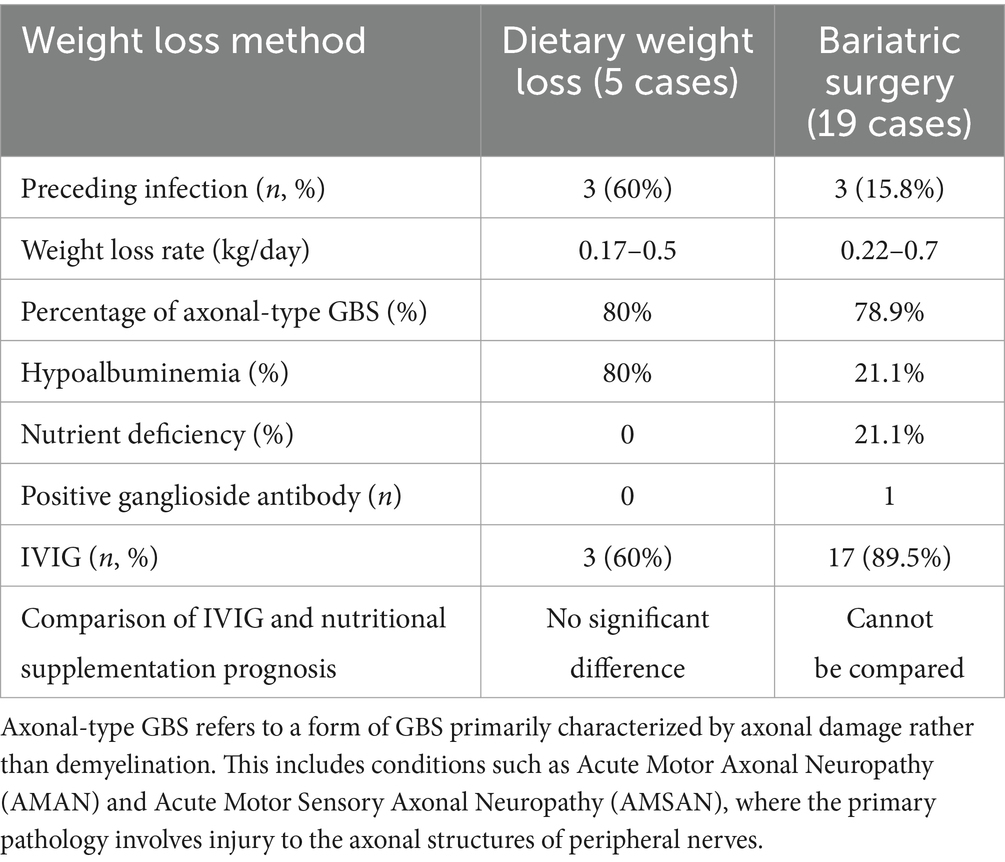
Table 3. Comparison of clinical characteristics and treatment outcomes between dietary weight loss and weight loss surgery in patients with GBS.
This study reviewed five cases of GBS associated with diet-induced weight loss and compared them with nineteen cases of bariatric surgery-related GBS reported in the literature. All patients exhibited acute, symmetrical limb weakness and diminished tendon reflexes.
In patients with diet-related GBS, 60% reported a preceding infection, while only 15.8% of those with bariatric surgery-related GBS had a similar history. However, despite this difference, it is important to note that all preceding infections in our study were cases of diarrhea, and both serological tests for Campylobacter jejuni antibodies and stool cultures were negative. Through stool culture, common diarrhea-associated pathogens were consistently not detected, suggesting that infection may not be the primary trigger in these cases of GBS. We acknowledge that diarrhea could be caused by other pathogens, and the detection of such pathogens may be limited by the range of tests used or variations in pathogen load. Nonetheless, the negative microbiological results do not entirely exclude an infectious trigger due to current diagnostic limitations.
Given these limitations in pathogen detection, alternative triggers must be considered. Furthermore, in the 19 cases of GBS related to weight loss surgery, there is insufficient direct evidence to suggest that a preceding infection triggered the onset of GBS. Among these, two patients had a history of preceding diarrhea, and one had an upper respiratory infection. However, no specific pathogen was identified, and only one case showed a low concentration of Campylobacter jejuni antibodies in the serum, with no history of preceding infection.
Moreover, our five diet-induced cases exhibited weight loss rates ranging from 0.17 to 0.5 kg/day, while the literature reports weight loss rates of 0.22 to 0.75 kg/day in bariatric surgery-related GBS cases—far exceeding the recommended safe weight loss rate of 0.5–1 kg per week (24, 25).
Although current evidence is limited, clinical experience suggests that the rapidity and method of weight loss, such as extreme restrictive diets, may predispose patients to nutritional deficiencies and nerve damage. Therefore, given this data, it is plausible that weight loss—whether through dieting or surgery—may itself act as a potential trigger for GBS, independent of infection.
In patients with diet-related GBS, 80% showed hypoalbuminemia, while only 21.1% of surgery-related GBS patients experienced hypoalbuminemia, and the same percentage had nutritional deficiencies. Despite this, both groups exhibited around 80% of cases with pronounced axonal involvement, and the majority maintained normal CSF protein levels, which is characteristic of axonal forms of GBS. This suggests a potential link between malnutrition and axonal damage in GBS. These findings are consistent with previous reports of peripheral neuropathy following bariatric surgery, where the underlying pathogenesis may differ from classical GBS (26).
Earlier studies have found that acute axonal neuropathy in weight-loss patients is often caused by nutritional deficiencies (27). Malnutrition and acute axonal neuropathy are closely associated, with improvements observed following weight gain and vitamin supplementation. Specifically, deficiencies in iron, calcium, vitamin B12, and fat-soluble vitamins (A, D, E, K) are thought to impair neural function directly, further supporting the idea that malnutrition plays a significant role in axonal injury (28, 29).
Malnutrition significantly impairs the immune defense mechanisms of the host, particularly in the gut epithelium. Nutritional deficiencies restrict the availability of complement components, thereby affecting the capacity of professional phagocytes to engulf and eliminate pathogens. In experimental protein-energy malnutrition (PEM) models in mice, macrophage phagocytosis, as well as the production of reactive oxygen intermediates (ROIs) and reactive nitrogen intermediates (RNIs), is diminished. Additionally, the antigen-presenting function of dendritic cells to T cells is suppressed. In experimental peritonitis in mice, short-term PEM leads to impaired immune cell migration and extravasation, as evidenced by a reduced number of CD11b/CD18-positive cells at the infection site. This impairment may be linked to a decrease in the chemokine MIP-2 concentration (42).
Both innate and adaptive immunity are compromised by malnutrition. Defects in innate immunity include impaired epithelial barrier function of the skin and gut, reduced granulocyte microbicidal activity, fewer circulating dendritic cells, and decreased complement proteins. However, leukocyte numbers and acute-phase responses remain intact. On the other hand, defects in adaptive immunity are characterized by reduced soluble IgA levels in saliva and tears, atrophy of lymphoid organs, diminished delayed-type hypersensitivity responses, a decrease in circulating B cells, a shift from Th1-associated cytokines to Th2-associated cytokines, and lymphocyte hyporesponsiveness to phytohemagglutinin. Nonetheless, lymphocyte and immunoglobulin levels in peripheral blood are preserved (30). Vitamin D deficiency is commonly observed in patients with GBS, and its immunoregulatory role, particularly in the modulation of T lymphocyte function, has been widely recognized (31).
We report five cases of diet-induced GBS, three of which were treated with IVIG and demonstrated favorable outcomes. The remaining two patients, with milder symptoms, received nutritional supplementation (Vitamin B1, B12, and folic acid) and achieved full recovery, despite normal serum levels of these vitamins. These findings suggest that IVIG treatment may not be absolutely necessary in diet-induced GBS patients. Similar to axonal peripheral neuropathy seen after bariatric surgery, favorable outcomes can often be achieved through nutritional supplementation alone (27, 32).
Currently, there are no guidelines or comparative studies supporting the necessity of IVIG treatment for diet-induced GBS. However, in bariatric surgery-related GBS, one case showed a positive anti-ganglioside antibody result (41), suggesting that an immune mechanism may be involved in the pathogenesis of GBS and supporting the rationale for IVIG treatment. Given that GBS can be life-threatening and lead to permanent disability, a combined treatment approach of IVIG and nutritional supplementation might be a prudent choice, depending on the severity of the symptoms, the rate of progression, and the patient’s preferences. Further large-scale comparative studies are needed to explore the necessity of IVIG treatment in diet-induced GBS.
Both diet-induced weight loss and bariatric surgery may trigger GBS. It is essential for clinicians to recognize the early signs of GBS in patients undergoing these weight loss interventions and provide prompt treatment to improve outcomes and prevent complications.
The primary limitations of this study include the small sample size and its retrospective, single-center design, which may introduce selection bias and incomplete data issues. It is important to note that our hospital is part of a larger regional network—including major institutions such as Xiangya Hospital—that treats a significantly higher number of GBS cases; thus, calculating incidence solely from our hospital’s data may not fully reflect the regional burden. Future studies should incorporate epidemiological expertise and involve multi-center data to better ascertain the relationship between rapid weight loss and GBS. Therefore, conclusions should be interpreted with caution and validated through larger-scale prospective studies.
The datasets presented in this article are not readily available because of ethical and privacy restrictions. Requests to access the datasets should be directed to the corresponding author.
The requirement of ethical approval was waived by the Ethics Committee of Changsha Central Hospital for the studies involving humans due to the retrospective nature of the study. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation in this study was provided by the participants' legal guardians/next of kin. Written informed consent was obtained from the individual(s), and minor(s)' legal guardian/next of kin, for the publication of any potentially identifiable images or data included in this article.
QW: Data curation, Investigation, Writing – original draft. F-YL: Methodology, Resources, Supervision, Writing – original draft, Writing – review & editing, Formal analysis. JH: Methodology, Supervision, Visualization, Writing – review & editing, Validation. WX: Investigation, Supervision, Validation, Writing – review & editing, Data curation. T-QF: Investigation, Methodology, Software, Writing – review & editing, Conceptualization. H-SZ: Formal analysis, Project administration, Resources, Writing – review & editing. ZW: Methodology, Project administration, Supervision, Validation, Writing – review & editing. W-GZ: Conceptualization, Formal analysis, Writing – review & editing.
The author(s) declare that no financial support was received for the research and/or publication of this article.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The authors declare that Gen AI was used in the creation of this manuscript. Generative AI tools were utilized to assist in polishing and correcting grammatical errors in the preparation of this manuscript. However, all scientific content, analysis, and conclusions are based on actual data and were authored solely by the researchers, not generated by AI.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
GBS, Guillain-Barré Syndrome; IVIG, Intravenous immunoglobulins; PE, Plasma exchange; CSF, Cerebrospinal fluid; SG, Sleeve Gastrectomy; LGRS, Laparoscopic Gastric Restriction Surgery; LGP, Laparoscopic Gastroplasty; LSG, Laparoscopic Sleeve Gastrectomy; RYGB, Roux-en-Y Gastric Bypass; Post-DG-GDA, Post-Distal Gastrectomy with Gastroduodenal Anastomosis; AMAN, Acute Motor Axonal Neuropathy; AMSAN, Acute Motor Sensory Axonal Neuropathy; PEM, Protein-energy malnutrition; ROIs, Reactive oxygen intermediates; RNIs, Reactive nitrogen intermediates.
1. Sarma, S, Sockalingam, S, and Dash, S. Obesity as a multisystem disease: trends in obesity rates and obesity-related complications. Diabetes Obes Metab. (2021) 23:3–16. doi: 10.1111/dom.14290
2. Yang, M, Liu, S, and Zhang, C. The related metabolic diseases and treatments of obesity. Healthcare. (2022) 10:1616. doi: 10.3390/healthcare10091616
3. Pi-Sunyer, FX. The medical risks of obesity. Obes Surg. (2002) 12:S6–S11. doi: 10.1007/BF03342140
4. Obesity Comorbidities. (2024). Clinical Guidance. Healio. Available online at: https://www.healio.com/clinical-guidance/obesity/obesity-related-comorbidities (Accessed December 22, 2024).
5. Lopez-Jimenez, F, Almahmeed, W, Bays, H, Cuevas, A, Di Angelantonio, E, le Roux, CW, et al. Obesity and cardiovascular disease: mechanistic insights and management strategies. A joint position paper by the world heart federation and world obesity federation. Eur J Prev Cardiol. (2022) 29:2218–37. doi: 10.1093/eurjpc/zwac187
6. Fruh, SM. Obesity: risk factors, complications, and strategies for sustainable long-term weight management. J Am Assoc Nurse Pract. (2017) 29:S3–S14. doi: 10.1002/2327-6924.12510
7. Memon, Y, Elfituri, S, ProfDrA, R, DrE, F, Aigbedion, G, Chaudhary, ZQ, et al. Patterns and consequences of obesity on cardiovascular concerns prevalence, fluctuations, and healthcare dilemmas. J Popul Ther Clin Pharmacol. (2024) 31:451–60. doi: 10.53555/jptcp.v31i1.4026
8. Drucker, DJ. Prevention of cardiorenal complications in people with type 2 diabetes and obesity. Cell Metab. (2024) 36:338–53. doi: 10.1016/j.cmet.2023.12.018
9. Cohen, R, Sforza, NS, and Clemente, RG. Impact of metabolic surgery on type 2 diabetes mellitus, cardiovascular risk factors, and mortality: a review. Curr Hypertens Rev. (2021) 17:159–69. doi: 10.2174/1573402116666200804153228
10. Biobaku, F, Ghanim, H, Monte, SV, Caruana, JA, and Dandona, P. Bariatric surgery: remission of inflammation, Cardiometabolic benefits, and common adverse effects. J Endocr Soc. (2020) 4:bvaa049. doi: 10.1210/jendso/bvaa049
11. Ji, Y, Lee, H, Kaura, S, Yip, J, Sun, H, Guan, L, et al. Effect of bariatric surgery on metabolic diseases and underlying mechanisms. Biomolecules. (2021) 11:1582. doi: 10.3390/biom11111582
12. Bjørklund, G, Semenova, Y, Pivina, L, and Costea, D-O. Follow-up after bariatric surgery: a review. Nutrition. (2020) 78:110831. doi: 10.1016/j.nut.2020.110831
13. Collazo-Clavell, ML, and Shah, M. Common and rare complications of bariatric surgery. Endocrinol Metab Clin. (2020) 49:329–46. doi: 10.1016/j.ecl.2020.02.003
14. Aljthalin, RA, and Al-Attas, AA. Guillain–Barré syndrome variants: a grave complication of bariatric surgery. Egypt J Neurol Psychiatry Neurosurg. (2022) 58:1–4. doi: 10.1186/s41983-022-00526-1
15. Şahi̇n, Ş, Ateş, MF, Çinar, N, and Karşidağ, S. A new etiology for variant of Guillain-Barré syndrome: bariatric surgery. Eur Res J. (2019) 5:1024–7. doi: 10.18621/eurj.461760
16. Chang, CG, Adams-Huet, B, and Provost, DA. Acute post-gastric reduction surgery (APGARS) neuropathy. Obes Surg. (2004) 14:182–9. doi: 10.1381/096089204322857537
17. Huang, C, Zhang, Y, Deng, S, Ren, Y, and Lu, W. Trauma-related Guillain-Barré Syndrome: systematic review of an emerging concept. Front Neurol. (2020) 11:588290. doi: 10.3389/fneur.2020.588290
18. Fokke, C, van den Berg, B, Drenthen, J, Walgaard, C, van Doorn, PA, and Jacobs, BC. Diagnosis of Guillain-Barré syndrome and validation of Brighton criteria. Brain J Neurol. (2014) 137:33–43. doi: 10.1093/brain/awt285
19. Ishaque, N, Khealani, BA, Shariff, AH, and Wasay, M. Guillain-Barré syndrome (demyelinating) six weeks after bariatric surgery: a case report and literature review. Obes Res Clin Pract. (2015) 9:416–9. doi: 10.1016/j.orcp.2015.02.001
20. Degterev, DA, Suponeva, NA, Bodunova, NA, Voronova, МV, Zorin, ЕA, Piradov, МA, et al. A rare complication of bariatric surgery: Polyradiculoneuropathy as a type of Guillain-Barré syndrome. Ter Arkh. (2016) 88:79–83. doi: 10.17116/terarkh201688579-83
21. Machado, FCN, Valério, BCO, Morgulis, RNF, Nunes, KF, and Mazzali-Verst, S. Acute axonal polyneuropathy with predominant proximal involvement: an uncommon neurological complication of bariatric surgery. Arq Neuropsiquiatr. (2006) 64:609–12. doi: 10.1590/S0004-282X2006000400017
22. Sunbol, AH, Almaghrabi, S, Al Aslany, SJ, Aldairi, MM, Makhdum, SA, Trabulsi, N, et al. Delayed Guillain-Barré syndrome after bariatric surgery: a report of three cases. Case Rep Surg. (2018) 2018:e8413206:1–5. doi: 10.1155/2018/8413206
23. Shahrizaila, N, Lehmann, HC, and Kuwabara, S. Guillain-Barré syndrome. Lancet. (2021) 397:1214–28. doi: 10.1016/S0140-6736(21)00517-1
24. Stubbs, J, Whybrow, S, and Lavin, J. Dietary and lifestyle measures to enhance satiety and weight control: practical weight-control solutions. Nutr Bull. (2010) 35:113–25. doi: 10.1111/j.1467-3010.2010.01827.x
25. Freedhoff, Y, Morin, M-P, and Langlois, MF, (2020). Adult MMC. Commercial products and programs in obesity management. Available online at: https://www.semanticscholar.org/paper/Commercial-Products-and-Programs-in-Obesity-i-Morin/5e9a5732485c4ac378d65d66835e7453f530e43f (Accessed February 26, 2025).
26. Keskin, AO, and Yerdelen, D. Acute polyneuropathies after bariatric surgery: does immunity play a role? Case series and literature review. Int J Neurosci. (2023) 133:1304–8. doi: 10.1080/00207454.2023.2263811
27. Hamel, J, and Logigian, EL. Acute nutritional axonal neuropathy. Muscle Nerve. (2018) 57:33–9. doi: 10.1002/mus.25702
28. Lombardo, M, Franchi, A, Biolcati Rinaldi, R, Rizzo, G, D’Adamo, M, Guglielmi, V, et al. Long-term Iron and vitamin B12 deficiency are present after bariatric surgery, despite the widespread use of supplements. Int J Environ Res Public Health. (2021) 18:4541. doi: 10.3390/ijerph18094541
29. Chamberlain, C, Terry, R, Shtayyeh, T, and Martinez, C. Recognizing postoperative nutritional complications of bariatric surgery in the primary care patient: a narrative review. J Osteopath Med. (2021) 121:105–12. doi: 10.7556/jaoa.2020.135
30. Bourke, CD, Berkley, JA, and Prendergast, AJ. Immune dysfunction as a cause and consequence of malnutrition. Trends Immunol. (2016) 37:386–98. doi: 10.1016/j.it.2016.04.003
31. Sirufo, MM, Magnanimi, LM, Ginaldi, L, and De Martinis, M. Guillain-Barré syndrome, the IL-33/ST2 axis, and vitamin D. Eur J Neurol. (2022) 29:e20. doi: 10.1111/ene.15379
32. Yasawy, ZM, and Hassan, A. Post bariatric surgery acute axonal polyneuropathy: doing your best is not always enough. Ann Indian Acad Neurol. (2017) 20:309–12. doi: 10.4103/aian.AIAN_24_17
33. Ates, MP, Dibek, DM, Guven, H, and Comoglu, SS. (2025). A rare complication of bariatric surgery; Guillain Barre Syndrome. Available online at: https://meddocsonline.org/journal-of-clinical-images/a-rare-complication-of-bariatric-surgery-guillain-barre-syndrome.html (Accessed February 15, 2025).
34. Aluka, KJ, Turner, PL, and Fullum, TM. Guillain-Barré syndrome and Postbariatric surgery polyneuropathies. JSLS. (2009) 13:250–3.
35. Quigley, S, Colledge, J, Mukherjee, S, and Patel, K. Bariatric surgery: a review of normal postoperative anatomy and complications. Clin Radiol. (2011) 66:903–14. doi: 10.1016/j.crad.2011.04.017
36. Shehabeldin, M, Sanchez, P, and Avila, M. Neurologic complications following bariatric surgery: a case report and literature review (P2.195). Neurology. (2017) 88:195. doi: 10.1212/WNL.88.16_supplement.P2.195
37. Chang, CG, Helling, TS, Black, WE, and Rymer, MM. Weakness after gastric bypass. Obes Surg. (2002) 12:592–7. doi: 10.1381/096089202762252415
38. Najjari, K, Gouravani, M, Talebpour, M, Kor, F, Iranmanesh, M, and Zabihi, MH. Gastric lumen obstruction after open VBG and guillain-Barré syndrome following revisional surgery: a case report. Obes Surg. (2021) 31:3324–6. doi: 10.1007/s11695-021-05351-8
39. Hamdeh, MA, Mounshar, AAMAA, Asad, RM, Shubietah, A, Basalat, NMAJ, Hassouneh, JS, et al. Guillain-Barré syndrome following laparoscopic sleeve gastrectomy: a tale of two cases. Obes Surg. (2025) 35:345–9. doi: 10.1007/s11695-024-07635-1
40. Mahwish, N, Omara, EIM, Rangraze, IR, and Qaeidy, AA. Post-bariatric Guillain-Barré syndrome: a case report emphasizing timely recognition and intervention. J Emerg Crit Care Med. (2024) 8:142. doi: 10.21037/jeccm-23-142
41. Landais, AF. Rare neurologic complication of bariatric surgery: acute motor axonal neuropathy (AMAN), a severe motor axonal form of the Guillain Barré syndrome. Surg Obes Relat Dis. (2014) 10:e85–7. doi: 10.1016/j.soard.2014.02.019
Keywords: Guillain-Barré Syndrome, weight loss, bariatric surgery, restrictive diets, nutritional deficiencies
Citation: Wu Q, Li F-Y, Hu J, Xu W, Feng T-Q, Zhou H-S, Wang Z and Zeng W-G (2025) Guillain-Barré Syndrome following weight loss: a review of five diet-induced cases and nineteen bariatric surgery cases. Front. Neurol. 16:1557515. doi: 10.3389/fneur.2025.1557515
Received: 10 January 2025; Accepted: 10 March 2025;
Published: 25 March 2025.
Edited by:
Mamede De Carvalho, University of Lisbon, PortugalReviewed by:
Isabel Conceição, Santa Maria Hospital, PortugalCopyright © 2025 Wu, Li, Hu, Xu, Feng, Zhou, Wang and Zeng. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Wen-Gao Zeng, d2d6OTk4OEAxNjMuY29t
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.