
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Neurol., 05 March 2025
Sec. Endovascular and Interventional Neurology
Volume 16 - 2025 | https://doi.org/10.3389/fneur.2025.1539127
Background: Symptomatic intracranial atherosclerotic stenosis (sICAS) is one of the common causes of ischemic stroke. However, the treatment of sICAS has remained a challenge in the past with unfavorable findings. This study aimed to evaluate the effectiveness and safety of different endovascular treatment methods for sICAS.
Methods: The study involved 154 patients with sICAS who received endovascular treatment at Qingdao University Hospital between January 2021 and October 2023. Based on the characteristics of the lesions, three different types of treatments were performed: bare metal stent group (BMS group), drug-coated balloon group (DCB group), and drug-eluting stent group (DES group). The primary endpoints included the incidence of in-stent restenosis (ISR) in the 6-month, periprocedural complications, the rate of stroke recurrence in the area of the stented artery during the follow-up period, and modified Rankin score (mRS) at discharge, at 1-month, at 3-month, at 6-month of patients after stenting.
Results: The incidence of perioperative complications did not differ significantly between groups (11.3% in the BMS group, 8.0% in the DCB group, and 6.1% in the DES group, p = 0.776). All patients (154/154) had successful reperfusion after endovascular treatment. The incidence of stroke during follow-up was 4.5% (7/154), with 5 (7.0%) patients in the BMS group, 1 (2.0%) patient in the DCB group, and 1 (3.0%) patient in the DES group. The restenosis rate in the BMS group [35.2% (25/71)] tended to be higher than that in the DCB group [6.0% (3/50)] and DES group [9.1% (3/33)]. In multivariate logistic regression analysis, endovascular treatment strategy and vessel distribution were significant independent risk factors for ISR within 6 months (p < 0.05).
Conclusion: Adverse events and success rates following stent implantation are comparable across therapy groups in individuals with sICAS. When compared to BMS, DES, and DCB reduce the risk of ISR, with the advantages of the DCB appearing to be greater for some high-risk patients with ICAS.
With 2.5 million stroke victims annually, stroke is the leading cause of death worldwide. The burden of stroke is most likely the largest in China. Intracranial atherosclerotic stenosis (ICAS), one of the main causes of ischemic stroke, is closely associated with a high incidence and death rate from stroke (1). Extracranial large artery atherosclerosis may be a common lesion in white persons in Europe and America. In contrast, atherosclerotic stenosis of the major intracranial arteries is found commonly among stroke patients of Asian, black, and Hispanic ancestry (2–4). Consequently, lowering the high incidence of cerebrovascular accidents requires both secondary prevention and efficient treatment (5). Even with aspirin treatment and standard management of vascular risk factors, patients who have recently experienced a transient ischemic attack (TIA) or stroke and have severe stenosis (70–99% of the diameter of a major intracranial artery) are particularly high risk for recurrent stroke in the territory of the stenotic artery (approximately 23% at 1 year) (6, 7). This suggests that medication-only treatment for symptomatic patients with cerebral artery stenosis might not be a practical means of preventing ischemic stroke. When traditional medical treatment is not working for Asian individuals with a high frequency of ICAS, endovascular therapy can be a helpful alternative. For the endovascular treatment of symptomatic intracranial atherosclerotic stenosis (sICAS), the Stenting vs. Aggressive Medical Management for Preventing Recurrent Stroke in Intracranial Stenosis (SAMMPRIS) and the Vitesse Intracranial Stent Study for Ischemic Stroke Therapy (VISSIT) trials did not demonstrate the benefits of endovascular treatment over medical therapy for the endovascular treatment of sICAS, with a high incidence of peri-operative ischemic or hemorrhagic stroke events (8, 9). Improved patient and device selection techniques were used in follow-up studies to lower the peri-operative event rate (10). Through the introduction of devices and improved neuro-interventional techniques, periprocedural complications have been adequately controlled. Intracranial stenting may be safe for use in some patients with sICAS, according to data from the Wingspan Stent System Post Market Surveillance (WEAVE) trial and the Registry Study of Stenting for Symptomatic Intracranial Artery Stenosis in China, which showed periprocedural complication rates of 2.6 and 4.3%, respectively (11–13). However, it has been observed that in-stent restenosis (ISR) caused by neointimal hyperplasia following stent implantation can reach 33%. This is a serious issue that needs to be resolved right once (14, 15). Consequently, it is imperative to prevent ISR in ICAS lesions. Drug-eluting stents (DES) and drug-coated balloons (DCB) can effectively lower the incidence of ISR by releasing antiproliferative drugs at the stenosis site to inhibit endothelial cell proliferation and vascular overactivity, and the initial application of DES and DCB in sICAS has demonstrated better safety and efficacy (16–20). However, the safety and efficaciousness of DES and DCB applied to intracranial arteries remain unclear, and the ideal course of treatment for sICAS patients is still unknown. The purpose of this paper is to evaluate the safety and effectiveness of several endovascular therapy modalities for sICAS to gather additional knowledge for clinical use.
Approved by the University Hospital of Qingdao’s institutional ethics council, this retrospective investigation was conducted at a tertiary stroke center. A total of 154 patients with for sICAS with endovascular recanalization between January 2021 and October 2023 were enrolled. Our inclusion criteria were: (1) patients over 18 years old; (2) patients with ≥70% stenosis of the main trunk of the middle cerebral artery (MCA), intracranial segment of the internal carotid artery (ICA), intracranial segment of the vertebral artery (VA), or basilar artery (BA) was confirmed by digital subtraction angiography (DSA) following Warfarin-Aspirin Symptomatic Intracranial Disease Study (WASID) criteria 21; (3) TIA or stroke in the territory of the target lesion area; (4) continuing, aggravated, or recurrent ischemic neurological deficits despite maximal medical therapy; (5) at least one atherosclerotic risk factor (hypertension, diabetes mellitus, hyperlipidemia, and smoking); (6) a modified Rankin score (mRS) ≤ 3; and (7) the previous TIA or ischemic stroke occurring more than 3 weeks before the endovascular procedures. The exclusion criteria were as follows: (1) non-atherosclerotic stenosis or concurrent intracranial pathology such as vasculitis and arterial dissection; (2) concurrent intracranial tumor, aneurysm, and cerebral arteriovenous malformation; (3) tandem ≥50% stenosis of extracranial carotid or vertebral artery; (4) a concomitant any bleeding disorder; (5) allergies to heparin, aspirin, clopidogrel, metal implants, or narcotic drugs; and (6) intolerance to general anesthesia, comorbidities of malignant tumor or severe liver and kidney dysfunction.
Pre-procedurally, aggressive medical therapy and intensive risk factor management were implemented. This included stringent blood glucose control, cigarette control, aspirin (100 mg/day), clopidogrel (75 mg/day), and atorvastatin calcium (20 mg/day). Endovascular treatments were performed by experienced neurointerventionists. DSA was performed for all patients, and the strategy of endovascular treatment was decided according to the site and characteristics of the target lesions and based on the operators’ experience and preference, and all the procedures were performed under general anesthesia. Technical success was defined as residual stenosis of the target vessel ≤30% after angioplasty.
The selected DES included NOVA (Tianjin Sanoshenchang Company, China) and NANO (Beijing Lepu Company, China), and the BMS included Apollo (Shanghai Microtronics Company, China) and Neuroform EZ (Boston Scientific Corporation, USA). The femoral artery was punctured and cannulated using the Seldinger technique, with a 6F Cook-long arterial sheath. An intermediate catheter was delivered proximal to the target vessel to measure the maximum stenosis rate of the diseased vessel. The stenosis degree was measured according to the criteria of the WASID study, and to observe the collateral circulation compensation. The micro guidewire was carefully passed through the stenotic vessel and placed in the distal vessel to stabilize the guidewire. A balloon of appropriate size was delivered along the micro guidewire to pre-dilate the stenotic vessel, and after dilatation, the balloon was carefully withdrawn under fluoroscopy, and the stenotic vessel was dilated by DSA imaging. The DES or BMS was delivered along the microguidewire, and the balloon was slowly pressurized with a pressure of 6–8 atm after accurate alignment. After the successful release of the stent, angiography was performed again to observe the residual stenosis of the target vessel and the blood flow status of the vessel after stent implantation. The diameter of the stent was chosen to be slightly smaller than the diameter of the adjacent normal vessel, and the length of the stent was chosen to cover at least 1–2 mm of both ends of the stenotic vessel.
A 6F shuttle sheath was inserted under systemic heparinization into the intended artery. Following measurements of the stenotic segment length and the artery’s diameter proximal or distal to the lesion, the dilatation and DCB catheter sizes were established. The balloon’s diameter has to be between 10 and 20% lower than the non-diseased artery’s diameter either proximal or distal to the stenotic segment to prevent arterial rupture. After the intermediate catheter was positioned, a 0.014-inch guidewire was steered through the stenotic segment into the distal circulation. After the wire was traced to the stenosis region, a quick exchange coronary balloon catheter was inflated until the nominal pressure was attained in 30 s. After another 30 s, the balloon catheter was deflated. Following angioplasty with a DCB was done right away if there was no severe dissection or considerable residual stenosis because of either calcified lesions or elastic recoil. The DCB catheter (SeQuent Please, B Braun, Melsungen, Germany) was navigated via the wire to cover the whole diseased segment. After gradually increasing to the nominal pressure, the DCB was left inflated for 60 s. Angiography was done both immediately following the DCB’s deflation and 15–20 min later to make sure there had been no increasing dissection, thrombus formation, or arterial rebound. If there is still a relatively serious residual stenosis rate or arterial dissection and blood flow instability after drug balloon dilation, stent angioplasty can be selected according to the intraoperative situation. Residual stenosis, perforator vessel occlusion, and distal perfusion were evaluated by postoperative angiography.
Immediately following surgery, a CT scan was done to rule out brain bleeding. Furthermore, all patients were evaluated with Transcranial Doppler (TCD) for immediate restenosis and hyper-perfusion syndrome (HPS) within 24 h following the surgery. Antihypertensive medications were used to keep blood pressure between 110 and 130/70 and 80 mmHg to avoid HPS. If the patient experienced worsening symptoms after surgery, or if any new ones surfaced, a head Magnetic Resonance Imaging (MRI) would be performed to determine whether the distant embolism was the cause. Oral administration of aspirin 100 mg/day and clopidogrel 75 mg/day was taken for 3 months following the procedure; clopidogrel was discontinued at the 3-month mark, and aspirin 100 mg/day was taken orally for long-term use. Rehabilitation treatment was recommended for patients with functional disability. Long-term management of individual medical risk factors such as blood pressure, cholesterol, and diabetes mellitus were implemented.
All patients were followed up regularly through hospitalization or outpatient visits. Six months after the procedure, DSA was taken out to assess ISR, Stenosis >50% of the luminal diameter was considered angiographic restenosis on DSA. With a total score of 0–6, larger scores denoting worse neurological performance, the mRS was used to evaluate functional outcomes at discharge and at the 1-, 3-, and 6-month follow-up (21). The following was the result endpoint: (1) the 6-month incidence of ISR; (2) peri-procedural complications 7 days post-revascularization, including branch embolization, ischemic stroke, stent thrombosis, high perfusion, or symptomatic hemorrhage; (3) the rate of stroke recurrence in the area of the stented artery during the follow-up period. Recurrent ischemic stroke was considered to be any focal neurological symptom of sudden onset that lasted for at least 24 h, was related to the corresponding vascular territory, was not associated with a hemorrhage on brain CT or MRI, and occurred within the follow-up period; and (4) mRS at discharge, at 1-month, at 3-month, at 6-month of patients after stenting. The incidence of ISR in the 6-month was taken as the main prognostic indicator.
Continuous variables were described by median (standard deviation) and interquartile range (interquartile range). The differences between groups were determined by a one-way analysis of variance or Kruskal-Wallis test. The count (n) and percentage (%) were used to describe categorical variables, for which the chi-square test or Fisher’s exact tests were used. The impact of the various clinical factors on each outcome node was examined using univariate logistic analysis. If the probability value for the bivariate relationship with the endpoint was less than 0.1, the factor was considered for multivariate logistic regression analysis. Compute the 95% confidence intervals (CI) after that. All analyses were performed using SPSS statistical software version 29.0 (SPSS Inc., Chicago, IL, USA) and R statistical software version 4.0.3 (R Foundation), Graphpadrism9.4 software for drawing. A two-sided p-value < 0.05 was considered statistically significant.
Between January 2021 and October 2023, endovascular treatment (EVT) was used to treat 154 patients (91 males, aged 60.7 ± 8.70) who had TIA or stroke due to severe atherosclerotic stenosis of the intracranial artery. The stenoses were located as follows: intracranial internal carotid artery (ICA; n = 25, 16.2%), middle cerebral artery (n = 76, 49.4%), basilar artery (n = 27, 17.5%), intracranial vertebral artery (n = 26, 16.9%) (Table 1). Based on the location and characteristics of the target lesions as well as the experience and choice of the operators, different endovascular therapy techniques were selected. 71 (46.1%) of the 154 patients received BMS treatment, 50 (32.5%) received DCB treatment, and 33 (21.4%) received DES treatment. Intracranial artery stenosis and poor collaterals were all confirmed by DSA before stenting. The clinical risk factors for stroke, characteristics of the target artery, and mRS scores of all enrolled patients were evenly distributed among the three treatment groups, as shown in Table 2. The most common risk factor was hypertension 97 (63.0%), followed by diabetes mellitus 66 (42.9%). 13.6% of patients have a family history of stroke and 31.2% of patients have previously experienced stroke or TIA. There was no significant difference in functional scale, common risk factors, or relative disease history between the three groups.
The perioperative outcome is displayed in Table 3. 100% of patients (154/154) had effective reperfusion following EVT. Eight patients [16.0%(8/50)] in the DCB group experienced dissection following dilatation, but forward blood flow remained unaffected. The surgery was terminated after 10 min of monitoring when follow-up angiography revealed that the forward blood flow was still unaffected. None of the eight patients who experienced dissection showed any related ischemic symptoms. There were no patients who underwent salvage stent placement due to flow restriction dissection after DCB expansion. Specifically, six patients in the BMS group developed symptomatic intracerebral hemorrhage, one patient experienced an ischemic stroke in the branch area, and one patient underwent branch embolization. Within the DCB group, one patient developed stent thrombosis within 24 h of EVT, two patients suffered symptomatic intracerebral hemorrhage, and one patient underwent symptomatic hemorrhagic transformation due to hyper-reperfusion. Two patients in the DES group had symptomatic intracerebral bleeding and one patient had branch embolization. The incidence of peri-procedural complications was not shown to have significant differences across the groups (p = 0.776; 11.3% in the BMS group, 8.0% in the DCB group, and 6.1% in the DES group).
All patients received clinical and imaging follow-ups for more than 6 months. Table 4 showed that stroke occurred at a rate of 4.5% (7/154) during follow-up, five patients (7.0%) in the BMS group, one patient (2.0%) in the DCB group, and one patient (3.0%) in the DES group. The 30-day mRS scores, 90-day mRS scores, and 180-day mRS scores showed no significant difference among the three groups. During follow-up, all patients received DSA. The rate of restenosis in the BMS group [35.2%(25/71)] tended to be higher than that in the DCB group [6.0% (3/50)] and the DES group [9.1%(3/33)], and there was a significant difference among the three groups. In the DES group, there was an approximately 50% higher rate of restenosis (3 [9.1%] vs. 3 [6.0%]) compared with the DCB group, with no significant difference.
Table 5 displays the findings of the univariate analysis of the perioperative and follow-up outcomes. The results of univariate analyses indicated that symptomatic hemorrhage during the perioperative period was related to length of stay, smoking history, alcohol history, total surgical time, and stenosis length; on the other hand, risk factors for the perioperative complications rate included length of stay, smoking history, triglyceride, total surgical time, and stenosis length. While the distribution of vessels and the method of endovascular therapies were linked to restenosis, the recurrence of stroke was related to coronary disease, low-density lipoprotein cholesterol, triglycerides, total cholesterol, and serum uric acid. Multivariate logistic regression analysis revealed that the endovascular treatment strategy, the distribution of vessels for restenosis, the length of stay and total surgical time for perioperative complications, and total surgical time for symptomatic hemorrhage were significant (p < 0.05) independent risk factors. The only independent risk factor for stroke recurrence within 6 months was coronary disease (Table 6). Also, it was discovered that these two factors were significant when used in the multivariable model of restenosis probability. A nomogram predicting restenosis probability within 6 months after EVT was constructed with these two parameters based on the multivariable model (Figure 1).
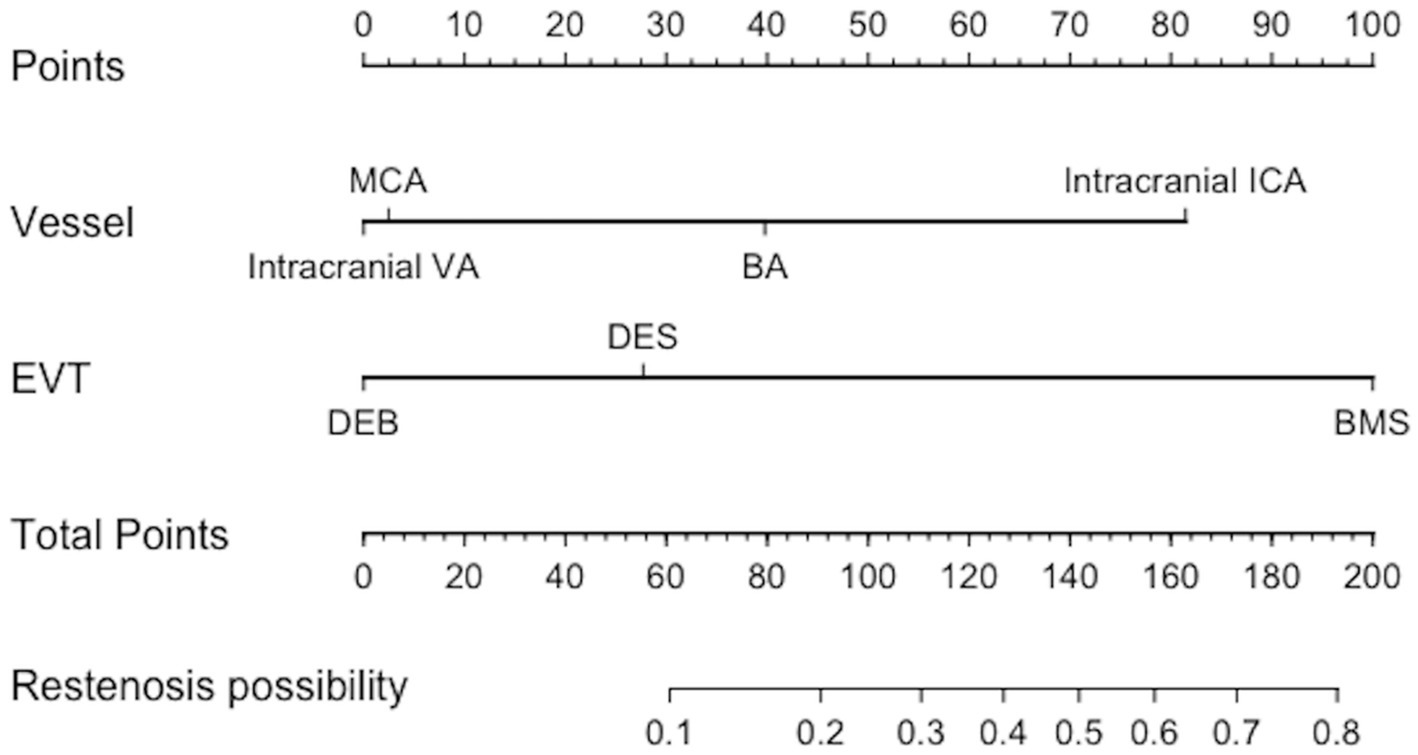
Figure 1. Nomogram predicting restenosis probability within 6 months after EVT. EVT, endovascular treatment; VA, Vertebral artery; BA, Basilar artery; MCA, Middle cerebral artery; ICA, Internal carotid artery; EVT, endovascular treatment; BMS, Bare metal stent; DCB, Drug-coated Balloon; DES, Drug-eluting stent.
In this study, we retrospectively investigated the safety and efficacy of different endovascular treatments for sICAS. Our research shows that: (1) the perioperative complication rate of patients with symptomatic intracranial atherosclerotic stenosis is 9.1, 4.5% of patients have at least one ischemic stroke in the ipsilateral intracranial artery area during the 6-month follow-up, and there is no significant difference in perioperative complications, improvement of cognitive function, and recurrent stroke within 6 months among the three groups; (2) In our study, the incidence of ISR in DES and DCB was lower than that in BMS, with statistical difference. The incidence of ISR in DCB was lower than that in BMS, although there was no statistical difference between the two groups. DCB is more likely to be the optimal treatment strategy.
Given the unfavorable outcomes of the SAMMPRIS and VISSIT studies, concerns have been raised regarding the safety and effectiveness of endovascular therapy for intracranial atherosclerotic stenosis. According to the SAMMPRIS study, the stenting arm using the Wingspan stent had a higher rate of 30-day stroke and death (14.7%) compared to the medical arm (12.6%), and a higher rate of 1-year stroke and death (19.7%) compared to the aggressive medical therapy (5.8%) (22). The VISSIT trial showed that the stenting group with a BMS had an even higher 30-day stroke/hard TIA rate (24.1%) compared to the medical group (9.4%) and that the stenting group’s 1-year stroke/hard TIA rate (36.2%) was much higher than the medical group’s (15.1%) (9). However, owing to the limitations of the trials, EVT is still seen by researchers and clinicians as a potentially effective way to prevent stroke in individuals with sICAS. Compared with the SAMMPRIS study, the incidence of perioperative complications in the bare stent group, drug-coated balloon group, and drug-coated stent group in this study were 11.3, 8.0, and 6.1%, respectively, all lower than 14.7%. The main reason could be that the surgeons in this study have a wealth of clinical experience, are more proficient at technical operations, and have a lower frequency of issues such as perforator obstruction and arterial dissection. Other reasons are strict preoperative screening and perioperative care, postoperative monitoring, stringent patient blood pressure and blood sugar control, and postoperative medication. Every patient group’s prognosis remains favorable throughout the follow-up procedure, and no deaths occur. Drug delivery devices such as drug-coated balloons and drug-eluting stents have been introduced in recent years. These devices attach drugs that prevent cell proliferation to their surface and release the drugs evenly onto the vascular wall. This reduces the risk of restenosis, endometrial hyperplasia, and cell proliferation. In this study, the incidence of 6-month restenosis was found to be 35.2% in the BMS group, 6.0% in the DCB group, and 9.1% in the DES group. Notably, the restenosis rate in the BMS group was significantly greater than that of the other two groups. In 2021, the results of the WANG et al. study showed that the incidence of perioperative complications was 2.9%, and the incidence of restenosis was 12%. The results of the RE-MONDA et al. study showed that the incidence of perioperative complications was 0%, and the incidence of restenosis 9 months after surgery was 12%. DCB treatment for sICAS has a decreased rate of restenosis, according to research findings, despite potential differences in the study’s design and use of DCB. It can be seen that DCB has a good effect in improving intracranial arterial ISR problems. Likewise, DES is beneficial in resolving intracranial artery ISR problems. In 2022, the results of the Jia et al. study showed that the incidence of perioperative complications was 7.6%, and the incidence of restenosis was 9.5% (17). Currently, there are few comparable studies of DCB and DES for symptomatic ICAS. In this study, the restenosis rate of the DES group was approximately 50% higher than that of the DCB group (3 [9.1%] vs. 3 [6.0%]), although there was no significant difference between the two groups. Compared to DES, DCB has greater pass ability, can increase the immediate success rate of surgery, and medication concentration at the lesion site, minimizes the danger of branch occlusion, prevents chronic inflammatory reactions of metal trabeculae and polymers due to the absence of stent foreign bodies (23, 24). On the other hand, DCB does not use a permanent implant, which lowers the risk of stent-related unfavorable biological reactions that cause thrombosis and restenosis, and promotes the vessel’s beneficial natural healing process (25–27). In addition to providing an antiproliferative medication, DCB facilitates mechanical expansion, which leads to positive vessel remodeling marked by the enlargement of the late lumen, the reduction of plaque, and the stabilization of plaque (28, 29). At the same time, DCB prevents foreign body placement and preserves the opportunity for subsequent treatment when necessary for the patient.
In addition, we found that under the same conditions, the restenosis rate of the intracranial internal carotid artery was higher than that of other intracranial vessels at a 6-month follow-up. Possible reasons are the intracranial ICA segments are more tortuous than other intracranial arteries, which can complicate endovascular device navigation or cause the stent to not fully expand during deployment, which can cause thrombus formation. The low adhesion ability of stents in tortuous arteries may cause more severe intimal hyperplasia, which in turn could cause more severe internal carotid artery stent restenosis. Because there are fewer tenuous perforators in the intracranial ICA, there are fewer problems from perforator obstruction or damage. Postoperative high perfusion was observed in only one patient in our study, and that patient’s case involved the intracranial internal carotid artery. One possible explanation for this could be the ICA being considerably larger than other intracranial arteries such as the middle cerebral artery, which can easily cause high perfusion syndrome after the stenotic lumen is resolved by stenting.
Vessel toxicity is a theoretical risk for DES and DCB that can be caused by the brain’s unique structural and functional complexity, as well as the specific response of cerebral blood vessels to drugs (30). A study that investigated the safety of drug-eluting DES in dog’s BA artery reveals no neurotoxic effects were observed in the intracranial vessel walls or brainstem tissue in which sirolimus-coated stents were implanted (31). Sun et al. (32) reported a long-term safety assessment of DES shows a safety profile similar to BMS, without any neurotoxic histological signs. Xu et al. (33) observed that rapamycin-eluting balloon is a safe and effective treatment for ICAS, respectively, and did not observe any sign of the toxic effect. We have checked arterial and cerebral toxicity by angiography and clinical status, respectively, and did not observe any sign of toxic effect. In addition, there are no reports of neurotoxic complications in other DES and DCB studies applied to cerebral artery stenosis (19, 34, 35). Further studies are needed to confirm whether vascular toxicity occurs.
This study has a few limitations. First, this was a retrospective, single-center, non-randomized study, and suffers from selection bias. Second, the sample size is relatively small for logistic regression analysis. Therefore, a multicenter, prospective, controlled trial is still needed to confirm our results.
In patients with symptomatic intracranial atherosclerotic stenosis, different treatment groups have similar success rates and adverse events in stent implantation. Compared to bare metal stents, drug-eluting stents, and drug-coated balloons can reduce the risk of ISR, and drug-coated balloons seem to show greater advantages for some high-risk patients with ICAS. However, this needs to be confirmed by further investigation, preferably in large multicenter randomized controlled clinical trials.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by the Medical Ethics Committee of the Affiliated Hospital of Qingdao University. The patients/participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
QL: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Validation, Writing – original draft. KZ: Data curation, Formal Analysis, Methodology, Writing – original draft. XP: Data curation, Formal analysis, Writing – original draft. CL: Formal analysis, Validation, Writing – original draft. XL: Investigation, Writing – original draft. NW: Investigation, Methodology, Resources, Supervision, Writing – review & editing.
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The authors declare that no Gen AI was used in the creation of this manuscript.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
ICAS, intracranial atherosclerotic stenosis; TIA, transient ischemic attack; sICAS, symptomatic intracranial atherosclerotic stenosis; ISR, in-stent restenosis; DES, drug-eluting stents; DCB, drug-coated balloons; BMS, bare metal stent; MCA, middle cerebral artery; ICA, internal carotid artery; VA, vertebral artery; BA, basilar artery; DSA, digital subtraction angiography; mRS, modified Rankin score; TCD, Transcranial Doppler; HPS, hyper-perfusion syndrome; MRI, Magnetic Resonance Imaging; EVT, endovascular treatment.
1. Gutierrez, J, Turan, TN, Hoh, BL, and Chimowitz, MI. Intracranial atherosclerotic stenosis: risk factors, diagnosis, and treatment. Lancet Neurol. (2022) 21:355–68. doi: 10.1016/S1474-4422(21)00376-8
2. Gorelick, PB, Caplan, LR, Hier, DB, Parker, SL, and Patel, D. Racial differences in the distribution of anterior circulation occlusive disease. Neurology. (1984) 34:54–9. doi: 10.1212/wnl.34.1.54
3. Sacco, RL, Kargman, DE, Gu, Q, and Zamanillo, MC. Race-ethnicity and determinants of intracranial atherosclerotic cerebral infarction. Northern Manhattan Stroke Study Stroke. (1995) 26:14–20. doi: 10.1161/01.str.26.1.14
4. Wong, LKS. Global burden of intracranial atherosclerosis. Int J Stroke Off J Int Stroke Soc. (2006) 1:158–9. doi: 10.1111/j.1747-4949.2006.00045.x
5. Qureshi, AI, and Caplan, LR. Intracranial atherosclerosis. Lancet Lond Engl. (2014) 383:984–98. doi: 10.1016/S0140-6736(13)61088-0
6. Kasner, SE, Chimowitz, MI, Lynn, MJ, Howlett-Smith, H, Stern, BJ, Hertzberg, VS, et al. Predictors of ischemic stroke in the territory of a symptomatic intracranial arterial stenosis. Circulation. (2006) 113:555–63. doi: 10.1161/CIRCULATIONAHA.105.578229
7. Zaidat, OO, Klucznik, R, Alexander, MJ, Chaloupka, J, Lutsep, H, Barnwell, S, et al. The NIH registry on use of the wingspan stent for symptomatic 70-99% intracranial arterial stenosis. Neurology. (2008) 70:1518–24. doi: 10.1212/01.wnl.0000306308.08229.a3
8. Derdeyn, CP, Chimowitz, MI, Lynn, MJ, Fiorella, D, Turan, TN, Janis, LS, et al. Aggressive medical treatment with or without stenting in high-risk patients with intracranial artery stenosis (SAMMPRIS): the final results of a randomised trial. Lancet Lond Engl. (2014) 383:333–41. doi: 10.1016/S0140-6736(13)62038-3
9. Zaidat, OO, Fitzsimmons, BF, Woodward, BK, Wang, Z, Killer-Oberpfalzer, M, Wakhloo, A, et al. Effect of a balloon-expandable intracranial stent vs medical therapy on risk of stroke in patients with symptomatic intracranial stenosis: the VISSIT randomized clinical trial. JAMA. (2015) 313:1240–8. doi: 10.1001/jama.2015.1693
10. Miao, Z. Intracranial angioplasty and stenting before and after SAMMPRIS: “from simple to complex strategy - the Chinese experience”. Front Neurol. (2014) 5:129. doi: 10.3389/fneur.2014.00129
11. Alexander, MJ, Zauner, A, Gupta, R, Alshekhlee, A, Fraser, JF, Toth, G, et al. The WOVEN trial: wingspan one-year vascular events and neurologic outcomes. J Neurointerventional Surg. (2021) 13:307–10. doi: 10.1136/neurintsurg-2020-016208
12. Ma, N, Zhang, Y, Shuai, J, Jiang, C, Zhu, Q, Chen, K, et al. Stenting for symptomatic intracranial arterial stenosis in China: 1-year outcome of a multicentre registry study. Stroke Vasc Neurol. (2018) 3:176–84. doi: 10.1136/svn-2017-000137
13. Miao, Z, Zhang, Y, Shuai, J, Jiang, C, Zhu, Q, Chen, K, et al. Thirty-day outcome of a multicenter registry study of stenting for symptomatic intracranial artery stenosis in China. Stroke. (2015) 46:2822–9. doi: 10.1161/STROKEAHA.115.010549
14. Derdeyn, CP, Fiorella, D, Lynn, MJ, Turan, TN, Cotsonis, GA, Lane, BF, et al. Nonprocedural symptomatic infarction and in-stent restenosis after intracranial angioplasty and stenting in the SAMMPRIS trial (stenting and aggressive medical Management for the Prevention of recurrent stroke in intracranial stenosis). Stroke. (2017) 48:1501–6. doi: 10.1161/STROKEAHA.116.014537
15. Jin, M, Fu, X, Wei, Y, Du, B, Xu, XT, and Jiang, WJ. Higher risk of recurrent ischemic events in patients with intracranial in-stent restenosis. Stroke. (2013) 44:2990–4. doi: 10.1161/STROKEAHA.113.001824
16. Abou-Chebl, A, Bashir, Q, and Yadav, JS. Drug-eluting stents for the treatment of intracranial atherosclerosis: initial experience and midterm angiographic follow-up. Stroke. (2005) 36:e165–8. doi: 10.1161/01.STR.0000190893.74268.fd
17. Jia, B, Zhang, X, Ma, N, Mo, D, Gao, F, Sun, X, et al. Comparison of drug-eluting stent with bare-metal stent in patients with symptomatic high-grade intracranial atherosclerotic stenosis. JAMA Neurol. (2022) 79:176–84. doi: 10.1001/jamaneurol.2021.4804
18. Si, JH, Ma, N, Gao, F, Mo, DP, Luo, G, and Miao, ZR. Effect of a drug-eluting stent vs. bare metal stent for the treatment of symptomatic intracranial and vertebral artery stenosis. Front Neurol. (2022) 13:854226. doi: 10.3389/fneur.2022.854226
19. Tang, Y, Li, T, Liu, W, He, Y, Zhu, L, Wang, ZL, et al. Comparison of drug-coated balloon with conventional balloon for angioplasty in symptomatic intracranial atherosclerotic stenosis. J Neurointerventional Surg. (2023) 15:e369–74. doi: 10.1136/jnis-2022-019685
20. Zhang, J, Zhang, X, Zhang, J, Song, Y, Zheng, M, Sun, L, et al. Drug-coated balloon dilation compared with conventional stenting angioplasty for intracranial atherosclerotic disease. Neurosurgery. (2020) 87:992–8. doi: 10.1093/neuros/nyaa191
21. Newcommon, NJ, Green, TL, Haley, E, Cooke, T, and Hill, MD. Improving the assessment of outcomes in stroke: use of a structured interview to assign grades on the modified rankin scale. Stroke. (2003) 34:377–8. doi: 10.1161/01.str.0000055766.99908.58
22. Chimowitz, MI, Lynn, MJ, Derdeyn, CP, Turan, TN, Fiorella, D, Lane, BF, et al. Stenting versus aggressive medical therapy for intracranial arterial stenosis. N Engl J Med. (2011) 365:993–1003. doi: 10.1056/NEJMoa1105335
23. Buccheri, D, Lombardo, RM, and Cortese, B. Drug-coated balloons for coronary artery disease: current concepts and controversies. Futur Cardiol. (2019) 15:437–54. doi: 10.2217/fca-2019-0009
24. Giacoppo, D, Alfonso, F, Xu, B, Claessen, BEPM, Adriaenssens, T, Jensen, C, et al. Paclitaxel-coated balloon angioplasty vs. drug-eluting stenting for the treatment of coronary in-stent restenosis: a comprehensive, collaborative, individual patient data meta-analysis of 10 randomized clinical trials (DAEDALUS study). Eur Heart J. (2020) 41:3715–28. doi: 10.1093/eurheartj/ehz594
25. Her, AY, Yuan, SL, Jun, EJ, Bhak, Y, Kim, MH, Garg, S, et al. Drug-coated balloon treatment for nonsmall de-novo coronary artery disease: angiographic and clinical outcomes. Coron Artery Dis. (2021) 32:534–40. doi: 10.1097/MCA.0000000000001006
26. Her, AY, Kim, B, Ahn, SH, Park, Y, Cho, JR, Jeong, YH, et al. Long-term clinical outcomes of drug-coated balloon treatment for De novo coronary lesions. Yonsei Med J. (2023) 64:359–65. doi: 10.3349/ymj.2022.0633
27. Shin, ES, Jun, EJ, Kim, S, Kim, B, Kim, TH, Sohn, CB, et al. Clinical impact of drug-coated balloon-based percutaneous coronary intervention in patients with multivessel coronary artery disease. JACC Cardiovasc Interv. (2023) 16:292–9. doi: 10.1016/j.jcin.2022.10.049
28. Her, AY, Ann, SH, Singh, GB, Kim, YH, Yoo, SY, Garg, S, et al. Comparison of paclitaxel-coated balloon treatment and plain old balloon angioplasty for De novo coronary lesions. Yonsei Med J. (2016) 57:337–41. doi: 10.3349/ymj.2016.57.2.337
29. Her, AY, Shin, ES, Chung, JH, Kim, YH, Garg, S, Lee, JM, et al. Plaque modification and stabilization after paclitaxel-coated balloon treatment for de novo coronary lesions. Heart Vessel. (2019) 34:1113–21. doi: 10.1007/s00380-019-01346-9
30. Fisher, JA, and Mikulis, DJ. Cerebrovascular reactivity: purpose, optimizing methods, and limitations to interpretation - a personal 20-year odyssey of (re)searching. Front Physiol. (2021) 12:629651. doi: 10.3389/fphys.2021.629651
31. Levy, EI, Hanel, RA, Howington, JU, Nemes, B, Boulos, AS, Tio, FO, et al. Sirolimus-eluting stents in the canine cerebral vasculature: a prospective, randomized, blinded assessment of safety and vessel response. J Neurosurg. (2004) 100:688–94. doi: 10.3171/jns.2004.100.4.0688
32. Sun, X, Wu, X, Yang, M, Deng, Y, Jia, B, Zhang, X, et al. Comprehensive assessment of drug kinetics, neurotoxicity, and safety of Sirolimus-eluting intracranial stents in canine basilar artery. Neurosurgery. (2024) 95:1199–208. doi: 10.1227/neu.0000000000003079
33. Xu, G, Dong, X, Tian, Y, Ma, L, Han, N, Yao, W, et al. Evaluation of safety and efficacy of rapamycin-eluting balloon in patients with intracranial atherosclerotic stenosis: a cohort study. J Cardiothorac Surg. (2023) 18:162. doi: 10.1186/s13019-023-02204-6
34. Hassan, AE, Mohammaden, MH, Rabah, RR, and Tekle, WG. Initial experience with the next-generation resolute Onyx Zotarolimus-eluting stent in symptomatic intracranial atherosclerotic disease. Front Neurol. (2020) 11:570100. doi: 10.3389/fneur.2020.570100
35. Qiao, H, Chang, CH, Wang, AYC, Li, S, Yang, W, Li, G, et al. Safety and efficacy of drug coated balloon angioplasty for intracranial atherosclerotic disease. J Neuro Interventional Surg. (2023) 15:e172–7. doi: 10.1136/jnis-2022-019122
Keywords: drug-eluding stent, drug-coated balloon, endovascular treatment, in-stent restenosis, bare metal stent
Citation: Lin Q, Zhong K, Pan X, Li C, Lu X and Wang N (2025) Comparison of safety and efficacy of different endovascular treatments for symptomatic intracranial atherosclerotic stenosis: results from a single center. Front. Neurol. 16:1539127. doi: 10.3389/fneur.2025.1539127
Received: 03 December 2024; Accepted: 24 January 2025;
Published: 05 March 2025.
Edited by:
Kaijun Zhao, Tongji University, ChinaReviewed by:
Qazi Zeeshan, Mayo Clinic Arizona, United StatesCopyright © 2025 Lin, Zhong, Pan, Li, Lu and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Naidong Wang, d2FuZ25haWRvbmcxNjNAMTYzLmNvbQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.