
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Neurol. , 11 March 2025
Sec. Neurorehabilitation
Volume 16 - 2025 | https://doi.org/10.3389/fneur.2025.1537635
 Guoping Qian1*
Guoping Qian1* Ewelina Perzanowska1
Ewelina Perzanowska1 Dominika Wilczyńska2
Dominika Wilczyńska2 Mirela Kozakiewicz1
Mirela Kozakiewicz1 Hongli Yu1,3
Hongli Yu1,3 Hejła Marcelina1
Hejła Marcelina1 Zbigniew Ossowski1*
Zbigniew Ossowski1*Background: Vojta therapy (VT) enhances postural control and improves gait abilities. However, there is limited evidence regarding the impact of home-based VT on individuals with Down syndrome (DS).
Objective: This study aimed to assess the feasibility and preliminary effects of a two-week home-based VT program on spatiotemporal gait parameters in individuals with DS.
Methods: Sixteen individuals with DS (mean age = 17.88 ± 4.57 years, 8 females) participated in a two-week home-based VT program. Feasibility was measured through adherence rates and the occurrence of adverse events. Spatiotemporal gait parameters were evaluated before and after the intervention using the Vicon motion capture system.
Results: All participants (100%) successfully completed the home-based VT program with no reported adverse events. Significant improvements were observed in walking speed, cadence, step time (left and right), stride time (left and right), step length (left and right), stride length (left and right), and single support (left and right) (p < 0.05).
Conclusion: This preliminary study suggests that home-based VT is a feasible approach and can lead to meaningful improvements in spatiotemporal gait parameters for individuals with DS. Further research with larger sample sizes, more robust designs, and extended follow-up periods is recommended.
Down syndrome (DS, OMIM #190685) or trisomy 21 is caused by the triplication of the whole or portion of chromosome 21 (1). It is the most prevalent genetic neurological disorder with intellectual disabilities and movement impairment, occurring in about 1 in 800 births globally (2). DS manifests clinically a variety of symptoms that include muscle hypotonia, short stature, atlantoaxial instability, intellectual disability, and congenital heart defects (2–4). Additionally, DS patients carry an increased risk of various medical conditions such as respiratory disease, gastrointestinal malformations, osteoporosis, thyroid dysfunction, epilepsy, and metabolic disorders (3, 5–8).
Musculoskeletal alteration of DS manifests as motor and coordination problems most frequently leading to abnormal gait patterns (9). Gait abnormalities often observed in DS individuals are reduced walk speeds, reduced stride length, increased stride width, increased hip flexion (10). Other typical characteristics include hip external rotation, increased knee flexion and valgus, and tibial external rotation (11). All these issues are mainly due to hypotonia, joint stiffness, and ligamentous laxity that disrupt intermuscular coordination and proprioceptive processing to ultimately impairing postural control and gait (12). These gait abnormalities continue into childhood, with studies demonstrating that DS children reach motor milestones, including independent walking, much later than typically developing children—often between 24 and 36 months—compared to the usual 11 to 15 months for typically developing children (9, 13–15).
These motor dysfunctions limit physical activity and quality of life, affecting daily living activities and increasing reliance on others (16). Consequently, rehabilitation programs, therapeutic exercises, and physiotherapy are essential for promoting independence and mobility in persons with DS. For example, studies have shown that massage treatment can significantly improve motor function in children with DS (17, 18), and whole-body vibration training has been shown to enhance balance after 20 weeks (19). Additionally, various reviews have confirmed the effectiveness of physiotherapy in improving the overall quality of life and aiding rehabilitation in people with DS (20, 21).
Vojta therapy (VT) is a widely used physiotherapy technique developed by Vaclav Vojta (22). This neuromodulative treatment based on reflex locomotion principles and aims to promote typical innate patterns, and improve postural control. VT is established on the theory of postural and gait control pattern generators, whereby distinct areas of the body are stimulated to provoke corrective movements. The “bottom-up” concept is believed to take advantage of neuroplasticity (23–25) to enable patient recovery. Experiments have determined VT effectiveness in enhancing motor function in healthy adults and cerebral palsy (CP) patients as measured by functional near-infrared spectroscopy or surface electromyography (26, 27).Therefore, routine application of VT in the treatment of neuromotor pathologies such as CP, multiple sclerosis (MS), and hypotonia, has revealed gait, sitting balance, balance, and gross motor function improvement (28–32). VT has also been effective in improving postural control in stroke patients (33), and a systematic review of 10 trials involving 522 subjects showed its impressive benefits in the management of respiratory distress syndrome in preterm infants (34).
Home-based physiotherapy has gained much popularity over the last few years due to its convenience and cost-effectiveness. By allowing patients to receive treatment in their own homes (35), this approach promotes greater independence compared to traditional in-clinic physiotherapy (36). Numerous studies suggest that home-based physiotherapy is effective in enhancing mobility and improving daily living activities (37–39). Although VT and home-based physiotherapy have both been demonstrated to have beneficial outcomes, feasibility and efficacy of home-based VT in persons with DS, and more specifically the effect on spatiotemporal gait parameter improvement, is a research gap. Since the study was of an exploratory nature, a pre-post intervention study design was undertaken to investigate the feasibility and initial effect of home-based VT on the improvement of spatiotemporal gait parameters in persons with DS. This study enables evaluation of within-subject change before and after home-based VT, which is highly applicable to research on novel rehabilitation interventions in a population where comparatively little research has been conducted. The aim of this research, therefore, was to determine the feasibility and preliminary effect of a home-based VT intervention on spatiotemporal gait parameters in adults with DS with preliminary evidence for the design of larger, controlled studies.
The study was a single-arm, prospective preliminary feasibility trial, employing a pre-post intervention design to evaluate the feasibility and effects of home-based VT on spatiotemporal gait parameters in individuals with DS. The study strictly adhered to the Transparent Reporting of Evaluations with Nonrandomised Designs (TREND) guidelines to ensure rigorous reporting standards (40).
Participants were recruited from different rehabilitation centers in Gdansk, Poland between April and May 2023. The inclusion criteria were: (1) individuals aged 10 to 30 years; (2) interest in participating in the home-based VT program; (3) the ability to follow instructions, with parental support to cooperate with the home-based VT. Exclusion criteria included: (1) participation in a structured physiotherapy program within 3 months prior to the study; (2) previous abdominal or head surgeries (due to risk associated with reflex creeping during treatment); (3) presence of inflammatory disease or acute fever; and (4) any pharmacological or surgical treatment affecting the nervous system.
Ethical approval was granted by the Bioethics Commission of the District Medical Chamber in Gdansk (KB/23–23). All procedures were carried out in compliance with the ethical standards established in the Declaration of Helsinki (1975) and its latest amendments. The study procedures, benefits, and potential risks were thoroughly explained to participants and their families. Written informed consent was acquired from all participants or their legal guardians if participants were unable to provide consent independently.
Participants underwent a two-week home-based VT program as the only intervention during the study, with the recommended frequency being once per day, ideally in the afternoon. Each session consisted of 2-min exercises on each side in the reflex creeping position (2 min × 2 times for both left and right sides), with a 2–3-min break between positions, totaling approximately 20 min per session. No additional physical therapy or exercises conducted before, during, or after the VT sessions. This ensures that the observed effects on gait parameters can be directly attributed to VT. According to VT recommendations, session duration typically ranges from 5 to 20 min per session (22, 41). Previous studies have demonstrated that a single 20-min VT session per day can effectively improve shoulder function in individuals with subacromial impingement syndrome (42), while being a feasible duration that helps prevent excessive fatigue, supporting the feasibility and effectiveness of our chosen intervention duration. In addition, given the home-based setting, a single daily session was chosen to balance efficacy and adherence while maintaining alignment with VT principles. A previous study emphasizes that session frequency must balance effectiveness with feasibility, as excessive sessions may reduce compliance (43). This study adopted a practical intervention duration and frequency suitable for home-based settings.
An initial fconsultation in person with an experienced physiotherapist carried out to illustrate the VT techniques, with comprehensive guidance to parents on how to use VT at home. Key points included maintaining symmetry during exercises, ensuring proper stabilization of support points, and positioning the head correctly. Parents practiced under direct supervision to ensure they could accurately apply VT at home. To enhance learning, Supplementary Material was provided, such as written information and video illustrations.
For maintaining quality and uniformity of home sessions, physiotherapist provided day-to-day supervision by video call with on-the-spot correction and feedback. Parents were also asked to videograph sessions for observation and modification whenever necessary. Systematic monitoring ensured adherence to protocol, reduced errors, and made adjustments in time based on individual response. VT sessions were conducted by the children in shorts only to allow visualization of muscle chain activity.
Spatiotemporal gait parameters, i.e., walking speed (m/s), cadence (steps/min), step time (sec), step length (m), stride time (sec), stride length (m), double support % of the gait cycle (% GC), and single support (% GC) were recorded at baseline and post-intervention. Baseline measurements were conducted 48 h before the home-based VT program to minimize the influence of physiological factors, such as fatigue or recent physical activity. Post-intervention gait data were recorded 48 h following the final home-based VT session to avoid interfering with the measurements by the acute reflex activation effects of VT.
Measurements were captured with a 10-camera Vicon motion capture system (VICON, Oxford Metrics Limited, UK), functioning at a frequency of 100 Hz. The Vicon system is recognized for its exceptional accuracy and precision (44), with a mean absolute error of 0.15 mm in static situations and under 2 mm during dynamic motions (44). The Vicon system captures marker positions during walking with an accuracy of −0.08 mm and an uncertainty of 0.33 mm (45). Sixteen reflective markers, each measuring 2.5 cm in diameter, were affixed to the left and right lower limbs and the spine. Joint centers were defined according to the plugin-gait model (46). The markers were positioned at the bilateral anterior superior iliac spines, posterior superior iliac spine, the middle and lower 1/3 of the femur, the lateral line of the knee joint, the lateral 1/3 of the tibia, lateral malleolus, calcaneus, and dorsal second metatarsophalangeal joint. Participants walked barefoot along a 10-meter path at a self-selected pace, and data from five repeated trials were collected.
Spatiotemporal gait data were processed using Vicon Nexus 2.9.3 software (Vicon, Oxford Metrics). Preliminary marker reconstruction and labeling followed standard Nexus procedures, and missing data were interpolated using the Woltring filter with default Vicon settings. Gait events, such as heel-strike (HS) and toe-off (TO), were automatically detected using Vicon Nexus 2.9.3 software via the Plug-in-Gait model, which applies kinematic-based detection algorithms. All gait events were manually reviewed and corrected when necessary to ensure accuracy. Spatiotemporal parameters were derived from detected gait events using standard temporal calculations within Vicon Nexus software. To minimize noise while preserving movement characteristics, raw marker trajectories were smoothed using a second-order zero-lag Butterworth low-pass filter. These measurements were performed at the Laboratory of Physical Effort and Genetics in Sport at the Gdansk University of Physical Education and Sport (GUPES) in Poland.
The feasibility of the two-week home-based VT program was evaluated based on three criteria: (1) participant adherence, described as the quantity of participants who completed the program; (2) safety, monitored through the reporting of any adverse events related to the therapy; and (3) preliminary effectiveness of the intervention (47).
Since this study was designed as a preliminary feasibility study, no formal power calculation was performed. The target sample size was set at 16 participants.
Descriptive statistics were employed to examine demographic characteristics and study outcomes, with continuous variables reported as mean ± standard deviation (S.D.) or median (interquartile range, IQR). Grubb’s test was used to identify outliers. The Shapiro–Wilk test was conducted to evaluate the normality of the differences between pre- and post-intervention spatiotemporal gait parameters. For normally distributed data, a paired-sample t-test was conducted to assess before and after intervention effects.
Data were analyzed using Microsoft Excel 2016 (Microsoft Corp.) and SPSS version 20.0 (IBM SPSS). Statistical significance was defined as p < 0.05 (two-tailed).
Sixteen participants (n = 8 female, 50%; n = 8 male, 50%) with a mean age of 17.88 ± 4.57 years were assessed by an experienced physiotherapist and recruited for this study. Among the participants, hypotonia was observed in 13 individuals (81%), and visual impairment was present in 12 individuals (75%). Additionally, 1 participant (6%) had a history of respiratory failure. Baseline characteristics for all participants are presented in Table 1, with further details for each subgroup provided in in Supplementary Table 1.
The Shapiro–Wilk test indicated that most variables followed a normal distribution. These variables—walking speed, cadence, step length (left and right), stride length (left and right), stride time (left and right), step time (left and right), single support (left), and double support—were examined with a paired samples t-test. Single support (right), which did not follow a normal distribution, was evaluated using the Wilcoxon signed-rank test. Changes in spatiotemporal gait parameters after home-based VT are reported in Table 2.
Following 2 weeks of home-based VT, participants with DS demonstrated a significant increase in walking speed, from a pre-assessment mean ± SD of 0.86 ± 0.24 m/s to a post-assessment mean of 1.17 ± 0.29 m/s (p < 0.01) (Table 2; Figure 1).
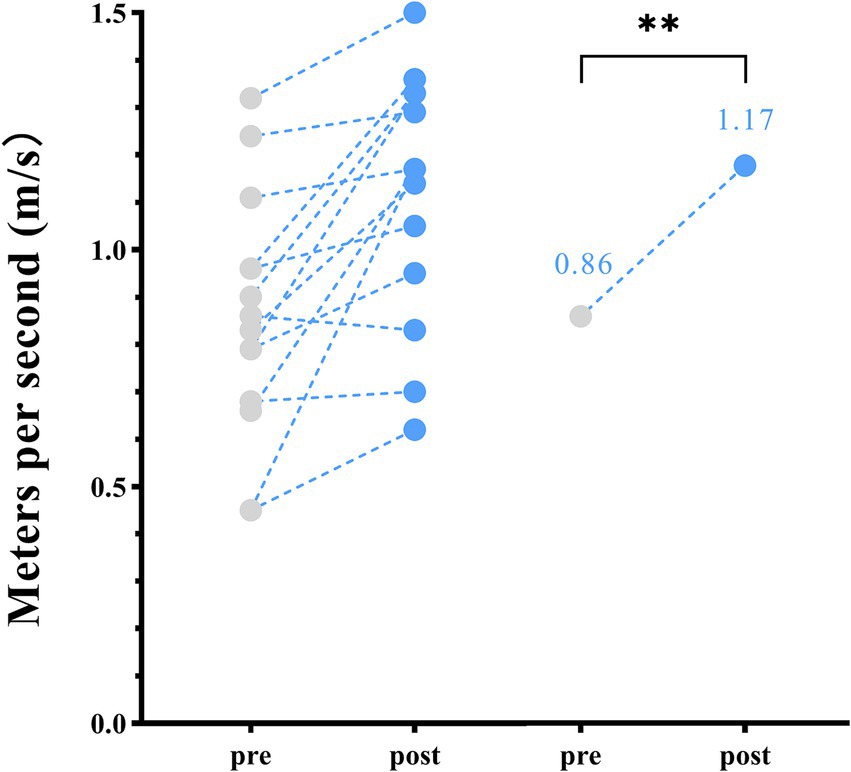
Figure 1. Spatiotemporal parameters—changes in walking speed between pre- and post-home-based vojta therapy (VT) program. The circles on the left represent individual participants; the circles on the right represent the group mean of pre- and post-intervention assessments.
Significant increase were observed for all spatial parameters from pre- to post-home-based VT, including: step length (left) increased by 0.09 m, step length (right) by 0.10 m, stride length (left) by 0.20 m, and stride length (right) by 0.16 m (p < 0.01) (Table 2; Figure 2).
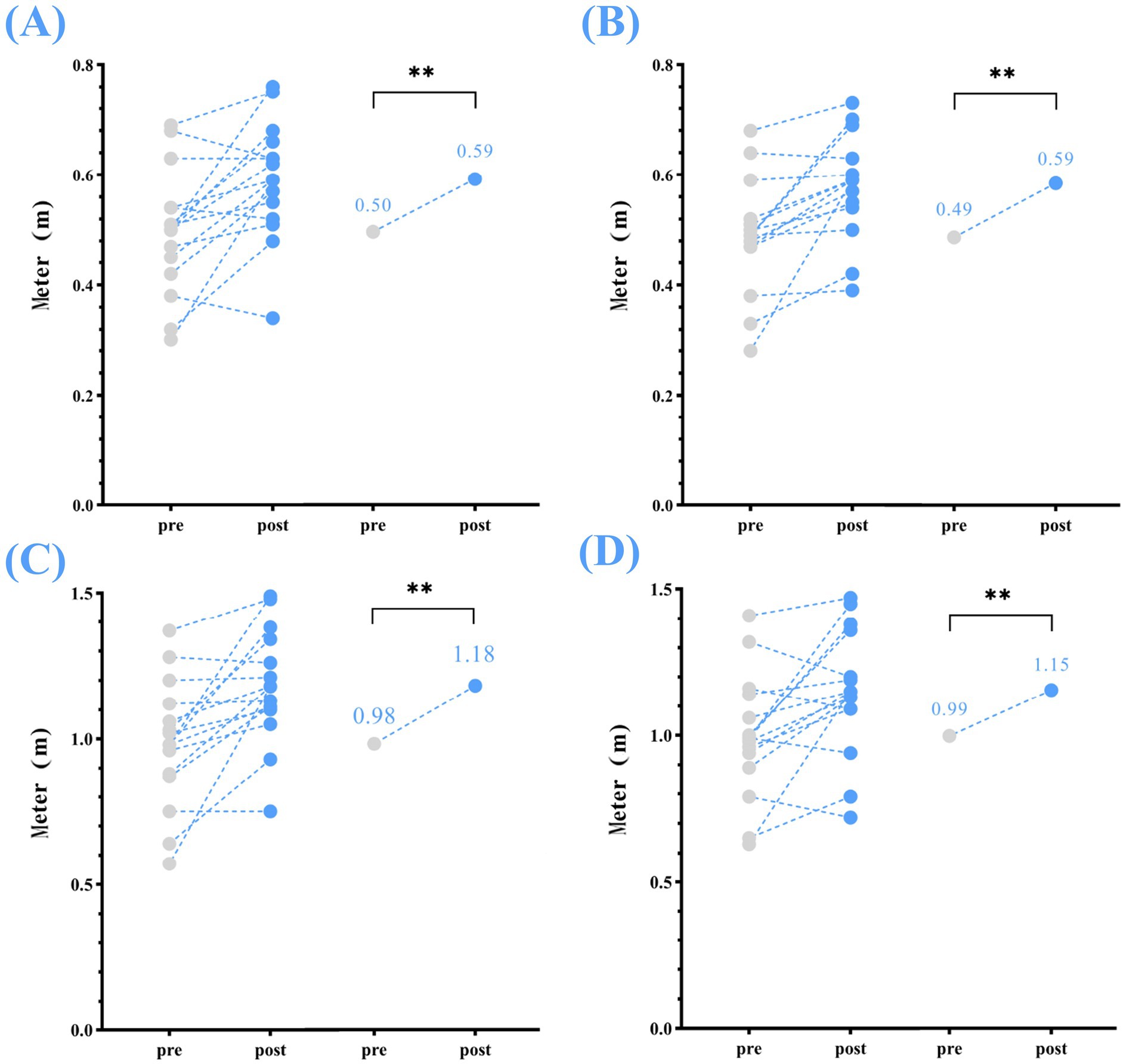
Figure 2. Spatial parameters—changes in step and stride length (left and right) between pre- and post-home-based VT. The following abbreviations are used: (A) step length (left); (B) step length (right); (C) stride length (left); (D) stride length (right); The circles on the left represent individual participants; the circles on the right represent the group mean for the pre- and post-intervention assessments.
Changes were also noted in temporal parameters, including: stride time (left) reduced from a mean (s) of 1.17 to 1.04, p < 0.01; stride time (right) reduced from 1.21 to 1.01, p < 0.01; step time (left) reduced from 0.61 to 0.53, p < 0.01; step time (right) reduced from 0.58 to 0.52, p < 0.05; and cadence increased from 102.57 steps/min to 119.36 steps/min, p < 0.01 (Table 2; Figure 3).
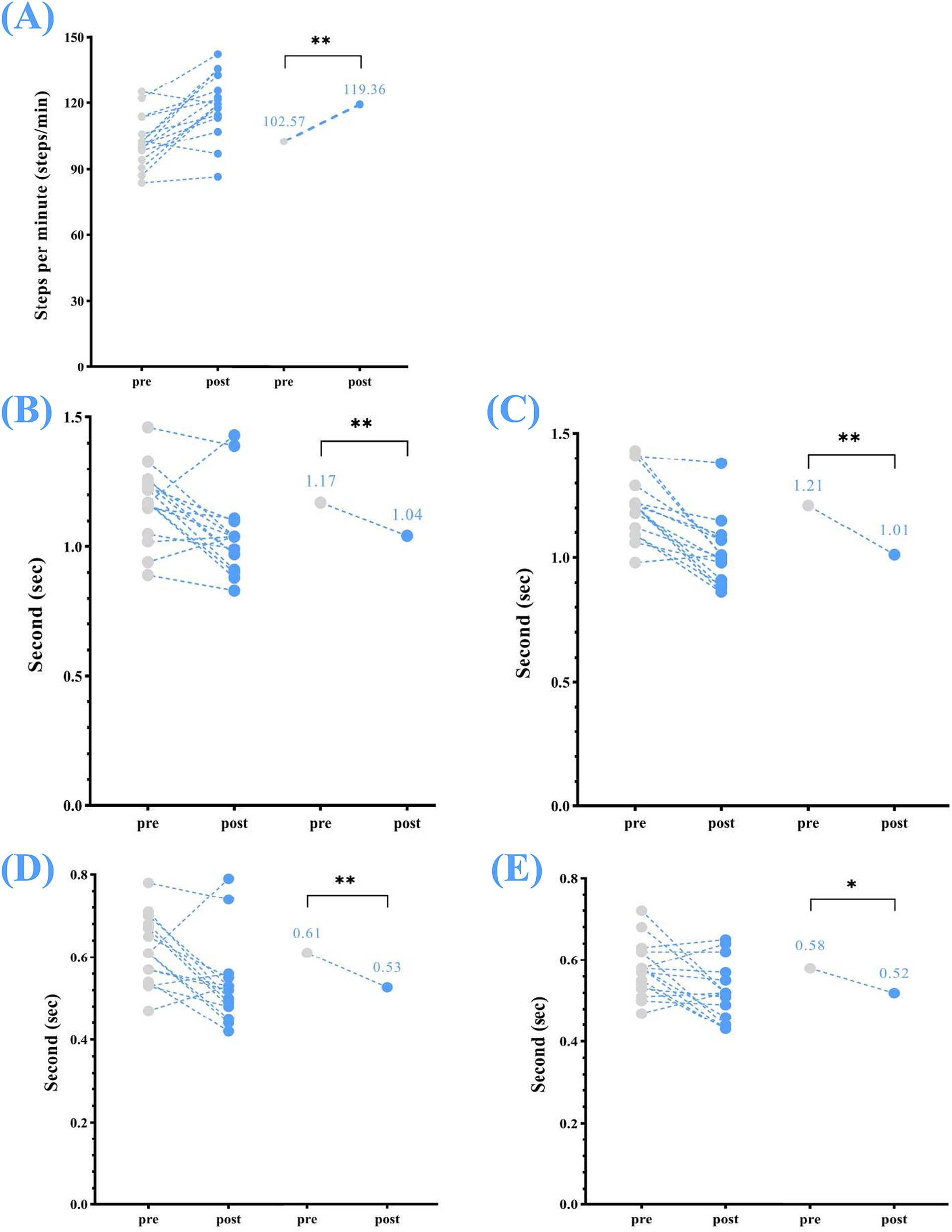
Figure 3. Temporal parameters—changes in step and stride times (left and right) and cadence between pre- and post-home-based VT. The following abbreviations are used: (A) cadence; (B) stride time (left); (C) stride time (right); (D) step time (left); (E) step time (right). The circles on the left represent the individual participants; the circles on the right represent the group mean for pre- and post-intervention. Home-based VT program.
Significant changes were also observed in single support (left), which reduced from 0.46 ± 0.04% GC at pre-assessment to 0.42 ± 0.05% GC at post-assessment (p < 0.05). Similarly, single support (right) from a median (IQR) of 0.50 (0.50, 0.50)% GC at pre-assessment to 0.42 (0.40, 0.40)% GC and at post-assessment (p < 0.01). While reduced in double support were noted after the home-based VT program, the changes were not statistically significant (double support: 0.24 ± 0.09% GC to 0.21 ± 0.07% GC, p = 0.392) (Table 2; Figure 4).
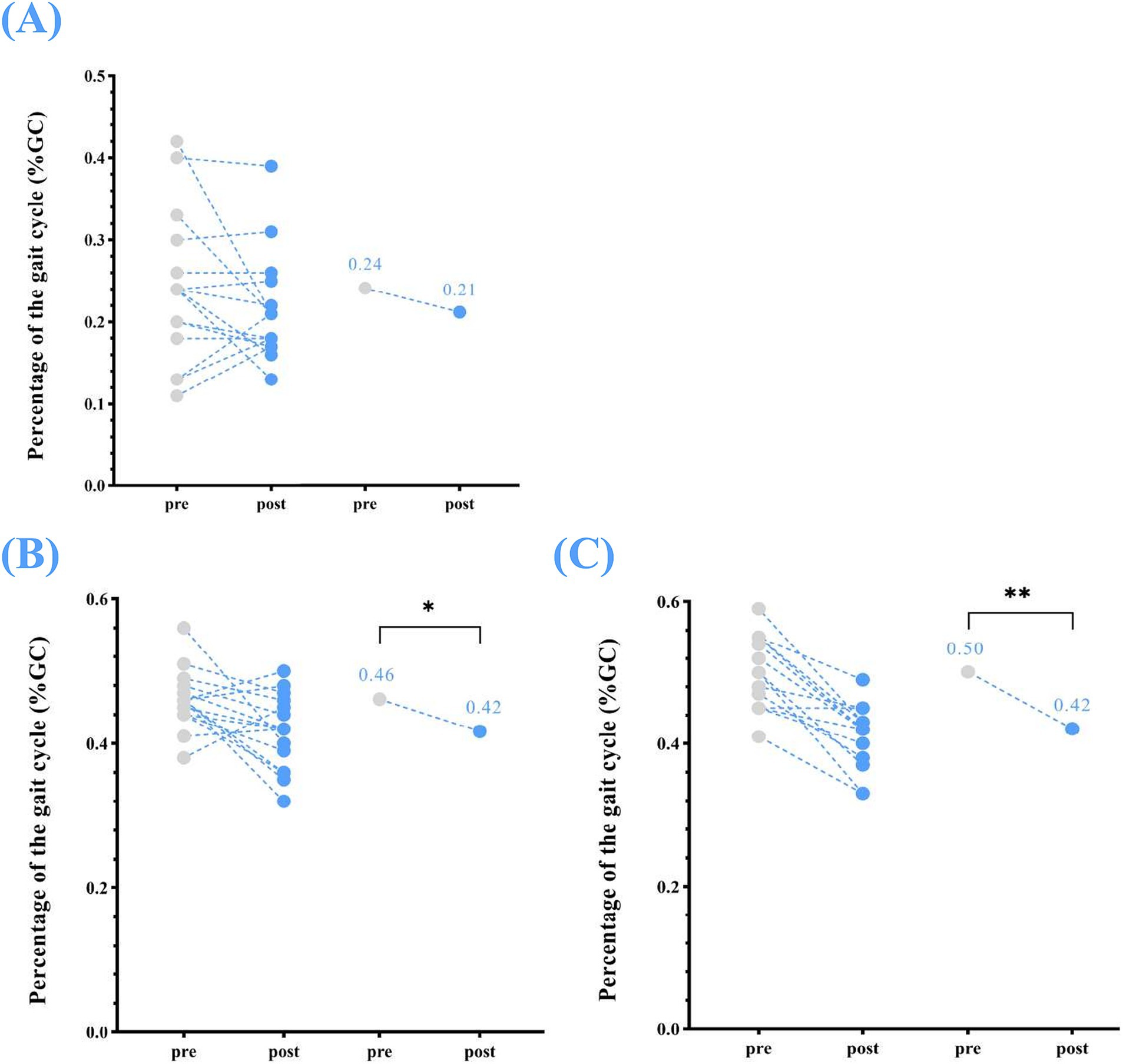
Figure 4. Temporophasic parameters—changes in single support (left and right) and double support between pre- and post-home-based VT. The following abbreviations are used: (A) double support (B) single support (left); (C) single support (right). The circles on the left represent individual participants; the circles on the right represent the group mean of pre- and post-intervention.
All participants completed the home-based VT program with 100% adherence, and no adverse events were reported.
Gait impairments in individuals with DS involve spatiotemporal gait parameters, angular changes in limb joints, and increased gait variability (48–50). Studies have shown that reduced gait parameters, such as walking speed and stride length, are often caused by ligament laxity and decreased muscle tone, leading to gait instability (51). As a result, individuals with DS tend to adopt a more cautious walking style, further reducing their stride length and gait speed. Since gait is fundamental to daily activities, normal gait development is crucial for overall healthy development. Unfortunately, from early childhood, the gait of individuals with DS is markedly different from that of typically developing individuals, leading to motor deficits that limit daily activities and discourage physical activity (15, 52). Studies of people with intellectual disabilities have shown lower levels of physical activity throughout their lives (53). Additionally, while gait typically improves as typically developing children grow, the gait of children with DS often remains impaired, emphasizing the need for sustained rehabilitation to address these deficits (10).
To our knowledge, this is the first study to explore the feasibility and effects of home-based VT in individuals with DS. Our findings show significant improvements in spatiotemporal gait parameters, including walking speed, stride time, step time, cadence, step length, stride length and single support following a two-week home-based VT program. However, changes in the gait cycle (double support) were not statistically significant. Importantly, no adverse events occurred, and all participants completed the program, demonstrating excellent adherence. These results align with previous studies that have reported improvements in gait function following VT (31). However, the existing research on VT primarily focuses on healthy adults, children, and individuals with neurological disorders such as multiple sclerosis, stroke, or cerebral palsy. Research on the use of VT in individuals with DS is extremely limited, highlighting the unique contribution of our study in this area.
One of the most critical gait indicators in our study was walking speed. There is substantial evidence supporting the importance of walking speed across the life span, as it is closely related to quality of life and overall mortality, earning it the designation of the “sixth vital sign” (54). Previous studies have demonstrated that reduced gait speed in individuals with DS leads to compensatory movement patterns during walking (48), further underscoring the need to improve gait speed to reduce these compensations (10, 55, 56). While other interventions like treadmill training, body-weight-supported gait training, and telehealth exercise programs have shown improvements in gait speed for children with DS (57), there has been a paucity of evidence on the effects of VT on spatiotemporal gait parameters in this population—until now. In our study, walking speed improved significantly (by 0.23 m/s) after the VT program, with effect sizes indicating moderate improvements. VT impact on walking speed may be explained by enhanced automatic postural reactions and increased muscle activation resulting from precisely guided reflex-based movements.
Although our findings indicate a significant improvement in walking speed, further study is required to determine if these results have clinical significance. For example, studies on stroke patients have suggested that improvements of 0.175 m/s or more in gait speed and increases of 0.08–0.14 m/s in older adults are considered clinically meaningful (58, 59). Thus, while our results are promising, larger studies are needed to confirm whether the improvements observed in individuals with DS meet the threshold for clinical significance.
In addition to walking speed, other spatiotemporal gait parameters—such as cadence, step length, stride length, stride time, and step time—also improved after VT. These improvements likely contributed to the overall enhancement in walking speed. For instance, previous research has shown that stride length is closely related to dynamic balance during walking (60). Individuals with DS often have shorter stride lengths due to poor dynamic balance, so improving stride length is crucial for enhancing gait quality (10). In our study, the increase in stride length following VT suggests that therapy not only improved walking speed but also enhanced overall gait quality. However, while improvements in the gait cycle (single and double support) were observed, they did not reach statistical significance. This may be due to the limited sample size, which limits the statistical power of the study.
Our study contributes to the limited evidence supporting the feasibility of home-based physiotherapy, particularly VT. The fact that all participants successfully completed the program without any adverse events suggests that home-based VT is a promising approach to improving gait function in individuals with DS. Given the accessibility of VT, incorporating it into early rehabilitation programs could improve positive effects. Future research should focus on longer intervention durations, comparative effectiveness studies, and personalized VT adjustments to maximize its clinical benefits.
While the sample size of this study was relatively small, it represents a crucial first step in evaluating home-based VT as a potential physical therapy method for improving gait function in individuals with DS. Our results demonstrated the feasibility of home-based VT and provide preliminary evidence that this therapy can be successfully implemented. These results will guide further research, particularly larger studies with longer follow-up periods, to further assess the impact of home-based VT on gait function.
However, several limitations must be acknowledged. First, the limited sample size and lack of a control group limit the generalizability of the findings and make it difficult to exclude potential confounding factors. Second, there may be age and gender differences in gait function within the sample, but sub-analysis could not be performed due to the limited number of participants. Future studies with larger sample sizes are needed to consider these factors and provide more reliable conclusions. Third, assessing gait performance 48 h after the intervention minimizes the immediate activation effect but may not fully capture the progression of VT-induced neuromuscular adaptations. Future studies should include multiple follow-up assessments at different time points, including short-term (e.g., 12, and 24 h after the intervention) and long-term (e.g., 1 week, 4 weeks after the intervention), to more fully understand the temporal dynamics and sustainability of the VT effect. Fourth, The high adherence rate observed may be partially attributed to the short two-week intervention. However, whether this adherence will persist in long-term programs remains uncertain. Future research should examine long-term adherence to assess the long-term feasibility of home VT. Last, while this study focused on spatiotemporal gait parameters, incorporation of kinematic assessments in future research will provide deeper insights into joint coordination, neuromuscular adaptations, and the biomechanical mechanisms underlying VT effects.
This first prospective study provides promising evidence that a 2-week home-based VT program can improve gait ability in persons with DS. While the results offer valuable preliminary insights into the potential benefits of home-based VT, the small sample size means these findings should be interpreted cautiously. Future research with larger sample sizes, longer treatment periods, and more robust designs can be essential to fully understanding the impact of home-based VT on gait ability in individuals with DS.
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding authors.
The studies involving humans were approved by the Bioethics Committee of the Medical Chamber of Gdańsk (Decision No. KB-23/23, May 9, 2023). The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation in this study was provided by the participants’ legal guardians/next of kin. Written informed consent was obtained from the individual(s), and minor(s)' legal guardian/next of kin, for the publication of any potentially identifiable images or data included in this article.
GQ: Writing – original draft, Writing – review & editing, Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Resources, Software, Validation, Visualization. EP: Conceptualization, Data curation, Funding acquisition, Investigation, Methodology, Resources, Software, Validation, Writing – review & editing. DW: Writing – review & editing. MK: Conceptualization, Investigation, Methodology, Project administration, Resources, Supervision, Writing – review & editing. HY: Writing – review & editing. HM: Writing – review & editing. ZO: Conceptualization, Data curation, Funding acquisition, Methodology, Resources, Writing – review & editing.
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This research was funded by the Gdansk University of Physical Education and Sport (GUPES).
We would like to acknowledge all students for their valuable help with the measurements and data acquisition; Further, we would like to thank all participants of this study and their legal guardians for their support.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The authors declare that no Gen AI was used in the creation of this manuscript.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fneur.2025.1537635/full#supplementary-material
1. Dierssen, M. Down syndrome: the brain in trisomic mode. Nat Rev Neurosci. (2012) 13:844–58. doi: 10.1038/nrn3314
3. Roizen, NJ, and Patterson, D. Down's syndrome. Lancet. (2003) 361:1281–9. doi: 10.1016/S0140-6736(03)12987-X
4. Antonarakis, SE, Skotko, BG, Rafii, MS, Strydom, A, Pape, SE, Bianchi, DW, et al. Down syndrome. Nat Rev Dis Primers. (2020) 6:9. doi: 10.1038/s41572-019-0143-7
5. Bull, MJ, Trotter, T, Santoro, SL, Christensen, C, and Grout, RWCouncil on Genetics, et al. Health supervision for children and adolescents with Down syndrome. Pediatrics. (2022) 149:e2022057010. doi: 10.1542/peds.2022-057010
6. Tsou, AY, Bulova, P, Capone, G, Chicoine, B, and Gelaro, BHarville TO, et al. Global Down syndrome Foundation medical care guidelines for adults with Down syndrome workgroup. Medical Care of Adults with Down syndrome: a clinical guideline. JAMA. (2020) 324:1543–56. doi: 10.1001/jama.2020.17024
7. Santoro, JD, Pagarkar, D, Chu, DT, Rosso, M, Paulsen, KC, Levitt, P, et al. Neurologic complications of Down syndrome: a systematic review. J Neurol. (2021) 268:4495–509. doi: 10.1007/s00415-020-10179-w
8. Engsner, S, Giang, KW, Dellborg, M, Fedchenko, M, Eriksson, P, and Mandalenakis, Z. Impact of Down syndrome on survival among patients with congenital heart disease. J Am Heart Assoc. (2024) 13:e031392. doi: 10.1161/JAHA.123.031392
9. Jain, PD, Nayak, A, Karnad, SD, and Doctor, KN. Gross motor dysfunction and balance impairments in children and adolescents with Down syndrome: a systematic review. Clin Exp Pediatr. (2022) 65:142–9. doi: 10.3345/cep.2021.00479
10. Zago, M, Duarte, NAC, Grecco, LAC, Condoluci, C, Oliveira, CS, and Galli, M. Gait and postural control patterns and rehabilitation in Down syndrome: a systematic review. J Phys Ther Sci. (2020) 32:303–14. doi: 10.1589/jpts.32.303
11. Rodríguez-Grande, EI, Vargas-Pinilla, OC, Torres-Narvaez, MR, and Rodríguez-Malagón, N. Neuromuscular exercise in children with Down syndrome: a systematic review. Sci Rep. (2022) 12:14988. doi: 10.1038/s41598-022-19086-8
12. Agiovlasitis, S, Mendonca, GV, McCubbin, JA, and Fernhall, B. Prediction of energy expenditure during walking in adults with Down syndrome. J Appl Res Intellect Disabil. (2018) 31:151–6. doi: 10.1111/jar.12392
13. Beqaj, S, Jusaj, N, and Živković, V. Attainment of gross motor milestones in children with Down syndrome in Kosovo - developmental perspective. Med Glas (Zenica). (2017) 14:189–98. doi: 10.17392/917-17
14. Frank, K, and Esbensen, AJ. Fine motor and self-care milestones for individuals with Down syndrome using a retrospective chart review. J Intellect Disabil Res. (2015) 59:719–29. doi: 10.1111/jir.12176
15. Vandoni, M, Giuriato, M, Pirazzi, A, Zanelli, S, Gaboardi, F, Carnevale Pellino, V, et al. Motor skills and executive functions in pediatric patients with Down syndrome: a challenge for tailoring physical activity interventions. Pediatr Rep. (2023) 15:691–706. doi: 10.3390/pediatric15040062
16. Muñoz-Llerena, A, Ladrón-de-Guevara, L, Medina-Rebollo, D, and Alcaraz-Rodríguez, V. Impact of physical activity on autonomy and quality of life in individuals with Down syndrome: a systematic review. Healthcare (Basel). (2024) 12:181. doi: 10.3390/healthcare12020181
17. Pinero-Pinto, E, Benítez-Lugo, ML, Chillón-Martínez, R, Rebollo-Salas, M, Bellido-Fernández, LM, and Jiménez-Rejano, JJ. Effects of massage therapy on the development of babies born with Down syndrome. Evid Based Complement Alternat Med. (2020) 2020:4912625. doi: 10.1155/2020/4912625
18. Paleg, G, Romness, M, and Livingstone, R. Interventions to improve sensory and motor outcomes for young children with central hypotonia: a systematic review. J Pediatr Rehabil Med. (2018) 11:57–70. doi: 10.3233/PRM-170507
19. Saquetto, MB, Pereira, FF, Queiroz, RS, da Silva, CM, Conceição, CS, and Gomes, NM. Effects of whole-body vibration on muscle strength, bone mineral content and density, and balance and body composition of children and adolescents with Down syndrome: a systematic review. Osteoporos Int. (2018) 29:527–33. doi: 10.1007/s00198-017-4360-1
20. Ruiz-González, L, Lucena-Antón, D, Salazar, A, Martín-Valero, R, and Moral-Munoz, JA. Physical therapy in Down syndrome: systematic review and meta-analysis. J Intellect Disabil Res. (2019) 63:1041–67. doi: 10.1111/jir.12606
21. Valentín-Gudiol, M, Mattern-Baxter, K, Girabent-Farrés, M, Bagur-Calafat, C, Hadders-Algra, M, and Angulo-Barroso, RM. Treadmill interventions in children under six years of age at risk of neuromotor delay. Cochrane Database Syst Rev. (2017) 2017:CD009242. doi: 10.1002/14651858.CD009242.pub3
22. Vojta, V. The basic elements of treatment according to Vojta. Management of the motor disorders of children with cerebral palsy, Suffolk: Lavenham Press Ltd. (1984): 128–160.
23. Khan, MH, Helsper, J, Farid, MS, and Grzegorzek, M. A computer vision-based system for monitoring Vojta therapy. Int J Med Inform. (2018) 113:85–95. doi: 10.1016/j.ijmedinf.2018.02.010
24. Selves, C, Stoquart, G, and Lejeune, T. Gait rehabilitation after stroke: review of the evidence of predictors, clinical outcomes and timing for interventions. Acta Neurol Belg. (2020) 120:783–90. doi: 10.1007/s13760-020-01320-7
25. Sanz-Esteban, I, Cano-de-la-Cuerda, R, San-Martín-Gómez, A, Jiménez-Antona, C, Monge-Pereira, E, Estrada-Barranco, C, et al. Cortical activity during sensorial tactile stimulation in healthy adults through Vojta therapy. A randomized pilot controlled trial. J Neuroeng Rehabil. (2021) 18:13. doi: 10.1186/s12984-021-00824-4
26. Pérez-Robledo, F, Sánchez-González, JL, Bermejo-Gil, BM, Llamas-Ramos, R, Llamas-Ramos, I, de la Fuente, A, et al. Electromyographic response of the abdominal muscles and stabilizers of the trunk to reflex locomotion therapy (RLT). A Preliminary Study. J Clin Med. (2022) 11:3866. doi: 10.3390/jcm11133866
27. Sánchez-González, JL, Díez-Villoria, E, Pérez-Robledo, F, Sanz-Esteban, I, Llamas-Ramos, I, Llamas-Ramos, R, et al. Synergy of muscle and cortical activation through Vojta reflex locomotion therapy in Young healthy adults: a pilot randomized controlled trial. Biomedicines. (2023) 11:3203. doi: 10.3390/biomedicines11123203
28. Carratalá-Tejada, M, Cuesta-Gómez, A, Ortiz-Gutiérrez, R, Molina-Rueda, F, Luna-Oliva, L, and Miangolarra-Page, JC. Reflex locomotion therapy for balance, gait, and fatigue rehabilitation in subjects with multiple sclerosis. J Clin Med. (2022) 11:567. doi: 10.3390/jcm11030567
29. Ha, S-Y, and Sung, Y-H. Vojta approach affects neck stability and static balance in sitting position of children with Hypotonia. Int Neurourol J. (2021) 25:S90–5. doi: 10.5213/inj.2142344.172
30. Sung, YH, and Ha, SY. The Vojta approach changes thicknesses of abdominal muscles and gait in children with spastic cerebral palsy: a randomized controlled trial, pilot study. Technol Health Care. (2020) 28:293–301. doi: 10.3233/THC-191726
31. Sánchez-González, JL, Sanz-Esteban, I, Menéndez-Pardiñas, M, Navarro-López, V, and Sanz-Mengíbar, JM. Critical review of the evidence for Vojta therapy: a systematic review and meta-analysis. Front Neurol. (2024) 15:1391448. doi: 10.3389/fneur.2024.1391448
32. Sanz-Mengibar, JM, Menendez-Pardiñas, M, and Santonja-Medina, F. Segíti-e a cerebralis paresisben szenvedő gyermekek bruttó motoros funkcióinak fejlődését a Vojta-módszer? [is the implementation of Vojta therapy associated with faster gross motor development in children with cerebral palsy?]. Ideggyogy Sz. (2021) 74:329–36. doi: 10.18071/isz.74.0329
33. Novak, I, Mcintyre, S, Morgan, C, Campbell, L, Dark, L, Morton, N, et al. Vojta therapy improves postural control in very early stroke rehabilitation: a randomised controlled pilot trial. Neurol Res Pract. (2020) 2:23. doi: 10.1186/s42466-020-00070-4
34. Igual Blasco, A, Piñero Peñalver, J, Fernández-Rego, FJ, Torró-Ferrero, G, and Pérez-López, J. Effects of chest physiotherapy in preterm infants with respiratory distress syndrome: a systematic review. Healthcare (Basel). (2023) 11:1091. doi: 10.3390/healthcare11081091
35. Jarbandhan, A, Toelsie, J, Veeger, D, Bipat, R, Vanhees, L, and Buys, R. Feasibility of a home-based physiotherapy intervention to promote post-stroke mobility: a randomized controlled pilot study. PLoS One. (2022) 17:e0256455. doi: 10.1371/journal.pone.0256455
36. Alharbi, AA, and Albalwi, AA. Exploring the influential factors impacting the provision of family-centered Care for Children with cerebral palsy in Saudi Arabia. Children (Basel). (2023) 10:1868. doi: 10.3390/children10121868
37. LWME, B, MME, G, Kleijnen, J, Rameckers, E, Schnackers, M, Smeets, R, et al. Feasibility and effectiveness of home-based therapy programmes for children with cerebral palsy: a systematic review. BMJ Open. (2020) 10:e035454. doi: 10.1136/bmjopen-2019-035454
38. Magaziner, J, Mangione, KK, Orwig, D, Baumgarten, M, Magder, L, Terrin, M, et al. Effect of a multicomponent home-based physical therapy intervention on ambulation after hip fracture in older adults: the CAP randomized clinical trial. JAMA. (2019) 322:946–56. doi: 10.1001/jama.2019.12964
39. Latham, NK, Harris, BA, Bean, JF, Heeren, T, Goodyear, C, Zawacki, S, et al. Effect of a home-based exercise program on functional recovery following rehabilitation after hip fracture: a randomized clinical trial. JAMA. (2014) 311:700–8. doi: 10.1001/jama.2014.469
40. Des Jarlais, DC, Lyles, C, and Crepaz, NTREND Group. Improving the reporting quality of nonrandomized evaluations of behavioral and public health interventions: the TREND statement. Am J Public Health. (2004) 94:361–6. doi: 10.2105/ajph.94.3.361
41. Orth, H. Das Kind in der Vojta-Therapie: Ein Begleitbuch für die Praxis 2nd ed. München: Urban & Fischer (2011).
42. Juárez-Albuixech, ML, Redondo-González, O, Tello-Díaz-Maroto, I, de la Guía, JLT, Villafañe, JH, and Jiménez-Antona, C. Feasibility and efficacy of the Vojta therapy in subacromial impingement syndrome: a randomized controlled trial. J Exerc Rehabil. (2021) 17:256–64. doi: 10.12965/jer.2142328.164
43. Argent, R, Daly, A, and Caulfield, B. Patient involvement with home-based exercise programs: can connected health interventions influence adherence? JMIR Mhealth Uhealth. (2018) 6:e47. doi: 10.2196/mhealth.8518
44. Merriaux, P, Dupuis, Y, Boutteau, R, Vasseur, P, and Savatier, X. A study of Vicon system positioning performance. Sensors (Basel). (2017) 17:1591. doi: 10.3390/s17071591
45. Eichelberger, P, Ferraro, M, Minder, U, Denton, T, Blasimann, A, Krause, F, et al. Analysis of accuracy in optical motion capture - a protocol for laboratory setup evaluation. J Biomech. (2016) 49:2085–8. doi: 10.1016/j.jbiomech.2016.05.007
46. Available online at:https://help.vicon.com/space/Nexus216/11607059/Plug-in+Gait+Reference+Guide
47. Orsmond, GI, and Cohn, ES. The distinctive features of a feasibility study: objectives and guiding questions. OTJR (Thorofare N J). (2015) 35:169–77. doi: 10.1177/1539449215578649
48. Galli, M, Rigoldi, C, Brunner, R, Virji-Babul, N, and Giorgio, A. Joint stiffness and gait pattern evaluation in children with Down syndrome. Gait Posture. (2008) 28:502–6. doi: 10.1016/j.gaitpost.2008.03.001
49. Smith, BA, Stergiou, N, and Ulrich, BD. Patterns of gait variability across the lifespan in persons with and without Down syndrome. J Neurol Phys Ther. (2011) 35:170–7. doi: 10.1097/NPT.0b013e3182386de1
50. Rigoldi, C, Galli, M, and Albertini, G. Gait development during lifespan in subjects with Down syndrome. Res Dev Disabil. (2011) 32:158–63. doi: 10.1016/j.ridd.2010.09.009
51. Kubo, M, and Ulrich, B. Coordination of pelvis-HAT (head, arms and trunk) in anterior-posterior and medio-lateral directions during treadmill gait in preadolescents with/without Down syndrome. Gait Posture. (2006) 23:512–8. doi: 10.1016/j.gaitpost.2005.06.007
52. Jung, HK, Chung, E, and Lee, BH. A comparison of the balance and gait function between children with Down syndrome and typically developing children. J Phys Ther Sci. (2017) 29:123–7. doi: 10.1589/jpts.29.123
53. Golubović, Š, Maksimović, J, Golubović, B, and Glumbić, N. Effects of exercise on physical fitness in children with intellectual disability. Res Dev Disabil. (2012) 33:608–14. doi: 10.1016/j.ridd.2011.11.003
54. Fritz, S, and Lusardi, M. White paper: "walking speed: the sixth vital sign". J Geriatr Phys Ther. (2009);32:46–49. Erratum in: J Geriatr Phys Ther. 2009;32(3):110.
55. Hocking, J, McNeil, J, and Campbell, J. Physical therapy interventions for gross motor skills in people with an intellectual disability aged 6 years and over: a systematic review. Int J Evid Based Healthc. (2016) 14:166–74. doi: 10.1097/XEB.0000000000000085
56. Rodríguez-Grande, EI, Buitrago-López, A, Torres-Narváez, MR, Serrano-Villar, Y, Verdugo-Paiva, F, and Ávila, C. Therapeutic exercise to improve motor function among children with Down syndrome aged 0 to 3 years: a systematic literature review and meta-analysis. Sci Rep. (2022) 12:13051. doi: 10.1038/s41598-022-16332-x
57. Lopes, JBP, Duarte, NAC, Lazzari, RD, and Oliveira, CS. Virtual reality in the rehabilitation process for individuals with cerebral palsy and Down syndrome: a systematic review. J Bodyw Mov Ther. (2020) 24:479–83. doi: 10.1016/j.jbmt.2018.06.006
58. Perera, S, Mody, SH, Woodman, RC, and Studenski, SA. Meaningful change and responsiveness in common physical performance measures in older adults. J Am Geriatr Soc. (2006) 54:743–9. doi: 10.1111/j.1532-5415.2006.00701.x
59. Fulk, GD, Ludwig, M, Dunning, K, Golden, S, Boyne, P, and West, T. Estimating clinically important change in gait speed in people with stroke undergoing outpatient rehabilitation. J Neurol Phys Ther. (2011) 35:82–9. doi: 10.1097/NPT.0b013e318218e2f2
Keywords: home-based Vojta therapy, spatiotemporal gait parameters, Down syndrome, VICON, feasibility study
Citation: Qian G, Perzanowska E, Wilczyńska D, Kozakiewicz M, Yu H, Marcelina H and Ossowski Z (2025) Exploring the impact of home-based Vojta therapy on gait performance in individuals with Down syndrome: a preliminary feasibility study. Front. Neurol. 16:1537635. doi: 10.3389/fneur.2025.1537635
Received: 04 December 2024; Accepted: 21 February 2025;
Published: 11 March 2025.
Edited by:
Juan Luis Sánchez González, University of Salamanca, SpainReviewed by:
Francisco Molina Rueda, Rey Juan Carlos University, SpainCopyright © 2025 Qian, Perzanowska, Wilczyńska, Kozakiewicz, Yu, Marcelina and Ossowski. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Guoping Qian, Z3VvcGluZy5xaWFuQGF3Zi5nZGEucGw=; Zbigniew Ossowski, emJpZ25pZXcub3Nzb3dza2lAYXdmLmdkYS5wbA==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.