- 1Shandong University of Traditional Chinese Medicine, Rehabilitation Medicine School, Jinan, China
- 2State University of New York at Buffalo, Albany, NY, United States
- 3The First Affiliated Hospital of Guangzhou University of Chinese Medicine, Rehabilitation Center, Guangzhou, China
- 4Affiliated Traditional Chinese Medicine Hospital of Guangzhou Medical University, Guangzhou, China
- 5Neck-Shoulder and Lumbocrural Pain Hospital of Shandong First Medical University, Shandong First Medical University & Shandong Academy of Medical Sciences, Jinan, China
- 6The Second Affiliated Hospital of Shandong University of Traditional Chinese Medicine, Rehabilitation Medicine Department, Jinan, China
- 7The Central Laboratory, Shandong Mental Health Center, Shandong University, Jinan, China
Background: Stroke is a significant health threat, and its complex interplay with fractures warrants further investigation. Depression, a critical psychological mediator in various health conditions, may also play a role. This study aims to clarify the intricate relationships among stroke, depressive symptoms, and fracture risk, potentially informing more holistic clinical strategies.
Methods: Utilizing the most recent data from the National Health and Nutrition Examination Survey (NHANES, 2017 to 2020), this study encompassed 4,979 valid samples. T-test and chi square test are conducted to compare the differences between fracture and non fracture subgroups. Subsequently, regression models were applied to assess the mediating impact of depression, with Sobel’s test and the bootstrap method deployed to substantiate the mediation pathways.
Results: In this study, we conducted subgroup and regression analyses to investigate factors influencing fractures in stroke patients using NHANES data. Subgroup analysis revealed significant associations with gender, race, osteoporosis, and depression. Female stroke patients had a higher fracture rate (73.86% vs. 47.78%, p < 0.001), and those with post-stroke depression (29.67% vs. 13.16%, p < 0.001) or osteoporosis (33.33% vs. 15.81%, p < 0.05) were at increased risk of fractures. Logistic regression models showed a positive association between stroke and fractures in the unadjusted (OR = 1.862, 95% CI: 1.348–2.573, p < 0.001) and adjusted I models (OR = 1.789, 95% CI: 1.240–2.581, p < 0.01), but not in the adjusted II model. Depression was significantly correlated with fractures in all models (unadjusted OR = 2.785, 95% CI: 1.271–6.101, p < 0.05; Model 1 OR = 3.737, 95% CI: 1.470–9.498, p < 0.01; Model 2 OR = 3.068, 95% CI: 1.026–9.175, p < 0.05). Mediation analysis using Sobel and bootstrap tests indicated that depression mediates 7.657% of the relationship between stroke and fractures (Z = 2.31, p < 0.05), with significant indirect (Z = 2.80, p < 0.01), direct (Z = 3.61, p < 0.001), and total effects (Z = 3.92, p < 0.01). The direct effect of stroke on fracture was 0.079 (95% CI: 0.036–0.121), the total effect was 0.085 (95% CI: 0.043–0.128), and the indirect effect mediated by depressive symptoms was 0.007 (95% CI: 0.002–0.011). These results suggest that depressive symptoms following stroke may contribute to an increased risk of fractures.
Conclusion: Depressive symptoms serve as a critical mediator in the link between stroke and fracture risk. Consequently, our study concludes that holistic prevention strategies for fractures in stroke patients must incorporate a focus on mental health to effectively address this complex clinical challenge.
1 Introduction
Stroke is now the second leading cause of death and disability worldwide (1). Data indicates a concerning trend of stroke incidence shifting toward younger demographics (2). Moreover, a staggering 70% of stroke-related fatalities and 87% of resulting disabilities are concentrated in low- and middle-income nations (3). This alarming situation not only leads to a significant loss of labor force but also imposes a substantial financial and healthcare burden on these countries (4). Fracture represents a perilous complication arising from stroke (5). The occurrence of fractures in the stroke population can hinder functional recovery by leading to prolonged hospitalization, reduced independence and delays in rehabilitation (6). Therefore, prevention of fractures is important for the recovery of stroke patients.
Depression affects approximately 71% of stroke survivors within the critical three-month post-stroke period (7). This mental health condition is a significant contributor to the exacerbation of physical decline and the decline in cognitive-psychological performance among individuals who have experienced a stroke (8–10). Notably, a negative correlation exists between the depression severity and bone density, with severe depression contributing to osteoporosis, and increased fracture risk (11, 12). The mechanisms underlying these phenomena are likely closely related to the regulation of the “brain-neuro-musculoskeletal” axis, which involves the bidirectional interaction between the brain’s neural control over the musculoskeletal system and the feedback regulation of brain function by the musculoskeletal system (13). For instance, the hypothalamic–pituitary–adrenal (HPA) axis, a critical pathway through which the nervous and endocrine systems interact, leads to the secretion of cortisol and other hormones upon activation (14). Depression often activates the HPA axis, increasing cortisol levels. Elevated cortisol not only exacerbates mood disorders like depression but also impairs musculoskeletal health by inhibiting osteoblast activity, reducing calcium absorption, and weakening bone structure. Cortisol plays a key role in stress responses, including fear, anxiety, and depression, while also inhibiting osteoblast activity, bone calcium absorption, and vascularization (15, 16). These effects indirectly disrupt bone microstructure and impedes bone turnover and metabolism (17). Therefore, depression, as a prevalent mood disorder, may significantly influence the link between stroke and fracture.
However, current research on the association between neuropsychiatric disorders and musculoskeletal diseases primarily focuses on adolescent and elderly populations (18–20). The relationship between depression and fracture incidence in stroke patients has not been well-defined. While previous studies have reported significant associations between depression and fall risk in stroke patients, most have been limited by small sample sizes and insufficient analytical depth, leaving the precise role of depression in the relationship between stroke and fracture poorly understood (21). To address these limitations, this study employs robust statistical methods, such as Sobel’s test and bootstrap analysis, which enhance the reliability and validity of the mediation findings. This provides a solid foundation for future research and clinical practice, supporting the development of more effective strategies (22, 23).
In summary, to delve deeper into the association between stroke and fracture incidence risk, we used data from the National Health and Nutrition Examination Survey (NHANES) 2017–2020 to (1) identify the influencing factors affecting fracture incidence in stroke and non-stroke populations; (2) investigate the relationship between stroke and fracture incidence and analyze the mediating role and extent of the influence of depression between stroke and fracture; (3) analyze stroke and fracture incidence-related Potential Mechanisms.
2 Materials and methods
2.1 Study population
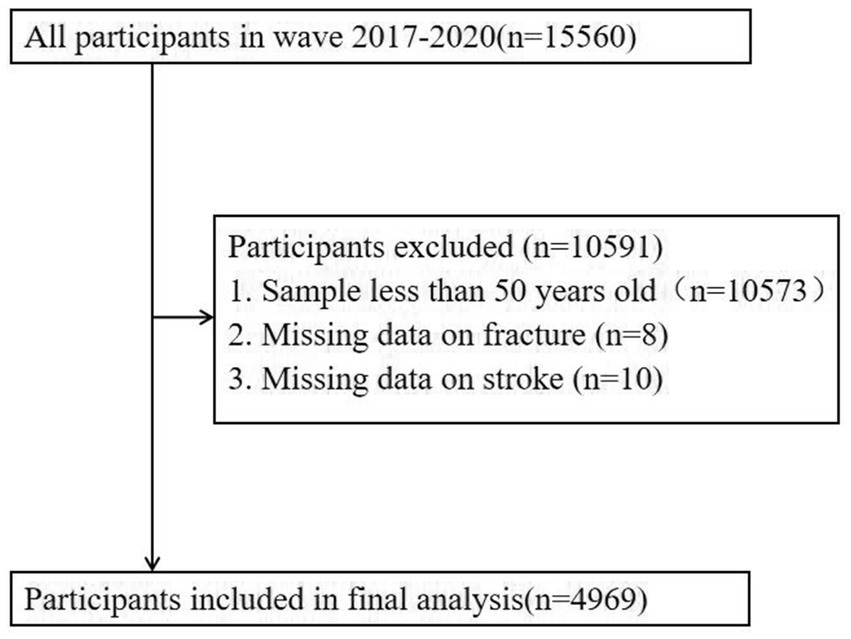
A cross-sectional study was conducted using data from the National Health and Nutrition Examination Survey (NHANES) official website: https://www.cdc.gov/nchs/nhanes/NHANES. First, we included the entire 2017–2020 NHANES population (n = 15,560). Samples younger than 50 (n = 104,573) and those with missing fracture information (n = 8) were then excluded. We finally analyzed the remaining sample of 4,979 cases. Due to the fact that Mexican American persons were oversampled and all Hispanic persons from 2007 onwards were oversampled, we adjusted all analyses for the complex sample design of NHANES using the sample weights from NHANES (24).
2.2 Outcome variable
The primary outcome variable is fracture. Participants were asked, “Has the doctor ever told you about a hip/wrist/spine fracture or fracture?” A fracture in any of these three areas is considered a separate issue; a ‘yes’ to any one of them is indicative of a fracture (25).
2.3 Evaluation of exposures
The exposure variable is stroke, which was diagnosed through self reporting by doctors and patients in face-to-face interviews. A person who answers “Yes” to the question “Ever told you had a stroke?” is defined as having a stroke. With the NHANES database’s limited details on stroke types and severity, it is reasonable to deduce that most stroke participants in this study likely suffer from ischemic stroke, aligning with the prevailing research within the NHANES database (26).
Depression is the main covariate. The Patient Health Questionnaire (PHQ-9) is used to diagnose depression, which is a self reporting assessment based on the fourth edition of the Diagnostic and Statistical Manual of Mental Disorders (DSM-IV) version, used to describe the nine signs and symptoms of depression. The scores were summed to give each participant a total score ranging from 0 to 27. As in previous studies, the present study defined depression as a total PHQ-9 score of ≥10 (27).
In addition, covariates also include: osteoporosis, dizziness, fasting glucose, alcohol, diabetes, High blood pressure, BMI, age, gender, race, marital status, education level, family monthly poverty level category, et al. An individual who affirms the query “Have you ever been diagnosed with osteoporosis or brittle bones?” is classified as having osteoporosis. Similarly, an individual who responds affirmatively to the question “Have you experienced dizziness or lightheadedness?” is categorized as experiencing dizziness. Furthermore, an individual who answers “Yes” to the question “Have you ever consumed any form of alcohol?” is identified as an alcohol consumer. Additionally, serum cotinine levels are a reliable biomarker for assessing tobacco exposure. Cotinine, a primary metabolite of nicotine, serves as an effective indicator of an individual’s level of exposure to tobacco smoke (28). All variables were obtained through standardized clinical evaluation and laboratory testing, and were selected based on previous literature and clinical expert opinions to control for potential confounding factors.
2.4 Ethic statement
The study protocol was reviewed and approved by the Research Ethics Review Board of the National Center for Health Statistics.
2.5 Statistical analysis
We conducted all statistical analyses using SAS version 9.4. Firstly, this study conducted a statistical description of the demographic characteristics and key variables of society. Categorical variables are described by frequency (percentage), while continuous variables are described by mean (standard deviation, SD). The differences between fractured and non fractured subgroups were compared using t-test or chi square test. Secondly, a univariate analysis was conducted on the fracture and non fracture subgroups of stroke patients using the aforementioned method. All samples were weighted to account for potential biases and to ensure that the study’s findings are representative of the broader population. This approach is crucial for enhancing the accuracy and generalizability of the research results (29). Then, we employed weighted logistic regression models to estimate the adjusted odds ratios (aOR) for fracture occurrence, accounting for various covariates and adjusting for independent variables across different demographic groups. Model 1 focused on the influence of age, gender, and race, while Model 2 expanded the analysis to include education, marital status, family monthly poverty level category, cotinine levels, and history of alcohol consumption. These additional variables in Model 2 aimed to provide a more comprehensive understanding of the factors associated with fracture risk. Both models were designed to control for potential confounding effects, ensuring that the estimated odds ratios were adjusted for the influence of these independent variables (30). Next, Judd and Kenny’s recommendations and Baron and Kenny’s causal step approach were employed to investigate the relationship between stroke, depression, and fracture among the population aged 50 and above.Therefore, the three regression processes would estimate the follow ing effects: (1) the effect of stroke on fracture; (2) the effect of stroke on depressive symptoms; (3) the effect of depressive symptoms on fracture when stroke were controlled; and (4) the effect of stroke on fracture when depressive symptoms were controlled (31). In our analyses, covariates were adjusted to enhance model estimation precision. The Sobel test was applied to assess the significance of the indirect effect, with a Z-value surpassing 1.96 indicating significance. Effect sizes and 95% confidence intervals were determined using bootstrapping with 5,000 resamples, considering effects significant if their intervals did not span zero. Statistical tests were two-tailed, with p < 0.05 set as the threshold for significance (25) (Figure 1).
3 Result
3.1 Univariate analysis
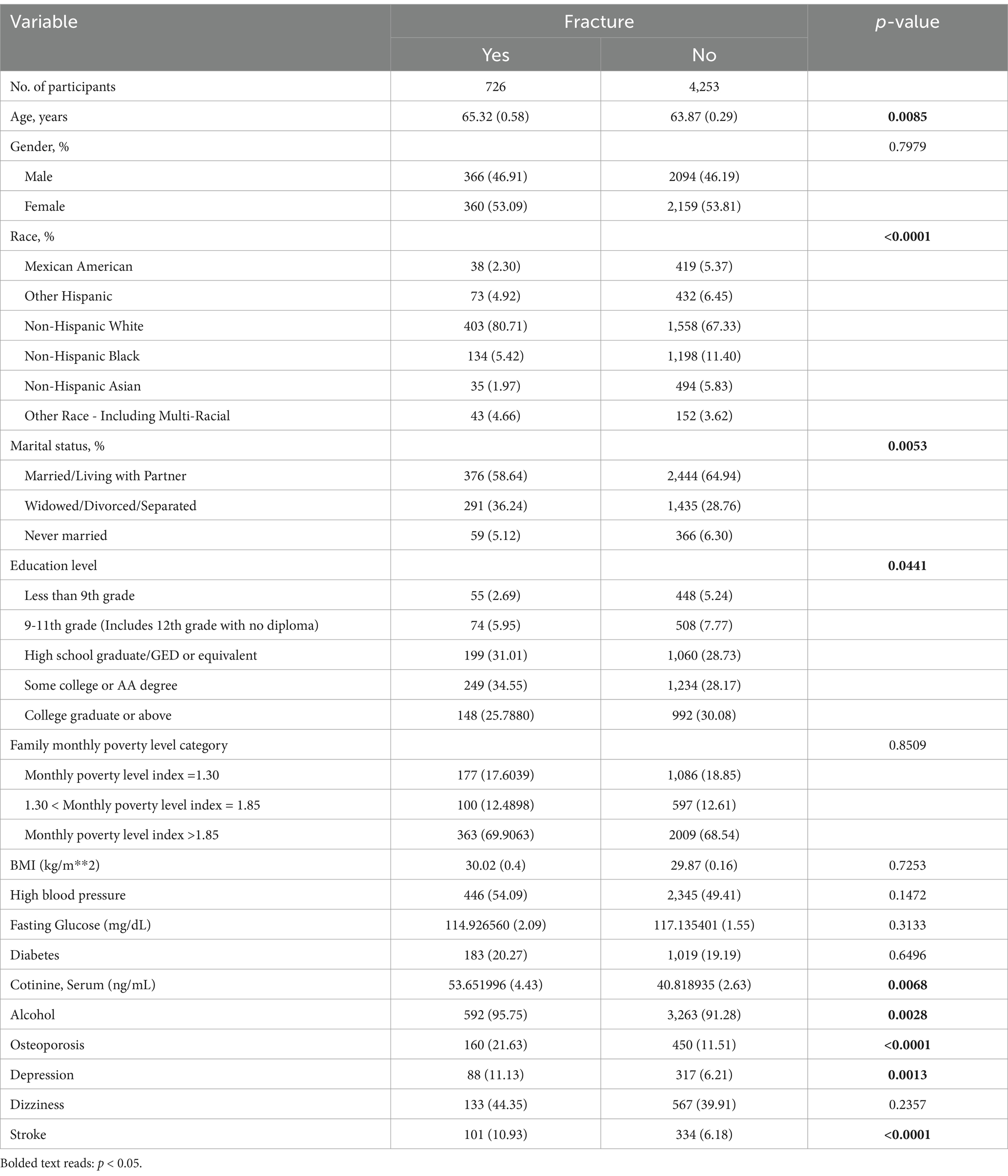
The results of univariate analysis are shown in Table 1. We found significant association between participants with and without fractures in terms of age, race, marital status, education level, tobacco smoke exposure, alcohol consumption, bone density level, depression status, and stroke. Compared with the non fracture group, the fracture group had a higher proportion of patients diagnosed with stroke (10.93% > 6.18%, p < 0.001), depression (11.13% > 6.21%, p < 0.05), and osteoporosis (21.63% > 11.51%, p < 0.001). However, there is no significant association between dizziness and fractures(44.35% > 39.91%, p > 0.05).
3.2 Subgroup analysis
Subgroup analysis was conducted to confirm which factors affect fractures in the stroke population. The results of subgroup analysis are shown in Table 2. We found a significant association between gender, race, osteoporosis, depression, and fractures in stroke patients. Specifically, among stroke patients, the proportion of male fractures is lower (26.14% < 52.22%, p < 0.001), while the probability of female fractures is higher (73.86% > 47.78%, p < 0.001). In addition, stroke patients with depression(29.67% > 13.16%, p < 0.001) and osteoporosis(33.33% > 15.81%, p < 0.05) are more likely to experience fractures.
3.3 Logistic regressive analysis
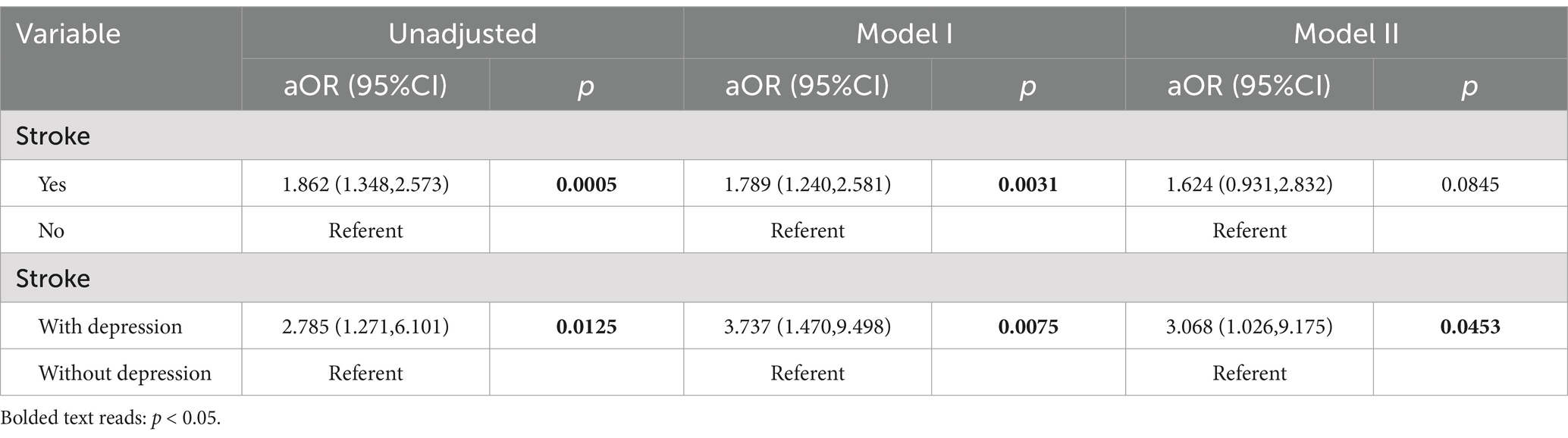
The associations between stroke, depression, and fracture risk were evaluated using Unadjusted, Model I, and Model II approaches. The effect sizes, ORs, and 95% CIs are presented in Table 3. The results showed that stroke was positively associated with fracture risk in both Unadjusted model (OR = 1.862, 95% CI: 1.348–2.573, p < 0.001) and Model I (OR = 1.789, 95% CI: 1.240–2.581, p < 0.01). However, there is no significant association in Model II. In the stroke population, using non depressed individuals as a reference, there was a significant positive association between depression and fractures among Unadjusted (OR = 2.785, 95% CI: 1.271–6.101, p < 0.05), Model I (OR = 3.737, 95% CI:1.470–9.498, p < 0.01) and Model II (OR = 3.068, 95% CI: 1.026–9.175, p < 0.05).
3.4 Mediation analysis
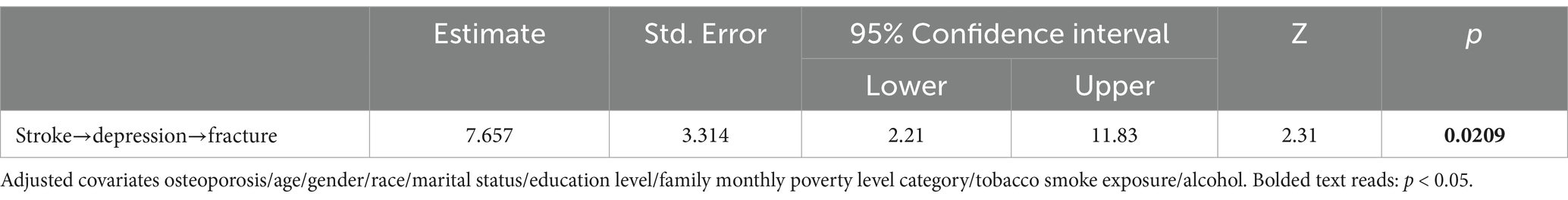
Regression analysis was utilized to explore the mediating role of depressive symptoms on the relationship between stroke and fracture. Meanwhile, we adjusted the covariate osteoporosis, age, gender, race, marital status, education level, family monthly poverty level category, tobacco exposure and alcohol. The Sobel and bootstrap tests were conducted to check for indirect, direct, and total effects. Table 4 shows that the percentage of depression mediated stroke and fracture is 7.657% (Z = 2.31, p < 0.05). As shown in Figure 2, Sobel test indicates that indirect effects (Z = 2.80, p < 0.01), direct effects (Z = 3.61, p < 0.001), and total effective rate (Z = 3.92, p < 0.001) are significant. This indicates that stroke can cause depressive symptoms, thereby increasing the risk of fractures. The boot strap method indicated that the direct effect of stroke on fracture was 0.079 (95% CI: 0.036–0.121), while the total effect was 0.085 (95% CI: 0.043–0.128). The indirect effect of stroke on fracture mediated by depressive symptoms was 0.007 (95% CI: 0.002–0.011).
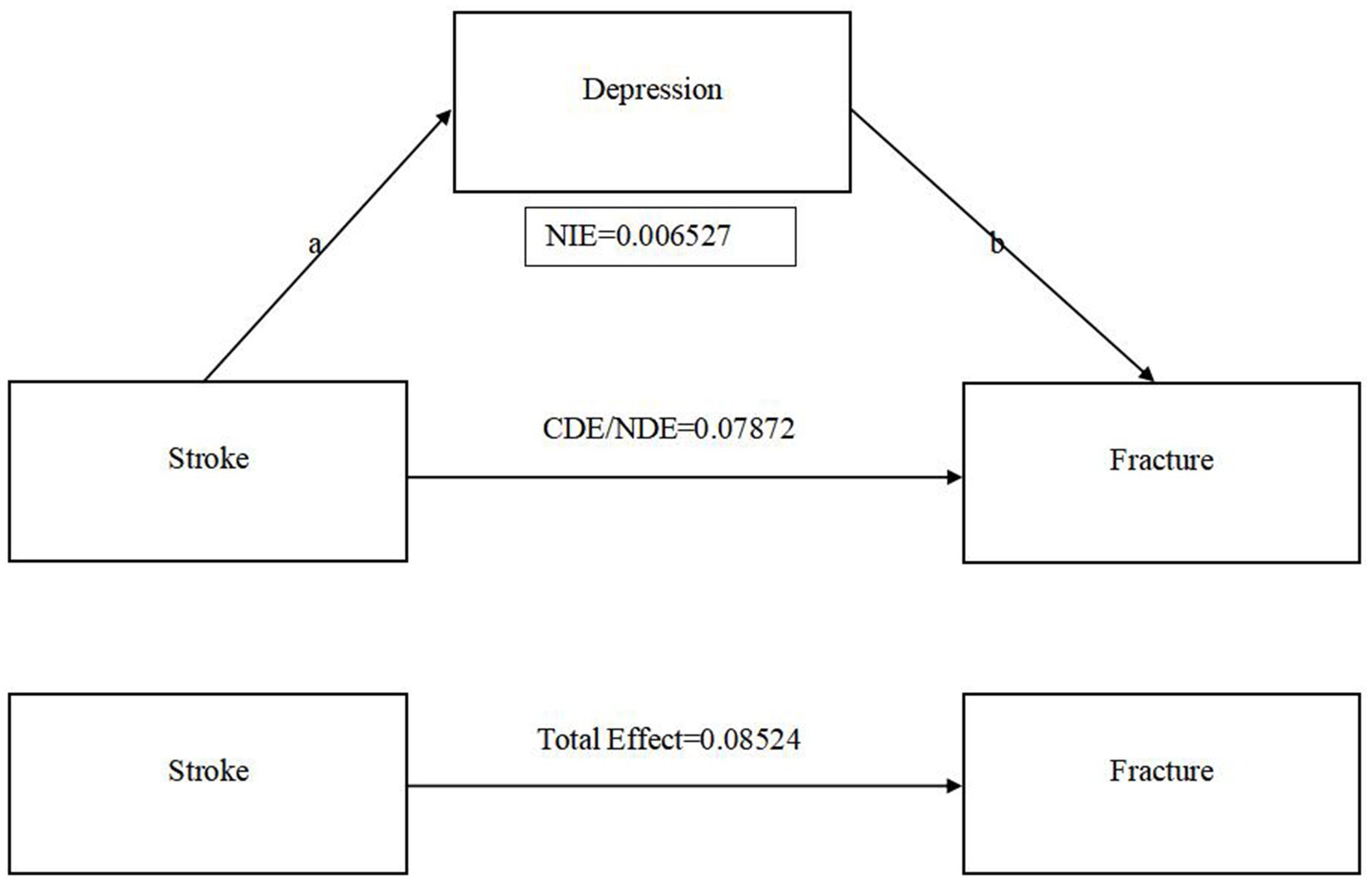
Figure 2. The relationship between stroke, depression and fracture. (a) CDE: Controlled Direct Effect; NDE: Natural Direct Effect; NIE(a*b): Natural Indirect Effect. (b) Adjusted covariates osteoporosis/age/gender/race/marital status/education level/family monthly poverty level category/tobacco smoke exposure/alcohol.
4 Discussion
Using data from 2017 to 2020 in the National Health and Nutrition Examination Survey (NHANES), the study found a significant association between stroke and fractures, with depressive symptoms mediating the risk of fractures in stroke patients (32). Sobel and Bootstrap methods supported the results of fundamental analysis, demonstrating the robustness of the results (33).
The relationship between stroke and fracture is influenced by multiple factors. While stroke survivors are prone to dizziness, often leading to falls and fractures (34, 35). However, the present study found no significant association between dizziness and fracture risk in this population. This suggests that dizziness may not be a primary factor in stroke-related fractures (36). As is well known, osteoporosis is the primary factor leading to fractures (37). The study identified a significant association between osteoporosis and fractures in stroke patients, likely due to limited mobility, reduced mechanical load from paralysis (38), and oxidative stress from cerebral ischemia–reperfusion injury. This stress disrupts the dynamic balance of osteoblast differentiation, apoptosis, and metabolism, contributing to osteoporosis (39). At the same time, gender emerges as an important influencing factor (40). Table 2 shows that in the stroke population, women in the stroke population are more prone to fractures than men. Yao et al. (41) reveal that estrogen fluctuations during menopause disrupt neurotransmitter secretion, affecting osteoblast and osteoclast balance and leading to osteoporosis. Furthermore, estrogen may contribute to osteoporosis progression by inducing oxidative stress, triggering inflammatory responses, and modulating microRNA expression, further increasing fracture susceptibility (42).
Existing research confirms that depression plays a pivotal mediating role in stroke and its associated complications (43), with a significant association between stroke and depression (44). In addition, osteoporosis can be triggered by increased life stress and depression (45). Hence, we cautiously posit that depression could serve as a critical intermediary in the relationship between stroke and fractures. Table 3 shows that the stroke and unadjusted fracture models showed significant associations. Nonetheless, following the partial adjustment for confounding factors, the association observed between stroke and fracture models became non-significant. This indicates that the association between stroke and fractures is likely due to a complex interplay of various contributing elements (46). However, stratified regression analyses of the stroke population yielded a significant association between depressive status and the fracture model in the stroke population, whether or not confounders were excluded (47). Therefore, depression plays an important role in the relationship between stroke and fracture. Table 4 indicates that while the mediated association between depression and the occurrence of stroke-related fractures is modest (7.57%, p < 0.05) but statistically significant (48). The Sobel test results, as illustrated in Figure 2, confirm the statistical significance of the indirect effects (Z = 2.80, p < 0.01), direct effects (Z = 3.61, p < 0.001), and total effects (Z = 3.92, p < 0.001) of stroke on depressive symptoms and subsequent fracture risk. Bootstrap analysis provides a more nuanced view, pinpointing the direct effect of stroke on fractures at 0.079 (95% CI: 0.036–0.121) and the total effect at 0.085 (95% CI: 0.043–0.128). The indirect effect, mediated by depression, is a modest but significant 0.007 (95% CI: 0.002–0.011). These insights highlight the pivotal role of depression as a mediator in the relationship between stroke and fracture risk, underscoring the need for integrated approaches in managing these conditions (49).
In addition to exploring depression as a mediator between stroke and fracture risk, the underlying neurophysiological mechanisms warrant further investigation. Stroke induces structural and functional impairments in critical brain regions, which can precipitate negative emotional states such as depression (50). This emotional disturbance is associated with dysregulation of neurotransmitter secretions (51). Specifically, the HPA axis increases cortisol levels while decreasing serotonin levels (52). These imbalances impair osteoblast function, hinder calcium absorption in bones, and impede skeletal angiogenesis (53). Consequently, bone microarchitecture is compromised, and normal bone turnover and metabolism are disrupted, predisposing individuals to osteoporosis and an increased risk of fractures. Conversely, fractures also impact stroke recovery (47). Research indicates that fractures can enhance inflammation in the peri-infarct area, thereby exacerbating ischemic stroke (54). Furthermore, inadequate post-fracture care leading to lower extremity deep vein thrombosis also increases the risk of stroke (55). Thus, stroke and fractures may have a reciprocal causal relationship, with regulatory mechanisms based on the “brain-neuro-musculoskeletal” axis.
During the analysis, we searched for papers published on PubMed before September 2024 using the keywords “stroke,” “fracture,” and “depression.” A total of 199 papers were retrieved, of which three were relevant to our study. Yeh et al. (56) found that post-stroke depression significantly contributes to hip fractures among stroke survivors, exerting a disproportionately negative effect on younger individuals, irrespective of gender and the presence of comorbid conditions. Kelly et al. (57) consider that the brain-bone axis plays a crucial role in the regulation of skeletal metabolism, sensory innervation, and endocrine crosstalk among these organs. Furthermore, a previous study identified a notable association between stroke and fractures in elderly patients undergoing rehabilitation post-stroke (58). However, unlike our findings, no significant disparities in depressive symptoms and functional status were observed upon admission and discharge among the compared patient cohorts. This discrepancy may be related to the failure to exclude confounding factors (59). Additionally, the previous study did not quantify depression’s influence on the stroke-fracture relationship. Moreover, it lacked an analysis of the underlying mechanisms at play.
Our research has to some extent overcome these shortcomings and has the following advantages. Firstly, we established a distinct association between stroke and fractures, particularly noting a significant association between fracture incidence and depression in stroke patients. Secondly, we employed a weighted regression model to scrutinize the relationship between depression and fractures in this patient population, effectively addressing the challenge of a limited sample size. Thirdly, through an in-depth analysis with depression as a mediating variable, we discovered that while depression’s contribution is not substantial, its statistical significance is marked. Lastly, we propose an interactive relationship exists between brain neurotransmitters and bone cells, potentially regulated by the ‘brain-bone’ axis.
However, our study has some limitations. The sample size is relatively small and the data is restricted to the American demographic, primarily composed of Hispanic Americans, the elderly, and children, which may affect the generalizability of the findings. Additionally, the stroke situation is obtained through self-report, which could easily cause recall bias. Moreover, NHANES only records fracture data for individuals over 50 years old, preventing us from observing stroke and fracture incidence in younger populations.
The cross-sectional design of our study limits the ability to establish a temporal sequence among stroke, depression, and fracture occurrence. Future studies should incorporate multicenter and demographically diverse cohorts to further validate the intricate relationships among these variables. A prospective study design would be beneficial, as it would allow for a more accurate assessment of the temporal relationships between stroke, depression, and fracture risk (60). This approach would help in understanding the causal pathways and potential differences in outcomes across various stroke subtypes, such as ischemic and hemorrhagic strokes. Additionally, intervention studies could be recommended to evaluate the impact of targeted mental health interventions on reducing fracture risk in stroke patients. Such studies could include randomized controlled trials to assess the efficacy of depression management programs in improving bone health outcomes and reducing fall-related injuries. Moreover, animal experiments exploring the mechanisms of the brain-bone axis could provide valuable insights into the underlying physiological processes. Studies using animal models can help elucidate the bidirectional communication between the brain and bone, involving factors such as neuroendocrine signals and extracellular vesicles. Such research could advance our understanding of neuroendocrine signals and extracellular vesicles, ultimately contributing to the development of therapeutic strategies for treating both neurological and musculoskeletal disorders. By exploring these mechanisms, researchers may pave the way for developing novel therapeutic strategies that target both neurological and musculoskeletal disorders.
5 Conclusion
This study indicates a positive association between stroke and fractures, highlighting depression as a substantial mediating factor. It suggests that clinicians should consider both conditions concurrently in their assessments.
Data availability statement
Publicly available datasets were analyzed in this study. This data can be found at: https://www.cdc.gov/nchs/nhanes/NHANES.
Ethics statement
Ethical review and approval was not required for the study on human participants in accordance with the local legislation and institutional requirements. Written informed consent from the patients/participants or patients/participants’ legal guardian/next of kin was not required to participate in this study in accordance with the national legislation and the institutional requirements.
Author contributions
YD: Conceptualization, Investigation, Methodology, Project administration, Software, Supervision, Writing – original draft, Writing – review & editing. XP: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. DX: Conceptualization, Data curation, Investigation, Methodology, Resources, Software, Supervision, Validation, Writing – original draft, Writing – review & editing. ZL: Conceptualization, Methodology, Project administration, Supervision, Visualization, Writing – original draft, Writing – review & editing. YW: Conceptualization, Formal analysis, Methodology, Visualization, Writing – original draft, Writing – review & editing. MY: Methodology, Validation, Visualization, Writing – original draft, Writing – review & editing. GY: Formal analysis, Funding acquisition, Project administration, Resources, Validation, Writing – original draft, Writing – review & editing. LL: Formal analysis, Funding acquisition, Project administration, Resources, Supervision, Validation, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This work was supported by the National Natural Science Foundation of China General Project (no. 82374556), the National Key R&D Program (no. 2018YFC1706005), the High Level Key Discipline Construction Project of the State Administration of Traditional Chinese Medicine (no. zyyzdxk-2013123), the Major Science and Technology Innovation Project of Shandong Province (no. 2022CXGC020510), the Integration Development Strategy Project of Jinan City and College (no. JNSX2024046), and the clinical-basic joint innovation team project of Shandong First Medical University (no. CX202408).
Acknowledgments
The author extends heartfelt thanks to the NHANES study staff and participants for their invaluable contributions. Gratitude is also expressed for the diligent efforts of the editors and reviewers in bringing this article to publication.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Saini, V, Guada, L, and Yavagal, DR. Global epidemiology of Stroke and access to acute ischemic Stroke interventions. Neurology. (2021) 97:S6–S16. doi: 10.1212/WNL.0000000000012781
2. Akinyemi, RO, Ovbiagele, B, Adeniji, OA, Sarfo, FS, Abd-Allah, F, Adoukonou, T, et al. Stroke in Africa: profile, progress, prospects and priorities. Nat Rev Neurol. (2021) 17:634–56. doi: 10.1038/s41582-021-00542-4
3. Ge, R, You, S, Zheng, D, Zhang, Z, Cao, Y, and Chang, J. Global, regional, and national temporal trends of diet-related ischemic stroke mortality and disability from 1990 to 2019. Int J Stroke. 19:665–75. doi: 10.1177/17474930241237932
4. Dalli, LL, Borschmann, K, Cooke, S, Kilkenny, MF, Andrew, NE, Scott, D, et al. Fracture risk increases after Stroke or transient ischemic attack and is associated with reduced quality of life. Stroke. (2023) 54:2593–601. doi: 10.1161/STROKEAHA.123.043094
5. Zhang, N, Guo, L, Yu, Y, Chen, S, Gao, L, Hou, X, et al. New-onset stroke on the risk of hip fracture: the Kailuan cohort study in China. BMC Public Health. (2023) 23:925. doi: 10.1186/s12889-023-15787-5
6. Chen, Y, Du, H, Song, M, Liu, T, Ge, P, Xu, Y, et al. Relationship between fear of falling and fall risk among older patients with stroke: a structural equation modeling. BMC Geriatr. (2023) 23:647. doi: 10.1186/s12877-023-04298-y
7. Viktorisson, A, Andersson, EM, Lundstrom, E, and Sunnerhagen, KS. Levels of physical activity before and after stroke in relation to early cognitive function. Sci Rep. (2021) 11:9078. doi: 10.1038/s41598-021-88606-9
8. Le Bozec, M, Tebeka, S, Dubertret, C, Sleurs, D, Mhanna, E, and Le Strat, Y. The association of stroke with mental and physical disorders in US adults: a nationally representative study. J Psychiatr Res. (2023) 168:45–51. doi: 10.1016/j.jpsychires.2023.10.035
9. Arevalo, M, Lopez-Medina, C, Moreno Martinez-Losa, M, Molto, A, Font, P, Collantes-Estevez, E, et al. Role of HLA-B27 in the comorbidities observed in axial Spondyloarthritis: data from COMOSPA. Joint Bone Spine. (2020) 87:445–8. doi: 10.1016/j.jbspin.2020.03.012
10. Liu, L, Xu, M, Marshall, IJ, Wolfe, CD, Wang, Y, and O'Connell, MD. Prevalence and natural history of depression after stroke: a systematic review and meta-analysis of observational studies. PLoS Med. (2023) 20:e1004200. doi: 10.1371/journal.pmed.1004200
11. Ma, M, Liu, X, Jia, G, Liu, Z, Zhang, K, He, L, et al. The association between depression and bone metabolism: a US nationally representative cross-sectional study. Arch Osteoporos. (2022) 17:113. doi: 10.1007/s11657-022-01154-1
12. Feuer, AJ, Demmer, RT, Thai, A, and Vogiatzi, MG. Use of selective serotonin reuptake inhibitors and bone mass in adolescents: an NHANES study. Bone. (2015) 78:28–33. doi: 10.1016/j.bone.2015.04.042
13. Shi, H, and Chen, M. The brain-bone axis: unraveling the complex interplay between the central nervous system and skeletal metabolism. Eur J Med Res. (2024) 29:317. doi: 10.1186/s40001-024-01918-0
14. Karavitaki, N, Bettinger, JJ, Biermasz, N, Christ-Crain, M, Gadelha, MR, Inder, WJ, et al. Exogenous opioids and the human endocrine system: an Endocrine Society scientific statement. Endocr Rev. (2024) 45:773–94. doi: 10.1210/endrev/bnae023
15. Chang, C, Greenspan, A, and Gershwin, ME. The pathogenesis, diagnosis and clinical manifestations of steroid-induced osteonecrosis. J Autoimmun. (2020) 110:102460. doi: 10.1016/j.jaut.2020.102460
16. Pang, JP, Hu, XP, Wang, YX, Liao, JN, Chai, X, Wang, XW, et al. Discovery of a novel nonsteroidal selective glucocorticoid receptor modulator by virtual screening and bioassays. Acta Pharmacol Sin. (2022) 43:2429–38. doi: 10.1038/s41401-021-00855-6
17. Kim, JM, Lin, C, Stavre, Z, Greenblatt, MB, and Shim, JH. Osteoblast-osteoclast communication and bone homeostasis. Cells. (2020) 9:2073. doi: 10.3390/cells9092073
18. Takeda, T, Imoto, Y, Nagasawa, H, Takeshita, A, and Shiina, M. Stress fracture and premenstrual syndrome in Japanese adolescent athletes: a cross-sectional study. BMJ Open. (2016) 6:e013103. doi: 10.1136/bmjopen-2016-013103
19. Alexiou, KI, Roushias, A, Varitimidis, SE, and Malizos, KN. Quality of life and psychological consequences in elderly patients after a hip fracture: a review. Clin Interv Aging. (2018) 13:143–50. doi: 10.2147/CIA.S150067
20. Rajha, HE, Abdelaal, R, Charfi, K, Alemadi, AO, Al-Sheraim, AS, Al-Maadid, MA, et al. Examining depression, antidepressants use, and class and their potential associations with osteoporosis and fractures in adult women: results from ten NHANES cohorts. J Affect Disord. (2025) 369:1223–32. doi: 10.1016/j.jad.2024.10.114
21. Jorgensen, L, Engstad, T, and Jacobsen, BK. Higher incidence of falls in long-term stroke survivors than in population controls: depressive symptoms predict falls after stroke. Stroke. (2002) 33:542–7. doi: 10.1161/hs0202.102375
22. Huang, IL, Liu, CY, and Chung, MH. Sleep quality and internet addiction among junior college students; the mediating role of depression: a cross-sectional study. Arch Psychiatr Nurs. (2023) 46:1–7. doi: 10.1016/j.apnu.2023.06.011
23. Pan, H, Liu, S, Miao, D, and Yuan, Y. Sample size determination for mediation analysis of longitudinal data. BMC Med Res Methodol. (2018) 18:32. doi: 10.1186/s12874-018-0473-2
24. Liu, X, Liu, X, Wang, Y, Zeng, B, Zhu, B, and Dai, F. Association between depression and oxidative balance score: National Health and nutrition examination survey (NHANES) 2005-2018. J Affect Disord. (2023) 337:57–65. doi: 10.1016/j.jad.2023.05.071
25. Zhang, X, Chen, Y, Zhu, W, and Xu, L. Reevaluating fracture prevalence trends in the U.S.: the importance of weighted analysis in NHANES data - Letter to the Editor regarding 'Trends in prevalence of fractures among adults in the United States, 1999-2020: A population-based study' by bin Xu et al. Int J Surg. 110:4002–3. doi: 10.1097/JS9.0000000000000964
26. Mao, Y, Weng, J, Xie, Q, Wu, L, Xuan, Y, Zhang, J, et al. Association between dietary inflammatory index and Stroke in the US population: evidence from NHANES 1999-2018. BMC Public Health. (2024) 24:50. doi: 10.1186/s12889-023-17556-w
27. Qi, X, Wang, S, Huang, Q, Chen, X, Qiu, L, Ouyang, K, et al. The association between non-high-density lipoprotein cholesterol to high-density lipoprotein cholesterol ratio (NHHR) and risk of depression among US adults: a cross-sectional NHANES study. J Affect Disord. (2024) 344:451–7. doi: 10.1016/j.jad.2023.10.064
28. Peng, L, Luo, X, Cao, B, and Wang, X. Unraveling the link: environmental tobacco smoke exposure and its impact on infertility among American women (18-50 years). Front Public Health. (2024) 12:1358290. doi: 10.3389/fpubh.2024.1358290
29. Xian, G, Chai, Y, Gong, Y, He, W, Ma, C, Zhang, X, et al. The relationship between healthy lifestyles and cognitive function in Chinese older adults: the mediating effect of depressive symptoms. BMC Geriatr. (2024) 24:299. doi: 10.1186/s12877-024-04922-5
30. Yao, T, Di, A, Li, J, Zhang, S, He, J, Xu, N, et al. Association between serum uric acid and intracranial arterial stenosis in a Korean population: a secondary analysis based on a cross-sectional study. Front Neurol. (2022) 13:791456. doi: 10.3389/fneur.2022.791456
31. Horlyck-Romanovsky, MF, and Haley, SJ. Increasing obesity odds among foreign-born new Yorkers are not explained by eating out, age at arrival, or duration of residence: results from NYC HANES 2004 and 2013/2014. BMC Public Health. (2021) 21:1453. doi: 10.1186/s12889-021-11351-1
32. Zou, J, Lin, R, Miao, Y, Xie, M, Wang, X, Gao, L, et al. Association between Life's simple 7 and post-stroke depression symptom from 2005-2016 NHANES survey: a cross-sectional study. J Psychiatr Res. (2024) 177:346–51. doi: 10.1016/j.jpsychires.2024.07.005
33. Zeng, Q, Ding, J, Tu, R, He, H, Wang, S, Huang, Y, et al. The mediating effect of depressive symptoms on the association between childhood friendship and physical function in middle-aged and older adults: evidence from the China health and retirement longitudinal study (CHARLS). J Affect Disord. (2024) 359:196–205. doi: 10.1016/j.jad.2024.05.087
34. Alamri, SH, Ghamri, RA, Alshehri, WH, Alhuthayli, RS, Alamoudi, NM, Alnufaei, RD, et al. Falls and correlations among community-dwelling older adults: a cross-sectional study in Jeddah, Saudi Arabia. Pak. J Med Sci. (2023) 39:109–16. doi: 10.12669/pjms.39.1.6993
35. Chen, J, Zhao, W, Yue, X, and Zhang, P. Risk factors for the occurrence of benign paroxysmal positional Vertigo: a systematic review and Meta-analysis. Front Neurol. (2020) 11. doi: 10.3389/fneur.2020.596454
36. Lee, D, Cho, IY, Chang, WH, Yoo, JE, Choi, HL, Park, J, et al. Fracture risk among Stroke survivors according to Poststroke disability status and Stroke type. Stroke. (2024) 55:1498–506. doi: 10.1161/STROKEAHA.123.044953
37. Clynes, MA, Harvey, NC, Curtis, EM, Fuggle, NR, Dennison, EM, and Cooper, C. The epidemiology of osteoporosis. Br Med Bull. (2020) 133:105–17. doi: 10.1093/bmb/ldaa005
38. Brooke-Wavell, K, Skelton, DA, Barker, KL, Clark, EM, De Biase, S, Arnold, S, et al. Strong, steady and straight: UK consensus statement on physical activity and exercise for osteoporosis. Br J Sports Med. (2022) 56:837–46. doi: 10.1136/bjsports-2021-104634
39. Li, J, Shi, L, and Sun, J. The pathogenesis of post-stroke osteoporosis and the role oxidative stress plays in its development. Front Med (Lausanne). (2023) 10:1256978. doi: 10.3389/fmed.2023.1256978
40. Tanislav, C, and Kostev, K. Factors associated with fracture after stroke and TIA: a long-term follow-up. Osteoporos Int. (2020) 31:2395–402. doi: 10.1007/s00198-020-05535-5
41. Yao, Y, Cai, X, Chen, Y, Zhang, M, and Zheng, C. Estrogen deficiency-mediated osteoimmunity in postmenopausal osteoporosis. Med Res Rev. (2024). doi: 10.1002/med.22081
42. Iantomasi, T, Romagnoli, C, Palmini, G, Donati, S, Falsetti, I, Miglietta, F, et al. Oxidative stress and inflammation in osteoporosis: molecular mechanisms involved and the relationship with microRNAs. Int J Mol Sci. (2023) 24. doi: 10.3390/ijms24043772
43. Li, J, Yang, L, Lv, R, Kuang, J, Zhou, K, and Xu, M. Mediating effect of post-stroke depression between activities of daily living and health-related quality of life: meta-analytic structural equation modeling. Qual Life Res. (2023) 32:331–8. doi: 10.1007/s11136-022-03225-9
44. Yang, Z, He, M, Zhang, Q, Li, S, Chen, H, and Liao, D. Exploring the bi-directional relationship and shared genes between depression and stroke via NHANES and bioinformatic analysis. Front Genet. (2023) 14:1004457. doi: 10.3389/fgene.2023.1004457
45. Wuertz-Kozak, K, Roszkowski, M, Cambria, E, Block, A, Kuhn, GA, Abele, T, et al. Effects of early life stress on bone homeostasis in mice and humans. Int J Mol Sci. (2020) 21. doi: 10.3390/ijms21186634
46. Lamo-Espinosa, JM, Mariscal, G, Gomez-Alvarez, J, and San-Julian, M. Incidence and risk factors for stroke after hip fracture: a meta-analysis. Sci Rep. (2023) 13:17618. doi: 10.1038/s41598-023-44917-7
47. Mijajlovic, MD, Aleksic, V, Stojanovski, N, and Bornstein, NM. Relationship between bone disorders and stroke. Neurol Sci. (2020) 41:3579–87. doi: 10.1007/s10072-020-04748-0
48. Yao, S, Zhang, M, Dong, SS, Wang, JH, Zhang, K, Guo, J, et al. Bidirectional two-sample Mendelian randomization analysis identifies causal associations between relative carbohydrate intake and depression. Nat Hum Behav. (2022) 6:1569–76. doi: 10.1038/s41562-022-01412-9
49. Zhao, L, Yang, F, Sznajder, KK, Zou, C, Jia, Y, and Yang, X. Resilience as the mediating factor in the relationship between sleep disturbance and post-stroke depression of Stroke patients in China: a structural equation modeling analysis. Front Psych. (2021) 12:625002. doi: 10.3389/fpsyt.2021.625002
50. Chang, X, He, Y, Liu, Y, Fei, J, Qin, X, Song, B, et al. Serum brain derived neurotrophic factor levels and post-stroke depression in ischemic stroke patients. J Affect Disord. (2024) 361:341–7. doi: 10.1016/j.jad.2024.06.050
51. Colita, D, Burdusel, D, Glavan, D, Hermann, DM, Colita, CI, Colita, E, et al. Molecular mechanisms underlying major depressive disorder and post-stroke affective disorders. J Affect Disord. (2024) 344:149–58. doi: 10.1016/j.jad.2023.10.037
52. Yang, JZ, Kang, CY, Yuan, J, Zhang, Y, Wei, YJ, Xu, L, et al. Effect of adverse childhood experiences on hypothalamic-pituitary-adrenal (HPA) axis function and antidepressant efficacy in untreated first episode patients with major depressive disorder. Psychoneuroendocrinology. (2021) 134:105432. doi: 10.1016/j.psyneuen.2021.105432
53. Yang, F, Liu, Y, Chen, S, Dai, Z, Yang, D, Gao, D, et al. A GABAergic neural circuit in the ventromedial hypothalamus mediates chronic stress-induced bone loss. J Clin Invest. (2020) 130:6539–54. doi: 10.1172/JCI136105
54. Wei, M, Lyu, H, Huo, K, and Su, H. Impact of bone fracture on ischemic Stroke recovery. Int J Mol Sci. (2018) 19. doi: 10.3390/ijms19051533
55. Zhang, L, Liu, X, Pang, P, Luo, Z, Cai, W, Li, W, et al. Incidence and risk factors of admission deep vein thrombosis in patients with traumatic fracture: a multicenter retrospective study. Clin Appl Thromb Hemost. (2023) 29:10760296231167143. doi: 10.1177/10760296231167143
56. Yeh, HF, Hsu, YC, Clinciu, DL, Tung, HH, Yen, YC, and Kuo, HC. Depression and young age impact on hip fracture subsequent to stroke: a population-based cohort study. Int J Nurs Pract. (2018) 24:e12665. doi: 10.1111/ijn.12665
57. Kelly, RR, Sidles, SJ, and LaRue, AC. Effects of neurological disorders on bone health. Front Psychol. (2020) 11:612366. doi: 10.3389/fpsyg.2020.612366
58. Lieberman, D, Friger, M, Fried, V, Grinshpun, Y, Mytlis, N, Tylis, R, et al. Characterization of elderly patients in rehabilitation: stroke versus hip fracture. Disabil Rehabil. (1999) 21:542–7. doi: 10.1080/096382899297198
59. Liu, H, Zhang, X, Zhou, Y, Nguyen, TN, Zhang, L, Xing, P, et al. Association between blood pressure and different antihypertensive drugs with outcome after ischemic stroke: a Mendelian randomization study. Int J Stroke. (2023) 18:1247–54. doi: 10.1177/17474930231185695
Keywords: stroke, fracture, depression, association, NHANES, mediation
Citation: Dan Y, Pei X, Xu D, Liu Z, Wang Y, Yin M, Li L and Yu G (2025) Association between stroke and fracture and the mediating role of depression: a cross-sectional study from NHANES 2017 to 2020. Front. Neurol. 16:1533565. doi: 10.3389/fneur.2025.1533565
Edited by:
Arthur Sá Ferreira, University Center Augusto Motta, BrazilReviewed by:
Shinsuke Hidese, Teikyo University, JapanChi Chen, Guizhou University of Traditional Chinese Medicine, China
Copyright © 2025 Dan, Pei, Xu, Liu, Wang, Yin, Li and Yu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Gongchang Yu, eXVnb25nY2hhbmdAc2RmbXUuZWR1LmNu; Li Li, bGlseS5qaW5hbkAxNjMuY29t
 Yuqin Dan
Yuqin Dan Xuewen Pei2
Xuewen Pei2 Danghan Xu
Danghan Xu Meng Yin
Meng Yin Li Li
Li Li Gongchang Yu
Gongchang Yu