
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Neurol., 24 February 2025
Sec. Neuromuscular Disorders and Peripheral Neuropathies
Volume 16 - 2025 | https://doi.org/10.3389/fneur.2025.1530286
Introduction: The aim of this study was to investigate the association between hepatitis B core antibody (HBcAb) positivity and the need of mechanical ventilation (MV) in patients with Guillain-Barré syndrome (GBS).
Methods: We retrospectively analyzed the clinical data of 159 patients who were diagnosed with GBS between December 2014 and April 2023 in the Affiliated Hospital of Xuzhou Medical University. Patients were categorized into two groups according to the need for MV. Variables that were significantly different between the two groups in univariate analysis were analyzed through multivariate logistic regression models.
Results: The final study population included 159 patients, 28 (17.6%) of whom need MV. In univariate analysis, Medical Research council sum score (MRC) on admission (p < 0.001), bulbar paralysis (p < 0.001), autonomic dysfunction (p < 0.001), HBcAb (p = 0.009), neutrophil/lymphocyte ratio (NLR) (p < 0.001), and Serum albumin (p = 0.016) were associated with MV. Multivariate logistic regression analysis showed lower MRC on admission (OR = 0.946, 95%CI: 0.908–0.985, p = 0.008), bulbar paralysis (OR = 3.726, 95%CI: 1.118–12.421, p = 0.032), autonomic dysfunction (OR = 3.804, 95%CI: 1.058–13.679, p = 0.041), HBcAb positivity (OR = 6.154, 95%CI: 1.253–30.229, p = 0.025), and higher NLR (OR = 1.214, 95%CI: 1.039–1.417, p = 0.014) were the risk factors for the need of MV in patients with GBS.
Conclusion: HBcAb positivity increased the risk of MV in patients with GBS. Lower MRC on admission, bulbar paralysis, autonomic dysfunction, and higher NLR were the risk factors for the need for MV.
Guillain-Barré syndrome (GBS) is a kind of acute inflammatory peripheral neuropathies that are mediated by the immune system (1). Even though the disease is self-limiting and immunotherapies like intravenous immunoglobulin (IVIG) and plasma exchange (PE) can help control the disease’s progression and reduce disability, a significant number of patients suffer from long-term disability or pass away from the disease (2). Respiratory complications are the main cause of death from the disease, and a recent review reported that the incidence of respiratory failure in patients with GBS ranged from 6 to 33% (3). That is why the prognosis of patients with GBS may be improved by early and precise prediction of those who will require mechanical ventilation (MV) and individualized therapy early in the course of treatment (4). The current study identified a number of factors that may be related to the need for MV in patients with GBS, such as higher neutrophil/lymphocyte ratio (NLR), bulbar paralysis, autonomic dysfunction, lower medical research council sum score (MRC) on admission, and the presence of a conduction block as demonstrated by neurophysiological assessment (5–11).
The pathomechanism of GBS is strongly correlated with prior infections, with approximately one-half to two-thirds of patients with Guillain-Barré syndrome having a history of previous infections (12). Some infectious agents that can cause GBS include Campylobacter jejuni, Cytomegalovirus, Epstein–Barr Virus, Mycoplasma pneumoniae, Haemophilus influenzae, Herpes simplex virus, Hepatitis B Virus (HBV), and Hepatitis E Virus (13). Japanese researchers conducted a large-scale epidemiological study showing that coexisting infectious diseases, mainly combined acute cytomegalovirus infection or herpes simplex virus, increased the risk of requiring MV in patients with GBS (14). HBV can induce the production of immune complexes or damage extrahepatic tissues through the direct viral response, resulting in various extrahepatic manifestations, with GBS being one such manifestation in the nervous system (15). GBS associated with HBV infection or HBV vaccination has been reported in numerous cases (16–18), and recurrence of GBS has been reported to be associated with reactivation or exacerbation of chronic HBV. These cases show that GBS patients who appear to be co-infected with HBV are prone to respiratory failure (19), and one study has shown that mothers infected with HBV have an increased susceptibility to respiratory disease in their offspring (20). There are no systematic studies evaluating the association between HBV infection and the need for MV in GBS patients. The hallmark antibody of HBV-infected patients is the hepatitis B core antibody (HBcAb), which is expressed during all phases of acute or chronic HBV infection (17). We conducted a retrospective study to investigate the relationship between HBcAb and the need for MV in patients who have GBS.
In this retrospective investigation, we enrolled patients who met the diagnostic criteria for GBS (21) and had been admitted to the neurology ward of the Affiliated Hospital of Xuzhou Medical University from December 2014 to April 2023. Patients with the following features should be excluded: (1) chronic inflammatory demyelinating polyradiculoneuropathy (CIDP); (2) peripheral neuropathies caused by other etiologies, such as poisoning and diabetes mellitus; (3) the presence of neurologic illness sequelae, which complicates the determination of the severity of GBS; (4) received hormone or IVIG treatment in other hospitals; (5) Absence of five indicators of HBV or inadequate medical records. This study was approved by the Ethics Committee of the Affiliated Hospital of Xuzhou Medical University.
For all included patients, we collected data on the following characteristics: sex, age, history of chronic diseases (hypertension, diabetes, coronary artery disease, cerebrovascular disease), antecedent events (mainly upper respiratory infections and diarrhea), time from onset to admission, time from onset to nadir, MRC on admission, facial nerve palsy, bulbar paralysis, tendon reflexes, sensory deficits, autonomic dysfunction (blood pressure fluctuations, tachyarrhythmias and bradycardia, and abnormal sweating), and treatment options.
Peripheral venous blood samples collected for the first time from patients on admission to the hospital were tested for five indicators of HBV, including HBV surface antigen (HBsAg), HBV surface antibody (HBsAb), HBV e antigen (HBeAg), HBV e antibody (HBeAb), and HBcAb, as well as for neutrophils, lymphocytes, aspartate transaminase, alanine transaminase, serum albumin, uric acid, serum sodium, serum chloride, serum potassium, and NLR. Cerebrospinal fluid protein results were collected from patients who had undergone complete lumbar punctures.
Patients who had complete neurophysiology were classified into acute inflammatory demyelinating polyradiculoneuropathy (AIDP), acute motor axonal neuropathy (AMAN), acute motor sensory axonal neuropathy (AMSAN) and uncertain according to Hadden’s criteria (22). Patients not fulfilling criteria for AIDP, AMAN, and AMSAN were classified as uncertain.
The MRC is used to evaluate patients’ impaired muscle strength by examining hip flexion, knee extension, foot dorsiflexion, as well as upper arm abduction, elbow flexion, and wrist extension (a total of 5 grades of score), which are then summed up to give a total score (23). The decision to apply MV was made by the doctor in charge. Patients should meet at least one of the following major criteria or two minor criteria in order to receive MV (24). Major criteria: (1) respiratory distress; (2) hypercapnia (PaCO2 > 48 mmHg); (3) hypoxaemia (PaO2 < 56 mmHg); (4) spirometry ≤15 mL/kg. Minor criteria: (1) inability to cough; (2) inability to remove bronchial secretions; (3) severe bulbar dysfunction. Patients were categorized into the MV group and the non-MV group according to the need for MV.
SPSS version 26.0 (IBM Corp, Armonk, New York) was used for data processing and analysis. Count data are expressed as percent or ratio, which were compared using the chi-square test or Fisher exact test. The normality test was conducted using the Kolmogorov–smirnov test. Measurement data conforming to a normal distribution were expressed as mean ± standard deviation, and the t test was used for comparison; measurement data not conforming to a normal distribution were expressed as median and interquartile range (IQR), and the Mann–Whitney U test was used for comparison. Multivariate logistic regression was used to analyze risk factors that affect MV in patients with GBS. Furthermore, we also performed subgroup analyses to explore the relationship between HBcAb and MV in different subgroups. Two-tailed p-values < 0.05 were considered statistically significant.
A total of 159 patients with GBS were enrolled (Figure 1), the mean age of the patients was (51.07 ± 17.48) years, 96 (60.38%) patients were male patients and 63 (39.62%) patients were female patients. Electromyography was completed in 126 of 159 patients, while the remaining 33 patients were unable to cooperate or refused to complete the Electromyography. The lumbar puncture was completed in 135 of 159 patients, and protein-cell separation was present in 76.30% (103/135) of the patients, the other 24 patients were unable to cooperate or refused to perfect lumbar puncture. The most common treatment was IVIG, which was applied in 146 (91.82%) patients, plasma exchange (PE) was applied in 3 (1.89%) patients, and both IVIG and PE were applied in 4 (2.52%) patients. For financial or allergy-related reasons, 10 (6.29%) patients received just general symptomatic treatment or hormone. The MV group included 28 patients (17.61%) who had respiratory failure requiring MV, the remaining 131 patients were included in the non-MV group.
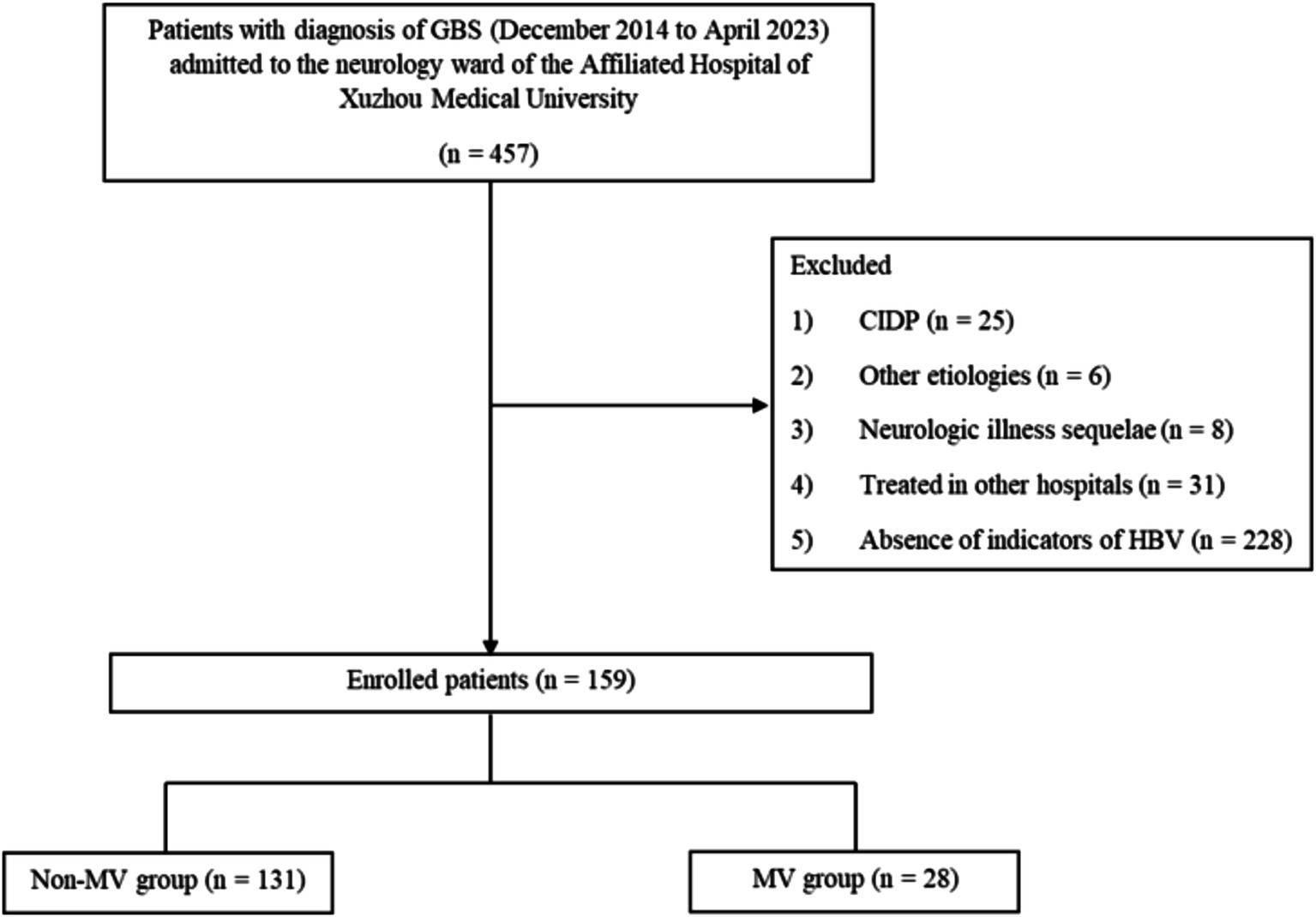
Figure 1. Flowchart showing the selection process of patients for the study. GBS, Guillain-Barré syndrome; CIDP, Chronic Inflammatory Demyelinating Polyneuropathy; HBV, hepatitis B virus; MV, mechanical ventilation.
Compared with the non-MV group, patients in the MV group had more common bulbar paralysis, autonomic dysfunction, HBcAb positivity, higher NLR, but lower MRC and serum albumin, while the rest of the indices were not statistically different between the two groups (Table 1).
The multivariate logistic regression analysis comprised indicators that showed statistically significant differences in the univariate analysis (Table 1). Multivariate logistic analysis revealed that lower MRC on admission, bulbar paralysis, autonomic dysfunction, HBcAb positivity, and higher NLR were the risk factors for the need of MV in patients with GBS (Table 2). The correlation between HBcAb and MV remained consistent across various subgroups, unaffected by factors such as age, gender, MRC on admission, bulbar paralysis, NLR, and serum albumin (all P for interaction > 0.05) (Figure 2).
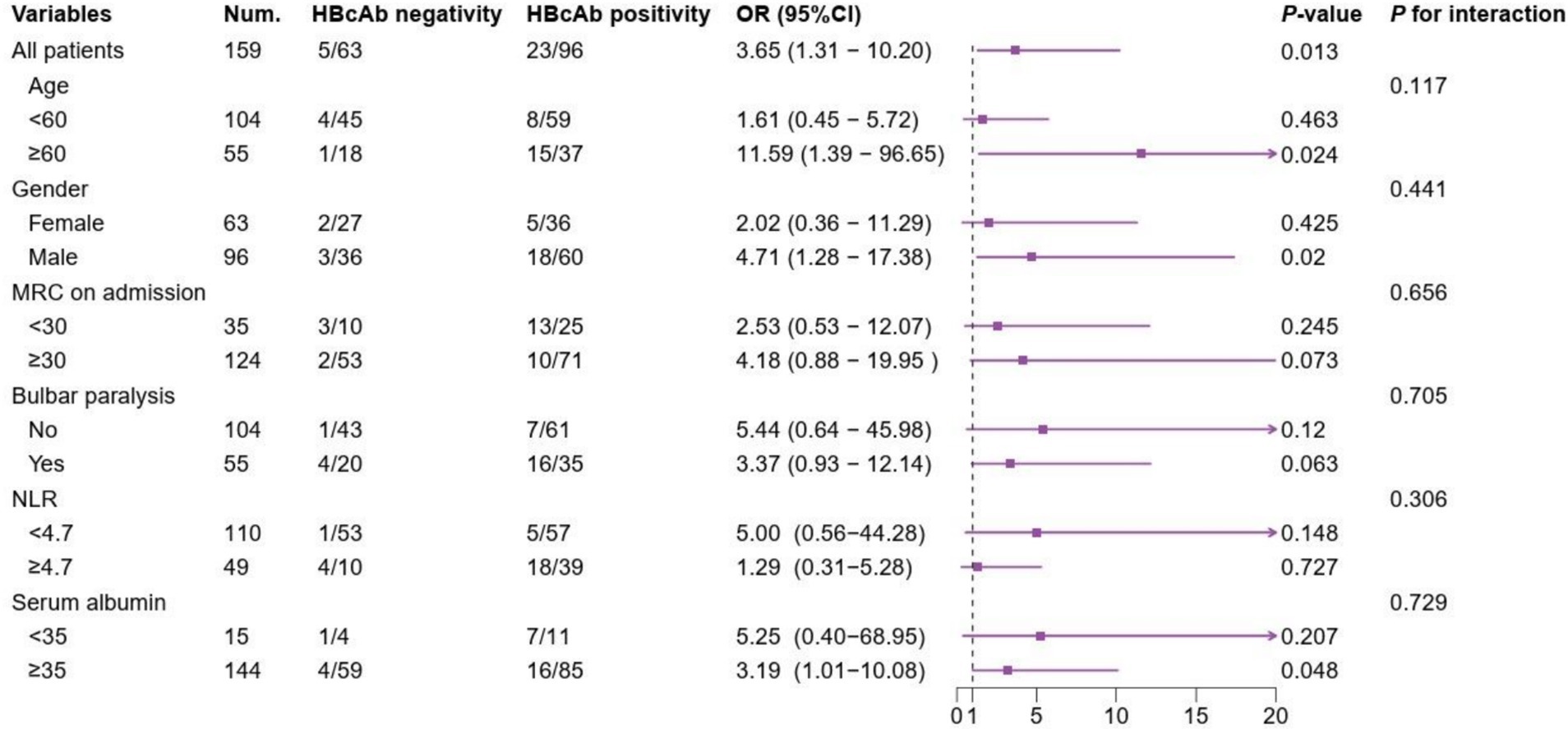
Figure 2. Subgroup analyses of the association between HBcAb and MV in patients with GBS. NUM, number; HBcAb, hepatitis B core antibody; MRC, Medical Research council sum score; NLR, neutrophil/lymphocyte ratio.
GBS is a self-limiting disease, the majority of patients can fully recover, but respiratory failure requiring MV is a serious complication of GBS. According to previous studies, the incidence of respiratory failure in patients with GBS ranges from 6 to 33% (3). The current study found that 17.61% of patients with GBS had respiratory failure, falling within this range. Multivariate logistic regression analysis in this study revealed that HBcAb positivity, low MRC on admission, bulbar paralysis, autonomic dysfunction, and higher NLR were risk factors for the requirement for MV in patients with GBS.
The major HBV serological markers include HBsAg, HBsAb, HBeAg, HBeAb, HBcAb. During different stages of HBV infection, the other four markers may be falsely negative, leading to the missed diagnosis of patients with HBV infection, while HBcAb is the hallmark antibody of HBV infection, which is expressed in the serum of all kinds of HBV-infected patients (25), hence, HBcAb positivity was selected as an observational marker of HBV infection in patients with GBS in this investigation. HBV infection induces the production and deposition of immune complexes, induced autoantibodies reacting with tissue antigens, or the presence of direct viral reactions, which may occur in extrahepatic tissues such as the nervous system, skin, muscles, joints, and kidneys. GBS is a rare neurologic complication of HBV infection (17). The definitive mechanism by which HBV induces GBS is unclear, but on the basis of published studies to date, there are three possible mechanisms. The first is immune complex damage caused by the virus; in prior case reports of GBS with HBV infection, immunofluorescence was used to detect the presence of hepatitis B virus surface antigen-positive markers on the nerve endothelium and small vessels of the nerve endothelium of patients with GBS (26). Serum and cerebrospinal fluid titers of hepatitis B virus surface antigen immune complexes increase in patients with GBS during the acute phase; these titers decrease following antiviral treatment and the recovery of neurologic function (26, 27). These findings suggest that HBV is likely to induce GBS through the formation of immune complexes leading to peripheral neurovasculitis and eventually to GBS. The second is direct viral damage; patients with GBS have HBV DNA found in their cerebrospinal fluid, thus, it is proposed that direct infection of the peripheral nerve system by the HBV may result in Guillain-Barré syndrome (28). The third is an indirect immune response known as “molecular mimicry,” in which the HBV infection may cause the production of antibodies and the activation of monocytes through the amino acid sequences of the HBV polymerase mimicking one of the polypeptides in the basic protein of the peripheral nerve myelin sheath (29). Studies on Campylobacter jejuni and Zika virus infections have shown this process, which allows patients with infection-related GBS to produce autoimmune antibodies through molecular mimicry that are specific to GBS, particularly ganglioside antibodies (1). When T cells and macrophages recognize cross-reactive antigens, B cells respond with an anti-ganglioside, which breaks down the blood–brain barrier, activates complement, activates macrophages in the nerve endothelium, and releases cytokines and free radicals (such as nitric oxide), which can cause axonal damage or loss of the myelin sheath. Activated T lymphocytes harm Schwann cells and nerve terminals simultaneously by activating complement and releasing pro-inflammatory cytokines (30).
HBV can cause lung damage in patients with infection by numerous mechanisms, including immune complex formation. The virus’s surface antigens can produce polyarteritis nodosa, when the virus deposits itself in the blood vessels of multiple organs. Likewise, these immune complexes have the ability to accumulate in the lungs’ tiny blood arteries and capillaries, which impedes gas exchange and results in obstructive ventilation (31). Immune complexes mediated by cryoglobulins are also deposited in the vascular and interstitial tissues of the lungs, which may be a possible mechanism contributing to lung dysfunction (20). Furthermore, HBV activates the release of inflammatory agents that disrupt the physiologic healing response to lung injury, thus making the lungs susceptible to injurious factors (32). Our study demonstrated for the first time by retrospective analysis that HBV infection is a risk factor for the need for mechanical ventilation in patients with GBS, and we speculate that this is due to the fact that patients infected with HBV are more likely to develop respiratory failure because their lung function is already impaired prior to the onset of GBS as compared to uninfected patients.
Some of the previously reported risk factors were also demonstrated in our study. We used the MRC to evaluate the degree of muscle weakness on admission, which shows that lower MRC on admission is a risk factor for the need for MV, meaning that the more severe the paralysis on admission, the higher the probability of needing MV. This might be since patients who have severe muscular weakness on admission tend to be bedridden longer, which increases their risk of suffering from respiratory conditions such aspiration pneumonia and eventual respiratory failure (3). Bulbar paralysis is characterized by dysphagia and dysarthria, which can lead to sputum inability to be coughed up, aspiration pneumonia, and even asphyxia, inhibiting the patient’s respiratory function (33). The autonomic dysfunction that occurs in many patients with GBS (34), which decreases airway vagal tone and the ventilatory response to hypoxia or hypercapnia, promotes the accumulation of respiratory mucus plugs (35), leading to respiratory failure and death in patients with GBS (36). The NLR is an effective indicator of the systemic inflammatory response and a rapid-responding biomarker of cellular immune activation. In GBS patients, the higher NLR is indicative of an underlying pro-inflammatory condition and immune system dysregulation (37). Neutrophils are central components of the innate immune system that enhance pro-inflammatory immune responses to fight pathogens and clear foreign substances from the body (38). Lymphocytes, which are central players in the adaptive immune system, attenuate pro-inflammatory responses and modulate immune responses. Patients with chronic respiratory failure have a higher NLR than healthy individuals (39), and the NLR has been recognized as a risk factor for the poor prognosis of ventilator-associated pneumonia (40). In addition, it has been argued that a shorter time from onset to admission or time from onset to nadir is a risk factor for the need for MV in patients with GBS (9). However, our study did not show the correlation, which may be related to the fact that the patients with GBS who were included in the analysis came to the hospital when their symptoms were minor and received treatment with IVIG earlier in our hospital.
There are several limitations to this study. First, HBcAb positivity represents the existence of HBV infection, but it does not indicate that HBV infection is in acute exacerbation, chronic exacerbation, or only occult infection; hence, quantitative HBV DNA testing is needed to determine the presence of viral replication in patients with GBS who have HBcAb positivity; but this study was a retrospective analysis and unable to quantitative HBV DNA testing was obtained. Second, because the study was retrospective and some patients were too severe to cooperate with the examination, not all the enrolled patients were perfected with the two examinations of electromyography and lumbar puncture, which might affect our comparative analysis of neurophysiology and cerebrospinal fluid. Thirdly, this study is based on a single center population with a small number of cases, which needs to be further expanded for in-depth study.
In conclusion, HBcAb positivity increased the risk of MV in patients with GBS. Lower MRC on admission, bulbar paralysis, autonomic dysfunction, and higher NLR were the risk factors for the need for MV.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving humans were approved by The Ethics Committee of the Affiliated Hospital of Xuzhou Medical University. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and institutional requirements.
WZ: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Software, Validation, Visualization, Writing – original draft, Writing – review & editing. QY: Data curation, Investigation, Supervision, Validation, Writing – original draft, Writing – review & editing. YW: Data curation, Investigation, Methodology, Validation, Visualization, Writing – original draft, Writing – review & editing. JY: Conceptualization, Data curation, Formal analysis, Funding acquisition, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. XY: Conceptualization, Data curation, Formal analysis, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing.
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This study was supported by the Sixth Phase of the “333 Talents” Project (third-level talent program) in Jiangsu Province and The first level of youth talent cultivation at Nanjing Brain Hospital (23-25-1R8-2).
We would like to express our deep gratitude to Affiliated Hospital of Xuzhou Medical University for allowing us to carry out this study.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The authors declare that no Generative AI was used in the creation of this manuscript.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Shahrizaila, N, Lehmann, HC, and Kuwabara, S. Guillain-Barré syndrome. Lancet. (2021) 397:1214–28. doi: 10.1016/S0140-6736(21)00517-1
2. Song, Y, Zheng, X, Fang, Y, Liu, S, Liu, K, Zhu, J, et al. Current status of Guillain-Barré syndrome (GBS) in China: a 10-year comprehensive overview. Rev Neurosci. (2023) 34:869–97. doi: 10.1515/revneuro-2023-0024
3. Shang, P, Zhu, M, Baker, M, Feng, J, Zhou, C, and Zhang, HL. Mechanical ventilation in Guillain-Barré syndrome. Expert Rev Clin Immunol. (2020) 16:1053–64. doi: 10.1080/1744666X.2021.1840355
4. Howard, RS. Respiratory failure because of neuromuscular disease. Curr Opin Neurol. (2016) 29:592–601. doi: 10.1097/WCO.0000000000000363
5. Green, C, Baker, T, and Subramaniam, A. Predictors of respiratory failure in patients with Guillain-Barré syndrome: a systematic review and meta-analysis. Med J Aust. (2018) 208:181–8. doi: 10.5694/mja17.00552
6. Wu, X, Li, C, Zhang, B, Shen, D, Li, T, Liu, K, et al. Predictors for mechanical ventilation and short-term prognosis in patients with Guillain-Barré syndrome. Crit Care. (2015) 19:310. doi: 10.1186/s13054-015-1037-z
7. Charoentanyarak, K, Singjam, A, and Saengsuwan, J. Clinical predictors and electrodiagnostic characteristics in patients with Guillain-Barré syndrome with respiratory failure: a retrospective, matched case-control study. PeerJ. (2022) 10:e12930. doi: 10.7717/peerj.12930
8. Vellipuram, AR, Cruz-Flores, S, Chaudhry, MRA, Rawla, P, Maud, A, Rodriguez, GJ, et al. Comparative outcomes of respiratory failure associated with common neuromuscular emergencies: myasthenia gravis versus Guillain-Barré syndrome. Medicina (Kaunas). (2019) 55:375. doi: 10.3390/medicina55070375
9. Toamad, U, Kongkamol, C, Setthawatcharawanich, S, Limapichat, K, Prabphal, K, and Sathirapanya, P. Clinical presentations as predictors of prolonged mechanical ventilation in Guillain-Barré syndrome in an institution with limited medical resources. Singapore Med J. (2015) 56:558–61. doi: 10.11622/smedj.2015152
10. Shangab, M, and Al, KM. Clinical predictors for mechanical ventilation and prognosis in patients with Guillian-Barre syndrome: a 10-year experience. Neurol Sci. (2021) 42:5305–9. doi: 10.1007/s10072-021-05251-w
11. Ning, P, Yang, B, Yang, X, Zhao, Q, Huang, H, Shen, Q, et al. A nomogram to predict mechanical ventilation in Guillain-Barré syndrome patients. Acta Neurol Scand. (2020) 142:466–74. doi: 10.1111/ane.13294
12. Jacobs, BC, Rothbarth, PH, van, FGA, Herbrink, P, Schmitz, P, de, M, et al. The spectrum of antecedent infections in Guillain-Barré syndrome: a case-control study. Neurology. (1998) 51:1110–5. doi: 10.1212/wnl.51.4.1110
13. Liu, H, and Ma, Y. Hepatitis E virus-associated Guillain-Barre syndrome: revision of the literature. Brain Behav. (2020) 10:e01496. doi: 10.1002/brb3.1496
14. Kobori, S, Kubo, T, Otani, M, Muramatsu, K, Fujino, Y, Adachi, H, et al. Coexisting infectious diseases on admission as a risk factor for mechanical ventilation in patients with Guillain-Barré syndrome. J Epidemiol. (2017) 27:311–6. doi: 10.1016/j.je.2016.07.003
15. Sonavane, AD, Saigal, S, Kathuria, A, Choudhary, NS, and Saraf, N. Guillain-Barré syndrome: rare extra-intestinal manifestation of hepatitis B. Clin J Gastroenterol. (2018) 11:312–4. doi: 10.1007/s12328-018-0847-3
16. Khamaisi, M, Shoenfeld, Y, and Orbach, H. Guillain-Barré syndrome following hepatitis B vaccination. Clin Exp Rheumatol. (2004) 22:767–70.
17. Sarin, SK, Kumar, M, Lau, GK, Abbas, Z, Chan, HLY, Chen, CJ, et al. Asian-Pacific clinical practice guidelines on the management of hepatitis B: a 2015 update. Hepatol Int. (2016) 10:1–98. doi: 10.1007/s12072-015-9675-4
18. Luis del Carpio Orantes. Síndrome de Guillain y Barré variedad miller fisher asociado a hepatitis viral B. Neurología Argentina. (2019) 11:40–3. doi: 10.1016/j.neuarg.2018.01.003
19. Wei, J, and Duan, S. Severe Guillain-Barré syndrome associated with chronic hepatitis B: a case report and literature review. Medicine (Baltimore). (2021) 100:e27989. doi: 10.1097/MD.0000000000027989
20. Govrin-Yehudain, Y, Wainstock, T, Abu-Freha, N, and Sheiner, E. Maternal hepatitis B virus and hepatitis C virus carrier status during pregnancy and long-term respiratory complications in the offspring. Early Hum Dev. (2020) 140:104904. doi: 10.1016/j.earlhumdev.2019.104904
21. van den Berg, B, Walgaard, C, Drenthen, J, Fokke, C, Jacobs, BC, and van Doorn, PA. Guillain-Barré syndrome: pathogenesis, diagnosis, treatment and prognosis. Nat Rev Neurol. (2014) 10:469–82. doi: 10.1038/nrneurol.2014.121
22. Hadden, RD, Cornblath, DR, Hughes, RA, Zielasek, J, Hartung, HP, Toyka, KV, et al. Electrophysiological classification of Guillain-Barré syndrome: clinical associations and outcome. Plasma exchange/Sandoglobulin Guillain-Barré syndrome trial group. Ann Neurol. (1998) 44:780–8. doi: 10.1002/ana.410440512
23. Kleyweg, RP, van der Meché, FG, and Schmitz, PI. Interobserver agreement in the assessment of muscle strength and functional abilities in Guillain-Barré syndrome. Muscle Nerve. (1991) 14:1103–9. doi: 10.1002/mus.880141111
24. Durand, MC, Porcher, R, Orlikowski, D, Aboab, J, Devaux, C, Clair, B, et al. Clinical and electrophysiological predictors of respiratory failure in Guillain-Barré syndrome: a prospective study. Lancet Neurol. (2006) 5:1021–8. doi: 10.1016/S1474-4422(06)70603-2
25. Grob, P, Jilg, W, Bornhak, H, Gerken, G, Gerlich, W, Günther, S, et al. Serological pattern "anti-HBc alone": report on a workshop. J Med Virol. (2000) 62:450–5. doi: 10.1002/1096-9071(200012)62:4<450::aid-jmv9>3.0.co;2-y
26. Tsukada, N, Koh, CS, Inoue, A, and Yanagisawa, N. Demyelinating neuropathy associated with hepatitis B virus infection. Detection of immune complexes composed of hepatitis B virus surface antigen. J Neurol Sci. (1987) 77:203–16. doi: 10.1016/0022-510x(87)90123-7
27. Tabor, E. Guillain-Barré syndrome and other neurologic syndromes in hepatitis a, B, and non-a, non-B. J Med Virol. (1987) 21:207–16. doi: 10.1002/jmv.1890210303
28. Ray, G, Ghosh, B, and Bhattacharyya, R. Acute hepatitis B presenting as Guillain-Barré syndrome. Indian J Gastroenterol. (2003) 22:228.
29. Caniello, M, Baxter, P, Lino, A, Lima, L, and Pinto, W. Confluent peripheral multiple mononeuropathy associated to acute hepatitis B: a case report. Rev Inst Med Trop Sao Paulo. (2002) 44:171–3. doi: 10.1590/s0036-46652002000300011
30. Rodríguez, Y, Rojas, M, Pacheco, Y, Acosta-Ampudia, Y, Ramírez-Santana, C, Monsalve, DM, et al. Guillain-Barré syndrome, transverse myelitis and infectious diseases. Cell Mol Immunol. (2018) 15:547–62. doi: 10.1038/cmi.2017.142
31. El Amrousy, D, Hassan, S, and El Ashry, H. Chronic hepatitis B infection in children and its relation to pulmonary function tests: a case-control study. Pediatr Infect Dis J. (2020) 39:192–6. doi: 10.1097/INF.0000000000002543
32. Spagnolo, P, Zeuzem, S, Richeldi, L, and du Bois, RM. The complex interrelationships between chronic lung and liver disease: a review. J Viral Hepat. (2010) 17:381–90. doi: 10.1111/j.1365-2893.2010.01307.x
33. Mengi, T, Seçil, Y, İncesu, TK, Arici, Ş, Akkiraz, ZÖ, Gürgör, N, et al. Guillain-Barré syndrome and swallowing dysfunction. J Clin Neurophysiol. (2017) 34:393–9. doi: 10.1097/WNP.0000000000000380
34. Zaeem, Z, Siddiqi, ZA, and Zochodne, DW. Autonomic involvement in Guillain-Barré syndrome: an update. Clin Auton Res. (2019) 29:289–99. doi: 10.1007/s10286-018-0542-y
35. Mazzeo, AT, La Monaca, E, Di Leo, R, Vita, G, and Santamaria, LB. Heart rate variability: a diagnostic and prognostic tool in anesthesia and intensive care. Acta Anaesthesiol Scand. (2011) 55:797–811. doi: 10.1111/j.1399-6576.2011.02466.x
36. Chakraborty, T, Kramer, CL, Wijdicks, EFM, and Rabinstein, AA. Dysautonomia in Guillain-Barré syndrome: prevalence, clinical Spectrum, and outcomes. Neurocrit Care. (2020) 32:113–20. doi: 10.1007/s12028-019-00781-w
37. Zahorec, R. Neutrophil-to-lymphocyte ratio, past, present and future perspectives. Bratisl Lek Listy. (2021) 122:474–88. doi: 10.4149/BLL_2021_078
38. Ley, K, Hoffman, HM, Kubes, P, Cassatella, MA, Zychlinsky, A, Hedrick, CC, et al. Neutrophils: new insights and open questions. Sci Immunol. (2018) 3:eaat4579. doi: 10.1126/sciimmunol.aat4579
39. Ocakli, B, Tuncay, E, Gungor, S, Sertbas, M, Adiguzel, N, Irmak, I, et al. Inflammatory markers in patients using domiciliary non-invasive mechanical ventilation: C reactive protein, procalcitonin, neutrophil lymphocyte ratio. Front Public Health. (2018) 6:245. doi: 10.3389/fpubh.2018.00245
Keywords: Guillain-Barré syndrome, hepatitis B virus, mechanical ventilation, predictors, risk factors
Citation: Zhang W, Yao Q, Wang Y, Yin J and Yang X (2025) Clinical study of the relationship between hepatitis B core antibody and mechanical ventilation in patients with Guillain-Barré syndrome. Front. Neurol. 16:1530286. doi: 10.3389/fneur.2025.1530286
Received: 18 November 2024; Accepted: 08 February 2025;
Published: 24 February 2025.
Edited by:
German Moris, SESPA, SpainReviewed by:
Luis Del Carpio-Orantes, Mexican Social Security Institute, MexicoCopyright © 2025 Zhang, Yao, Wang, Yin and Yang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xinxin Yang, bmV1cm9sb2d5YW5nQDEyNi5jb20=; Junxiong Yin, eGlvbmcyMDFAMTYzLmNvbQ==
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.