- 1Fujian University of Traditional Chinese Medicine, Fujian, China
- 2The Second Affiliated People's Hospital of Fujian University of Traditional Chinese Medicine, Fujian, China
- 3People's Hospital Affiliated to Fujian University of Traditional Chinese Medicine, Fujian, China
- 4Rehabilitation Hospital Affiliated to Fujian University of Traditional Chinese Medicine, Fujian, China
Background: The present study explored the risk factors for cerebral infarction perioperative moyamoya disease by meta-analysis.
Methods: The PubMed, Embase, Cochrane library, Web of science databases were searched for case–control/cohort studies on risk factors for the emergence of cerebral infarction perioperative moyamoya disease, the search was done from the database creation to June 1, 2024, and the data was analyzed by using stata15.0.
Result: Ten retrospective cohort studies (N = 3,239) were included. Meta-analysis results suggested posterior cerebral artery involvement [OR = 2.62, 95%CI (1.36, 5.06)], preoperative magnetic resonance angiography [OR = 2.81, 95%CI (1.27, 6.22)], previous infarction [OR = 2.52, 95% CI (1.69, 3.75)] were risk factor for the development of cerebral infarction perioperative moyamoya disease.
Conclusion: This study proves that posterior cerebral artery involvement and grade of preoperative magnetic resonance angiography is higher, and the previous infarction happened moyamoya disease a risk factor for cerebral infarction. Therefore, people with these risk factors should be intervened in advance to prevent the occurrence of perioperative cerebral infarction.
Introduction
Moyamoya disease (MMD) is a chronic cerebrovascular disease with an unknown cause that involves progressive stenosis or occlusion of the ends of the internal carotid arteries (IC), anterior cerebral arteries (ACA), and the beginnings of the middle cerebral arteries (MCA) bilaterally, with the secondary formation of a smoky blood vessel network (1, 2). The age distribution of MMD has two peaks: around 10 years old and around 40 years old, but it is more common in adults (3). MMD can be categorized into ischemic and hemorrhagic types, with ischemic type being the most common. The main treatment means for this disease is intracranial and extracranial revascularization, which mainly includes direct bypass revascularization, indirect bypass revascularization and the combination of the two (4, 5). Bypass surgery can rapidly increase the cerebral blood flow in the anastomotic blood vessel supply area, improve the symptoms of cerebral ischemia, and reduce the probability of cerebral hemorrhage caused by rupture of collateral bypass vessels (6), which is significantly better than other treatment modalities in preventing cerebral infarction or cerebral hemorrhage, and previous studies have pointed out that the efficacy of direct bypass surgery or direct combined with indirect bypass surgery (referred to as combined bypass surgery) for patients with MMD is better compared with indirect bypass surgery (7, 8), so it has become the main treatment method for MMD. Therefore, it has become the preferred treatment option for MMD. Some studies (9–11) have pointed out that the most common complication after bypass surgery in MMD patients is cerebral infarction, the incidence of which ranges from 1.7 to 13.0%, which seriously affects the quality of life of the patients after the operation, and the personalized perioperative blood pressure management can effectively reduce the incidence and severity of cerebral infarction in the patients after the operation (12). Study (13) have suggested that the posterior cerebral artery increases the risk of cerebral palsy in patients with MMD, other (14) has found the opposite conclusion, the risk factors of perioperative cerebral infarction in Moyamoya disease are still controversial (15), therefore, this study aims to explore the risk factors of perioperative cerebral infarction in Moyamoya disease by meta-analysis, and to provide guidance for the improvement of the prognosis and quality of life of these patients.
Materials and methods
Literature retrieval
Two authors searched PubMed, Embase, Cochrane library, and web of science databases from database creation to June 1, 2024, with the search terms cerebral Infarction, risk factors, and moyamoya disease, and the specific search strategy is described in Supplementary Table S1.
Literature selection
Inclusion criteria: adults who met the diagnostic criteria for moyamoya disease, exposure factor was perioperative cerebral infarction, primary outcome indicator was multivariate risk factors, and study type was case–control study or case–control study.
Exclusion criteria: duplicate articles, protocols, animal experiments, reviews, inaccessible full text, articles without usable data.
Data extraction
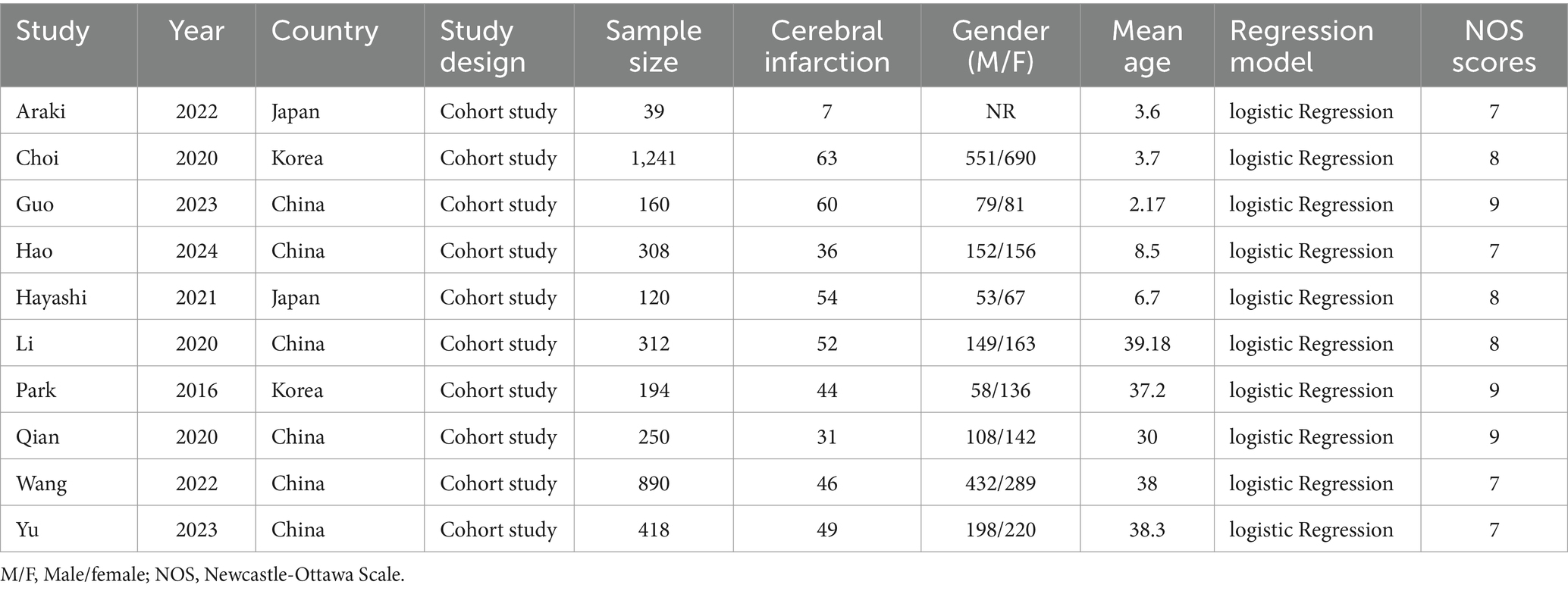
Two authors rigorously selected the literature based upon predetermined inclusion and exclusion criteria. In case of any disagreement, they resolved it through discussion or sought the opinion of a third party to negotiate and reach consensus. Information extracted from the included studies included the following key details: study, year, country, study design, sample size, cerebral infarction, gender (M/F), mean age, regression model.
Quality evaluation
The Newcastle-Ottawa Scale (NOS) (16) was used to evaluate case–control studies, including the selection of the study population (4 points), comparability between groups (2 points), and measurement of exposure factors or results (3 points). The total score of the scale is 9, with ≤4 indicating low quality, 5–6 indicating medium quality, and ≥ 7 indicating high quality. If the two researchers disagree on the evaluation process, they will discuss the decision or ask a third party to decide.
Statistical analysis
The data were statistically analyzed using Stata 15.0 software. The OR (Odds ratio) and 95% CI (confidence interval) of the risk factors included in the articles were extracted. Heterogeneity test (Q test) and I2 statistic were used to select the appropriate model to calculate the combined OR. If I2 ≥ 50%, a random effects model was used; if I2 ≤ 50%, a fixed effects model was used. For I2 > 50%, we assessed the sensitivity of the literature using the leave-one-out method. In addition, we performed publication bias using the Egger test with the significance level set at α = 0.05. p-value <0.05 was considered statistically significant.
Result
Literature screening results
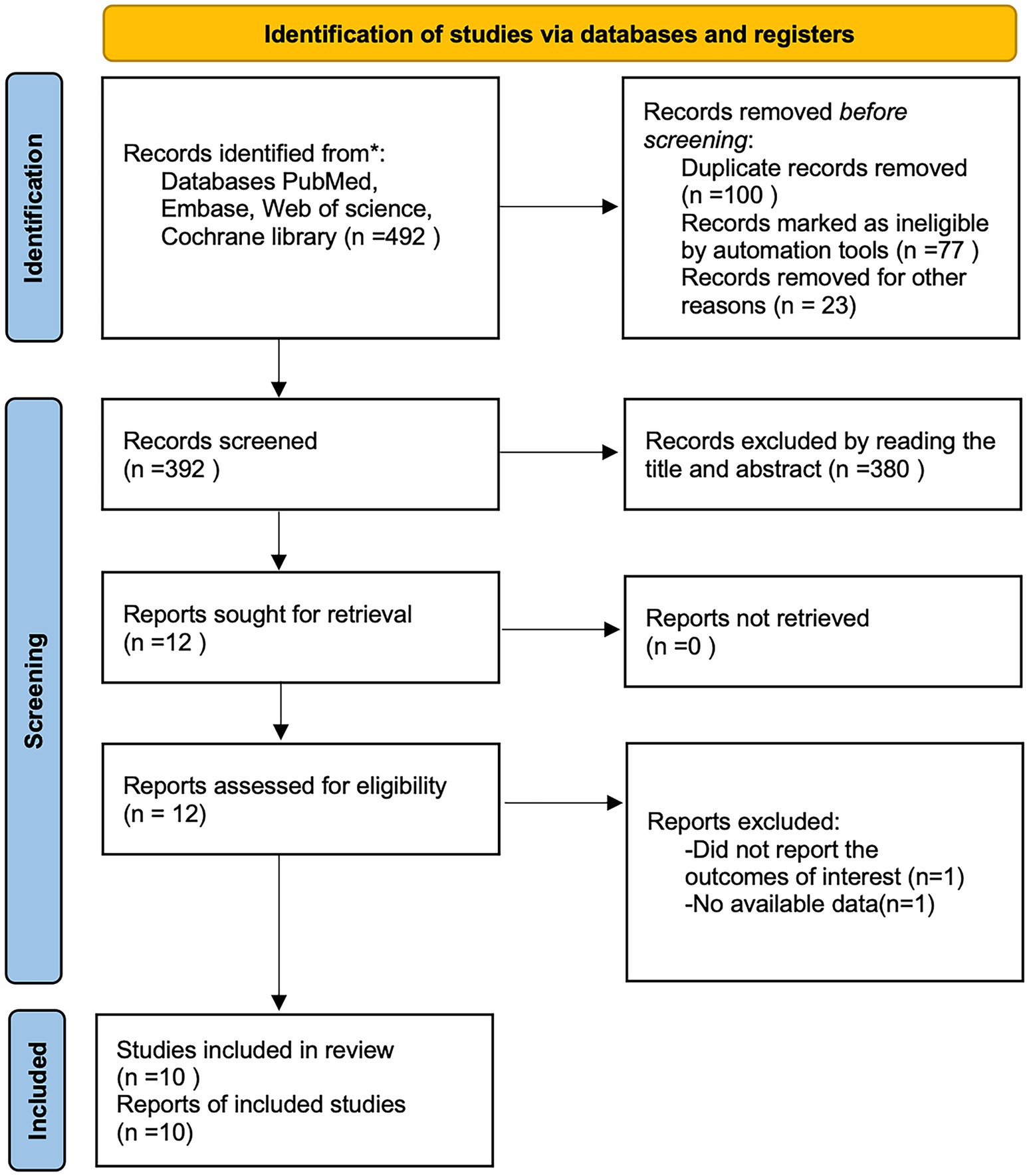
A total of 492 articles were obtained by searching PubMed, Embase, Cochrane library, and web of science databases, and 10 retrospective cohort studies (14, 17–25) were finally included by removing 100 duplicates, 380 articles by reading the title and abstract, and 2 articles by reading the full text (Figure 1), of which all 10 studies were cohort studies, all from Asian. The NOS scores of the included studies were 7 for 4 articles (17, 20, 24, 25), 8 for 3 articles (18, 21, 22), and 9 for 3 studies (14, 19, 23). The basic characteristics of the included studies are shown in Table 1.
Meta-analysis results
Age
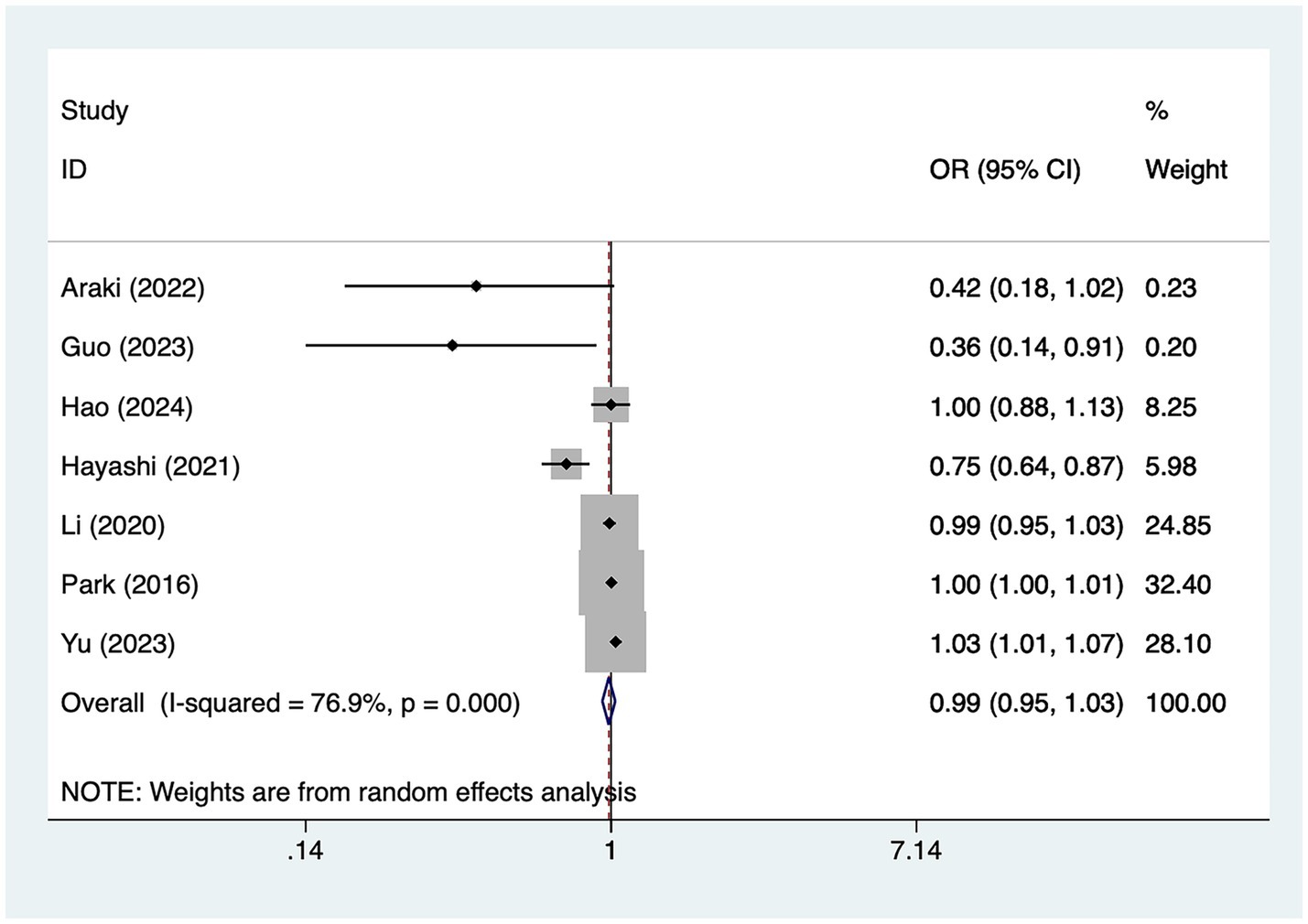
A total of seven studies mentioned age, in which heterogeneity was tested (I2 = 76.9%, p = 0.001) and therefore analyzed using a random-effects model, and the results of the analysis (Figure 2) suggested that age was not a risk factor for the development of cerebral infarction perioperative moyamoya disease [OR = 0.99, 95% CI (0.95, 1.03), p = 0.14].
Posterior cerebral artery involvement
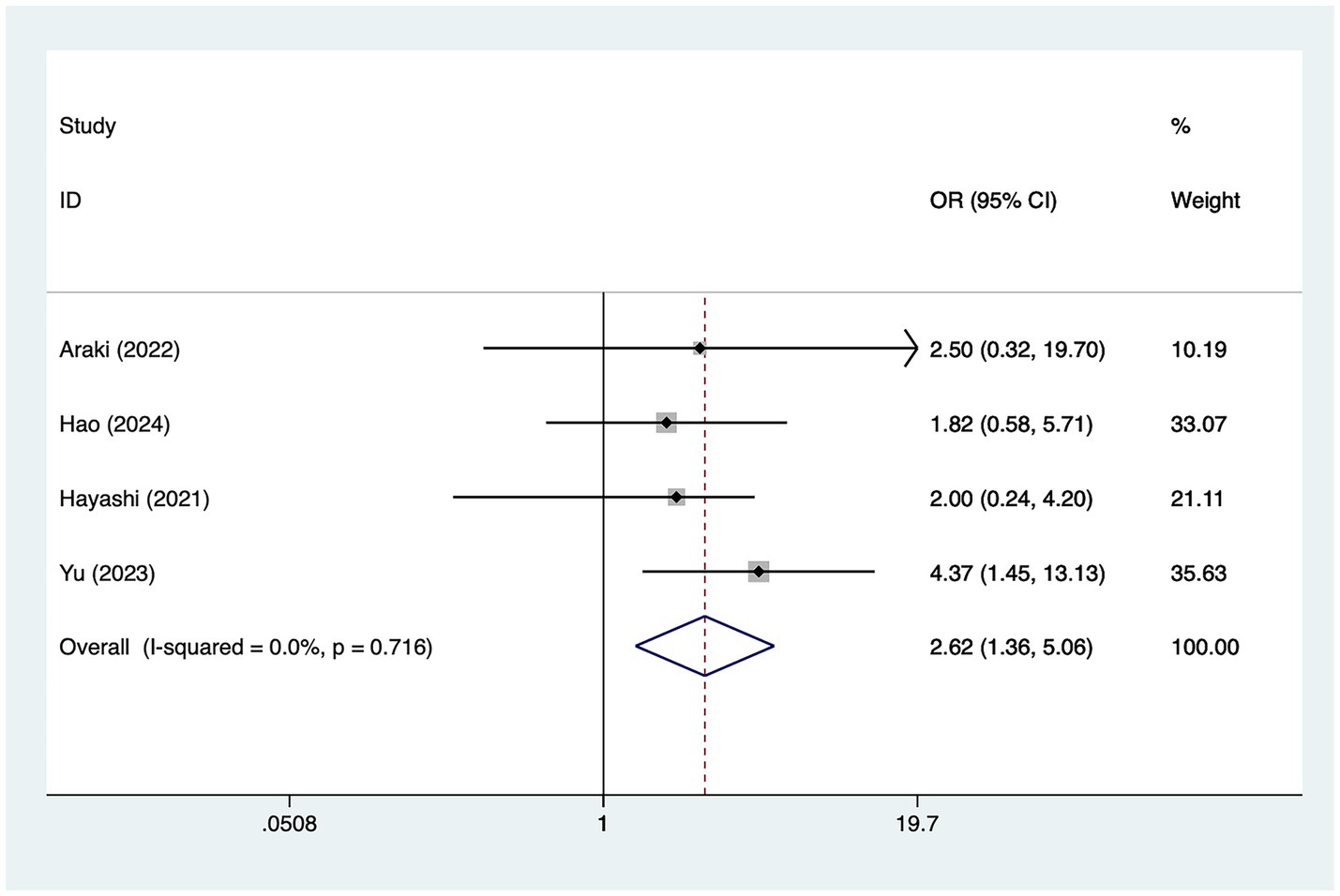
A total of three studies mentioned posterior cerebral artery involvement, in which heterogeneity was tested (I2 = 0.0%, p = 0.716) and therefore analyzed using a fixed-effects model, and the results of the analysis (Figure 3) suggested that posterior cerebral artery involvement was a risk factor for the development of cerebral infarction perioperative moyamoya disease [OR = 2.62, 95% CI (1.36, 5.06), p = 0.002].
Preoperative MRA grade
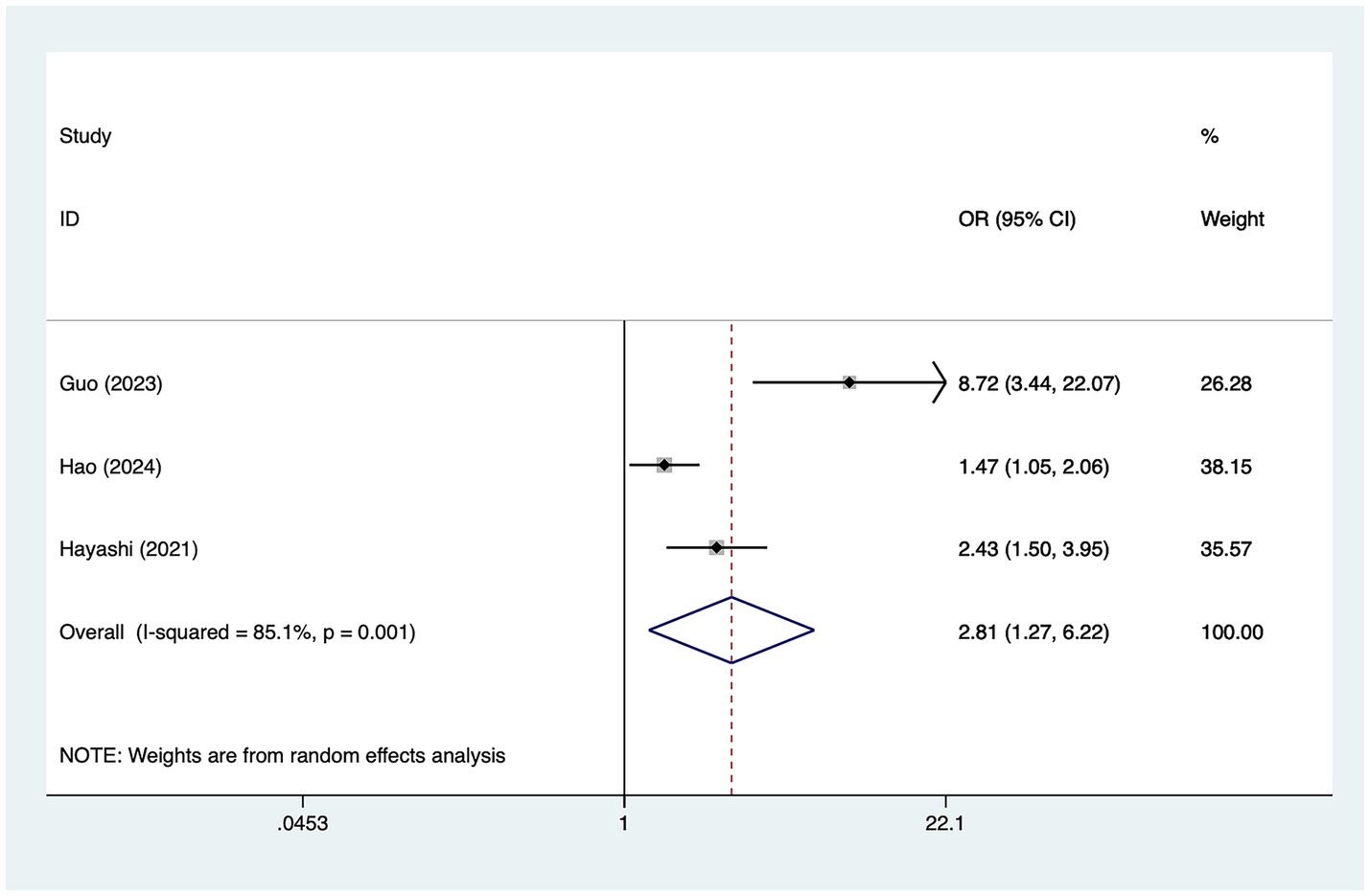
A total of five studies mentioned preoperative magnetic resonance angiography (MRA) grade, in which heterogeneity was tested (I2 = 85.1%, p = 0.001) and therefore analyzed using a random-effects model, and the results of the analysis (Figure 4) suggested that preoperative MRA grade was a risk factor for the development of cerebral infarction perioperative moyamoya disease [OR = 2.81, 95% CI (1.27, 6.22), p = 0.003].
Previous infarction
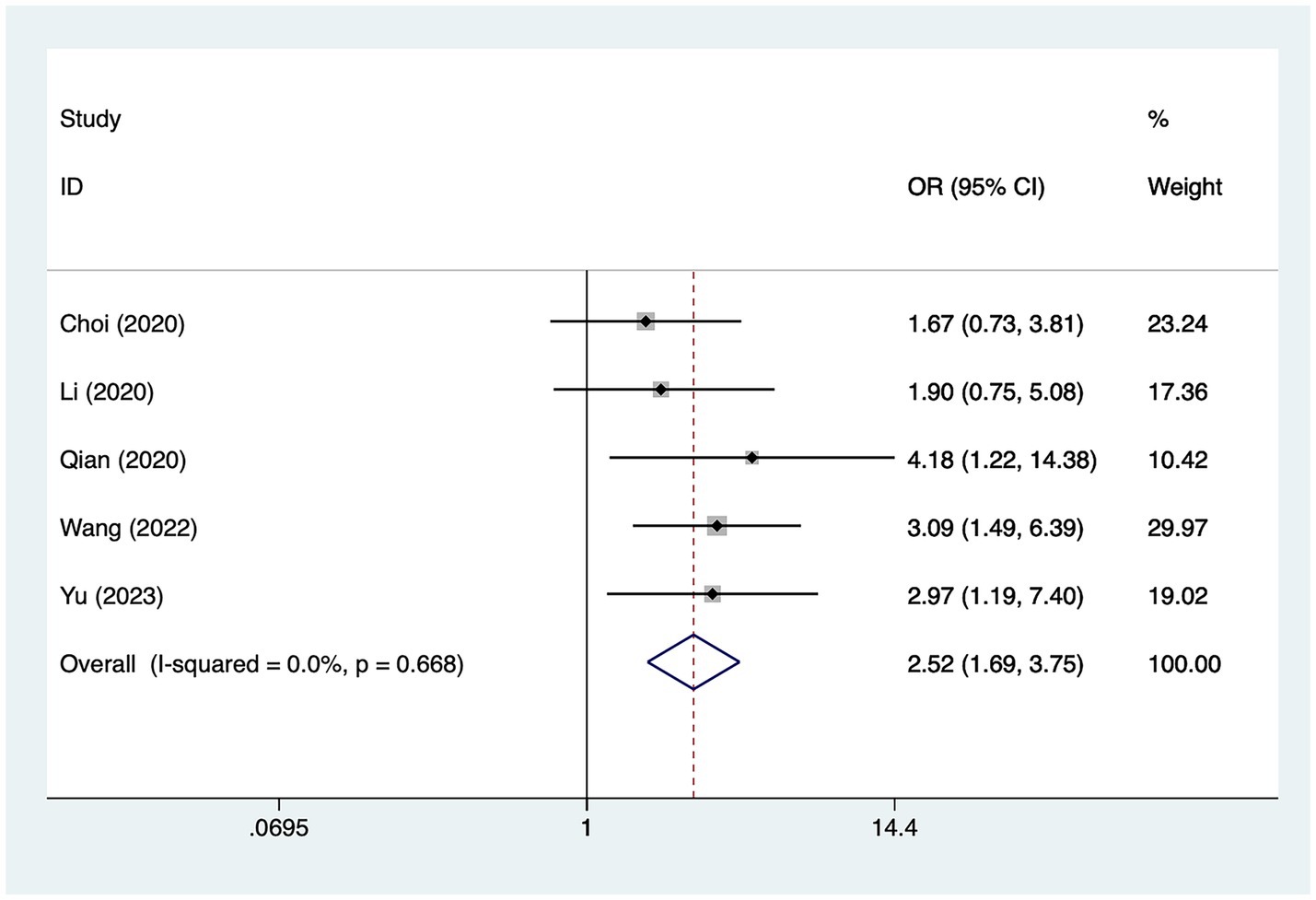
A total of five studies mentioned previous infarction, in which heterogeneity was tested (I2 = 0.0%, p = 0.668) and therefore analyzed using a fixed-effects model, and the results of the analysis (Figure 5) suggested that previous infarction was a risk factor for the development of cerebral infarction perioperative moyamoya disease [OR = 2.52, 95% CI (1.69, 3.75), p = 0.01].
Female
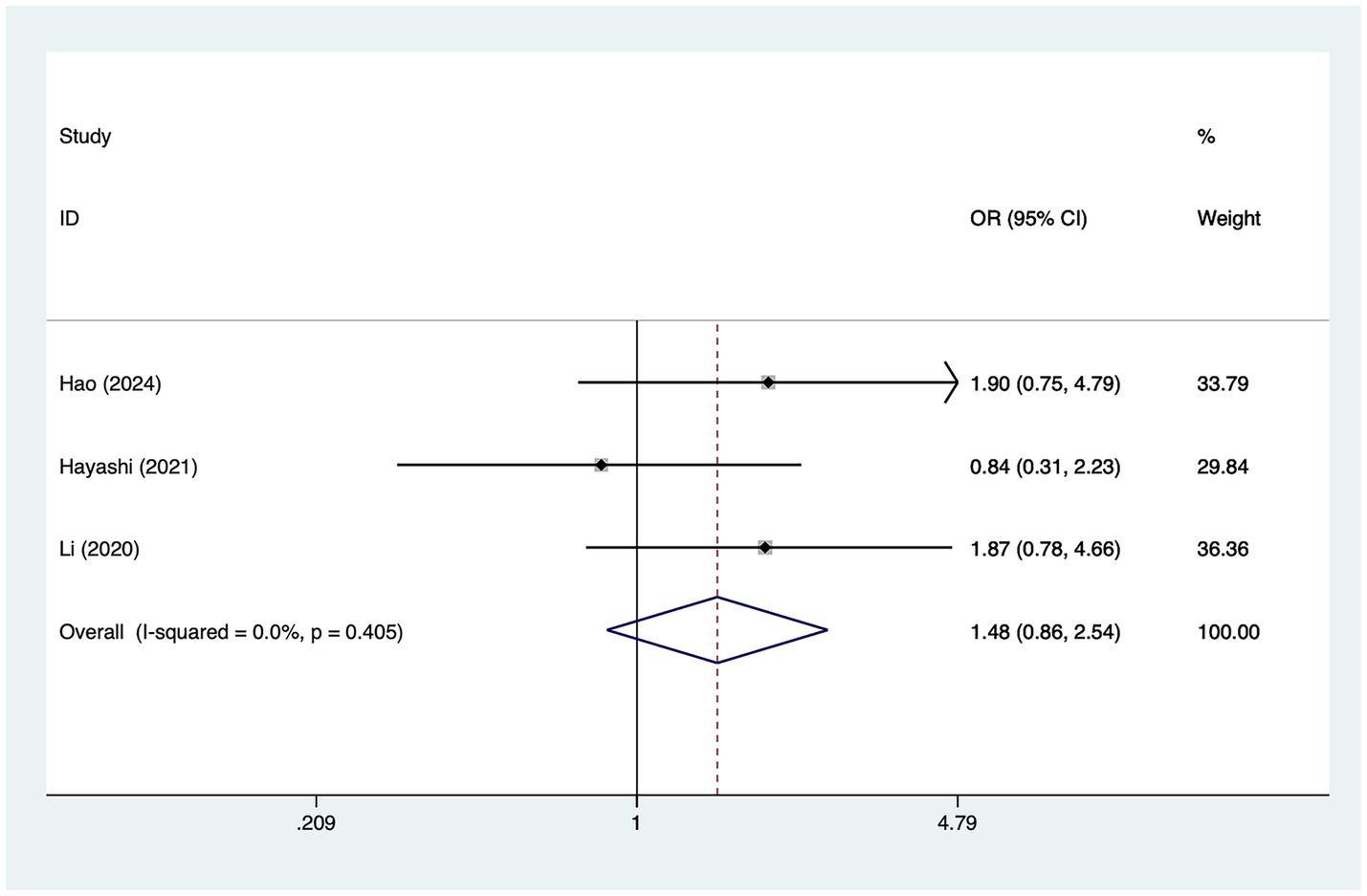
A total of three studies mentioned female, in which heterogeneity was tested (I2 = 0.0%, p = 0.405) and therefore analyzed using a fixed-effects model, and the results of the analysis (Figure 6) suggested that female was not a risk factor for the development of cerebral infarction perioperative moyamoya disease [OR = 1.48, 95% CI (0.86, 2.54), p = 0.53].
Hypertension
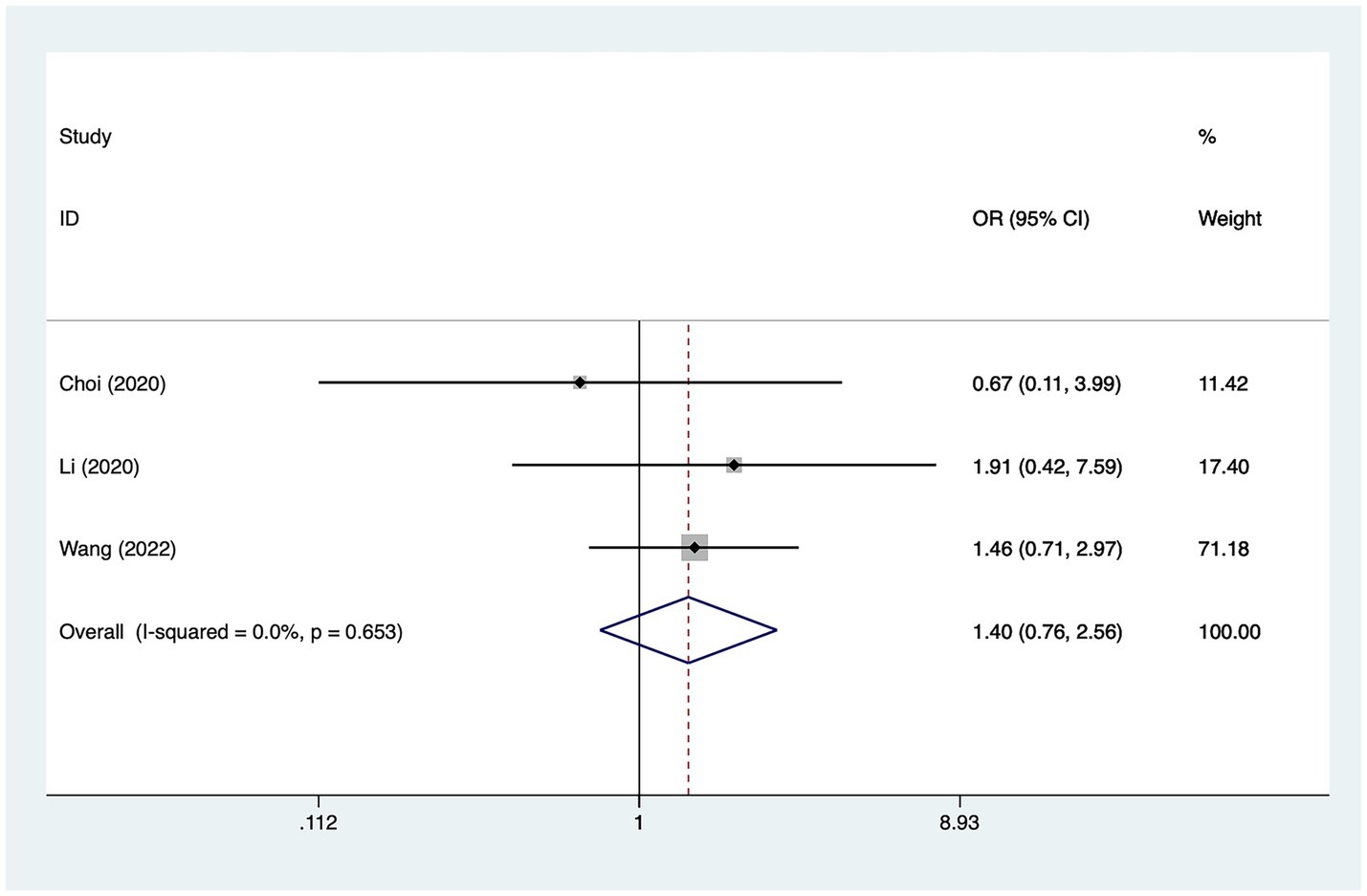
A total of three studies mentioned hypertension, in which heterogeneity was tested (I2 = 0.0%, p = 0.653) and therefore analyzed using a fixed-effects model, and the results of the analysis (Figure 7) suggested that hypertension was not a risk factor for the development of cerebral infarction perioperative moyamoya disease [OR = 1.40, 95% CI (0.76, 2.56), p = 0.12].
Publication bias
The included studies were analyzed for publication bias using Egger’s test, which suggested the possibility of publication bias for age (p = 0.51), posterior cerebral artery involvement (p = 0.13), preoperative magnetic resonance angiography (p = 0.45), previous infarction (p = 0.24), gender (p = 0.06), hypertension (p = 0.07), previous infarction(p = 0.24), female(p = 0.06), hypertension(p = 0.07) were less likely to have publication bias.
Discussion
MMD blood flow reconstruction surgery is difficult to operate and has a high risk of bleeding; surgical stress, depth of anesthesia, operator proficiency, degree of refinement, and the physical quality of the patient may all have an impact on blood pressure (26, 27); in addition, arteries need to be blocked in the course of the surgery, which further reduces the cerebral perfusion; and it is difficult to control the fluctuation of the patient’s blood pressure in the course of the operation, making it difficult to ensure a stable perfusion of the brain tissue (28), and the patient’s incidence of cerebral infarction in the perioperative period is risk is significantly elevated (29, 30).
This study is the first to explore the risk factors for cerebral infarction after MMD, and it was found that MMD patients with posterior cerebral artery involvement are more likely to have cerebral infarction. First, stenosis or occlusion of the posterior cerebral artery occurs in the late Suzuki stage, and patients with this late stage have more severe intracranial stenosis, poorer cerebral perfusion, and more unstable hemodynamics (31, 32). Secondly, stenosis or occlusion of posterior cerebral artery involvement affects the compensatory capacity of collateral circulation in MMD patients, which is an important factor in maintaining cerebral perfusion in MMD patients, and furthermore, stenosis or occlusion of posterior cerebral artery involvement increases the risk of white blood clots (33). Furthermore, the relationship between posterior cerebral artery involvement stenosis or occlusion and revascularization is not only that posterior cerebral artery involvement stenosis leads to an increased risk of cerebral infarction in patients, but also that revascularization in patients with MMD may cause posterior cerebral artery involvement stenosis (34), therefore, for the population with posterior cerebral artery involvement, the progress of the disease should be closely monitored in clinical practice, and surgical intervention should be performed as early as possible to prevent the occurrence of cerebral infarction.
The study found that preoperative infarction is a risk factor for perioperative infarction in patients with MMD. Zhao et al. (35) proved that patients with preoperative ischemic manifestations had a significantly higher risk of perioperative ischemic complications through a study of 500 patients with MMD; Muraoka et al. (13) also proved that patients with preoperative cerebral infarction are more likely to have perioperative cerebral infarction than other, in fact, patients with preoperative ischemic attacks or cerebral infarction often had insufficient compensation of the side branches and extremely unstable cerebral hemodynamics, and mechanical damage to the compensated side branches during the surgical procedure and the sudden change of cerebral hemodynamics after revascularization were more likely to induce perioperative cerebral infarction (2, 36). In addition, transient ischemic attack can not only increase the occurrence of intravascular micro thrombosis but also increase the activity of serum inflammatory cytokines as well as related proteases, which further aggravates the occurrence of cerebral infarction (37), for MMD patients with preoperative infarction, life and exercise therapy should be carried out as early as possible to prevent postoperative cerebral infarction (38).
The current study found that higher Preoperative MRA grading was a risk factor for perioperative cerebral infarction in patients with MMD. The higher MRA stage on the non-operative side implies that the stenosis and occlusion of blood vessels on the non-operative side are more severe, and the ability of the non-operative side to compensate to the operative side is reduced, which is prone to cause perioperative cerebral infarction (39). Zhao et al. (35) showed that advanced MRA stage is a risk factor for ischemic complications in adult patients, therefore, for patients with higher grade of MRA before operation, early intervention and physical intervention should be performed to prevent the occurrence of cerebral infarction after operation (40).
The study still has the following limitations: first, fewer studies were included and the cut included studies were from Asia, which may affect the extrapolation of our conclusions; second, a part of the study population belonged to children and a part of the study population belonged to adults, which may be the source of our heterogeneity; third, some of the studies had a greater degree of heterogeneity but the test for heterogeneity was not able to find out the source of the heterogeneity.
Conclusion
The present study demonstrated that posterior cerebral artery involvement, higher preoperative magnetic resonance angiography grading, and previous infarction are risk factors for the development of cerebral infarction perioperative moyamoya disease. Therefore, people with a combination of these risk factors should be intervened in advance to prevent perioperative cerebral infarction. However, since study limitations exist, In the future, we hope to have more studies with different regions, multi-centers and large samples to support our views.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding authors.
Author contributions
JW: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. SL: Conceptualization, Formal analysis, Investigation, Project administration, Resources, Software, Validation, Writing – original draft. RL: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Resources, Supervision, Visualization, Writing – original draft. YW: Conceptualization, Formal analysis, Funding acquisition, Investigation, Methodology, Supervision, Validation, Writing – original draft. FS: Conceptualization, Data curation, Investigation, Project administration, Validation, Writing – original draft. XP: Conceptualization, Funding acquisition, Investigation, Methodology, Supervision, Validation, Writing – original draft. XC: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Resources, Validation, Writing – original draft.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Acknowledgments
We would like to thank the researchers and study participants for their contributions.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fneur.2025.1530137/full#supplementary-material
References
1. Asselman, C, Hemelsoet, D, Eggermont, D, Dermaut, B, and Impens, F. Moyamoya disease emerging as an immune-related angiopathy. Trends Mol Med. (2022) 28:939–50. doi: 10.1016/j.molmed.2022.08.009
2. Ihara, M, Yamamoto, Y, Hattori, Y, Liu, W, Kobayashi, H, Ishiyama, H, et al. Moyamoya disease: diagnosis and interventions. Lancet Neurol. (2022) 21:747–58. doi: 10.1016/s1474-4422(22)00165-x
3. Zhang, X, Xiao, W, Zhang, Q, Xia, D, Gao, P, Su, J, et al. Progression in Moyamoya disease: clinical features, neuroimaging evaluation, and treatment. Curr Neuropharmacol. (2022) 20:292–308. doi: 10.2174/1570159x19666210716114016
4. Chiang, CC, Shahid, AH, Harriott, AM, Tietjen, GE, Savastano, LE, Klaas, JP, et al. Evaluation and treatment of headache associated with moyamoya disease - a narrative review. Cephalalgia. (2022) 42:542–52. doi: 10.1177/03331024211056250
5. Zhang, G, Liu, E, Tan, X, Liu, C, and Yang, S. Research progress on moyamoya disease combined with thyroid diseases. Front Endocrinol (Lausanne). (2023) 14:1233567. doi: 10.3389/fendo.2023.1233567
6. Starke, RM, Komotar, RJ, and Connolly, ES. Optimal surgical treatment for moyamoya disease in adults: direct versus indirect bypass. Neurosurg Focus. (2009) 26:E8. doi: 10.3171/2009.01.Focus08309
7. Moussouttas, M, and Rybinnik, I. A critical appraisal of bypass surgery in moyamoya disease. Ther Adv Neurol Disord. (2020) 13:1756286420921092. doi: 10.1177/1756286420921092
8. Reynolds, MR, Derdeyn, CP, Grubb, RL Jr, Powers, WJ, and Zipfel, GJ. Extracranial-intracranial bypass for ischemic cerebrovascular disease: what have we learned from the carotid occlusion surgery study? Neurosurg Focus. (2014) 36:E9. doi: 10.3171/2013.10.Focus13427
9. Kawaguchi, S, Okuno, S, and Sakaki, T. Effect of direct arterial bypass on the prevention of future stroke in patients with the hemorrhagic variety of moyamoya disease. J Neurosurg. (2000) 93:397–401. doi: 10.3171/jns.2000.93.3.0397
10. Miyamoto, S, Yoshimoto, T, Hashimoto, N, Okada, Y, Tsuji, I, Tominaga, T, et al. Effects of extracranial-intracranial bypass for patients with hemorrhagic moyamoya disease: results of the Japan adult Moyamoya trial. Stroke. (2014) 45:1415–21. doi: 10.1161/strokeaha.113.004386
11. Sato, S, Kojima, D, Shimada, Y, Yoshida, J, Fujimato, K, Fujiwara, S, et al. Preoperatively reduced cerebrovascular contractile reactivity to hypocapnia by hyperventilation is associated with cerebral hyperperfusion syndrome after arterial bypass surgery for adult patients with cerebral misery perfusion due to ischemic moyamoya disease. J Cereb Blood Flow Metab. (2018) 38:1021–31. doi: 10.1177/0271678x18757621
12. Tian, B, Xu, B, Liu, Q, Hao, Q, and Lu, J. Adult Moyamoya disease: 320-multidetector row CT for evaluation of revascularization in STA-MCA bypasses surgery. Eur J Radiol. (2013) 82:2342–7. doi: 10.1016/j.ejrad.2013.09.006
13. Muraoka, S, Araki, Y, Kondo, G, Kurimoto, M, Shiba, Y, Uda, K, et al. Postoperative cerebral infarction risk factors and postoperative Management of Pediatric Patients with Moyamoya disease. World Neurosurg. (2018) 113:e190–9. doi: 10.1016/j.wneu.2018.01.212
14. Park, W, Ahn, JS, Lee, HS, Park, JC, and Kwun, BD. Risk factors for newly developed cerebral infarction after surgical revascularization for adults with Moyamoya disease. World Neurosurg. (2016) 92:65–73. doi: 10.1016/j.wneu.2016.03.053
15. Lai, PMR, Gomez-Paz, S, Patel, NJ, Frerichs, KU, Thomas, AJ, Aziz-Sultan, MA, et al. Asymptomatic Moyamoya disease in a north American adult cohort. World Neurosurg. (2022) 161:e146–53. doi: 10.1016/j.wneu.2022.01.076
16. Stang, A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. (2010) 25:603–5. doi: 10.1007/s10654-010-9491-z
17. Araki, Y, Uda, K, Yokoyama, K, Kanamori, F, Kurimoto, M, Shiba, Y, et al. Risk factors for cerebral infarction early after revascularization in children younger than 5 years with Moyamoya disease. World Neurosurg. (2022) 160:e220–6. doi: 10.1016/j.wneu.2021.12.115
18. Choi, JW, Chong, S, Phi, JH, Lee, JY, Kim, HS, Chae, JH, et al. Postoperative symptomatic cerebral infarction in pediatric Moyamoya disease: risk factors and clinical outcome. World Neurosurg. (2020) 136:e158–64. doi: 10.1016/j.wneu.2019.12.072
19. Guo, Q, Pei, S, Wang, QN, Li, J, Han, C, Liu, S, et al. Risk factors for preoperative cerebral infarction in infants with Moyamoya disease. Transl Stroke Res. (2023) 15:795–804. doi: 10.1007/s12975-023-01167-z
20. Hao, FB, Gao, G, Guo, QB, Liu, S, Wang, M, Chang, Z, et al. Risk factors for massive cerebral infarction in pediatric patients with Moyamoya disease. Pediatr Neurol. (2024) 153:159–65. doi: 10.1016/j.pediatrneurol.2024.01.001
21. Hayashi, T, Kimiwada, T, Karibe, H, Shirane, R, Sasaki, T, Metoki, H, et al. Preoperative risks of cerebral infarction in pediatric Moyamoya disease. Stroke. (2021) 52:2302–10. doi: 10.1161/strokeaha.120.032699
22. Li, JX, Zhao, YH, Zhao, M, Cao, P, Liu, X, Ren, H, et al. High variance of intraoperative blood pressure predicts early cerebral infarction after revascularization surgery in patients with Moyamoya disease. Neurosurg Rev. (2020) 43:759–69. doi: 10.1007/s10143-019-01118-z
23. Qian, Y, Huang, B, Hu, ZM, Wang, J, Zhao, P, and Li, XG. Analysis of factors related to cerebral infarction after direct bypass surgery in adults with Moyamoya disease. Cerebrovasc Dis. (2020) 49:55–61. doi: 10.1159/000504743
24. Wang, JX, Jiang, HQ, Tang, JW, Lin, C, Ni, W, and Gu, YX. Postoperative cerebral infarction after revascularization in patients with moyamoya disease: incidence and risk factors. Front Neurol. (2022) 13:131053193. doi: 10.3389/fneur.2022.1053193
25. Yu, T, Ye, X, Zeng, C, Chen, X, and Zhao, Y. Angioarchitectural risk factors associated with postoperative cerebral infarction in patients of ischemic moyamoya disease. J Neurosurg. (2023) 39:451–5. doi: 10.3760/cma.j.cn112050-20220910-00437
26. Azadgoli, B, Leland, HA, Wolfswinkel, EM, Bakhsheshian, J, Russin, JJ, and Carey, JN. Combined direct and indirect cerebral revascularization using local and flow-through flaps. J Reconstr Microsurg. (2018) 34:103–7. doi: 10.1055/s-0037-1606552
27. Egashira, Y, Yoshimura, S, Enomoto, Y, Nakayama, N, and Iwama, T. Single-stage direct revascularization for bilateral anterior cerebral artery regions in pediatric Moyamoya disease: a technical note. World Neurosurg. (2018) 118:324–8. doi: 10.1016/j.wneu.2018.07.081
28. Guo, Z, Yan, Z, Qu, F, Cheng, D, Wang, C, and Feng, Y. The value of indocyanine green-FLOW800 in microvasculature for predicting cerebral hyperperfusion syndrome in moyamoya disease patients. Sci Rep. (2023) 13:18352. doi: 10.1038/s41598-023-45676-1
29. Lu, L, Huang, Y, Han, Y, Li, Y, Wan, X, Chen, J, et al. Clinical effect of a modified superficial temporal artery-middle cerebral artery bypass surgery in Moyamoya disease treatment. Front Neurol. (2023) 14:1273822. doi: 10.3389/fneur.2023.1273822
30. Rennert, RC, Ravina, K, Strickland, BA, Bakhsheshian, J, Carey, J, and Russin, JJ. Radial artery fascial flow-through free flap for complex cerebral revascularization: technical notes and long-term neurologic and radiographic outcomes. Oper Neurosurg (Hagerstown). (2019) 16:424–34. doi: 10.1093/ons/opy124
31. Ozaki, S, Inoue, A, Miyazaki, H, Onoue, S, Ichikawa, H, Fukumoto, S, et al. Long-term outcome over five years after surgical revascularization in adult Moyamoya disease. No Shinkei Geka. (2016) 44:823–34. doi: 10.11477/mf.1436203386
32. Ren, B, Zhang, ZS, Liu, WW, Bao, XY, Li, DS, Han, C, et al. Surgical outcomes following encephaloduroarteriosynangiosis in adult moyamoya disease associated with type 2 diabetes. J Neurosurg. (2016) 125:308–14. doi: 10.3171/2015.7.Jns15218
33. Kim, YS, Kim, TS, Yang, IC, and Joo, SP. Staged, combined Management of Ruptured Vertebral Artery Dissecting Aneurysms Involving the posterior inferior cerebellar artery: report of 4 cases and review of the literature. World Neurosurg. (2019) 128:444–7. doi: 10.1016/j.wneu.2019.05.146
34. Tsutsui, T, Ikedo, T, Kitazawa, Y, Otsuka, R, Nishiwaki, T, Kushi, Y, et al. Impact of morphological factors on the future growth of Unruptured posterior communicating artery aneurysms. World Neurosurg. (2023) 175:e897–903. doi: 10.1016/j.wneu.2023.04.039
35. Zhao, M, Deng, X, Zhang, D, Wang, S, Zhang, Y, Wang, R, et al. Risk factors for and outcomes of postoperative complications in adult patients with moyamoya disease. J Neurosurg. (2018) 130:531–42. doi: 10.3171/2017.10.Jns171749
36. Gonzalez, NR, Amin-Hanjani, S, Bang, OY, Coffey, C, du, R, Fierstra, J, et al. Adult Moyamoya disease and syndrome: current perspectives and future directions: a scientific statement from the American Heart Association/American Stroke Association. Stroke. (2023) 54:e465–79. doi: 10.1161/str.0000000000000443
37. Kazumata, K, Ito, M, Tokairin, K, Ito, Y, Houkin, K, Nakayama, N, et al. The frequency of postoperative stroke in moyamoya disease following combined revascularization: a single-university series and systematic review. J Neurosurg. (2014) 121:432–40. doi: 10.3171/2014.1.Jns13946
38. Zhu, Z, Zhang, Z, and Li, Q. Successful perioperative management of a patient undergoing laparoscopic colon surgery with high-risk of cerebral ischemia due to moyamoya disease. Asian J Surg. (2024) 47:2928–9. doi: 10.1016/j.asjsur.2024.02.101
39. Parray, T, Martin, TW, and Siddiqui, S. Moyamoya disease: a review of the disease and anesthetic management. J Neurosurg Anesthesiol. (2011) 23:100–9. doi: 10.1097/ANA.0b013e3181f84fac
Keywords: risk factors, cerebral infarction, meta-analysis, moyamoya disease, perioperative
Citation: Wu J, Li S, Liang R, Wang Y, Shi F, Pan X and Chen X (2025) Risk factors for perioperative cerebral infarction in moyamoya disease: a meta-analysis. Front. Neurol. 16:1530137. doi: 10.3389/fneur.2025.1530137
Edited by:
Zhaolan Hu, Central South University, ChinaReviewed by:
Shihao He, Peking Union Medical College Hospital (CAMS), ChinaZhihang Tang, Guangzhou University of Chinese Medicine, China
Copyright © 2025 Wu, Li, Liang, Wang, Shi, Pan and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jincan Wu, MjQ1MTg0MTQ5N0BxcS5jb20=; Shiju Li, MTQxNzE2NzA4NkBxcS5jb20=
†These authors have contributed equally to this work
 Jincan Wu
Jincan Wu Shiju Li2*†
Shiju Li2*†