
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
BRIEF RESEARCH REPORT article
Front. Neurol., 25 February 2025
Sec. Neuromuscular Disorders and Peripheral Neuropathies
Volume 16 - 2025 | https://doi.org/10.3389/fneur.2025.1526453
 Nathan W. Fielder1
Nathan W. Fielder1 Michael Frazure2
Michael Frazure2 Ivan Poliacek3,4
Ivan Poliacek3,4 Donald C. Bolser3
Donald C. Bolser3 Kimberly E. Iceman5*
Kimberly E. Iceman5* Teresa Pitts5
Teresa Pitts5Baclofen is a GABAB receptor agonist used clinically to manage spasticity. It has also been associated with increased duration of mechanical ventilation and rates of aspiration pneumonitis. We hypothesized that baclofen would impair pharyngeal swallow, a vital airway protective reflex. Electromyography (EMG) activity was recorded in four spontaneously breathing, sodium pentobarbital-anesthetized cats. Swallow was stimulated by oral water infusion before and after administration of 3 μg/kg and 10 μg/kg (±)baclofen doses. Swallow-related thyrohyoid EMG amplitude increased after 3 μg/kg and 10 μg/kg (±)baclofen, while parasternal EMG amplitude decreased after 3 μg/kg (±)baclofen. Geniohyoid, thyroarytenoid, and thyropharyngeus EMG amplitudes increased on average, but did not reach significance. Clinically, increased thyrohyoid activation may extend duration of laryngeal abduction. Decreased parasternal activation could impair development of the negative intrathoracic pressure that aids bolus propulsion during swallow. These changes may reflect increased risk of aspiration, and more work is needed to study the effects of baclofen on airway protection.
Swallow is a complex and highly coordinated behavior consisting of oral, pharyngeal, and esophageal phases (1). A bolus passes through the oral cavity, reaching the oropharynx and stimulating swallow initiation. Airway closure and concomitant contraction of pharyngeal constrictor muscles transports the bolus into the esophagus, aided by inspiratory muscle activity (diaphragm and parasternal) which produces negative intrathoracic pressure during swallow (called “Schluckatmung”) (2, 3). The esophagus then directs the bolus into the stomach. The circuits that orchestrate motor control of the numerous muscles required for this behavior consist of various brainstem nuclei (2, 4). A web of brainstem premotor nuclei communicates to multiple motor nuclei in the brainstem and spinal cord and their corresponding motor units via trigeminal, hypoglossal, vagal, phrenic, and other nerves. The pharyngeal phase of swallow is noteworthy because it centers on the intersection the of airway and digestive tracts at the level of the larynx. This necessitates intricate coordination between swallow and breathing (5). Without proper coordination, dysphagia and aspiration can occur, increasing infection risk and decreasing quality of life and health outcomes (6).
Baclofen, a GABAB agonist with a poorly understood mechanism of action, has a wide range of uses as an antispastic medication: clonus, flexor spasms, sequalae of spinal cord injury, cough, and alcohol withdrawal syndrome (7). It has also been used clinically as an antitussive agent because of its antispastic properties (8), and has been demonstrated to alter muscle activity during cough in cats (9). Straus et al. (10) speculate that overall, baclofen worsened breathing instability in humans by increasing variability of ventilatory periods and tidal volume. Baclofen dose is correlated with aspiration pneumonitis and both the length of mechanical ventilation and the length of hospital stay (11), even in patients who did not undergo surgery. Baclofen may also modulate the threshold for bronchoconstriction (12). There are few studies on the effects of baclofen on swallow, particularly the pharyngeal motor components, thus we recorded activity of swallow-related pharyngeal muscles to test the hypothesis that baclofen modulates swallow.
Experiments were performed on 4 spontaneously breathing adult male cats (5.1 ± 0.6 kg). The protocol was approved by both the University of Florida and University of Louisville Institutional Animal Care and Use Committees (IACUC). The animals were initially anesthetized with sodium pentobarbital (Lundbeck, Inc., Deerfield, IL) (35 mg/kg i.v.); supplementary doses were given as needed (1–3 mg/kg i.v.). The right femoral artery and vein were cannulated to monitor blood pressure and administer intravenous fluids, respectively, and a tracheostomy was performed. Physiologic levels of end-tidal CO2 (4–4.5%; Datax Engstrom; Datax Ohmeda, Inc.; Madison, WI), body temperature (Homeothermic Blanket Control Unit; Harvard Apparatus; Holliston, MA), and arterial blood gas composition (i-STAT1; Abaxis; Union City, CA) were monitored and maintained (13).
Electromyograms (EMGs) were recorded using bipolar insulated fine wire electrodes (A-M Systems stainless steel Teflon coated 0.003″, bare 0.0055″, half hard), with two independent wires inserted in into each muscle for differential recordings, using the technique of Basmajian and Stecko (14). Five muscles were used to evaluate swallow motor pattern: mylohyoid, geniohyoid, thyrohyoid, thyroarytenoid, and thyropharyngeus; the parasternal muscle evaluated breathing. Both EMG placement and detection of swallow were conducted as previously described in detail (13). Swallow was induced by introducing 3ccs of water into the oropharynx via a 1-inch long, thin polyethylene catheter (outer diameter 0.5–1.0 mm) attached to a syringe.
“Spike2” was used to record and analyze EMG activity (Cambridge Electronic Design, United Kingdom). EMGs were rectified and moving averages (time constant 20 ms) obtained. Durations were measured between the swallow onset and the point where the signal returned to baseline (ms). EMG amplitude measures were normalized to the maximum amplitude that each muscle displayed during swallow and are presented as percent of maximum (i.e., maximum amplitude is 100%).
There were 3 conditions for all animals: the control condition with no baclofen, 3 μg/kg IA, and 10 μg/kg IA (+7 μg/kg for cumulative dosing) (±)baclofen. Doses were separated by 10 min to allow for distribution of the drug and completion of all stimulus trials.
Means ± standard deviations were calculated and averaged for each condition across animals. Statistical differences were assessed with ANOVA (one-way repeated measures) and Tukey’s multiple comparison tests. Swallow number per trial and swallow-breathing coordination were assessed with Wilcoxon tests. An assigned coding system was used for the breathing phase in which the swallow occurred: inspiration (I; start to peak parasternal activity) as “1”; early expiration (E1; peak to end parasternal activity) as “2”; and late expiration (E2; end of parasternal activity to start of next breath parasternal activity) as “3.” For all tests, p < 0.05 was considered significant.
Swallow was reliably elicited during the entire protocol in all animals. Figure 1 illustrates the effect of (±)baclofen on EMG amplitudes of swallow-related muscles. It significantly increased the maximum EMG amplitude of the thyrohyoid (p = 0.004) with 3 μg/kg (p = 0.007) and 10 μg/kg (p = 0.04) doses compared to control, and decreased parasternal EMG amplitude with the 3 μg/kg dose (p = 0.009) compared to control. Results are reported in Table 1. Geniohyoid, thyroarytenoid, and thyropharyngeus EMG amplitudes increased on average, but did not reach significance. Following baclofen administration, there were no significant changes in muscle EMG durations, swallow duration, nor swallow number per stimulation trial. A Wilcoxon test showed no difference in swallow-breathing coordination between control and 3 μg/kg (Z = −0.17, p = 0.9) or 10 μg/kg (Z = −1.16, p = 0.25) (±)baclofen conditions.
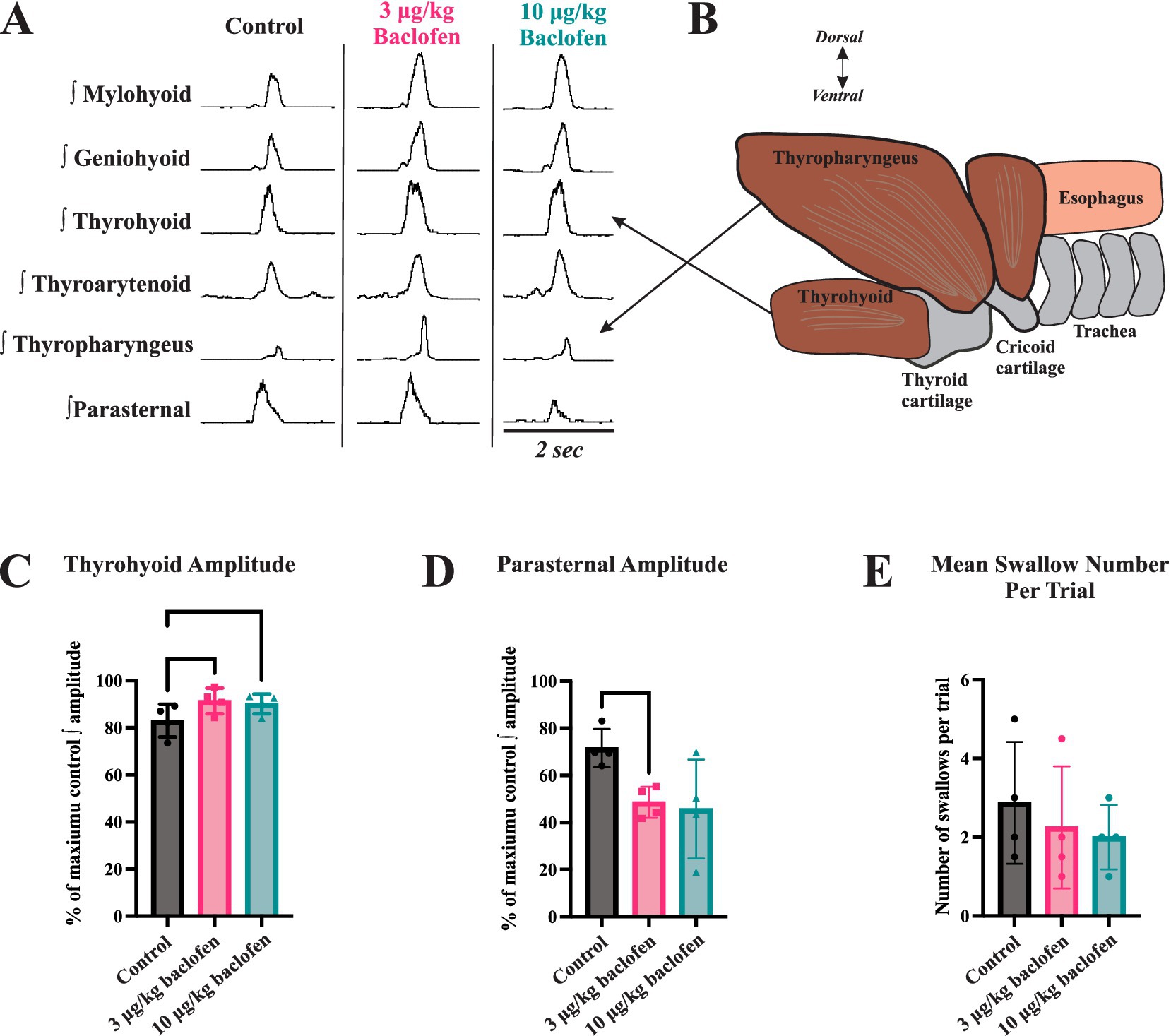
Figure 1. The effects of baclofen on swallow-related muscle activity. (A) Representative traces of muscle electromyography (EMG) recordings during swallow before (control) and after administration of 3 μg/kg and 10 μg/kg doses of (+)baclofen (signals have been rectified and integrated). (B) The thyrohyoid muscle attaches to the hyoid bone superiorly and thyroid cartilage inferiorly. Thyrohyoid contraction elevates the larynx and aids in closure of the epiglottis over the airway. (C) Thyrohyoid EMG amplitude increased following administration of 3 μg/kg and 10 μg/kg (±)baclofen. (D) Parasternal EMG amplitude decreased following administration of 3 μg/kg (±)baclofen. EMG amplitudes were normalized to maximum for statistical comparisons. (E) Swallow number per stimulus trial showed a decreasing trend with baclofen, but this was not significant.
Our results demonstrate that swallow motor patterns are altered following baclofen administration. To our knowledge, our study is the first to evaluate effects of baclofen on the motor pattern and durations of oropharyngeal muscles during swallow. Clinical literature demonstrates increased rates of aspiration pneumonitis and extension of mechanical ventilation among patients on baclofen (10, 11). Baclofen-induced dysphagia may play a role in these cases, but the explicit mechanism is not well studied. Typically, the thyrohyoid muscle achieves an elevation of the larynx to close the epiglottis over the airway. Increased thyrohyoid activation may discoordinate the swallow pattern to allow pharyngeal contents to pass into the larynx.
We previously reported that baclofen decreased the rectus abdominis EMG amplitude during coughing, the inspiratory and active expiratory (E1) phases of cough, and cough number per stimulus. Baclofen did not affect EMG amplitudes of laryngeal muscles or parasternal muscles, nor the duration of the passive expiratory (E2) phase during cough. This suggested that laryngeal and inspiratory motor drives during cough are controlled differentially. The current results confirm this, based on the fact that thyrohyoid amplitude increased while parasternal amplitude decreased. This is relevant for spinal cord injury wherein spinal tracts would be disrupted, while cranial pathways left intact. Collectively, these results provide evidence for a control system that regulates laryngeal activity, inspiratory spinal drive, and expiratory spinal motoneurons separately. It is possible that the increase in upper airway muscle activity during swallow that we observed in this study is a secondary consequence of reduced activity of inspiratory muscles, rather than a result of direct action on GABA receptors. The influence of baclofen on cough has also been studied in spinal cord injury (15); subjects treated with baclofen had a significantly higher cough threshold (diminished cough reflex sensitivity) than control subjects.
Baclofen exerts different effects on components of breathing drive, and this can differ by species [discussed in Straus et al. (10)]. Its overall effect in many mammal species seems to be ventilatory depression. However, baclofen has been shown to have excitatory (increased diaphragm discharge) or disinhibitory (lung inflation reflex) effects in rats (16, 17). In cat, small doses of baclofen can increase phrenic nerve discharge and tidal volume (18). Depending on the dose, baclofen can have inhibitory or excitatory effect in motoneurons (19), and does not appear to exert its antispastic action postsynaptically at a clinical dose. While GABA signaling is known to be important in central control of breathing, the central respiratory effects of baclofen have not been well studied in mammals. The site(s) of action of baclofen on breathing and in the current study are unknown. However, Straus et al. (10) concluded that baclofen interfered with central respiratory patterning in their human study, in which it worsened breathing instability.
A small number of other studies have investigated the effects of baclofen on swallow, primarily the esophageal phase. One study reported the effects of baclofen and another GABAB agonist on transient lower esophageal sphincter relaxations and swallow in conscious dogs (20). Both compounds inhibited transient lower esophageal sphincter relaxations and spontaneous swallow. When swallowing was stimulated with oral water injection, both compounds also inhibited primary peristalsis. The authors concluded that these agents acted by inhibiting the swallow central pattern generator, but this could also be related to a more direct effect on esophageal autonomic circuits. Hung and colleagues (21) posit that baclofen acts to block sensory inputs to the central swallow pattern generator without altering the pharyngeal swallow motor pattern. They conducted a manometry study in healthy humans who received oral baclofen or placebo and concluded that while baclofen has little to no effect on volitional swallow, it may impair upper esophageal sphincter contractility, and impair pharyngeal swallow by reducing the effective swallowing of large volumes (piecemeal swallow). However, in another study in cats, baclofen selectively blocked the reflex responses to rapid esophageal distension and reduced water-evoked pharyngeal swallow frequency (22). We did not observe any changes in swallow number or duration or swallow-breathing coordination in the current study.
The general GABA mimetic muscimol and the GABAA agonist diazepam both inhibit electrically-stimulated swallow in cat (23), and GABAA activity is thought to mediate both pharyngeal and esophageal deglutitory inhibition (24). In a study in anesthetized rats, swallows were evoked by pharyngeal distension, punctate mechanical stimulation, capsaicin, distilled water applied topically to the vocal folds, or by electrical stimulation of the superior laryngeal nerve (25). Infusion of either baclofen or diazepam inhibited swallows evoked by mechanical, chemical, and electrical stimulation, and these effects were reversed by the respective antagonists. However, there was no significant effect of baclofen on EMG burst durations of suprahyoid and thyrohyoid muscles; burst amplitude was not reported. Collectively, these data indicate that diazepam and baclofen both centrally inhibit components of swallow.
The GABAB agonist baclofen enhanced thyrohyoid activation during swallow, and decreased parasternal activation. Along with our previous study of the effects of baclofen on cough, our results support the conjecture that laryngeal activity, inspiratory spinal drive, and expiratory spinal motoneurons are controlled separately. It is possible that the divergence reveals that baclofen’s greatest effects are on spinal motoneurons. In spinal cord injury, spinal tracts are disrupted, while cranial pathways are left intact, and cervical spinal cord injury produces laryngeal dysregulation and dysphagia, but the underlying mechanisms are unknown (26). Patients with spinal cord injuries are commonly treated with baclofen to reduce spasticity; however, it decreases cough reflex sensitivity (15), and can inhibit swallow even in healthy cats (23) and rats (23, 25). Future studies may investigate GABAB brainstem receptors, various animal models, or patients experiencing baclofen-induced dysphagia or dystussia. Findings of disordered activation during airway protective behaviors may play a clinical role in screening/therapy for dysphagic complications and invite future studies.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The animal study was approved by the University of Florida Institutional Animal Care and Use Committee and the University of Louisville Institutional Animal Care and Use Committee. The study was conducted in accordance with the local legislation and institutional requirements.
NF: Formal analysis, Writing – original draft, Writing – review & editing. MF: Formal analysis, Methodology, Supervision, Writing – original draft, Writing – review & editing. IP: Conceptualization, Investigation, Methodology, Writing – review & editing. DB: Conceptualization, Funding acquisition, Investigation, Methodology, Project administration, Resources, Supervision, Writing – review & editing. KI: Formal analysis, Methodology, Supervision, Writing – original draft, Writing – review & editing. TP: Conceptualization, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Supervision, Writing – review & editing.
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This work was supported by NIH grants NS110169, HL HL155721, HL163008. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
The author(s) declare that no Gen AI was used in the creation of this manuscript.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Miller, AJ. Neurophysiological basis of swallowing. Dysphagia. (1986) 1:91–100. doi: 10.1007/BF02407121
2. Pitts, T, and Iceman, KE. Deglutition and the regulation of the swallow motor pattern. Physiology (Bethesda). (2023) 38:10–24. doi: 10.1152/physiol.00005.2021
3. Marckwald, M. The movements of respiration: And their innervation in the rabbit. With a supplement on the relation of respiration to deglutition, and on the question of the existence of respiratory Centres in the spinal cord. London, UK: Blackie & Son (1888).
4. Bieger, D. Rhombencephalic pathways and neurotransmitters controlling deglutition. Am J Med. (2001) 111:85–9. doi: 10.1016/S0002-9343(01)00824-5
5. Martin-Harris, B, Brodsky, MB, Price, CC, Michel, Y, and Walters, B. Temporal coordination of pharyngeal and laryngeal dynamics with breathing during swallowing: single liquid swallows. J Appl Physiol. (2003) 94:1735–43. doi: 10.1152/japplphysiol.00806.2002
6. Ekberg, O, Hamdy, S, Woisard, V, Wuttge-Hannig, A, and Ortega, P. Social and psychological burden of dysphagia: its impact on diagnosis and treatment. Dysphagia. (2002) 17:139–46. doi: 10.1007/s00455-001-0113-5
7. Kent, CN, Park, C, and Lindsley, CW. Classics in chemical neuroscience: baclofen. ACS Chem Neurosci. (2020) 11:1740–55. doi: 10.1021/acschemneuro.0c00254
8. Dicpinigaitis, PV, and Dobkin, JB. Antitussive effect of the GABA-agonist baclofen. Chest. (1997) 111:996–9. doi: 10.1378/chest.111.4.996
9. Castillo, D, and Pitts, T. Influence of baclofen on laryngeal and spinal motor drive during cough in the anesthetized cat. Laryngoscope. (2013) 123:3088–92. doi: 10.1002/lary.24143
10. Straus, C, Teulier, M, Morel, S, Wattiez, N, Hajage, D, Giboin, C, et al. Baclofen destabilises breathing during sleep in healthy humans: a randomised, controlled, double-blind crossover trial. Br J Clin Pharmacol. (2021) 87:1814–23. doi: 10.1111/bcp.14569
11. Pommier, P, Debaty, G, Bartoli, M, Viglino, D, Carpentier, F, Danel, V, et al. Severity of deliberate acute baclofen poisoning: a nonconcurrent cohort study. Basic Clin Pharmacol Toxicol. (2014) 114:360–4. doi: 10.1111/bcpt.12161
12. Dicpinigaitis, PV, Nierman, DM, and Miller, A. Baclofen-induced bronchoconstriction. Ann Pharmacother. (1993) 27:883–4. doi: 10.1177/106002809302700713
13. Pitts, T, Rose, MJ, Mortensen, AN, Poliacek, I, Sapienza, CM, Lindsey, BG, et al. Coordination of cough and swallow: a meta-behavioral response to aspiration. Respir Physiol Neurobiol. (2013) 189:543–51. doi: 10.1016/j.resp.2013.08.009
14. Basmajian, JV, and Stecko, G. A new bipolar electrode for electromyography. J Appl Physiol. (1962) 17:849. doi: 10.1152/jappl.1962.17.5.849
15. Dicpinigaitis, PV, Grimm, DR, and Lesser, M. Baclofen-induced cough suppression in cervical spinal cord injury. Arch Phys Med Rehabil. (2000) 81:921–3. doi: 10.1053/apmr.2000.5612
16. Seifert, E, and Trippenbach, T. Effects of baclofen on the Hering-Breuer inspiratory-inhibitory and deflation reflexes in rats. Am J Phys Regul Integr Comp Phys. (1998) 274:R462–9. doi: 10.1152/ajpregu.1998.274.2.R462
17. Trippenbach, T, and Lake, N. Excitatory cardiovascular and respiratory effects of baclofen in intact rats. Can J Physiol Pharmacol. (1994) 72:1200–7. doi: 10.1139/y94-170
18. Pierrefiche, O, Foutz, AS, and Denavit-Saubié, M. Effects of GABAB receptor agonists and antagonists on the bulbar respiratory network in cat. Brain Res. (1993) 605:77–84. doi: 10.1016/0006-8993(93)91358-Y
19. Li, Y, Li, X, Harvey, PJ, and Bennett, DJ. Effects of baclofen on spinal reflexes and persistent inward currents in motoneurons of chronic spinal rats with spasticity. J Neurophysiol. (2004) 92:2694–703. doi: 10.1152/jn.00164.2004
20. Lehmann, A, Bremner-Danielsen, M, Brändén, L, and Kärrberg, L. Inhibitory effects of GABA(B) receptor agonists on swallowing in the dog. Eur J Pharmacol. (2002) 448:67–70. doi: 10.1016/S0014-2999(02)01907-6
21. Hung, JS, Liang, SW, Omari, T, Wong, MW, Lei, WY, Yi, CH, et al. Effects of the GABA(B) agonist baclofen on volitional swallowing in normal subjects. Kaohsiung J Med Sci. (2023) 39:80–6. doi: 10.1002/kjm2.12607
22. Lang, IM, Medda, BK, and Shaker, R. Mechanisms of reflexes induced by esophageal distension. Am J Physiol Gastrointest Liver Physiol. (2001) 281:G1246–63. doi: 10.1152/ajpgi.2001.281.5.G1246
23. Hockman, CH, Weerasuriya, A, and Bieger, D. GABA receptor-mediated inhibition of reflex deglutition in the cat. Dysphagia. (1996) 11:209–15. doi: 10.1007/BF00366388
24. Wang, YT, and Bieger, D. Role of solitarial GABAergic mechanisms in control of swallowing. Am J Phys. (1991) 261:R639–46. doi: 10.1152/ajpregu.1991.261.3.R639
25. Tsujimura, T, Sakai, S, Suzuki, T, Ujihara, I, Tsuji, K, Magara, J, et al. Central inhibition of initiation of swallowing by systemic administration of diazepam and baclofen in anaesthetized rats. Am J Physiol Gastrointest Liver Physiol. (2017) 312:G498–507. doi: 10.1152/ajpgi.00299.2016
Keywords: swallow, baclofen, GABAB, laryngeal, diaphragm
Citation: Fielder NW, Frazure M, Poliacek I, Bolser DC, Iceman KE and Pitts T (2025) Effects of baclofen on swallow motor pattern. Front. Neurol. 16:1526453. doi: 10.3389/fneur.2025.1526453
Received: 11 November 2024; Accepted: 10 February 2025;
Published: 25 February 2025.
Edited by:
Giuseppe Piscosquito, Ospedali Riuniti San Giovanni di Dio e Ruggi d’Aragona, ItalyReviewed by:
Silvestro Roatta, University of Turin, ItalyCopyright © 2025 Fielder, Frazure, Poliacek, Bolser, Iceman and Pitts. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Kimberly E. Iceman, ay5pY2VtYW5AaGVhbHRoLm1pc3NvdXJpLmVkdQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.